Abstract
Background:
Physical exercise has been found to impact neurophysiological and structural aspects of the human brain. However, most research has used animal models, which yields much confusion regarding the real effects of exercise on the human brain, as well as the underlying mechanisms.
Objective:
To present an update on the impact of physical exercise on brain health; and to review and analyze the evidence exclusively from human randomized controlled studies from the last six years.
Methods:
A search of the literature search was conducted using the MEDLINE (via PubMed), EMBASE, Web of Science and PsycINFO databases for all randomized controlled trials published between January 2014 and January 2020.
Results:
Twenty-four human controlled trials that observed the relationship between exercise and structural or neurochemical changes were reviewed.
Conclusions:
Even though this review found that physical exercise improves brain plasticity in humans, particularly through changes in brain-derived neurotrophic factor (BDNF), functional connectivity, basal ganglia and the hippocampus, many unanswered questions remain. Given the recent advances on this subject and its therapeutic potential for the general population, it is hoped that this review and future research correlating molecular, psychological and image data may help elucidate the mechanisms through which physical exercise improves brain health.
Keywords: Physical Activity, Exercise, Brain, Mental Health, Review
RESUMO
Introdução:
Evidências das últimas décadas têm mostrado que o exercício físico impacta de forma significativa aspectos neurofisiológicos e estruturais do cérebro humano. No entanto, a maioria das pesquisas emprega modelos animais, o que gera confusão no que diz respeito aos efeitos reais do exercício no cérebro humano, assim como os mecanismos adjacentes.
Objetivo:
Apresentar uma atualização sobre o impacto do exercício no cérebro; revisar e analisar sistematicamente as evidências provenientes exclusivamente de estudos randomizados controlados em humanos, dos últimos seis anos.
Métodos:
Foi conduzida uma busca na literatura usando as bases de dados MEDLINE (via PubMed), EMBASE, Web of Science e PsycINFO, para todos os estudos randomizados e controlados publicados entre janeiro de 2014 e janeiro de 2020.
Resultados:
Foram revisados 24 estudos randomizados controlados em humanos, que observavam a relação entre exercício físico e alterações neuroquímicas e estruturais no cérebro.
Conclusões:
Ainda que esta revisão tenha observado que o exercício físico melhora a plasticidade cerebral em humanos, particularmente por meio de alterações no fator neurotrófico derivado do cérebro (BDNF), conectividade funcional, núcleos da base e hipocampo, muitas questões ainda precisam ser respondidas. Dados os avanços recentes nessa temática e seu potencial terapêutico para a população em geral, espera-se que este manuscrito e pesquisas futuras que correlacionem estudos moleculares e variáveis psicológicas e de imagem possam ajudar na elucidação dos mecanismos pelos quais o exercício físico melhora a saúde cerebral.
Palavras-chave: Atividade Física, Exercício Físico, Cérebro, Saúde Mental, Revisão
INTRODUCTION
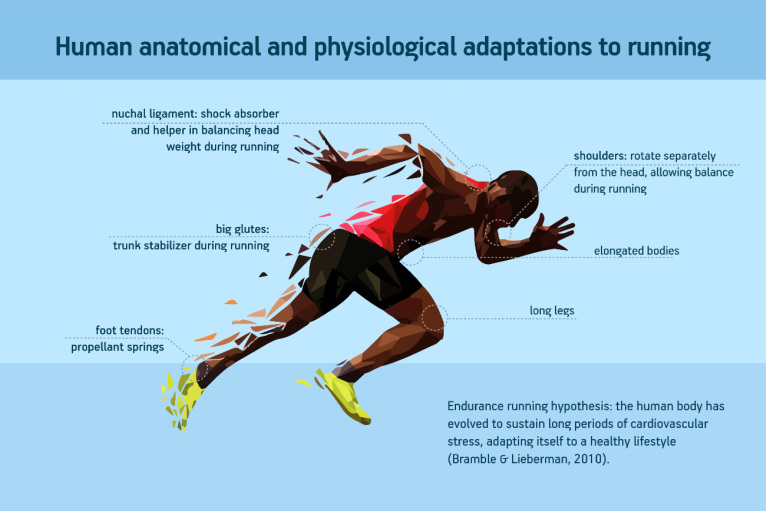
In 2004, Bramble and Lieberman suggested that humans evolved from monkey-like ancestors, specifically due to their ability to run long distances. According to these authors1, strong selection for running was crucial in shaping the body of modern man and was an essential factor in the appearance of specific anatomical features. Figure 1 shows typical human anatomical and physiological features that are adaptations to running, according to the endurance running theory from Bramble and Lieberman1. The close connection between movement (exercise) and human evolution is shown by the fact that inactivity makes people physically and mentally ill2. Studies have shown that movement is so essential for humans that the brain not only benefits from it, but also requires it in order to function properly3.
Figure 1. Human anatomical and physiological features that are adaptations to running, according to the endurance running theory.
The basic neurobiological mechanisms associated with physical exercise can occur at two levels: extracellular, with exercise inducing angiogenesis from pre-existing vessels; and intracellular, through increasing hippocampus neurogenesis4. The functional significance of this effect is still uncertain, but it has been suggested that newly formed neurons can be integrated into the existing neural network and become fully functional5. Exercise also seems to induce the growth of new synapses (synaptogenesis)5. In addition, animal studies have shown that exercise increases the synthesis of growth factors such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor (IGF-1), proteins that play a crucial role in neuroplasticity, neuroprotection and neurogenesis4. There is also evidence that neuromodulation and neurotransmission are regulated by physical exercise6,7. Lastly, an emerging concept suggests that brain health and cognitive functions are modulated by the interrelationship between central and peripheral factors8. Systemic inflammatory processes, which are present in metabolic diseases such as hypertension or insulin resistance, increase central nervous system inflammation and are associated with cognitive decline8. Human randomized controlled trials have shown that exercise upregulates neurotransmitters9, boosts neurotrophic factor synthesis10,11, increments functional connectivity12,13 and increases basal ganglion14 and hippocampus15,16 volume.
Studies have indicated that physical exercise reduces symptoms associated with different mental disorders, such as depression and anxiety9, and neurodegenerative diseases such as Alzheimer's and Parkinson's8. Thus, exercise forms an effective neuroprotective strategy against the deleterious effects of aging17,18.
Although the understanding of exercise-related molecular and cellular changes in humans is relatively limited, imaging technologies have enabled observation of changes in brain structure and function as a result of exercise in humans. Diamond19, for example, found that fitness training had robust but selective benefits for cognition, among which the largest benefits related to executive control processes. Other studies found that highly fit or aerobically trained participants showed better behavioral performance and greater task-related activity in the prefrontal and parietal cortices, i.e., in regions consistently implicated in attentional selection and resolution of response conflict20,21.
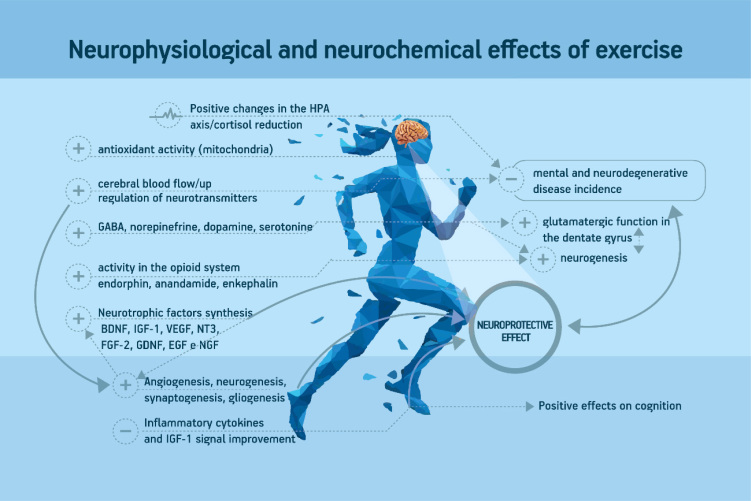
Neuroimaging studies have suggested that physical exercise has a protective role in preventing age-related decline and disorders, especially brain atrophy. Colcombe et al.22 observed significant increases in both gray matter and white matter volumes (primarily in prefrontal and temporal areas) in older adults (60–79 years), as a result of an exercise program. Erickson et al.23 found that aerobically trained subjects showed preservation of and increased hippocampus volume and better spatial memory performance. Erickson et al.23 also observed increased anterior hippocampus volume in older adults, following a long-term exercise program. Interestingly, a 1.4% decline in the control group was also observed. Other studies showed that increases in total physical activity were positively related to increases in gray matter volume in the prefrontal and cingulate cortices24, as well as greater white matter integrity in the frontal and temporal lobes25. Among the many possible mechanisms through which physical exercise yields the abovementioned improvements are the following: downregulation of the HPA axis4; upregulation of different neurotransmitters and neuromodulators16; increased neurogenesis11, synaptogenesis5 and neurotrophic factors4,6,7; and the interrelationship between central and peripheral factors8. However, at the cellular level, most of this evidence comes from animal studies5,6,7,8. Figure 2 summarizes the neurophysiological and neurochemical effects of exercise.
Figure 2. Summary of neurophysiological and neurochemical effects of physical exercise.
It is relevant to note that since the approach of human neuroscience is basically noninvasive, it does not allow direct measurement of exercise effects on the brain at the cellular and molecular levels. To overcome this limitation, research uses animal models12. However, as previously stated, this yields significant confusion regarding the real effects of exercise on human brain structures and neurochemistry, as well as regarding the underlying mechanisms involved. In addition, many human studies have methodological limitations, such as the lack of control groups or randomization. For this reason, it is crucial to elucidate the real impact of physical exercise on the human brain by examining the evidence specifically from human randomized controlled trials.
Therefore, the aims of the current article were: 1) to present an update on the impact of physical exercise on brain health; and 2) to review and analyze evidence exclusively from human randomized controlled studies from the last six years.
METHODS
Registration and protocol
This review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the CRD # 4202015989. It was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), a 27-item checklist that includes the title, abstract, methods, results, discussion and funding, which is designed to help authors of systematic reviews and meta-analyses.
Search strategy
The search of the literature was conducted independently by two reviewers (CFV and SL) using MEDLINE (via PubMed), EMBASE, Web of Science and PsycINFO databases for all randomized control trials published between January 2014 and January 2020 (last six years). Studies that examined the relationship between physical exercise, structural, and neurochemical changes were scrutinized. One strategy was to frame the search in the form of a question, while allowing clarifications needed for selecting relevant results: Does exercise or physical activity cause structural, neurochemical and neurophysiological changes in the brain of healthy adults, according exclusively to RCTs?
Another strategy used for creating a searchable question was to put it in the form of a PICO question. PICO only partially applied to our research question, but the principle of breaking the question into searchable parts is useful and has been applied:
P: Population: healthy adults.
I: Intervention: exercise and physical activity.
C: Comparison: control.
O: Outcome: structural, neurochemical and neurophysiological changes in the brain.
We searched the databases using mainly keywords and controlled vocabularies. Because of the diverse nature of the relationship between exercise and the nervous system, different keywords can be applied. So the choice of specific keyword was based on the current literature in the field of exercise neuroscience. A simple chart was set up in order to help organize the searching. A column representing each idea and two correlated rows was created: one row for the controlled vocabulary terms and the other for the synonyms and phrases that express the idea in a keyword search. The terms within the column were combined with OR, while different columns were combined with AND. Consequently, a Boolean logic using the three most common operators (AND, OR and NOT) was applied. Studies that examined the relationship between exercise or physical activity and brain changes were scrutinized. The search strategy included studies, abstracts, titles and keywords, as follows:
((exercise OR physical activity OR exercise program OR exercise intervention OR physical activity intervention OR physical activity program) AND (brain OR brain changes OR brain volume OR structural changes) AND (healthy) AND (adults)) NOT children.
((exercise OR physical activity OR exercise program OR exercise intervention OR physical activity intervention OR physical activity program) AND (neurochemical changes OR neurophysiological changes OR gray matter OR white matter OR connectivity OR cerebral blood flow OR hippocampus OR cortex OR prefrontal cortex OR cortical activity OR neurotransmitters OR neurotrophic factors) AND (healthy) AND (adults)) NOT children.
Study selection criteria
Studies that investigated the relationship between physical exercise and structural or neurochemical changes were included in the systematic review. Studies were considered eligible only if: (1) they were human randomized controlled trials (RCTs); (2) they investigated healthy adults; (3) they were published or accepted for publication in a peer-reviewed journal; (4) interventions included an aerobic exercise program; (5) intervention programs included other types of physical activity, such as dance, sports and resistance training; (6) interventions included acute or chronic exercise; (7) interventions included observation of the impact of exercise on any brain structure (not function), volume, connectivity and blood flow; and (8) interventions included observation of the impact of exercise on the brain's neurochemistry (neurotransmitters, neuromodulators or neurotrophic factors).
Studies were excluded if: (1) they were not randomized controlled trials (RCTs); (2) they investigated individuals suffering from any diseases; (2) they were conducted on children or adolescents; (3) they were cross-sectional, reviews or study protocols; (4) they were animal studies; (5) the outcome variable was not the impact of physical exercise on brain structures or neurochemistry; (6) they were published in any language other than English; and (7) they were published before 2014.
Search data extraction
Two authors (EHMD and AB) separately screened abstracts, titles, and texts of the retrieved studies. They removed duplicates and excluded those that did not meet the selection criteria. Subsequently, two other authors (MD and HV) collected the following data from each article that had been selected: (1) year of publication; (2) sample; (3) intervention characteristics; (4) variables of interest; and (6) outcomes.
Risk of bias
After the phases of search strategy, selection criteria and data extraction, the author CFV assessed the methodological risk of bias of the studies through the Quality Assessment Tool for Quantitative Studies (QATQS), which was developed by the Effective Public Health Practice Project (EPHPP, 1998). QATQS is a tool that provides a standardized means to assess study quality and develop recommendations for study findings. This quality appraisal tool was developed as an important step within the systematic review process. The final results from using the QATQS gave rise to overall methodological ratings of strong (no weak ratings), moderate (one weak rating) or weak (two or more weak ratings) in eight sections: 1) selection bias; 2) study design; 3) confounders; 4) blinding; 5) data collection methods; 6) withdrawals and dropouts; 7) intervention integrity; and 8) analysis. Any disagreements were resolved by a third researcher (RV). Table 1 shows the assessment of study quality through the QATQS.
Table 1. Effective public healthcare practice quality assessment (quality assessment tool for quantitative studies).
| Study | Selection bias | Study design | Confounders | Blinding | Data collection methods | Withdrawals and drop-outs | Intervention integrity | Analyses | Overall rating Strong: no weak ratings, Moderate: one weak rating, Weak: two or more weak ratings |
|---|---|---|---|---|---|---|---|---|---|
| Nagamatsu et al., 201634 | 1 | 1 | 3 | 3 | 1 | 3 | 1 | 1 | WEAK |
| Neimann et al., 201435 | 1 | 1 | 3 | 3 | 1 | 3 | 1 | 1 | WEAK |
| Best et al., 2015 26 | 1 | 1 | 1 | 3 | 1 | 3 | 1 | 1 | WEAK |
| Nocera et al., 201636 | 1 | 1 | 3 | 3 | 1 | 3 | 1 | 1 | WEAK |
| Demiracka et al., 201527 | 1 | 1 | 2 | 3 | 1 | 3 | 1 | 1 | WEAK |
| Suwabe et al., 201838 | 1 | 1 | 2 | 3 | 1 | 3 | 1 | 1 | WEAK |
| Oliveira et al., 201937 | 1 | 1 | 2 | 3 | 1 | 3 | 1 | 1 | WEAK |
| Church et al., 201610 | 1 | 1 | 2 | 3 | 1 | 3 | 1 | 1 | WEAK |
| Hriv et al., 201729 | 1 | 1 | 2 | 3 | 1 | 3 | 1 | 1 | WEAK |
| Gregoire et al., 201928 | 1 | 1 | 2 | 3 | 1 | 3 | 1 | 1 | WEAK |
| Kim J et al., 201830 | 1 | 1 | 2 | 3 | 1 | 3 | 1 | 1 | WEAK |
| Marston et al., 201933 | 1 | 1 | 2 | 3 | 1 | 3 | 1 | 1 | WEAK |
| Vaughan et al., 201411 | 1 | 1 | 2 | 1 | 1 | 3 | 1 | 1 | WEAK |
| Forti et al., 20156 | 1 | 1 | 2 | 1 | 1 | 3 | 1 | 1 | WEAK |
| Magon et al., 201632 | 1 | 1 | 3 | 3 | 1 | 1 | 1 | 1 | WEAK |
| Maddock et al., 20169 | 1 | 1 | 2 | 3 | 1 | 1 | 1 | 1 | MODERATE |
| Matura et al., 201731 | 1 | 1 | 3 | 1 | 1 | 3 | 1 | 1 | WEAK |
| Zschucke et al., 201441 | 1 | 1 | 3 | 3 | 1 | 3 | 1 | 1 | WEAK |
| Tamura et al., 201439 | 1 | 1 | 1 | 3 | 1 | 3 | 1 | 1 | WEAK |
| Wagner et al., 201712 | 1 | 1 | 2 | 1 | 1 | 3 | 1 | 1 | WEAK |
| Varma et al., 201514 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | MODERATE |
| Rosano et al., 201740 | 1 | 1 | 2 | 3 | 1 | 1 | 1 | 1 | MODERATE |
| Kleemeyer et al., 201513 | 1 | 1 | 2 | 3 | 1 | 1 | 1 | 1 | MODERATE |
| Kim L et al., 201717 | 1 | 1 | 2 | 3 | 1 | 1 | 1 | 1 | MODERATE |
RESULTS
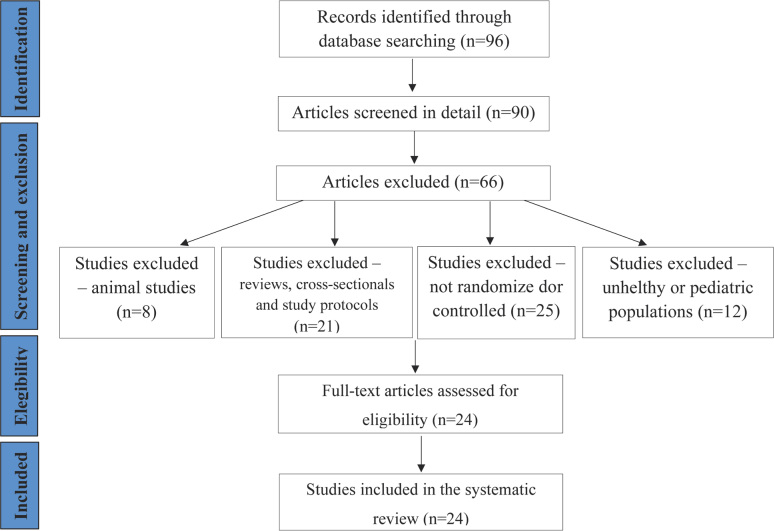
The search yielded a total of 96 potentially eligible articles: 52 from Medline, 14 from Embase, 24 from Web of Science and 6 from PsycINFO. After removing 6 duplicates, 90 were screened in detail. A total of 66 studies were excluded from the review because: a) they were animal studies (8); b) they were reviews, meta-analysis, study protocols or cross-sectional studies (21); c) they were not randomized or controlled (25); and d) they were conducted on non-healthy or pediatric populations (12). In the end, a total of 24 studies met the inclusion criteria (S126, S210, S327, S46, S528, S629, S717, S830, S913, S109, S1131, S1232, S1333, S1434, S1535, S1636, S1737, S1838, S1939, S2040, S2114, S2211, S2312 and S2441). These were assessed for eligibility and later included in this review. The study extraction flow is demonstrated in the PRISMA diagram (Figure 3).
Figure 3. PRISMA diagram showing the study extraction flow.
The studies included were published between 2014 and 2020 and were all randomized controlled trials, with sample sizes ranging from 20 to 155 subjects, aged between 21 and 65 years. The main interventions used in these studies were aerobic exercise15, resistance training6, coordination exercises3 or a combination of coordination and cognitive exercises1, calisthenics1 or a mixed program3.
The risks of bias of the studies included are displayed in Table 1. The risk of performance bias was high in these studies because it was difficult to blind participants or exercise coaches, but six studies reported blinding of the outcome assessors (S4, S11, S18, S21, S22 and S23). The risk of attrition bias was high in most studies (due to unreported data), except for six (S7, S9, S10, S12, S20 and S21) Nine studies used an active control group (S1, S2, S4, S5, S9, S14, S15, S16 and S24), instead of a no-intervention control and four studies did not inform the type of control used (S8, S19, S21 and S23).
The total duration of the interventions ranged from one session (acute) (S10, S18 and S24) to 6 to 52 weeks (S1, S2, S3, S4, S5, S6, S7, S8, S9, S11, S12, S13, S14, S15, S16, S17, S19, S20, S21, S22 and S23) and to two years (S19 and S20).
The total number of minutes (volume) spent on the interventions ranged from 10 to 30 minutes during acute protocols (S10, S18 and S24) to approximately 40 to 180 minutes/week (S1, S2, S3, S4, S5, S6, S7, S8, S11, S12, S13, S14, S15, S16, S17, S19, S20, S21, S22 and S23). The overall frequency ranged from 1 to 7 times per week.
Some studies provided data regarding changes in neurotransmitters (S10, S17 and S23) or neurotrophic synthesis (S2, S4, S5, S6, S8, S11, S13, S22 and S23). In these studies, positive correlations were observed between exercise and increased neurotrophic factors (S2, S4, S5, S8, S13, S22 and S23) and between exercise and upregulation of neurotransmitters (S10). Four studies found that exercise did not increase the levels of neurotrophic factors (S6, S11 and S17) or neurotransmitters (S17). Studies also observed increases (S18 and S23) or no differences in functional connectivity (S12) or in gray matter volume (S11). One study found that exercise increased white matter volume (S19) or reduced white matter atrophy (S1). Brain activity in the hippocampus area was found to increase after exercise (S24) or decrease but correlate with better cognition (S16). With regard to structural changes, seven studies investigated the effects of exercise on basal ganglion volume (S14 and S15) and on hippocampus volume (S7, S9, S20, S21 and S23). One study (S14) found no differences between groups regarding basal ganglion volume, but that individuals with declines in mobility levels had significant decreases in left putamen volume. Another study found that motor fitness, but not cardiovascular fitness, was positively related to the volume of the putamen and the globus pallidus (S15). All studies that investigated changes in the hippocampus found a positive correlation between exercise and increased hippocampus volume (S7, S9, S20, S21 and S23). Table 2 summarizes the information in the studies.
Table 2. Summary of the characteristics of the randomized controlled trials.
| Study | Sample | Intervention | Control condition | Intensity of intervention | Volume and frequency | Duration | Variables of interest | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Best et al., 2015 (S1)26 | n=155 | Resistance training | Balance and toning exercises | High | 60 min 3x/week | 52 weeks | Brain volume, mood and cognition. | 1. Resistance training improves memory, reduces cortical white matter atrophy and increases peak muscle power executive function, compared with balance-and-toning. 2. These effects persisted at 2-year follow-up, relative to balance-and-toning. |
| Church et al., 2016 (S2)10 | n=20 | High-intensity low-volume (HI) | Low-intensity high-volume (HV) | Moderate to high | Duration not informed 4x/week | 8 weeks | Plasma BDNF | 1. BDNF response is significantly elevated after both high-intensity and high-volume training protocols. |
| Demirakca et al., 2016 (S3)37 | n=21 | Coordination exercises + cognitive training | Rest | n/a | 60 min, 1x/week | 13 weeks | Functional connectivity (rs-fMRI) | 1. Significant connectivity alterations occurred between the visual cortex and parts of the superior parietal area (BA7). Premotor area and cingulate gyrus were also affected. |
| Forti et al., 2015 (S46 | n=56 | High and low resistance training | Mixed low resistance training | Low and high | Duration not informed 3x/week | 12 weeks | Plasma BDNF | 1. Only the mixed-low-resistance training program (high number of repetitions at a sufficiently high external resistance) was able to increase circulating BDNF in older male participants. 2. Training to volitional fatigue might be necessary to obtain optimal results. |
| Gregoire et al., 2019 (S5)28 | n=34 | Lower body strength + aerobic training (LBS-A) and upper body strength + aerobic training (UBS-A) | Gross motor activities (GMA). | Not informed | 60 min, 3x/week | 8 weeks | Plasma BDNF and cognition | 1. All interventions resulted in improved cognitive functions but the GMA intervention induced a larger increase in plasma BDNF concentration (cognition improvement could occur without concomitant detectable changes in BDNF). 2. No correlation was observed between changes in BDNF concentrations and cognitive performances. |
| Hvid et al., 2017 (S6)29 | n=47 | Progressive high-intensity power training | No intervention | Moderate to high | Approx. 45 min, 2x/week | 12 weeks | Serum BDNF (mature and total) | 1. Baseline systemic levels of serum mBDNF and tBDNF were not affected by exercise training. |
| Kim L et al., 2017 (S7)17 | n=21 | Strength training | No intervention | Moderate | 50-60 min, 3x/week | 24 weeks | Hippocampus volume | 1. Hippocampus volume was significantly increased in the strength exercise group, but decreased in the control group. |
| Kim J et al., 2018 (S8)30 | n=26 | Aquarobic exercise program | Not informed | Moderate | 60 min, 2x/week | 12 weeks | Plasma BDNF and irisin | 1. Significantly higher serum irisin and BDNF levels in the exercise group than in the control group were found. 2. Serum irisin and BDNF levels were significantly higher 30 min after the first exercise session and 30 min after the last exercise session. |
| Kleemeyer et al., 2015 (S9)13 | n=52 | High-intensity aerobic exercise | Low-intensity aerobic exercise | Low-vigorous | 25-55 min, 2-3x/week | 6 months | Hippocampus volume and microstructure | 1. More positive changes in fitness were associated with more positive changes in tissue density and more positive changes in tissue density were associated with more positive changes in volume. 2. Fitness-related changes in hippocampus volume may be brought about by changes in tissue density. |
| Maddock et al., 2016 (S10)9 | n=38 | Aerobic exercise | Rest | Vigorous | 30 min | Acute | Cortical glutamate and GABA levels (MRS) | 1. Results showed that glutamate and GABA signals increased significantly in the visual cortex following exercise. In addition, there was an increase in glutamate following exercise in the anterior cingulate cortex. 2. The results are consistent with an exercise-induced expansion of the cortical pools of glutamate and GABA. |
| Matura et al., 2017 (S11)31 | n=53 | Aerobic exercise | Waiting list | Moderate | 30 min, 3x/week | 12 weeks | Brain metabolism, gray matter (GM) volume and cognition. | 1. Cerebral choline concentrations remained stable in the exercise group, while increasing in the control group. 2. No effect of training was seen on cerebral N-acetyl aspartate or BDNF levels and no changes in cortical GM volume in response to aerobic exercise. 3. Stable choline concentrations in the intervention group might indicate a neuroprotective effect of aerobic exercise. |
| Magon et al., 2016 (S12)32 | n=28 | Slackline training | Educational Sessions | n/a | 90 min, 3x/week | 6 weeks | Functional connectivity (MRI) | 1. MRI analysis did not reveal morphological or functional connectivity differences before or after the training between the intervention and control groups. 2. However, subsequent analysis in subjects with improved slackline performance showed a decrease of connectivity between the striatum and other brain areas during the training period, which means an increased efficiency of the striatal network. |
| Marston et al., 2019 (S13)33 | n=45 | High-load resistance training and moderate-load resistance training | No intervention | Moderate to high | Duration not informed 2x/week | 12 weeks | Peripheral growth factors and homocysteine | 1. High-load or moderate-load resistance training twice per week for 12 weeks has no effect on peripheral growth factors or homocysteine in healthy late middle-aged adults. |
| Nagamatsu et al., 2016 (S14)34 | n=101 | Aerobic exercise | Toning exercises | Moderate | 40 min, 1x/week | 12 months | Mobility and basal ganglion volume | 1. In both groups, no differences were observed in the putamen volume regardless of change in mobility. 2. However, those who declined in mobility levels significantly decreased in left putamen volume. |
| Neimann et al., 2014 (S15)35 | n=92 | Aerobic exercise and coordination training | Stretching and relaxation | Moderate | 45-60 min, 3x/week | 12 months | Basa ganglion volume | 1. Motor fitness but not cardiovascular fitness was positively related with the volume of the putamen and the globus pallidus. 2. Coordination training increased caudate and globus pallidus volume. |
| Nocera et al., 2017 (S16)36 | n=32 | Aerobic exercise | Balance exercises | Moderate to vigorous | 20-45 min, 3x/week | 12 weeks | Brain activity during cognitive tasks | 1. Cognition (verbal fluency) was improved in the aerobic exercise group, compared with controls. 2. fMRI comparisons of IFG (inferior frontal gyrus) activity showed lower activity in the right IFG following the intervention in the aerobic group, compared with controls. |
| Oliveira et al., 2019 (S17)37 | n=34 | Aerobic exercise | Waiting list | Moderate | 40 min, 3x/week | 12 weeks | Plasma anandamide (AEA), mood and body weight | 1. Regular moderate aerobic exercise reduces plasma AEA levels. 2. This reduction was associated with weight loss and improved mood, in particular, reduced anger. |
| Suwabe et al., 2018 (S18)38 | n=36 | Aerobic exercise | Rest | Low | 10 min | Acute | Functional connectivity during cognitive task | 1. A single 10-min bout of exercise increased functional connectivity between hippocampus DG/CA3 and cortical regions. 2. The magnitude of the enhanced functional connectivity predicted the extent of memory improvement. |
| Tamura et al., 2014 (S19)39 | n=110 | Calisthenics | Not informed | Moderate | 10 min/day, everyday | 2 years | Brain volume and cognition | 1. The exercise group showed significant improvements in attentional shift. 2. Neuroimaging analysis revealed the significant preservation of bilateral prefrontal volume in the exercise group. 3. The longitudinal changes in attentional shift and memory were positively correlated with the prefrontal volumetric changes. |
| Rosano et al., 2017 (S20)40 | n=27 | Aerobic exercise | Health education | Moderate | Not reported | 2 years | Hippocampus volume | 1. Increased volume of the left hippocampus, left cornu ammonis and right hippocampus in the intervention group. |
| Varma et al 2015 (S21)14 | n=92 | Aerobic exercise (walking) | Not informed | Low to vigorous | 10,000 steps/day threshold (pedometer) | Not informed | Hippocampus volume | 1. A greater amount, duration, and frequency of total daily walking activity were each associated with larger hippocampus volume among older women, but not men. 2. These relationships were specific to hippocampus volume, compared to the thalamus, used as a control brain region, and remained significant for low-intensity walking activity, independent of moderate to vigorous-intensity activity and self-reported exercise |
| Vaughan et al., 2014 (S22)11 | n=49 | Multimodal exercise program (cardiovascular, strength and motor fitness) | Waiting list | Not reported | 60 min, 2x/week | 16 weeks | BDNF and cognition | 1. The exercise program resulted in neurocognitive and physical performance improvements and increased levels of plasma BDNF, in older women, compared with controls. 2. Increases in BDNF levels imply neurogenesis may be a component of the mechanism underpinning the cognitive improvements associated with exercise. |
| Wagner et al., 2015 (S23)12 | n=34 | Aerobic exercise | Not informed | Moderate | 60 min, 3x/week | 6 weeks | Hippocampus volume, hippocampus glutamate/ glutamine and NAA (N-acetyl aspartate). | 1. A positive correlation between the degree of fitness improvement and increased BDNF levels was found. 2. A volume decrease of about 2% of the hippocampus was negatively correlated with fitness improvement and increased BDNF levels; and positively correlated with increased TNF-α concentrations. 3. A decrease in glutamate-glutamine levels was observed in the right anterior hippocampus in the exercise group only. |
| Zschucke et al., 2014 (S24)41 | n=40 | Aerobic exercise | Light stretching exercises | Moderate | 30 min | Acute | Brain activation (fMRI) during stress task (MIST) cortisol and amylase | 1. Participants of the aerobic group showed a significantly reduced cortisol response to the MIST, which was inversely related to the previous exercise-induced amylase and cortisol fluctuations. 2. Higher bilateral hippocampus activity and lower prefrontal cortex (PFC) activity was observed in the aerobic group. |
DISCUSSION
The aim in this study was to review data exclusively from human randomized controlled studies conducted among healthy adults. The review systematically examined the literature from the last six years with regard to the effects of physical exercise on brain volume, structures, functional connectivity and neurochemical factors such as neurotransmitters, BDNF and the HPA axis, control groups or randomization.
Among the studies that observed changes in neurotrophic factors (S2, S4, S5, S6, S8, S11, S13, S22 and S23), all of them used exercise programs that lasted more than six weeks (regular exercise). Seven studies found a positive correlation between exercise and increased plasma or serum BDNF (S2, S4, S5, S8, S13, S22 and S23). These results are in agreement with an extensive meta-analysis conducted by Szuhany et al.42, which demonstrated the strength of the association between exercise and increased BDNF levels in humans. The review showed a moderate effect size for increases in BDNF after acute exercise. In addition, the effect of an exercise session on BDNF levels was intensified by regular exercise. These authors explained that each episode of exercise results in a “dose” of BDNF activity and that the magnitude of this “dose” can be enhanced over time by regular exercise.
In the present review, most studies that found a correlation between physical exercise and increased plasma BDNF (pBDNF), used moderate to high-intensity exercise as part of the main intervention. Unfortunately, several other studies did not report the intensity level used. In the literature, other studies have also found that exercise-induced BDNF effects in humans follow a dose-dependent relationship with regard to duration and intensity of exercise, such that the best outcomes are linked to moderate exercise43. A recent review by Knaepen et al.44 found that high intensities and graded exercise tests elicited the greatest exercise-induced increases in pBDNF concentration in healthy participants. In acute protocols, this increase has been shown to last post-exercise, to some extent. Another interesting fact is that many of the studies reviewed here not only used aerobic exercise, but also used multimodal protocols, resistance training and coordinative exercises (S2, S4, S5 and S22). Accordingly, there is evidence that increases in pBDNF concentrations can be observed in response to a variety of exercise protocols and types45,46. Rasmussen et al.47 and Tang et al.48 also observed effects that indicate that an acute exercise-induced increase in pBDNF is stable in response to different exercise types and protocols. According to the evidence reviewed here, moderate-intensity multimodal exercises are more effective in promoting increases in peripheral levels of BDNF, although it is still not possible to draw definite conclusions or to establish recommendation protocols for the type and intensity of exercises in a multimodal program that would be required in order to produce an increase in BDNF levels. Vedovelli et al.49, observed that a combined intervention for increased muscle strength and aerobic conditioning can increase BDNF levels, and that aerobic conditioning is at least partially responsible for that improvement. These authors also stated that BDNF could be a key component of the beneficial effects of physical activity on cognitive functioning, since this neurotrophin can modulate neurogenesis, neuroplasticity and neuronal survival. In addition, Vaughan et al.11, observed that human studies had found that motor fitness (balance, flexibility, co-ordination, agility and reaction time ability) was associated with brain activation patterns that differed from those related to cardiovascular fitness. Motor fitness training entails complexity that requires sustained attention and concentration, thereby increasing the cognitive load and evoking positive neuroplasticity. Although promising, a greater number of studies, with larger samples and less methodological biases, are needed in order to better elucidate the relationship between BDNF and multimodal exercise.
In the present review, one study (S17) observed a negative correlation between moderate regular exercise and decreased peripheral anandamide levels (AEA). It was also observed that this reduction was associated with weight loss and improved mood. Other data corroborate these findings. In a study by Matias et al.50, salivary AEA levels were positively correlated with body mass index, waist circumference and fasting insulin levels. Preclinical studies have also indicated that AEA has a negative effect on peripheral metabolism by impairing insulin signaling and mitochondrial function51. Although acute aerobic exercise has been shown to increase circulating AEA50, Gasperi et al.52 found increased upregulation and activity of resting fatty acid amide hydrolase (FAAH) — a major enzyme responsible for AEA breakdown — in the lymphocytes of physically active young men, compared with sedentary young men. The observed lower circulating AEA levels associated with improved mood, however, seems to contradict the current understanding of the relationship between exercise-related mood enhancement and endocannabinoids53,54. Several studies have indicated that endocannabinoids have stress-buffering, anxiolytic and antidepressant effects via CB1 receptors55. On the other hand, Antunes et al.56 showed that reduced resting plasma AEA in exercise-addicted runners was accompanied by higher negative mood scores. Such discrepancies might be due to the distinct effects of acute versus chronic exercise, measures used during exercise versus resting conditions and heterogeneity among the samples. Endocannabinoid responses to acute and chronic exercise among healthy people deserve further investigation.
In this review, two studies observed the effects of acute and regular physical exercise on other neurotransmitters. One study (S10) found that acute exercise increases the levels of GABA and glutamate in the anterior cingulate and visual cortices. During acute aerobic exercise, in the process of aerobic glycolysis, glucose is broken down to pyruvate, which then further breaks down to lactate or lactic acid. When exercise transitions from an aerobic to an anaerobic nature, the “anaerobic threshold” is met. Beyond this point, lactic acidosis occurs. Lactate is able to cross the blood-brain barrier, but is independently made by astrocytes in the brain, where it serves as a precursor of glutamate. Glutamate is then taken up by astrocytes and converted to glutamine in the glutamate-glutamine cycle57. Two recent studies used proton magnetic resonance spectroscopy (H1MRS) to investigate brain-level changes in lactate, glutamate and glutamine58 and revealed that lactate, glutamate and glutamine levels transiently increased by approximately 20% in the human cortex. Acute exercise has been known to increase peripheral lactate levels and, even though direct quantification of acute exercise-induced brain lactate levels in humans is difficult, these results were also observed in S10.
Another study analyzed in this review (S23) provided interesting results: a decrease in glutamate-glutamine levels in the right anterior hippocampus in the exercise group that seemed to be correlated with a volume decrease in the hippocampus of about 2%. The authors of that study stated that the observed volume changes were not a consequence of a neuronal loss in the right hippocampus, but rather, resulted from potential changes in gliogenesis and/or fiber organization. Astroglia are actively involved in the uptake, metabolism and recycling of glutamate, and the glutamate-glutamine cycle between neurons and glia is a major metabolic pathway that reflects the synaptic release of glutamate. Therefore, changes in glutamate metabolism might be linked indirectly to the observed structural changes, in particular those of glial morphology59. Further investigations regarding changes in peripheral and central neurotransmitter levels after exercise in humans are necessary to better elucidate related mechanisms.
Two studies in this review observed increases in functional connectivity as a consequence of acute aerobic (S18) and long-term coordinative exercise (S3). One long-term study found that resistance exercise reduced white matter atrophy (S1) and a 12-week study found no differences in gray matter volume in the aerobic exercise group, compared with the control (S11). Most of these results were congruent with other studies in the literature that showed alterations in white matter and connectivity as a result of exercise. Colcombe et al.60 reported an anterior cluster of increased white matter after six months of exercise, in a group of elderly adults. Indeed, investigators have been witnessing significant advancements in the ability to study the connectivity between brain areas embodied by white matter (see Smith et al.61 for review). One recent study62 found a correlation between white matter integrity and changes in VO2 max scores in frontal and temporal white matter tracts. Interestingly, the change in white matter integrity for the aerobic training group did not significantly differ from that of a control group that participated in one year of non-aerobic exercise, thus suggesting that aspects other than aerobic exercise contributed to the observed change. Voss et al.63 also observed that differences in resting functional connectivity were associated with fitness level. The S3 study of this review specifically observed increased connectivity in brain areas associated with the default mode network (DMN), such as the anterior cingulate cortex and the prefrontal cortex, in the intervention group. These brain regions show a decrease in activity when external processing demands are increased. Voss et al.63 demonstrated that some of the functional connections within the DMN exhibit a positive correlation with VO2 max score and spatial memory.
Even though these are promising results, it remains necessary for future research to test whether there is specificity in exercise training-induced plasticity of brain networks.
With regard to structural changes, all the studies found significant changes in the volume of the basal ganglia and the hippocampus. Three studies found that moderate to vigorous aerobic activity was associated with a greater increase in hippocampus volume (S9, S20 and S21). These results are in agreement with those from other studies in the current literature that correlated exercise with structural changes in hippocampus volume and vasculature64. Erikson et al.65 showed that subjects with higher VO2 max scores had larger hippocampus volumes than those with lower VO2 max scores. Erickson et al.66 showed a correlation between the volume of the hippocampus and cardiovascular fitness in older adults. A follow-up study64 demonstrated that long-term aerobic exercise increased the volume of the hippocampus by 2% in elderly adults, while controls who underwent one year of stretching exercises exhibited a 1.4% decrease in hippocampus volume. Similarly, Pajonk et al.67 reported that there was a 12–16% increase in hippocampus size in a small group of exercising schizophrenic patients, as well as in matched controls.
Interestingly, one study in this review that used strength training also observed greater hippocampus volume (S7). The effects of resistance training on other neuroplastic factors, such as neurogenesis or BDNF level, are not clear yet68,69, but these results suggest that the improved communication between muscle fibers and the brain, as a result of strength training, may serve a protective role in slowing down age-related declines in hippocampus volume70. However, further studies are needed to confirm the mechanism of variations in hippocampus volume according to the type of exercise.
With regard to basal ganglia, one study (S14) found no differences in putamen volume in the intervention group, after 12 weeks of aerobic exercise. However, the authors of that study observed that individuals with significant declines in mobility levels also showed decreases in left putamen volume. Another study (S15) showed increased caudate and globus pallidus volume in subjects who underwent a coordinative training. Indeed, better motor fitness goes together with more frequent execution of motor-demanding exercise, thus resulting in more frequent stimulation of the corresponding sensorimotor (dorsal) part of the striatum (dorsal putamen) and output structure (globus pallidus) of the basal ganglion nuclei. Coordination training, which constantly requires adapting to new tasks, can be very similar to the early stages of motor learning, and is consequently associated with improvements in performance and activation of the striatum71,72. Therefore, it can be assumed that the observed volume increase found in the basal ganglia among subjects who attended the coordination training resulted in experience-dependent plasticity73. Another study in the literature found an association between cardiovascular fitness and caudate volume74 but, based on the functions of the basal ganglia, it seems reasonable to assume that the association between motor fitness and basal ganglion volume might be higher than the one between cardiovascular fitness and basal ganglion volume. Much research is still needed in order to elucidate this association. Different tools for statistical analyses, basal ganglion volume determinations, numbers of samples and intervention characteristics need to be taken into consideration.
In conclusion, studying the effects of physical exercise on brain structure and neurochemistry is still recent. While robust animal research protocols have demonstrated that aerobic exercise is a powerful modulator of structural brain plasticity, human trials have primarily focused on neuroimaging and cognitive studies, and have yielded conflicting results. The lack of methodological accuracy and the use of different types of exercise, frequency, intensity and duration hinders the meaning of results. Even though this short review found that exercise improves brain plasticity in humans, particularly through changes in BDNF, functional connectivity, basal ganglia and the hippocampus, many unanswered questions remain. Therefore, future studies in humans are needed in order to demonstrate the full potential of physical exercise (or movement in general) among healthy individuals and as a therapeutic strategy to remediate a variety of mental and neurological diseases or to lessen the burden of cognitive decline associated with aging. It is hoped that future studies correlating basic research with psychological variables and imaging studies may better elucidate the mechanisms through which physical exercise improves brain health in humans.
References
- 1.1. Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004 Nov;432(7015):345-52. https://doi.org/10.1038/nature03052 [DOI] [PubMed]
- 2.2. Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012 Apr;2(2):1143-211. https://doi.org/10.1002/cphy.c110025 [DOI] [PMC free article] [PubMed]
- 3.3. Duzel E, van Praag H, Sendtner M. Can physical exercise in old age improve memory and hippocampal function? Brain. 2016 Mar;139(Pt 3):662-73. https://doi.org/10.1093/brain/awv407 [DOI] [PMC free article] [PubMed]
- 4.4. Mello MT, Boscolo RA, Esteves AM, Tufilk SO. Exercício físico e os aspectos psicobiológicos. Rev Bras Med Esporte. 2005 Jun;11(3):195-9. https://doi.org/10.1590/S1517-86922005000300010
- 5.5. Zhao C, Teng EM, Summers RG Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006 Jan;26(1):3-11. https://doi.org/10.1523/JNEUROSCI.3648-05.2006 [DOI] [PMC free article] [PubMed]
- 6.6. Forti LN, Van Roie E, Njemini R, Coudyzer W, Beyer I, Delecluse C, et al. Dose-and gender-specific effects of resistance training on circulating levels of brain derived neurotrophic factor (BDNF) in community-dwelling older adults. Exp Gerontol. 2015 Oct;70:144-9. https://doi.org/10.1016/j.exger.2015.08.004 [DOI] [PubMed]
- 7.7. Deslandes A, Moraes H, Ferreira C, Veiga H, Silveira H, Mouta R, et al. Exercise and mental health: many reasons to move. Neuropsychobiology. 2009 Aug;59(4):191-8. https://doi.org/10.1159/000223730 [DOI] [PubMed]
- 8.8. Kim B, Feldman EL. Insulin resistance as a key link for the increased risk of cognitive impairment in themetabolic syndrome. Exp Mol Med. 2015 Mar;47(3):e149. https://doi.org/10.1038/emm.2015.3 [DOI] [PMC free article] [PubMed]
- 9.9. Maddock RJ, Casazza GA, Fernandez DH, Maddock MI. Acute modulation of cortical glutamate and GABA content by physical activity. J Neurosci. 2016 Feb;36(8):2449-57. https://doi.org/10.1523/JNEUROSCI.3455-15.2016 [DOI] [PMC free article] [PubMed]
- 10.10. Church DD, Hoffman JR, Mangine GT, Jajtner AR, Townsend JR, Beyer KS, et al. Comparison of high-intensity vs. high-volume resistance training on the BDNF response to exercise. J Appl Physiol (1985). 2016 Jul;121(1):123-8. https://doi.org/10.1152/japplphysiol.00233.2016 [DOI] [PubMed]
- 11.11. Vaughan S, Wallis M, Polit D, Steele M, Shum D, Morris N. The effects of multimodal exercise on cognitive and physical functioning and brain-derived neurotrophic factor in older women: a randomised controlled trial. Age Ageing. 2014 Sep;43(5):623-9. https://doi.org/10.1093/ageing/afu010 [DOI] [PubMed]
- 12.12. Wagner G, Herbsleb M, de la Cruz F, Schumann A, Brünner F, Schachtzabel C, et al. Hippocampal structure, metabolism, and inflammatory response after a 6-week intense aerobic exercise in healthy young adults: a controlled trial. J Cereb Blood Flow Metab. 2015 Oct;35(10):1570-8. https://doi.org/10.1038/jcbfm.2015.125 [DOI] [PMC free article] [PubMed]
- 13.13. Kleemeyer MM, Kühn S, Prindle J, Bodammer NC, Brechtel L, Garthe A, et al. Changes in fitness are associated with changes in hippocampal microstructure and hippocampal volume among older adults. Neuroimage. 2016 May;131:155-61. https://doi.org/10.1016/j.neuroimage.2015.11.026 [DOI] [PubMed]
- 14.14. Varma VR, Chuang YF, Harris GC, Tan EJ, Carlson MC. Low-intensity daily walking activity is associated with hippocampal volume in older adults. Hippocampus. 2015 May;25(5):605-15. https://doi.org/10.1002/hipo.22397 [DOI] [PMC free article] [PubMed]
- 15.15. Kandola A, Hendrikse J, Lucassen PJ, Yücel M. Aerobic exercise as a tool to improve hippocampal plasticity and function in humans: practical implications for mental health treatment. Front Hum Neurosci. 2016 Jul;10:373. https://doi.org/10.3389/fnhum.2016.00373 [DOI] [PMC free article] [PubMed]
- 16.16. Den Ouden L, Kandola A, Suo C, Hendrikse J, Costa R, Watt MJ, et al. The Influence of Aerobic Exercise on Hippocampal Integrity and Function: Preliminary Findings of a Multi-Modal Imaging Analysis. Brain Plast. 2018 Dec;4(2):211-6. https://doi.org/10.3233/BPL-170053 [DOI] [PMC free article] [PubMed]
- 17.17. Kim YS, Shin SK, Hong SB, Kim HJ. The effects of strength exercise on hippocampus volume and functional fitness of older women. Exp Gerontol. 2017 Oct;97:22-8. https://doi.org/10.1016/j.exger.2017.07.007 [DOI] [PubMed]
- 18.18. Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006 Jan;144(2):73-81. https://doi.org/10.7326/0003-4819-144-2-200601170-00004 [DOI] [PubMed]
- 19.19. Diamond A. Effects of physical exercise on executive functions: going beyond simply moving to moving with thought. Ann Sports Med Res. 2015 Jan;2(1):1011. PMID: 26000340 PMCID: PMC4437637 [PMC free article] [PubMed]
- 20.20. Tsujii T, Komatsu K, Sakatani K. Acute effects of physical exercise on prefrontal cortex activity in older adults: a functional near-infrared spectroscopy study. In: Welch WJ, Palm F, Bruley DF, Harrison DK, editors. Oxygen Transport to Tissue XXXIV. Advances in Experimental Medicine and Biology. V. 765. New York, NY: Springer; 2012. https://doi.org/10.1007/978-1-4614-4989-8_41 [DOI] [PubMed]
- 21.21. Hashimoto N, Yokogawa M, Kojima H, Tanaka S, Nakagawa T. Effect of moderate exercise intensities on the cortical activity in young adults. J Phys Ther Sci. 2018 Oct;30(10):1257-61. https://doi.org/10.1589/jpts.30.1257 [DOI] [PMC free article] [PubMed]
- 22.22. Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004 Mar;101(9):3316-21. https://doi.org/10.1073/pnas.0400266101 [DOI] [PMC free article] [PubMed]
- 23.23. Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011 Feb;108(7):3017-22. https://doi.org/10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed]
- 24.24. Wittfeld K, Jochem C, Dörr M, Schminke U, Gläser S, Bahls M, et al. Cardiorespiratory Fitness and Gray Matter Volume in the Temporal, Frontal, and Cerebellar Regions in the General Population. Mayo Clin Proc. 2020 Jan;95(1):44-56. https://doi.org/10.1016/j.mayocp.2019.05.030 [DOI] [PubMed]
- 25.25. Ruotsalainen I, Gorbach T, Perkola J, Renvall V, Syväoja HJ, Tammelin T, et al. Physical activity, aerobic fitness, and brain white matter: Their role for executive functions in adolescence. Dev Cogn Neurosci. 2020 Apr;42:100765. https://doi.org/10.1016/j.dcn.2020.100765 [DOI] [PMC free article] [PubMed]
- 26.26. Best JR, Chiu BK, Liang Hsu C, Nagamatsu LS, Liu-Ambrose T. Long-Term effects of resistance exercise training on cognition and brain volume in older women: results from a randomized controlled trial. J Int Neuropsychol Soc. 2015 Nov;21(10):745-56. https://doi.org/10.1017/S1355617715000673 [DOI] [PubMed]
- 27.27. Demirakca T, Cardinale V, Dehn S, Ruf M, Ende G. The exercising brain: changes in functional connectivity induced by an integrated multimodal cognitive and whole-body coordination training. Neural Plast. 2015 Dec;2016:8240894. https://doi.org/10.1155/2016/8240894 [DOI] [PMC free article] [PubMed]
- 28.28. Grégoire CA, Berryman N, St-Onge F, Vu T, Bosquet L, Arbour N, et al. Gross motor skills training leads to increased brain-derived neurotrophic factor levels in healthy older adults: a pilot study. Front Physiol. 2019 Apr;10:410. https://doi.org/10.3389/fphys.2019.00410 [DOI] [PMC free article] [PubMed]
- 29.29. Hvid LG, Nielsen MKF, Simonsen C, Andersen M, Caserotti P. Brain-derived neurotrophic factor (BDNF) serum basal levels is not affected by power training in mobility-limited older adults - a randomized controlled trial. Exp Gerontol. 2017 Jul;93:29-35. https://doi.org/10.1016/j.exger.2017.03.019 [DOI] [PubMed]
- 30.30. Kim JH, Kim DY. Aquarobic exercises improve the serum blood irisin and brain-derived neurotrophic factor levels in elderly women. Exp Gerontol. 2018 Apr;104:60-5. https://doi.org/10.1016/j.exger.2018.01.024 [DOI] [PubMed]
- 31.31. Matura S, Fleckenstein J, Deichmann R, Engeroff T, Füzéki E, Hattingen E, et al. Effects of aerobic exercise on brain metabolism and grey matter volume in older adults: results of the randomised controlled SMART trial. Transl Psychiatry. 2017 Jul;7(7):e1172. https://doi.org/10.1038/tp.2017.135 [DOI] [PMC free article] [PubMed]
- 32.32. Magon S, Donath L, Gaetano L, Thoeni A, Radue EW, Faude O, et al. Striatal functional connectivity changes following specific balance training in elderly people: MRI results of a randomized controlled pilot study. Gait Posture. 2016 Sep;49:334-9. https://doi.org/10.1016/j.gaitpost.2016.07.016 [DOI] [PubMed]
- 33.33. Marston KJ, Peiffer JJ, Rainey-Smith SR, Gordon N, Teo SY, Laws SM, et al. Resistance training enhances delayed memory in healthy middle-aged and older adults: A randomised controlled trial. J Sci Med Sport. 2019 Nov;22(11):1226-31. https://doi.org/10.1016/j.jsams.2019.06.013 [DOI] [PubMed]
- 34.34. Nagamatsu LS, Weinstein AM, Erickson KI, Fanning J, Awick EA, Kramer AF, et al. Exercise mode moderates the relationship between mobility and basal ganglia volume in healthy older adults. J Am Geriatr Soc. 2016 Jan;64(1):102-8. https://doi.org/10.1111/jgs.13882 [DOI] [PMC free article] [PubMed]
- 35.35. Niemann C, Godde B, Staudinger UM, Voelcker-Rehage C. Exercise-induced changes in basal ganglia volume and cognition in older adults. Neuroscience. 2014 Dec;281:147-63. https://doi.org/10.1016/j.neuroscience.2014.09.033 [DOI] [PubMed]
- 36.36. Nocera J, Crosson B, Mammino K, McGregor KM. Changes in Cortical Activation Patterns in Language Areas following an Aerobic Exercise Intervention in Older Adults. Neural Plast. 2017;2017:6340302. https://doi.org/10.1155/2017/6340302 [DOI] [PMC free article] [PubMed]
- 37.37. Oliveira AB, Ribeiro RT, Mello MT, Tufik S, Peres M. Anandamide Is related to clinical and cardiorespiratory benefits of aerobic exercise training in migraine patients: a randomized controlled clinical trial. Cannabis Cannabinoid Res. 2019 Dec;4(4):275-284. https://doi.org/10.1089/can.2018.0057 [DOI] [PMC free article] [PubMed]
- 38.38. Suwabe K, Byun K, Hyodo K, Reagh ZM, Roberts JM, Matsushita A, et al. Rapid stimulation of human dentate gyrus function with acute mild exercise. Proc Proc Natl Acad Sci U S A. 2018 Oct;115(41):10487-92. https://doi.org/10.1073/pnas.1805668115 [DOI] [PMC free article] [PubMed]
- 39.39. Tamura M, Nemoto K, Kawaguchi A, Kato M, Arai T, Kakuma T, et al. Long-term mild-intensity exercise regimen preserves prefrontal cortical volume against aging. Int J Geriatr Psychiatry. 2015 Jul;30(7):686-94. https://doi.org/10.1002/gps.4205 [DOI] [PubMed]
- 40.40. Rosano C, Guralnik J, Pahor M, Glynn NW, Newman AB, Ibrahim TS, et al. Hippocampal Response to a 24-Month Physical Activity Intervention in Sedentary Older Adults. Am J Geriatr Psychiatry. 2017 Mar;25(3):209-17. https://doi.org/10.1016/j.jagp.2016.11.007 [DOI] [PMC free article] [PubMed]
- 41.41. Zschucke E, Renneberg B, Dimeo F, Wüstenberg T, Ströhle A. The stress-buffering effect of acute exercise: Evidence for HPA axis negative feedback. Psychoneuroendocrinology. 2015 Jan;51:414-25. https://doi.org/10.1016/j.psyneuen.2014.10.019 [DOI] [PubMed]
- 42.42. Szuhany KL, Bugatti M, Otto MW. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J Psychiatr Res. 2015 Jan;60:56-64. https://doi.org/10.1016/j.jpsychires.2014.10.003 [DOI] [PMC free article] [PubMed]
- 43.43. Ruscheweyh R, Willemer C, Krüger K, Duning T, Warnecke T, Sommer J, et al. Physical activity and memory functions: an interventional study. Neurobiol Aging. 2011 Jul;32(7):1304-19. https://doi.org/10.1016/j.neurobiolaging.2009.08.001 [DOI] [PubMed]
- 44.44. Knaepen K, Goekint M, Heyman ME, Meeusen R. Neuroplasticity - exercise-induced response of peripheral brain-derived neurotrophic factor: a systematic review of experimental studies in human subjects. Sports Med. 2010 Sep;40(9):765-801. https://doi.org/10.2165/11534530-000000000-00000 [DOI] [PubMed]
- 45.45. Rosano C, Venkatraman VK, Guralnik J, Newman AB, Glynn NW, Launer L, et al. Psychomotor speed and functional brain MRI 2 years after completing a physical activity treatment. J Gerontol A Biol Sci Med Sci. 2010 Jun;65(6):639-47. https://doi.org/10.1093/gerona/glq038 [DOI] [PMC free article] [PubMed]
- 46.46. Winter B, Breitenstein C, Mooren FC, Voelker K, Fobker M, Lechtermann A et al. High impact running improves learning. Neurobiol Learn Mem. 2007 May;87(4):597-609. https://doi.org/10.1016/j.nlm.2006.11.003 [DOI] [PubMed]
- 47.47. Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol. 2009 Oct;94(10):1062-9. https://doi.org/10.1113/expphysiol.2009.048512 [DOI] [PubMed]
- 48.48. Tang SW, Chu E, Hui T, Helmeste D, Law C. Influence of exercise on serum brain-derived neurotrophic factor concentrations in healthy human subjects. Neurosci Lett. 2008 Jan;431(1):62-5. https://doi.org/10.1016/j.neulet.2007.11.019 [DOI] [PubMed]
- 49.49. Vedovelli K, Giacobbo BL, Corrêa MS, Wieck A, Argimon IIL, Bromberg E. Multimodal physical activity increases brain-derived neurotrophic factor levels and improves cognition in institutionalized older women. Geroscience. 2017 Aug;39(4):407-17. https://doi.org/10.1007/s11357-017-9987-5 [DOI] [PMC free article] [PubMed]
- 50.50. Matias I, Gatta-Cherifi B, Tabarin A, Clark S, Leste-Lasserre T, Marsicano G, et al. Endocannabinoids measurement in human saliva as potential biomarker of obesity. PLoS One. 2012;7(7):e42399. https://doi.org/10.1371/journal.pone.0042399 [DOI] [PMC free article] [PubMed]
- 51.51. Tantimonaco M, Ceci R, Sabatini S, Rossi A, Gasperi V, Maccarrone M. Physical activity and the endocannabinoid system: An overview. Cell Mol Life Sci. 2014 Jul;71(14):2681-98. https://doi.org/10.1007/s00018-014-1575-6 [DOI] [PMC free article] [PubMed]
- 52.52. Gasperi V, Ceci R, Tantimonaco M, Talamonti E, Battista N, Parisi A, et al. The fatty acid amide hydrolase in lymphocytes from sedentary and active subjects. Med Sci Sports Exerc. 2014 Jan;46(1):24-32. https://doi.org/10.1249/MSS.0b013e3182a10ce6 [DOI] [PubMed]
- 53.53. Brellenthin AG, Crombie KM, Hillard CJ, Koltyn KF. Endocannabinoid and mood responses to exercise in adults with varying activity levels. Med Sci Sports Exerc. 2017 Aug;49(8):1688-96. https://doi.org/10.1249/MSS.0000000000001276 [DOI] [PubMed]
- 54.54. Sparling P, Giuffrida A, Piomelli D, Rosskopf L, Dietrich A. Exercise activates the endocannabinoid system. Neuroreport. 2003 Dec;14(17):2209-11. https://doi.org/10.1097/00001756-200312020-00015 [DOI] [PubMed]
- 55.55. Crosby KM, Bains JS. The intricate link between glucocorticoids and endocannabinoids at stress-relevant synapses in the hypothalamus. Neuroscience. 2012 Mar;204:31-7. https://doi.org/10.1016/j.neuroscience.2011.11.049 [DOI] [PubMed]
- 56.56. Antunes HKM, Leite GSF, Lee KS, Barreto AT, Dos Santos RVT, Souza HS, et al. Exercise deprivation increases negative mood in exercise-addicted subjects and modifies their biochemical markers. Physiol Behav. 2016 Mar;156:182-90. https://doi.org/10.1016/j.physbeh.2016.01.028 [DOI] [PubMed]
- 57.57. Basso JC, Suzuki WA. The effects of acute exercise on mood, cognition, neurophysiology and neurochemical pathways: a review. Brain Plast. 2017 Feb;2(2):127-52. https://doi.org/10.3233/BPL-160040 [DOI] [PMC free article] [PubMed]
- 58.58. Dennis A, Thomas AG, Rawlings NB, Near J, Nichols TE, Clare S, et al. An ultra-high field magnetic resonance spectroscopy study of post exercise lactate, glutamate and glutamine change in the human brain. Front Physiol. 2015;6:351. https://doi.org/10.3389/fphys.2015.00351 [DOI] [PMC free article] [PubMed]
- 59.59. Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994 Oct;91(22):10625-9. https://doi.org/10.1073/pnas.91.22.10625 [DOI] [PMC free article] [PubMed]
- 60.60. Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003 Mar;14(2):125-30. https://doi.org/10.1111/1467-9280.t01-1-01430 [DOI] [PubMed]
- 61.61. Smith SM, Miller KL, Salimi-Khorshidi G, Webster M, Beckmann CF, Nichols TE, et al. Network modelling methods for FMRI. Neuroimage. 2011 Jan;54(2):875-91. https://doi.org/10.1016/j.neuroimage.2010.08.063 [DOI] [PubMed]
- 62.62. Heo S, Kramer AF. The Influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention [master's thesis]. Urbana-Campaign: University of Illinois; 2010. [DOI] [PMC free article] [PubMed]
- 63.63. Voss MW, Erickson KI, Prakash RS, Chaddock L, Malkowski E, Alves H, et al. Functional connectivity: a source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia. 2010 Apr;48(5):1394-406. https://doi.org/10.1016/j.neuropsychologia.2010.01.005 [DOI] [PMC free article] [PubMed]
- 64.64. Thomas AG, Dennis A, Bandettini PA, Johansen-Berg H. The effects of aerobic activity on brain structure. Front Psychol. 2012 Mar;3:86. https://doi.org/10.3389/fpsyg.2012.00086 [DOI] [PMC free article] [PubMed]
- 65.65. Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009 Oct;19(10):1030-9. https://doi.org/10.1002/hipo.20547 [DOI] [PMC free article] [PubMed]
- 66.66. Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, Mcauley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U.S.A. 2011; 108:3017–3022. [DOI] [PMC free article] [PubMed]
- 67.67. Pajonk FG, Wobrock T, Gruber O, Scherk H, Berner D, Kaizl I, et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry. 2010 Feb;67(2):133-43. https://doi.org/10.1001/archgenpsychiatry.2009.193 [DOI] [PubMed]
- 68.68. Gomes FGN, Fernandes J, Campos DV, Cassilhas RC, Viana GM, D'Almeida V, et al. The beneficial effects of strength exercise on hippocampal cell proliferation and apoptotic signaling is impaired by anabolic androgenic steroids. Psychoneuroendocrinology. 2014 Dec;50:106-17. https://doi.org/10.1016/j.psyneuen.2014.08.009 [DOI] [PubMed]
- 69.69. Nokia MS, Lensu S, Ahtiainen JP, Johansson PP, Koch LG, Britton SL, et al. Physical exercise increases adult hippocampal neurogenesis in male rats provided it is aerobic and sustained. J Physiol. 2016 Apr;594(7):1855-73. https://doi.org/10.1113/JP271552 [DOI] [PMC free article] [PubMed]
- 70.70. Kim YS, Shin SK, Hong SB, Kim HJ. The effects of strength exercise on hippocampus volume and functional fitness of older women. Exp Gerontol. 2017; 97:22-28. https://doi.org/10.1016/j.exger.2017.07.007 [DOI] [PubMed]
- 71.71. Puttemans V, Wenderoth N, Swinnen SP. Changes in brain activation during the acquisition of a multifrequency bimanual coordination task: from the cognitive stage to advanced levels of automaticity. J Neurosci. 2005 Apr;25(17):4270-8. https://doi.org/10.1523/JNEUROSCI.3866-04.2005 [DOI] [PMC free article] [PubMed]
- 72.72. Steele CJ, Penhune VB. Specific increases within global decreases: a functional magnetic resonance imaging investigation of five days of motor sequence learning. J Neurosci. 2010 Jun;30(24):8332-41. https://doi.org/10.1523/JNEUROSCI.5569-09.2010 [DOI] [PMC free article] [PubMed]
- 73.73. Taubert M, Villringer A, Ragert P. Learning-related gray and white matter changes in humans: an update. Neuroscientist. 2012 Aug;18(4):320-5. https://doi.org/10.1177/1073858411419048. [DOI] [PubMed]
- 74.74. Verstynen TD, Lynch B, Miller DL, Voss MW, Prakash RS, Chaddock L, et al. Caudate nucleus volume mediates the link between cardiorespiratory fitness and cognitive flexibility in older adults. J Aging Res. 2012 Jul;2012:939285. https://doi.org/10.1155/2012/939285 [DOI] [PMC free article] [PubMed]