Abstract
Background
This is an update of the Cochrane Review published in Issue 4, 2015. Cervical cancer is one of the most frequent cause of death from gynaecological cancers worldwide. Many new cervical cancer cases in low‐income countries present at an advanced stage. Standard care in Europe and the US for locally advanced cervical cancer (LACC) is chemoradiotherapy. In low‐income countries, with limited access to radiotherapy, LACC may be treated with chemotherapy and hysterectomy. It is not certain if this improves survival. It is important to assess the value of hysterectomy with radiotherapy or chemotherapy, or both, as an alternative.
Objectives
To determine whether hysterectomy, in addition to standard treatment with radiotherapy or chemotherapy, or both, in women with LACC (Stage IB2 to III) is safe and effective compared with standard treatment alone.
Search methods
We searched CENTRAL, MEDLINE via Ovid, Embase via Ovid, LILACS, trial registries and the grey literature up to 3 February 2022.
Selection criteria
We searched for randomised controlled trials (RCTs) that compared treatments involving hysterectomy versus radiotherapy or chemotherapy, or both, in women with LACC International Federation of Gynecology and Obstetrics (FIGO) Stages IB2 to III.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We independently assessed study eligibility, extracted data and assessed the risk of bias. Where possible, we synthesised overall (OS) and progression‐free (PFS) or disease‐free (DFS) survival in a meta‐analysis using a random‐effects model. Adverse events (AEs) were incompletely reported and we described the results of single trials in narrative form. We used the GRADE approach to assess the certainty of the evidence.
Main results
From the searches we identified 968 studies. After deduplication, title and abstract screening, and full‐text assessment, we included 11 RCTs (2683 women) of varying methodological quality. This update identified four new RCTs and three ongoing RCTs.
The included studies compared: hysterectomy (simple or radical) with radiotherapy or chemoradiotherapy or neoadjuvant chemotherapy (NACT) versus radiotherapy alone or chemoradiotherapy (CCRT) alone or CCRT and brachytherapy. There is also one ongoing study comparing three groups: hysterectomy with CCRT versus hysterectomy with NACT versus CCRT.
There were two comparison groups for which we were able to do a meta‐analysis.
Hysterectomy (radical) with neoadjuvant chemotherapy versus chemoradiotherapy alone
Two RCTs with similar design characteristics (620 and 633 participants) found no difference in five‐year OS between NACT with hysterectomy versus CCRT. Meta‐analysis assessing 1253 participants found no evidence of a difference in risk of death (OS) between women who received NACT plus hysterectomy and those who received CCRT alone (HR 0.94, 95% CI 0.76 to 1.16; moderate‐certainty evidence). In both studies, the five‐year DFS in the NACT plus surgery group was worse (57%) compared with the CCRT group (65.6%), mostly for Stage IIB.
Results of single trials reported no apparent difference in long‐term severe complications, grade 3 acute toxicity and severe late toxicity between groups (very low‐quality evidence).
Hysterectomy (simple or radical) with neoadjuvant chemotherapy versus radiotherapy alone
Meta‐analysis of three trials of NACT with hysterectomy versus radiotherapy alone, assessing 571 participants, found that women who received NACT plus hysterectomy had less risk of death (OS) than those who received radiotherapy alone (HR 0.71, 95% CI 0.55 to 0.93; I2 = 0%; moderate‐quality evidence). However, a significant number of participants who received NACT plus hysterectomy also had radiotherapy. There was no difference in the proportion of women with disease progression or recurrence (DFS and PFS) between NACT plus hysterectomy and radiotherapy groups (RR 0.75, 95% CI 0.53 to 1.05; I2 = 20%; moderate‐quality evidence).
The certainty of the evidence was low or very‐low for all other comparisons for all outcomes.
None of the trials reported quality of life outcomes.
Authors' conclusions
From the available RCTs, we found insufficient evidence that hysterectomy with radiotherapy, with or without chemotherapy, improves the survival of women with LACC who are treated with radiotherapy or CCRT alone. The overall certainty of the evidence was variable across the different outcomes and was universally downgraded due to concerns about risk of bias. The certainty of the evidence for NACT and radical hysterectomy versus radiotherapy alone for survival outcomes was moderate. The same occurred for the comparison involving NACT and hysterectomy compared with CCRT alone. Evidence from other comparisons was generally sparse and of low or very low‐certainty. This was mainly based on poor reporting and sparseness of data where results were based on single trials. More trials assessing medical management with and without hysterectomy may test the robustness of the findings of this review as further research is likely to have an important impact on our confidence in the estimate of effect.
Keywords: Female; Humans; Chemoradiotherapy; Chemoradiotherapy/adverse effects; Chemoradiotherapy/methods; Chemotherapy, Adjuvant; Chemotherapy, Adjuvant/methods; Hysterectomy; Neoadjuvant Therapy; Neoadjuvant Therapy/methods; Uterine Cervical Neoplasms; Uterine Cervical Neoplasms/drug therapy; Uterine Cervical Neoplasms/radiotherapy
Plain language summary
Hysterectomy with medical management for cervical cancer that has spread to nearby tissues only
The issue
Cancer of the neck of the womb (cervical cancer) is the most common cancer among women up to 65 years of age. A high proportion of women in poor countries are diagnosed with locally advanced cervical cancer (spread to nearby tissues, but no obvious distant spread). They are usually treated with radiotherapy, with or without chemotherapy (medical treatment). Hysterectomy (surgery to remove the womb and the cervix) with medical treatment is also used, especially in poor countries where access to radiotherapy is limited.
The aim of the review
Is hysterectomy with medical treatment more beneficial compared to medical treatment alone in women with locally advanced cervical cancer?
How did we conduct the review?
A literature search from 1966 to February 2022 identified 11 clinical trials at moderate to high risk of bias. These included 2683 women and compared: hysterectomy with radiotherapy versus radiotherapy alone; hysterectomy with chemoradiotherapy (chemotherapy plus radiotherapy) versus chemoradiotherapy alone; hysterectomy with chemoradiotherapy versus internal radiotherapy (brachytherapy) with chemoradiotherapy; and hysterectomy preceded by chemotherapy (neoadjuvant, to reduce the size of the cancer) versus radiotherapy alone. We also identified three ongoing trials.
What are the main findings?
Hysterectomy (simple (womb and cervix) or radical (womb, cervix and surrounding tissues)) with neoadjuvant chemotherapy versus radiotherapy alone
By combining results from three studies that assessed 571 women, we found that fewer women who received neoadjuvant chemotherapy plus hysterectomy died than those who received radiotherapy alone. However, many women in the first group also had radiotherapy. There was no difference in the number of women who were disease‐free after treatment.
Hysterectomy (radical) with neoadjuvant chemotherapy versus chemoradiotherapy alone
We combined the results of two studies that assessed 1253 women. We found no difference in the risk of death between women who received hysterectomy with neoadjuvant chemotherapy and those who received chemoradiotherapy alone.
Side effects were not well reported. Results of single trials showed no differences in severe side effects between groups in any comparison. Limited data suggested that the interventions appeared to be reasonably well tolerated, although more evidence is needed.
Studies did not report how women's quality of life was affected.
What are the conclusions?
We found insufficient evidence that hysterectomy added to radiotherapy and chemoradiation improved survival, quality of life or side effects in women with locally advanced cervical cancer compared with medical treatment alone. Overall, the quality of the evidence was variable and we had concerns about risk of bias. More trials assessing medical management with and without hysterectomy may test the robustness of the findings of this review. Further data from carefully planned trials assessing medical management with and without hysterectomy are likely to impact on how confident we are about these findings.
Summary of findings
Summary of findings 1. Hysterectomy (radical) with neoadjuvant chemotherapy versus chemoradiotherapy alone for women with locally advanced cervical cancer.
| Hysterectomy (radical) with neoadjuvant chemotherapy versus with chemoradiotherapy alone for women with locally advanced cervical cancer | ||||
|
Patient or population: women with locally advanced cervical cancer Settings: outpatient Intervention: NACT + hysterectomy Comparison: CCRT | ||||
| Outcomes | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence(GRADE) | Comments |
|
Overall survival Median follow‐up 58.5–98.4 months in the 2 trials |
HR 0.94 (0.76 to 1.16) | 1253 (2 RCTs) |
⊕⊕⊕⊝ Moderatea |
I2 = 0% |
|
DFS Median follow‐up 58.5–98.4 months in the 2 trials |
HR 1.38 (1.02 to 1.87) | 633 (1 RCT) |
⊕⊕⊝⊝ Lowb |
— |
| 5‐year DFS in the NACT + surgery group was 57% vs 65.6% in the chemoradiotherapy group (P = 0.021) | 620 (1 RCT) |
|||
| Quality of life | — | — | — | Not reported. |
| SAEs and toxicity |
SAEs In first trial, there were no toxic deaths reported. 198 SAEs occurred: 145 in the NACT + surgery arm vs 53 in the CCRT arm. In the second trial there were 114 grade 3 or 4 SAEs: 92 in the NACT + surgery arm vs 22 in the CCRT arm |
198 (1 RCT) 114 (1 RCT) |
⊕⊝⊝⊝ Very lowc |
— |
|
Toxicity In 1 trial, NACT + surgery group, compared with the chemoradiotherapy group, there was a lower rate of rectal (5.7% with NACT + surgery vs 13.3% with chemoradiotherapy; P = 0.002), bladder (2.8% with NACT + surgery vs 7.3% with chemoradiotherapy; P = 0.017), and vaginal (19.9% with NACT + surgery vs 36.9% with chemoradiotherapy; P = 0.001) toxicity occurring or persisting 90 days after treatment completion. However, 24 months after treatment completion, there was no difference in rectal and bladder toxicities between groups, whereas vaginal toxicity continued to occur at a lower rate in the NACT + surgery group (12.0% with NACT + surgery vs 25.6% with chemoradiotherapy; P = 0.001). |
114 (1 RCT) |
|||
|
Treatment‐related morbidity No treatment‐related deaths in either chemoradiotherapy or NACT + surgery arm. Overall, 89% of participants in the chemoradiotherapy arm and 73% in the NACT + surgery arm had complications, with 18% in NACT + surgery arm experiencing recurrence and requiring adjuvant radiotherapy. |
111 (1 RCT) |
|||
| CI: confidence interval; CCRT: concurrent chemoradiotherapy; DFS: disease‐free survival; HR: hazard ratio; NACT: neoadjuvant chemotherapy; SAE: serious adverse event. | ||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||
aDowngraded one level due to concerns regarding the uncertainty of risk of bias in individual trials and only two trials in meta‐analysis (although it is arguable whether the number of included participants represented relatively sparse data). bDowngraded two levels due to risk of bias and sparse data. cDowngraded three levels due to incomplete and poor reporting of important adverse events and toxicities, sparseness of data and risk of bias concerns.
Summary of findings 2. Hysterectomy (simple or radical) with neoadjuvant chemotherapy versus radiotherapy alone for women with locally advanced cervical cancer.
| Hysterectomy (simple or radical) with neoadjuvant chemotherapy versus radiotherapy alone for women with locally advanced cervical cancer | ||||
|
Patient or population: women with locally advanced cervical cancer Settings: outpatient Intervention: neoadjuvant chemotherapy + radical hysterectomy Comparison: radiotherapy alone | ||||
| Outcomes | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments |
|
Overall survival Median follow‐up 39–60 months in the 3 trials |
HR 0.71 (0.55 to 0.93) | 571 (3 RCTs) | ⊕⊕⊕⊝ Moderatea | — |
|
Disease‐ or progression‐free survival Median follow‐up 39–60 months in the 3 trials |
HR 0.75 (0.53 to 1.05) | 571 (3 RCTs) | ⊕⊕⊕⊝ Moderatea | There were varying definitions of disease‐ and progression‐free survival. However, we did not consider this merited further downgrading to low‐certainty evidence. |
| Quality of life | — | — | — | Not reported. |
| Severe adverse events and toxicity |
Acute severe toxicity RR 1.32 (0.47 to 3.71) |
118 (1 RCT) |
⊕⊕⊝⊝ Lowb | — |
|
Long‐term severe complications RR 0.86 (0.49 to 1.50) |
409 (1 RCT) |
|||
|
Severe late toxicity RR 0.60 (0.27 to 1.34) |
118 (1 RCT) |
|||
| CI: confidence interval; HR: hazard ratio; RCT: randomised controlled trial; RR: risk ratio. | ||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||
aDowngraded one level due to concerns regarding the uncertainty of risk of bias in individual trials. bDowngraded two levels due to incomplete and poor reporting of important adverse events and toxicities and sparseness of data.
Summary of findings 3. Hysterectomy (simple or radical) with radiotherapy versus radiotherapy alone.
| Hysterectomy (simple or radical) with radiotherapy versus radiotherapy alone | ||||
|
Patient or population: women with locally advanced cervical cancer Settings: outpatient Intervention: radiotherapy + hysterectomy (simple or radical) Comparison: radiotherapy alone | ||||
| Outcomes | Relative effect (95% CI) | No of participants | Certainty of the evidence (GRADE) | Comments |
|
Overall survival Median follow‐up 9.6 years |
HR 0.89 (0.61 to 1.29) | 256 (1 RCT) |
⊕⊕⊖⊖ Lowa,b |
12 participants in each regimen (10% with radiotherapy + hysterectomy vs 9% with radiotherapy) were lost to follow‐up by 5 years. |
|
Progression‐free survival Median follow‐up 9.6 years |
HR 0.77 (0.54 to 1.10) |
256 (1 RCT) |
⊕⊕⊖⊖ Lowa,b |
12 participants in each regimen (10% with radiotherapy + hysterectomy vs 9% with radiotherapy) were lost to follow‐up by 5 years. |
| Tumour‐free actuarial survival at 5 years | 5‐year, tumour‐free actuarial survival for women with Stage IB was 80% in the preoperative radiotherapy + hysterectomy group and 89% in the radiotherapy group. In Stage IIA, these rates were 79% in the preoperative radiotherapy + hysterectomy group and 56% in the radiotherapy group. | 118 (1 RCT) |
⊕⊖⊖⊖ Very lowa,b,c |
— |
| Quality of life | — | — | — | Not reported. |
| Severe/serious adverse events | 1 trial stated that both treatment programmes were well tolerated and there were no differences between groups in adverse effects. There were 18/129 women with a grade 3 or 4 adverse effect in the radiotherapy + hysterectomy group and 19 cases in 18/121 women of severe adverse effects in the radiotherapy group. In another trial, only 1/48 (2%) women with Stage IB disease experienced a severe complication (grade 3) in the radiotherapy + hysterectomy group (ureteral stricture) whereas 5/40 experienced severe complications in the radiotherapy group (including rectovaginal fistula, vesicovaginal fistula, ureteral stricture and pelvic infection) (P > 0.05). Similarly in women with Stage IIA disease, 5/14 (40%) women experienced a severe complication in the radiotherapy + hysterectomy group (including proctitis, rectal stricture, small bowel stricture and ureteral stricture) whereas only 1/16 women experienced a severe complication in the radiotherapy group (rectal stricture) (P > 0.05). |
374 (2 RCTs) |
⊕⊕⊖⊖ Lowa,b |
Relative effect measures were not presented due to the crude combining of adverse events or sparse data, or both. |
| CI: confidence interval; HR: hazard ratio; RCT: randomised controlled trial. | ||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||
aDowngraded one level due to sparse data leading to imprecision. bDowngraded one level due to small number of trials and a lack of representation. cDowngraded one level due to inadequate reporting of results.
Summary of findings 4. Hysterectomy (simple or radical) with chemoradiotherapy versus chemoradiotherapy alone.
| Hysterectomy (simple or radical) with chemoradiotherapy versus chemoradiotherapy alone | ||||
|
Patient or population: women with locally advanced cervical cancer Settings: outpatient Intervention: chemoradiotherapy + hysterectomy (simple or radical) Comparison: chemoradiotherapy alone | ||||
| Outcomes | Relative effect | No of participants | Certainty of the evidence (GRADE) | Comments |
|
Overall survival Median follow‐up 3.8 years |
Overall survival was inadequately reported and it was not possible to calculate a hazard ratio. Overall survival time in the chemoradiotherapy + hysterectomy group was 6–40 months, median survival time was 23 months, and 3‐year survival rate was 82.7%. Total survival time in the chemoradiotherapy group was 5–41 months, median survival time was 22.5 months and 3‐year survival rate was 81.8%. Trial authors reported differences between arms were not statistically significant (P = 0.56). | 102 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
|
Progression or event‐free survival Median follow‐up 3.8 years |
Progression‐free survival was inadequately reported in both trials and it was not possible to calculate a hazard ratio. In 1 trial, progression‐free survival time in the chemoradiotherapy + hysterectomy group was 3–40 months, median survival time was 23 months and 3‐year survival rate was 73.1%. The progression‐free survival time in the chemoradiotherapy alone group was 5–41 months, median survival time was 22 months and 3‐year survival rate was 64.8%. There was no significant difference between arms (P = 0.76). Another trial included 61 women and compared chemoradiotherapy + simple or radical hysterectomy vs chemoradiotherapy alone. There was no difference in 3‐year event‐free (death) survival rate (86% in the chemoradiotherapy + hysterectomy group vs 97% in the chemoradiotherapy alone group; log rank P = 0.15). |
163 (2 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
| Quality of life | — | — | — | Not adequately reported. |
| Severe/serious adverse events | — | — | — | Not adequately reported. |
| RCT: randomised controlled trial. | ||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||
aDowngraded two levels due to sparse data leading to imprecision. bDowngraded one level due to small number of trials and a lack of representation.
Summary of findings 5. Hysterectomy (simple or radical) with chemoradiotherapy versus internal radiotherapy (brachytherapy) with chemoradiotherapy.
| Hysterectomy (simple or radical) with chemoradiotherapy versus internal radiotherapy (brachytherapy) with chemoradiotherapy | ||||
|
Patient or population: women with locally advanced cervical cancer Settings: outpatient Intervention: chemoradiotherapy + hysterectomy (simple or radical) Comparison: chemoradiotherapy alone | ||||
| Outcomes | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments |
|
Overall survival Median follow‐up 3 years |
HR 0.65 (95% CI 0.35 to 1.21) | 211 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | — |
|
Progression or event‐free survival Median follow‐up 3 years |
HR 0.70 (95% CI 0.31 to 1.34) | 211 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | — |
| Quality of life | — | — | — | Not reported. |
| Severe late complications | There was no difference in the proportion of women with severe late complications in the brachytherapy and radical hysterectomy groups (P = 0.53). There were 4 cases of grade 3 or 4 proctitis in the brachytherapy group vs 2 cases in the radical hysterectomy group; 3 cases of severe cystitis in the brachytherapy group vs 0 in the radical hysterectomy group; 0 cases of grade 3 or 4 hydronephrosis in either group. Of the 211 participants, chemoradiotherapy with cisplatin and gemcitabine appeared to be reasonably well tolerated, although nearly a third of women experienced severe neutropenia (most grade 3). Of the 86 women who received a radical hysterectomy, the number of intraoperative and early surgical complications appeared to be reasonably low, with bleeding (9/86) being the most common. |
211 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | — |
| CI: confidence interval; RCT: randomised controlled trial. | ||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||
aDowngraded one level due to sparse data leading to imprecision. bDowngraded one level due to small number of trials and a lack of representation.
Background
Description of the condition
Cervical cancer is the fourth most frequently diagnosed cancer and the fourth leading cause of cancer death in women, with an estimated 604,000 new cases and 342,000 deaths worldwide in 2020 (Global Cancer Statistics 2020). Rates remain disproportionately high in transitioning (countries with a low or medium Human Development Index (a measure that considers income, health and education to assess how well economies are doing)) versus transitioned (countries with a high or very high Human Development Index) countries (incidence: 18.8 per 100,000 in transitioning versus 11.3 per 100,000 in transitioned; mortality: 12.4 per 100,000 in transitioning versus 5.2 per 100,000 in transitioned). Mortality from cervical cancer has declined in many high‐income countries, particularly in countries with organised cervical cancer screening programmes; however, in low‐income countries, mortality has increased and it remains a major international problem (Global Cancer Statistics 2020). Cervical cancer is considered nearly completely preventable with primary and secondary measures such as human papillomavirus vaccination and screening methods; however, these measures have not been equitably implemented across and within countries, resulting in the above inequality on incidence and mortality. The introduction in 1988 of a national cervical screening programme in the UK within a decade led to a halving in the incidence of cervical cancer, from an age‐standardised incidence rate of 16.2 per 100,000 to an age‐standardised incidence rate of 8.3 per 100,000 in 2008 (NCIN 2010). In many transitioning countries, access to health services is limited and screening for cervical cancer is either absent or reaches few of the women who need it. In these areas, cervical cancer is the most common cancer in women and the leading cause of cancer death (Global Cancer Statistics 2020; Mathers 2008).
In 2018, the World Health Organization called for global action to reduce incidence of cervical cancer (to 4 per 100,000 or less worldwide) by adopting a triple intervention strategy of vaccinating young girls by the age of 15 years, screening of women twice in the age range of 35 to 45 years and treating at least 90% of the precancer lesions detected through screening (Global Cancer Statistics 2020). In the meantime, many women are diagnosed at an advanced stage that is more difficult to treat. Naga and colleagues reported that more than two‐thirds of women have advanced disease at diagnosis and approximately 85% occur in transitioning countries (Naga 2018). Another report from a transitioning country has shown that over 80% of new cervical cancer cases are found at advanced stages (Stage IB2 or more), and over half of these are Stage III to IV (Khuhaprema 2010).
Cervical cancer is staged according to the International Federation of Gynecology and Obstetrics (FIGO) system (Appendix 1). Since the publication of the first review, the FIGO staging of cervical cancer has changed (Singh 2019; Table 6). Prior to this, FIGO staging for cervical cancer was based mainly on clinical examination (Percorelli 2009). In 2018, this approach was revised to allow imaging (r) and pathology (p) findings, where available, to assign stage (Bhatla 2018). The most important changes were as follows.
1. Comparison of 2009 and 2018 FIGO staging of cervical cancer.
| Stage I (2018): carcinoma strictly confined to the cervix (extension to the uterine corpus should be disregarded) | ||
| 2009 FIGO stage: description | 2018 FIGO stage: description | Comment |
| IA: invasive carcinoma diagnosed only by microscopy, with maximum depth of invasion </= 5 mm and largest extension </= 7 mm | IA: invasive carcinoma diagnosed only by microscopy, with maximum depth of invasion < 5 mm |
– Lateral extent of the carcinoma is no longer considered in distinguishing between FIGO Stage IA and IB carcinomas – If margins of loop are involved patient is allocated to Stage IB1 |
|
|
|
|
|
|
| IB: clinically visible lesions limited to the cervix or preclinical cancers greater than Stage IA | IB: invasive carcinoma with measured deepest invasion >/= 5 mm (greater than Stage IA), lesion limited to the cervix uteri |
– See above – LVSI must be commented upon, although does not affect FIGO stage |
|
IB1: invasive carcinoma >/= 5 mm depth of stromal invasion, and < 2 cm in greatest dimension | – New stage category |
| IB2: invasive carcinoma >/= 2 cm and < 4 cm in greatest dimension | – New stage category | |
| IB2: invasive carcinoma > 4 cm in greatest dimension | IB3: invasive carcinoma >/= 4 cm in greatest dimension | – New stage category |
Adapted from Singh 2019. LVSI: lymphovascular space invasion.
The horizontal dimension is no longer considered in defining the upper boundary of a Stage IA carcinoma.
The diagnosis of Stage IA1 and IA2 carcinomas is made on microscopic examination of a surgical specimen, which includes the entire lesion. The margins of an excision specimen should be reported to be negative for disease.
If the margins of the cone biopsy are positive for invasive cancer, the patient is assigned to Stage IB1.
Stage IB has been subdivided into IB1, IB2 and IB3 based on maximum tumour size.
The revised 2018 system includes nodal status; the presence of nodal involvement in a tumour of any size upstages the case to Stage IIIC, with IIIC1 indicating pelvic and IIIC2 indicating para‐aortic nodal involvement. The revised FIGO classification is thereby now more closely aligned with the structure of the TNM classification, which is a classification system of cancer that describes the size of the tumour and any spread of cancer into nearby tissue (T); it describes spread of cancer to nearby lymph nodes (N); and it also describes metastasis (spread of cancer to other parts of the body) (M) (Bhatla 2018).
Remaining or recurrent disease frequently occurs after initial treatment in more than 50% of Stage III to IVA cervical cancer, leading to mortality (Appendix 1).
In this review, women with locally advanced cervical cancer (LACC) were the population of interest. It is likely that the included studies used the 2009 FIGO classification system (Percorelli 2009), as the new system was introduced in 2018 (Bhatla 2018).
Description of the intervention
Treatment decisions for invasive cervical cancer should be individualised and based on factors such as age, medical condition of the women, stage of disease and other tumour‐related factors in order to yield the best cure with minimum complications (Kesic 2006). As a general rule, multiple treatment modalities have more potential complications and adverse effects than one treatment modality.
For Stage IA1, local cervical treatments (large loop or needle excision of the transformation zone (LLETZ/NETZ), knife cone biopsy) or total hysterectomy (surgery to remove the womb and the neck of the womb) can be used, depending on women's preferences and fertility aspirations.
For Stage IA2 to IB1, radical hysterectomy with pelvic lymphadenectomy or chemoradiotherapy have been the accepted treatment modalities with reported similar efficacies (Eifel 1993). This finding was supported mainly by a randomised controlled trial (RCT) before the concurrent chemoradiotherapy (CCRT) era (Landoni 1997). In younger women, surgery is preferred, partly because of the advantage of preservation of ovarian function.
Radical hysterectomy, and bilateral pelvic lymphadenectomy, involves the removal of the uterus, the cervix, the upper part of the vagina and the tissues around the cervix (parametrial tissue), as well as the lymph nodes (glands) in the pelvis to determine if they contain cancer cells (pelvic lymphadenectomy). Although this type of surgery has excellent results, it can result in adverse effects such as organ injury (bladder, bowel, blood vessel, nerve) and long‐term adverse effects such as sexual or bladder dysfunction, pelvic cyst formation and lymphoedema (swelling) of the legs.
Radical trachelectomy may be an alternative to radical hysterectomy in women who want to preserve their fertility, provided they meet certain criteria. These are tumour size 2 cm or less and no metastasis to regional lymph nodes (Shepherd 2012). Radical trachelectomy involves removing the cervix, the upper part of the vagina and the parametrial tissue and the pelvic lymph glands. This treatment is well‐established, appears to be safe and effective in preserving fertility, and has a high chance of conception. Late miscarriage and premature labour are the most serious adverse effects in pregnancies where the women have had a trachelectomy.
For Stage IB2 tumours and above, the incidence of lymph node metastasis increases significantly, as well as the incidence of central, regional and distant recurrences (Alvarez 1989; Burghardt 1978; Chung 1980; Delgado 1990; Piver 1975). If a surgical approach is chosen, there may be difficulties in removing all of the tumour with a margin of normal tissue (that is adequate surgery), therefore there is a high probability of requiring additional treatment (radiotherapy with or without chemotherapy) with the increased morbidity of combined treatment. For women with FIGO Stage IB2 disease and higher, chemoradiotherapy is now standard care; it has been shown to improve disease‐free survival (DFS), progression‐free survival (PFS) and overall survival (OS) (CCCMAC 2010; NCI 1999). It involves administration of cisplatin‐based chemotherapy during the course of radiotherapy, all delivered within seven weeks. The chemotherapy makes the cancer cells more sensitive to the radiotherapy and therefore improves the treatment results.
However, surgery in LACC (Stage IB2 to III) has been considered in the following cases:
after chemoradiotherapy, for those in whom no complete remission is achieved within two to three months following treatment, the tumour is oncologically operable and the woman is clinically fit to undergo additional surgery;
after either radiotherapy or chemotherapy are used to shrink the cervical tumour to a size where it can be removed with normal margins;
following neoadjuvant chemotherapy (NACT; chemotherapy given before other treatments to reduce the size of the tumour), especially in transitioning countries with limited access to radiotherapy, but it remains unclear whether it offers a benefit over surgery alone or chemoradiotherapy (Rydzewska 2012).
Stage IVA cervical cancer, where the cancer has spread to the adjacent bladder or rectum, is usually treated with chemoradiotherapy (CCCMAC 2010). Some authors have suggested that NACT plus radical surgery (including removal of the affected bladder or rectum) might be a valid alternative to standard treatment (Benedetti Panici 2007).
For Stage IVB, the aim of treatment is generally palliative radiotherapy and chemotherapy (Kesic 2006; NCI 2014).
How the intervention might work
Although surgical resection of advanced non‐metastatic forms of cervical cancer is controversial, it may help improve local control (Houvenaeghel 1998). Many studies report favourable outcomes of hysterectomy for women with advanced cervical cancer after radiotherapy (Classe 2006; Kornovski 2007; Leino 1994; Noterman 2006; Potish 1990; Tsuda 2001; Wang 2002). Whether simple total hysterectomy (Leino 1994; Potish 1990; Wang 2002) or radical hysterectomy (Classe 2006; Kornovski 2007; Noterman 2006; Tsuda 2001; Wang 2002) is needed is unclear (Noterman 2006).
Multimodal treatment of hysterectomy combined with chemotherapy or radiotherapy, or both, may improve survival; but it may (Hequet 2013) or may not (Classe 2006; Perez 1987) cause significantly worse adverse events compared with radiotherapy or chemoradiotherapy alone.
Why it is important to do this review
It is important to assess the value of hysterectomy in addition to chemotherapy, radiotherapy or chemoradiotherapy in the treatment of LACC.
Objectives
To determine whether hysterectomy, in addition to standard treatment with radiotherapy or chemotherapy, or both, in women with LACC (Stage IB2 to III) is safe and effective compared with standard treatment alone.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Women (aged 18 years or older) with LACC (Stage IB2 to III).
Types of interventions
We compared hysterectomy in combination with neoadjuvant, concurrent or adjuvant therapy versus non‐surgical interventions.
Hysterectomy (radical) with NACT versus chemoradiotherapy alone.
Hysterectomy (simple or radical) with NACT versus radiotherapy alone.
Hysterectomy (simple or radical) with radiotherapy versus radiotherapy alone.
Hysterectomy (simple or radical) with chemoradiotherapy versus chemoradiotherapy alone.
Hysterectomy (radical) with chemoradiotherapy versus internal radiotherapy (brachytherapy) with chemoradiotherapy.
Hysterectomy (radical) with chemoradiotherapy versus hysterectomy (radical) with NACT versus chemoradiotherapy alone.
Types of outcome measures
Primary outcomes
Overall survival (OS): survival until death from all causes assessed from the time when women were enrolled in the study, or as defined by the trial authors.
Secondary outcomes
Progression‐free survival (PFS).
If authors reported disease‐free survival (DFS) rather than PFS then this was assessed.
Quality of life measures using a scale that had been validated through reporting of norms against a validated scale in a peer‐reviewed publication.
-
Severe adverse events
-
Surgery‐related complications: measured as the proportion of women who developed one of the items below (according to the study definition) within 12 weeks. These were classified as either early (before discharge from hospital or within seven days of surgery), late (from seven days to within 12 weeks of surgery), or total complications (early and late):
any postoperative infection;
surgery‐related injuries (blood vessel, nerve, bladder, bowel);
excessive blood loss (according to the study definition);
thromboembolic events;
any anaesthesiological complications;
other severe adverse event;
fistula formation;
voiding or bladder dysfunction;
lymphocysts or lymphoedema;
psychosexual dysfunction.
-
Chemotherapy‐ and radiotherapy‐related complications: grades of chemotherapeutic and radiotherapeutic toxicity were extracted and grouped as:
haematological (leukopenia, anaemia, thrombocytopenia, neutropenia, haemorrhage);
gastrointestinal (nausea, vomiting, anorexia, diarrhoea, liver toxicity, proctitis);
genitourinary;
skin (stomatitis, mucositis, alopecia, allergy);
neurological (peripheral and central); and
pulmonary.
-
Search methods for identification of studies
We sought papers in all languages and conducted translations where necessary.
Electronic searches
See: Cochrane Gynaecological Cancer Group methods used in reviews (gnoc.cochrane.org).
For this update, we searched the following electronic databases on 3 February 2022:
the Cochrane Register of Controlled Trials (CENTRAL; 2022, Issue 2), in the Cochrane Library Appendix 2;
MEDLINE via Ovid (1946 to 4 February 2022) Appendix 3;
Embase via Ovid (1980 to 2022 week 4) Appendix 4;
LILACS (February 2022) Appendix 5.
All relevant articles that were found were identified on PubMed and, using the 'related articles' feature, we carried out further searches for newly published articles.
Searching other resources
Unpublished and grey literature
We conducted a Google search for Internet‐based resources and open‐access publications. We searched Metaregister (www.controlled-trials.com/rct), Physicians Data Query (www.nci.nih.gov), ClinicalTrials.gov (www.clinicaltrials.gov), and the National Cancer Institute (www.cancer.gov/clinicaltrials) for ongoing trials. One trial was identified through these searches; this trial closed to recruitment in September 2014. We contacted the principal investigator for more details and preliminary results, but have not yet received a reply. Therefore, we have added this trial to the Ongoing studies section.
We searched conference proceedings and abstracts through ZETOC (zetoc.mimas.ac.uk) and WorldCat Dissertations.
Handsearching
We handsearched the citation lists of included studies, key textbooks and previous systematic reviews.
We handsearched reports/websites/conferences of the following:
International Gynecological Cancer Society (IGCS);
European Society of Gynaecological Oncology (ESGO);
Society of Gynecologic Oncologists (SGO);
British Gynaecological Cancer Society (BGCS);
Australian Society of Gynaecologic Oncologists (ASGO);
American Society of Clinical Oncology (ASCO);
European Society of Medical Oncology (ESMO);
Clinical Oncological Society of Australia (COSA).
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to the reference management database, Endnote. We removed duplicates and two review authors (AB, FK) independently examined the remaining references. We excluded those studies that clearly did not meet the inclusion criteria and obtained the full texts of potentially relevant references. Two review authors (AB, FK) independently assessed the eligibility of the retrieved papers. We resolved disagreements by discussion between the two review authors with a final review by the other authors (EB, MP, DO). Reasons for exclusion are documented.
Data extraction and management
For included studies, we abstracted data as recommended in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The data included the following:
author, year of publication and journal citation (including language);
country;
setting;
inclusion and exclusion criteria;
study design, methodology;
-
study population:
total number enrolled;
patient characteristics;
age;
comorbidities;
type of initial or primary treatment (chemotherapy, chemoradiation, or radiotherapy), including details on dose, duration and combination;
performance status;
-
advanced cervical cancer details at diagnosis:
stage;
grade;
histology;
-
intervention (hysterectomy) details:
type of hysterectomy (total or subtotal, simple or radical with/without pelvic lymphadenectomy);
timing of hysterectomy;
prevention of complications (prophylactic antibiotics or any other measures);
grade or prior training of surgeon;
-
comparison details:
details of dose and duration of chemotherapeutic, radiotherapeutic or a combination treatment used;
method of primary treatment administration;
drug regimen;
local control, for example, bleeding, pressure symptoms, pain;
risk of bias in study (assessment of risk of bias in included studies);
duration of follow‐up;
-
outcomes, OS and PFS, quality of life and severe adverse events:
for each outcome, outcome definition (with diagnostic criteria if relevant);
unit of measurement (if relevant);
for scales, upper and lower limits, and whether high or low score is good;
results, number of participants allocated to each intervention group and
for each outcome of interest, sample size, missing participants.
We extracted data on outcomes as follows.
For time to event (OS and PFS) data, we extracted the log of the hazard ratio (HR) (log(HR)) and its standard error from trial reports. If these were not reported, we attempted to estimate them from other reported statistics using the methods of Parmar 1998.
For dichotomous outcomes (e.g. adverse events), we extracted the number of participants in each treatment arm who experience the outcome of interest and the number of participants.
These were assessed at the endpoint in order to estimate a risk ratio (RR) and 95% confidence interval (CI).
Where possible, all the data extracted were those relevant to an intention‐to‐treat analysis in which the participants were analysed in the groups to which they were assigned. We noted the time points at which outcomes were collected and reported.
Two review authors (AB, FK) abstracted data independently onto a data abstraction form specially designed for the review. We resolved differences between review authors by discussion.
For continuous outcomes (e.g. quality of life), we had planned to extract the final value and standard deviation of the outcome of interest and the number of women assessed at the endpoint in each treatment arm at the end of follow‐up, in order to estimate the mean difference (if trials measured outcomes on the same scale) or standardised mean difference (if trials measured outcomes on different scales) between treatment arms and its standard error. However, none of the trials reported continuous outcome data for the quality of life.
Assessment of risk of bias in included studies
We assessed the risk of bias in the included RCTs in accordance with the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions using the Cochrane’s RoB 1 tool and the criteria specified in Chapter 8 (Higgins 2011). This included assessment of:
sequence generation;
allocation concealment;
blinding (restricted to blinding of outcome assessors as it was not possible to blind participants or investigators to these treatment modalities);
-
incomplete outcome data, we recorded the proportion of participants whose outcomes were not reported at the end of the study. We coded the satisfactory level of losses to follow‐up for each outcome as:
yes, if less than 20% of participants were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment arms;
no, if 20% or greater of participants were lost to follow‐up or reasons for loss to follow‐up were different between treatment arms;
unclear, if loss to follow‐up was not reported;
selective reporting of outcomes;
other possible sources of bias.
Two review authors (FK, AB) applied the risk of bias tool independently and resolved differences by discussion. Results were summarised in a risk of bias summary and graph (Figure 1; Figure 2). Results of meta‐analyses were interpreted depending on the findings with respect to risk of bias.
1.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Measures of treatment effect
We used the following measures of the effect of treatment:
for time‐to‐event data, we used HRs with 95% CI, where possible;
for dichotomous outcomes, we used RRs with 95% CI.
Unit of analysis issues
There were no unit of analysis issues.
Dealing with missing data
We did not impute missing outcome data for any of the outcomes.
Assessment of heterogeneity
We assessed heterogeneity between studies by visual inspection of forest plots, by estimation of the percentage heterogeneity between trials that could not be ascribed to sampling variation (Higgins 2003), and by a formal statistical test of the significance of the heterogeneity (Deeks 2001). If there was evidence of substantial heterogeneity, the possible reasons for the heterogeneity were investigated and reported.
Assessment of reporting biases
We did not examine funnel plots corresponding to meta‐analysis of the primary outcome to assess the potential for small‐study effects such as publication bias, as stated in the protocol a priori, due to the fact that meta‐analyses of only three trials were possible.
Data synthesis
If sufficient clinically similar trials were available, we pooled their results in meta‐analyses.
For time to event data, we pooled HRs using the generic inverse variance facility of Review Manager 5 (Review Manager 2014).
We used random‐effects models with inverse variance weighting for all meta‐analyses (DerSimonian 1986).
Subgroup analysis and investigation of heterogeneity
We had intended to conducted subgroup analysis in the meta‐analysis of progression and DFS, grouping trials by whether the trial measured progression or DFS. However, this was not possible due to the lack of statistical heterogeneity and sparse data, but it may be considered in update of the review (see Differences between protocol and review).
Sensitivity analysis
We performed a meta‐analysis of three trials assessing NACT and hysterectomy versus radiotherapy alone.
We performed a meta‐analysis of two trials assessing NACT and hysterectomy versus chemoradiotherapy alone.
Summary of findings and assessment of the certainty of the evidence
We presented the overall certainty of the evidence for each outcome according to the GRADE approach, which takes into account issues related to internal validity (risk of bias, inconsistency, imprecision, publication bias) and external validity (such as directness of results) (Langendam 2013; Schünemann 2020). We created summary of findings tables based on the methods described the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020) and using GRADEpro GDT (GRADEpro GDT). We used the GRADE checklist and GRADE Working Group certainty of evidence definitions (Meader 2014). We downgraded the evidence from 'high' certainty by one level for serious (or by two for very serious) concerns for each limitation and grade as follows.
High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate‐certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low‐certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low‐certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
We presented summary of findings tables reporting the following outcomes listed in order of priority:
OS.
PFS or DFS.
Quality of life.
Severe adverse events and toxicity.
Results
Description of studies
We searched for RCTs assessing the role of hysterectomy in combination with chemotherapy or radiotherapy, or both, versus chemoradiotherapy alone.
Results of the search
The search strategy identified 968 unique references. We read the abstracts and excluded those that did not meet the inclusion criteria at this stage. We retrieved 20 articles in full and after full‐text screening excluded six references for the reasons described in the Characteristics of excluded studies table. Eleven studies met our inclusion criteria and are described in the Characteristics of included studies table (Benedetti‐Panici 2002; Cetina 2013; Chang 2000; EORTC 2019; Gupta 2018; Keys 2003; Khan 2014; Morice 2012; Noriyuki 2010; Perez 1987; Zheng 2017). See PRISMA flow chart for further details of study selection process (Figure 3). Three studies are ongoing (CSEM 006 study; Reis Fihlo 2018; Shanmugam 2019).
3.
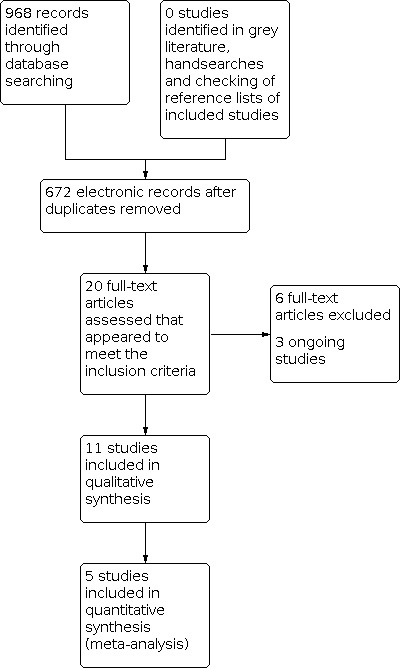
Study flow diagram.
Searches of the grey literature did not identify any additional studies.
Included studies
The 11 included studies randomised 2683 women, all of whom were assessed for primary survival outcomes at the end of the studies (Benedetti‐Panici 2002; Cetina 2013; Chang 2000; EORTC 2019; Gupta 2018; Keys 2003; Khan 2014; Morice 2012; Noriyuki 2010; Perez 1987; Zheng 2017).
The review identified the following treatment comparisons for the 11 included studies.
Hysterectomy (radical) with NACT versus chemoradiotherapy alone (EORTC 2019; Gupta 2018; Khan 2014).
Hysterectomy (simple or radical) with NACT versus radiotherapy alone (Benedetti‐Panici 2002; Chang 2000; Noriyuki 2010).
Hysterectomy (simple or radical) with radiotherapy versus radiotherapy alone (Keys 2003; Perez 1987).
Hysterectomy (simple or radical) with chemoradiotherapy versus chemoradiotherapy alone (Morice 2012; Zheng 2017).
Hysterectomy (radical) with chemoradiotherapy versus internal radiotherapy (brachytherapy) with chemoradiotherapy (Cetina 2013).
The duration of follow‐up of participants varied from 36 to 98 months. In Keys 2003, the women who were last seen alive had a median follow‐up of 9.6 years (range 0.3 to 16.1 years). In EORTC 2019, the median follow‐up was 8.2 years (95% CI 7.8 to 8.6).
The certainty of the evidence in this review was low or very low for all comparisons of outcomes other than for NACT and radical hysterectomy versus radiotherapy alone. The certainty of the evidence for OS and progression or DFS was moderate and was mainly downgraded due to concerns regarding risk of bias in individual trials. The trials in all the comparisons were at high or moderate risk of bias.
Hysterectomy (radical) with neoadjuvant chemotherapy versus chemoradiotherapy alone
One multicentre RCT included 620 participants with FIGO Stage IB2, IIA (greater than 4 cm) or IIB cervical cancer (EORTC 2019). Both patient groups received cisplatin‐based chemotherapy. In the NACT plus surgery group, women received neoadjuvant cisplatin‐based chemotherapy on day one. Treatment was repeated every 21 days. Within six weeks after the last chemotherapy course, and with a cumulative minimum of 225 mg/m2, women underwent a type III to V Piver‐Rutledge radical hysterectomy. Women with positive lymph nodes or tumour invasion into the parametria or less than 5 mm from the resection borders after surgery received standard adjuvant external‐beam radiotherapy once daily, five days a week, for 5.0 to 5.6 weeks (25 to 28 treatment days) followed by external boost radiotherapy or brachytherapy for one or two days In the concurrent radiotherapy group, radiation consisted of 45 Gy to 50 Gy plus boost concurrent with weekly cisplatin chemotherapy (40 mg/m2 per week). Adjuvant hysterectomy was allowed, but not recommended, in cases of histologically confirmed residual tumour. Participants in both groups were evaluated for OS at five years (primary endpoint) and OS, PFS, toxicity and quality of life (secondary endpoints).
Gupta 2018 was a single‐centre RCT that included women with cervical cancer Stage 1B2, IIA or IIB disease. The NACT plus surgery group received three cycles of paclitaxel (175 mg/m2) and carboplatin (dosed to an area under curve of 5 to 6) once every three weeks. Participants underwent clinical response assessment after the second and third cycles of chemotherapy. Participants who had no response or disease progression at these time points crossed over to receive definitive CCRT, whereas responders underwent surgery three to four weeks after the third cycle of chemotherapy.
Participants assigned to the NACT plus surgery group underwent Piver‐Rutledge class III radical abdominal hysterectomy, bilateral pelvic lymphadenectomy and lower para‐aortic lymph node sampling by expert gynaecological oncologists. Surgery was abandoned in participants with intraoperative findings of either unresectable primary tumour or lymph node disease, and these participants were treated with definitive concurrent chemoradiation. Participants assigned to the concurrent chemoradiation group and those who were crossed over from the NACT plus surgery group received standard external‐beam radiation to the whole pelvis and brachytherapy. They received an external radiation dose of 40 Gy in 20 fractions with 2 Gy per fraction and a midline shield at 20 Gy, followed by intracavitary radiation to 'point A' as follows: either two applications of a low‐dose rate of 30 Gy each or five applications of a high‐dose rate of 7 Gy each. Radiation doses were modified to respect tumour, rectal and bladder constraints. These women also received five cycles of cisplatin (40 mg/m2), administered once every week starting with external‐beam radiotherapy. Participants in the NACT plus surgery group who underwent radical hysterectomy were given adjuvant therapy (radiotherapy or CCRT) as per protocol‐defined criteria, in accordance with published evidence. On the basis of histopathological evaluation of the surgical specimen, adjuvant chemoradiation was given in the presence of any one of the following features: lymph node metastasis, positive surgical margins or parametrial involvement. Adjuvant radiotherapy alone was given based on the presence of any two of the following features: deep cervical stromal invasion, lymphovascular invasion or tumour size greater than 4 cm. Participants in both groups were evaluated at protocol‐defined time points to evaluate response, monitor for relapse and assess toxicity.
In Khan 2014, both patient groups received platinum‐based chemotherapy. Participants assigned to the chemoradiotherapy group received external beam radiotherapy (EBRT; 45 Gy to 50 Gy) followed by brachytherapy. Participants assigned to the NACT plus surgery group, had radical hysterectomy and pelvic lymphadenectomy. Participants in both groups were evaluated for short‐term complications within 30 days of completion of treatment and long‐term complications that were reported within two years after treatment.
Hysterectomy (simple or radical) with neoadjuvant chemotherapy versus radiotherapy alone
Three RCTs randomised 571 women compared NACT and hysterectomy (simple or radical) versus radiotherapy alone (Benedetti‐Panici 2002; Chang 2000; Noriyuki 2010). Benedetti‐Panici 2002 included women with Stage IB2 to III cervical cancer, Chang 2000 included women with IB to IIA bulky disease and Noriyuki 2010 had only women with Stage IIIB disease. The median age in each arm was similar in Benedetti‐Panici 2002 and Chang 2000 (range 46 to 52 years), whereas in Noriyuki 2010, women were significantly older in the radiotherapy arm (mean age 53 in the NACT and hysterectomy arm versus 60 years in the radiotherapy arm). All women in Benedetti‐Panici 2002 and Noriyuki 2010 and most in Chang 2000 had squamous cell cancers. In Benedetti‐Panici 2002 and Chang 2000, the Eastern Cooperative Oncology Group performance status was zero for most eligible women. Noriyuki 2010 did not report performance status.
In Benedetti‐Panici 2002, the NACT regimen was not predetermined; minimal requirements were a cisplatin‐containing regimen with a 240 mg/m2 or greater total cisplatin dose with a maximum of two additional drugs, administered over six to eight weeks. After NACT, the women were clinically reassessed and classified as suitable or unsuitable for radical surgery. Participants who were unsuitable for radical surgery were treated with radiotherapy. Surgery consisted of radical hysterectomy (type III to V) plus systematic (at least 20 nodes to be resected) pelvic lymphadenectomy (aortic lymphadenectomy was optional). Postoperative radiotherapy was given in participants with positive surgical resection margins or metastatic nodes, or both. In the case of node metastasis, the choice of adjuvant treatment was based on the institution's policy (e.g. chemotherapy, external‐beam radiotherapy or no further therapy). Adjuvant treatment was given to 48 (29%) participants in the surgical group; 38 (23%) participants in the surgical group underwent adjuvant radiotherapy.
Conventional radiotherapy consisted of external‐beam, megavoltage radiotherapy (45 Gy to 50 Gy) to the whole pelvis over five to six weeks. In the presence of metastatic pelvic nodes an extra dose of 5 Gy to 7 Gy was administered. Low‐dose rate brachytherapy (20 Gy to 30 Gy to the tumour volume) was provided two to four weeks after external radiotherapy. Aortic node metastases, when present, were irradiated (45 Gy per five weeks, followed by a 5 Gy boost if residual disease was eventually detected) with extended fields encompassing pelvic and aortic volume or at the end of pelvic irradiation, in the case of a pelvic complete remission. Salvage treatments were allowed in women who showed progressive disease.
In Chang 2000, the NACT was cisplatin and vincristine, followed by bleomycin. Two to four weeks after the completion of NACT, participants underwent a type III radical abdominal hysterectomy and pelvic lymphadenectomy. The adnexae were usually left in women aged 40 years or less if the gross appearance of the adnexae was normal.
The radiotherapy usually included a combination of external radiotherapy and high‐dose rate brachytherapy; with 40 Gy to 44 Gy whole pelvic irradiation. The para‐aortic lymph nodes were not routinely included in the treatment field. Parametria received up to 50 Gy. If bulky tumour persisted after 44 Gy of irradiation, external‐beam doses to the lower pelvis were increased to 50 Gy to 54 Gy without central block followed by brachytherapy, or to 70 Gy without brachytherapy. The median cumulative dose to 'point A' in this treatment protocol was 70 Gy. Thirty‐seven participants were treated using this method. The postoperative radiotherapy was given by using techniques similar to those described above. The dose to the whole pelvis was 44 Gy to 45 Gy, and that to the true pelvis was 50 Gy to 54 Gy. After external radiotherapy, brachytherapy was given in two to three fractions with a total dose of 4 Gy/0.5 cm to 6 Gy/0.5 cm below the vaginal mucosa.
Participants in the NACT arm had a higher incidence of receiving adjuvant therapy with either radiotherapy or chemotherapy after the scheduled treatment than those in the radiotherapy arm, who received radical hysterectomy as the adjuvant therapy. Of the 68 women in the NACT arm, 62 underwent hysterectomy and 19 of those had adjuvant radiotherapy, six had adjuvant chemotherapy and two had chemoradiotherapy.
In Noriyuki 2010, the NACT regimen consisted of cisplatin, bleomycin and mitomycin for three courses every four weeks. If the tumour was surgically removable, a radical hysterectomy was performed with bilateral salpingo‐oophorectomy and pelvic lymphadenectomy, and then radiotherapy was given at 40 Gy to the whole pelvic region. If the tumour progressed or relapsed, combined chemotherapy of bleomycin, vincristine, mitomycin and cisplatin (BOPM) was given, and then irinotecan with cisplatin as the third line. If the local tumour was inoperable, radiotherapy was given at 40 Gy to the whole pelvic region with 20 Gy brachytherapy, followed by BOPM chemotherapy.
The radiotherapy group received radiotherapy to the whole pelvic region in 20 fractions totalling 40 Gy. The total dose delivered to 'point B' as a boost dose with midline shield coverage was 20 Gy. The total dose delivered by brachytherapy was 24 Gy to 30 Gy. The pelvic field extended from the upper margin of L5 to the mid‐portion of the obturator foramen or the lowest level of disease, with a 3 cm margin, and laterally 1.5 cm to 2 cm beyond the lateral margins of the bony pelvic wall. The duration of the radiotherapy was four weeks. In cases with local recurrence or progression of the primary lesion, chemotherapy was added, which included BOMP, irinotecan with cisplatin, and cisplatin or carboplatin alone. When distant metastasis occurred, the researchers added radiotherapy, or the single lesion was surgically removed.
All three studies assessed OS (Benedetti‐Panici 2002; Chang 2000; Noriyuki 2010), one study also assessed PFS (Benedetti‐Panici 2002) and two studies also assessed DFS (Chang 2000; Noriyuki 2010). However, the definition of DFS in Chang 2000 was absence of persistent or recurrent disease, so this appeared to be a combination of progression and DFS. It was possible to include all three studies in a meta‐analyses of OS and PFS or DFS as HR estimates were either explicitly reported, deduced (Parmar 1998), or obtained via personal correspondence (Noriyuki 2010). Benedetti‐Panici 2002 reported severe toxicity and complications and Chang 2000 reported tumour response to treatment and toxicity. Noriyuki 2010 did not report adverse events. None of the three studies reported quality of life outcomes.
Hysterectomy (simple or radical) with radiotherapy versus radiotherapy alone
Two studies, which included 374 women, compared preoperative radiotherapy and radical hysterectomy versus radiotherapy alone (Keys 2003; Perez 1987). Keys 2003 included 256 eligible women with Stage IB2 disease (tumour size 4 cm to 8 cm); Perez 1987 included 118 women with Stage IIA disease as well as Stage IB (but women with a tumour more than 5 cm were excluded). The age distribution was comparable in the two groups in both trials, but additional information was not reported in Perez 1987. In Keys 2003, just over 75% of the women were 50 years old or under. Most women in both trials had squamous cell carcinoma of the cervix. Additional baseline information was not reported in the Perez 1987 trial; but the performance status of women in Keys 2003 was generally good (more than 76% in both arms with performance status of 0 and more than 20% with performance status of 1). Keys 2003 did not mention whether the participants were evaluated clinically or radiologically after radiotherapy in order to assess the tumour response and residual disease.
In Keys 2003, the daily fraction size was 180 Gy and external treatment carried to a total dose of 40 Gy for the radiation alone and 45 Gy for the adjuvant hysterectomy regimens. Both groups received brachytherapy one to two weeks after completing external treatment. The brachytherapy dose prescription was different between the treatment arms; the radiation alone group received 40 Gy with a total dose of 80 Gy to 'point A', while those who had hysterectomy received only 30 Gy with a total dose of 75 Gy to 'point A'. A minimum dose of 55 Gy was prescribed to 'point B' for both regimens. All irradiation was completed within 10 weeks. The surgical group then underwent simple hysterectomy with removal of tubes and ovaries, if present, two to six weeks after completion of all irradiation.
In Perez 1987, participants who were treated with preoperative radiotherapy and surgery received 20 Gy whole pelvis irradiation and one brachytherapy for 5000 to 6000 milligram‐hours (approximately 5 Gy to 6 Gy low‐dose rate given over six days), followed two to six weeks later by a radical hysterectomy and bilateral pelvic lymphadenectomy (up to the bifurcation of the common iliac vessels). The dose to the cervix was about 70 Gy and to the pelvic lymph nodes 30 Gy.
Treatment with irradiation alone in Perez 1987 consisted of 10 Gy to 20 Gy delivered to the whole pelvis and an additional parametrial dose to total of 50 Gy to the external iliac lymph nodes combined with two brachytherapy insertions for a total of approximately 7500 milligram‐hours (65 Gy to 70 Gy to 'point A'). The dose to the paracervical tissues was about 85 Gy and to the pelvic lymph nodes 60 Gy.
Keys 2003 assessed OS, pelvic‐free survival and the rate of pelvic recurrence. Perez 1987 assessed the five‐year tumour‐free actuarial survival, the sites of failure after therapy and treatment complications.
Hysterectomy (simple or radical) with chemoradiotherapy versus chemoradiotherapy alone
Morice 2012 included 61 women with FIGO Stage IB2 or II cervical cancer with a complete clinical and radiological response after chemoradiotherapy, randomly allocated to the treatment arms: hysterectomy or no hysterectomy. The median age and stage distribution was similar in both groups (45 years in the chemoradiotherapy and hysterectomy arm and 44 years in the chemoradiotherapy and no hysterectomy arm). Half of the women had FIGO Stage IB2 and half Stage II disease. More than 80% of participants in each group had squamous cell cancer. The performance status of the included women was not described.
Radiotherapy was delivered to the pelvis for a total dose of 45 Gy to 50 Gy, in five fractions of 1.8 Gy to 2 Gy per week, followed one to two weeks later by brachytherapy. Most women in both groups had one application of brachytherapy at 15 Gy. CCRT was cisplatin during external radiotherapy. A complete clinical and radiological response (based on magnetic resonance imaging (MRI)) was evaluated six to eight weeks after brachytherapy.
Hysterectomy could be performed via laparotomy or a laparoscopy and could be extrafascial or radical (type II according to the Piver classification) according to the preoperative examination. A selective or complete pelvic lymphadenectomy was optional and could be performed if lymphadenopathy was detected during surgery.
The trial gave HRs for OS and recurrence‐free survival as well as reporting the site of first recurrence. Morbidity was not reported after confirmation from the study authors. The median duration of follow‐up was 3.8 years (range 0.4 to 5.8 years) when the trial was closed early because of poor accrual.
Zheng 2017 included 102 participants with LACC and compared chemoradiotherapy and radical hysterectomy and pelvic lymph node dissection versus chemoradiotherapy alone. Fifty‐two participants were included in the hysterectomy arm and 50 in the chemoradiotherapy alone arm.
Women who met the inclusion criteria were first treated with CCRT.
The radiotherapy plan was as follows: the linear accelerator was used for external pelvic irradiation, and the intracavitary 192Ir was used for radiotherapy. Stage 1: the whole basin irradiation before and after the field or left and right field irradiation, four or five times a week, each time 2.25 Gy or 1.8 Gy, pelvic centre total dose 30 Gy. Stage 2: the lead block protected the uterus, and the uterus continued to be irradiated from the front and back, five times a week, 1.8 Gy to 2.0 Gy each time, and the total periuterine dose was 15 Gy to 20 Gy. At the beginning of the second stage of external irradiation, intracavitary and the back of the cavity were performed at the same time once a week, with 4.6 Gy to 7.0 Gy at 'point A' and total of 35 Gy to 42 Gy at 'point A'. Chemotherapy regimen: cisplatin alone: 35 mg/m2 to 40 mg/m2, once a week. Surgical treatment: participants in the experimental group underwent radical surgery four to six weeks after the completion of CCRT. For radical total hysterectomy and pelvic lymphadenectomy, 3 cm of paracervical and vaginal tissues were removed.
The outcomes of the study included recurrence and OS rate using. As outcome indicators they used: short‐term efficacy evaluation: evaluate the efficacy according to the tumour regression before and after treatment; complete remission: complete regression of the tumour by gynaecological examination and imaging examination; partial remission: tumour volume reduction of 50% or less; stability of disease: tumour volume reduction of 50% or less; progression of disease: tumour enlargement or presence of new lesions. Complete remission plus partial remission indicated effective treatment.
Hysterectomy (radical) with chemoradiotherapy versus internal radiotherapy (brachytherapy) with chemoradiotherapy
Cetina 2013 included 211 women aged 18 to 70 years with a histological diagnosis of untreated FIGO Stage IB2 to IIB cervical cancer and no evidence of para‐aortic lymph node involvement. It was reported that these 211 women were randomly allocated to either brachytherapy after external‐beam radiotherapy with chemotherapy or radical hysterectomy after external‐beam radiotherapy with chemotherapy. Women were ineligible for the study if they had previously received chemotherapy or radiotherapy. The median age, and stage distribution, was similar in both groups (44 years in the brachytherapy arm and 45 years in the hysterectomy arm). The median performance status (Karnofsky's) score was 90 and the median tumour size was 32 mm in both arms. Most participants in each treatment arm had FIGO Stage IIB disease (70% of participants in the brachytherapy arm and 74% in the hysterectomy arm). More than 80% of participants in each group had squamous cell cancer.
Participants received 50.4 Gy external‐beam radiotherapy to the entire pelvic region in 28 sessions of 1.8 Gy/day, five days/week, over the six weeks of chemotherapy. Immediately after completion of external‐beam radiotherapy with chemotherapy, participants in the brachytherapy underwent low‐dose rate brachytherapy of 30 Gy to 35 Gy delivered to 'point A', to result in a cumulative dose of 80 Gy to 85 Gy combining external‐beam radiotherapy and brachytherapy. The cumulative external‐beam radiotherapy and brachytherapy dose to 'point B' (the pelvic wall) was 55 Gy to 65 Gy.
Within four to six weeks after the external‐beam radiotherapy with chemotherapy, participants in the hysterectomy group were submitted to type III radical hysterectomy and bilateral pelvic lymph node dissection and para‐aortic lymph node sampling, if the multidisciplinary team judged the disease could be resected obtaining margins free of disease. Postoperative low‐dose rate brachytherapy was mandated in participants in the hysterectomy are only if the surgical specimen revealed positive surgical margins and was administered within four weeks after surgery at a median dose of 30 Gy to the vaginal mucosa delivered to a depth of 0.5 cm.
The trial gave HRs for OS and PFS. The trial also reported pathological response; operative complications; toxicity to chemoradiation with cisplatin and gemcitabine; long‐term complications; and late complications including proctitis, cystitis and hydronephrosis. Only late complications were reported in a breakdown by treatment arm. The median duration of follow‐up was 36 months (range 3 to 80 months).
Excluded studies
We excluded six full‐text studies.
Five studies included women who received hysterectomy or surgical staging in both arms (Katsumata 2013; Keys 1999; Sardi 1997; Sun 2013; Yang 2016).
One study compared surgery versus radiotherapy in women with early‐stage carcinoma of the cervix (Sundfor 1996).
Studies awaiting classification
We found no studies awaiting classification.
Ongoing studies
Hysterectomy (radical) with neoadjuvant chemotherapy versus chemoradiotherapy alone
Reis Fihlo 2018 is an ongoing study and the final results will be available in 2023 (personal communication with the authors). Both participant groups (Stage IB2, IIA, or IIB) will receive platinum‐based chemotherapy. Participants assigned to the NACT and radical hysterectomy group will receive chemotherapy every 21 days for six weeks. Within six weeks after the last chemotherapy course, women will undergo a type III to V Piver‐Rutledge radical hysterectomy. Women with positive lymph nodes or tumour invasion into the parametria or less than 5 mm from the resection borders after surgery will receive standard adjuvant external‐beam radiotherapy once daily, five days a week, for 5.0 to 5.6 weeks (25 to 28 treatment days) followed by external boost radiotherapy or brachytherapy for one or two days. Participants assigned to the CCRT group will receive standard therapy comprising cisplatin‐based chemotherapy once weekly for six weeks. Adjuvant hysterectomy will be allowed, but not recommended, in cases of histologically confirmed residual tumour. Participants in both groups will be evaluated for OS, PFS, toxicity of the regimens and quality of life.
CSEM 006 study is an ongoing study assessing DFS of women with Stage IIB cervical cancer randomised to NACT combined with surgery versus CCRT.
Hysterectomy (radical) with chemoradiotherapy versus hysterectomy with neoadjuvant chemotherapy versus chemoradiotherapy alone
Shanmugam 2019 is an ongoing RCT where all three participant groups with LACC will receive cisplatin and paclitaxel chemotherapy. Women assigned to the CCRT group will receive cisplatin (75 mg/m2) and paclitaxel (175 mg/m2) given within a three‐week interval between the two cycles along with concurrent radiotherapy of EBRT 50 Gy (2 Gy for 25 doses) followed by brachytherapy of 21 Gy (7 Gy for 3 doses) completed within eight weeks. Women assigned to the preoperative chemoradiation plus radical hysterectomy group will receive cisplatin (75 mg/m2) and paclitaxel (175 mg/m2) given within a three‐week interval between the two cycles along with concurrent radiotherapy of 50 Gy EBRT (2 Gy for 25 doses) followed by radical hysterectomy within three weeks after completion of radiotherapy. Women assigned to the preoperative chemotherapy plus radical hysterectomy group will receive cisplatin (75 mg/m2) and paclitaxel (175 mg/m2) given within a three‐week interval for three cycles followed by radical hysterectomy within three weeks after completion of chemotherapy. The outcomes of the study will be OS, PFS, overall response rate, complete clinical response, partial clinical response and quality of life.
For further details of the excluded studies see the Characteristics of excluded studies table.
Risk of bias in included studies
Nine studies were at overall high risk of bias (Cetina 2013; Chang 2000; EORTC 2019; Keys 2003; Khan 2014; Morice 2012; Noriyuki 2010; Perez 1987; Zheng 2017), and Benedetti‐Panici 2002 (which satisfied four of the criteria that we used to assess risk of bias) and Chang 2000 (which satisfied three items) were at moderate to high risk of bias (see Figure 1).
Allocation
Only Gupta 2018, Keys 2003, and Perez 1987 reported the method of generation of the sequence of random numbers used to allocate women to the treatment arms, but they did not report concealment of this allocation sequence from participants and the healthcare professionals involved in the trials. The other eight trials did not report on the method of sequence generation, although two trials reported adequate concealment of allocation (Benedetti‐Panici 2002; Chang 2000). Allocation concealment was unclear in nine trials (Cetina 2013; EORTC 2019; Gupta 2018; Keys 2003; Khan 2014; Morice 2012; Noriyuki 2010; Perez 1987; Zheng 2017).
Blinding
Since it was not possible to blind participants and clinicians to these particular interventions, performance bias may have been an issue in all 11 included trials. Only one trial reported adequate blinding (low‐risk of detection bias; Benedetti‐Panici 2002), so the other 10 trials may have been prone to detection bias.
Incomplete outcome data
At least 80% of eligible women who were randomised were assessed at the endpoint in nine trials (Benedetti‐Panici 2002; Cetina 2013; Chang 2000; Gupta 2018; Keys 2003; Morice 2012; Perez 1987; Noriyuki 2010; Zheng 2017), but this was unclear in two trials (EORTC 2019; Khan 2014). Two trials did not use intention‐to‐treat analyses (Morice 2012; Perez 1987).
Selective reporting
Five studies reported pertinent outcomes, although none reported quality of life outcomes (low risk of reporting bias; Benedetti‐Panici 2002; Cetina 2013; Chang 2000; Gupta 2018; Keys 2003). Two studies did not report adverse events or toxicity (high risk of reporting bias; Morice 2012; Noriyuki 2010). One study did not report OS despite the fact that the number of women who had died would have been known since disease progression was defined as the number of women whose disease had progressed or died (high risk of reporting bias; Perez 1987). This raised concern about a significant reporting bias. EORTC 2019, Khan 2014, and Zheng 2017 were either in abstract form only or inadequately reported primary survival outcomes, so it was difficult to judge whether outcomes were selectively reported (unclear risk of reporting bias).
Other potential sources of bias
It was unclear whether any additional forms of bias may have been present in all studies so this item was scored at unclear risk of bias, although over 25% of participants in each arm deviated from the protocol in Benedetti‐Panici 2002 and so this study was at high risk of bias for this item.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Hysterectomy (radical) with neoadjuvant chemotherapy versus chemoradiotherapy alone
Three trials included 1364 women and compared NACT and hysterectomy versus chemoradiotherapy alone (EORTC 2019; Gupta 2018; Khan 2014). See Table 1.
Overall survival
Two studies, assessing 1253 participants, reported OS (EORTC 2019; Gupta 2018). There was no evidence of a difference in risk of death between women who received NACT plus hysterectomy and those who received chemoradiotherapy alone (HR 0.94, 95% CI 0.76 to 1.16; moderate‐certainty evidence; Analysis 1.1). The percentage of the variability in effect estimates that was due to heterogeneity rather than sampling error (chance) was not important (I2 = 0%).
1.1. Analysis.

Comparison 1: Hysterectomy (radical) with neoadjuvant chemotherapy (NACT) versus chemoradiotherapy alone, Outcome 1: Overall survival
EORTC 2019 included 620 participants with Stage IB2 to IIB cervical cancer and compared NACT plus surgery (314 participants) with standard chemoradiotherapy (312 participants). Five‐year OS was 72% in the NACT plus surgery arm and 76% in the standard chemoradiotherapy arm (difference 4.0%, 95% CI –4% to 12%; HR 0.87, 95% CI 0.65 to 1.17; P = 0.332). The trial reported 191 (31%) deaths with a median follow‐up of 8.2 years (interquartile range (IQR) 7.8 to 8.6). Five‐year OS was 72% in the NACT plus surgery arm and 76% in the standard chemoradiotherapy arm (difference 4.0%, 95% CI –4% to 12%). Additional radiotherapy was given to 113 (36.3%) participants in the NACT plus surgery arm. Additional surgery was performed in nine (2.9%) participants in the standard chemoradiotherapy arm.
Gupta 2018 included 633 women aged 18 to 65 years with histologically confirmed squamous cell carcinoma of the cervix with 1994 FIGO Stage IB2, IIA or IIB disease. It compared NACT plus surgery with chemoradiotherapy. Five‐year OS rates in the NACT plus surgery group was 75.4% compared with 74.7% in the concurrent chemoradiation group (HR 1.03, 95% CI 0.75 to 1.40; P = 0.87).
Disease‐free survival
In Gupta 2018, five‐year DFS in the NACT plus surgery group was 69.3% compared with 76.7% in the concurrent chemoradiation group (HR 1.38, 95% CI 1.02 to 1.87; P = 0.038). In subgroup analyses, there was evidence of a difference in DFS detriment in the NACT plus surgery group in participants with FIGO Stage IIB disease, with a significant test of interaction between treatment effect and Stages IIA and IIB disease. In women with Stage IIB disease, the five‐year DFS rates in the NACT plus surgery group was 67.2% and concurrent chemoradiation group was 79.3% (unadjusted HR for DFS in the NACT plus surgery group 1.90, 95% CI 1.25 to 2.89; P = 0.003).
In EORTC 2019, the five‐year DFS in the NACT plus surgery group was 56.9% compared with 65.6% in the CCRT group (P = 0.021), mostly for Stage IIB disease. However, the authors noted that the first imaging measurement occurred systematically earlier in the neoadjuvant plus surgery arm than in the chemoradiotherapy arm.
Quality of life
None of the studies reported quality of life.
Severe adverse events
EORTC 2019 reported no toxic deaths. Short‐term severe adverse events (G3 or higher) occurred more frequently with NACT plus surgery (35%) than with chemoradiotherapy (21%; P < 0.001). In total, there were 198 serious adverse events (SAEs): 145 in the NACT plus surgery arm versus 53 in the standard chemoradiotherapy arm. Within the group of SAEs, there were 109 serious adverse reactions (SARs) with NACT plus surgery, and 35 SARs with chemoradiotherapy. Nearly all were chemotherapy related. In the NACT plus surgery arm, 238 (76%) women underwent surgery.
Main reasons for not having surgery as per protocol were toxicity (25/74, 34%), progressive disease (18/74, 24%) and insufficient response to NACT (12/74, 16%).
Toxicity
In Gupta 2018, in the NACT plus surgery group compared with the concurrent chemoradiation group, there was a lower rate of rectal (5.7% with NACT plus surgery versus 13.3% with chemoradiotherapy; P = 0.002), bladder (2.8% with NACT plus surgery versus 7.3% with chemoradiotherapy; P = 0.017), and vaginal (19.9% with NACT plus surgery versus 36.9% with chemoradiotherapy; P = 0.001) toxicity occurring or persisting 90 days after treatment completion. However, 24 months after treatment completion, there was no difference in rectal and bladder toxicities between groups, whereas vaginal toxicity continued to occur at a lower rate in the NACT plus surgery group (12.0% with NACT plus surgery versus 25.6% with chemoradiotherapy; P = 0.001).
Treatment‐related morbidity
Khan 2014 included 111 women with Stage IB2 to III cervical cancer comparing the toxicity‐related morbidity of CCRT with NACT plus surgery. Participants were evaluated for short‐term complications within 30 days of completion of treatment and long‐term complications that were reported within two years after treatment. There were no treatment‐related deaths. Overall 89% of participants in the chemoradiotherapy arm and 73% in the NACT plus surgery arm had complications, with 18% in the NACT plus surgery arm experiencing recurrence and requiring adjuvant radiotherapy.
In EORTC 2019 there were grade 3/4 complications related to surgery: 8 (3.3%) participants had bleeding, 10 (4.2%) operative lesions to ureter or bladder, 3 (1.2%) fistula, 7 (2.9%) others (sepsis, urinary tract infection and wound dehiscence).
Hysterectomy (simple or radical) with neoadjuvant chemotherapy versus radiotherapy alone
Three studies compared hysterectomy (simple or radical) with NACT versus radiotherapy alone (Benedetti‐Panici 2002; Chang 2000; Noriyuki 2010). See Table 2.
Overall survival
Meta‐analysis of three studies, assessing 571 participants, found that women who received NACT plus hysterectomy had a lower risk of death compared with women who received radiotherapy alone (HR 0.71, 95% CI 0.55 to 0.93; moderate‐certainty evidence; Analysis 2.1) (Benedetti‐Panici 2002; Chang 2000; Noriyuki 2010). The percentage of the variability in effect estimates that was due to heterogeneity rather than chance was not important (I2 = 0%).
2.1. Analysis.

Comparison 2: Hysterectomy (simple or radical) with neoadjuvant chemotherapy (NACT) versus radiotherapy alone, Outcome 1: Overall survival
Progression‐free survival and disease‐free survival
Meta‐analysis of three studies, assessing 571 participants, found no difference in the risk of disease progression between women who received NACT plus hysterectomy and those who received radiotherapy alone (HR 0.75, 95% CI 0.53 to 1.05; moderate‐certainty evidence; Analysis 2.2) (Benedetti‐Panici 2002; Chang 2000; Noriyuki 2010). The percentage of the variability in effect estimates that was due to heterogeneity rather than chance might not have been important (I2 = 20%).
2.2. Analysis.

Comparison 2: Hysterectomy (simple or radical) with neoadjuvant chemotherapy (NACT) versus radiotherapy alone, Outcome 2: Disease‐ or progression‐free survival
Severe adverse events
Acute toxicity
Benedetti‐Panici 2002 reported short‐term complications but it was not possible to make comparisons because participants were compared in terms of those who received NACT, hysterectomy and radiotherapy separately, and participants may have experienced more than one toxicity in each category (low‐certainty evidence).
Chang 2000 reported 9/68 (13%) cases of grade 3 acute toxicity in the NACT plus hysterectomy group and 7/50 (22%) cases of severe acute toxicity (5/7 were grade 3) in the radiotherapy alone group. There was no evidence of a difference between groups (RR 1.32, 95% CI 0.47 to 3.71; low‐certainty evidence). Acute toxicities included nausea, vomiting, diarrhoea, liver and dermatological adverse effects.
Long‐term complications and toxicity
In Benedetti‐Panici 2002, long‐term severe complications occurred in 32 (19.5%) women in the NACT arm and late severe morbidity with radiotherapy was observed in 39 (22%) women. There was no evidence of a difference in long‐term severe complications between NACT plus hysterectomy and radiotherapy alone (RR 0.86, 95% CI 0.49 to 1.50; low‐certainty evidence).
Chang 2000 reported 9/68 (13%) cases of severe late toxicity (8/9 were grade 3) in the NACT plus hysterectomy group and 11/50 (22%) grade 3 cases in the radiotherapy alone group. There was no evidence of a difference between groups (RR 0.60, 95% CI 0.27 to 1.34; low‐certainty evidence). Late toxicities included intestinal obstruction, radiation cystitis, radiation proctitis and lower leg oedema.
Hysterectomy (simple or radical) with radiotherapy versus radiotherapy alone
Keys 2003 and Perez 1987 included 374 women and compared preoperative radiotherapy and radical hysterectomy versus radiotherapy alone. The trialists gave a breakdown by FIGO Stage and intervention group in Perez 1987 but did not report OS or use appropriate survival techniques to allow the trial to be pooled. See Table 3.
Overall survival
In Keys 2003, there was no evidence of a difference in the risk of death between women who received radiotherapy plus extrafascial hysterectomy and those who received radiotherapy alone (HR 0.89, 95% CI 0.61 to 1.29; low‐certainty evidence).
Progression‐free survival
In Keys 2003, there was evidence of a difference in the risk of disease progression or death between women who received radiotherapy plus hysterectomy and those who received radiotherapy alone (HR 0.77, 95% CI 0.54 to 1.10; low‐certainty evidence).
Tumour‐free actuarial survival at five years
In Perez 1987, five‐year, tumour‐free actuarial survival for women with Stage IB was 80% with preoperative radiotherapy and surgery and 89% with radiotherapy alone. It was not reported how many women were Stage IB1 and how many Stage IB2 (the Stage IB2 group was of interest for this Cochrane Review). Women with barrel‐shaped cervix (endocervical lesion with cervix diameter larger than 5 cm) were excluded. In Stage IIA, the five‐year tumour‐free survival was 79% in the preoperative radiotherapy and surgery group and 56% in the radiotherapy alone group. These differences were not significant (very low‐certainty evidence).
Quality of life
None of the studies reported quality of life.
Severe/serious adverse events
In the women with Stage IB disease in Perez 1987, only 1/48 (2%) women experienced a severe complication (grade 3) in the radiotherapy and surgery group (ureteral stricture) whereas 5/40 experienced severe complications in the radiotherapy alone group (including rectovaginal fistula, vesicovaginal fistula, ureteral stricture and pelvic infection). This difference was not significant. Similarly in women with Stage IIA disease, 5/14 (40%) women experienced a severe complication in the radiotherapy and surgery group (including proctitis, rectal stricture, small bowel stricture and ureteral stricture) whereas only 1/16 experienced a severe complication in the radiotherapy alone group (rectal stricture). This difference was not significant (low‐certainty evidence).
Keys 2003 stated that both treatment programmes were well tolerated and there did not appear to be a difference between groups in terms of adverse effects. There were 18/129 women with a grade 3 or 4 adverse effect in the hysterectomy and radiotherapy group and 19 cases in 18/121 women of severe adverse effects in the radiotherapy alone group. Two women in each group received no radiotherapy and were not included (low‐certainty evidence).
Hysterectomy (simple or radical) with chemoradiotherapy versus chemoradiotherapy alone
Two studies compared hysterectomy (simple or radical) with chemoradiotherapy versus chemoradiotherapy alone (Morice 2012; Zheng 2017)
Morice 2012 included 61 women and compared chemoradiotherapy and simple or radical hysterectomy versus chemoradiotherapy alone. Zheng 2017 included 102 women with LACC and compared chemoradiotherapy and radical hysterectomy with pelvic lymph node dissection versus chemoradiotherapy alone. Fifty‐two participants were included in the hysterectomy arm and 50 in the chemoradiotherapy alone arm. See Table 4.
Overall survival
OS was inadequately reported in Zheng 2017 and it was not possible to calculate an HR. OS time of the chemoradiotherapy plus surgery group was 6 to 40 months, median survival time was 23 months and three‐year survival rate was 82.7%. Total survival time of the chemoradiotherapy group was 5 to 41 months, the median survival time was 22.5 months and the three‐year survival rate was 81.8%. The trial authors reported no evidence of a difference between arms (Chi2 = 0.338, P = 0.56; very low‐certainty evidence).
The postoperative pathological data in the hysterectomy arm showed that the residual rate of non‐cancer was 82.7%, and the residual rate of cancer was 5.8%.
Progression‐free survival
PFS was inadequately reported in Zheng 2017 and it was not possible to calculate an HR. PFS time of the chemoradiotherapy plus surgery group was 3 to 40 months, median survival time was 23 months and three‐year survival rate was 73.1%. PFS time of the chemoradiotherapy alone group was 5 to 41 months, median survival time was 22 months and three‐year survival rate was 64.8%. There was no evidence of a difference between groups (Chi2 = 0.092, P = 0.76; very low‐certainty evidence).
Morice 2012 reported no evidence of a difference in three‐year event‐free (death) survival rate (86% in the chemoradiotherapy plus hysterectomy group and 97% in the chemoradiotherapy alone group; log rank P = 0.15; very low‐certainty evidence).
Recurrence‐free survival at three years
Morice 2012 reported no evidence of a difference in three‐year event‐free (recurrence) survival rate (72% in the chemoradiotherapy plus hysterectomy group and 89% in the chemoradiotherapy alone group; log rank P = 0.17; low‐certainty evidence). We did not attempt to calculate an HR using the methods of Parmar 1998 due to this being a single trial analysis (very low‐certainty evidence).
The authors reported that morbidity was studied in a further publication, but when we contacted them, they could not provide data on morbidity.
Quality of life
The trial did not report quality of life.
Severe adverse events
The trial did not adequately report SAEs.
Hysterectomy (radical) with chemoradiotherapy versus internal radiotherapy (brachytherapy) with chemoradiotherapy
One trial included 211 women and compared brachytherapy versus radical hysterectomy in women who had already received external‐beam chemoradiotherapy with gemcitabine plus cisplatin (Cetina 2013). See Table 5.
Overall survival
There was no evidence of a difference in the risk of death between women in the brachytherapy group and those in the radical hysterectomy group (HR 0.65, 95% CI 0.35 to 1.21; P = 0.19; low‐certainty evidence).
Progression‐free survival
There was no evidence of a difference in the risk of disease progression or death between women in the brachytherapy group and those in the radical hysterectomy group (HR 0.70, 95% CI 0.31 to 1.34; P = 0.24; low‐certainty evidence).
Quality of life
The study did not report quality of life.
Severe adverse events
Severe late complications
There was no evidence of a difference in the proportion of women with severe late complications between groups (P = 0.53; low‐certainty evidence). There were four cases of grade 3 or 4 proctitis in the brachytherapy group and two cases in the radical hysterectomy group. There were three cases of severe cystitis in the brachytherapy group and none in the radical hysterectomy group, and there were no reported cases of grade 3 or 4 hydronephrosis in either group (low‐certainty evidence).
Of the 211 participants in the trial, chemoradiotherapy with cisplatin and gemcitabine appeared to be reasonably well tolerated, although nearly a third of women experienced severe neutropenia (most grade 3). Of the 86 women who received a radical hysterectomy, the number of intraoperative and early surgical complications appeared to be reasonably low, with bleeding (9/86) being the most common (low‐certainty evidence).
Discussion
Summary of main results
We found 11 studies including 2683 women that met our inclusion criteria. These studies assessed the role of hysterectomy (radical or simple) in combination with chemotherapy or radiotherapy, or both, in the treatment of LACC.
The RCTs were of varying methodological quality; most were at high risk of bias. These trials compared the following treatments for women with LACC (Stage IB2 to III).
Hysterectomy (radical) with NACT versus chemoradiotherapy alone (EORTC 2019; Gupta 2018; Khan 2014).
Hysterectomy (simple or radical) with NACT versus radiotherapy alone (Benedetti‐Panici 2002; Chang 2000; Noriyuki 2010).
Hysterectomy (simple or radical) with radiotherapy versus radiotherapy alone (Keys 2003; Perez 1987).
Hysterectomy (simple or radical) with chemoradiotherapy versus chemoradiotherapy alone (Morice 2012; Zheng 2017).
Hysterectomy (radical) with chemoradiotherapy versus internal radiotherapy (brachytherapy) with chemoradiotherapy (Cetina 2013).
Three trials included 1364 women and compared NACT and hysterectomy versus chemoradiotherapy alone (EORTC 2019; Gupta 2018; Khan 2014).
EORTC 2019 compared NACT plus surgery with standard CCRT. The preliminary results of this study revealed no difference in five‐year OS between NACT plus hysterectomy and CCRT, indicating that quality of life and long‐term toxicity are important to decide optimal treatment. Overall toxicity was acceptable, occurred more frequently in the NACT plus surgery arm and was mainly related to NACT. The five‐year DFS in the NACT plus surgery group was worse compared with the concurrent chemoradiation group (P = 0.021), mostly for Stage IIB disease. However. the authors noted that the first imaging measurement occurred systematically earlier in the neoadjuvant plus surgery arm than in the chemoradiation arm and this may have affected this result. These first data also indicate that in the surgery arm short‐term toxicity due to NACT has influenced further treatment. Of note, additional treatment was given to a significantly greater number of participants in the NACT and surgery group versus the number of participants in the concurrent chemoradiation group.
Gupta 2018, similarly to the EORTC trial, compared NACT plus surgery with standard CCRT. The five‐year DFS in the NACT plus surgery group was worse compared with the concurrent chemoradiation group, whereas there was no evidence of a difference in the corresponding five‐year OS rates. In subgroup analyses, the DFS detriment in the NACT plus surgery group was significant in women with FIGO Stage IIB disease, with a significant test of interaction between treatment effect and Stages IIA and IIB disease.
Meta‐analysis of three studies, assessing 571 women, found that women who received NACT plus hysterectomy had less risk of death than those who received radiotherapy alone, but there was no difference in the proportion of women with disease progression or recurrence between groups (Benedetti‐Panici 2002; Chang 2000; Noriyuki 2010).
Benedetti‐Panici 2002 reported no difference in long‐term severe complications between NACT plus hysterectomy and radiotherapy alone. Moreover, it has to be considered that 38 (23%) women who were operated on also underwent adjuvant radiotherapy and that 30% of these women were likely to present with severe late complications.
Chang 2000 found no difference in grade 3 acute toxicity and severe late toxicity between the NACT plus hysterectomy group and the radiotherapy alone group.
In summary these data demonstrate a possible beneficial effect of NACT plus hysterectomy versus radiotherapy in terms of survival, but no difference in DFS. This difference may be due to the NACT, the adjuvant treatment,]or both, rather than hysterectomy since these also differed between groups.
Keys 2003 and Perez 1987 included 374 women and compared preoperative radiotherapy and hysterectomy versus radiotherapy alone. These two trials reported no differences in the risk of death or disease progression, five‐year tumour‐free actuarial survival and severe complications between women who received radiotherapy plus hysterectomy and those who received radiotherapy alone.
Only Keys 2003 described the OS and PFS in the subgroup of women with residual disease in the hysterectomy specimen. Women with grossly positive hysterectomy specimens progressed and died at almost seven times the rate compared to those with negative specimens.
Morice 2012 included 61 women and reported no difference in overall and recurrence‐free survival at three years between chemoradiotherapy and hysterectomy (simple or radical) versus chemoradiotherapy alone. The study did not report adverse events and morbidity data.
Similarly, Cetina 2013 compared brachytherapy versus radical hysterectomy in 211 women who had already received chemoradiotherapy with gemcitabine plus cisplatin. They found no difference in the risk of death, disease progression or severe late complications between women in the brachytherapy group and those in the hysterectomy group.
Harms of treatment, especially in terms of quality‐of‐life data, were poorly reported. Studies comparing the more modern standard treatment of chemoradiotherapy did not demonstrate a benefit with the addition of hysterectomy.
Overall completeness and applicability of evidence
All 11 included studies are relevant in terms of the patient population, types of interventions, effectiveness and outcomes. However, in six studies, the role of hysterectomy as adjuvant treatment is more difficult to assess because the women received different types of primary or neoadjuvant treatment compared with the group who had a hysterectomy (Benedetti‐Panici 2002; Chang 2000; EORTC 2019; Gupta 2018; Khan 2014; Noriyuki 2010). Three trials that compared NACT and hysterectomy versus radiotherapy alone appeared to have external validity and represented a wide geographic area including Italy, China and Japan (Benedetti‐Panici 2002; Chang 2000; Noriyuki 2010). Benedetti‐Panici 2002 was a multicentre trial. The generalisability of other studies was less strong as the comparisons differed across studies and only results of single studies could be reported, although some were from multiple centres, which strengthens their representativeness.
Morice 2012 included women who had a complete response after chemoradiotherapy. The remaining studies did not provide an assessment of the response before surgery. It is important to note that women with a complete response to treatment before surgery potentially have a better prognosis compared to women with residual disease, therefore, the role of adjuvant hysterectomy should be assessed in subgroups with similar prognostic factors (Gadducci 2013; Hequet 2013; Landoni 2014). EORTC 2019 is a multicentre, RCT comparing neoadjuvant hysterectomy plus surgery compared to chemoradiotherapy alone; however, at the moment we only have preliminary results.
Overall, studies reported survival data well, although data on harms were poorly reported. These data are insufficient to recommend, outside of clinical trials, adding hysterectomy to chemoradiotherapy/radiotherapy in women with LACC.
Currently, the standard treatment for LACC is platinum‐based CCRT (CCCMAC 2010; NCI 1999). Although in this review we included older RCTs that did not use chemotherapy, the more relevant studies in modern clinical practice are those that use platinum‐based treatment.
The evidence appears to be of low or very low‐certainty for all comparison outcomes other than for NACT and radical hysterectomy versus radiotherapy alone (GRADE Working Group 2004). The certainty of the evidence for overall and progression or DFS was moderate and was mainly downgraded due to concerns regarding risk of bias in individual trials. The trials in all of the comparisons were at high or moderate risk of bias. More trials that assess identical medical management with and without hysterectomy may test the robustness of the findings of this review as further research is likely to have an important impact on our confidence in the estimates of effect. The meta‐analyses in the review found that women who received NACT plus hysterectomy had less risk of death (so prolonged survival) than those who received radiotherapy alone (HR 0.71, 95% CI 0.55 to 0.93; see Analysis 2.1), but there was no difference in disease progression. However, it is difficult to assess the impact of the hysterectomy given that it was in combination with NACT, since much of this difference may have been due to the chemotherapy component controlling microscopic distant disease rather than improving local control. Using the GRADE approach (GRADE Working Group 2004), the evidence summarised by this review is not sufficient to drive changes in clinical practice. Uncertainty about the additive effects of hysterectomy on a number of different outcomes justifies its evaluation in addition to chemoradiotherapy in future clinical trials.
Quality of the evidence
We reviewed 11 heterogeneous studies, assessing 2683 women, that evaluated the role of hysterectomy with radiotherapy or chemotherapy, or both, in women with LACC. Losses to follow‐up were small but the trials generally scored poorly for other risk of bias items and were potentially at high risk of bias. The number of women in the trials varied considerably with the largest including 633 women (Gupta 2018), and the smallest including only 42 women (Noriyuki 2010).
We included trials with LACC but these trials had a different number of cases for each stage of disease (Stage IB2 to IIIB), therefore, the results may have differed across trials.
The type and dose of medical treatment (chemotherapy, radiotherapy or both) were heterogeneous across the trials.
The baseline indicators to measure the general health of participants in the studies were incompletely reported. Six studies mentioned the performance status of women (Benedetti‐Panici 2002; Cetina 2013; Chang 2000; EORTC 2019; Gupta 2018; Keys 2003), and not all studies reported important clinical details such as the size of the tumour and information on residual disease.
Primary survival outcomes were largely well reported in the trials included in the first publication of this review (Kokka 2015). HRs were reported explicitly, deduced or obtained via correspondence so time‐to‐event data were analysed using appropriate survival methods in meta‐analyses or, where possible, in single study reports. However, updated trials included in this update of the review were inadequately reported to include survival outcomes in meta‐analyses.
There was incomplete reporting of harms and not all studies reported quality of life. Eight studies reported morbidity by treatment arm (Benedetti‐Panici 2002; Cetina 2013; Chang 2000; EORTC 2019; Gupta 2018; Keys 2003; Khan 2014; Perez 1987), but three trials did not (Morice 2012; Noriyuki 2010; Zheng 2017). It is important to describe the adverse effects of adjuvant hysterectomy in women with LACC receiving multiple treatments as the available literature suggests severe morbidity (Hequet 2013; Mabuchi 2017).
In Benedetti‐Panici 2002, 28% of women deviated from the protocol in each arm (58/210 in the NACT and hysterectomy arm, 55/199 in the radiotherapy arm), which is high. An additional concern was that of the 210 women in the NACT and hysterectomy arm, 75 received radiotherapy, 37 due to not being suitable for hysterectomy and 38 after hysterectomy. Only 164/210 (78%) in the NACT and hysterectomy arm had surgery. This cross‐over and the protocol deviation are potentially highly significant sources of bias.
Four studies did not mention the route of hysterectomy, that is open or laparoscopic (Benedetti‐Panici 2002; Cetina 2013; Chang 2000; Keys 2003). Morice 2012 indicated how many cases had laparoscopic or open procedures; however the study outcomes, including morbidity, were not subgrouped regarding this factor. Evidence suggests that laparoscopic procedures may have less morbidity than open procedures when performed for the appropriate group of women (Bijen 2009; Colombo 2009; Park 2013). Future studies should ideally consider this factor.
In Keys 2003, surgical staging of lymph nodes was optional and was performed on 57 (22%) women equally divided between the two study arms. Any women with metastasis to the para‐aortic nodes was ineligible for the RCT. Of the 103 women who did not have prerandomisation surgical staging, 54 (52%) had a hysterectomy and lymph node sampling procedure. Of these, seven (13%) had positive para‐aortic nodes. Since the surgical staging of lymph nodes was optional and it was performed in a subgroup of women, it is likely that this study was biased as far as the homogeneity of the staging and the prognosis of included women.
In Chang 2000, there were three different dose ranges for brachytherapy during the study period.
For the update of this review, we identified two RCTs with similar design characteristics (EORTC 2019; Gupta 2018). The EORTC trial results have been given so far in abstracts and oral presentations; the full paper is yet to be published. We understand there is a plan for a meta‐analysis of these two studies.
We identified three ongoing trials that may provide more evidence (CSEM 006 study; Reis Fihlo 2018; Shanmugam 2019).
The overall certainty of the evidence for NACT and radical hysterectomy versus radiotherapy alone was moderate for survival outcomes and low for adverse events. All other comparisons provided low‐certainty evidence, mainly because of poor reporting of outcomes and sparse data where results were based on single trials. The imprecision in single trials may be due to there being no significant difference between two treatments or an absence of evidence, which may come to light with greater statistical power. None of the studies reported quality of life and adverse events were incompletely reported, so the certainty of the evidence was low or very low for these outcomes in all comparisons. The trials in all comparisons were at high or moderate risk of bias. Further research is likely to have an important impact on our confidence in the estimates of effects and may change the estimates in the treatment comparisons based on single trial results and for outcomes that were incompletely reported; but we are quite confident of the reliability of the meta‐analysis of NACT and hysterectomy versus radiotherapy alone (571 participants) for the assessment of survival outcomes.
The certainty of the evidence in all other comparisons was low or very low for all outcomes.
This review identified that more evidence is needed and there is justification for evaluating the role of hysterectomy in combination with other adjuvant and neoadjuvant treatment options in clinical trials.
Potential biases in the review process
We performed a comprehensive search including electronic databases and the grey literature. Two review authors independently assessed references and extracted data. We restricted the included studies to RCTs, which provide the strongest level of evidence available. Hence, we have attempted to reduce bias in the review process.
One significant threat to the validity of the review is the possibility of publication bias, that is, studies that had negative results did not find the treatments to have been effective may not have been published. We were unable to assess this possibility as the meta‐analyses included just three studies and the review 11 studies in total.
Agreements and disagreements with other studies or reviews
Treatment of LACC should be individualised and limited to the minimum number of treatment modalities to yield the best cure with minimal complications. The US National Cancer Institute alert in February 1999 stated that chemoradiotherapy should be considered for all women with cervical cancer. This was based on significant improvements in PFS and OS when cisplatin‐based chemotherapy was administered during radiotherapy for various stages of cervical cancer (Morris 1999; NCI 1999; Rose 1999; Whitney 1999).
Chemoradiotherapy is considered by many groups (in North America, Europe) as the standard treatment for LACC (CCCMAC 2010; Green 2001). This includes pelvic external‐beam radiotherapy with concurrent platinum‐based chemotherapy followed by brachytherapy to boost the central disease response. Alternatively, LACC has been treated with primary radiotherapy alone.
In other countries, the lack of access to radiotherapy and the presumed poor control of metastatic disease has necessitated the use of NACT and hysterectomy. Chemotherapy is administered before other treatments to reduce the tumour volume and, therefore, to make women with clinically inoperable disease amenable to surgery (Sardi 1990; Sardi 1997).
With adjuvant hysterectomy the primary site of cervical cancer is removed. This approach may be preferred by women and the physicians as the 'initial site' of the tumour is removed. However, it is not certain if this approach results in improved survival. This systematic review of the currently available published trials found no evidence that adjuvant hysterectomy improves OS in women with LACC treated with radiotherapy or chemotherapy, or both. In women with a complete response to chemoradiotherapy or radiotherapy there is no obvious benefit. Women with a partial response to chemoradiotherapy or radiotherapy represent a poorer prognostic group; the role of adjuvant hysterectomy in this group of women is still debated (Azria 2005; Hequet 2013; Houvenaeghel 2007; Ota 2008; Sun 2014).
In Keys 2003, women with grossly positive hysterectomy specimens progressed and died at almost seven‐times the rate of those with negative specimens. None of the rest of the included RCTs provided information or appeared to have analysed the subgroup of women with residual disease following concurrent chemotherapy; this subgroup of women is quite challenging to treat and involves debate at the multidisciplinary team meetings. Hysterectomy after radiotherapy/concurrent chemotherapy is not the standard of care in most high‐income countries. In cases of suboptimal chemoradiotherapy, due to poor radiotherapy resources, adjuvant hysterectomy may have a role (Kundargi 2013), as it is impossible to deliver a curative dose of radiation via external beam alone. In other settings, hysterectomy for residual central (i.e. cervix or uterus) disease after completion of radiotherapy/concurrent chemotherapy (external beam and brachytherapy) the usual practice is to wait as a minimum of 12 weeks as it is possible that the disease will respond slowly and surgery after radiotherapy can be challenging. In situations where it has not been possible to place a brachytherapy applicator (the brachytherapy may have failed because there is still very bulky disease), then careful consideration of surgical excision is required (whether one will achieve complete clearance surgically before proceeding). In such cases, surgery may involve an exenteration; management in all such cases needs to be individualised as exenterative surgery may not be appropriate even if technically feasible. One of the concerns regarding surgery following chemoradiotherapy is surgery‐related morbidity. The CCCMAC 2010 meta‐analysis showed that chemoradiotherapy alone can cause severe adverse effects; chemotherapy can cause significant acute toxicity and radiotherapy can cause late complications that are difficult to reverse. Surgery following these modalities can be challenging as the quality of the tissues and the potential for healing are adversely affected by the preceding treatments. Hequet 2013 and Ferrandina 2014 found that the morbidity following surgery was high, suggesting an under‐reporting of morbidity data in the included studies. Well‐designed studies are required to assess the adverse effects of adjuvant hysterectomy following concurrent chemotherapy/radiotherapy of women with LACC, including studies where advanced radiotherapy techniques such as intensity‐modulated radiotherapy are used; intensity‐modulated radiotherapy has been associated with reduced toxicity (Baojuan 2012; Lin 2019; Marjanovic 2019), hence adjuvant hysterectomy may cause less morbidity.
In five included studies there was no difference in the rate of local and distant recurrences between the two arms (Cetina 2013; Keys 2003; Morice 2012; Noriyuki 2010; Perez 1987). In Chang 2000, there was a reduction in the local recurrence rate (9% versus 21%) and a slight decrease in the distant recurrence rate (10% versus 13%) in women who received concurrent radiation and cisplatin and radical hysterectomy compared with those who received radiotherapy alone. The beneficial role of hysterectomy on this difference is unclear because, as mentioned earlier, evidence suggests that it is the addition of cisplatin that reduces the risk of local and distant recurrence (Morris 1999; NCI 1999; Peters 2000; Rose 1999; Whitney 1999).
The most recent randomised studies have highlighted a possible worse result of NACT and surgery versus CCRT in the women with Stage IIB disease (EORTC 2019; Gupta 2018). Meta‐analyses of these two studies and the results of the ongoing trial, CSEM 006 study, should provide more evidence about the best practice in this subgroup of women.
Authors' conclusions
Implications for practice.
From the available randomised controlled trials (RCTs), we found insufficient evidence to suggest that hysterectomy improves the survival of women with locally advanced cervical cancer (LACC) who were treated with radiotherapy or chemoradiotherapy. We did find that women who received neoadjuvant chemotherapy plus hysterectomy had less risk of death than those who received radiotherapy alone, but it is unclear whether that survival benefit was attributable to the hysterectomy or chemotherapy, or because a significant number of these women also received adjuvant radiotherapy. The five‐year disease‐free survival in the neoadjuvant chemotherapy plus surgery group may have been slightly lower than in the concurrent chemoradiation group.
The trials were at moderate or high risk of bias. The overall certainty of the evidence is variable across the different comparisons and outcomes and was often downgraded due to concerns over the risk of bias and incomplete reporting of outcomes. This downgrading was mainly based on poor reporting and sparseness of data for some of the comparisons, where results were based on a single trial. The imprecision in single trials may be due to the small sample sizes and few events.
The decision to offer adjuvant hysterectomy (simple or radical, by open or laparoscopic procedure, with or without lymphadenectomy) needs to be individualised or performed in the context of a clinical trial.
Implications for research.
None of the trials reported quality of life trials and adverse events were incompletely reported, so the quality of the evidence was low or very low for these outcomes across all comparisons. The trials in all of the comparisons were at high or moderate risk of bias. Further research is likely to have an important impact on our confidence in the estimates of effect and may change the estimates in the treatment comparisons based on single trial results and for outcomes that were incompletely reported.
What's new
| Date | Event | Description |
|---|---|---|
| 24 February 2022 | New citation required but conclusions have not changed | Four new studies have been included, making a total of 11 and three ongoing studies identified. Overall conclusions unchanged. |
| 24 February 2022 | New search has been performed | The searches were updated to 4 February 2022. |
History
Protocol first published: Issue 1, 2013 Review first published: Issue 4, 2015
Acknowledgements
We thank Jo Morrison for clinical and editorial advice, Jane Hayes for designing the original search strategy, Jo Platt for running searches, and Gail Quinn and Clare Jess for their contribution to the editorial process. We thank the referees for many helpful suggestions and comments. We also thank the authors of the Morice 2012 and Noriyuki 2010 for providing additional information on outcomes. We thank Jonathan Baker and his team at the Queen Elizabeth Queen Mother Hospital, for their support with the literature search.
With many thanks to Yulan Shen (Clinical Research RN, Australia) and Si Jia Zhu for (Chinese medical student at Beijing University of Chinese Medicine) for translation work through Cochrane TaskExchange.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
Appendices
Appendix 1. FIGO staging classification for cervical cancer
| Stage | Characteristics |
| I | The carcinoma is strictly confined to the cervix (extension to the corpus should be disregarded). |
| IA | Invasive cancer identified only microscopically. All gross lesions, even with superficial invasion, are Stage IB cancers. Invasion is limited to a measured stromal invasion with a maximal depth of 5.0 mm and a horizontal extension of ≤ 7.0 mm. Depth of invasion should be ≤ 5.0 mm taken from the base of the epithelium, either surface or glandular, from which it originates. Vascular space involvement, either venous or lymphatic, should not alter the staging. |
| IA1 | Measured stromal invasion of ≤ 3.0 mm in depth and extension of ≤ 7.0 mm. |
| IA2 | Measured stromal invasion of > 3.0 mm and ≤ 5.0 mm in depth with an extension of ≤ 7.0 mm |
| IB | Clinical lesions confined to the cervix or preclinical lesions > IA. All gross lesions even with superficial invasion are Stage IB cancers |
| IB1 | Clinical lesions ≤ 4 cm in size. |
| IB2 | Clinical lesions > 4 cm in size. |
| II | The carcinoma extends beyond the cervix, but has not extended onto the pelvic wall; the carcinoma involves the vagina, but not as far as the lower third. |
| IIA | No obvious parametrial involvement. Involvement of up to the upper two‐thirds of the vagina. |
| IIB | Obvious parametrial involvement, but not into the pelvic sidewall. |
| III | The carcinoma has extended onto the pelvic wall; on rectal examination there is no cancer‐free space between the tumour and the pelvic wall; the tumour involves the lower third of the vagina; all cases with a hydronephrosis or non‐functioning kidney should be included, unless they are known to be due to other causes. |
| IIIA | No extension onto the pelvic wall, but involvement of the lower third of the vagina. |
| IIIB | Extension onto the pelvic wall or hydronephrosis or non‐functioning kidney. |
| IV | The carcinoma has extended beyond the true pelvis or has clinically involved the mucosa of the bladder or rectum. |
| IVA | Spread of the growth to adjacent organs. |
| IVB | Spread to distant organs. |
Appendix 2. CENTRAL search strategy
MeSH descriptor Uterine Cervical Neoplasms explode all trees
(cervi* near/5 (cancer* or neoplas* or carcinom* or malignan* or tumor* or tumour*))
(#1 OR #2)
MeSH descriptor Hysterectomy explode all trees
hysterectom*
(#4 OR #5)
MeSH descriptor Antineoplastic Agents explode all trees
MeSH descriptor Antineoplastic Combined Chemotherapy Protocols explode all trees
MeSH descriptor Chemotherapy, Adjuvant, this term only
chemotherap*
cisplatin
carboplatin
gemcitabine
paclitaxel
etoposide
fluorouracil
bleomycin
ifosphamide
(#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18)
MeSH descriptor Radiotherapy explode all trees
radiotherap*
radiation
(#20 OR #21 OR #22)
chemoradi* or radiochemo*
(#19 OR #23 OR #24)
(#3 AND #6 AND #25)
Appendix 3. MEDLINE (Ovid) search strategy
exp Uterine Cervical Neoplasms/
(cervi* adj5 (cancer* or neoplas* or carcinom* or malignan* or tumor* or tumour*)).mp.
1 or 2
exp Hysterectomy/
hysterectom*.mp.
4 or 5
exp Antineoplastic Agents/
exp Antineoplastic Combined Chemotherapy Protocols/
Chemotherapy, Adjuvant/
chemotherap*.mp.
cisplatin.mp.
carboplatin.mp.
gemcitabine.mp.
paclitaxel.mp.
etoposide.mp.
fluorouracil.mp.
belomycin.mp.
ifosphamide.mp.
7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18
exp Radiotherapy/
radiotherap*.mp.
radiation.mp.
20 or 21 or 22
(chemoradi* or radiochemo*).mp.
19 or 23 or 24
3 and 6 and 25
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
clinical trials as topic.sh.
randomly.ab.
trial.ti.
27 or 28 or 29 or 30 or 31 or 32 or 33
26 and 34
key:
mp = title, original title, abstract, name of substance word, subject heading word, unique identifier pt = publication type ab = abstract sh = subject heading
Appendix 4. Embase (Ovid) search strategy
exp uterine cervix tumor/
(cervi* adj5 (cancer* or neoplas* or carcinom* or malignan* or tumor* or tumour*)).mp.
1 or 2
exp hysterectomy/
hysterectom*.mp.
4 or 5
exp antineoplastic agent/
exp chemotherapy/
chemotherap*.mp.
cisplatin.mp.
carboplatin.mp.
gemcitabine.mp.
paclitaxel.mp.
etoposide.mp.
fluorouracil.mp.
belomycin.mp.
ifosphamide.mp.
7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17
exp radiotherapy/
radiotherap*.mp.
radiation.mp.
19 or 20 or 21
(chemoradi* or radiochemo*).mp.
18 or 22 or 23
3 and 6 and 24
crossover procedure/
double‐blind procedure/
randomized controlled trial/
single‐blind procedure/
random*.mp.
factorial*.mp.
(crossover* or cross over* or cross‐over*).mp.
placebo*.mp.
(double* adj blind*).mp.
(singl* adj blind*).mp.
assign*.mp.
allocat*.mp.
volunteer*.mp.
26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38
25 and 39
key:
[mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
Appendix 5. LILACS search strategy
(MH:hysterectomy or hysterectom$) AND ((cervi$ and (cancer$ or tumor$ or tumour$ or malignan$ or carcinoma$ or neoplas$)) or MH:Uterine Cervical Neoplasms)
Data and analyses
Comparison 1. Hysterectomy (radical) with neoadjuvant chemotherapy (NACT) versus chemoradiotherapy alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Overall survival | 2 | Hazard Ratio (IV, Random, 95% CI) | 0.94 [0.76, 1.16] |
Comparison 2. Hysterectomy (simple or radical) with neoadjuvant chemotherapy (NACT) versus radiotherapy alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Overall survival | 3 | Hazard Ratio (IV, Random, 95% CI) | 0.71 [0.55, 0.93] | |
| 2.2 Disease‐ or progression‐free survival | 3 | Hazard Ratio (IV, Random, 95% CI) | 0.75 [0.53, 1.05] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Benedetti‐Panici 2002.
| Study characteristics | ||
| Methods | Multicentre RCT conducted in Italy | |
| Participants | 409 eligible women with Stage IB2–III cervical cancer. 210 women received NACT + RH and 199 received radiotherapy. No difference in baseline characteristics between arms. Histopathology type Squamous cell cancer of the cervix. Median age 49 years (range 25–70 years) in the NACT + RH arm and 52 years in the radiotherapy arm (range 28–69 years). Performance status 0 in most eligible women: 94% in the NACT + RH arm and 91% in the radiotherapy arm. FIGO Stage FIGO Stage 1B2 to IIA > 4 cm: 41% of women in the NACT + RH arm and 44% in the radiotherapy arm. FIGO Stage IIB: 35% of women in the NACT + RH arm and 38% in the radiotherapy arm. FIGO Stage III: 24% of women in the NACT + RH arm and 18% in the radiotherapy arm. Tumour size Tumour size > 5 cm: 54% in the NACT + RH arm and 58% in the radiotherapy arm. Tumour grade Grade 1–2: 63% of women in the NACT + RH arm and 62% in the radiotherapy arm. Grade 3: 34% of women in the NACT + RH arm and 32% in the radiotherapy arm. Ungraded: 3% in the NACT + RH arm and 6% in the radiotherapy arm. Lymph node status Negative: 69% in the NACT + RH arm and 74% in the radiotherapy arm. Positive: 23% in the NACT + RH arm and 22% in the radiotherapy arm. Positive aortic: 5% in the NACT + RH arm and 3% in the radiotherapy arm. Unknown: 8% in the NACT + RH arm and 4% in the radiotherapy arm. |
|
| Interventions |
The NACT regimen was not predetermined, but minimal requirements were a cisplatin‐containing regimen with < 240 mg/m2 total cisplatin dose with ≤ 2 additional drugs, administered over 6–8 weeks. After NACT, women were clinically reassessed and classified as suitable or unsuitable for RH. Women unsuitable for RH were treated by radiotherapy. Surgery consisted of RH (type III–V) + systematic (≥ 20 nodes to be resected) pelvic lymphadenectomy (aortic lymphadenectomy was optional). Postoperative radiotherapy given in women with positive surgical resection margins or metastatic nodes, or both. In the case of node metastasis, the choice of adjuvant treatment was based on the institution's policy (i.e. chemotherapy, external‐beam radiotherapy or no further therapy).
External‐beam, megavoltage radiotherapy (45–50 Gy) to the whole pelvis over 5–6 weeks. In the presence of metastatic pelvic nodes, detected by CT, MRI or lymphangiography, an extra dose of 5–7 Gy was administered. Low‐dose rate brachytherapy (20–30 Gy to the tumour volume) was provided 2–4 weeks after external radiotherapy. Aortic node metastases, when present, were irradiated (45 Gy/5 weeks, followed by a 5‐Gy boost to residual disease eventually detected) with extended fields encompassing pelvic and aortic volume or at the end pelvic irradiation, in the case of a pelvic complete remission. Salvage treatments were allowed in women who showed progressing disease. |
|
| Outcomes | OS PFS Severe morbidity |
|
| Notes | Median follow‐up of the overall population was 40 months (range 1–107 months). When the analysis was restricted to surviving women, the median duration of follow‐up was 53 months (range 3–107 months). Type III and type IV radical hysterectomies were performed. Authors described assigned treatment as inadequate in 49 (23%) in the NACT + RH arm and 33 women (17%) in the radiotherapy arm. Reasons for inadequate treatment.
The RR of long‐term severe complications for chemosurgery vs radiotherapy alone was 0.86 (95% CI 0.49 to 1.50). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were randomly assigned to NACT + RH or radiotherapy by telephoning the trial data centre. Women were stratified at randomisation by disease stage, age and institution but it was unclear whether or not this sequence generation was done adequately. |
| Allocation concealment (selection bias) | Low risk | Women were randomly assigned to NACT + RH or radiotherapy by telephoning the trial data centre. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind participants and clinicians to these interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 409/409 eligible women were analysed for primary survival outcomes using appropriate statistical techniques. |
| Selective reporting (reporting bias) | Low risk | All case report forms were reviewed first by 2 study members and further verified by 2 independent investigators (1 radiotherapist and 1 surgeon). All pertinent outcomes appeared to have been reported by the trial authors. |
| Other bias | High risk | Large deviations from protocol; % of women deviated from protocol and this may have had impact on results. 58/210 (26%) women in NACT + RH arm (of those eligible), 55/199 (28%) in radiotherapy (of those eligible). |
Cetina 2013.
| Study characteristics | ||
| Methods | RCT conducted in Mexico. Unclear whether this was a single or multicentre trial. | |
| Participants | Between May 2004 and June 2009, 211 eligible women with a histological diagnosis of untreated FIGO Stage IB2–IIB cervical cancer with no evidence of cancer in para‐aortic lymph nodes (as evaluated by CT scan). 100 participants were assigned to brachytherapy (brachytherapy) and 111 to RH. Aged 18–70 years. Women were ineligible for the study if they had previously received chemotherapy or radiotherapy. No difference in baseline characteristics between arms. Median age 44 years (range 23–66 years) in the brachytherapy group and 45 years (range 25–62 years) in the RH group. Karnofsky's performance status Median score was 90 in both arms (range 80–100 in the brachytherapy arm and 70–100 in the RH arm). Mean tumour size 32 mm in both arms (range 12–64 mm in the brachytherapy arm and 12–81 mm in the RH arm). Table 1 in Cetina 2013 stated that the median tumour size in each group was 32 cm but we assumed this should have been 32 mm. FIGO Stage FIGO Stage IB: 218 (18%) women in the brachytherapy arm and 18 (16%) in the RH arm. FIGO Stage IIA2: 12 (12%) women in the brachytherapy arm and 11 (10%) in the RH arm. FIGO Stage IIB: 70 (70%) women in the brachytherapy arm and 82 (74%) in the RH arm. Histopathological type Squamous cell cancer: 83 (83%) women in the brachytherapy arm and 100 (90%) in the RH arm Adenocarcinoma: 14 (14%) women in the brachytherapy arm and 8 (7%) in the RH arm. Adenosquamous carcinoma: 3 (3%) women in the brachytherapy arm and 3 (3%) in the RH arm. Haemoglobin Median haemoglobin concentration: 13.3 g/dL (range 10.1–18 g/dL) in the brachytherapy group and 12.8 g/dL (range 10–16 g/dL) in the RH group. |
|
| Interventions |
Immediately after completion of external‐beam radiotherapy and chemotherapy, women in the brachytherapy group underwent low‐dose rate brachytherapy. An brachytherapy dose of 30–35 Gy was delivered to 'point A' to result in a cumulative dose of 80–85 Gy combining external‐beam radiotherapy and brachytherapy. The cumulative external‐beam radiotherapy and brachytherapy dose to 'point B' (the pelvic wall) was 55–65 Gy.
Within 4–6 weeks after the EBRT and chemotherapy, women in the RH group were submitted to type III RH and bilateral pelvic lymph node dissection and para‐aortic lymph node sampling if the multidisciplinary team judged the disease could be resected obtaining margins free of disease. Postoperative low‐dose rate brachytherapy was mandated in the surgical arm women only if the surgical specimen revealed positive surgical margins and was administered within 4 weeks after surgery at a median dose of 30 Gy to the vaginal mucosa delivered to a depth of 0.5 cm. External‐beam chemoradiotherapy with gemcitabine + cisplatin External‐beam radiotherapy 50.4 Gy to the entire pelvic region in 28 sessions of 1.8 Gy/day, 5 days/week, over the 6 weeks of chemotherapy. |
|
| Outcomes | OS PFS Pathological response: defined as complete with the absence of viable malignant cells in the surgical specimen Operative complications: defined as intraoperative including: bladder, ureteral, bowel and vascular injuries and estimated blood loss > 1000 mL Early postoperative and long‐term complications: defined as any adverse event occurring within or after 30 days from surgery Late complications: including proctitis, cystitis and hydronephrosis Toxicity to chemoradiotherapy with cisplatin and gemcitabine (not reported by treatment arm) |
|
| Notes | Median length of follow‐up was 36 months (3–80 months). Dose modification was not allowed for any of the drugs. In the brachytherapy arm, 86 (86%) completed treatment as per protocol. 3 (3%) women abandoned treatment during external‐beam radiotherapy and 11 (11%) had residual tumours after external‐beam radiotherapy that prevented application of brachytherapy. In the surgical arm, 86/111 (77%) women received the full intervention. 15 (13.5%) abandoned treatment during external‐beam radiotherapy; 7 (6%) were judged not to be resectable and 3 (3%) had medical contraindication to surgery. No differences between the median dose and days to complete external‐beam radiotherapy between arms. Both arms received a median of 5 cycles (1–6) of cisplatin + gemcitabine. Among the 86 women who received surgery, 62 (72%) had pathological complete response and 24 (28%) had pathological partial response. In 16/24 women, there were only microscopic foci. The median number of pelvic and para‐aortic lymph nodes removed was 30 (range 8–60) in the brachytherapy arm and 14 (range 3–32) in the surgical arm. 9 women (10%) had positive pelvic lymph nodes (median 3; range 1–7) and 6 of these also had positive para‐aortic lymph nodes (median 2; range 1–4), and they received para‐aortic radiotherapy. PFS rates in the brachytherapy arm were 75% vs 72% in the surgical arm. OS was 76% in the brachytherapy vs 74.5% in the surgical arm. In the univariate analysis, none of the factors analysed (time to complete external‐beam radiotherapy, < 45 vs > 45 days; histology, squamous vs non‐squamous; clinical Stage IB2–IIA vs IIB; age < 50 vs > 50 years and haemoglobin > 12 vs > 12 g/dL) for either PFS or OS were significant. The multivariate analysis also showed none to be significant. Toxicity to chemoradiotherapy: the most frequent toxic effects were haematological and gastrointestinal. Grade 3 leukopenia occurred in 30% and neutropenia in 25% of women. Acute complications in the surgical arm Median hospital stay was 5 days (range 4–6 days). Median surgical time was 4 hours (range 4–6 hours). Median blood loss was 450 mL (range 150–1600 mL). 12 women (14%) were transfused. 3 women (3.5%) had vascular laceration; 1 (1.5%) had a urethral tear and 2 (2%) had section of the ureter. 1 woman (1.5%) had wound dehiscence and 1 (1.5%) woman had infection in the surgical wound. Late toxicity In the brachytherapy arm, grade 1 and 2 proctitis and cystitis were registered in nearly half of the women; however, grade 3 occurred in only 2% and grade 4 in only 2%. In the surgical arm, 6 women had infection after 30 days from surgery. 3 (3.4%) women had unilateral lymphocysts that required treatment with percutaneous drainage in 2 and lymphocyst resection and drainage in 1. In addition, 2 (2.3%) women had uretero‐cutaneous fistulae treated with surgery and double J‐stent positioning. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Quote: "After signing an informed consent, women were stratified according to FIGO stage IB2–IIA or IIB and randomly assigned before chemoradiation". |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind participants and clinicians to these interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 211/211 (100%) eligible women were analysed for efficacy and toxicity and survival outcomes were analysed using appropriate statistical techniques. 18/211 women were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All pertinent outcomes appeared to have been reported by the trial authors. |
| Other bias | Unclear risk | Insufficient information to assess whether an additional risk of bias existed. |
Chang 2000.
| Study characteristics | ||
| Methods | RCT conducted in China. Unclear whether this was a single or multicentre trial. | |
| Participants | 120 eligible women with FIGO Stage IB (bulky) to IIA (bulky) cervical cancer. No difference in baseline characteristics between arms. Histopathological type Squamous cell cancer: 91% of women in the NACT + RH arm and 88% in the radiotherapy arm. Adenocarcinoma: 4% of women in the NACT + RH arm and 8% in the radiotherapy arm. Adenosquamous carcinoma: 4% of women in the NACT + RH arm and 4% in the radiotherapy arm. Age Median age 46 years in the NACT + RH group and 47 years in radiotherapy group. Performance status 0: 85% in the NACT + RH arm and 83% in the radiotherapy arm. 1: 15% in the NACT + RH arm and 15% in the radiotherapy arm. 2: 0% in the NACT + RH arm and 2% in the radiotherapy arm. FIGO Stage FIGO Stage IB bulky: 53% of women in the NACT + RH arm and 50% in the radiotherapy arm. FIGO Stage IIA bulky: 47% of women in the NACT + RH arm and 50% in the radiotherapy arm. Mean tumour size Mean tumour size was 5.0 (SD 0.8) cm in the NACT + RH arm and 4.9 (SD 0.7) cm in the radiotherapy arm. Mean tumour size was > 6 cm in 10% in the NACT + RH arm and 10% in the radiotherapy arm. Tumour type Endophytic: 51% in the NACT + RH arm and 54% in the radiotherapy arm. Exophytic: 49% in the NACT + RH arm and 46% in the radiotherapy arm. Tumour grade Grade I: 3% in the NACT + RH arm and 6% in the radiotherapy arm. Grade II: 40% in the NACT + RH arm and 27% in the radiotherapy arm. Grade III: 44% in the NACT + RH arm and 40% in the radiotherapy arm. Undifferentiated grade: 9% in the NACT + RH arm and 6% in the radiotherapy arm. Unspecified grade: 4% in the NACT + RH arm and 21% in the radiotherapy arm. |
|
| Interventions |
NACT was cisplatin and vincristine, followed by bleomycin. 2–4 weeks after the completion of NACT, women underwent a type III radical abdominal hysterectomy and pelvic lymphadenectomy. The adnexae were usually left in women aged < 40 years if they were grossly normal in appearance.
Radiotherapy usually included external irradiation + high‐dose rate brachytherapy. Women received 40–44 Gy of whole pelvic irradiation; the para‐aortic lymph nodes were not routinely included in the treatment field. Parametria received up to 50 Gy. Daily fraction was 1.8–2 Gy, 5 fractions per week. If bulky tumour persisted after 44 Gy of irradiation, external‐beam doses to the lower pelvis were increased to 50–54 Gy without central block, followed by brachytherapy, or to 70 Gy without brachytherapy. 7 women received external irradiation alone.
3 different dose ranges for brachytherapy during this period. Before April 1992, brachytherapy given as 3 fractions with 2‐week intervals between each fraction; dose to 'point A' was 6.5–7.2 Gy/fraction. Of the women in the radiotherapy arm, 1 received this method. Between July 1992 and September 1993, 5 women were transferred to another hospital for brachytherapy because the remote control after‐loading system of the main hospital was out of order. Women received a total of 4 fractions of high‐dose brachytherapy by 2 applicator insertions; on each insertion, 2 fractions of 7–7.5 Gy to 'point A' were given during the same day with an interval of 4–6 hours. The median cumulative dose to 'point A' was 72 Gy. After August 1993, brachytherapy performed in the main hospital and given in 6 fractions at 2 fractions per week; the dose to 'point A' was 4.3 Gy/fraction. The median cumulative dose to 'point A' in this treatment protocol was 70 Gy. 37 women received this method. Response to radiotherapy was evaluated by a radiation oncologist and a gynaecological oncologist weekly during treatment. Postoperative radiotherapy given using techniques similar to those described above, the dose to the whole pelvis was 44–45 Gy, and that to the true pelvis was 50–54 Gy. After external irradiation, intravaginal brachytherapy was given in 2–3 fractions with a total dose of 4–6 Gy/0.5 cm below vaginal mucosa. |
|
| Outcomes | OS DFS Response Toxicity |
|
| Notes | Women with enlarged para‐aortic lymph nodes on image study were ineligible unless results of cytological or histological studies were negative. Type III radical hysterectomies were performed. Median duration of follow‐up was 39 months. Median DFS for the radiotherapy arm was 68 months, but median for the NACT + RH arm could not be calculated as more than half of women did not experience a relapse. There was no difference in DFS between arms (P = 0.8). 2‐year survival rate was 81% (95% CI 71% to 91%) in the NACT + RH arm and 84% (95% CI 72% to 95%) in the radiotherapy arm, and the estimated 5‐year survival rates were 70% (95% CI 56% to 83%) in the NACT + RH arm and 62% (95% CI 43% to 80%) in the radiotherapy arm. DFS and OS of the women who underwent NACT + RH did not differ from those of women treated with radiotherapy alone. Women in the NACT + RH arm had a higher incidence of receiving adjuvant therapy, with either radiotherapy or chemotherapy, after the scheduled treatment than those in the radiotherapy arm, who received RH as the adjuvant therapy. Of the 68 women in the NACT + RH arm, 62 underwent RH and 19 of those had adjuvant radiotherapy, 6 had adjuvant chemotherapy and 2 had chemoradiotherapy. The NACT + RH group had higher incidence of local (21%) vs distant (9%) relapse, whereas the radiotherapy had equal incidence of local vs distant relapse (12%). Higher incidences of relapse over the vagina and over the lung were noted in the NACT + RH arm, whereas the radiotherapy arm showed a higher rate of para‐aortic node relapse. Incidence of acute toxicity, mainly mild‐to‐moderate gastrointestinal and haematological toxicity and urinary retention, was higher in the NACT + RH arm than in the radiotherapy arm. Incidence of cystitis (due to radiation), proctitis (due to radiation) and lymphoedema was higher in the radiotherapy arm. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was conducted by the Biostatistics Consulting Center of Chang Gung Memorial Hospital". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind participants and clinicians to these interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Authors stated that to allocate more women to the presumably favourable treatment arm, they allocated 60% of the women to the NACT + RH arm and 40% to the radiotherapy arm. This could potentially have introduced bias when clinicians assessed subjective outcomes such as classification of response and toxicity. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 120/120 eligible women were analysed for primary survival outcomes using appropriate statistical techniques. |
| Selective reporting (reporting bias) | Low risk | All pertinent outcomes appeared to have been reported by the trial authors. |
| Other bias | Unclear risk | Insufficient information to assess whether an additional risk of bias existed. |
EORTC 2019.
| Study characteristics | ||
| Methods | Randomised phase III study (EORTC55994). Multicentre, multinational (Austria, Belgium, France, Italy, Netherlands, Portugal, Spain, the UK). Between May 2002 and June 2014 620 women with FIGO Stage IB2–IIB were randomised to NACT + surgery (311 women) or standard CCRT (309 women). Estimated primary completion date July 2019. |
|
| Participants | Women with FIGO Stage IB2, IIA > 4 cm or IIB cervical cancer. Histologically confirmed cervical cancer, including the following subtypes:
Age Range: 18–75 years Performance status WHO 0–2 |
|
| Interventions |
Women received neoadjuvant cisplatin‐based chemotherapy on day 1. Treatment repeats every 21 days. Within 6 weeks after the last chemotherapy course, and with a cumulative minimum of 225 mg/m2, women underwent a type III‐V Piver‐Rutledge RH. Women with positive lymph nodes or tumour invasion into the parametria or < 5 mm from the resection borders after surgery receive standard adjuvant external‐beam radiotherapy once daily, 5 days/week, for 5.0–5.6 weeks (25–28 treatment days) followed by external boost radiotherapy or brachytherapy for 1 or 2 days.
Radiotherapy 45–50 Gy + boost concurrent with weekly cisplatin chemotherapy (40 mg/m2/week). Adjuvant hysterectomy allowed, but not recommended, in cases of histologically confirmed residual tumour. |
|
| Outcomes |
Primary endpoint OS at 5 years Secondary endpoints OS PFS Toxicity Quality of life |
|
| Notes |
Results Median follow‐up was 8.2 years (95% CI 7.8 to 8.6) and similar between arms. Total of 191 deaths (31%) occurred. Age, stage and histological cell type were balanced between arms. Protocol treatment was completed in 459 (74%) women (71% for NACT + surgery and 82% for concurrent chemotherapy). In NACT + surgery arm, 238 (76%) women underwent surgery. Main reasons for not having surgery as per‐protocol were toxicity (25/74, 34%), progressive disease (18/74, 24%) and insufficient response to NACT (12/74, 16%). 198 SAEs occurred: 145 in the NACT + surgery arm vs 53 in the concurrent chemotherapy arm. Within the group of SAEs, there were 109 serious adverse reactions in the NACT + surgery arm and 35 in the concurrent chemotherapy arm. Nearly all were chemotherapy related. Protocol treatment was discontinued in 41 women due to toxicity (6.5%). In the NACT + surgery arm, surgery in 23 women was abandoned due to NACT‐related toxicity. Most frequent reported were nausea and vomiting, infections, metabolism disturbance, and renal and urinary disorders. There were grade 3/4 complications related to surgery in the following number of women: 8 (3.3%) bleeding, 10 (4.2%) operative lesions to ureter or bladder, 3 (1.2%) fistula, 7 (2.9%) others (sepsis, urinary tract infection and wound dehiscence). Additional radiotherapy given to 113 (36.3%) women in the NACT + surgery arm. Additional surgery was performed in 9 (2.9%) women in the concurrent chemotherapy arm. Short‐term severe adverse events (≥ grade 3) occurred more frequently in the NACT + surgery arm (35%) than in the concurrent chemotherapy arm (21%) (P < 0.001). 5‐year OS was 72% in the NACT + surgery arm and 76% in the concurrent chemotherapy arm (difference 4.0%, 95% CI –4% to 12%; HR 0.87, 95% CI 0.65 to 0.15; P = 0.332). 5‐year DFS in the NACT + surgery group was 56.9% compared with 65.6% in the CCRT arm, mostly for Stage IIB disease. However. the authors noted that the first imaging measurement occurred systematically earlier in the NACT + surgery arm than in the CCRT arm. Conclusions: these preliminary results revealed no difference in 5‐year OS between NACT + surgery and CCRT, indicating that quality of life and long‐term toxicity are important to decide optimal treatment. Overall toxicity was acceptable, occurred more frequently in the NACT + surgery arm and was mainly related to NACT. The final results were expected by April 2019, including long‐term toxicity and treatment effect across prognostic factors. Clinical trial information: NCT00039338. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not possible but should not have introduced bias for objectives outcomes such as OS and PFS. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear. |
| Selective reporting (reporting bias) | Unclear risk | In abstract form only, so unclear. |
| Other bias | Unclear risk | Insufficient information to permit judgement. |
Gupta 2018.
| Study characteristics | ||
| Methods | Single‐centre RCT conducted in India. | |
| Participants | 633 women aged 18–65 years with histologically confirmed squamous cell carcinoma of the cervix with 1994 FIGO Stage IB2, IIA or IIB disease. The baseline characteristics did not show any significant differences between the 2 arms. Histopathological type Squamous cell carcinoma Median age 50 years in the NACT + RH group and 48 years in the CCRT group. Performance status 0: 91.8% in the NACT + RH arm and 92.4% in the CCRT arm. 1: 8.2% in the NACT + RH arm and 7.6% in the CCRT arm. FIGO Stage FIGO Stage IB2 bulky: 18% of women in the NACT + RH arm and 17.7% in the CCRT arm. FIGO Stage IIA bulky: 25.3% of women in the NACT + RH arm and 24.6% in the CCRT arm. FIGO Stage IIB bulky: 56.7% of women in the NACT + RH arm and 57.7% in the CCRT arm. Tumour grade Not specified |
|
| Interventions |
3 cycles of paclitaxel 175 mg/m2 and carboplatin (dosed to an area under curve of 5–6) once every 3 weeks. Women underwent clinical response assessment after the second and third cycles of chemotherapy. Women who had no response or disease progression at these times were crossed over to receive definitive CCRT, whereas responders underwent surgery 3–4 weeks after the third cycle of chemotherapy. Women assigned to the NACT + surgery group underwent Piver‐Rutledge class III radical abdominal hysterectomy, bilateral pelvic lymphadenectomy and lower para‐aortic lymph node sampling by expert gynaecological oncologists. Surgery was abandoned in women with intraoperative findings of either unresectable primary tumour or lymph node disease, and these women were treated with definitive CCRT.
Women assigned to the CCRT group and those who crossed over from the NACT + surgery group received standard external‐beam radiotherapy to the whole pelvis + brachytherapy. They received an external radiation dose of 40 Gy in 20 fractions with 2 Gy per fraction and a midline shield at 20 Gy, followed by intracavitary radiation to 'point A' as follows: either 2 applications of a low‐dose rate of 30 Gy each or 5 applications of a high‐dose rate of 7 Gy each. Radiation doses were modified to respect tumour, rectal and bladder constraints. These women also received 5 cycles of cisplatin 40 mg/m2, administered once per week starting with external‐beam radiotherapy.
Women in the NACT + surgery group who underwent RH were given adjuvant therapy (radiotherapy or CCRT) as per protocol defined criteria, in accordance with published evidence. On the basis of histopathological evaluation of the surgical specimen, adjuvant chemoradiotherapy was given in the presence of any 1 of the following features: lymph node metastasis, positive surgical margins or parametrial involvement. Adjuvant radiotherapy alone was given based on the presence of any 2 of the following features: deep cervical stromal invasion, lymphonodular invasion, or tumour size > 4 cm. Women in both groups were evaluated at protocol defined time points to evaluate response, monitor for relapse and assess toxicity. |
|
| Outcomes | DFS (primary endpoint) OS (secondary endpoint) Toxicity |
|
| Notes | Written informed consent was obtained from all women before inclusion in the study. Women were randomly assigned, after stratification by stage. Between September 2003 and February 2015, 635 women were randomly assigned, of whom 633 (316 in the NACT + surgery group and 317 in the CCRT group) were included in the final analysis, with a median follow‐up of 58.5 months. There was good compliance to planned treatment in both arms, including doses and duration of chemotherapy and radiotherapy. In the NACT + surgery group, 2 (0.63%) women received CCRT, and in the CCRT group, 2 (0.63%) women underwent surgical resection (protocol non‐adherence). In the NACT + surgery group, 68 (21.5%) women crossed over (presurgery crossover and intraoperative unresectable disease) to receive definitive CCRT, 42 (13.3%) women received postoperative adjuvant chemoradiotherapy, and 31 (9.8%) women received postoperative adjuvant radiotherapy, according to protocol‐defined criteria. 5‐year DFS in the NACT + surgery group was 69.3% compared with 76.7% in the CCRT group (HR 1.38, 95% CI 1.02 to 1.87; P = 0.038), whereas the corresponding 5‐year OS rates were 75.4% ‐in the NACT + surgery group and 74.7% in the CCRT group (HR 1.025, 95% CI 0.752 to 1.398; P = 0.87). In subgroup analyses, the DFS detriment in the NACT + surgery group was statistically significant in women with FIGO Stage IIB disease, with a significant test of interaction between treatment effect and Stages IIA and IIB disease. In women with Stage IIB disease, 5‐year DFS rates in the NACT + surgery was 67.2% and CCRT group was 79.3% (unadjusted HR for DFS in the NACT + surgery group, 1.90, 95% CI 1.25 to 2.89; P = 0.003). In the NACT + surgery group, compared with the CCRT group, there was a lower rate of rectal, bladder and vaginal toxicity occurring or persisting 90 days after treatment completion (rectal: 5.7% with NACT + surgery vs 13.3% with CCRT; P = 0.002; bladder: 2.8% with NACT + surgery vs 7.3% with CCRT; P = 0.017); vaginal: 19.9% with NACT + surgery vs 36.9% with CCRT; P = 0.001). However, 24 months after treatment completion, there was no difference in rectal and bladder toxicities between groups, whereas vaginal toxicity continued to occur at a lower rate in the NACT + surgery group (12.0% with NACT + surgery vs 25.6% with CCRT; P = 0.001). Despite adequate NACT, surgery was possible in only 227 (72.15%) women in the NACT + surgery group. The 2 chemotherapy regimens were different in the 2 groups with respect to platinum and paclitaxel dose and administration schedule. Thus, the choice of platinum drug is unlikely to be a critical factor in the outcome of the study. Quality of life was not prospectively measured. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible women were randomly assigned to either study group in a 1:1 ratio using a computerised block design with a block size of 4. |
| Allocation concealment (selection bias) | Unclear risk | Random assignment was performed by the Clinical Trials Unit of Tata Memorial Centre. Women were stratified according to clinically determined 1994 FIGO Stage IB2, IIA, or IIB disease before random assignment. There were only 4 block sizes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study investigators and participants were not blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study investigators and participants were not blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 635 women combined and 29 lost to follow‐up but only 2/635 were not included in final analysis. |
| Selective reporting (reporting bias) | Low risk | DFS was reported in first instance without the inclusion of participants who died from any cause, but this is additionally reported (albeit not statistically significant whereas the former was significant and this is presented as main result in the abstract). However, in our review, we used the 1 including death from any cause so no bias to our review. They also included OS and other morbidity outcomes. |
| Other bias | Unclear risk | Insufficient information to permit judgement. |
Keys 2003.
| Study characteristics | ||
| Methods | Multicentre RCT conducted in the US (GOG #71, RTOG #84‐12). Primary study objective was to determine whether adjuvant hysterectomy following radiotherapy for women with bulky Stage IB cervical cancer improved survival. |
|
| Participants | 256 eligible women. It was not mentioned whether the women were evaluated clinically or radiologically after radiotherapy in order to assess the tumour response and residual disease. Surgical staging of lymph nodes was optional and was performed on 57 (22%) women, equally divided between the study arms. Any women with metastasis to the para‐aortic nodes were ineligible for the RCT. Histopathology type Adenocarcinoma: 6% in the radiotherapy group and 7% in the radiotherapy + hysterectomy group. Adenosquamous carcinoma: 7% in the radiotherapy group and 7% in the radiotherapy + hysterectomy group. Squamous cell carcinoma: 86% in the radiotherapy group and 86% in the radiotherapy + hysterectomy group. Age ≤ 30 years: 9% in the radiotherapy group and 9% in the radiotherapy + hysterectomy group. 31–40 years: 37% in the radiotherapy group and 29% in the radiotherapy + hysterectomy group. 41–50 years: 32% in the radiotherapy group and 35% in the radiotherapy + hysterectomy group. 51–60 years: 17% in the radiotherapy group and 18% in the radiotherapy + hysterectomy group. 61–70 years: 4% in the radiotherapy group and 8% in the radiotherapy + hysterectomy group. 71–80 years: 1% in the radiotherapy group and 2% in the radiotherapy + hysterectomy group. Race White: 49% in the radiotherapy group and 52% in the radiotherapy + hysterectomy group. African American: 35% in the radiotherapy group and 27% in the radiotherapy + hysterectomy group. Other: 15% in the radiotherapy group and 21% in the radiotherapy + hysterectomy group. GOG performance grade 0: 77% in the radiotherapy group and 76% in the radiotherapy + hysterectomy group. 1: 22% in the radiotherapy group and 20% in the radiotherapy + hysterectomy group. 2: 1% in the radiotherapy group and 4% in the radiotherapy + hysterectomy group. Tumour size 4 cm: 9% in the radiotherapy group and 13% in the radiotherapy + hysterectomy group. 5 cm: 36% in the radiotherapy group and 33% in the radiotherapy + hysterectomy group. 6 cm: 32% in the radiotherapy group and 27% in the radiotherapy + hysterectomy group. 7 cm: 10% in the radiotherapy group and 18% in the radiotherapy + hysterectomy group. ≥ 8 cm: 12% in the radiotherapy group and 9% in the radiotherapy + hysterectomy group. Tumour type Exophytic: 48% in the radiotherapy group and 43% in the radiotherapy + hysterectomy group. Barrel: 52% in the radiotherapy group and 57% in the radiotherapy + hysterectomy group. |
|
| Interventions |
Radiotherapy Radiotherapy was delivered externally. Daily fraction size was 180 Gy and external treatment carried to a total dose of 40 Gy for the radiation alone regimen and 45 Gy for the adjuvant hysterectomy regimens. Dose distribution could not vary > 5% across the treatment volume. These parameters were the same for both treatment arms. The intracavitary treatment dose prescription was different between the treatment arms. Both groups were to have an intracavitary treatment 1–2 weeks after completing external treatment. The radiotherapy alone group received 40 Gy to 'point A', while those who were to have hysterectomy received only 30 Gy. Interstitial therapy was not permitted on this protocol. A total dose of 80 Gy to 'point A' was prescribed for the radiotherapy alone regimen and 75 Gy for the adjuvant hysterectomy regimen. A minimum dose of 55 Gy was prescribed to 'point B' for both regimens. A parametrial boost was permitted if necessary to achieve this dose. All irradiation was to be completed within 10 weeks. Hysterectomy The radiotherapy + hysterectomy group was then to undergo extrafascial hysterectomy with removal of tubes and ovaries, if present, 2–6 weeks after completion of all irradiation. This operation was described to include the removal of the corpus and cervix without contiguous parametrial tissue. |
|
| Outcomes | OS Pelvic‐free survival Pelvic recurrence |
|
| Notes | 256 eligible women with exophytic or 'barrel' shaped tumours measuring 4 cm were randomised to either external and intracavitary radiotherapy (124 women) or attenuated radiotherapy + extrafascial hysterectomy (132 women).
Of the women randomised to receive radiotherapy alone, 87% received a total 'point A' dose of 78 Gy or more while 49% had a total duration of treatment of ≤ 60 days. For the radiotherapy + hysterectomy treatment regimen, 90% received a total 'point A' dose of ≥ 71 Gy while 80% had a total duration of treatment of ≤ 60 days. Regarding the proportion of received to prescribed dose to 'point A', 96% of women had proportions > 0.80 from the radiotherapy alone group and 95% of women from the radiotherapy + hysterectomy regimen. 22% of women (28/124 (23%) women in radiotherapy alone group and 29/132 (22%) women in radiotherapy + hysterectomy group) received the optional up‐front surgical staging. Of the 103 women who did not have prerandomisation surgical staging on the hysterectomy regimen, 54 (52%) had a hysterectomy and lymph node sampling procedure. Of these, 7 (13%) had positive para‐aortic nodes. Both treatment programmes were well tolerated. Hysterectomy did not increase the frequency of reported grade 3 and 4 adverse effects (13 (10%) women for each regimen). 18/26 of these serious adverse effects were from the gastrointestinal or genitourinary tract exclusively. The frequency of any reported adverse effect was higher for the radiotherapy + hysterectomy group (56% with radiotherapy alone vs 63% with radiotherapy + hysterectomy). 57 (46%) women had progression of disease among the radiotherapy alone group and 49 (37%) women had progressions in the radiotherapy + hysterectomy group. The PRS results included, as failures, those with disease progression and deaths that occurred without progression (8 with radiotherapy alone vs 11 with radiotherapy + hysterectomy). Significance level for the log‐rank test was P = 0.07 (1‐tail). Reduction in the risk of progression/death for the radiotherapy + hysterectomy group to the radiotherapy alone group was 23% (i.e. RR 0.77, 90% CI –3 to 43). Median PFS was 7.4 years for the radiotherapy alone regimen and no estimate was available for the radiotherapy + hysterectomy regimen (PFS was 53% at 8.4 years). There were 2 treatment‐related deaths in the radiotherapy alone group and 1 in the radiotherapy + hysterectomy group. 8 women died of intercurrent disease and 12 died of unknown causes. Those who were last seen alive had a median follow‐up time of 9.6 years (range 0.3 to 16.1 years). 12 women in each regimen (10% in radiotherapy alone group vs 9% in radiotherapy + hysterectomy group) were lost to follow‐up by 5 years. There was no apparent difference in the survival experience of women by treatment regimen (P = 0.26, 1‐tail). The relative risk estimate of the combined‐treatment group to the radiotherapy alone group was 0.89 (90% CI 0.65 to 1.21). 56 women died in each treatment regimen. The modelling of survival identified the same prognostic factors with very similar relative risk estimates. Adjusting for tumour size, performance status and age, the relative risk of progression for the combined treatment group to the radiotherapy alone group was statistically significant (i.e. RR 0.72; P = 0.04, 1‐tail). The adjusted relative risk estimate of death was 0.84, which was not statistically significant. At 5 years, the strictly local relapse incidence was up to 27% for the radiotherapy alone regimen vs 14% for the radiotherapy + hysterectomy regimen, although the radiotherapy + hysterectomy group was slightly more likely to have distal progression (16% with radiotherapy alone vs 20% with radiotherapy + hysterectomy). These rates agreed with the distribution of progression site. To determine if there was any differential effect of adjuvant hysterectomy by tumour size, a test for interaction was performed. The interaction term, when quantified using the whole centimetre tumour size, was of borderline significant (P = 0.06) for PFS but was statistically significant (P = 0.007) for survival. The results indicated that women in the radiotherapy + hysterectomy group had a lower risk of progression and death than the radiotherapy‐alone group for tumour sizes of 4 cm, 5 cm and 6 cm. Combined, the RR for death was 0.60 (relative risk of progression 0.58) for the radiotherapy + hysterectomy group to the radiotherapy alone group. Women with tumour sizes of ≥ 7 cm in the radiotherapy + hysterectomy group progressed at a higher rate after 14 months; the relative risk estimate was 1.27 (relative risk of death 2.03). The crossover from lower to higher risk for the radiotherapy + hysterectomy regimen occurred between 6 cm and 7 cm for both PFS and survival. Histological evaluation of the cervical specimens for the 123 women who underwent hysterectomy identified 59 (48%) with no detectable malignancy, while 49 (40%) were microscopically positive and 15 (12%) were grossly positive. Median time to surgery from study entry was 3.0 months (10th percentile 2.4 and 90th percentile 3.8 months). Comparing PFS and survival of women with negative vs positive specimens were statistically significant. Women with grossly positive hysterectomy specimens progressed and died at almost 7 times the rate compared to those with negative specimens. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization with equal probability of assignment to each treatment regimen was carried out by a block arrangement balancing the treatment assignment within major GOG institutions and the option of para‐aortic lymph node sampling". |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants and clinicians to these interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 256/256 eligible women were analysed for primary survival outcomes using appropriate statistical techniques. 2 women in each arm did not receive radiotherapy and were not examined for adverse effects. |
| Selective reporting (reporting bias) | Low risk | All pertinent outcomes appeared to have been reported by the trial authors. |
| Other bias | Unclear risk | Insufficient information to assess whether an additional risk of bias existed. |
Khan 2014.
| Study characteristics | ||
| Methods | Randomised controlled trial conducted in Nigeria. No information if single or multicentre study. | |
| Participants | Women with squamous cell carcinoma of the cervix Stage 1B2–III (not specified which subcategories). 111 women randomised but numbers per group not reported. |
|
| Interventions |
All participants received cisplatin‐based chemotherapy. Participants received EBRT 45–50 Gy + brachytherapy 20–30 Gy.
All participants received platinum‐based chemotherapy. Surgery was RH + pelvic lymphadenectomy. |
|
| Outcomes | Treatment‐related morbidity: short‐term complications within 30 days of completion of treatment and long‐term complications that were reported within 2 years after treatment. | |
| Notes | Abstract only Treatment was delivered as per protocol in both arms. There were no treatment‐related deaths. Overall 89% of participants in chemoradiotherapy and 73% in NACT + surgery had complications. 18% in NACT + surgery had recurrence and required adjuvant radiotherapy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not possible but should not have introduced bias for objective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Unclear risk | Insufficient information to permit judgement. |
Morice 2012.
| Study characteristics | ||
| Methods | RCT conducted in France. Unclear whether single or multicentre trial, but appeared multicentre. FIGO Stage IB2 or II cervical cancer received pelvic external radiotherapy + concurrent cisplatin chemotherapy with or without RH. |
|
| Participants | 61 eligible women having achieved a complete clinical and radiological response after CCRT were randomly allocated to hysterectomy or no hysterectomy. Histopathology type Squamous cell cancer: 90% of women in the CCRT + hysterectomy arm and 80% in the CCRT alone arm. Adenocarcinoma: 10% of women in the CCRT + hysterectomy arm and 20% in the CCRT alone arm. Median age 45 years in the CCRT + hysterectomy arm and 44 in the CCRT alone arm. FIGO Stage FIGO Stage IB2: 52% of women in the CCRT + hysterectomy arm and 50% in the CCRT alone arm. FIGO Stage II: 48% of women in the CCRT + hysterectomy arm and 50% in the CCRT alone arm. External radiotherapy Median dose was 45 Gy in the CCRT + hysterectomy arm and 46 in the CCRT alone arm. Brachytherapy 1 application: 24 women in the CCRT + hysterectomy arm and 25 in the CCRT alone arm. 2 applications: 4 women in the CCRT + hysterectomy arm and 4 in the CCRT alone arm. Unknown applications: 3 women in the CCRT + hysterectomy arm and 3 in the CCRT alone arm. Median dose of brachytherapy 15 Gy in both groups. Unknown in 9 women. Lateropelvic external‐beam radiotherapy boost 23% in the CCRT + hysterectomy arm and 31% in the CCRT alone arm. Median duration of radiotherapy 51 days in the CCRT + hysterectomy arm and 51.5 days in the CCRT alone arm. Unknown in 5 women. Courses of concurrent chemotherapy 5 in each group. Unknown in 6 women. Initial characteristics 26 women had clinical spread to the parametria during initial management and 10 women had clinical spread to the vagina during initial management. During pretherapeutic examinations, 18 women had suspicious pelvic nodes (on MRI or CT scan, or both) 0 women had suspicious para‐aortic nodes. 13 women underwent an initial para‐aortic lymphadenectomy (associated with pelvic node dissection in 1). 2 women had involved para‐aortic nodes and 1 woman had positive pelvic nodes (of 17 removed) but without para‐aortic involvement. |
|
| Interventions |
Radiotherapy Delivered to the pelvis for a total dose of 45–50 Gy in 5 fractions of 1.8–2 Gy per week followed 1–2 weeks later by brachytherapy. Radiotherapy was combined with concurrent chemotherapy using cisplatin during external radiotherapy. Use of a sixth course of concurrent chemotherapy during brachytherapy was optional. Complete clinical and radiological response (based on MRI) evaluated 6–8 weeks after brachytherapy Chemotherapy Concurrent cisplatin chemotherapy. Hysterectomy Performed using a laparotomy or a laparoscopic approach and extrafascial or radical (type II according to the Piver classification) according to the preoperative examination. A selective or complete pelvic lymphadenectomy was optional and could have been performed if lymphadenopathy was detected during surgery. If a para‐aortic lymphadenectomy was not performed initially using a laparoscopic approach, it could have been done at the time of pelvic surgery in women randomised to CCRT + hysterectomy. It was possible for some of the women randomised to CCRT alone to undergo surgery after brachytherapy to carry out a laparotomy or a laparoscopic para‐aortic lymphadenectomy, but without pelvic surgery. |
|
| Outcomes | OS EFS (event referring to recurrence or death) Site of first recurrence Morbidity (the results not published in this or other publication) |
|
| Notes | This trial was closed because of poor accrual: only 61 women were enrolled (in 2003–2006) and were reported in this study: 31 in the CCRT + hysterectomy arm and 30 in the CCRT alone arm. Median duration of follow‐up 3.8 years (range 0.4–5.8 years). 1 woman initially included in the CCRT + hysterectomy arm did not undergo hysterectomy and 1 women included in in the CCRT alone arm did undergo hysterectomy. After the end of CCRT + brachytherapy all women, except 4, had a normal clinical examination (but this status was unknown for 8 women). In the CCRT + hysterectomy arm, a laparoscopic hysterectomy was performed in 7 cases and a RH was performed in 10 cases. 16 women underwent para‐aortic lymphadenectomy. 6 women underwent complete bilateral pelvic lymphadenectomy, 3 had a unilateral pelvic lymphadenectomy with contralateral pelvic selective lymphadenectomy and 4 had a selective pelvic lymphadenectomy. In the CCRT alone arm, 17 women underwent a para‐aortic lymphadenectomy (via a laparoscopic approach for 6 of them). No woman underwent a complete pelvic lymphadenectomy and 1 woman had a selective pelvic adenectomy. In the CCRT + hysterectomy arm, 11 women had residual disease after histological analysis (isolated cells for 6 women, ≤ 1 cm for 3 women, and > 1 cm for 2 women). Only 1 of them (with residual disease > 1 cm) had an abnormal pre‐randomisation MRI (ambiguous endocervical lesion measuring 8 mm). In the CCRT + hysterectomy arm, 3 women had metastatic pelvic nodes (2 had 1 involved node and 1 had 3 involved nodes) and 2 women had metastatic para‐aortic nodes (1 had 2 involved nodes and 1 had 8 involved nodes). In the CCRT alone arm, 1 woman had metastatic pelvic nodes and 0 women had metastatic para‐aortic nodes. 3‐year EFS rates were 72% (95% CI 53 to 85) in the CCRT + hysterectomy arm and 89% (95% CI 75 to 96) in the CCRT alone arm (not significant). 3‐year OS rates were 86% (95% CI 69 to 55) in the CCRT + hysterectomy arm and 97% (95% CI 83 to 99) in the CCRT alone arm (not significant). The log‐rank test found no difference between groups in terms of OS and EFS. 12 women relapsed (5 of them died): 8 in the CCRT + hysterectomy arm and 4 in the CCRT alone arm. Location of recurrent disease was known in 11 women. Among 2 women having a pelvic node recurrence, 0 had a suspicious pelvic node during initial imaging and 1 had lateropelvic boost for parametrial spread. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants and clinicians to these interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 61/61 randomised women were analysed. |
| Selective reporting (reporting bias) | High risk | After contacting the authors, we were informed that the morbidity results were not published or available. |
| Other bias | Unclear risk | Insufficient information to assess whether an additional risk of bias existed. |
Noriyuki 2010.
| Study characteristics | ||
| Methods | Single‐centre RCT conducted in Japan. | |
| Participants | 42 women were enrolled: 20 received NACT + RH and 22 received radiotherapy. All were exclusively Stage IIIB cervical cancers. Age Mean age: 53.2 (SD 1.7) years in the NACT + RH group and 59.9 (SD 1.7) years in the radiotherapy group. Mean age during enrolment was statistically younger in the NACT + RH group compared to the radiotherapy group (P < 0.01). Age range: 36–69 years in the NACT + RH group and 42–70 years in the radiotherapy group (unpaired t‐test). Histopathology Histopathology type was squamous cell cancer only. Keratinising: 8 women in the NACT + RH group and 4 in the radiotherapy group. Non‐keratinising: 12 women in the NACT + RH group and 18 in the radiotherapy group (Fisher's test). Hydronephrosis Prevalence of hydronephrosis was similar between groups (2 in each group). Parametrium involvement Bilateral parametrium involvement was significantly higher in the radiotherapy group (5 in the NACT + RH group and 13 in the radiotherapy group; P < 0.05). |
|
| Interventions |
NACT Intra‐arterial infusion chemotherapy consisting of cisplatin, bleomycin and mitomycin for 3 courses every 4 weeks. Evaluations were done after each course of chemotherapy to determine the local tumour size and whether there were free surgical margins of the involved parametrium in the pelvis. Radical hysterectomy If the tumour was surgically removable, a RH was performed with bilateral salpingo‐ oophorectomy and pelvic lymphadenectomy, and then radiotherapy at 40 Gy to the whole pelvic region. If the tumour progressed or relapsed, a combined chemotherapy of BOPM was given, and then irinotecan with cisplatin as the third line. If the local tumour was inoperable (1 woman) radiotherapy 40 Gy given to the whole pelvic region and 20 Gy to intracavity, followed by BOPM chemotherapy.
Radiotherapy to the whole pelvic region in 20 fractions totalling 40 Gy. Total dose delivered to 'point B' as a boost dose with midline shield coverage was 20 Gy. Total dose delivered by brachytherapy was 24–30 Gy. Duration of radiotherapy was 4 weeks. Radiotherapy was discontinued if white blood cell count was < 3000/mm3. In cases with local recurrence or progression of the primary lesion, chemotherapy was added, which included BOMP, irinotecan with cisplatin, and cisplatin or carboplatin alone. When distant metastasis occurred, radiotherapy was added or the single lesion was surgically removed. |
|
| Outcomes | 5‐year survival 5‐year DFS The trial authors supplied HR estimates for OS and DFS for inclusion in meta‐analyses. |
|
| Notes | In the NACT + RH arm, if the tumour became operable within 3 courses, RH was performed. The radiotherapy alone group received radiotherapy to the whole pelvis of 40 Gy, midline shield coverage of 20 Gy, and intracavitary of 24–30 Gy. Median duration of follow‐up 60 months. The keratinising type of cervical squamous cell carcinoma responded more poorly to radiotherapy than the non‐keratinising type. However, both keratinising and non‐keratinising types responded similarly to NACT + RH. After 15 years of enrolment, the study had only 42 eligible women and, furthermore, the recent meta‐analysis showed that CCRT was superior to sequential chemotherapy. Thus, it was decided to stop this study before the expected number of women were enrolled and a follow‐up study performed. There were no differences in the recurrence rates and recurrence sites between groups. 5‐year survival rate was 47% for the NACT + RH group and 48% for the radiotherapy group. No statistical difference in OS between groups. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants and clinicians to these interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 42/42 randomised women were analysed and 0 women were lost to follow‐up (including the 2 women who died). |
| Selective reporting (reporting bias) | High risk | Adverse event and toxicity data not reported. |
| Other bias | Unclear risk | Insufficient information to assess whether an additional risk of bias existed. |
Perez 1987.
| Study characteristics | ||
| Methods | Single‐centre RCT conducted in Washington University, USA. Women were randomised to radiotherapy + RH or radiotherapy alone. |
|
| Participants | 118 eligible women with Stage IB to IIA cervical cancer. It was not reported how many women were Stage IB1 and how many Stage IB2 (the latter group is area of interest for this Cochrane Review). Women with barrel‐shaped cervix (endocervical lesion with cervix diameter > 5 cm) were excluded. Age distribution was comparable between groups. No additional information given regarding the age (i.e. range, median age, mean age) of the participating women. In women treated with radiotherapy + RH (62 women) Stage IB women: 43 had squamous cell carcinoma, 4 had adenocarcinoma and 1 had adenosquamous carcinoma. Stage IIA women: 14 had squamous cell carcinoma. In women treated with radiotherapy alone (56 women) Stage IB women: 35 had squamous cell carcinoma, 4 had adenocarcinoma and 1 had adenosquamous carcinoma. Stage IIA women: 16 had squamous cell carcinoma. |
|
| Interventions |
2000 Gy whole pelvis irradiation and 1 intracavitary insertion for 5000–6000 milligram‐hours, followed 2–6 weeks later by a RH and bilateral pelvic lymphadenectomy (up to the bifurcation of the common iliac chain). The dose to the cervix was about 7000 Gy and to the pelvic lymph nodes 3000 Gy.
1000–2000 Gy delivered to the whole pelvis and an additional parametrial dose to total of 5000 Gy to the external iliac lymph nodes combined with 2 intracavitary insertions for a total of approximately 7500 milligram‐hours (6500–7000 Gy to 'point A'). The dose to the paracervical tissues was about 8500 Gy and to the pelvic lymph nodes 6000 Gy. |
|
| Outcomes | 5‐year tumour‐free actuarial survival Sites of failure after therapy Complications |
|
| Notes | All women were available for 5‐year follow‐up and the median period of observation was 6 years. No women were lost to follow‐up. In the women with Stage IB, 48 received preoperative radiotherapy + RH and 40 received radiotherapy alone. In women with Stage IIA, 14 received preoperative radiotherapy + RH and 16 received radiotherapy alone. 5‐year, tumour‐free actuarial survival for Stage IB women was 80% in the preoperative radiotherapy + RH group and 89% in the radiotherapy alone group. In Stage IIA, 5‐year survival was 79% in the preoperative radiotherapy + RH group and 56% in the radiotherapy alone group. The difference was not statistically significant. An analysis of the chronological distribution of recurrences showed that 85% of the failures occurred within 3 years from therapy, at about the same rate in both groups. The overall incidence of major complications was 11% in the preoperative radiotherapy + RH group and 16% in the radiotherapy alone group. This difference was not statistically significant. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were … randomised by the flip of a coin". |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants and clinicians to these interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 118/118 randomised women were analysed and 0 women were lost to follow‐up. |
| Selective reporting (reporting bias) | High risk | 5‐year tumour‐free survival was reported but OS was not, even though the number of deaths would have been known. |
| Other bias | Unclear risk | Insufficient information to assess whether an additional risk of bias existed. |
Zheng 2017.
| Study characteristics | ||
| Methods | Single‐centre RCT conducted in China with recruitment by 2 departments. The paper was translated, originally written in Mandarin. RCT study on chemoradiotherapy with adjuvant surgery in later‐stage cervical cancer since June 2012. These are the preliminary results of the study. We emailed the corresponding author to ask for any more up‐to‐date results, no reply to date. Primary efficacy endpoint of the chemoradiotherapy group was 5‐year survival rate > 60%. Authors chose lower point of the 5‐year survival rate (60–70%) according to NCI series of studies, because Stage IIIA and IIIB cervical cancer will be included in a further study. 5‐year survival rate was set to be ≥ 70%, and increased by 10% compared to chemoradiotherapy group to be statistically significant (significance level 5% with 80% power). Recruiting period was set for 3 years; follow‐up was 5 years. Lost to follow‐up rate was set for 10%. Median survival rates for chemoradiotherapy + surgery group was set for 9.7 years and for chemoradiotherapy group was 6.8 years. Power and sample size calculation program software was used to calculate the sample size for each group, which was 327. If sample size is not reached before end of recruiting period, this study will be terminated, but statistical analysis will be performed. Inclusion criteria: women with cervical cancer; stage confirmed by 2 gynaeoncologists (oncologist specialised in gynaecology): Stage IB (tumour dimension > 4 cm), Stage IIA–IIIB (2009 FIGO cancer stage system). All participants were followed up for > 5 years, and the case lost to follow‐up from the date of loss to follow‐up was calculated as death. Statistical analysis using SPSS 20.0 software. The Chi2 test or Fisher exact probability method was used to compare the number of data sets. The rate was calculated by Kaplan‐Meier method and the survival curve was drawn. The inspection level was set as α = 0.05. Intentionality score was used for statistical analysis (intention‐to‐treat analysis). |
|
| Participants | 102 eligible participants were included before this interim analysis, including 52 in the chemoradiotherapy + surgery group and 50 in the chemoradiotherapy group. All the participants were numbered according to the enrolment order. Currently, 102 participants enrolled in study, with the numbers ranging from 1 to 102. Each participant was randomly assigned to 1 and 2 using a random number generator, with 1 as the chemoradiotherapy + surgery group and 2 as the chemoradiotherapy group. |
|
| Interventions |
Chemoradiotherapy Women who met the inclusion criteria were first treated with CCRT in the radiotherapy department. Linear accelerator was used for external pelvic irradiation, and the intracavitary 192Ir was used for radiotherapy. Stage 1: the whole basin irradiation before and after the field or left and right field irradiation, 4 or 5 times a week, each time 2.25 Gy or 1.8 Gy, pelvic centre total dose 30 Gy. Stage 2: the lead block protected the uterus, and the uterus continued to be irradiated from the front and back, 5 times a week, 1.8–2.0 Gy each time, and the total periuterine dose 15–20 Gy. At the beginning of the second stage of external irradiation, intracavitary and the back of the cavity were performed at the same time once a week, with 4.6–7.0 Gy at 'point A' and total of 35–42 Gy at 'point A'. Chemotherapy regimen: cisplatin alone: 35–40 mg/m2, once a week. Surgical treatment Participants in the chemoradiotherapy + surgery group underwent radical surgery in the hospital 4–6 weeks after the completion of CCRT. For radical total hysterectomy and pelvic lymphadenectomy, 3 cm of parastatal and vaginal tissues were removed. |
|
| Outcomes | Recurrence OS rate Outcome indicators Short‐term efficacy evaluation: evaluate the efficacy according to the tumour regression before and after treatment: complete remission: complete regression of the tumour by gynaecological examination and imaging examination; partial remission: tumour volume reduction ≥ 50%; stability of disease: tumour volume reduction ≤ 50%; progression of disease: tumour enlargement or presence of new lesions. Complete and partial response means an effective treatment. |
|
| Notes | 6 participants in the chemoradiotherapy + surgery group refused surgery. In the chemoradiotherapy group, 2 participants chose surgery due to tumour regression, 4 participants automatically requested surgery. Short‐term efficacy evaluation at the end of CCRT Chemoradiotherapy + surgery group: complete remission rate 80.8% (42/52); partial remission rate 13.5% (7/52); stability of disease rate 5.8% (3/52); progression of disease rate 0%. Chemoradiotherapy group: complete response rate 72.0% (36/50); partial response rate 20.0% (10/50), stability of disease rate 4.0% (2/50); progression of disease rate 4.0% (2/50). Chi2 analysis of Kendall's Tau‐b scale data showed no statistically significant difference between groups (P = 0.290). The postoperative pathological results of the chemoradiotherapy + surgery group showed that after radiotherapy, the residual rate of non‐cancer was 82.7%, and the residual rate of cancer was 5.8%. PFS time PFS time was defined as the survival time from the end of chemoradiotherapy to the end of follow‐up without local tumour progression or recurrence. Since it is impossible to judge the progression of the lost follow‐up cases, to avoid overestimating the therapeutic effect, such participants were treated as if they had no progression. The PFS time of the chemoradiotherapy + surgery group was 3–40 months, median survival time was 23 months and 3‐year survival rate was 73.1%. PFS time in the chemoradiotherapy group was 5–41 months, median survival time was 22 months and 3‐year survival rate was 64.8%. Kaplan‐Meier Survival analysis showed that PFS rate was similar between groups (Chi2 = 0.092, P = 0.761). Total survival time OS was defined as the survival time of participants from the enrolment time to the end of follow‐up, and the participants lost to follow‐up were treated as deaths. OS time in the chemoradiotherapy + surgery group was 6–40 months, median survival time was 23 months and 3‐year survival rate was 82.7%. Total survival time in the chemoradiotherapy group was 5–41 months, median survival time was 22.5 months and 3‐year survival rate was 81.8%. Kaplan‐Meier survival analysis showed that the OS rate was similar between groups (Chi2 = 0.338, P = 0.561). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Each participant was randomly assigned to 1 and 2 using a random number generator. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not possible but should not have introduced bias for objective outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 102/102 (100%) women analysed. |
| Selective reporting (reporting bias) | Unclear risk | HRs were not reported in the paper. |
| Other bias | Unclear risk | Insufficient information to permit judgement. |
BOPM: bleomycin, vincristine, mitomycin and cisplatin; CCRT: concurrent chemoradiotherapy; CI: confidence interval; CT: computed tomography; DFS: disease‐free survival; EBRT: external beam radiotherapy; EFS: event‐free survival; FIGO: International Federation of Gynecology and Obstetrics; GOG: Gynecologic Oncology Group; HR: hazard ratio; MRI: magnetic resonance imaging; NACT: neoadjuvant chemotherapy; NCI: National Cancer Institute; OS: overall survival; PFS: progression‐free survival; RCT: randomised controlled trial; RH: radical hysterectomy; RR: risk ratio; SAE: serious adverse event; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Katsumata 2013 | Women in both arms received hysterectomy. |
| Keys 1999 | Women in both arms received hysterectomy. |
| Sardi 1997 | Women in both arms received hysterectomy or surgical staging. |
| Sun 2013 | Women in both arms received hysterectomy. |
| Sundfor 1996 | Compared surgery or radiotherapy in women with early‐stage carcinoma of the cervix. |
| Yang 2016 | Study sought to evaluate the toxicity and curative effect of irinotecan + cisplatin neoadjuvant chemotherapy for women with Stage IB2, IIA2 and IIB cervical cancer. The participants were randomly assigned to 2 groups: 1–2 cycles of chemotherapy + surgery or surgery alone. |
Characteristics of ongoing studies [ordered by study ID]
CSEM 006 study.
| Study name | |
| Methods | Randomised controlled trial |
| Participants | Women with Stage IIB cervical cancer |
| Interventions | NACT + surgery vs CCRT |
| Outcomes | 2‐year disease‐free survival |
| Starting date | Not specified. Enrolment is expected to be completed by December 2022. |
| Contact information | Professor Jihong Liu. linjih@mail.sysu.edu.cn |
| Notes |
Reis Fihlo 2018.
| Study name | The final results will be available in 2023 (personal communication with the authors). |
| Methods | Abstract only RCT Not possible to define if single or multicentre trial. |
| Participants | Disease characteristics: women aged ≥ 18 years with invasive squamous cell carcinoma of the cervix; FIGO Stage IB2, IIA or IIB; ECOG Performance Status 0–1 and adequate organ function are required. |
| Interventions | 244 women will be randomly assigned to 1 of 2 arms.
Standard chemoradiotherapy (cisplatin 40 mg/m2 intravenously: days 1, 8, 15, 29 and 36 concurrent with external radiotherapy 50.4 Gy fractionated in 28 sessions of 1.8 Gy followed by brachytherapy in 4 insertions of 7 Gy).
Intravenous NACT (cisplatin 75 mg/m2 day 1 + paclitaxel 80 mg/m2) day 1, 8 and 15 of each 21 days for 3 cycles. After each cycle, the participant will be evaluated to verify toxicity and tumour response. After the third cycle, the participants with a complete clinical response or substantial tumour reduction (tumour restricted to cervix 4 cm), confirmed by pelvic MRI will undergo Piver‐Rutledge class III abdominal hysterectomy and pelvic lymphadenectomy 3–6 weeks after the last cycle. Participants with tumour progression or severe toxicity after any cycle of NACT or with inoperable tumour after the third cycle of NACT will be treated with definitive standard chemoradiotherapy. |
| Outcomes |
Primary endpoint 5‐year overall survival Secondary endpoints Disease‐free survival Rate of operability (in the NACT + radical hysterectomy arm) Complete pathological response (in the NACT + radical hysterectomy arm) |
| Starting date | |
| Contact information | |
| Notes | No results to date. Clinical Trial Identification UTN: U1111‐1213‐5169 |
Shanmugam 2019.
| Study name | Comparison of neoadjuvant chemotherapy followed by radical hysterectomy and neoadjuvant chemoradiation followed by radical hysterectomy with concurrent chemoradiation in locally advanced carcinoma cervix (FIGO Stages IB2, IIA2, IIB): interim results of a randomized control study |
| Methods | Single‐centre RCT conducted in India. Exploring the therapeutic efficacy, toxicity profile and quality of life in locally advanced cervical carcinoma receiving 3 treatments: CCRT, preoperative chemoradiotherapy + radical hysterectomy and preoperative chemotherapy + radical hysterectomy. Age range: 18–65 years. Women included had histologically confirmed squamous cell carcinoma. Women with non‐squamous histologies were excluded. |
| Participants | 100 women with locally advanced cervical cancer (FIGO Stages IB2, IIA2 and IIB) were treated between June 2014 and March 2018. 33 women received CCRT (EBRT 50 Gy and brachytherapy 21 Gy). 33 women received with preoperative chemoradiotherapy (EBRT 50 Gy) + radical hysterectomy. 34 women received preoperative chemotherapy using 3 weekly cisplatin 75 mg/m2 and paclitaxel 175 mg/m2 + radical hysterectomy. |
| Interventions |
Cisplatin 75 mg/m2 and paclitaxel 175 mg/m2 given within 3‐week interval between the 2 cycles along with concurrent radiotherapy of EBRT 50 Gy (2 Gy in 25 sessions) followed by brachytherapy of 21 Gy (7 Gy for 3 doses) completed within 8 weeks.
Cisplatin 75 mg/m2 and paclitaxel 175 mg/m2 given within 3‐week interval between the 2 cycles along with concurrent radiotherapy of EBRT 50 Gy (2 Gy of 25 sessions) followed by radical hysterectomy within 3 weeks after completion of radiotherapy.
Cisplatin 75 mg/m2 and paclitaxel 175 mg/m2 given within 3 weeks interval for 3 cycles followed by radical hysterectomy within 3 weeks after completion of chemotherapy. |
| Outcomes | Overall survival Progression‐free survival Overall response rate Complete clinical response Partial clinical response Quality of life |
| Starting date | July 2014 |
| Contact information | Subbiah Shanmugam, Professor and Head, Department of Surgical Oncology, Government Royapettah Hospital, Kilpauk Medical College, Chennai, Tamil Nadu, India; e‐mail: subbiahshanmugam67@gmail.com |
| Notes | This paper is an interim response of the study. The trial will be included in a review update when final analyses are reported. Participants with advancing disease were termed as treatment failure and taken care of with conventional chemoradiotherapy. Quality of life was assessed using 2 validated questionnaires (EORTC QLQ‐C30 and EORTC QLQ‐CX24). Of 33 participants randomised to CCRT arm, 6% defaulted during EBRT and 6% defaulted for brachytherapy. Of 33 participants randomised to preoperative chemoradiotherapy + radical hysterectomy arm, 6% defaulted for surgical treatment (after completing EBRT and 2 cycles of chemotherapy) Of 34 participants randomised to preoperative chemotherapy + radical hysterectomy arm, 3% defaulted for surgery. Follow‐up period to June 2018 with a median follow‐up of 28 months. At the time of this interim analysis Clinical characteristics were reported to be well adjusted at baseline between arms. Oncological outcomes Overall response rate: CCRT arm: 97%; preoperative chemoradiotherapy + radical hysterectomy arm: 94%; preoperative chemotherapy + radical hysterectomy arm: 88%. Complete clinical response rate: CCRT arm: 72%; preoperative chemoradiotherapy + radical hysterectomy arm: 45%; preoperative chemotherapy + radical hysterectomy arm: 26%. Partial clinical response rate: CCRT arm: 12%; preoperative chemoradiotherapy + radical hysterectomy arm: 42%; preoperative chemotherapy + radical hysterectomy arm: 59%. At time of response assessment 3% of CCRT, 6% of preoperative chemoradiotherapy + radical hysterectomy and 12% of preoperative chemotherapy + radical hysterectomy arm participants had progressive disease and changed over to radical chemoradiotherapy treatment. Clinical response rates among the treatment arms were statistically significant (P = 0.0022). Survival outcomes Participants alive without disease: CCRT arm: 70%; preoperative chemoradiotherapy + radical hysterectomy arm: 88%; preoperative chemotherapy + radical hysterectomy arm: 85%. Participants dead: CCRT arm: 12%; preoperative chemoradiotherapy + radical hysterectomy arm: 6%; preoperative chemotherapy + radical hysterectomy arm: 0%. Recurrences in alive participants: CCRT: 2 participants (due to distant recurrence); preoperative chemoradiotherapy + radical hysterectomy arm: 4 participants (9% had local and 3% had distant recurrence and were treated with surgical/medical methods); preoperative chemotherapy + radical hysterectomy: 0. Clinical response rates among the treatment arms were statistically significant (P = 0.0022). Toxicity profile No treatment‐related deaths. Quality of life 81/100 participants completed quality of life questions. 1 question‐form was analysed per participant by the end of 3 months after completion of respective treatment method. For items relating to sexual performance, only sexually active women replied to the questions. For items relating to functioning scales, women in the CCRT arm disclosed lower score for physical and social functioning and women in the preoperative chemotherapy + radical hysterectomy arm revealed reduced emotional functioning and global health quality of life. There was no significant difference among symptom scales, but women in the CCRT arm disclosed an increased degree of symptom experience, difficulties in relation to sexual functioning/vaginal functioning and sexual agony. Women in the preoperative chemotherapy + radical hysterectomy arm disclosed more sexual activity and lymphoedema was more frequently recorded in women in the preoperative chemoradiotherapy + radical hysterectomy arm. Peripheral neuropathy was experienced more by women in the preoperative chemotherapy + radical hysterectomy arm. |
CCRT: concurrent chemoradiotherapy; EBRT: external beam radiotherapy; ECOG: Eastern Cooperative Oncology Group; EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer Quality of Life of Cancer Patients; EORTC QLQ‐CX24: European Organisation for Research and Treatment of Cancer Quality of Life – Cervical Cancer; FIGO: International Federation of Gynecology and Obstetrics; MRI: magnetic resonance imaging; NACT: neoadjuvant chemotherapy; RCT: randomised controlled trial.
Differences between protocol and review
The objective of the original protocol was, 'To assess the effectiveness and safety (surgery‐related complications) of hysterectomy with radiotherapy, chemotherapy or both in locally advanced cervical cancer (Stages IB2 to III)' (Kokka 2013), but was modified to: 'To determine whether hysterectomy, in addition to standard treatment with radiotherapy or chemotherapy, or both, in women with locally advanced cervical cancer (Stage IB2 to III), is safe and effective compared with standard treatment alone'. The original objective was reworded for clarity.
Types of outcome measures
Primary outcomes
The primary outcome definition was expanded to incorporate definitions by trialists.
Overall survival: survival until death from all causes assessed from the time when women were enrolled in the study, or as defined by the trial authors.
Secondary outcomes
Progression‐free (PFS) and disease‐free survival were listed as separate outcomes in the protocol but in the review PFS was preferred. We subsequently defined trials using disease‐free survival and PFS as a subgroup analysis that was of interest (see below). Local control was specified as an outcome of interest in the protocol but was omitted in the review after discussion about its importance and our wanting to restrict outcomes to those that are important and pertinent.
Progression‐free survival.
If authors reported disease‐free survival (DFS) rather than PFS then this was assessed.
Quality of life measured using a scale that has been validated through reporting of norms against a validated scale in a peer‐reviewed publication.
Severe adverse events.
Complications of chemotherapy and radiotherapy were added to the review as only surgery‐related complications were listed in the protocol but complications from these medical agents are important.
-
Chemotherapy‐ and radiotherapy‐related complications: grades of chemotherapeutic and radiotherapeutic toxicity were extracted and grouped as:
haematological (leukopenia, anaemia, thrombocytopenia, neutropenia, haemorrhage);
gastrointestinal (nausea, vomiting, anorexia, diarrhoea, liver toxicity, proctitis);
genitourinary;
skin (stomatitis, mucositis, alopecia, allergy);
neurological (peripheral and central); and
pulmonary.
Search methods for identification of studies
Some of the methods for searching were not implemented, namely approaching major co‐operative trials groups active in this area.
Continuous outcome data were not reported in any of the trials so the following sections in the protocol which discussed the handling of the data of such outcomes were removed as they were unnecessary (dichotomous data were not meta‐analysed so was removed in the data synthesis section).
Data extraction and management
We extracted data on outcomes as below:
for continuous outcomes (e.g. quality of life), we planned to extract the final value and standard deviation of the outcome of interest and the number of women assessed at the endpoint in each treatment arm at the end of follow‐up, in order to estimate the mean difference (if trials measured outcomes on the same scale) or standardised mean difference (if trials measured outcomes on different scales) between treatment arms and its standard error.
Measures of treatment effect
We used the following measures of the effect of treatment:
for continuous outcomes, we used the mean difference between treatment arms.
Data synthesis
If sufficient clinically similar trials were available, we pooled their results in meta‐analyses.
For any dichotomous outcomes, we calculated the RR for each study and pooled them.
For continuous outcomes, we pooled the mean differences between the treatment arms at the end of follow‐up if all trials measured the outcome on the same scale, otherwise we planned to pool standardised mean differences.
If any trials had multiple treatment groups, we planned to divide the 'shared' comparison group into the number of treatment groups and comparisons between each treatment group and treat the split comparison group as independent comparisons.
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analyses according to:
type of hysterectomy undergone (simple versus radical);
type of radiotherapy used;
chemotherapeutic regimen used;
histopathological types of cervical cancer cases;
whether the trial measured progression or disease‐free survival. This was added after the protocol after going through the searches; it was likely to be important; however, subgroup analysis by outcome definition was not possible.
Sensitivity analysis
If a sufficient number of trials were included in the review, we planned to conduct sensitivity analyses to examine the possible contribution of other clinical or methodological differences between the trials, specifically:
trials at low risk of bias versus those at high and unclear risk of bias;
trials that seemed to differ from the others in their clinical criteria for defining survival.
Contributions of authors
FK and AB searched for relevant trials and individually examined each potentially relevant full text reference.
AB extracted data on risk of bias items.
FK and AB drafted the full review. AB drafted methodological and statistical sections of the review as well as various sections of the discussion; FK drafted clinical sections of the review, added expertise and drafted some of the discussion.
EB, MP, DO and AO reviewed the final version of the review.
Sources of support
Internal sources
-
New source of support, Other
N/A
External sources
-
Department of Health, UK
NHS Cochrane Collaboration programme Grant Scheme CPG‐10/4001/12
Declarations of interest
FK: none.
AB: none.
AO: none.
EB: none.
MP: none.
DO: none.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Benedetti‐Panici 2002 {published data only}
- Benedetti-Panici P, Greggi S, Colombo A, Amoroso M, Smaniotto D, Giannarelli D, et al. Neoadjuvant chemotherapy and radical surgery versus exclusive radiotherapy in locally advanced squamous cell cervical cancer: results from the Italian multicenter randomized study. Journal of Clinical Oncology 2002;20(1):179-88. [DOI] [PubMed] [Google Scholar]
Cetina 2013 {published data only}
- Cetina L, González-Enciso A, Cantú D, Coronel J, Pérez-Montiel D, Hinojosa J, et al. Brachytherapy versus radical hysterectomy after external beam chemoradiation with gemcitabine plus cisplatin: a randomized, phase III study in IB2–IIB cervical cancer patients. Annals of Oncology 2013;24:2043-7. [DOI: 10.1093/annonc/mdt142] [DOI] [PubMed] [Google Scholar]
Chang 2000 {published data only}
- Chang T-C, Lai C-H, Hong J-H, Hsueh S, Huang K-G, Chou H-H, et al. Randomized trial of neoadjuvant cisplatin, vincristine, bleomycin, and radical hysterectomy versus radiation therapy for bulky stage IB and IIA cervical cancer. Journal of Clinical Oncology 2000;18(8):1840-7. [DOI] [PubMed] [Google Scholar]
EORTC 2019 {published data only}
- Kenter G, Greggi S, Vergote I, Katsaros D, Kobierski J, Massuger L, et al. Results from neoadjuvant chemotherapy followed by surgery compared to chemoradiation for stage IB2–IIB cervical cancer: EORTC55994. Journal of Gynecologic Cancer 2019;37(15 suppl):5503-5503. [Google Scholar]
Gupta 2018 {published data only}
- Gupta S, Maheswari A, Parab P, Mahantshetty U, Hawaldar R, Sastri S, et al. Neoadjuvant chemotherapy followed by radical surgery versus concomitant chemotherapy and radiotherapy in patients with Stage IB2, IIA, or IIB squamous cervical cancer: a randomized controlled trial. Journal of Clinical Oncology 2018;36:1548-55. [DOI] [PubMed] [Google Scholar]
Keys 2003 {published data only}
- Keys HM, Bundy BN, Stehman FB, Okagaki T, Gallup DG, Burnett AF, et al. Radiation therapy with and without extrafascial hysterectomy for bulky stage IB cervical carcinoma: a randomized trial of the Gynecologic Oncology Group. Gynecologic Oncology 2003;89(3):343-53. [DOI] [PubMed] [Google Scholar]
Khan 2014 {published data only}
- Khan N. Chemoradiation versus neoadjuvant chemotherapy and surgery in locally advanced squamous cell cervical cancer: a randomized study in a north east Nigerian center. 15th Biennial Meeting of the International Gynecologic Cancer Society; 2014 Nov 8-11; Melbourne, Australia 2014.
Morice 2012 {published data only}
- Morice P, Rouanet P, Rey A, Romestaing P, Houvenaeghel G, Boulanger JC, et al. Results of the GYNECO 02 study, an FNCLCC phase III trial comparing hysterectomy with no hysterectomy in patients with a (clinical and radiological) complete response after chemoradiation therapy for stage IB2 or II cervical cancer. Oncologist 2012;17(1):64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Noriyuki 2010 {published data only}
- Noriyuki Y, Hiroshi S, Kazuko F, Kimihiro N, Tsuyomu I. A randomized trial of neoadjuvant chemotherapy followed by radical surgery versus radiotherapy alone in stage IIIb carcinoma of the uterine cervix. Journal Of Gynecologic Surgery 2010;26(2):105-13. [Google Scholar]
Perez 1987 {published data only}
- Perez CA, Camel HM, Kao MS, Hederman MA. Randomized study of preoperative radiation and surgery or irradiation alone in the treatment of stage IB and IIA carcinoma of the uterine cervix: final report. Gynecologic Oncology 1987;27(2):129-40. [DOI] [PubMed] [Google Scholar]
Zheng 2017 {published data only}
- Zheng J, Huang B, Zhou Y, Huang S, Chen S, Liet L. Concomitant chemoradiation followed by radical surgery for locally advanced cervical cancer patients preliminary result from RCT. Chinese Journal of Evidence-Based Medicine 2017;17(1):1-6. [Google Scholar]
References to studies excluded from this review
Katsumata 2013 {published data only}
- Katsumata N, Yoshikawa H, Kobayashi H, Saito T, Kuzuya K, Nakanishi T, et al. Phase III randomised controlled trial of neoadjuvant chemotherapy plus radical surgery vs radical surgery alone for stages IB2, IIA2, and IIB cervical cancer: a Japan Clinical Oncology Group trial (JCOG 0102). British Journal of Cancer 2013;108(10):1957-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keys 1999 {published data only}
- Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. New England Journal of Medicine 1999;340(15):1154-61. [DOI] [PubMed] [Google Scholar]
Sardi 1997 {published data only}
- Sardi JE, Giaroli A, Sananes C, Ferreira M, Soderini A, Bermudez A, et al. Long-term follow-up of the first randomized trial using neoadjuvant chemotherapy in stage Ib squamous carcinoma of the cervix: the final results. Gynecologic Oncology 1997;67(1):61-9. [DOI] [PubMed] [Google Scholar]
Sun 2013 {published data only}
- Sun H, Xin J, Lu Z, Wang N, Liu N, Guo Q. Potential molecular mechanisms for improved prognosis and outcome with neoadjuvant chemotherapy prior to laparoscopical radical hysterectomy for patients with cervical cancer. Cellular Physiology and Biochemistry 2013;32(5):1528-40. [DOI] [PubMed] [Google Scholar]
Sundfor 1996 {published data only}
- Sundfor K, Trope CG, Kjorstad KE. Radical radiotherapy versus brachytherapy plus surgery in carcinoma of the cervix 2A and 2B. Long-term results from a randomized study 1968-1980. Acta Oncologica 1996;35(Suppl 8):99-107. [DOI] [PubMed] [Google Scholar]
Yang 2016 {published data only}
- Yang Z, Chen D, Zhang J, Yao D, Kun G, Wang H, et al. The efficacy and safety of neoadjuvant chemotherapy in the treatment of locally advanced cervical cancer: a randomized multicenter study. Gynecologic Oncology 2016;141(2):231-9. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
CSEM 006 study {published data only}
- Tu H, Huang H, Quyang Y, Liu Q, Xian B, Song K, et al. Neoadjuvant chemotherapy followed by radical surgery versus concurrent chemoradiotherapy in patients with FIGO stage IIB cervical cancer: the CSEM 006 study. International Journal of Gynecological Cancer 2021;31:129-33. [DOI] [PubMed] [Google Scholar]
Reis Fihlo 2018 {published data only}
- Reis Fihlo PT, Andrade JM, Batista MP, Sousa CB, Oliveira TH, Arruda GV, et al. Neoadjuvant chemotherapy and radical surgery versus chemoradiation for stage IB2, IIA2, IIB cervical cancer: a randomized controlled trial. Annals of Oncology 2018;29(Suppl 8):VIII357-8.
Shanmugam 2019 {published data only}
- Shanmugam S, Govindasamy G, Hussain S, Mani J. Comparison of neoadjuvant chemotherapy followed by radical hysterectomy and neoadjuvant chemoradiation followed by radical hysterectomy with concurrent chemoradiation in locally advanced carcinoma cervix (FIGO Stage IB2, IIA2, IIB): interim results of a randomized control study. Journal of South Asian Federation of Obstetrics and Gynecology 2019;11(1):35-43. [Google Scholar]
Additional references
Alvarez 1989
- Alvarez RD, Soong SJ, Kinney WK, Reid GC, Schray MF, Podratz KC, et al. Identification of prognostic factors and risk groups in patients found to have nodal metastasis at the time of radical hysterectomy for early-stage squamous carcinoma of the cervix. Gynecologic Oncology 1989;36:130-5. [DOI] [PubMed] [Google Scholar]
Azria 2005
- Azria E, Morice P, Haie-Meder C, Thoury A, Pautier P, Lhomme C, et al. Results of hysterectomy in patients with bulky residual disease at the end of chemoradiotherapy for stage IB2/II cervical carcinoma. Annals of Surgical Oncology 2005;12:332-7. [DOI] [PubMed] [Google Scholar]
Baojuan 2012
- Baojuan Y, Lin Z, Haiyan C, Qi L, Yunyan Z, Yashuang Z. Dosimetric comparison of intensity modulated radiotherapy and three-dimensional conformal radiotherapy in patients with gynecologic malignancies: a systematic review and meta-analysis. Radiation Oncology 2012;7:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benedetti Panici 2007
- Benedetti Panici P, Bellati F, Manci N, Pernice M, Plotti F, Di Donato V, et al. Neoadjuvant chemotherapy followed by radical surgery in patients affected by FIGO stage IVA cervical cancer. Annals of Surgical Oncology 2007;14(9):2643-8. [DOI] [PubMed] [Google Scholar]
Bhatla 2018
- Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri. International Journal of Gynaecology and Obstetrics 2018;143(Suppl 2):22-36. [DOI] [PubMed] [Google Scholar]
Bijen 2009
- Bijen CB, Vermeulen KM, Mourits MJ, Bock GH. Costs and effects of abdominal versus laparoscopic hysterectomy: systematic review of controlled trials. PLoS One 2009;4(10):e7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burghardt 1978
- Burghardt E, Pickel H. Local spread and lymph node involvement in cervical cancer. Obstetrics and Gynecology 1978;52:138-45. [PubMed] [Google Scholar]
CCCMAC 2010
- Chemoradiotherapy for Cervical Cancer Meta-analysis Collaboration (CCCMAC). Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: individual patient data meta-analysis. Cochrane Database of Systematic Reviews 2010, Issue 1. Art. No: CD008285. [DOI: 10.1002/14651858.CD008285] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chung 1980
- Chung CK, Nahhas WA, Stryker JA, Curry SL, Abt AB, Mortel R. Analysis of factors contributing to treatment failures in stage IB and IIA carcinoma of the cervix. American Journal of Obstetrics and Gynecology 1980;138:550-6. [DOI] [PubMed] [Google Scholar]
Classe 2006
- Classe JM, Rauch P, Rodier JF, Morice P, Stoeckle E, Lasry S, et al. Surgery after concurrent chemoradiotherapy and brachytherapy for the treatment of advanced cervical cancer: morbidity and outcome: results of a multicenter study of the GCCLCC (Groupe des Chirurgiens de Centre de Lutte Contre le Cancer). Gynecologic Oncology 2006;102:523-9. [DOI] [PubMed] [Google Scholar]
Colombo 2009
- Colombo PE, Bertrand MM, Gutowski M, Mourregot A, Fabbro M, Saint-Aubert B, et al. Total laparoscopic radical hysterectomy for locally advanced cervical carcinoma (stages IIB, IIA and bulky stages IB) after concurrent chemoradiation therapy: surgical morbidity and oncological results. Gynecologic Oncology 2009;114(3):404-9. [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors(s). Systematic Reviews in Health Care: Meta-Analysis in Context. 2nd edition. London (UK): BMJ Publication Group, 2001. [Google Scholar]
Delgado 1990
- Delgado E, Bundy B, Zaino R, Sevin BU, Creasman WT, Major F. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecologic Oncology 1990;38:352-7. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI] [PubMed] [Google Scholar]
Eifel 1993
- Eifel PJ. Radiotherapy versus radical surgery for gynecologic neoplasms: carcinomas of the cervix and vulva. Frontiers of Radiation Therapy and Oncology 1993;27:130-42. [DOI] [PubMed] [Google Scholar]
Ferrandina 2014
- Ferrandina G, Ercoli A, Fagotti A, Fanfani F, Gallotta V, Margariti AP, et al. Completion surgery after concomitant chemoradiation in locally advanced cervical cancer: a comprehensive analysis of pattern of postoperative complications. Annals of Surgical Oncology 2014;21(5):1692-9. [DOI] [PubMed] [Google Scholar]
Gadducci 2013
- Gadducci A, Sartori E, Maggino T, Zola P, Cosio S, Zizioli V, et al. Pathological response on surgical samples is an independent prognostic variable for patients with Stage IB2–IIB cervical cancer treated with neoadjuvant chemotherapy and radical hysterectomy: an Italian multicenter retrospective study (CTF Study). Gynecologic Oncology 2013;131(3):640-4. [DOI: 10.1016/j.ygyno.2013.09.029] [DOI] [PubMed] [Google Scholar]
Global Cancer Statistics 2020
- Hyuna S, Jacques F, Rebecca LS, Mathieu L, Isabelle S, Ahmedin J, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of Incidence and mortality worldwide for 36 cancers in 185 countries. CA: a Cancer Journal for Clinicians 2021;71(3):209-49. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 1 February 2022. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
GRADE Working Group 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Green 2001
- Green JA, Kirwan JM, Tierney JF, Symonds P, Fresco L, Collingwood M, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet 2001;358(9284):781-6. [DOI] [PubMed] [Google Scholar]
Hequet 2013
- Hequet D, Marchand E, Place V, Fourchotte V, De La Rochefordière A, Dridi S, et al. Evaluation and impact of residual disease in locally advanced cervical cancer after concurrent chemoradiation therapy: results of a multicenter study. European Journal of Surgical Oncology 2013;39(12):1428-34. [DOI: 10.1016/j.ejso.2013.10.006] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2020
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Houvenaeghel 1998
- Houvenaeghel G, Martino M, Bladou F, Moutardier V, Ternier F, Delpero JR, et al. Value of surgery in the treatment of advanced cervical cancers. Annales de Chirurgie 1998;52:425-33. [PubMed] [Google Scholar]
Houvenaeghel 2007
- Houvenaeghel G, Lelievre L, Buttarelli M, Jacquemier J, Carcopino X, Viens P, et al. Contribution of surgery in patients with bulky residual disease after chemoradiation for advanced cervical carcinoma. European Journal of Surgical Oncology 2007;33:498-503. [DOI] [PubMed] [Google Scholar]
Kesic 2006
- Kesic V. Management of cervical cancer. European Journal of Surgical Oncology 2006;32:832-7. [DOI] [PubMed] [Google Scholar]
Khuhaprema 2010
- Khuhaprema T, Srivatanakul P, Sriplung H, Wiangnon S, Sumitsawan Y, Attasara P. Cancer in Thailand 2001–2003. Bangkok: National Cancer Institute 2010;V:3-76. [Google Scholar]
Kornovski 2007
- Kornovski Y, Gorchev G. Histopathological findings in postoperative specimens in cervical cancer patients with stages IB2–IVA after neoadjuvant chemotherapy and preoperative plus postoperative radiotherapy. Journal of the Balkan Union of Oncology 2007;12:57-63. [PubMed] [Google Scholar]
Kundargi 2013
- Kundargi RS, Guruprasad B, Hanumantappa N, Rathod PS, Devi UK, Bafna UD. The role of surgery in locally advanced carcinoma of cervix after sub-optimal chemoradiation: Indian scenario. South Asian Journal of Cancer 2013;2(3):137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Landoni 1997
- Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet 1997;350(9077):535-40. [DOI] [PubMed] [Google Scholar]
Landoni 2014
- Landoni F, Sartori E, Maggino T, Zola P, Zanagnolo V, Cosio S, et al. Is there a role for postoperative treatment in patients with stage IB2-IIB cervical cancer treated with neo-adjuvant chemotherapy and radical surgery? An Italian multicenter retrospective study. Gynecologic Oncology 2014;132(3):611-7. [DOI: 10.1016/j.ygyno.2013.12.010] [DOI] [PubMed] [Google Scholar]
Langendam 2013
- Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schünemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews. Systematic Reviews 2013;23(2):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leino 1994
- Leino R, Grenman S, Rantanen V, Kiilholma P, Salmi T. Operative treatment of advanced cervical cancer after full pelvic irradiation. Annales Chirurgiae et Gynaecologiae 1994;208(Suppl):50-3. [PubMed] [Google Scholar]
Lin 2019
- Lin AJ, Kidd E, Dehbashtu F, Siegel B, Mutic S, Thaker PH, et al. Intensity modulated radiation therapy and image guided adapted brachytherapy for cervix cancer. International Journal of Radiation Oncology, Biology, Physics 2019;103(5):1088-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mabuchi 2017
- Mabuchi S, Kozasa K, Kimura T. Radical hysterectomy after radiotherapy for recurrent or persistent cervical cancer. International Journal of Gynaecology and Obstetrics 2017;139(2):185-91. [DOI] [PubMed] [Google Scholar]
Marjanovic 2019
- Marjanovic D, Karapandzic VP, Rundic SS, Tomasevic A, Saric M, Miskovic I, et al. Implementation of intensity-modulated radiotherapy and comparison with three-dimensional conformal radiotherapy in the postoperative treatment of cervical cancer. Journal of B.U.ON. 2019;24(5):2028-34. [PubMed] [Google Scholar]
Mathers 2008
- Mathers C, Boerma T, Fat DM. The global burden of disease: 2004 update. www.who.int/publications/i/item/9789241563710 (accessed 29 April 2020).
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morris 1999
- Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. New England Journal of Medicine 1999;340(15):1137-43. [DOI] [PubMed] [Google Scholar]
Naga 2018
- Naga Ch P, Gurram L, Chopra S, Mahantshetty U. The management of locally advanced cervical cancer. Current Opinions in Oncology 2018;30(5):323-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
NCI 1999
- National Cancer Institute. NCI clinical announcement. United States Department of Health and Human Services, Public Health Service, National Institutes of Health February 1999; Bethesda (MD).
NCI 2014
- National Cancer Institute. Stage IVB cervical cancer treatment. www.cancer.gov/cancertopics/pdq/treatment/cervical/HealthProfessional/page9 (accessed prior to 29 April 2022).
NCIN 2010
- National Cancer Intelligence Network. Cervical cancer incidence and screening coverage. www.ncin.org.uk/publications/data_briefings/cervical_incidence_and_screening (accessed prior to 29 April 2022).
Noterman 2006
- Noterman D, Philippson C, Hertens D, Veys I, Schobbens JC, Nogaret JM. Neoadjuvant radiochemotherapy and surgery of locally advanced cervical carcinoma: review of 22 patients treated at the Bordet Institute. Journal de Gynecologie Obstetrique et Biologie de la Reproduction 2006;35:23-7. [DOI] [PubMed] [Google Scholar]
Ota 2008
- Ota T, Takeshima N, Tabata T, Hasumi K, Takizawa K. Adjuvant hysterectomy for treatment of residual disease in patients with cervical cancer treated with radiation therapy. British Journal of Cancer 2008;99(8):1216-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2013
- Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Laparoscopic versus open radical hysterectomy in patients with stage IB2 and IIA2 cervical cancer. Journal of Surgical Oncology 2013;108(1):63-9. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815-34. [DOI] [PubMed] [Google Scholar]
Percorelli 2009
- Pecorelli S, Zigliani L, Odicino F. Revised FIGO staging for carcinoma of the cervix. International Journal Gynecology and Obstetrics 2009;205(2):107-8. [DOI] [PubMed] [Google Scholar]
Peters 2000
- Peters WA 3rd, Liu PY, Barrett RJ 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. Journal of Clinical Oncology 2000;18(8):1606-13. [DOI] [PubMed] [Google Scholar]
Piver 1975
- Piver MS, Chung WS. Prognostic significance of cervical lesion size and pelvic node metastases in cervical carcinoma. Obstetrics and Gynecology 1975;46(5):507-10. [PubMed] [Google Scholar]
Potish 1990
- Potish RA, Twiggs LB, Prem KA, Carson LF, Adcock LL. Surgical intervention following multimodality therapy for advanced cervical cancer. Gynecologic Oncology 1990;38:175-80. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rose 1999
- Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. New England Journal of Medicine 1999;340(15):1144-53. [DOI] [PubMed] [Google Scholar]
Rydzewska 2012
- Rydzewska L, Tierney J, Vale CL, Symonds PR. Neoadjuvant chemotherapy plus surgery versus surgery for cervical cancer. Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No: CD007406. [DOI: 10.1002/14651858.CD007406.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sardi 1990
- Sardi J, Sananes C, Giaroli A, Maya G, di Paola G. Neoadjuvant chemotherapy in locally advanced carcinoma of the cervix uteri. Gynecologic Oncology 1990;38(3):486-93. [DOI] [PubMed] [Google Scholar]
Schünemann 2020
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Shepherd 2012
- Shepherd JH. Cervical cancer. Best Practice & Research. Clinical Obstetrics & Gynaecology 2012;26(3):293-309. [DOI] [PubMed] [Google Scholar]
Singh 2019
- Singh N, Rous B, Ganesan R. 2018 FIGO staging system for cervical cancer: summary and comparison with 2009 FIGO staging system. www.bgcs.org.uk/wp-content/uploads/2019/05/BAGP-2018-FIGO-Cervix-Ca-staging-doc-v1.2.pdf (accessed prior to 4 January 2021).
Sun 2014
- Sun L, Sheng X, Jiang J, Li X, Liu N, Liu Y, et al. Surgical morbidity and oncologic results after concurrent chemoradiation therapy for advanced cervical cancer. International Journal of Gynaecology and Obstetrics 2014;125(2):111-5. [DOI: 10.1016/j.ijgo.2013.07.041] [DOI] [PubMed] [Google Scholar]
Tsuda 2001
- Tsuda H, Tanaka M, Manabe T, Nakata S, Ishiko O, Yamamoto K. Phase I–II study of neoadjuvant chemoradiotherapy followed by radical surgery in locally advanced cervical cancer. Anticancer Drugs 2001;12:853-8. [DOI] [PubMed] [Google Scholar]
Wang 2002
- Wang Y, Cao P, Zhang X, Zeng Q. Combined treatment for locally advanced carcinoma of uterine cervix. Zhonghua Zhong Liu Za Zhi 2002;24:508-10. [PubMed] [Google Scholar]
Whitney 1999
- Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC Jr, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. Journal of Clinical Oncology 1999;17(5):1339-48. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kokka 2013
- Kokka F, Bryant A, Oram D, Powell M. Hysterectomy for patients with locally advanced cervical cancer after primary radiotherapy and/or chemotherapy. Cochrane Database of Systematic Reviews 2013, Issue 1. Art. No: CD010260. [DOI: 10.1002/14651858.CD010260] [DOI] [Google Scholar]
Kokka 2015
- Kokka F, Bryant A, Brockbank E, Powell M, Oram D. Hysterectomy with radiotherapy or chemotherapy or both for women with locally advanced cervical cancer. Cochrane Database of Systematic Reviews 2015, Issue 4. Art. No: CD010260. [DOI: 10.1002/14651858.CD010260.pub2] [DOI] [PubMed] [Google Scholar]


