Abstract
The meningococcal lactoferrin receptor is composed of the integral outer membrane protein LbpA and the peripheral lipoprotein LbpB. Homooligomeric complexes of LbpA and heterooligomers consisting of LbpA and LbpB were identified. Furthermore, five cell surface-exposed loops of LbpA were identified, which partially confirms a previously proposed topology model.
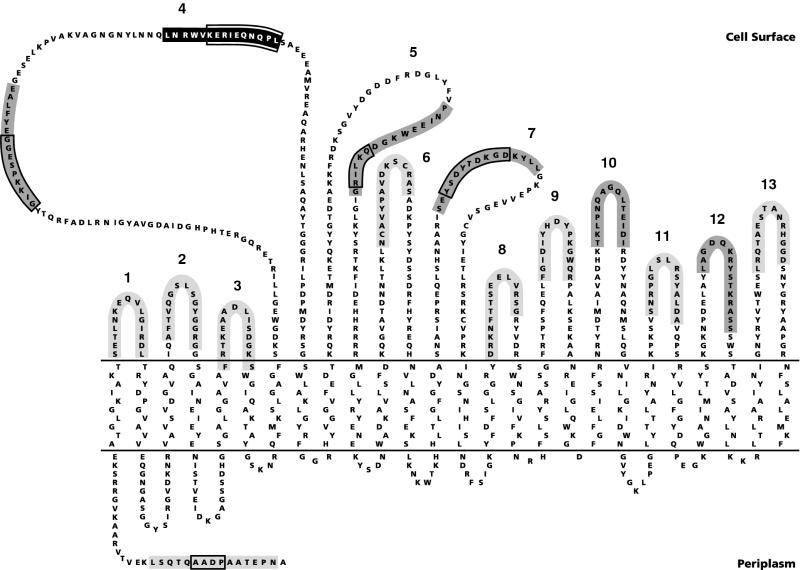
The meningococcal lactoferrin receptor is thought to be formed by the transmembranous lactoferrin-binding protein A (LbpA) and the peripheral lipoprotein LbpB (10, 12). The deduced amino acid sequence of LbpA (9, 10) shows homology to those of the neisserial transferrin receptor TbpA and of the TonB-dependent siderophore receptors of Escherichia coli. A topology model for LbpA, according to which the protein traverses the outer membrane 26 times in a β-sheet conformation, thereby exposing 13 loops to the bacterial surface (Fig. 1), has been proposed elsewhere (10). Recently, the crystal structures of the siderophore receptors FhuA (5, 6) and FepA (2) revealed β-barrel structures consisting of only 22 β-strands with the first 150 amino acids forming a plug that closes the barrel. Since the homology between LbpA and the siderophore receptors is particularly high in this N-terminal part, the first four β-strands and their flanking regions in the proposed LbpA model (Fig. 1) are likely to form a similar plug, leaving a 22-stranded β-barrel for LbpA as well. The lipoprotein LbpB showed homology to the transferrin receptor TbpB (10, 12). Therefore, it was assumed that the lactoferrin receptor, like the transferrin receptor (3), consists of two proteins, LbpA and LbpB. Whereas evidence for an interaction between TbpA and TbpB has been reported elsewhere (1, 4), such evidence is lacking for LbpA and LbpB. The goal of the present study was to verify the proposed topology model for LbpA as well as the putative interaction between LbpA and LbpB.
FIG. 1.
Topology model of LbpA of strain BNCV (10). The epitope recognized by two MAbs (9) is indicated in black. Antisera against synthetic peptides marked in dark or light gray reacted or failed to react, respectively, with intact cells. Numbers indicate the postulated cell surface-exposed loops. For the synthesis of the peptide corresponding to loop 6, the internal cysteines were replaced by 2-aminobutyric acid. The most variable parts of the LbpA are boxed.
Oligomeric complexes of LbpA and LbpB.
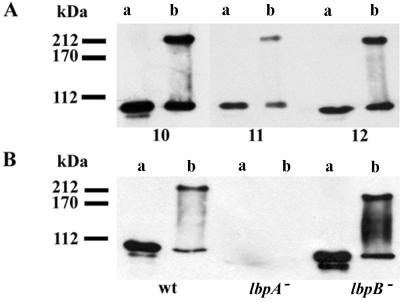
To verify the topology model of LbpA, 14 synthetic peptides corresponding to (parts of) the putative exposed loops and to the N terminus of LbpA (Fig. 1) were used to immunize mice as described elsewhere (12). To test the reactivity of the antisera with LbpA, cell envelopes from iron-limited cells (9) were isolated by ultracentrifugation (170,000 × g, 4°C, 5 min) after ultrasonic disintegration of the cells and dissolved in sample buffer (7) without β-mercaptoethanol. After sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting, all antisera appeared to react with the expected 103-kDa band in the cell envelopes of wild-type strain BNCV (see Fig. 2A, lane a, for example). Interestingly, when the samples were not heated at 100°C before electrophoresis, an additional reaction of the antisera and of the LbpA-specific monoclonal antibody (MAb) mn98K2 (9) was observed with a band of approximately 200 kDa (Fig. 2, lanes b), indicating that LbpA is part of an oligomeric complex. The 103- and 200-kDa bands were not detected in samples of the lbpA mutant strain CE1457 (Fig. 2B), demonstrating that both bands represent forms of LbpA. Both bands were detected in a sample of the lbpB mutant CE1454 (Fig. 2B). Therefore, the 200-kDa band does not represent a heterooligomeric complex of LbpA and LbpB but, probably, a homooligomer of LbpA.
FIG. 2.
Western blot analysis of cell envelope proteins incubated at 100°C (lanes a) or 0°C (lanes b) before SDS-PAGE at 10 mA and 4°C. (A) Cell envelopes of strain BNCV probed with antisera (1:200 dilution) against synthetic LbpA peptides corresponding to loops 10, 11, and 12 in the topology model. (B) Cell envelopes of wild-type strain BNCV (wt), lbpA mutant CE1457 (8), and lbpB mutant CE1454 (12) probed with an LbpA-specific MAb. The binding of the primary antibody was monitored by incubation with a horseradish peroxidase-conjugated goat anti-mouse immunoglobulin antiserum (GAMPO; Jackson ImmunoResearch Laboratories, Inc.) and enhanced chemiluminescence (ECL) detection (Amersham). Molecular mass markers are indicated at the left.
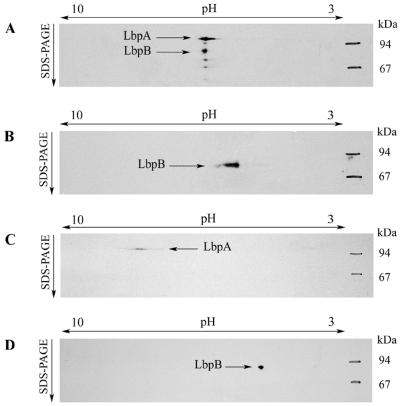
To investigate the existence of a heterooligomeric complex, two-dimensional electrophoresis with nondenaturing isoelectric focusing (IEF) in the first and denaturing SDS-PAGE in the second dimension was applied. Both proteins comigrated in the first dimension (Fig. 3A), supporting the hypothesis that they form a complex. Under denaturing IEF, LbpA and LbpB did not migrate together but, in accordance with their isoelectric points, to the basic and the acidic area, respectively (Fig. 3C and D). Furthermore, in samples from the lbpA mutant (Fig. 3B), LbpB migrated in the first dimension to a different position than that in samples from the wild-type strain. Hence, in samples of the wild-type strain, both proteins comigrate in the first dimension on the basis of a mutual interaction.
FIG. 3.
Two-dimensional electrophoresis of cell envelope proteins with native IEF in the first dimension and denaturing SDS-PAGE in the second dimension (A and B) or with both dimensions denaturing (C and D). (A, C, and D) Cell envelopes of strain BNCV. (B) Cell envelopes of an lbpA mutant of BNCV. The rehydration solution for the native IEF contained cell envelopes (315 μg of total protein), 1.25% Elugent (Calbiochem), 0.5% Pharmalytes (Pharmacia), and 18.75% glycerol. For denaturing IEF, the rehydration solution, containing cell envelopes (90 μg of total protein), was prepared as described elsewhere (14) but with 1.25% Elugent instead of CHAPS (3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate). The IPG gels (pH 3 to 10 L; Pharmacia) were focused with a constant current of 1 mA and a constant temperature of 15°C until 50 V · h in total was reached. After electrophoresis, proteins were blotted and LbpA and LbpB were detected with the LbpA-specific MAb mn98K2 and with an LbpB-specific antiserum raised against the synthetic peptide C1 (12).
Topology of LbpA.
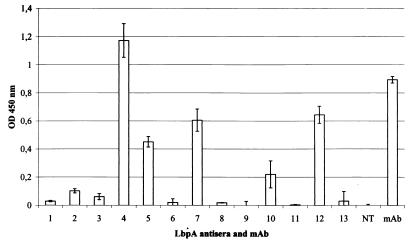
The accessibility of the putative cell surface-exposed loops of LbpA in intact cells was tested in enzyme-linked immunosorbent assays (ELISAs) by using the antisera directed against the synthetic LbpA peptides. The antisera directed against the peptides of loops 4, 5, 7, 10, and 12 reacted with intact cells (Fig. 4), thus confirming the cell surface exposure of these loops (Fig. 1). The peptides corresponding to loops 4, 5, and 7 overlap with highly variable regions in LbpA (10), consistent with the notion that such variable domains of outer membrane proteins are, in general, cell surface exposed. Furthermore, consistent with the model, the epitope for two MAbs was previously localized in loop 4 (Fig. 1). All the other antisera failed to show significant binding. In the case of the antisera directed against the putative loops 6, 8, 9, 11, and 13, this lack of reactivity may be explained by low titers of the antisera, since they also reacted only weakly with purified denatured LbpA (13) in an ELISA (data not shown). In the case of the antiserum against the loop 6 peptide, the low reactivity may be explained by the substitution of 2-aminobutyric acid for the two cysteines in the peptide. In contrast, the sera directed against the peptides of loops 1, 2, and 3 and of the N terminus were highly reactive in an ELISA with purified LbpA but not with cell envelopes as immobilized antigen (data not shown). These epitopes are apparently hidden within the membrane or shielded by other parts of the protein. Hence, these data are consistent with the idea that the N-terminal segment of LbpA forms a plug within the β-barrel domain as has been reported for the three-dimensional structures of the siderophore receptors (2, 5, 6). Interestingly, the sera against the peptides of loops 1 and 2 showed a strong reaction with cells that were killed by heat inactivation for 30 min at 56°C prior to coating (data not shown). The heat treatment does not result in a total denaturation of the protein, since cells that were inactivated in this way are still able to bind lactoferrin (11). Apparently, these epitopes are unmasked by the heat treatment, which is routinely used to kill the meningococci.
FIG. 4.
Binding of various LbpA peptide antisera and MAb mn98K2 to strain BNCV in whole-cell ELISAs, performed as described elsewhere (12). Meningococci were killed by incubation for 1 h with 400 μg of tetracycline per ml prior to coating. The reactions were quantified by reading the optical density (OD) at 450 nm. Results are averages from three independent determinations with standard deviations indicated by error bars. The binding of the antisera to whole cells of the lbpA mutant strain CE1457 was determined as well, and the resulting values were subtracted from those obtained with the wild-type strain. Numbers indicate the cell surface-exposed loops, against which the sera were directed. NT, N-terminal peptide.
To identify a ligand-binding site in LbpA, competition ELISAs were performed (data not shown). Iron-limited meningococcal cells, applied as a coating to the wells of a microtiter plate, were first incubated with various dilutions of human lactoferrin (11% iron saturated; generously provided by Agennix) and subsequently with the antipeptide sera or the MAb. However, no inhibition of antibody binding by preincubation with lactoferrin was observed.
In this study, we demonstrated that the lactoferrin receptor consists of a macromolecular complex of LbpA and LbpB. LbpA is probably present in this complex as a dimer. Moreover, we could confirm experimentally the cell surface exposure of five postulated loops of the LbpA protein. However, we propose that LbpA, like FhuA and FepA, forms a 22-stranded, rather than a 26-stranded, β-barrel, with 11 cell surface-exposed loops and with a plug formed by the N-terminal 150 residues that closes the β-barrel. Since LbpA is an important candidate for a serogroup B vaccine, this structural information is important regarding the location of potentially useful antigenic determinants.
Acknowledgments
We thank Peter Hoogerhout for peptide synthesis and Jenny van der Biezen for technical assistance.
These investigations were supported by the Netherlands Foundation for Chemical Research (SON) with financial aid from the Netherlands Technology Foundation (STW).
REFERENCES
- 1.Boulton I C, Gorringe A R, Carr R, Gorinsky B, Joannou C L, Evans R W. Characterisation of the meningococcal transferrin binding protein complex by photon correlation spectroscopy. FEBS Lett. 1997;414:409–413. doi: 10.1016/s0014-5793(97)01025-9. [DOI] [PubMed] [Google Scholar]
- 2.Buchanan S K, Smith B S, Venkatramani L, Xia D, Esser L, Palnitkar M, Chakraborty R, van der Helm D, Deisenhofer J. Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat Struct Biol. 1999;6:56–63. doi: 10.1038/4931. [DOI] [PubMed] [Google Scholar]
- 3.Cornelissen C N, Sparling P F. Iron piracy: acquisition of transferrin-bound iron by bacterial pathogens. Mol Microbiol. 1994;14:843–850. doi: 10.1111/j.1365-2958.1994.tb01320.x. [DOI] [PubMed] [Google Scholar]
- 4.Cornelissen C N, Sparling P F. Binding and surface exposure characteristics of the gonococcal transferrin receptor are dependent on both transferrin-binding proteins. J Bacteriol. 1996;178:1437–1444. doi: 10.1128/jb.178.5.1437-1444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson A D, Hofmann E, Coulton J W, Diederichs K, Welte W. Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science. 1998;282:2215–2220. doi: 10.1126/science.282.5397.2215. [DOI] [PubMed] [Google Scholar]
- 6.Locher K P, Rees B, Koebnik R, Mitschler A, Moulinier L, Rosenbusch J P, Moras D. Transmembrane signaling across the ligand-gated FhuA receptor: crystal structure of free and ferrichrome-bound states reveal allosteric changes. Cell. 1998;95:771–778. doi: 10.1016/s0092-8674(00)81700-6. [DOI] [PubMed] [Google Scholar]
- 7.Lugtenberg B, Meijers J, Peters R, van der Hoek P, van Alphen L. Electrophoretic resolution of the ‘major outer membrane protein’ of Escherichia coli K12 into four bands. FEBS Lett. 1975;58:254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- 8.Pettersson, A., and J. Tommassen. Unpublished data.
- 9.Pettersson A, van der Ley P, Poolman J T, Tommassen J. Molecular characterization of the 98-kilodalton iron-regulated outer membrane protein of Neisseria meningitidis. Infect Immun. 1993;61:4724–4733. doi: 10.1128/iai.61.11.4724-4733.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pettersson A, Klaarenbeek V, van Deurzen J, Poolman J T, Tommassen J. Molecular characterization of the structural gene for the lactoferrin receptor of the meningococcal strain H44/76. Microb Pathog. 1994;17:395–408. doi: 10.1006/mpat.1994.1085. [DOI] [PubMed] [Google Scholar]
- 11.Pettersson A, Maas A, Tommassen J. Identification of the iroA gene product of Neisseria meningitidis as a lactoferrin receptor. J Bacteriol. 1994;176:1764–1766. doi: 10.1128/jb.176.6.1764-1766.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pettersson A, Prinz T, Umar A, van der Biezen J, Tommassen J. Molecular characterization of LbpB, the second lactoferrin-binding protein of Neisseria meningitidis. Mol Microbiol. 1998;27:599–610. doi: 10.1046/j.1365-2958.1998.00707.x. [DOI] [PubMed] [Google Scholar]
- 13.Prinz, T., N. Schroten, and J. Tommassen. Unpublished data.
- 14.Rabilloud T, Adessi C, Giraudel A, Lunardi J. Improvement of the solubilization of proteins in two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis. 1997;18:307–316. doi: 10.1002/elps.1150180303. [DOI] [PMC free article] [PubMed] [Google Scholar]