Abstract
This scoping review of shear wave elastography (SWE) articles in musculoskeletal soft tissue and nerve research demonstrates methodological heterogeneity resulting from a lack of standardized data collection and reporting requirements. Seven literature databases were searched for original articles published in English from 2004–2020 that examine human skeletal muscles, tendons, and nerves in vivo. Although 5,868 records were initially identified, only 375 reports met inclusion criteria. Of the 375 articles, 260 examined 89 unique muscles, 94 examined 14 unique tendons, and 43 examined 8 unique nerves. Cohorts were often small (n=11–20) and young (mean=20–29 years), and participants were typically tested in the prone position. Regarding equipment, a variety of ultrasound systems (n=11), ultrasound models (n=18), and transducers (n=19) were identified. Only 11% of articles contained information on the use of electromyography to confirm absence of muscle activity, and only 8% reported measurement depth. Since musculoskeletal soft tissue and nerve stiffness can vary significantly based on data collection methods, it is essential to standardize SWE collection and reporting procedures. This will allow SWE to serve as a valid and reproducible tool for assessing tissue pathology, disease progression, and response to intervention within a variety of musculoskeletal and nerve-related disorders.
Keywords: elastic modulus, sonoelastography, elastogram, shear wave elastography, neuromuscular ultrasound, musculoskeletal ultrasound
1. Introduction3
Conventional B-mode and Doppler ultrasonography are used routinely as a first-line approach in the evaluation of musculoskeletal soft tissues and nerves in various traumatic and pathologic conditions (Taljanovic et al., 2017). One limitation of conventional ultrasound (US) is that the sonoacoustic tissue changes (e.g., increased/decreased echogenicity (Ihnatsenka and Boezaart, 2010); disorganized/abnormal/altered internal fascicular architecture (Van Hooren et al., 2020)) associated with disease or injury may not be visible early in the course of disease or immediately post injury (Taljanovic et al., 2017). However, recent advances in sonoelastographic techniques have improved the ability to identify tissue abnormalities and changes through the evaluation of elastic properties. Tissue elasticity is the ability to resist deformation from an applied force (Sigrist et al., 2017) and is determined by the structure and composition of the tissue itself (Shiina et al., 2002). Thus, changes in the elasticity of musculoskeletal soft tissues (e.g., muscles, tendons) and nerves may reflect underlying pathophysiology (Klauser et al., 2014). This is because injuries or diseases of the musculoskeletal system can lead to alterations in the biomechanical properties of the tissue (Drakonaki et al., 2012; Ryu and Jeong, 2017). For instance, collagen fiber disruption in tendon tears (Klauser et al., 2013) and soft tissue edema and atrophy in muscles (Alfuraih et al., 2019) are associated with a decrease in tissue elasticity. Therefore, the use of sonoelastography has increased in recent decades due to its potential for enhancing diagnostic capabilities (e.g., pathologic tendons, ruptured tendons, tendons with tendinopathy, and systemic diseases (Prado-Costa et al., 2018), tracking disease progression (e.g., Duchenne muscular dystrophy (Yu et al., 2022); inflammatory rheumatic diseases (Farrow et al., 2020)), and assessing response to treatment in various musculoskeletal conditions (e.g., botulinum toxin A treatment in spastic muscles (Dağ et al., 2020; Zúñiga et al., 2021); manual stretching treatment in congenital muscular torticollis (Kwon and Park, 2012)).”
Two primary sonoelastography methods are used in musculoskeletal research and practice: strain elastography in which a mechanical force compresses the tissues axially, and shear wave elastography (SWE) in which compressive acoustic waves dynamically stress the tissues (Ooi et al., 2014). Strain elastography is the original form of elastography most often used to detect tumors or malignancy in breast, thyroid, and liver tissues (Garra, 2015; Lin et al., 2019; Sigrist et al., 2017). Using strain elastography requires the application of an external compressive force by the operator or internal forces created by pulsatile structures within the body (e.g., arteries) to cause tissue deformation. When forces are applied, a semi-transparent color elastogram (color map) is generated and overlaid on the B-mode grey-scale image. This elastogram provides the operator with qualitative information about the tissue, as specific colors indicate varying levels of tissue elasticity (Sigrist et al., 2017). At best, only pseudo-quantitative information can be obtained from strain elastography by calculating strain ratios, which compare tissue strain in the region of interest with adjacent healthy tissue (strain ratio >1 indicates target tissue is stiffer than normal reference tissue) (Ozturk et al., 2018).
SWE provides a more quantitative and reproducible approach for assessing the stiffness of tissues and is less operator-dependent than strain elastography (Drakonaki et al., 2012; Klauser et al., 2014; Ryu and Jeong, 2017). It uses a focused US beam that leverages acoustic radiation forces emitted by an US transducer to induce shear waves throughout a tissue. These shear waves propagate perpendicular to and at a slower velocity than the US beam, resulting in particle displacements that can be measured as shear wave velocity (meters per second). The distribution of shear wave velocities at each pixel is directly related to the shear modulus (ratio of stress to strain) which is an absolute measure of the tissue’s elastic properties in units of pressure (kilopascals) (Taljanovic et al., 2017). Shear wave velocity and shear modulus are used to calculate Young’s modulus, which is a measure of tissue elasticity, using the equation E=3μ=3pcs2 (where E=Young’s modulus, μ=shear modulus, p=tissue density, and cs=shear wave velocity) (Nowicki and Dobruch-Sobczak, 2016). In SWE, as in strain elastography, a color elastogram may be overlaid on the standard B-mode image. Specific colors indicate soft or stiff tissues and are based on how fast shear waves propagate through the tissue (faster speeds for stiff tissues). In contrast to strain elastography, SWE allows for quantitative measurements to be obtained from anywhere within the color elastogram due to the sequencing of particle displacements made possible by ultrafast analysis (Yoon et al., 2013).
The addition of SWE to many commercially available US systems over the past decade has resulted in a rapid increase of published articles on its use in evaluating various musculoskeletal soft tissues and nerves (Harmon et al., 2019; Lin et al., 2019; Ryu and Jeong, 2017; Snoj et al., 2020; Taljanovic et al., 2017; Wee and Simon, 2019). This surge in SWE use is of particular importance because there are several factors to consider when applying SWE to muscles, tendons, and nerves. First, elastography was designed with the intent to image homogenous tissues (e.g., breast, thyroid, and liver). This is why shear modulus and Young’s modulus, as measures of tissue elasticity (Taljanovic et al., 2017), are based upon the assumption that tissues are isotropic, homogenous, incompressible, and elastic (Ryu and Jeong, 2017). Since musculoskeletal soft tissues (e.g., muscle and nerve) are heterogeneous, anisotropic, and have highly distinct borders and shapes (Wee and Simon, 2019), this presents inherent challenges to calculating tissue elasticity. For this reason, and because tissue elasticity is sensitive to transducer orientation (Davis et al., 2019; Eby et al., 2013), many researchers believe that SWE results should be reported in terms of shear wave velocity (meters/second), as an accurate measure in isotropic tissues (Davis et al., 2019; Royer et al., 2011; Ryu and Jeong, 2017), rather than tissue elasticity (kilopascals). Due to the multifarious tissue composition of musculoskeletal soft tissue and nerves, stiffness can vary significantly depending on the regions examined, which adds to the complexity of collecting, interpreting, and reporting SWE data. Second, in addition to these tissue considerations, emerging evidence suggests that the US system and presets, transducer frequency, depth of measurement, and patient positioning, may also influence SWE stiffness (Brandenburg et al., 2014; Drakonaki et al., 2012; Ewertsen et al., 2016; Ruby et al., 2019; Sadeghi et al., 2019; Sigrist et al., 2017). This highlights the need for SWE users to consistently conduct and clearly report SWE data collection methods and procedures.
The absence of standardized procedures for collecting and reporting SWE data makes it particularly challenging to compare data across articles, reproduce and replicate findings, and establish normative reference values. Since specific technical factors (i.e., tissue type, testing position, ultrasound machine, transducer frequency, measurement depth, and muscle activity) may affect the reproducibility and accuracy of SWE data, the purpose of this review is to summarize the methodological heterogeneity of published articles using SWE for the assessment of soft tissues (categories include muscles, tendons, and nerves) in humans in vivo. The aims of this review were to: (1) encapsulate the methods used across all articles and within each soft tissue category, (2) identify current gaps in the literature, and (3) provide guidelines and a checklist for reporting SWE data collection procedures in publications to improve standardization, reproduction, and replication. Standardizing SWE data collection and reporting procedures is essential to advance the diagnostic and prognostic capabilities of this relatively new technology.
2. Methods
2.1. Eligibility Criteria
The eligibility criteria used for study selection is listed in Table 1. Records were limited by publication year because the application of SWE to musculoskeletal structures occurred within the last decade. Language was restricted to English because the review team did not have the ability to translate from other languages. Publication type was limited to original research to reduce the likelihood of including duplicate information published from secondary sources (e.g., review articles). If a record contained only a title (i.e., no abstract), the title text was required to pass inclusion criterion #4 to move on to full text screening. During full text screening, articles were required to contain SWE data (reported in kilopascals or meters per second) to ensure that only original research articles/primary sources were included. Articles were excluded in the order criteria are listed in Table 1. Please note that although ligaments were not one of the musculoskeletal soft tissues included in the purpose statement (and therefore the search strategy), articles on ligaments that resulted from this search were still included in this scoping review. Therefore, the ligament results for this review represent a constrained sample of published SWE articles on ligaments.
Table 1.
Eligibility criteria for title and abstract and full text screening.
| INCLUSION CRITERIA (ALL criteria must be true to pass) | EXCLUSION CRITERIA (at least ONE criterion must be true to exclude) |
|---|---|
|
| |
| TITLE & ABSTRACT SCREENING | |
|
| |
| Article passes criteria if: | Article fails criteria if: |
| 1. Publication year is 2004 or later | 1. Published prior to 2004 |
| 2. Written language is English | 2. Not written in English |
| 3. Publication type is original research, such as: | 3. Not original research, such as: |
| • Brief or Short Communication/Report | • Book/Book Chapter |
| • Clinical Article/Research/Study/Trial | • Case Report/Series |
| • Observational Study | • Commentary/Opinion Piece |
| • Original Article/Communication/Contribution/Investigation/Paper/Research/Work | • Conference Paper |
| • Editorial | |
| • Randomized Controlled Trial | • Innovative Methodology/Technical Note |
| • Research or Scientific Article/Paper/Report | • Lecture |
| • Literature/Narrative Review | |
| Note: pass article through if publication type cannot be discerned but article meets all other inclusion criteria. | • Modeling Study |
| 4. SWE type includes at least one of the following: | 4. Does not include SWE types listed in inclusion criterion #4 AND includes one of following: |
| • Acoustic Radiation Force Impulse | |
| • Comb Push Ultrasound Shear Wave | • Contrast-Enhanced Ultrasonography |
| • Dynamic Elastography with Transient Stimuli | • Dynamic Elastography Harmonic Stimuli |
| • Elastic Modulus | • Focused Ultrasound Ablation |
| • Elastography | • High Intensity Focused Ultrasound |
| • Elastosonography | • M-Mode |
| • Guided Wave | • Magnetic Resonance Elastography |
| • Shear Elastic Modulus | • Magnetic Resonance Imaging |
| • Shear Wave Elastography | • Non-Ultrasound Elastography |
| • Shear Wave Velocity | • Shear Wave Dispersion Ultrasound Vibrometry |
| • Siemens/Shear Wave Elasticity Imaging | • Shear Wave Induced Resonance Elastography |
| • Sonoelastography | • Static Compression |
| • Strain Ratio | • Strain Elastography |
| • SuperSonic Shear Imaging | • Vibration Elastography |
| • Ultrasonic Shear Wave Elastography | • Vibroacoustics |
| • Ultrasound Real-Time Tissue Elastography | |
| 5. Participant sample includes humans in vivo | 5. Does not include humans in vivo in sample, and instead includes other groups, such as: |
| • Animals | |
| • Cadavers | |
| • In vitro | |
| • Phantom | |
| 6. Structures include at least one of the following: | 6. Does not include one structures listed in inclusion criterion #6 |
| • Muscle/Skeletal Muscle | |
| • Tendon | |
| • Nerve • Ligament |
Note: exclude article if structures only consist of cartilage, connective tissue, fascia, joint capsule, meniscus, smooth muscle, or a specific tumor/tissue mass within or arising from muscles (sarcoma), ligaments, nerves (neurofibroma, neuroma, schwannoma), or tendons |
| Note: pass article through if one of the above structures is not specified but article meets all other inclusion criteria. | |
|
| |
| FULL TEXT SCREENING | |
|
| |
| 7. Must contain SWE data (i.e., reported in kilopascals or meters per second) | 7. Does not contain SWE data |
Abbreviations: SWE=shear wave elastography
2.2. Information Sources and Search Strategies
A biomedical librarian developed the search strategies and conducted the literature searches in July 2020 in seven databases (vendors): CINAHL Plus (EbscoHost), Cochrane Library: Database of Systematic Reviews (Wiley & Sons), Embase (Elsevier), Physiotherapy Evidence Database (PEDro) (University of Sydney, Australia), PubMed (United States National Library of Medicine), Scopus (Elsevier), and Web of Science: Core Collection (Clarivate Analytics). Relevant keywords and controlled vocabularies — Subject Headings (CINAHL), EMTREE (Embase), and MeSH (PubMed and Cochrane Library) — were used to search for each concept of interest (see Appendix A for final search strategies by database). The searches were limited to records published in English from 2004–2020, and a search strategy was used to exclude specific publication types. EndNote X9 (Clarivate Analytics) was used to manage all citations and identify duplicate references.
2.3. Study Selection Process
Unique records were exported from EndNote X9 and imported into Covidence (Veritas Health Innovation, Melbourne, Australia) for study selection. The research team conducted study selection at two levels: title and abstract and full text screening (see Appendix B for a list of author names and their respective contributions during study selection and data charting).
2.3.1. Pilots of Study Selection
Eleven team members participated in study selection pilots for title and abstract and full text screening prior to executing the official review. A random sample of citations was selected by the biomedical librarian at both screening levels. For the title and abstract screening pilot, the first round contained 25 citations. After task completion, the team met to review the records lacking 100% agreement and revised the eligibility criteria accordingly. Then, a second pilot round was conducted on an additional 13 citations. Based on group performance and discussion, the team made further changes to the criteria prior to beginning study selection. For the full text screening pilot, one round of screening was performed on 8 citations, and corresponding changes were made to the eligibility criteria before starting study selection.
2.3.2. Study Selection
Eight team members participated in the title and abstract screening and nine team members in the full text screening for study selection. For both screening levels, each unique record was independently screened by any two reviewers using the established eligibility criteria. When two reviewers’ votes were in conflict, a senior team member with extensive SWE experience made the final decision to include or exclude.
2.4. Data Charting Process
Two team members jointly developed the first draft of the data charting form and finalized the draft after receiving feedback from co-authors. The extraction team conducted data charting using Qualtrics XM, an internet-based survey tool (Qualtrics XM, Provo, UT).
2.4.1. Pilot of Data Charting
The pilot was conducted in two phases. In the first phase, nine team members extracted data from the same two articles. After group discussion regarding resulting discrepancies, the data analyst made changes to the data charting form in preparation for phase two. In the second phase, eight team members were placed into pairs and assigned a third person (senior-level) reviewer. Each pair of reviewers extracted data from five articles unique to each pair. After discussion with third person reviewers regarding resulting discrepancies, the data analyst made additional changes to the extraction form.
2.4.2. Data Charting
Each extraction pair was assigned approximately 94 articles for data charting (89 of which remained after the second phase of the pilot), and both members within each pair independently extracted data from each article for the variables listed below. One exception was that only the data analyst extracted data for the variable ‘Body Position’, as this variable was added after initial extraction was complete. The following information was extracted for each variable:
- Soft Tissue
- Select soft tissue(s) ‘Ligament’, ‘Muscle’, ‘Nerve’, and/or ‘Tendon’
- Specific Tissue
- Select/enter specific type of ligament(s), muscle(s), nerve(s), and/or tendon(s)
- Sample Information
- Enter the following for each group in the article:
- Sample Group Name and Condition Description
- Sample Size (n) or ‘Not Reported’
- Sample Mean Age (years) or ‘Not Reported’
- Sample Standard Deviation Age (years) or ‘Not Reported’
- Body Position
- Enter a detailed description regarding SWE testing position(s) with particular emphasis on body position(s) (e.g., seated, prone, supine) or enter ‘Not Reported’
- US System Brand
- Select/enter brand name of US system(s) (e.g., ‘SuperSonic Imagine’, ‘Verasonic, Inc.’) or select ‘Not Reported’
- US System Model
- Select/enter model name and number of US system(s) (e.g., ‘Acuson S2000’, ‘Aplio 300’) or select ‘Not Reported’
- US Transducer Type
- Enter description of transducer(s), including shape and frequency range, or ‘Not Reported’
- Measurement Depth
- Enter depth(s) of measurement in centimeters or select ‘Not Reported’
- Use of Electromyography (EMG)
- Select ‘Surface EMG (Explicitly Stated)’, ‘Surface EMG (Not Explicitly Stated)’, or ‘No EMG/Not Reported’
- Note: the answer option ‘Surface EMG (Not Explicitly Stated)’ means that the article described the use of EMG without identifying it as Surface EMG
- Primary Aim
- Enter main aim(s) related to SWE
- Primary Finding
- Enter main finding(s) or conclusion(s) corresponding to primary aim(s)
Once a pair completed data extraction for their respective articles, the data analyst exported the data from Qualtrics into a Microsoft Excel spreadsheet and used a formula (e.g., IF(A1=A2,A2)) to identify variables with identical responses. For those cells without identical responses, ‘FALSE’ was generated based on the formula, and conditional formatting was used to highlight those discrepancies. The data analyst sent the discrepancies to the third person reviewer to resolve. The third person reviewer referenced the full text articles to resolve discrepancies, discussed resulting decisions with the two extractors, and sent the final agreed-upon extractions to the data analyst. This process took place over several sessions — each group extracted and reviewed approximately 20–25 articles at a time.
A quality data assurance check was performed after all third person reviewers addressed extraction discrepancies. As such, the accuracy of data extraction was verified for all variables (excluding Primary Aim and Primary Finding since these variables were not coded or subsequently analyzed) by a fourth team member who verified the extracted data against the full text articles. If all information was accurate after additional review, the data extracted were marked as final. If any information was not accurate upon review, the fourth team member made necessary changes, and a fifth team member confirmed the accuracy of those changes. If the fifth team member agreed with the changes made, revised data were marked as final. If the fourth and fifth team members did not agree on the changes, a sixth team member made a decision and the revised data were marked as final.
2.5. Data Recoding
2.5.1. Recoded Variables
The following variables were recoded after data extraction in order to summarize the data, with the original extractions and recodes included in Appendix C (see Appendix C, Table C.1 for Sample Information and Table C.2 for all remaining variables):
- Sample Information
- The original text entry for Sample Group Name and Condition Description (Table C.1, Column E) was recoded (Table C.1, Columns F & G) into the following two variables and corresponding subgroups:
- Sample Group: ‘Athletes’, ‘Healthy Volunteers’, ‘Patients’
- Sample Condition: ‘Arthritis’, ‘Disease/Disorder/Condition’, ‘Dystonia’, ‘Entrapment Neuropathy’, ‘Fracture’, ‘Headache/Migraine’, ‘Musculoskeletal Condition’, ‘Muscular Dystrophy’, ‘None (Healthy)’, ‘Pain’, ‘Plantar Fasciitis/Fasciopathy’, ‘Spinal Cord Injury’, ‘Tendinosis/Tendon Rupture’
- Body Position
- The original text entry (Table C.2, Column H) was recoded (Table C.2, Columns I–M) according to the following subgroups: ‘Seated Upright’, ‘Seated Reclined’, ‘Seated Forward Lean’, ‘Lying Prone’, ‘Lying Supine’, ‘Lying Laterally’, ‘Standing’, and ‘Not Reported or Unclear’. These subgroups were coded across all articles as well as for articles by Soft Tissue category.
- US Transducer Type
- The original text entry (Table C.2, Column P) was recoded (Table C.2, Column Q) into the following subgroups based on the highest frequency and shape of the probe (where C=curved, SC= supercurved; L=linear, SL=superlinear; SLV=superlinear volumetric)
- ‘4C’; ‘5C’; ‘6C/SC’; ‘7C’; ‘7L/SL’; ‘7.5L’; ‘8SL’; ‘9C’; ‘9L’; ‘10L/SL’; ‘11L/SL’; ‘11SC’; ‘12L/SL’; ‘12SC’; ‘13SL’; ‘14L/SL’; ‘15L/SL’; ‘16–5SLV’; ‘18L/SL’; ‘Not Reported’
2.5.2. Coding Notes
The code ‘Discrepancy’ was used to indicate uncertainty regarding the correct value when an article stated a value differently in at least two locations.
The code ‘Not Reported’ was used to indicate any information that could not be found in the original article.
- For the variable Body Position:
- The code ‘Seated Upright’ was used for hip/trunk angles designated as 85–90 degrees. The code ‘Seated Reclined’ was used when (1) angles were outside of the 85–90 degree range if participants were seated on an isokinetic dynamometer, or (2) angles were <85 degrees and chair type was unknown.
- The code ‘Not reported or unclear’ was used for (1) articles that were lacking a testing position description altogether, (2) articles that had testing position descriptions but body position was unclear or not able to be replicated based on the information provided, and/or (3) testing position was described but it was unclear if used for SWE testing.
- For the variable US System Model, we made the following assumption:
- When an article only said ‘S2000’ or ‘S3000’, we treated this as ‘Acuson S2000’ and ‘Acuson S3000’, respectively.
- For the variable US Transducer Type, we made the following assumptions:
- For all SuperSonic Imagine machines/Aixplorer models, curved probes were coded as SC and linear probes as SL per their standard designations.
- If either the probe shape or highest frequency were not indicated in the article, the entry was coded as ‘Not Reported’.
3. Results
3.1. Literature Search and Screening
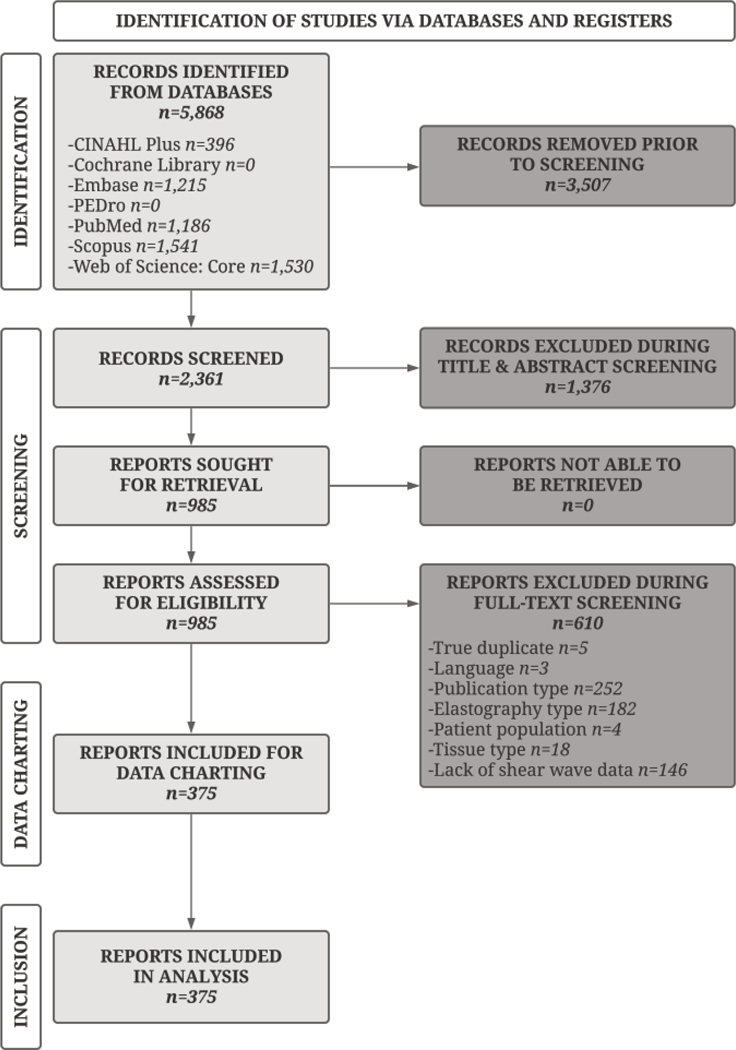
A flow diagram encapsulates the literature search and screening process (Figure 1). The literature search resulted in 5,868 records across seven databases. After removing 3,507 duplicates, 2,361 records remained for screening. Title and abstract screening reduced the number of reports to 985, and full text screening to 375, all of which were included in the final analysis. Of the 610 excluded during full text screening, the primary exclusion reasons were incorrect publication type (n=252), wrong elastography type (n=182), or absence of unique shear wave data (n=146).
Figure 1.
Flow Diagram of Study Selection Process. This diagram portrays the flow of information through the various phases of the scoping review by indicating the number of records identified from various databases, excluded at each stage of review, and included in the final analysis.
3.2. Data Charting
3.2.1. Variable Description — All Articles
Data extraction results for the 375 articles are provided for all variables in Appendix C (see Table C.1 for Sample Information and Table C.2 for all remaining variables). A descriptive summary of the variables and their subcategories are presented in Table 2 (Column 1) with the most common subcategories highlighted in bold font (see Table 2a for Sample Information, Table 2b for Body Position, and Table 2c for all remaining variable data). For Sample Information, out of 602 participant groups across the 375 articles, the ‘most common subcategories’ (by variable), were: ‘Patients’ (Sample Group), ‘None (Healthy)’ (Sample Condition), ‘11 to 20’ (Sample Size), and ‘20 to 29 years’ (Sample Mean Age). For Body Position, out of 450 body positions across all articles, ‘Lying Prone’ was the most common subcategory. For all other variables, out of 375 articles, the ‘most common subcategories’ (by variable) were: ‘SuperSonic Imagine’ (US System Brand), ‘Aixplorer’ (US System Model), ‘15L/15SL’ (US Transducer Type), ‘Not Reported’ (Measurement Depth), and ‘No EMG/Not Reported’ (Use of EMG). Table 2 includes a summary of all variables, except for ‘Sample Standard Deviation Age’ (located in Table C.1), ‘Primary Aim’ (Table C.2), and ‘Primary Finding’ (Table C.2).
Table 2.
Descriptive summary of variables. For each extracted variable (listed in capital letters in first column), the number (n) and corresponding percentage (%) of articles are provided across all articles combined as well as by soft tissue category (articles examining muscles, tendons, nerves, or ligaments). The most common subcategory for each tissue type is indicated in bold font. Table (a) contains Sample Information data, (b) contains Body Position data, and (c) contains data from the remaining variables.
| a) | ALL GROUPS n=602 | MUSCLE GROUPS n=401 | TENDON GROUPS n=156 | NERVE GROUPS n=79 | LIGAMENT GROUPS n=34 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| SAMPLE GROUPS | ||||||||||
|
| ||||||||||
| Athletes | 18 | 3% | 12 | 3% | 10 | 6% | 0 | 0% | 2 | 6% |
| Healthy Volunteers | 139 | 23% | 103 | 26% | 27 | 17% | 13 | 16% | 7 | 21% |
| Patients | 445 | 74% | 286 | 71% | 119 | 76% | 66 | 84% | 25 | 74% |
| SAMPLE CONDITIONS | ||||||||||
|
| ||||||||||
| Arthritis | 7 | 1% | 3 | 1% | 4 | 3% | 0 | 0% | 0 | 0% |
| Disease/Disorder/Condition | 77 | 13% | 46 | 11% | 10 | 6% | 24 | 30% | 0 | 0% |
| Dystonia | 1 | <1% | 1 | <1% | 0 | 0% | 0 | 0% | 0 | 0% |
| Entrapment Neuropathy | 10 | 2% | 0 | 0% | 0 | 0% | 10 | 13% | 0 | 0% |
| Fracture | 2 | <1% | 2 | <1% | 0 | 0% | 0 | 0% | 0 | 0% |
| Headache/Migraine | 4 | 1% | 3 | 1% | 0 | 0% | 1 | 1% | 0 | 0% |
| Musculoskeletal Condition | 28 | 5% | 20 | 5% | 5 | 3% | 4 | 5% | 3 | 9% |
| Muscular Dystrophy | 3 | <1% | 3 | 1% | 0 | 0% | 0 | 0% | 0 | 0% |
| None (Healthy) | 404 | 67% | 293 | 73% | 101 | 65% | 39 | 49% | 28 | 82% |
| Pain | 19 | 3% | 17 | 4% | 2 | 1% | 1 | 1% | 0 | 0% |
| Plantar Fasciitis/Fasciopathy | 4 | 1% | 1 | <1% | 0 | 0% | 0 | 0% | 3 | 9% |
| Spinal Cord Injury | 2 | <1% | 2 | <1% | 0 | 0% | 0 | 0% | 0 | 0% |
| Tendinosis/Tendon Rupture | 41 | 7% | 10 | 2% | 34 | 22% | 0 | 0% | 0 | 0% |
| SAMPLE SIZE (n) | ||||||||||
|
| ||||||||||
| 1 to 10 | 120 | 20% | 97 | 24% | 25 | 16% | 6 | 8% | 15 | 44% |
| 11 to 20 | 237 | 39% | 171 | 43% | 58 | 37% | 21 | 27% | 8 | 24% |
| 21 to 30 | 118 | 20% | 67 | 17% | 36 | 23% | 24 | 30% | 6 | 18% |
| 31 to 40 | 60 | 10% | 28 | 7% | 16 | 10% | 20 | 25% | 1 | 3% |
| 41 to 50 | 19 | 3% | 8 | 2% | 7 | 4% | 5 | 6% | 1 | 3% |
| 51 to 100 | 33 | 5% | 20 | 5% | 10 | 6% | 3 | 4% | 1 | 3% |
| 101 + | 12 | 2% | 9 | 2% | 4 | 3% | 0 | 0% | 0 | 0% |
| Not Reported/Discrepancy | 3 | <1% | 1 | <1% | 0 | 0% | 0 | 0% | 2 | 6% |
| SAMPLE MEAN AGE (years) | ||||||||||
|
| ||||||||||
| 0 to 9 | 19 | 3% | 19 | 5% | 0 | 0% | 0 | 0% | 0 | 0% |
| 10 to 19 | 24 | 4% | 22 | 5% | 2 | 1% | 0 | 0% | 0 | 0% |
| 20 to 29 | 223 | 37% | 171 | 43% | 59 | 38% | 9 | 11% | 16 | 47% |
| 30 to 39 | 66 | 11% | 39 | 10% | 19 | 12% | 11 | 14% | 6 | 18% |
| 40 to 49 | 63 | 10% | 35 | 9% | 19 | 12% | 16 | 20% | 4 | 12% |
| 50 to 59 | 72 | 12% | 29 | 7% | 21 | 13% | 24 | 30% | 4 | 12% |
| 60 + | 44 | 7% | 28 | 7% | 9 | 6% | 11 | 14% | 1 | 3% |
| Not Reported/Discrepancy | 91 | 15% | 58 | 14% | 27 | 17% | 8 | 10% | 3 | 9% |
| b) | ALL POSITIONS n=450 | MUSCLE POSITIONS n=320 | TENDON POSITIONS n=106 | NERVE POSITIONS n=45 | LIGAMENT POSITIONS n=19 | |||||
| n | % | n | % | n | % | n | % | n | % | |
| BODY POSITION | ||||||||||
|
| ||||||||||
| Seated Upright | 94 | 21% | 67 | 21% | 15 | 14% | 14 | 31% | 5 | 26% |
| Seated Reclined | 14 | 3% | 12 | 4% | 2 | 2% | 1 | 2% | 0 | 0% |
| Seated Forward Lean | 1 | <1% | 1 | <1% | 0 | 0% | 0 | 0% | 0 | 0% |
| Lying Prone | 137 | 30% | 93 | 29% | 48 | 45% | 5 | 11% | 9 | 47% |
| Lying Supine | 131 | 29% | 90 | 28% | 25 | 24% | 21 | 47% | 5 | 26% |
| Lying Laterally | 9 | 2% | 6 | 2% | 1 | 1% | 2 | 4% | 0 | 0% |
| Standing | 9 | 2% | 6 | 2% | 3 | 3% | 0 | 0% | 0 | 0% |
| Not Reported or Unclear | 55 | 12% | 45 | 14% | 12 | 11% | 2 | 4% | 0 | 0% |
| c) | ALL ARTICLES n=375 | MUSCLE ARTICLES n=260 | TENDON ARTICLES n=94 | NERVE ARTICLES n=43 | LIGAMENT ARTICLES n=19 | |||||
| n | % | n | % | n | % | n | % | n | % | |
| US SYSTEM BRAND | ||||||||||
|
| ||||||||||
| Canon/Toshiba Medical Systems | 23 | 6% | 11 | 4% | 3 | 3% | 6 | 14% | 3 | 16% |
| Esaote SpA | 2 | 1% | 2 | 1% | 1 | 1% | 0 | 0% | 0 | 0% |
| GE Healthcare Systems | 28 | 7% | 13 | 5% | 6 | 6% | 10 | 23% | 0 | 0% |
| Hitachi | 1 | <1% | 1 | <1% | 0 | 0% | 0 | 0% | 0 | 0% |
| Mindray | 5 | 1% | 3 | 1% | 1 | 1% | 1 | 2% | 0 | 0% |
| Philips Healthcare US Solutions | 1 | <1% | 0 | 0% | 1 | 1% | 0 | 0% | 0 | 0% |
| Samsung US Systems | 2 | 1% | 2 | 1% | 0 | 0% | 0 | 0% | 0 | 0% |
| Siemens Medical Solutions | 83 | 22% | 58 | 22% | 21 | 22% | 10 | 23% | 7 | 37% |
| SuperSonic Imagine | 226 | 60% | 168 | 65% | 59 | 63% | 16 | 37% | 9 | 47% |
| Ultrasonix/BK Medical | 1 | <1% | 0 | 0% | 1 | 1% | 0 | 0% | 0 | 0% |
| Verasonic, Inc. | 4 | 1% | 4 | 2% | 0 | 0% | 0 | 0% | 0 | 0% |
| Not Reported | 2 | 1% | 1 | <1% | 2 | 2% | 0 | 0% | 0 | 0% |
| US SYSTEM MODEL | ||||||||||
|
| ||||||||||
| Acuson Oxana 2 | 1 | <1% | 1 | <1% | 0 | 0% | 0 | 0% | 0 | 0% |
| Acuson S2000 | 41 | 11% | 28 | 11% | 6 | 6% | 8 | 19% | 5 | 26% |
| Acuson S3000 | 42 | 11% | 29 | 11% | 16 | 17% | 2 | 5% | 2 | 11% |
| Aixplorer | 217 | 58% | 165 | 63% | 54 | 57% | 15 | 35% | 8 | 42% |
| Aplio 300 | 2 | 1% | 2 | 1% | 0 | 0% | 0 | 0% | 0 | 0% |
| Aplio 500 | 15 | 4% | 8 | 3% | 3 | 3% | 4 | 9% | 0 | 0% |
| Aplio i800 | 4 | 1% | 1 | <1% | 0 | 0% | 0 | 0% | 3 | 16% |
| Aplio i900 | 2 | 1% | 0 | 0% | 0 | 0% | 2 | 5% | 0 | 0% |
| EPIQ 7 | 1 | <1% | 0 | 0% | 1 | 1% | 0 | 0% | 0 | 0% |
| EUB-7500 | 1 | <1% | 1 | <1% | 0 | 0% | 0 | 0% | 0 | 0% |
| Logiq e | 1 | <1% | 0 | 0% | 1 | 1% | 0 | 0% | 0 | 0% |
| Logiq E9 | 26 | 7% | 12 | 5% | 5 | 5% | 10 | 23% | 0 | 0% |
| Logiq S8 | 1 | <1% | 1 | <1% | 0 | 0% | 0 | 0% | 0 | 0% |
| MyLab9 | 2 | 1% | 2 | 1% | 1 | 1% | 0 | 0% | 0 | 0% |
| Resona 7 | 4 | 1% | 3 | 1% | 0 | 0% | 1 | 2% | 0 | 0% |
| Resona device | 1 | <1% | 0 | 0% | 1 | 1% | 0 | 0% | 0 | 0% |
| RS80A | 2 | 1% | 2 | 1% | 0 | 0% | 0 | 0% | 0 | 0% |
| SonixMDP Q+ | 1 | <1% | 0 | 0% | 1 | 1% | 0 | 0% | 0 | 0% |
| Not Reported | 14 | 4% | 8 | 3% | 6 | 6% | 1 | 2% | 1 | 5% |
| US TRANSDUCER TYPE | ||||||||||
|
| ||||||||||
| 4C | 1 | <1% | 1 | <1% | 0 | 0% | 0 | 0% | 0 | 0% |
| 5C | 1 | <1% | 1 | <1% | 0 | 0% | 0 | 0% | 0 | 0% |
| 6C/6SC | 6 | 2% | 5 | 2% | 0 | 0% | 1 | 2% | 0 | 0% |
| 7C | 2 | 1% | 2 | 1% | 0 | 0% | 0 | 0% | 0 | 0% |
| 7L/7SL | 3 | 1% | 3 | 1% | 0 | 0% | 0 | 0% | 0 | 0% |
| 7.5L | 1 | <1% | 1 | <1% | 0 | 0% | 0 | 0% | 0 | 0% |
| 8SL | 2 | 1% | 1 | <1% | 1 | 1% | 0 | 0% | 0 | 0% |
| 9C | 1 | <1% | 0 | 0% | 0 | 0% | 1 | 2% | 0 | 0% |
| 9L | 94 | 25% | 63 | 24% | 24 | 26% | 13 | 30% | 6 | 32% |
| 10L/10SL | 54 | 14% | 45 | 17% | 10 | 11% | 5 | 12% | 4 | 21% |
| 11L/11SL | 5 | 1% | 5 | 2% | 0 | 0% | 0 | 0% | 0 | 0% |
| 11SC | 1 | <1% | 1 | <1% | 0 | 0% | 0 | 0% | 0 | 0% |
| 12L/12SL | 6 | 2% | 2 | 1% | 4 | 4% | 0 | 0% | 0 | 0% |
| 12SC | 1 | <1% | 1 | <1% | 0 | 0% | 0 | 0% | 0 | 0% |
| 13SL | 1 | <1% | 1 | <1% | 1 | 1% | 0 | 0% | 0 | 0% |
| 14L/14SL | 17 | 5% | 9 | 3% | 1 | 1% | 6 | 14% | 1 | 5% |
| 15L/15SL | 161 | 43% | 113 | 43% | 44 | 47% | 16 | 37% | 3 | 16% |
| 16–5SLV | 1 | <1% | 1 | <1% | 0 | 0% | 0 | 0% | 0 | 0% |
| 18L/18SL | 14 | 4% | 4 | 2% | 4 | 4% | 3 | 7% | 3 | 16% |
| Not Reported | 16 | 4% | 11 | 4% | 8 | 9% | 0 | 0% | 2 | 11% |
| MEASUREMENT DEPTH | ||||||||||
|
| ||||||||||
| Depth Provided | 30 | 8% | 23 | 9% | 3 | 3% | 4 | 9% | 1 | 5% |
| Not Reported | 345 | 92% | 237 | 91% | 91 | 97% | 39 | 91% | 18 | 95% |
| USE OF EMG | ||||||||||
|
| ||||||||||
| Surface EMG (Explicit) | 37 | 10% | 36 | 14% | 2 | 2% | 3 | 7% | 0 | 0% |
| Surface EMG (Not Explicit) | 5 | 1% | 5 | 2% | 1 | 1% | 0 | 0% | 0 | 0% |
| No EMG/Not Reported | 333 | 89% | 219 | 84% | 91 | 97% | 40 | 93% | 19 | 100% |
Note:
a) <1% was used for values between 0 and .5% to separate these values from 0% and values that rounded up to 1%, and
b) some articles fit into multiple variable subcategories accounting for overlap in the category percentages (i.e., exceeding 100%).
Abbreviations: for US Transducer Type: C=curved, SC= supercurved; L=linear, SL=superlinear; SLV=superlinear volumetric; EMG=electromyography; SWE=shear wave elastography; US=ultrasound.
Some data were not reported, were unclear, or were reported with discrepancies, with a small portion (<5%) occurring for Sample Size, US System Brand, US System Model, and US Transducer Type, and a larger portion occurring for Sample Mean Age (15%), Sample Standard Deviation Age (22%), Body Position (12%), Measurement Depth (92%), and Use of EMG (89%).
Some variables had multiple answers/entries:
Soft Tissue — 36 articles (10%) examined multiple soft tissue categories
Specific Tissue — 169 articles (45%) examined multiple specific tissues
Sample Information — 176 articles (47%) contained multiple samples
Body Position — 60 articles (16%) used multiple body positions
US System Brand — 3 articles (1%) used multiple US systems
US System Model — 3 articles (1%) used multiple US models
US Transducer Type — 11 articles (3%) used multiple transducers
Measurement Depth — 11 articles (3%) contained multiple depths (i.e., a range)
3.2.2. Variable Description — Articles by Soft Tissue Category
Data extraction results are also provided by soft tissue category (for articles examining muscles, tendons, nerves, and ligaments). Figure 2 provides the percentage of included articles that examined each of the four soft tissue categories. Table 2 (columns 2–5) provides a descriptive summary of the variables and their subcategories (see Table 2a for Sample Information, Table 2b for Body Position, and Table 2c for all remaining variable data), with the most common subcategories highlighted in bold font. For Sample Information, the ‘most common subcategories’ described in Section 3.2.1 for all articles held true for muscle, tendon, nerve, and ligament articles, except for Sample Size and Sample Mean Age. For Sample Size, ‘21 to 30’ was most common for nerve articles and ‘1 to 10’ for ligament articles. For Sample Mean Age, ‘50 to 59 years’ was the most common for nerve articles. For Body Position, ‘lying prone’ was the most common position for muscle, tendon, and ligament articles, but ‘lying supine’ was most common for nerve articles. For all other variables, the ‘most common subcategories’ described in Section 3.2.1 also held true, with the exception of transducer type (‘9L’ was most common) for ligament articles.
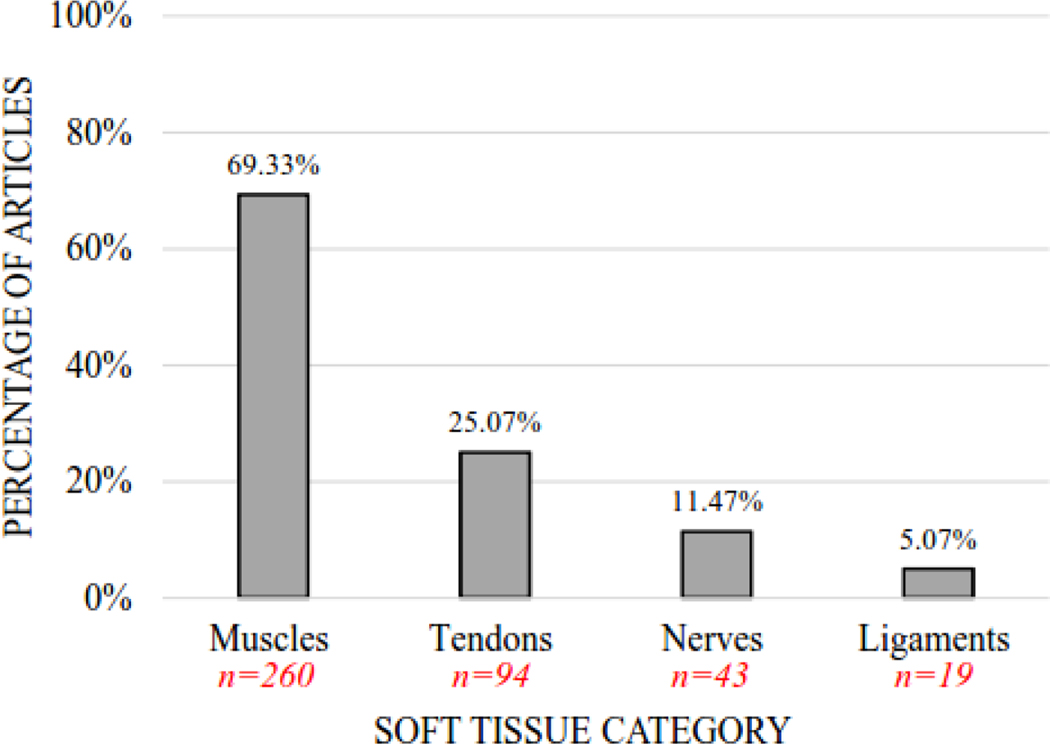
Figure 2.
Percentage of Articles by Soft Tissue Category. The vertical bar graph depicts the percentage of articles (n=375) that included muscles, tendons, nerves, and ligaments for shear wave elastography examination. Note: Some articles examined multiple soft tissue categories, accounting for overlap in the category percentages (i.e., exceeding 100%).
3.2.3. Variable Description — Articles by Specific Tissues
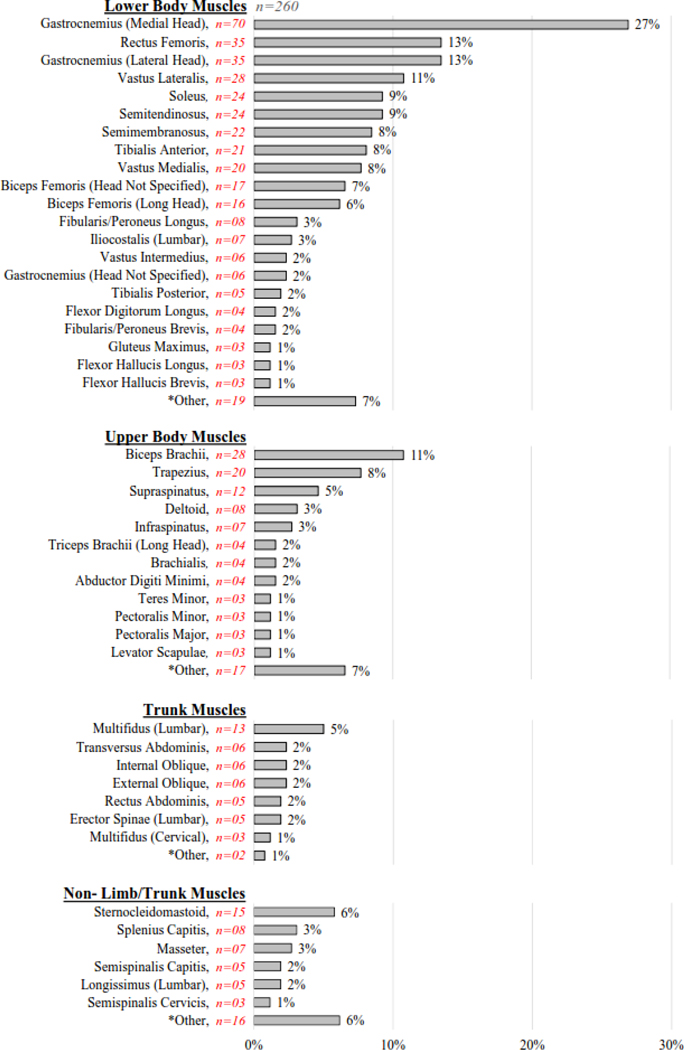
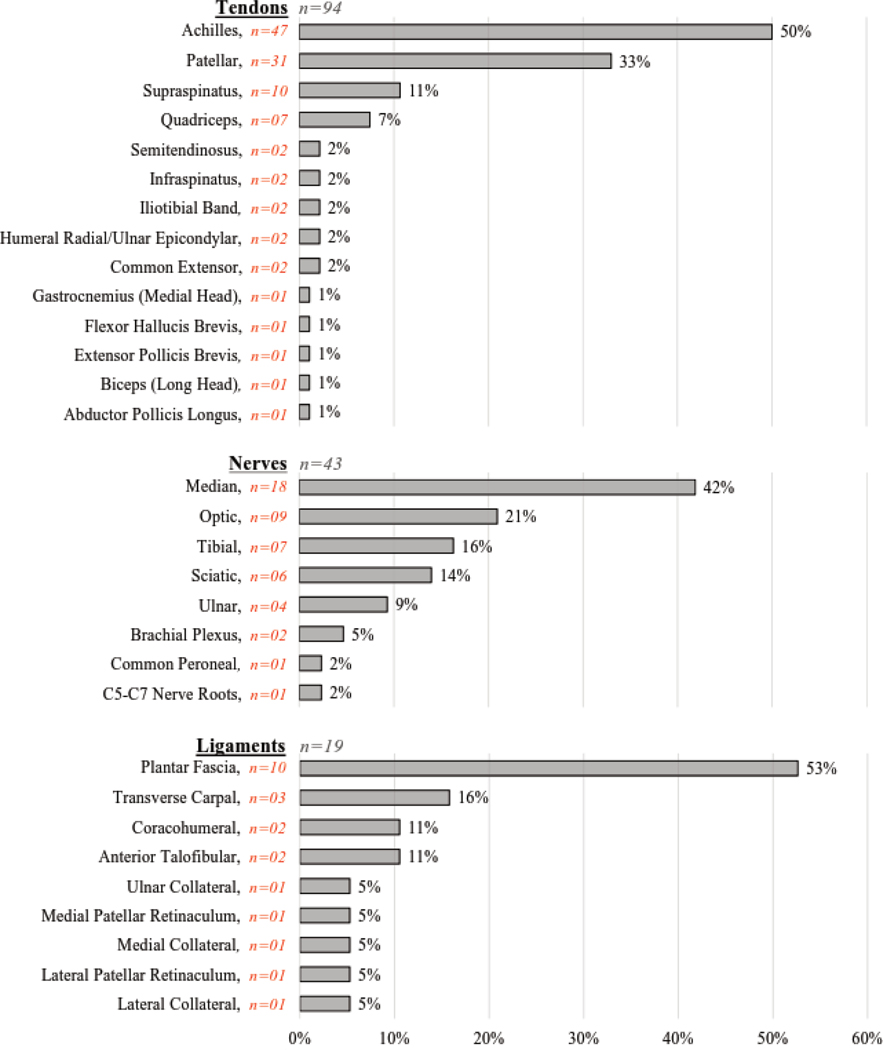
The percentage of articles examining each specific tissue is provided for each soft tissue category (Figure 3), with muscles presented in Figure 3a and tendons, nerves, and ligaments presented in Figure 3b. Of the 375 articles included in this review, 89 specific muscles were examined across 260 articles, 14 specific tendons across 94 articles, 8 specific nerves across 43 articles, and 9 specific ligaments across 19 articles (although ligaments were not part of this review’s intended purpose or search criteria). Across all specific tissues (regardless of soft tissue category), the ten most common specific tissues examined (n=number of articles) were: gastrocnemius muscle (medial head) (n=70), Achilles tendon (n=47), gastrocnemius muscle (lateral head) (n=35), rectus femoris muscle (n=35), patellar tendon (n=31), biceps brachii muscle (n=28), vastus lateralis muscle (n=28), soleus muscle (n=24), semitendinosus muscle (n=24), and semimembranosus muscle (n=22). For a list of the articles corresponding to each specific tissue, please refer to Supplementary Table S1 for the hyperlinked references organized by specific tissue category. This table is intended to be a helpful resource for researchers and clinicians as they review the literature in preparation for designing their studies.
Figure 3a.
- Lower Body Muscles: Adductor Magnus, Biceps Femoris (Short Head), Extensor Digitorum Longus, Flexor Digitorum Brevis, Gluteus Medius, Gracilis, Iliacus, Iliopsoas, Psoas Major, Quadricep (Unspecified), Quadriceps Femoris, Sartorius, Tensor Fasciae Latae, Triceps Surae
- Upper Body Muscles: Abductor Hallucis, Brachioradialis, Extensor Carpi Radialis Brevis, Extensor Carpi Ulnaris, Flexor Carpi Radialis, Flexor Digitorum Profundus, Flexor Digitorum Superficialis, Forearm Flexor, Hypothenar, Latissimus Dorsi, Pronator Quadratus, Teres Major, Thenar, Triceps Brachii (Head Not Specified)
- Trunk Muscles: Multifidus (Sacral), Serratus Anterior
- Non-Limb/Trunk Muscles: Anterior Scalene, Diaphragm, Digastric (Anterior Belly), Geniohyoid, Lateral Rectus, Levator Ani, Medial Rectus, Musculus Rectus Inferior, Musculus Rectus Medialis, Musculus Rectus Superior, Musculus Rectus Temporalis, Spinalis Capitis, Urethral Rhabdosphincter
Note: Some muscle articles examined multiple specific tissues, accounting for overlap in the category percentages (i.e., exceeding 100%).
Figure 3b.
Percentage of Specific Tissues for Articles Examining Tendons, Nerves, and Ligaments. These horizontal bar graphs depict the percentage of specific tissues examined for articles on tendons (n=94), nerves (n=43), and ligaments (n=19).
Note: Some tendon, nerve, and ligament articles examined multiple specific tissues, accounting for overlap in the category percentages (i.e., exceeding 100%).
4. Discussion
4.1. Review’s Purpose and Aims
Shear wave elastography (SWE) is an advanced US imaging modality recently used to investigate musculoskeletal soft tissues and nerves. Accordingly, guidelines are still lacking for the use of SWE in musculoskeletal practice and research, which contributes to the heterogeneity of published data collection procedures and findings. Since specific technical factors (i.e., tissue type, testing position, ultrasound machine, transducer frequency, measurement depth, and muscle activity) may affect the reproducibility and accuracy of SWE data, the purpose of this scoping review was to summarize the variety of SWE methods used across articles that examine human soft tissues (categories include muscles, tendons, and nerves) in vivo. The aims of this review were to: (1) encapsulate the methods used across all articles and within each soft tissue category, (2) identify current gaps in the literature, and (3) provide guidelines and a checklist for reporting SWE collection procedures in publications to improve standardization.
4.2. Summary of Methods Used Across Articles
In this scoping review, 375 articles were identified that use SWE to assess musculoskeletal soft tissues and nerves in humans in vivo published between 2004 and 2020. As expected, methodological procedures varied widely across articles due to the absence of reporting guidelines in musculoskeletal practice and research. Not surprisingly, the soft tissue category explored most often was muscles (69% of all articles), followed sequentially by tendons, nerves, and ligaments. The specific tissues examined most often (i.e., gastrocnemius muscle (medial head), Achilles tendon, gastrocnemius muscle (lateral head)…see Section 3.2.3) were ones commonly impacted by disease and relatively easy to image; thus it was not surprising these were among the top selected tissues. The most common sample group examined was patients (74%), as opposed to athletes and healthy volunteers, which may be because SWE users often recruit patients from their own clinics or hospitals. It was not surprising that the sample condition most often included was none (healthy) (67%), which is likely because much of the field is trying to establish reference values with the recent application of SWE to musculoskeletal soft tissues and nerves. Sample sizes were most commonly small (n=11–20, 39%) and composed of young adults (ages 20 to 29 years, 37%). The most common body position for SWE testing was lying prone (30%), followed closely by lying supine (29%). The SuperSonic Imagine ultrasound brand (60%) and Aixplorer model (58%) were used most, which may be because this machine is marketed specifically for shear wave elastography, and the 15L/15SL transducer was most common (43%). Surprisingly, the majority of articles (92%) did not report measurement depth and did not use or report the use of surface EMG (89%) (see Section 4.3 for more information).
4.3. Important Reporting Omissions
Several of the articles included in this review lacked a description of study procedures, such as the number of times SWE was collected, the order in which outcome measures were tested, or when SWE was collected relative to a task (e.g., during, before, after). In addition, descriptions for study design were frequently absent, such as the number of visits that occurred or whether the study was cross-sectional or longitudinal, prospective or retrospective, or interventional or non-interventional. Important details regarding the sample descriptions were often missing as well, including participant age, sex, race, height, weight, health history, hand- or leg-dominance, participation in treatment or therapy, level of physical activity, and duration exercise was restricted prior to SWE data collection. Often times, the operator’s level of experience with collecting SWE data was not described which is problematic because operators with more experience achieve higher reliability and greater SWE data accuracy (Wang et al., 2020). Perhaps the most surprising gap was that many articles’ testing position descriptions were too vague, and/or lacked demonstrative pictures or illustrations, to understand or replicate what was done. Furthermore, the number of testing positions performed and participant instructions related to testing position were often nebulous or missing entirely. It is particularly problematic that measurement depth was not reported in the vast majority of articles, as deeper structures yield less accurate results (Alfuraih et al., 2018; Alrashed and Alfuraih, 2021; Martiartu et al., 2021). Additionally, muscle stiffness is known to vary depending upon the location of measurement along the length of the muscle (e.g., proximal, mid, distal) and region of interest selection (Alfuraih et al., 2017; Lee et al., 2020; Martiartu et al., 2021), and this information was often missing or vague. Lastly, it is concerning that the majority of articles, particularly those that examined muscles, did not use (or report the use of) surface EMG to confirm the absence of muscle activity during testing, since muscle activity impacts SWE results (Lima et al., 2018).
Another important factor for SWE users to consider is the upper limits of the US system(s) and/or transducers used in their studies. As the stiffness of a particular tissue approaches the upper limit of the system/transducer, a ceiling effect can occur, which can lead to saturated SWE measurements. It was our original intention to collect this information from articles during data extraction. However, we quickly realized this information is missing from articles. This is likely because this information is not available online or included in materials (e.g., user manuals) provided by the manufacturers. A senior author contacted the manufacturers of each of the US systems/transducers used in the reviewed studies but was unable to obtain a complete list of upper limit information. Therefore, we recommend that the upper limits of US systems be made readily available, such as via the manufacturer’s webpages and in the user manuals, so that sonographers are aware of the limitations of their respective US systems.
4.4. Guidelines for Standardizing SWE Data Collection Reporting Procedures
Although the articles included in this review are an important contribution to the literature, we identified significant omissions of important methodological components (outlined in Section 4.3). In addition to the reporting guidelines recently published for conventional ultrasound (B-Mode and Doppler) by the European League Against Rheumatism (Costantino et al., 2021), we believe it is necessary to create SWE-specific guidelines for authors to use to standardize SWE data collection and reporting procedures in the published literature. In Table 3 we present a list of reporting recommendations for SWE studies examining muscles, tendons, nerves, and ligaments as a solution to the problems identified earlier. This list and accompanying checklist (Appendix D) are directed to SWE users, journal peer reviewers, and journal editorial staff to help improve future SWE research. The recommendations are based upon information found or missing across the 375 published articles contained within this review. Our recommendations are SWE-specific and intended to be used in addition to or in conjunction with existing reporting guidelines in research and medicine (e.g., PRISMA for systematic reviews/meta-analyses, CONSORT for clinical trials, AGREE for practice guidelines, STROBE for observational studies, CARE for case reports, etc.).
Table 3.
Recommendations for reporting SWE methodological methods and results in publications.
| PARTICIPANT HISTORY & DEMOGRAPHICS |
|
|
| Report relevant information regarding the study participants, including: |
| • Number of participants by group, gender/sex, race/ethnicity (and/or report per participant) |
| • Mean and standard deviation of group height, weight, and body mass index (and/or report per participant) |
| • Relevant health history (e.g., diagnoses, previous injuries, surgeries/procedures) |
| • Treatment or therapy (i.e., type, duration, amount) |
| • Level of physical activity (e.g., typical aerobic and/or strength training activity) |
| STUDY DESIGN & PROCEDURE |
|
|
| Describe study design, collection procedures, and specify the number of SWE measurements, including: |
| • Whether study design was cross-sectional or longitudinal, prospective or retrospective, and interventional (e.g., needle EMG, stretching) or non-interventional |
| • A description of data collection procedures, including when SWE was collected |
| • Number of total study visits as well as which visits SWE was collected and number of times SWE was collected per visit |
| • Number of structures examined with SWE per participant, and number of SWE measurements per structure |
| • Range and/or average depth of SWE measurement per structure |
| PRE-COLLECTION INFORMATION |
|
|
| Provide any relevant information regarding participant restrictions, routines, or instructions prior to data collection, including: |
| • Whether participants were asked to avoid exercise, and if so, for how long |
| • Whether participants were allowed to exercise, and if so, specify type (aerobic versus anaerobic), intensity, and duration of activity as well as when activity occurred |
| • Any relaxation, warmup, or stretching routine prior to data collection |
| • Instructions given regarding protocol |
| ULTRASOUND INFORMATION |
|
|
| Specify all relevant information regarding ultrasound system(s) used in this study, including: |
| • Number of ultrasound systems used |
| • Brand and model type of each ultrasound system |
| • Location of each ultrasound system manufacturer |
| • Upper limits of ultrasound system (maximum kilopascals and/or meters/second) |
| PROBE-RELATED INFORMATION |
|
|
| Describe probe-related information for each structure and testing position so that others can easily replicate your setup, including: |
| • Scanner presets used for imaging (e.g., musculoskeletal, nerve, nerve block) |
| • Number of probes used |
| • Probe orientation (e.g., long axis/longitudinal; short axis/transverse) relative to structure, shape (e.g., convex, linear array, superlinear array), and frequency |
| • Probes used for B-mode versus SWE |
| • Structures examined with each probe |
| • Whether unilateral or bilateral SWE measurements were taken |
| • Whether the dominant or nondominant side was tested, how dominant was defined, and whether that corresponded to the left or right side of each participant |
| • Anatomical measurement location or site of measurement (e.g., proximal third of lower limb, popliteal fossa, muscle mid-belly) |
| • How measurement location was identified (e.g., anatomical landmarks) and maintained across measurements (e.g., skin marked) |
| • Pictures or illustrations of probe location for each structure |
| • Who (e.g., operator) or what (e.g., custom-made device) held the probe |
| • Amount of coupling gel/agent used (e.g., copious amount; a gel standoff was created) |
| • Degree of probe pressure applied |
| • Upper limits of transducer (frequency, maximum kilopascals and/or meters/second) |
| OPERATOR TRAINING |
|
|
| Describe the qualifications and relevant SWE experience of all operators, including: |
| • Number of operators performing measurements |
| • Degree or role of operator(s) as well as amount of experience using SWE and B-mode ultrasound (e.g., years, number patients) |
| • Number of collections performed by each operator |
| TESTING POSITIONS |
|
|
| Describe and provide images of testing position(s) and provide clear, detailed instructions given to participants so others can easily replicate your setup, including: |
| • Any testing position instructions given |
| • Number of testing positions |
| • *Body position (e.g., seated upright or reclined, lying prone or supine, standing) |
| • *Limb position on bed/table/plinth or in space (e.g., elbow flexed, pronated, resting on abdomen) |
| • *Joint position (e.g., degrees of flexion/extension, adduction/abduction, pronation/supination, protraction/retraction, inversion/eversion, internally/externally rotated) |
| • Weight/resistance (e.g., gravity eliminated, anti-gravity, anti-gravity + amount of weight) |
| • Muscles relaxed or contracted |
| • Use of equipment to support/hold testing position, ranging from the type of furniture used for body support (e.g., table, bed, chair with or without back support) to what materials were used to hold body parts in place (e.g., non-compliant straps) |
| • Pictures or illustrations of each testing position |
| MONITORING MUSCLE ACTIVITY |
|
|
| Describe all relevant factors for monitoring muscle activity, including: |
| • Room temperature during M collection |
| • Whether surface EMG was collected during SWE, and if so, which muscle(s) were monitored, and location of placement of surface EMG electrodes |
| • Process for ensuring that no muscle activity occurred during muscle SWE examination |
| REGIONS OF INTEREST |
|
|
| Describe region of interest standardization and selection process, including: |
| • Whether regions of interest were standardized/consistent across participants |
| • Number, size (range in diameter), and position (e.g., superficial, deep, or at a set depth) of regions of interest acquired from each participant |
| SWE RESULTS |
|
|
| For each structure and testing position, report relevant information used to obtain the reported shear modulus results, including: |
| • Algorithms or calculations performed (e.g., averages) |
| • **Mean and standard deviation of group shear modulus in kilopascals (and/or report per participant) |
| • **Mean and standard deviation of group shear modulus in meters/second (and/or report per participant) |
| • If multiple SWE measurements were taken, report reliability (e.g., intra- or inter-class correlation coefficients) and specify intra-day, inter-day, intra-operator, or inter-operator results for: |
| • each structure |
| • each testing position |
| • each depth |
Note: if provide body, limb, or joint position in degrees, define what is neutral.
Note: if provide this data in graph form, provide raw data in table form in supplemental information.
Abbreviations: EMG=electromyography; SWE=shear wave elastography.
Although Table 3 and Appendix D contain detailed descriptions of our suggested reporting recommendations, here is a summary of the recommendations we consider to be most important: (1) indicate the SWE operator’s amount of experience with SWE and B-mode ultrasound, (2) describe any restrictions placed on participants’ exercise prior to SWE testing, (3) specify when and how often SWE was collected, (4) indicate the brand, model type, and upper limits (maximum kilopascals and/or meters/second) of the ultrasound system, (5) describe the transducer orientation, pressure, and upper limits of frequency, (6) describe in detail or provide pictures of the probe location for each structure/site of measurement, including whether the dominant or nondominant side was tested (if relevant), (7) report the depth at which measurements were obtained, (8) describe in detail or provide pictures of testing positions (including specifics on body, limb, and joint position, whether weight or resistance was used, and whether muscles were relaxed or contracted) in order for others to easily replicate your setup, (9) describe the process for ensuring that no muscle activity occurred during SWE examination (ideally with the use of surface electromyography), and (10) report the region of interest standardization and selection process.
4.5. Limitations of this Scoping Review
Despite this extensive review of the literature, it is possible this report contains an incomplete retrieval of articles due to the selection of databases searched, search terms, and restrictions/filters placed on the searches. For example, one filter was language — we made the decision to only include articles written in (or already translated to) English. This means that the findings here may not be representative of SWE methods or findings from other countries, contexts, or demographics. Another limitation of this review is that our results only represent a limited portion of current SWE research on ligaments, since ligaments were not part of our original research question or search strategy. In addition, the results for this review are only up to date as of July 2020 which excludes more currently published literature. Future reviews could consider extracting additional information, such as the following variables: factors related to study design, whether participants were instructed to avoid exercise prior to SWE testing, the inclusion of warm-up/stretching routines prior to SWE testing, the level of operator training for SWE, whether room temperature was controlled, whether regions of interest were standardized across participants, and specifics on testing position, such as limb position, (e.g., elbow flexed, pronated, resting on abdomen), joint position (e.g., degrees of flexion/extension, adduction/abduction, pronation/supination, protraction/retraction, inversion/eversion, internally/externally rotated), or the use of weight/resistance (e.g., gravity eliminated, anti-gravity, anti-gravity + amount of weight).
4.6. Future Directions
This scoping review highlights that there is high variability in the way that researchers and clinicians are currently conducting SWE measurements. Thus, it is important to emphasize that standardized methods need to be employed in SWE studies (based on our suggested recommendations contained herein) before we can appropriately determine the factors that lead to consistent (or inconsistent) SWE results. Once standardized data collection procedures become the norm, new studies will need to be conducted before the field can (1) determine the extent to which various factors influence SWE results, (2) calculate the reliability of SWE measurements when controlling for such factors, and/or (3) determine the utility of SWE as a diagnostic tool by calculating the sensitivity and specificity of SWE measurements for specific diseases. Once the factors, reliability, and sensitivity and specificity are established with standardized SWE procedures, we are optimistic that SWE will eventually be used to aid in disease diagnosis, monitor disease progression, and assess treatment response and response to intervention.
4.7. Conclusions
This scoping review provides evidence of the heterogeneity of methods used across SWE articles in musculoskeletal soft tissue and nerve research that have resulted from the absence of data collection and reporting standards. While SWE may eventually serve as an objective tool for assessing tissue pathology, disease progression, and response to intervention, the methodological variability across current SWE research limits its potential and warrants the need for guidelines. Therefore, our recommendations attempt to address these important limitations and should be used when planning and reporting SWE data collection procedures to improve future research and ensure its reproducibility and replicability.
Supplementary Material
Highlights.
High methodological heterogeneity was found across 375 shear wave elastography (SWE) articles due to lack of data collection and reporting standards.
Two surprising findings were that most studies did not contain information on depth of measurement or use of surface electromyography.
Adherence to our reporting recommendations/checklist will advance the diagnostic and prognostic capabilities of this technology.
Acknowledgements
We want to thank Emma J. Leone, MS for her assistance in the creation of Table S1.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. However, this work was supported by the Intramural Research Program at the National Institutes of Health Clinical Center, Bethesda, MD. This funding source did not have any involvement in the study design, in the collection, analysis or interpretation of data, or in the writing of the report.
Footnotes
Declaration of Interest
None of the authors have potential conflicts of interest to be disclosed.
Abbreviations: C=curved; EMG=electromyography; L=linear; SC=supercurved; SL=superlinear; SLV=superlinear volumetric; SWE=shear wave elastography; US=ultrasound
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kevin J. Cipriano, Department of Rehabilitation, Georgetown University Hospital-MedStar National Rehabilitation Hospital, Washington, DC, USA.
Jordan Wickstrom, Rehabilitation Medicine Department, Clinical Center, National Institutes of Health, Bethesda, MD, USA.
Michael Glicksman, University of Pittsburgh Medical Center, Department of Physical Medicine and Rehabilitation, Pittsburgh, PA, USA.
Lauren Hirth, Rehabilitation Medicine Department, Clinical Center, National Institutes of Health, Bethesda, MD, USA.
Michael Farrell, Department of Rehabilitation, Georgetown University Hospital-MedStar National Rehabilitation Hospital, Washington, DC, USA.
Alicia A. Livinski, National Institutes of Health Library, Office of Research Services, National Institutes of Health, Bethesda, MD, USA.
Sogol Attaripour Esfahani, Canadian Advances for Neuro-Orthopedics for Spasticity Congress (CANOSC), Kingston, Ontario, Canada.
Robert J. Maldonado, Department of Rehabilitation, Georgetown University Hospital-MedStar National Rehabilitation Hospital, Washington, DC, USA.
Jared Astrow, Department of Rehabilitation, Georgetown University Hospital-MedStar National Rehabilitation Hospital, Washington, DC, USA.
William A. Berrigan, Department of Orthopaedics, Emory University School of Medicine, Atlanta, GA, USA.
Antonia M. H. Piergies, Department of Psychology, University of California, Davis, Davis, CA, USA; MIND Institute, University of California, Davis, Sacramento, CA, USA.
Lisa D. Hobson-Webb, Department of Neurology, Neuromuscular Division, Duke University, Durham, NC, USA.
Katharine E. Alter, Rehabilitation Medicine Department, Clinical Center, National Institutes of Health, Bethesda, MD, USA.
References
- Alfuraih AM, O’Connor P, Hensor E, Tan AL, Emery P, Wakefield RJ. The effect of unit, depth, and probe load on the reliability of muscle shear wave elastography: variables affecting reliability of SWE. J Clin Ultrasound 2018;46(2):108–15. 10.1002/jcu.22534 [DOI] [PubMed] [Google Scholar]
- Alfuraih AM, O’Connor P, Tan AL, Hensor E, Emery P, Wakefield RJ. An investigation into the variability between different shear wave elastography systems in muscle. Med Ultrason 2017;19(4):392–400. 10.11152/mu-1113 [DOI] [PubMed] [Google Scholar]
- Alfuraih AM, O’Connor P, Tan AL, Hensor EMA, Ladas A, Emery P, et al. Muscle shear wave elastography in idiopathic inflammatory myopathies: a case-control study with MRI correlation. Skeletal Radiol 2019;48(8):1209–19. 10.1007/s00256-019-03175-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alrashed AI, Alfuraih AM. Reproducibility of shear wave elastography among operators, machines, and probes in an elasticity phantom. Ultrasonography 2021;40(1):158–66. 10.14366/usg.20011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenburg JE, Eby SF, Song P, Zhao H, Brault JS, Chen S, et al. Ultrasound elastography: the new frontier in direct measurement of muscle stiffness. Arch Phys Med Rehabil 2014;95(11):2207–19. 10.1016/j.apmr.2014.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantino F, Carmona L, Boers M, Backhaus M, Balint PV, Bruyn GA, et al. EULAR recommendations for the reporting of ultrasound studies in rheumatic and musculoskeletal diseases (RMDs). Ann Rheum Dis 2021;80(7):840–7. 10.1136/annrheumdis-2020-219816 [DOI] [PubMed] [Google Scholar]
- Dağ N, Cerit MN, Şendur HN, Zinnuroğlu M, Muşmal BN, Cindil E, et al. The utility of shear wave elastography in the evaluation of muscle stiffness in patients with cerebral palsy after botulinum toxin A injection. J Med Ultrason (2001) 2020;47(4):609–15. 10.1007/s10396-020-01042-6 [DOI] [PubMed] [Google Scholar]
- Davis LC, Baumer TG, Bey MJ, Holsbeeck MV. Clinical utilization of shear wave elastography in the musculoskeletal system. Ultrasonography 2019;38(1):2–12. 10.14366/usg.18039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakonaki EE, Allen GM, Wilson DJ. Ultrasound elastography for musculoskeletal applications. Br J Radiol 2012;85(1019):1435–45. 10.1259/bjr/93042867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eby SF, Song P, Chen S, Chen Q, Greenleaf JF, An KN. Validation of shear wave elastography in skeletal muscle. J Biomech 2013;46(14):2381–7. 10.1016/j.jbiomech.2013.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewertsen C, Carlsen JF, Christiansen IR, Jensen JA, Nielsen MB. Evaluation of healthy muscle tissue by strain and shear wave elastography - dependency on depth and ROI position in relation to underlying bone. Ultrasonics 2016;71:127–33. 10.1016/j.ultras.2016.06.007 [DOI] [PubMed] [Google Scholar]
- Farrow M, Biglands J, Alfuraih AM, Wakefield RJ, Tan AL. Novel muscle imaging in inflammatory rheumatic diseases - a focus on ultrasound shear wave elastography and quantitative MRI. Front Med (Lausanne) 2020;7:434. 10.3389/fmed.2020.00434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garra BS. Elastography: history, principles, and technique comparison. Abdom Imaging 2015;40(4):680–97. 10.1007/s00261-014-0305-8 [DOI] [PubMed] [Google Scholar]
- Harmon B, Wells M, Park D, Gao J. Ultrasound elastography in neuromuscular and movement disorders. Clin Imaging 2019;53:35–42. 10.1016/j.clinimag.2018.10.008 [DOI] [PubMed] [Google Scholar]
- Ihnatsenka B, Boezaart AP. Ultrasound: basic understanding and learning the language. Int J Shoulder Surg 2010;4(3):55–62. 10.4103/0973-6042.76960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauser AS, Miyamoto H, Bellmann-Weiler R, Feuchtner GM, Wick MC, Jaschke WR. Sonoelastography: musculoskeletal applications. Radiology 2014;272(3):622–33. 10.1148/radiol.14121765 [DOI] [PubMed] [Google Scholar]
- Klauser AS, Miyamoto H, Tamegger M, Faschingbauer R, Moriggl B, Klima G, et al. Achilles tendon assessed with sonoelastography: histologic agreement. Radiology 2013;267(3):837–42. 10.1148/radiol.13121936 [DOI] [PubMed] [Google Scholar]
- Kwon DR, Park GY. Diagnostic value of real-time sonoelastography in congenital muscular torticollis. J Ultrasound Med 2012;31(5):721–7. 10.7863/jum.2012.31.5.721 [DOI] [PubMed] [Google Scholar]
- Lee H, Kim K, Lee Y. Development of stiffness measurement program using color mapping in shear wave elastography. Diagnostics (Basel) 2020;10(6). 10.3390/diagnostics10060362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima K, Costa-Júnior JFS, Pereira WCA, Oliveira LF. Assessment of the mechanical properties of the muscle-tendon unit by supersonic shear wave imaging elastography: a review. Ultrasonography 2018;37(1):3–15. 10.14366/usg.17017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CP, Chen IJ, Chang KV, Wu WT, Özçakar L. Utility of ultrasound elastography in evaluation of carpal tunnel syndrome: a systematic review and meta-analysis. Ultrasound Med Biol 2019;45(11):2855–65. 10.1016/j.ultrasmedbio.2019.07.409 [DOI] [PubMed] [Google Scholar]
- Martiartu NK, Nambiar S, Kirchner IN, Paverd C, Cester D, Frauenfelder T, et al. Sources of variability in shear wave speed and dispersion quantification with ultrasound elastography: a phantom study. Ultrasound Med Biol 2021;47(12):3529–42. 10.1016/j.ultrasmedbio.2021.08.013 [DOI] [PubMed] [Google Scholar]
- Nowicki A, Dobruch-Sobczak K. Introduction to ultrasound elastography. J Ultrason 2016;16(65):113–24. 10.15557/JoU.2016.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi CC, Malliaras P, Schneider ME, Connell DA. “Soft, hard, or just right?” Applications and limitations of axial-strain sonoelastography and shear-wave elastography in the assessment of tendon injuries. Skeletal Radiol 2014;43(1):1–12. 10.1007/s00256-013-1695-3 [DOI] [PubMed] [Google Scholar]
- Ozturk A, Grajo JR, Dhyani M, Anthony BW, Samir AE. Principles of ultrasound elastography. Abdom Radiol (NY) 2018;43(4):773–85. 10.1007/s00261-018-1475-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado-Costa R, Rebelo J, Monteiro-Barroso J, Preto AS. Ultrasound elastography: compression elastography and shear-wave elastography in the assessment of tendon injury. Insights Imaging 2018;9(5):791–814. 10.1007/s13244-018-0642-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer D, Gennisson JL, Deffieux T, Tanter M. On the elasticity of transverse isotropic soft tissues (L). J Acoust Soc Am 2011;129(5):2757–60. 10.1121/1.3559681 [DOI] [PubMed] [Google Scholar]
- Ruby L, Mutschler T, Martini K, Klingmüller V, Frauenfelder T, Rominger MB, et al. Which confounders have the largest impact in shear wave elastography of muscle and how can they be minimized? An elasticity phantom, ex vivo porcine muscle and volunteer study using a commercially available system. Ultrasound Med Biol 2019;45(10):2591–611. 10.1016/j.ultrasmedbio.2019.06.417 [DOI] [PubMed] [Google Scholar]
- Ryu J, Jeong WK. Current status of musculoskeletal application of shear wave elastography. Ultrasonography 2017;36(3):185–97. 10.14366/usg.16053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi S, Quinlan K, Eilertson KE, Billy GG, Bible J, Sions M, et al. Changes in shear modulus of the lumbar multifidus muscle during different body positions. J Biomech Eng 2019. 10.1115/1.4043443 [DOI] [PubMed] [Google Scholar]
- Shiina T, Nitta N, Ueno E, Bamber JC. Real time tissue elasticity imaging using the combined autocorrelation method. J Med Ultrason (2001) 2002;29(3):119–28. 10.1007/bf02481234 [DOI] [PubMed] [Google Scholar]
- Sigrist RMS, Liau J, Kaffas AE, Chammas MC, Willmann JK. Ultrasound elastography: review of techniques and clinical applications. Theranostics 2017;7(5):1303–29. 10.7150/thno.18650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoj Ž, Wu CH, Taljanovic MS, Dumić-Čule I, Drakonaki EE, Klauser AS. Ultrasound elastography in musculoskeletal radiology: past, present, and future. Semin Musculoskelet Radiol 2020;24(2):156–66. 10.1055/s-0039-3402746 [DOI] [PubMed] [Google Scholar]
- Taljanovic MS, Gimber LH, Becker GW, Latt LD, Klauser AS, Melville DM, et al. Shear-wave elastography: basic physics and musculoskeletal applications. Radiographics 2017;37(3):855–70. 10.1148/rg.2017160116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hooren B, Teratsias P, Hodson-Tole EF. Ultrasound imaging to assess skeletal muscle architecture during movements: a systematic review of methods, reliability, and challenges. J Appl Physiol (1985) 2020;128(4):978–99. 10.1152/japplphysiol.00835.2019 [DOI] [PubMed] [Google Scholar]
- Wang H, Zheng P, Sang L, Wang X. Does operator experience and the Q-Box diameter affect the repeatability of liver stiffness measurements obtained by 2-dimensional shear wave elastography? J Ultrasound Med 2020;39(4):741–7. 10.1002/jum.15153 [DOI] [PubMed] [Google Scholar]
- Wee TC, Simon NG. Ultrasound elastography for the evaluation of peripheral nerves: a systematic review. Muscle Nerve 2019;60(5):501–12. 10.1002/mus.26624 [DOI] [PubMed] [Google Scholar]
- Yoon JH, Ko KH, Jung HK, Lee JT. Qualitative pattern classification of shear wave elastography for breast masses: how it correlates to quantitative measurements. Eur J Radiol 2013;82(12):2199–204. 10.1016/j.ejrad.2013.08.047 [DOI] [PubMed] [Google Scholar]
- Yu HK, Liu X, Pan M, Chen JW, Liu C, Wu Y, et al. Performance of passive muscle stiffness in diagnosis and assessment of disease progression in Duchenne muscular dystrophy. Ultrasound Med Biol 2022;48(3):414–21. 10.1016/j.ultrasmedbio.2021.09.003 [DOI] [PubMed] [Google Scholar]
- Zúñiga LDO, López CAG, González ER. Ultrasound elastography in the assessment of the stiffness of spastic muscles: a systematic review. Ultrasound Med Biol 2021;47(6):1448–64. 10.1016/j.ultrasmedbio.2021.01.031 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.