Abstract
Purpose
The aim of this study was to understand the resistance mechanism of ceftazidime/avibactam (CZA) in carbapenem-resistant Klebsiella pneumoniae under antibiotic selection pressure.
Patients and Methods
Four CZA-resistant Klebsiella pneumoniae strains were isolated from two patients, and six CZA-resistant strains that were produced in vitro were screened from 25 carbapenem-resistant Klebsiella pneumoniae strains. The mechanisms of resistance to CZA of these strains were characterized by PCR and Sanger sequencing.
Results
CZA-resistant Klebsiella pneumoniae with different resistance mechanisms (including upregulation of the expression of efflux pumps and KPC variants (KPC-14, KPC-44)) were isolated from the same patient (patient 1). In patient 2, the resistance mechanism of CZA-resistant Klebsiella pneumoniae was the mutation of KPC-2 to KPC-33. In addition, among the CZA-resistant Klebsiella pneumoniae that were produced in vitro, we found 3 new KPC variants: KPC-86 (D179G), KPC-87 (GT241A) and KPC-88 (G523T).
Conclusion
In this study, although the CZA-resistant bacteria originated from only two clinical patients, four different mechanisms of CZA resistance were detected. In the in vitro induction experiment, the mechanisms of resistance to CZA in strains from different patients were also different. The above result implies that the mechanisms of resistance to CZA are generally random and diverse. Therefore, elucidating the mechanism of resistance to CZA can provide a certain theoretical basis for the effective response of CZA-resistant strains and the selection of antibiotics.
Keywords: ceftazidime/avibactam-resistance, KPC mutants, antibiotic pressure, Klebsiella pneumoniae, resistance mechanisms
Introduction
Due to restrictions on antibiotic options, carbapenem-resistant Enterobacterales (CRE) has become a serious threat to human health. Ceftazidime/avibactam (CZA) is a new β-lactam antibacterial combination (2 g ceftazidime and 0.5 g avibactam) that was approved by the United States Food and Drug Administration in 2015 for the treatment of infections caused by CRE.1 CZA could effectively inhibit the enterobacteria that produce A-, C-, and D-type β-lactamases (include KPCs, AmpC and OXA-48).2
According to clinical research reports, the application of CZA has significantly improved the clinical cure rate and survival rate of patients with CRE infection, and CZA is superior to other drug treatment options.3 According to a survey of 210 hospitals released by the United States in 2020, the number of CZA hospital prescriptions increased nearly 18-fold from 2015 to 2017. However, the first case of CZA-resistant bacteria was reported in the United States in 2016. From 2016 to 2021, multiple emergent cases of strains resistant to CZA were reported worldwide. Among them, Klebsiella pneumoniae is one of the main resistant bacteria.4–6 Klebsiella pneumoniae was considered an opportunistic nosocomial pathogen and had attracted widespread attention due to its disease severity and antibiotic resistance. Klebsiella pneumoniae was highly virulent and multidrug-resistant and could cause life-threatening infections.7,8
In China, carbapenem-resistant Klebsiella pneumoniae increased nearly 10-fold during the decade from 2010 to 2021. CZA was approved by the China National Medical Products Administration (NMPA) to enter the Chinese clinic on May 21, 2019. In the first half of 2020, the drug resistance rate of CZA reached 14.9% according to the analysis of the drug data from 4395 cases of carbapenem-resistant Klebsiella pneumoniae obtained by the China Bacterial Resistance Surveillance Network (CHINET) from more than 50 hospitals in China.9–11 The emergence of CZA-resistant strains indicates that human health could be seriously threatened, and it also brings new challenges for clinicians.
Recent studies have reported that the resistance mechanisms of carbapenem-resistant Klebsiella pneumoniae against CZA mainly include mutations in KPCs (KPC-2, KPC-3), mutations in porins, the effects of efflux pumps, and the variation of the copy number of the KPC-encoding plasmid.12–14 However, due to the relatively short time that CZA has been used in clinical practice in China, the number of collections and studies of clinically resistant strains in China is small. Therefore, the resistance mechanism of Klebsiella pneumoniae against CZA and new medication regimens in China urgently need to be studied.
In this study, CZA-resistant strains from 2 patients were collected, and the resistance mechanism was analysed. The result showed the same specimen of the same patient could produce CZA resistant strains with multiple resistance mechanism. And the resistance mechanisms of the CZA-resistance Klebsiella pneumoniae isolated from the two patients included KPC mutantions (KPC-2 was mutated to KPC-44, KPC-14 and KPC-33) and upregulated efflux pumps. However, we discovered that inducing CZA-resistant Klebsiella pneumoniae in vitro not only showed the same KPC mutations (KPC-2 was mutated to KPC-44) as the in-vivo CZA-resistant strain, but also three new KPC variants (KPC-86, KPC-87 and KPC-88) were discovered. The above results indicate the diversity of the resistance mechanisms of Klebsiella pneumoniae against CZA.
Materials and Methods
Bacterial Strains
Four CZA-resistant Klebsiella pneumoniae strains were collected, which were derived from different body fluid samples (ascites, blood and sputum) of two clinical patients in 2020. There was no significant difference in the growth rate and morphology of these strains. The patients were admitted to the Second Affiliated Hospital of Chongqing Medical University of China.
Induction of CZA-Resistant Strains
Twenty-five Klebsiella pneumoniae strains, used to induce CZA resistance in vitro, also came from clinical patients. There was no significant difference in the growth rate and morphology of these strains. The initial Klebsiella pneumoniae isolates were cultured in 5 mL nutrient broth medium respectively. Fifty microliters of the bacterial solution and CZA (ceftazidime: avibactam 3:2, 50 μg) were added to 1 mL broth medium and cultured for 24 hours in a 35 °C bacterial incubator to induce CZA-resistant strains. Bacterial strains were passaged every day for 10 generations.
Antimicrobial Susceptibilities
The antibiotics imipenem (IMP), meropenem (MER), tigecycline (TGC) and CZA were used to test the sensitivity to drugs of the bacterial strains. Antimicrobial susceptibilities were determined with the broth microdilution method, as previously described by CLSI.15
Detection of Carbapenemase Type
The detection of the carbapenemase type used the APB(3-aminophenylboronic acid)/EDTA method.16 This method is based on disk diffusion method in the CLSI guidelines.15 Briefly, a 0.5 McFarland turbidity of Klebsiella pneumoniae was spread on a Mueller-Hinton agar plate, and then 4 tablets of imipenem (10 μg/tablet) were placed on the agar plate. APB (300 μg) and EDTA (292 μg) are KPC-type carbapenemase and metallo β-lactamases inhibitors, respectively, and they were dropped on two pieces of imipenem tablets. A mixture of APC and EDTA was dropped on another imipenem tablet, and nothing was dropped on the last imipenem tablet. If the difference in the inhibition zone diameter between the enzyme inhibitor mixture and the single drug was more than 5 mm, it could be determined that the tested strain produces class A carbapenemase (mainly KPC enzyme), class B metallo β-lactamases, or both types of carbapenemase.
The detection of the carbapenemase type was also determined with the method of modified carbapenem inactivation method (mCIM) and EDTA-CIM (eCIM), as previously described by CLSI.15 Briefly, 1 μL loopful of isolates were mixed in 2mL of tryptone soya broth (TSB). One meropenem disc (10μg) was immersed in the suspension and incubated in a 35°C incubator for 4 hours. The E. coli ATCC 25922 with 0.5 McFarland turbidity was inoculated on the MHA plate, and the meropenem tablet was taked out of the TSB broth and sticked it on the MHA plate. The plate was incubated at 35 degrees Celsius for 18–24 hours. If the meropenem inhibition zone was 6–15mm, or the diameter of the inhibition zone was 16–18mm and there are scattered colonies, the strain was tested produces carbapenemase. If the mCIM test was positive, the eCIM test was performed. Another 2mL TSB broth tube was added 20μL 0.5M EDTA. The rest of the operation steps were the same as mCIM. If the diameter of the inhibition zone of meropenem was ≥5mm compared with mCIM, the metallo-beta lactamase was considered positive.
Extraction of Bacterial DNA and Screening of Drug Resistance Genes
Bacterial genomic DNA was extracted using a bacterial genome extraction kit (Thermo Scientific, China). The KPC resistance genes of drug-resistant strains were detected by PCR according to the research of Dallenne et al.17 The positive products amplified by PCR were sent to Shanghai Sangon Biological Engineering Technology Co., Ltd. for Sanger sequencing. The sequences were compared with those deposited in GenBank database (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Multi-Locus Sequence Typing (MLST)
MLST typing could provide a valuable method and clear data for epidemiological studies of Klebsiella pneumoniae isolates and could represent different spatial and temporal dynamics of Klebsiella pneumoniae. Seven housekeeping genes (tonB (plasma perimeter energy sensor), gapA (glyceraldehyde 3-phosphate dehydrogenase), mdh (malate dehydrogenase), infB (translation initiation factor 2), rpoB (beta subunit of RNA polymerase), pgi (phosphoglucose isomerase) and phoE (phospho E)) were used for MLST PCR amplification, and the PCR products were sequenced and analyzed according to Pasteur MLST site (https://bigsdb.pasteur.fr/klebsiella/klebsiella.html).18
MIC Determination of the Efflux Pump Inhibitors (CCCP)
Carbonyl cyanochlorophenylhydrazone (CCCP) is an efflux pump inhibitor that was purchased from Sigma–Aldrich (St Louis, USA). MIC determination of CCCP was performed with the broth microdilution method as previously described.19 Briefly, CCCP solutions with concentrations of 256, 128, 64, 32, 16, 8, 4, 2, 1, 0.5, 0.25, and 0 μg/mL were added to 96-well plates in sequence. Then, 50 μL of bacterial suspension with optical density equivalent to 0.5 McFarland was added dropwise to each well of 96-well plates containing different concentrations of CCCP. The 96-well plate was placed in a 37 °C CO2 incubator and incubated for 16–20 hours. The highest CCCP concentration (8 μg/mL) with obvious bacterial growth was selected as the optimal concentration for the efflux pump inhibition experiment.
CZA-CCCP Disc Synergy Test
Then, CCCP (32 μg/mL, 50 μL/well) was mixed with different concentrations of CZA (64, 32, 16, 8, 4, 2, 1, 0.5, 0.25, and 0 μg/mL, 50 μL/well) and configured as a mixed solution. At this time, the final concentration of CCCP was 16 μg/mL. Then, a mixed solution (50 μL) was added to the bacterial suspension (0.5 McFarland, 50 μL/well) and mixed thoroughly. At this time, the final concentration of CCCP was 8 μg/mL. Compared with the control group (CZA), the MIC value of the experimental group (CZA and CCCP) decreased by more than two gradients, and thus, the result was determined to be positive.
Immune-Enzymatic Test
KPC was detected with a colloidal gold immunochromatographic assay using the NG-test CARBA5 kit (NG-biotech, France), and the experimental protocol was performed according to the product manual.
Conjugation Experiment
The CZA-resistant strains with KPC mutants (KPC-86, KPC-87 and KPC-88) were used as donors. And rifampicin-resistant E. coli (EC600) was used as the recipient. The CZA-resistant strains (KPC-86, KPC-87 and KPC-88) also exhibited ampicillin resistance. Transconjugants were selected on MH agar plates supplemented with ampicillin (128 μg/mL) and rifampicin (256 μg/mL). E. coli EC600 transconjugants were identified using the mass spectrum system, and KPC mutations were further confirmed by PCR and Sanger sequencing. The conjugation efficiency was measured and calculated following the protocol in https://openwetware.org/wiki/conjugation.
Results
Clinical Case Report
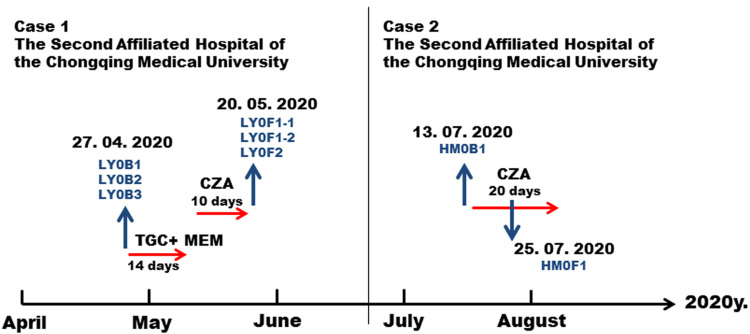
A 40-year-old male patient was admitted to the Second Affiliated Hospital of Chongqing Medical University on April 27, 2020 due to upper abdominal pain for more than one month and fever for three days. He was diagnosed with acute severe pancreatitis and sepsis. Carbapenem-resistant and CZA-sensitive Klebsiella pneumoniae were isolated from the peritoneal ascites, blood and sputum (strain number: ascites LY0B1, blood LY0B2 and sputum LY0B3, Table 1). The three primary isolates all produced carbapenemase and the enzyme type was KPC-2. Tigecycline plus meropenem was used to fight infection for 14 days from April 27 to May 10, but the patient’s condition did not improve. On May 10, the patient was treated with CZA (ceftazidime/avibactam, 2 g/0.5 g, tid). On May 20, the ascites and sputum were taken again for culture (Figure 1, left panel).
Table 1.
Antimicrobial Susceptibility and Resistance Mechanism of Klebsiella pneumoniae from Patients
| Isolate | MIC (μg/mL) | Carbapenemases | MLST | ESBL | AmpC | Efflux Pump | |||
|---|---|---|---|---|---|---|---|---|---|
| IMP | MEM | TGC | CZA | ||||||
| LY0B1 | ≥64 | ≥64 | 0.5 | 0.5 | KPC-2 | ST11 | – | – | / |
| LY0B2 | ≥64 | ≥64 | 0.5 | 0.5 | KPC-2 | ST11 | – | – | / |
| LY0B3 | ≥64 | ≥64 | 0.5 | 0.5 | KPC-2 | ST11 | – | – | / |
| LY0F1-1 | ≥64 | ≥64 | 1 | 128 | KPC-2 | ST11 | – | – | ↑ |
| LY0F1-2 | 0.25 | 2 | 0.5 | 128 | KPC -14 | ST11 | TEM | – | / |
| LY0F2 | 8 | 32 | 0.5 | 128 | KPC-44 | ST11 | – | – | / |
| HM0B1 | ≥64 | ≥64 | 1 | 0.5 | KPC-2 | ST11 | – | – | / |
| HM0F1 | 0.25 | 2 | 1 | 256 | KPC-33 | ST11 | TEM | – | / |
Abbreviations: IMP, imipenem; MEM, meropenem; TGC, tigecycline; CZA, ceftazidime/avibactam; MLST, Multiple-locus sequence typing; ESBL, extended-spectrum beta-lactamase; AmpC, AmpC beta lactamase.
Figure 1.
Schedule of antibacterial treatment and isolation of Klebsiella pneumoniae strains. The red arrows represent the time period of medication. The blue arrow represents the specimen collection at the corresponding time.
Abbreviations: IMP, imipenem; MEM, meropenem; CZA, ceftazidime/avibactam.
Another case was a 75-year-old male patient who was admitted to the Second Affiliated Hospital of Chongqing Medical University on July 13, 2020 due to severe pneumonia. The patient’s alveolar lavage fluid was cultured with carbapenem-resistant Klebsiella pneumonia (strain number: HM0B1), and the type of carbapenemase was KPC-2. From July 15 to August 5, the patient was treated with CZA (ceftazidime/avibactam, 2 g/0.5 g, tid) for anti-infection. On July 25, CZA-resistant Klebsiella pneumoniae (strain number: HM0F1) was cultured from sputum (Figure 1, right panel).
The Mechanisms of Resistance to CZA of the Clinical Isolates
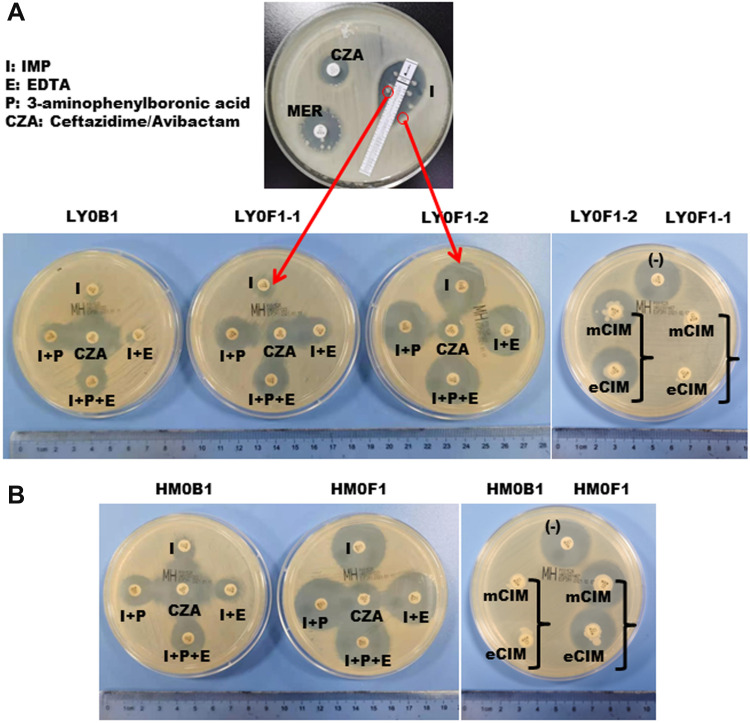
Interestingly, three CZA-resistant strains (LY0F1-1, LY0F1-2 and LY0F2) with different resistance mechanisms were isolated from patient 1. LY0F1-1 and LY0F1-2 were isolated from the same drainage sample (Figure 2A). The antimicrobial susceptibility results are shown in Table 1. LY0F1-1 exhibited IMP and MER resistance (MIC, 64 μg/mL), while LY0F1-2 was susceptible to IMP and intermediate to MER (MIC, 0.25 μg/mL and 2 μg/mL, respectively) (Table 1). The strain cultured from the sputum specimens of patient 1 (LY0F2) was also resistant to CZA, but the MICs of IMP and MER were 8 μg/mL and 32 μg/mL, respectively (Table 1). Therefore, the three CZA-resistant strains isolated from the same patient have different MICs for IMP and MER. The antimicrobial susceptibility results indicated that the HM0F1 isolate from patient 2 was resistant to CZA, and the MIC values of IMP and MER were 0.25 μg/mL and 2 μg/mL, respectively (Table 1).
Figure 2.
Isolation of CZA-resistant Klebsiella pneumoniae and detection of the enzyme type of the strain. (A) The top panel shows that CZA-resistant Klebsiella pneumonia was isolated from the puncture fluid sample of patient 1 after 10 days of CZA administration, and the sensitivity of CZA, MEM, and IMP was tested by the K-B method. The bottom panel in (A) and (B) shows that the enzyme types of isolates LY0F1-1, LY0F1-2 and HM0F1 were detected by enzyme inhibitor enhancement experiments and mCIM and eCIM experiments.
Abbreviations: MEM, meropenem; TGC, tigecycline; CZA, ceftazidime/avibactam; mCIM, modified carbapenem inactivation method; eCIM, EDTA-modified carbapenem inactivation method.
To research the mechanism of CZA resistance in the above four KPC-producing Klebsiella pneumoniae strains, the activity and genotype of carbapenemase were detected. The results showed that the carbapenemase type of the CZA-resistant strain LY0F1-1 was KPC-2, and the expression of KPC-2 did not increase (Figure 2A). However, the efflux pump was upregulated in the LY0F1-1 strain (Table 1,). We speculated that LY0F1-1 may also have other resistance mechanisms, which need to be further studied. The carbapenemase types in the CZA-resistant strains LY0F1-2, LY0F2 and HM0F1 were mutated from the original KPC-2 to KPC-14, KPC-44 and KPC-33, respectively, which was the mechanism of CZA resistance in the three isolates (Table 1). The mCIM and eCIM tests did not find that LY0F1-2, LY0F2 and HM0F1 produced serine carbapenemase and metallo β-lactamases (Figure 2A and B), but the immune-enzymatic test for the KPC enzyme was positive. KPC gene was detected to be positive in both primary strains and CZA-resistant strains by PCR (Supplementary Figure 1A).
The above results indicate that the same specimen (LY0F1-1 and LY0F1-2) from the same patient could produce CZA-resistant strains with multiple resistance mechanisms (upregulation of the efflux pump, KPC-14), which shows the diversity of CZA resistance mechanisms.
The Resistance Mechanism of CZA-Resistant Klebsiella pneumoniae That Were Produced in vitro
To verify the diversity of KPC mutations in CZA-resistant Klebsiella pneumoniae under CZA drug selection. Twenty-five strains of carbapenem-resistant Klebsiella pneumoniae were induced with CZA resistance in vitro. The results showed that 6 strains developed CZA resistance, and the resistance rate was as high as 24%. And blaKPC was detected by PCR (Supplementary Figure 1B). As shown in Table 2, the carbapenemase genotypes of the primary strains were all blaKPC-2, and the main mechanism for the induced strains to acquire CZA resistance was mutation of the KPC-2 protein. Among them, KPC-86, KPC-87 and KPC-88 were first discovered by us and have been submitted to PubMed (Accession numbers: MZ067229, MZ067230, MZ067231). The variant KPC-86 was derived from the LY0F1 strain of patient 1, which was completely different from the KPC variant (KPC-14) that formed the CZA-resistant strain (LY0F1-1, LY0F1-2) in vivo. KPC-86 presented the nucleotide A533G mutation, resulting in the substitution of aspartic acid by a glycine at position 179 (D179G). In addition, the mutant KPC-87 of the 10B isolate showed deletion of the nucleotides GCA after position 721, which causes the amino acid substitution of GT241A. Isolate 207B acquired CZA resistance through variant KPC-88, which was associated with a nucleotide substitution at position 523 (G523T). This substitution results in the amino acid change D176Y in the KPC-2 protein. In order to demonstrate the association between CZA resistance and mutated enzymes, the conjugation experiments was performed. The results showed that the receptor bacteria (E. coli EC600 strain) developed CZA resistance (Supplementary Figure 2) and the gene sequencing results showed that there were KPC genes (KPC-86, KPC-87, KPC-88) in the corresponding bacteria. The above results further illustrate the diversity of KPC variants in CZA-resistant Klebsiella pneumoniae.
Table 2.
Antimicrobial Susceptibility and Resistance Mechanism of CZA-Resistant Klebsiella pneumoniae Induced in vitro
| Isolate | MIC (μg/mL) | Carbapenemases | MLST | ESBL | AMPc | Efflux Pump | |||
|---|---|---|---|---|---|---|---|---|---|
| IMP | MEM | TGC | CZA | ||||||
| LY0B1 | ≥64 | ≥64 | 0.5 | 0.5 | KPC-2 | ST11 | – | / | |
| LY0F1Y | 1 | 4 | 1 | 128 | KPC-86 | ST11 | TEM | – | / |
| LY0B2 | ≥64 | ≥64 | 0.5 | 0.5 | KPC-2 | ST11 | – | / | |
| LY0F2Y | ≥16 | ≥64 | 1 | 128 | KPC-44 | ST11 | – | / | |
| LY0B3 | ≥64 | ≥64 | 0.5 | 0.5 | KPC-2 | ST11 | – | / | |
| LY0F3Y | 8 | 32 | 0.5 | 128 | KPC-44 | ST11 | – | / | |
| 10B | ≥16 | ≥64 | 0.5 | ≤2 | KPC-2 | ST11 | – | / | |
| 10F | ≥16 | 16 | 1 | 64 | KPC-87 | ST11 | – | / | |
| 207B | ≥16 | ≥64 | 0.25 | 4 | KPC-2 | ST11 | – | / | |
| 207F | 2 | 2 | 0.5 | 64 | KPC-88 | ST11 | TEM | – | ↑ |
| 14B | ≥16 | ≥64 | 2 | ≤2 | KPC-2 | ST11 | – | / | |
| 14F | ≥16 | 32 | 0.5 | 256 | KPC-2 | ST11 | – | ↑ | |
Abbreviations: IMP, imipenem; MEM, meropenem; TGC, tigecycline; CZA, ceftazidime/avibactam; MLST, Multiple-locus sequence typing; ESBL, extended-spectrum beta-lactamase; AmpC, AmpC beta lactamase.
To further explore the relationship between the changes in drug susceptibility and the characteristics of the resistant phenotypes for the different KPC mutant types of CZA-resistant strains, we analyzed the results of drug susceptibility tests and detected other drug-resistant phenotypes of the strains. The results showed that the carbapenemase (KPC-2) variants KPC-14, KPC-33, KPC-86, and KPC-88 of the CZA-resistant strains were all sensitive to IMP and MEM and were positive for ESBL (Table 3, Figure 3). However, the variants KPC-44 and KPC-87 of the CZA-resistant strains showed resistance to IMP and MEM and were negative for ESBL (Table 3). The above results indicate that the KPC-14, KPC-33, KPC-86 and KPC-88 variants tend to have similar IMP and MEM drug resistance characteristics and resistance phenotypes. The KPC-44 and KPC-87 variants tended to have similar IMP and MEM resistance characteristics and resistance phenotypes.
Table 3.
Antimicrobial Susceptibility Changes and the Characteristics of Resistant Phenotypes of Different KPC Mutant Types of CZA Resistant Strains
| Carbapenemases | Isolate | Interpretive Categories | ESBL | AMPc | Efflux Pump | |||
|---|---|---|---|---|---|---|---|---|
| IMP | MEM | TGC | CZA | |||||
| KPC-2 | LY0B1 | R | R | S | S | – | – | – |
| KPC-33/86/88 | LYOF1 HMOF1 207F |
S/I | S/I | S | R | + | – | – |
| KPC-44/87 | LYOF2 LYOF2Y LYOF3Y |
R | R | S | R | – | – | – |
Abbreviations: IMP, imipenem; MEM, meropenem; TGC, tigecycline; CZA, ceftazidime/avibactam; MLST, Multiple-locus sequence typing; ESBL, extended-spectrum beta-lactamase; AmpC, AmpC beta lactamase.
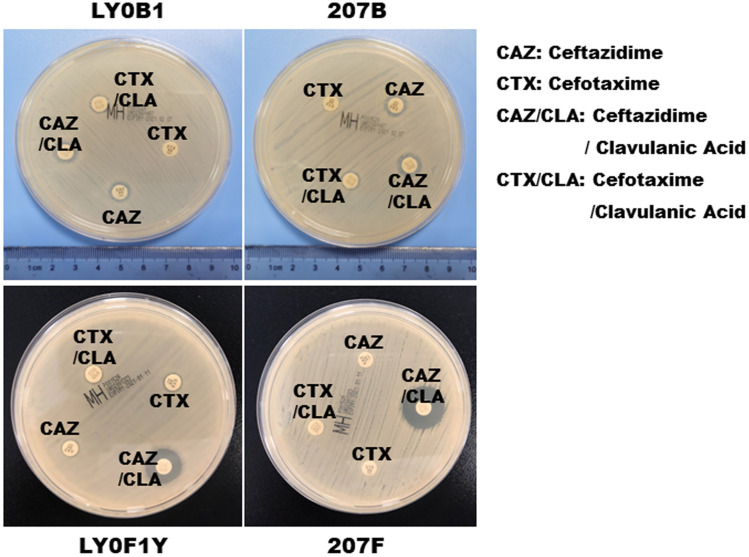
Figure 3.
ESBL confirmation test of the CZA-resistant Klebsiella pneumoniae (LY0F1Y and 207F) that were produced in vitro.
Abbreviations: CAZ, ceftazidime; CTX, cefotaxime; CAZ/CLA, ceftazidime/clavulanic acid; CTX/CLA, cefotaxime/ clavulanic acid.
Discussion
In this study, we isolated CZA-resistant Klebsiella pneumoniae with different resistance mechanisms from clinical patients treated with CZA. We also found that CZA-resistant strains with different resistance mechanisms could appear in the same clinical patient sample. In addition, we discovered three new CZA resistance mechanisms of new KPC variants (KPC-86, KPC-87 and KPC-88).
In patient 1, we found carbapenem-resistant Klebsiella pneumoniae in ascites, sputum and blood. However, after 10 days of CZA medication, two CZA-resistant strains with different resistance mechanisms were isolated from the same strain of ascites. CZA-resistant strains with different resistance mechanisms also appeared in sputum specimens. Shields et al also reported that three different KPC-3 mutant CZA strains appeared in the same patient.20 The widespread appearance of different resistant strains in the same patient indicates that CZA-resistant mutations are diverse. However, in our research, KPC mutant CZA-resistant strains were isolated from ascites, and strains with upregulated pump protein expression as the mechanism of resistance were also found. These findings fully demonstrate the diversity of drug resistance. In addition, although CZA-resistant Klebsiella pneumoniae was isolated in the ascites and sputum of patient 1, the blood isolate did not develop CZA resistance. This result may be related to the highest drug concentration in the blood, which was higher than the mutation concentration, leading to the direct killing of all bacteria. The abdominal cavity, which has a large amount of bacteria, was the primary site of infection. In the abdominal cavity, the drug concentration was lower than that in the blood, and the drug concentration in the lung was lower. Therefore, bacteria are more likely to acquire drug-resistant mutations in the abdominal cavity and sputum.
In this study, we found that the resistance mechanism of CZA-resistant strains isolated from patients was mainly due to KPC variants. The mutants are KPC-14, KPC-44 and KPC-33. The MIC values of IMP and MEM of bacteria with these KPC variants (KPC-14, KPC-44 and KPC-33) were consistent with the results of other research teams.21–24 In addition, we were surprised to find that the mechanism of resistance to CZA that was induced in vitro by the bacterial isolates in the ascites specimen in patient 1 was different from the mechanism of resistance of the drug-resistant strains in vivo. After induction of CZA resistance in vitro, a new KPC variant, KPC-86 (D179G), appeared. The MIC values of IMP and MEM of this variant were significantly reduced (Table 2), which may be due to the location of the mutation site on the Ω-loop.25 However, the change in MIC value was not as significant as that of KPC-33 (D179Y),21 which also had a mutation at position 179. Compared with the KPC-14 variant (2-amino-acid 242Gly and 243Thr deletion) that occurred in resistant strains in vivo, the MIC values IMP and MEM of KPC-86 were not significantly lower than that of KPC-14.24 Another new KPC variant, KPC-87 (GT242A), appeared in isolate 10B, and the MIC values of IMP and MEM were not significantly reduced. Although the mutation site of KPC-87 was the same as that of KPC-14, the difference in the mutation mode resulted in a large difference in the MIC values of IMP and MEM between KPC-87 and KPC-14 cells. The MIC values of IMP and MEM of another new KPC variant, KPC-88 (D176Y), found in this study were similar to those of KPC-33, which may also be related to the location of the mutation site on the Ω-loop.25 The above results fully indicate that the CZA resistance mechanism of these strains are random, diverse, and uncertain.
Conclusion
In this study, we detected different resistance mechanisms (KPC-14, KPC-44 and KPC-33) of CZA-resistant Klebsiella pneumoniae from clinical patients treated with CZA for approximately 10 days. We discovered three new KPC variants (KPC-86, KPC-87 and KPC-88) of these CZA-resistant strains that were induced by CZA in vitro. These findings indicate that the resistance mechanisms of CZA are generally random and diverse and provide a reference basis for the clinical use of CZA. These findings indicate that the resistance mechanisms of CZA are generally random and diverse, and that CZA-resistant strains with different resistance mechanisms may also appear in the same patient. This provides a reference basis for the clinical use of CZA and the selection of antibiotic after CZA resistance. This also emphasizes the importance of preventing the spread of CZA-resistant strains and the importance of drug sensitivity testing.
Funding Statement
The work was supported by funds (to Di Mu) from the National Natural Science Foundation of China (82102376) and Kuanren Talents Program of the second affiliated hospital of Chongqing Medical University. This work was supported by funds (to Bin Sun) from the National Natural Science Foundation of China (81802062).
Ethical Approval
The research was conducted in accordance with the Declaration of Helsinki and according to the recommendations of the Ethics Committee of The Second Affiliated Hospital of Chongqing Medical University. The clinical samples were part of the routine hospital laboratory procedure, the patient data came from the medical record system, and confidentiality was assured throughout the study. The written informed consent has been provided by the patients to have the case details published.
Author Contributions
Min Jiang and Bin Sun share first authorship. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Yahav D, Giske CG, Grāmatniece A, Abodakpi H, Tam VH, Leibovici L. New β-lactam-β-lactamase inhibitor combinations. Clin Microbiol Rev. 2020;34(1):e00115–20. doi: 10.1128/CMR.00115-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma R, Park TE, Moy S. Ceftazidime-Avibactam: a Novel Cephalosporin/β-Lactamase Inhibitor Combination for the Treatment of Resistant Gram-negative Organisms. Clin Ther. 2016;38(3):431–444. doi: 10.1016/j.clinthera.2016.01.018 [DOI] [PubMed] [Google Scholar]
- 3.Jorgensen SCJ, Trinh TD, Zasowski EJ, et al. Real-world experience with ceftazidime-avibactam for multidrug-resistant gram-negative bacterial infections. Open Forum Infect Dis. 2019;6(12):ofz522. doi: 10.1093/ofid/ofz522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strich JR, Ricotta E, Warner S, et al. Pharmacoepidemiology of ceftazidime-avibactam use: a retrospective cohort analysis of 210 US hospitals. Clin Infect Dis. 2021;72(4):611–621. doi: 10.1093/cid/ciaa061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isler B, Ezure Y, Romero JLG, Harris P, Stewart AG, Paterson DL. Is ceftazidime/avibactam an option for serious infections due to extended-spectrum-β-lactamase- and AmpC-producing Enterobacterales?: a systematic review and meta-analysis. Antimicrob Agents Chemother. 2020;65(1):e01052–20. doi: 10.1128/AAC.01052-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietl B, Martínez LM, Calbo E, Garau J. Update on the role of ceftazidime-avibactam in the management of carbapenemase-producing Enterobacterales. Future Microbiol. 2020;15(7):473–484. doi: 10.2217/fmb-2020-0012 [DOI] [PubMed] [Google Scholar]
- 7.Mirzaie A, Ranjbar R. Antibiotic resistance, virulence-associated genes analysis and molecular typing of Klebsiella pneumoniae strains recovered from clinical samples. AMB Express. 2021;11(1):122. doi: 10.1186/s13568-021-01282-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmadi M, Ranjbar R, Behzadi P, et al. Virulence factors, antibiotic resistance patterns, and molecular types of clinical isolates of Klebsiella pneumoniae. Expert Rev Anti Infect Ther. 2022;20(3):463–472. doi: 10.1080/14787210.2022.1990040 [DOI] [PubMed] [Google Scholar]
- 9.Wang G, Zhao G, Chao X, Xie L, Wang H. The characteristic of virulence, biofilm and antibiotic resistance of Klebsiella pneumoniae. Int J Environ Res Public Health. 2020;17(17):6278. doi: 10.3390/ijerph17176278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhen X, Stålsby Lundborg C, Sun X, Zhu N, Gu S, Dong H. Economic burden of antibiotic resistance in China: a national level estimate for inpatients. Antimicrob Resist Infect Control. 2021;10(1):5. doi: 10.1186/s13756-020-00872-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zong Z, Wu A, Hu B. Infection control in the era of antimicrobial resistance in China: progress, challenges, and opportunities. Clin Infect Dis. 2020;71(Suppl 4):S372–S378. doi: 10.1093/cid/ciaa1514 [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Wang J, Wang R, Cai Y. Resistance to ceftazidime-avibactam and underlying mechanisms. J Glob Antimicrob Resist. 2020;22:18–27. doi: 10.1016/j.jgar.2019.12.009 [DOI] [PubMed] [Google Scholar]
- 13.Karakonstantis S, Kritsotakis EI, Gikas A. Treatment options for K. pneumoniae, P. aeruginosa and A. baumannii co-resistant to carbapenems, aminoglycosides, polymyxins and tigecycline: an approach based on the mechanisms of resistance to carbapenems. Infection. 2020;48(6):835–851. doi: 10.1007/s15010-020-01520-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coppi M, Di Pilato V, Monaco F, Giani T, Conaldi PG, Rossolini GM. Ceftazidime-avibactam resistance associated with increased blaKPC-3 gene copy number mediated by pKpQIL plasmid derivatives in sequence type 258 Klebsiella pneumoniae. Antimicrob Agents Chemother. 2020;64(4):e01816–19. doi: 10.1128/AAC.01816-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Humphries RM, Ambler J, Mitchell SL, et al. CLSI methods development and standardization working group best practices for evaluation of antimicrobial susceptibility tests. J Clin Microbiol. 2018;56(4):e01934–17. doi: 10.1128/JCM.01934-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding L, Shi Q, Han R, et al. Comparison of four carbapenemase detection methods for blaKPC-2 variants. Microbiol Spectr. 2021;9(3):e0095421. doi: 10.1128/Spectrum.00954-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dallenne C, Da Costa A, Decré D, Favier C, Arlet G. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J Antimicrob Chemother. 2010;65(3):490–495. doi: 10.1093/jac/dkp498 [DOI] [PubMed] [Google Scholar]
- 18.Tan D, Zhang Y, Cheng M, et al. Characterization of Klebsiella pneumoniae ST11 isolates and their interactions with lytic phages. Viruses. 2019;11(11):1080. doi: 10.3390/v11111080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson K, Hemarajata P, Sun D, et al. Resistance to ceftazidime-avibactam is due to transposition of KPC in a porin-deficient strain of Klebsiella pneumoniae with increased efflux activity. Antimicrob Agents Chemother. 2017;61(10):e00989–17. doi: 10.1128/AAC.00989-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shields RK, Chen L, Cheng S, et al. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother. 2017;61(3):e02097–16. doi: 10.1128/AAC.02097-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li D, Li K, Dong H, et al. Ceftazidime-avibactam resistance in Klebsiella pneumoniae sequence type 11 due to a mutation in plasmid-borne blakpc-2 to blakpc-33, in Henan, China. Infect Drug Resist. 2021;14:1725–1731. doi: 10.2147/IDR.S306095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galani I, Karaiskos I, Angelidis E, et al. Emergence of ceftazidime-avibactam resistance through distinct genomic adaptations in KPC-2-producing Klebsiella pneumoniae of sequence type 39 during treatment. Eur J Clin Microbiol Infect Dis. 2021;40(1):219–224. doi: 10.1007/s10096-020-04000-9 [DOI] [PubMed] [Google Scholar]
- 23.Bianco G, Boattini M, Iannaccone M, et al. Carbapenemase detection testing in the era of ceftazidime/avibactam-resistant KPC-producing Enterobacterales: a 2-year experience. J Glob Antimicrob Resist. 2021;24:411–414. doi: 10.1016/j.jgar.2021.02.008 [DOI] [PubMed] [Google Scholar]
- 24.Niu S, Chavda KD, Wei J, et al. A ceftazidime-avibactam-resistant and carbapenem-susceptible Klebsiella pneumoniae strain harboring blaKPC-14 isolated in New York City. mSphere. 2020;5(4):e00775–20. doi: 10.1128/mSphere.00775-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winkler ML, Papp-Wallace KM, Bonomo RA. Activity of ceftazidime/avibactam against isogenic strains of Escherichia coli containing KPC and SHV β-lactamases with single amino acid substitutions in the Ω-loop. J Antimicrob Chemother. 2015;70(8):2279–2286. doi: 10.1093/jac/dkv094 [DOI] [PMC free article] [PubMed] [Google Scholar]