Abstract
Persistent COVID-19 symptoms may be related to residual inflammation, but no preventive treatment has been evaluated. This study aimed to analyze, in a prospective cohort, whether corticosteroid use in the acute phase of COVID-19 in hospitalized patients may reduce the risk of persistent COVID-19 symptoms. A total of 306 discharged patients, including 112 (36.6%) from the ICU, completed a structured face-to-face assessment 4 months after admission. Of these, 193 patients (63.1%) had at least one persistent symptom, mostly dyspnea (38.9%) and asthenia (37.6%). One-hundred and four patients have received corticosteroids. In multivariable adjusted regression analysis, corticosteroid use was not associated with the presence of at least one symptom (OR=1.00, 95% CI: 0.58–1.71, p=0.99) or with the number of persistent symptoms (p=0.74). Corticosteroid use remained ineffective when analyzing the ICU subpopulation separately. Our study suggests that corticosteroid use had no impact on persistent symptoms after COVID-19 in discharged patients.
Keywords: COVID-19, persistent symptoms, corticosteroid use, long COVID-19, asthenia
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has exposed patients to a life-threatening infectious disease. The burden of this infection is worsened by long-term persistent symptoms, which are experienced by up to 60% of patients who have been discharged from hospital.1,2 Studies have suggested that persistent symptoms could be related to residual inflammation.3–5 Corticosteroids reduced mortality in patients who required oxygen,6,7 by mitigation of the cytokine storm in the acute phase, and could prevent the prolonged inflammatory response involved in persistent symptoms following COVID-19. The impact of corticosteroid use during the acute phase on persistent symptoms remains poorly studied.8
We aimed to analyze whether corticosteroids given in the acute infection phase of COVID-19 in hospitalized patients could prevent persistent symptoms 4 months after hospital admission, after adjusting for confounders.
Materials and Method
We conducted a prospective cohort study of hospitalized adult patients with COVID-19, who were discharged from the Amiens Picardie University Hospital, France, from 2nd February 2020 to 28th December 2020. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection was confirmed by polymerase chain reaction (PCR) in nasopharyngeal swabs. We excluded patients who died before the follow-up visit, those for whom assessment would be difficult owing to psychotic disorder or dementia, and those who declined to participate or who were assessed in another center. Data on clinical characteristics in the acute phase of infection were collected from standardized and comprehensive medical records. Clinical decisions on medical management, medications, and referral to the intensive care unit (ICU) were made by the physician team based on current practice guidelines at the time of acute COVID-19 infection. Patients in the corticosteroid group received at least 6 days of dexamethasone 6 mg daily or equivalent. All patients were interviewed facetoface by trained physicians. During the follow-up visit, open questions were asked to detect symptoms experienced at 4 months, and the interview was completed by direct assessment of the most frequently reported symptoms and questionnaires to evaluate depression and anxiety. Patients also underwent clinical examination, blood analysis, and lung CT scan. The study protocol was approved by the institutional review board and CHU Amiens-Picardie ethics committee (PI2018_843_0049), and was conducted in accordance with institutional policies and the revised Declaration of Helsinki.
Multivariable adjusted logistic regression models were used to investigate associations between corticosteroid use and categorical outcomes, and multivariable Poisson regression models were used for continuous outcomes. Multiple models were constructed to account for potential confounders, including age, sex, cigarette smoking, hypertension, diabetes, cardiovascular diseases, chronic lung disease, number of symptoms at presentation, length of stay in hospital, and use of other treatment for COVID-19. All tests were two sided, and a p-value less than 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 27.0 software (IBM, Armonk, New York).
Results
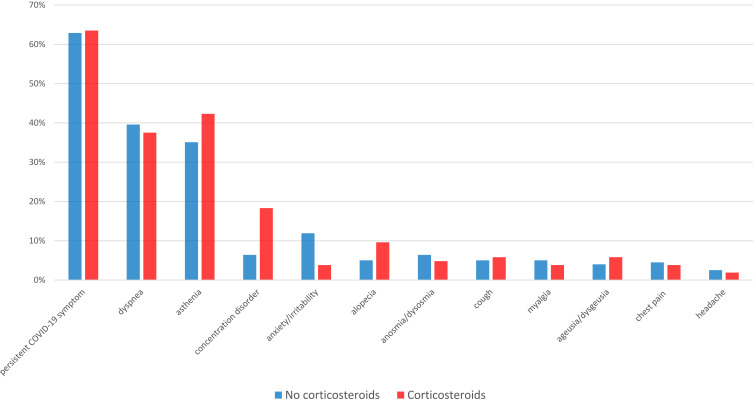
Among 586 patients discharged from hospital, structured 4-month follow-up visits were achieved in 306 patients (patients who declined or had visits conducted in other hospitals [n=148], patients with pre-existing cognitive or psychotic disorder [n=98], and patients who had died [n=34] were excluded). The demographic and clinical characteristics of participants are shown in Table 1. The median duration from hospital admission to the follow-up visit was 115 days (IQR: 103–130 days). One-hundred and four patients had received corticosteroids, with a median duration of administration of 10 days (IQR: 8–10 days). The prevalences of comorbid conditions and chronic medication were similar between the two groups. In each group, patients reported five (IQR: 3–6) symptoms at admission. Fever and anosmia were less frequent in the group with corticosteroid use. As expected, patients who received corticosteroids had more severe presentation and required supplemental oxygen and ICU admission more frequently (54.8% vs 27.2%). One-hundred and ninety-three patients (63.6%) had at least one symptom at the follow-up visit. The most frequent persistent symptoms were dyspnea (38.9%) and asthenia (37.6%) (Figure 1).
Table 1.
Baseline Characteristics of Study Population by Corticosteroid Use During the Active Phase of COVID-19
| Overall (n=306) | No Corticosteroids (n=202) | Corticosteroids (n=104) | p-Value | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age (years) | 64.4 ± 14.2 | 65.4 ± 15.1 | 62.4 ± 12.1 | 0.08 |
| >65 years | 153 (50.0) | 110 (54.5) | 43 (41.3) | 0.03 |
| Male sex | 179 (58.5) | 120 (59.4) | 59 (56.7) | 0.74 |
| BMI (kg/m2) | 30.25 ± 6.6 | 29.71 ± 6.7 | 31.29 ± 6.0 | 0.05 |
| Obesity (>30 kg/m2) | 142 (46.9) | 88 (44.2) | 54 (51.9) | 0.20 |
| Comorbidities | ||||
| Hypertension | 161 (52.6) | 111 (55.0) | 50 (48.1) | 0.25 |
| Diabetes | 84 (27.5) | 48 (23.8) | 36 (34.6) | 0.06 |
| Smoking | 117 (38.2) | 71 (35.1) | 46 (44.2) | 0.12 |
| Cardiovascular disease | 94 (30.7) | 65 (32.2) | 29 (27.9) | 0.44 |
| Chronic lung disease | 67 (21.9) | 45 (22.3) | 22 (21.2) | 0.82 |
| Chronic kidney disease | 17 (5.6) | 12 (5.9) | 5 (4.8) | 0.68 |
| Neoplasia | 11 (3.6) | 9 (4.5) | 2 (1.9) | 0.34 |
| Pregnancy | 8 (2.6) | 4 (2.0) | 4 (3.8) | 0.45 |
| Medication | ||||
| Chronic corticosteroid use | 16 (5.2) | 9 (4.5) | 7 (6.7) | 0.40 |
| Immunosuppressive medication | 19 (6.2) | 11 (5.4) | 8 (7.7) | 0.44 |
| ACE inhibitors | 67 (21.9) | 48 (23.8) | 19 (18.3) | 0.27 |
| ARBs | 39 (12.7) | 27 (13.4) | 12 (11.5) | 0.65 |
| Beta-blocker | 63 (20.6) | 45 (22.3) | 18 (17.3) | 0.31 |
| Metformin | 50 (16.3) | 27 (13.4) | 23 (22.1) | 0.07 |
| Disease characteristics | ||||
| Number of initial symptoms | 5 (3–6) | 5 (3–6) | 5 (3–6) | 0.27 |
| Asthenia | 106 (34.6) | 67 (33.2) | 39 (37.5) | 0.45 |
| Dyspnea | 186 (60.8) | 123 (60.9) | 63 (60.6) | 0.96 |
| Hypo/hyperthermia | 241 (78.8) | 170 (84.2) | 71 (68.3) | <0.01 |
| Myalgia | 154 (50.3) | 97 (48.0) | 57 (54.8) | 0.26 |
| Chest pain | 59 (19.3) | 42 (20.8) | 17 (16.3) | 0.35 |
| Cough | 215 (70.3) | 139 (68.8) | 76 (73.1) | 0.44 |
| Anosmia/dysosmia | 85 (27.8) | 65 (32.2) | 20 (19.2) | 0.02 |
| Ageusia/dysgeusia | 102 (33.3) | 72 (35.6) | 30 (28.8) | 0.23 |
| Headache | 59 (19.3) | 34 (16.8) | 25 (24.0) | 0.13 |
| Malaise/dizziness | 25 (8.2) | 20 (9.9) | 5 (4.8) | 0.18 |
| Diarrhea | 93 (34.1) | 64 (31.7) | 29 (27.9) | 0.49 |
| Clinical course | ||||
| Length of stay in hospital (days) | 10 (6–19) | 10 (6–17) | 12 (8–25) | 0.02 |
| ICU | 112 (36.6) | 55 (27.2) | 57 (54.8) | <0.001 |
| Oxygenation | ||||
| No oxygen therapy | 39 (12.7) | 37 (18.3) | 2 (1.9) | <0.001 |
| O2 <4 L | 129 (42.2) | 96 (47.5) | 33 (31.7) | 0.01 |
| O2 >4 L | 43 (14.1) | 26 (12.9) | 17 (12.9) | 0.41 |
| HFNC or NIV | 36 (11.7) | 11 (5.5) | 25 (24.0) | <0.001 |
| IMV | 51 (16.7) | 26 (12.9) | 25 (24.0) | 0.01 |
| Specific treatment (apart from corticosteroids) | ||||
| Hydroxychloroquine | 45 (14.7) | 30 (14.9) | 15 (14.4) | 0.92 |
| Remdisivir | 11 (3.6) | 4 (2) | 7 (6.7) | 0.07 |
| Lopinavir/ritonavir | 50 (16.3) | 45 (22.3) | 5 (4.8) | <0.001 |
| Infectious complications | 55 (18.0) | 31 (15.3) | 24 (23.1) | 0.10 |
| Need for antibiotic therapy | 196 (64.1) | 125 (61.9) | 71 (68.3) | 0.27 |
| Thrombotic complications | 22 (7.2) | 15 (7.4) | 7 (6.7) | 0.99 |
| Follow-up | ||||
| Time from symptom onset to re-evaluation (days) | 121 (109–139) | 122 (110–139) | 118 (109–137) | 0.02 |
| Time from hospitalization admission to follow-up (days) | 115 (103–130) | 115 (104–131) | 111 (101–130) | 0.07 |
Notes: Data are n (%), mean ± sd, or median (IQR).
Abbreviations: BMI, body mass index; ARB, angiotensin II receptor blocker; ACE, angiotensin-converting enzyme; CRP, C-reactive protein; HFNC, high-flow nasal cannula; NIV, non-invasive ventilation; IMV, invasive mechanical ventilation.
Figure 1.
Presence of symptoms at 4 months from hospital admission for COVID-19, according to administration of corticosteroids.
In univariate analysis, no significant interaction was found between corticosteroid use in the active phase of COVID-19 and persistent COVID-19 symptoms at 4 months (OR=1.03, 95% CI: 0.63–1.69, p=0.92). When additionally adjusting for differences in “classical” determinants for COVID-19 mortality and persistent symptoms (age, sex, cigarette smoking, hypertension, diabetes, cardiovascular diseases, chronic lung disease, number of symptoms at presentation, and length of stay in hospital), corticosteroid use remained ineffective in preventing persistent symptoms (OR=1.00, 95% CI: 0.58–1.71, p=0.99). The results were also similar when analyzing separately the ICU subpopulation (n=112) (OR=1.26, 95% CI: 0.51–3.11, p=0.62), patients with non-invasive ventilation (n=51) (OR=0.49, 95% CI: 0.13–1.88, p=0.30), and those receiving mechanical ventilation (n=36) (OR=2.25, 95% CI: 0.21–23.87, p=0.50). A similar result was found when taking other treatments into account (OR=1.06, 95% CI: 0.60–1.88, p=0.84). Antiviral and hydroxychloroquine treatment were also analyzed specifically, and were not found to have any effect on prolonged symptoms.
Corticosteroid use was not associated with the number of persistent COVID-19 symptoms in Poisson regression (incidence rate ratio [IRR]=1.08, 95% CI: 0.88–1.32, p=0.45). After adjustment, corticosteroid use remained ineffective (aIRR=1.04, 95% CI: 0.84–1.27, p=0.74) in the overall population and subgroups.
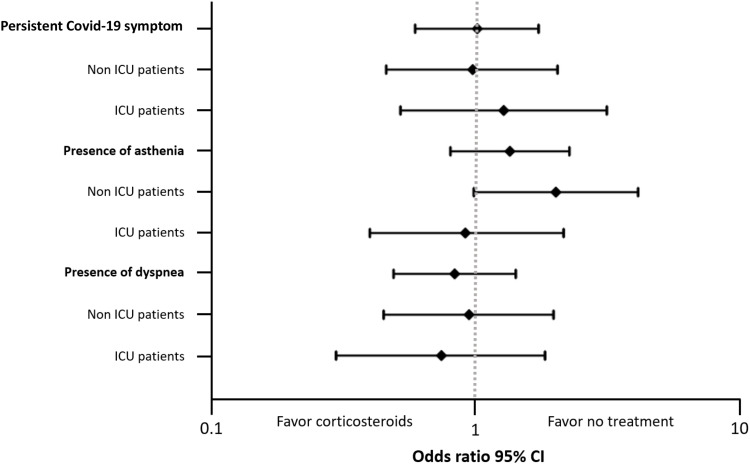
In analysis restricted to the most common persistent symptoms, no association was found for asthenia (OR=1.33, 95% CI: 0.79–2.24, p=0.28) or dyspnea (OR=0.82, 95% CI: 0.48–1.40, p=0.46) for the overall population and subgroups. All adjusted odd ratios are presented in Figure 2.
Figure 2.
Forest plot of impact of corticosteroid use in acute phase of COVID-19 on persistent symptoms 4 months after hospitalization. For associations of corticosteroids with outcome measure, the variables, including age (per 1-year increase), sex, obesity, hypertension, diabetes, cardiovascular disease, chronic lung disease, smoking, length of stay in hospital (per 1-day increase), and number of initial symptoms (per one-symptom increase), were all included in the models.
Abbreviations: ICU, intensive care unit; CI, confidence interval.
Discussion
In our large cohort of hospitalized patients with COVID-19, corticosteroid use during the acute phase of COVID-19 had no impact on persistent symptoms at 4 months after admission.
Similarly to other cohorts, more than 50% of discharged patients had persistent symptoms in our study.1,9–11 Risk factors for severe COVID-19, including older age, male sex, and pre-existing comorbidities, are well described,12 but those associated with long-term persistent symptoms remains less appreciated in hospitalized patients. A cross-sectional study identified an association between the severity of acute COVID-19 infection and persistent symptoms in people who have had COVID-19.13 Taking all these data into account, multivariable analysis was performed in our study with adjustment for age, sex, determinants of COVID-19 severity and severity of acute infection based on number of symptoms at presentation, and length of stay in hospital. In doing so, we have not documented any impact of corticosteroid use on persistent symptoms. This result is mitigated by the widely recognized benefit of dexamethasone on mortality rate in patients with oxygen requirement.6,7 The approach of physicians to COVID-19 has changed over time following the accumulation of evidence and guidelines. We also provide analysis taking into account other treatments, such as hydroxychloroquine or antiviral therapy. To account for potential selection bias caused by post-intensive care syndrome, we provided an analysis of hospitalized patients and patients needing supportive care separately, and the main finding was robust in the various sensitivity analyses that we performed.
Little is known about the pathophysiology underlying persistent symptoms of COVID-19. Patients who tested positive retained a significant inflammatory response (ie, elevated biomarkers) 2 months after the acute phase3 and inflammatory biomarkers remained elevated for patients with persistent symptoms.14 In a cohort of 58 patients, radiological abnormalities and persistent symptoms were correlated with persistent serum markers of inflammation and acute disease severity,15 suggesting that the inflammatory response may be associated with persistent symptoms. A conflicting finding was reported by Townsend et al in a cohort of 128 participants, where no association between immune cell turnover or proinflammatory molecules and post-COVID-19 fatigue was found.16
The role of persisting viral replication on long-term symptoms has been also suggested. In line with our results, a retrospective study showed that dexamethasone had no effect on concentration kinetics and antibody response in hospitalized COVID-19 patients.17 Otherwise, microvascular impairment18 and autoimmunity19 have also been discussed as being implicated in persistent symptoms.
To our knowledge, the influence of treatment with systemic corticosteroids during the acute phase of the illness has been documented in only one observational study, including 72 patients.8 Persistent symptoms, assessed by a telephone survey, were less frequent in the group that received corticosteroids. Unfortunately, owing to the small cohort in that study, adjustment taking into account COVID-19 severity was not performed, and the larger proportion of men (75.0% vs 52.3%, p=0.004) and people with hypertension (71.9% vs 40.9%, p=0.007) in the steroid group may have had an impact on the results.
Strengths of our study are the completeness of data, the structured face-to-face assessment intervention including chest radiographs, and the detailed analysis focused on corticosteroid use and persistent symptoms. However, our study had several limitations. First, this study was monocentric, conducted in a tertiary center. Second, since reliable analysis of persistent symptoms could not be performed in elderly patients with dementia or those who had died, our analysis may not represent all discharged patients. Also, some patients who were discharged were followed up in other public hospitals or private practices by the referring physician and were not included in our study. Third, some residual symptoms such as neuropsychological symptoms did not occur frequently enough to enable specific analysis to be performed. Finally, despite statistical adjustment on clinical factors of severity, residual confounders may still exist between groups.
Our study suggested that corticosteroid use had no impact on persistent symptoms in hospitalized patients. Long COVID-19 is composed of heterogeneous symptoms that often affect multiple organ systems, probably implying different mechanisms. Further studies are needed to better understand the pathophysiology of long-term persistent symptoms.
Funding Statement
The authors did not receive support from any organization for the submitted work.
Data Transparency
The dataset generated and analyzed during the current study is available from the corresponding author on reasonable request.
Ethics Approval
The study protocol was approved by institutional review board and CHU Amiens-Picardie ethics committee (PI2018_843_0049). This study complies with the Declaration of Helsinki.
Consent to Participate
Written information on the study was given to each patient. In case of opposition, the patient was not included (according to the French law for this type of study; Article 1121-1 of the Public Health Code).
Disclosure
Drs Chan Sui Ko and Candellier are joint first authors. Dr Cedric Joseph reports congress participation support from MSD, outside the submitted work. The authors declare that they have no other conflicts of interests to disclose.
References
- 1.Ghosn J, Piroth L, Epaulard O, et al. Persistent COVID-19 symptoms are highly prevalent 6 months after hospitalization: results from a large prospective cohort. Clin Microbiol Infect. 2021;27(7):1041.e1–1041.e4. doi: 10.1016/j.cmi.2021.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blomberg B, Mohn KG, Brokstad KA, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021;27(9):1607–1613. doi: 10.1038/s41591-021-01433-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doykov I, Hällqvist J, Gilmour KC, Grandjean L, Mills K, Heywood WE. ‘The long tail of Covid-19’ - The detection of a prolonged inflammatory response after a SARS-CoV-2 infection in asymptomatic and mildly affected patients. F1000Res. 2020;9:1349. doi: 10.12688/f1000research.27287.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergamaschi L, Mescia F, Turner L, et al. Longitudinal analysis reveals that delayed bystander CD8+ T cell activation and early immune pathology distinguish severe COVID-19 from mild disease. Immunity. 2021;54(6):1257–1275.e8. doi: 10.1016/j.immuni.2021.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myall KJ, Mukherjee B, Castanheira AM, et al. Persistent post-COVID-19 interstitial lung disease. an observational study of corticosteroid treatment. Ann Am Thorac Soc. 2021;18(5):799–806. doi: 10.1513/AnnalsATS.202008-1002OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horby P, Lim WS, Emberson JR, et al.; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran VT, Mahévas M, Bani-Sadr F, Robineau O, Perpoint T, Perrodeau E; COCORICO Collaborative Group. Corticosteroids in patients hospitalized for COVID-19 pneumonia who require oxygen: observational comparative study using routine care data. Clin Microbiol Infect. 2021;27(4):603–610. doi: 10.1016/j.cmi.2020.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catalán IP, Martí CR, Sota DP, et al. Corticosteroids for COVID-19 symptoms and quality of life at 1 year from admission. J Med Virol. 2021;94(1):205–210. doi: 10.1002/jmv.27296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreno-Pérez O, Merino E, Leon-Ramirez JM, et al.; COVID19-ALC research group. Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect. 2021;82(3):378–383. doi: 10.1016/j.jinf.2021.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crook H, Raza S, Nowell J, Young M, Edison P. Long covid-mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648 [DOI] [PubMed] [Google Scholar]
- 13.Kamal M, Abo Omirah M, Hussein A, Saeed H. Assessment and characterisation of post-COVID-19 manifestations. Int J Clin Pract. 2021;75(3):e13746. doi: 10.1111/ijcp.13746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raman B, Cassar MP, Tunnicliffe EM, et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021;31:100683. doi: 10.1016/j.eclinm.2020.100683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandal S, Barnett J, Brill SE, et al.; ARC Study Group. ‘Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2021;76(4):396–398. doi: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15(11):e0240784. doi: 10.1371/journal.pone.0240784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mühlemann B, Thibeault C, Hillus D, et al. Impact of dexamethasone on SARS-CoV-2 concentration kinetics and antibody response in hospitalized COVID-19 patients: results from a prospective observational study. Clin Microbiol Infect. 2021;27(10):1520.e7–1520.e10. doi: 10.1016/j.cmi.2021.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kommoss FKF, Schwab C, Tavernar L, et al. The pathology of severe COVID-19-related lung damage. Dtsch Arztebl Int. 2020;117(29–30):500–506. doi: 10.3238/arztebl.2020.0500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seeßle J, Waterboer T, Hippchen T, et al. Persistent symptoms in adult patients one year after COVID-19: a prospective cohort study. Clin Infect Dis. 2021:ciab611. doi: 10.1093/cid/ciab611. [DOI] [PMC free article] [PubMed] [Google Scholar]