Abstract
Background
Aerosols and spatter are generated in a dental clinic during aerosol‐generating procedures (AGPs) that use high‐speed hand pieces. Dental healthcare providers can be at increased risk of transmission of diseases such as tuberculosis, measles and severe acute respiratory syndrome (SARS) through droplets on mucosae, inhalation of aerosols or through fomites on mucosae, which harbour micro‐organisms. There are ways to mitigate and contain spatter and aerosols that may, in turn, reduce any risk of disease transmission. In addition to personal protective equipment (PPE) and aerosol‐reducing devices such as high‐volume suction, it has been hypothesised that the use of mouth rinse by patients before dental procedures could reduce the microbial load of aerosols that are generated during dental AGPs.
Objectives
To assess the effects of preprocedural mouth rinses used in dental clinics to minimise incidence of infection in dental healthcare providers and reduce or neutralise contamination in aerosols.
Search methods
We used standard, extensive Cochrane search methods. The latest search date was 4 February 2022.
Selection criteria
We included randomised controlled trials and excluded laboratory‐based studies. Study participants were dental patients undergoing AGPs. Studies compared any preprocedural mouth rinse used to reduce contaminated aerosols versus placebo, no mouth rinse or another mouth rinse. Our primary outcome was incidence of infection of dental healthcare providers and secondary outcomes were reduction in the level of contamination of the dental operatory environment, cost, change in mouth microbiota, adverse events, and acceptability and feasibility of the intervention.
Data collection and analysis
Two review authors screened search results, extracted data from included studies, assessed the risk of bias in the studies and judged the certainty of the available evidence. We used mean differences (MDs) and 95% confidence intervals (CIs) as the effect estimate for continuous outcomes, and random‐effects meta‐analysis to combine data
Main results
We included 17 studies with 830 participants aged 18 to 70 years. We judged three trials at high risk of bias, two at low risk and 12 at unclear risk of bias.
None of the studies measured our primary outcome of the incidence of infection in dental healthcare providers.
The primary outcome in the studies was reduction in the level of bacterial contamination measured in colony‐forming units (CFUs) at distances of less than 2 m (intended to capture larger droplets) and 2 m or more (to capture droplet nuclei from aerosols arising from the participant's oral cavity). It is unclear what size of CFU reduction represents a clinically significant amount.
There is low‐ to very low‐certainty evidence that chlorhexidine (CHX) may reduce bacterial contamination, as measured by CFUs, compared with no rinsing or rinsing with water. There were similar results when comparing cetylpyridinium chloride (CPC) with no rinsing and when comparing CPC, essential oils/herbal mouthwashes or boric acid with water. There is very low‐certainty evidence that tempered mouth rinses may provide a greater reduction in CFUs than cold mouth rinses. There is low‐certainty evidence that CHX may reduce CFUs more than essential oils/herbal mouthwashes. The evidence for other head‐to‐head comparisons was limited and inconsistent.
The studies did not provide any information on costs, change in micro‐organisms in the patient's mouth or adverse events such as temporary discolouration, altered taste, allergic reaction or hypersensitivity. The studies did not assess acceptability of the intervention to patients or feasibility of implementation for dentists.
Authors' conclusions
None of the included studies measured the incidence of infection among dental healthcare providers. The studies measured only reduction in level of bacterial contamination in aerosols. None of the studies evaluated viral or fungal contamination. We have only low to very low certainty for all findings. We are unable to draw conclusions regarding whether there is a role for preprocedural mouth rinses in reducing infection risk or the possible superiority of one preprocedural rinse over another. Studies are needed that measure the effect of rinses on infectious disease risk among dental healthcare providers and on contaminated aerosols at larger distances with standardised outcome measurement.
Keywords: Humans; Chlorhexidine; Chlorhexidine/therapeutic use; Communicable Diseases; Communicable Diseases/drug therapy; Health Personnel; Mouthwashes; Mouthwashes/therapeutic use; Oils, Volatile; Respiratory Aerosols and Droplets; Severe Acute Respiratory Syndrome; Water
Plain language summary
Does use of mouth rinse before a dental procedure reduce the risk of infection transmission from patient to health professional?
Why is this question important?
Many dental procedures generate droplets that settle on a surface quickly. If high‐speed instruments, such as a drill, are used, aerosols are generated, which consist of tiny particles that remain suspended in the air and that can be inhaled or that settle farther away on surfaces. These aerosols contain a variety of micro‐organisms and may transmit infections either through direct contact or indirectly through the contaminated surfaces. To prevent the spread of infection, it may help to reduce the number of micro‐organisms that are present in these aerosols. The use of mouth rinses before a dental procedure ('preprocedural mouth rinse') has been suggested as a possible way to reduce the amount of contamination of these aerosols. Chlorhexidine, povidone iodine and cetylpyridinium chloride (CPC) are some of the commonly used mouth rinses. They act by killing or inactivating the micro‐organisms in the mouth and thereby reducing the level of contamination in the aerosol that is generated. We wanted to find out whether rinsing the mouth before a dental procedure reduces the contamination of aerosols produced during dental procedures in practice and helps prevent the transmission of infectious diseases.
How did we identify and evaluate the evidence?
We searched for all relevant studies that compared mouth rinses used before dental procedures against placebo (fake treatment), no intervention or another mouth rinse considered to be inactive. We then compared the results, and summarised the evidence from all the studies. Finally, we assessed our confidence in the evidence. To do this, we considered factors such as the way studies were conducted, study sizes and consistency of findings across studies.
What did we find?
We found 17 studies that met our inclusion criteria. These studies used chlorhexidine, CPC, essential oil/herbal mouth rinses, povidone iodine and boric acid in comparison to no rinsing, or rinsing with water, saline (salt water) or another mouth rinse. None of the studies measured the how often dental healthcare providers became infected with micro‐organisms. All the included studies measured the level of bacterial contamination in droplets or aerosols in the dental clinic. They did not examine contamination with viruses or fungi.
Most rinses decreased bacterial contamination in aerosols to some extent, but there was considerable variation in the effects and we do not know what size of reduction is necessary to reduce infection risk.
The studies did not provide any information on costs, change in micro‐organisms in the patient's mouth or side effects such as temporary discolouration, altered taste, allergic reaction or hypersensitivity. The studies did not assess whether patients were happy to use a mouth rinse or whether it was easy for dentists to implement.
Overall, the results suggest that using a preprocedural mouth rinse may reduce the level of bacterial contamination in aerosols compared with no rinsing or rinsing with water, but we have only low or very low certainty that the evidence is reliable and we do not know how this reduction in contamination relates to the risk of infection.
What does this mean?
We have very little confidence in the evidence, and further studies may change the findings of our review. No studies measured infection risk or investigated viral or fungal contamination.
How up‐to‐date is this review?
The evidence in this Cochrane Review is current to February 2022.
Summary of findings
Summary of findings 1. Chlorhexidine compared to no rinsing for preventing risk of infection in dental healthcare providers and contamination of aerosols in dental clinic.
| Chlorhexidine compared with no rinsing for preventing risk of infection in dental healthcare providers and contamination of aerosols in dental clinic | ||||||
|
Population: dental patients undergoing aerosol‐generating procedures and dental healthcare professionals treating them Settings: university hospital Intervention: CHX Comparison: no rinsing | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no rinsing | Risk with CHX | |||||
| Incidence of infection of dental healthcare providers | Not reported | |||||
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – operator level | High heterogeneity in the meta‐analysis (I² = 89%; 2 studies) | |||||
| Reduction in level of contamination by particles ≤ 5 µm (droplet nuclei) at distances of ≥ 2 m from patient's oral cavity in the operative environment | Not reported | |||||
| Adverse events such as transient discolouration, altered taste, allergic reaction, hypersensitivity | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony‐forming unit; CHX: chlorhexidine; CI: confidence interval. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect. | ||||||
Summary of findings 2. Chlorhexidine compared to water for preventing risk of infection in dental healthcare providers and contamination of aerosols in dental clinic.
| Chlorhexidine compared to water for preventing transmission of infectious diseases through aerosols in dental healthcare providers | ||||||
|
Population: dental patients undergoing aerosol‐generating procedures and dental healthcare professionals treating them Setting: university hospital Intervention: CHX Comparison: water (distilled) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with distilled water | Risk with CHX | |||||
| Incidence of infection of dental healthcare providers | Not reported | |||||
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – combined patient and operator level | The range of mean reduction in level of contamination was 114.7 to 1963 CFUs | MD 632.94 CFUs lower (1267.33 lower to 1.45 higher) | — | 80 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | Since heterogeneity was high (I² = 92%), we repeated the sensitivity analysis by removing Reddy 2012, which used 0.2% CHX (the other 2 studies used 0.12% CHX). The result was MD 956.11 CFUs lower (95% CI 1626.04 lower to 286.19 lower). |
| Reduction in the level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – combined operator, assistant and patient level | The mean reduction in the level of contamination was 114.7 CFUs | MD 101.1 CFUs lower (107.01 lower to 95.19 lower) | — | 20 (1 RCT) | ⊕⊕⊝⊝ Lowb,c | Study compared tempered CHX vs distilled water. |
| Reduction in level of contamination by particles ≤ 5 µm (droplet nuclei) at distances of ≥ 2 m from patient's oral cavity in the operative environment | Not reported | |||||
| Adverse events such as transient discolouration, altered taste, allergic reaction, hypersensitivity | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony‐forming unit; CHX: chlorhexidine; CI: confidence interval; MD: mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect. | ||||||
aDowngraded two levels due to inconsistency. bDowngraded one level due to imprecision. cDowngraded one level due to risk of bias.
Summary of findings 3. Chlorhexidine compared to saline for preventing risk of infection in dental healthcare providers and contamination of aerosols in dental clinic.
| Chlorhexidine compared to saline for preventing transmission of infectious diseases through aerosols in dental healthcare providers | ||||||
|
Population: dental patients undergoing aerosol‐generating procedures and dental care professionals treating them Setting: university hospital Intervention: CHX Comparison: saline | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with saline | Risk with CHX | |||||
| Incidence of infection of dental healthcare providers | Not reported | |||||
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – operator level | The mean reduction in the level of contamination was 46.5 CFUs | MD 21.33 CFUs lower (36.80 lower to 5.86 lower) | — | 12 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
| Reduction in the level of contamination by particles ≤ 5 µm (droplet nuclei) at distances of ≥ 2 m from patient's oral cavity in the operative environment | Not reported | |||||
| Adverse events such as transient discolouration, altered taste, allergic reaction, hypersensitivity | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony‐forming unit; CHX: chlorhexidine; CI: confidence interval; MD: mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect. | ||||||
aDowngraded two levels due to serious risk of bias. bDowngraded two levels due to serious imprecision.
Summary of findings 4. Chlorhexidine compared to essential oils/herbal mouthwashes for preventing risk of infection in dental healthcare providers and contamination of aerosols in dental clinic.
| Chlorhexidine compared to essential oils/herbal mouthwashes for preventing transmission of infectious diseases through aerosols in dental healthcare providers | ||||||
|
Population: dental patients undergoing aerosol‐generating procedures and dental care professionals treating them Setting: university hospital Intervention: CHX Comparison: essential oils/herbal mouthwash | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with essential oils/herbal mouthwash | Risk with CHX | |||||
| Incidence of infection of dental healthcare providers | Not reported | |||||
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – operator level | The mean reduction in level of contamination ranged from 24.68 to 56.2 CFUs |
MD 23.09 CFUs lower (34.4 lower to 11.78 lower) |
— | 76 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b | The studies compared 0.2% CHX to tea tree essential oil, herbal mouthwash A and herbal mouthwash B (see Table 5). |
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – assistant level | The mean reduction in the level of contamination ranged from 19.25 to 26 CFUs | MD 12.21 CFUs lower (15.58 lower to 8.83 lower) | — | 76 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b | The studies compared 0.2% CHX to tea tree essential oil, herbal mouthwash A and herbal mouthwash B (see Table 5). |
| Reduction in level of contamination by particles ≤ 5 µm (droplet nuclei) at distances of ≥ 2 m from patient's oral cavity in the operative environment | Not reported | |||||
| Adverse events such as transient discolouration, altered taste, allergic reaction, hypersensitivity | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony‐forming unit; CHX: chlorhexidine; CI: confidence interval; MD: mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect. | ||||||
aDowngraded one level due to risk of bias. bDowngraded one level due to imprecision.
1. Details of essential oil and herbal mouth rinses.
| Study | Intervention name in study | Composition |
| Bay 1993 | Essential oil mouthwash C | Listerine (active ingredients: menthol (mint) 0.042%, thymol (thyme) 0.064%, methyl salicylate (wintergreen) 0.06%, and eucalyptol (eucalyptus) 0.092%) |
| Gupta 2014 | Herbal mouthwash B | Natural herb bibhitaki (terminilia bellirica) 10 mg, Nagavalli (Piper betle) 10 mg, peelu (Salvadora persica) 5 mg, powders peppermint satva (mentha spp) 1.6 mg, yavani satva (caraway) 0.4 mg, oils gandhapura taila (wintergreen) 1.2 mg and cardamom 0.2 mg |
| Nayak 2020 | Herbal mouthwash A | Befresh, which contains cinnamomum zeylanicum, mentha spicata, syzygium aromaticum, and eucalyptusglobulus |
| Shetty 2013 | Essential oil mouthwash A | Tea tree oil |
| Suresh 2011 | Essential oil mouthwash B | Eucalyptol + thymol + methyl salicylate + menthol |
Summary of findings 5. Chlorhexidine compared to povidone iodine for preventing risk of infection in dental healthcare providers and contamination of aerosols in dental clinic.
| Chlorhexidine compared to povidone iodine for reduction in the level of contamination in aerosols | ||||||
|
Population: dental patients undergoing aerosol‐generating procedures and dental care professionals treating them Setting: university hospital Intervention: CHX Comparison: povidone iodine | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with povidone iodine | Risk with CHX | |||||
| Incidence of infection of dental healthcare providers | Not reported | |||||
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – operator level (aerobic cultures) | The range of mean reduction in level of contamination was 30 to 52 CFUs | MD 10.75 CFUs lower (26.24 lower to 4.74 higher) | — | 52 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | — |
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – operator level (aerobic and anaerobic cultures combined) | The mean reduction in level of contamination was 35.4 CFUs | MD 4.7 CFUs lower (7.01 lower to 2.39 lower) | — | 30 (1 RCT) | ⊕⊝⊝⊝ Very lowb,c | — |
| Reduction in level of contamination by particles ≤ 5 µm (droplet nuclei) at distances of ≥ 2 m from patient's oral cavity in the operative environment – aerobic cultures | The mean reduction in level of contamination was 18.5 CFUs | MD 0.20 CFUs higher (7.28 lower to 7.68 higher) | — | 40 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
| Reduction in level of contamination by particles ≤ 5 µm (droplet nuclei) at distances of ≥ 2 m from patient's oral cavity in the operative environment – anaerobic cultures | The mean reduction in level of contamination was 19.5 CFUs | MD 0.8 CFUs lower (11.65 lower to 10.05 higher) | — | 40 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
| Adverse events such as transient discolouration, altered taste, allergic reaction, hypersensitivity | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony‐forming unit; CHX: chlorhexidine; CI: confidence interval; MD: mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect. | ||||||
aDowngraded two levels due to risk of bias. bDowngraded two levels due to imprecision. c Downgraded one level due to risk of bias.
Summary of findings 6. Chlorhexidine compared to cetylpyridinium chloride (CPC) for preventing risk of infection in dental healthcare providers and contamination of aerosols in dental clinic.
| Chlorhexidine compared to cetylpyridinium chloride (CPC) for preventing transmission of infectious diseases through aerosols in dental healthcare providers | ||||||
|
Population: dental patients undergoing aerosol‐generating procedures and dental care professionals treating them Setting: university hospital Intervention: CHX Comparison: CPC | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with CPC | Risk with CHX | |||||
| Incidence of infection of dental healthcare providers | Not reported | |||||
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – operator | The mean reduction in level of contamination was 62.2 CFUs | MD 2.20 CFUs lower (9.07 lower to 4.67 higher) | — | 20 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | Tempered CHX vs tempered CPC |
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – assistant | The mean reduction in level of contamination was 38.7 CFUs | MD 0.20 CFUs lower (6.30 lower to 5.90 higher) | — | 20 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | Tempered CHX vs tempered CPC |
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – operator | The range of mean reduction in level of contamination was 6.9 to 71.5 CFUs | MD 4.43 CFUs higher (2.27 lower to 11.13 higher) | — | 50 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | Cold CHX vs cold CPC |
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – assistant | The mean reduction in level of contamination was 47.3 CFUs | MD 8.80 CFUs higher (2.70 higher to 14.90 higher) | — | 20 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | Cold CHX vs cold CPC |
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – operator | The mean reduction in level of contamination was 71.5 CFUs | MD 11.50 CFUs lower (18.37 lower to 4.63 lower) | — | 20 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | Tempered CHX vs cold CPC |
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – assistant | The mean reduction in level of contamination was 47.3 CFUs | MD 8.80 CFUs lower (14.90 lower to 2.70 lower) | — | 20 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | Tempered CHX vs cold CPC |
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – operator | The mean reduction in level of contamination was 62.2 CFUs | MD 13.60 CFUs higher (6.73 higher to 20.47 higher) | — | 20 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | Cold CHX vs tempered CPC |
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – assistant | The mean reduction in level of contamination was 38.7 CFUs | MD 17.40 CFUs higher (11.30 higher to 23.50 higher) | — | 20 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | Cold CHX vs tempered CPC |
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – operator level | The mean reduction in level of contamination was 61 CFUs | MD 30 CFUs lower (85.86 lower to 25.86 higher) | — | 30 (1 RCT) | ⊕⊝⊝⊝ Very lowb,c | CHX vs CPC+Zn+F (CPC plus zinc and sodium fluoride) |
| Reduction in level of contamination by particles ≤ 5 µm (droplet nuclei) at distances of ≥ 2 m from patient's oral cavity in the operative environment | Not reported | |||||
| Adverse events such as transient discolouration, altered taste, allergic reaction, hypersensitivity | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony‐forming unit; CHX: chlorhexidine; CI: confidence interval; CPC: cetylpyridinium chloride; MD: mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect. | ||||||
aDowngraded one level due to risk of bias. bDowngraded two levels due to imprecision. cDowngraded one level due to inconsistency,
Summary of findings 7. Tempered chlorhexidine compared to non‐tempered chlorhexidine for preventing risk of infection in dental healthcare providers and contamination of aerosols in dental clinic.
| Tempered chlorhexidine compared to non‐tempered chlorhexidine for preventing transmission of infectious diseases through aerosols in dental healthcare providers | ||||||
|
Population: dental patients undergoing aerosol‐generating procedures and dental care professionals treating them Setting: university hospital Intervention: tempered CHX Comparison: non‐tempered CHX | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with non‐tempered CHX | Risk with tempered CHX | |||||
| Incidence of infection of dental healthcare providers | Not reported | |||||
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – operator level | The mean reduction in level of contamination was 75.8 CFUs | MD 15.8 CFUs lower (22.67 lower to 8.93 lower) | — | 20 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – assistant level | The mean reduction in level of contamination was 56.1 CFUs | MD 17.60 CFUs lower (23.70 lower to 11.50 lower) | — | 20 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – combined | The mean reduction in level of contamination was 24.4 CFUs | MD 10.80 CFUs lower (15.15 lower to 6.45 lower) | — | 20 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
| Reduction in the level of contamination by particles ≤ 5 µm (droplet nuclei) at distances of ≥ 2 m from patient's oral cavity in the operative environment | Not reported | |||||
| Adverse events such as transient discolouration, altered taste, allergic reaction, hypersensitivity | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony‐forming unit; CI: confidence interval; MD: mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect. | ||||||
aDowngraded one level due to risk of bias. bDowngraded two levels due to imprecision.
Background
The use of high‐speed hand pieces, ultrasonic scalers and similar devices in dental clinics contributes to the generation of contaminated aerosols and spatter (Meng 2020), which have the potential to harbour harmful viral, bacterial and fungal organisms, and can be a source of infection (Mosaddad 2019). Over 700 microbial species have been detected in saliva, including the recent coronavirus‐19 (COVID‐19) virus; though they become diluted in the water during aerosol‐generating procedures (AGPs), they may pose a risk of infection in dental healthcare providers (Li 2020; To 2020). Such contaminated aerosols and spatter generated during dental procedures can also remain suspended in the air or settle on surfaces in a dental clinic (Laheij 2012), through which the transmission of diseases such as tuberculosis, measles and severe acute respiratory syndrome (SARS) can occur directly through inhalation of these droplets or aerosols, or indirectly from contact with contaminated surfaces (Harrel 2004). The droplets generated during coughing, sneezing and speaking are usually larger than 5 μm in diameter and settle in the near vicinity (less than 1 m), whereas bioaerosols produced during AGPs remain afloat for a considerable amount of time and spread virulent pathogens over a distance of 1.8 m, causing potential risk to dental healthcare providers and patients (Jones 2015).
In March 2020, the highly pathogenic and transmissible COVID‐19 (severe acute respiratory syndrome coronavirus (SARS‐CoV‐2)) virus emerged and spread rapidly around the world. Due to the unknown modes of transmission and uncertainty in the management of the viral infection, most dental practices were suspended across the globe (Fallahi 2020; Hughes 2020). Clinical studies with a small sample size have evaluated body fluids of people with COVID‐19 and reported remarkably high levels of SARS‐CoV‐2 in saliva and the oropharynx (Jeong 2020; Yoon 2020), pointing towards a potential source of infective aerosols during dental treatment of SARS‐CoV‐2‐positive people.
One study on the impact of adhering to infection control guidelines by 2195 dentists in the USA showed that the existing infection control recommendations may be sufficient to prevent COVID‐19 infection in dental settings (Estrich 2020). This could be attributed to the adherence of dentists to currently recommended Centers for Disease Control and Prevention (CDC) infection prevention and control guidelines such as disinfection (99.1%), using personal protective equipment (PPE) (85.2%), COVID‐19 screening (98.5%), use of masks by staff members (99.1%), social distancing (98.9%), preprocedural mouth rinse (12%) and use of extraoral suction (4%). It can be assumed that any intervention to reduce the ambient microbial load during AGPs would further mitigate the risk of infection transmission.
In view of this, organisations such as the UK's Faculty of General Dental Practice (FGDP) and the CDC published strict guidelines for safe and effective practice including preprocedural mouth rinsing (CDC 2020; FGDP 2020). Yoon 2020 reported a short‐term reduction of SARS‐CoV‐2 viral load in saliva following chlorhexidine (CHX) mouth rinse. One randomised control trial in 2021 involving 16 SARS‐CoV‐2‐positive patients showed sustained reduction in viral loads with the use of two commercially available mouth rinses (Seneviratne 2021).
In vitro studies have provided some evidence on commercially available oral rinses reducing the viral load including that of SARS‐CoV‐2 (Bidra 2020; Meister 2020), and other enveloped viruses such as SARS‐CoV, MERS‐CoV, influenza A, parainfluenza, cytomegalovirus, hepatitis B, herpes simplex virus type‐1, herpes simplex virus type‐2, human immunodeficiency virus‐1 and human papilloma virus (HPV) (Reis 2021). Effect of mouth rinses on viral load may not always correlate with their effect on the infectivity (replicating virus) of the viral culture (Gottsauner 2020). This advocates for a systematic evaluation of available clinical trials utilising these oral rinse formulations in prevention of infection transmission through contaminated aerosols.
Interventions that could possibly reduce the volume of aerosols generated during dental procedures, or their level of contamination, are evaluated in one Cochrane rapid review (Nagraj 2020). Our planned Cochrane Review will complement Nagraj 2020 by exploring the available evidence on the effectiveness and safety of preprocedural mouth rinses for preventing transmission of infectious diseases in a dental clinic.
Description of the condition
Dental AGPs are used during endodontic treatment, periodontal and restorative procedures involving the use of high‐speed hand pieces, ultrasonic scalers and high‐pressure air syringes, which are responsible for most aerosols generated in a dental clinic (Barnes 1998; Eliades 2020; Gross 1992; Harrel 2004; Veena 2015). The Scottish Dental Clinical Effectiveness Programme (SDCEP) have categorised AGPs as those procedures that will produce aerosol particles such as ultrasonic scaling, using high‐speed air rotor and air polishing (Group A), that may produce aerosol particles such as slow‐speed/electric hand piece, prophylaxis with pumice, 3‐in‐1 syringe (either air or water) and diathermy (Group B) and that may produce spatter but are unlikely to produce aerosol particles such as dental examination without the use of 3‐in‐1 syringe, dental extraction, impression and intraoral radiography (Group C) (SDCEP 2021). However, there is no global consensus on the list of dental procedures that can be considered AGPs (Jackson 2020).
Some dental procedures can indirectly generate aerosols by agitating the airway (e.g. intraoral radiography or maxillary impressions). These procedures may induce the patient to cough forcibly, thereby releasing aerosols filled with microbes (Mair 2020). However, AGPs have been more commonly attributed to cross‐infection in dentistry (Chanpong 2020; Harrel 2004). While performing AGPs, the quantifiable risk of disease transmission is based on patient factors (i.e. whether they are infected with, or the carrier of, any disease), virulence of the pathogenic micro‐organism, type of procedure, duration of procedure, ability to employ mitigation factors such as aerosol‐reducing interventions and the probability of success of the intervention (Koletsi 2020). Bacteria can multiply rapidly by replicating their DNA, unlike most viruses that need entry into a living cell (such as a human cell) to be able to reproduce (Munita 2016). This is of significance in a compromised host that has increased susceptibility to allow micro‐organisms to colonise, replicate and survive.
Considering that dental treatment involves close contact between the patient and the treating dentist, any type of aerosol that is generated can lead to cross‐infection, and minimising the number of contaminated aerosols generated is key to infection control (Eliades 2020; Zemouri 2017). The aerosols generated can be differentiated into spatter, droplet and droplet nuclei, based on the particle size (Harrel 2004; Mair 2020). 'Spatter' are the larger droplets that are greater than 50 µm in diameter and are airborne for only a brief period of time (Wells 1934). The smaller 'droplets' remain suspended in the air for varying amounts of time, after which the water content evaporates, leaving the solid particles called 'droplet nuclei' (5 µm or less) to freely float in the air (Harrel 2004; James 2016; Mair 2020). The smaller droplets and droplet nuclei are responsible for airborne transmission of infectious diseases (James 2016) (Figure 1). In addition to pathogenic and non‐pathogenic bacteria, viruses and other micro‐organisms, bioaerosols also contain components of plaque, nasopharyngeal and oral secretions, and traces of materials used in dental procedures (Eliades 2020; Zemouri 2017).
1.
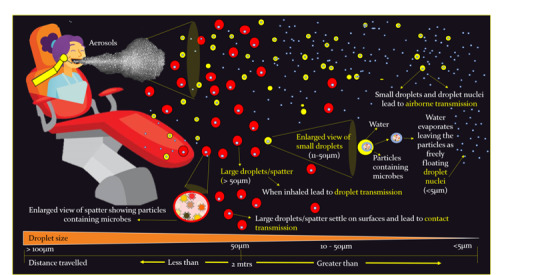
Aerosols and spatter generated in the dental clinic. mtrs: metres.
Description of the intervention
The chain of spread of infection consists of a reservoir, portal of exit from the reservoir (such as aerosols), an infectious agent, a susceptible host and a portal of entry into the host through a nasal or oral cavity (Mupparapu 2019). 'Infective dose' is of relevance when it comes to transmission of infectious diseases, and any technique that mitigates this dose would be beneficial in dental procedures (Li 2004). Therefore, it is reasonable to assume that any strategy used for reducing the viable microbial content of aerosols could lower the risk of cross‐contamination in the dental setting (Fine 1992). Preprocedural mouth rinses would supplement this endeavour, along with PPE, rubber dam isolation, high‐volume suction, high‐efficiency particulate air (HEPA) filters, UV lights, and ozonisation. The layering of infection control steps, as described by Harrel 2004, reduces risk with ultimate aim of breaking the transmission chain, preventing cross‐infection, and ensuring safe and effective dental practice. Most of the tested oral rinses have shown broad‐spectrum antibacterial, antimicrobial and virucidal properties (Corner 1988; Meister 2020). A detailed infographic is presented in Figure 2 (Nagraj 2020).
2.
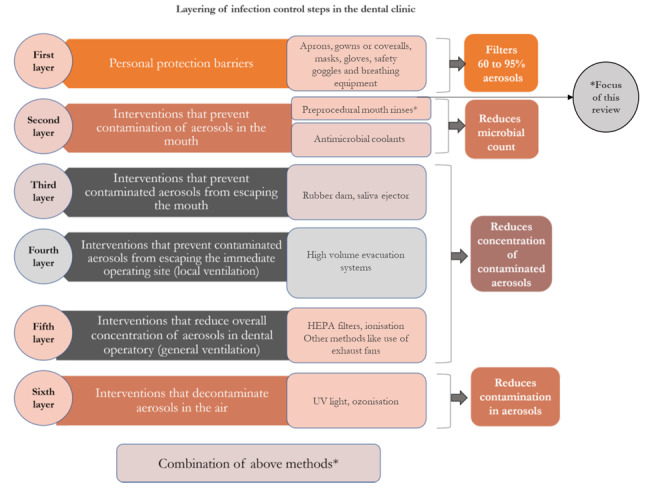
Layering of infection control steps in the dental clinic. HEPA: high‐efficiency particulate air; UV: ultraviolet.
Layering of infection control steps
Layer 1: personal protective barriers
PPE includes masks or respirators, gowns or coveralls, protective eye wear and face shields that reduce and protect from exposure to aerosols. A Cochrane Review evaluated the use of PPE for infection control (Verbeek 2020). Hence this intervention is not included in this review.
Layer 2: preprocedural rinses and antimicrobial coolants
Layer 2 involves interventions such as preprocedural use of mouth rinses and antimicrobial coolants that prevent contamination of aerosols in the mouth. Antimicrobial agents such as CHX and povidone iodine are used as coolants along with ultrasonic scalers. The effectiveness of antimicrobial coolants is presented in a Cochrane Review (Nagraj 2020). The use of preprocedural mouth rinses is included in this review and is described in the next section of this review.
Layers 3, 4, 5 and 6
Layers 3, 4, 5 and 6 are interventions that prevent contaminated aerosols from escaping the mouth (e.g. rubber dam and saliva ejector), prevent contaminated aerosols from escaping the immediate operatory (e.g. high‐volume evacuation), reduce overall concentration of aerosol in the operatory using HEPA filters, and that decontaminate aerosols in air (such as UV light and ozonisation), respectively. The effectiveness of these interventions to reduce contaminated aerosols in a dental operatory is evaluated in another Cochrane Review (Nagraj 2020).
How the intervention might work
Chlorhexidine
CHX, chemically a bisbiguanide, is considered as a gold standard disinfectant in dentistry (Mandel 1994). It is a superior antiplaque agent with broad antimicrobial spectrum that includes gram‐positive and gram‐negative organisms, certain bacterial spores, lipophilic viruses, yeasts and dermatophytes (Jones 1997; Mandel 1994). This mouth rinse is commercially available in concentrations of 0.12% to 0.2% of CHX gluconate, with or without alcohol (Ciancio 2000). Although some studies on the virucidal effects of CHX against SARS‐CoV and related viral infections under biologically mimicked conditions have shown proven benefit (Yoon 2020), other studies have reported no antiviral property with the use of CHX (Jones 1997; Marui 2019; Meister 2020).
The most important property of CHX is the intrinsic ability to be retained by the oral surfaces and released gradually into the oral cavity over a period of time (Carrilho 2010). Staining of teeth and gingiva has been reported with use of CHX for more than seven days, except in formulations that contain polyvinylpyrrolidone in addition to CHX (Tartaglia 2019). Taste alteration and increased calculus formation are other reported adverse effects (Ciancio 2000).
Cetylpyridinium chloride
Cetylpyridinium chloride (CPC) is a cationic quaternary ammonium with broad‐spectrum antimicrobial and antiviral properties (Popkin 2017). It acts by penetrating the cell wall of organisms causing lysis and destruction. It is a surface‐active agent specifically active against gram‐positive organisms and yeast. It is commercially available in concentrations ranging from 0.05% to 0.10%. It is an established antiplaque agent and is used as an adjuvant in mild‐to‐moderate gingivitis (Ciancio 2000).
Essential oils
Essential oils are plant extracts obtained by distillation or cold press and are mixed with a carrier oil base to be used commercially. They are powerful antioxidants with regenerating, oxygenating and immune‐strengthening properties (Araujo 2015). Tea tree oil, helichrysum oil, chamomile oil, clove oil, eucalyptus oil and lavender oil are commonly used. Generally, mouth rinses contain oil of thymol, menthol, eucalyptol, and methyl salicylate in a hydroalcohol solution (Claffey 2003). Essential oils have antimicrobial properties and work by killing planktonic and biofilm‐associated bacteria and a broad spectrum of bacteria and yeasts associated with halitosis, gingivitis and periodontitis. They disrupt bacterial cell membranes and cell walls, inhibit bacterial growth and development, inhibit glucosyltransferase enzymes, reduce extracellular polysaccharide formation, and reduce plaque endotoxin levels (Alshehri 2018; Araujo 2015). Essential oils have also shown antiviral properties by causing capsid disintegration and viral expansion and thus making them ineffective (Wani 2021).
Herbal mouth rinses
Herbal mouth rinses are available as non‐prescription medications, and are widely used due to the belief that alternative or complementary therapy offers no or minimal adverse effects (Sridharan 2016). Botanical sources such as neem, basil, lemon grass, chamomile, linseed, leaf extracts such as from Carica papaya or guava leaf, peppermint, turmeric (Cai 2020), and green propolis (Pedrazzi 2015) have been incorporated into aqueous solutions to be used as mouth rinses. These are phytotherapeutic plants, containing a mixture of active agents such as catechins, tannins and sterols (Manipal 2016). These mouth rinses from botanical sources have been shown to have significant effects on both gram‐positive and gram‐negative organisms, including Escherichia coli, Streptococcus species and Salmonella species (Dua 2015). They possess significant antimicrobial and superoxide scavenging properties, leading to reduction in the bacterial load, decrease in overall inflammation and reduction in oxidative stress, ultimately leading to healing and recovery (Dua 2015; Mathur 2018).
Povidone iodine
Povidone iodine is a broad‐spectrum antimicrobial agent with established in vitro efficacy against many gram‐positive and gram‐negative bacteria including mycobacteria; bacterial spores; and a wide range of enveloped and non‐enveloped viruses, fungi and protozoa (Kanagalingam 2015). The microbicidal action of povidone iodine is related to the non‐complexed, freely mobile elemental iodine (Kanagalingam 2015). This activated iodine reacts in electrophilic reactions with enzymes of the respiratory chain as well as with amino acids from the cell membrane proteins both located in the cell wall. As a result, the well‐balanced tertiary structure necessary for maintaining the respiratory chain is destroyed and the micro‐organism is irreversibly damaged (Eggers 2018).
There were significant reductions in SARS‐CoV‐2 viral load when povidone iodine mouth rinse was tested under biologically mimicked conditions (Meister 2020). Povidone iodine as gargles and throat sprays demonstrated rapid virucidal activity against coronavirus strains, especially SARS‐CoV, after 10 seconds' exposure (Kariwa 2004). The exposure of 120 seconds was effective against Candida albicans that reduced the overall treatment cost of fungal infections (Kanagalingam 2015).
C31G
C31G is an equimolar mixture of alkyl dimethylglycine and alkyl dimethyl amine oxide that is buffered with citric acid (Corner 1988). It is a surface‐active agent effective against gram‐positive and gram‐negative bacteria and is used in a concentration of 0.5%. It acts by inhibition of bacterial glycolysis, bacterial adherence and low minimum inhibitory concentration (MIC) against a host of microbes (Corner 1988).
Hydrogen peroxide
Hydrogen peroxide in low concentrations is used in mouth rinses, dentifrices and teeth‐whitening agents (Mandel 1994; Walsh 2000). Commercially available mouth rinses contain 1% to 3% of hydrogen peroxide (Hossainian 2011). Being a strong oxidising agent, it exerts good antimicrobial effects against gram‐positive and gram‐negative organisms. Nevertheless, there is no evidence to support the use of hydrogen peroxide mouth rinses as an antiviral agent (Ortega 2020).
Saline
Saline is known to have antibacterial properties. One study using home‐made saline with a concentration of 2% and 5.8% showed marked activity on gram‐positive bacteria and the antibacterial effect for a 2% solution lasted three hours and for a 5.8% solution lasted five hours (Nokam Kamdem 2022). Nokam Kamdem and colleagues found the 5.8% saline to be as effective as 0.1% CHX.
Ozonated water
Ozone (O3) is an allotropic form of oxygen and has the ability to oxidise organic material. Because of this property, it has been topically used to reduce infections affecting the oral cavity (Nagayoshi 2004).
Boric acid
Boric acid 0.75% to 3% has been used as antimicrobial and antifungal agent because of its potential fungicidal and bacteriostatic action. It contains AN0128, a boron‐containing compound that has demonstrated antimicrobial and anti‐inflammatory properties (Luan 2008), and has been successfully used in the field of dentistry as irrigation agent to reduce the periodontal pocket depth in people with periodontitis (Sağlam 2013).
Why it is important to do this review
Although aerosols produced during AGPs in the dental clinic have always been considered a potential threat for transmission of diseases among dentists and dental personnel, there are virtually no documented transmission episodes of any of these through dental practices. This concern of transmission has become even more important in the context of the current pandemic challenge posed by COVID‐19. Epidemics such as those of SARS, Middle East respiratory syndrome (MERS) and now COVID‐19 warrant superior infection control practices in dental clinics to sustain and provide necessary services during challenging times (Meng 2020). People attending dental clinics can cough, sneeze or undergo dental procedures that aerosolise dental materials, saliva and blood along with micro‐organisms. People who are infected can be in the incubation period, be asymptomatic or choose to conceal their infection (Meng 2020). Hence, it is the duty of healthcare professionals to take necessary precautions to the highest possible level.
Following the suspension of most dental practices due to the COVID‐19 pandemic, strict infection control guidelines are being proposed by major governing bodies across the world in order to resume routine practices, without which people with oral health problems will go untreated, and dental practices will have financial impact (CDC 2020; FGDP 2020). CDC guidelines recommend that dental settings need to balance providing necessary services to patients while minimising the risk of transmission of infectious diseases in the dental clinic. The Occupational Safety and Health Administration (OSHA) classified dentists as in the very high‐risk category due to the potential exposure to pathogenic micro‐organisms through AGPs. Also, according to the US Department of Labor, dental hygienists have the highest occupational risk at 99.7%, followed by the dental assistants at 92.5% and general dentists at 92.1%.
With current concerns about the transmission of infectious diseases and cross‐contamination in the dental setting, several methods have been proposed to reduce the risk of infection spread. Use of mouth rinses by the patient before the commencement of any dental procedure has been proposed and tested in various clinical and laboratory studies as a possible measure to reduce the amount of contamination in the aerosols generated during AGPs (Fine 1996; Hunter 2014; Joshi 2017). In one recently published rapid review on AGPs and their mitigation in International Dental Guidance documents, 82% (51/63) of documents recommended preprocedural mouth rinse for people without COVID; however, only 10/51 documents provided references to support the recommendation (Clarkson 2020). Different types of mouth rinses are commercially available, but there is uncertainty regarding the use of these preprocedural mouth rinses as we do not know if they are effective for preventing the risk of infection in dental healthcare providers and contamination of aerosols, and if so, whether one might work better than another. This review may help dental professionals prepare themselves to adopt best practices to reduce the risk of infection and help in the reduction of contaminated aerosols.
Objectives
To assess the effects of preprocedural mouth rinses used in dental clinics to minimise incidence of infection in dental healthcare providers and reduce or neutralise contamination in aerosols.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) either with parallel arm or cluster‐RCT design in dental patients. We included cross‐over design for secondary objectives but planned to include first‐period data from cross‐over trials as parallel‐arm studies. We excluded quasi‐RCTs, split‐mouth studies, controlled clinical trials and experimental studies conducted in a laboratory environment.
Types of participants
We included studies with dental patients undergoing AGPs.
Types of interventions
We included any preprocedural rinse that aimed to reduce contaminated aerosols in dental clinics, compared to placebo, no mouth rinse or another mouth rinse. We excluded studies that combined preprocedural rinse with other methods to reduce the aerosols, as this is beyond the scope of the review.
Types of outcome measures
We could not find any recommended core outcomes related to this review published in the Core Outcome Measures in Effectiveness Trials (COMET) initiative (COMET 2020). Hence, the review authors decided on the outcomes by consensus.
Primary outcomes
Incidence of infection of dental healthcare providers (dentists, dental surgery assistants, dental hygienists, dental technologists, dental laboratory staff, dental aides or trainee students)
Measurement of outcomes: whether a participant infected any dental healthcare provider; if so, we planned to count it as one event.
Secondary outcomes
Reduction in the level of contamination by particles larger than 5 µm (droplets) at distances of less than 2 m from patient's oral cavity in the operative environment
Reduction in the level of contamination by particles of 5 µm or less (droplet nuclei) at distances of 2 m or more from patient's oral cavity in the operative environment
Cost
Change of microbiota in the patient's mouth
Adverse events such as transient discolouration, altered taste, allergic reaction, hypersensitivity
Acceptability and feasibility of the intervention to patients and dental healthcare providers
Measurement of outcomes: we planned to measure the reduction in the level of contamination using colony‐forming units (CFUs), where the samples could be collected with culture plates, or the results of polymerase chain reaction (PCR) or index of microbial air contamination (IMA). We planned to measure cost in GBP or USD, and change of microbiota using CFUs or viral load. Adverse events are described qualitatively. Acceptability and feasibility of the interventions was to be measured using ordinal (e.g. Likert scale) or dichotomous (e.g. yes/no) data.
Search methods for identification of studies
Electronic searches
Cochrane Oral Health's Information Specialist conducted systematic searches in the following databases for RCTs and controlled clinical trials. There were no language, publication year or publication status restrictions:
Cochrane Oral Health's Trials Register (searched 4 February 2022) (Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Register of Studies (searched 4 February 2022) (Appendix 2);
MEDLINE Ovid (1946 to 4 February 2022) (Appendix 3);
Embase Ovid (1980 to 4 February 2022) (Appendix 4);
World Health Organization COVID‐19 Global Literature on Coronavirus Disease Database (search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov) (searched 4 February 2022) (Appendix 5);
Cochrane COVID‐19 Study Register (covid-19.cochrane.org/) (searched via the Cochrane Register of Studies) (searched 4 February 2022) (Appendix 6).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid, presented in Appendix 3. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategies designed by Cochrane for identifying RCTs as described in the Cochrane Handbook for Systematic Reviews of Interventions, Technical Supplement to Chapter 4 (Lefebvre 2020). We also used a search filter to identify non‐randomised studies, as presented in Waffenschmidt 2020.
Searching other resources
Cochrane Oral Health's Information Specialist searched the following trials registries to identify ongoing studies:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov/) (searched 4 February 2022) (Appendix 7);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch) (searched 4 February 2022) (Appendix 8).
We searched the reference lists of included studies and relevant systematic reviews for further studies.
We did not perform a separate search for adverse effects.
We checked to ensure that none of the included studies were retracted due to error or fraud.
We contacted the original authors for clarification and further data if trial reports were unclear.
Data collection and analysis
Selection of studies
Two review authors independently screened the titles and abstracts. The search was designed to be sensitive and include controlled clinical trials, these were filtered out early in the selection process if they were not randomised. We resolved any conflicts during the screening process by discussion. If this was not possible, we consulted a third review author (arbiter) and reached consensus through discussion. We used online Rayyan software to screen the titles and abstracts (Rayyan 2016).
Two review authors independently screened the full‐text articles. We resolved any conflicts during the screening by mutual discussion. If this was not possible, we consulted a third review author (arbiter) and reached consensus through discussion. For included studies, we extracted useful information and data from the full‐text articles. These are presented in the Characteristics of included studies table. We also present the Characteristics of excluded studies tables, stating the reasons for the exclusion of studies.
Data extraction and management
One review author designed the data extraction form and another review author tested its suitability. Two review authors independently extracted the data using the data extraction form. We limited the data extraction to a minimal set of required data items.
Where studies had multiple publications, we collated the reports of the same study, so that each study, rather than each report, was the unit of interest for the review. Such studies had a single identifier with multiple references.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias using the Cochrane RoB 1 tool for RCTs, the results of which were reported in a table; Higgins 2011). For RCTs, we classified each domain as being at high, low or unclear risk of bias. We attempted to contact the trial authors if there was insufficient information or it was unclear. We resolved any disagreements by discussion between the review authors. If consensus was not reached, we consulted a third review author (arbiter).
Measures of treatment effect
We reported continuous outcomes as means differences (MDs) with 95% confidence intervals (CIs). If the included trials reported continuous outcomes obtained from different instruments, we used the standardised mean difference (SMD) as the effect measure, with 95% CIs.
We planned to qualitatively describe the costs for the interventions used. For ordinal data, we planned to dichotomise the data and present the effect sizes as risk ratios (RRs) with 95% CIs. We planned to present the effect sizes for dichotomous outcomes as RRs with 95% CIs. However, none of the included studies reported costs for the interventions, dichotomous and ordinal data.
Unit of analysis issues
For the primary outcome, the unit of analysis was the event of infection in any of the dental healthcare providers. For the secondary outcomes, the unit of analysis was the participant undergoing the AGP. In multi‐arm trials, we selected the relevant arms for inclusion in our analyses. If more than two arms were relevant to this review, we planned to split the control group between multiple comparisons so that participants were not double‐counted in a meta‐analysis. However, none of the meta‐analyses had this issue.
Dealing with missing data
When we encountered trials with missing data, we contacted the investigators or sponsors of these studies, if the contact author's email address was available. We calculated missing data from other data, such as standard deviations (SDs) and P values. We planned to re‐analyse the data according to the intention‐to‐treat (ITT) principle whenever possible. However, we did not encounter such trials.
Assessment of heterogeneity
We assessed heterogeneity by visually inspecting the forest plots to determine closeness of point estimates with each other and overlap of CIs. We used the Chi² test with a P value of 0.1 to indicate statistical significance. We also used the I² statistic, following the interpretation recommended in the Cochrane Handbook for Systematic Reviews of Interventions (0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100% represents considerable heterogeneity) (Higgins 2019).
Assessment of reporting biases
If we had included 10 or more studies, we planned to construct a funnel plot to investigate any potential reporting bias. However, most of the analyses were based on fewer than 10 studies.
Data synthesis
We analysed the data using Review Manager 5 (Review Manager 2020). In the absence of substantial clinical or methodological heterogeneity, we performed meta‐analysis using the random‐effects model. If there was substantial or considerable heterogeneity, we investigated this using a subgroup analysis. Where a meta‐analysis was not appropriate, we discussed the data narratively.
Subgroup analysis and investigation of heterogeneity
We planned to investigate heterogeneity by performing the following subgroup analyses.
Type of AGP (e.g. ultrasonic and sonic scaling, tooth preparation using air turbine hand piece or air abrasion, three‐way syringe).
Duration or dosage (or both) of mouth rinse.
Position of the culture plates (for CFU).
Type of culture media used (aerobic and anaerobic).
Type of air sampler used (Reynier's, Andersen cascade, Surface Air System sampler, Active Casella slit sampler).
Of these, we had sufficient data to conduct subgroup analyses based on position of the culture plates and type of culture media used.
Sensitivity analysis
To explore the possible effect of losses to follow‐up on the effect estimates for the secondary outcomes, we planned to perform sensitivity analyses. We planned to remove those studies at high risk of bias (studies assessed to have high risk of bias in at least one domain) and report if any there were significant differences between the results of these analyses. However, most comparisons were based on single studies, or all the included studies were at high risk of bias or we could not meta‐analyse the data due to high heterogeneity for which we had no explanation.
For dichotomous outcomes, we planned to vary the event rate within the missing participants from intervention and control groups within plausible limits. However, none of the included studies reported the outcomes dichotomously.
Summary of findings and assessment of the certainty of the evidence
We planned to summarise the results of the analyses in summary of findings tables for the following outcomes, for all comparisons.
Incidence of infection of dental healthcare providers.
Reduction in the level of contamination by particles larger than 5 µm (droplets) at distances of less than 2 m from patient's oral cavity in the operative environment.
Reduction in the level of contamination by particles 5 µm or less (droplet nuclei) at distances of 2 m or more from patient's oral cavity in the operative environment.
Adverse events.
However, we did not find any included studies reporting the primary outcome (incidence of infection of dental healthcare providers). Hence, we summarised the results of the analyses in summary of findings tables for the other three outcomes for seven comparisons only.
To identify the most important seven comparisons, we engaged stakeholders (four general dental practitioners and six specialists) who ranked the comparisons. We have presented the top seven comparisons identified by stakeholders as summary of findings tables (Table 1; Table 2; Table 3; Table 4; Table 6; Table 7; Table 8). For the other nine comparisons, we presented the summary of findings as additional tables (Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17).
2. Cetylpyridinium chloride compared to no rinsing for preventing risk of infection in dental healthcare providers and contamination of aerosols in dental clinic.
| Cetylpyridinium chloride compared to no rinsing for preventing transmission of infectious diseases through aerosols in dental healthcare providers | ||||||
|
Population: dental patients undergoing aerosol‐generating procedures and dental healthcare professionals treating them Setting: university hospital Intervention: CPC Comparison: no rinsing | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no rinsing | Risk with CPC | |||||
| Incidence of infection of dental healthcare providers | Not reported | |||||
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – operator level | The mean reduction in level of contamination was 41.7 CFUs | MD 34.8 CFUs lower (65.92 lower to 3.68 lower) | — | 30 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – operator level | The mean reduction in level of contamination was 644 CFUs | MD 583 CFUs lower (958.36 lower to 207.64 lower) | — | 30 (1 RCT) | ⊕⊕⊝⊝ Lowb | CPC+Zn+F vs no rinsing |
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – combined | The range of mean reduction in level of contamination was 803.3 to 875 CFUs | MD 0.75 CFUs lower (1.32 lower to 0.18 lower) | — | 200 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | CPC formulation vs no rinsing |
| Reduction in level of contamination by particles ≤ 5 µm (droplet nuclei) at distances of ≥ 2 m from patient's oral cavity in the operative environment | Not reported | |||||
| Adverse events such as transient discolouration, altered taste, allergic reaction, hypersensitivity | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony‐forming unit; CI: confidence interval; CPC: cetylpyridinium chloride; CPC+Zn+F: cetylpyridinium chloride + zinc + fluoride; MD: mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect. | ||||||
aDowngraded one level due to risk of bias. bDowngraded two levels due to imprecision.
3. Cetylpyridinium chloride compared to water for preventing risk of infection in dental healthcare providers and contamination of aerosols in dental clinic.
| Cetylpyridinium chloride compared to water for preventing transmission of infectious diseases through aerosols in dental healthcare providers | ||||||
|
Population: dental patients undergoing aerosol‐generating procedures and dental healthcare professionals treating them Setting: university hospital Intervention: CPC Comparison: water | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with water | Risk with CPC | |||||
| Incidence of infection of dental healthcare providers | Not reported | |||||
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – operator level | The mean reduction in level of contamination was 55.6 CFUs | MD 48.7 CFUs lower (67.82 lower to 29.58 lower) | — | 30 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | CPC vs water |
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – operator level | The mean reduction in level of contamination was 307 CFUs | MD 246 CFUs lower (437.96 lower to 54.04 lower) | — | 30 (1 RCT) | ⊕⊕⊝⊝ Lowb | CPC+ZN+F vs water |
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – combined | The mean reduction in level of contamination was 569 CFUs | MD 0.78 CFUs lower (1.24 lower to 0.31 lower) | — | 105 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | CPC formulation vs water |
| Reduction in level of contamination by particles ≤ 5 µm (droplet nuclei) at distances of ≥ 2 m from patient's oral cavity in the operative environment | Not reported | |||||
| Adverse events such as transient discolouration, altered taste, allergic reaction, hypersensitivity | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony‐forming unit; CI: confidence interval; CPC: cetylpyridinium chloride; MD: mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect. | ||||||
aDowngraded one level due to risk of bias. bDowngraded two levels due to imprecision.
4. Tempered cetylpyridinium chloride compared to cold cetylpyridinium chloride for preventing risk of infection in dental healthcare providers and contamination of aerosols in dental clinic.
| Tempered cetylpyridinium chloride compared to cold cetylpyridinium chloride for preventing transmission of infectious diseases through aerosols in dental healthcare providers | ||||||
|
Population: dental patients undergoing aerosol‐generating procedures and dental healthcare professionals treating them Setting: university hospital Intervention: tempered CPC Comparison: cold CPC | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with cold CPC | Risk with tempered CPC | |||||
| Incidence of infection of dental healthcare providers | Not reported | |||||
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – operator | The mean reduction in level of contamination was 71.5 CFUs | MD 9.3 CFUs lower (16.17 lower to 2.43 lower) | — | 20 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – assistant | The mean reduction in level of contamination was 47.3 CFUs | MD 8.6 CFUs lower (14.7 lower to 2.5 lower) | — | 20 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
| Reduction in level of contamination by particles ≤ 5 µm (droplet nuclei) at distances of ≥ 2 m from patient's oral cavity in the operative environment | Not reported | |||||
| Adverse events such as transient discolouration, altered taste, allergic reaction, hypersensitivity | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony‐forming unit; CI: confidence interval; CPC: cetylpyridinium chloride; MD: mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect. | ||||||
aDowngraded one level due to risk of bias. bDowngraded two levels due to imprecision.
5. Povidone iodine compared to saline for preventing transmission of infectious diseases through aerosols in dental healthcare providers.
| Povidone iodine compared to saline for preventing transmission of infectious diseases through aerosols in dental healthcare providers | ||||||
|
Population: dental patients undergoing aerosol‐generating procedures and dental care professionals treating them Setting: university hospital Intervention: povidone iodine Comparison: saline | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with saline | Risk with povidone iodine | |||||
| Incidence of infection of dental healthcare providers | Not reported | |||||
| Reduction in the level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – operator level | The mean reduction was 46.5 CFUs | MD 16.5 CFUs lower (32.65 lower to 0.35 lower) | — | 12 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
| Reduction in the level of contamination by particles ≤ 5 µm (droplet nuclei) at distances of ≥ 2 m from patient's oral cavity in the operative environment | Not reported | |||||
| Adverse events such as transient discolouration, altered taste, allergic reaction, hypersensitivity | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony‐forming unit; CI: confidence interval; MD: mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect. | ||||||
aDowngraded two levels due to risk of bias. bDowngraded two levels due to imprecision.
6. Essential oil/herbal mouthwash compared to water for preventing risk of infection in dental healthcare providers and contamination of aerosols in dental clinic.
| Essential oil/herbal mouthwash compared to water for preventing transmission of infectious diseases through aerosols in dental healthcare providers | ||||||
|
Population: dental patients undergoing aerosol‐generating procedures and dental healthcare professionals treating them Setting: university hospital Intervention: essential oil/herbal mouthwash Comparison: water | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with water | Risk with essential oil/herbal mouthwash | |||||
| Incidence of infection of dental healthcare providers | Not reported | |||||
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – operator level | High heterogeneity in the meta‐analysis (3 studies) | |||||
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – assistant level | High heterogeneity in the meta‐analysis (3 studies) | |||||
| Reduction in level of contamination by particles ≤ 5 µm (droplet nuclei) at distances of ≥ 2 m from patient's oral cavity in the operative environment | Not reported | |||||
| Adverse events such as transient discolouration, altered taste, allergic reaction, hypersensitivity | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony‐forming unit; CHX: chlorhexidine; CI: confidence interval; MD: mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect. | ||||||
7. Chlorhexidine compared to ozonated water for preventing risk of infection in dental healthcare providers and contamination of aerosols in dental clinic.
| Chlorhexidine compared to ozonated water for reduction in the level of contamination of aerosols | ||||||
|
Population: dental patients undergoing aerosol‐generating procedures and dental healthcare professionals treating them Setting: university hospital Intervention: CHX Comparison: ozonated water | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with ozonated water | Risk with CHX | |||||
| Incidence of infection of dental healthcare providers | Not reported | |||||
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – operator level aerobic assessed with CFUs | The mean reduction in level of contamination was 48.5 CFUs | MD 17.8 lower (33.70 lower to 1.90 lower) | — | 40 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
| Reduction in level of contamination by particles ≤ 5 µm (droplet nuclei) at distances of ≥ 2 m from patient's oral cavity in the operative environment – aerobic assessed with CFUs | The mean reduction in level of contamination was 31 CFUs | MD 12.3 lower (27.12 lower to 2.52 higher) | — | 40 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | — |
| Reduction in level of contamination by particles ≤ 5 µm (droplet nuclei) at distances of ≥ 2 m from patient's oral cavity in the operative environment – anaerobic assessed with CFUs | The mean reduction in level of contamination was 22.1 CFUs | MD 3.4 lower (14.66 lower to 7.86 higher) | — | 40 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | — |
| Reduction in level of contamination by particles ≤ 5 µm (droplet nuclei) at distances of ≥ 2 m from patient's oral cavity in the operative environment | Not reported | |||||
| Adverse events such as transient discolouration, altered taste, allergic reaction, hypersensitivity | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony‐forming unit; CI: confidence interval; MD: mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect. | ||||||
aDowngraded two levels due to high risk of bias. bDowngraded two levels due to imprecision. cDowngraded one level due to inconsistency.
8. Povidone iodine compared to ozonated water for preventing risk of infection in dental healthcare providers and contamination of aerosols in dental clinic.
| Povidone iodine compared to ozonated water for reduction in the level of contamination in aerosols | ||||||
|
Population: dental patients undergoing aerosol‐generating procedures and dental healthcare professionals treating them Setting: university hospital Intervention: povidone iodine Comparison: ozonated water | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with ozonated water | Risk with povidone iodine | |||||
| Incidence of infection of dental healthcare providers | Not reported | |||||
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – operator level aerobic assessed with CFUs | The mean reduction in level of contamination was 48.5 | MD 3.5 higher (17.1 lower to 24.1 higher) | — | 40 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | — |
| Reduction in level of contamination by particles ≤ 5 µm (droplet nuclei) at distances of ≥ 2 m from patient's oral cavity in the operative environment – aerobic assessed with CFUs | The mean reduction in level of contamination aerobic was 31 | MD 12.5 lower (26.33 lower to 1.33 higher) | — | 40 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | — |
| Reduction in level of contamination by particles ≤ 5 µm (droplet nuclei) at distances of ≥ 2 m from patient's oral cavity in the operative environment – anaerobic assessed with CFUs | The mean reduction in level of contamination was 22.1 | MD 2.6 lower (9.54 lower to 4.34 higher) | — | 40 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | — |
| Adverse events such as transient discolouration, altered taste, allergic reaction, hypersensitivity | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony‐forming unit; CHX; chlorhexidine; CI: confidence interval; MD: mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect. | ||||||
aDowngraded two levels due to high risk of bias. bDowngraded one level due to inconsistency. cDowngraded two levels due to imprecision.
9. Chlorhexidine compared to boric acid for reduction in the level of contamination in aerosols.
| Chlorhexidine compared to boric acid for reduction in the level of contamination in aerosols | ||||||
|
Population: dental patients undergoing aerosol‐generating procedures and dental healthcare professionals treating them Setting: university hospital Intervention: CHX Comparison: boric acid | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with boric acid | Risk with CHX | |||||
| Incidence of infection of dental healthcare providers | Not reported | |||||
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – operator level | The mean reduction in level of contamination was 49.8 CFUs | MD 26.6 CFUs lower (28.24 lower to 24.96 lower) | — | 60 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – assistant level | The mean reduction in level of contamination was 20.9 CFUs | MD 8.03 CFUs lower (9.65 lower to 6.41 lower) | — | 60 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
| Reduction in level of contamination by particles ≤ 5 µm (droplet nuclei) at distances of ≥ 2 m from patient's oral cavity in the operative environment | Not reported | |||||
| Adverse events such as transient discolouration, altered taste, allergic reaction, hypersensitivity | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony‐forming unit; CHX: chlorhexidine; CI: confidence interval; MD: mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect. | ||||||
aDowngraded one level due to unclear risk of bias. bDowngraded two levels due to imprecision.
10. Boric acid compared to water for reduction in the level of contamination in aerosols.
| Boric acid compared to water for reduction in the level of contamination in aerosols | ||||||
|
Population: dental patients undergoing aerosol‐generating procedures and dental healthcare professionals treating them Setting: university hospital Intervention: boric acid Comparison: water | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with water | Risk with boric acid | |||||
| Incidence of infection of dental healthcare providers | Not reported | |||||
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – operator level | The mean reduction in level of contamination was 79.1 CFUs | MD 29.3 CFUs lower (32.65 lower to 25.95 lower) | — | 60 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
| Reduction in level of contamination by particles > 5 µm (droplets) at distances of < 2 m from patient's oral cavity in the operative environment – assistant level | The mean reduction in level of contamination was 34.13 CFUs | MD 14.04 CFUs lower (16.47 lower to 11.61 lower) | — | 60 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — |
| Reduction in level of contamination by particles ≤ 5 µm (droplet nuclei) at distances of ≥ 2 m from patient's oral cavity in the operative environment | Not reported | |||||
| Adverse events such as transient discolouration, altered taste, allergic reaction, hypersensitivity | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFU: colony‐forming unit; CI: confidence interval; MD: mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect. | ||||||
aDowngraded one level due to unclear risk of bias. bDowngraded two levels due to imprecision.
We used the GRADE framework to evaluate the certainty of evidence for each outcome as high, moderate, low or very low, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We justified all decisions to downgrade the certainty of the evidence using footnotes and provided comments to aid readers' understanding where necessary.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies tables.
Results of the search
The electronic search strategies identified 6356 records, and five records from cross‐references of the included studies and non‐Cochrane systematic reviews on this topic. We had 4319 records after deduplication. We excluded 4269 records after screening the abstracts as they were irrelevant and requested full‐text copies of 50 articles. From the 50 articles, we excluded 23 studies (25 reports) and identified four ongoing studies. The remaining 17 studies (21 reports) met the inclusion criteria of this review. See Figure 3 for the selection process.
3.
Study flow diagram.
Included studies
We included 17 studies with 830 participants. See the Characteristics of included studies table for further details.
Countries of origin
Eleven trials were from India (Gupta 2014; Joshi 2017; Kaur 2014; Mohan 2016; Nayak 2020; Nisha 2021; Reddy 2012; Shetty 2013; Suresh 2011; Tasneem 2017; Verma 2017); three from the USA (Bay 1993; Fine 1993a; Mohammed 1964); two from Brazil (Feres 2010; Retamal‐Valdes 2017); and one from Puerto Rico (Mohammed 1970).
All the included studies were published in the English language.
Trial design
All included studies were RCTs with parallel‐arm designs except one, which had a cross‐over design (Fine 1993a). Five studies had two arms (Fine 1993a; Mohammed 1970; Mohan 2016; Suresh 2011; Tasneem 2017); nine had three arms (Bay 1993; Gupta 2014; Kaur 2014; Mohammed 1964; Nayak 2020; Nisha 2021; Reddy 2012; Shetty 2013; Verma 2017); and three had four arms (Feres 2010; Joshi 2017; Retamal‐Valdes 2017).
Sample size
The minimum sample size was 18 (Fine 1993a; Verma 2017); and the maximum sample size was 185 (Mohammed 1964). None of the included studies mentioned the sample size calculation or power of the study.
Aerosol‐generating procedures tested
Fourteen studies tested interventions during ultrasonic scaling procedures (Feres 2010; Fine 1993a; Gupta 2014; Joshi 2017; Kaur 2014; Mohan 2016; Nayak 2020; Nisha 2021; Reddy 2012; Retamal‐Valdes 2017; Shetty 2013; Suresh 2011; Tasneem 2017; Verma 2017). Of these 14, four studies used a magnetostrictive ultrasonic scaler (Feres 2010; Fine 1993a; Retamal‐Valdes 2017; Suresh 2011); four studies used a piezoelectric scaler (Gupta 2014; Joshi 2017; Nayak 2020; Verma 2017); and the remaining six studies did not report the type of scaler used.
One study tested interventions during air‐abrasive polishing (Bay 1993); and two studies during tooth preparation with a high‐speed hand piece (Mohammed 1964; Mohammed 1970).
Funding
Private companies funded five trials were funded by private companies that manufactured the tested mouth rinses or the tested mouth rinses were sponsored by those companies (Bay 1993; Feres 2010; Fine 1993a; Nayak 2020; Retamal‐Valdes 2017). One study had university funding (Nisha 2021). Four trials did not disclose any funding details or conflict of interest (Gupta 2014; Mohammed 1964; Mohammed 1970; Mohan 2016), and the remaining seven trials reported that they had not received any funding and had no conflicts of interest (Joshi 2017; Kaur 2014; Reddy 2012; Shetty 2013; Suresh 2011; Tasneem 2017; Verma 2017).
Clinical set‐up
Disinfection of the operating room
Three trials fumigated the operatory used in the trial before starting the procedure (Joshi 2017; Nisha 2021; Verma 2017), one of which, Joshi 2017, used 34% formaldehyde. Other studies did not report any details regarding fumigation.
Dental unit waterlines
Two trials flushed the dental unit waterlines before the procedure (Fine 1993a; Nayak 2020). Bay 1993 flushed the polisher waterlines for two minutes before each use. Two trials flushed the ultrasonic scaler with distilled water before treatment (Feres 2010; Shetty 2013).
Participants
The maximum participant age reported was 70 years (Retamal‐Valdes 2017); the minimum was 18 years (Retamal‐Valdes 2017). Eleven studies recruited adults only (Fine 1993a; Gupta 2014; Joshi 2017; Kaur 2014; Mohan 2016; Nayak 2020; Nisha 2021; Retamal‐Valdes 2017; Shetty 2013; Suresh 2011; Tasneem 2017). Mohammed 1964 recruited university students. Reddy 2012 recruited people of any age. The remaining four studies did not mention any details about the age group (Bay 1993; Feres 2010; Mohammed 1970; Verma 2017).
One trial recruited only males (Mohammed 1970). Six studies recruited both males and females (Bay 1993; Gupta 2014; Mohammed 1964; Nisha 2021; Reddy 2012; Retamal‐Valdes 2017). The remaining 10 trials did not mention the sex of the study participants (Feres 2010; Fine 1993a; Joshi 2017; Kaur 2014; Mohan 2016; Nayak 2020; Shetty 2013; Suresh 2011; Tasneem 2017; Verma 2017).
Inclusion/exclusion criteria
Eight studies recruited medically healthy people (Feres 2010; Fine 1993a; Joshi 2017; Kaur 2014; Nisha 2021; Retamal‐Valdes 2017; Tasneem 2017; Verma 2017); and seven studies recruited people who were not taking any antibiotic treatment or with a recent history of antibiotic treatment (Gupta 2014; Mohan 2016; Nayak 2020; Nisha 2021; Reddy 2012; Shetty 2013; Suresh 2011). Two studies did not mention any such inclusion/exclusion criteria (Mohammed 1964; Mohammed 1970).
Outcomes
The included studies did not measure our primary outcome, incidence of infection of dental healthcare providers. The outcomes reported in the included studies were the reduction in the level of contamination in particles larger than 5 µm at distances of less than 2 m in the operative environment and reduction in the level of contamination in particles 5 µm or less at distances of 2 m or more in the operative environment. Both these outcomes were measured in CFUs. None of the included studies used automatic colony counters for the assessment of the outcome. Four trials used manual colony counters (Bay 1993; Feres 2010; Joshi 2017; Retamal‐Valdes 2017). Fine 1993a counted colonies using a dissecting microscope, with results reported as total CFUs per filter. Other trials did not report the details of colony counting.
Eleven trials reported the mean CFUs either at operator or assistant levels. One trial reported the mean CFUs at 3 feet (91 cm) distance from the patient and did not mention the precise location of the culture media (Mohan 2016). Four trials reported only the combined mean CFUs from the culture media placed at different locations in the dental clinic (Mohammed 1964; Mohammed 1970; Reddy 2012; Tasneem 2017).
Sixteen studies used passive aerosol sampling where the aerosols drop down to settle on the culture media. Bay 1993 used active aerosol sampling from the oropharynx of patients undergoing dental air‐abrasive polishing.
Interventions
Ten trials compared CHX to either water or saline (or both) or no rinsing reduction in the level of contamination in particles larger than 5 µm (droplets) at distances of less than 2 m from patient's oral cavity in the operative environment.
1. Chlorhexidine rinsing versus no rinsing
Two trials compared 0.12% CHX to no rinsing at operator mouth‐mask level (operator level) (Feres 2010; Retamal‐Valdes 2017).
Feres 2010 used a one‐minute rinsing protocol and Retamal‐Valdes 2017 did not mention the rinsing duration.
2. Chlorhexidine versus water
Six trials compared CHX to distilled water at the operator level (Feres 2010; Gupta 2014; Nayak 2020; Nisha 2021; Retamal‐Valdes 2017; Shetty 2013). Of these, three trials used 0.2% CHX (Gupta 2014; Nayak 2020; Shetty 2013), and three used 0.12% CHX (Feres 2010; Nisha 2021; Retamal‐Valdes 2017). Four trials also evaluated the outcome at assistant level (Gupta 2014; Nayak 2020; Nisha 2021; Shetty 2013).
One trial compared non‐tempered 0.2% CHX and tempered 0.2% CHX with sterile water at operator level and assistant level; however, the combined data was presented (Reddy 2012).
Five studies used a one‐minute rinsing protocol (Feres 2010; Gupta 2014; Nayak 2020; Nisha 2021; Reddy 2012). Gupta 2014 performed rinsing 10 minutes prior to the dental procedure and Nayak 2020 30 minutes prior to treatment. Two studies did not mention the rinsing time (Retamal‐Valdes 2017; Shetty 2013).
Bay 1993 used a 30‐second rinsing time directly prior to treatment and evaluated the outcome at the patient level. Therefore, the data from this study were not included in the meta‐analysis.
3. Chlorhexidine versus saline
One trial compared 0.2% CHX to saline for the same outcome at 3 feet (91 cm) away from patient's mouth (exact location of the culture plate placement was not reported) (Mohan 2016), and another trial compared 0.2% CHX to saline at the operator level (Verma 2017).
Mohan 2016 followed a one‐minute rinsing protocol and Verma 2017 used a two‐minute rinsing time.
4. Chlorhexidine versus essential oils/herbal mouthwash
We combined trials using essential oil mouth rinse or herbal mouth rinse versus CHX mouth rinse under this comparison as herbal mouth rinse is prepared using the essential oil from the plant extracts (Cai 2020). We presented the composition of the essential oil mouth washes in Table 5.
Five trials compared CHX mouth rinse to essential oil/herbal mouthwash for the outcome reduction in the level of contamination in particles larger than 5 µm (droplets) at distances of less than 2 m from the patient's oral cavity in the operative environment (Bay 1993; Gupta 2014; Nayak 2020; Shetty 2013; Suresh 2011). Four trials used 0.2% CHX (Gupta 2014; Nayak 2020; Shetty 2013; Suresh 2011), and Bay 1993 used 0.12% CHX. Three trials assessed the contamination at the operator and assistant levels (Gupta 2014; Nayak 2020; Shetty 2013). One trial assessed at the assistant level and at 6 inches (30 cm) from the patient's mouth (Suresh 2011). Another trial assessed at the Andersen's level 2 (oropharynx) (Bay 1993).
Shetty 2013 compared 0.2% CHX with essential oil mouthwash A (tea tree oil). Suresh 2011 trial compared 0.2% CHX with essential oil mouthwash B (combination of eucalyptol, thymol, methyl salicylate and menthol essential oil). Bay 1993 compared 0.12% CHX with essential oil mouthwash C (Listerine), which contains essential oils (such as 0.042% menthol (mint), 0.064% thymol (thyme), 0.06% methyl salicylate (wintergreen) and 0.092% eucalyptol (eucalyptus)).
Nayak 2020 compared 0.2% CHX mouthwash with herbal mouthwash A (Befresh, which contains Cinnamomum zeylanicum, Mentha spicata, Syzygium aromaticum and Eucalyptus globulus) and Gupta 2014 compared 0.2% CHX mouthwash with herbal mouthwash B (which contains Terminilia bellirica, Piper betle, Salvadora persica, powders peppermint (Mentha spp) and Caraway, Wintergreen oil and cardamom).
Three studies used a one‐minute rinsing time (Gupta 2014; Nayak 2020; Suresh 2011). Shetty 2013 did not mention the rinsing time. Gupta 2014 performed rinsing 10 minutes prior to the dental procedure and Nayak 2020 30 minutes prior to the dental procedure. Bay 1993 used a 30‐second rinsing time directly prior to treatment.
5. Chlorhexidine versus povidone iodine
For reduction in the level of contamination in particles larger than 5 µm (droplets) at distances of less than 2 m from patient's oral cavity in the operative environment at operator level, two trials compared 0.2% CHX mouthwash to povidone iodine mouthwash using aerobic culture (Tasneem 2017; Verma 2017), and one trial assessed using both aerobic and anaerobic cultures (Kaur 2014). Kaur 2014 used 1% povidone Iodine while Verma 2017 used 5%.
For reduction in the level of contamination in particles 5 µm or less (droplet nuclei) at distances of 2 m or more from patient's oral cavity in the operative environment, Kaur 2014 compared the same mouthwashes using both aerobic and anaerobic cultures.
Kaur 2014 used a one‐minute rinsing protocol and Verma 2017 used a two‐minute rinsing protocol. Tasneem 2017 did not mention the rinsing time. None of the studies mentioned when the rinsing was performed prior to the procedure.
6. Chlorhexidine versus cetylpyridinium chloride
Three trials reported reduction in the level of contamination in particles larger than 5 µm (droplets) at distances of less than 2 m from patient's oral cavity in the operative environment. Feres 2010 compared cold 0.12% CHX with cold 0.05% CPC at operator level. Joshi 2017 compared tempered 0.2% CHX with tempered 0.05% CPC, cold 0.2% CHX with cold 0.05% CPC, tempered 0.2% CHX with cold 0.05% CPC and cold 0.2% CHX with tempered 0.05% CPC at operator and assistant level. Retamal‐Valdes 2017 compared 0.12% CHX and 0.075% CPC plus 0.28% zinc + 0.05% sodium fluoride (CPC+Zn+F) at operator level.
Two studies used the specified mouth rinse for one minute (Feres 2010; Joshi 2017). Joshi 2017 used the mouth rinse 10 minutes prior to the dental procedure. Retamal‐Valdes 2017 did not mention rinsing time.
7. Tempered chlorhexidine versus non‐tempered chlorhexidine
Two trials compared tempered 0.2% CHX versus non‐tempered 0.2% CHX and reported reduction in the level of contamination in particles larger than 5 µm (droplets) at distances of less than 2 m from patient's oral cavity in the operative environment. Joshi 2017 compared the outcome at operator and assistant levels and Reddy 2012 reported the outcome as combined reduction in the level of contamination.
Both trials reported the comparison after one minute of rinsing (Joshi 2017; Reddy 2012). Joshi 2017 used the mouth rinse 10 minutes prior to dental procedure.
8. Cetylpyridinium chloride versus no rinsing
Four trials reported reduction in the level of contamination in particles larger than 5 µm (droplets) at distances of less than 2 m from patient's oral cavity in the operative environment at operator level. Feres 2010 compared 0.05% CPC versus no rinsing. Retamal‐Valdes 2017 compared CPC+Zn+F versus no rinsing. Two trials compared CPC plus domiphen bromide plus quaternary ammonium compounds with water and reported the same outcome as the combined results (Mohammed 1964; Mohammed 1970).
One study used the mouth rinse for one minute (Feres 2010). Three trials did not report the timing of rinsing (Mohammed 1964; Mohammed 1970; Retamal‐Valdes 2017).
9. Cetylpyridinium chloride versus water
Three trials reported reduction in the level of contamination in particles larger than 5 µm (droplets) at distances of less than 2 m from patient's oral cavity in the operative environment at operator level. Feres 2010 compared 0.05% CPC with distilled water. Retamal‐Valdes 2017 compared CPC+Zn+F with distilled water. Mohammed 1964 compared CPC plus domiphen bromide plus quaternary ammonium compounds with water and reported the same outcome as the combined results.
One study used the specified mouth rinse for one minute (Feres 2010). Three trials did not report the timing of rinsing (Mohammed 1964; Mohammed 1970; Retamal‐Valdes 2017).
10. Tempered cetylpyridinium chloride versus cold cetylpyridinium chloride
One trial compared tempered 0.05% CPC and cold 0.05% CPC and reported reduction in the level of contamination in particles larger than 5 µm (droplets) at distances of less than 2 m from patient's oral cavity in the operative environment at operator and assistant levels (Joshi 2017).
Joshi 2017 used the mouth rinse for one minute, 10 minutes prior to the dental procedure.
11. Povidone iodine versus saline
One trial compared 5% povidone iodine versus saline and reported reduction in the level of contamination in particles larger than 5 µm (droplets) at distances of less than 2 m from patient's oral cavity in the operative environment at operator level (Verma 2017).
Verma 2017 used a two‐minute rinsing time.
12. Essential oil/herbal mouthwash versus water
Four trials reported reduction in the level of contamination in particles larger than 5 µm (droplets) at distances of less than 2 m from the patient's oral cavity in the operative environment under this comparison. Nayak 2020 compared herbal mouthwash A with water and Gupta 2014 compared herbal mouthwash B with distilled water at operator and assistant levels. Shetty 2013 compared essential oil mouthwash A with distilled water at operator and assistant levels. Bay 1993 compared essential oil mouthwash C with distilled water and reported the combined outcome. We presented the composition of the essential oil mouth washes in Table 5.
Two studies used a one‐minute rinsing protocol (Gupta 2014; Nayak 2020). Gupta 2014 performed rinsing 10 minutes prior to the dental procedure and Nayak 202030 minutes prior. Bay 1993 used a 30‐second rinsing time directly prior to the treatment. Shetty 2013 did not report rinsing time.
13. Chlorhexidine versus ozonated water
One trial reported two outcomes, reduction in the level of contamination in particles larger than 5 µm (droplets) at distances of less than 2 m and more than 2 m from patient's oral cavity in the operative environment (Kaur 2014). Kaur 2014 used 0.2% CHX rinse or ozonated water rinse with 0.082 mg/hour ozone output as preprocedural rinse before ultrasonic scaling procedure at operator level (mask of the operator) and 9 feet behind the patient.
Kaur 2014 used a one‐minute rinsing time.
14. Povidone iodine versus ozonated water
One trial reported two outcomes, reduction in the level of contamination in particles larger than 5 µm (droplets) at distances of less than 2 m and more than 2 m from patient's oral cavity in the operative environment (Kaur 2014). Kaur 2014 used 1% povidone iodine rinse or ozonated water rinse with 0.082 mg/hour ozone output as preprocedural rinse after ultrasonic scaling procedure at operator level (mask of the operator) and 9 feet behind the patient.
This study used a one‐minute rinsing time.
15. Chlorhexidine versus boric acid
One trial reported reduction in the level of contamination in particles larger than 5 µm (droplets) at distances of less than 2 m from patient's oral cavity in the operative environment at operator and assistant levels (Nisha 2021). Nisha 2021 used 0.12% CHX and 0.75% boric acid mouth rinse as preprocedural rinses 15 minutes before an ultrasonic scaling procedure at operator level (mask of the operator).
Nisha 2021 used a one‐minute rinsing time.
16. Boric acid versus water
One trial reported reduction in the level of contamination in particles larger than 5 µm (droplets) at distances of less than 2 m from patient's oral cavity in the operative environment at operator and assistant levels (Nisha 2021). Nisha 2021 used 0.75% boric acid mouth rinse and water as preprocedural rinses 15 minutes before an ultrasonic scaling procedure at operator level (mask of the operator).
Nisha 2021 used a one‐minute rinsing time.
17. Essential oil versus hydroalcohol mouthwash
One study compared essential oil mouthwash C (Listerine) with coloured and flavoured 5% hydroalcohol mouthwash in a cross‐over study design for reduction in the level of contamination in particles larger than 5 µm (droplets) at distances less than 2 m from patient's oral cavity at operator level using aerobic culture method during ultrasonic scaling (Fine 1993a). The study reported the mean log 10 values in a graph format and the data for the first phase were not reported. Hence, we could not include the results of the study in a meta‐analysis.
The participants followed a 30‐second rinsing protocol 40 minutes before the ultrasonic scaling procedure.
Excluded studies
We excluded 23 studies (25 reports) for the reasons listed in the Characteristics of excluded studies table. Of these, we could not access two trials from the clinical trial registry (CTRI/2017/10/010189; CTRI/2018/05/013935). Eight trials were not RCTs (Altonen 1976; Ciancio 1994; Dawson 2016; Litsky 1970; Paul 2020; Rajachandrasekaran 2019; Toroglu 2001; Worrall 1987). Six trials had split‐mouth design (Devker 2012; Fine 1992; Fine 1993b; Klyn 2001; Narayana 2016; Sawhney 2015). One trial checked bacterial contamination in the bone debris collected from the osteotomy site (Young 2002). One trial had a crossover design and the crossover sequence was not randomised (Santos 2014). NCT02319668 used toothpaste as the control and NCT04659928 used different high‐volume evacuation devices and hydrogen peroxide mouth rinse in combination. Three trials used high‐volume evacuation device in combination with preprocedural rinse (Logothetis 1995; Ramesh 2015; Swaminathan 2014).
Studies awaiting classification
There are no studies awaiting classification.
Ongoing studies
We identified four ongoing trials (CTRI/2021/03/031653; CTRI/2021/10/037528; NCT03839719; NCT04717063; see Characteristics of ongoing studies).
Risk of bias in included studies
We assessed three trials at high risk of bias overall (Bay 1993; Kaur 2014; Verma 2017), and 12 trials as unclear risk of bias overall because they each had at least one risk of bias domains that we judged to be unclear (Feres 2010; Fine 1993a; Gupta 2014; Joshi 2017; Mohammed 1964; Mohammed 1970; Mohan 2016; Nisha 2021; Reddy 2012; Shetty 2013; Suresh 2011; Tasneem 2017) (Figure 4; Figure 5). Two trials had low risk of bias overall (Nayak 2020; Retamal‐Valdes 2017).
4.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
5.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Only four trials gave the details of random sequence generation and allocation concealment (Fine 1993a; Kaur 2014; Nayak 2020; Retamal‐Valdes 2017). Four trials had low risk of bias in random sequence generation and had not provided any information on allocation concealment (Joshi 2017; Nisha 2021; Shetty 2013; Tasneem 2017). One trial provided no information on random sequence generation and had low risk of bias for allocation concealment (Gupta 2014), and one trial had provided the details of random sequence generation and was assessed to have high risk of bias for allocation concealment based on the details provided through personal communication (Verma 2017). The other seven trials did not provide any information on random sequence generation and allocation concealment and thus we assessed them to have unclear risk of bias (Bay 1993; Feres 2010; Mohammed 1964; Mohammed 1970; Mohan 2016; Reddy 2012; Suresh 2011).
Blinding
We assessed two trials to have high risk of performance bias as the dentists who carried out the AGPs were not blinded (Bay 1993; Verma 2017). Five trials did not give any details on blinding and were assessed to have unclear risk of bias (Mohammed 1964; Mohammed 1970; Mohan 2016; Reddy 2012; Shetty 2013), and nine trials were at low risk of bias (Feres 2010; Fine 1993a; Gupta 2014; Joshi 2017; Kaur 2014; Nayak 2020; Nisha 2021; Retamal‐Valdes 2017; Tasneem 2017). None of the trials used automated colony counters. Therefore, we assessed trials to have low risk of bias if they used manual colony counting with assessor blinding. One trial had unclear performance bias and low risk of detection bias (Suresh 2011).
Incomplete outcome data
Three trials did not provide any details regarding the dropouts and hence we assessed these trials to have unclear risk of bias (Bay 1993; Feres 2010; Shetty 2013). We assessed the other 13 trials, which had no or minimal dropouts, at low risk of bias.
Selective reporting
Only two trials followed the reporting according to their registered protocols and thus were at low risk of bias (Nayak 2020; Retamal‐Valdes 2017). One trial had registered the trial retrospectively and the sample size mentioned was 10 per group (Nisha 2021). We are not sure if the outcomes reported were same as the planned ones and thus judged this at unclear risk of bias. Another two trials had deviated from the protocol, thus being assessed at high risk of bias (Kaur 2014; Verma 2017). The remaining trials provided no details of the trial registration and hence we could not evaluate if all the planned outcomes were adequately reported. Therefore, these trials were at unclear risk of bias.
Other potential sources of bias
We identified no other potential sources of bias and thus assessed all studies at low risk of other bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 6; Table 7; Table 8
We presented the results for 16 comparisons for two outcomes (reduction in the level of contamination at distances less than 2 m and 2 m or more from patient's oral cavity). We combined similar interventions with similar control in the analyses (e.g. different concentrations of CHX (0.12% and 0.2%), water and distilled water as 'water'). The unit of measurement in all 16 analyses was CFUs.
We analysed the level of contamination at operator and assistant levels and omitted the patient‐level data. If the studies reported the combined data for level of contamination (i.e. mean CFUs from operator and assistant levels), we analysed it.
Wherever appropriate, we analysed similar interventions as subgroups. For example, CHX and tempered CHX and CPC, CPC+Zn+F and CPC plus domiphen bromide plus quaternary ammonium compounds.
As the active ingredient in any herbal mouthwash is the essential oil, we analysed different herbal mouthwashes and essential oil mouthwashes as subgroups.
We imputed data or calculated MDs or SDs either using the graphs or based on the available information (Table 18; Table 19; Table 20; Table 21). We imputed the P value (0.001) based on the description of results and calculated the SE in the Mohammed 1964 and Mohammed 1970 trials.
11. Data imputed in Feres 2010.
| Dentist | n | Mean (mm) | SE (mm) | Meana | SEa | SDa |
| No intervention | 15 | 3 | 1 | 41.7 | 13.9 | 53.83 |
| Water | 15 | 4 | 0.5 | 55.6 | 6.9 | 26.72 |
| CHX | 15 | 1 | 1 | 13.9 | 13.9 | 53.83 |
| CPC | 15 | 0.5 | 0.5 | 6.9 | 6.9 | 26.72 |
| Participant | n | Mean (mm) | SE (mm) | Meana | SEa | SDa |
| No intervention | 15 | 71 | 10 | 986.1 | 138.9 | 537.96 |
| Water | 15 | 44 | 10 | 611.1 | 138.9 | 537.96 |
| CHX | 15 | 15 | 3 | 208.3 | 41.7 | 161.5 |
| CPC | 15 | 17 | 4 | 236.1 | 55.6 | 215.34 |
| Combined (all locations) | n | Mean (mm) | SE (mm) | Meana | SEa | SDa |
| No intervention | 15 | 99 | 12 | 1375.0 | 166.7 | 645.63 |
| Water | 15 | 70 | 12 | 972.2 | 166.7 | 645.63 |
| CHX | 15 | 21 | 3 | 291.7 | 41.7 | 161.5 |
| CPC | 15 | 22 | 4.5 | 305.6 | 62.5 | 242.06 |
Data (mean and standard error (SE)) are derived from graph. SE was converted to standard deviation (SD) using the SD calculator. We used data reported at patient level, dentist level and all locations (combined). Authors also discussed the CFUs collected at the support board, which was not considered in analysis. aData are derived; CFU: colony‐forming unit; CHX: chlorhexidine; CPC: cetylpyridinium chloride; n: number of participants.
12. Data imputed in Joshi 2017.
| Area | Mean | SE | SDa |
| Patient | Tempered CPC 76.4 Cold CPC 103.6 Tempered CHX 70.2 Cold CHX 102.9 |
± 4.08 | ± 12.9 |
| Assistant | Tempered CPC 38.7 Cold CPC 47.3 Tempered CHX 38.5 Cold CHX 56.1 |
± 2.20 | ± 6.96 |
| Operator | Tempered CPC 62.2 Cold CPC 71.5 Tempered CHX 60 Cold CHX 75.8 |
± 2.48 | ± 7.84 |
Data given as mean and standard error (SE); SE converted to standard deviation (SD) using SD calculator based on the sample size of 10. aData were derived. CHX: chlorhexidine; CPC: cetylpyridinium chloride.
13. Data imputed in Retamal‐Valdes 2017.
| Clinician | Mean (cm) | SE (cm) | Meana | SDa |
| No rinsing | 2.1 | 2.4 | 644 | 736 |
| Water | 1 | 1.2 | 307 | 368 |
| CPC+ZN+F | 0.2 | 0.3 | 61 | 92 |
| CHX | 0.1 | 0.2 | 31 | 61 |
| Volunteer/patient | Mean (cm) | SE (cm) | Meana | SDa |
| No rinsing | 2.9 | 1.8 | 890 | 552 |
| Water | 3 | 1.2 | 920 | 368 |
| CPC+ZN+F | 1.3 | 1 | 399 | 307 |
| CHX | 1.2 | 0.6 | 368 | 184 |
| Combined (all locations) | Mean (cm) | SE (cm) | Meana | SDa |
| No rinsing | 8.2 | 7.5 | 2515 | 2301 |
| Water | 6.4 | 4.5 | 1963 | 1380 |
| CPC+ZN+F | 2.5 | 1.85 | 767 | 567 |
| CHX | 1.9 | 1 | 583 | 307 |
Data (mean and standard error (SE)) were derived from graph. SE was converted to standard deviation (SD) using the Review Manager 5 calculator. We used the data reported at patient level, dentist level and all locations (combined). Authors also discussed the CFU collected at the support board, which was not considered in analysis. aData were derived; CHX: chlorhexidine; CPC+Zn+F: cetylpyridinium chloride + zinc + fluoride.
14. Data imputed in Shetty 2013.
| Site | CHX (mean ± SDa) | Distilled water (mean ± SDa) |
| Operator | 10.35 ± 107.13 | 131.15 ± 107.13 |
| Patient | 1.95 ± 43.28 | 50.75 ± 43.28 |
| Assistant | 8 ± 100.08 | 120.85 ± 100.08 |
| Site | CHX (mean ± SDa) | Essential oil mouthwash A (tea tree oil) (mean ± SDa) |
| Operator | 10.35 ± 12.71 | 24.68 ± 12.71 |
| Patient | 1.95 ± 7.54 | 10.45 ± 7.54 |
| Assistant | 8 ± 10.9 | 20.29 ± 10.9 |
| Site | Essential oil mouthwash A (tea tree oil) (mean ± SDa) | Distilled water (mean ± SDa) |
| Operator | 24.68 ± 94.42 | 131.15 ± 94.42 |
| Patient | 10.45 ± 35.74 | 50.75 ± 35.74 |
| Assistant | 20.29 ± 89.18 | 120.85 ± 89.18 |
Data given in graph. Mean for chlorhexidine (CHX) and distilled water was given in graph. However, for essential oil mouthwash A (tea tree oil), mean was derived from digital graph analyser. Standard deviation (SD) calculated using mean and P value (P < 0.001). We used data reported at operator level, patient level and assistant level. aData were derived.
1. Chlorhexidine versus no rinsing
Two studies with 60 participants evaluated reduction in the level of contamination in particles larger than 5 µm (droplets) at distances less than 2 m from patient's oral cavity using a 0.12% CHX preprocedural rinse compared to no rinsing (Feres 2010; Retamal‐Valdes 2017). They evaluated the level of contamination at operator level but not assistant level.
At operator level
At the operator level, because of the high heterogeneity (I² = 89%), which we could not explain, we decided not to meta‐analyse the results (Analysis 1.1; Table 1).
1.1. Analysis.

Comparison 1: Chlorhexidine versus no rinsing, Outcome 1: Reduction in the level of contamination at < 2 m
2. Chlorhexidine versus water
At operator level
Six studies with 196 participants evaluated reduction in the level of contamination in particles larger than 5 µm (droplets) at distances of less than 2 m from patient's oral cavity using a CHX preprocedural rinse compared to distilled water at operator level (Feres 2010; Gupta 2014; Nayak 2020; Nisha 2021; Retamal‐Valdes 2017; Shetty 2013). Because of the high heterogeneity (I² = 98%), which we could not explain, we decided not to meta‐analyse the results (Analysis 2.1).
2.1. Analysis.

Comparison 2: Chlorhexidine versus water, Outcome 1: Reduction in the level of contamination at < 2 m
At assistant level
Four studies with 136 participants evaluated reduction in the level of contamination in particles larger than 5 µm (droplets) at distances less than 2 m from patient's oral cavity using a 0.2% CHX preprocedural rinse compared to distilled water at assistant level (Gupta 2014; Nayak 2020; Nisha 2021; Shetty 2013). Because of the high heterogeneity (I² = 96%), which we could not explain, we decided not to meta‐analyse the results (Analysis 2.1).
Bay 1993 evaluated the outcome at patient level and thus the data from this study were not included in the meta‐analysis.
Because of the high heterogeneity in these three analyses, we decided to perform sensitivity analysis by excluding studies in which the SDs were derived. However, this did not explain the heterogeneity.
Combined levels
Three studies with 80 participants evaluated reduction in the level of contamination in particles larger than 5 µm (droplets) at distances less than 2 m from patient's oral cavity using a CHX preprocedural rinse compared to distilled water (Feres 2010; Reddy 2012; Retamal‐Valdes 2017). The outcome was assessed as a combination of mean CFUs at different levels (patient, operator and assistant levels) (MD –632.94 CFUs, 95% CI –1267.33 to 1.45; I² = 92%; 3 studies, 80 participants; Analysis 2.1). Because of the high heterogeneity, we decided to perform a sensitivity analysis. Feres 2010 and Retamal‐Valdes 2017 used 0.12% CHX and presented the combined data from patient and operator level whereas Reddy 2012 used 0.2% CHX and presented the combined data from all three levels. After exclusion of the data from Reddy 2012, heterogeneity was less and the meta‐analysis suggested a benefit from CHX as a preprocedural rinse compared to distilled water (MD –956.11 CFUs, 95% CI –1626.04 to –286.19; I² = 67%; 2 studies, 60 participants; Analysis 2.1). See Table 2.
Tempered chlorhexidine
One study with 20 participants evaluated reduction in the level of contamination in particles larger than 5 µm (droplets) at distances less than 2 m from patient's oral cavity using tempered 0.2% CHX preprocedural rinse compared to rinsing with distilled water (Reddy 2012). The outcome was assessed as a combination of mean CFUs different levels (operator, assistant and patient levels). The results showed a benefit from the tempered CHX as a preprocedural rinse compared to distilled water (MD –101 CFUs, 95% CI –107.01 to –95.19; 1 study, 20 participants; Analysis 2.1). See Table 2.
3. Chlorhexidine versus saline
Two studies with 32 participants evaluated reduction in the level of contamination in particles larger than 5 µm (droplets) at distances less than 2 m from patient's oral cavity using a 0.2% CHX preprocedural rinse compared to saline (Mohan 2016; Verma 2017). See Table 3. The outcome was assessed as mean CFUs at different levels (Analysis 3.1).
3.1. Analysis.

Comparison 3: Chlorhexidine versus saline, Outcome 1: Reduction in the level of contamination at < 2 m
At three feet away from patient's mouth
One study compared 0.2% CHX preprocedural mouth rinse with saline on contamination at distances of 3 feet from the patient's mouth (exact location of the culture plate placement was not given in the report) (MD –668.40 CFUs, 95% CI –1044.45 to –292.35; 20 participants; Analysis 3.1) (Mohan 2016).
At operator level
One study evaluated this outcome at the operator level and the meta‐analysis suggested a benefit from the 0.2% CHX preprocedural mouth rinse compared to saline (MD –21.33 CFUs, 95% CI –36.80 to –5.86; 12 participants; Analysis 3.1) (Mohan 2016).
4. Chlorhexidine versus essential oils/herbal mouthwash
Five trials compared CHX with different types of essential oil/herbal preprocedural mouth rinse for reduction in the level of contamination in particles larger than 5 µm (droplets) at distances less than 2 m from patient's oral cavity (Bay 1993; Gupta 2014; Nayak 2020; Shetty 2013; Suresh 2011). See Table 4. The outcome was assessed as a combination of mean CFUs at different levels (operator, assistant, pharynx, or a combination) (Analysis 4.1).
4.1. Analysis.

Comparison 4: Chlorhexidine versus essential oils or herbal mouthwash, Outcome 1: Reduction in the level of contamination at < 2 m
Three trials compared 0.2% CHX mouthwash with essential oil/herbal mouthwashes at operator level (Gupta 2014; Nayak 2020; Shetty 2013). The effect estimates at both levels showed benefit from CHX compared to essential oil/herbal mouthwash (MD –23.09 CFUs, 95% CI –34.40 to –11.78; I² = 67%; 76 participants; Analysis 4.1).
Three trials compared 0.2% CHX mouthwash with essential oil/herbal mouthwashes at assistant level (Gupta 2014; Nayak 2020; Shetty 2013). The effect estimates at both levels showed benefit from CHX compared to essential oil/herbal mouthwash (MD –12.21 CFUs, 95% CI –15.58 to –8.83; I² = 7%; 76 participants; Analysis 4.1).
One trial compared 0.2% CHX with essential oil mouthwash B at 15 cm from the participant's mouth (exact location of the culture plate placement is not given in the report) (Suresh 2011). The effect estimates showed benefit from the CHX mouthwash compared to essential oil mouthwash B (MD –44.10 CFUs, 95% CI –59.12 to –29.08; 20 participants; Analysis 4.1).
Bay 1993 compared 0.12% CHX with essential oil mouthwash C (Listerine) at pharynx level (level 2), which was considered as patient level and hence was not analysed.
5. Chlorhexidine versus povidone iodine
Three studies compared CHX with povidone iodine for reduction in level of contamination in particles larger than 5 µm (droplets) at distances less than 2 m from patient's oral cavity (Kaur 2014; Tasneem 2017; Verma 2017). See Table 6.
At operator level
Aerobic
Two trials compared 0.2% CHX with 1% and 5% povidone iodine preprocedural mouth rinses at operator level using aerobic culture media (povidone iodine: 1%: Kaur 2014; 5%: Verma 2017). The analysis showed 95% CI crossing the line of no effect and the effect estimate was thus not robust (MD –10.75 CFUs, 95% CI –26.24 to 4.74; I² = 44%; 52 participants; Analysis 5.1). Both studies had high risk of bias and hence we did not perform a sensitivity analysis.
5.1. Analysis.

Comparison 5: Chlorhexidine versus povidone iodine, Outcome 1: Reduction in the level of contamination at < 2 m
Aerobic and anaerobic combined
Tasneem 2017 compared 0.2% CHX with povidone iodine preprocedural mouth rinses at operator level using both aerobic and anaerobic culture media (presented as combined mean CFUs). The effect estimate shows benefit from the CHX mouthwash compared with povidone iodine mouthwash (MD –4.70 CFUs, 95% CI –7.01 to –2.39; 30 participants; Analysis 5.1).
One of the studies compared CHX with povidone iodine for reduction in level of contamination in particles 5 µm or less (droplet nuclei) at distances of 2 m or more from patient's oral cavity (Kaur 2014). They compared 0.2% CHX with 1% povidone iodine preprocedural mouth rinse using aerobic and anaerobic culture media at 9 feet behind the patient. The analysis showed 95% CI crossing the line of no effect and the effect estimates are thus not robust (aerobic: MD 0.20 CFUs, 95% CI –7.28 to 7.68; 40 participants; anaerobic: MD –0.80 CFUs, 95% CI –11.65 to 10.05; 40 participants; Analysis 5.2).
5.2. Analysis.

Comparison 5: Chlorhexidine versus povidone iodine, Outcome 2: Reduction in level of contamination at ≥ 2 m
6. Chlorhexidine versus cetylpyridinium chloride
Three trials compared CHX with CPC mouthwash for reduction in the level of contamination in particles larger than 5 µm (droplets) at distances less than 2 m from patient's oral cavity (Feres 2010; Joshi 2017; Retamal‐Valdes 2017). See Table 7.
Tempered 0.2% chlorhexidine versus tempered 0.05% cetylpyridinium chloride
Joshi 2017 reported the effect estimates at operator and assistant levels for this comparison. The analysis showed the 95% CI crossing the line of no effect (operator: MD –2.20 CFUs, 95% CI –9.07 to 4.67; 20 participants; assistant: MD –0.20 CFUs, 95% CI –6.30 to 5.90; 20 participants; Analysis 6.1).
6.1. Analysis.

Comparison 6: Chlorhexidine versus cetylpyridinium chloride (CPC), Outcome 1: Reduction in the level of contamination at < 2 m
Cold 0.12% or 0.2% chlorhexidine versus cold 0.05% cetylpyridinium chloride
Two trials reported the effect estimates at operator level for this comparison using 0.12% (Feres 2010) and 0.2% (Joshi 2017) CHX. The analysis showed the 95% CI crossing the line of no effect (MD 4.43 CFUs, 95% CI –2.27 to 11.13; I² = 0%; 50 participants; Analysis 6.1). Joshi 2017 reported the effect estimate at assistant level only, which showed benefit of using cold CPC (MD 8.80, 95% CI 2.70 to 14.90; 20 participants; Analysis 6.1).
Tempered 0.2% chlorhexidine versus cold 0.05% cetylpyridinium chloride
Joshi 2017 reported the effect estimates at operator and assistant levels for this comparison. The meta‐analyses showed benefit in the tempered CHX group at both the levels (operator: MD –11.50 CFUs, 95% CI –18.37 to –4.63; 20 participants; assistant: MD –8.80 CFUs, 95% CI –14.90 to –2.70; 20 participants; Analysis 6.1).
Cold 0.2% chlorhexidine versus tempered 0.05% cetylpyridinium chloride
Joshi 2017 reported the effect estimates at operator and assistant levels for this comparison. The meta‐analyses showed benefit in the tempered CPC group at both the levels (operator: MD 13.60 CFUs, 95% CI 6.73 to 20.47; 20 participants; assistant: MD 17.40 CFUs, 95% CI 11.30 to 23.50; 20 participants; Analysis 6.1).
Chlorhexidine 0.12% versus cetylpyridinium chloride plus zinc plus sodium fluoride
Retamal‐Valdes 2017 reported the effect estimates at operator level for this comparison. The analysis showed 95% CI crossing the line of no effect (MD –30.00 CFUs, 95% CI –85.86 to 25.86; 30 participants; Analysis 6.1).
7. Tempered chlorhexidine versus non‐tempered chlorhexidine
Two trials compared tempered 0.2% CHX with non‐tempered CHX for reduction in the level of contamination in particles larger than 5 µm (droplets) at distances less than 2 m from patient's oral cavity (Joshi 2017; Reddy 2012). See Table 8.
Joshi 2017 reported the effect estimates at operator and assistant levels for this comparison. The meta‐analyses showed benefit in the tempered CHX group at operator and assistant levels (operator: MD –15.80 CFUs, 95% CI –22.67 to –8.93; 20 participants; 1 study; assistant: MD –17.60 CFUs, 95% CI –23.70 to –11.50; 20 participants; Analysis 7.1).
7.1. Analysis.

Comparison 7: Tempered chlorhexidine versus non‐tempered chlorhexidine, Outcome 1: Reduction in the level of contamination at < 2 m
Reddy 2012 reported the effect estimate as combined mean CFUs (patient, operator and assistant levels). The meta‐analyses showed benefit in the tempered CHX group (MD –10.80 CFUs, 95% CI –15.15 to –6.45; 20 participants; 1 study; Analysis 7.1).
8. Cetylpyridinium chloride versus no rinsing
One study compared 0.05% CPC with no rinsing (Feres 2010), and another study compared CPC+Zn+F with no rinsing (Retamal‐Valdes 2017). Two studies compared, CPC plus domiphen bromide plus quaternary ammonium compounds with no rinsing (Mohammed 1964; Mohammed 1970). All comparisons were for reduction in the level of contamination in particles larger than 5 µm (droplets) at distances less than 2 m from patient's oral cavity. Feres 2010 and Retamal‐Valdes 2017 reported the mean CFUs at the operator level. The other two studies combined the CFUs and expressed the outcomes as count data. Therefore, we decided to use the generic inverse variance method to analyse the data. See Table 9 for summary of findings.
At the operator level, Feres 2010 and Retamal‐Valdes 2017 studies showed benefit from using CPC or CPC+Zn+F versus no rinsing (CPC: MD –34.80 CFUs, 95% CI –65.92 to –3.68; 30 participants; 1 study; CPC+Zn+F: MD –583.00 CFUs, 95% CI –958.36 to –207.64; 30 participants; 1 study; Analysis 8.1).
8.1. Analysis.

Comparison 8: Cetylpyridinium chloride (CPC) versus no rinsing, Outcome 1: Reduction in level of contamination at < 2 m [CFUs]
The meta‐analysis of Mohammed 1964 and Mohammed 1970 showed benefit from using CPC plus domiphen bromide plus quaternary ammonium compounds (MD –0.75 CFUs, 95% CI –1.32 to –0.18; 200 participants; I² = 60%; Analysis 8.1).
9. Cetylpyridinium chloride versus water
Feres 2010 compared 0.05% CPC with water rinsing, and Retamal‐Valdes 2017 compared CPC+Zn+F with water rinsing. Mohammed 1964 compared CPC plus domiphen bromide plus quaternary ammonium compounds with water rinsing. All comparisons were for reduction in the level of contamination in particles larger than 5 µm (droplets) at distances less than 2 m from patient's oral cavity. Feres 2010 and Retamal‐Valdes 2017 reported the mean CFUs at the operator level. Mohammed 1964 combined the CFUs and expressed the outcomes as count data. Therefore, we decided to use the generic inverse variance method to analyse the data. See Table 10 for summary of findings.
At the operator level, two trials showed benefit from using CPC (Feres 2010) and CPC+Zn+F (Retamal‐Valdes 2017) (CPC: MD –48.70 CFUs, 95% CI –67.82 to –29.58; 1 study, 30 participants; CPC+Zn+F: MD –246.00 CFUs, 95% CI –437.96 to –54.04; 1 study, 30 participants; Analysis 9.1).
9.1. Analysis.

Comparison 9: Cetylpyridinium chloride (CPC) versus water, Outcome 1: Reduction in level of contamination at < 2 m [CFUs]
Mohammed 1964 showed benefit from using CPC plus domiphen bromide plus quaternary ammonium compounds (MD –0.78 CFUs, 95% CI –1.24 to –0.31; 105 participants; Analysis 9.1).
10. Tempered cetylpyridinium chloride versus cold cetylpyridinium chloride
Joshi 2017 compared tempered 0.05% CPC with cold 0.05% CPC for reduction in the level of contamination in particles larger than 5 µm (droplets) at distances less than 2 m from patient's oral cavity at operator and assistant levels. See Table 11 for summary of findings.
The effect estimates showed benefit from using the tempered CPC at both the levels (operator: MD –9.30 CFUs, 95% CI –16.17 to –2.43; 20 participants; assistant: MD –8.60 CFUs, 95% CI –14.70 to –2.50; 20 participants; Analysis 10.1).
10.1. Analysis.

Comparison 10: Tempered cetylpyridinium chloride (CPC) versus cold CPC, Outcome 1: Reduction in the level of contamination at < 2 m
11. Povidone iodine versus saline
Verma 2017 compared 5% povidone iodine with saline as preprocedural mouth rinse for reduction in the level of contamination in particles larger than 5 µm (droplets) at distances less than 2 m from patient's oral cavity at operator level. See Table 12 for a summary of findings.
The effect estimates showed benefit from using the povidone iodine (MD –16.50 CFUs, 95% CI –32.65 to –0.35; 12 participants; Analysis 11.1).
11.1. Analysis.

Comparison 11: Povidone iodine versus saline, Outcome 1: Reduction in the level of contamination at < 2 m
12. Essential oil/herbal mouthwash versus water
Three studies compared mouthwashes containing different essential oils versus water at operator and assistant level for reduction in the level of contamination in particles larger than 5 µm (droplets) at distances less than 2 m from patient's oral cavity (Gupta 2014; Nayak 2020; Shetty 2013). One study compared another mouthwash containing essential oils versus water and reported CFUs at the different levels using Andersen's air sampler (Bay 1993). Level 2 (pharynx level) is considered as patient level and was not analysed.
At both the operator and assistant levels, because of the high heterogeneity (operator: I² = 98%; assistant: I² = 99%), which we could not explain, we decided not to meta‐analyse the results.
See Analysis 12.1 and Table 13 for a summary of findings.
12.1. Analysis.

Comparison 12: Essential oils/herbal mouthwash versus water, Outcome 1: Reduction in the level of contamination at < 2 m
13. Chlorhexidine versus ozonated water
One study of 40 participants comparing 0.2% CHX rinsing versus ozonated water rinsing reported two outcomes: reduction in the level of contamination in particles larger than 5 µm (droplets) at distances less than 2 m from patient's oral cavity at operator level using aerobic culture method and reduction in the level of contamination in particles 5 µm or less (droplet nuclei) at distances 2 m or more from patient's oral cavity using aerobic and anaerobic culture methods (Kaur 2014). See Table 14 for a summary of findings.
For reduction in the level of contamination in particles larger than 5 µm (droplets) at distances less than 2 m from patient's oral cavity, the study showed benefit from CHX rinsing at operator level compared to ozonated water (MD –17.80 CFUs, 95% CI –33.70 to –1.90). However, for reduction in the level of contamination in particles 5 µm or less (droplet nuclei) at distances 2 m or more from patient's oral cavity, the 95% CI crossed the line of no effect for aerobic and anaerobic types of culture methods (aerobic: MD –12.30 CFUs, 95% CI –27.12 to 2.52; anaerobic: MD –3.40 CFUs, 95% CI –14.66 to 7.86; Analysis 13.1; Analysis 13.2).
13.1. Analysis.

Comparison 13: Chlorhexidine versus ozonated water, Outcome 1: Reduction in the level of contamination at < 2 m
13.2. Analysis.

Comparison 13: Chlorhexidine versus ozonated water, Outcome 2: Reduction in the level of contamination at ≥ 2 m
14. Povidone iodine versus ozonated water
One study of 40 participants comparing 1% povidone iodine rinsing versus ozonated water rinsing reported two outcomes: reduction in the level of contamination in particles larger than 5 µm (droplets) at distances less than 2 m from patient's oral cavity at operator level using an aerobic culture method and reduction in the level of contamination in particles 5 µm or less (droplet nuclei) at distance 2 m or more from patient's oral cavity using aerobic and anaerobic culture methods (Kaur 2014). See Table 15 for a summary of findings.
The study found no benefit for either outcome from povidone iodine rinsing at operator level compared to ozonated water as the 95% CI crossed the line of no effect (operator level at less than 2 m: MD 3.50 CFUs, 95% CI –17.10 to 24.10; more than 2 m with aerobic culture method: MD –12.50 CFUs, 95% CI –26.33 to 1.33; more than 2 m with anaerobic culture method: MD –2.60 CFUs, 95% CI –9.54 to 4.34; Analysis 14.1; Analysis 14.2).
14.1. Analysis.

Comparison 14: Povidone iodine versus ozonated water, Outcome 1: Reduction in the level of contamination at < 2 m
14.2. Analysis.

Comparison 14: Povidone iodine versus ozonated water, Outcome 2: Reduction in the level of contamination at ≥ 2 m
15. Chlorhexidine versus boric acid
One study of 60 participants compared 0.12% CHX versus 0.75% boric acid as preprocedural mouth rinse for reduction in the level of contamination in particles larger than 5 µm (droplets) at distances less than 2 m from patient's oral cavity at operator level and assistant level (Nisha 2021). The effect estimates showed benefit from using the CHX mouth rinse compared to boric acid mouth rinse (operator level: MD –26.60 CFUs, 95% CI –28.24 to –24.96; assistant level: MD –8.03 CFUs, 95% CI –9.65 to –6.41; Analysis 15.1). See Table 16 for a summary of findings.
15.1. Analysis.

Comparison 15: Chlorhexidine versus boric acid, Outcome 1: Reduction in the level of contamination at < 2 m
16. Boric acid versus water
One study of 60 participants compared 0.75% boric acid versus water as preprocedural mouth rinse for reduction in the level of contamination in particles larger than 5 µm (droplets) at distances less than 2 m from patient's oral cavity (Nisha 2021). The effect estimates showed benefit from using the boric acid mouth rinse compared to water (operator: MD –29.30 CFUs, 95% CI –32.65 to –25.95; assistant: MD –14.04 CFUs, 95% CI –16.47 to –11.61; Analysis 16.1). See Table 17 for a summary of findings.
16.1. Analysis.

Comparison 16: Boric acid versus water, Outcome 1: Reduction in the level of contamination at < 2 m
Discussion
Summary of main results
We found 17 studies that evaluated 16 comparisons in this Cochrane Review. None of the studies measured the incidence of infection of dental healthcare providers. Sixteen studies reported the results for reduction in the level of bacterial contamination by particles larger than 5 µm (droplets) in the operative environment.
Two trials compared CHX versus no rinsing at operator (dentist) level. One of the two trials showed beneficial effect; however, the 95% CI crossed the midline in one trial and the meta‐analysis showed high heterogeneity (Table 1).
Five trials compared CHX versus water at operator and assistant level but showed high heterogeneity that prevented meta‐analysis. All trials showed a beneficial effect, but the MDs ranged from –1380 CFUs to –16 CFUs (Table 2).
One trial compared CHX versus saline at operator level and found a beneficial effect in the CHX group to reduce the level of contamination (MD –21.33 CFUs, 95% CI –36.80 to –5.86; 12 participants) (Table 3).
Five trials compared CHX versus three different essential oils. At operator level, CHX reduced the level of contamination more than essential oil/herbal mouthwash (MD –23.09 CFUs, 95% CI –34.40 to –11.78; 3 studies, 76 participants; very low‐certainty evidence) and at assistant level (MD –12.21 CFUs, 95% CI –15.58 to –8.83; 3 studies, 76 participants; very low‐certainty evidence) (Table 4).
There was no evidence of an effect of CHX on contamination at less than 2 m compared to povidone iodine in two trials measured as aerobic cultures only (MD –10.75 CFUs, 95% CI –26.24 to 4.74; 52 participants; very low‐certainty evidence), but there was a small effect when measured with combined aerobic and anaerobic cultures in a third trial (MD –4.70 CFUs, 95% CI –7.01 to –2.39; 30 participants; very low‐certainty evidence). There was also no effect at more than 2 m distance measured in aerobic or anaerobic cultures (aerobic: MD 0.20 CFUs, 95% CI –7.28 to 7.68; 1 study, 40 participants; anaerobic: MD –0.80 CFUs, 95% CI –11.65 to 10.05; 1 study, 40 participants; both very low‐certainty evidence) (Table 6).
Three trials compared CHX to CPC or CPC+Zn+F. There was no evidence of a difference between CHX and CPC at operator level in two trials (MD 4.43 CFUs, 95% CI –2.27 to 11.13; 50 participants; very low‐certainty evidence), but there was an effect at the assistant level (MD 8.80 CFUs, 95% CI 2.70 to 14.90; 20 participants; very low‐certainty evidence). There was no evidence of an effect compared to CPC+Zn+F at operator level (MD –30.00 CFUs, 95% CI –85.86 to 25.86; 30 participants; very low‐certainty evidence). One trial compared various combinations of tempered CHX and CPC and found that the tempered rinse was better than the cold regardless of the content (Table 7).
Tempered (47 °C) CHX reduced contamination more than non‐heated CHX at operator level in one study (MD –15.80 CFUs, 95% CI –22.67 to –8.93; 20 participants; very low‐certainty evidence) with similar results for the assistant level and for the combined operator and assistant level in another study (Table 8).
None of the 17 studies provided data on costs or change of microbiota in the patient's mouth or adverse events such as transient discolouration, altered taste, allergic reaction, hypersensitivity, or acceptability and feasibility of the intervention to patients.
The certainty of the evidence was low to very low for all comparisons due to risk of bias, imprecision, inconsistency, or a combination of these.
Overall completeness and applicability of evidence
‐The primary objective of this review was to measure the incidence of infection among dental healthcare providers who were exposed to aerosols. We understand that it is difficult to conduct trials to focus on this primary objective. Dental healthcare providers could become infected in many ways other than aerosols produced during the AGPs in a dental clinic and practically it is difficult to differentiate the origin of infection.
All included studies reported reduction in the level of contamination in the operative environment at operator and assistant levels. The outcome measure was bacterial CFUs, which were counted in an aerobic or anaerobic culture media or both. However, none of these studies checked for the viral or fungal organisms in the aerosols. This could be because of the difficulties in viral culture or comparatively lower prevalence of aerosol‐borne fungal infections. The CFU is a surrogate measure for this outcome and may not represent the reduction of aerosol in the operative environment. In addition to this, we found no information on minimally important clinical difference (MICD) for CFUs in order to determine the effectiveness of the preprocedural mouth rinse interventions. For these reasons, we are unsure how these results can be interpreted.
The acceptable bacterial load (total viable count) in dental unit water lines must be between 100 CFU/mL and 200 CFU/mL (DoH 2013), and a heterotrophic bacterial load of 500 CFU or less for infection control in dental healthcare system (ADA 1996; CDC 2003). However, none of the included studies tested or reported this.
One study used active aerosol sampling (Bay 1993). The other 16 studies used passive aerosol sampling where the aerosols drop down to settle on the culture media. Such measurements are useful in a surgical theatre where wound contamination is possible due to the contaminated aerosols. However, in a dental clinic, it would be more apt to measure both the active aerosols (using devices such as slit sampler and the N6‐Andersen sampler in combination with DG18 (dichloran 18% glycerol agar) and MEA (malt extract agar) (Pasquarella 2000)) and passive aerosols.
There is no standardisation of outcome measurement methods. The most commonly agreed measure, index of microbial air contamination (IMA), was not established until 2000. The IMA for a dental clinic is 25 (which equates to 10 CFU/dm²/hour to 39 CFU/dm²/hour), which is a good performance indicator at a high‐risk venue such as a dental clinic. The IMA is based on the microbial count on Petri dishes of 9 cm in diameter left open to the air for one hour, 1 m from the floor, at least 1 m away from walls or any obstacle (Pasquarella 2000). Even after 2000, many trials did not use this standard. The varied duration, height and distances used in the included studies could be one of the possible reasons for high heterogeneity in meta‐analyses in our review.
Patarakulwiwat 2018, in their experimental study, showed that magnetostrictive ultrasonic scalers produce more droplets compared to piezoelectric scalers. As we included four studies using each type of scaler, we explored the possibility of heterogeneity due to the difference in the type of scaler. However, this was not the reason for heterogeneity in the meta‐analyses in this review.
All 17 included studies tested the aerosols generated during ultrasonic scaling, tooth preparation procedures using high‐speed air rotor instruments or air polishing. No studies tested other AGPs such as cavity preparation of carious teeth or surgical extractions. Procedures such as impressions or intraoral radiography that can induce cough were also not tested. Therefore, it is difficult to extrapolate the results of this review to all procedures in dentistry.
Only one included study tested the contamination due to particles 5 µm or less (droplet nuclei) at 2 m or more from patient's oral cavity. Dental surgeon and dental assistants are usually seated at less than 2 m from the patient's mouth and it is probable that this is the reason few studies have tested the contamination at greater distances.
There is also a possibility of contamination in the dental operative environment that could have been cultured along with the bacteria from the aerosols. Only three included studies had fumigated the rooms where the procedures were carried out (Joshi 2017; Nisha 2021; Verma 2017). Another possibility of contamination is through the bacteria present in the waterlines. Only five included studies addressed this issue by flushing the water for few minutes before starting the AGP (Bay 1993; Feres 2010; Fine 1993a; Nayak 2020; Shetty 2013). This issue of contamination could have been investigated by using the control culture media (baseline data). However, only seven included studies reported such baseline data (Bay 1993; Feres 2010; Fine 1993a; Kaur 2014; Reddy 2012; Retamal‐Valdes 2017; Verma 2017).
Although we had 17 trials included in this review, most of the comparisons were based on the results of single trials and could not be combined in meta‐analyses. It appears that tempered or tempered CHX and CPC mouthwashes show better reduction in contamination compared with colder versions of the same mouthwashes in individual trials. However, the evidence was of very low certainty for all comparisons. High‐quality RCTs are required to test the contamination of the dental operative environment with different micro‐organisms (aerobic and anaerobic bacteria, fungi and viruses).
We could not meta‐analyse a cross‐over trial as the authors did not report the data from the first phase of the study (Fine 1993a). We could not include Bay 1993 in the meta‐analysis as the aerosols were sampled directly from the patient's oropharynx and thus did not measure the contamination at operator or assistant level.
Quality of the evidence
The certainty of the evidence for all comparisons was low or very low for the considered outcomes. Most comparisons were evaluated by single trials with a relatively small number of participants and low event rates. We downgraded the certainty of the evidence mainly for high/unclear risk of bias, imprecision and inconsistency.
Potential biases in the review process
There is a possibility of unpublished data that we could have missed in this review. The Analyses 1.1.2 (Analysis 1.1), Analysis 2.1, Analysis 3.1.1 (Analysis 3.1) and 5.1.1 (Analysis 5.1) showed considerable heterogeneity and we were unable to explain this. There were huge differences in the CFUs between the included studies, which could be related multiple factors such as varying inclusion criteria of participants in the studies, oral hygiene status of those participants, different rinsing times, different AGPs, waterline contamination, varying fallow time and disinfection of dental clinic. Moreover, the included studies did not provide any details on the variation in the duration and speed of using high‐speed rotary instruments or ultrasonic scalers between different dentists and combination of AGPs in a single dental procedure (e.g. use of 3‐in‐1 syringe with both air and water and use of high‐speed rotary instruments), which could influence the quantity of droplets and droplet nuclei generated.
Agreements and disagreements with other studies or reviews
There are several systematic reviews on the use of preprocedural mouthwashes to reduce viral load in dental aerosols.
Samaranayake 2021 investigated the efficacy of preprocedural rinses, high‐volume evacuators and rubber dam in reducing bioaerosols produced during AGPs. They included 17 clinical trials, of which seven tested the efficacy of preprocedural mouth rinses (we included three of these studies in our review (Feres 2010; Fine 1993a; Retamal‐Valdes 2017) and excluded four (Dawson 2016; Fine 1992; Klyn 2001; Logothetis 1995)). All but one study included in their review showed a significant bacterial reduction after preprocedural rinsing with either CHX, CPC or essential oils. In contrast to our review, their review analysed the effect of the interventions at patient level also. We also analysed the outcome at various distances from the point of aerosol generation.
Mohd‐Said 2021 conducted a systematic review on the effectiveness of preprocedural mouth rinses in reducing aerosol contamination during periodontal prophylaxis. The review included 21 RCTs and reported significant reductions in bacterial contaminations measured in CFUs. Unlike our review, Mohd‐Said 2021 included split‐mouth trials and cross‐over trials (although cross‐over trials were not excluded from our review, we planned to include only first‐period data). We judged two of the included studies in that review to be non‐RCTs (Paul 2020; Rajachandrasekaran 2019), and it had a cross‐over trial that we excluded as the cross‐over sequence was not randomised (Santos 2014). The review authors used the Cochrane RoB 2 tool.
The Moosavi 2020 review included five original studies that used mouth washes as preprocedural or as a regular home use mouth rinse, after brushing. The review also included studies that used mouthwashes in people with COVID‐19. The outcome of interest was the reduction of viral load in saliva in people using mouthwashes, reduction of viral load in people with COVID‐19 and prevention of ventilator‐assisted pneumonia. Most included studies used C31G, povidone iodine and CHX as mouth rinses. Other mouth rinses were not discussed in this review. This is in contrast to our review which included all the other mouth rinses. The authors concluded that the use of mouthwash before dental procedures reduces the risk of transmission of the virus through the aerosol and saliva to the dental team and the use of this mouthwash in people with COVID‐19 improves systemic problems associated with oral microbial flora.
A systematic review by Marui 2019 also investigated the effect of preprocedural mouth rinses on the reduction of CFUs in dental aerosols. It included 13 RCTs that tested the efficacy of CHX, essential oils, CPC and herbal products. Meta‐analysis of 12 studies showed that mouth rinses with CHX, essential oils and CPC significantly reduced percentage of CFU reduction. The authors concluded that there is moderate evidence that preprocedural mouth rinses significantly reduce the number of micro‐organisms in the dental aerosols. In contrast to our review, this meta‐analysis did not include subgroups at various levels such as assistant, operator or patient, neither did they considered distance. They used 'mean percentage of CFU reduction' as the outcome measure and did not explain at which levels they had taken the CFU counts, which could be the reason for disagreement with our results.
Two Cochrane Reviews carried out by Burton and colleagues focused specifically on COVID‐19. They aimed to evaluate the RCT evidence for patient use of antimicrobial mouth washes before any AGP to prevent possible transmission of COVID‐19 to healthcare workers (Burton 2020a; Burton 2020b). They identified no trials, but these reviews are currently being updated.
Authors' conclusions
Implications for practice.
None of the included studies reported incidence of infection among dental healthcare providers. The analyses suggest that chlorhexidine, especially when tempered, and other mouth rinses may reduce bacterial contamination in aerosols, but it is not possible to draw any reliable conclusions because we have only low‐ to very low‐certainty evidence and we do not know how colony‐forming unit (CFU) measurement relates to infection risk. None of the included trials tested the reduction in level of viral or fungal contamination in droplets or aerosols. Overall, we are unable to draw conclusions regarding whether there is a role for preprocedural mouth rinses in reducing infection risk or the possible superiority of one preprocedural rinse over another.
Implications for research.
Research could determine the most effective methods to reduce contaminated aerosols generated during dental procedures (Group A, B and C aerosol‐generating procedures (AGPs); SDCEP 2021) by conducting well‐planned randomised controlled trials (RCTs) including the outcomes developed through any of the core outcome consortiums such as COMET (COMET 2020) and reporting following CONSORT 2010 recommendations.
In designing such clinical trials, we suggest the following be considered.
Evidence:
trials focusing on testing similar mouth rinses (concentration and dose);
trials focusing on measuring contamination through droplets and droplet nuclei;
studies measuring patient‐reported outcomes (acceptability and feasibility) and cost‐effectiveness.
Population:
trials testing different types of AGPs (Group A, B and C (SDCEP 2021));
trials of participants' head position while conducting the AGPs to allow standardisation and reporting.
Intervention:
focusing on similar mouth rinses used in earlier studies such as chlorhexidine 0.2% rinsing for one minute. This will supplement the existing evidence base allowing us to draw more robust conclusions;
methods to eliminate all possible contamination such as fumigation of dental operative environment and waterline disinfection;
tempered mouthwashes compared with the colder versions to evaluate the role of temperature;
standardisation of participant inclusion criteria, oral hygiene status, rinsing time, fallow time and disinfection methods.
Comparison:
trials comparing the active intervention to rinsing with saline or water as the best placebo.
Outcomes:
testing contamination of droplets near the operating dental surgeon and dental assistant seating positions rather than in other different directions;
testing contamination of aerosols at distances further than 2 m;
undertaking a consensus exercise to establish what to measure as the contamination outcome and how it should be measured, for example through a COMET initiative. This could include active air sampling and the standard index of microbial air contamination;
experiments or calculations on how contamination outcomes can be transformed to reflect infection risk.
History
Protocol first published: Issue 12, 2020
Acknowledgements
We are thankful to Cochrane Oral Health: Anne Littlewood, Information Specialist; Laura MacDonald, Managing Editor; Prof Anne Marie Glenny and Prof Jan Clarkson, Co‐ordinating Editors; Dr Phil Riley, Deputy Co‐ordinating Editor; Prof Ana Jeroncic, Statistical Editor and Prof Helen Worthington, Emeritus Co‐ordinating Editor. We also thank Derek Richards and Nicola Innes for comments, and Jessica Sharp and Anne Lawson for copy editing. We thank Prof Dr Patrick Kee Peng Kong, Chief Executive and Vice‐Chancellor and Prof Dr Jaspal Singh Sahota, Faculty of Medicine, Manipal University College Malaysia and Professor Dr Abdul Rashid Hj Ismail, Dean, Faculty of Dentistry, Manipal University College Malaysia for constant support to undertake Cochrane Reviews. We are grateful to Professor Dr Adinegara Lutfi Abas, Pro Vice‐Chancellor, Manipal University College Malaysia for statistical advice.
We thank Dr Sheema Tasneem, Dr Amruta Joshi, Prof Dr Vandana R Desai, Dr Neha Verma and Prof Dr Balaji Manohar, the authors of the included studies who promptly responded to our queries. We are grateful to our stakeholders Dr Kedar Kulkari (Australia), Dr Anajali Madan (Bahrain), Dr Shaima Al‐mashhadani (Dubai), Prof Dr Vandana R Desai and Prof Dr Ashok Lingappa (India), Dr Ahmad Sofi Mahmudi and Dr Erfan Shamsoddin (Iran), Dr Dalia Abdullah and Prof Dr Sunil Kumar Nettem (Malaysia), and Dr Mark‐Steven Howe and Dr Alexander Legge (UK), who helped us with prioritising the top seven comparisons that were presented as summary of findings tables.
Appendices
Appendix 1. Cochrane Oral Health's Trials Register search strategy
Cochrane Oral Health’s Trials Register is available via the Cochrane Register of Studies. For information on how the register is compiled, see https://oralhealth.cochrane.org/trials
1 MESH DESCRIPTOR Air Microbiology AND INREGISTER 2 MESH DESCRIPTOR Air Pollution, Indoor AND INREGISTER 3 MESH DESCRIPTOR Aerosols AND INREGISTER 4 MESH DESCRIPTOR Inhalation Exposure AND INREGISTER 5 (aerosol* or bioaerosol*) AND INREGISTER 6 (droplet* or splatter* or spatter* or microbe* or bacillus or germ* or microorganism* or virus* or viral or coronavirus* or COVID* or "middle east? respiratory syndrome*" or MERS or MERS‐CoV or "camel flu" or SARS or "sudden acute respiratory syndrome*" or "Wuhan virus*" or 2019‐nCoV or SARS‐CoV‐2 or SARS‐CoV or SARS‐CoV‐1 or SARS‐1) AND INREGISTER 7 (air near5 (pollut* or quality or impur*)) AND INREGISTER 8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 9 MESH DESCRIPTOR Mouthwashes EXPLODE ALL AND INREGISTER 10 (mouthwash* or gargl* or mouthrins*) AND INREGISTER 11 ((oral or mouth or larynx* or pharynx* or intraoral) and (irrigat* or lavag* or wash* or rins* or decontaminat* or mist or clean*)) AND INREGISTER 12 MESH DESCRIPTOR Chlorhexidine EXPLODE ALL AND INREGISTER 13 MESH DESCRIPTOR Povidone‐Iodine EXPLODE ALL AND INREGISTER 14 MESH DESCRIPTOR Cetylpyridinium EXPLODE ALL AND INREGISTER 15 MESH DESCRIPTOR Hexetidine EXPLODE ALL AND INREGISTER 16 MESH DESCRIPTOR Anti‐Infective Agents, Local EXPLODE ALL AND INREGISTER 17 MESH DESCRIPTOR Hydrogen Peroxide EXPLODE ALL AND INREGISTER 18 MESH DESCRIPTOR Carbamide Peroxide EXPLODE ALL AND INREGISTER 19 MESH DESCRIPTOR Triclosan EXPLODE ALL AND INREGISTER 20 MESH DESCRIPTOR Oils, Volatile EXPLODE ALL AND INREGISTER 21 MESH DESCRIPTOR Plant oils EXPLODE ALL AND INREGISTER 22 MESH DESCRIPTOR Plant Extracts EXPLODE ALL AND INREGISTER 23 MESH DESCRIPTOR Menthol AND INREGISTER 24 MESH DESCRIPTOR Lavandula AND INREGISTER 25 MESH DESCRIPTOR Thymus plant AND INREGISTER 26 MESH DESCRIPTOR Mentha piperita AND INREGISTER 27 MESH DESCRIPTOR Eugenol AND INREGISTER 28 MESH DESCRIPTOR Cinnamomum verum AND INREGISTER 29 MESH DESCRIPTOR Muramidase AND INREGISTER 30 MESH DESCRIPTOR Lactoferrin AND INREGISTER 31 MESH DESCRIPTOR Glucose oxidase AND INREGISTER 32 MESH DESCRIPTOR Lactoperoxidase AND INREGISTER 33 MESH DESCRIPTOR Benzethonium AND INREGISTER 34 MESH DESCRIPTOR Teas, Herbal AND INREGISTER 35 MESH DESCRIPTOR Cymbopogon AND INREGISTER 36 MESH DESCRIPTOR Sesame Oil AND INREGISTER 37 (povidone or chlorhexidine or CHX or PVP or Polyvinylpyrrolidone or Betadine* or Providine* or Disadine* or Isodine* or Pharmadine* or Alphadine* or Betaisodona or Tubulicid or Novalsan or Sebidin or MK‐412A or MK412A) AND INREGISTER 38 (Chlorhexamed or Corsodyl or Curasept or Dyna‐Hex or Eludril or Gibitan or Hexidine or Hibiclens or Hibident or Hibiscrub or Hibisol or Hibitane or Peridex or avagard) AND INREGISTER 39 (Hexadecylpyridinium or Cetylpyridium or Biosept or Ceepryn or Cetamium or Catamium or Sterogenol or Dobendan or Merocets or Pristacin or Pyrisept or Angifonil or Cetylyre) AND INREGISTER 40 (Hexigel or Steri‐sol or "Steri sol" or Hextril or Oraldine or Oralspray or Hexoral or Bactidol or Elsix or Duranil or Doreperol or Hexetidine) AND INREGISTER 41 (Hydrogen Peroxide or H2O2 or Hydroperoxide or Superoxol or Oxydol or Perhydrol or Urea Peroxide or Perhydrol Urea) AND INREGISTER 42 (Methyl salicylate or methylsalicylate or Rheumabal or Metsal Liniment or Hewedolor or Linsal) AND INREGISTER 43 (Tricolsan or Hydroxydiphenyl or trichlorodiphenyl or Clearasil or Cliniclean or Irgasan or Trisan or Oxy Skin Wash or pHisoHex or Sapoderm or Tersaseptic or Aquasept or Ster‐Zac or Manusept or Microshield) AND INREGISTER 44 ((irrigat* or rins* or wash* or lavag* or intraoral* or topical) and (antimicrobial or anti‐microbial or disinfect* or antisept* or anti‐infect* or herbal)) AND INREGISTER 45 ("essential oil*" or "plant oil*" or menthol or menthyl or (mint near2 oil*) or lavender or thyme or peppermint or "mentha piperita" or eugenol or eucalyptus or "blue gum*" or cajeput or clove or cinnamon) AND INREGISTER 46 (muramidase or lysozyme* or leftose or lactoferrin or lactotransferrin or "glucose oxidase" or lactoperoxidase or "saliva substitute") AND INREGISTER 47 (Listerine or Biotene) AND INREGISTER 48 ("chlorine dioxide" or "Benzethonium chloride" or "Bencetonium Chloride" or "Formula Magic" or "Hyamine 1622" or "Orchid Fresh II" or Phemeride or Phemerol or Phemethryn or Puri‐Clens or Quatrachlor or Solamin or "Vital Oxide") AND INREGISTER 49 ("zinc salt*" or "green tea" or "lemon grass" or lemongrass or citronella or "Cymbopogon nardus" or "Andropogon nardus" or sesame or Triphala or Ela) AND INREGISTER 50 #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 51 #8 AND #50
Appendix 2. Cochrane Central Register of Controlled Clinical Trials (CENTRAL) search strategy
1 MESH DESCRIPTOR dentistry EXPLODE ALL AND CENTRAL:TARGET 2 MESH DESCRIPTOR dental facilities EXPLODE ALL AND CENTRAL:TARGET 3 MESH DESCRIPTOR infection control, dental AND CENTRAL:TARGET 4 MESH DESCRIPTOR dentists EXPLODE ALL AND CENTRAL:TARGET 5 MESH DESCRIPTOR dental staff AND CENTRAL:TARGET 6 MESH DESCRIPTOR Dental Auxiliaries EXPLODE ALL AND CENTRAL:TARGET 7 (dental or dentist* or hygienist*) AND CENTRAL:TARGET 8 ((oral or maxillofacial) and (care* or procedure* or surgery or surgical or medicine)) AND CENTRAL:TARGET 9 orthodonti* AND CENTRAL:TARGET 10 periodont* AND CENTRAL:TARGET 11 (tooth or teeth or gum* or endodont* or plaque* or pulpotom* or pulpectom* or "cavity prep*" or molar* or bicuspid* or premolar* or pre‐molar* or incisor* or canine* or eyetooth or eyeteeth or cuspid*) AND CENTRAL:TARGET 12 ((scal* near2 polish*) or "root canal" or (root near6 resect*) or (root* near3 planing) or apicectom* or apicoectom*) AND CENTRAL:TARGET 13 ((root* or periodont* or dental or subgingiv* or gingiv* or supragingiv*) and (scale or scaling or scaler* or curettage)) AND CENTRAL:TARGET 14 MESH DESCRIPTOR Dental High‐Speed Equipment AND CENTRAL:TARGET 15 ("high speed air rotor*" or "low speed handpiece*" or "low speed hand piece*" or micromotor* or "turbine handpiece*" or "electrosurgery unit" or "air polisher*" or "prophy angle*" or "air‐water syringe*" or "high speed hand piece*" or "high speed handpiece*" or "three‐way air syringe*" or "threeway air syringe*" or "ultrasonic scaler*" or "hard‐tissue laser*" or "dental drill*" or "piezo unit*" or "piezo hand piece*" or "piezo handpiece*" or "rotary instrument*" or "air abrasion" or "water spray*") AND CENTRAL:TARGET 16 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 17 MESH DESCRIPTOR Air microbiology AND CENTRAL:TARGET 18 MESH DESCRIPTOR Air Pollution, Indoor AND CENTRAL:TARGET 19 MESH DESCRIPTOR Aerosols AND CENTRAL:TARGET 20 MESH DESCRIPTOR Inhalation Exposure AND CENTRAL:TARGET 21 (aerosol* or bioaerosol*) AND CENTRAL:TARGET 22 (droplet* or splatter* or spatter* or microbe* or bacillus or germ* or microorganism* or virus* or viral or coronavirus* or COVID* or "middle east? respiratory syndrome*" or MERS or MERS‐CoV or "camel flu" or SARS or "sudden acute respiratory syndrome*" or "Wuhan virus*" or 2019‐nCoV or SARS‐CoV‐2 or SARS‐CoV or SARS‐CoV‐1 or SARS‐1) AND CENTRAL:TARGET 23 (air near5 (pollut* or quality or impur*)) AND CENTRAL:TARGET 24 #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 25 MESH DESCRIPTOR Mouthwashes EXPLODE ALL AND CENTRAL:TARGET 26 (mouthwash* or gargl* or mouthrins*) AND CENTRAL:TARGET 4319 27 ((oral or mouth or larynx* or pharynx* or intraoral) and (irrigat* or lavag* or wash* or rins* or decontaminat* or mist or clean*)) AND CENTRAL:TARGET 28 MESH DESCRIPTOR Chlorhexidine EXPLODE ALL AND CENTRAL:TARGET 29 MESH DESCRIPTOR Povidone‐Iodine EXPLODE ALL AND CENTRAL:TARGET 30 MESH DESCRIPTOR Cetylpyridinium EXPLODE ALL AND CENTRAL:TARGET 31 MESH DESCRIPTOR Hexetidine EXPLODE ALL AND CENTRAL:TARGET 32 MESH DESCRIPTOR Anti‐Infective Agents, Local EXPLODE ALL AND CENTRAL:TARGET 33 MESH DESCRIPTOR Hydrogen Peroxide EXPLODE ALL AND CENTRAL:TARGET 34 MESH DESCRIPTOR Carbamide Peroxide EXPLODE ALL AND CENTRAL:TARGET 35 MESH DESCRIPTOR Triclosan EXPLODE ALL AND CENTRAL:TARGET 36 MESH DESCRIPTOR Oils, volatile EXPLODE ALL AND CENTRAL:TARGET 37 MESH DESCRIPTOR Plant oils EXPLODE ALL AND CENTRAL:TARGET 38 MESH DESCRIPTOR Plant extracts EXPLODE ALL AND CENTRAL:TARGET 39 MESH DESCRIPTOR Menthol EXPLODE ALL AND CENTRAL:TARGET 40 MESH DESCRIPTOR Lavandula EXPLODE ALL AND CENTRAL:TARGET 41 MESH DESCRIPTOR Thymus plant AND CENTRAL:TARGET 42 MESH DESCRIPTOR Mentha piperita AND CENTRAL:TARGET 43 MESH DESCRIPTOR Eugenol AND CENTRAL:TARGET 44 MESH DESCRIPTOR Cinnamomum verum AND CENTRAL:TARGET 45 MESH DESCRIPTOR Muramidase AND CENTRAL:TARGET 46 MESH DESCRIPTOR Lactoferrin AND CENTRAL:TARGET 47 MESH DESCRIPTOR Glucose oxidase AND CENTRAL:TARGET 48 MESH DESCRIPTOR Lactoperoxidase AND CENTRAL:TARGET 49 MESH DESCRIPTOR Benzethonium AND CENTRAL:TARGET 50 MESH DESCRIPTOR Teas, Herbal AND CENTRAL:TARGET 51 MESH DESCRIPTOR Cymbopogon AND CENTRAL:TARGET 52 MESH DESCRIPTOR Sesame oil AND CENTRAL:TARGET 53 (povidone or chlorhexidine or CHX or PVP or Polyvinylpyrrolidone or Betadine* or Providine* or Disadine* or Isodine* or Pharmadine* or Alphadine* or Betaisodona or Tubulicid or Novalsan or Sebidin or MK‐412A or MK412A) AND CENTRAL:TARGET 54 (Chlorhexamed or Corsodyl or Curasept or Dyna‐Hex or Eludril or Gibitan or Hexidine or Hibiclens or Hibident or Hibiscrub or Hibisol or Hibitane or Peridex or avagard) AND CENTRAL:TARGET 55 (Hexadecylpyridinium or Cetylpyridium or Biosept or Ceepryn or Cetamium or Catamium or Sterogenol or Dobendan or Merocets or Pristacin or Pyrisept or Angifonil or Cetylyre) AND CENTRAL:TARGET 56 (Hexigel or Steri‐sol or "Steri sol" or Hextril or Oraldine or Oralspray or Hexoral or Bactidol or Elsix or Duranil or Doreperol or Hexetidine) AND CENTRAL:TARGET 57 (Hydrogen Peroxide or H2O2 or Hydroperoxide or Superoxol or Oxydol or Perhydrol or Urea Peroxide or Perhydrol Urea) AND CENTRAL:TARGET 58 (Methyl salicylate or methylsalicylate or Rheumabal or Metsal Liniment or Hewedolor or Linsal) AND CENTRAL:TARGET 59 (Tricolsan or Hydroxydiphenyl or trichlorodiphenyl or Clearasil or Cliniclean or Irgasan or Trisan or Oxy Skin Wash or pHisoHex or Sapoderm or Tersaseptic or Aquasept or Ster‐Zac or Manusept or Microshield) AND CENTRAL:TARGET 60 ((irrigat* or rins* or wash* or lavag* or intraoral* or topical) and (antimicrobial or anti‐microbial or disinfect* or antisept* or anti‐infect* or herbal)) AND CENTRAL:TARGET 61 ("essential oil*" or "plant oil*" or menthol or menthyl or (mint near2 oil*) or lavender or thyme or peppermint or "mentha piperita" or eugenol or eucalyptus or "blue gum*" or cajeput or clove or cinnamon) AND CENTRAL:TARGET 62 (muramidase or lysozyme* or leftose or lactoferrin or lactotransferrin or "glucose oxidase" or lactoperoxidase or "saliva substitute") AND CENTRAL:TARGET 63 (Listerine or Biotene) AND CENTRAL:TARGET 64 ("chlorine dioxide" or "Benzethonium chloride" or "Bencetonium Chloride" or "Formula Magic" or "Hyamine 1622" or "Orchid Fresh II" or Phemeride or Phemerol or Phemethryn or Puri‐Clens or Quatrachlor or Solamin or "Vital Oxide") AND CENTRAL:TARGET 65 ("zinc salt*" or "green tea" or "lemon grass" or lemongrass or citronella or "Cymbopogon nardus" or "Andropogon nardus" or sesame or Triphala or Ela) AND CENTRAL:TARGET 66 #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 67 #16 AND #24 AND #66
Appendix 3. MEDLINE Ovid search strategy
1 exp dentistry/ 2 exp dental facilities/ 3 infection control, dental/ 4 exp dentists/ 5 dental staff/ 6 exp dental auxiliaries/ 7 (dental or dentist$ or hygienist$).mp. 8 ((oral or maxillofacial) adj5 (care$ or procedure$ or surgery or surgical or medicine)).mp. 9 orthodonti$.mp. 10 periodont$.mp. 11 (tooth or teeth or gum$ or endodont$ or plaque$ or pulpotom$ or pulpectom$ or "cavity prep$" or molar$ or bicuspid$ or premolar$ or pre‐molar$ or incisor$ or canine$ or eyetooth or eyeteeth or cuspid$).mp. 12 ((scal$ adj2 polish$) or "root canal" or (root adj6 resect$) or (root$ adj3 planing) or apicectom$ or apicoectom$).mp. 13 ((root$ or periodont$ or dental or subgingiv$ or gingiv$ or supragingiv$) adj5 (scale or scaling or scaler$ or curettage)).mp. 14 Dental high speed equipment/ 15 ("high speed air rotor$" or "low speed handpiece$" or "low speed hand piece$" or micromotor$ or "turbine handpiece$" or "electrosurgery unit" or "air polisher$" or "prophy angle$" or "air‐water syringe$" or "high speed hand piece$" or "high speed handpiece$" or "three‐way air syringe$" or "threeway air syringe$" or "ultrasonic scaler$" or "hard‐tissue laser$" or "dental drill$" or "piezo unit$" or "piezo hand piece$" or "piezo handpiece$" or "rotary instrument$" or "air abrasion" or "water spray$").mp. 16 or/1‐15 17 Air microbiology/ 18 Air pollution, indoor/ 19 Aerosols/ 20 Inhalation exposure/ 21 (aerosol$ or bioaerosol$).mp. 22 (droplet$ or splatter$ or spatter$ or microbe$ or bacillus or germ$ or microorganism$ or virus$ or viral or coronavirus$ or COVID$ or "middle east? respiratory syndrome$" or MERS or MERS‐CoV or "camel flu" or SARS or "sudden acute respiratory syndrome$" or "Wuhan virus$" or 2019‐nCoV or SARS‐CoV‐2 or SARS‐CoV or SARS‐CoV‐1 or SARS‐1).mp. 23 (air adj5 (pollut$ or quality or impur$)).mp. 24 or/17‐23 25 exp Mouthwashes/ 26 (mouthwash$ or gargl$ or mouthrins$).ab,ti. 27 ((oral or mouth larynx$ or pharynx$ or intraoral) adj3 (irrigat$ or lavag$ or wash$ or rins$ or decontaminat$ or mist or clean$)).ab,ti. 28 exp Chlorhexidine/ 29 exp Povidone‐Iodine/ 30 exp Cetylpyridinium/ 31 exp Hexetidine/ 32 exp Anti‐Infective Agents, Local/ 33 exp Hydrogen Peroxide/ 34 exp Carbamide Peroxide/ 35 exp Triclosan/ 36 exp Oils, volatile/ 37 exp Plant oils/ 38 exp Plant extracts/ 39 Menthol/ 40 Lavandula/ 41 Thymus plant/ 42 Mentha piperita/ 43 Eugenol/ 44 Cinnamomum verum/ 45 Muramidase/ 46 Lactoferrin/ 47 Glucose oxidase/ 48 Lactoperoxidase/ 49 Benzethonium/ 50 Teas, herbal/ 51 Cymbopogon/ 52 Sesame oil/ 53 (povidone or chlorhexidine or CHX or PVP or Polyvinylpyrrolidone or Betadine$ or Providine$ or Disadine$ or Isodine$ or Pharmadine$ or Alphadine$ or Betaisodona or Tubulicid or Novalsan or Sebidin or MK‐412A or MK412A).ab,ti. 54 (Chlorhexamed or Corsodyl or Curasept or Dyna‐Hex or Eludril or Gibitan or Hexidine or Hibiclens or Hibident or Hibiscrub or Hibisol or Hibitane or Peridex or avagard).ab,ti. 55 (Hexadecylpyridinium or Cetylpyridium or Biosept or Ceepryn or Cetamium or Catamium or Sterogenol or Dobendan or Merocets or Pristacin or Pyrisept or Angifonil or Cetylyre).ab,ti. 56 (Hexigel or Steri‐sol or "Steri sol" or Hextril or Oraldine or Oralspray or Hexoral or Bactidol or Elsix or Duranil or Doreperol or Hexetidine).ab,ti. 57 (Hydrogen Peroxide or H2O2 or Hydroperoxide or Superoxol or Oxydol or Perhydrol or Urea Peroxide or Perhydrol Urea).ab,ti. 58 (Methyl salicylate or methylsalicylate or Rheumabal or Metsal Liniment or Hewedolor or Linsal).ab,ti. 59 (Tricolsan or Hydroxydiphenyl or trichlorodiphenyl or Clearasil or Cliniclean or Irgasan or Trisan or Oxy Skin Wash or pHisoHex or Sapoderm or Tersaseptic or Aquasept or Ster‐Zac or Manusept or Microshield).ab,ti. 60 ((irrigat$ or rins$ or wash$ or lavag$ or intraoral$ or topical) adj3 (antimicrobial or anti‐ microbial or disinfect$ or antisept$ or anti‐infect$ or herbal)).ab,ti. 61 ("essential oil$" or "plant oil$" or menthol or menthyl or (mint adj2 oil$) or lavender or thyme or peppermint or "mentha piperita" or eugenol or eucalyptus or "blue gum$" or cajeput or clove or cinnamon).ab,ti. 62 (muramidase or lysozyme$ or leftose or lactoferrin or lactotransferrin or "glucose oxidase" or lactoperoxidase or "saliva substitute").ab,ti. 63 (Listerine or Biotene).ab,ti. 64 ("chlorine dioxide" or "Benzethonium chloride" or "Bencetonium Chloride" or "Formula Magic" or "Hyamine 1622" or "Orchid Fresh II" or Phemeride or Phemerol or Phemethryn or Puri‐Clens or Quatrachlor or Solamin or "Vital Oxide").ab,ti. 65 ("zinc salt$" or "green tea" or "lemon grass" or lemongrass or citronella or "Cymbopogon nardus" or "Andropogon nardus" or sesame or Triphala or Ela).ab,ti. 66 or/25‐65 67 16 and 24 and 66
The above subject search was linked with the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials in MEDLINE (as described in Lefebvre 2020, box 3c).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
In addition, to identify non‐randomized studies, we linked the search to a study design filter designed by Waffenschmidt and colleagues (Waffenschmidt 2020):
1. exp cohort studies/or exp epidemiologic studies/or exp clinical trial/or exp evaluation studies as topic/or exp statistics as topic/ 2. ((control and (group* or study)) or (time and factors) or program or survey* or ci or cohort or comparative stud* or evaluation studies or follow‐up*).mp. 3. or/1–2 4. (animals/not humans/) or comment/or editorial/or exp review/or meta analysis/or consensus/or exp guideline/ 5. hi.fs. or case report.mp. 6. or/4–5 7. 3 not 6
Appendix 4. Embase Ovid search strategy
1. exp dentistry/ 2. dental facility/ 3. exp dentist/ 4. exp dental personnel/ 5. (dental or dentist$ or hygienist$).mp. 6. ((oral or maxillofacial) adj5 (care$ or procedure$ or surgery or surgical or medicine)).mp. 7. orthodonti$.mp. 8. periodont$.mp. 9. (tooth or teeth or gum$ or endodont$ or plaque$ or pulpotom$ or pulpectom$ or "cavity prep$" or molar$ or bicuspid$ or premolar$ or pre‐molar$ or incisor$ or canine$ or eyetooth or eyeteeth or cuspid$).mp. 10. ((scal$ adj2 polish$) or "root canal" or (root adj6 resect$) or (root$ adj3 planing) or apicectom$ or apicoectom$).mp. 11. ((root$ or periodont$ or dental or subgingiv$ or gingiv$ or supragingiv$) adj5 (scale or scaling or scaler$ or curettage)).mp. 12. ("high speed air rotor$" or "low speed handpiece$" or "low speed hand piece$" or micromotor$ or "turbine handpiece$" or "electrosurgery unit" or "air polisher$" or "prophy angle$" or "air‐water syringe$" or "high speed hand piece$" or "high speed handpiece$" or "three‐way air syringe$" or "threeway air syringe$" or "ultrasonic scaler$" or "hard‐tissue laser$" or "dental drill$" or "piezo unit$" or "piezo hand piece$" or "piezo handpiece$" or "rotary instrument$" or "air abrasion" or "water spray$").mp. 13. or/1‐12 14. Indoor air pollution/ 15. Aerosol/ 16. exp Environmental exposure/ 17. (aerosol$ or bioaerosol$).mp. 18. (droplet$ or splatter$ or spatter$ or microbe$ or bacillus or germ$ or microorganism$ or virus$ or viral or coronavirus$ or COVID$ or "middle east? respiratory syndrome$" or MERS or MERS‐CoV or "camel flu" or SARS or "sudden acute respiratory syndrome$" or "Wuhan virus$" or 2019‐nCoV or SARS‐CoV‐2 or SARS‐CoV or SARS‐CoV‐1 or SARS‐1).mp. 19. (air adj5 (pollut$ or quality or impur$)).mp. 20. or/14‐19 21. exp Mouthwash/ 22. (mouthwash$ or gargl$ or mouthrins$).ab,ti. 23. ((oral or mouth larynx$ or pharynx$ or intraoral) adj3 (irrigat$ or lavag$ or wash$ or rins$ or decontaminat$ or mist or clean$)).ab,ti. 24. Chlorhexidine/ 25. Povidone‐Iodine/ 26. Cetylpyridinium salt/ 27. Hexetidine/ 28. exp Anti‐Infective Agent/ 29. Hydrogen Peroxide/ 30. Carbamide Peroxide/ 31. Triclosan/ 32. exp Vegetable oil/ 33. exp Plant extract/ 34. Menthol/ 35. Eugenol/ 36. exp Isozyme/ 37. Lactoferrin/ 38. Glucose oxidase/ 39. Lactoperoxidase/ 40. Benzethonium chloride/ 41. Herbal tea/ 42. exp Cymbopogon/ 43. Sesame seed oil/ 44. (povidone or chlorhexidine or CHX or PVP or Polyvinylpyrrolidone or Betadine$ or Providine$ or Disadine$ or Isodine$ or Pharmadine$ or Alphadine$ or Betaisodona or Tubulicid or Novalsan or Sebidin or MK‐412A or MK412A).ab,ti. 45. (Chlorhexamed or Corsodyl or Curasept or Dyna‐Hex or Eludril or Gibitan or Hexidine or Hibiclens or Hibident or Hibiscrub or Hibisol or Hibitane or Peridex or avagard).ab,ti. 46. (Hexadecylpyridinium or Cetylpyridium or Biosept or Ceepryn or Cetamium or Catamium or Sterogenol or Dobendan or Merocets or Pristacin or Pyrisept or Angifonil or Cetylyre).ab,ti. 47. (Hexigel or Steri‐sol or "Steri sol" or Hextril or Oraldine or Oralspray or Hexoral or Bactidol or Elsix or Duranil or Doreperol or Hexetidine).ab,ti. 48. (Hydrogen Peroxide or H2O2 or Hydroperoxide or Superoxol or Oxydol or Perhydrol or Urea Peroxide or Perhydrol Urea).ab,ti. 49. (Methyl salicylate or methylsalicylate or Rheumabal or Metsal Liniment or Hewedolor or Linsal).ab,ti. 50. (Tricolsan or Hydroxydiphenyl or trichlorodiphenyl or Clearasil or Cliniclean or Irgasan or Trisan or Oxy Skin Wash or pHisoHex or Sapoderm or Tersaseptic or Aquasept or Ster‐Zac or Manusept or Microshield).ab,ti. 51. ((irrigat$ or rins$ or wash$ or lavag$ or intraoral$ or topical) adj3 (antimicrobial or anti‐microbial or disinfect$ or antisept$ or anti‐infect$ or herbal)).ab,ti. 52. ("essential oil$" or "plant oil$" or menthol or menthyl or (mint adj2 oil$) or lavender or thyme or peppermint or "mentha piperita" or eugenol or eucalyptus or "blue gum$" or cajeput or clove or cinnamon).ab,ti. 53. (muramidase or lysozyme$ or leftose or lactoferrin or lactotransferrin or "glucose oxidase" or lactoperoxidase or "saliva substitute").ab,ti. 54. (Listerine or Biotene).ab,ti. 55. ("chlorine dioxide" or "Benzethonium chloride" or "Bencetonium Chloride" or "Formula Magic" or "Hyamine 1622" or "Orchid Fresh II" or Phemeride or Phemerol or Phemethryn or Puri‐Clens or Quatrachlor or Solamin or "Vital Oxide").ab,ti. 56. ("zinc salt$" or "green tea" or "lemon grass" or lemongrass or citronella or "Cymbopogon nardus" or "Andropogon nardus" or sesame or Triphala or Ela).ab,ti. 57. or/21‐56 58. 13 and 20 and 57
The above subject search was linked with the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials in Embase (as described in Lefebvre 2020, box 3e).
Randomized controlled trial/
Controlled clinical study/
random$.ti,ab.
randomization/
intermethod comparison/
placebo.ti,ab.
(compare or compared or comparison).ti.
((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab.
(open adj label).ti,ab.
((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab.
double blind procedure/
parallel group$1.ti,ab.
(crossover or cross over).ti,ab.
((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab.
(assigned or allocated).ti,ab.
(controlled adj7 (study or design or trial)).ti,ab.
(volunteer or volunteers).ti,ab.
human experiment/
trial.ti.
or/1‐19
random$ adj sampl$ adj7 ("cross section$" or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.)
Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.)
(((case adj control$) and random$) not randomi?ed controlled).ti,ab.
(Systematic review not (trial or study)).ti.
(nonrandom$ not random$).ti,ab.
"Random field$".ti,ab.
(random cluster adj3 sampl$).ti,ab.
(review.ab. and review.pt.) not trial.ti.
"we searched".ab. and (review.ti. or review.pt.)
"update review".ab.
(databases adj4 searched).ab.
(rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/
Animal experiment/ not (human experiment/ or human/)
or/21‐33
20 not 34
Appendix 5. World Health Organization COVID‐19 Global Literature on Coronavirus Disease Database search strategy
(tw:(((mouthwash* or gargl* or mouthrins*)))) AND (tw:(((aerosol or bioaerosol))))
Appendix 6. Cochrane COVID‐19 Study Register search strategy
1 MESH DESCRIPTOR dentistry EXPLODE ALL 2 MESH DESCRIPTOR dental facilities EXPLODE ALL 3 MESH DESCRIPTOR infection control, dental 4 MESH DESCRIPTOR dentists EXPLODE ALL 5 MESH DESCRIPTOR dental staff 6 MESH DESCRIPTOR Dental Auxiliaries EXPLODE ALL 7 (dental or dentist* or hygienist*) 8 ((oral or maxillofacial) and (care* or procedure* or surgery or surgical or medicine)) 9 orthodonti* 10 periodont* 11 (tooth or teeth or gum* or endodont* or plaque* or pulpotom* or pulpectom* or "cavity prep*" or molar* or bicuspid* or premolar* or pre‐molar* or incisor* or canine* or eyetooth or eyeteeth or cuspid*) 12 ((scal* near2 polish*) or "root canal" or (root near6 resect*) or (root* near3 planing) or apicectom* or apicoectom*) 13 ((root* or periodont* or dental or subgingiv* or gingiv* or supragingiv*) and (scale or scaling or scaler* or curettage)) 14 MESH DESCRIPTOR Dental High‐Speed Equipment 15 ("high speed air rotor*" or "low speed handpiece*" or "low speed hand piece*" or micromotor* or "turbine handpiece*" or "electrosurgery unit" or "air polisher*" or "prophy angle*" or "air‐water syringe*" or "high speed hand piece*" or "high speed handpiece*" or "three‐way air syringe*" or "threeway air syringe*" or "ultrasonic scaler*" or "hard‐tissue laser*" or "dental drill*" or "piezo unit*" or "piezo hand piece*" or "piezo handpiece*" or "rotary instrument*" or "air abrasion" or "water spray*") 16 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 17 MESH DESCRIPTOR Air microbiology 18 MESH DESCRIPTOR Air Pollution, Indoor 19 MESH DESCRIPTOR Aerosols 20 MESH DESCRIPTOR Inhalation Exposure 21 (aerosol* or bioaerosol*) 22 (droplet* or splatter* or spatter* or microbe* or bacillus or germ* or microorganism* or virus* or viral or coronavirus* or COVID* or "middle east? respiratory syndrome*" or MERS or MERS‐CoV or "camel flu" or SARS or "sudden acute respiratory syndrome*" or "Wuhan virus*" or 2019‐nCoV or SARS‐CoV‐2 or SARS‐CoV or SARS‐CoV‐1 or SARS‐1) 23 (air near5 (pollut* or quality or impur*)) 24 #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 25 MESH DESCRIPTOR Mouthwashes EXPLODE ALL 26 (mouthwash* or gargl* or mouthrins*) 27 ((oral or mouth or larynx* or pharynx* or intraoral) and (irrigat* or lavag* or wash* or rins* or decontaminat* or mist or clean*)) 28 MESH DESCRIPTOR Chlorhexidine EXPLODE ALL 29 MESH DESCRIPTOR Povidone‐Iodine EXPLODE ALL 30 MESH DESCRIPTOR Cetylpyridinium EXPLODE ALL 31 MESH DESCRIPTOR Hexetidine EXPLODE ALL 32 MESH DESCRIPTOR Anti‐Infective Agents, Local EXPLODE ALL 33 MESH DESCRIPTOR Hydrogen Peroxide EXPLODE ALL 34 MESH DESCRIPTOR Carbamide Peroxide EXPLODE ALL 35 MESH DESCRIPTOR Triclosan EXPLODE ALL 36 MESH DESCRIPTOR Oils, volatile EXPLODE ALL 37 MESH DESCRIPTOR Plant oils EXPLODE ALL 38 MESH DESCRIPTOR Plant extracts EXPLODE ALL 39 MESH DESCRIPTOR Menthol EXPLODE ALL 40 MESH DESCRIPTOR Lavandula EXPLODE ALL 41 MESH DESCRIPTOR Thymus plant 42 MESH DESCRIPTOR Mentha piperita 43 MESH DESCRIPTOR Eugenol 44 MESH DESCRIPTOR Cinnamomum verum 45 MESH DESCRIPTOR Muramidase 46 MESH DESCRIPTOR Lactoferrin 47 MESH DESCRIPTOR Glucose oxidase 48 MESH DESCRIPTOR Lactoperoxidase 49 MESH DESCRIPTOR Benzethonium 50 MESH DESCRIPTOR Teas, Herbal 51 MESH DESCRIPTOR Cymbopogon 52 MESH DESCRIPTOR Sesame oil 53 (povidone or chlorhexidine or CHX or PVP or Polyvinylpyrrolidone or Betadine* or Providine* or Disadine* or Isodine* or Pharmadine* or Alphadine* or Betaisodona or Tubulicid or Novalsan or Sebidin or MK‐412A or MK412A) 54 (Chlorhexamed or Corsodyl or Curasept or Dyna‐Hex or Eludril or Gibitan or Hexidine or Hibiclens or Hibident or Hibiscrub or Hibisol or Hibitane or Peridex or avagard) 55 (Hexadecylpyridinium or Cetylpyridium or Biosept or Ceepryn or Cetamium or Catamium or Sterogenol or Dobendan or Merocets or Pristacin or Pyrisept or Angifonil or Cetylyre) 56 (Hexigel or Steri‐sol or "Steri sol" or Hextril or Oraldine or Oralspray or Hexoral or Bactidol or Elsix or Duranil or Doreperol or Hexetidine) 57 (Hydrogen Peroxide or H2O2 or Hydroperoxide or Superoxol or Oxydol or Perhydrol or Urea Peroxide or Perhydrol Urea) 58 (Methyl salicylate or methylsalicylate or Rheumabal or Metsal Liniment or Hewedolor or Linsal) 59 (Tricolsan or Hydroxydiphenyl or trichlorodiphenyl or Clearasil or Cliniclean or Irgasan or Trisan or Oxy Skin Wash or pHisoHex or Sapoderm or Tersaseptic or Aquasept or Ster‐Zac or Manusept or Microshield) 60 ((irrigat* or rins* or wash* or lavag* or intraoral* or topical) and (antimicrobial or anti‐microbial or disinfect* or antisept* or anti‐infect* or herbal)) 61 ("essential oil*" or "plant oil*" or menthol or menthyl or (mint near2 oil*) or lavender or thyme or peppermint or "mentha piperita" or eugenol or eucalyptus or "blue gum*" or cajeput or clove or cinnamon) 62 (muramidase or lysozyme* or leftose or lactoferrin or lactotransferrin or "glucose oxidase" or lactoperoxidase or "saliva substitute") 63 (Listerine or Biotene) 64 ("chlorine dioxide" or "Benzethonium chloride" or "Bencetonium Chloride" or "Formula Magic" or "Hyamine 1622" or "Orchid Fresh II" or Phemeride or Phemerol or Phemethryn or Puri‐Clens or Quatrachlor or Solamin or "Vital Oxide") 65 ("zinc salt*" or "green tea" or "lemon grass" or lemongrass or citronella or "Cymbopogon nardus" or "Andropogon nardus" or sesame or Triphala or Ela) 66 #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 67 #16 AND #24 AND #66
Appendix 7. US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) search strategy
Expert search: ((mouthrinse OR mouthrinses OR mouthrinsing OR mouthwash OR mouthwashes OR mouthwashing OR gargle OR gargles OR gargling) AND (aerosol OR aerosols OR bioaerosol OR bioaerosols))
Appendix 8. World Health Organization International Clinical Trials Registry Platform search strategy
mouthrinse AND aerosol OR mouthrinses AND aerosol OR mouthrinsing AND aerosol OR mouthrinse AND aerosols OR mouthrinses AND aerosols OR mouthrinsing AND aerosols
mouthrinse AND bioaerosol or mouthrinses AND bioaerosol OR mouthrinsing AND bioaerosol OR mouthrinse AND bioaerosols OR mouthrinses AND bioaerosols OR mouthrinsing AND bioaerosols
mouthwash AND aerosol OR mouthwashes AND aerosol OR mouthwashing AND aerosol OR mouthwash AND aerosols OR mouthwashes AND aerosols OR mouthwashing AND aerosols
mouthwash AND bioaerosol OR mouthwashes AND bioaerosol OR mouthwashing AND bioaerosol OR mouthwash AND bioaerosols OR mouthwashes AND bioaerosols OR mouthwashing AND bioaerosols
Data and analyses
Comparison 1. Chlorhexidine versus no rinsing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Reduction in the level of contamination at < 2 m | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1.1 Operator level | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 2. Chlorhexidine versus water.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Reduction in the level of contamination at < 2 m | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1.1 Operator level | 6 | 196 | Mean Difference (IV, Random, 95% CI) | ‐105.46 [‐146.70, ‐64.22] |
| 2.1.2 Assistant level | 4 | 136 | Mean Difference (IV, Random, 95% CI) | ‐38.19 [‐55.66, ‐20.72] |
| 2.1.3 Combined levels | 3 | 80 | Mean Difference (IV, Random, 95% CI) | ‐632.94 [‐1267.33, 1.45] |
| 2.1.4 Tempered chlorhexidine versus water – combined levels | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐101.10 [‐107.01, ‐95.19] |
Comparison 3. Chlorhexidine versus saline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Reduction in the level of contamination at < 2 m | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1.1 3 feet from patient's mouth | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐668.40 [‐1044.45, ‐292.35] |
| 3.1.2 Operator level | 1 | 12 | Mean Difference (IV, Random, 95% CI) | ‐21.33 [‐36.80, ‐5.86] |
Comparison 4. Chlorhexidine versus essential oils or herbal mouthwash.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Reduction in the level of contamination at < 2 m | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1.1 At operator level | 3 | 76 | Mean Difference (IV, Random, 95% CI) | ‐23.09 [‐34.40, ‐11.78] |
| 4.1.2 At assistant level | 3 | 76 | Mean Difference (IV, Random, 95% CI) | ‐12.21 [‐15.58, ‐8.83] |
| 4.1.3 At 15 cm from patient's mouth | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐44.10 [‐59.12, ‐29.08] |
Comparison 5. Chlorhexidine versus povidone iodine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Reduction in the level of contamination at < 2 m | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1.1 Operator level – aerobic | 2 | 52 | Mean Difference (IV, Random, 95% CI) | ‐10.75 [‐26.24, 4.74] |
| 5.1.2 Operator level (aerobic and anaerobic combined) | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐4.70 [‐7.01, ‐2.39] |
| 5.2 Reduction in level of contamination at ≥ 2 m | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.2.1 Aerobic | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.2.2 Anaerobic | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 6. Chlorhexidine versus cetylpyridinium chloride (CPC).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Reduction in the level of contamination at < 2 m | 3 | 220 | Mean Difference (IV, Random, 95% CI) | 2.41 [‐4.52, 9.33] |
| 6.1.1 Tempered chlorhexidine versus tempered CPC – operator | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐9.07, 4.67] |
| 6.1.2 Tempered chlorhexidine versus tempered CPC – assistant | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐6.30, 5.90] |
| 6.1.3 Cold chlorhexidine versus cold CPC – operator | 2 | 50 | Mean Difference (IV, Random, 95% CI) | 4.43 [‐2.27, 11.13] |
| 6.1.4 Cold chlorhexidine versus cold CPC – assistant | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 8.80 [2.70, 14.90] |
| 6.1.5 Tempered chlorhexidine versus cold CPC – operator | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐11.50 [‐18.37, ‐4.63] |
| 6.1.6 Tempered chlorhexidine versus cold CPC – assistant | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐8.80 [‐14.90, ‐2.70] |
| 6.1.7 Cold chlorhexidine versus tempered CPC – operator | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 13.60 [6.73, 20.47] |
| 6.1.8 Cold chlorhexidine versus tempered CPC – assistant | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 17.40 [11.30, 23.50] |
| 6.1.9 Chlorhexidine versus CPC + zinc (Zn) + fluoride (F) – operator level | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐30.00 [‐85.86, 25.86] |
Comparison 7. Tempered chlorhexidine versus non‐tempered chlorhexidine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Reduction in the level of contamination at < 2 m | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1.1 Operator level | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐15.80 [‐22.67, ‐8.93] |
| 7.1.2 Assistant level | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐17.60 [‐23.70, ‐11.50] |
| 7.1.3 Combined | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐10.80 [‐15.15, ‐6.45] |
Comparison 8. Cetylpyridinium chloride (CPC) versus no rinsing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Reduction in level of contamination at < 2 m [CFUs] | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1.1 CPC versus no rinsing – operator level | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐34.80 [‐65.92, ‐3.68] |
| 8.1.2 CPC + zinc + fluoride versus no rinsing – operator level | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐583.00 [‐958.36, ‐207.64] |
| 8.1.3 CPC formulation versus no rinsing – combined | 2 | 200 | Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.32, ‐0.18] |
Comparison 9. Cetylpyridinium chloride (CPC) versus water.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Reduction in level of contamination at < 2 m [CFUs] | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1.1 CPC versus water – operator level | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐48.70 [‐67.82, ‐29.58] |
| 9.1.2 CPC + zinc + fluoride versus water – operator level | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐246.00 [‐437.96, ‐54.04] |
| 9.1.3 CPC formulation versus water – combined | 1 | 105 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.24, ‐0.31] |
Comparison 10. Tempered cetylpyridinium chloride (CPC) versus cold CPC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Reduction in the level of contamination at < 2 m | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1.1 Operator | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐9.30 [‐16.17, ‐2.43] |
| 10.1.2 Assistant | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐8.60 [‐14.70, ‐2.50] |
Comparison 11. Povidone iodine versus saline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Reduction in the level of contamination at < 2 m | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1.1 Operator level | 1 | 12 | Mean Difference (IV, Random, 95% CI) | ‐16.50 [‐32.65, ‐0.35] |
Comparison 12. Essential oils/herbal mouthwash versus water.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Reduction in the level of contamination at < 2 m | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12.1.1 At operator level | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12.1.2 At assistant level | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 13. Chlorhexidine versus ozonated water.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Reduction in the level of contamination at < 2 m | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1.1 Operator level aerobic | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐17.80 [‐33.70, ‐1.90] |
| 13.2 Reduction in the level of contamination at ≥ 2 m | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.2.1 Aerobic | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐12.30 [‐27.12, 2.52] |
| 13.2.2 Anaerobic | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐3.40 [‐14.66, 7.86] |
Comparison 14. Povidone iodine versus ozonated water.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Reduction in the level of contamination at < 2 m | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1.1 Operator level aerobic | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 3.50 [‐17.10, 24.10] |
| 14.2 Reduction in the level of contamination at ≥ 2 m | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.2.1 Aerobic | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐12.50 [‐26.33, 1.33] |
| 14.2.2 Anaerobic | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐2.60 [‐9.54, 4.34] |
Comparison 15. Chlorhexidine versus boric acid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 15.1 Reduction in the level of contamination at < 2 m | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1.1 Operator level | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐26.60 [‐28.24, ‐24.96] |
| 15.1.2 Assistant level | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐8.03 [‐9.65, ‐6.41] |
Comparison 16. Boric acid versus water.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 16.1 Reduction in the level of contamination at < 2 m | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1.1 Operator level | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐29.30 [‐32.65, ‐25.95] |
| 16.1.2 Assistant level | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐14.04 [‐16.47, ‐11.61] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bay 1993.
| Study characteristics | ||
| Methods | Title: effectiveness of antimicrobial mouth rinses on aerosols produced by air polisher Trial design: randomised 3‐arm parallel‐group design Trial registration: not reported Location: Kansas City, US Setting: university dental clinic Language: English Number of centres: 1 Study period: not reported, probably 1990–1992 Funding source: Procter and Gamble sponsored the Peridex mouth rinse Dental aerosol procedure tested: air‐abrasive polisher |
|
| Participants | Age: adults Total number of participants: 45 (30 women, 15 men) Inclusion criteria: ≥ 16 teeth; mean score of 2.5 on Turesky's modification of the Quigley‐Hein plaque index using Ramfjords teeth Exclusion criteria: use of anticoagulant or antibiotic medication; sodium restricted diet, heart murmur; rheumatic disease of valve replacement; gross caries; significant oral pathology; contagious disease; uncontrolled hypertension Number randomised: 45 |
|
| Interventions |
Intervention 1 Group name: essential oil Number randomised: 15 Description of intervention: 30 sec rinsing with 15 mL essential oil directly prior to treatment; Listerine (contained essential oils such as 0.042% menthol (mint), 0.064% thymol (thyme), 0.06% methyl salicylate (wintergreen) and 0.092% eucalyptol (eucalyptus); Warner‐Lambert, Morris Plains, NJ, US Any co‐interventions: no Intervention 2 Group name: CHX Number randomised: 15 Description of intervention: 30 sec rinsing with 15 mL 0.12% CHX rinse directly prior to treatment; Peridex, Procter Gamble, Cincinnati, OH, US Any co‐interventions: no Comparator Group name: water Number randomised: 15 Description of control: 30 sec rinsing with tap water directly prior to treatment |
|
| Outcomes | Outcome name: CFU per ft³ at 3 levels of inhalation and the total CFUs/ft³ across the levels Outcome measurement: Andersen air sampler, a cascade impactor system with 6 filters representing human respiratory tract of which 3 were used representing the pharynx (> 5.5 μm), secondary bronchi, (2–5.5 μm, alveoli (< 2 μm) with a constant air flow of 1 ft³ per min. Measurements taken at baseline (5 min), using the air abraser (3 min) and after treatment (2 min). Agar plates were incubated for 24 hours at 37 °C Effect estimate: mean difference in CFU Outcome name: total CFUs/ft³ across the levels Outcome measurement: see above Effect estimate: mean difference in CFU Adverse events: not reported |
|
| Notes |
Key study conclusions Lack of effectiveness for all rinses. No difference between treatment groups in reducing bacterial aerosols. Neither mouth rinses nor water were consistently more effective in reducing bacterial aerosol. Contact information: Nina Louise Bay, Assistant Professor and Director, School of Dental Hygiene, Dental branch, University of Texas Health Science Centre, Houston, Texas, USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or providers. There is a possibility of clinicians altering their behaviour. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "examiner‐blinded." Comment: those who counted the CFUs were blind to the participant and the treatment conditions. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported. We assumed no loss of data. |
| Selective reporting (reporting bias) | Unclear risk | Clinical trial registration details not available. |
| Other bias | Low risk | None detected. |
Feres 2010.
| Study characteristics | ||
| Methods | Title: the effectiveness of a preprocedural mouth rinse containing cetylpyridinium chloride in reducing bacteria in the dental office Trial design: randomised parallel‐group placebo‐controlled trial Trial registration: not reported Location: São Paulo, Brazil Setting: university clinic (periodontal clinic at Guarulhos University) Language: English Number of centres: 1 Study period: not reported, probably 2008–2010 Funding source: supported financially and through the donation of products by Colgate‐Palmolive, Sao Paulo. In addition, 2 authors are employees of this company. Dental aerosol procedure tested: full‐mouth oral prophylaxis using an ultrasonic scaler (Magnetostrictive) |
|
| Participants | Age: not reported (inclusion criteria range 30–70 years) Total number of participants: 60 Inclusion criteria: aged 30–70 years, available for duration of study, ≥ 20 natural teeth present, ≥ 80% of tooth surfaces had to have visible supragingival plaque, < 10% had to have visible supragingival calculus, and < 30% had to have PD and CAL of ≥ 5 mm Exclusion criteria: people with orthodontic bands, partial removable dentures, tumours of the soft or hard tissues of the oral cavity, advanced periodontal disease, ≥ 5 carious lesions requiring immediate restorative treatment, history of allergy to CPC or any of the personal care products used during the study, any medical condition that could compromise the participant's safety or the quality of the study results; people who had undergone a professional oral prophylaxis within 1 month of entering study and pregnant or breastfeeding women |
|
| Interventions |
Intervention 1 Group name: test group (0.05% CPC) Number randomised: 15 Description of intervention: rinse for 1 min with 15 mL of 0.05% CPC and expectorate all remaining liquid Any co‐interventions: none Intervention 2 Group name: CHX Number randomised: 15 Description of intervention: rinse for 1 min with 15 mL of 0.12% CHX and expectorate all remaining liquid Any co‐interventions: no Intervention 3 Group name: water Number randomised: 15 Description of intervention: rinse for 1 min with 15 mL of water and expectorate all remaining liquid Any co‐interventions: no Comparator Group name: A (no rinsing) Number randomised: 15 Description of control: no rinsing performed (no intervention) |
|
| Outcomes | Outcome name: mean CFU Outcome measurement: plates containing 5% sheep blood agar supplemented with N‐acetyl muramic acid and menadione on a counter within the operatory for 30 min was used to collect and count CFU. Placed at 3 locations: patient's chest, examiner's forehead and support board attached to the chair tray. Effect estimate: data given in graph Outcome name: mean decrease in CFU Outcome measurement: as above Effect estimate: data given in graph Outcome name: specific microbes Outcome measurement: checkerboard DNA–DNA hybridisation Effect estimate: mean difference as percentage of the total DNA probe counts Adverse events: not reported |
|
| Notes |
Key study conclusions CPC and CHX were equally effective in decreasing the total number of spatter micro‐organisms compared with no rinsing (P < 0.05, Kruskal‐Wallis and Mann‐Whitney U tests) for all locations. Contact information: Dr Feres, Universidade Guarulhos, Centro de Pós‐Graduação e Pesquisa–CEPPE, São Paulo, Brazil; e‐mail mferes@ung.br Comments: E‐mail sent on 30 March 2021 requesting the missing details and reply awaited. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The examiner, study coordinator and investigator did not know which mouth rinse each participant used." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The technician was blinded to which participants received which treatment solutions. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported. |
| Selective reporting (reporting bias) | Unclear risk | Clinical trial registration details not available. |
| Other bias | Low risk | None detected. |
Fine 1993a.
| Study characteristics | ||
| Methods | Title: reducing bacteria in dental aerosols: pre procedural use of an antiseptic mouth rinse Trial design: randomised cross‐over trial Trial registration: not reported Location: New York, NY, USA Setting: university dental clinic Language: English Number of centres: 1 Study period: not reported, probably 1991–1992 Funding source: this study was supported by a grant from the Warner‐Lambert Co. Co‐authors Barnett, Vincent and Mr Olshan are employees of the Warner‐Lambert Company. Dental aerosol procedure tested: air sampled during ultrasonic scaling (Magnetostrictive) of random max quadrant for 5 min at baseline; after 40 min of treatment, air was resampled during the baseline scaling that was repeated for 5 min |
|
| Participants | Age: adults Total number of participants: 18 Inclusion criteria: ADA Periodontal Case Type I (gingivitis) or II (incipient periodontitis) as determined by clinical probing and radiographs; no history of diabetes, blood dyscrasia, hepatic or renal disease, or immunosuppression; no history of rheumatic fever, cardiac murmur or defect, or any other condition requiring prophylactic antibiotics before invasive dental procedures; no current antibiotic, anticoagulant or steroidal therapy; no periodontal therapy including scaling, root planing or dental prophylaxis during the previous 6 months; presence of ≥ 20 sound natural teeth with a mean plaque index > 1.5 and a mean gingival index > 1.5 Exclusion criteria: no details given Number randomised: 18 Number evaluated (withdrawals/missing participants): 17; 1 participant was excluded since they initiated antibiotic therapy during the study. |
|
| Interventions |
Intervention 1 Group name: antiseptic mouth rinse (Listerine) Number randomised: 18 Description of intervention: rinsed with 20 mL of assigned rinse for 30 sec under supervision Any co‐interventions: no Washout period: 1 week Comparator Group name: coloured and flavoured 5% hydroalcohol Number randomised: no details given Description of control: rinsed with 20 mL of assigned rinse for 30 sec under supervision. Identical clinical procedures were repeated after 1 week with another 24‐hour no‐oral hygiene period. Washout period: 1 week |
|
| Outcomes | Outcome name: CFU expressed as log10 scores Outcome measurement: air sampling with a vacuum air sampling device (Mattson‐Garvin model 200) at 5 cm from patient's mouth at 55 ft³ per hour with a 0.45 μm filter. For each period, a filter was put on enriched trypticase soy agar incubated at 37 °C for 24–72 hours. CFU counted with dissecting microscope. Effect estimate: Listerine: before: mean 2.79 (SE 0.28), 17 participants; after: mean 1.60 (SE 0.30); hydroalcohol: before: mean 2.80 (SE 0.27); after: mean 2.63 (SE 0.32) Adverse events: not reported |
|
| Notes |
Key study conclusions Preprocedural rinse reduced levels of bacteria in aerosols produced during routine dental procedures. Rinsing with an antiseptic at outset of a simulated dental visit can reduce level of viable bacteria in an aerosol produced by ultrasonic scaling 40 min later. Contact information: Dr Fine, Professor of Dentistry and Director, Division of Oral Infectious Diseases, Columbia University, School of Dental and Oral Surgery, New York, New York, USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned schedule with a computer‐generated random code. |
| Allocation concealment (selection bias) | Low risk | The personnel dispensing the test rinses did not otherwise participate in the study to avoid potential bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participant, dentist and dental assistant were unaware of treatment code. Quote: "Double‐blinding was maintained by assigning the rinse schedule with a computer generated random code." Quote: "The control rinse was coloured and flavoured to resemble an actual product." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant, dentist and dental assistant were aware of the treatment code. Quote: "Double‐blinding was maintained by assigning the rinse schedule with a computer generated random code" Quote: "The control rinse was coloured and flavoured to resemble an actual product." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 dropout. |
| Selective reporting (reporting bias) | Unclear risk | Clinical trial registration details not available. |
| Other bias | Low risk | None detected. |
Gupta 2014.
| Study characteristics | ||
| Methods | Trial design: 3‐armed parallel‐group RCT Trial registration: not reported Location: Jodhpur, India Setting: dental school clinic Language: English Number of centres: 1 Study period: March 2012 to April 2012 (45 days) Funding source: not reported Dental aerosol procedure tested: piezoelectric ultrasonic scaling for 30 min |
|
| Participants | Age: mean 40 years, range 25–55 years Total number of participants: 24 (16 men, 8 women) Inclusion criteria: ≥ 20 permanent teeth; mean plaque score 2.0–3.0 on Plaque Index. ≥ 4 sites with pocket PD > 4 mm; non‐smokers; systemically healthy people Exclusion criteria: systemic or topical antibiotics; oral prophylaxis within last 3 months; ≥ 5 caries lesions requiring immediate restorative treatment; pregnant or breastfeeding Number randomised and evaluated: 24 |
|
| Interventions |
Intervention 1 Group name: group A: CHX mouth rinse Number randomised: 8 Description of intervention: rinse for 1 min with 10 mL of 0.2% CHX, 10 min before ultrasonic scaling Intervention 2 Group name: group B: herbal mouth rinse Number randomised: 8 Description of intervention: rinse for 1 min with 10 mL of herbal mouth rinse, 10 min before ultrasonic scaling; contained Terminilia bellirica, Piper betle, Salvadora persica, powders peppermint (Mentha spp) and Caraway, Wintergreen oil and cardamom Any co‐interventions: none Comparator Group name: group C: water Number randomised: 8 Description of control: rinse for 1 min with 10 mL of water, 10 min before ultrasonic scaling |
|
| Outcomes | Outcome name: total CFU at different locations Outcome measurement: blood agar plates at patients' chest; at doctor's chest and at the assistant's chest area; mean 30 cm from patient's mouth. Agar plates were open for 30 min during treatment and 30 min after treatment. Agar plates were incubated for 48 hours at 37 °C. CFUs were counted. Effect estimate: data given in graph Adverse events: not reported |
|
| Notes |
Key study conclusions CHX mouth rinse for 1 min, 10 min before ultrasonic scaling resulted in consistently fewer CFUs than when herbal mouth rinse or water. Herbal mouth rinse was effective in reducing aerosol contamination produced by ultrasonic scaling, though less potent than 0.2% CHX. Contact information: Dr Gunjan Gupta, Vyas Dental College, Jodhpur, Rajasthan, India; E‐mail: drgunjan_arun@yahoo.co.in Comments: mail sent on 30 March 2021 requesting the missing details and reply awaited. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were recruited in chronologic order, by systematic sampling." |
| Allocation concealment (selection bias) | Low risk | An independent examiner who was not involved in the study allotted the mouth rinse. Quote: "Treatment group was concealed from the patient, operator, and microbiologist." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment group was concealed from the patient, operator and microbiologist. Quote: "double masked." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The operator was not involved in any evaluations before or after. Treatment group was concealed from the patient, operator and microbiologist. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Clinical trial registration details not available. |
| Other bias | Low risk | None detected. |
Joshi 2017.
| Study characteristics | ||
| Methods | Title: efficacy of two pre‐procedural rinses at two different temperatures in reducing aerosol contamination produced during ultrasonic scaling in a dental set‐up – a microbiological study Trial design: 4‐arm randomised parallel‐group controlled trial Location: Mumbai, India Setting: dental school hospital Language: English Number of centres: 1 Study period: 60 days from September 2015 to October 2015 Funding source: not reported Dental aerosol procedure tested: ultrasonic scaling (piezoelectric) |
|
| Participants | Age (mean): group A1: 32.5 years; A2: 32.7 years; B1: 32.64 years; B2: 33.0 years Total number of participants: 40 Inclusion criteria: ≥ 20 natural teeth present excluding third molars; diagnosed with chronic gingivitis having PD ≤ 3 mm; modified Gingival Index ≥ 1 and Gingival Bleeding Index > 30% of the sites examined Exclusion criteria: allergies to constituents found in conventional mouth rinses; untreated/grossly carious teeth; having undergone non‐surgical or surgical periodontal therapy and antibiotic or anti‐inflammatory (or both) therapy within the past 6 months; systemically compromised; pregnant and breastfeeding women Number randomised: 40 Number evaluated (withdrawals/missing participants): all 40 evaluated |
|
| Interventions | 4 groups with 10 participants in each Intervention 1 Group A1: 0.05% CPC at 47 °C Number randomised: 10 Description of intervention: rinsing for 60 sec with 10 mL of assigned mouth rinse 10 min prior to treatment Any co‐interventions: none Intervention 2 Group A2: 0.05% CPC at 18 °C Number randomised: 10 Description of intervention: rinsing for 60 sec with 10 mL of assigned mouth rinse 10 min prior to treatment Any co‐interventions: none Intervention 3 Group B1: 0.2% CHX at 47 °C Number randomised: 10 Description of intervention: rinsing for 60 sec with 10 mL of assigned mouth rinse 10 min prior to treatment Any co‐interventions: none Intervention 4 Group B2: 0.2% CHX at 18 °C Number randomised: 10 Description of intervention: rinsing for 60 sec with 10 mL of assigned mouth rinse 10 min prior to treatment Any co‐interventions: none |
|
| Outcomes | Exposure: oral prophylaxis for all participants was carried out in a standardised dental chair using distilled water with controlled frequency (30 KHz) and water pressure (0.3 MPa). Scaling for 30 min Outcome name: CFU count Outcome measurement: blood agar plates at the neck of the patient, the assistant and the operator 30 cm from patient's mouth. Labelled plates were exposed at start of scaling procedure performed for 30 min, and left uncovered on the operator's stool, assistant's stool and the back rest of the dental chair for additional 30 min. Incubated for 48 hours at 37 °C. CFUs counted using a colony counter Effect estimate: mean CFUs at patient, assistant or operator Adverse events: not reported |
|
| Notes |
Key study conclusions Mouth rinses equally effective Groups A1 and B1 (47 °C) showed maximum reduction in bacterial counts in all 3 areas compared to their cold counterparts A2 and B2 at 18 °C Contact information: Amruta Arun Joshi, Department of Periodontics, MGM Dental College and Hospital, Maharashtra, India; E‐mail: amrutajoshi2611@gmail.com |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. Quote: "Computer‐generated random numbers were used for randomization." |
| Allocation concealment (selection bias) | Unclear risk | Quote: mouth rinses "were procured from manufacturers and were transferred into identical opaque white bottles labelled as A1, B1, A2 and B2 for the purpose of blinding, by an investigator not involved in the study." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: mouth rinses "were procured from manufacturers and were transferred into identical opaque white bottles labelled as A1, B1, A2 and B2 for the purpose of blinding, by an investigator not involved in the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: mouth rinses "were procured from manufacturers and were transferred into identical opaque white bottles labelled as A1, B1, A2 and B2 for the purpose of blinding, by an investigator not involved in the study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The non‐compliance rate for our study was zero and there were no dropouts." |
| Selective reporting (reporting bias) | Unclear risk | We are unsure if all planned outcomes were reported as the trial was registered retrospectively. |
| Other bias | Low risk | No other bias observed. |
Kaur 2014.
| Study characteristics | ||
| Methods | Title: effect of chlorhexidine, povidone iodine, and ozone on microorganisms in dental aerosols: randomized double‐blind clinical trial Trial design: randomised, parallel‐group trial Trial registration: not reported Location: Karnataka, India Setting: dental school hospital Language: English Number of centres: 1 Study period: October 2006 to March 2008 – personal communication Funding source: no funding received – personal communication Dental aerosol procedure tested: ultrasonic scaling (type of scaler not reported) |
|
| Participants | Age (range): 20–50 years Total number of participants: 60 Inclusion criteria: ≥ 20 permanent teeth present Exclusion criteria: presence of periodontitis, medical conditions or taking medications that would contraindicate treatment Number randomised: 60 Number evaluated (withdrawals/missing participants): 60 (no dropouts – personal communication) |
|
| Interventions |
Intervention 1 Group name: 0.2% CHX Number randomised: 20 Description of intervention: 0.2% CHX rinse; volume unclear, for 1 min Any co‐interventions: none Intervention 2 Group name: 1% povidone iodine Number randomised: 20 Description of intervention: rinsing for 1 min Any co‐interventions: none Comparator Group name: ozonated water with 0.082 mg/hour ozone output Number randomised: 20 Description of intervention: rinsing for 1 min Any co‐interventions: none |
|
| Outcomes | Exposure: after baseline sampling for 30 min, prerinse scaling was performed for 10 min by operator, with universal tip attached to the ultrasonic scaler and prerinse sampling for 30 min; after this, patients rinsed and were again subjected to 10 min of scaling plus postrinse sampling for 30 min. Outcome name: CFUs aerobic Outcome measurement: 3 agar plates at chest of patient, mask of the operator and 9 ft behind patient at 3 measurement moments: baseline = 30 min before procedure; during prerinse scaling 10 min and postscaling 30 min; during postrinse scaling 10 min and postscaling 30 min. Agar plates incubated aerobically at 37 °C for 48 hours and anaerobically in an increased carbon dioxide chamber for 48 hours. CFU counted with a counter and examiner blind to treatment. Effect estimate: mean difference Outcome name: anaerobic CFU Outcome measurement: see above Effect estimate: mean difference Adverse events: no adverse effects seen – personal communication |
|
| Notes |
Key study conclusions Significant reduction of bacterial CFU in all the 3 groups, showing the maximum reduction of up to 57%. At mask position, reduction in aerobic colonies from prerinse to postrinse was 57% for CHX group (57%), 54% for placebo group, and 47% for ozonated group. Highest anaerobic CFU reduction at chest position and 9 ft for CHX group followed by placebo and ozonated groups. Contact information: Dr Vandana KL, Departments of Oral and Maxillofacial Surgery, Christian Dental College, Ludhiana, Punjab, India; E‐mail: vanrajs@gmail.com Comments: mail sent on 30 March 2021 requesting missing details and reply received on 3 May 2021 via WhatsApp. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used random number table. Quote: "randomization was done on an alternate basis." Quote: "using a randomization table." |
| Allocation concealment (selection bias) | Low risk | Quote: "To maintain full blinding of the results, the randomization code was held by one of the authors remotely from all assessments and wasn't broken until all data had been collected and analyzed." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "To maintain full blinding of the results, the randomization code was held by one of the authors remotely from all assessments and wasn't broken until all data had been collected and analyzed." Quote: operator was "blinded of both, the mouth rinse and irrigation provided to the subjects." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Colonies were counted using the colony counter device by the examiner, who was blinded to the rinse provided. Quote: operator was "blinded of both, the mouth rinse and irrigation provided to the subjects." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | High risk | Non‐significant results not reported. |
| Other bias | Low risk | None detected. |
Mohammed 1964.
| Study characteristics | ||
| Methods | Title: efficacy of preoperative oral rinsing to reduce air contamination during use of air turbine handpieces Trial design: 3‐arm randomised parallel‐group trial Trial registration: not reported Location: Puerto Rico, USA Setting: university dental clinic Language: English Number of centres: 1 Study period: no details given, 1963 assumed Funding source: not reported Dental aerosol procedure tested: high‐speed drilling for cavity preparation |
|
| Participants | Age: not reported but all were male or female students Total number of participants: 185 Inclusion criteria: all participants had moderate gingivitis and, for better control, all cavities were prepared in the lower molar region Exclusion criteria: not reported Number randomised: 185 Number evaluated (withdrawals/missing participants): 185 |
|
| Interventions |
Intervention Group name: group 2 (mouthwash) Number randomised: 80 Description of intervention: sampling done after rinsing with 20 mL of mouthwash formulation; CPC + domiphen bromide + quaternary ammonium compounds Any co‐interventions: none Comparator 1 Group name: group 1 (no rinse) Number randomised: 80 Description of control: no rinsing before cavity preparation Comparator 2 Group name: group 3 (distilled water) Number randomised: 25 Description of control: sampling performed after rinsing with 20 mL of distilled water |
|
| Outcomes | Outcome name: CFU Outcome measurement: mean bacterial counts. Used aerosol monitoring equipment made available by the Millipore Filter Corporation. Sterile impinger mounted rigidly in an upright position; top plug removed from the clinical field monitor, and adapter end of a sterile sampling tube inserted. 30 mL of sterile impingement fluid added to impinger. Vacuum source connected, and air sample was drawn into and through impinger. Effect estimate: mean difference |
|
| Notes |
Key study conclusions Oral rinse before operative procedures with high‐speed drills was effective for the clearance of debris and the clearance of oral microorganisms. Mouthwash formulation seemed superior to water (76% reduction). Contact information: Clive I Mohammed, University of Puerto Rico, School of Dentistry, San Juan, Puerto Rico |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. Quote: "allotted at random, divided as to sex and number." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | Unclear risk | No measure of variance reported and clinical trial registration details not available. |
| Other bias | Low risk | None detected. |
Mohammed 1970.
| Study characteristics | ||
| Methods | Title: preoperative oral rinsing as a means of reducing air contamination during use of air turbine handpieces Trial design: randomised controlled trial Trial registration: not reported Location: School of Dentistry, University of Puerto Rico Setting: university hospital Language: English Number of centres: 1 Study period: not reported Funding source: not reported Dental aerosol procedure tested: drilling tooth with air turbine handpiece |
|
| Participants | Age: not reported Total number of participants: 40 males Inclusion criteria: participants who needed class I cavity preparation in lower molar region Exclusion criteria: not reported Number randomised: 20 participants per group |
|
| Interventions |
Intervention Group name: group II (preprocedural mouth rinse) Number randomised: 20 Description of intervention: CPC + domiphen bromide + quaternary ammonium antiseptics and cationic surfactants, alcohol Any co‐interventions: none Comparator Group name: group I (no preprocedural mouth rinse) Number randomised: 20 Description of control: none Any co‐interventions: none |
|
| Outcomes | Outcome name: contamination in aerosols Outcome measurement: mean colony count; used trypticase soy broth culture medium and sample collected after 1 min of the procedure using a sterile impinger from 10 inches in front of and above the patient's mouth Effect estimate: group I: 803.3 (total 16,066; 20 participants); group II: 348.3 (total 6967; 20 participants). SD not reported Adverse events: not reported |
|
| Notes |
Key study conclusions Mouthwash formulation appeared to reduce contamination. Contact information: Victor Monserrate, Formerly Assistant Professor, Department of Histopathology, University of Puerto Rico School of Dentistry, Puerto Rico |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | Unclear risk | No measure of variance reported and clinical trial registration details unavailable. |
| Other bias | Low risk | None detected. |
Mohan 2016.
| Study characteristics | ||
| Methods | Title: the efficacy of pre‐procedural mouth rinse on bacterial count in dental aerosol following oral prophylaxis. Trial design: randomised controlled trial Trial registration: not reported Location: Chennai, India Setting: university hospital Language: English Number of centres: 1 Study period: not reported Funding source: not reported Dental aerosol procedure tested: oral prophylaxis using ultrasonic scaler (type of scaler not reported) |
|
| Participants | Age (range): 25–40 years Total number of participants: 20 Inclusion criteria: ≥ 20 permanent teeth present; lack of any dental treatment for previous 8 months; Plaque Index 1–3 Exclusion criteria: people with systemic disorders such as hypertension, rheumatic disorders, pregnancy; receiving antibiotic or immunosuppressive treatment Number randomised: 10 per group Number evaluated (withdrawals/missing participants): 10 per group (0) |
|
| Interventions |
Intervention Group name: group II Number randomised: 10 Description of intervention: 0.2% CHX mouth rinse for 1 min Any co‐interventions: none Comparator Group name: group I Number randomised: 10 Description of control: saline mouth rinse for 1 min Any co‐interventions: none |
|
| Outcomes | Outcome name: contamination in aerosols Outcome measurement: mean CFU; blood agar medium; 3 ft away from patient's mouth Effect estimate (mean CFU): group I: 891.1 (SD 595.19); group II: 222.7 (SD 117.8) Adverse events: not reported |
|
| Notes |
Key study conclusions CHX had a significant effect as an antimicrobial preprocedural mouth rinse in reducing number of CFUs in the aerosol produced by the ultrasonic scaling units Contact information: Meenakshi Mohan, Saveetha Dental College, Chennai 77, India; E‐mail: drmeena.mohan23@gmail.com Comments: mail sent on 29 March 2021 requesting the missing details and reply awaited |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. Quote: "subjects were randomly distributed into two groups of ten subjects each." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Clinical trial registration details not available. |
| Other bias | Low risk | None detected. |
Nayak 2020.
| Study characteristics | ||
| Methods | Title: comparative evaluation of efficacy of chlorhexidine and herbal mouthwash as a preprocedural rinse in reducing dental aerosols: a microbiological study Trial design: blinded, placebo‐controlled, randomised, 3‐group parallel design Trial registration: CTRI/2019/01/017038 Location: Mangalore, India Setting: university hospital Language: English Number of centres: 1 Study period: not reported Funding source: Warren, a division of Indoco remedies, India, and Sagar Pharmaceuticals, BPRL PVT Ltd., India, provided free Rexidin (CHX mouthwash) and Befresh (herbal mouthwash containing Cinnamomum zeylanicum, Mentha spicata, Syzygium aromaticum, and Eucalyptus globulus) samples Dental aerosol procedure tested: ultrasonic scaling (piezoelectric) |
|
| Participants | Age (mean): group A (CHX): 42.43 (SD 6.3) years; group B (Herbal mouthwash): 43.73 (SD 5.5) years and group C (control): 44 (SD 6) years Total number of participants: 30 (10 per group) Inclusion criteria: systemically healthy people; aged > 18 years; ≥ 20 teeth; diagnosed with mild–moderate form of chronic periodontitis Exclusion criteria: smokers, pregnant and breastfeeding women, underwent any periodontal treatment in last 6 months or received antibiotics or non‐steroidal anti‐inflammatory drugs in past 8 weeks and people using mouthwash or known allergy to CHX mouthwash Number randomised: 30 Number evaluated (withdrawals/missing participants): 30 (0) |
|
| Interventions |
Intervention 1 Group name: group A Number randomised: 10 Description of intervention: 0.2% CHX herbal mouthwash (10 mL for 1 min as a preprocedural rinse 30 min before procedure) Any co‐interventions: none Intervention 2 Group name: group B Number randomised: 10 Description of intervention: Befresh herbal mouthwash (10 mL for 1 min as a preprocedural rinse 30 min before procedure; contained Cinnamomum zeylanicum, Mentha spicata, Syzygium aromaticum and Eucalyptus globulus Any co‐interventions: none Comparator Group name: group C Number randomised: 10 Description of control: preprocedural rinse with water (10 mL for 1 min as a preprocedural rinse 30 min before procedure) Any co‐interventions: none |
|
| Outcomes | Outcome name: contamination in aerosols Outcome measurement: mean CFUs/plate at 30 cm from patient's mouth, at assistant chest area, operator chest area and patient chest area (postscaling for 10 min; blood agar plates used to collect samples) Effect estimate: mean (SD) Adverse events: not reported |
|
| Notes |
Key study conclusions Reduction in bacteria present in aerosol during ultrasonic scaling found with herbal rinse (Befresh) and it was as effective as CHX. Contact information: Sangeeta Umesh Nayak, Department of Periodontology, Manipal College of Dental Sciences, Mangalore; Manipal Academy of Higher Education, Manipal, Karnataka, India; E‐mail: sangeeta.nayak@manipal.edu |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used computer‐generated table. |
| Allocation concealment (selection bias) | Low risk | Quote: "The enrollment and allocation were done by a masked examiner." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All assigned mouthwashes were colourless. Assigned mouthwash was dispensed in a similar opaque bottle and by a blinded operator. Operator who performed scaling was also blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded researchers performed the microbial analysis. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the trial registration document were reported in the article. |
| Other bias | Low risk | None detected. |
Nisha 2021.
| Study characteristics | ||
| Methods | Title: efficacy of preprocedural boric acid mouth rinse in reducing viable bacteria in dental aerosols produced during ultrasonic scaling Trial design: 3‐arm parallel‐group randomised controlled trial Trial registration: CTRI/2017/10/010189 (registered retrospectively) Location: Mysuru, India Setting: university hospital Language: English Number of centres: 1 Study period: March 2016 to August 2016 Funding source: JSS Academy of higher education and Research University, Mysuru, Karnataka (No.REG/DIR(R)/URG/5 4/2011‐12/7279/9) Dental aerosol procedure tested: ultrasonic scaling (type of scaler not reported) |
|
| Participants | Age (mean): 37 years Total number of participants: 96 (32 in each group) Inclusion criteria: systemically healthy men or women aged 25–55 years; diagnosed with moderate chronic periodontitis having ≥ 20 natural teeth with a mean Plaque Index of 2–3, probing pocket depth ≥ 4 mm in ≥ 4 sites (probing pocket depth criteria not mentioned in the trial register) Exclusion criteria: people with respiratory infection, smokers, pregnant/breastfeeding women, administration of any mouth rinses or oral irrigation, any systemic or topical antimicrobial drug intake in last 6 months, hypersensitivity to CHX gluconate or boric acid and other formula ingredients Number randomised: 96 Number evaluated (withdrawals/missing participants): 30 in each group (2 dropouts from each group) |
|
| Interventions |
Intervention 1 Group name: group A: CHX Number randomised: 32 Description of intervention: 0.12% CHX (Periogard, Colgate‐Palmolive) Any co‐intervention: none Intervention 2 Group name: group B: boric acid Number randomised: 32 Description of intervention: boric acid 0.75% Any co‐intervention: none Comparator Group name: group C (control group): water Number randomised: 32 Description of intervention: water Any co‐intervention: none Participants rinsed for 1 min with 15 mL of 0.12% CHX, 10 mL boric acid or 10 mL water 15 min prior to procedure. |
|
| Outcomes | Outcome name: contamination of aerosols at 30 cm from patient's mouth on patient's chest, operator's chest and dental assistant's chest. Blood agar plates used for culture. Outcome measurement: mean CFUs Effect estimate: mean (SD) Adverse events: not reported |
|
| Notes |
Key study conclusion Study recommends routine use of preprocedural mouth rinse to reduce bacterial aerosols generated during ultrasonic scaling and that 0.12% CHX gluconate is more effective than 0.75% BA mouthwash in reducing CFUs count. Contact information: Dr Avinash Bettahalli Shivamallu, Department of Periodontology, J.S.S Dental College and Hospital, JSS Academy of Higher Education and Research, Mysuru, Karnataka, India; E‐mail: avinashbs@gmail.com Comments: type of scaler, method of allocation concealment, reason for dropout, any adverse events were not reported. Trial registration document mentions the method as cross‐over trial and sample size as 10 per group. The trial was registered retrospectively. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomly allotted using computer‐generated random sequence table (GraphPad) to one of the three groups by one examiner." |
| Allocation concealment (selection bias) | Unclear risk | Not reported in main publication. Trial registration document quote: "an open list of random numbers." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the treatment was performed by another examiner (S Nisha) unknown of the pre rinse used before scaling." Quote: "Since the study protocol involved triple masking, the operator, microbiologist, and the patients were unaware of allocated groups." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Since the study protocol involved triple masking, the operator, microbiologist, and the patients were unaware of allocated groups." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 dropouts from each group; reason for dropout not reported, but intention‐to‐treat analysis appears to have been used. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in trial registration document were reported in the article; however, the trial registration was done retrospectively and for a sample of 10 participants. |
| Other bias | Low risk | None observed. |
Reddy 2012.
| Study characteristics | ||
| Methods | Title: efficacy of 0.2% tempered chlorhexidine as a pre‐procedural mouth rinse: a clinical study Trial design: 3‐arm parallel‐group randomised controlled trial Trial registration: not reported Location: Bengaluru, India Setting: university hospital Language: English Number of centres: 1 Study period: not reported Funding source: none declared Dental aerosol procedure tested: ultrasonic scaling (type of scaler not reported) |
|
| Participants | Age: not reported Total number of participants: 30 Inclusion criteria: not reported Exclusion criteria: history of treatment for moderate‐to‐severe periodontitis in past 6 months, having systemic disease, receiving systemic antibiotics Number randomised: 30 Number evaluated (withdrawals/missing participants): 30 (0) |
|
| Interventions |
Intervention 1 Group name: group II: 0.2% non‐tempered CHX Number randomised: 10 Description of intervention: 0.2% non‐tempered CHX Any co‐interventions: none Intervention 2 Group name: group III: 0.2% tempered CHX mouthwash Number randomised: 10 Description of intervention: 0.2% tempered CHX mouthwash rinsing for 60 sec (tempering = warming to 47 °C) Any co‐interventions: none Comparator Group name: group I Number randomised: 10 Description of control: rinse with sterile water for 60 sec Any co‐interventions: none |
|
| Outcomes | Outcome name: contamination of aerosols at a distance of 4 ft at 3, 6 and 12 o'clock positions from patient's mouth. Blood agar plates used for culture Outcome measurement: mean CFUs Effect estimate: mean (SD) Adverse events: not reported |
|
| Notes |
Key study conclusions CHX had a significant effect as an antimicrobial preprocedural mouth rinse in reducing number of CFUs in aerosol produced by ultrasonic scaling units. Both tempered and non‐tempered forms of 0.2% were effective in reducing the bacterial load in aerosol, but tempered CHX was slightly better. Contact information: Dr MGS Prasad, Department of Periodontics, Dr Syamala Reddy Dental College, Karnataka, India; E‐mail: prasadmgs@indiatimes.com Comments: we mailed the contact author for missing details on 29 March 2021 and the mail was returned as unavailable. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. Quote: "divided randomly into 3 groups." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout. |
| Selective reporting (reporting bias) | Unclear risk | Clinical trial registration details not available. |
| Other bias | Low risk | None detected. |
Retamal‐Valdes 2017.
| Study characteristics | ||
| Methods | Title: effectiveness of a pre‐procedural mouthwash in reducing bacteria in dental aerosols: randomized clinical trial Trial design: 4‐arm single‐blind randomised controlled trial Trial registration: NCT02875769 Location: Sao Paulo, Brazil Setting: university hospital Language: English Number of centres: 1 Study period: February to April 2015 Funding source: supported by Colgate Palmolive Company (Piscataway, NJ, USA) and Latin America Oral Health Association (LAOHA) Dental aerosol procedure tested: dental prophylaxis using ultrasonic scaler (Magnetostrictive) |
|
| Participants | Age (mean): no rinsing group: 46.27 (SD 6.63) years; water group: 36.13 (SD 8.71); CPC+Zn+F: 45.47 (SD 11.79) years; CHX group: 41.40 (SD 10.93) years Total number of participants: 60 Inclusion criteria: men or women aged 18–70 years, ≥ 20 natural teeth, ≥ 80% of the sites with visible supragingival plaque, < 10% of sites with visible supragingival calculus, < 30% of sites with PD ≥ 5 mm Exclusion criteria: presence of orthodontic bands; partial removable dentures; lesions of the soft or hard tissues of the oral cavity; carious lesions requiring immediate restorative treatment; history of allergy to CHX, CPC, zinc lactate or sodium fluoride; participation in any other clinical study within 1 month period prior to entering study; professional tooth cleaning procedure (oral prophylaxis) during 1 month prior to entering study; pregnant or breastfeeding women; antibiotic therapy in previous 6 months; continuous use of oral mouthwashes and any systemic condition that may require prophylactic medication for dental treatment (e.g. mitral valve prolapse) Number randomised: 60 Number evaluated (withdrawals/missing participants): 60 (0) |
|
| Interventions |
Intervention Group name: test: CPC+Zn+F (0.075% CPC + 0.28% zinc + 0.05% sodium fluoride) mouth rinse Number randomised: 15 per group Description of intervention: 20 mL CPC+Zn+F solution dispensed in opaque bottles Any co‐interventions: none Comparator 1 Group name: A (no rinsing) Number randomised: 15 per group Description of control: group A volunteers did not rinse their mouth before the dental prophylaxis. Any co‐interventions: none Comparator 2 Group name: B (water) Number randomised: 15 per group Description of control: group B volunteers rinsed their mouth with 20 mL water Any co‐interventions: none Comparator 3 Group name: CHX digluconate 0.12% Number randomised: 15 per group Description of control: volunteers rinsed their mouth with 20 mL of CHX, dispensed in an opaque bottle Any co‐interventions: none |
|
| Outcomes | Outcome name: contamination of aerosols at the support board, volunteer's chest (immediately in front of the volunteer's mouth) and on the clinician's forehead. HNK agar (tryptic soy agar with yeast extract enriched with 5% menadione, 5% sheep blood and 1% N‐acetylmuramic acid) plates were used for the sample collection and incubated under anaerobic conditions. Outcome measurement: mean CFUs Effect estimate: mean (SD) Adverse events: none of the participants reported any adverse events. |
|
| Notes |
Key study conclusions CPC+Zn+F was effective in reducing viable bacteria in oral aerosol after a dental prophylaxis with ultrasonic scaler. Contact information: Magda Feres, Universidade de Guarulhos, Department of Periodontology, Dental Research Division, São Paulo, Brazil; E‐mail: mferes@ung.br |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used computer‐generated random numbers table. |
| Allocation concealment (selection bias) | Low risk | Centralised allocation performed by a researcher who was not involved in any other phases of the trial. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Opaque bottles used for test and CHX mouth rinses. No rinse and rinse with water group were not blinded. However, the outcome measurement was objective (colony count) and was not influenced by the blinding of participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinician and the laboratory technician who counted the colonies were blinded to test or comparator. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout. |
| Selective reporting (reporting bias) | Low risk | In the results section, CHX group showed fewer number of microbial CFUs compared to CPC+Zn+F group. However, the conclusion of the report highlights the reduction in only the CPC+Zn+F group. |
| Other bias | Low risk | None detected. |
Shetty 2013.
| Study characteristics | ||
| Methods | Title: compare the efficacy of two commercially available mouth rinses in reducing viable bacterial count in dental aerosol produced during ultrasonic scaling when used as a preprocedural rinse. Trial design: randomised single‐blind, placebo‐controlled parallel‐group study Trial registration: not reported Location: Mangalore, India Setting: university hospital Language: English Number of centres: 1 Study period: not reported Funding source: none declared Dental aerosol procedure tested: ultrasonic scaling (type of scaler not reported) |
|
| Participants | Age (range): 25–45 years Total number of participants: 60 Inclusion criteria: ≥ 20 permanent teeth, an oral hygiene score (Green and Vermillion 1960) 1.3–3, Plaque Index (Silness and Loe 1964) 1–2, gingival index score (Loe and Silness 1963) 1–2 Exclusion criteria: cardiac pacemakers, resin restorations, conditions requiring prophylactic antibiotics, prior to dental procedures or currently using antibiotics or history of antibiotic usage in past 6 months and with any history of systemic diseases Number randomised: 60 Number evaluated (withdrawals/missing participants): not reported |
|
| Interventions |
Intervention 1 Group name: group II Number randomised: 20 per group Description of intervention: 0.2% CHX digluconate (Rexidine) Any co‐interventions: none Intervention 2 Group name: group III Number randomised: 20 per group Description of intervention: group III: tea tree oil (Emoform) Any co‐interventions: none Comparator Group name: group I Number randomised: 20 Description of control: rinse with distilled water Any co‐interventions: none |
|
| Outcomes | Outcome name: contamination of aerosols at 15 cm from operator's nose level, 15 cm from dental assistant's nose level and 30 cm from patient's chest level. Trypticase soy agar plates used for aerobic culture Outcome measurement: mean CFUs Effect estimate: mean (SD) Adverse events: not reported |
|
| Notes |
Key study conclusions Antiseptic mouthwashes reduced bacterial CFUs in aerosol samples. CHX rinses were superior to tea tree rinses when used preprocedurally in reducing aerosolised bacteria. Contact information: Shamila K Shetty, Assistant Professor, Department of Periodontics, AB Shetty Memorial Institute of Dental Sciences, Nitte University, Mangalore, Karnataka India; telephone: +919901121270; e‐mail: shetty.shamila@gmail.com Comments: mail sent on 29 March 2021 requesting the missing details and reply awaited |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported. |
| Selective reporting (reporting bias) | Unclear risk | Clinical trial registration details not available. |
| Other bias | Low risk | None detected. |
Suresh 2011.
| Study characteristics | ||
| Methods | Title: comparison of efficacy of preprocedural rinsing with chlorhexidine and essential oil mouthwash in reducing viable bacteria in dental aerosols – a microbiological study Trial design: 2‐arm, parallel‐group randomised controlled trial Trial registration: not reported Location: Chennai, India Setting: dental school hospital Language: English Number of centres: 1 Study period: not reported Funding source: none reported Dental aerosol procedure tested: oral prophylaxis (piezoelectric scaler) |
|
| Participants | Age (range): 20–45 years Total number of participants: 20 Inclusion criteria: ≥ 20 permanent teeth; mean Oral Hygiene Index – simplified and Gingival Index score 2.0–3.0 Exclusion criteria: medical conditions contradicting the use of ultrasonic scalers and those on systemic or topical antibiotics and antiseptic mouth rinses for the past 6 months Number randomised: 20 Number evaluated (withdrawals/missing participants): all 20 participants evaluated |
|
| Interventions |
Intervention Group name: group 1 CHX Number randomised: 10 Description of intervention: 0.2% mouth rinse CHX – 10 mL with 1‐min rinsing time Any co‐intervention: none Comparator Group name: group 2 (essential oil mouth rinse) Number randomised: 10 Description of intervention: 20 mL with 1 min rinsing time; mouth rinse contained eucalyptol, thymol, methyl salicylate and menthol essential oil Any co‐intervention: none |
|
| Outcomes | Exposure: oral prophylaxis with a piezoelectric scaler Outcome name: CFU number Outcome measurement: blood agar plates placed at patient's chest area (location A) and at the dental chair's tray 15 cm from the patient's mouth (location B). Used 5% sheep blood agar plates; plates incubated at 37 °C in an increased carbon dioxide chamber for 48 hours Effect estimate: data given in graph Outcome name: mean CFU Outcome measurement: as above Effect estimate: data given in graph |
|
| Notes |
Key study conclusions When CHX was used as a prerinse before ultrasonic scaling there were consistently fewer CFUs in the 2 standard locations than when an essential oil‐containing mouth rinse was used. Contact information: Dr Snophia Suresh MDS, Reader, Department of Periodontics, Thaimoogambigai Dental College & Hospital, Chennai, India; E‐mail: Suresh_sno@yahoo.com Comments: mail sent on 29 March 2021 requesting missing details and reply awaited |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "laboratory technician was not aware which plate was exposed during the two test procedures." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Clinical trial registration details not available. |
| Other bias | Low risk | None detected. |
Tasneem 2017.
| Study characteristics | ||
| Methods | Title: effectiveness of preprocedural rinse with chlorhexidine and povidone iodine in preventing bioaerosol contamination during ultrasonic scaling – a clinical and microbiological study Trial design: double‐blind parallel‐group randomised controlled trial Trial registration: not reported Location: Srikakulam, India Setting: university hospital Language: English Number of centres: 1 Study period: June to December 2016 (personal communication) Funding source: no funding received (personal communication) Dental aerosol procedure tested: ultrasonic scaling (type of scaler not reported) |
|
| Participants | Age (range): 20–50 years Total number of participants: 30 Inclusion criteria: ≥ 20 permanent teeth Exclusion criteria: presence of periodontitis, medical conditions or taking medications that would contraindicate treatment Number randomised: 30 Number evaluated (withdrawals/missing participants): 30 (0) |
|
| Interventions |
Intervention Group name: group I Number randomised: 15 Description of intervention: 0.2% CHX mouth rinse Any co‐interventions: none Comparator Group name: group II Number randomised: 15 Description of intervention: povidone iodine mouth rinse (concentration not reported) Any co‐interventions: none |
|
| Outcomes | Outcome name: contamination of aerosols at patient's chest level (15 cm) and on the mask of the operator (45 cm). Blood agar plates used for aerobic and anaerobic culture. Outcome measurement: mean CFUs Effect estimate: mean (SD) Adverse events: no adverse events (personal communication) |
|
| Notes |
Key study conclusions CHX was superior to povidone iodine in reducing the number of viable bacteria confirmed by decreased number of CFUs. Contact information: Dr Sheema Tasneem (PG Student), Department of Periodontics and Oral Implantology, SreeSai Dental College and Research Institute, Chapuram, Srikakulam, India; E‐mail: sheema.tasneem@gmail.com Comments: mail sent on 29 March 2021 requesting missing details and reply received on 7 April 2021. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used random numbers table. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Allocation of patients for either of the groups was done by flip coin method of randomization." (personal communication) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Operator who performed the scaling was blinded for the mouth rinse used. Participants were not blinded. However, participant blinding would have not influenced the study outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Laboratory technician was blinded for the agar plates from group I or II. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Clinical trial registration details not available. |
| Other bias | Low risk | None detected. |
Verma 2017.
| Study characteristics | ||
| Methods | Title: evaluation of aerosol contamination during ultrasonic procedures Trial design: 3‐arm parallel‐group randomised controlled trial Trial registration: not reported Location: Udaipur, India Setting: university hospital Language: English Number of centres: 1 Study period: not reported Funding source: not funded Dental aerosol procedure tested: ultrasonic scaling (piezoelectric) |
|
| Participants | Age: not reported Total number of participants: 18 Inclusion criteria: presence of full complement of maxillary and mandibular anterior teeth; absence of any dental treatment for past 1 year; people with plaque and gingival score 1–2 Exclusion criteria: history of systemic disease, cardiac pacemakers or respiratory complication; pregnancy; conditions requiring prophylactic antibiotics, prior to dental procedure and those currently on medication Number randomised: 18 Number evaluated (withdrawals/missing participants): 18 (0) |
|
| Interventions |
Intervention 1 Group name: group II Number randomised: 6 Description of intervention: 0.2% CHX mouth rinse for 2 min Any co‐interventions: none Intervention 2 Group name: group III Number randomised: 6 Description of intervention: 5% povidone iodine mouth rinse for 2 min Any co‐interventions: none Comparator Group name: group I Number randomised: 6 Description of control: 0.9% saline used as mouth rinse for 2 min |
|
| Outcomes | Outcome name: contamination in aerosols Outcome measurement: mean CFUs measured at 3 locations; operator's eye level, participant's eye level and participant's chest level. Blood agar plates used for aerobic culture. Effect estimate: mean (SD) Adverse events: none reported |
|
| Notes |
Key study conclusions Antiseptic mouth rinses reduce the bacterial CFU in the aerosol. Povidone iodine was superior to CHX when used preprocedurally. Contact information: Dr Neha Verma, Department of Periodontics, Pacific Dental College and Hospital, Udaipur, India; E‐mail: nehavarma_0731@yahoo.com Comments: mail sent on 29 March 2021 requesting the missing details and reply received on 4 May 2021. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised selection (from personal communication). |
| Allocation concealment (selection bias) | High risk | Quote: "All the procedure was carried by a single examiner. This was done to avoid inter‐examination bias." (from personal communication) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not performed (from personal communication). There is a possibility of clinicians altering their behaviour. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding not done (from personal communication). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | High risk | Povidone iodine showed no difference in the mean CFUs compared to the CHX group on plate 3 (participant's eye level). However, the results section stated, "The present study also demonstrates maximum reduction of aerobic colonies using Povidone Iodine at subject's eye." The conclusion section mentioned it as, "Povidone iodine was found to be superior to chlorhexidine when used pre‐procedurally." |
| Other bias | Low risk | None detected. |
ADA: American Dental Association; CAL: clinical attachment level; CFU: colony‐forming units; CHX: chlorhexidine; CPC: cetylpyridinium chloride; ft: feet; min: minutes PD: probing depth; RCT: randomised controlled trial; SD: standard deviation; SE: standard error; sec: second.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Altonen 1976 | Not an RCT. |
| Ciancio 1994 | Not an RCT. |
| CTRI/2017/10/010189 | Unable to access. |
| CTRI/2018/05/013935 | Unable to access. |
| Dawson 2016 | Not an RCT. |
| Devker 2012 | Split‐mouth design. |
| Fine 1992 | Split‐mouth design. |
| Fine 1993b | Split‐mouth design. |
| Klyn 2001 | Split‐mouth design |
| Litsky 1970 | Not an RCT. |
| Logothetis 1995 | High‐velocity suction used with the preprocedural rinse. |
| Narayana 2016 | Split‐mouth design. |
| NCT02319668 | Mouthwash was compared with toothpaste. |
| NCT04659928 | High‐volume evacuation used with the preprocedural rinse. |
| Paul 2020 | Not an RCT. |
| Rajachandrasekaran 2019 | Not an RCT. |
| Ramesh 2015 | High‐volume suction used with the preprocedural rinse. |
| Santos 2014 | Cross‐over sequence not randomised. |
| Sawhney 2015 | Split‐mouth design. |
| Swaminathan 2014 | High‐speed evacuator used with the preprocedural rinse. |
| Toroglu 2001 | Not an RCT. |
| Worrall 1987 | Not an RCT. |
| Young 2002 | Bacterial contamination was checked in the bone debris collected from the osteotomy site. |
RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
CTRI/2021/03/031653.
| Study name | Efficiency of a herbal mouthwash in decreasing infection from splatter production during dental treatment procedures |
| Methods | Type of study: randomised, parallel‐group, placebo‐controlled trial Method of generating random sequence: coin toss, lottery, toss of dice, shuffling cards, etc. Method of concealment: open list of random numbers Blinding: participant blinded |
| Participants | Age (range): 18–60 years Gender: both Sample size: 30 Inclusion criteria: age 18–60 years; with minimum of 20 permanent teeth; Plaque Index Score 2–3; probing pocket depth ≥ 4 mm for > 2 sites; People who were systemically healthy Exclusion criteria: history of systemic or topical antibiotics use within last 3 months; smokers and people dependent on alcohol; pregnant and lactating women; history of oral prophylaxis or mouthwash use within last 3 months |
| Interventions | Intervention: turmix mouth rinse. Participant will be asked to rinse mouth using turmix mouthwash and then have ultrasonic scaling performed to check the efficacy of turmix mouthwash in reducing the aerosol contamination Comparator 1: chlorhexidine mouthwash. Participants will be asked to rinse mouth using chlorhexidine mouthwash and then have ultrasonic scaling performed to check the efficacy of chlorhexidine in reducing the aerosol contamination Comparator 2: placebo. Participants will be asked to rinse mouth using water and then have ultrasonic scaling performed |
| Outcomes | Aim: to determine the effectiveness of turmix mouthwash as preprocedural rinse in reducing aerosol contamination during ultrasonic scaling in people with chronic periodontitis. Extent of aerosol contamination during ultrasonic scaling and root planing Efficacy of intervention during ultrasonic scaling and root planing |
| Starting date | 4 March 2021 |
| Contact information | Nidhi Pandey, PG Student, Institute of Dental Sciences Bareilly, Department of Periodontology and Implantology Institute of Dental Sciences, Rohilkhand Medical College, Bareilly, UTTAR PRADESH, 243006, India; telephone: 8218251306; E‐mail: docpandey.n19@gmail.com |
| Notes | Open for recruitment |
CTRI/2021/10/037528.
| Study name | Comparison of providone iodine and chlorhexidine as a mouth rinse before cleaning treatment to reduce the bacterial count during treatment |
| Methods | Study design: randomised, parallel‐group trial Method of random sequence generation: computer‐generated randomisation Allocation: on‐site computer system Blinding: not applicable |
| Participants | Age (range): 17–54 years Gender: male and female Sample size: 42 Inclusion criteria: systemically healthy people; with ≥ 20 permanent teeth present; give informed consent to participate in the study Exclusion criteria: people who are asthmatic, have infectious disease or are immunocompromised; pregnant and lactate women |
| Interventions | Comparator: povidone iodine Intervention: chlorhexidine gluconate There was some inconsistency in the descriptions of the concentration used in each intervention. |
| Outcomes | Efficacy as a preprocedural mouth rinse in reducing aerosol contamination and bacterial count during ultrasonic scaling |
| Starting date | 5 November 2021 |
| Contact information | Naskath Sabana, PG student, Yenepoya Dental College, Department of Periodontology, Yenepoya University Deralakatte, Karnataka, India; telephone 9003493961; E‐mail: naskathsabana651@gmail.com |
| Notes | Not yet recruiting |
NCT03839719.
| Study name | Efficacy of ocimum sanctum as a pre‐procedural mouth rinse in reducing aerosol contamination produced by ultrasonic scaler: a clinical and microbiological study |
| Methods | Allocation: randomised Intervention model: cross‐over assignment Intervention model description: ultrasonic scaling done in 1st and 4th quadrant without any mouth rinse and fall out samples were collected in the blood agar plates kept at a distance of 0.5 m and 1 m from the oral cavity. After ultrasonic scaling of the 1st and 4th quadrant minimum 30‐min interval maintained. After 30 min rinsing the mouth with the ocimum sanctum mouth rinse, chlorhexidine gluconate mouth rinse or placebo for 60 seconds. Ultrasonic scaling was repeated in 2nd and 3rd quadrant and fall out samples were collected in the newly placed blood agar plates kept at 0.5 m and 1 m away from the patient. Treatment was carried out by placing 3 sterile agar plates uncovered at predesignated sites to collect samples of aerosolised bacteria. Single‐centre, double‐blind, placebo‐controlled study conducted over 2 months. Blinding: triple (participant, investigator, outcomes assessor) Blinding description: participants and investigator are blinded about mouth rinse use and assigned quadrant Primary purpose: prevention |
| Participants | Age (range): 18–40 years Gender: men and women Inclusion criteria: people with generalised chronic gingivitis or mild generalised chronic periodontitis of either sex; systemically healthy people, have ≥ 20 teeth; aged 18–40 years; who abide by approved protocol guidelines and willing to give written informed consent Exclusion criteria: any known systemic disease that affects periodontium, such as diabetes, cardiovascular disease, cancer, etc.; receiving anti‐inflammatory, perioceutics, antibiotics, steroids, cytotoxic and drugs for 3 months; known allergy to the material used; pregnant and breastfeeding women; have undergone any type of non‐surgical or surgical periodontal therapy (or both) earlier, in the past 6 months; tobacco users (smoke and smokeless) and people dependent on alcohol |
| Interventions | Placebo; before rinsing with the mouth rinse, ultrasonic scaling was performed in the 1st and 4th quadrant without any mouth rinse (placebo mouth rinse) and fall out samples were collected in the blood agar plates kept at a distance of 0.5 m and 1 m from the oral cavity. Ocimum sanctum mouth rinse. After ultrasonic scaling of the 1st and 4th quadrant minimum 30‐min interval maintained. After 30 min rinsing the mouth with ocimum sanctum mouth rinse for 60 seconds, ultrasonic scaling was repeated in 2nd and 3rd quadrant and fall out samples were collected in the newly placed blood agar plates kept at 0.5 m and 1 m from the patient. Chlorhexidine gluconate mouth rinse. After ultrasonic scaling of the 1st and 4th quadrant minimum 30 min interval maintained. After 30 min rinsing the mouth with chlorhexidine gluconate mouth rinse for 60 seconds. Ultrasonic scaling was repeated in the 2nd and 3rd quadrant and fall out samples were collected in the newly placed blood agar plates kept at 0.5 m and 1 m from the patient. |
| Outcomes | Counting of CFU up to 48 hours Preformed 10% blood agar plates were incubated at 37 °C for 48 hour after collecting the sample. Counting of CFUs was performed by a microbiologist who was blinded regarding the time of exposure and location of agar plate. The microbial counting was performed afterwards. |
| Starting date | 9 January 2019 |
| Contact information | Dr Pratik Kumar Ashok Bhai Chaudhari, Government College of Dentistry, Indore, India |
| Notes | Study completed 26 April 2019 |
NCT04717063.
| Study name | Clinical efficacy of single‐use of peroxyl mouthwash for reducing bacteria saliva and bioaerosol contamination phase 2 |
| Methods | Allocation: randomised Intervention model: parallel assignment Intervention model description: each participants will be randomised to receive 1 of 2 possible study products Blinding: triple (participant, investigator, outcomes assessor) Blinding description: triple (participant, investigator, outcome assessor) Primary purpose: treatment |
| Participants | Gender: men and women Sample size: 100 Inclusion criteria: ALL of the following criteria: ages 18–70 years inclusive; availability for the duration of the study; good general health; ≥ 20 natural teeth; Gingivitis Index 1.0 (Loe and Silness); signed informed consent form Exclusion criteria: must NOT HAVE ANY of the following conditions: symptoms consistent with COVID‐19 or have tested positive; presence of orthodontic bands; tumour(s) of the soft or hard tissues of the oral cavity; advanced periodontal disease (purulent exudate, tooth mobility, extensive loss of periodontal attachment or alveolar bone, or a combination) or peri‐implantitis; ≥ 5 carious lesions requiring immediate restorative treatment; use of antibiotic 1 month prior to entry into the study; participation in any other clinical study or test panel within 1 month prior to entry into study; dental prophylaxis during the past 2 weeks prior to baseline examinations; history of allergies to oral care/personal care consumer products or their ingredients; receiving any prescription medicines that might interfere with the study outcome; an existing medical condition that prohibits not eating or drinking for periods up to 4 hours; history of alcohol or drug abuse; pregnant or breastfeeding women |
| Interventions | Drug: peroxyl mouth wash 1.5% Placebo: placebo mouth wash |
| Outcomes | Antibacterial efficacy of mouthwash in reducing the levels of bacteria in bioaerosols generated by dental prophylaxis at 30 min |
| Starting date | 16 November 2020 |
| Contact information | Yiming Li, Loma Linda University |
| Notes | Recruitment completed |
CFU: colony‐forming unit; min: minute.
Differences between protocol and review
We performed sensitivity analysis in those meta‐analyses that had imputed data to check for differences between the results of these analyses.
We asked 11 dental surgeons to prioritise the comparisons for presentation in the summary of findings tables, as we had not planned this in our protocol. We used purposive sampling to select these dental surgeons based on geographic locations and their experience in using rinses before performing AGPs. We shared a Google form with all the comparisons and the dental surgeons ranked the comparisons (from rank 1 for the most important comparison to rank 10 for the least important comparison). One dental surgeon was unable to complete the prioritisation exercise within the given deadline and the other 10 dental surgeons prioritised the comparisons. We calculated the total of these Likert scales for each comparison from the 10 dental surgeons and identified the seven lowest scored comparisons as the most important ones.
Contributions of authors
SKN: obtaining full‐text articles, data extraction, GRADE and final review draft preparation, and review update.
PE: data analysis, GRADE and final review draft preparation.
MP: screening of titles and abstracts, screening full texts and final review draft preparation.
MN: arbiter, data extraction, analysis and final review draft preparation.
GS: screening of titles and abstracts, screening full texts and final review draft preparation.
TF: screening of titles and abstracts, data extraction and final review draft preparation.
JHV: data extraction and analysis, writing results and discussion.
Sources of support
Internal sources
-
Division of Dentistry, School of Medical Sciences, Faculty of Biology, Medicine and Health, The University of Manchester, UK
Support to Cochrane Oral Health
-
Manchester Academic Health Sciences Centre (MAHSC) and the NIHR Manchester Biomedical Research Centre, UK
Support to Cochrane Oral Health
External sources
-
Cochrane Oral Health Global Alliance, Other
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships-alliances). Contributors in recent years have been the American Association of Public Health Dentistry, USA; AS‐Akademie, Germany; the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; and Swiss Society of Endodontology, Switzerland.
-
NIHR, Other
This project was supported by the National Institute for Health and Care Research (NIHR), via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, the National Health Service or the Department of Health and Social Care.
Declarations of interest
SKN: none.
PE: none.
MP: none.
MN: none.
GS: none.
TF: none.
JHV: none.
New
References
References to studies included in this review
Bay 1993 {published data only}
- Bay NL, Overman PR, Krust-Bray K, Cobb C, Gross KB. Effectiveness of antimicrobial mouthrinses on aerosols produced by an air polisher. Journal of Dental Hygiene 1993;67(6):312-7. [PubMed] [Google Scholar]
Feres 2010 {published data only}
- Feres M, Figueiredo LC, Faveri M, Stewart B, Vizio W. Pre-procedural mouthrinse reduces viable bacteria in dental aerosols. 87th General Session and Exhibition of IADR/AADR/CADR; 2009 Apr 1-4; Miami (FL) 2009.
- Feres M, Figueiredo LC, Faveri M, Stewart B, De Vizio W. The effectiveness of a preprocedural mouthrinse containing cetylpyridiniuim chloride in reducing bacteria in the dental office. Journal of the American Dental Association 2010;141(4):415-22. [DOI] [PubMed] [Google Scholar]
Fine 1993a {published data only}
- Fine DH, Yip J, Furgang D, Barnett ML, Olshan AM, Vincent J. Reducing bacteria in dental aerosols: pre-procedural use of an antiseptic mouthrinse. Journal of the American Dental Association 1993;124(5):56-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gupta 2014 {published data only}
- Gupta G, Mitra D, Ashok KP, Gupta A, Soni S, Ahmed S, et al. Efficacy of preprocedural mouth rinsing in reducing aerosol contamination produced by ultrasonic scaler: a pilot study. Journal of Periodontology 2014;85(4):562-8. [DOI] [PubMed] [Google Scholar]
Joshi 2017 {published data only}
- Joshi AA, Padhye AM, Gupta HS. Efficacy of two pre-procedural rinses at two different temperatures in reducing aerosol contamination produced during ultrasonic scaling in a dental set-up – a microbiological study. Journal of International Academy of Periodontology 2017;19(4):138-44. [PubMed] [Google Scholar]
Kaur 2014 {published data only}
- Desai VR [pers comm]. Re – study details: effect of chlorhexidine, povidone iodine, and ozone on microorganisms in dental aerosols: randomized double-blind clinical trial. WhatsApp to Sumanth Kumbargere Nagraj 3 May 2021. [DOI] [PubMed]
- Kaur R, Singh I, Vandana KL, Desai R. Effect of chlorhexidine, povidone iodine, and ozone on microorganisms in dental aerosols: randomized double-blind clinical trial. Indian Journal of Dental Research 2014;25(2):160-5. [DOI] [PubMed] [Google Scholar]
Mohammed 1964 {published data only}
- Mohammed CI, Manhold JH. Efficacy of preoperative oral rinsing to reduce air contamination during use of air turbine handpieces. Journal of the American Dental Association (1939) 1964;69(6):715. [DOI] [PubMed] [Google Scholar]
Mohammed 1970 {published data only}
- Mohammed CI, Monserrate V. Preoperative oral rinsing as a means of reducing air contamination during use of air turbine handpieces. Oral Surgery, Oral Medicine, Oral Pathology 1970;29(2):291-4. [DOI] [PubMed] [Google Scholar]
Mohan 2016 {published data only}
- Mohan M, Jagannathan N. The efficacy of pre-procedural mouth rinse on bacterial count in dental aerosol following oral prophylaxis. Dental and Medical Problems 2016;53(1):78-82. [Google Scholar]
Nayak 2020 {published data only}
- CTRI/2019/01/017038. Comparison of efficacy of 0.2% chlorhexidine and herbal mouth wash as a pre-procedural mouth rinsing in reducing aerosol contamination produced by ultrasonic scaler – microbiology study. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2019/01/017038 (first received 10 January 2019).
- Nayak SU, Kumari A, Rajendran V, Singh VP, Hegde A, Pai KK. Comparative evaluation of efficacy of chlorhexidine and herbal mouthwash as a preprocedural rinse in reducing dental aerosols: a microbiological study. International Journal of Dentistry 2020;2020:2021082. [DOI: 10.1155/2020/2021082] [DOI] [Google Scholar]
Nisha 2021 {published data only}
- CTRI/2017/10/010189. Efficacy of pre-procedural boric acid mouth rinse in reducing viable bacteria in dental aerosols produced during ultrasonic scaling – a pilot study. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=11806 (first received 5 October 2017).
- Nisha S, Shivamallu AB, Gujjari SK, Shashikumar P, Ali NM, Kulkarni M. Efficacy of preprocedural boric acid mouthrinse in reducing viable bacteria in dental aerosols produced during ultrasonic scaling. Contemporary Clinical Dentistry 2021;12(3):282-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reddy 2012 {published data only}
- Reddy S, Prasad MG, Kaul S, Satish K, Kakarala S, Bhowmik N. Efficacy of 0.2% tempered chlorhexidine as a pre-procedural mouth rinse: a clinical study. Journal of Indian Society of Periodontology 2012;16(2):213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Retamal‐Valdes 2017 {published data only}
- NCT02875769. Pre-procedural mouthwash in reducing bacteria in dental aerosols. clinicaltrials.gov/ct2/show/NCT02875769 (first received 23 August 2016).
- Retamal-Valdes B, Soares GM, Stewart B, Figueiredo LC, Faveri M, Miller S, et al. Effectiveness of a pre-procedural mouthwash in reducing bacteria in dental aerosols: randomized clinical trial. Pesquisa Odontologica Brasileira [Brazilian Oral Research] 2017;31:e21. [DOI] [PubMed] [Google Scholar]
Shetty 2013 {published data only}
- Shetty SK, Sharath K, Shenoy S, Sreekumar C, Shetty RN, Biju T. Compare the efficacy of two commercially available mouthrinses in reducing viable bacterial count in dental aerosol produced during ultrasonic scaling when used as a preprocedural rinse. Journal of Contemporary Dental Practice 2013;14(5):848-51. [DOI] [PubMed] [Google Scholar]
Suresh 2011 {published data only}
- Suresh S, Manimegalai M, Sudhakar S, Sopia. Comparison of efficacy of preprocedural rinsing with chlorhexidine and essential oil mouthwash in reducing viable bacteria in dental aerosols – a microbiological study. International Journal of Contemporary Dentistry 2011;2(6):1-6. [Google Scholar]
Tasneem 2017 {published data only}
- Tasneem S [pers comm]. Re: study details: effectiveness of preprocedural rinse with chlorhexidine and povidone iodine in preventing bioaerosol contamination during ultrasonic scaling – a clinical and microbiological study. E-mail to Sumanth Kumbargere Nagraj 7 April 2021.
- Tasneem SM, Prasad SS, Srinivas BV, Anil Kumar K, Yadav RK. Effectiveness of pre procedural rinse with chlorhexidine and povidone-iodine in preventing bio aerosol contamination during ultrasonic scaling – a clinical and microbiological study. Journal of Contemporary Medicine and Dentistry 2017;5(2):60-4. [Google Scholar]
Verma 2017 {published data only}
- Verma N, Baidya D, Makhijani B, Shetty N, Mathur A, Manohar B. Evaluation of aerosol contamination during ultrasonic procedures. Journal of Nepalese Society of Periodontology and Oral Implantology 2017;1:17-22. [Google Scholar]
- Verma N [pers comm]. Re – study details: effectiveness of preprocedural rinse with chlorhexidine and povidone iodine in preventing bioaerosol contamination during ultrasonic scaling – a clinical and microbiological study. E-mail to Sumanth Kumbargere Nagraj 4 May 2021.
References to studies excluded from this review
Altonen 1976 {published data only}
- Altonen M, Saxen L, Kosunen T, Ainamo J. Effect of two antimicrobial rinses and oral prophylaxis on preoperative degerming of saliva. International Journal of Oral Surgery 1976;5(6):276-84. [DOI] [PubMed] [Google Scholar]
Ciancio 1994 {published data only}
- Ciancio S. Expanded and future uses of mouthrinses. Journal of the American Dental Association 1994;125:29S-32S. [DOI] [PubMed] [Google Scholar]
CTRI/2017/10/010189 {published data only}
- CTRI/2017/10/010189. Use of boric acid mouth rinse in decreasing aerosols levels during scaling. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2017/10/010189 (first received 25 October 2017).
CTRI/2018/05/013935 {published data only}
- CTRI/2018/05/013935. Comparison of two mouthrinses in reducing aerosol contamination during ultrasonic scaling. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2018/05/013935 (first received 17 May 2018).
Dawson 2016 {published data only}
- Dawson M, Soro V, Dymock D, Price R, Griffiths H, Dudding T, et al. Microbiological assessment of aerosol generated during debond of fixed orthodontic appliances. American Journal of Orthodontics and Dentofacial Orthopedics 2016;150(5):831-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Devker 2012 {published data only}
- Devker NR, Mohitey J, Vibhute A, Chouhan VS, Chavan P, Malagi S, et al. A study to evaluate and compare the efficacy of preprocedural mouthrinsing and high volume evacuator attachment alone and in combination in reducing the amount of viable aerosols produced during ultrasonic scaling procedure. Journal of Contemporary Dental Practice 2012;13(5):681-9. [DOI] [PubMed] [Google Scholar]
Fine 1992 {published data only}
- Fine DH, Mendieta C, Barnett ML, Furgang D, Meyers R, Olshan A, et al. Efficacy of preprocedural rinsing with an antiseptic in reducing viable bacteria in dental aerosols. Journal of Periodontology 1992;63(10):821-4. [DOI] [PubMed] [Google Scholar]
Fine 1993b {published data only}
- Fine DH, Furgang D, Korik I, Olshan A, Barnett ML, Vincent JW. Reduction of viable bacteria in dental aerosols by preprocedural rinsing with an antiseptic mouthrinse. American Journal of Dentistry 1993;6(5):219-21. [PubMed] [Google Scholar]
- Fine DH, Mendieta C, Furgang D, Yip J, Lieb R, Olshan A, et al. Reduction of aerosolized bacteria by a pre-procedural rinse. Journal of Dental Research 1991;70:411, 1165. [Google Scholar]
Klyn 2001 {published data only}
- Klyn SL, Cummings DE, Richardson BW, Davis RD. Reduction of bacteria-containing spray produced during ultrasonic scaling. General Dentistry 2001;49(6):648-52. [PMID: ] [PubMed] [Google Scholar]
Litsky 1970 {published data only}
- Litsky BY, Mascis JD, Litsky W. Use of an antimicrobial mouthwash to minimize the bacterial aerosol contamination generated by the high-speed drill. Oral Surgery, Oral Medicine, Oral Pathology 1970;29(1):25-30. [DOI] [PubMed] [Google Scholar]
Logothetis 1995 {published data only}
- Logothetis DD, Martinez-Welles JM. Reducing bacterial aerosol contamination with a chlorhexidine gluconate pre-rinse. Journal of the American Dental Association 1995;126(12):1634-9. [DOI] [PubMed] [Google Scholar]
- Logothetis DD, Martinez-Welles JM. Reduction of bacterial aerosol contamination following a prerinse with chlorhexidine. Journal of Dental Research 1994;73:432. [Google Scholar]
Narayana 2016 {published data only}
- Narayana TM, Mohanty L, Sreenath G, Vidhyadhari P. Role of preprocedural rinse and high volume evacuator in reducing bacterial contamination in bioaerosols. Journal of Oral and Maxillofacial Pathology 2016;20(1):59-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02319668 {published data only}
- NCT02319668. Antimicrobial agent for reducing bacteria in aerosols and oral cavity. clinicaltrials.gov/show/NCT02319668 (first received 18 December 2014).
NCT04659928 {published data only}
- NCT04659928. Evaluation of aerosol in a dental clinic. clinicaltrials.gov/show/NCT04659928 (first received 9 December 2020).
Paul 2020 {published data only}
- Paul B, Baiju RM, Raseena NB, Godfrey PS, Shanimole PI. Effect of aloe vera as a preprocedural rinse in reducing aerosol contamination during ultrasonic scaling. Journal of Indian Society of Periodontology 2020;24(1):37-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rajachandrasekaran 2019 {published data only}
- Rajachandrasekaran Y, Valiathan M, Jayaraman BG, Mahalakshmi K, Padmavathy K. An herbal alternative to control nosocomial pathogens in aerosols and splatter during ultrasonic scaling. Pesquisa Brasileira em Odontopediatria e Clinica Integrada 2019;19(1):e4807. [Google Scholar]
Ramesh 2015 {published data only}
- Ramesh A, Thomas JT, Muralidharan NP, Varghese SS. Efficacy of adjunctive usage of hydrogen peroxide with chlorhexidine AS preprocedural mouthrinse on dental aerosol. National Journal of Physiology, Pharmacy and Pharmacology 2015;5(5):431-5. [Google Scholar]
Santos 2014 {published data only}
- Santos IR, Moreira AC, Costa MG, Castellucci e Barbosa Md. Effect of 0.12% chlorhexidine in reducing microorganisms found in aerosol used for dental prophylaxis of patients submitted to fixed orthodontic treatment. Dental Press Journal of Orthodontics 2014;19(3):95-101. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sawhney 2015 {published data only}
- Sawhney A, Venugopal S, Girish Babu RJ, Garg A, Mathew M, Yadav M, et al. Aerosols how dangerous they are in clinical practice. Journal of Clinical and Diagnostic Research 2015;9(4):ZC52-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Swaminathan 2014 {published data only}
- Swaminathan Y, Thomas JT, Muralidharan NP. The efficacy of preprocedural mouth rinse of 0.2% chlorhexidine and commercially available herbal mouth containing salvadora persica in reducing the bacterial load in saliva and aerosol produced during scaling. Asian Journal of Pharmaceutical and Clinical Research 2014;7:71-4. [Google Scholar]
Toroglu 2001 {published data only}
- Toroglu MS, Haytac MC, Koksal F. Evaluation of aerosol contamination during debonding procedures. Angle Orthodontist 2001;71(4):299-306. [DOI] [PubMed] [Google Scholar]
Worrall 1987 {published data only}
- Worrall SF, Knibbs PJ, Glenwright HD. Methods of reducing bacterial contamination of the atmosphere arising from use of an air-polisher. British Dental Journal 1987;163(4):118-9. [DOI] [PubMed] [Google Scholar]
Young 2002 {published data only}
- Young MP, Carter DH, Worthington HV, McCord JF, Korachi M, Drucker DB. The effects of an immediately pre-surgical chlorhexidine oral rinse on the bacterial contaminants of bone debris collected during dental implant surgery. Clinical Oral Implants Research 2002;13(1):20-9. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
CTRI/2021/03/031653 {published data only}
- Efficiency of a herbal mouthwash in decreasing infection from splatter production during dental treatment procedures. Ongoing study. 4 March 2021. Contact author for more information.
CTRI/2021/10/037528 {published data only}
- CTRI/2021/10/037528. Comparison of providone iodine and chlorhexidine as a mouth rinse before cleaning treatment to reduce the bacterial count during treatment. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2021/10/037528 (first received 25 October 2021).
NCT03839719 {published data only}
- NCT03839719. OSE as a pre-procedural mouth rinse: a clinical and microbiological study. clinicaltrials.gov/show/nct03839719 (first received 15 February 2019).
NCT04717063 {published data only}
- NCT04717063. Clinical efficacy of single-use of peroxyl mouthwash for reducing bacteria saliva and bioaerosol contamination phase 2. www.clinicaltrials.gov/ct2/show/NCT04717063 (first received 20 January 2021).
Additional references
ADA 1996
- American Dental Association. ADA statement on dental unit waterlines. Journal of American Dental Association 1996;127:185-6. [Google Scholar]
Alshehri 2018
- Alshehri FA. The use of mouthwash containing essential oils (LISTERINE®) to improve oral health: a systematic review. Saudi Dental Journal 2018;30(1):2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Araujo 2015
- Araujo MW, Charles CA, Weinstein RB. Meta-analysis of the effect of an essential oil-containing mouthrinse on gingivitis and plaque. Journal of American Dental Association 2015;146(8):610-22. [DOI] [PubMed] [Google Scholar]
Barnes 1998
- Barnes JB, Harrel SK, Rivera-Hidalgo F. Blood contamination of the aerosols produced by in vivo use of ultrasonic scalers. Journal of Periodontology 1998;69(4):434-8. [DOI] [PubMed] [Google Scholar]
Bidra 2020
- Bidra AS, Pelletier JS, Westover JB, Frank S, Brown SM, Tessema B. Rapid in-vitro inactivation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) using povidone-iodine oral antiseptic rinse. Journal of Prosthodontics 2020;29(6):529-33. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burton 2020a
- Burton MJ, Clarkson JE, Goulao B, Glenny A-M, McBain AJ, Schilder AG, et al. Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID-19 infection to improve patient outcomes and to protect healthcare workers treating them. Cochrane Database of Systematic Reviews 2020, Issue 9. Art. No: CD013627. [DOI: 10.1002/14651858.CD013627.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burton 2020b
- Burton MJ, Clarkson JE, Goulao B, Glenny A-M, McBain AJ, Schilder AG, et al. Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID-19 infection to improve patient outcomes and to protect healthcare workers treating them. Cochrane Database of Systematic Reviews 2020, Issue 9. Art. No: CD013627. [DOI: 10.1002/14651858.CD013627.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cai 2020
- Cai H, Chen J, Panagodage PN, Liang X. Effects of herbal mouthwashes on plaque and inflammation control for patients with gingivitis: a systematic review and meta-analysis of randomised controlled trials. Evidence-based Complementary and Alternative Medicine : ECAM 2020;2020:2829854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carrilho 2010
- Carrilho MR, Carvalho RM, Sousa EN. Substantivity of chlorhexidine to human dentin. Dental Materials 2010;26(8):779-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2003
- Centers for Disease Control and Prevention. Guidelines for infection control in dental health-care settings. Morbidity and Mortality Weekly Report. Surveillance Summaries : MMWR 2003;52(RR-17):29, 45. [PubMed]
CDC 2020
- Centers for Disease Control and Prevention. Dental settings: interim infection prevention and control guidance for dental settings during the COVID-19 response. cdc.gov/coronavirus/2019-ncov/hcp/dental-settings.html (accessed 6 August 2020).
Chanpong 2020
- Chanpong B, Tang M, Rosenczweig A, Lok P, Tang R. Aerosol-generating procedures and simulated cough in dental anesthesia. Anesthesia Progress 2020;67(3):127-34. [DOI: 10.2344/anpr-67-03-04] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ciancio 2000
- Ciancio SG. Antiseptics and antibiotics as chemotherapeutic agents for periodontitis management. Compendium Continuing Education in Dentistry 2000;21(1):59-78. [PubMed] [Google Scholar]
Claffey 2003
- Claffey N. Essential oil mouthwashes: a key component in oral health management. Journal of Clinical Periodontology 2003;30:22-4. [DOI] [PubMed] [Google Scholar]
Clarkson 2020
- Clarkson J, Ramsay C, Richards D, Robertson C, Aceves-Martins M, on behalf of the CoDER Working Group. Aerosol generating procedures and their mitigation in international dental guidance documents – a rapid review. oralhealth.cochrane.org/news/aerosol-generating-procedures-and-their-mitigation-international-guidance-documents (accessed prior to 27 July 2022).
COMET 2020
- COMET Initiative. Core outcome measures in effectiveness trials. www.comet-initiative.org/ (accessed prior to 27 July 2022).
CONSORT 2010
- CONSORT Group. CONSORT Statement. www.consort-statement.org/ (accessed 19 June 2021).
Corner 1988
- Corner AM, Dolan MM, Yankell SL, Malamud D. C31G, a new agent for oral use with potent antimicrobial and antiadherence properties. Antimicrobial Agents and Chemotherapy 1988;32(3):350-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
DoH 2013
- Department of Health. Decontamination health technical memorandum 01-05: decontamination in primary care dental practices. www.england.nhs.uk/publication/decontamination-in-primary-care-dental-practices-htm-01-05/ (accessed prior to 27 July 2022).
Dua 2015
- Dua K, Sheshala R, Al-Waeli HA, Gupta G, Chellappan DK. Antimicrobial efficacy of extemporaneously prepared herbal mouthwashes. Recent Patents on Drug Delivery & Formulation 2015;9(3):257-91. [DOI] [PubMed] [Google Scholar]
Eggers 2018
- Eggers M, Koburger-Janssen T, Eickmann M, Zorn J. In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/mouthwash against respiratory and oral tract pathogens. Infectious Diseases and Therapy 2018;7(2):249-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eliades 2020
- Eliades T, Koletsi D. Minimizing the aerosol-generating procedures in orthodontics in the era of a pandemic: current evidence on the reduction of hazardous effects for the treatment team and patients. American Journal of Orthodontics and Dentofacial Orthopedics 2020;158(3):330-42. [DOI: 10.1016/j.ajodo.2020.06.002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Estrich 2020
- Estrich CG, Milkenssen M, Morrisey R, Geisinger MA. Estimating COVID-19 prevalence and infection control practices among US dentists. Journal of American Dental Association 2020;151(11):815-24. [DOI: 10.1016/j.adaj.2020.09.005] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fallahi 2020
- Fallahi HR, Keyhan SO, Zandian D, Kim SG, Cheshmi B. Being a front-line dentist during the COVID-19 pandemic: a literature review. Maxillofacial Plastic and Reconstructive Surgery 2020;42(1):12. [DOI: 10.1186/s40902-020-00256-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
FGDP 2020
- Faculty of General Dental Practice. Implications of COVID-19 for the safe management of general dental practice – a practical guide. www.fgdp.org.uk/implications-covid-19-safe-management-general-dental-practice-practical-guide (accessed August 2020).
Fine 1996
- Fine DH, Korik I, Furgang D. Assessing pre-procedural subgingival irrigation and rinsing with an antiseptic mouthrinse to reduce bacteremia. Journal of American Dental Association 1996;127(5):641-6. [DOI] [PubMed] [Google Scholar]
Gottsauner 2020
- Gottsauner MJ, Michaelides I, Schmidt B, Scholz KJ, Buchalla W, Widbiller M, et al. A prospective clinical pilot study on the effects of a hydrogen peroxide mouthrinse on the intraoral viral load of SARS-CoV-2. Clinical Oral Investigations 2020;24(10):3707-13. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gross 1992
- Gross KB, Overman PR, Cobb C, Brockmann S. Aerosol generation by two ultrasonic scalers and one sonic scaler. A comparative study. Journal of Dental Hygiene 1992;66(7):314-8. [PubMed] [Google Scholar]
Harrel 2004
- Harrel SK, Molinari J. Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. Journal of the American Dental Association 2004;135(4):429-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019. [Google Scholar]
Hossainian 2011
- Hossainian N, Slot DE, Afennich F, Weijden GA. The effects of hydrogen peroxide mouthwashes on the prevention of plaque and gingival inflammation: a systematic review. International Journal of Dental Hygiene 2011;9(3):171-81. [DOI] [PubMed] [Google Scholar]
Hughes 2020
- Hughes S, Troise O, Donaldson H, Mughal N, Moore LS. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clinical Microbiology and Infection 2020;26(10):1395-9. [DOI: 10.1016/j.cmi.2020.06.025] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hunter 2014
- Hunter A, Kalathingal S, Shrout M, Plummer K, Looney S. The effectiveness of a pre-procedural mouthrinse in reducing bacteria on radiographic phosphor plates. Imaging Science in Dentistry 2014;44(2):149-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jackson 2020
- Jackson T, Deibert D, Wyatt G, Durand-Moreau Q, Adisesh A, Khunti K, et al. Classification of aerosol-generating procedures: a rapid systematic review. BMJ Open Respiratory Research 2020;7(1):e000730. [DOI: 10.1136/bmjresp-2020-000730.] [DOI] [PMC free article] [PubMed] [Google Scholar]
James 2016
- James R, Mani A. Dental aerosols: a silent hazard in dentistry. International Journal of Scientific Research 2016;5:1761-3. [Google Scholar]
Jeong 2020
- Jeong HW, Kim SM, Kim HS, Kim YI, Kim JH, Cho JY, et al. Viable SARS-CoV-2 in various specimens from COVID-19 patients. Clinical Microbiology and Infection 2020;26(11):1520-4. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 1997
- Jones CG. Chlorhexidine: is it still the gold standard? Periodontology 2000 1997;15:55-62. [DOI: 10.1111/j.1600-0757.1997.tb00105.x] [DOI] [PubMed] [Google Scholar]
Jones 2015
- Jones RM, Brosseau LM. Aerosol transmission of infectious disease. Journal of Occupational and Environmental Medicine 2015;57(5):501-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kanagalingam 2015
- Kanagalingam J, Feliciano R, Hah JH, Labib H, Le TA, Lin JC. Practical use of povidone-iodine antiseptic in the maintenance of oral health and in the prevention and treatment of common oropharyngeal infections. International Journal of Clinical Practice 2015;69(11):1247-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kariwa 2004
- Kariwa H, Fujii N, Takashima I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions, and chemical reagents. Japanese Journal of Veterinary Research 2004;52(3):105-12. [PubMed] [Google Scholar]
Koletsi 2020
- Koletsi D, Belibasakis GN, Eliades T. Interventions to reduce aerosolized microbes in dental practice: a systematic review with network meta-analysis of randomized controlled trials. Journal of Dental Research 2020;99(11):1228-30. [DOI: 10.1177/0022034520943574] [DOI] [PubMed] [Google Scholar]
Laheij 2012
- Laheij AM, Kistler JO, Belibasakis GN, Välimaa H, deSoet JJ. Healthcare-associated viral and bacterial infections in dentistry. Journal of Oral Microbiology 2012;4:4. [PMID: DOI: 10.3402/jom.v4i0.17659] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2020
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. training.cochrane.org/handbook/archive/v6.1.
Li 2004
- Li RW, Leung KW, Sun FC, Samaranayake LP. Severe acute respiratory syndrome (SARS) and the GDP. Part II: implications for GDPs. British Dental Journal 2004;197(3):130-4. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2020
- Li Y, Ren B, Peng X, Hu T, Li J, Gong T, et al. Saliva is a non-negligible factor in the spread of COVID-19. Molecular Oral Microbiology 2020;35(4):141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Luan 2008
- Luan Q, Desta T, Chehab L, Sanders VJ, Plattner J, Graves DT. Inhibition of experimental periodontitis by a topical boron-based antimicrobial. Journal of Dental Research 2008;87(2):148-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mair 2020
- Mair AD, Korne PH. Decoding dental aerosols. secibonline.com/wp-content/uploads/2020/05/Decoding-Dental-Aerosols_May-212020.pdf (accessed 16 August 2020).
Mandel 1994
- Mandel ID. Antimicrobial mouthrinses: overview and update. Journal of American Dental Association 1994;125(8 Suppl 2):2S-10S. [DOI: 10.1016/s0002-8177(94)14001-x] [DOI] [PubMed] [Google Scholar]
Manipal 2016
- Manipal S, Hussain S, Wadgave U, Duraiswamy P, Ravi K. The mouthwash war – chlorhexidine vs. herbal mouth rinses: a meta-analysis. Journal of Clinical and Diagnostic Research 2016;10(5):ZC81-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marui 2019
- Marui VC, Souto ML, Rovai ES, Romito GA, Chambrone L, Pannuti CM. Efficacy of preprocedural mouthrinses in the reduction of microorganisms in aerosol: a systematic review. Journal of American Dental Association 2019;150(12):1015-26. [DOI] [PubMed] [Google Scholar]
Mathur 2018
- Mathur A, Gopalakrishnan D, Mehta V, Rizwan SA, Shetiya SH, Bagwe S. Efficacy of green tea-based mouthwashes on dental plaque and gingival inflammation: a systematic review and meta-analysis. Indian Journal of Dental Research 2018;29(2):225-32. [DOI] [PubMed] [Google Scholar]
Meister 2020
- Meister TL, Brüggemann Y, Todt D, Conzelmann C, Müller JA, Groß R, et al. Virucidal efficacy of different oral rinses against SARS-CoV-2. Journal of Infectious Diseases 2020;222(8):1289-92. [DOI: 10.1093/infdis/jiaa471] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meng 2020
- Meng L, Hua F, Bian Z. Coronavirus disease 2019 (COVID-19): emerging and future challenges for dental and oral medicine. Journal of Dental Research 2020;99(5):481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohd‐Said 2021
- Mohd-Said S, Mohd-Dom TN, Suhaimi N, Rani H, McGrath C. Effectiveness of pre-procedural mouth rinses in reducing aerosol contamination during periodontal prophylaxis: a systematic review. Frontiers in Medicine 2021;8:600769. [PMID: ] [DOI] [PMC free article] [PubMed]
Moosavi 2020
- Moosavi MS, Aminishakib P, Ansari M. Antiviral mouthwashes: possible benefit for COVID-19 with evidence-based approach. Journal of Oral Microbiology 2020;12(1):1794363. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mosaddad 2019
- Mosaddad SA, Tahmasebi E, Yazdanian A, Rezvani MB, Seifalian A, Yazdanian M, et al. Oral microbial biofilms: an update. European Journal of Clinical Microbiology & Infectious Diseases 2019;38(11):2005-19. [PMID: ] [DOI] [PubMed] [Google Scholar]
Munita 2016
- Munita JM, Arias CA. Mechanisms of antibiotic resistance. Microbiology Spectrum 2016;4(2):1-37. [DOI: 10.1128/microbiolspec.VMBF-0016-2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mupparapu 2019
- Mupparapu M, Kothari KR. Review of surface disinfection protocols in dentistry: a 2019 update. Quintessence International 2019;50(1):58-65. [DOI] [PubMed] [Google Scholar]
Nagayoshi 2004
- Nagayoshi M, Fukuizumi T, Kitamura C, Yano J, Terashita M, Nishihara T. Efficacy of ozone on survival and permeability of oral microorganisms. Oral Microbiology and Immunology 2004;19(4):240-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nagraj 2020
- Nagraj SK, Eachempati P, Paisi M, Nasser M, Sivaramakrishnan G, Verbeek JH. Interventions to reduce contaminated aerosols produced during dental procedures for preventing infectious diseases. Cochrane Database of Systematic Reviews 2020, Issue 10. Art. No: CD013686. [DOI: 10.1002/14651858.CD013686.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nokam Kamdem 2022
- Nokam Kamdem GS Jr, Toukam M, Ntep Ntep DB, Kwedi KG, Zilefac BN, Fokam ST, et al. Comparison of the effect of saline mouthwash versus chlorhexidine on oral flora. Advances in Oral and Maxillofacial Surgery 2022;6:100273. [Google Scholar]
Ortega 2020
- Ortega KL, Rech BO, El Haje GL, Gallo CB, Pérez-Sayáns M, Braz-Silva PH. Do hydrogen peroxide mouthwashes have a virucidal effect? A systematic review. Journal of Hospital Infection 2020;106(4):657-62. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pasquarella 2000
- Pasquarella C, Pitzurra O, Savino A. The index of microbial air contamination. Journal of Hospital Infection 2000;46(4):241-56. [PMID: ] [DOI] [PubMed] [Google Scholar]
Patarakulwiwat 2018
- Patarakulwiwat P, Rodanant P. Importance of patient's position during oral prophylaxis: a simulated study in phantom head. Mahidol Dental Journal 2018;38:39-44. [Google Scholar]
Pedrazzi 2015
- Pedrazzi V, Leite MF, Tavares RC, Sato S, Nascimento GC, Issa JP. Herbal mouthwash containing extracts of baccharis dracunculifolia as agent for the control of biofilm: clinical evaluation in humans. Scientific World Journal 2015;2015:6. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Popkin 2017
- Popkin DL, Zilka S, Dimaano M, Fujioka H, Rackley C, Salata R, et al. Cetylpyridinium chloride (CPC) exhibits potent, rapid activity against influenza viruses in vitro and in vivo. Pathogens & Immunity 2017;2(2):252-69. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rayyan 2016 [Computer program]
- Rayyan QCRI, the Systematic Reviews web app. Qatar Computing Research Institute, Hamad Bin Khalifa University. Doha, Qatar: Qatar Computing Research Institute, Hamad Bin Khalifa University, 2016.
Reis 2021
- Reis IN, do Amaral GC, Mendoza AA, das Graças YT, Mendes-Correa MC, Romito GA, et al. Can preprocedural mouthrinses reduce SARS-CoV-2 load in dental aerosols? Medical Hypotheses 2021;146:110436. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Sağlam 2013
- Sağlam M, Arslan U, Buket Bozkurt Ş, Hakki SS. Boric acid irrigation as an adjunct to mechanical periodontal therapy in patients with chronic periodontitis: a randomized clinical trial. Journal of Periodontology 2013;84(9):1297-308. [PMID: ] [DOI] [PubMed] [Google Scholar]
Samaranayake 2021
- Samaranayake LP, Fakhruddin KS, Buranawat B, Panduwawala C. The efficacy of bio-aerosol reducing procedures used in dentistry: a systematic review. Acta Odontologica Scandinavica 2021;79(1):69-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
SDCEP 2021
- SDCEP 2021. Mitigation of aerosol generating procedures in dentistry – a rapid review. www.sdcep.org.uk/wp-content/uploads/2021/04/SDCEP-Mitigation-of-AGPs-in-Dentistry-Rapid-Review-v1.2-April-2021.pdf (accessed prior to 27 July 2022).
Seneviratne 2021
- Seneviratne CJ, Balan P, Ko KK, Udawatte NS, Lai D, Ng DH, et al. Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore. Infection 2021;49(2):305-11. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sridharan 2016
- Sridharan K, Sivaramakrishnan G. Clinical trials in Ayurveda: analysis of clinical trial registry of India. Journal of Ayurveda and Integrative Medicine 2016;7(3):141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tartaglia 2019
- Tartaglia GM, Tadakamadla SK, Connelly ST, Sforza C, Martín C. Adverse events associated with home use of mouthrinses: a systematic review. Therapeutic Advances in Drug Safety 2019;10:1-16. [DOI: 10.1177/2042098619854881] [DOI] [PMC free article] [PubMed] [Google Scholar]
To 2020
- To KK, Tsang OT, Yip CC, Chan K-H, Wu TC, Chan JM, et al. Consistent detection of 2019 novel coronavirus in saliva. Clinical Infectious Diseases 2020;71(15):841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Veena 2015
- Veena HR, Mahantesha S, Joseph PA, Patil SR, Patil SH. Dissemination of aerosol and splatter during ultrasonic scaling: a pilot study. Journal of Infection and Public Health 2015;8(3):260-5. [DOI: 10.1016/j.jiph.2014.11.004] [DOI] [PubMed] [Google Scholar]
Verbeek 2020
- Verbeek JH, Rajamaki B, Ijaz S, Sauni R, Toomey E, Blackwood B, et al. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD011621. [DOI: 10.1002/14651858.CD011621.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Waffenschmidt 2020
- Waffenschmidt S, Navarro-Ruan T, Hobson N, Hausner E, Sauerland S, Haynes RB. Development and validation of study filters for identifying controlled non-randomized studies in PubMed and Ovid MEDLINE. Research Synthesis Methods 2020;11(5):617-26. [DOI] [PubMed] [Google Scholar]
Walsh 2000
- Walsh LJ. Safety issues relating to the use of hydrogen peroxide in dentistry. Australian Dental Journal 2000;45(4):257-89. [DOI] [PubMed] [Google Scholar]
Wani 2021
- Wani AR, Yadav K, Khursheed A, Rather MA. An updated and comprehensive review of the antiviral potential of essential oils and their chemical constituents with special focus on their mechanism of action against various influenza and coronaviruses. Microbial Pathogenesis 2021;152:104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wells 1934
- Wells WF. On air-borne infection: study II. Droplets and droplet nuclei. American Journal of Epidemiology 1934;20:611-8. [DOI: 10.1093/oxfordjournals.aje.a118097] [DOI] [Google Scholar]
Yoon 2020
- Yoon JG, Yoon J, Song JY, Soo-Young Y, Lim C, Hye S, et al. Clinical significance of a high SARS-CoV-2 viral load in the saliva. Journal of Korean Medical Sciences 2020;35(20):e195. [DOI: 10.3346/jkms.2020.35.e195] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zemouri 2017
- Zemouri C, deSoet H, Crielaard W, Laheij A. A scoping review on bio-aerosols in healthcare and the dental environment. PloS One 2017;12(5):e0178007. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Kumbargere Nagraj 2020
- Kumbargere Nagraj S, Eachempati P, Paisi M, Nasser M, Sivaramakrishnan G, Verbeek JH, et al. Preprocedural mouth rinses for preventing transmission of infectious diseases through aerosols in dental healthcare providers. Cochrane Database of Systematic Reviews 2020, Issue 12. Art. No: CD013826. [DOI: 10.1002/14651858.CD013826] [DOI] [PMC free article] [PubMed] [Google Scholar]


