Abstract
BACKGROUND
The optimal treatment for posthemorrhagic hydrocephalus in newborns has not been established yet. Moreover, despite many valid therapeutic alternatives, unfavorable neurodevelopmental outcomes are frequent. According to recent literature, these discouraging results could be related to secondary inflammatory damage of the white matter due to the gradual dissolution of the intraventricular hematoma, which should be removed.
OBSERVATIONS
Neuroendoscopic lavage (NEL) has proven to be a safe and reliable procedure, able to adequately remove the intraventricular clots and the products of blood degradation. To increase surgical control of the entire ventricular system, the authors illustrated a case in which they associated real-time transfontanellar ultrasound monitoring with NEL.
LESSONS
Coupling these two techniques, the authors performed a rapid ventricular wash and obtained intraoperative confirmation of complete and accurate clot removal.
Keywords: endoscopic lavage, intraventricular hemorrhage, hydrocephalus, neonates, transfontanellar ultrasound, neuroendoscopy
ABBREVIATIONS : CSF = cerebrospinal fluid; CT = computed tomography; DRIFT = drainage, irrigation, and fibrinolytic therapy; IVH = intraventricular hemorrhage; MRI = magnetic resonance imaging; NEL = neuroendoscopic lavage; PHH = posthemorrhagic hydrocephalus; rtPA = recombinant tissue plasminogen activator; US = ultrasound
The optimal treatment for posthemorrhagic hydrocephalus (PHH) in newborns is still unclear, and recommendations in the literature are not based on strong clinical evidence derived from randomized controlled trials or prospective cohort studies.1,2 Despite several valid therapeutic alternatives (i.e., ventricular access devices, external ventricular drains, ventriculosubgaleal shunts, or seriate lumbar punctures), unfavorable neurodevelopmental outcomes and shunt dependency are still common results. According to recent literature, poor outcomes could be related not only to primary ventricular dilation but also to secondary white matter damage due to the presence of intraventricular hematoma. In fact, gradual dissolution of the clot triggers a secondary injury caused by free radicals damage and chronic inflammation, as described previously.3–6
To overcome this pathophysiological phenomenon, neuroendoscopic lavage (NEL), which allows an effective wash of blood components and residual clot suction, has been recently introduced by several pediatric neurosurgery centers in Europe.7–12 This technique has proven able to arrest hydrocephalus progression, especially in cases in which adequate removal of the hematoma was achieved.9,10,12 However, in more complex cases with extensive hemorrhage or subacute presentation, endoscopic visibility could be inadequate to obtain complete suction of the clot. A case involving a full-term newborn affected by univentricular congenital heart disease, complicated by subacute PHH after an endovascular stenting procedure, gave us the opportunity to associate real-time ultrasound (US) monitoring with NEL. The aim of intraoperative US control was to facilitate removal of the intraventricular hematoma in a patient with abnormal hemodynamic and coagulative conditions before an irreversible progression of the hydrocephalus. US monitoring seemed a feasible, rapid, and inexpensive way to increase surgical control over the entire ventricular system during the neuroendoscopic procedure.
Illustrative Case
A 28-day-old full-term newborn (gestational age 39 weeks) with univentricular congenital heart disease was affected by tetraventricular PHH caused by a grade III intraventricular hemorrhage (IVH)13 after percutaneous endovascular stenting that required antiaggregant and anticoagulant therapy. The patient suffered a generalized seizure with gaze deviation, and magnetic resonance imaging (MRI) showed tetraventricular hydrocephalus sustained by IVH (Fig. 1). Immediately after the reversal of coagulative conditions, we decided on US-assisted NEL.
FIG. 1.
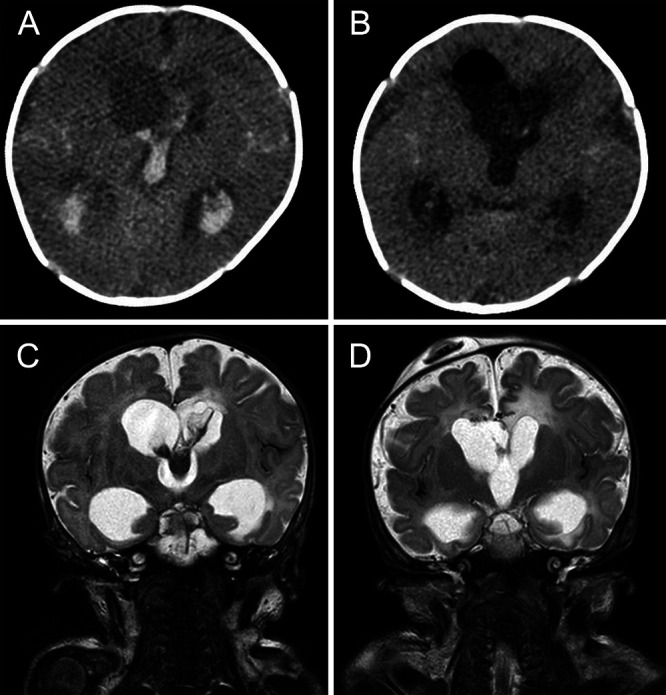
Preoperative (A and C) and postoperative (B and D) imaging. A: Axial CT scan shows a large clot inside the lateral and third ventricles. B: CT confirms complete removal of the clot, and the pneumoventricle resolved spontaneously within a few days. C: Coronal T2-weighted MRI offers better visualization of intraventricular clot and its extension. D: T2-weighted MRI shows the patency of the ventricular system, the absence of residual hemorrhage, and the reduction of ventricular dilatation. Ommaya reservoir is correctly positioned.
After induction of general anesthesia and intubation, the patient was supine with the head straight. On the right side, a short, precoronaric half-moon incision was created, and a small burr hole was made first by rotating a scalpel and then by using a rongeur. The second surgeon performed transfontanellar US (C11×, 8-5 MHz, Sonosite M-Turbo) to evaluate the ventricular dilation and clearly display the clot distribution before opening the dura (Fig. 2). The dura was coagulated and opened, and a minimal corticotomy was realized. The flexible endoscopy unit (4 mm, Karl Storz-Endoskope) was inserted under US guidance, and continuous irrigation was performed with Ringer’s solution. After removal of the clot from the right lateral ventricle and the foramen of Monro, the contralateral clot could be visualized and removed by performing an interventricular septostomy. Subsequently, the tip of the endoscope was guided backward into the third ventricle to achieve complete hemorrhage suction under constant US monitoring. During this step, the probe’s sagittal projection ensured better visualization of the third ventricle and the hematoma embedded in the sylvian aqueduct, providing a chance to verify its patency after aspiration, which was performed in safe and monitored conditions (Fig. 3). Before removing the endoscope, a final transfontanellar US scan of the ventricular system was performed to ensure its complete wash and exclude acute complications. At the end of the procedure, a ventricular catheter connected to an Ommaya reservoir was placed in the right ventricle. The burr hole was sealed with a gelatin sponge and glue. Soft-tissue incisions were meticulously closed.
FIG. 2.
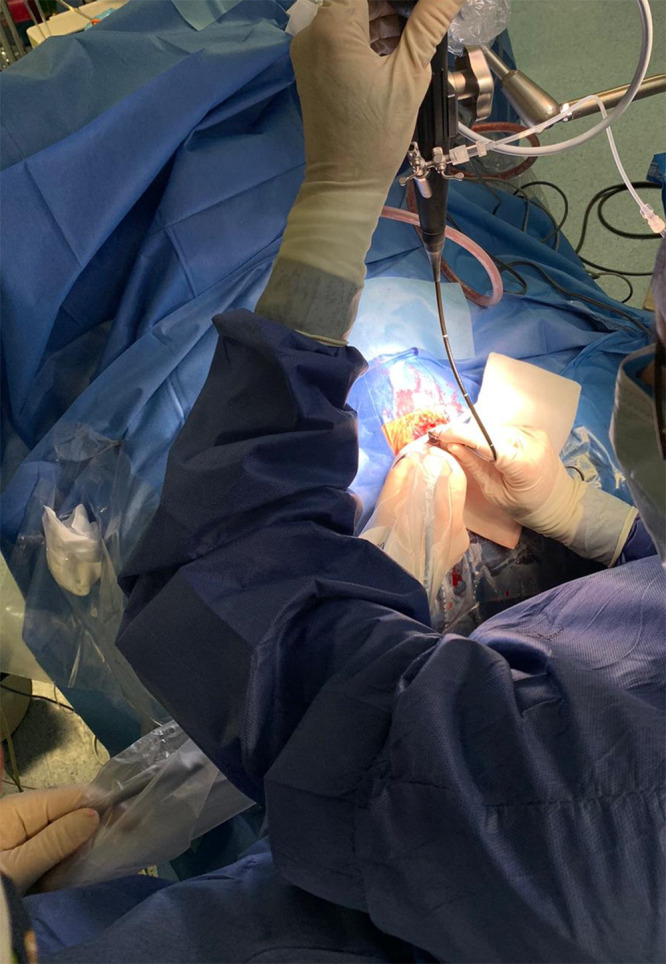
Superior view of operative field prepared with accurate exposure of anterior fontanelle. One surgeon handles the endoscopic unit, and a second surgeon, standing laterally, performs the real-time transfontanellar US after coating the probe with sterile transmission gel. A third surgeon (not visible) controls irrigation and aspiration from the endoscope.
FIG. 3.
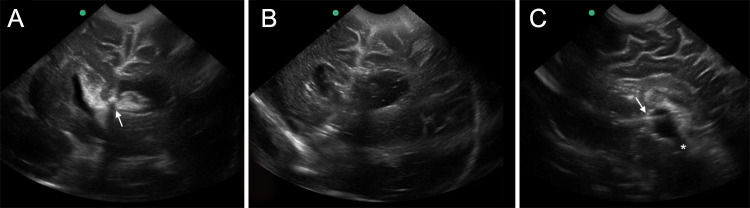
Frames from intraoperative US monitoring video. A and B: Coronal views of lateral ventricles during and after clot aspiration. The white arrow shows the endoscope’s tip over the clot near the right foramen of Monro. C: Sagittal view of third ventricle at the end of procedure. The supraoptic and the infundibular recesses are visible at the intersection of the floor and the anterior wall. The white arrow indicates the endoscope directed toward the sylvian aqueduct (asterisk), which is completely patent.
Discussion
Conventional temporary treatments of neonatal IVH have led to variable results, and a fervent debate in the literature confirms the lack of well-defined guidelines. Therefore, this uncertain climate and the fragility of this patient population have pushed the neurosurgical community to find new therapeutic alternatives.
In 2007, Whitelaw et al. proposed a randomized clinical trial entitled DRIFT (drainage, irrigation, and fibrinolytic therapy), in which they treated PHH with fibrinolytic therapy using recombinant tissue plasminogen activator (rtPA) and continuous cerebrospinal fluid (CSF) flushing.14 The study showed a reduction of severe disability and an improvement of neurocognitive development in the intervention group at 2 years,15 but it stopped recruitment because of a significant increase in early rebleeding (which was not reported by Park et al. with the administration of urokinase instead of rtPA16). However, despite the adverse event, DRIFT had the merit to move the treatment of PHH toward direct washing of CSF to eliminate blood components and cytokines from the ventricular system, thus limiting the progression of hydrocephalus. Indeed, a few years later, Schulz et al. reported the first series of 19 newborns treated with NEL, demonstrating that this procedure is a feasible and safe alternative for complete CSF wash.8 This endoscopic treatment has been evaluated only in short case series, indicating that the principal benefit of this procedure is the significant reduction of shunt dependency, which varies from 57% to 61% compared to 60% to 87% with standard therapeutic alternatives.7–9,11,12,17 Moreover, similar results have already been demonstrated in adult patients undergoing endoscopic treatment of IVH, in which the most significant prognostic factors are the amount of intraventricular blood and its complete removal.18–21 At present, an international, prospective multicenter study is in progress, and its results may enforce the benefit of NEL in neurodevelopmental outcome.22
Although described as safe and effective, endoscopic treatment of IVH in newborns can present intraoperative pitfalls and complications, especially if performed in less experienced centers. For example, the achievement of complete removal of the clot can lengthen surgical times and expose a patient to the risk of longer time under general anesthesia. To potentiate the safety and efficacy of this surgery in these fragile and often pluripathological infants, we propose an association between intraoperative transfontanellar US and NEL.
Observations
In our case, US helped to check blind spots not directly visible from the endoscope and ensure complete removal of the hemorrhage, despite suboptimal vision. Indeed, the gradual degradation of the clot caused cloudy CSF, which made navigation burdensome (Fig. 4). Real-time US monitoring was useful for overcoming this obstacle, and by flipping the probe on different axes, the US could be a valuable “second eye” guiding the surgeon toward safe aspiration of the clot. As with our patient, the clot generally is adherent to the choroid plexus of the lateral ventricles8 and is connected to the third ventricle plexus. Therefore, when the endoscope sucks out the clot from the foramen of Monro, a simultaneous effect on the third ventricle component must be considered because it could be firmly attached to the endothelium and rebleed. By monitoring distal indirect motions of the clot in real time, we modulated the lavage and suction force, thus controlling its fragmentation (Video 1).
VIDEO 1. Clip showing three moments of intraoperative US monitoring through the anterior fontanel. In coronal and sagittal views, the tip of the endoscope is easily recognizable while removing the clots. Click here to view.
FIG. 4.
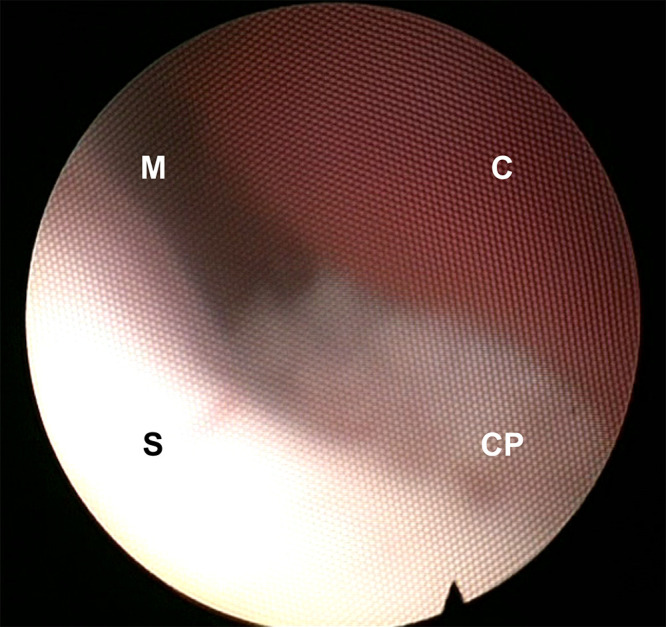
Intraoperative view during NEL for a massive IVH in our patient. Despite copious irrigation with Ringer’s solution, the cloudy CSF offers bad visibility of hematoma and anatomical structures, making navigation more difficult. C = intraventricular clot; CP = choroid plexus; M = right foramen of Monro; S = septum.
A surgeon, especially if less experienced, can feel more confident when using the endoscope by taking advantage of the US global view of the ventricular system. By expanding the operative vision, it is possible to plan the most effective surgical strategy in real time and guide the aspiration step by step. Furthermore, US ensures a final check that excludes the presence of residual clots immediately after the procedure. Recently, Tirado-Caballero et al. published a series of 46 newborns with IVH treated using NEL in which the presence of residual clots, observed in 54% of patients, was associated with hydrocephalus progression that required further lavage. In this case series, multiple NELs were associated with a higher infection rate, poorer cognitive results, and a worse shunt dependency rate.12 Therefore, because the goal of this surgery should be removal of the clot in a single procedure, we assume that intraoperative US can reduce the risk of a second lavage by improving the outcome. In our case, we achieved the goal without lengthening operating times (Fig. 5).
FIG. 5.
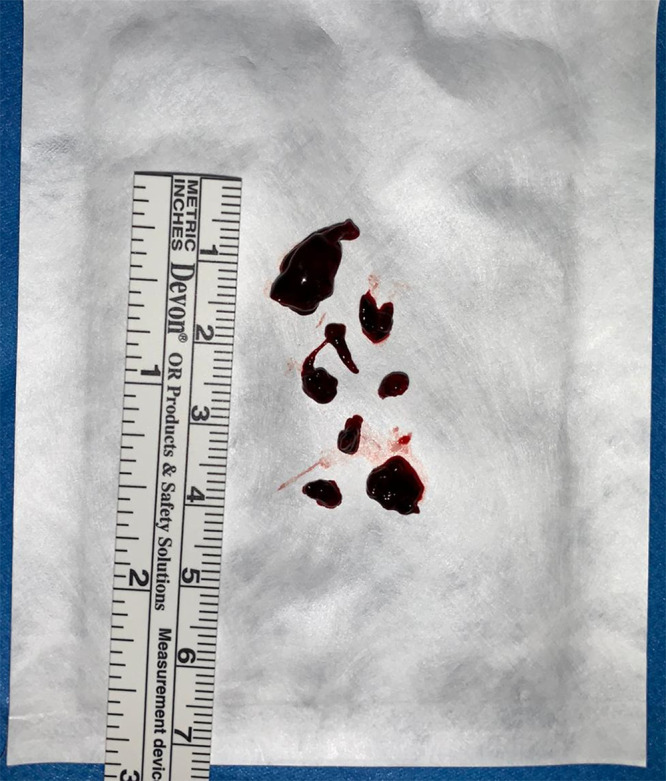
Postoperative photograph of the fragmented removed clot.
The presence of an additional surgeon dedicated to US was not uncomfortable and did not affect the management of the endoscope by the first surgeon. The probe, placed at the level of the anterior fontanel, which must be widely exposed during preparation of the sterile field, did not come into conflict with the endoscope. Lastly, US is a quick and inexpensive resource that can be easily used multiple times. However, a surgeon requires adequate training to become familiar with brain anatomy as seen with US.
In our patient, postoperative computed tomography (CT) confirmed complete removal of the intraventricular hematoma (Fig. 1). Head circumference and US ventricular index were monitored daily and remained stable in the weeks after surgery. Postoperative infection or intraventricular rebleeding did not occur. Subgaleal fluid collection above the entry point was the only complication, but it did not require any treatment. The patient’s neurological conditions are progressively recovering with intensive physiotherapy and neurocognitive rehabilitation. Unfortunately, a second coagulation disorder 2 months after the NEL procedure caused a dorsal spinal epidural hematoma that required neurosurgical evacuation, leaving a condition of areflexic paraplegia. However, the most recent neurological evaluation indicated complete head control and mild muscle tone of the trunk in the sitting position that requires use of the hands for support. In a supine position, the infant grabs and manipulates objects and shows correct progressive psychomotor acquisitions. At present, he receives antiepileptic drugs and hydrocortisone replacement therapy, but he did not suffer further generalized seizures, and electroencephalogram controls confirmed good organization of electrical activity by age. Approximately 8 months after surgery, the patient still does not need a ventricular shunt, and the last MRI study showed stable ventricular sizes with no white matter injuries. The patient’s head circumference is in the 25th to 50th percentile.
Lessons
In our still limited preliminary experience, US monitoring during NEL was technically feasible and safe, ensuring a second continuous point of view on the operative field to facilitate clot removal. In the future, we are confident that US monitoring will become a common accompaniment to NEL in the treatment of pediatric PHH.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Sartori, Furlanis, Baro, Denaro. Acquisition of data: Sartori, Furlanis. Analysis and interpretation of data: Sartori, Furlanis, Caliri, Garbin. Drafting the article: Sartori, Furlanis, Baro. Critically revising the article: Caliri, Garbin, Denaro. Reviewed submited version of manuscript: Sartori. Approved the final version of the manuscript on behalf of all authors: Sartori, Furlanis. Administrative/technical/material support: Sartori. Study supervision: Denaro. Surgeon: Denaro, Baro, Sartori.
Supplemental Information
Videos
Video 1. https://vimeo.com/522394096.
References
- 1. Mazzola CA, Choudhri AF, Auguste KI, et al. Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 2: Management of posthemorrhagic hydrocephalus in premature infants. J Neurosurg Pediatr. 2014;14(suppl 1):8–23. doi: 10.3171/2014.7.PEDS14322. [DOI] [PubMed] [Google Scholar]
- 2. El-Dib M, Limbrick DD, Jr, Inder T, et al. Management of post- hemorrhagic ventricular dilatation in the infant born preterm. J Pediatr. doi: 10.1016/j.jpeds.2020.07.079. Published online July 30, 2020, doi:10.1016/j.jpeds.2020.07.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mahaney KB, Buddhala C, Paturu M, et al. Intraventricular hemorrhage clearance in human neonatal cerebrospinal fluid: associations with hydrocephalus. Stroke. 2020;51(6):1712–1719. doi: 10.1161/STROKEAHA.119.028744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gao C, Du H, Hua Y, et al. Role of red blood cell lysis and iron in hydrocephalus after intraventricular hemorrhage. J Cereb Blood Flow Metab. 2014;34(6):1070–1075. doi: 10.1038/jcbfm.2014.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gao F, Liu F, Chen Z, et al. Hydrocephalus after intraventricular hemorrhage: the role of thrombin. J Cereb Blood Flow Metab. 2014;34(3):489–494. doi: 10.1038/jcbfm.2013.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gram M, Sveinsdottir S, Cinthio M, et al. Extracellular hemoglobin: mediator of inflammation and cell death in the choroid plexus following preterm intraventricular hemorrhage. J Neuroinflammation. 2014;11(1):200. doi: 10.1186/s12974-014-0200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schulz M, Bührer C, Spors B, et al. Endoscopic neurosurgery in preterm and term newborn infants: a feasibility report. Childs Nerv Syst. 2013;29(5):771–779. doi: 10.1007/s00381-012-2003-6. [DOI] [PubMed] [Google Scholar]
- 8. Schulz M, Bührer C, Pohl-Schickinger A, et al. Neuroendoscopic lavage for the treatment of intraventricular hemorrhage and hydrocephalus in neonates. J Neurosurg Pediatr. 2014;13(6):626–635. doi: 10.3171/2014.2.PEDS13397. [DOI] [PubMed] [Google Scholar]
- 9. d’Arcangues C, Schulz M, Bührer C, et al. Extended experience with neuroendoscopic lavage for posthemorrhagic hydrocephalus in neonates. World Neurosurg. 2018;116:e217–e224. doi: 10.1016/j.wneu.2018.04.169. [DOI] [PubMed] [Google Scholar]
- 10. Cavalheiro S, Dastoli P, Suriano I, et al. Brain wash in premature neonate with intraventricular hemorrhage. Childs Nerv Syst. 2007;23(9):1051. [Google Scholar]
- 11. Etus V, Kahilogullari G, Karabagli H, Unlu A. Early endoscopic ventricular irrigation for the treatment of neonatal posthemorrhagic hydrocephalus: a feasible treatment option or not? A multicenter study. Turk Neurosurg. 2018;28(1):137–141. doi: 10.5137/1019-5149.JTN.18677-16.0. [DOI] [PubMed] [Google Scholar]
- 12. Tirado-Caballero J, Rivero-Garvia M, Arteaga-Romero F, et al. Neuroendoscopic lavage for the management of posthemorrhagic hydrocephalus in preterm infants: safety, effectivity, and lessons learned. J Neurosurg Pediatr. 2020;26(3):1–10. doi: 10.3171/2020.2.PEDS2037. [DOI] [PubMed] [Google Scholar]
- 13. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 14. Whitelaw A, Evans D, Carter M, et al. Randomized clinical trial of prevention of hydrocephalus after intraventricular hemorrhage in preterm infants: brain-washing versus tapping fluid. Pediatrics. 2007;119(5):e1071–e1078. doi: 10.1542/peds.2006-2841. [DOI] [PubMed] [Google Scholar]
- 15. Whitelaw A, Jary S, Kmita G, et al. Randomized trial of drainage, irrigation and fibrinolytic therapy for premature infants with posthemorrhagic ventricular dilatation: developmental outcome at 2 years. Pediatrics. 2010;125(4):e852–e858. doi: 10.1542/peds.2009-1960. [DOI] [PubMed] [Google Scholar]
- 16. Park YS, Kotani Y, Kim TK, et al. Efficacy and safety of intraventricular fibrinolytic therapy for post-intraventricular hemorrhagic hydrocephalus in extreme low birth weight infants: a preliminary clinical study. Childs Nerv Syst. 2021;37(1):69–79. doi: 10.1007/s00381-020-04766-5. [DOI] [PubMed] [Google Scholar]
- 17. Behrens P, Tietze A, Walch E, et al. Neurodevelopmental outcome at 2 years after neuroendoscopic lavage in neonates with posthemorrhagic hydrocephalus. J Neurosurg Pediatr. 2020;26(5):465–602. doi: 10.3171/2020.5.PEDS20211. [DOI] [PubMed] [Google Scholar]
- 18. Longatti PL, Martinuzzi A, Fiorindi A, et al. Neuroendoscopic management of intraventricular hemorrhage. Stroke. 2004;35(2):e35–e38. doi: 10.1161/01.STR.0000113736.73632.F6. [DOI] [PubMed] [Google Scholar]
- 19. Longatti P, Fiorindi A, Martinuzzi A. Neuroendoscopic aspiration of hematocephalus totalis: technical note. Neurosurgery. 2005;57(suppl 4):E409. doi: 10.1227/01.neu.0000176702.26810.b7. [DOI] [PubMed] [Google Scholar]
- 20. Basaldella L, Marton E, Fiorindi A, et al. External ventricular drainage alone versus endoscopic surgery for severe intraventricular hemorrhage: a comparative retrospective analysis on outcome and shunt dependency. Neurosurg Focus. 2012;32(4):E4. doi: 10.3171/2012.1.FOCUS11349. [DOI] [PubMed] [Google Scholar]
- 21. Rohde V, Schaller C, Hassler WE. Intraventricular recombinant tissue plasminogen activator for lysis of intraventricular haemorrhage. J Neurol Neurosurg Psychiatry. 1995;58(4):447–451. doi: 10.1136/jnnp.58.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thomale UW, Cinalli G, Kulkarni AV, et al. TROPHY registry study design: a prospective, international multicenter study for the surgical treatment of posthemorrhagic hydrocephalus in neonates. Childs Nerv Syst. 2019;35(4):613–619. doi: 10.1007/s00381-019-04077-4. [DOI] [PubMed] [Google Scholar]