Abstract
Although TroA (Tromp1) was initially reported to be a Treponema pallidum outer membrane protein with porin-like properties, subsequent studies have suggested that it actually is a periplasmic substrate-binding protein involved in the transport of metals across the treponemal cytoplasmic membrane. Here we conducted additional physicochemical studies to address the divergent viewpoints concerning this protein. Triton X-114 phase partitioning of recombinant TroA constructs with or without a signal sequence corroborated our prior contention that the native protein’s amphiphilic behavior is due to its uncleaved leader peptide. Whereas typical porins are trimers with extensive β-barrel structure, size exclusion chromatography and circular dichroism spectroscopy revealed that TroA was a monomer and predominantly alpha-helical. Neutron activation, atomic absorption spectroscopy, and anomalous X-ray scattering all demonstrated that TroA binds zinc in a 1:1 molar stoichiometric ratio. TroA does not appear to possess structural features consistent with those of bacterial porins.
The quest for outer membrane proteins of Treponema pallidum as potential vaccine candidates and virulence determinants is a formidable but exceedingly important area of contemporary syphilis research (22). In this regard, Blanco et al. (3, 4) recently reported on the identification of a 31-kDa protein (designated T. pallidum rare outer membrane protein 1, or Tromp1) in isolated T. pallidum outer membranes that formed ion-conducting channels in planar lipid bilayers. However, others have noted that Tromp1 has extensive sequence homology with a novel family of periplasmic substrate-binding proteins (designated cluster 9) involved in metal (i.e., iron, zinc, or manganese) transport in other prokaryotes (1, 2, 10, 14, 15, 17, 19) and that the tromp1 gene is contiguous to and transcriptionally linked with homologs for additional ATP-binding cassette (ABC) transporter components (11, 14). Moreover, Akins and coworkers (1) showed that Tromp1 contains an uncleaved signal sequence, is very hydrophilic downstream of the signal peptide, does not form aqueous channels in liposomes, and is not surface exposed in T. pallidum. Although these studies weigh heavily in favor of TroA (transport-related operon A) as the appropriate nomenclature for this protein (11), additional data are needed to resolve this controversy. Here we present physicochemical evidence that TroA is a zinc-containing protein with structural features inconsistent with those of bacterial porins.
An uncleaved signal sequence confers amphiphilicity on TroA.
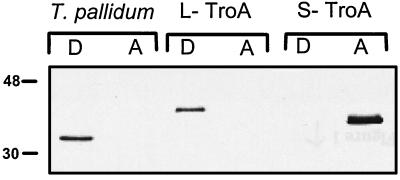
In a prior study (1), we used an in vitro-coupled transcription-translation system to demonstrate that native TroA contains an uncleaved N-terminal signal sequence. We also showed that recombinant TroA lacking this signal sequence was hydrophilic, while the native protein was amphiphilic (1). Based upon these findings, we proposed that the signal sequence alone is responsible for TroA’s amphiphilic character (1). To garner further support for this notion, we directly compared the Triton X-114 phase partitioning behaviors of recombinant TroA constructs which differed only by the presence or absence of the native polypeptide’s 22-amino-acid signal sequence (14). The larger construct, L-TroA, was PCR-amplified from T. pallidum DNA with the forward and reverse primers 5′-CGCGGATCCTTGATACGTGAAAGAATATGTGCCTGC-3′ and 5′-CGGAATTCTTACTAGCGAGCCAACGCAGCAACGATCG-3′, respectively, while the smaller construct, S-TroA, was amplified with the forward primer 5′-CGCGGATCCTTCGGTAGCAAGGATGCCGCAGCGGA C-3′ and the reverse primer used for L-TroA. The PCR products were cloned into the BamHI and EcoRI sites of the expression vector pProEx1 (Gibco BRL, Gaithersburg, Md.); the resulting polypeptides both contain a 28-amino-acid N-terminal extension which includes the His6 tag used for affinity purification. Interestingly, L-TroA was very poorly expressed in Escherichia coli and required solubilization in 8 M urea for affinity purification on the Ni-nitrilotriacetic acid agarose matrix, whereas S-TroA was abundantly expressed and quantitatively recovered from the E. coli cell supernatant (data not shown). As shown in Fig. 1, L-TroA, like its native counterpart, partitioned exclusively into the detergent-enriched phase following solubilization in 2% Triton X-114; S-TroA, in contrast, partitioned exclusively into the aqueous phase (Fig. 1). Identical phase-partitioning results were obtained using L-TroA and S-TroA constructs lacking the N-terminal extension (data not shown). It should be noted that porins, unlike TroA, are amphiphilic even without their leader peptides and completely partition into the Triton X-114 detergent-enriched phase (1). These results also support our proposed topology, which places TroA within the periplasmic space where it is anchored by its uncleaved leader peptide to the treponemal cytoplasmic membrane (1).
FIG. 1.
An uncleaved signal sequence confers amphiphilicity on TroA. Detergent-enriched (D) and aqueous (A) phases were collected following Triton X-114 phase partitioning of T. pallidum, and recombinant constructs possessed (L-TroA) or lacked (S-TroA) the native polypeptide’s 22-amino-acid leader peptide. Proteins were visualized by immunoblot analysis by using a TroA-specific murine monoclonal antibody. Note that both L-TroA and S-TroA contain 28-amino-acid N-terminal extensions derived from the pProEx1 expression vector. Numbers at the left correspond to molecular mass markers.
TroA is a monomer with predominant alpha-helical secondary structure.
Typical porins, such as E. coli OmpF, are multistranded β-barrels that form highly stable trimers (20, 25). It was of interest, therefore, to examine the oligomeric state and secondary structure of TroA. Because porins attain their final conformation and trimeric state following removal of their signal peptides (24, 25), we reasoned that it was appropriate to use S-TroA in the present studies. S-TroA (calculated molecular mass, 34,417 Da) resolved as a single peak with a deduced molecular mass of 34,457 Da by size exclusion chromatography (Superdex-S75 exclusion column) under nondenaturing conditions (20 mM Tris-HCl, pH 7.5, 20 mM NaCl) (data not shown). Circular dichroism spectroscopy, performed on an AVIV (Lakewood, N.J.) model 62DS spectrophotometer and analyzed by using the self-consistent algorithm of Sreerama and Woody (29), revealed that the protein (with or without the 28-amino-acid N-terminal extension created by the cloning vector) was 60% alpha-helix and only 3% beta-sheet, a result consistent with the large amount of alpha-helical structure predicted by both the Chou-Fasman algorithm (8) and the algorithm of Garnier et al. (12). Also noteworthy was the observation that cleaved S-TroA was fully water soluble. While these results do not entirely preclude the possibility that native TroA is multimeric and predominantly a β-barrel, it seems unlikely that the native and recombinant forms of the protein should adopt such different conformations.
TroA binds zinc in a 1:1 molar ratio.
As noted earlier, TroA shares sequence homology with known periplasmic metal-binding proteins (1, 2, 10, 14, 15, 17, 19). Unlike most of the other bacteria containing TroA orthologs, genetic approaches cannot be used to analyze metal uptake by T. pallidum. For this reason, we applied to TroA physical methods which recently were used to examine heavy-metal binding by Pzp1, the Haemophilus influenzae TroA ortholog (17). Neutron activation of S-TroA (following His-tag removal and extensive dialysis) revealed that zinc was present at a molar ratio of 1:1 and at a level markedly above that found in the control dialysate; other elements were present in only trace or undetectable quantities. Because neutron activation has limited sensitivity, particularly for iron, we also examined S-TroA by atomic absorption spectroscopy. This technique also detected zinc in a 1:1 stoichiometric ratio, while iron was not detected (Table 1).
TABLE 1.
Atomic absorption analysis of Zn and Fe contents in 4.72 μM TroA (His-tag removed) and the corresponding dialysate
| Element | μM concn in protein | μM concn in dialysate | metal:protein molar ratio |
|---|---|---|---|
| Zn | 4.66 | <0.15 | 4.51:4.72 = 0.96 |
| Fe | <1.00 | <1.00 | N/Aa |
N/A, not applicable.
Anomalous scattering of S-TroA crystals.
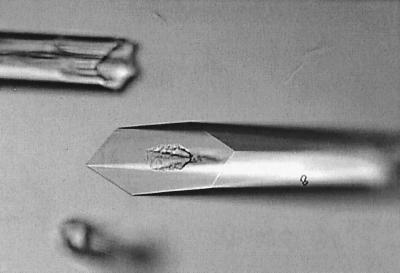
As a final confirmation of the metal-binding data, we analyzed the anomalous scattering signal at the X-ray wavelength of the zinc K absorption edge with S-TroA crystals. The crystals were grown from hanging drops of a 1:1 mixture of reservoir solution comprised of 30% polyethylene glycol, 1 M LiCl, and 0.1 M HEPES-Cl (pH 7.0) and containing 30 to 40 mg of protein/ml. Hexagonal rods with a size of 0.1 by 0.1 by 0.6 mm grew after 1 week of incubation (Fig. 2) and diffracted to a resolution of 2.1 Å when measured by using a Rigaku RU200 (Molecular Structure Corporation, The Woodlands, Tex.) rotating copper anode X-ray generator and R-axis II detector. The crystals were found to belong to a P61 space group with a unit cell dimension of a = b = 125.2 Å, c = 74.5 Å, α = β = 90°, and γ = 120°. Based on protein crystal volume considerations, there are two copies of S-TroA in the crystallographic asymmetric unit. That crystals with a high degree of order were readily obtained with S-TroA is further proof that this construct is in a conformationally native state.
FIG. 2.
Large hexagonal rod S-TroA crystals used for the anomalous zinc scattering experiment.
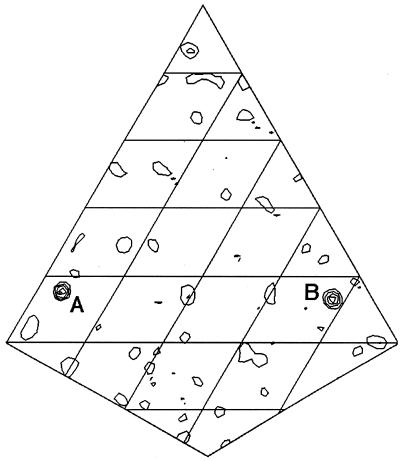
Anomalous scattering data were collected at the BL5.0.2 terminal of The Advanced Light Source (Berkeley Laboratory, Berkeley, Calif.) at a wavelength of 1.2824 Å fixed by a double Si crystal monochromator. Data were processed using HKL2000 (HKL Research, Charlottesville, Va.), and the anomalous difference Patterson map was calculated by using the CCP4 program suite. As shown in Fig. 3, two anomalous scatterers (signal six times the standard deviation from the mean) were found in the asymmetric unit of the crystal, consistent with one zinc atom bound per protein monomer. Interestingly, TroA lacks the H-, D-, and E-rich central domain which is thought to be essential for zinc binding by this group of metalloproteins (19). It does, however, contain ten histidine residues, three of which (His-68, -133, and -199), along with surrounding amino acids, are highly conserved between TroA and cluster 9 zinc-binding TroA homologs (data not shown). Complete determination of the TroA crystal structure will elucidate the contribution of these three amino acids, as well as the other histidines, to zinc coordination.
FIG. 3.
A zinc anomalous difference Patterson map of TroA at section Z = 1/6. The Zn anomalous dispersion data at resolutions from 20.0 to 2.7 Å were used. Two Zn sites (A and B), reflecting two protein molecules in one asymmetric unit, are shown. Each contour represents a standard deviation from the mean.
TroA and the quest for T. pallidum outer membrane proteins.
The many difficulties inherent in the molecular characterization of T. pallidum outer membrane proteins (22) have spawned a variety of experimental approaches for accomplishing this elusive objective (5–7, 11, 13, 23, 30). Of these, isolation of T. pallidum outer membranes seemed particularly promising because it permitted direct identification of candidate outer membrane proteins. More recently, however, there has been increasing evidence that most of the uncharacterized polypeptides in outer membrane fractions are periplasmic or cytoplasmic membrane contaminants rather than authentic outer membrane proteins (26–28). This conclusion also appears to pertain to TroA despite its relative abundance in outer membrane preparations (6, 23).
Although bacterial porins have limited primary amino acid sequence homology, their three-dimensional structures are similar (9). Periplasmic substrate-binding proteins also have limited sequence homology but a high degree of similarity in their tertiary structures (21). Most importantly, the tertiary structures of porins and substrate-binding proteins are highly dissimilar. Porins consist of 16 anti-parallel strands in a β-barrel configuration, whereas substrate-binding proteins consist of two distinct globular domains bisected by a cleft or groove for ligand binding (21). In this regard, it is interesting to note that the three-dimensional structure of PsaA, the pneumococcal TroA homologue and a known virulence determinant, was recently shown to be that of a substrate-binding protein with zinc as the probable metal ligand (16). The results described here, therefore, lay the groundwork for definitively resolving TroA structure and, in so doing, the divergent viewpoints concerning its function and cellular location in T. pallidum. An equally important implication of our work concerns the fact that Tro-like ABC transporters in other bacterial pathogens have been shown to influence transport-nonrelated virulence-related processes (10, 15, 18). Thus, our findings also set the stage for an investigation of the role of zinc, an essential trace element, and zinc transporters in T. pallidum physiology and host-pathogen interactions during syphilitic infection.
Acknowledgments
This research was supported in part by U.S. Public Health Service grants AI-26756 to J.D.R., AI-16692 to M.V.N., and DK-52089 to C.A.H. and by Robert A. Welch Foundation grants I-0940 to M.V.N. and J.D.R. and I-1318 to C.A.H.
We also are indebted to Martin Goldberg for technical assistance, to Ken Bourell for assistance with graphics and figure preparation, and to Eric Hansen for helpful suggestions.
REFERENCES
- 1.Akins D R, Robinson E, Shevchenko D V, Elkins C, Cox D L, Radolf J D. Tromp1, a putative rare outer membrane protein, is anchored by an uncleaved leader peptide to the Treponema pallidum cytoplasmic membrane. J Bacteriol. 1997;179:576–586. doi: 10.1128/jb.179.16.5076-5086.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartsevich V V, Pakrasi H B. Molecular identification of an ABC transporter complex for manganese: analysis of a cyanobacterial mutant strain impaired in the photosynthetic oxygen evolution process. EMBO J. 1995;14:1845–1853. doi: 10.1002/j.1460-2075.1995.tb07176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanco D R, Champion C I, Exner M M, Erdjument-bromage H, Hancock R W, Tempst P, Miller J N, Lovett M A. Porin activity and sequence analysis of a 31-kilodalton Treponema pallidum subsp. pallidum rare outer membrane protein (Tromp1) J Bacteriol. 1995;177:3556–3562. doi: 10.1128/jb.177.12.3556-3562.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanco D R, Champion C I, Exner M M, Shang E S, Skare J T, Hancock R E W, Miller J N, Lovett M A. Recombinant Treponema pallidum rare outer membrane protein 1 (Tromp1) expressed in Escherichia coli has porin activity and surface antigenic exposure. J Bacteriol. 1996;178:6685–6692. doi: 10.1128/jb.178.23.6685-6692.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanco D R, Giladi M, Champion C I, Haake D A, Chikami G K, Miller J N, Lovett M A. Identification of Treponema pallidum subspecies pallidum genes encoding signal peptides and membrane-spanning sequences using a novel alkaline phosphatase expression vector. Mol Microbiol. 1991;5:2405–2415. doi: 10.1111/j.1365-2958.1991.tb02086.x. [DOI] [PubMed] [Google Scholar]
- 6.Blanco D R, Reimann K, Skare J, Champion C I, Foley D, Exner M M, Hancock R E W, Miller J N, Lovett J N. Isolation of the outer membranes from Treponema pallidum and Treponema vincentii. J Bacteriol. 1994;176:6088–6099. doi: 10.1128/jb.176.19.6088-6099.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centurion-Lara A, Castro C, Barrett L, Cameron C, Mostowfi M, Van Voorhis W C, Lukehart S A. Treponema pallidum major sheath protein homologue Tpr K is a target of opsonic antibody and the protective immune response. J Exp Med. 1999;189:647–656. doi: 10.1084/jem.189.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou P Y, Fasman G D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- 9.Cowan S W, Schirmer T, Rummel G, Steiert M, Ghosh R, Pauptit R A, Jansonius J N, Rosenbusch J P. Crystal structures explain functional properties of two E. coli porins. Nature. 1992;358:727–733. doi: 10.1038/358727a0. [DOI] [PubMed] [Google Scholar]
- 10.Dintilhac A, Alloing G, Granadel C, Claverys J-P. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol Microbiol. 1997;25:727–739. doi: 10.1046/j.1365-2958.1997.5111879.x. [DOI] [PubMed] [Google Scholar]
- 11.Fraser C M, Norris S J, Weinstock G M, White O, Sutton G C, Dodson R, Gwinn M, Hickey E K, Clayton R, Ketchum K A, Sodergren E, Hardham J M, McLeod M P, Salzberg S, Peterson J, Khalak H, Richardson D, Howell J K, Chidambaram M, Utterback T, McDonald L, Artiach P, Bowman C, Cotton M D, Fujii C, Garland S, Hatch B, Horst K, Roberts K, Sandusky M, Weidman J, Smith H O, Venter J C. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science. 1998;281:375–388. doi: 10.1126/science.281.5375.375. [DOI] [PubMed] [Google Scholar]
- 12.Garnier J, Osguthorpe D J, Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978;120:97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- 13.Hardham J M, Stamm L V. Identification and characterization of the Treponema pallidum tpn50 gene, an ompA homolog. Infect Immun. 1994;62:1015–1025. doi: 10.1128/iai.62.3.1015-1025.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardham J M, Stamm L V, Porcella S F, Frye J G, Barnes N Y, Howell J K, Mueller S L, Radolf J D, Weinstock G M, Norris S J. Identification and transcriptional analysis of a Treponema pallidum operon encoding a putative ABC transporter system, an iron-activated repressor protein homolog, and a glycolytic pathway enzyme homolog. Gene. 1997;197:47–64. doi: 10.1016/s0378-1119(97)00234-5. [DOI] [PubMed] [Google Scholar]
- 15.Kolenbrander P E, Andersen R N, Baker R A, Jenkinson H F. The adhesion-associated sca operon in Streptococcus gordonii encodes an inducible high-affinity ABC transporter for Mn2+ uptake. J Bacteriol. 1997;180:290–295. doi: 10.1128/jb.180.2.290-295.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrence M C, Pilling P A, Epa V C, Berry A M, Ogunniyi A D, Paton J C. The crystal structure of pneumococcal surface antigen PsaA reveals a metal-binding site and a novel structure for a putative ABC-type protein. Structure. 1999;6:1553–1561. doi: 10.1016/s0969-2126(98)00153-1. [DOI] [PubMed] [Google Scholar]
- 17.Lu D, Boyd B, Lingwood C A. Identification of the key protein for zinc uptake in Haemophilus influenzae. J Biol Chem. 1998;272:29033–29038. doi: 10.1074/jbc.272.46.29033. [DOI] [PubMed] [Google Scholar]
- 18.Novak R, Braun J S, Charpentier E, Tuomanen E. Penicillin tolerance genes of Streptococcus pneumoniae: the ABC-type manganese permease complex Psa. Mol Microbiol. 1998;29:1285–1296. doi: 10.1046/j.1365-2958.1998.01016.x. [DOI] [PubMed] [Google Scholar]
- 19.Patzer S I, Hantke K. The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol Microbiol. 1998;28:1199–1210. doi: 10.1046/j.1365-2958.1998.00883.x. [DOI] [PubMed] [Google Scholar]
- 20.Phale P S, Philippsen A, Kiefhaber T, Koebnik R, Phale V P, Schirmer T, Rosenbusch J P. Stability of trimeric OmpF porin: the contribution of the latching loop. Biochemistry. 1998;37:15663–15670. doi: 10.1021/bi981215c. [DOI] [PubMed] [Google Scholar]
- 21.Quiocho F, Ledvina P S. Atomic structure and specificity of bacterial periplasmic receptors for active transport and chemotaxis: variation of common themes. Mol Microbiol. 1996;20:17–25. doi: 10.1111/j.1365-2958.1996.tb02484.x. [DOI] [PubMed] [Google Scholar]
- 22.Radolf J D. Treponema pallidum and the quest for outer membrane proteins. Mol Microbiol. 1995;16:1067–1073. doi: 10.1111/j.1365-2958.1995.tb02332.x. [DOI] [PubMed] [Google Scholar]
- 23.Radolf J D, Robinson E J, Bourell K W, Akins D R, Porcella S F, Weigel L M, Jones J D, Norgard M V. Characterization of outer membranes isolated from Treponema pallidum, the syphilis spirochete. Infect Immun. 1995;63:4244–4252. doi: 10.1128/iai.63.11.4244-4252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reid J, Fung H, Gehring K, Klebba P E, Nikaido H. Targeting of porin to the outer membrane of Escherichia coli. Rate of trimer assembly and identification of a dimer intermediate. J Biol Chem. 1988;263:7753–7759. [PubMed] [Google Scholar]
- 25.Sen K, Nikaido H. In vitro trimerization of OmpF porin secreted by spheroplasts of Escherichia coli. Proc Natl Acad Sci USA. 1990;87:743–747. doi: 10.1073/pnas.87.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shevchenko D V, Akins D R, Robinson E, Li M, Popova T G, Cox D L, Radolf J D. Molecular characterization and cellular localization of TpLRR, a processed leucine-rich repeat protein of Treponema pallidum, the syphilis spirochete. J Bacteriol. 1997;179:3188–3195. doi: 10.1128/jb.179.10.3188-3195.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shevchenko D V, Akins D R, Robinson E J, Li M, Shevchenko O V, Radolf J D. Identification of homologs for thioredoxin, peptidyl prolyl cis-trans isomerase, and glycerophosphodiester phosphodiesterase in outer membrane fractions from Treponema pallidum, the syphilis spirochete. Infect Immun. 1997;67:4179–4189. doi: 10.1128/iai.65.10.4179-4189.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shevchenko D V, Sellati T J, Cox D L, Shevchenko O V, Robinson E J, Radolf J D. Membrane topology and cellular location of the Treponema pallidum glycerophosphodiester phosphodiesterase (GlpQ) ortholog. Infect Immun. 1999;67:2266–2276. doi: 10.1128/iai.67.5.2266-2276.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sreerama N, Woody R W. A self-consistent method for the analysis of protein secondary structure from circular dichroism. Anal Biochem. 1996;209:32–44. doi: 10.1006/abio.1993.1079. [DOI] [PubMed] [Google Scholar]
- 30.Stebeck C E, Shaffer J M, Arroll T W, Lukehart S A, Van Voorhis W C. Identification of the Treponema pallidum subsp. pallidum glycerophosphodiester phosphodiesterase homologue. FEMS Microbiol Lett. 1997;154:303–310. doi: 10.1111/j.1574-6968.1997.tb12660.x. [DOI] [PubMed] [Google Scholar]