ABSTRACT
Lipid profile management is one of the crucial components to optimize outcomes in patients with chronic kidney disease (CKD). CKD is associated with poor cardiovascular outcomes due to both a direct cardiovascular impact of CKD and the presence of metabolic comorbidities. Low-density lipoprotein cholesterol is the main target of current lipid-lowering drugs. However, the derangement of lipid metabolism in CKD is more complex. The recently described triglyceride–glucose index (TyG) is associated with cardiovascular outcomes in the general population. In recent studies, the TyG was associated with CKD progression in CKD patients and with cardiovascular death in patients on peritoneal dialysis. Quiroga et al. now show that the TyG is associated with the occurrence of major cardiovascular events in individuals free from diabetes with non-dialysis-dependent CKD.
Keywords: cardiovascular events, chronic kidney disease, dyslipidemia, triglyceride glucose index
In a prospective multicentric cohort study, Quiroga et al. [1] assessed whether the recently described triglyceride–glucose index (TyG) predicted the occurrence of major adverse cardiovascular events (MACE) in nondiabetic patients with chronic kidney disease (CKD). The TyG was higher in patients with stage 3–5 CKD than in controls and the TyG was independently associated with the occurrence of severe MACE in non-diabetic CKD patients. These findings provide new insights into the management of dyslipidemia in CKD patients [2].
The prevalence of CKD is increasing, in part due to the progressive aging of the population and the increased prevalence of age-associated metabolic and cardiovascular comorbidities. Obesity and hyperlipidemia are among the most common independent risk factors for CKD, implying that lipid build-up may be deleterious to renal function [3]. Excess free fatty acids can harm podocytes, proximal tubular epithelial cells and tubulointerstitial tissue via a variety of mechanisms, including the increased production of reactive oxygen species (ROS) and lipid peroxidation, which promote mitochondrial damage and cause tissue inflammation, all of which lead to glomerular and tubular lesions [3].
Major cardiovascular disease is a key cause of death in CKD. The risk of cardiovascular disease in CKD increases with increasing disease stage: it is already increased in stage 1–3 CKD patients, but is dramatically increased in CKD stage 4–5 [4]. Non-dialysis-dependent CKD patients are up to 10-fold more likely to die from cardiovascular disease, while the risk may be up to 100-fold higher in dialysis-dependent patients [5]. The increased risk of atherosclerosis in CKD is multifactorial [6]. Both conventional and CKD-related mechanisms may be involved. Conventional risk factors such as hypertension, dyslipidemia, diabetes, smoking, physical inactivity and obesity contribute to atherosclerosis in CKD patients and should be assessed and addressed [7, 8]. The latter include chronic systemic inflammation, uremic toxins, loss of Klotho, oxidative stress and CKD metabolic bone disease/hyperparathyroidism [9].
Hypertriglyceridemia and low high-density lipoprotein (HDL) cholesterol levels are common lipid patterns in patients with severe CKD, yet low-density lipoprotein (LDL) cholesterol levels are frequently normal. There is a clear link between LDL cholesterol and major atherosclerotic events in the general population. However, in patients with CKD, LDL cholesterol has a negative relationship with these outcomes when LDL cholesterol levels are below average and a slightly positive relationship with mortality when LDL cholesterol levels are higher [6]. In CKD patients, lipid levels may change with disease progression or in response to management of the disease [10]. In general, research suggests that reducing LDL cholesterol can help individuals with CKD not on dialysis to decrease the incidence of major atherosclerotic events [6, 10].
Current guidelines recommend the use of lipid-lowering agents in nondialysis CKD patients, but not in patients undergoing dialysis [11]. Specifically, Kidney Disease: Improving Global Outcomes recommends prescription of statins to CKD patients in stages 1–5 not on dialysis [2] and emphasizes that patients with a decreased estimated glomerular filtration rate (eGFR) have lower LDL-cholesterol levels yet cardiovascular risk is high [2]. In the large 4D and AURORA (A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Hemodialysis: An Assessment of Survival and Cardiovascular Events) trials, statins did not decrease cardiovascular events in dialysis patients.
Statins decrease LDL cholesterol levels in patients with normal kidney function and with CKD [12]. However, lipid-lowering treatment should be modified according to cardiovascular disease risk and to kidney function and promising novel agents such as proprotein convertase subtilisin/Kexin type 9 inhibitors (PCSK9i) should be considered [13]. Sodium-glucose cotransporter-2 (SGLT-2) inhibitors, the most recent family of kidney protective drugs [14], may mildly increase cholesterol, LDL cholesterol and HDL cholesterol levels, but they decrease glycemia and triglycerides and may reduce the atherogenic small dense LDL particle levels [15]. Thus they may be predicted to decrease the TyG and indeed, early studies suggest that they do, at least in a diabetic non-CKD population [16]. Given the beneficial effect of SGLT-2 inhibitors on kidney and cardiovascular outcomes, these findings merit an in-depth analysis.
CKD patients usually have high plasma triglycerides and low HDL levels, which is associated with an increased risk of atherosclerosis [1]. Impaired lecithin–cholesterol acyltransferase (LCAT) function decreases HDLC and apoprotein A-1 levels. Deficient apoprotein A-1 levels limit free cholesterol efflux from macrophages into HDL cholesterol, leading to free cholesterol accumulation in macrophages and atherosclerotic plaque progression [17]. CKD is also associated with monocyte activation and monocytosis, which may further accelerate the atherosclerosis process. Kanbay et al. [18] found that the monocyte:HDL ratio increases as eGFR decreases. Since low HDL values are related to CKD itself, HDL levels respond poorly to exercise in CKD patients compared with healthy people [13]. As CKD progresses, HDL decreases. In CKD, high levels of triglycerides result from increased production and decreased catabolism. Triglyceride levels increase in the early stages of CKD and peak in patients undergoing dialysis or suffering from nephrotic syndrome. Lipoprotein A is an atherogenic molecule whose plasma levels are increased up to 4-fold in patients with nephrotic syndrome. LDL levels and total cholesterol levels vary in CKD patients and are high in patients with nephrotic syndrome [17].
The TyG has been used as a marker for insulin resistance. It is calculated via a natural logarithm function of fasting blood glucose and fasting blood triglycerides. The logic behind this formula is that insulin resistance frequently causes increased triglyceride and glucose levels in healthy people [19]. The TyG is a quick and feasible method because it only requires basic blood tests and can be included as a further result in routine clinical biochemistry labs. Since insulin resistance plays a role in the pathogenesis of atherosclerotic cardiovascular diseases, TyG was hypothesized to be associated with a higher risk of atherosclerosis. Indeed, a higher TyG was independently associated with the occurrence of atherosclerotic cardiovascular disease in a meta-analysis of eight cohort studies comprising 5 731 294 diabetic and nondiabetic participants [20]. However, no prior study assessed nondiabetic and non-dialysis-dependent CKD patients, which was the population studied by Quiroga et al. [1].
Quiroga et al. [1] performed an observational study to assess the association between the TyG and MACE in nondiabetic patients with CKD not on dialysis. The study included 1142 CKD participants without previous cardiovascular events and 460 control participants. The TyG was higher in CKD patients (median value 8.63) than in controls [1]. Overall, the TyG increased with increasing albuminuria categories but was similar across GFR categories. Indeed, TyG was higher in patients with a urinary albumin:creatinine ratio >300 mg/g (P = .009) (Figure 1) and median values were higher than 8.63 in CKD stages 3–5 (P < .001), but there was no significant difference between stage 2 CKD and controls. The TyG was also higher in patients with imaging evidence of subclinical atherosclerosis.
Figure 1:
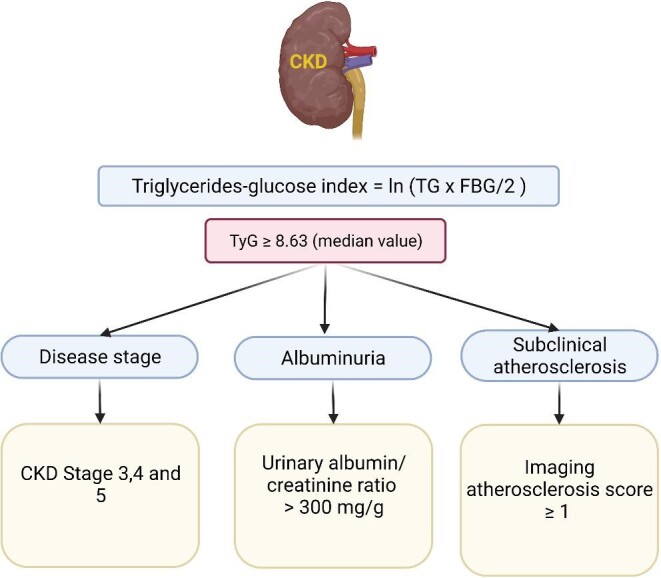
In nondiabetic patients with CKD not on dialysis and without a prior history of cardiovascular disease, higher values for the TyG were found in CKD categories G3–G5 and A3, as well as in patients with imaging evidence of subclinical atherosclerosis at baseline. Data from Quiroga et al. [1]. TyG = ln(TG [mg/dL] × glucose [mg/dL]/2). TG: triglycerides; FBG: fasting blood glucose; Ln: natural logarithm.
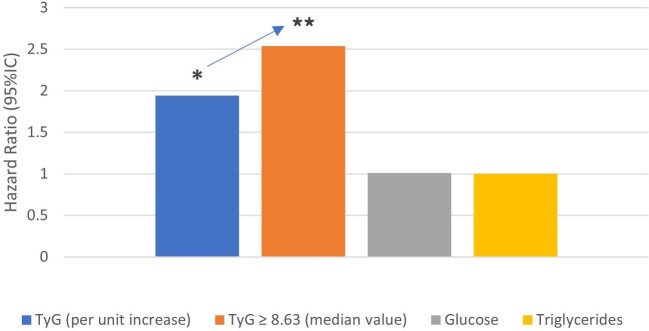
The TyG independently predicted major cardiovascular events in diabetes-free and dialysis-free CKD patients [1]. The 4-point MACE-free survival over 72 months was lower in patients with a TyG >8.63 (log rank = 10.5 and P = 0.001). In an adjusted model, each unit increase in TyG was associated with a 94% higher risk of MACE {hazard ratio [HR] 1.94 [95% confidence interval (CI) 1.01–3.73], P = 0.046} while a TyG >8.63 was associated with a 2.54 higher risk of MACE (95% CI 1.27–5.07; P = 0.008) and serum glucose or triglycerides alone were not associated with a higher risk (Figure 2).
Figure 2:
In nondiabetic patients with CKD not on dialysis and without a prior history of cardiovascular disease, higher values for the TyG predicted a composite outcome of 4-point MACE (cardiovascular death, nonfatal myocardial infarction, nonfatal stroke and hospitalization for unstable angina). Hazard ratio shown for a model adjusted for age, sex, hypertension and imaging atherosclerotic score and for each unit increase in TyG, a TyG >8.63 and each mg/dL increase in serum glucose or triglycerides. Adjustment for age, sex, hypertension and dyslipidemia yielded similar results (not shown). Data from Quiroga et al. [1].
Since a high TyG was associated with the risk of MACE in stage 2–5 CKD patients without diabetes or dialysis, the next question is how to incorporate this information into routine clinical practice. The TyG may eventually be used as a biomarker for the identification of higher-risk populations among CKD patients initially considered to be lower risk based on the absence of diabetes or cardiovascular disease or dialysis. There is no consensus about when to start triglyceride-lowering agents or on the threshold for treatment in patients with CKD. Future studies should assess whether intervention based on the TyG improves outcomes [18].
One limitation of Quiroga et al.’s study [1] was that the design was biased and was not highly representative of the general CKD population, as only patients without key risk factors (e.g. diabetes) or without a history of cardiovascular disease were enrolled. Another limitation was that TyG measurement was cross-sectional, which limits the interpretation of TyG measurement predictivity on CKD disease progression, as potential changes in TyG over time were not assessed.
In conclusion, the TyG could be a useful tool to further refine the risk of cardiovascular events in stage 2–5 CKD patients without diabetes or dialysis. Threshold values of TyG should be confirmed in further studies in additional international CKD populations. In this regard, TyG is an easy-to-access method that may help to evaluate the risk of cardiovascular complications and to prevent the development of cardiovascular events through early intervention. Further observational studies can investigate the significance of repeated TyG measurements and trends in CKD patients and eventual clinical trials should assess the impact of interventions initiated based on abnormal TyG indexes.
Contributor Information
Fatma Yildirim, Department of Medicine, Koc University School of Medicine, Istanbul, Turkey.
Abdullah B Yildiz, Department of Medicine, Koc University School of Medicine, Istanbul, Turkey.
Mehmet Kanbay, Division of Nephrology, Department of Medicine, Koc University School of Medicine, Istanbul, Turkey.
FUNDING
This study received no funding.
CONFLICT OF INTEREST STATEMENT
M.K. is a member of the Editorial Board of CKJ. The other authors declare that they have no conflicts of interest.
DATA AVAILABILITY STATEMENT
No new data were generated or analyzed in support of this research.
REFERENCES
- 1. Quiroga B, Ramos PM, Horrillo ASet al. . Triglycerides-glucose index and the risk of cardiovascular events in persons with non-diabetic chronic kidney disease. Clin Kidney J 2022; 10.1093/ckj/sfac073 (22 February 2022, date last accessed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wanner C, Tonelli M. KDIGO Clinical Practice Guideline for Lipid Management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int 2014; 85: 1303–1309 [DOI] [PubMed] [Google Scholar]
- 3. Gai Z, Wang T, Visentin Met al. . Lipid accumulation and chronic kidney disease. Nutrients 2019; 11: 722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gisterå A, Hansson GK. The immunology of atherosclerosis. Nat Rev Nephrol 2017; 13: 368–380 [DOI] [PubMed] [Google Scholar]
- 5. Iwai T, Kataoka Y, Otsuka Fet al. . Chronic kidney disease and coronary atherosclerosis: evidences from intravascular imaging. Expert Rev Cardiovasc Ther 2019; 17: 707–716 [DOI] [PubMed] [Google Scholar]
- 6. Ferro CJ, Mark PB, Kanbay Met al. . Lipid management in patients with chronic kidney disease. Nat Rev Nephrol 2018; 14: 727–749 [DOI] [PubMed] [Google Scholar]
- 7. Uhlig K, Levey AS, Sarnak MJ. Traditional cardiac risk factors in individuals with chronic kidney disease. Semin Dial 2003; 16: 118–127 [DOI] [PubMed] [Google Scholar]
- 8. Jankowski J, Floege J, Fliser Det al. . Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation 2021; 143: 1157–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Afsar B, Turkmen K, Covic Aet al. . An update on coronary artery disease and chronic kidney disease. Int J Nephrol 2014; 2014: 767424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hager MR, Narla AD, Tannock LR. Dyslipidemia in patients with chronic kidney disease. Rev Endocr Metab Disord 2017; 18: 29–40 [DOI] [PubMed] [Google Scholar]
- 11. Thobani A, Jacobson TA. Dyslipidemia in patients with kidney disease. Cardiol Clin 2021; 39: 353–363 [DOI] [PubMed] [Google Scholar]
- 12. Kanbay M, Turgut F, Covic Aet al. . Statin treatment for dyslipidemia in chronic kidney disease and renal transplantation: a review of the evidence. J Nephrol 2009; 22: 598–609 [PubMed] [Google Scholar]
- 13. Dincer N, Dagel T, Afsar Bet al. . The effect of chronic kidney disease on lipid metabolism. Int Urol Nephrol 2019; 51: 265–277 [DOI] [PubMed] [Google Scholar]
- 14. Fernandez-Fernandez B, Sarafidis P, Kanbay Met al. . SGLT2 inhibitors for non-diabetic kidney disease: drugs to treat CKD that also improve glycaemia. Clin Kidney J 2020; 13: 728–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Filippas-Ntekouan S, Tsimihodimos V, Filippatos Tet al. . SGLT-2 inhibitors: pharmacokinetics characteristics and effects on lipids. Expert Opin Drug Metab Toxicol 2018; 14: 1113–1121 [DOI] [PubMed] [Google Scholar]
- 16. Imre E, Gunhan HG, Erel Pet al. . SGLT2 inhibitors improve plasma atherogenic biomarkers in patients with type 2 diabetes: a real world retrospective observational study. Minerva Endocrinol (Torino) 2021; doi: 10.23736/S2724-6507.21.03465-5 [DOI] [PubMed] [Google Scholar]
- 17. Bulbul MC, Dagel T, Afsar Bet al. . Disorders of lipid metabolism in chronic kidney disease. Blood Purif 2018; 46: 144–152 [DOI] [PubMed] [Google Scholar]
- 18. Kanbay M, Solak Y, Unal HUet al. . Monocyte count/HDL cholesterol ratio and cardiovascular events in patients with chronic kidney disease. Int Urol Nephrol 2014; 46: 1619–1625 [DOI] [PubMed] [Google Scholar]
- 19. Alizargar J, Bai CH, Hsieh NCet al. . Use of the triglyceride–glucose index (TyG) in cardiovascular disease patients. Cardiovasc Diabetol 2020; 19: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ding X, Wang X, Wu Jet al. . Triglyceride–glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta-analysis of cohort studies. Cardiovasc Diabetol 2021; 20: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.