ABSTRACT
Background
Sodium and calcium polystyrene sulfonate (SPS/CPS) cation-exchange resins have had long-standing clinical use for hyperkalemia in patients with chronic kidney disease (CKD). However, uncertainty exists regarding the real-world usage of SPS/CPS for acute and chronic management of hyperkalemia. We evaluated the prescription patterns of SPS/CPS and their impact on renin–angiotensin–aldosterone system inhibitor (RAASi) treatment in patients with CKD Stages G3–G5 after an episode of de novo hyperkalemia.
Methods
We conducted a retrospective cohort study using population-level administrative databases in Manitoba, Canada, which included adults with CKD and a RAASi prescription who had an episode of de novo hyperkalemia (≥5.5 mmol/L) between January 2007 and December 2017.
Results
A total of 10 009 individuals were included in our study cohort. Among the study population, 4% received an SPS/CPS prescription within 30 days of their hyperkalemia episode. Of those, 22% received a 1-day supply of SPS/CPS and 7% received a prescription for more than 30 days. There were 8145 patients using RAASi at baseline who survived 90 days after their first hyperkalemia episode. Of those, 1447 (18%) discontinued their RAAS inhibitor and 339 (5%) received a prescription of SPS/CPS. Also, the proportion of patients who discontinued their RAASi was similar among those who did and did not receive a prescription of SPS/CPS.
Conclusion
In patients with CKD receiving RAASi therapy, there is a low frequency of SPS/CPS prescription after an episode of hyperkalemia. RAASi discontinuation or downtitration is the most used pharmacologic approach for the management of hyperkalemia, a strategy that deprives patients of the cardiac and renal protective benefits of RAASi. New options for the management of hyperkalemia in this population are needed.
Keywords: cation-exchange resin, hyperkalemia, kidney disease, population, sodium polystyrene sulfonate
INTRODUCTION
Hyperkalemia (serum potassium levels ≥5.5 mmol/L) is a common, potentially life-threatening, electrolyte disorder that is an independent risk factor for mortality in patients with chronic kidney disease (CKD) [1]. Risk factors for hyperkalemia in patients with CKD include comorbidities frequently encountered in this population such as diabetes mellitus and cardiovascular disease, and medications such as renin–angiotensin–aldosterone system inhibitors (RAASi) and mineralocorticoid receptor antagonists (MRA) [2, 3].
Guidelines for the management of acute hyperkalemia are well described and are a combination of three major strategies: stabilization of the myocardium, intracellular shifting and enhanced elimination via urinary or fecal excretion [4, 5]. Chronic hyperkalemia management is based on the discontinuation of medications associated with hyperkalemia, prescription of cation-exchange resins and diuretics, and adherence to a decreased potassium diet [6]. Downtitration or discontinuation of RAASi, such as angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers, is a common strategy for the management of chronic hyperkalemia [3]. However, RAASi are first-line medications for the treatment of CKD due to their renal and cardiovascular protective effects [7]. Therefore, this strategy of reducing or eliminating RAASi in response to hyperkalemia is suboptimal, as doing so deprives many patients at high risk of CKD progression from receiving the renoprotective effects of the RAASi [8, 9].
Alternatives to discontinuing RAASi remain few and of unclear efficacy. Studies evaluating the treatment of hyperkalemia with loop diuretics, for example, have shown that the degree and predictability of response are uncertain [10]. There is poor evidence to support dietary potassium restriction as studies have shown a low adherence to the dietary recommendations [10, 11]. Although available for more than 50 years, cation-exchange resins, such as sodium polystyrene sulfonate (SPS) and calcium polystyrene sulfonate (CPS), which bind potassium in the gastrointestinal tract and enhance fecal elimination, have an unclear safety, effectiveness and tolerability profile. Only small trials exist addressing the efficacy of SPS, the most commonly used cation-exchange resin [12, 13]. However, there are concerns about a poor side-effect profile and the risk of colonic necrosis when mixed with sorbitol [14, 15]. As newer treatments such as patiromer and sodium zirconium cyclosilicate emerge, several authors have highlighted the need for studies evaluating SPS/CPS prescriptions and practice patterns [12]. We therefore examined the treatment patterns of SPS and CPS in the chronic management of hyperkalemia for patients with CKD Stages G3–G5 receiving RAASi in Manitoba, Canada.
MATERIALS AND METHODS
The study protocol was approved by the Health Research Ethics Board (HREB) at the University of Manitoba [HREB#: H2020:193 (HS23873)]. This study was conducted using a prespecified protocol and adheres to the Reporting of Studies Conducted Using Observational Routinely Collected Health Data guidelines (Supplementary data, Table S1).
Data source
Using provincial administrative health databases in Manitoba, Canada, we performed a retrospective cohort study. Databases accessed are housed at the Manitoba Centre for Health Policy [16, 17], and included the Manitoba Health Insurance Registry (list of all Manitoba residents), Medical Services and Claims (medical claims data), Canadian Institute for Health Information Discharge Abstract Database (hospitalization events and discharge diagnoses), Shared Health Diagnostic Services (laboratory tests), Drug Program Information Network (medications) and Vital Statistics (deaths). Deidentified information in the databases is linked to each individual through a unique scrambled personal health identification number.
Study population
Our study population consisted of adults (age ≥18 years), both inpatients and outpatients, with CKD who were being treated with a RAASi at the time of their hyperkalemia diagnosis. A patient with CKD was defined as an individual who had an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 on at least two occasions ≥90 days apart. Serum creatinine was converted into eGFR using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. Hyperkalemia was defined as a serum potassium level ≥5.5 mmol/L between 1 January 2007 and 31 December 2017 but no prior tests showing hyperkalemia (to exclude recurrent hyperkalemia). The date of the first qualifying serum potassium for hyperkalemia was set as the index date. All subjects were followed from the index date until death, migration out of the province or 31 March 2018. Using the medication database, a current RAASi user was defined as someone with a RAASi prescription that overlapped with the date of the de novo hyperkalemia episode. To allow for late refills, we allowed a grace period for the overlap of the prescription date plus the number of days supplied multiplied by 1.5. Patients were excluded if they had fewer than 365 days of observation time prior to the index date, if they had received dialysis or a kidney transplant before their index date or if the incident serum potassium level was ≥10.0 mmol/L (to exclude likely spurious results).
Exposure
The primary study exposure was a prescription of SPS or CPS within 30 days of the hyperkalemia episode. This was determined using the Drug Program Information Network database, which captures all fulfilled outpatient prescriptions.
Variables
Demographic and clinical variables known or hypothesized to be associated with hyperkalemia were assessed at the index date and included demographics (age and sex), comorbidities (hypertension, diabetes mellitus, stroke, heart failure, unstable angina and atrial fibrillation) and medications (NSAIDs, beta-blockers, potassium-sparing and non-potassium-sparing diuretics, aldosterone receptor antagonists, low-molecular-weight heparin, azole antifungals, calcineurin inhibitors, digoxin, potassium supplements and trimethoprim; Supplementary data, Tables S2 and S3). Comorbidities were identified using their International Classification of Diseases, 9th or 10th revision, codes from the Medical Services and Claims and hospital discharge databases. Medication use was separated into current user (the patient fulfilled a prescription that overlaps with the study period) and nonuser (no prescription that overlaps with the study period). Baseline comorbidities were defined using validated local case definitions.
Study outcomes
The primary outcome was the frequency of SPS/CPS prescriptions defined as the number of SPS/CPS prescriptions during the study period (within 30 days of the de novo hyperkalemia episode). We further examined whether patients received a subsequent prescription of SPS or CPS as well as the number of days prescribed for the initial and subsequent prescriptions. A repeated prescription for SPS or CPS was defined as a new prescription any time after the initial prescribed potassium-binding resin. Among patients with a recurrence of hyperkalemia, defined as a new episode of serum potassium ≥5.5 mmol/L, we evaluated prescription patterns of SPS/CPS with the same methods as used for the initial episode.
A secondary outcome was the discontinuation of RAASi 90 days after the de novo hyperkalemia episode. Discontinuation was defined as an absence of a new prescription after the 90 days mark. This 90-day addition allowed us to offset immortal time bias. Among those who were considered continuers of RAASi prescriptions, we determined if they continued at the maximal recommended dose or if they continued at a submaximal dose based on the guidelines for dosing for patients with heart failure as current clinical guidelines lack a description of maximal doses for patients with CKD [18].
Statistical analysis
When reporting baseline characteristics, continuous variables were reported as means with standard deviations and categorical variables were reported as frequencies (percentages). Means of continuous variables were compared with the independent t-test and categorical variables were compared with the Chi-squared test. We reported the number and frequency of patients in our cohort who were prescribed SPS/CPS within 30 days of the index date. Among those patients, we reported the frequency of different categories of number of days’ supply of those prescriptions (1, 2–10, 11–20, 21–30, >30), the time from the de novo hyperkalemia episode to an SPS/CPS prescription by median number of days with interquartile range (IQR), a breakdown between repeated and acute prescriptions and the mean serum potassium of the earliest test following the SPS/CPS prescription. Among users who received more than one prescription after the initial SPS/CPS prescription, we reported the frequency of different categories of number of days’ supply of those prescriptions, and the median number of days to a new prescription. Among patients who survived 30 days after the index hyperkalemia episode, we reported the number of patients who had a new episode (recurrence) of hyperkalemia and evaluated whether SPS or CPS was prescribed within 30 days of that recurrent hyperkalemia episode. We waited 30 days after the de novo hyperkalemia episode to assess recurrences and subsequent SPS/CPS prescriptions to align with the end of our assessment period of immediate prescriptions. We assessed continuation versus discontinuation of RAASi using an intention-to-treat approach. On Day 90 post-index date, surviving patients were assigned to their treatment arm (continuation or discontinuation). Those with a new prescription of RAASi that overlapped with the 90-day mark were considered continuers. Those who continued RAASi therapy were included in a dose analysis using an intention-to-treat approach, with their initial new RAASi prescriptions classified according to whether their daily dose was equivalent to the maximal recommended dose or was a submaximal dose. A comparison of discontinuation and dosing among those who did and did not receive SPS/CPS therapy was done using the Chi-squared test. All statistical analyses were performed using SAS Version 9.4 (SAS Institute, Inc., Cary, NC, USA). P-values <0.05 were considered statistically significant.
RESULTS
Patient characteristics
We identified 42 040 patients who had an episode of de novo hyperkalemia out of 670 707 patients with serum potassium tests during the study period from 1 January 2007 to 31 December 2017. Of these, 17 657 patients met our inclusion criteria and had CKD at baseline (Figure 1). Among these patients with CKD, 57% (n = 10 009) were receiving a RAASi prescription at the time of the de novo hyperkalemia episode. Baseline characteristics of the cohort are presented in Table 1. Mean age was 74 years (SD 13) and 48.53% of the cohort was female (n = 4857). The mean serum potassium at first hyperkalemia episode was 5.85 ± 0.49 mmol/L, and most patients in both groups had a serum potassium value between 5.5 and 6 mmol. The mean eGFR overall was 42.12 (13.26). The most common comorbid condition in our cohort was hypertension (98.93%), and 6174 (61.68%) had a diagnosis of diabetes mellitus, 4570 (45.66%) had heart failure and 3604 (36.01%) had atrial fibrillation. Patients who received an SPS/CPS prescription were younger, had lower eGFR and a higher frequency of diabetes, and were less likely to have heart failure or atrial fibrillation.
FIGURE 1:
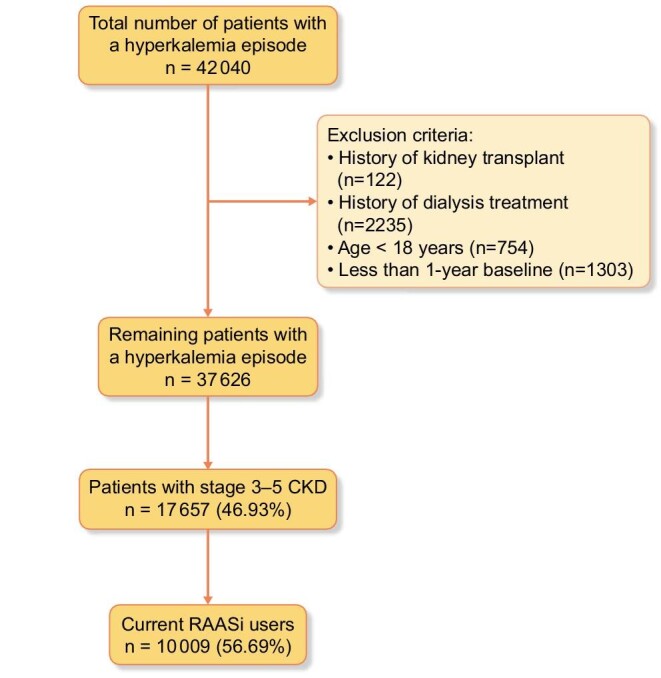
Study cohort flow diagram.
Table 1.
Characteristics of the study cohort overall and by whether patients did or did not receive a prescription of SPS/CPS for their de novo episode of hyperkalemia
| Overall, N = 10 009 | Patients who received an SPS/CPS prescription, N = 429 | Patients who did not receive an SPS/CPS prescription, N = 9580 | P-value | |
|---|---|---|---|---|
| Females | 4857 (48.53%) | 186 (43.36%) | 4671 (48.76%) | 0.03 |
| Age, mean (SD) | 74.48 (12.77) | 70.06 (14.30) | 74.68 (12.66) | <0.01 |
| Outpatient | 9965 (99.56%) | 428 (99.77%) | 9537 (99.55%) | 0.51 |
| Serum K+ test, median (IQR) ≥5.5 and <6 mmol/L ≥6 and ≤6.5 mmol/L >6.5 mmol/L |
5.70 (5.5–10) 7429 (74.22) 1817 (18.15%) 763 (7.62%) |
5.80 (5.50–9.10) 284 (66.20%) 109 (25.41%) 36 (8.39%) |
5.70 (5.50–10) 7145 (74.58%) 1708 (17.83%) 727 (7.59%) |
0.06 |
| Age group | <0.01 | |||
| 18–44 years 45–54 years 55–64 years 65–74 years ≥75 years |
206 (2.06%) 528 (5.28%) 1385 (13.84%) 2394 (23.92%) 5496 (54.91%) |
22 (5.13%) 46 (10.72%) 64 (14.92%) 118 (27.51%) 179 (41.72%) |
184 (1.92%) 482 (5.03%) 1321 (13.79%) 2276 (23.76%) 5317 (55.50%) |
|
| eGFR | <0.01 | |||
| <15 15–29 30–44 45–59 |
385 (3.85%) 1581 (15.80%) 3108 (31.05%) 4935 (49.31%) |
37 (8.62%) 92 (21.45%) 144 (33.57%) 156 (36.36%) |
348 (3.63%) 1489 (15.54%) 2964 (30.94%) 4779 (49.89%) |
|
| Comorbidities | ||||
| Diabetes mellitus Hypertension Heart failure Unstable angina Stroke Atrial fibrillation |
6174 (61.68%) 9902 (98.93%) 4570 (45.66%) 1987 (19.85%) 2048 (20.46%) 3604 (36.01%) |
301 (70.16%) 425 (99.07%) 146 (34.03%) 72 (16.78%) 67 (15.62%) 100 (23.31%) |
5873 (61.30%) 9477 (98.92%) 4424 (46.18%) 1915 (19.99%) 1981 (20.68%) 3504 (36.58%) |
<0.01 0.78 <0.01 0.10 0.01 <0.01 |
| Medications Azole Current users Non-users Beta-blockers Current users Non-users Calcineurin inhibitors Current users Non-users Digoxin Current users Non-users Heparin Current users Non-users Prescription NSAIDs Current users Non-users K supplements Current users Non-users Trimethoprim Current users Non-users MRA Current users Non-users Other diuretics Current users Non-users |
34 (0.34%) 9975 (99.66%) 4886 (48.82%) 5123 (51.18%) 0 10009 (100%) 880 (8.79%) 9129 (91.21%) 68 (0.68%) 9941 (99.33%) 444 (4.44%) 9565 (95.57%) 615 (6.14%) 9394 (93.85%) 433 (4.33%) 9576 (95.67%) 1675 (16.73%) 8334 (83.27%) 4343 (43.39%) 5666 (56.61%) |
0 429 (100%) 194 (45.22%) 235 (54.78%) 0 429 (100%) 36 (8.39%) 393 (91.61%) a 423 (98.60%) 16 (3.73%) 413 (96.28%) 13 (3.03%) 416 (96.97%) 22 (5.13%) 407 (94.87%) 77 (17.95%) 352 (82.05%) 199 (46.39%) 230 (43.61%) |
34 (0.36%) 9546 (99.64%) 4692 (48.98%) 4888 (51.02%) 0 9580 (100%) 844 (8.81%) 8736 (91.19%) 62 (0.65%) 9518 (99.35%) 428 (4.47%) 9152 (95.54%) 602 (6.28%) 8978 (93.72%) 411 (4.29%) 9169 (95.71%) 1598 (16.68%) 7982 (83.32%) 4144 (43.26%) 5436 (56.70%) |
0.21 0.13 – 0.77 0.09 0.47 <0.01 0.40 0.49 0.21 |
Data are presented as n (%) unless otherwise indicated.
aNot reported due to small numbers.
There were no significant differences in medication usage between participants who did and did not receive an SPS/CPS prescription except in current and nonusers of potassium supplements.
SPS/CPS prescription
Among 10 009 patients in the study cohort, 429 (4.29%) patients received an SPS or CPS prescription within 30 days of the index hyperkalemia episode. Most prescribers were general practitioners (54.20%) with the rest being nephrologists (11.01%), general internists (10.43%) and cardiologists (6.67%), among others (17.69%). Among those who received an SPS/CPS prescription, most of the patients received a prescription for ≥10 days (Table 2). Approximately 22% received a prescription for 1 day and only 6.76% received treatment for more than 30 days in the initial SPS/CPS prescription. The median time from the index hyperkalemia episode to an SPS/CPS prescription was 3 days (IQR: 1–11). The median number of days from SPS/CPS prescription to a new serum potassium test was 7 days (IQR: 3–29). The mean serum potassium value after SPS/CPS prescription was 4.72 mmol/L (SD 0.70), while the mean of first serum potassium after index hyperkalemia without a prescription was 4.84 mmol/L (SD 0.71).
Table 2.
Number of days’ supply of SPS/CPS prescriptions by frequency of prescribed days for the first episode of hyperkalemia and for repeated users
| Days of SPS/CPS therapy | Patients with at least one SP/CPS prescription, N = 429 | Repeated users, N = 206a |
|---|---|---|
| 1 | 93 (21.68%) | 39 (18.93%) |
| 2–10 | 187 (43.59%) | 60 (29.13%) |
| 11–20 | 37 (8.62%) | 24 (11.65%) |
| 21–30 | 83 (19.35%) | 61 (29.61%) |
| >30 | 29 (6.76%) | 22 (10.68%) |
Data are presented as n (%).
aRepeated prescription was defined as a new prescription of SPS/CPS any time after the first prescription.
Among those who received an SPS/CPS prescription, 206 (48.01%) received a subsequent prescription of SPS/CPS and 223 (51.98%) did not. Among those with a repeated prescription, 48.05% received a first prescription for ≤10 days (Table 2). The median time from the first SPS/CPS prescription to a subsequent prescription was 124 days (IQR: 32–409 days). The mean serum potassium value after the first SPS/CPS prescription was 4.77 mmol/L (SD 0.61) in those who received subsequent SPS/CPS prescriptions, while the mean of first serum potassium after the first SPS/CPS prescription was 4.55 mmol/L (SD 0.77) in those who did not receive subsequent SPS/CPS prescriptions (P-value = 0.01). Among those with repeated SPS/CPS prescriptions, 28.16% received only 1 other subsequent prescription, 33.50% received between 2 and 4 subsequent prescriptions, 17.48% received between 5 and 9 subsequent prescriptions and 20.97% received 10 or more prescriptions.
RAASi prescription patterns and SPS/CPS use
Of 10 009 at baseline, 8145 people (81.38%) survived 90 days after the hyperkalemia episode. A total of 1447 (17.77%) discontinued their RAASi and 6698 (82.23%) continued the medication on Day 90 (Table 3). The proportion of discontinuation was similar among those who received an SPS/CPS prescription and those who did not (P = 0.98). Among those who received repeated SPS/CPS prescriptions, 21 (9.29%) had discontinued their RAAS inhibitors and 205 (90.71%) continued the medication. At baseline, 26.45% (2647) of all patients were receiving a maximal dose of their RAASi. Among participants who continued their RAASi, 18.01% (n = 1467) continued receiving RAASi at a maximal dose and 64.22% (n = 5231) continued at a submaximal dose. The proportions of maximal and submaximal dosages of RAASi 90 days after the index hyperkalemia episode were not significantly different between those who did and who did not receive an SPS/CPS prescription (P = 0.53).
Table 3.
RAASi prescription pattern among patients who survived 90 days after the index hyperkalemia episode
| RAASi | Total, N = 8145 | Received SPS/CPS prescription, N = 399 | No SPS/CPS prescription, N = 7746 | P-value |
|---|---|---|---|---|
| Discontinuation | 1447 (17.77%) | 71 (17.79%) | 1376 (17.76%) | 0.98 |
| Continuation | 6698 (82.23%) | 328 (82.21%) | 6370 (82.24%) | |
| Maximum dose | 1467 (18.01%) | 80 (20.05%) | 1387 (17.91%) | 0.53 |
| Submaximal dose | 5231 (64.22%) | 248 (62.16%) | 4983 (64.33%) |
Data are presented as n (%).
DISCUSSION
In this retrospective cohort study of patients with CKD and hyperkalemia who were receiving RAASi at baseline, we found that only 4.29% of people received treatment for hyperkalemia in the form of a cation-exchange resin. Of those patients, only half received a subsequent prescription for SPS/CPS and >60% received treatment for ≤10 days. Discontinuation of RAASi was four times more likely than an SPS/CPS prescription after a de novo hyperkalemia episode in our cohort (17.77% versus 4.29%), suggesting it is a much more common strategy to treat hyperkalemia in our cohort.
Our study results are consistent with other cohort studies that have also reported a low incidence of SPS/CPS prescriptions among patients with CKD. In a Swedish cohort study of patients with CKD but regardless of hyperkalemia, 18.89% patients received an SPS prescription during the observation period [19]. In subsequent SPS prescriptions, 41% of individuals had a single isolated dispensation, and the remaining 59% of SPS users had, on average, three dispensations/year. Furthermore, they evaluated the dose prescribed and noted that only 13% received SPS at the per-label dose [19]. A chart review of a single center in Israel examined patients with CKD receiving RAASi who were treated chronically with a low dose of SPS after episodes of hyperkalemia (defined as serum potassium ≥6 mmol/L) [13]. Of 113 included patients, only 12% of patients received SPS treatment after at least one episode of hyperkalemia [13]. In another cohort in France, among patients with a baseline potassium >5 mmol/L, only 16.9% were on a potassium-binding resin while 50.6% were on a non-potassium-sparing diuretic [20]. An important difference between our cohort and the Swedish and French cohorts is that they included only patients followed by a nephrologist, while our cohort had a broader definition of CKD including all patients with hyperkalemia in our province. Those studies also aimed to evaluate the efficacy and safety of SPS in patients with CKD, while we specifically examined the pattern of prescriptions of potassium binders and RAASi.
For many years, SPS and CPS were the only medications available using the enteric route for the treatment of chronic hyperkalemia. However, the long-term efficacy of these two medications has not been evaluated in large randomized, placebo-controlled trials. SPS has been found in cohort studies to have a possibly dose-dependent association with serious adverse gastrointestinal effects such as intestinal ischemia, thrombosis, ulceration and perforation [14, 19]. Moreover, concomitant administration of SPS with sorbitol may increase the risk of intestinal necrosis [21]. Secondary to these concerns, in 2009, the US Food and Drug Administration posted a warning regarding adverse gastrointestinal events related to SPS [15]. Like SPS, there have been several case reports of colonic necrosis in patients using CPS [22, 23]. Furthermore, less serious gastrointestinal side effects such as constipation, nausea, and vomiting are also more common in patients receiving SPS and a possible reason for intolerance [19]. Thus, physicians are more likely to use RAASi downtitration or discontinuation to treat CKD patients who experience hyperkalemia as found in our study and other cohort studies [8, 9]. An American cohort study examined RAASi prescription patterns in the general population and found that, after a hyperkalemic event, RAASi dose was downtitrated in 21% of patients and discontinued in 27% [9]. This constitutes a therapeutic barrier as RAASi therapy plays a crucial role in renal and cardioprotection in patients with CKD [24, 25]. Effective well-tolerated potassium-lowering therapies that enable the continuation of RAASi would be desirable, but the low real-world usage of SPS/CPS compared with RAASi discontinuation following a hyperkalemia event suggests that these medications do not currently satisfy this need.
Our study has several strengths. First, the analysis of large, population-level databases used provides a complete capture of healthcare use in an entire province and adds to the generalizability of our results. Given the closed healthcare system, we would not have missed any prescriptions for resins or RAASi and can therefore accurately describe usage patterns for the general population. Limitations of the study include its observational nature and inability with current databases to co-interventions, including changes in over-the-counter medications related to potassium homeostasis such as NSAIDs, changes in acid/base status and dietary changes related to hyperkalemia. Additionally, the medication database does not register medications given to hospitalized patients, we could not ascertain compliance with treatment as prescribed and because SPS and CPS are available in a powder/liquid presentation, it is difficult to quantify dosage/month as compared with tablet-based treatments.
CONCLUSION
Our population-level study shows that SPS/CPS is not commonly prescribed in patients with CKD who experience hyperkalemia events, and when used, it is prescribed for short periods and not used as chronic therapy to prevent recurrent hyperkalemia. The more common strategy is to discontinue RAASi, which can deprive patients of important therapeutics to prevent CKD progression and cardiovascular events. These findings highlight the need for effective, well-tolerated treatments for hyperkalemia in patients with CKD.
Supplementary Material
ACKNOWLEDGEMENTS
This study was approved by the University of Manitoba Health Research Ethics Board [ethics file number HS23873 (H2020:193)]. The authors acknowledge the Manitoba Centre for Health Policy for use of data contained in the Manitoba Population Research Data Repository under project #2020-042 (HIPC#2020/2021-06). The results and conclusions are those of the authors and no official endorsement by the Manitoba Centre for Health Policy, Manitoba Health or other data providers is intended or should be inferred. Data used in this study are from the Population Health Research Data Repository housed at the Manitoba Centre for Health Policy, University of Manitoba, and were derived from data provided by Manitoba Health, Seniors and Active Living, Vital Statistics and Shared Health Diagnostic Services.
Contributor Information
Hongru Ren, Department of Internal Medicine, Max Rady College of Medicine, University of Manitoba, Winnipeg, Manitoba, Canada.
Silvia J Leon, Chronic Disease Innovation Centre, Seven Oaks General Hospital, Winnipeg, Manitoba, Canada.
Reid Whitlock, Chronic Disease Innovation Centre, Seven Oaks General Hospital, Winnipeg, Manitoba, Canada.
Claudio Rigatto, Department of Internal Medicine, Max Rady College of Medicine, University of Manitoba, Winnipeg, Manitoba, Canada; Chronic Disease Innovation Centre, Seven Oaks General Hospital, Winnipeg, Manitoba, Canada.
Paul Komenda, Department of Internal Medicine, Max Rady College of Medicine, University of Manitoba, Winnipeg, Manitoba, Canada; Chronic Disease Innovation Centre, Seven Oaks General Hospital, Winnipeg, Manitoba, Canada.
Clara Bohm, Department of Internal Medicine, Max Rady College of Medicine, University of Manitoba, Winnipeg, Manitoba, Canada; Chronic Disease Innovation Centre, Seven Oaks General Hospital, Winnipeg, Manitoba, Canada.
David Collister, Department of Internal Medicine, Max Rady College of Medicine, University of Manitoba, Winnipeg, Manitoba, Canada; Chronic Disease Innovation Centre, Seven Oaks General Hospital, Winnipeg, Manitoba, Canada.
Navdeep Tangri, Department of Internal Medicine, Max Rady College of Medicine, University of Manitoba, Winnipeg, Manitoba, Canada; Chronic Disease Innovation Centre, Seven Oaks General Hospital, Winnipeg, Manitoba, Canada.
FUNDING
Research reported in this publication was supported by Otsuka Canada Pharmaceutical Inc. The funding sources had no role in the design and conduct of the study, analysis or interpretation of the data and preparation or final approval of the manuscript before publication.
AUTHORS’ CONTRIBUTIONS
N.T. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design was carried out by N.T. Acquisition, analysis or interpretation of data was performed by all authors. Drafting of the manuscript was done by all authors. Critical revision of the manuscript for important intellectual content was by P.K., D.C., C.R., C.B. and N.T. Statistical analysis was performed by S.J.L. and R.W. N.T. obtained funding and provided supervision.
CONFLICT OF INTEREST STATEMENT
N.T. has received research support and honoraria from Otsuka Canada. N.T. reports personal fees from Roche Inc., other from ClinPredict Inc., grants and personal fees from Astra Zeneca Inc., grants and personal fees from Janssen, personal fees from Boehringer Ingelheim/Eli LIlly, grants, personal fees and other from Tricida Inc., other from PulseData and other from Mesentech, outside the submitted work. The other authors have no conflicts of interest to declare.
REFERENCES
- 1. Kovesdy CP, Regidor DL, Mehrotra Ret al. Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol 2007; 2: 999–1007 [DOI] [PubMed] [Google Scholar]
- 2. Sarafidis PA, Blacklock R, Wood Eet al. Prevalence and factors associated with hyperkalemia in predialysis patients followed in a low-clearance clinic. Clin J Am Soc Nephrol 2012; 7: 1234–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kovesdy CP. Updates in hyperkalemia: outcomes and therapeutic strategies. Rev Endocr Metab Disord 2017; 18: 41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahee P, Crowe AV.. The management of hyperkalaemia in the emergency department. J Accid Emerg Med 2000; 17: 188–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mushiyakh Y, Dangaria H, Qavi Set al. Treatment and pathogenesis of acute hyperkalemia. J Community Hosp Intern Med Perspect 2011; 1: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leon SJ, Harasemiw O, Tangri N.. New therapies for hyperkalemia. 2019; 28: 238–244 [DOI] [PubMed] [Google Scholar]
- 7. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013; 3: 1–150 [DOI] [PubMed] [Google Scholar]
- 8. Yildirim T, Arici M, Piskinpasa Set al. Major barriers against renin-angiotensin-aldosterone system blocker use in chronic kidney disease stages 3-5 in clinical practice: a safety concern? Ren Fail 2012; 34: 1095–1099 [DOI] [PubMed] [Google Scholar]
- 9. Epstein M, Reaven NL, Funk SEet al. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin- aldosterone system inhibitors. Am J Manag Care 2015; 21: S212–S220 [PubMed] [Google Scholar]
- 10. Clase CM, Carrero J-J, Ellison DHet al. Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2020; 97: 42–61 [DOI] [PubMed] [Google Scholar]
- 11. St-Jules R, Woolf K, Pompeii Met al. Exploring problems in following the hemodialysis diet, and their relation to energy and nutrient intakes: The Balance Wise Study. J Ren Nutr 2016; 26: 118–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lepage L, Dufour A, Doiron Jet al. Randomized clinical trial of sodium polystyrene sulfonate for the treatment of mild hyperkalemia in CKD. Clin J Am Soc Nephrol 2015; 10: 2136–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chernin G, Gal-oz A, Ben-assa Eet al. Secondary prevention of hyperkalemia with sodium polystyrene sulfonate in cardiac and kidney patients on renin-angiotensin-aldosterone system inhibition therapy. Clin Cardiol 2012; 35: 32–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Noel JA, Bota SE, Petrcich Wet al. Risk of hospitalization for serious adverse gastrointestinal events associated with sodium polystyrene sulfonate use in patients of advanced age. JAMA Intern Med 2019; 179: 1025–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sterns RH, Rojas M, Bernstein Pet al. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol 2010; 21: 733–735 [DOI] [PubMed] [Google Scholar]
- 16. Roos LL, Nicol JP.. A research registry : uses, development, and accuracy. J Clin Epidemiol 1999; 52: 39–47 [DOI] [PubMed] [Google Scholar]
- 17. Roos LL. Using administrative data for longitudinal research : comparisons with primary data collection. J Chronic Dis 1987; 40: 41–49 [DOI] [PubMed] [Google Scholar]
- 18. Ezekowitz JA, Meara EO, Mcdonald MAet al. 2017 Comprehensive update of the Canadian Cardiovascular Society Guidelines for the Management of Heart Failure. Can J Cardiol 2017; 33: 1342–1433. [DOI] [PubMed] [Google Scholar]
- 19. Laureati P, Xu Y, Trevisan Met al. Initiation of sodium polystyrene sulphonate and the risk of gastrointestinal adverse events in advanced chronic kidney disease: a nationwide study. Nephrol Dial Transplant 2020; 35: 1518–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wagner S, Metzger M, Flamant Met al. Association of plasma potassium with mortality and end-stage kidney disease in patients with chronic kidney disease under nephrologist care—The NephroTest study. BMC Nephrol 2017; 18: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McGowan CE, Saha S, Chu Get al. Intestinal necrosis due to sodium polystyrene sulfonate (Kayexalate) in sorbitol. South Med J 2009; 102: 493–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Joo M, Bae WK, Kim NHet al. Colonic mucosal necrosis following administration of calcium polystryrene sulfonate (Kalimate) in a uremic patient. J Korean Med Sci 2009; 24: 1207–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Castillo-Cejas MD, Alonso-Cotoner C. Colonic necrosis due to calcium polystyrene sulfonate (Kalimate) not suspended in sorbitol. Rev Esp Enferm Dig 2013; 105: 232–234 [DOI] [PubMed] [Google Scholar]
- 24. Schrier RW. Hyperkalemia: a threat to RAAS inhibition? Nat Rev Nephrol 2010; 6: 245–246 [DOI] [PubMed] [Google Scholar]
- 25. Weir MR, Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol 2010; 5: 531–548 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


