ABSTRACT
Background
Evidence linking chronic kidney disease (CKD) and sleep duration is inconsistent. This study examined whether sleep duration is associated with a long-term risk of kidney function decline.
Methods
This retrospective, longitudinal cohort study included 82 001 participants who visited a primary care centre in Japan. Participants were categorized into CKD risk groups and sleep duration categories according to their self-reported average nightly sleep duration. The relationship between average nightly sleep duration and the incidence of composite renal outcome comprised a ≥40% reduction in estimated glomerular filtration rate (eGFR) from baseline and a decline in eGFR to <15 mL/min/1.73 m² was evaluated.
Results
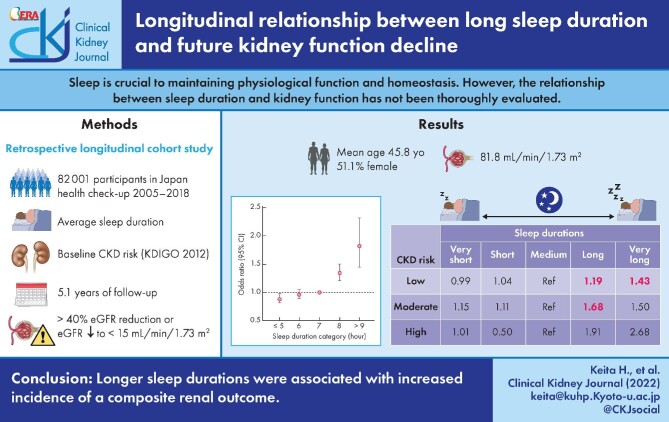
The mean age and eGFR (±standard deviation) of the patient cohort were 45.8 (±12.4) years and 81.8 (±15.4) mL/min/1.73 m², respectively. A total of 41 891 participants (51.1%) were women. During the median follow-up of 5.1 years [interquartile range 2.2–9.6], 4214 (5.1%) participants achieved the composite renal outcome. Only the long and very long sleep durations (≥8 h/night) were associated with an increased incidence of the composite renal outcome compared with the reference duration (7 h/night) [adjusted odds ratio (OR) 1.22 and 1.44; 95% confidence interval (CI) 1.09–1.36 and 1.13–1.84, for long and very long sleep durations, respectively]. Furthermore, this association was significant for both long and very long sleep durations in the low CKD risk group but only for long sleep duration in the intermediate CKD risk group. The results of the sex-specific analysis showed that men had a decreased risk of achieving the composite renal outcome (OR 0.91; 95% CI 0.79–1.06), while there was an increased risk for women (OR 1.14; 95% CI 1.02–1.28).
Conclusions
Average sleep durations ≥8 h/night were associated with an increased incidence of poor renal outcomes over time. However, a longitudinal cohort study is required to confirm whether sleep duration can prevent poor renal outcomes.
Keywords: chronic kidney disease, epidemiology, generalized estimating equation, kidney function, sleep duration
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Chronic kidney disease (CKD) is a very common condition characterized by a gradual decline in kidney function, and causes significant morbidity and mortality worldwide [1]. CKD often has no clear onset of symptoms. Even when the disease progresses, patients often report only vague complaints such as slight oedema or becoming fatigued easily due to renal anaemia, which can delay the detection of the disease. In addition, many individuals suffer from CKD, and the medical and economic costs to society are substantial and increase as the disease progresses [2]. Thus, an improved understanding of the factors contributing to kidney function decline in CKD is needed.
Sleep is crucial to maintaining physiological function and homeostasis, as it provides the opportunity to repair and restore tissues. An average individual spends approximately one-third of their lifetime sleeping; however, evidence reveals that many individuals find it difficult to achieve sufficient sleep quality and duration in modern society [3]. Accordingly, sleep duration has been identified as a potential lifestyle risk factor, particularly when considering short sleep duration. Epidemiological studies have identified a relationship between sleep duration and several diseases including hypertension, metabolic syndrome, diabetes and cardiovascular disease; short sleep duration has also been associated with increased mortality [4–9]. However, a recent meta-analysis showed that long sleep duration is associated with various increased health risks compared with short sleep duration [10].
The evidence linking CKD and sleep duration has often been conflicting, and risk estimates have been inconsistent. In 2016, a large prospective study of 4238 middle-aged women with an 11-year follow-up period reported that short sleep duration was significantly and independently associated with a more rapid decline in kidney function than appropriate sleep duration [11]. In a more recent study, Yamamoto et al. [12] performed a prospective cohort study of 1601 patients with CKD and reported that both short and prolonged sleep durations were significantly associated with a decline in kidney function. Importantly, the association persisted even after adjusting for age and known CKD risk factors, such as a history of hypertension and diabetes. However, despite the great variability in sleep duration over time, most studies have based their analysis on sleep duration evaluated at baseline. Therefore, further investigation of the relationship between sleep duration and kidney function is required and should consider changes in average sleep duration over time.
To assess the relationship between sleep duration and kidney function, we conducted a retrospective longitudinal cohort study with a long follow-up period in a population of adults undergoing routine health examinations. Specifically, we evaluated the relationship between average annual sleep duration and kidney function decline over 14 years in participants who may be at risk of CKD. Our analysis was performed using the generalized estimating equation (GEE) with a logit function and a binomial distribution. We hypothesized that individuals with shorter (≤6 h/night) or longer (≥8 h/night) sleep durations would experience greater kidney function decline over time than those who had an appropriate sleep duration (7 h/night).
MATERIALS AND METHODS
Study Design
We conducted a retrospective study using longitudinal data collected from St Luke's International Hospital in Tokyo, Japan. All adult participants (>18 years) who visited the hospital's Centre for Preventive Medicine for a health check-up between 1 January 2005 and 31 December 2018 were included. Participants with an estimated glomerular filtration rate (eGFR) <15 mL/min/1.73 m² during their first visit were excluded from the analysis. In addition, participants who only visited the health centre once were excluded, as were participants who opted out of having their anonymized data used in the study. We grouped the remaining participants by average nightly sleep duration and baseline CKD risk, and compared the outcomes between groups. This study was approved by the St Luke's Ethics Committee (REB No. 18-R203).
Calculation of eGFR and determination of the composite renal outcome
As a part of standard health check-ups, participants provided a blood sample at each visit that was analysed for serum creatinine. Then, eGFR was calculated according to the following Japanese formulas [13]:
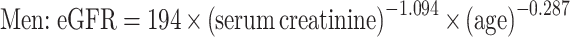 |
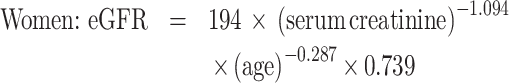 |
In this study, the primary outcome was a composite renal outcome comprised of a ≥40% reduction in eGFR from baseline and a decline in eGFR to <15 mL/min/1.73 m² [14]. Based on the calculated eGFR and a proteinuria evaluation, participants were categorized into baseline CKD risk groups in accordance with the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 clinical practice guidelines [15]. Information relating to dialysis status was collected from medical records or self-reported by the participants.
Sleep duration categorization
All participants self-reported their average nightly sleep duration during check-ups to a member of the research team who was present at each visit. Sleep duration data were analysed at each visit as a time-series. Participants were categorized into one of five nightly sleep duration categories—very short (≤5 h), short (6 h), medium (7 h), long (8 h) and very long (≥9 h)—consistent with previous studies [16, 17]. In our study, we defined the medium (7 h) sleep duration as the reference group for the analyses.
Covariates
To account for covariates that may influence the relationship between sleep duration and the composite renal outcome, we collected information relating to the participants’ demographics, health habits and medical history, including data relating to alcohol consumption (abstinent, occasional drinking, regular drinking), smoking status (never smoked, former smoker and current smoker) and exercise status (≤1 time/week, 1–2 times/week, 3–5 times/week and ≥5 times/week). Body mass index (BMI) was calculated using height and weight data collected during the check-ups. In accordance with the recommended BMI ranges for determining obesity in Asian populations, participants were categorized into one of the three groups: underweight (≤18.5 kg/m2), normal weight (18.5–24.9 kg/m2), or obese/overweight (≥25.0 kg/m2) [18, 19]. Medical history information, including a history of hypertension, diabetes, dyslipidaemia and any confirmed cancer diagnosis, was also collected. Participants were classified into one of four groups relating to their level of CKD risk, as described above. All data were self-reported at each visit.
GEE in longitudinal data analysis
The GEE is often used in clinical research and is a common statistical approach to fitting marginal models for longitudinal data analysis [20]. The participants’ baseline characteristics were first compared between sleep duration categories. Models were then used to assess the incidence of the composite renal outcome by sleep duration category, using a GEE with a logit function and binomial distribution. We evaluated all the covariates included in the GEE as time-varying covariates.
Using this approach, it is possible to adjust time-series changes in covariates and minimize the effect of missing values. For example, when using Cox proportional hazard analysis, participants with missing data at baseline must be excluded. However, using GEE analysis, it is possible to adjust the variables if they are available at some point during the study period, even if there are missing values at baseline. Three models were adjusted for various potential covariates to identify key parameters. Model 1 was adjusted for patient age, sex, baseline eGFR, proteinuria and BMI. Model 2 was adjusted for health habits (alcohol consumption, smoking status and exercise status) in addition to Model 1 covariates. Model 3 was adjusted for comorbidities (hypertension, diabetes, dyslipidaemia and all types of cancer) in addition to Model 2 covariates. We then performed a sub-analysis where participants were stratified according to their baseline CKD risk category, and the relationship between sleep duration and the composite renal outcome was re-assessed. Sensitivity analyses were performed using the stages of CKD based on eGFR and the degree of proteinuria. All analyses were performed using STATA 15 (STATA Corp., College Station, TX, USA).
RESULTS
The baseline characteristics of the participants across sleep duration categories are presented in Table 1. A total of 82 001 participants were included in this study, with a mean age [±standard deviation (SD)] of 45.8 (±12.4) years; 41 891 (51.1%) participants were women (Figure 1). The median observation period was 1856 [interquartile range (IQR) 797–3506] days, and the median number of visits was 5 (IQR 3–9). Participants with a sleep duration ≥8 h/night tended to be older (>35 years) and exercised more frequently (>1–2 times/week). The missing values for sleep duration and eGFR are 11.40% and 0.021%, respectively. The relationship between sleep duration and obesity was U-shaped, such that participants with very short or very long sleep durations were more frequently obese. In terms of medical history, the prevalence of comorbidities tended to increase with sleep duration. During the follow-up, 4214 (5.1%) participants reached the composite renal outcome. When considering patient characteristics by sex it was observed that male participants had higher BMI, higher prevalence of hypertension and diabetes, and more severe dyslipidaemia including higher levels of low-density lipoprotein and triglycerides (Supplementary data, Table S1).
Table 1.
Baseline participant characteristics stratified by average nightly sleep duration category
| Very short, 5 h or less (n = 20 455) | Short, 6 h (n = 33 897) | Middle, 7 h (n = 20 691) | Long, 8 h (n = 6237) | Very long, 9 h or more (n = 712) | Total (n = 82 001) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age in years, mean (SD) | 42.6 | (11.0) | 45.2 | (11.8) | 48.3 | (12.8) | 50.8 | (14.5) | 52.4 | (15.7) | 45.8 | (12.4) |
| Women, n (%) | 10 377 | (50.7) | 17 367 | (51.2) | 10 587 | (51.2) | 3200 | (51.3) | 360 | (49.9) | 41 891 | (51.1) |
| Alcohol consumption, n (%) | ||||||||||||
| Abstainer | 8100 | (39.6) | 13 295 | (39.2) | 8309 | (40.2) | 2654 | (42.6) | 320 | (44.4) | 32 678 | (39.9) |
| Occasional drinker | 3706 | (18.1) | 6082 | (17.9) | 3462 | (16.7) | 942 | (15.1) | 85 | (11.8) | 14 277 | (17.4) |
| Regular drinker | 8649 | (42.3) | 14 520 | (42.8) | 8920 | (43.1) | 2641 | (42.3) | 316 | (43.8) | 35 046 | (42.7) |
| Smoking status, n (%) | ||||||||||||
| Never | 12 729 | (62.2) | 20 957 | (61.8) | 12 540 | (60.6) | 3622 | (58.1) | 384 | (53.3) | 50 232 | (61.3) |
| Former | 3949 | (19.3) | 7501 | (22.1) | 5024 | (24.3) | 1623 | (26.0) | 217 | (30.1) | 18 314 | (22.3) |
| Current | 3777 | (18.5) | 5439 | (16.1) | 3127 | (15.1) | 992 | (15.9) | 120 | (16.6) | 13 455 | (16.4) |
| Exercise, n (%) | ||||||||||||
| Almost none | 9265 | (45.3) | 13 202 | (39.0) | 6989 | (33.8) | 2064 | (33.1) | 253 | (35.1) | 31 773 | (38.8) |
| 1–2 times a week | 7146 | (34.9) | 12 778 | (37.7) | 7871 | (38.0) | 2162 | (34.7) | 239 | (33.2) | 30 196 | (36.8) |
| 3–5 times a week | 2477 | (12.1) | 4969 | (14.7) | 3706 | (17.9) | 1255 | (20.1) | 128 | (17.8) | 12 535 | (15.3) |
| Almost everyday | 1567 | (7.7) | 2948 | (8.7) | 2125 | (10.3) | 756 | (12.1) | 101 | (14.0) | 7497 | (9.1) |
| Body mass index, n (%) | ||||||||||||
| Underweight | 1923 | (9.4) | 3359 | (9.9) | 2132 | (10.3) | 693 | (11.1) | 96 | (13.3) | 8203 | (10.0) |
| Normal weight | 14 154 | (69.2) | 24 106 | (71.1) | 14 940 | (72.2) | 4395 | (70.5) | 485 | (67.3) | 58 080 | (70.8) |
| Obese/overweight | 4378 | (21.4) | 6432 | (19.0) | 3619 | (17.5) | 1148 | (18.4) | 140 | (19.4) | 15 717 | (19.2) |
| Medical history, n (%) | ||||||||||||
| Any cancer | 544 | (2.7) | 1273 | (3.8) | 1027 | (5.0) | 414 | (6.6) | 50 | (6.9) | 3308 | (4.0) |
| Hypertension | 1126 | (5.5) | 2366 | (7.0) | 1934 | (9.4) | 765 | (12.3) | 105 | (14.6) | 6296 | (7.7) |
| Systolic BP, mmHg (SD) | 116.3 | (16.2) | 117.1 | (16.6) | 118.6 | (17.3) | 120.1 | (18.2) | 120.7 | (18.7) | 117.6 | (16.8) |
| Diastolic BP, mmHg (SD) | 71.4 | (11.3) | 72.0 | (11.3) | 73.1 | (11.5) | 73.7 | (11.7) | 73.7 | (12.0) | 72.3 | (11.4) |
| Diabetes | 265 | (1.3) | 586 | (1.7) | 517 | (2.5) | 204 | (3.3) | 35 | (4.9) | 1607 | (2.0) |
| Fasting blood glucose, mg/dL (SD) | 98.5 | (15.1) | 98.7 | (14.3) | 99.6 | (15.2) | 100.4 | (16.4) | 101.0 | (16.9) | 99.0 | (14.9) |
| Haemoglobin A1c, % (SD) | 5.1 | (0.6) | 5.1 | (0.5) | 5.2 | (0.6) | 5.2 | (0.6) | 5.2 | (0.6) | 5.1 | (0.6) |
| Dyslipidaemia | 672 | (3.3) | 1456 | (4.3) | 1157 | (5.6) | 428 | (6.9) | 61 | (8.5) | 3774 | (4.6) |
| LDL cholesterol, mg/dL (SD) | 114.1 | (30.5) | 115.2 | (30.2) | 116.6 | (30.0) | 117.8 | (31.0) | 117.4 | (32.2) | 115.5 | (30.3) |
| HDL cholesterol, mg/dL (SD) | 62.8 | (15.7) | 62.9 | (15.5) | 62.6 | (15.6) | 62.2 | (15.4) | 62.6 | (17.0) | 62.8 | (15.6) |
| Triglyceride, mg/dL (SD) | 94.8 | (76.2) | 96.1 | (74.9) | 98.5 | (73.2) | 102.3 | (80.8) | 109.7 | (123.1) | 97.0 | (75.9) |
| Kidney function | ||||||||||||
| eGFR, mL/min/1.73 m2 (SD) | 83.2 | (15.1) | 82.1 | (15.3) | 80.7 | (15.6) | 79.9 | (16.3) | 80.1 | (17.7) | 81.8 | (15.4) |
| Blood urea nitrogen, mg/dL (SD) | 12.3 | (3.9) | 12.5 | (4.0) | 12.8 | (4.1) | 12.9 | (4.2) | 12.8 | (4.7) | 12.5 | (4.0) |
| Serum creatinine, mg/dL (SD) | 0.7 | (0.2) | 0.7 | (0.2) | 0.7 | (0.2) | 0.7 | (0.2) | 0.7 | (0.2) | 0.7 | (0.2) |
| Proteinuria, n (%) | ||||||||||||
| Normal | 20 235 | (98.9) | 33 575 | (99.1) | 20 509 | (99.1) | 6176 | (99.0) | 707 | (98.1) | 81 202 | (99.0) |
| Mild | 214 | (1.1) | 316 | (1.0) | 178 | (0.9) | 59 | (1.0) | 14 | (1.9) | 781 | (1.0) |
| Severe | 6 | (0.0) | 6 | (0.0) | 4 | (0.0) | 2 | (0.0) | 0 | (0.0) | 18 | (0.0) |
| CKD risk category, n (%) | ||||||||||||
| Low | 19 421 | (95.0) | 31 903 | (94.1) | 19 128 | (92.5) | 5618 | (90.1) | 635 | (88.1) | 76 705 | (93.5) |
| Moderate | 963 | (4.7) | 1861 | (5.5) | 1429 | (6.9) | 545 | (8.7) | 70 | (9.7) | 4868 | (5.9) |
| High | 58 | (0.3) | 112 | (0.3) | 107 | (0.5) | 58 | (0.9) | 12 | (1.7) | 347 | (0.4) |
| Very high | 13 | (0.1) | 21 | (0.1) | 27 | (0.1) | 16 | (0.3) | 4 | (0.6) | 81 | (0.1) |
| Composite renal outcome | ||||||||||||
| ≥40% reduction in eGFR | 898 | (4.4) | 1664 | (4.9) | 1134 | (5.5) | 409 | (6.6) | 69 | (9.7) | 4174 | (5.1) |
| eGFR <15 mL/min/1.73 m² | 2 | (0.0) | 6 | (0.0) | 3 | (0.0) | 4 | (0.1) | 1 | (0.1) | 16 | (0.0) |
BP, blood pressure; eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
FIGURE 1:
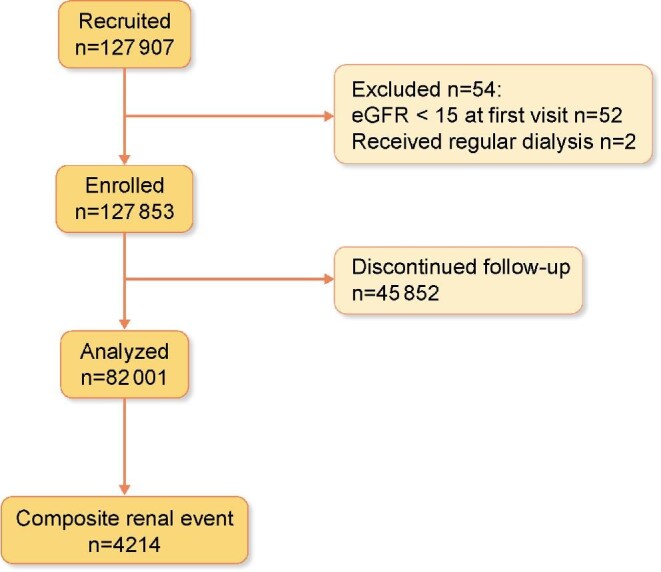
Flow diagram of patient cohorts used in the study.
We identified a relationship between sleep duration and the incidence of the composite renal outcome (Figure 2). Specifically, participants with longer average nightly sleep durations were at a significantly higher risk of developing the composite renal outcome at the median follow-up of 5.1 years. The adjusted odds ratio (OR) of the composite renal outcome in each sleep duration category was compared with the reference (7 h/night) group (Table 2). Very long [≥9 h/night; adjusted OR 1.44; 95% confidence interval (CI) 1.13–1.84 in Model 3] and long sleep durations (8 h/night; adjusted OR 1.22; 95% CI 1.09–1.36 in Model 3) were associated with an increased incidence of the composite renal outcome compared with the medium (reference) sleep duration. The incidence of the composite renal outcome in participants with short (6 h/night) or very short (≤5 h/night) sleep duration was comparable with that of the reference group across all models. Specifically, very short sleep duration was associated with a lower composite renal outcome in the unadjusted model; however, this effect disappeared in the covariate-adjusted model. The results of the sex-specific analysis showed that very short sleep duration was associated with a decrease in the incidence of the composite renal outcome in men (OR 0.91; 95% CI 0.79–1.06), while it was associated with an increased incidence of the composite renal outcome in women (OR 1.14; 95% CI 1.02–1.28) (Supplementary data, Table S2). In addition, we also performed an analysis of the incidence of the individual renal outcomes as a sensitivity analysis. Overall, the results showed that the OR was higher for the ‘eGFR <15 mL/min/1.73m²’ outcome than for the ‘≥40% reduction in eGFR’ outcome (Supplementary data, Table S3). However, CIs of ‘eGFR <15 mL/min/1.73m²’ outcome were wide due to the small sample size.
FIGURE 2:
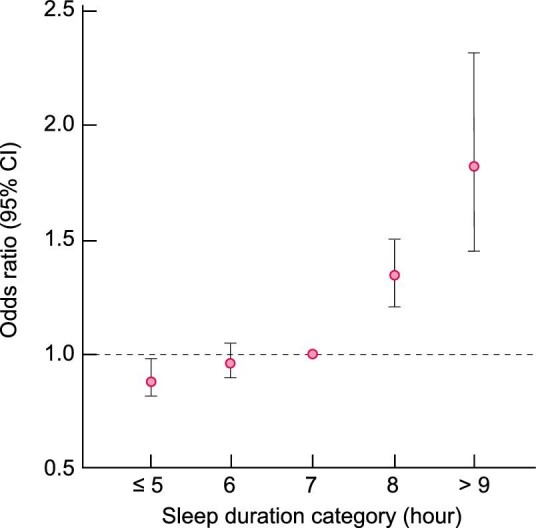
Association between sleep duration and incidence of the composite renal outcome.
Table 2.
Comparison of the composite renal outcome incidence by sleep duration category as determined by the multivariable generalized estimating equation
| Composite renal outcome incidence, adjusted odds ratio (95% confidence interval) | |||||
|---|---|---|---|---|---|
| Very short, 5 h or less | Short, 6 h | Medium, 7 h | Long, 8 h | Very long, 9 h or more | |
| Unadjusted | 0.88d (0.81–0.96) | 0.96 (0.89–1.03) | Reference | 1.33 (1.19–1.49) | 1.80 (1.42–2.29) |
| Model 1a | 1.05 (0.96–1.15) | 1.06 (0.98–1.14) | Reference | 1.22 (1.09–1.37) | 1.46 (1.14–1.86) |
| Model 2b | 1.04 (0.95–1.14) | 1.05 (0.97–1.14) | Reference | 1.22 (1.10–1.37) | 1.47 (1.15–1.88) |
| Model 3c | 1.03 (0.95–1.14) | 1.05 (0.97–1.14) | Reference | 1.22 (1.09–1.36) | 1.44 (1.13–1.84) |
aModel 1 was adjusted for age, sex, baseline eGFR, proteinuria and body mass index.
bModel 2 was adjusted for health habits (alcohol consumption, smoking status and exercise habit) in addition to the covariates in Model 1.
cModel 3 wad adjusted for comorbidities (hypertension, diabetes, dyslipidaemia and any type of cancer) in addition to the covariates in Model 2.
dNumbers in bold are statistically significant (P < 0.05).
When participants were stratified by their baseline CKD risk category in our sub-analysis, the findings were similar to those of the main analysis (Table 3). In the low CKD risk sub-analysis, participants in the very long (adjusted OR 1.43; 95% CI 1.10–1.86) and long sleep duration categories (adjusted OR 1.19; 95% CI 1.05–1.34) had an increased incidence of the composite renal outcome compared with participants in the reference sleep duration category. In the intermediate and high CKD risk sub-analysis, participants in the very long and long sleep duration categories tended to have an increased incidence of the composite renal outcome; however, this association was only significant for intermediate CKD risk participants in the long sleep duration category (adjusted OR 1.68; 95% CI 1.10–2.56).
Table 3.
Adjusted odds ratio for the composite renal outcome by sleep duration group, stratified by baseline chronic kidney disease risk category
| Composite renal outcome odds ratio, adjusted odds ratio (95% confidence interval) | |||||
|---|---|---|---|---|---|
| Chronic kidney disease risk categorya, n (%) | Very short, 5 h or less | Short, 6 h | Medium, 7 h | Long, 8 h | Very long, 9 h or more |
| Low risk, 8594 (11.2)b | 0.99 (0.90–1.09) | 1.04 (0.96–1.12) | Reference | 1.19d (1.05–1.34) | 1.43 (1.10–1.86) |
| Intermediate risk, 329 (6.8) | 1.15 (0.73–1.80) | 1.11 (0.76–1.62) | Reference | 1.68 (1.10–2.56) | 1.59 (0.71–3.56) |
| High risk, 77 (22.2) | 1.01 (0.40–2.55) | 0.50 (0.21–1.23) | Reference | 1.91 (0.84–4.34) | 2.68 (0.80–9.01) |
| Very high risk, 53 (65.4) | ―c | ― | Reference | ― | ― |
aModel was adjusted for age, sex, body mass index, health habits (alcohol consumption, smoking status and exercise habit) and comorbidities (hypertension, diabetes, dyslipidaemia and any type of cancer).
bThe number and proportion of composite renal events in each CKD risk categories.
cAnalyses were not concaved; thus, no odds ratio was calculated.
dNumbers in bold are statistically significant (P < 0.05).
DISCUSSION
We conducted a large-scale retrospective cohort study of adults with a median follow-up of 5.1 years to evaluate the relationship between kidney function and sleep duration using the GEE method for longitudinal data analysis. In our study, we observed that long (8 h) and very long (≥9 h) average nightly sleep durations were associated with an increased incidence of the composite renal outcome compared with the reference sleep duration (7 h). Interestingly, participants in the short and very short sleep duration categories had a similar incidence of the composite renal outcome compared with the reference group. Additionally, participants in the intermediate and high CKD risk categories with very long or long sleep durations tended to have an increased incidence of the composite renal outcome. However, this association was only significant for intermediate CKD risk participants in the long sleep duration category. Low CKD risk participants in the long and very long sleep duration categories had a significantly increased incidence of the composite renal outcome compared with the reference group.
A recent large prospective cohort study found that short (<5 h/night) or long (>8 h/night) sleep duration and poor sleep quality were significantly associated with a higher incidence of end-stage kidney disease [11]. In our study, longer sleep duration was associated with a deterioration of kidney function; however, no significant deterioration in kidney function was observed in participants in the short sleep duration category. Sub-analyses by CKD risk category also confirmed that longer sleep duration was generally associated with a greater incidence of kidney function decline. Our data suggest that short-term sleep duration information collected from individuals with normal kidney function is not an effective marker of future susceptibility to CKD. While previous studies have pointed to a relationship between short sleep duration and CKD, obesity, hypertension, diabetes and dyslipidaemia [21] they have typically assessed medical history only at baseline. By contrast, the strength of our study was its longitudinal design and the collection of data from participants at each visit. This approach allowed ongoing assessment of sleep duration until the composite renal outcome was achieved, which provides more specific insight into the relationship between sleep duration and renal outcomes. Interestingly, in a sub-analysis, we found that both longer sleep durations and very short sleep durations were associated with an increased incidence of the composite renal outcome for women, consistent with previous studies. On the contrary, very short sleep duration was associated with a reduced incidence of the composite renal outcome for men, even in models that adjusted for covariates. This result may contribute to the fact that short sleep duration was not a predictor of worsening renal function in our study. It is unclear why the effect of short sleep duration on the composite renal outcome incidence varied between men and women. It is possible that confounding factors that were not adjusted for in this study, such as social status, may have an effect on renal function; however, further investigation is needed. Future work should also determine whether long-term sleep trends may be more predictive of CKD development.
Longer sleep duration may have been associated with kidney impairment as sleep disorder may cause genetically driven alterations in kidney phenotypes. According to the UK Biobank cohort, long sleep duration is affected by an increased evening chronotype and circadian rhythm disturbances [22]. Notably, circadian rhythm abnormalities are thought to cause non-dipper hypertension and may contribute to the increased incidence of the composite renal outcome in participants who sleep for longer sleep durations. In addition, preclinical data suggest that circadian rhythm dysfunction and loss of diurnal rhythm may promote renal function decline [23]. Furthermore, prolonged sleep may be secondary to the excessive daytime sleepiness caused by obstructive sleep apnoea and central sleep apnoea [24], which are known CKD risk factors and may have contributed to renal function decline. In addition, recent studies have suggested that prolonged sleep is an independent risk factor for poor health outcomes [25–27]. Although these findings offer insight into the potential mechanisms linking sleep duration and CKD risk, determining whether these findings translate into clinical populations merits further investigation.
This study has some limitations. First, we only assessed information regarding sleep duration from a questionnaire and did not adjust data according to sleep quality. For example, the classification of sleep duration remains unclear for participants who may have an average nightly sleep duration between the categories, which were separated on the questionnaire by 1 h. However, a previous study shows that the correlation between self-reported sleep duration and actual sleep duration was 0.47, indicating sleep duration categorization was likely accurate [28]. In addition, our cohort consisted of a large number of participants, and we relied on a strictly controlled electronic medical record database, which may mitigate potential bias. Second, some participants were lost to follow-up and the number of participants in each of the four CKD risk groups was not well-balanced. Losing participants to follow-up and unbalanced groups may contribute to selection bias since longitudinal studies require participants to make multiple visits. Third, the number of participants in the very long sleep duration category was smaller than that in the other sleep duration categories. Fourth, our study was performed at a single centre in the national capital, which is populated by many relatively young and active adults who may be unable to sleep for long durations because of their lifestyles and commitments. Fourth, in GEE analysis, covariates can be treated as longitudinal data, but measurements close to the occurrence of an event can have the opposite causal effect. For example, poor kidney function may affect sleep duration. Finally, we initially adopted an eGFR reduction of ≥40% as the primary outcome, as this was reported as a surrogate marker for renal prognosis [29]. However, a ≥40% reduction in eGFR is possible due to measurement errors or short-term conditions, such as acute kidney injury. To account for this possibility, we instead used a composite renal outcome that also included an eGFR <15 mL/min/1.73 m². Taken together, we believe that our research provides robust insight into the relationship between sleep duration and CKD risk.
In conclusion, our study demonstrated that longer sleep durations were associated with an increased incidence of a composite renal outcome composed of a reduction in eGFR and the need for dialysis. However, a longitudinal cohort study is still needed to identify individuals at risk of poor renal outcomes based on their sleep duration and to determine whether sleep duration information can be used to guide clinical management to prevent kidney function decline. Determining a reference sleep duration that may contribute to the prevention of CKD will be invaluable and may have clinical consequences not only for individuals with CKD but also for other systemic disorders that have known associations with sleep duration.
Supplementary Material
ACKNOWLEDGEMENTS
We gratefully acknowledge the Centre for Preventive Medicine at St Luke's International Hospital for their data contribution and the St Luke's Ethics Committee for their approval.
Contributor Information
Keita Hirano, Department of Nephrology, St Luke's International Hospital, Tokyo, Japan; Department of Nephrology, Kyoto University Graduate School of Medicine, Kyoto, Japan; Department of Healthcare Quality and Safety, Gunma University Graduate School of Medicine, Gunma, Japan.
Yasuhiro Komatsu, Department of Nephrology, St Luke's International Hospital, Tokyo, Japan; Department of Healthcare Quality and Safety, Gunma University Graduate School of Medicine, Gunma, Japan.
Takuro Shimbo, Department of Internal Medicine, Ohta Nishinouchi Hospital, Fukushima, Japan.
Hirosuke Nakata, Department of Nephrology, Kyoto University Graduate School of Medicine, Kyoto, Japan.
Daiki Kobayashi, Division of General Internal Medicine, Department of Medicine, Tokyo Medical University Ibaraki Medical Center, Ibaraki, Japan; Division of General Internal Medicine, Department of Medicine, St Luke's International Hospital, Tokyo, Japan; Department of Internal Medicine, Fujita Health University, Aichi, Japan; Department of General Medicine, Juntendo University Faculty of Medicine, Tokyo, Japan.
CONFLICT OF INTEREST STATEMENT
None declared.
DATA AVAILABILITY STATEMENT
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.
REFERENCES
- 1. Gansevoort RT, Correa-Rotter R, Hemmelgarn BRet al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013; 382: 339–352 [DOI] [PubMed] [Google Scholar]
- 2. Wang V, Vilme H, Maciejewski MLet al. The economic burden of chronic kidney disease and end-stage renal disease. Semin Nephrol 2016; 36: 319–330 [DOI] [PubMed] [Google Scholar]
- 3. Wong K, Chan AHS, Ngan SC. The effect of long working hours and overtime on occupational health: a meta-analysis of evidence from 1998 to 2018. Int J Environ Res Public Health 2019; 16: 2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu Y, Zhai L, Zhang D. Sleep duration and obesity among adults: a meta-analysis of prospective studies. Sleep Med 2014; 15: 1456–1462 [DOI] [PubMed] [Google Scholar]
- 5. Xi B, He D, Zhang Met al. Short sleep duration predicts risk of metabolic syndrome: a systematic review and meta-analysis. Sleep Med Rev 2014; 18: 293–297 [DOI] [PubMed] [Google Scholar]
- 6. Shan Z, Ma H, Xie Met al. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care 2015; 38: 529–537 [DOI] [PubMed] [Google Scholar]
- 7. Wang Y, Mei H, Jiang Y-Ret al. Relationship between duration of sleep and hypertension in adults: a meta-analysis. J Clin Sleep Med 2015; 11: 1047–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang X, Chen H, Li Set al. Association of sleep duration with the morbidity and mortality of coronary artery disease: a meta-analysis of prospective studies. Heart Lung Circ 2015; 24: 1180–1190 [DOI] [PubMed] [Google Scholar]
- 9. Shen X, Wu Y, Zhang D. Nighttime sleep duration, 24-hour sleep duration and risk of all-cause mortality among adults: a meta-analysis of prospective cohort studies. Sci Rep 2016; 6: 21480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. García-Perdomo HA, Zapata-Copete J, Rojas-Cerón CA. Sleep duration and risk of all-cause mortality: a systematic review and meta-analysis. Epidemiol Psychiatr Sci 2018; 28: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McMullan CJ, Curhan GC, Forman JP. Association of short sleep duration and rapid decline in renal function. Kidney Int 2016; 89: 1324–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamamoto R, Shinzawa M, Isaka Yet al. Sleep quality and sleep duration with CKD are associated with progression to ESKD. Clin J Am Soc Nephrol 2018; 13: 1825–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matsuo S, Imai E, Horio Met al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992 [DOI] [PubMed] [Google Scholar]
- 14. Matsushita K, Chen J, Sang Yet al. Risk of end-stage renal disease in Japanese patients with chronic kidney disease increases proportionately to decline in estimated glomerular filtration rate. Kidney Int 2016; 90: 1109–1114 [DOI] [PubMed] [Google Scholar]
- 15. Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members . Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158: 825. [DOI] [PubMed] [Google Scholar]
- 16. Kobayashi D, Takahashi O, Deshpande GAet al. Association between weight gain, obesity, and sleep duration: a large-scale 3-year cohort study. Sleep Breath 2012; 16: 829–833 [DOI] [PubMed] [Google Scholar]
- 17. Kobayashi D, Takahashi O, Deshpande GAet al. Relation between metabolic syndrome and sleep duration in Japan: a large scale cross-sectional study. Intern Med 2011; 50: 103–107 [DOI] [PubMed] [Google Scholar]
- 18. WHO Expert Consultation . Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363: 157–163 [DOI] [PubMed] [Google Scholar]
- 19. Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int 2014; 85: 49–61 [DOI] [PubMed] [Google Scholar]
- 20. Wang M. Generalized estimating equations in longitudinal data analysis: a review and recent developments. Adv Stat 2014; 2014: 1–11 [Google Scholar]
- 21. Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med 2010; 71: 1027–1036 [DOI] [PubMed] [Google Scholar]
- 22. Lane JM, Vlasac I, Anderson SGet al. Genome-wide association analysis identifies novel loci for chronotype in 100,420 individuals from the UK Biobank. Nat Comm 2016; 7: 10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Motohashi H, Tahara Y, Whittaker DSet al. The circadian clock is disrupted in mice with adenine-induced tubulointerstitial nephropathy. Kidney Int 2020; 97: 728–740 [DOI] [PubMed] [Google Scholar]
- 24. Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest 2014; 146: 1387–1394 [DOI] [PubMed] [Google Scholar]
- 25. Song Q, Liu X, Hu Wet al. Long sleep duration is an independent risk factor for incident atrial fibrillation in a chinese population: a prospective cohort study. Sci Rep 2017; 7: 3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matsubayashi H, Nagai M, Dote Ket al. Long sleep duration and cardiovascular disease: Associations with arterial stiffness and blood pressure variability. J Clin Hypertens 2021; 23: 496–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jike M, Itani O, Watanabe Net al. Long sleep duration and health outcomes: A systematic review, meta-analysis and meta-regression. Sleep Med Rev 2018; 39: 25–36 [DOI] [PubMed] [Google Scholar]
- 28. Lauderdale DS, Knutson KL, Yan LLet al. Self-reported and measured sleep duration: how similar are they? Epidemiology 2008; 19: 838–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Levey AS, Inker LA, Matsushita Ket al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis 2014; 64: 821–835 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.