ABSTRACT
Background
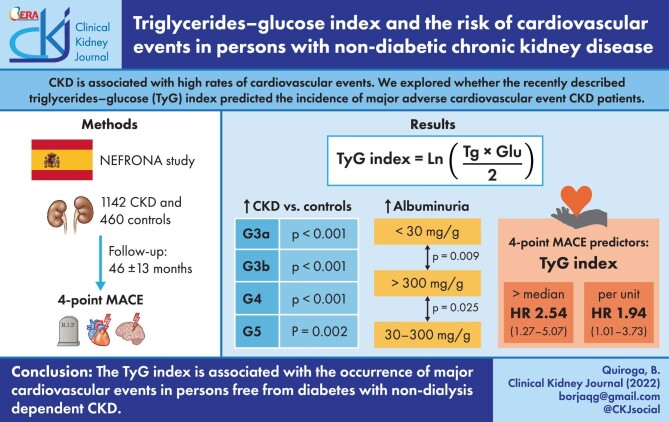
Chronic kidney disease (CKD) is associated with high rates of cardiovascular events. We here explored whether the recently described triglycerides–glucose index (TyG) predicted the incidence of major adverse cardiovascular events (MACE) in these patients.
Methods
This observationa study was undertaken of 1142 persons with CKD and free from diabetes and 460 controls from the prospective NEFRONA study. The study exposure was the TyG index at cohort inclusion. The study outcome was MACE (cardiovascular death, nonfatal myocardial infarction, nonfatal stroke and hospitalization for unstable angina). Covariates included demographics, comorbidities, lipid profile, renal function and glycaemic control. Cox regression models evaluated the association between TyG index and 4-point MACE in patients with CKD.
Results
TyG was higher [median 8.63 (interquartile range 8.32–8.95)] in patients with CKD compared with controls (P < 0.001). TyG increased across albuminuria categories but was similar for glomerular filtration rate categories among patients with CKD stages 3–5. During 46 ± 13 months of follow-up, 49 (4.3%) MACE were registered. TyG predicted the occurrence of MACE {hazard ratio (HR) 1.95 [95% confidence interval (CI) 1.11–3.40] per TyG unit increase; and HR 2.29 (95% CI 1.24–4.20] for TyG values above the median of 8.63 units}. Sensitivity analysis for subgroups of participants according to age, kidney function, body mass index and imaging evidence of atherosclerosis yielded similar results, as did adjusted analysis. Neither triglycerides nor glucose alone was associated with MACE.
Conclusions
The TyG index is associated with the occurrence of major cardiovascular events in persons free from diabetes with non-dialysis dependent CKD.
Keywords: CKD, dyslipidemia, MACE, triglycerides–glucose index
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Patients with chronic kidney disease (CKD) have an increased risk of major adverse cardiovascular events (MACE). Cardiovascular morbi-mortality increases as CKD progresses [1]. The increased cardiovascular risk has been attributed to the concomitant prevalence of traditional (such as dyslipidemia, hypertension, diabetes mellitus, etc.) and non-traditional (uraemia, inflammation, oxidative stress, etc.) risk factors [2].
Abnormalities in lipids are common in patients with CKD, and statins (w/wo ezetimibe) decrease cardiovascular events in them [3]. High levels of low-density lipoprotein cholesterol (LDL-C) have been also associated with high risk of cardiovascular events [4], but the role of other lipids in predicting cardiovascular disease (CVD) risk is less studied. Triglycerides levels are high in CKD, especially in advanced stages. However, and in contrast to LDL-C or high-density lipoprotein cholesterol (HDL-C), evidence on the relation between triglycerides and cardiovascular prognosis is heterogeneous [5, 6]. Treatment with classical drugs for hypertriglyceridemia [i.e. peroxisome proliferator-activated receptor α (PPARα) agonists] have yielded a beneficial effect on cardiovascular prognosis in patients with CKD [7]. However, PPARα agonist may exert adverse events (increase in creatinine, myopathy or rhabdomyolysis) that limit their use in these patients. Indeed, the threshold for starting a triglyceride-lowering treatment has not been established, and the benefit seems to be specific to high-risk populations [6].
The recently described triglyceride–glucose index (TyG) may predict cardiovascular events in non-CKD populations. Specifically, TyG has been independently associated with the incidence of coronary artery disease and stroke in high-risk and in atherosclerosis-free populations [8]. Additionally, one study showed that TyG predicts the incidence of CKD in the general population [9]. In patients with CKD, sporadic studies have associated higher TyG index values with the progression to end-stage kidney disease [10]. In one study of patients undergoing peritoneal dialysis patients, TyG was associated with the risk of cardiovascular death [11].
To the best of our knowledge, no studies have explored the possible value of TyG in identifying patients with non-dialysis dependent CKD at higher risk of MACE, and this was the objective of our study.
MATERIALS AND METHODS
Subjects
NEFRONA is a prospective multicentric cohort which between 2010 and 2012 enrolled 3004 persons from 81 Spanish hospitals: 2445 of them were classified as having CKD stages 3–5 with glomerular filtration rate (GFR) <60 mL/min/1.73 m2 at inclusion estimated using the 4-variable Modification of Diet in Renal Disease (MDRD) equation [12]. The remaining 559 individuals were classified as controls. The control group included adults with GFR >60 mL/min/1.73 m2 without any evidence of kidney damage. Controls were recruited from primary care centres, as described in Arroyo et al. [13]. NEFRONA excluded CKD or control participants who had active infections, pregnancy, history of cardiovascular disease or organ transplant [13].
For this study, we excluded patients and controls with diabetes mellitus, leaving 1142 patients with non-dialysis CKD and 460 controls, free from diabetes or history of cardiovascular events (Figure 1). Although the inclusion criteria for controls was an estimated GFR (eGFR) >60 mL/min/1.73 m2, 16 patients were reclassified as stage 2 CKD as they had positive albuminuria at baseline.
Figure 1:
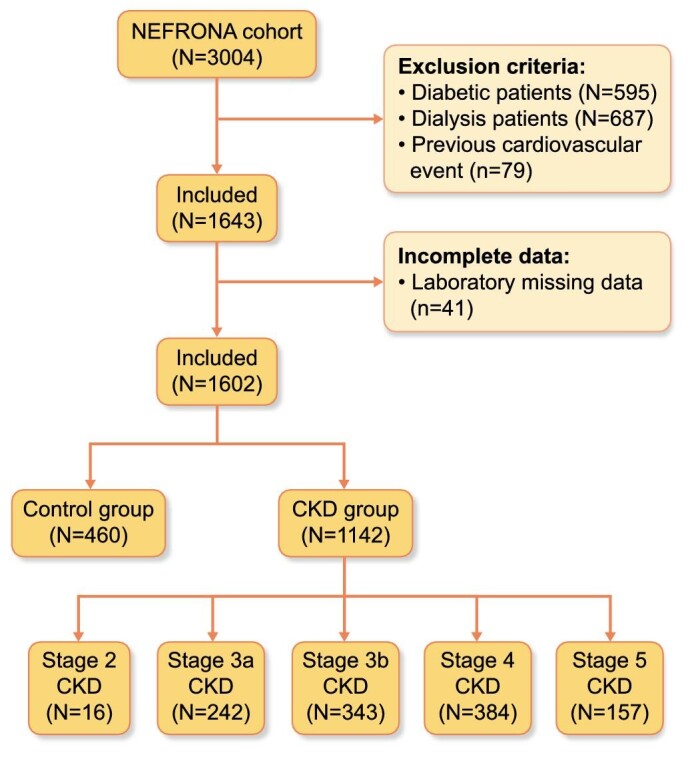
Study flow chart. CKD: chronic kidney disease.
Exposure
The exposure is TyG calculated at study inclusion, with both measurements performed in fasting conditions and calculated as Ln (fasting TG [mg/dL] × fasting blood glucose [mg/dL]/2) [14].
Covariates
At inclusion, demographic data (sex and birth date) and classical cardiovascular risk factors [body mass index (BMI), smoking habits, hypertension and dyslipidemia] were collected. In patients with CKD, aetiology of the renal disease was registered if available in clinical registries. Renal [eGFR and urinary albumin–creatinine ratio (uACR)], lipid (LDL-C, HDL-C, triglycerides, total cholesterol) and metabolic [fasting glucose and glycated haemoglobin (Hb1Ac)] laboratory values were also obtained at baseline. CKD stages and uACR categories were established according to Kidney Disease: Improving Global Outcomes (KDIGO) guidelines [15]. Dyslipidemia was defined according to current guidelines at the moment of the protocol approval [16]. Atherosclerosis was evaluated as described previously [17]. Briefly, based on carotid ultrasound and ankle-brachial index performed at baseline, an atherosclerosis score (AS) was defined in four categories. AS = 0 corresponded to the absence of atherosclerosis, AS = 1 to mild atherosclerosis, AS = 2 to moderate atherosclerosis and AS = 3 to severe atherosclerosis.
Outcomes
Patients were followed for the occurrence of MACE, defined as the composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke and hospitalization for unstable angina.
Ethical concerns
The study was approved by each local ethics committee and subjects were included after providing informed consent.
Statistics
Data are expressed as mean (standard deviation) or median (IQR) depending on the distribution as assessed with the Kolgomorov–Smirnov test. First, we identified covariates associated with higher values of the TyG index (using the median as cutoff). Considering the low number of total events (4-point MACE), we used median TyG index values (8.63) to categorize the population, as it was close to the cutoff of 8.60 units, identified by the Youden’s J statistics as the threshold with best performance in terms of sensitivity and specificity for this outcome. The comparison with other qualitative variables was performed with chi-squared or Fisher test depending on their parametric characteristics. Inference between TyG index and dichotomic quantitative variables was performed using t-test or Mann–Whitney, and for non-dichotomic quantitative variables using ANOVA or Kruskal–Wallis tests. The Spearman test was used for correlations of the TyG index as a continuous variable with other quantitative variables.
Next, we assessed factors associated with the risk of MACE using Cox regression. Adjusted models for the independent association between the TyG index (as a dichotomic or as a continuous variable) and MACE were constructed based on modifier effect variables and confounders. To avoid overfitted models, the number of variables in each model was not higher than the total number of events divided by 10. The statistical analysis was performed using SPSS version 26.0 (IBM Corp., Armonk, NY). Plots were drawn using GraphPad Prism version 9.02 (GraphPad 155 Holdings, LLC, San Diego, CA, USA).
RESULTS
Baseline characteristics
We included 1142 patients with CKD, with a mean age of 59 ± 12 years and 40% (458) of them being women. Their median eGFR was 31 (20–44) mL/min/1.73 m2 and uACR 91 (11–392) mg/g. Sixteen patients (1%) had stage 2, 242 (21%) had stage 3a, 343 (30%) had stage 3b, 384 (34%) had stage 4 and 147 (14%) had stage 5 CKD. Among the 670 patients with registered urinary albumin-to-creatinine ratio data, 235 (21%) has <30 mg/g (A1), 243 (21%) has 30–300 mg/g (A2) and 192 (17%) has >300 mg/g (A3). The main CKD aetiologies were hypertensive (25%) and glomerular (19%). Based on the AS, 170 (20%) of the patients with CKD did not have atherosclerosis, 127 (14%) had mild atherosclerosis, 547 (63%) had moderate atherosclerosis and 30 (3%) had severe atherosclerosis (Table 1), although none had had a prior MACE. Baseline characteristics of CKD and control patients are shown in Supplementary data, Table S1.
Table 1.
Baseline characteristics of included participants with CKD according to the median triglycerides–glucose index value
| Overall (n = 1142) | TyG < 8.63 (n = 571) | TyG ≥ 8.63 (n = 571) | Pa | |
|---|---|---|---|---|
| Sex (women, %) | 458 (40) | 257 (45) | 201 (35) | 0.001 |
| Age (years) | 59 ± 12 | 58 ± 13 | 59 ± 11 | 0.498 |
| BMI (kg/m2) | 28 ± 5 | 28 ± 5 | 29 ± 5 | <0.001 |
| Smoking habits (n, %) Non-smoker Former smoker Current smoker |
551 (48) 409 (36) 222 (19) |
279 (49) 206 (36) 86 (15) |
232 (41) 203 (36) 136 (24) |
<0.001 |
| Hypertension (n, %) | 1096 (96) | 540 (95) | 556 (97) | 0.023 |
| Dyslipidemia (n, %) | 765 (67) | 331 (58) | 434 (76) | <0.001 |
| eGFR (mL/min/1.73 m2) | 31 (20–44) | 31 (20–44) | 31 (20–43) | 0.912 |
| uACR (mg/g) (n = 670) | 91 (11–392) | 66 (10–300) | 118 (12–466) | 0.041 |
| CKD G category (n, %): 2 3a 3b 4 5 |
16 (1) 242 (21) 343 (30) 384 (34) 157 (14) |
12 (2) 118 (21) 173 (30) 180 (32) 88 (15) |
4 (1) 124 (22) 170 (30) 204 (35) 69 (12) |
0.093 |
| CKD aetiology (n, %) Glomerular Hypertensive Tubulointerstitial Hereditary Unknown Other |
219 (19) 286 (25) 161 (14) 144 (13) 139 (12) 193 (17) |
106 (20) 121 (21) 93 (16) 82 (14) 77 (13) 92 (16) |
113 (20) 165 (29) 68 (12) 62 (11) 62 (11) 101 (17) |
0.008 |
| Total cholesterol (mg/dL) | 185 (162–211) | 176 (157–200) | 192 (168–217) | <0.001 |
| LDL-C (mg/dL) | 107 (88–129) | 104 (85–124) | 111 (90–134) | <0.001 |
| HDL-C (mg/dL) | 50 (41–61) | 54 (46–66) | 45 (37–53) | <0.001 |
| Triglycerides (mg/dL) | 118 (163–89) | 89 (73–104) | 162 (137–202) | <0.001 |
| ASb (n, %) AS = 0 AS = 1 AS = 2 AS = 3 |
170 (20) 127 (14) 547 (63) 30 (3) |
99 (23) 65 (15) 258 (59) 13 (3) |
71 (16) 62 (14) 289 (66) 17 (4) |
0.073 |
| Fasting glucose (mg/dL) | 94 (87–102) | 91 (85–99) | 97 (90–105) | <0.001 |
| Lipid-lowering therapies (n, %) Statins Ezetimibe Fibrates |
686 (60) 51 (5) 58 (5) |
314 (55) 17 (3) 14 (3) |
372 (65) 34 (6) 44 (8) |
<0.0010.021<0.001 |
| Hb1Ac (%) | 5.5 ± 0.4 | 5.5 ± 0.4 | 5.6 ± 0.4 | 0.009 |
| TyG index | 8.63 (8.32–8.95) | 8.32 (8.11–8.48) | 8.95 (8.78–9.19) | <0.001 |
Data are displayed as mean ± standard deviation or median (IQR).
TyG: triglycerides–glucose index; BMI: body mass index; eGFR: estimated glomerular filtration rate; uACR: urinary albumin–creatinine ratio; CKD: chronic kidney disease; Hb1Ac: glycated haemoglobin; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; AS: atherosclerotic score.
a P-value for the comparison between patients with CKD according to TyG median.
bBased on carotid ultrasound and ankle-brachial index.
Factors associated with the triglycerides and glucose index
TyG was 8.63 (8.32–8.95) in patients with CKD, a median concentration significantly higher than in controls [8.38 (8.05–8.80)] (P < 0.001), but we did not observe differences across CKD stages (Figure 2). TyG differed across uACR categories, being higher in patients with macroalbuminuria versus microalbuminuria (P = 0.025) or normoalbuminuria (P = 0.009) (Figure 3). Among patients with CKD, 874 had a registered AS. TyG was lower in patients with absence of atherosclerosis (AS = 0) compared with pathological AS (AS ≥ 1) [8.55 (8.24–8.89) versus 8.66 (8.33–8.97)] (P = 0.016).
Figure 2:
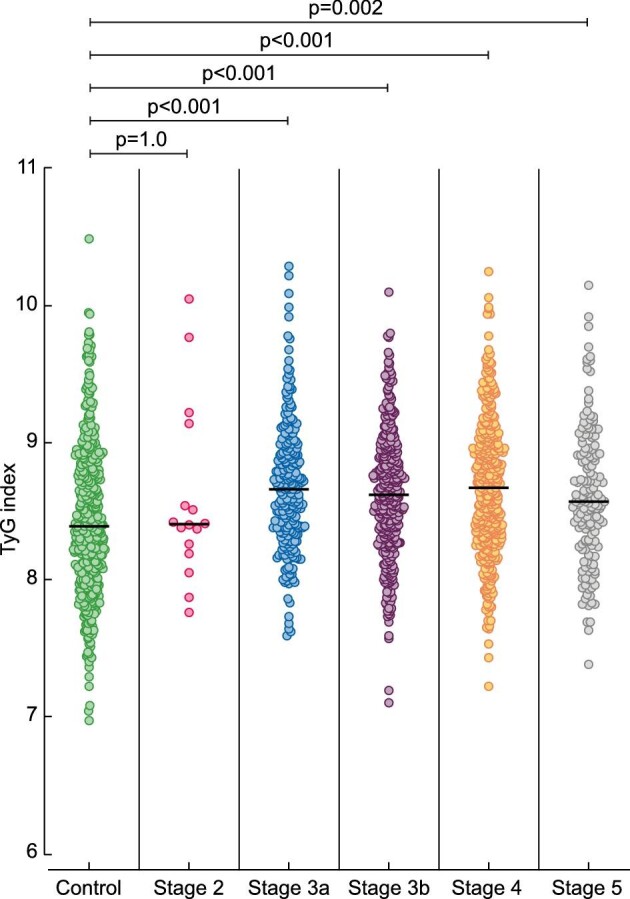
TyG levels in controls and in patients with CKD stages 2–5.
Figure 3:
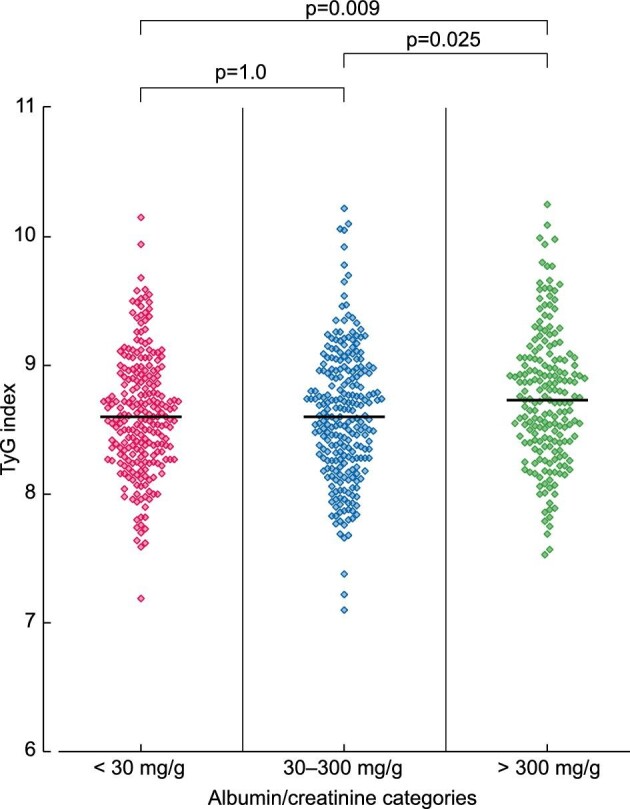
TyG levels according to urinary albumin-to-creatinine ratio categories (n = 670).
TyG was categorized based on the median value (8.63). Patients with elevated TyG were more often men, smokers, with higher BMI, prevalence of hypertension, dyslipidemia and higher Hb1Ac (Table 1). In univariate analyses, TyG showed weak but significant correlations with total cholesterol, LDL-C, HDL-C, fasting glucose, Hb1Ac and uACR, and a strong expected correlation with triglycerides (Table 2). There was no association with eGFR. Similarly, in the control group, TyG correlated to total cholesterol, LDL-C, HDL-C, fasting glucose, Hb1Ac and triglycerides (Supplementary data, Table S2).
Table 2.
Univariate correlation between TyG and glucose, renal and lipid parameters in patients with CKD (n = 1142)
| ρ (Spearman) | P | |
|---|---|---|
| Total cholesterol (mg/dL) | 0.204 | <0.001 |
| LDL-C (mg/dL) | 0.089 | 0.090 |
| HDL-C (mg/dL) | −0.423 | <0.001 |
| Triglycerides (mg/dL) | 0.962 | <0.001 |
| Fasting glucose (mg/dL) | 0.328 | <0.001 |
| Hb1Ac (%) | 0.150 | 0.002 |
| eGFR (mL/min/1.73 m2) | 0.001 | 0.971 |
| uACR (mg/g) (n = 670) | 0.109 | 0.005 |
Correlations were assessed using Spearman test. LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; uACR: urinary albumin–creatinine ratio.
TyG and the risk of MACE
During median 46 ± 13 months of follow-up, 49 (4.3%) patients with CKD suffered from MACE, explained by stroke (37% of cases), unstable angina (24%), nonfatal myocardial infarction (27%) and cardiovascular death (12%).
Univariate analysis showed that age, BMI, eGFR, HDL-C, fasting glucose, AS, TyG (continuous variable) and TyG ≥8.63 were associated with the risk of MACE, while triglycerides or Hb1Ac were not (Table 3).
Table 3.
Univariate Cox regression model depicting HR and 95% CI of baseline covariates associated with the risk of developing MACE in patients with CKD (n = 1142)
| HR (95% CI) | P | |
|---|---|---|
| Sex (women) | 0.69 (0.38–1.26) | 0.231 |
| Age (per year) | 1.06 (1.03–1.09) | 0.001 |
| BMI (per kg/m2) | 1.08 (1.03–1.13) | 0.002 |
| Smoking habit (current smoker) | 1.38 (0.64–2.98) | 0.412 |
| Hypertension (yes) | 21.3 (0.4–11 899.5) | 0.342 |
| Dyslipidemia (yes) | 1.47 (0.77–2.83) | 0.241 |
| eGFR (per mL/min/1.73m2) | 0.97 (0.95–0.99) | 0.010 |
| uACR (per mg/g) (n = 670) | 1.00 (1.00–1.00) | 0.065 |
| Total cholesterol (per mg/dL) | 1.00 (0.99–1.01) | 0.663 |
| LDL-C (per mg/dL) | 1.00 (0.99–1.01) | 0.965 |
| HDL-C (per mg/dL) | 0.97 (0.95–0.99) | 0.033 |
| Triglycerides (per mg/dL) | 1.00 (1.00–1.00) | 0.099 |
| Statins (yes) | 1.35 (0.75–2.46) | 0.319 |
| AS (per 1 point) | 1.97 (1.21–3.21) | 0.006 |
| Fasting glucose (per mg/dL) | 1.02 (1.00–1.05) | 0.048 |
| Hb1Ac (per 1%) | 0.47 (0.17–1.25) | 0.129 |
| TyG (per unit) | 1.95 (1.11–3.40) | 0.018 |
| TyG ≥ 8.63 | 2.29 (1.24–4.20) | 0.008 |
Univariate regression Cox analysis for 4-point MACE as a dependent variable. TyG: triglycerides–glucose index; HR: hazard ratio; 95% CI: 95% confidence interval; uACR: urinary albumin–creatinine ratio; TyG = Ln (TG [mg/dL] × glucose [mg/dL]/2).
In multivariable-adjusted Cox regression, TyG (per unit of increase) and TyG ≥8.63 (median value) maintained their independent predictive value (Table 4). Sensitivity analysis showed absence of interaction between TyG and age, AS, BMI and eGFR (P > 0.05 for all). Subgroup analyses suggested similar association across strata of age, sex, presence of AS and eGFR (Supplementary data, Table S3). Survival curves depict a clear separation for patients below and above the median TyG (Figure 4).
Table 4.
Association between TyG, triglycerides and fasting glucose to 4-point MACE in patients with CKD (n = 1142)
| Unadjusted MACE 4-pt | Model 1a | Model 2b | Model 3c | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| TyG (per unit increase) | 1.95 (1.11–3.40) | 0.018 | 2.14 (1.18–3.90) | 0.012 | 2.01 (1.09–3.70) | 0.025 | 1.94 (1.01–3.73) | 0.046 |
| TyG ≥ 8.63 (median value) | 2.29 (1.24–4.20) | 0.008 | 2.22 (1.21–4.09) | 0.010 | 2.09 (1.13–3.88) | 0.019 | 2.54 (1.27–5.07) | 0.008 |
| Glucose (per mg/dL) | 1.02 (1.00–1.05) | 0.048 | 1.02 (0.99–1.04) | 0.246 | 1.01 (0.99–1.04) | 0.308 | 1.01 (0.98–1.03) | 0.608 |
| Triglycerides (per mg/dL) | 1.00 (1.00–1.00) | 0.099 | 1.00 (1.00–1.01) | 0.021 | 1.00 (1.00–1.01) | 0.040 | 1.00 (0.99–1.01) | 0.143 |
The association of each parameter [TyG (per unit increase), TyG (≥8.63), glucose and triglycerides] to 4-point MACE is assessed separately in three different adjusted models.
TyG: triglycerides–glucose index; HR: hazard ratio; 95% CI: 95% confidence interval. TyG = Ln (TG [mg/dL] × glucose [mg/dL]/2).
aModel 1: Cox regression adjusted for age and gender.
bModel 2: Cox regression adjusted for age, sex, hypertension and dyslipidemia.
cModel 3. Cox regression adjusted for age, sex, hypertension and atherosclerotic score.
Figure 4:
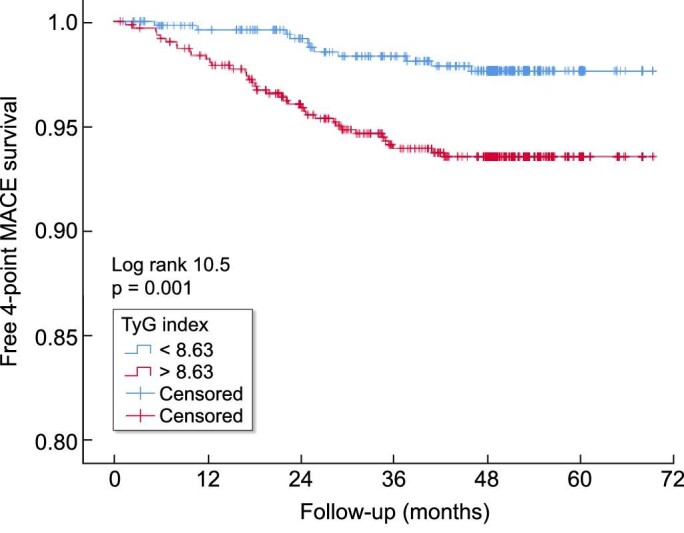
MACE-free survival curves according to median TyG. TyG: triglycerides-glucose index; MACE: major adverse cardiovascular events.
DISCUSSION
Special features on lipid metabolism in patients with CKD may contribute to atherogenesis. This may render monitoring for high LDL-C and low HDL-C levels insufficient to correctly assess cardiovascular risk. As an example, lipoprotein size and concentration have been established as stronger predictors for adverse outcomes in CKD [18]. However, they cannot be assessed in routine clinical practice in most centres [19]. NEFRONA recruited persons with no previous history of CVD and free from diabetes, rendering a low-risk population. Still, in our study, we observe a strong direct association between TyG and the risk of MACE in a large population of persons with CKD stages 3–5 not requiring dialysis and free from diabetes. We should highlight that this risk association was not observed for either triglycerides or glucose alone. This easy-to-measure index may perhaps then be relevant for risk stratification in this population.
Although triglycerides are the most frequent abnormality in CKD [20], their role in atherogenesis and, consequently, in cardiovascular prognosis is not well known. Population-based studies have shown that higher triglyceride values are associated with an increased cardiovascular risk [21]. However, the heterogeneous results in trials of triglyceride-lowering therapies on hard outcomes, and the lack of specific therapies (beyond fibrates) and consensus-threshold levels for starting triglyceride-lowering therapy has led to therapeutic nihilism in dyslipidemia guidelines [22]. Beyond the efficacy of interventions, other factors may contribute to the lack of positive results in patients treated with triglyceride-lowering therapies. On one hand, the initiation of these drugs in CKD could be late as triglycerides can promote atherosclerotic plaques even with normal lipid parameters [23]. On the other hand, the laboratory parameters to assess the relationship between triglyceride metabolism and cardiovascular events may be suboptimal [24]. In this regard, TyG can be considered as a new biomarker for identifying patients that might benefit from early intervention and be used to enrich future clinical trials of patients at high CV risk.
Our study extends to persons with CKD in previous population-based studies [25] showing the usefulness of TyG in predicting subclinical atherosclerosis. In our cohort, TyG was associated with early evidence of subclinical atherosclerosis (assessed with the AS) as well as other cardiovascular risk factors including albuminuria [26]. The addition of new biomarkers for stratifying patient risk that can be easily measured in clinical practice could help in improving cardiovascular prognosis with the prescription of highly effective protecting drugs. TyG can easily and automatically be calculated from routine lab analysis and presented in lab reports. Interestingly, recent observational studies have shown that TyG can respond to interventions, being reduced upon initiation of treatment with sodium–glucose cotransporter 2 (SGLT2) inhibitors [27]. SGLT2 inhibitors provide impressive renal and cardiovascular beneficial effects, beyond the glycaemic control, in patients with diabetes and free from diabetes with CKD or heart failure [28]. In the field of lipoprotein-lowering therapies, proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) reduce cardiovascular events in patients with history of atherosclerotic disease [29] and regress atheroma plaques, a very prevalent condition in patients with CKD [30]. We speculate that linking early detection of atheroma plaques with easily measured biomarkers in CKD should change the concept of prescribing new and potent drugs that reduce cardiovascular risk only in advanced stages. In an observational study [31], PCSK9i reduced TyG in patients with familial hypercholesterolemia. Obviously, before TyG can be used to guide clinical decisions, validation of its usefulness in interventional studies or trials is needed.
Several limitations should be acknowledged in the interpretation of our findings: the observational design has inherent biases that preclude causality in the associations observed. The population was of low risk with no history of CVD, which may reduce confounding in the association between TyG and outcomes, but at the same time can limit representativeness. Because of this low risk, the incidence of MACE was low (49 events, 4.3%) and this conditioned the number of variables we could use to adjust our models to avoid overfitting. Finally, our study is based on one single TyG measurement and the potential cardiovascular implications of changes in TyG (for example, in response to changes in lifestyle or drug therapy) over time require further investigation.
CONCLUSION
We show TyG strongly associates with the risk of MACE in a population with non-dialysis CKD at low CV risk. Because TyG is an easily measured and inexpensive index, we propose that its routine evaluation could help risk-stratify patients and inform the choice of prevention strategies for primary CVD prevention.
Supplementary Material
ACKNOWLEDGEMENTS
J.J.C. is supported by the Swedish Research Council (#2019-01059), the Swedish Heart and Lung Foundation (#20190587) and the Westman Foundation.
Contributor Information
Borja Quiroga, IIS-La Princesa, Nephrology Department, Hospital Universitario de la Princesa, Madrid, Spain.
Patricia Muñoz Ramos, Nephrology Department, Hospital Universitario Infanta Leonor, Madrid, Spain.
Ana Sánchez Horrillo, IIS-La Princesa, Nephrology Department, Hospital Universitario de la Princesa, Madrid, Spain.
Alberto Ortiz, IIS-Fundación Jimenez Diaz, School of Medicine, Universidad Autónoma de Madrid, Fundación Renal Iñigo Alvarez de Toledo-IRSIN, REDinREN, Instituto de Investigación Carlos III, Madrid, Spain.
José Manuel Valdivielso, Vascular and Renal Translational Research Group, UDETMA, REDinREN del ISCIII, IRBLleida, Lleida, Spain.
Juan Jesús Carrero, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden.
FUNDING
The NEFRONA study was funded by a research grant from AbbVie and promoted by the Spanish Society of Nephrology (S.E.N.).
AUTHORS’ CONTRIBUTIONS
B.Q. obtained the data, conceptualized the study and wrote the manuscript. P.M.R. obtained the data and wrote the manuscript. A.S.H. obtained the data and revised the manuscript. A.O. conceptualized the study, performed statistical analysis and wrote the manuscript. J.M.V. obtained the data and revised the manuscript. J.J.C. conceptualized the study, performed statistical analysis and revised the manuscript.
CONFLICT OF INTEREST STATEMENT
B.Q. has received honoraria for conferences, consulting fees and advisory boards from Vifor-Pharma, Astellas, Amgen, Bial, Ferrer, Novartis, AstraZeneca, Sandoz, Laboratorios Bial, Esteve, Sanofi-Genzyme and Otsuka. A.O. has received consultancy or speaker fees or travel support from Astellas, AstraZeneca, Amicus, Amgen, Fresenius Medical Care, Bayer, Sanofi-Genzyme, Menarini, Kyowa Kirin, Alexion, Otsuka and Vifor Fresenius Medical Care Renal Pharma, and is the director of the Catedra Mundipharma-UAM of diabetic kidney disease and the Catedra AstraZeneca-UAM of CKD and electrolytes. A.O. is the editor-in-chief of ckj. None declared for P.M.R., J.M.V., A.S.H. and J.J.C.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
REFERENCES
- 1. Levin A, Djurdjev O, Barrett Bet al. . Cardiovascular disease in patients with chronic kidney disease: getting to the heart of the matter. Am J Kidney Dis 2001; 38: 1398–1407 [DOI] [PubMed] [Google Scholar]
- 2. Quiroga B, Verdalles Ú, Reque Jet al. . Cardiovascular events and mortality in chronic kidney disease (stages I-IV). Nefrologia 2013; 33: 539–545 [DOI] [PubMed] [Google Scholar]
- 3. Baigent C, Landray MJ, Reith Cet al. . The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet North Am Ed 2011; 377: 2181–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tonelli M, Muntner P, Lloyd Aet al. . Association between LDL-C and risk of myocardial infarction in CKD. J Am Soc Nephrol 2013; 24: 979–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bermudez-Lopez M, Forne C, Amigo Net al. . An in-depth analysis shows a hidden atherogenic lipoprotein profile in non-diabetic chronic kidney disease patients. Expert Opin Ther Targets 2019; 23: 619–630 [DOI] [PubMed] [Google Scholar]
- 6. Bajaj A, Xie D, Cedillo-Couvert Eet al. . Lipids, apolipoproteins, and risk of atherosclerotic cardiovascular disease in persons with CKD. Am J Kidney Dis 2019; 73: 827–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jun M, Zhu B, Tonelli Met al. . Effects of fibrates in kidney disease: a systematic review and meta-analysis. J Am Coll Cardiol 2012; 60: 2061–2071 [DOI] [PubMed] [Google Scholar]
- 8. Ding X, Wang X, Wu Jet al. . Triglyceride-glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta-analysis of cohort studies. Cardiovasc Diabetol 2021; 20: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okamura T, Hashimoto Y, Hamaguchi M. et al. Triglyceride-glucose index is a predictor of incident chronic kidney disease: a population-based longitudinal study. Clin Exp Nephrol 2019; 23: 948–955 [DOI] [PubMed] [Google Scholar]
- 10. Fritz J, Brozek W, Concin Het al. . The triglyceride–glucose index and obesity-related risk of end-stage kidney disease in Austrian adults. JAMA Network Open 2021; 4: e212612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yan Z, Yu D, Cai Yet al. . Triglyceride glucose index predicting cardiovascular mortality in Chinese initiating peritoneal dialysis: a cohort study. Kidney Blood Press Res 2019; 44: 669–678 [DOI] [PubMed] [Google Scholar]
- 12. Levey AS, Bosch JP, Lewis JBet al. . A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461–470 [DOI] [PubMed] [Google Scholar]
- 13. Arroyo D, Betriu A, Martinez-Alonso Met al. . Observational multicenter study to evaluate the prevalence and prognosis of subclinical atheromatosis in a Spanish chronic kidney disease cohort: baseline data from the NEFRONA study. BMC Nephrol 2014; 15: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F.. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord 2008; 6: 299–304 [DOI] [PubMed] [Google Scholar]
- 15. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150. [Google Scholar]
- 16. K/DOQI Group . K/DOQI clinical practice guidelines for managing dyslipidemia in chronic kidney disease. Am J Kidney Dis 2003; 41: S1–S9212751048 [Google Scholar]
- 17. Junyent M, Martínez M, Borràs Met al. . Predicting cardiovascular disease morbidity and mortality in chronic kidney disease in Spain. The rationale and design of NEFRONA: a prospective, multicenter, observational cohort study. BMC Nephrol 2010; 11: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bermúdez-López M, Arroyo D, Betriu Àet al. . New perspectives on CKD-induced dyslipidemia. Expert Opin Ther Targets 2017; 21: 967–976 [DOI] [PubMed] [Google Scholar]
- 19. Theofilis P, Vordoni A, Koukoulaki Met al. . Dyslipidemia in chronic kidney disease: contemporary concepts and future therapeutic perspectives. Am J Nephrol 2021; 52: 693–701 [DOI] [PubMed] [Google Scholar]
- 20. Chan DT, Dogra GK, Irish ABet al. . Chronic kidney disease delays VLDL-apoB-100 particle catabolism: potential role of apolipoprotein C-III. J Lipid Res 2009; 50: 2524–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lamprea-Montealegre JA, Staplin N, Herrington WGet al. ; SHARP Collaborative Group . Apolipoprotein B, triglyceride-rich lipoproteins, and risk of cardiovascular events in persons with CKD. Clin J Am Soc Nephrol 2020; 15: 47–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kidney Disease: Improving Global Outcomes (KDIGO) Lipid Work Group . KDIGO clinical practice guideline for lipid management in chronic kidney disease. Kidney Int Suppl 2013; 3: 259–305 [Google Scholar]
- 23. Fernández-Friera L, Fuster V, López-Melgar Bet al. . Normal LDL-cholesterol levels are associated with subclinical atherosclerosis in the absence of risk factors. J Am Coll Cardiol 2017; 70: 2979–2991 [DOI] [PubMed] [Google Scholar]
- 24. Bermúdez-López M, Betriu À, Valdivielsoa JMet al. . Beyond the traditional lipid parameters in chronic kidney disease. Más allá de los parámetros lipídicos tradicionales en la enfermedad renal crónica. Nefrología (English Edition) 2018; 38: 109–113 [DOI] [PubMed] [Google Scholar]
- 25. Lambrinoudaki I, Kazani MV, Armeni Eet al. . The TyG index as a marker of subclinical atherosclerosis and arterial stiffness in lean and overweight postmenopausal women. Heart Lung Circ 2018; 27: 716–724 [DOI] [PubMed] [Google Scholar]
- 26. Lu YW, Chang CC, Chou RHet al. . Gender difference in the association between TyG index and subclinical atherosclerosis: results from the I-Lan Longitudinal Aging Study. Cardiovasc Diabetol 2021; 20: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Imre E, Gunhan HG, Erel Pet al. . SGLT2 inhibitors improve plasma atherogenic biomarkers in patients with type 2 diabetes: a real world retrospective observational study. Minerva Endocrinol 2021; [in press] [DOI] [PubMed] [Google Scholar]
- 28. Zelniker TA, Wiviott SD, Raz Iet al. . SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet North Am Ed 2019; 393: 31–39 [DOI] [PubMed] [Google Scholar]
- 29. Ma W, Guo X, Ma Yet al. . Meta-analysis of randomized clinical trials comparing PCSK9 monoclonal antibody versus ezetimibe/placebo in patients at high cardiovascular risk. Atherosclerosis 2021; 326: 25–34 [DOI] [PubMed] [Google Scholar]
- 30. Masson W, Lobo M, Siniawski Det al. . Role of non-statin lipid-lowering therapy in coronary atherosclerosis regression: a meta-analysis and meta-regression. Lipids Health Dis 2020; 19: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scicali R, Di Pino A, Urbano Fet al. . Analysis of steatosis biomarkers and inflammatory profile after adding on PCSK9 inhibitor treatment in familial hypercholesterolemia subjects with nonalcoholic fatty liver disease: a single lipid center real-world experience. Nutr Metab Cardiovasc Dis 2021; 31: 869–879 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.