Abstract
Background
Tenofovir-diphosphate (TFV-DP) measured in dried blood spots (DBS) and tenofovir (TFV) measured in urine/plasma have been used to measure TFV-based oral pre-exposure prophylaxis (PrEP) adherence. However, there are limited data comparing these 3 metrics and their appropriate use for PrEP adherence monitoring.
Methods
We collected DBS, urine, and plasma samples from HIV-negative adults randomized to a low (2 doses/week), moderate (4 doses/week), or perfect (7 doses/week) adherence group (via directly observed therapy) of tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) for 6 weeks, followed by a 4-week washout phase. Drug concentrations were measured using liquid chromatography tandem mass spectrometry. Linear mixed-effects modeling was used to examine associations between drug concentrations and dosing time.
Results
Among 28 participants, the median age was 33 years, and 12 (43%) were female. At steady state, 25th percentile TFV-DP concentrations were 466, 779, and 1375 fmol/3 mm punch in the low, moderate, and perfect adherence group, respectively. Correlation was stronger between quantifiable TFV-DP and plasma TFV (r = 0.65; P < .01) than between TFV-DP and urine TFV (r = 0.50; P < .01). Among all participants, each additional week of cumulative dosing on average led to a mean increase of 158 fmol/3 mm punch (P < .001) in TFV-DP during the dosing phase. Each additional day after the last dose was associated with 43 fmol/3 mm punch lower TFV-DP (P = .07).
Conclusions
TFV-DP levels in DBS provide valuable insight into both dosing recency and cumulative doses from variable adherence patterns. Our observed benchmark TFV-DP concentrations were slightly higher than prior predicted estimates based on convenience samples.
Keywords: tenofovir-diphosphate, HIV, adherence, preexposure prophylaxis, tenofovir
Daily preexposure prophylaxis (PrEP) of oral tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC) reduces the risk of human immunodeficiency virus (HIV) acquisition [1] and is recommended by the World Health Organization (WHO) for high-risk individuals [2–4]. PrEP can also be taken as an event-driven (ED) regimen for men who have sex with men (MSM), offering more flexibility and convenience for people with infrequent or predictable risks [5]. The 2016 United Nations’ Declaration on Ending AIDS by 2030 included a commitment to ensure 3 million eligible people access to PrEP. PrEP use is projected to rise at an increasing speed as more countries continue to adopt and implement PrEP policies [1].
The efficacy of oral PrEP greatly depends on maintaining sufficient drug concentrations from consistent and timely medication adherence [6, 7]. Therefore, oral PrEP adherence monitoring is critical and can help identify people with suboptimal adherence who might benefit from enhanced support or intervention. Accurate measurement of oral PrEP adherence is challenging. Methods such as diaries, clinic visit attendance, self-report, pill counts, and electronic pill bottles/caps do not measure real-time drug concentrations and have only demonstrated limited accuracy and reliability [8–10]. The recent approval of the long-acting injectable cabotegravir (CAB) for HIV prevention offers a potentially easier alternative for people with demonstrated poor adherence to oral PrEP. Yet, tenofovir-based oral PrEP regimens remain the recommended HIV prevention approach for high-risk populations, particularly in resource-limited areas [11].
Direct pharmacological measurement of drug levels in biological samples is more objective but often difficult to interpret regarding the relationship between efficacy, adherence, and drug concentrations. Tenofovir-diphosphate (TFV-DP) accumulates appreciably in red blood cells with repeated daily doses and has a long half-life of 17 days in this cellular compartment [12]. Measurement of TFV-DP in dried blood spots (DBS) is useful to assess cumulative patterns of adherence over longer time periods. High concentrations of TFV-DP measured in DBS (>700 fmol/punch) were found to be associated with substantial reduction in HIV infection risks [13]. These “benchmarks” of TFV-DP levels in DBS were simulated using statistical models based on samples conveniently collected without regard to time since last dose taken and were mostly from populations where Asians and/or women were underrepresented [12, 14]. Additional data defining adherence benchmarks for different cumulative dosing patterns across different populations, coupled with timed sample collection, would be reassuring. Urine and plasma tenofovir (TFV) concentrations are short-term adherence monitoring metrics due to their short half-life (∼15 hours) [15–17]. Short-term metrics may provide valuable information about whether TDF was ingested recently, which could be potentially helpful to understand ED PrEP adherence when cumulative dosing patterns are less informative than the recency of dosing [18].
Prior studies evaluating urine/plasma TFV or DBS TFV-DP have primarily focused on only one aspect of “dosing time” in PrEP adherence monitoring: either dosing recency or total cumulative time of dosing. Data are still limited on how TFV levels in urine and plasma and TFV-DP levels in DBS correlate with each other. Our objectives were to assess the associations between different dosing time (dosing recency and cumulative dosing time) and drug concentrations of TFV-DP in DBS and TFV in urine/plasma in an Asian population and to estimate the correlations between the three metrics.
METHODS
Study Design
The Tenofovir Adherence to Rapidly Guide and Evaluate PrEP and HIV Therapy (TARGET) study (ClinicalTrials.gov #NCT0301260) was a three-arm randomized, open-label pharmacokinetic clinical trial among healthy adults enrolled in Thailand with controlled levels of PrEP adherence. The study protocol has been published previously [19]. Eligible volunteers without HIV or hepatitis B infection, aged 18–49 years, were randomized to receive 2 doses/week (Monday and Thursday), 4 doses/week (Monday, Wednesday, Friday, and Saturday), or 7 doses/week (once daily) of TDF 300 mg/FTC 200 mg (Truvada, Gilead Sciences) for 6 weeks, representing low, moderate, and perfect PrEP adherence, respectively. During the 6-week dosing phase, we performed directly observed therapy (DOT) for each study drug intake. All participants ingested their last dose at the beginning of week 7 and were followed for another 4 weeks during the drug washout phase.
Collection of Urine, Plasma, and DBS Samples
We collected predose time-matched spot urine, plasma, and DBS specimens before the second dose in week 1/3/5 and before the last dose in week 7. During the washout phase, blood and urine samples were collected on the Monday morning of week 8/9/10/11. We also conducted weekly liver and renal function assessment for all participants at week 1/3/5/7/10.
Measurement of Drug Concentrations
Urine, plasma, and DBS samples were stored at –70°C until analysis. TFV concentrations in urine and plasma, as well as TFV-DP in DBS, were measured using validated liquid chromatography tandem mass spectrometry (LC-MS/MS) assays over the concentration ranges of 50–50 000 ng/mL in urine and 3–2500 ng/mL in plasma. An indirect LC-MS/MS assay to quantify the intracellular concentration of TFV-DP in red blood cells was validated in Thailand based on a previously published assay [14, 20]. Briefly, whole blood was spotted on Whatman Protein Saver 903 cards (50 µL/spot), air-dried overnight at room temperature, and then stored at –70°C until analysis. For analysis, a 3-mm whole punch was taken from a single DBS, red blood cells were lysed from the paper, and then TFV-DP was separated from partially dephosphorylated TFV analytes by solid-phase extraction (SPE). TFV-DP was subsequently dephosphorylated using alkaline phosphatase. Finally, TFV was separated using SPE, and the eluted sample was dried, reconstituted in mobile phase, and injected into the LC-MS/MS system. The range of the standard curve was linear over the range 200–3000 fmol/3mm punch.
Statistical Analyses
We calculated Spearman correlation coefficients (γ) to estimate the correlations between time-matched predose TFV-DP levels in DBS and TFV concentrations in urine/plasma during the dosing phase. Log transformation (natural base) was used to account for the different scales, ranges, and units between 3 measurements. Concentrations in DBS, plasma, and urine below the lower limit of quantification (LLOQ) were imputed with half of the LLOQ values for each metric, namely 100 fmol/3mm punch for DBS, 1.5 ng/mL for plasma, and 25 ng/mL for urine. A sensitivity analysis was also performed by restricting the analysis among samples above the LLOQ. We used linear mixed-effects modeling (LMM) to evaluate the effect of cumulative dosing time and dosing recency on drug concentrations detected within each clinical metric. To assess the associations between different metrics and dosing time for participants who were still on PrEP, we restricted our LMM among samples collected during the dosing phase only. Cumulative dosing time was included in models as the number of weeks into study after the baseline visit. Dosing recency was represented with the number of days since last dose for each participant. All statistical analyses were performed using R (R Core Team).
RESULTS
Twenty-eight participants (43% female) with 224 DBS samples were included in the analyses. Half of the samples were collected from 4 visits during the dosing phase, and the other half from 4 visits during the washout phase. Overall, 212 of the DBS samples were time-matched with predose trough plasma and spot urine samples. The median age of participants (interquartile range [IQR]) was 33 (28–40) years (Table 1), and the moderate adherence group had fewer female participants (2/10 [20.0%]) compared with the low (4/9 [44.4%]) or perfect (6/9 [66.7%]) adherence group. Participants in the moderate adherence group also had a slightly higher median (IQR) estimated glomerular filtration rate of 124 (97–149) than the low and perfect adherence groups. Other clinical/hematologic/lab measurements were similar between the 3 adherence groups.
Table 1.
Baseline Characteristics by Adherence Groups a
| … | Adherence Groupb | ||
|---|---|---|---|
| Low (n = 9) | Moderate (n = 10) | Perfect (n = 9) | |
| Age, years | 38 (21–45) | 32 (20–49) | 34 (25–44) |
| Body mass index, kg/m2 | 23.1 (20.2–28.4) | 24.0 (22.1–25.1) | 20.2 (19.1–24.3) |
| Female, No. (%) | 4 (44.4) | 2 (20) | 6 (66.7) |
| Hemoglobin, g/dL | 14.7 (9.9–15.4) | 14.5 (12.6–16.2) | 12.4 (10.1–15.8) |
| Hematocrit, % | 43 (33–47) | 44 (39–48) | 38 (32–48) |
| eGFR,c mL/min | 108 (60–126) | 124 (97–149) | 91 (83–116) |
Abbreviations: eGFR, estimated glomerular filtration rate.
Data are presented as median (interquartile range) unless otherwise specified.
Adherence groups: perfect: 7 doses/week; moderate: 4 doses/week; low: 2 doses/week.
Estimated with the Cockcroft-Gault equation.
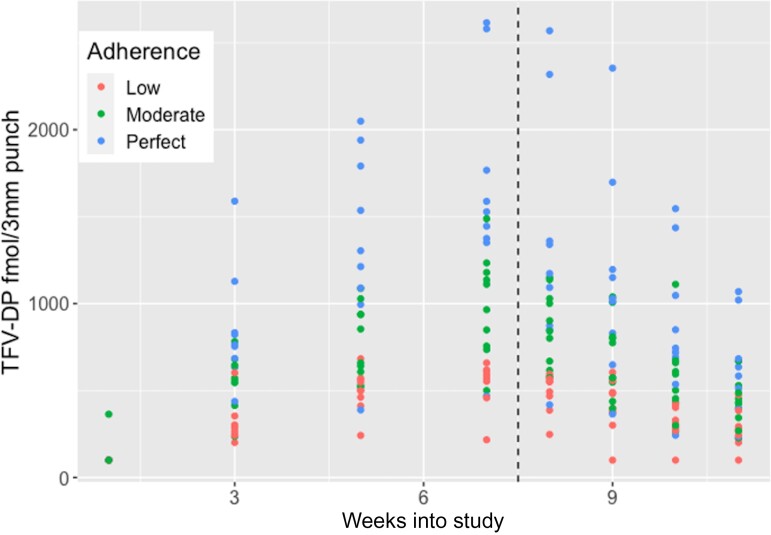
The median trough TFV-DP levels in predose DBS samples during the dosing phase (IQR) were 329 (176–555) fmol/3 mm punch in the low adherence group, 622 (332–875) fmol/3 mm punch in the moderate adherence group, and 914 (317–1530) fmol/3 mm punch in the perfect adherence group (Table 2). Female participants showed a ∼40 fmol/3 mm punch higher mean, but a similar median TFV-DP concentration as male participants during the dosing phase (Table 3). When we restricted our analysis to TFV-DP concentrations above the LLOQ (30 samples excluded, with 15 from each gender), the median TFV-DP (IQR) was 640 (395–1047) and 584 (438–902) fmol/3 mm punch among females and males, respectively, for the entire study phase (dosing + washout). Figure 1 shows the observed TFV-DP concentrations accumulated during the dosing phase until week 7, when the last dose of each regimen was administered. The 25th quantiles of TFV-DP levels in DBS samples before the last dose at the start of week 7 were 466, 779, and 1375 fmol/3 mm punch for the low, moderate, and perfect adherence group, respectively.
Table 2.
TFV-DP Concentrations (fmol/3 mm punch) by Adherence Groups
| Dosing Phase | No.a | Mean | SD | Median | 25th Quantile | 75th Quantile |
|---|---|---|---|---|---|---|
| Low adherence | 36 | 359 | 202 | 329 | 176 | 555 |
| Moderate adherence | 40 | 616 | 378 | 622 | 332 | 875 |
| Perfect adherence | 36 | 988 | 734 | 914 | 317 | 1530 |
| Week 7 (after the last dose) | ||||||
| Low adherence | 9 | 527 | 134 | 571 | 466 | 610 |
| Moderate adherence | 10 | 995 | 290 | 1038 | 779 | 1168 |
| Perfect adherence | 9 | 1635 | 655 | 1528 | 1375 | 1767 |
| Washout phase | ||||||
| Low adherence | 36 | 373 | 143 | 383 | 276 | 485 |
| Moderate adherence | 40 | 638 | 259 | 585 | 437 | 803 |
| Perfect adherence | 36 | 997 | 562 | 862 | 645 | 1179 |
Abbreviations: TFV-DP, tenofovir-diphosphate; SD, standard deviation.
No.: total number of DBS samples.
Table 3.
TFV-DP Levels in DBS by Gender
| Dosing Phase | No.a | Mean | SD | Median | 25th Quantile | 75th Quantile |
|---|---|---|---|---|---|---|
| Male | 64 | 636 | 521 | 557 | 241 | 800 |
| Female | 48 | 675 | 582 | 554 | 176 | 1088 |
| Washout phase | ||||||
| Male | 64 | 667 | 390 | 560 | 419 | 844 |
| Female | 48 | 670 | 503 | 566 | 351 | 835 |
Abbreviations: DBS, dried blood spots; TFV-DP, tenofovir-diphosphate; SD, standard deviation.
No.: total number of DBS samples.
Figure 1.
TFV-DP concentrations in DBS (y-axis) by adherence groups (low vs moderate vs perfect) over weeks into the study for the dosing and washout phases (separated by the dashed vertical line). Abbreviations: TFV-DP, tenofovir-diphosphate.
Compared with the low adherence group (2 doses/week), moderate (4 doses/week) adherence was on average associated with an estimated 250 fmol/3 mm punch higher TFV-DP level during the dosing phase [Standard Error (SE), 108; P = .02], and the corresponding estimate was 622 fmol/3 mm punch higher for the perfect adherence (7 doses/week) group (SE, 144; P < .01). Each additional cumulative week of TDF dosing was on average associated with 158 fmol/3mm punch (SE, 12; P < .001) higher TFV-DP concentration for participants with the same weekly PrEP regimen (in the same adherence group). In another mixed model examining the association between “days since last dose” (dosing regency) and TFV-DP levels in DBS during the dosing phase, we found that each additional day since last dosing was on average associated with 43 fmol/3 mm punch lower TFV-DP in DBS (SE, 24; P = .07) for participants with the same length of cumulative dosing.
Separate mixed models to evaluate the effect of dosing time (dosing recency & cumulative dosing time) on urine or plasma TFV concentrations showed that each additional day since last dosing on average contributed to 2435 (SE, 456; P < .001) and 9.01 (SE, 0.98; P < .001) ng/mL lower TFV levels in urine and plasma, respectively. Each additional week of cumulative dosing time was also associated with an increase of 192 (SE, 129; P = .14) and 0.95 (SE, 0.36; P = .013) ng/mL in urine and plasma TFV during the dosing phase. Correlation was stronger between quantifiable TFV-DP and plasma TFV during the dosing phase (n = 79; Spearman r = 0.65; P < .01; 95% Confidence Interval (CI), 0.51–0.76) than that between TFV-DP and urine TFV (n = 85; Spearman r = 0.50; P < .01; 95% CI, 0.30–0.64). The difference between 2 correlation coefficients was significant (P < .01) with a bootstrapped 95% CI at 0.08–0.32.
DISCUSSION
In a study of directly observed administration of TDF/FTC among HIV-negative participants in Thailand, we found that the steady state adherence benchmarks for TFV-DP concentrations in DBS were 466, 779, and 1375 fmol/3 mm punch for participants taking 2, 4, and 7 doses per week, respectively. TFV-DP levels in DBS were associated with both dosing recency and cumulative dosing time. Urine and plasma metrics of TFV (which both measure recent adherence) were modestly correlated with TFV-DP levels in DBS.
Participants with perfect (7 doses/week) and moderate (4 doses/week) adherence had higher TFV-DP concentrations than those with low (2 doses/week) adherence, which is consistent with former studies that demonstrated dose proportionality of DBS TFV-DP measurement [14]. Drug concentrations have been increasingly used to assess PrEP or ART adherence and corresponding outcomes [21–23]. However, most prior studies focused on only one aspect of adherence in relation to dosing time: dosing recency or cumulative time. Also, biological samples tested in prior studies were usually collected at “convenient” times without regard to time since last dosing. Our study utilized directly observed dosing and predose samples to assess the effect of both cumulative weeks of dosing and days since last dose on the concentrations of TFV-DP in DBS and TFV in urine/plasma among an Asian population.
Based on statistical models, previous studies estimated clinically meaningful TFV-DP thresholds of 350, 700, and 1250 fmol/punch in DBS at steady state (week 8 to 12) for 2, 4, and 7 doses/week [12, 14]. These benchmarks showed strong correlations with PrEP effectiveness among MSM and have been used to assess gradients of adherence [6, 24–26]. TFV-DP levels of >700 fmol/punch has been considered equivalent to oral TDF-FTC dosing of ≥ 4 doses/week and correlated with a 100% reduction in HIV risk [13]. However, this benchmark was estimated with the rounded 25th percentiles of TFV-DP concentrations of participants taking ≥ 4 doses/week. In other words, there were also at least 25% of participants taking ≥ 4 doses/week who had TFV-DP levels ≤700 fmol/punch. For such individuals, the clinical implication of TFV-DP benchmarks as proxies for sufficient weekly doses offering good enough protection is yet unclear.
Our benchmark TFV-DP concentrations were slightly higher but comparable to prior predicted estimates. Several reasons might have contributed to the observed differences. First, the participants in the prior study were on 33% vs 67% vs 100% weekly dosing regimens. Predictions were made for dosing rates at 2 and 4 doses/week on average based on a mixed model with log(TFV-DP) levels as a smooth, nonlinear function of time and weekly doses, while our benchmarks were directly observed from participants with 2, 4, and 7 doses/week. In addition, all DBS samples in the prior study were collected every 2 weeks during the 12-week dosing phase conveniently without regard to time since last dose. In the current analysis, trough TFV-DP concentrations were tested with predose DBS samples collected before the second dose in week 1/3/5 and before the last dose in week 7. Second, our study population was entirely Asian from Thailand, whereas the prior study included no Asian participants, and race was recently found to be associated with different TFV-DP concentrations, possibly driven by pharmacogenomics [14, 27]. Finally, our study used the drug concentrations at week 7 as steady state levels, whereas the prior benchmarks were calculated with drug concentrations between week 8 and week 12 (steady state). There was concern that the DBS TFV-DP levels at week 7 in our study had not yet reached steady state levels; however, the median TFV-DP concentration from daily dosing at week 7 in our study was 1528 fmol/3 mm punch, which was comparable to median concentrations reported at week 8 (1493 fmol/punch) and during the entire steady state (1534 fmol/punch) in the prior study. Consistent with previous findings, our study also showed higher quantifiable median TFV-DP concentrations in females than males [14]. A greater number of doses received per week among females than males might have contributed to the observed sex differences. In our study, more females were randomly assigned to the perfect adherence group (7 doses/week) than males. Females on average received 4.83 doses per week during the dosing phase, while males only received 3.94 doses per week. Whether there would be higher drug concentrations among females than males with the same number of weekly doses warrants further research.
Pharmacologic measurements for PrEP adherence monitoring are generally grouped into short-term and long-term metrics based on their half-lives in different biological specimens. Though both sets of metrics have demonstrated strong associations with PrEP efficacy/effectiveness, most prior studies have only assessed short-terms metrics for “dosing recency” or long-term metrics for “cumulative dosing.” For instance, plasma TFV concentrations can inform drug adherence within the preceding 2 days up to a week [15]. Plasma TFV concentrations of at least 35.5, 4.2, 2.5, and 0.5 ng/mL also indicated adherence consistent with at least 7, 4, 2, and 1 dose in the prior week, respectively, from the HPTN 066 study [28]. With similar half-life to plasma TFV, urine TFV concentrations are much higher and more variable. Urine TFV levels ≥1500 ng/mL have accurately classified 98% of participants who took their last dose within the past 24 hours [29] and are gaining in interest as point-of-care urine-based tests become available. Although short-term metrics can be susceptible to the “white-coat” effect where nonadherent participants may appear to be adherent by ingesting a dose right before drug level testing, a recent analysis examining emtricitabine triphosphate (FTC-TP, short-term metric) and TFV-DP levels in study participants who knew their blood was being monitored for adherence testing did not show that effect [30]. Long-term metrics such as TFV-DP are therefore increasingly preferred in clinical trials to evaluate established adherence patterns over a longer period of time. The resulting drug concentrations in DBS and peripheral blood mononuclear cells (PBMCs) [6] and hair samples [31, 32] have provided important benchmarks for interpreting the adherence–drug concentration–efficacy relationship. For example, a TFV-DP concentration of 700 fmol/punch in DBS and a TFV level of 0.023 ng/mg in hair were found to be commensurate with a median adherence of 4.1 doses/week, which is highly protective against HIV infection among MSM [32].
Since the endorsement of the ED PrEP “2-1-1” regimen from the International Antiviral Society-USA and the WHO as an alternative to the daily regimen, there is growing acceptance of ED PrEP among MSM [33]. However, current adherence benchmarks for short- or long-term metrics have mostly been based on established dosing patterns (1 dose per day, 1–7 doses/week), and their interpretations in the setting of ED-PrEP is unclear. Because ED PrEP is normally taken at irregular intervals due to the sex act–dependent nature of ED PrEP, drug concentrations are very likely not to reach steady state levels when tested for adherence. A recent study of ED-PrEP adherence in Amsterdam reported a median TFV-DP concentration of 591 fmol/punch, suggesting that half of the participants on ED-PrEP offering good protection were below the 700 fmol/punch threshold [33]. Hence, a metric to measure both dosing recency and cumulative dosing information may be critical when assessing ED PrEP adherence. More data are urgently needed to assess the association between cumulative dosing time/time since last dose and the concentrations of both short- and long-term metrics for PrEP adherence monitoring.
Contrary to expectations that TFV-DP in DBS, a long-term metric, can only provide information about cumulative doing, our results showed that each additional day since last dose taken (dosing recency) was also associated with lower TFV-DP in DBS. Though the finding was not statistically significant, the association between TFV-DP and dosing recency was further supported by its moderate to strong correlations with urine and plasma TFV. Both urine and plasma TFV were associated with dosing recency but not necessarily cumulative dosing according to our data. The higher correlation coefficient between DBS TFV-DP & plasma TFV vs that between DBS TFV-DP & urine TFV may partly be explained by the much higher concentration and variability of TFV in urine than in plasma. Of note, the associations between drug concentrations and dosing time (either dosing recency or cumulative doses) in our study should be limited to the dosing phase only. Future studies with time since last dosing information available are needed to elucidate the relationship between long-term metrics such as TFV-DP in DBS and dosing recency.
In conclusion, urine/plasma TFV levels modestly correlate with TFV-DP concentrations in DBS. The latter metric provides valuable insight into both dosing recency and cumulative doses from variable adherence patterns. Our study provides new information on defining benchmarks of adherence from a direct-observed therapy study. Benchmarks of TFV-DP concentrations observed from our study will help guide PrEP adherence monitoring and interventions.
Acknowledgments
We thank the participants in the studies for their contribution and the staff who worked on the trial.
Financial support. This work was supported by research grants from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH; grant numbers R21AI127200 and R01AI143340). Gilead Sciences donated Truvada.
Patient consent. A, The participants’ written consent was obtained. B, The design of the work was approved by ethics committees at the Institute for the Development of Human Research Protections at the Medical Sciences Department, Thai Ministry of Public Health (TARGET/IHRP); the ethics committee of Sanpatong Hospital (SPT REC 007/60); the Faculty of Associated Medical Sciences, Chiang Mai University (AMSEC-FB-009); and the University of Washington Institutional Review Board in Seattle (STUDY00000058).
Contributor Information
Xin Niu, Department of Epidemiology, University of Washington, Seattle, Washington, USA.
Rachel W Kubiak, Department of Epidemiology, University of Washington, Seattle, Washington, USA.
Oraphan Siriprakaisil, Sanpatong Hospital, Chiang Mai, Thailand.
Virat Klinbuyaem, Sanpatong Hospital, Chiang Mai, Thailand.
Pra ornsuda Sukrakanchana, AMS/IRD Research Collaboration, Department of Medical Technology, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai, Thailand.
Ratchada Cressey, Division of Clinical Chemistry, Department of Medical Technology, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai, Thailand.
Hideaki Okochi, Department of Medicine, University of California San Francisco, San Francisco, California, USA; Department of Bioengineering and Therapeutic Sciences, University of California San Francisco, San Francisco, California, USA.
Monica Gandhi, Department of Medicine, University of California San Francisco, San Francisco, California, USA.
Tim R Cressey, AMS/IRD Research Collaboration, Department of Medical Technology, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai, Thailand; Department of Molecular and Clinical Pharmacology, University of Liverpool, Liverpool, United Kingdom.
Paul K Drain, Department of Epidemiology, University of Washington, Seattle, Washington, USA; Department of Global Health, University of Washington, Seattle, Washington, USA; Department of Medicine, University of Washington, Seattle, Washington, USA.
References
- 1. Chou R, Evans C, Hoverman A, et al. . Preexposure prophylaxis for the prevention of HIV infection: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2019; 321:2214–30. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. 2nd ed. Geneva: World Health Organization; 2016. [PubMed] [Google Scholar]
- 3. Holmes D. FDA paves the way for pre-exposure HIV prophylaxis. Lancet 2012; 380:325. [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention . Recommendations for HIV prevention with adults and adolescents with HIV in the United States. 2014. Available at: https://stacks.cdc.gov/view/cdc/44064. Accessed October 6, 2021.
- 5. Molina J-M, Capitant C, Spire B, et al. . On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 2015; 373:2237–46. [DOI] [PubMed] [Google Scholar]
- 6. Anderson PL, Glidden D V, Liu A, et al. . Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012; 4:151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Donnell D, Baeten JM, Bumpus NN, et al. . HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr 2014; 66:340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bärnighausen T, Chaiyachati K, Chimbindi N, Peoples A, Haberer J, Newell M-L. Interventions to increase antiretroviral adherence in Sub-Saharan Africa: a systematic review of evaluation studies. Lancet Infect Dis 2011; 11:942–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Checchi KD, Huybrechts KF, Avorn J, Kesselheim AS. Electronic medication packaging devices and medication adherence: a systematic review. JAMA 2014; 312:1237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fogarty L, Roter D, Larson S, Burke J, Gillespie J, Levy R. Patient adherence to HIV medication regimens: a review of published and abstract reports. Patient Educ Couns 2002; 46:93–108. [DOI] [PubMed] [Google Scholar]
- 11. Landovitz RJ, Donnell D, Clement ME, et al. . Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med 2021; 385:595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Castillo-Mancilla JR, Zheng J-H, Rower JE, et al. . Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses 2013; 29:384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grant RM, Anderson PL, McMahan V, et al. . Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 2014; 14:820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anderson PL, Liu AY, Castillo-Mancilla JR, et al. . Intracellular tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following directly observed therapy. Antimicrob Agents Chemother 2018; 62:e01710–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koenig HC, Mounzer K, Daughtridge GW, et al. . Urine assay for tenofovir to monitor adherence in real time to tenofovir disoproxil fumarate/emtricitabine as pre-exposure prophylaxis. HIV Med 2017; 18:412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fonsart J, Saragosti S, Taouk M, et al. . Single-dose pharmacokinetics and pharmacodynamics of oral tenofovir and emtricitabine in blood, saliva and rectal tissue: a sub-study of the ANRS IPERGAY trial. J Antimicrob Chemother 2017; 72:478–85. [DOI] [PubMed] [Google Scholar]
- 17. Seifert SM, Chen X, Meditz AL, et al. . Intracellular tenofovir and emtricitabine anabolites in genital, rectal, and blood compartments from first dose to steady state. AIDS Res Hum Retroviruses 2016; 32:981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dai JY, Hendrix CW, Richardson BA, et al. . Pharmacological measures of treatment adherence and risk of HIV infection in the VOICE study. J Infect Dis 2016; 213:335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cressey TR, Siriprakaisil O, Klinbuayaem V, et al. . A randomized clinical pharmacokinetic trial of tenofovir in blood, plasma and urine in adults with perfect, moderate and low PrEP adherence: the TARGET study. BMC Infect Dis 2017; 17:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zheng JH, Rower C, McAllister K, et al. . Application of an intracellular assay for determination of tenofovir-diphosphate and emtricitabine-triphosphate from erythrocytes using dried blood spots. J Pharm Biomed Anal 2016; 122:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brooks KM, Anderson PL. Pharmacologic-based methods of adherence assessment in HIV prevention. Clin Pharmacol Ther 2018; 104:1056–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spinelli MA, Haberer JE, Chai PR, Castillo-Mancilla J, Anderson PL, Gandhi M. Approaches to objectively measure antiretroviral medication adherence and drive adherence interventions. Curr HIV/AIDS Rep 2020; 17:301–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Drain PK, Bardon AR, Simoni JM, et al. . Point-of-care and near real-time testing for antiretroviral adherence monitoring to HIV treatment and prevention. Curr HIV/AIDS Rep 2020; 17:487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Castillo-Mancilla JR, Morrow M, Coyle RP, et al. . Tenofovir diphosphate in dried blood spots is strongly associated with viral suppression in individuals with human immunodeficiency virus infections. Clin Infect Dis 2019; 68:1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morrow M, MaWhinney S, Coyle RP, et al. . Predictive value of tenofovir diphosphate in dried blood spots for future viremia in persons living with HIV. J Infect Dis 2019; 220:635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koss CA, Bacchetti P, Hillier SL, et al. . Differences in cumulative exposure and adherence to tenofovir in the VOICE, iPrEx OLE, and PrEP demo studies as determined via hair concentrations. AIDS Res Hum Retroviruses 2017; 33:778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu AY, Cohen SE, Vittinghoff E, et al. . Preexposure prophylaxis for HIV infection integrated with municipal- and community-based sexual health services. JAMA Intern Med 2016; 176:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hendrix CW, Andrade A, Bumpus NN, et al. . Dose frequency ranging pharmacokinetic study of tenofovir-emtricitabine after directly observed dosing in healthy volunteers to establish adherence benchmarks (HPTN 066). AIDS Res Hum Retroviruses 2016; 32:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gandhi M, Wang G, King R, et al. . Development and validation of the first point-of-care assay to objectively monitor adherence to HIV treatment and prevention in real-time in routine settings. AIDS 2020; 34:255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morrow M, MaWhinney S, Coyle RP, et al. . Emtricitabine triphosphate in dried blood spots predicts future viremia in persons with HIV and identifies mismatch with self-reported adherence. AIDS 2021; 35:1949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gandhi M, Glidden D V, Liu A, et al. . Strong correlation between concentrations of tenofovir (TFV) emtricitabine (FTC) in hair and TFV diphosphate and FTC triphosphate in dried blood spots in the iPrEx open label extension: implications for pre-exposure prophylaxis adherence monitoring. J Infect Dis 2015; 212:1402–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu AY, Yang Q, Huang Y, et al. . Strong relationship between oral dose and tenofovir hair levels in a randomized trial: hair as a potential adherence measure for pre-exposure prophylaxis (PrEP). PLoS One 2014; 9:e83736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoornenborg E, Achterbergh RC, van der Loeff MFS, et al. . Men who have sex with men more often chose daily than event-driven use of pre-exposure prophylaxis: baseline analysis of a demonstration study in Amsterdam. J Int AIDS Soc 2018; 21:e25105. [DOI] [PMC free article] [PubMed] [Google Scholar]