Keywords: blood pressure, cardiorespiratory fitness, exercise, heat therapy, sauna bathing
Abstract
Regular exercise and sauna bathing have each been shown to improve cardiovascular function in clinical populations. However, experimental data on the cardiovascular adaptations to regular exercise in conjunction with sauna bathing in the general population are lacking. Therefore, we compared the effects of exercise and sauna bathing to regular exercise using a multi-arm randomized controlled trial. Participants (n = 47) aged 49 ± 9 with low physical activity levels and at least one traditional cardiovascular disease (CVD) risk factor were randomly assigned (1:1:1) to guideline-based regular exercise and 15-min postexercise sauna (EXS), guideline-based regular exercise (EXE), or control (CON) for 8 wk. The primary outcomes were blood pressure (BP) and cardiorespiratory fitness (CRF). Secondary outcomes included fat mass, total cholesterol levels, and arterial stiffness. EXE had a greater change in CRF (+6.2 mL/kg/min; 95% CI, +4.2 to +8.3 mL/kg/min) and fat mass but no differences in BP when compared with CON. EXS displayed greater change in CRF (+2.7 mL/kg/min; 95% CI, +0.2 to +5.3 mL/kg/min), lower systolic BP (−8.0 mmHg; 95% CI, −14.6 to −1.4 mmHg), and lower total cholesterol levels compared with EXE. Regular exercise improved CRF and body composition in sedentary adults with CVD risk factors. However, when combined with exercise, sauna bathing demonstrated a substantially supplementary effect on CRF, systolic BP, and total cholesterol levels. Sauna bathing is a valuable lifestyle tool that complements exercise for improving CRF and decreasing systolic BP. Future research should focus on the duration and frequency of exposure to ascertain the dose-response relationship.
INTRODUCTION
Physical activity and exercise training are well-documented strategies to prevent ailments (1) and various diseases (2). The current health and exercise guidelines (3) recommend 150–300 min of moderate-intensity physical activity spread across three to five sessions per week. In addition, resistance exercise should be performed at least twice a week (3, 4). Evidently, we have come to a firm understanding of exercise and how it can be used to improve cardiovascular health. However, unlike exercise, heat therapy and the health benefits of Finnish sauna bathing are still not well understood, despite its increasing use throughout the world (5), even though observational cohort studies (6–8) have found regular use of the sauna to be positively associated with numerous cardiovascular outcomes.
Indeed, most studies investigating the efficacy of sauna bathing have either been acute (0–30 min after sauna) or short-term (2–4 wk long) (9), and as pointed out by a recent review, long-term experimental evidence in heat therapy (10) based on the Finnish sauna is needed. The efficacy of sauna use on a regular basis when combined with exercise has been shown in both extremes of the population; in well-trained cyclists (11) and runners (12), as well as patients with heart failure (13) and other diseases (14). However, these data are somewhat limited for the general population.
A significant portion of the general population today has at least one cardiovascular disease (CVD) risk factor (obesity, elevated blood pressures, elevated cholesterol, family history of coronary heart disease, and smoking or a history of smoking). This includes the majority of the population in Australia (15), Canada (16), Europe (17), United States (18), and a substantial percentage of people in China (19). This underscores the problems of public health in our modern society. Thus, it is important to develop interventional strategies that target these groups, as they form a larger part of the general population. Furthermore, these groups often stand to benefit most from lifestyle-related interventions (20).
One traditional CVD risk factor that warrants consideration is elevated blood pressure (BP) level, as increases in BP have been associated with an increase in CVD risk (21). BP has also been well documented to respond favorably to regular physical activity, which plays a pivotal role in the nonpharmacological management of hypertension (22). However, recent evidence suggests that regular heat therapy can lower BP to a comparable, if not larger degree (23). As such, adding regular sauna bathing to exercise could potentially yield even greater benefits than regular exercise alone. In addition to traditional CVD risk factors, cardiorespiratory fitness (CRF) has also been recently highlighted as a strong predictor of health outcomes (24) and is indicative of functional capacity and overall physical health (25). CRF can be measured directly using maximal testing or estimated via submaximal testing and is a significant prognosticator regardless of the method by which it is derived (26).
Our previously published works showed promising results through sauna use (27), as well as via a combination of exercise followed by sauna (28) in acute responses. Our objective for the current experiment was thus to expand these findings and explore the likelihood of cardiovascular adaptations, using BP and CRF as primary outcomes. We seek to provide fundamental and valuable information to the study of heat therapy and sauna use and its potential as a lifestyle intervention that could be prescribed alongside exercise effectively.
As such, we conducted an 8-wk multi-arm randomized controlled trial (RCT) using the current recommended guidelines on physical activity, in a population with CVD risk factors. The primary focus was to compare the cardiovascular adaptations of regular exercise alone (EXE) to regular exercise and sauna bathing (EXS), with a sedentary control (CON) group serving as a comparator against the EXE group to ascertain the efficacy of the 8-wk exercise intervention. To the best of our knowledge, this is the first multi-arm RCT investigating the long-term effects of exercise and sauna use in a nonathletic and nonclinical general population.
MATERIALS AND METHODS
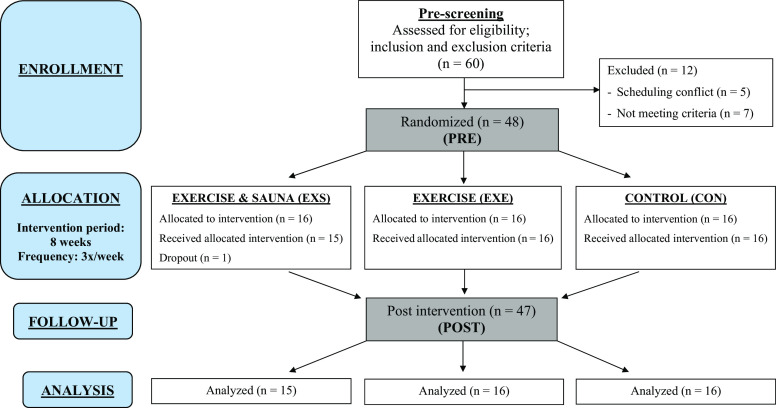
A multi-arm, parallel-group (allocation ratio 1:1:1) RCT (Unique identifier: NCT04540718) was conducted in accordance with Consolidated Standards of Reporting Trials (29) guidelines (Fig. 1). The institutional review board of the Central Finland Hospital District ethical committee, Jyväskylä, Finland, approved this study (Dnro 3 U/2019). All participants provided written informed consent. The data that support the findings of this study are available to researchers upon reasonable request to the corresponding author.
Figure 1.
Experimental design (adapted and modified according to CONSORT guidelines template). n, number of human volunteers/participants.
Study Population
Female and male participants between 30 and 64 yr were recruited through medium-to-large organizations (City council of Jyväskylä, Jyväskylä Energy, Central Finland hospital) via email. The inclusion criteria of study participants consisted of a sedentary lifestyle and at least one traditional CVD risk factor. Sedentary lifestyle was identified as having a desk-bound job and less than 30 min of total physical activity per week. The CVD risk factors were elevated cholesterol, family history of coronary heart disease (CHD), hypertension, obesity, and smoking.
Total cholesterol level >239 mg/dL was considered elevated. Family history of CHD was positive if father (<55 yr) or mother (<65 yr) had premature CHD. Prestudy resting systolic BP (SBP) >139 mmHg and/or diastolic (DBP) >89 mmHg was considered elevated (30). Obesity was defined as body mass index (BMI) >30 kg/m2. Exclusion criteria were 1) sauna bathing more than once a week within the past 6 mo, 2) commuting to work via activities such as running or cycling, 3) previous CHD and/or diabetes, and 4) any diagnosed and/or symptomatic CVD, musculoskeletal injury, or any other physical or mental condition within 6 mo before the commencement of the study. Participants were also excluded if they had resting SBP <100 mmHg or >159 mmHg, BMI over 40 kg/m2, or if they were on any CVD medication.
Before the trial commencement, participants (n = 60) attended an information session where they were briefed about the research purposes, measurement procedures, and intervention period. Five participants dropped out. Subsequently, a prescreening session was conducted to collect baseline information (anthropometric data, resting electrocardiogram, and brachial BP) and ensure that the remaining 55 met the study eligibility based on the inclusion and exclusion criteria. Seven participants who did not meet the criteria were excluded, leading to a final sample size of 48 participants.
Randomization and Design
After the successful completion of prescreening procedures, participants were randomized into the EXS, EXE, or the CON group (Fig. 1). The randomization sequence was created using Excel 2016 (Microsoft, Redmond, WA) with a 1:1:1 allocation using simple randomization with stratification by a researcher with no clinical involvement in the trial. Biological sex was used for stratification, as there was a disproportionate number of female to male participants. Forty-eight participants (42 females, 6 males) were enrolled into the trial by the corresponding author. Participants were assigned to their respective interventions (16 per group, 14 females, and 2 males) by a member of the research team who was uninvolved with the data collection and analysis process. To ensure that the statistical analyses were nonbiased, the statistician was blinded to the assignment and completely uninvolved in the participant recruitment and data collection processes.
Participants in the CON group were informed that a similar 8-wk supervised exercise training program would be offered to them after the trial. This was done to minimize dropout rates and increase adherence to preexisting lifestyle and physical activity habits during the trial period to reduce potential confounding factors. The study consisted of two measurement days completed by all groups and an 8-wk intervention for the EXS and EXE groups. Participants recorded and submitted a food diary the day before their preintervention (PRE) measurement. This was returned to them 48 h before the postintervention (POST) measurement, and they were carefully instructed to follow the same food intake before their measurement days. All participants were reminded regularly to maintain their regular daily activities and diet to minimize the possible influence of external variables on the outcome measures.
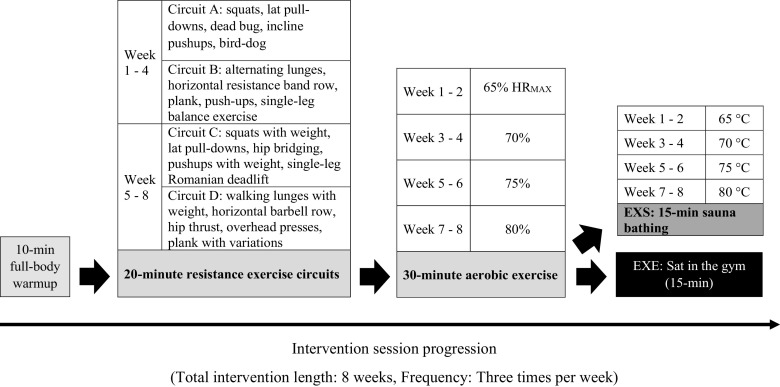
The intervention groups exercised three times a week (Monday, Wednesday, and Friday) in the evenings, between 1600 and 2100. Training sessions were carried out in groups of 1–5 participants with two qualified instructors. A predetermined adherence rate of 95% for 24 training sessions was successfully achieved. One participant from the EXS group dropped out during the first week due to undisclosed personal reasons. The exercise intervention was based on the Finnish national exercise guidelines (31), which are adapted from the guidelines of the American College of Sports Medicine (ACSM) and reflect current recommendations (3). Each exercise session lasted 60 min and was performed in the following order: a 10-min full-body warm-up, 20 min of resistance exercise, and 30 min of aerobic exercise. Details of the intervention are shown in Fig. 2.
Figure 2.
Details of the intervention. Loads were increased for resistance exercises when participants were able to complete the movement comfortably with good form. More challenging variations for the bodyweight exercises were introduced when the participant completed the basic movement with no noticeable difficulties. For example, resistance bands were used for dead bugs, bird-dog were executed with eyes closed, etc.
Resistance training was a mixture of body weight and basic resistance training exercises. Starting loads for each resistance exercise were determined individually on a separate day before the intervention. The exercises were performed in a circuit fashion, with the aim of providing a full-body workout. Each circuit consisted of five movements, and each movement was performed for 45 s with a 15-s break between them. Completion of a circuit took 5 min, followed by a 1-min break. The circuits were completed three times each session. Circuits A and B were used alternately in each training for the first 4 wk, and circuits C and D were used in the final 4 wk. Harder variations of body weight movements and greater resistance exercise loads for each individual were introduced as performance improved, based on the assessment of the exercise instructors.
Aerobic exercise was performed using Monark cycle ergometers (Monark 828 E, Varberg, Sweden). Individual maximum heart rates were calculated (32) and used thereafter to prescribe aerobic exercise intensity, starting from 65% of maximum heart rate with a fortnightly increase of 5%. Aerobic exercise heart rate was closely monitored and verified every 5 min. Participants maintained a constant pedaling frequency of 65–70 revolutions per minute (rpm), while the magnetic resistance of the bike ergometer was adjusted to achieve the required exercise intensity. After aerobic exercise, participants in the EXS group proceeded to the sauna room, whereas those in the EXE group waited in the gym until the participants in the EXS group completed 15 min of sauna exposure. The temperature of sauna exposure started from 65°C and was increased by 5°C fortnightly and was monitored and recorded every minute via a commercially available wireless thermometer unit (Wireless thermometer 7410; Suomen Lämpömittari Oy, Helsinki, Finland). Relative humidity of the sauna room was between 10% and 20%. Participants were allowed to leave the sauna at any time if they felt uncomfortable, but all participants in the EXS group completed all 15 min of every postexercise sauna exposure successfully without leaving the sauna room.
Measurement of Outcomes
All measurements (PRE and POST) took place in the exercise and health laboratory, at the Faculty of Sport and Health Sciences, of the University of Jyväskylä. The primary outcomes were estimated relative maximal oxygen uptake (V̇o2max) as a measure of CRF and brachial BP. The CRF tests were completed in the evening between 1600 and 2000, and participants were instructed to refrain from heavy physical activity for 48 h and abstain from alcohol and nicotine for 12 h before all CRF tests. A multistage test similar to the YMCA submaximal bicycle test (33) was used. The test consisted of four stages of incremental submaximal workloads lasting 4 min each. Cadence was kept at 50–60 rpm (34), and heart rate was measured via a heart rate monitor (Polar V800; Polar Electro Oy, Kempele, Finland). After the test was completed, a regression line was plotted using the four points corresponding to each stage and extrapolated to the maximal heart rate. A perpendicular line was subsequently formed down to the x-axis and absolute oxygen uptake was read off from the graph (33, 34). From the resulting value, relative V̇o2max was then calculated using individual body mass.
Two separate brachial BP measurements were taken on the right upper arm using automated oscillometric devices. The first measurement was taken with the Omron HEM-7320-LA (Omron Healthcare Co., Ltd, Kyoto, Japan), followed by the Arteriograph (TensioMed, Budapest, Hungary) after a 10-min rest in a supine position, according to the established guidelines (30, 35). If the difference in SBP between the two measurements was larger than 10 mmHg, another measurement was taken after a 5-min rest. The two measured values that differed the least were averaged and used for applanation tonometry analysis. Arterial stiffness indices of pulse-wave velocity (PWV) and augmentation index (AIx) as secondary outcomes were recorded noninvasively with the Pulsepen device (DiaTecne s.r.l., Milan, Italy; www.pulsepen.com) according to published guidelines (36). BP and tonometer measurements were taken by a single trained operator to ensure consistency. Intraclass correlation coefficient (ICC) estimates and their 95% CI were calculated using statistical software R (36), based on a mean-rating (k = 2), absolute-agreement, two-way mixed-effects model (ICC 2.1: 0.81 with 95% CI = 0.77–0.85, SE = 0.4).
Body composition measurements and blood samples were collected in the morning between 0630 and 0930 in fasted conditions. Participants were instructed to abstain from food, drinks, alcohol, and nicotine for 12 h and to refrain from heavy physical activity for 48 h before all measurements. Body composition was determined using dual-energy X-ray absorptiometry, and venous blood samples were collected by a qualified technician from the antecubital vein into Vacuette SST 6-mL tubes using sterile needles. The sample was centrifuged for 10 min at 2,000 rpm after which serum was removed and stored at −80°C until chemical analyses. Serum samples were subsequently analyzed with colorimetric assay, Konelab 20 analyzer (Thermo, Vantaa, Finland) to determine total cholesterol levels. Sensitivities and coefficients of variation (with-in-assay CV % average) were 3.86 mg/dL and 1.00%.
Statistical Analyses
Categorical variables are presented as number (%), whereas continuous variables are presented as means ± SD; 95% CIs are presented where appropriate. Statistical power of 80% with α = 0.05 for a difference of one standard deviation in means can be achieved with n = 15.68 per group when a two-tailed independent samples t test is used. Thus, the final sample size of 15, 16, and 16 (EXS, EXE and CON, respectively, with two males per group) in the three groups was expected to be adequate to detect most clinically significant differences.
Distributions of responses were tested for normality with the Shapiro–Wilk test for each group separately. After the family-wise error rate was controlled by using a Bonferroni correction, the null hypothesis of normality was not rejected for any variable of any group. Between-group differences PRE and POST intervention were analyzed using independent t tests. The comparisons were done between CON and EXE groups and between EXE and EXS groups. The level for statistical significance was set at P ≤ 0.05. For completeness, we also fitted a mixed linear model for all three groups and each response variable. The details and results of these exploratory analyses are shown in Supplemental Table S1 (see https://doi.org/10.6084/m9.figshare.20160479.v1). The calculations were implemented with the statistical software R (37), with graphics done using the ggplot2 package (38).
RESULTS
Characteristics of Participants
The characteristics of the participants are presented in Table 1. The three most commonly present traditional CVD risk factors were obesity (54%), family history of CHD (37%), and elevated BP (35%). Baseline V̇o2max, SBP, and DBP were 28.3 ± 5.6 mL/kg/min, 133 ± 12 mmHg, and 79 ± 10 mmHg, respectively. No sex-based or race/ethnicity-based differences were present.
Table 1.
Baseline participant characteristics
| Characteristics | Total n = 47 | Control n = 16, 14 Females |
Exercise Only n = 16, 14 Females |
Exercise + Sauna n = 15, 13 Females |
|---|---|---|---|---|
| Age, yr | 49 ± 9 | 49 ± 8 | 51 ± 9 | 47 ± 8 |
| Body mass, kg | 89.0 ± 14.3 | 86.5 ± 15.6 | 87.3 ± 13.0 | 93.5 ± 13.2 |
| Body mass index, kg/m2 | 31.3 ± 4.1 | 31.1 ± 4.7 | 31.3 ± 4.2 | 32.2 ± 3.6 |
| Estimated V̇o2max, mL/kg/min | 28.3 ± 5.6 | 30.1 ± 4.8 | 29.4 ± 5.7 | 26.4 ± 6.3 |
| Systolic BP, mmHg | 133 ± 12 | 129 ± 9 | 134 ± 14 | 134 ± 14 |
| Diastolic BP, mmHg | 79 ± 10 | 78 ± 5 | 79 ± 11 | 80 ± 13 |
| Risk Factors* | Number (%) | Number/Group Total | ||
|---|---|---|---|---|
| Obesity (BMI >30 kg/m2) | 25 (54%) | 8/16 | 9/16 | 8/15 |
| Family history of CHD | 18 (38%) | 8/16 | 5/16 | 5/15 |
| Elevated BP | 16 (35%) | 7/16 | 5/16 | 4/15 |
| Elevated cholesterol (>239 mg/dL) | 10 (22%) | 4/16 | 3/16 | 3/15 |
| Smoker (history of smoking) | 6 (13%) | 2/16 | 2/16 | 2/15 |
Values are means ± SD. No significant differences were found at baseline between groups for all the parameters. BMI, body mass index; BP, brachial blood pressure; CHD, coronary heart disease; V̇o2max, maximal oxygen consumption. *Number of participants with one, two, and three risk factors was 23, 20, and 4, respectively. No participant had more than three risk factors.
Exercise versus Control
Significant PRE-POST differences were found between the control (CON) and exercise (EXE) groups for V̇o2max and fat mass. Comparatively, EXE had greater increases in V̇o2max and decreases in fat mass. No significant differences were found for BP, arterial stiffness indices, and total cholesterol (Table 2). Female-only data are found in Supplemental Table S2 (see https://doi.org/10.6084/m9.figshare.19575979).
Table 2.
PRE-POST comparison of means between the EXE and CON groups
| Outcome Variable | EXE (n = 16, 14 Females) |
CON (n = 16, 14 Females) |
||||
|---|---|---|---|---|---|---|
| PRE | POST | PRE | POST | Mean Difference, 95% CI | P Value | |
| Estimated V̇o2max, mL/kg/min | 29.4 ± 5.7 | 32.0 ± 6.6 | 30.1 ± 4.8 | 26.8 ± 4.6 | 6.2 (4.1, 8.3) | 0.00000211 |
| SBP, mmHg | 134 ± 14 | 134 ± 14 | 130 ± 9 | 130 ± 10 | 0.5 (−4.6, 5.6) | 0.841 |
| DBP, mmHg | 79 ± 11 | 80 ± 9 | 79 ± 5 | 82 ± 6 | −1.9 (−5.5, 1.7) | 0.295 |
| Fat mass, kg | 37.8 ± 10.5 | 36.5 ± 10.1 | 38.0 ± 12.4 | 38.0 ± 12.3 | −1.3 (−2.3, -0.3) | 0.0125 |
| Total cholesterol, mg/dL | 203 ± 34 | 208 ± 30 | 215 ± 34 | 211 ± 29 | 12 (−8, 27) | 0.215 |
| PWV, m/s | 9.2 ± 1.7 | 9.2 ± 1.4 | 8.5 ± 1.5 | 8.7 ± 2.4 | −0.2 (−1.2, 0.8) | 0.662 |
| AIx, % | 16.1 ± 11.9 | 17.3 ± 10.0 | 15.5 ± 11.0 | 15.4 ± 8.7 | 1.2 (−6.5, 8.9) | 0.760 |
Values are means ± SD. Data were analyzed using independent t tests. AIx, augmentation index; CI, confidence interval; CON, control; DBP, brachial diastolic blood pressure; EXE, exercise; PRE, preintervention; POST, postintervention; PWV, pulse-wave velocity; SBP, brachial systolic blood pressure; V̇o2max, maximal oxygen consumption.
Exercise and Sauna versus Exercise
PRE-POST differences in V̇o2max, SBP, and total cholesterol levels were significant between the EXE and exercise and sauna (EXS) groups (Table 3). Specifically, V̇o2max was greater (Fig. 3), whereas SBP (Fig. 4) and total cholesterol levels (Fig. 5) were lower in the EXS than the EXE group after the 8-wk intervention period. There were no differences in any other outcome variables. Female-only data are found in Supplemental Table S3 (see https://doi.org/10.6084/m9.figshare.19576030).
Table 3.
PRE-POST comparison of means between the EXS and EXE groups
| Outcome Variable | EXS (n = 15, 13 Females) |
EXE (n = 16, 14 Females) |
||||
|---|---|---|---|---|---|---|
| PRE | POST | PRE | POST | Mean Difference, 95% CI | P Value | |
| Estimated V̇o2max, mL/kg/min | 26.4 ± 6.3 | 32.0 ± 6.4 | 29.4 ± 5.7 | 32.0 ± 6.6 | 2.7 (0.2, 5.3) | 0.0343 |
| SBP, mmHg | 134 ± 14 | 126 ± 11 | 134 ± 14 | 134 ± 14 | −8.0 (−14.6, −1.4) | 0.0198 |
| DBP, mmHg | 80 ± 13 | 80 ± 14 | 79 ± 11 | 80 ± 9 | −0.6 (−6.0, 4.8) | 0.823 |
| Fat mass, kg | 39.6 ± 8.2 | 37.7 ± 8.5 | 37.8 ± 10.5 | 36.5 ± 10.1 | −0.6 (−1.9, 0.7) | 0.339 |
| Total cholesterol, mg/dL | 200 ± 32 | 188 ± 33 | 203 ± 34 | 208 ± 30 | −19 (−35, 0) | 0.0467 |
| PWV, m/s | 9.6 ± 1.9 | 9.2 ± 1.7 | 9.2 ± 1.7 | 9.2 ± 1.4 | −0.4 (−1.1, 0.3) | 0.249 |
| AIx, % | 17.7 ± 10.6 | 12.6 ± 14.1 | 16.1 ± 11.9 | 17.3 ± 10.0 | −6.3 (−14.8, 2.2) | 0.142 |
Values are means ± SD. Data were analyzed using independent t tests. AIx, augmentation index; CI, confidence interval; DBP, brachial diastolic blood pressure; EXE, exercise; EXS, exercise + sauna; PRE, preintervention; POST, postintervention; PWV, pulse wave velocity; SBP, brachial systolic blood pressure; V̇o2max, maximal oxygen consumption.
Figure 3.
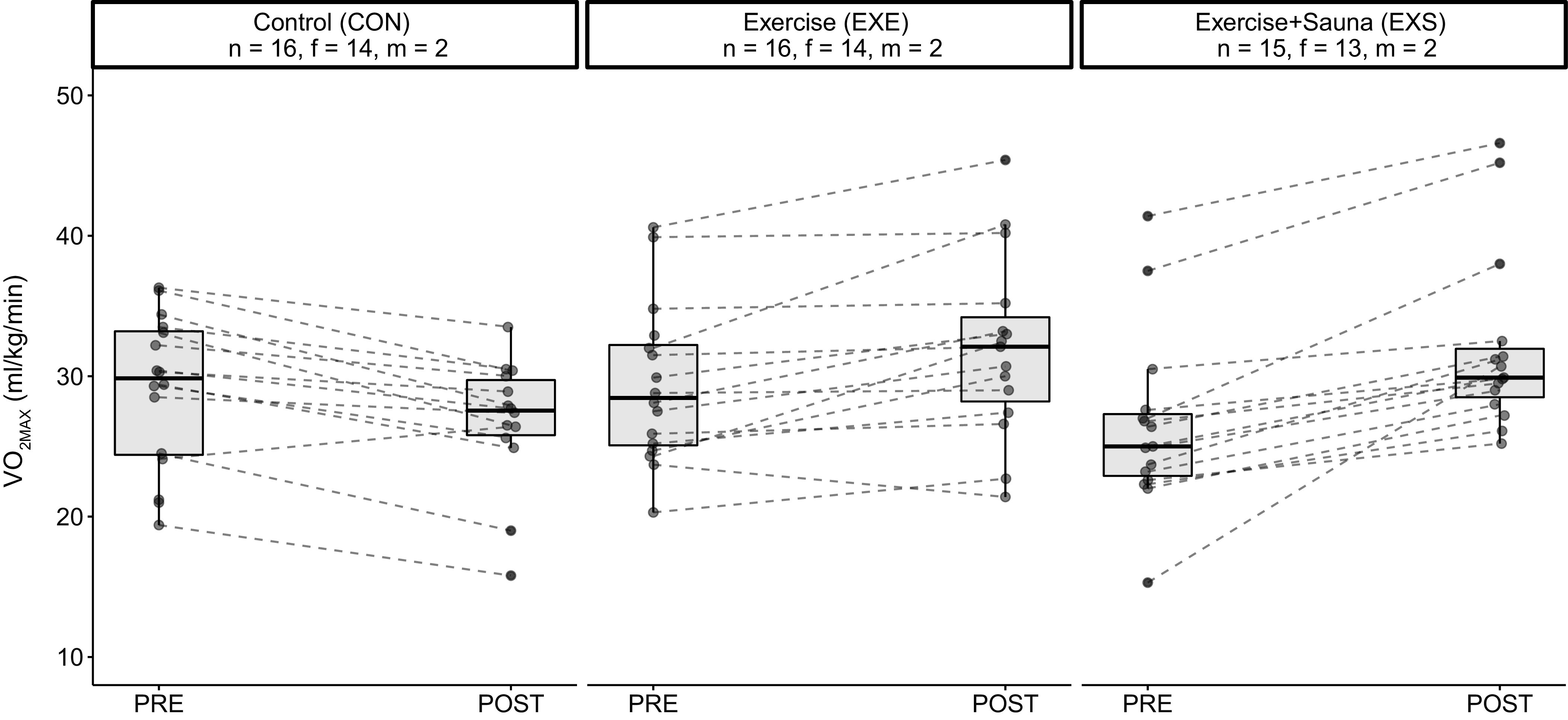
Graphical representation of the PRE-POST changes in CRF (relative V̇o2max) of the three groups. CRF, cardiorespiratory fitness; f, female; m, male; PRE, preintervention; POST, postintervention.
Figure 4.
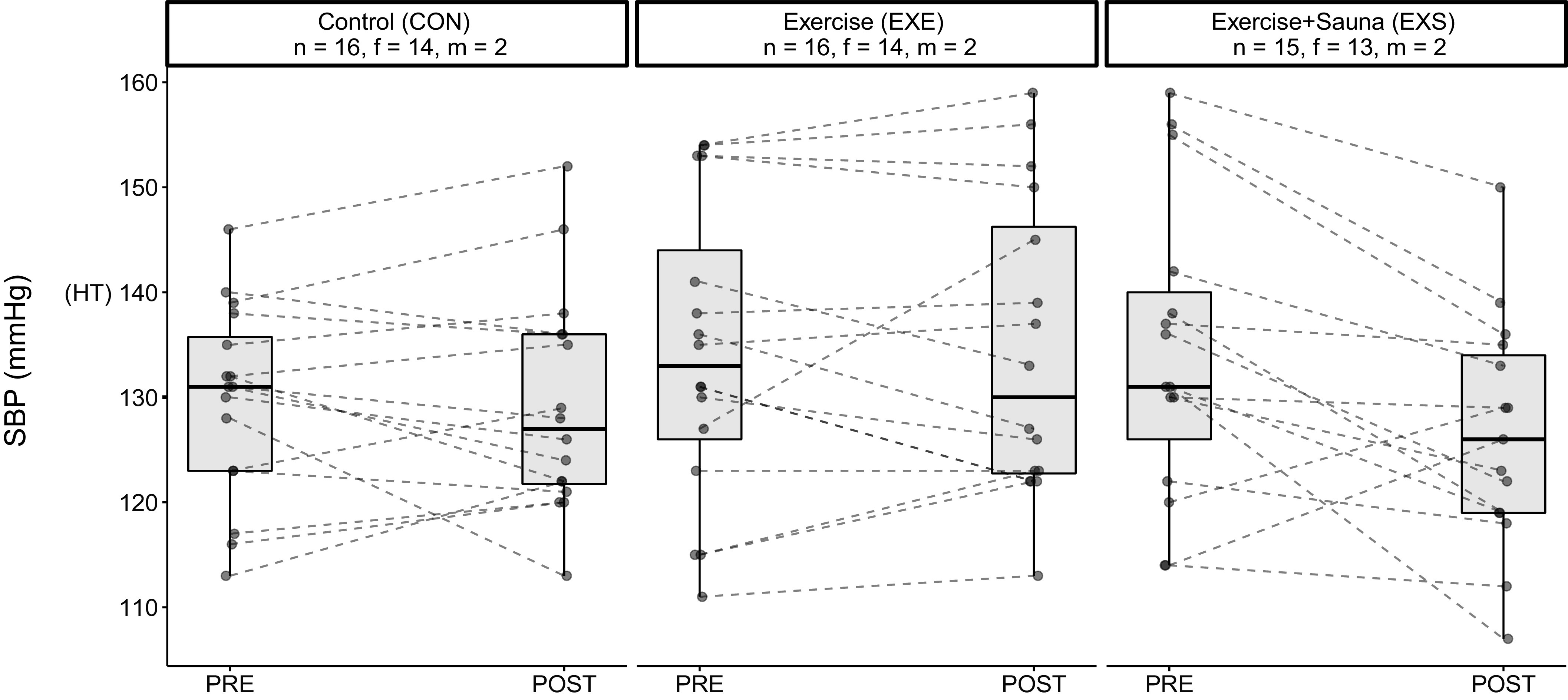
Graphical representation of the PRE-POST changes in SBP of the three groups. HT, Grade 1 hypertension classification; f, female; m, male; PRE, preintervention; POST, postintervention; SBP, systolic blood pressure.
Figure 5.
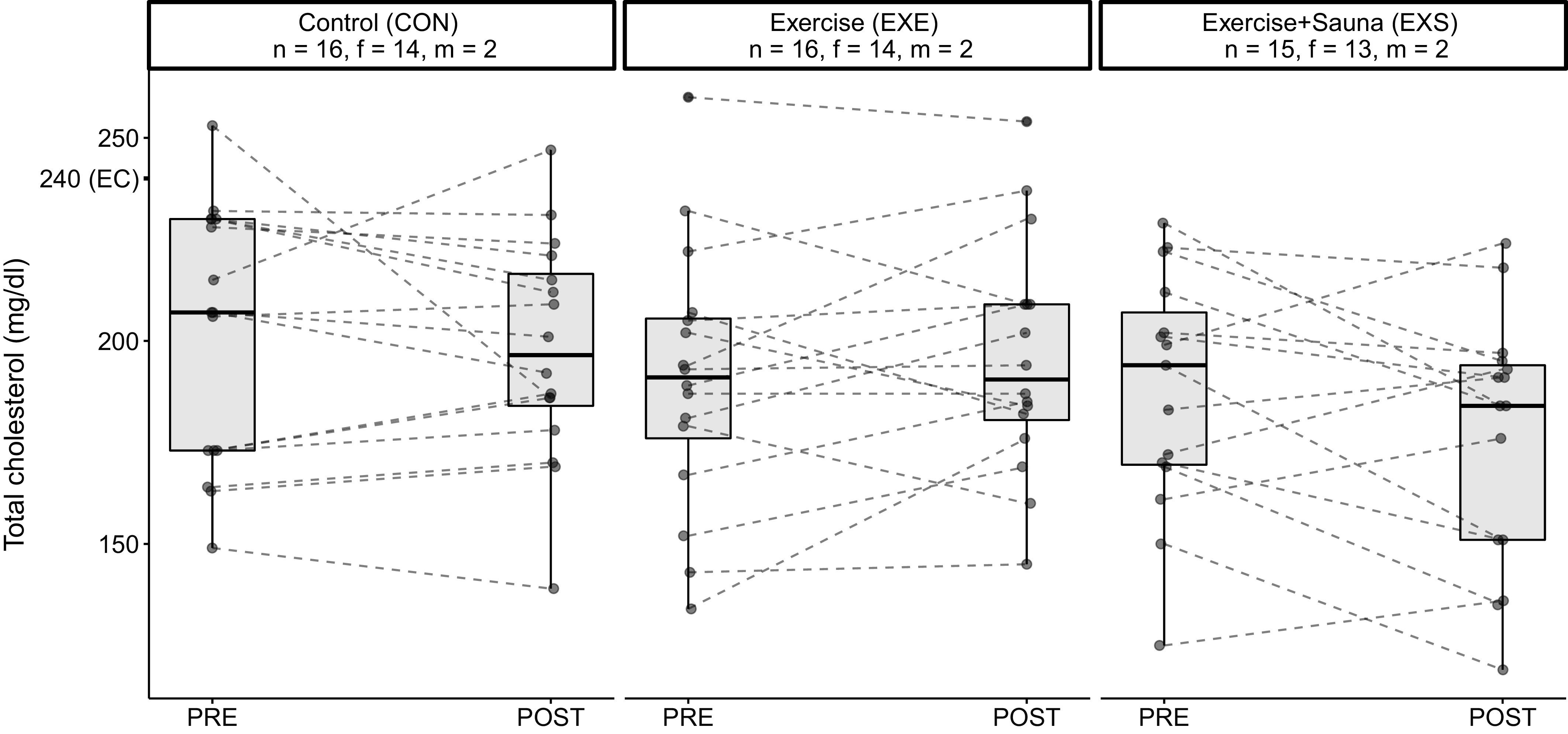
Graphical representation of the PRE-POST changes in total cholesterol levels of the three groups. EC, elevated cholesterol; f, female; m, male; PRE, preintervention; POST, postintervention.
DISCUSSION
In this multi-arm RCT, we compared the effects of an 8-wk exercise and sauna intervention (EXS) to regular exercise without sauna (EXE), using a sedentary population with relatively low physical activity levels and at least one traditional CVD risk factor. A control group (CON) was included to validate the efficacy of the exercise intervention. Our results show improvements in CRF based on the estimated V̇o2max and lower fat mass for the EXE group compared with the CON group. More importantly, the EXS group demonstrated a greater increase in CRF, and greater decreases in SBP and total cholesterol levels, when compared with the EXE group.
To a reasonable extent, the differences seen between the CON and EXE groups were expected. Physical activity guidelines supported by research evidence suggest that 150 min of moderate-intensity exercise per week is sufficient to induce beneficial health adaptations (4). As such, our 8-wk exercise intervention was constructed to adhere as closely as possible to the recommendations (31). Every supervised session included a full-body warm-up, followed by resistance, then aerobic exercise. In addition, exercise intensity was progressively increased throughout the 8 wk, for both resistance and aerobic exercise.
The long-term benefits of exercise training on physical health have been well established (3, 39), and regular aerobic training has been shown to improve both body composition (39) and CRF (40) even at relatively lower intensities, which is consistent with the main findings of the current study. Furthermore, it has been well documented that performing resistance exercise before aerobic exercise leads to higher energy expenditure and fat mass loss (41–43), which was how our training sessions were designed. The results from this experiment are in support of the literature and are indicative that the 8-wk exercise intervention provided an adequate stimulus for physiological adaptations to both CRF and body composition.
Despite these adaptations, however, there were no differences in changes to BP and other secondary variables such as arterial stiffness between the EXE and CON groups. This may have been partially due to the length of the present intervention, as training interventions are typically longer in duration. Although cardiovascular adaptations such as arterial remodeling and capillary growth have been well documented to occur within the first few weeks of exercise training (44), this did not appear to be the case. The structure of the exercise training likely contributed to this lack of response in the other variables, as the divergent nature of resistance and aerobic exercise has been reasonably established (45). Moreover, combined resistance and aerobic training have been documented to be less effective than aerobic training alone in reducing arterial stiffness (46) and BP (47).
One of the objectives of the current study was to elucidate the synergistic effects of sauna exposure and exercise on the primary variables of BP and CRF, in a sedentary population with traditional CVD risk factors. Specifically, our data suggest that the addition of 15-min sauna exposure, regularly after every exercise session, three times a week for 8 wk was able to improve CRF, SBP, and total cholesterol levels significantly, when compared with performing the same exercise intervention alone. Indeed, previous studies have shown that sauna exposure is an effective additive tool to an exercise program for both clinical (13, 48) and athletic populations (10, 49).
Studies have found positive acute cardiovascular responses from the use of passive heat (50), sauna exposure (27, 51), and sauna exposure with exercise (52, 53). A recent study also showed that postexercise sauna exposure had an augmentative effect, thereby increasing the overall training stress (54). However, to our best knowledge, this is the first multi-arm RCT investigating the cardiovascular and health effects of long-term sauna exposure in the general population with CVD risk factors. The current results suggest that the addition of regular sauna exposure was able to increase CRF and led to a decrease in SBP when comparing the EXS and EXE groups.
Heat acclimation studies have shown the efficacy of using heat to improve aerobic fitness (55, 56). Moreover, the use of heat has been shown to induce a greater level of acute physiological strain and cellular response at a lower relative workload than hypoxia (57). Therefore, it is more likely that the present cardiovascular adaptations seen in this study may be the result of functional enhancements rather than structural changes in the arteries (58), as there were no significant changes to PWV and AIx as measures of arterial stiffness. Nevertheless, this needs to be further investigated, as heat shock proteins, muscle endothelial nitric oxide synthase content, and capillary density were not measured in the present study.
It is worth noting that CRF adaptations to passive heat exposure in the form of sauna bathing have yet to be thoroughly investigated mechanistically. However, cardiovascular adaptations to heat acclimation have been well documented (59), which provides us with a vital framework that may explain the difference in CRF between the EXE and EXS groups. Exercise training and passive heat have been shown to have additive effects that lead to improved myocardial contractility, over exercise training or heat alone, in animal models (60). Eight weeks of heat acclimation has been shown to improve myocardial compliance, rendering it more efficient (60, 61). It is thus plausible that cardiovascular stability may have been augmented by the addition of regular sauna exposure to exercise, and that functional, rather than morphological adaptations were responsible for the differences in CRF between the intervention groups, particularly in the absence of changes to arterial stiffness parameters. Moreover, infrared sauna therapy (13) has also been shown to augment increases in CRF via functional changes, which is consistent with our present findings.
A recent systematic review showed that compared with controls, heat therapy decreased both SBP and DBP by an average of 4 mmHg (23). Based on the latest guidelines on BP (30, 35), it was postulated that a reduction in BP by ∼5 mmHg would improve individual BP categorization (23, 62). Although our study did not find differences in DBP between interventions, SBP levels for the EXS group were 8 mmHg lower than in the EXE group postintervention, which is almost an entire BP category. This is a clinically important new finding to highlight, as a recent meta-analysis (63) reported a nearly linear relationship between a 5-mmHg decrease in SBP and a lowered risk of all-cause mortality across all BP categories.
Although higher resting BP before an intervention has been suggested as a potential mitigating factor in the therapeutic effects seen in heat-related studies (23), only 35% of the participants in the current experiment had an elevated resting BP. In addition, there was no difference in the PRE values of SBP and DBP between all three groups. One mechanism that may have contributed to the lower SBP in the EXS group was the concomitant lowering of total cholesterol levels. This agrees with the findings from an earlier study (64), which found that heat therapy was able to improve the blood lipid profile, specifically total cholesterol levels, in a sedentary obese population. In addition, there were improvements to BP but no changes to BMI or body composition, which is remarkably comparable to the findings from our current study. Even though the authors used hot water immersion as opposed to the sauna, there were similarities between our experimental designs worth noting, such as the intervention period and the frequency of exposure. These are crucial factors to consider for future research in the area, as has been pointed out by several others (23, 58).
Some limitations of this study ought to be noted. The trial lacked a sauna-only group, which would have allowed us to determine if the benefits seen could have been solely attributable to the sauna. Diet of the participants was not controlled for during the trial, which could have influenced the results. However, participants followed the same diet a day before each measurement was taken, which improves the consistency and reliability of our data. Maximal oxygen consumption was not measured directly, but the indirect method better suited the study population and added external validity. The study sample had only six male participants. However, we addressed this issue using stratified randomization and included separate tables of results (Supplemental Tables S2 and S3, Supplemental Fig. S1; see https://doi.org/10.6084/m9.figshare.19582801.v1, Supplemental Fig. S2; see https://doi.org/10.6084/m9.figshare.19582813.v1, Supplemental Fig. S3; see https://doi.org/10.6084/m9.figshare.19582822.v1) that excludes the males for a more accurate and complete perspective. Moreover, insufficient female data have been a long-standing problem in scientific research; therefore, this could be viewed as a strength, rather than a limitation of this study.
Indeed, our study does have several strengths. We extended the findings of our earlier research in the acute setting (28, 65), with an 8-wk interventional study using a sedentary population, who were nonfrequent sauna users, to investigate the complementary effects of exercise followed by sauna exposure. Body composition was determined using dual-energy X-ray absorptiometry, while arterial stiffness and BP measurements adhered closely to established guidelines (30, 35, 36). Compliance of the intervention groups was excellent, with only 2 participants each missing a single session out of 24. All other participants completed the 24 sessions successfully with only 1 dropout for the entire study. Moreover, a statistician that was blind to the intervention assignment performed the data analysis using coded variables.
In conclusion, regular exercise using the recommended guidelines three times a week, for 50 min each time, can effectively improve CRF and body composition. The addition of a regular 15-min typical Finnish sauna after exercise supplemented the gains in CRF, reductions in SBP, and lowered total cholesterol levels considerably. Future research should adopt a more systematic approach in the study of heat exposure and seek to understand the optimal exposure durations, frequencies, modalities, and temperatures for various beneficial adaptations.
Perspectives and Significance
The design of this experiment allowed us to ascertain to a reasonable extent the additive effect of regular sauna exposure to exercise on cardiovascular health outcomes such as BP and CRF. These beneficial changes seen are promising, given that the essential methodological parameters of sauna exposure, such as duration and frequency were not only relatively short and tolerable, but practically feasible and replicable as well. Taken into context with mechanistic studies from molecular physiology, this is indicative of the noteworthy potential that passive heat therapy has. In addition, this study opens up opportunities to investigate shorter bouts of regular exercise in conjunction with sauna use and lends support for regular sauna bathing to be a possible therapeutic alternative, particularly for those with compromised exercise capacities, and possibly other rehabilitation settings as well. Sauna bathing is a safe and simple lifestyle modification and steps should be taken to make it more accessible worldwide.
DATA AVAILABILITY
The data that support the findings of this study are available to researchers upon reasonable request to the corresponding author.
SUPPLEMENTAL DATA
Supplemental Table S1; https://doi.org/10.6084/m9.figshare.20160479.v1.
Supplemental Table S2 https://doi.org/10.6084/m9.figshare.19575979.v1.
Supplemental Table S3 https://doi.org/10.6084/m9.figshare.19576030.v1.
Supplemental Fig. S1 https://doi.org/10.6084/m9.figshare.19582801.v1.
Supplemental Fig. S2 https://doi.org/10.6084/m9.figshare.19582813.v1.
Supplemental Fig. S3: https://doi.org/10.6084/m9.figshare.19582822.v1.
GRANTS
The Finnish Cultural Foundation (SKR) partially funded the corresponding author’s salary (Grant No.: 00190620 to E. Lee).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.L. conceived and designed research; E.L. and I.K. performed experiments; J.K. analyzed data; E.L., J.P.A., E.A.H., and J.A.L. interpreted results of experiments; J.K. prepared figures; E.L. and J.K. drafted manuscript; E.L., J.K., J.P.A., E.A.H., P.W., S.K.K., and J.A.L. edited and revised manuscript; E.L., I.K., J.K., J.P.A., E.A.H., P.W., S.K.K., and J.A.L. approved final version of manuscript.
REFERENCES
- 1. Kruk J. Physical activity in the prevention of the most frequent chronic diseases: an analysis of the recent evidence. Asian Pac J Cancer Prev 8: 325–338, 2007. [PubMed] [Google Scholar]
- 2. Myers J, McAuley P, Lavie CJ, Despres J-P, Arena R, Kokkinos P. Physical activity and cardiorespiratory fitness as major markers of cardiovascular risk: their independent and interwoven importance to health status. Prog Cardiovasc Dis 57: 306–314, 2015. doi: 10.1016/j.pcad.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 3. Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The physical activity guidelines for Americans. JAMA 320: 2020–2028, 2018. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Authors/Task Force Members; Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, , et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur J Prev Cardiol. 23: NP1–NP96, 2016. doi: 10.1177/2047487316653709. [DOI] [PubMed] [Google Scholar]
- 5. Hussain JN, Greaves RF, Cohen MM. A hot topic for health: results of the Global Sauna Survey. Complement Ther Med 44: 223–234, 2019. doi: 10.1016/j.ctim.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 6. Laukkanen T, Khan H, Zaccardi F, Laukkanen JA. Association between sauna bathing and fatal cardiovascular and all-cause mortality events. JAMA Intern Med 175: 542–548, 2015. doi: 10.1001/jamainternmed.2014.8187. [DOI] [PubMed] [Google Scholar]
- 7. Kunutsor SK, Khan H, Zaccardi F, Laukkanen T, Willeit P, Laukkanen JA. Sauna bathing reduces the risk of stroke in Finnish men and women: a prospective cohort study. Neurology 90: e1937–e1944, 2018. doi: 10.1212/WNL.0000000000005606. [DOI] [PubMed] [Google Scholar]
- 8. Laukkanen JA, Laukkanen T. Sauna bathing and systemic inflammation. Eur J Epidemiol 33: 351–353, 2018. doi: 10.1007/s10654-017-0335-y. [DOI] [PubMed] [Google Scholar]
- 9. Li Z, Jiang W, Chen Y, Wang G, Yan F, Zeng T, Fan H. Acute and short-term efficacy of sauna treatment on cardiovascular function: a meta-analysis. Eur J Cardiovasc Nurs 20: 96–105, 2021. doi: 10.1177/1474515120944584. [DOI] [PubMed] [Google Scholar]
- 10. Brunt VE, Minson CT. Heat therapy: mechanistic underpinnings and applications to cardiovascular health. J Appl Physiol (1985) 130: 1684–1704, 2021. doi: 10.1152/japplphysiol.00141.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stanley J, Halliday A, D'Auria S, Buchheit M, Leicht AS. Effect of sauna-based heat acclimation on plasma volume and heart rate variability. Eur J Appl Physiol 115: 785–794, 2015. doi: 10.1007/s00421-014-3060-1. [DOI] [PubMed] [Google Scholar]
- 12. Kirby NV, Lucas SJE, Armstrong OJ, Weaver SR, Lucas RAI. Intermittent post-exercise sauna bathing improves markers of exercise capacity in hot and temperate conditions in trained middle-distance runners. Eur J Appl Physiol 121: 621–635, 2021. doi: 10.1007/s00421-020-04541-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ohori T, Nozawa T, Ihori H, Shida T, Sobajima M, Matsuki A, Yasumura S, Inoue H. Effect of repeated sauna treatment on exercise tolerance and endothelial function in patients with chronic heart failure. Am J Cardiol 109: 100–104, 2012. doi: 10.1016/j.amjcard.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 14. Matsumoto S, Shimodozono M, Etoh S, Miyata R, Kawahira K. Effects of thermal therapy combining sauna therapy and underwater exercise in patients with fibromyalgia. Complement Ther Clin Pract 17: 162–166, 2011. doi: 10.1016/j.ctcp.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 15. Collins HP, AM Q, Kalisch D. Key Indicators of Progress for Chronic Disease and Associated Determinants. Canberra: Australian Institute of Health and Welfare (AIHW), Australian Government. 2011. https://www.aihw.gov.au/reports/chronic-disease/key-indicators-of-progress-for-chronic-disease/summary. [Google Scholar]
- 16.Public Health Agency of Canada. Risk factor atlas (Online). https://www.canada.ca/en/public-health/services/chronic-diseases/risk-factor-atlas.html [2021 May 5].
- 17. Timmis A, Townsend N, Gale C, Grobbee R, Maniadakis N, Flather M, Wilkins E, Wright L, Vos R, Bax J, Blum M, Pinto F, Vardas P; ESC Scientific Document Group. European Society of Cardiology: Cardiovascular Disease Statistics 2017. Eur Heart J 39: 508–579, 2018. doi: 10.1093/eurheartj/ehx628. [DOI] [PubMed] [Google Scholar]
- 18. Fryar CD, Chen T-C, Li X. Prevalence of uncontrolled risk factors for cardiovascular disease: United States, 1999–2010. NCHS Data Brief 103: 1–8, 2012. [PubMed] [Google Scholar]
- 19. Li X, Wu C, Lu J, Chen B, Li Y, Yang Y, Hu S, Li J. Cardiovascular risk factors in China: a nationwide population-based cohort study. Lancet Public Health 5: e672–e681, 2020. [Erratum in Lancet Public Health 6: e271, 2021] doi: 10.1016/S2468-2667(20)30191-2. [DOI] [PubMed] [Google Scholar]
- 20. Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, , et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129: S76–S99, 2014. [Erratum in Circulation 129 25 Suppl 2: S100–S101, 2014]. doi: 10.1161/01.cir.0000437740.48606.d1. [DOI] [PubMed] [Google Scholar]
- 21. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, , et al. Heart Disease and Stroke Statistics-2022 Update: a report from the American Heart Association. Circulation 145: e153–e639, 2022. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 22. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr.. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 138: e484–e594, 2018. doi: 10.1161/CIR.000000000000059610.1016/j.jacc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 23. Pizzey FK, Smith EC, Ruediger SL, Keating SE, Askew CD, Coombes JS, Bailey TG. The effect of heat therapy on blood pressure and peripheral vascular function: a systematic review and meta-analysis. Exp Physiol 106: 1317–1334, 2021. doi: 10.1113/EP089424. [DOI] [PubMed] [Google Scholar]
- 24. Ross R, Blair SN, Arena R, Church TS, Després J-P, Franklin BA, Haskell WL, Kaminsky LA, Levine BD, Lavie CJ, Myers J, Niebauer J, Sallis R, Sawada SS, Sui X, Wisløff U; American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Cardiovascular and Stroke Nursing; Council on Functional Genomics and Translational Biology; Stroke Council. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation 134: e653–e699, 2016. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 25. Harber MP, Kaminsky LA, Arena R, Blair SN, Franklin BA, Myers J, Ross R. Impact of cardiorespiratory fitness on all-cause and disease-specific mortality: advances since 2009. Prog Cardiovasc Dis 60: 11–20, 2017. doi: 10.1016/j.pcad.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 26. Celis-Morales CA, Lyall DM, Anderson J, Iliodromiti S, Fan Y, Ntuk UE, Mackay DF, Pell JP, Sattar N, Gill JMR. The association between physical activity and risk of mortality is modulated by grip strength and cardiorespiratory fitness: evidence from 498 135 UK-Biobank participants. Eur Heart J 38: 116–122, 2017. doi: 10.1093/eurheartj/ehw249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee E, Laukkanen T, Kunutsor SK, Khan H, Willeit P, Zaccardi F, Laukkanen JA. Sauna exposure leads to improved arterial compliance: findings from a non-randomised experimental study. Eur J Prev Cardiol 25: 130–138, 2018. doi: 10.1177/2047487317737629. [DOI] [PubMed] [Google Scholar]
- 28. Lee E, Willeit P, Laukkanen T, Kunutsor SK, Zaccardi F, Khan H, Laukkanen JA. Acute effects of exercise and sauna as a single intervention on arterial compliance. Eur J Prev Cardiol 27: 1104–1107, 2020. doi: 10.1177/2047487319855454. [DOI] [PubMed] [Google Scholar]
- 29. Boutron I, Altman DG, Moher D, Schulz KF, Ravaud P, CONSORT NPT Group. CONSORT statement for randomized trials of nonpharmacologic treatments: a 2017 update and a CONSORT extension for nonpharmacologic trial abstracts. Ann Intern Med 167: 40–47, 2017. doi: 10.7326/M17-0046. [DOI] [PubMed] [Google Scholar]
- 30. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension [Erratum in Eur Heart J 40: 475, 3021–3104, 2018]. Eur Heart J 39: 3021–3104, 2018. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 31.UKK-instituutti. Liikkumalla terveyttä – askel kerrallaan. Viikoittainen liikkumisen suositus 18-64 vuotiaille, 2020. 2020, accessed 13 April 2022. https://ukkinstituutti.fi/liikkuminen/liikkumisen-suositukset/aikuisten-liikkumisen-suositus/. [Google Scholar]
- 32. Nes BM, Janszky I, Wisløff U, Støylen A, Karlsen T. Age-predicted maximal heart rate in healthy subjects: the HUNT fitness study. Scand J Med Sci Sports 23: 697–704, 2013. doi: 10.1111/j.1600-0838.2012.01445.x. [DOI] [PubMed] [Google Scholar]
- 33. Golding LA, Myers CR. Y’s Way to Physical Fitness: The Complete Guide to Fitness Testing and Instruction. Champaign, Illinois: Human Kinetics, 1989. [Google Scholar]
- 34. Andersen KL, Shephard RJ, Denolin H, Varnauskas E, Masironi R, et al. Fundamentals of Exercise Testing. Geneva: World Health Organization, 1971, p. 54–56. https://apps.who.int/iris/handle/10665/40145. [Google Scholar]
- 35. Muntner P, Shimbo D, Carey RM, Charleston JB, Gaillard T, Misra S, Myers MG, Ogedegbe G, Schwartz JE, Townsend RR, Urbina EM, Viera AJ, White WB, Wright JT Jr.. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension 73: e35–e66, 2019. doi: 10.1161/HYP.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tomlinson LA. Methods for assessing arterial stiffness: technical considerations. Curr Opin Nephrol Hypertens 21: 655–660, 2012. doi: 10.1097/MNH.0b013e32835856e3. [DOI] [PubMed] [Google Scholar]
- 37.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria; 2022. http://www.R-project.org/. [Google Scholar]
- 38. Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag, 2016. https://ggplot2.tidyverse.org. [Google Scholar]
- 39. Wilson MG, Ellison GM, Cable NT. Basic science behind the cardiovascular benefits of exercise. Heart 101: 758–765, 2015. doi: 10.1136/heartjnl-2014-306596. [DOI] [PubMed] [Google Scholar]
- 40. Milanović Z, Sporiš G, Weston M. Effectiveness of high-intensity interval training (HIT) and continuous endurance training for VO2max improvements: a systematic review and meta-analysis of controlled trials. Sports Med 45: 1469–1481, 2015. doi: 10.1007/s40279-015-0365-0. [DOI] [PubMed] [Google Scholar]
- 41. Gravelle BL, Blessing DL. Physiological adaptation in women concurrently training for strength and endurance. J Strength Cond Res 14: 5–13, 2000. doi:. [DOI] [Google Scholar]
- 42. Kang J, Rashti SL, Tranchina CP, Ratamess NA, Faigenbaum AD, Hoffman JR. Effect of preceding resistance exercise on metabolism during subsequent aerobic session. Eur J Appl Physiol 107: 43–50, 2009. doi: 10.1007/s00421-009-1100-z. [DOI] [PubMed] [Google Scholar]
- 43. Kang J, Ratamess N. Which comes first? Resistance before aerobic exercise or vice versa? ACSM's Health & Fitness Journal 18: 9–14, 2014. doi: 10.1249/FIT.0000000000000004. [DOI] [Google Scholar]
- 44. Hellsten Y, Nyberg M. Cardiovascular adaptations to exercise training. Compr Physiol 6: 1–32, 2015. doi: 10.1002/cphy.c140080. [DOI] [PubMed] [Google Scholar]
- 45. Fyfe JJ, Bishop DJ, Stepto NK. Interference between concurrent resistance and endurance exercise: molecular bases and the role of individual training variables. Sports Med 44: 743–762, 2014. doi: 10.1007/s40279-014-0162-1. [DOI] [PubMed] [Google Scholar]
- 46. Montero D, Vinet A, Roberts CK. Effect of combined aerobic and resistance training versus aerobic training on arterial stiffness. Int J Cardiol 178: 69–76, 2015. doi: 10.1016/j.ijcard.2014.10.147. [DOI] [PubMed] [Google Scholar]
- 47. Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc 2: e004473, 2013. doi: 10.1161/JAHA.112.004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Haseba S, Sakakima H, Kubozono T, Nakao S, Ikeda S. Combined effects of repeated sauna therapy and exercise training on cardiac function and physical activity in patients with chronic heart failure. Disabil Rehabil 38: 409–415, 2016. doi: 10.3109/09638288.2015.1044032. [DOI] [PubMed] [Google Scholar]
- 49. Scoon GSM, Hopkins WG, Mayhew S, Cotter JD. Effect of post-exercise sauna bathing on the endurance performance of competitive male runners. J Sci Med Sport 10: 259–262, 2007. doi: 10.1016/j.jsams.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 50. Caldwell AR, Robinson FB, Tucker MA, Arcement CH, Butts CL, McDermott BP, Ganio MS. Effect of passive heat stress and exercise in the heat on arterial stiffness. Eur J Appl Physiol 117: 1679–1687, 2017. doi: 10.1007/s00421-017-3658-1. [DOI] [PubMed] [Google Scholar]
- 51. Kukkonen-Harjula K, Oja P, Laustiola K, Vuori I, Jolkkonen J, Siitonen S, Vapaatalo H. Haemodynamic and hormonal responses to heat exposure in a Finnish sauna bath. Eur J Appl Physiol Occup Physiol 58: 543–550, 1989. doi: 10.1007/BF02330710. [DOI] [PubMed] [Google Scholar]
- 52. Gayda M, Paillard F, Sosner P, Juneau M, Garzon M, Gonzalez M, Bélanger M, Nigam A. Effects of sauna alone and postexercise sauna baths on blood pressure and hemodynamic variables in patients with untreated hypertension. J Clin Hypertens (Greenwich) 14: 553–560, 2012. doi: 10.1111/j.1751-7176.2012.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sutkowy P, Woźniak A, Boraczyński T, Mila-Kierzenkowska C, Boraczyński M. The effect of a single Finnish sauna bath after aerobic exercise on the oxidative status in healthy men. Scand J Clin Lab Invest 74: 89–94, 2014. doi: 10.3109/00365513.2013.860616. [DOI] [PubMed] [Google Scholar]
- 54. Skorski S, Schimpchen J, Pfeiffer M, Ferrauti A, Kellmann M, Meyer T. Effects of postexercise sauna bathing on recovery of swim performance. Int J Sports Physiol Perform 15: 934–940, 2019. doi: 10.1123/ijspp.2019-0333. [DOI] [PubMed] [Google Scholar]
- 55. Cheung SS, McLellan TM. Heat acclimation, aerobic fitness, and hydration effects on tolerance during uncompensable heat stress. J Appl Physiol (1985) 84: 1731–1739, 1998. doi: 10.1152/jappl.1998.84.5.1731. [DOI] [PubMed] [Google Scholar]
- 56. Heathcote SL, Hassmén P, Zhou S, Stevens CJ. Passive heating: reviewing practical heat acclimation strategies for endurance athletes. Front Physiol 9: 1851, 2018. doi: 10.3389/fphys.2018.01851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee BJ, Emery-Sinclair EL, Mackenzie RW, Hussain A, Taylor L, James RS, Thake CD. The impact of submaximal exercise during heat and/or hypoxia on the cardiovascular and monocyte HSP72 responses to subsequent (post 24 h) exercise in hypoxia. Extrem Physiol Med 3: 15, 2014. doi: 10.1186/2046-7648-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cullen T, Clarke ND, Hill M, Menzies C, Pugh CJA, Steward CJ, Thake CD. The health benefits of passive heating and aerobic exercise: to what extent do the mechanisms overlap? J Appl Physiol (1985) 129: 1304–1309, 2020. doi: 10.1152/japplphysiol.00608.2020. [DOI] [PubMed] [Google Scholar]
- 59. Taylor NAS. Human heat adaptation. Compr Physiol 4: 325–365, 2014. doi: 10.1002/cphy.c130022. [DOI] [PubMed] [Google Scholar]
- 60. Levy E, Hasin Y, Navon G, Horowitz M. Chronic heat improves mechanical and metabolic response of trained rat heart on ischemia and reperfusion. Am J Physiol Heart Circ Physiol 272: H2085–H2094, 1997. doi: 10.1152/ajpheart.1997.272.5.H2085. [DOI] [PubMed] [Google Scholar]
- 61. Horowitz M, Shimoni Y, Parnes S, Gotsman MS, Hasin Y. Heat acclimation: cardiac performance of isolated rat heart. J Appl Physiol (1985) 60: 9–13, 1986. doi: 10.1152/jappl.1986.60.1.9. [DOI] [PubMed] [Google Scholar]
- 62. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, , et al. Heart Disease and Stroke Statistics-2019 update: a report from the American Heart Association. Circulation 139: e56–e528, 2019. [Erratum in Circulation 141: e33, 2020]. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 63. Bundy JD, Li C, Stuchlik P, Bu X, Kelly TN, Mills KT, He H, Chen J, Whelton PK, He J. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol 2: 775–781, 2017. doi: 10.1001/jamacardio.2017.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ely BR, Francisco MA, Halliwill JR, Bryan SD, Comrada LN, Larson EA, Brunt VE, Minson CT. Heat therapy reduces sympathetic activity and improves cardiovascular risk profile in women who are obese with polycystic ovary syndrome. Am J Physiol Regul Integr Comp Physiol 317: R630–R640, 2019. doi: 10.1152/ajpregu.00078.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lee E, Kostensalo J, Willeit P, Kunutsor SK, Laukkanen T, Zaccardi F, Khan H, Laukkanen JA. Standalone sauna vs exercise followed by sauna on cardiovascular function in non-naïve sauna users: a comparison of acute effects. Health Sci Rep 4: e393, 2021. doi: 10.1002/hsr2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1; https://doi.org/10.6084/m9.figshare.20160479.v1.
Supplemental Table S2 https://doi.org/10.6084/m9.figshare.19575979.v1.
Supplemental Table S3 https://doi.org/10.6084/m9.figshare.19576030.v1.
Supplemental Fig. S1 https://doi.org/10.6084/m9.figshare.19582801.v1.
Supplemental Fig. S2 https://doi.org/10.6084/m9.figshare.19582813.v1.
Supplemental Fig. S3: https://doi.org/10.6084/m9.figshare.19582822.v1.
Data Availability Statement
The data that support the findings of this study are available to researchers upon reasonable request to the corresponding author.