Keywords: claudin-2, endocannabinoid system, enteric nervous system, Ussing chambers, zonula occludens-1
Abstract
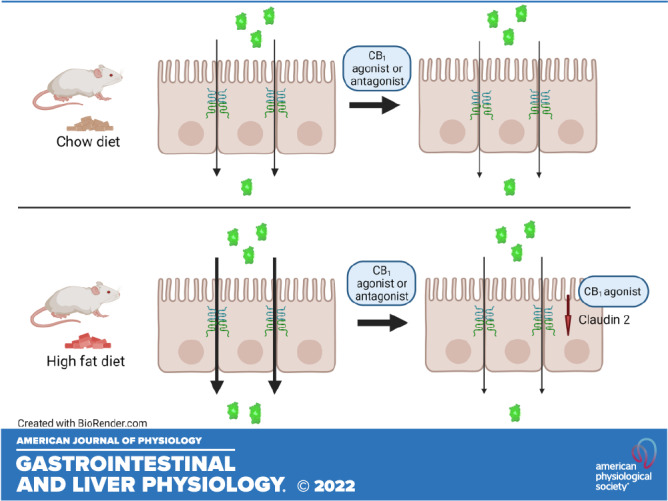
The endocannabinoid system of the gastrointestinal tract is involved in the control of intestinal barrier function. Whether the cannabinoid 1 (CB1) receptor is expressed on the intestinal epithelium and acutely regulates barrier function has not been determined. Here, we tested the hypothesis that ligands of the CB1 receptor acutely modulate small intestinal permeability and that this is associated with altered distribution of tight junction proteins. We examined the acute effects of CB1 receptor ligands on small intestinal permeability both in chow-fed and 2-wk high-fat diet (HFD)-fed mice using Ussing chambers. We assessed the distribution of CB1 receptor and tight junction proteins using immunofluorescence and the expression of CB1 receptor using PCR. A low level of CB1 expression was found on the intestinal epithelium. CB1 receptor was highly expressed on enteric nerves in the lamina propria. Neither the CB1/CB2 agonist CP55,940 nor the CB1 neutral antagonist AM6545 altered the flux of 4kDa FITC dextran (FD4) across the jejunum or ileum of chow-fed mice. Remarkably, both CP55,940 and AM6545 reduced FD4 flux across the jejunum and ileum in HFD-fed mice that have elevated baseline intestinal permeability. These effects were absent in CB1 knockout mice. CP55,940 reduced the expression of claudin-2, whereas AM6545 had little effect on claudin-2 expression. Neither ligand altered the expression of ZO-1. Our data suggest that CB1 receptor on the intestinal epithelium regulates tight junction protein expression and restores barrier function when it is increased following exposure to a HFD for 2 wk.
NEW & NOTEWORTHY The endocannabinoid system of the gastrointestinal tract regulates homeostasis by acting as brake on motility and secretion. Here we show that when exposed to a high fat diet, intestinal permeability is increased and activation of the CB1 receptor on the intestinal epithelium restores barrier function. This work further highlights the role of the endocannabinoid system in regulating intestinal homeostasis when it is perturbed.
INTRODUCTION
The gastrointestinal (GI) tract is lined by a single layer of epithelial cells and provides a critical interface separating the contents of the lumen from the rest of the body (1, 2). The intestinal epithelium is a selectively permeable barrier that facilitates the paracellular and transcellular transport of nutrients, water and electrolytes, while simultaneously inhibiting the passage of harmful entities such as toxins, foreign antigens, pathogens, and commensal bacteria (1, 3–5). Paracellular transport across the epithelium is dynamically regulated by protein complexes located between epithelial cells known as tight junctions (5–7). There are four groups of transmembrane proteins that together form the tight junction complexes: occludin (8), claudins (9), junctional adhesion molecules (10), and tricellulin (11). Scaffold proteins, such as zonula occludens (ZO) proteins (12), and cingulin (13), anchor the intracellular domains of the transmembrane proteins to the actin cytoskeleton (3).
The regulation of epithelial permeability is critically important for health; a breakdown in epithelial barrier function is associated with diseases that include inflammatory bowel disease (5, 14), celiac disease (5, 15, 16), irritable bowel syndrome (17, 18), obesity (19), and several others (5, 16, 20–22). There is an established relationship between exposure to high-fat diet (HFD) for varying durations (e.g., 4 or 12 wk) and increased intestinal permeability (23–28). However, it remains somewhat unclear whether the observed alterations in permeability are a direct impact of the diet itself or are a result of body weight gain and/or metabolic consequences associated with exposure to the HFD. Several studies point to changes in the distribution and localization of tight junction proteins in mediating increased permeability following exposure to HFD or in genetic models of obesity; however, the vast majority of studies on permeability have been conducted in the large intestine (19, 24, 29).
The molecular and cellular mediators that locally regulate epithelial tight junctions include vasoactive intestinal peptide expressing submucosal enteric neurons (30), enteric glial cells (31), and the immune mediators tumor necrosis factor and interferon γ, which act through myosin light chain kinase activation (1, 32, 33). Recent evidence has suggested the lipid mediators anandamide and 2-arachidonoylglycerol (2-AG), components of the endocannabinoid system, may also be involved in intestinal barrier regulation (34). The endocannabinoid system consists of these, and other, lipid mediators that act at the two canonical cannabinoid (CB) receptors, CB1 and CB2, and of the biosynthetic and degradative enzymes for these ligands (35, 36). Endocannabinoids are generally made on demand when the cells that produce them are activated (37); however, there appears also to be a tonic activation of the endocannabinoid system where endocannabinoid tone is enhanced, notably in pathophysiological conditions including inflammatory and metabolic diseases, as well as after exposure to HFD (34, 38, 39).
The endocannabinoid system has been implicated in the regulation of intestinal permeability primarily in conditions where baseline endocannabinoid tone is elevated. Both Muccioli et al. and Mehrpouya-Bahrami et al. demonstrated in vivo that chronic CB1 antagonism with rimonabant in obese mice reduces intestinal permeability (25, 26). Consistent with this, Muccioli et al. showed that the CB1 agonist HU-210 increases intestinal permeability, however, Tajik et al. found that the CB1 agonist arachidonyl-2'-chloroethylamide reduced permeability (26, 40). Furthermore, studies of Caco-2 cell monolayers in vitro have consistently supported a role for the CB1 receptor in regulating colonic permeability, although opposing effects of luminal and systemic endocannabinoids have been described (26, 41–43). The involvement of the endocannabinoid system may regulate intestinal permeability through alterations in tight junction proteins. Genetically obese Ob/Ob mice treated with the CB1 antagonist rimonabant had improved intestinal permeability and this was associated with increased expression of ZO-1 and occludin (29).
Given the evidence implicating CB1 receptor signaling in the control of intestinal permeability and the inconsistencies reported with the use of CB1 agonists in vivo, we sought to explore the acute effects of CB1 receptor ligands on small intestinal permeability both in chow-fed and short-term HFD-fed mice ex vivo, using Ussing Chambers. We tested the hypothesis that ligands of the CB1 receptor acutely modulate small intestinal permeability and that this change in permeability is associated with altered distribution of tight junction proteins. We show that CB1 receptor ligands regulate small intestinal permeability in animals exposed to a HFD through changes in the distribution of the tight junction protein claudin-2.
METHODS
Animals
Male CD1 mice (Charles River, Montreal, Quebec, Canada) aged 5–6 wk and male CB1 knockout (C57BL/6 background) and wild-type littermates (bred in house) (44) were used in this study. Mice were group housed (up to 5 mice per cage), under a 12-h light-dark cycle (lights off at 19:00) in a temperature- and humidity-controlled room. Mice were allowed free access to food and tap water and were acclimatized to the housing facility for at least 7 days before the experimental manipulation. This study was approved by the University of Calgary Animal Care Committee (Protocol No.: AC19-0124), and experimental protocols were carried out in accordance with the guidelines of the Canadian Council on Animal Care. These studies followed the ARRIVE guidelines for reporting animal use. Animals were euthanized by cervical dislocation under deep isoflurane anesthesia.
Diet
Mice were allowed access to a standard chow diet (Pico-Vac Lab Rodent Diet no. 5061, 13.2% kcal from fat; Lab Diet, St. Louis, MO) or high-fat diet (HFD; D12451, 45% kcal from fat; Research Diets, Inc., New Brunswick, NJ) for 2 wk. The 2-wk HFD timepoint was chosen to dissociate the effects of the diet itself from the body weight gain and/or metabolic consequences associated with exposure to a HFD. The 45% HFD model was chosen because it resembles the fat content of the widely consumed Western diet, where ∼40%–45% of calories are derived from fat (45). The standard chow and high-fat diets do not contain added endocannabinoids and endocannabinoids are not found to any detectable level on the specifications sheet of both diets. Body weight of each mouse was measured three times per week. No animals were excluded from studies following the 2-wk chow or HFD exposure.
Oral Glucose Tolerance Test
An oral glucose tolerance test (OGTT) was performed in awake CD1 mice after 2 wk of standard chow or HFD feeding (46). After an overnight fast (13–14 h), a 2 g/kg body wt dose of 40% glucose solution (0.4 mg/mL) was administered by oral gavage. Blood glucose was determined by removing 1–2 mm of the tail tip with sharp scissors and a small blood sample was drawn and measured with a glucose meter (Bayer, Leverkusen, Germany). Blood glucose was measured at 0, 5, 15, 30, 60, 90, and 120 min after oral glucose administration. Mice were euthanized following this procedure as described earlier and used in Ussing chamber studies at least 24 h after the OGTT.
Ussing Chamber Studies
Full-thickness segments of jejunum and ileum were opened along the mesenteric border, cleaned of luminal contents, and mounted in 4-mL Ussing chambers (Physiologic Instruments, San Diego, CA) with an exposed area of 0.3 cm2. The tissues were bathed at 37°C in oxygenated (95% O2–5% CO2) Krebs buffer (115 mM NaCl, 2 mM KH2PO4, 2.4 mM MgCl2·6H2O, 25 mM NaHCO3, 8 mM KCl, and 1.3 mM CaCl2; pH 7.4) containing 10 mM mannitol and 10 mM glucose in the mucosal and serosal compartments, respectively. BSA was not included in the chamber solutions to prevent loss of material due to excess foaming. Tissues were allowed to equilibrate for 20–30 min. Net electrogenic movement of ions across the epithelium was recorded as short-circuit current (Isc, μA/cm2). The measurement of transepithelial potential and Isc allowed for the calculation of transepithelial electrical resistivity (TEER, Ωcm2) according to Ohm’s law [TEER = voltage (V)/current (Isc)]. For permeability measurements, fluorescein isothiocyanate-dextran 4 kDa (FD4; Sigma FD4; Sigma Aldrich, St. Louis, MO) at 100 mg/mL was added to the mucosal compartment for a final concentration of 1 mg/mL. This was followed by an immediate sampling of the serosal compartment in duplicate to serve as baseline fluorescence. One hundred microliters were sampled in duplicate from the serosal compartment every 10 min for 50 min and loaded into a 96-well plate (Greiner Bio-One, Kremsmüster, Austria). The volume sampled from the serosal compartment (100 μL in duplicate) was replaced with 200 μL of Krebs solution. FD4 fluorescence was measured using a VictorX3 2030 plate reader (Perkin-Elmer, Waltham, MA) (excitation/emission 485/535 nm) (47).
Drugs
CP55,940 (100 nM; Bio-Techne Canada, Toronto, ON, Canada) AM6545 (1 μM; synthesized at the Center for Drug Discovery, Northeastern University), AM251 (1 μM; Bio-Techne Canada, Toronto, ON, Canada) (48–50) or the same volume of vehicle were added to both the mucosal and serosal compartments. The vehicle consisted of 1% Tween 80 and 2% DMSO in Krebs buffer.
Immunofluorescence
To ensure that tissues used for immunofluorescence were treated similarly to those used for the permeability studies, full-thickness segments of CD1 mouse jejunum and ileum were mounted in Ussing chambers as described above and allowed to equilibrate for 20 min. Vehicle (1% Tween 80 and 2% DMSO in Krebs buffer), CP55,940 (100 nM), AM251 (1 μM), and AM6545 (1 μM) (48, 49) were added to the mucosal and serosal compartments at the indicated concentration. FD4 was not added in these experiments. After 20 min of incubation with drug or vehicle, tissues were removed from the Ussing chambers and fixed in Zamboni’s fixative overnight at 4°C. Next, samples were washed in phosphate-buffered saline (PBS) solution (3 × 10 min) and placed in 20% sucrose + PBS solution overnight for cryoprotection. Specimens were embedded in optimum cutting temperature compound (OCT, Sakura Finetek USA, Torrance, CA) and flash frozen. Frozen samples were cryostat-sectioned (10–14 μm), and thaw mounted onto adhesive-coated slides. Sections were then washed with phosphate-buffered saline with 0.1% Triton-X100 (Sigma; PBST, 3 × 10 min) and incubated with a primary antibody at 4°C for 48 h [anti-CB1, 1:1,000; Cat. No. pAb001 (RRID: AB_2813823); ImmunoGenes, Budapest, Hungary; anti-PGP9.5, 1:500; Cat. No. RA95101 (RRID: AB_2313685); Ultraclone, Wellow, Isle of Wight, UK; anti-claudin-2 (51), 1:500; Cat. No. ab53032 (RRID: AB_869174); Abcam, Cambridge, UK; anti-ZO-1 (51), 1:500, Cat. No. 61–7300 (RRID: AB_2522938); Invitrogen, Waltham, MA]. We were unable to obtain a completely selective anti-CB2 receptor antibody for use in immunofluorescence for these studies. The sections were then washed in PBST (3 × 10 min) and incubated with a secondary antibody for 2 h at room temperature [donkey anti-rabbit CY3; 1:100, Jackson Immunoresearch; Cat. No. 711165185 (RRID:AB_2307443); goat anti-rabbit Alexa fluor 555, 1:100; Cat. No. A21429 (RRID: AB_2535850); Invitrogen]. The samples were washed in PBS and mounted with Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA). CB1 receptor knockout mice were used as a negative control for the anti-CB1 primary antibody. Sections were stained without a primary antibody to serve as a control for the secondary antibody. Furthermore, tissues taken directly from mice without experimental manipulation were also used as controls. CB1 immunoreactivity in the lamina propria of the mucosa of all tissues appeared to be in mucosal nerve fibers, we therefore endeavored to double label the sections with a pan-neuronal marker. After a thorough analysis of numerous neuronal markers that gave strong immunoreactivity in the submucosal and myenteric plexuses few were found to give strong positive mucosal nerve fibers. The pan-neuronal marker rabbit anti-PGP9.5 did, however, label both plexuses, as well as mucosal nerve fibers. Although double labelling on the same section would have been preferable, this was not possible since both antibodies are raised in rabbit. As a result, we stained consecutive 10-µm sections with anti-CB1 and PGP9.5 and compared the localization of the immunoreactivity of the two markers in the mucosa of the adjacent tissues.
In all cases, sections were visualized by an observer blinded to the treatment groups using a Nikon A1R confocal microscope with NIS Element AR imaging software and photographed as z-stacks using Nyquist settings.
Epithelial Cell Isolation
Segments of CD1 mouse jejunum and ileum were removed, opened longitudinally, and rinsed with ice-cold PBS. Epithelial cells were isolated from the underlying tissue. Briefly, tissues were washed for 30 min in HEPES-buffered Hanks’ Balanced Salt Solution containing Penicillin-Streptomycin on a 3 D orbital shaker at 4°C (cold room). Tissues were cut into small pieces and incubated in a cell recovery solution (2 mM EDTA, 43.4 mM sucrose, 0.5 mM dithiothreitol) for 2 h at 4°C (52–54). Forceps were used to gently press the tissue to facilitate the dissociation of epithelial cells. Remaining tissue was flash frozen in 1 mL QIAzol lysis reagent and stored at −80°C for further analyses. Cells were collected in the cell recovery solution and centrifuged at 400 g for 10 min at 4°C. The supernatant was discarded, and samples were flash frozen in 1-mL QIAzol lysis reagent and stored at −80°C.
Gene Expression Analysis
Gene expression was assessed by qPCR on isolated epithelial cells and in the remaining epithelium-denuded intestinal wall. RNA extraction was performed using QIAzol (QIAGEN, Hilden, Germany) and purified with the RNEasy Plus Mini Kit (QIAGEN) according to the manufacturer’s instructions. Briefly, samples were homogenized with stainless steel beads (QIAGEN) for 10 min at 50 Hz (TissueLyser LT, QIAGEN). Next, samples were mixed with chloroform and centrifuged (15,700 g for 15 min at 4°C). The upper aqueous layer was removed and used in the RNEasy Plus Mini Kit for final RNA isolation. The RNA concentration was measured using a NanoDrop (Thermo Fisher Scientific, Waltham, MA) and samples containing 1 μg/mL of RNA were prepared for reverse transcriptase reactions. cDNA was synthesized using the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific) with RNaseOut Recombinant Ribonuclease Inhibitor (Thermo Fisher Scientific) according to the manufacturer’s instructions. Quantitative real time PCR was performed using the StepOnePlus (Thermo Fisher Scientific). Reactions containing 20 ng cDNA, PerfeCTa SYBR Green FastMix ROX (Quanta Bio, Beverly, MA) (contains dNTPs, Taq Polymerase and SYBR Green I dye), and 500 nM of forward and reverse primers were loaded into a MicroAmp DNA/RNA/RNase-free 96-well PCR reaction plate (Applied Biosystems by Life Technologies) in duplicate. A no template control that did not contain cDNA was used to control for contaminated PCR reagents. The PCR cycling protocol was programmed as follows: 95°C for 10 min followed by 40 cycles 95°C for 3 s and 60°C for 30 s. Gene-specific primer sequences were synthesized by Integrated DNA Technologies (Coralville, IA). Forward and reverse primer sequences are in Table 1. Rpl19 and Actb were used as reference genes. The ΔCt values of the reference genes were averaged before the comparative analyses were performed. Comparative analyses were done using the 2−ΔCt method.
Table 1.
Primers for PCR
| Name and Function | Gene | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|---|
| Cannabinoid 1 receptor (26, 55, 56) | CB1 | CTGATGTTCTGGATCGGAGTC | TCTGAGGTGTGAATGATGATGC |
| Cannabinoid 2 receptor (26, 56) | CB2 | TGACAAATGACACCCAGTCTTCT | ACTGCTCAGGATCATGTACTCCTT |
| 60S Ribosomal protein L19 (26, 55) | Rpl19 | GAAGGTCAAAGGGAATGTGTTCA | CCTTGTCTGCCTTCAGCTTGT |
| β-Actin cytoskeletal protein (57, 58) | Actb | AAAGACCTGTACGCCAACACAGTGCTGTCTGG | CGTCATACTCCTGCTTGCTGATCCACATCTGC |
Statistical Analysis
Data were analyzed using GraphPad Prism version 6.0 (GraphPad, San Diego, CA). Data are presented as individual data points and as the mean ± SE. Sample size was determined based on previously published data (47). Statistical analyses for parametric data comparing two groups were performed using a Student’s t test. Statistical analyses for nonparametric data were performed using a Mann–Whitney test for unpaired data. Comparisons between several groups were performed using a two-way repeated measures ANOVA with Bonferroni posttest for multiple comparisons. In data sets where the assumptions for a two-way ANOVA were not met, data were log10-transformed to normalize the variances. A two-way ANOVA with Bonferroni posttest for multiple comparisons was performed on log10-transformed data. P < 0.05 was set as the level of statistical significance.
RESULTS
Short-Term HFD Leads to Glucose Intolerance Independently of Body Weight Gain
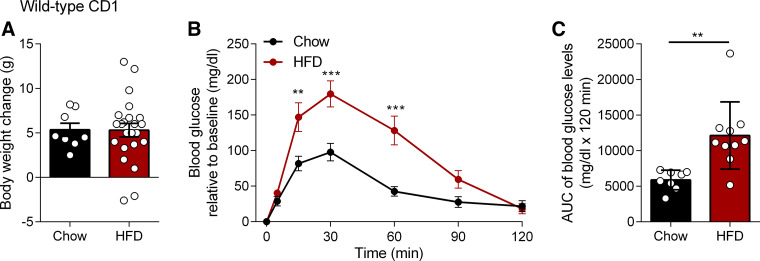
We observed no significant differences in body weight change between chow and HFD-fed CD1 mice during the studies (Fig. 1A), and this is consistent with a previous study by Hamilton et al. where no difference in body weight change was observed following short-term exposure to HFD (59). However, the HFD-fed mice developed a degree of glucose intolerance as evidenced by the significant increase in area under the curve (AUC) of blood glucose levels compared with chow (Fig. 1, B and C).
Figure 1.
Effects of short-term exposure to a high-fat diet (HFD) on body weight and glucose tolerance. Male CD1 mice were fed chow or HFD (45% kcal from fat) for 2 wk. A: body weight change in chow (n = 8) and HFD (n = 10) mice. No significant differences were observed. B: oral glucose tolerance test (OGTT) in mice fed chow (n = 8) or HFD (n = 10). After an overnight fast, glucose levels (mg/dL) were measured in a fasting state at 5, 15, 30, 60, and 120 min after administration of 2 mg/kg body wt glucose solution (0.4 mg/mL) by oral gavage. Blood glucose relative to baseline was significantly higher in HFD compared with chow at 15 min (P = 0.0019, t = 3.766, df = 16), 30 min (P < 0.0001, t = 4.712, df = 16), and 60 min (P < 0.0001, t = 4.942, df = 16). ***P < 0.001, **P < 0.01 HFD vs. chow in two-way ANOVA followed by Bonferroni correction for multiple comparisons. C: baseline corrected area under the curve (AUC) of blood glucose levels calculated by subtracting baseline glucose (t = 0) from all timepoints for each mouse individually, followed by AUC calculation. AUC of blood glucose in HFD was significantly different from chow (P = 0.0014, U = 6). **P < 0.01 in Mann–Whitney U test. Data are mean ± SE, n = 8–10/group. Groups are representative of all chow and HFD groups used in this study.
Cannabinoid Receptor mRNA and Protein Expression in Chow- and HFD-Fed Mice
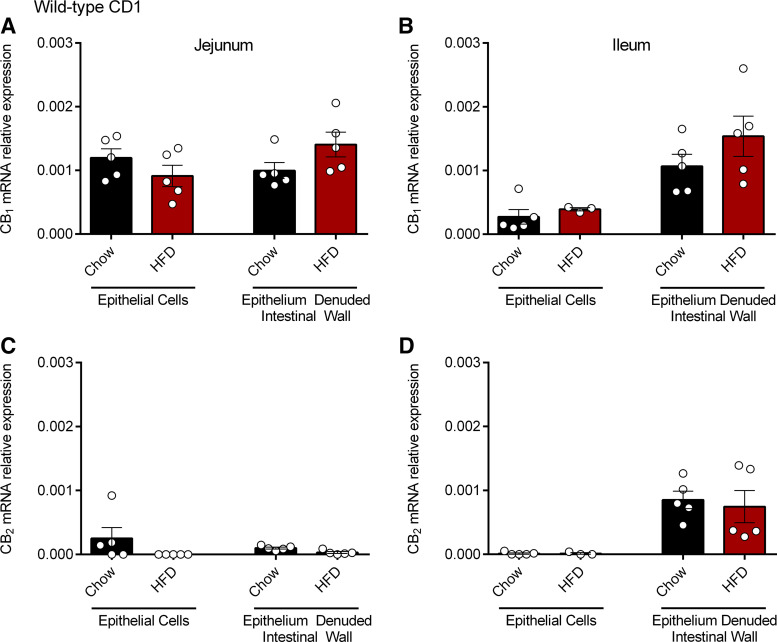
We next sought to assess cannabinoid receptor expression in the gut by examining the mRNA and protein expression of the CB1 and CB2 receptors in the small intestine of chow and HFD-fed CD1 mice. We used qPCR to determine the mRNA expression of the cannabinoid receptors on isolated epithelial cells and in the remaining epithelium-denuded intestinal wall. CB1 receptor was expressed at low levels in epithelial cells in the jejunum of both chow and HFD groups (Fig. 2A) and to a lesser extent in the ileum (Fig. 2B). It is known that the CB1 receptor is highly expressed on enteric neurons (35) and consistent with previously published reports, we demonstrated CB1 receptor expression in the epithelium denuded intestinal wall sections from the jejunum and ileum of chow and HFD-fed CD1 mice (Fig. 2, A and B). There were no significant differences in CB1 mRNA expression between chow and HFD groups. We found negligible CB2 expression in epithelial cells in jejunum and ileum of both chow and HFD-fed mice and low levels of CB2 receptor expression in the epithelium denuded intestinal wall in the ileum (Fig. 2D), but not in the jejunum (Fig. 2C).
Figure 2.
CB1 and CB2 receptor mRNA expression in the small intestine. CB1 and CB2 mRNA expression was measured by qPCR in the jejunum (A and C) and ileum (B and D) of male CD1 mice fed a chow or high-fat diet (HFD) for 2 wk. Data are mean ± SE; CB1 mRNA expression relative to Actb and Rpl19; n = 3–5/group.
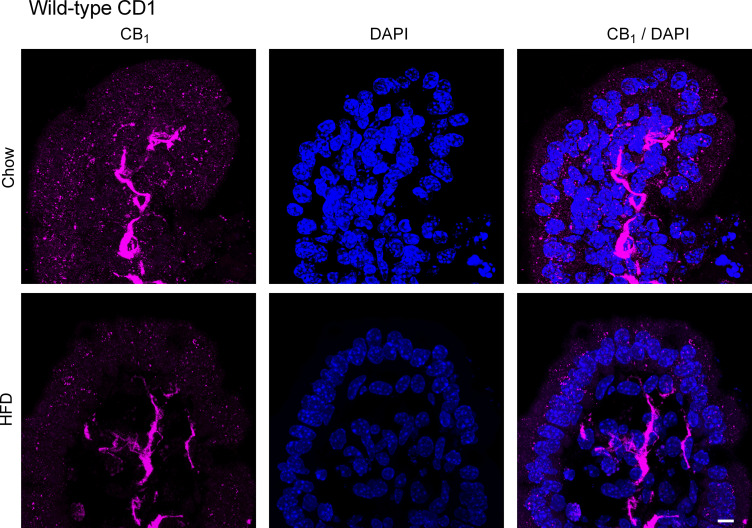
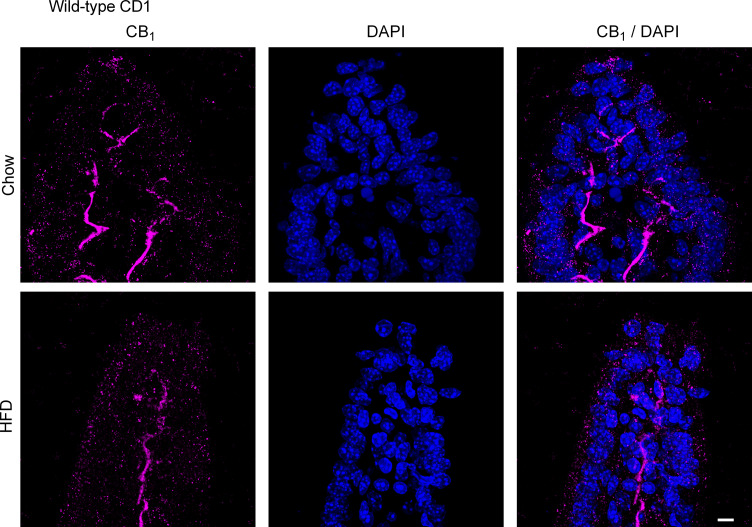
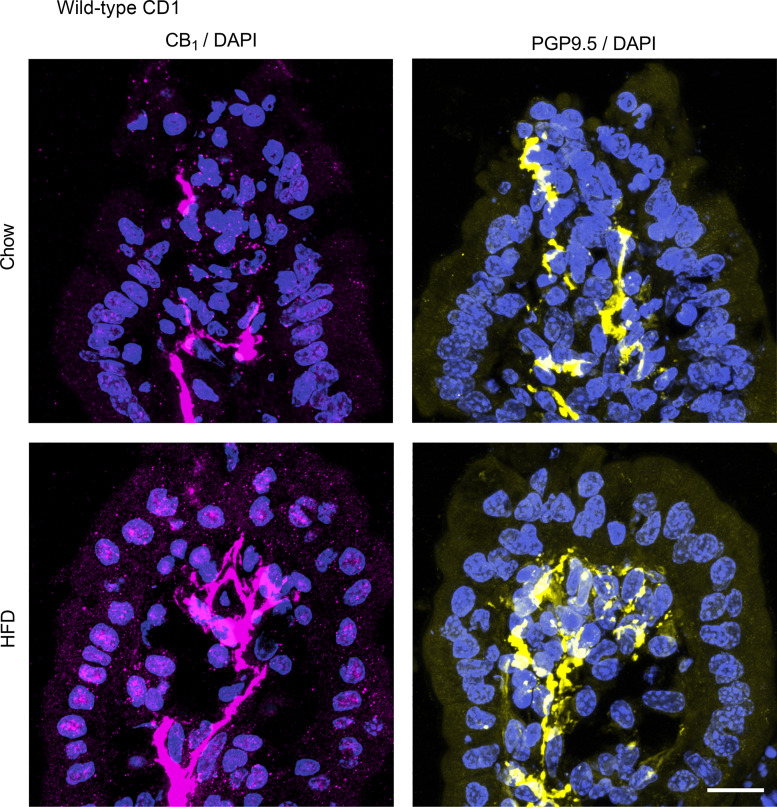
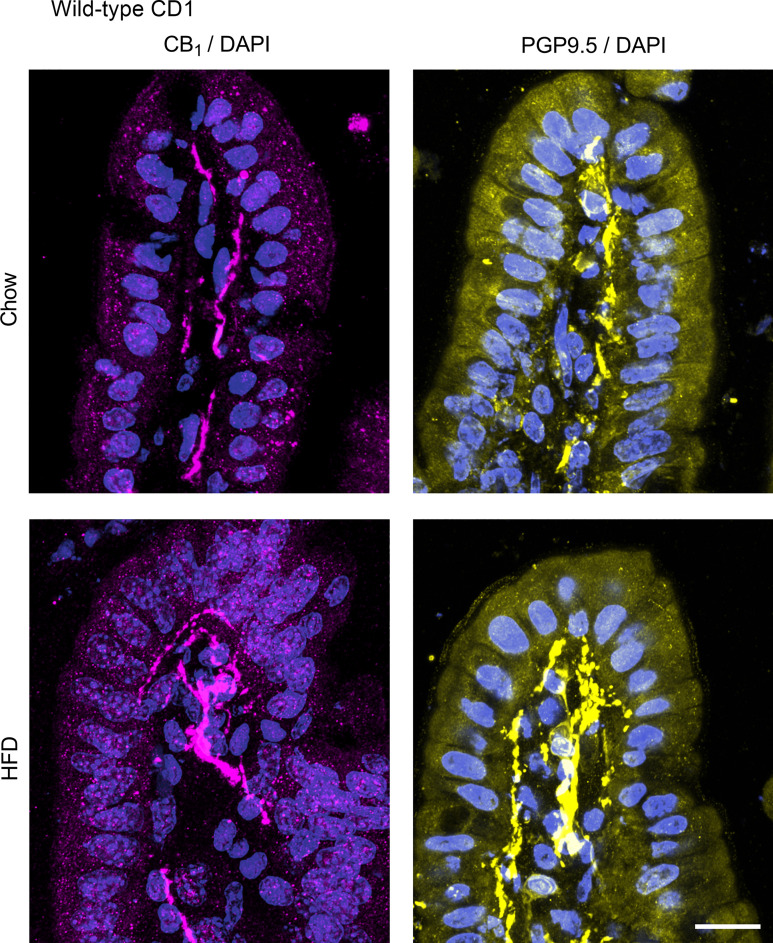
We next used immunofluorescence to evaluate the expression of the CB1 receptor in the small intestine of chow and HFD-fed CD1 mice. The CB1 receptor is highly expressed on enteric nerves in the mucosa in both the jejunum (Fig. 3) and ileum (Fig. 4) in chow and HFD-fed mice. We saw no differences in the expression pattern or intensity of this labeling between the groups. To confirm that CB1 immunoreactivity in the lamina propria of the mucosa is indeed in mucosal nerve fibers, we used the pan-neuronal marker PGP9.5 to compare the localization of the CB1 immunoreactivity in adjacent tissues. Indeed, the localization of PGP9.5 and CB1 immunoreactivity appeared to be similar in both the jejunum (Fig. 5) and ileum (Fig. 6) in chow and HFD-fed mice. In the jejunum of chow-fed mice, we noted some faint punctate fluorescence in the epithelium that was virtually absent in the HFD-fed mice (Fig. 3). A similar pattern of labeling was seen in the ileum of HFD-fed mice (Fig. 4). The exact nature of this labeling remains to be determined. To confirm that this pattern of labeling was not due to the experimental procedures used, we used tissues from animals with no experimental manipulation and confirmed the same results. We noted the expression of the CB1 receptor on enteric neurons, consistent with previously published reports (data not shown) (35). In CB1 receptor knockout mice, no labeling was observed in either region of the gut (data not shown). Similarly, no labeling was present in the absence of a primary antibody (data not shown).
Figure 3.
CB1 receptor expression in the jejunum. CB1 receptor expression was examined by immunofluorescence in male CD1 mice fed a standard chow or high-fat diet (HFD) for 2 wk (n = 5/group). Tissues were stained for CB1 with a rabbit anti-CB1 primary antibody followed by a goat anti-rabbit Alexa Fluor 555 secondary antibody. The CB1 receptor is highly expressed on enteric nerves in the mucosa. We observed no differences in the expression pattern or intensity of this labeling between groups. We also noted faint punctate staining in chow-fed mice that was virtually absent in the HFD-fed mice. Scale bar: 10 μm.
Figure 4.
CB1 receptor expression in the ileum. CB1 receptor expression was examined by immunofluorescence in male CD1 mice fed a standard chow or high-fat diet (HFD) for 2 wk (n = 5/group). Tissues were stained for CB1 with a rabbit anti-CB1 primary antibody followed by a goat anti-rabbit Alexa Fluor 555 secondary antibody. The CB1 receptor is highly expressed on enteric nerves in the mucosa. We observed no differences in the expression pattern or intensity of this labeling between groups. We also noted faint punctate staining in HFD-fed mice that was virtually absent in the chow-fed mice. Scale bar: 10 μm.
Figure 5.
PGP9.5 and CB1 receptor expression in the jejunum. PGP9.5 and CB1 receptor expression was examined by immunofluorescence in male CD1 mice fed a standard chow or high-fat diet (HFD) for 2 wk (n = 5/group). Adjacent tissue sections were stained for CB1 with a rabbit anti-CB1 primary antibody and for PGP9.5 with a rabbit anti-PGP9.5 primary antibody followed by a donkey anti-rabbit CY3 secondary antibody. PGP9.5 and CB1 had a similar expression pattern. No differences were observed between groups.
Figure 6.
PGP9.5 and CB1 receptor expression in the ileum. PGP9.5 and CB1 receptor expression was examined by immunofluorescence in male CD1 mice fed a standard chow or high-fat diet (HFD) for 2 wk (n = 5/group). Adjacent tissue sections were stained for CB1 with a rabbit anti-CB1 primary antibody and for PGP9.5 with a rabbit anti-PGP9.5 primary antibody followed by a donkey anti-rabbit CY3 secondary antibody. PGP9.5 and CB1 had a similar expression pattern. No differences were observed between groups.
HFD and Baseline Permeability in the Small Intestine
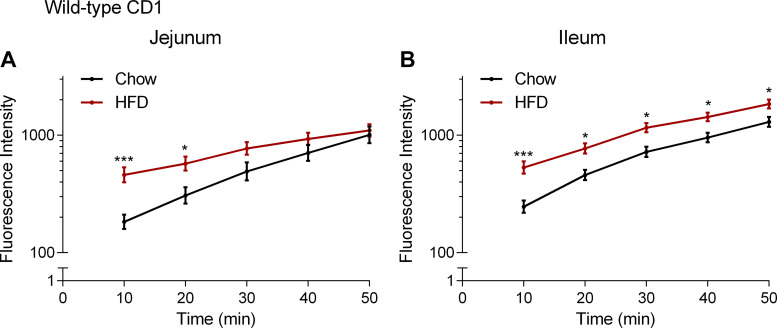
We then assessed the effect of HFD on baseline paracellular permeability by measuring the flux of FD4 across the jejunum and ileum over 50 min in CD1 mice-fed chow or HFD. Exposure to HFD led to a transient increase in FD4 flux across the jejunum (Fig. 7A) and ileum (Fig. 7B). The increase in FD4 flux across the ileum was sustained for 50 min.
Figure 7.
Effect of high-fat diet (HFD) on small intestinal permeability. Measurement of the flux of 4-kDa FITC-dextran over time across the jejunum (A) and ileum (B) after application of vehicle (1% Tween 80 and 2% DMSO in Krebs solution) in male CD1 mice fed chow or high-fat diet (HFD) for 2 wk. FD4 flux across the jejunum was significantly higher in HFD compared with chow at 10 min (P = 0.0007, t = 3.972, df = 20) and 20 min (P = 0.0388, t = 2.712, df = 20). FD4 flux across the ileum was significantly higher in HFD compared with chow at 10 min (P = 0.0004, t = 4.126, df = 20), 20 min (P = 0.0109, t = 3.146, df = 20), 30 min (P = 0.0123, t = 3.104, df = 20), 40 min (P = 0.0256, t = 2.859, df = 20), and 50 min (P = 0.0408, t = 2.693, df = 20). ***P < 0.001, *P < 0.05 compared with chow in two-way ANOVA followed by Bonferroni correction for multiple comparisons on log10-transformed data. Data are mean ± SE, n = 8–14 per group.
Role of the CB1 Receptor in the Regulation of Small Intestinal Permeability in Chow-Fed Mice
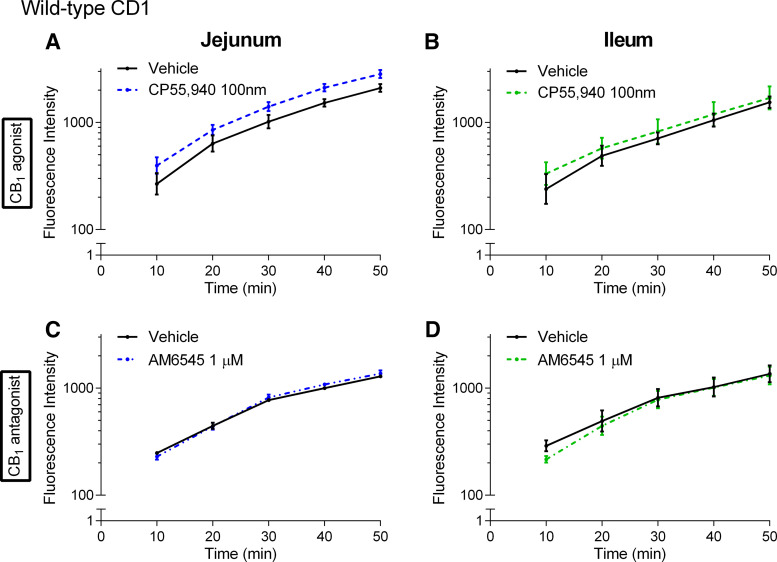
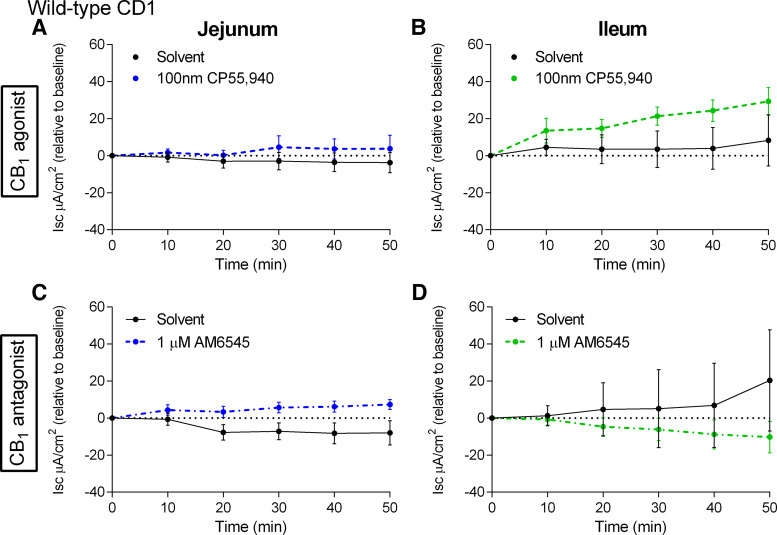
To investigate whether the CB1 receptor is involved in the regulation of paracellular permeability in chow-fed CD1 mice, we used a CB1/CB2 agonist (CP55,940) and a neutral CB1 antagonist (AM6545) (49) of the CB1 receptor and measured the flux of FD4 across the jejunum and ileum over 50 min. CP55,940 had no effect on FD4 flux across the jejunum (Fig. 8A) and ileum (Fig. 8B). Similarly, the CB1 antagonist AM6545 had no effect on FD4 flux across the jejunum (Fig. 8C) and ileum (Fig. 8D).
Figure 8.
Effects of CB1 receptor ligands on the acute regulation of small intestinal permeability in chow-fed mice. Measurement of the flux of 4-kDa FITC-dextran over time across the jejunum (A and C) and ileum (B and D) relative to vehicle (1% Tween 80 and 2% DMSO in Krebs solution) of male CD1 mice fed a standard chow diet following acute treatment in Ussing chambers with the CB1/CB2 receptor agonist CP55,940 (A and B) or the CB1 neutral antagonist AM6545 (C and D). No significant differences were observed. Data are log10-transformed and shown as mean ± SE, n = 4–5/group.
Role of the CB1 Receptor in the Regulation of Small Intestinal Permeability in HFD
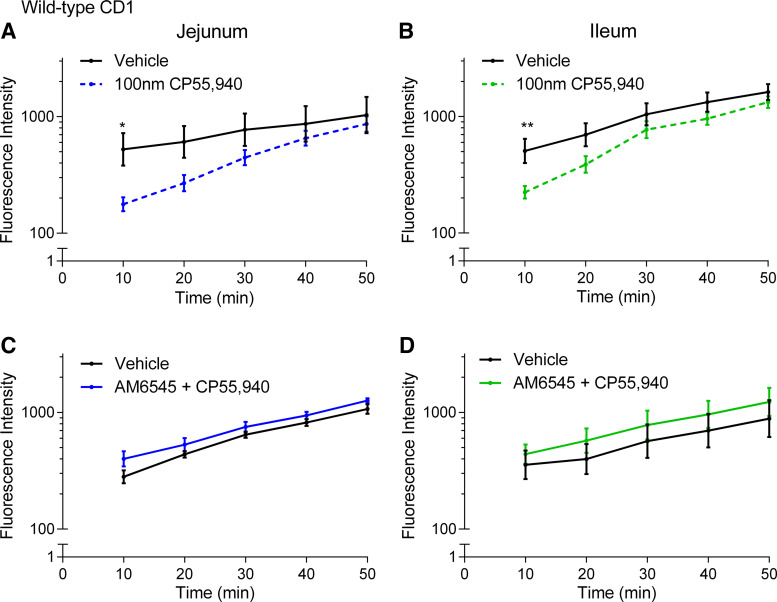
We next sought to investigate whether the CB1 receptor is involved in the acute regulation of paracellular permeability in HFD-fed CD1 mice. CP55,940 reduced the elevated FD4 flux across the jejunum (Fig. 9A) and ileum (Fig. 9B) in HFD-fed mice. To further understand if the reduction in FD4 flux following application of CP55,940 in HFD was CB1 receptor mediated, we pretreated tissues for 5 min with the CB1 antagonist AM6545 followed immediately by application of CP55,940. In the presence of AM6545, CP55,940 had no effect on FD4 flux across the jejunum (Fig. 9C) or the ileum (Fig. 9D). Thus, as expected, AM6545 blocks the effects of CP55,940 on small intestinal permeability.
Figure 9.
Effects of a CB1 receptor agonist on the acute regulation of small intestinal permeability in 2-wk high-fat diet (HFD)-fed mice. Measurement of the flux of 4-kDa FITC-dextran over time across the jejunum (A and C) and ileum (B and D) relative to vehicle (1% Tween 80 and 2% DMSO in Krebs solution) of male CD1 mice fed a HFD for 2 wk after acute treatment in Ussing chambers with the CB1/CB2 receptor agonist CP55,940 (A and B) or pretreatment with the CB1 antagonist AM6545 for 5 min followed by application of CP55,940 at t = 0 (C and D). FD4 flux across the jejunum was significantly lower in CP55,940 compared with vehicle at 10 min (A, P = 0.0212, t = 2.953, df = 14). FD4 flux across the ileum was significantly lower in CP55,940 compared with vehicle at 10 min (B, P = 0.0089, t = 3.248, df = 14). There was no difference in FD4 flux across the jejunum or ileum when tissues were pretreated with AM6545 before application of CP55,940. **P < 0.01, *P < 0.05 compared with vehicle in two-way ANOVA followed by Bonferroni correction for multiple comparisons on log10-transformed data. Data are mean ± SE, n = 4–8/group.
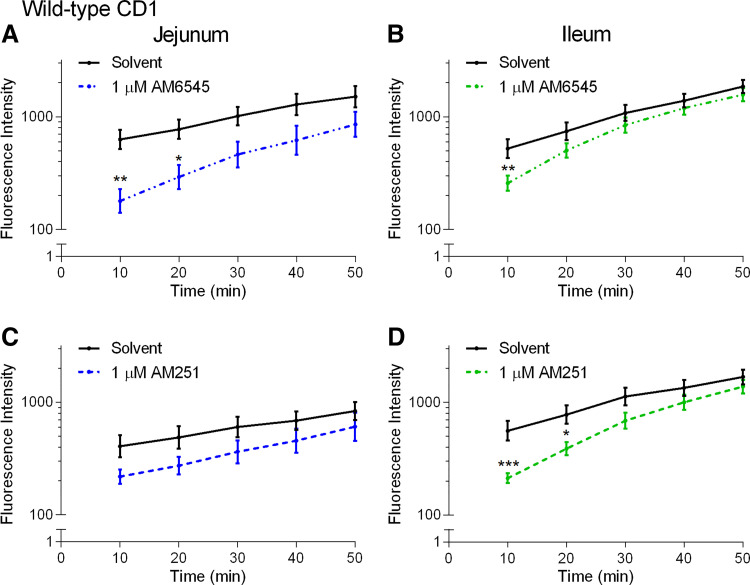
We then investigated the effect of CB1 neutral antagonist AM6545 administered on its own. AM6545 also reduced FD4 flux across the jejunum (Fig. 10A) and ileum (Fig. 10B) in HFD-fed CD1 mice. To explore this effect further, we investigated whether it occurred with a second antagonist and used the well characterized antagonist/inverse agonist AM251 (48). AM251 also reduced FD4 flux across the ileum (Fig. 10D) and there was a tendency for a reduction in the jejunum (Fig. 10C).
Figure 10.
Effects of CB1 receptor antagonists on the acute regulation of small intestinal permeability in 2-wk high-fat diet (HFD)-fed mice. Measurement of the flux of 4-kDa FITC-dextran over time across the jejunum (A and C) and ileum (B and D) relative to vehicle (1% Tween 80 and 2% DMSO in Krebs solution) of male CD1 mice fed a HFD for 2 wk after acute treatment in Ussing chambers with the neutral CB1 receptor antagonist AM6545 (A and B) and inverse agonist AM251 (C and D). FD4 flux across the jejunum was significantly lower with AM6545 compared with vehicle at 10 min (A, P = 0.0016, t = 3.790, df = 14) and 20 min (A, P = 0.0226, t = 2.934, df = 14). FD4 flux across the ileum was significantly lower than vehicle with AM251 at 10 min (D, P = 0.0003, t = 4.275, df = 14) and 20 min (D, P = 0.0150, t = 3.075, df = 14), and with AM6545 at 10 min (A, P = 0.0089, t = 3.249, df = 70). **P < 0.01, *P < 0.05 compared with vehicle in two-way ANOVA followed by Bonferroni correction for multiple comparisons on log10-transformed data. Data are mean ± SE, n = 4–8/group.
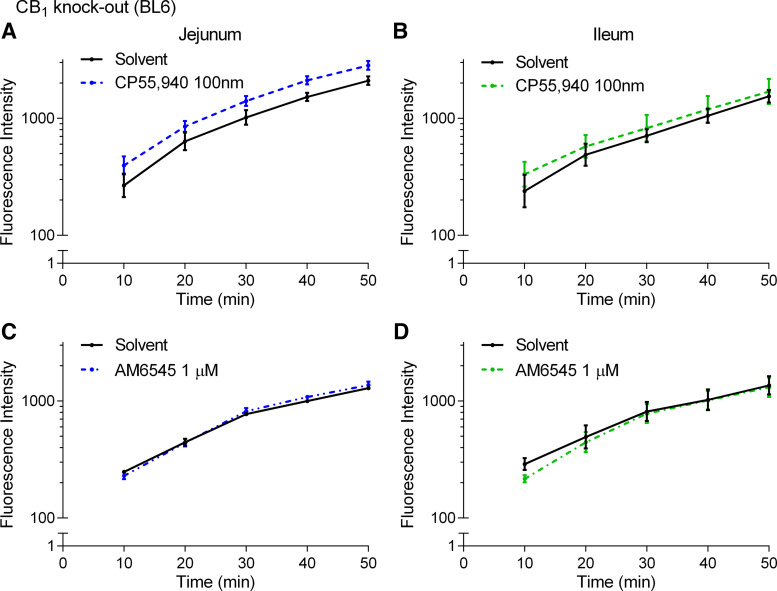
To further investigate the CB1 receptor, we assessed FD4 flux across the jejunum and ileum in CB1 knockout mice fed HFD. Neither the CP55,940 or AM6545 altered the flux of FD4 across the jejunum (Fig. 11, A and C) or ileum (Fig. 11, B and D) of HFD-fed CB1 knockout mice.
Figure 11.
The effects of CB1 receptor ligands on the acute regulation of small intestinal permeability in CB1 knockout mice fed a high-fat diet (HFD) for 2 wk. Measurement of the flux of 4-kDa FITC-dextran over time across the jejunum (A and C) and ileum (B and D) relative to vehicle (1% Tween 80 and 2% DMSO in Krebs solution) in male CB1 KO mice fed a HFD for 2 wk after acute treatment in Ussing chambers with the CB1/CB2 agonist CP55,940 (A and B) or the CB1 neutral antagonist AM6545 (C and D). No significant differences were observed. Data are log10-transformed and shown as mean ± SE, n = 3–8/group.
Role of the CB1 Receptor in the Regulation of Isc and TEER in the Small Intestine
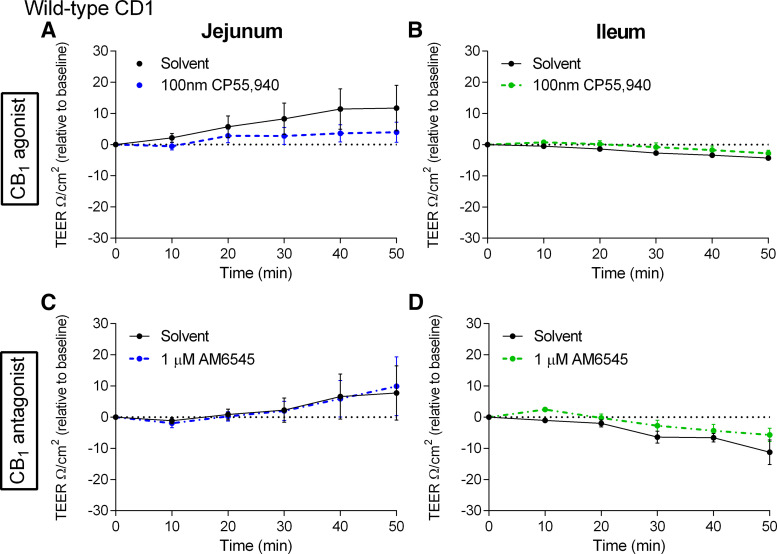
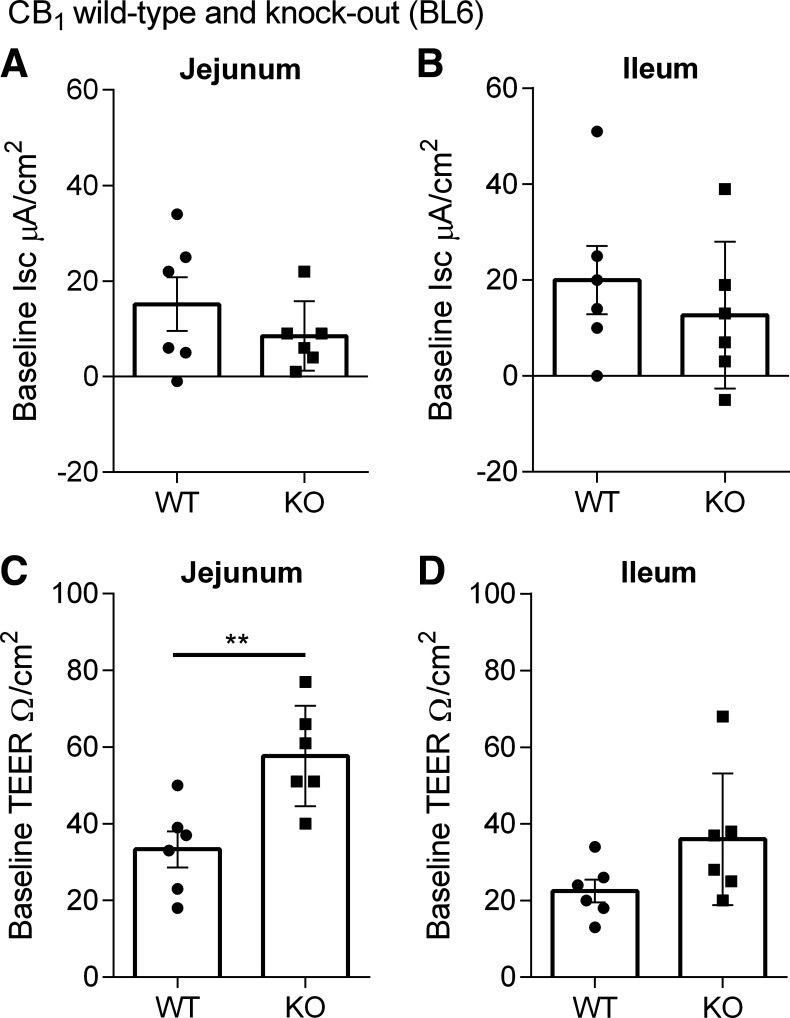
Given that CB1 receptor activation and inhibition reduced fluorescence intensity in the jejunum and ileum of mice fed a HFD for 2 wk, we sought to assess whether there were any changes in TEER. The CB1/CB2 agonist CP55,940 and CB1 antagonist AM6545 did not alter TEER (Fig. 12) (60). In addition, CB1 receptor knockout mice fed a HFD have a higher baseline TEER than wild-type in the jejunum and there was a tendency toward an increase in baseline TEER in the ileum (Fig. 14).
Figure 12.
Effects of a CB1 receptor agonist and antagonist on the acute regulation of transepithelial electrical resistivity in 2-wk high-fat diet (HFD)-fed mice. Measurement of transepithelial electrical resistivity (TEER) changes over time in the jejunum (A and C) and ileum (B and D) relative to vehicle (1% Tween 80 and 2% DMSO in Krebs solution) of male CD1 mice fed a HFD for 2 wk after acute treatment in Ussing chambers with the CB1/CB2 receptor agonist CP55,940 (A and B) or the CB1 receptor antagonist AM6545 (C and D). No significant differences were observed. Data are mean ± SE, n = 5–6/group.
Figure 14.
Baseline short-circuit current and transepithelial electrical resistivity (TEER) in 2-wk high-fat diet (HFD)-fed CB1 receptor wild-type and knockout (KO) mice. Measurement of baseline Isc in the jejunum (A and C) and ileum (B and D) in male CB1+/+ and CB1−/− mice fed a HFD for 2 wk. Baseline TEER was significantly higher in the jejunum of CB1−/− mice compared with CB1+/+ mice (P = 0.0043). **P < 0.01 compared with WT in Mann–Whitney test. Data are mean ± SE, n = 6/group. WT, wild type.
We also evaluated ion flux by measuring short-circuit current (Isc) in the jejunum and ileum of mice fed a HFD for 2 wk. Neither CP55,940 or AM6545 leads to any changes in Isc over the 50-min time course of the experiment (Fig. 13). Moreover, there was no significant difference in baseline Isc between CB1 wild-type and knockout mice fed a HFD in the jejunum and ileum (Fig. 14).
Figure 13.
Effects of a CB1 receptor agonist and antagonist on the acute regulation of short-circuit current in 2-wk high-fat diet (HFD)-fed mice. Measurement of short-circuit current (Isc) changes over time in the jejunum (A and C) and ileum (B and D) relative to vehicle (1% Tween 80 and 2% DMSO in Krebs solution) of male CD1 mice fed a HFD for 2 wk after acute treatment in Ussing chambers with the CB1/CB2 receptor agonist CP55,940 (A and B) or the CB1 receptor antagonist AM6545 (C and D). No significant differences were observed. Data are mean ± SE, n = 5–6/group.
Cannabinoid Ligands and the Regulation of Small Intestinal Permeability through Tight Junctions
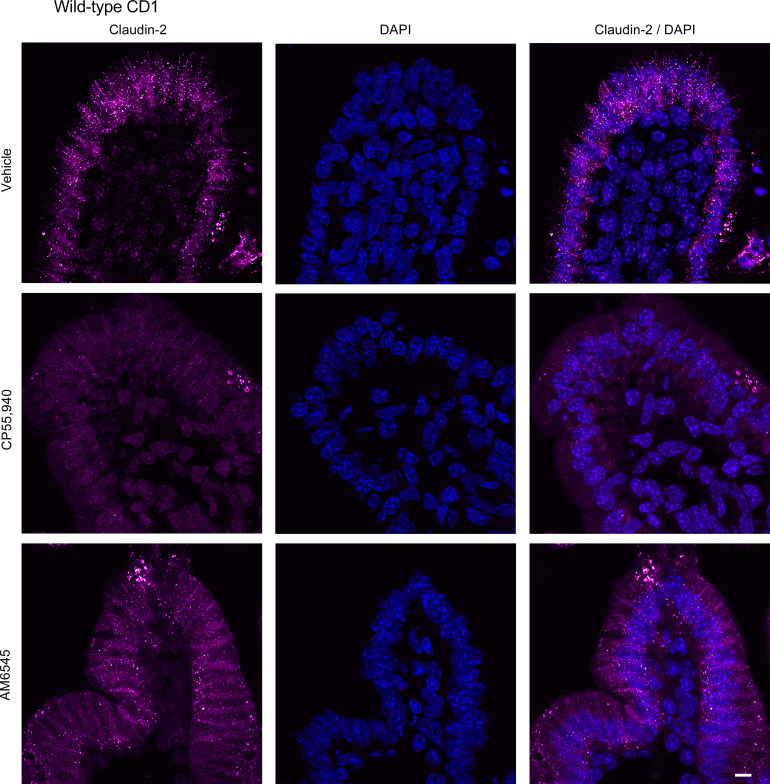
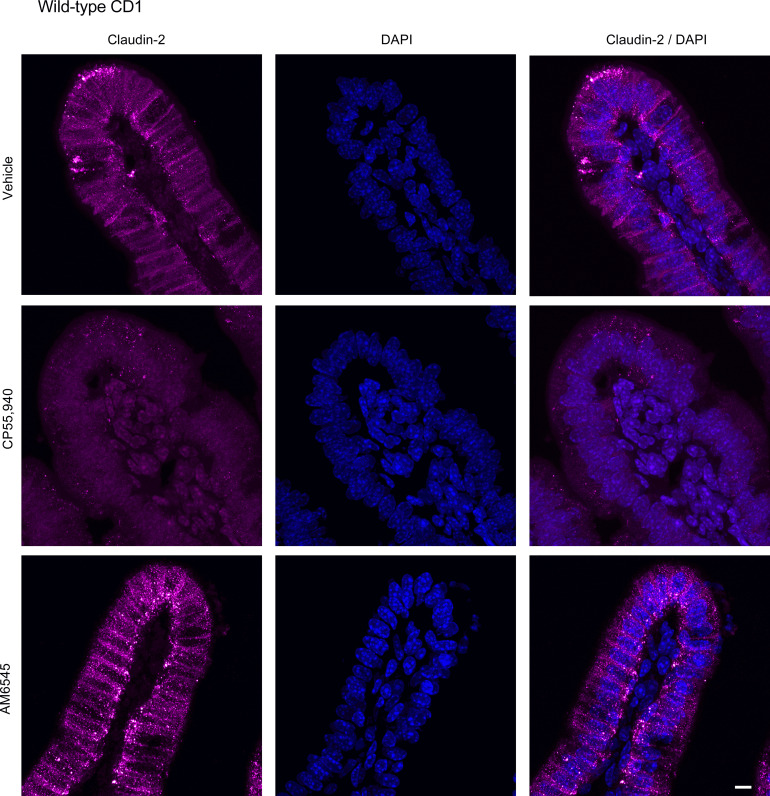
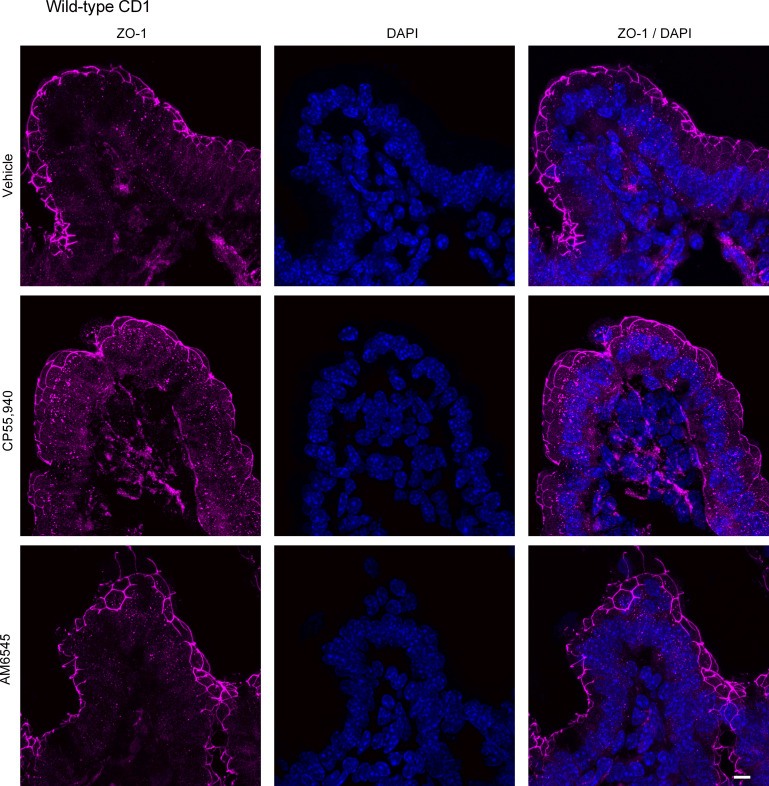
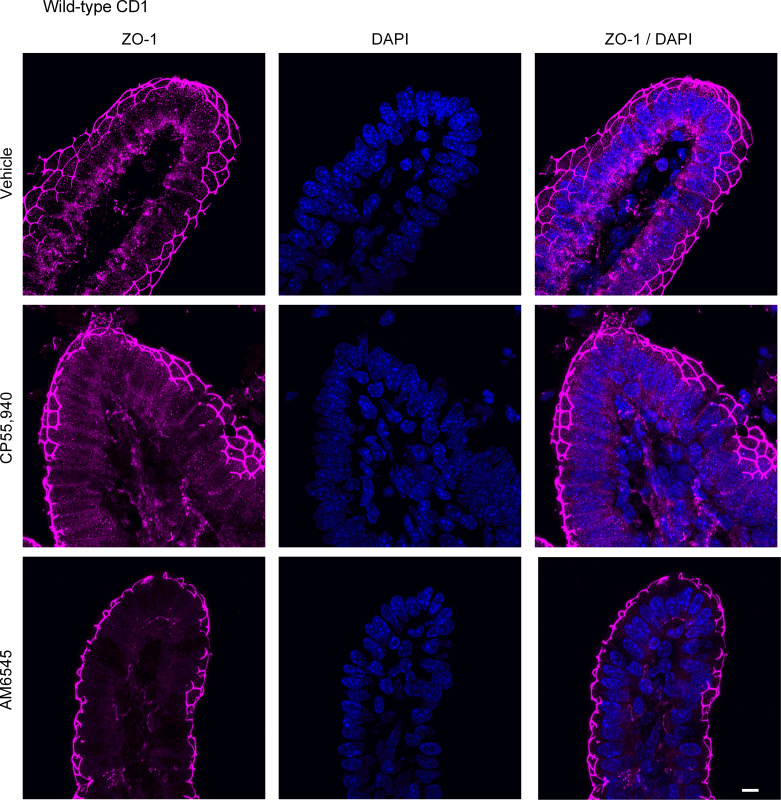
To evaluate if changes in small intestinal permeability are associated with altered expression of tight junction proteins, we examined the expression of claudin-2 and ZO-1 in HFD-fed mice. Small intestinal segments from HFD mice were mounted in Ussing chambers to mimic the experimental design of intestinal permeability measurements and were treated with vehicle, CP55,940 or AM6545. CP55,940 markedly reduced the expression of claudin-2 in the jejunum (Fig. 15; see also Supplemental Fig. S1; https://doi.org/10.6084/m9.figshare.19892572.v1) and ileum (Fig. 16), whereas the CB1 antagonist AM6545 somewhat reduced the degree of labeling in the jejunum, but had no effect on claudin-2 expression in the ileum (Figs. 15 and 16). AM6545 had no effect on the expression of ZO-1 in the jejunum (Fig. 17; see also Supplemental Fig. S1) or ileum (Fig. 18) of HFD mice. CP55,940 may have caused a slight increase in the amount of ZO-1 immunofluorescence in the cytoplasm of epithelial cells; however, there still remains a substantial amount of ZO-1 staining in the apical cell membrane. As such, it is unlikely that the movement of ZO-1 from the membrane to the cytoplasm was sufficient to appreciably decrease epithelial permeability.
Figure 15.
Expression of claudin-2 in the jejunum after treatment with CB1 receptor ligands. Tissues from male CD1 mice were stained for claudin-2 with a rabbit anti-claudin-2 primary antibody followed by a goat anti-rabbit Alexa Fluor 555 secondary antibody (n = 5/group). Representative immunofluorescence images of claudin-2 in the jejunum of high-fat diet (HFD)-fed mice after acute application of vehicle (1% Tween 80 and 2% DMSO in Krebs solution), CB1/CB2 receptor agonist (CP55,940), or CB1 receptor antagonist (AM6545) in Ussing chambers. Nuclei were counterstained with DAPI. Scale bar: 10 μm.
Figure 16.
Expression of claudin-2 in the ileum after treatment with CB1 receptor ligands. Tissues from male CD1 mice were stained for claudin-2 with an anti-claudin-2 primary antibody followed by a goat anti-rabbit Alexa Fluor 555 secondary antibody (n = 5/group). Representative immunofluorescence images of claudin-2 in the ileum of high-fat diet (HFD)-fed mice after acute application of vehicle (1% Tween 80 and 2% DMSO in Krebs solution), CB1/CB2 receptor agonist (CP55,940), or CB1 receptor antagonist (AM6545) in Ussing chambers. Nuclei were counterstained with DAPI. Scale bar: 10 μm.
Figure 17.
Expression of ZO-1 in the jejunum after treatment with CB1 receptor ligands. Tissues from male CD1 mice were stained for ZO-1 with a rabbit anti-ZO-1 primary antibody followed by a goat anti-rabbit Alexa Fluor 555 secondary antibody (n = 5/group). Representative immunofluorescence images of ZO-1 in the jejunum of high-fat diet (HFD)-fed mice after acute application of vehicle (1% Tween 80 and 2% DMSO in Krebs solution), CB1/CB2 receptor agonist (CP55,940), or CB1 receptor antagonist (AM6545) in Ussing chambers. Nuclei were counterstained with DAPI. Scale bar: 10 μm.
Figure 18.
Expression of ZO-1 in the ileum after treatment with CB1 receptor ligands. Tissues from male CD1 mice were stained for ZO-1 with a rabbit anti-ZO-1 primary antibody followed by a goat anti-rabbit Alexa Fluor 555 secondary antibody (n = 5/group). Representative immunofluorescence images of ZO-1 in the ileum of high-fat diet (HFD)-fed mice after acute application of vehicle (1% Tween 80 and 2% DMSO in Krebs solution), CB1/CB2 receptor agonist (CP55,940), or CB1 receptor antagonist (AM6545) in Ussing chambers. Nuclei were counterstained with DAPI. Scale bar: 10 μm.
DISCUSSION
The CB1 receptor is involved in several critical aspects of gut function, including the control of motility and fluid secretion, and is known to be widely expressed on enteric nerves (35, 61–63). Here we studied the distribution and function of the CB1 receptor in regulating small intestinal permeability in chow-fed and 2-wk HFD-fed mice. We found that there was a low level of CB1 gene expression on the intestinal epithelium, but CB1 receptors were highly expressed on enteric nerves in the lamina propria. Remarkably, we demonstrated that both CB1 receptor agonists and antagonists regulate small intestinal permeability in animals exposed to a HFD, but not those fed on regular chow. Although changes in the distribution of the tight junction protein claudin-2 explain the effects of the CB1 agonist, how the CB1 receptor antagonist exerts its effects remains to be determined. A limitation of our work was that we used only male mice. Further studies are required to determine if there are sex differences in the regulation of permeability by CB1 ligands.
This study recapitulated findings demonstrating CB1 receptor expression on enteric nerves in the submucosal and myenteric plexuses in the small intestine (35). Moreover, in the jejunum of chow-fed mice, we noted faint punctate labeling in the epithelium that was absent in the HFD-fed mice. Although the specific nature of this labelling is not clear, it was absent in CB1 knockout mice. Further studies are required to determine the exact nature of this labelling.
Although a single cell analysis of the mouse intestinal epithelium did not reveal CB1 receptor expression (64), previous studies have demonstrated CB1 receptor expression on K cells and CCK-producing enteroendocrine cells in the upper small intestine (65, 66). We demonstrated a low level of CB1 receptor gene expression in the jejunum of chow and HFD mice and CB1 mRNA expression in the epithelium denuded intestinal wall, which is consistent with previously published reports (26). The results of our studies contrast to some extent with those of Muccioli et al. (26), who demonstrated increased CB1 expression in the colon after 14 wk of 50% HFD. To this end, there appears to be a time- and region-specific effect of HFD on intestinal CB1 receptor expression, suggesting that signaling through CB1 may also impact epithelial physiology in a diet-dependent manner. This is not the first report of altered CB1 receptor protein expression without a matched alteration in mRNA expression. A postmortem analysis of the prefrontal cortex of individuals with schizophrenia treated with antipsychotics demonstrated a reduction in CB1 receptor protein expression and no change in mRNA expression (67). Urigüen et al. (67) attributed these findings to a downregulation of the receptor, suggesting that short-term exposure to HFD could lead to CB1 receptor downregulation and subsequent upregulation with prolonged exposure. We found no evidence for CB2 receptor expression on the epithelium of the mouse small intestine.
Our understanding of the endocannabinoid system is expanding to include a potential role in the regulation of intestinal permeability through the CB1 receptor. Here we demonstrated that CB1 receptor activation with the CB1/CB2 agonist CP55,940 and CB1 receptor blockade with the neutral CB1 antagonist AM6545 both lead to a transient reduction in small intestinal permeability in HFD-fed mice. Our in vitro findings are in line with previous work conducted in vivo by Muccioli et al. and Mehrpouya-Bahrami et al. (25, 26) who showed that CB1 antagonism reduced epithelial permeability. We also found that a CB1 agonist did the same, which was surprising. The ability of agonists and antagonists to elicit the same physiological response is intriguing. However, this is not the first time that cannabinoid receptor agonists and antagonists have produced similar physiological responses in the GI tract. López-Redondo et al. (68) demonstrated that a CB1 antagonist (rimonabant) and CB1 agonists (WIN55,212-2 and CP55,940) reduced the amplitude of fast excitatory postsynaptic potentials in the myenteric plexus. Further investigation will be required to elucidate the precise signaling mechanism behind these effects.
Biased agonism has been described in cannabinoid receptor signaling and might explain the ability of CP55,940 to produce the same physiological outcomes as AM6545 (69, 70). Biased agonism is an important consideration in cannabinoid pharmacology because the outcome of CB1 or CB2 receptor activation depends not only on the target receptor but also on the downstream signaling pathways preferentially activated by certain ligands. The three-state model of receptor function suggests that different agonists are able to stabilize different active receptor conformations and preferentially couple to specific heterotrimeric G proteins or β-arrestins (69, 71). Given the structural diversity of cannabinoid agonists (e.g., classical cannabinoids, nonclassical cannabinoids, eicosanoids, and indoles), it seems reasonable that they could preferentially stabilize different receptor conformations and consequently couple to diverse downstream signaling pathways (72). Moreover, there are examples in the literature where cannabinoid agonists lead to opposite behavioral effects. One study looked at the cannabinoid system in addictive behavior and found that priming injections with WIN55,212-2 and CP55,940 reinstated drug-seeking behavior in rats that had undergone extinction, but Δ9-tetrahydrocannabinol did not (73). Whether CP55,940 behaves as a biased agonist in the intestine remains to be shown.
Interestingly, Alhamoruni et al. (41) showed claudin-1 expression was sensitive to anandamide and 2-AG, but not Δ9-tetrahydrocannabinol. Although it is possible that this effect could be attributed to Δ9-tetrahydrocannabinol being a partial agonist, it cannot be ruled out that biased agonism led to downstream signaling that produced opposite responses. Thus, the intrinsic efficacy of a ligand not only depends on the target receptor, but also on the downstream signaling pathways and it is indeed possible for a single ligand to have multiple intrinsic affinities for a single receptor.
We showed that CP55,940 markedly reduced the expression of claudin-2 in the jejunum and ileum, which may have contributed to the observed reduction in permeability to FD4. Unlike most claudins in the intestinal epithelial tight junction, which contribute to epithelial barrier function, claudin-2 acts as a cation-selective channel that has been shown to increase epithelial permeability (74). Claudin-2 expression is increased during intestinal inflammation (75) and inactivation of claudin-2 pharmacologically or through genetic knockdown reduces inflammation in mouse models of colitis (76). Thus, it is plausible that CB1 receptor mediates decreased intestinal permeability through downregulation of claudin-2 expression, although the mechanism by which this occurs remains to be elucidated. Interestingly, 2-AG has been shown to reduce the enhanced permeability associated with a rat model of stress (77) through upregulation of claudins, including claudin-2. The effect was blocked by the CB1 antagonist rimonabant (77), suggesting that in the rat stress model, CB1 activation enhances claudin-2. Thus, the relationship between CB1 receptor activation and claudin-2 is unclear and will require further study.
The ability of CB1 agonists and antagonists to reduce intestinal permeability could also be explained through receptor internalization. Several lines of evidence exist for agonist-induced CB1 receptor internalization (78–80). Phosphorylation of the proximal COOH terminus by G protein receptor kinases following agonist binding leads to β-arrestin2 recruitment and subsequent clathrin-mediated receptor internalization (80). CB1 agonist-induced receptor internalization does not require G protein signaling through Gαi, Gαo, or Gαs (78, 79). Binding of CB1 agonists CP55,940, WIN55,212-2 and HU-210 to the CB1 receptor leads to internalization through clathrin-coated pits in AtT20 cells (78). Although it is well understood that CB1 agonist binding can lead to receptor internalization, there is evidence to suggest that antagonists at other G protein-coupled receptors can also cause receptor internalization. For example, antagonists of the vasopressin V2 receptor, bradykinin B2 receptor, and type 1 cholecystokinin receptor have all demonstrated antagonist induced receptor internalization (81–83). Therefore, it is possible that antagonist binding to the CB1 receptor stabilizes a receptor conformation that leads to a change in function to facilitate receptor internalization independent of G protein or β-arrestin signaling. CB1 receptor internalization following binding of both agonists and antagonists essentially produces a functional knockout, which aligns with our finding that CB1 knockout mice have reduced baseline permeability in the small intestine. Furthermore, the CB1 agonists and antagonists only effectively reduced permeability in the 2-wk HFD where CB1 receptor expression is functionally upregulated.
The role of the endocannabinoid system of the GI tract in the regulation of intestinal permeability is incompletely understood, but previous studies indicated that it was involved when there was elevated endocannabinoid tone (27, 29, 84). Our findings confirm and extended these observations to the acute regulation of intestinal permeability. We demonstrated that both CB1 receptor agonists and antagonists regulate small intestinal permeability in animals exposed to a HFD, but not those fed a regular chow diet. However, although changes in the distribution of the tight junction protein claudin-2 explain the effects of the CB1 agonist, exactly how the CB1 receptor antagonist exerts its effects remains to be determined. With the increased consumption of Western diet coupled with the usage of cannabis for recreational and/or medicinal purposes, this work highlights the critical importance of understanding the interaction between diet and cannabinoids in the regulation of the gut barrier, which is implicated in numerous diseases and is essential for the maintenance of health.
SUPPLEMENTAL DATA
Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.19892572.v1.
GRANTS
This work was supported by grants from the Canadian Institutes of Health Research [to W.K.M. (PJT-153290) and K.A.S. (FDN148380)], the Natural Sciences and Engineering Research Council of Canada [to W.K.M. (RGPIN/04321-2018)], and the National Institute on Drug Abuse [to A.M. (DA023142 and DA009158)]. H.C. was a recipient of a Cumming School of Medicine Graduate Scholarship. J.-B.C. was the recipient of Alberta Innovates and Human Frontier Science Program (LT000052/2017-L) Postdoctoral Fellowships.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.C., J.-B.C., W.K.M., and K.A.S. conceived and designed research; H.C., J.-B.C., C.M.K., L.E.W., and K.V. performed experiments; H.C., J.-B.C., C.M.K., and L.E.W. analyzed data; H.C., J.-B.C., C.M.K., W.K.M., and K.A.S. interpreted results of experiments; H.C., L.E.W., and K.A.S. prepared figures; H.C., W.K.M., and K.A.S. drafted manuscript; H.C., J.-B.C., C.M.K., L.E.W., K.V., A.M., W.K.M., and K.A.S. edited and revised manuscript; H.C., J.-B.C., C.M.K., L.E.W., K.V., A.M., W.K.M., and K.A.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Cristiane Baggio for assistance and support of these studies. The confocal microscopy was carried out in the Snyder Institute for Chronic Diseases Live Cell Imagining Laboratory, Cumming School of Medicine, University of Calgary. Online Graphical Abstract was created with BioRender and published with permission.
REFERENCES
- 1. Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9: 799–809, 2009. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 2. Turner HL, Turner JR. Good fences make good neighbors. Gut Microbes 1: 22–29, 2010. doi: 10.4161/gmic.1.1.11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee SH. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest Res 13: 11–18, 2015. doi: 10.5217/ir.2015.13.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Loh G, Blaut M. Role of commensal gut bacteria in inflammatory bowel diseases. Gut Microbes 3: 544–555, 2012. doi: 10.4161/gmic.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol 14: 9–21, 2017. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nusrat A, Turner JR, Madara JL. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am J Physiol Gastrointest Liver Physiol 279: G851–G857, 2000. doi: 10.1152/ajpgi.2000.279.5.G851. [DOI] [PubMed] [Google Scholar]
- 7. Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol 73: 283–309, 2011. doi: 10.1146/annurev-physiol-012110-142150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 123: 1777–1788, 1993. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol 141: 1539–1550, 1998. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martìn-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, Simmons D, Dejana E. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol 142: 117–127, 1998. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol 171: 939–945, 2005. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (Zonula Occludens) in a variety of epithelia. J Cell Biol 103: 755–766, 1986. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Citi S, Sabanay H, Jakes R, Geiger B, Kendrick-Jones J. Cingulin, a new peripheral component of tight junctions. Nature 333: 272–276, 1988. doi: 10.1038/333272a0. [DOI] [PubMed] [Google Scholar]
- 14. Michielan A, D'Incà R. Intestinal permeability in inflammatory bowel disease: pathogenesis, clinical evaluation, and therapy of leaky gut. Mediators Inflamm 2015: 628157, 2015. doi: 10.1155/2015/628157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Elburg RM, Uil JJ, Mulder CJJ, Heymans HSA. Intestinal permeability in patients with coeliac disease and relatives of patients with coeliac disease. Gut 34: 354–357, 1993. doi: 10.1136/gut.34.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fasano A. All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Res 9: 69, 2020. doi: 10.12688/f1000research.20510.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, Spiller RC. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol 101: 1288–1294, 2006. [Erratum in Am J Gastroenterol 101: 1944, 2006]. doi: 10.1111/j.1572-0241.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 18. Keita ÅV, Söderholm JD. Mucosal permeability and mast cells as targets for functional gastrointestinal disorders. Curr Opin Pharmacol 43: 66–71, 2018. doi: 10.1016/j.coph.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 19. Brun P, Castagliuolo I, Di Leo V, Buda A, Pinzani M, Palù G, Martines D. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol 292: G518–G525, 2007. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 20. Bosi E, Molteni L, Radaelli MG, Folini L, Fermo I, Bazzigaluppi E, Piemonti L, Pastore MR, Paroni R. Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia 49: 2824–2827, 2006. doi: 10.1007/s00125-006-0465-3. [DOI] [PubMed] [Google Scholar]
- 21. Sharpstone D, Neild P, Crane R, Taylor C, Hodgson C, Sherwood R, Gazzard B, Bjarnason I. Small intestinal transit, absorption, and permeability in patients with AIDS with and without diarrhoea. Gut 45: 70–76, 1999. doi: 10.1136/gut.45.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yacyshyn B, Meddings J, Sadowski D, Bowen-Yacyshyn MB. Multiple sclerosis patients have peripheral blood CD45RO+ B cells and increased intestinal permeability. Dig Dis Sci 41: 2493–2498, 1996. doi: 10.1007/BF02100148. [DOI] [PubMed] [Google Scholar]
- 23. Lam YY, Ha CWY, Campbell CR, Mitchell AJ, Dinudom A, Oscarsson J, Cook DI, Hunt NH, Caterson ID, Holmes AJ, Storlien LH. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS One 7: e34233, 2012. doi: 10.1371/journal.pone.0034233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57: 1470–1481, 2008. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 25. Mehrpouya-Bahrami P, Chitrala KN, Ganewatta MS, Tang C, Murphy EA, Enos RT, Velazquez KT, McCellan J, Nagarkatti M, Nagarkatti P. Blockade of CB1 cannabinoid receptor alters gut microbiota and attenuates inflammation and diet-induced obesity. Sci Rep 7: 15645, 2017. doi: 10.1038/s41598-017-15154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Muccioli GG, Naslain D, Bäckhed F, Reigstad CS, Lambert DM, Delzenne NM, Cani PD. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol 6: 392, 2010. doi: 10.1038/msb.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Teixeira TFS, Collado MC, Ferreira CLLF, Bressan J, Peluzio MDCG. Potential mechanisms for the emerging link between obesity and increased intestinal permeability. Nutr Res 32: 637–647, 2012. doi: 10.1016/j.nutres.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 28. Araújo JR, Tomas J, Brenner C, Sansonetti PJ. Impact of high-fat diet on the intestinal microbiota and small intestinal physiology before and after the onset of obesity. Biochimie 141: 97–106, 2017. doi: 10.1016/j.biochi.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 29. Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, Muccioli GG, Delzenne NM. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58: 1091–1103, 2009. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Neunlist M, Toumi F, Oreschkova T, Denis M, Leborgne J, Laboisse CL, Galmiche J-P, Jarry A. Human ENS regulates the intestinal epithelial barrier permeability and a tight junction-associated protein ZO-1 via VIPergic pathways. Am J Physiol Gastrointest Liver Physiol 285: G1028–G1036, 2003. doi: 10.1152/ajpgi.00066.2003. [DOI] [PubMed] [Google Scholar]
- 31. Yu YB, Li YQ. Enteric glial cells and their role in the intestinal epithelial barrier. World J Gastroenterol 20: 11273–11280, 2014. doi: 10.3748/wjg.v20.i32.11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zolotarevsky Y, Hecht G, Koutsouris A, Gonzalez DE, Quan C, Tom J, Mrsny RJ, Turner JR. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology 123: 163–172, 2002. [Erratum in Gastroenterology 123: 1412, 2002]. doi: 10.1053/gast.2002.34235. [DOI] [PubMed] [Google Scholar]
- 33. Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-γ and tumor necrosis factor-α synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol 166: 409–419, 2005. doi: 10.1016/S0002-9440(10)62264-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cani PD, Plovier H, Van Hul M, Geurts L, Delzenne NM, Druart C, Everard A. Endocannabinoids—at the crossroads between the gut microbiota and host metabolism. Nat Rev Endocrinol 12: 133–143, 2016. doi: 10.1038/nrendo.2015.211. [DOI] [PubMed] [Google Scholar]
- 35. Sharkey KA, Wiley JW. The role of the endocannabinoid system in the brain–gut axis. Gastroenterology 151: 252–266, 2016. doi: 10.1053/j.gastro.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cristino L, Bisogno T, Di Marzo V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat Rev Neurol 16: 9–29, 2020. doi: 10.1038/s41582-019-0284-z. [DOI] [PubMed] [Google Scholar]
- 37. Lutz B. On-demand activation of the endocannabinoid system in the control of neuronal excitability and epileptiform seizures. Biochem Pharmacol 68: 1691–1698, 2004. doi: 10.1016/j.bcp.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 38. Toczek M, Malinowska B. Enhanced endocannabinoid tone as a potential target of pharmacotherapy. Life Sci 204: 20–45, 2018. doi: 10.1016/j.lfs.2018.04.054. [DOI] [PubMed] [Google Scholar]
- 39. Howlett AC, Reggio PH, Childers SR, Hampson RE, Ulloa NM, Deutsch DG. Endocannabinoid tone versus constitutive activity of cannabinoid receptors. Br J Pharmacol 163: 1329–1343, 2011. doi: 10.1111/j.1476-5381.2011.01364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tajik N, Frech M, Schulz O, Schälter F, Lucas S, Azizov V, Dürholz K, Steffen F, Omata Y, Rings A, Bertog M, Rizzo A, Iljazovic A, Basic M, Kleyer A, Culemann S, Krönke G, Luo Y, Überla K, Gaipl US, Frey B, Strowig T, Sarter K, Bischoff SC, Wirtz S, Cañete JD, Ciccia F, Schett G, Zaiss MM. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat Commun 11: 1995, 2020. doi: 10.1038/s41467-020-15831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alhamoruni A, Lee AC, Wright KL, Larvin M, O'Sullivan SE. Pharmacological effects of cannabinoids on the Caco-2 cell culture model of intestinal permeability. J Pharmacol Exp Ther 335: 92–102, 2010. doi: 10.1124/jpet.110.168237. [DOI] [PubMed] [Google Scholar]
- 42. Alhamoruni A, Wright KL, Larvin M, O'Sullivan SE. Cannabinoids mediate opposing effects on inflammation-induced intestinal permeability. Br J Pharmacol 165: 2598–2610, 2012. doi: 10.1111/j.1476-5381.2011.01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Karwad MA, Couch DG, Theophilidou E, Sarmad S, Barrett DA, Larvin M, Wright KL, Lund JN, O'Sullivan SE. The role of CB1 in intestinal permeability and inflammation. FASEB J 31: 3267–3277, 2017. doi: 10.1096/fj.201601346R. [DOI] [PubMed] [Google Scholar]
- 44. Duncan M. Cannabinoid 1 receptors are critical for the innate immune response to TLR4 stimulation. Am J Physiol Regul Integr Comp Physiol 305: R224–R231, 2013. doi: 10.1152/ajpregu.00104.2013. [DOI] [PubMed] [Google Scholar]
- 45. Kopp W. How western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes Metab Syndr Obes 12: 2221–2236, 2019. doi: 10.2147/DMSO.S216791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zuurbier CJ, Koeman A, Houten SM, Hollmann MW, Florijn WJ. Optimizing anesthetic regimen for surgery in mice through minimization of hemodynamic, metabolic, and inflammatory perturbations. Exp Biol Med (Maywood). 239: 737–746, 2014. doi: 10.1177/1535370214524877. [DOI] [PubMed] [Google Scholar]
- 47. Cavin JB, Cuddihey H, MacNaughton WK, Sharkey KA. Acute regulation of intestinal ion transport and permeability in response to luminal nutrients: the role of the enteric nervous system. Am J Physiol Gastrointest Liver Physiol 318: G254–G264, 2020. doi: 10.1152/ajpgi.00186.2019. [DOI] [PubMed] [Google Scholar]
- 48. Storr MA, Bashashati M, Hirota C, Vemuri VK, Keenan CM, Duncan M, Lutz B, Mackie K, Makriyannis A, Macnaughton WK, Sharkey KA. Differential effects of CB1 neutral antagonists and inverse agonists on gastrointestinal motility in mice. Neurogastroenterol Motil 22: 787-e223, 2010. doi: 10.1111/j.1365-2982.2010.01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cluny NL, Vemuri VK, Chambers AP, Limebeer CL, Bedard H, Wood JT, Lutz B, Zimmer A, Parker LA, Makriyannis A, Sharkey KA. A novel peripherally restricted cannabinoid receptor antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents. Br J Pharmacol 161: 629–642, 2010. doi: 10.1111/j.1476-5381.2010.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tam J, Vemuri VK, Liu J, Bátkai S, Mukhopadhyay B, Godlewski G, Osei-Hyiaman D, Ohnuma S, Ambudkar SV, Pickel J, Makriyannis A, Kunos G. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest 120: 2953–2966, 2010. [Erratum in J Clin Invest 120: 3735, 2010]. doi: 10.1172/JCI42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nascimento JC, Matheus VA, Oliveira RB, Tada SFS, Collares-Buzato CB. High-fat diet induces disruption of the tight junction-mediated paracellular barrier in the proximal small intestine before the onset of type 2 diabetes and endotoxemia. Dig Dis Sci 66: 3359–3374, 2021. doi: 10.1007/s10620-020-06664-x. [DOI] [PubMed] [Google Scholar]
- 52. Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal α-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol 1: 113–118, 2000. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 53. Hirota SA. NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm Bowel Dis 17: 1359–1372, 2011. doi: 10.1002/ibd.21478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zeineldin M, Neufeld K. Isolation of epithelial cells from mouse gastrointestinal tract for western blot or RNA analysis. Bio Protoc 2: e292, 2012. doi: 10.21769/BioProtoc.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Geurts L, Muccioli GG, Delzenne NM, Cani PD. Chronic endocannabinoid system stimulation induces muscle macrophage and lipid accumulation in type 2 diabetic mice independently of metabolic endotoxaemia. PLoS One 8: e55963, 2013. doi: 10.1371/journal.pone.0055963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hamtiaux L, Hansoulle L, Dauguet N, Muccioli GG, Gallez B, Lambert DM. Increasing antiproliferative properties of endocannabinoids in n1e-115 neuroblastoma cells through inhibition of their metabolism. PLoS One 6: e26823, 2011. doi: 10.1371/journal.pone.0026823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Notarnicola M, Tutino V, De Nunzio V, Dituri F, Caruso M, Giannelli G. Dietary ω-3 polyunsaturated fatty acids inhibit tumor growth in transgenic ApcMin/+ mice, correlating with CB1 receptor up-regulation. Int J Mol Sci 18: 485, 2017. doi: 10.3390/ijms18030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Notarnicola M, Messa C, Refolo MG, Tutino V, Miccolis A, Caruso MG. Polyunsaturated fatty acids reduce fatty acid synthase and hydroxy-methyl-glutaryl CoA-reductase gene expression and promote apoptosis in HepG2 cell line. Lipids Health Dis 10: 10, 2011. doi: 10.1186/1476-511X-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hamilton MK, Boudry G, Lemay DG, Raybould HE. Changes in intestinal barrier function and gut microbiota in high-fat diet-fed rats are dynamic and region dependent. Am J Physiol Gastrointest Liver Physiol 308: G840–G851, 2015. doi: 10.1152/ajpgi.00029.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Overman EL, Rivier JE, Moeser AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-α. PLoS One 7: e39935, 2012. doi: 10.1371/journal.pone.0039935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hasenoehrl C, Taschler U, Storr M, Schicho R. The gastrointestinal tract—a central organ of cannabinoid signaling in health and disease. Neurogastroenterol Motil 28: 1765–1780, 2016. doi: 10.1111/nmo.12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. MacNaughton WK, Van Sickle MD, Keenan CM, Cushing K, Mackie K, Sharkey KA. Distribution and function of the cannabinoid-1 receptor in the modulation of ion transport in the guinea pig ileum: relationship to capsaicin-sensitive nerves. Am J Physiol Gastrointest Liver Physiol 286: G863–G871, 2004. doi: 10.1152/ajpgi.00482.2003. [DOI] [PubMed] [Google Scholar]
- 63. Tyler K, Hillard CJ, Greenwood-Van Meerveld B. Inhibition of small intestinal secretion by cannabinoids is CB1 receptor-mediated in rats. Eur J Pharmacol 409: 207–211, 2000. doi: 10.1016/S0014-2999(00)00843-8. [DOI] [PubMed] [Google Scholar]
- 64. Haber AL, Biton M, Rogel N, Herbst RH, Shekhar K, Smillie C, Burgin G, Delorey TM, Howitt MR, Katz Y, Tirosh I, Beyaz S, Dionne D, Zhang M, Raychowdhury R, Garrett WS, Rozenblatt-Rosen O, Shi HN, Yilmaz O, Xavier RJ, Regev A. A single-cell survey of the small intestinal epithelium. Nature 551: 333–339, 2017. doi: 10.1038/nature24489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Glass LL, Calero-Nieto FJ, Jawaid W, Larraufie P, Kay RG, Göttgens B, Reimann F, Gribble FM. Single-cell RNA-sequencing reveals a distinct population of proglucagon-expressing cells specific to the mouse upper small intestine. Mol Metab 6: 1296–1303, 2017. doi: 10.1016/j.molmet.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Argueta DA, Perez PA, Makriyannis A, Di Patrizio NV. Cannabinoid CB1 receptors inhibit gut-brain satiation signaling in diet-induced obesity. Front Physiol 10: 704, 2019. doi: 10.3389/fphys.2019.00704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Urigüen L, García-Fuster MJ, Calladó LF, Morentin B, La Harpe R, Casadó V, Lluis C, Franco R, García-Sevilla JA, Meana JJ. Immunodensity and mRNA expression of A2A adenosine, D 2 dopamine, and CB1 cannabinoid receptors in postmortem frontal cortex of subjects with schizophrenia: effect of antipsychotic treatment. Psychopharmacology (Berl) 206: 313–324, 2009. doi: 10.1007/s00213-009-1608-2. [DOI] [PubMed] [Google Scholar]
- 68. López-Redondo F, Lees GM, Pertwee RG. Effects of cannabinoid receptor ligands on electrophysiological properties of myenteric neurones of the guinea-pig ileum. Br J Pharmacol 122: 330–334, 1997. doi: 10.1038/sj.bjp.0701393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Glass M, Northup JK. Agonist selective regulation of G proteins by cannabinoid CB1 and CB2 receptors. Mol Pharmacol 56: 1362–1369, 1999. doi: 10.1124/mol.56.6.1362. [DOI] [PubMed] [Google Scholar]
- 70. Diez-Alarcia R, Ibarra-Lecue I, Lopez-Cardona ÁP, Meana J, Gutierrez-Adán A, Callado LF, Agirregoitia E, Urigüen L. Biased agonism of three different cannabinoid receptor agonists in mouse brain cortex. Front Pharmacol 7: 415, 2016. doi: 10.3389/fphar.2016.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bosier B, Muccioli GG, Hermans E, Lambert DM. Functionally selective cannabinoid receptor signalling: therapeutic implications and opportunities. Biochem Pharmacol 80: 1–12, 2010. doi: 10.1016/j.bcp.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 72. Hudson BD, Hébert TE, Kelly MEM. Ligand- and heterodimer-directed signaling of the CB1 cannabinoid receptor. Mol Pharmacol 77: 1–9, 2010. doi: 10.1124/mol.109.060251. [DOI] [PubMed] [Google Scholar]
- 73. Fattore L, Spano MS, Cossu G, Deiana S, Fratta W. Cannabinoid mechanism in reinstatement of heroin-seeking after a long period of abstinence in rats. Eur J Neurosci 17: 1723–1726, 2003. doi: 10.1046/j.1460-9568.2003.02607.x. [DOI] [PubMed] [Google Scholar]
- 74. Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci 115: 4969–4976, 2002. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 75. Weber CR, Nalle SC, Tretiakova M, Rubin DT, Turner JR. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab Invest 88: 1110–1120, 2008. doi: 10.1038/labinvest.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Raju P, Shashikanth N, Tsai P-Y, Pongkorpsakol P, Chanez-Paredes S, Steinhagen PR, Kuo W-T, Singh G, Tsukita S, Turner JR. Inactivation of paracellular cation-selective claudin-2 channels attenuates immune-mediated experimental colitis in mice. J Clin Invest 130: 5197–5208, 2020. doi: 10.1172/JCI138697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang J, Zhang X, Yang C, Zhao S. Effect of monoacylglycerol lipase inhibition on intestinal permeability in chronic stress model. Biochem Biophys Res Commun 525: 962–967, 2020. doi: 10.1016/j.bbrc.2020.02.173. [DOI] [PubMed] [Google Scholar]
- 78. Hsieh C, Brown S, Derleth C, Mackie K. Internalization and recycling of the CB1 cannabinoid receptor. J Neurochem 73: 493–501, 1999. doi: 10.1046/j.1471-4159.1999.0730493.x. [DOI] [PubMed] [Google Scholar]
- 79. Coutts AA, Anavi-Goffer S, Ross RA, MacEwan DJ, Mackie K, Pertwee RG, Irving AJ. Agonist-induced internalization and trafficking of cannabinoid CB1 receptors in hippocampal neurons. J Neurosci 21: 2425–2433, 2001. doi: 10.1523/JNEUROSCI.21-07-02425.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Daigle TL, Kearn CS, Mackie K. Rapid CB1 cannabinoid receptor desensitization defines the time course of ERK1/2 MAP kinase signaling. Neuropharmacology 54: 36–44, 2008. doi: 10.1016/j.neuropharm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pfeiffer R, Kirsch J, Fahrenholz F. Agonist and antagonist-dependent internalization of the human vasopressin V2 receptor. Exp Cell Res 244: 327–339, 1998. doi: 10.1006/excr.1998.4159. [DOI] [PubMed] [Google Scholar]
- 82. Houle S, Larrivée JF, Bachvarova M, Bouthillier J, Bachvarov DR, Marceau F. Antagonist-induced intracellular sequestration of rabbit bradykinin B2 receptor. Hypertension 35: 1319–1325, 2000. doi: 10.1161/01.hyp.35.6.1319. [DOI] [PubMed] [Google Scholar]
- 83. Cawston EE, Harikumar KG, Miller LJ. Ligand-induced internalization of the type 1 cholecystokinin receptor independent of recognized signaling activity. Am J Physiol Cell Physiol 302: C615–C627, 2012. doi: 10.1152/ajpcell.00193.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Frazier TH, DiBaise JK, McClain CJ. Gut microbiota, intestinal permeability, obesity-induced inflammation, and liver injury. JPEN J Parenter Enteral Nutr 35: 14S–20S, 2011. doi: 10.1177/0148607111413772. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.19892572.v1.