Abstract
Substantial genetic correlations have been reported across psychiatric disorders and numerous cross-disorder genetic variants have been detected. To identify the genetic variants underlying general psychopathology in childhood, we performed a genome-wide association study using a total psychiatric problem score. We analyzed 6,844,199 common SNPs in 38,418 school-aged children from 20 population-based cohorts participating in the EAGLE consortium. The SNP heritability of total psychiatric problems was 5.4% (SE = 0.01) and two loci reached genome-wide significance: rs10767094 and rs202005905. We also observed an association of SBF2, a gene associated with neuroticism in previous GWAS, with total psychiatric problems. The genetic effects underlying the total score were shared with common psychiatric disorders only (attention-deficit/hyperactivity disorder, anxiety, depression, insomnia) (rG > 0.49), but not with autism or the less common adult disorders (schizophrenia, bipolar disorder, or eating disorders) (rG < 0.01). Importantly, the total psychiatric problem score also showed at least a moderate genetic correlation with intelligence, educational attainment, wellbeing, smoking, and body fat (rG > 0.29). The results suggest that many common genetic variants are associated with childhood psychiatric symptoms and related phenotypes in general instead of with specific symptoms. Further research is needed to establish causality and pleiotropic mechanisms between related traits.
Introduction
Psychiatric disorders are moderately heritable, on average about 30–50% of the variability in symptoms can be explained by genetic differences between individuals [1]. The joint effect of common single nucleotide polymorphisms (SNP heritability) explains 5% to 30% of the variance in psychiatric disorders in adults [2]. Similar levels have been reported for behavioral and emotional symptoms in children, although there is large variability depending on child age and informant [3, 4]. A focus on childhood problems is particularly important, as many adult disorders can be traced back to problems in childhood [5].
Recent family and molecular genetic studies demonstrated that much of the genetic effects underlying psychiatric disorders are not unique to particular diagnoses, but rather shared across several psychiatric diagnoses and symptoms [2, 6–10]. This phenomenon is known as cross-phenotype association and suggests pleiotropy, i.e. the influence of a genetic variant on multiple traits, [11] and may be an explanation for the extensive co-occurrence of mental disorders [12]. Several lines of evidence support this notion. First, the SNP based genetic correlations between disorders from different domains, such as major depression, attention-deficit/hyperactivity disorder (ADHD), bipolar disorder and schizophrenia are moderate to high, [2] averaging 0.41 [10]. Second, measures of global psychopathology in children showed a common SNP heritability between 16% and 38% [8, 13]. Third, a genome-wide association meta-analysis (GWAS) of eight psychiatric disorders (ADHD, anorexia, autism, bipolar, depression, obsessive compulsive disorder, schizophrenia and Tourette’s) identified 23 loci associated with at least four of these disorders [14].
GWAS derived polygenic risk scores (PRS) for single disorders are good predictors of general psychopathology. For instance, a PRS for ADHD was more strongly associated with a general psychopathology factor than with specific hyperactivity or attention problems adjusted for general psychopathology [15]. In another study a composite PRS based on eight GWAS was associated with general psychopathology in childhood [16]. These cross-phenotype associations present a challenge in interpreting GWAS results that typically target a single disorder, raising the question of whether a multi-disorder approach would be more informative.
Previous GWAS of childhood disorders, such as autism spectrum disorders, ADHD, aggression and internalizing disorders, [4, 17–19] have provided insights into the genetic architecture of child psychiatric problems and into the genetic correlations between childhood psychiatric problems. However, with notable exceptions of a large recent ADHD study [20] and a GWAS on autism spectrum disorder, [17] these studies mostly failed to identify individual genome-wide significant loci. Besides increasing the sample size, some researchers propose the inclusion of related phenotypes in analyses to increase power [21, 22]. Genetic loci with pleiotropic effects may be missed in a GWAS of single psychiatric disorders. If a variant only modestly increases the risk of symptoms from different domains, any association with a specific disorder may be too weak to be detected. A focus on global psychopathology increases the power to detect unspecific genetic loci, which are associated with global psychiatric vulnerabilities. A previous GWAS [14] examined multiple disorder simultaneously, but analyses of multiple dimensional measures of psychiatric problems in childhood are lacking. This approach is arguably particularly promising in childhood given the less clearly expressed symptoms and the low homotypic but high heterotypic stability of problems, [23] i.e. the changing of symptoms from one domain to another.
Our aim was to identify genetic loci associated with a total psychiatric problem score representing a variety of psychiatric problems including internalizing, externalizing, attention, neurodevelopmental and other psychiatric problems. To identify these genetic variants, we performed a GWAS meta-analysis within the EArly Genetics and Lifecourse Epidemiology (EAGLE) consortium (https://www.eagle-consortium.org/). Finally, we estimated genetic correlations of the total psychiatric problem score with various single child and adult psychiatric, psychological, neurological and lifestyle or educational characteristics.
Methods and materials
Participants
Cohorts from the EAGLE consortium with parent-rated measures of psychiatric symptoms in the age range 5–16 years were invited to participate in the project Twenty cohorts from Europe, the US and Australia contributed data to this meta-analysis. See Table 1 and S1 File for cohort descriptions. Parents provided written informed consent for their children’s participation and the study was approved by the Ethics Committee of Erasmus MC, as well as by local ethics committees at each site, see S1 File for full ethics statements per participating cohort. The study was performed in accordance with the Declaration of Helsinki. We restricted the analysis to children of European ancestry to avoid population stratification bias. In total data from 38,418 participants with a mean age of 9.9 years (SD = 2.02) were meta-analyzed. This study was originally planned with a discovery-replication design. However, the obtained sample-size was not sufficiently large to split the sample, and we opted for maximizing power in discovery analyses.
Table 1. Phenotype characteristics.
| Cohort | n | Instrument | Domains | Informant | Age years | Age SD | Score Mean | Score SD | % Female |
|---|---|---|---|---|---|---|---|---|---|
| 1958BC-T1DGC | 2170 | Rutter | Int,Ext | Maternal | 11.3 | 0.1 | 6.2 | 3.4 | 51 |
| 1958BC-WTCCC | 2261 | Rutter | Int,Ext | Maternal | 11.3 | 0.2 | 6.2 | 3.4 | 48 |
| ALSPAC | 5461 | SDQ | Int,Ext | Maternal | 9.6 | 0.1 | 6.7 | 4.8 | 49 |
| BREATHE | 1618 | SDQ | Int,Ext | Both | 8.3 | 3.9 | 8.1 | 5.1 | 48 |
| CADD | 358 | CBCL 4–18 | Int,Ext,Sleep,TP,EP,PDD | Both | 13.0 | 2.6 | 16.2 | 21.9 | 28 |
| CATSS | 6498 | A-TAC | Int,Ext,EP,PDD | Both | 12.0 | 0.0 | 5.4 | 7.5 | 49 |
| COPSAC2010 | 547 | SDQ 4–10 | Int,Ext | Both | 6.0 | 0.3 | 7.1 | 4.7 | 48 |
| FinnTwin12 | 959 | MPNI | Int,Ext | Both | 11.4 | 0.3 | 11.3 | 6.8 | 53 |
| GenR | 1847 | CBCL 6–18 | Int,Ext,Sleep,TP,EP,PDD | Maternal | 9.7 | 0.3 | 17.3 | 15.2 | 51 |
| Gini-Lisa | 1389 | SDQ | Int,Ext | Maternal | 10.0 | 0.2 | 7.3 | 5.2 | 48 |
| Glaku | 312 | CBCL 6–18 | Int,Ext,Sleep,TP,EP,PDD | Maternal | 12.1 | 1.0 | 21.7 | 16.8 | 52 |
| INMA | 745 | SDQ | Int,Ext | Both | 5.1 | 0.8 | 8.9 | 5.0 | 38 |
| MUSP | 1156 | CBCL 6–18 | Int,Ext,Sleep,TP,EP,PDD | Maternal | 13.9 | 0.3 | 30.5 | 19.8 | 61 |
| NFBC1986 | 3346 | Rutter | Int,Ext | Maternal | 7.8 | 0.2 | 2.6 | 2.1 | 51 |
| NTR I | 2563 | CBCL 6–18 | Int,Ext,Sleep,TP,EP,PDD | Maternal | 9.9 | 1.0 | 19.3 | 15.9 | 52 |
| NTR II | 2960 | CBCL 6–18 | Int,Ext,Sleep,TP,EP,PDD | Maternal | 9.6 | 1.0 | 19.1 | 16.6 | 53 |
| RAINE | 1366 | CBCL 4–18 | Int,Ext,Sleep,TP,EP,PDD | Both | 10.6 | 0.2 | 21.1 | 18.6 | 48 |
| TCHAD | 2111 | CBCL 6–18 | Int,Ext,Sleep,TP,EP,PDD | Both | 13.0 | 0.0 | 11.7 | 12.5 | 51 |
| TEDS | 2707 | SDQ | Int,Ext | Both | 11.3 | 0.7 | 7.0 | 5.0 | 54 |
| TRAILS | 1283 | CBCL 6–18 | Int,Ext,Sleep,TP,EP,PDD | Maternal | 11.1 | 0.6 | 0.2 | 0.2 | 52 |
| YFS | 1352 | HES | Int,Ext | Maternal | 10.6 | 3.3 | 14.7 | 6.8 | 54 |
n sample size
Domains covered by instrument: Internalizing (Int), Externalizing (Ext), Sleep, Thought Problems (TP), Eating Problems (EP), Pervasive Developmental Disorder Score (PDD)
Informant questionnaire filled in by only mothers (maternal) or by either father or mother (both)
SD standard deviation
Outcome
Psychiatric problems were assessed with parent-rated questionnaires at the assessment wave closest to age 10 years. All items of a broad psychiatric questionnaire were summed into a single total psychiatric sum score. In all cohorts internalizing, externalizing and attention problems were assessed; in some questionnaires items on sleep, thought, eating problems, and pervasive developmental disorders were included in the total problem score (Table 1). Instruments included the Child Behavior Checklist (CBCL), [24] Strengths and Difficulties Questionnaire (SDQ), [25] parental version of the Multidimensional Peer Nomination Inventory (MPNI), [26] Rutter Children’ Behaviour Questionnaire, [27] the Autism–Tics, AD/HD and other Comorbidities inventory (A-TAC) [28], and items derived from the Health Examination Survey [29].
We applied a log transformation plus 1 to avoid bias due to non-normal residuals and influential observations. Because different scales were used, the log-transformed scores were converted to a z-score within cohorts to make units comparable across cohorts.
Genotyping and QC
Genotyping was performed using genome-wide arrays. Cohort-specific pre-imputation quality control (QC) was performed using established protocols. In all cohorts, SNPs were imputed to the 1000 Genomes Phase 1 or Phase 3 reference panel [30]. Each cohort performed a GWAS and summary results were collected for meta-analysis. We omitted the X-chromosome from further analysis as most cohorts had no information available on X-linked SNPs. Pre-meta-analysis QC was performed with EasyQC and QCGWAS [31–33]. The QC steps are summarized in S1 Fig. After meta-analysis, we excluded SNPs with low minor allele frequency (MAF < 5%), sample size (<5000), or with data from a small number of cohorts (<5). Finally, we checked the pooled results for spurious inflation by examining QQ-plots of the p-value distribution and by examining the LD score regression intercept (see statistical analysis). Full genetic methods and quality control per cohort can be found in S1 and S2 Tables.
Statistical analysis
Single SNP associations and meta-analysis
The z-scores of the total psychiatric problems scores were related to the SNP dosages in a linear model. Covariates included gender, age at assessment and principal components of ancestry. The number of dimensions (1–10) were specified by each cohort. CATSS and TCHAD additionally used a random effect to account for familial relatedness. FinnTwin12 and NTR applied a mixed model with two random effects to control for population stratification and relatedness. We pooled the results from the individual cohorts using an inverse-variance weighted fixed-effects meta-analysis. R 3.4.3 was used for QC, data preparation and analysis of results [34]. Meta-soft 2.0.1 was used for the meta-analysis of single SNP associations [35]. The individual cohort results after quality control were examined and meta-analyzed independently by the first and second author with consistent results. Genome-wide significance was set at p<5E-08. We had good power (>80%) to detect effect sizes between 0.05 for MAF = 50% and 0.11 for MAF = 5%. Effect size (Beta) was defined as change in log(total problems+1) in SD per effect allele. Power was calculated with gwas-power (https://github.com/kaustubhad/gwas-power) [36].
We also used the FUMA web tool [37] to explore potential functional implications of any identified variants. We reviewed positional mapping, eQTL analyses and chromatin interactions with all available databases (date: 2019-06-30). We also performed a lookup in the mQTL [38] database, to check for potential influences on gene expression via DNA methylation.
Gene-based and expression analysis
We performed gene-based tests using MAGMA [39] in FUMA. MAGMA estimates the joint effect of all SNPs within a gene, while accounting for the LD structure and gene size. We tested 18,168 protein coding genes and thus the p-value significance threshold was set at 3e-6 based on Bonferroni correction.
Second, we tested, whether the results from the gene-based tests were related to gene expression in several tissues. Specifically, we used MAGMA to test whether the strength of association between genes and the total psychiatric problem score was related to the mean gene expression level in a specific tissue, while considering average expression levels. Given that we expected gene variants to act via brain pathways, we tested expression in 13 brain regions (S3 Table). However, as gene effects may impact the brain indirectly via other tissues, we also investigated gene expression levels on an organ level (S4 Table). Gene expression levels were obtained from the GTEx 7 database [40].
Third, we further examined whether the predicted gene expression of selected genes was related to total psychiatric problems. We selected genes, that were (functionally) annotated to genome-wide hits, or that were genome-wide significant according to gene-based tests. To correlate gene expression with total psychiatric problems, we used a transciptome-wide association study (TWAS) approach [41]. In short, gene expression in a tissue is imputed based on expression information from the GTEx 7 database for a specific tissue and then correlated with a phenotype, as inferred from GWAS summary statics. We chose to examine the expression in the basal ganglia post-hoc, as the loci most strongly associated with total psychiatric problems were related to genes expressed in the basal ganglia regions, see section “results: gene expression” below. We also performed a lookup on TWAS hub, to examine whether gene expression by a gene identified in this study has previously been associated with other phenotypes [42, 43].
SNP heritability and genetic correlations
We estimated the SNP heritability of total psychiatric problem scores with LD score regression [44]. We used the online tool LD Hub [45] to estimate common SNP heritability and genetic correlations with various psychiatric, psychological, neurological and lifestyle or educational characteristics. To compute the genetic correlations we selected published GWAS summary statistics available on LD Hub, except genetic correlations with anxiety symptoms, [46] which were computed locally with ldsc 1.0.0.
Sensitivity analyses
The GWAS meta-analysis included cohorts using different instruments to measure total psychiatric problems with mean age ranges from 5 to 13 years. While our inclusion criteria maximized sample sizes and also increased the potential for generalizability, this approach may have increased the study heterogeneity. We therefore performed two sensitivity analyses to investigate effect consistency. In the first, we performed three separate meta-analyses for each of the major instrument groups. These meta-analyses included only cohorts using the CBCL, SDQ or Rutter questionnaires, respectively. We then tested consistency of genetic effects by computing the genetic correlation between each instrument.
In the second sensitivity analyses, we tested, whether the genetic correlation between total psychiatric problems and thought disorders changed depending on the age at which total psychiatric problems were assessed. Specifically, we hypothesized, that total psychiatric problems scores assessed during early adolescence would correlate more strongly with schizophrenia and bipolar disorders, than total psychiatric problems occurring before the onset of puberty. We therefore performed two meta-analyses consisting of the cohorts assessing youths below and equal or above age 12 years. We then examined the genetic correlation of total psychiatric problems in both age groups with schizophrenia and bipolar disorder.
Results
Spurious inflation and SNP Heritability
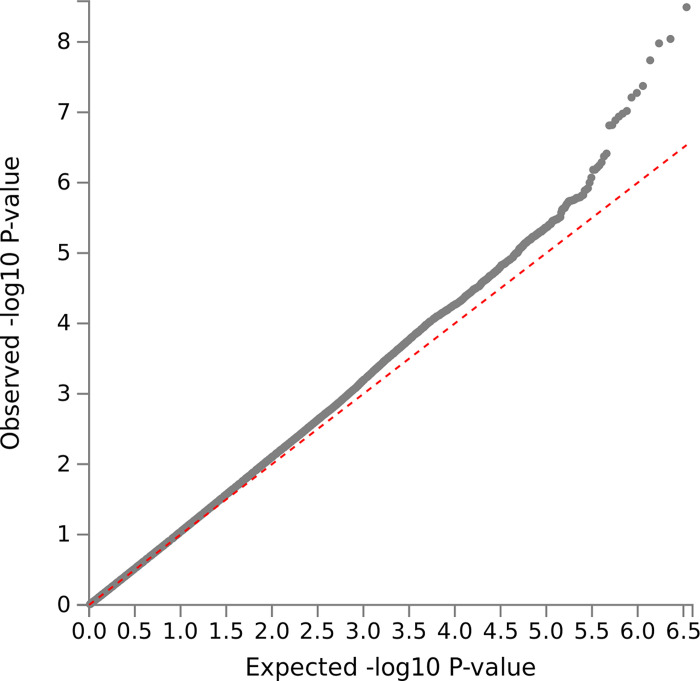
We tested 6,844,199 SNPs after quality control. The QQ-plot (Fig 1) showed some inflation, however, the LD score intercepts was close to 1 (β0 = 1.01, SE = 0.01), suggesting that the inflation was due to a true signal rather than spurious associations. The SNP heritability was estimated at 5.4% (SE = 0.013).
Fig 1. QQ-plot.
Quantile-quantile plot of observed -log 10 p values vs expected -log 10 p values assuming chance findings in single SNP analysis. Diagonal line indicates a p value distribution compatible with chance finding. Upward deviations indicate p values more significant than expected.
SNP based tests
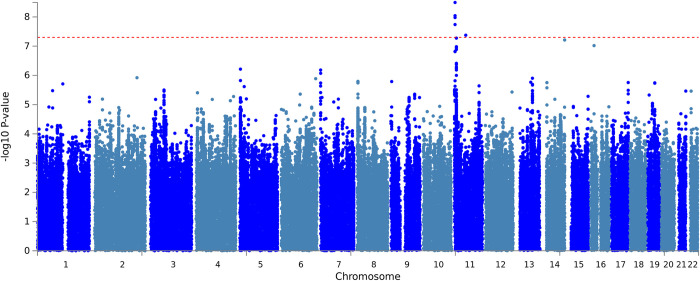
Two loci on chromosome 11 were genome-wide significant, see Fig 2. One locus is located around lead SNP rs10767094, which showed an increase of 0.08SD in total psychiatric problems per A allele (SE = 0.01, p = 3E-09, n = 8,216) (S2 Fig). The A allele is very common with an average frequency of 48% across the cohorts, but the SNP’s average imputation quality was a moderate 50% (Info/R2). Information on this locus was only available in 27% of participants (6 cohorts). The SNP showed a moderate amount of effect heterogeneity (I2 = 47.6%). Also on chromosome 11 an insertion/deletion variant (InDel) was genome-wide significant. A deletion of the A allele at rs202005905 was associated with an increase of 0.08SD in total psychiatric problems (SE = 0.01, p = 4E-08, n = 15,886, S3 Fig). Deletion prevalence was on average 16%, but again the imputation quality was modest with 52%, information was available in 41% of participants (9 cohorts) and the genetic variant showed moderate effect heterogeneity (I2 = 59.6%).
Fig 2. Manhattan plot.
Manhattan plot of -log 10 p values vs SNP position for single SNP analysis. SNPs above the red horizontal line indicate genome-wide significant findings.
The SNP rs10767094 lies in the intron of the long non-coding RNA Loc105379880 and rs202005905 lies in an intergenic region with no nearby genes. A FUMA eQTL and chromatin interaction analysis did not reveal any interactions with genes. The mQTL database did not list any associations with DNA methylation.
The third top locus did not reach genome-wide significance, but is of interest for its location in a gene previously implicated in neuroticism [47, 48] as well as being very close to genome-wide significance. The SNP rs72854494 lies within the gene SBF2. The T allele was associated with 0.05SD lower total psychiatric problems (SE = 0.01, p = 5E-08, n = 38,330) (S4 Fig). This association showed no heterogeneity (I2 = 0.0%) among the cohorts. The T allele occurred on average in 14% across cohorts, with a very good imputation quality of 96%. FUMA eQTL and chromatin interaction analysis, as well as a lookup in mQTL DB did not reveal any further information on functional association. Results for all SNPs with genome-wide suggestive p-values (p<5E-06) can be found in Table 2. The full summary statistics can be found at https://doi.org/10.6084/m9.figshare.17170994.v1.
Table 2. SNPs with genome-wide significant (p<5E-08) and suggestive (p<5E-07) results.
| SNP | Chr | BP | EA | OA | EAF | nstu | n | β | SE | p | I2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| rs10767094 | 11 | 3477509 | A | G | 0.48 | 6 | 8216 | 0.08 | 0.01 | 3E-09 | 47.6 |
| rs12098951 | 11 | 3478953 | A | G | 0.48 | 8 | 10417 | 0.08 | 0.01 | 9E-09 | 52.3 |
| rs10767093 | 11 | 3477421 | T | A | 0.48 | 8 | 10408 | 0.08 | 0.01 | 1E-08 | 40.3 |
| rs10767096 | 11 | 3477891 | T | C | 0.53 | 8 | 10382 | 0.07 | 0.01 | 2E-08 | 47.6 |
| rs202005905 | 11 | 54733705 | I | D | 0.84 | 9 | 15886 | -0.08 | 0.01 | 4E-08 | 59.6 |
| rs72854494 | 11 | 9946312 | T | C | 0.86 | 21 | 38330 | -0.05 | 0.01 | 5E-08 | 0.0 |
| rs188216744 | 14 | 106478354 | T | C | 0.55 | 5 | 7045 | 0.08 | 0.01 | 6E-08 | 87.2 |
| rs115749482 | 16 | 16754648 | A | G | 0.81 | 5 | 6930 | 0.08 | 0.01 | 1E-07 | 85.6 |
| rs59076561 | 11 | 9951438 | G | T | 0.86 | 20 | 35645 | -0.05 | 0.01 | 1E-07 | 0.0 |
| rs113227893 | 11 | 9944120 | D | I | 0.86 | 18 | 33850 | -0.05 | 0.01 | 1E-07 | 0.2 |
| rs67456791 | 11 | 9944108 | G | A | 0.86 | 20 | 35533 | -0.05 | 0.01 | 1E-07 | 0.0 |
| rs10767095 | 11 | 3477568 | G | A | 0.48 | 8 | 10413 | 0.07 | 0.01 | 2E-07 | 43.8 |
| rs10834158 | 11 | 3477887 | A | G | 0.52 | 9 | 11682 | 0.07 | 0.01 | 2E-07 | 56.1 |
| rs116657155 | 11 | 9954242 | A | G | 0.85 | 20 | 35619 | -0.05 | 0.01 | 4E-07 | 0.0 |
| rs60713856 | 11 | 9955418 | G | C | 0.85 | 20 | 35579 | -0.05 | 0.01 | 4E-07 | 0.0 |
| rs140557414 | 11 | 9953387 | A | C | 0.85 | 20 | 35632 | -0.05 | 0.01 | 5E-07 | 0.0 |
| rs57331333 | 11 | 9956272 | T | C | 0.85 | 20 | 35570 | -0.05 | 0.01 | 6E-07 | 0.0 |
| rs34543113 | 5 | 3339568 | G | A | 0.70 | 20 | 35612 | -0.04 | 0.01 | 6E-07 | 0.0 |
| rs11042555 | 11 | 9957159 | T | C | 0.85 | 20 | 35571 | -0.05 | 0.01 | 7E-07 | 0.0 |
| rs36189439 | 7 | 323206 | A | G | 0.58 | 6 | 7814 | 0.07 | 0.01 | 7E-07 | 69.2 |
Chr Chromosome
BP Basepair Position (Build 37 map)
EA Effect Allele
OA Other Allele
EAF Effect Allele Frequency
nstu Number of Studies
n Sample Size
β Beta
SE standard error
p p-value
I2 Effect heterogeneity
Gene-based test
Next we tested the association of 18,290 protein coding genes with the child total psychiatric problem score. None of the genes reached genome-wide significance (S3 Table, S5 and S6 Figs). We also post-hoc looked up the association of SBF2. The aggregate of 1,508 SNPs in SBF2 showed a nominal significance of p = 0.0004 (n = 35,736).
Gene expression
We performed a MAGMA tissue expression analysis in 13 specific brain tissues (S4 Table). Genes more strongly associated with total psychiatric problems tended to express particularly in four subcortical structures: caudate, putamen, anterior cingulate cortex and amygdala. However, these associations were not significant after correction for multiple testing. In addition, we analyzed tissue expression for 30 tissues on an organ level, see S5 Table. None of the organs had statistically significant associations, however, expression in the brain showed the strongest association (p = 0.06).
The top two genome-wide significant loci were not linked to a characterized gene, thus we decided to perform a TWAS analysis only for SBF2. We found that higher predicted levels of SBF2 in the basal ganglia were related to higher scores of total psychiatric symptoms (Z = +2.33, p = 0.02) based on the best linear unbiased predictions (BLUP) of a random variable representing 489 SNPs. A lookup in the TWAS Hub database revealed, that predicted levels of SBF2 gene products associate most with following phenotypes: neuroticism, body fat measures, red blood cell count, nervous feelings and worrying (http://twas-hub.org/genes/SBF2/).
Genetic correlation
Next we quantified the extent to which the genetic associations of child psychiatric problems scores were shared with other phenotypes. After adjustment for false discovery rate, insomnia, depressive symptoms, neuroticism, cigarettes smoked per day, body fat, body mass index, number of children, and age of smoking initiation all showed positive genetic correlations between 0.29 and 0.60 with the total psychiatric problem score (Table 3) based on the results of independent GWAS in adults. The highest correlation of total psychiatric problems was with ADHD, but this association did not survive multiple testing correction (rG = 0.86, SE = 0.39, p = 0.03, q = 0.06). Subjective wellbeing, childhood IQ, college completion, years of schooling, intelligence and age of smoking initiation showed significant negative correlations with the total psychiatric problem score, ranging from -0.66 to -0.42. Of the psychiatric phenotypes tested, the less common psychiatric disorders like schizophrenia, bipolar disorder, autism spectrum disorder, and anorexia were not genetically correlated with the total psychiatric problem score (rG < 0.01).
Table 3. Genetic correlations based on LD score regression.
| Correlated trait | PMID | rG | SE | p | q | h2 |
|---|---|---|---|---|---|---|
| Psychiatry | ||||||
| ADHD | 20732625 | 0.86 | 0.39 | 3E-02 | 6E-02 | 0.19 |
| Depressive symptoms | 27089181 | 0.60 | 0.13 | 1E-06 | 9E-06 | 0.05 |
| Anxiety symptoms | 26754954 | 0.60 | 0.26 | 3E-01 | 4E-01 | 0.26 |
| Insomnia | 28604731 | 0.49 | 0.15 | 9E-04 | 3E-03 | 0.05 |
| Major depressive disorder | 22472876 | 0.22 | 0.17 | 2E-01 | 3E-01 | 0.14 |
| PGC cross-disorder analysis | 23453885 | 0.07 | 0.11 | 5E-01 | 6E-01 | 0.16 |
| Autism spectrum disorder | 28540026 | 0.01 | 0.15 | 9E-01 | 1E+00 | 0.37 |
| Schizophrenia | 25056061 | -0.03 | 0.07 | 7E-01 | 8E-01 | 0.45 |
| Bipolar disorder | 21926972 | -0.16 | 0.11 | 1E-01 | 2E-01 | 0.43 |
| Anorexia Nervosa | 24514567 | -0.17 | 0.12 | 1E-01 | 2E-01 | 0.31 |
| Neurology | ||||||
| Amyotrophic lateral sclerosis | 27455348 | 0.30 | 0.23 | 2E-01 | 3E-01 | 0.04 |
| Parkinsons disease | 19915575 | 0.14 | 0.12 | 2E-01 | 3E-01 | 0.37 |
| Alzheimers disease | 24162737 | -0.10 | 0.17 | 6E-01 | 6E-01 | 0.05 |
| Personality and Wellbeing | ||||||
| Neuroticism | 27089181 | 0.41 | 0.09 | 1E-05 | 8E-05 | 0.09 |
| Neo-conscientiousness | 21173776 | 0.05 | 0.23 | 8E-01 | 9E-01 | 0.07 |
| Neo-openness to experience | 21173776 | 0.01 | 0.18 | 1E+00 | 1E+00 | 0.11 |
| Subjective well being | 27089181 | -0.46 | 0.12 | 1E-04 | 4E-04 | 0.02 |
| Intelligence and educational attainment | ||||||
| Childhood IQ | 23358156 | -0.42 | 0.16 | 8E-03 | 2E-02 | 0.27 |
| Years of schooling | 25201988 | -0.56 | 0.11 | 3E-07 | 2E-06 | 0.11 |
| Intelligence | 28530673 | -0.63 | 0.11 | 1E-08 | 2E-07 | 0.20 |
| College completion | 23722424 | -0.66 | 0.11 | 3E-09 | 8E-08 | 0.08 |
| Brain volume | ||||||
| Mean Hippocampus | 25607358 | 0.01 | 0.18 | 1E+00 | 1E+00 | 0.15 |
| Mean Thalamus | 25607358 | -0.06 | 0.20 | 8E-01 | 8E-01 | 0.11 |
| Infant head circumference | 22504419 | -0.13 | 0.18 | 5E-01 | 6E-01 | 0.22 |
| Intracranial Volume | 25607358 | -0.15 | 0.20 | 4E-01 | 6E-01 | 0.17 |
| Mean Pallidum | 25607358 | -0.17 | 0.17 | 3E-01 | 4E-01 | 0.17 |
| Mean Caudate | 25607358 | -0.18 | 0.14 | 2E-01 | 3E-01 | 0.25 |
| Mean Accumbens | 25607358 | -0.24 | 0.25 | 4E-01 | 5E-01 | 0.09 |
| Mean Putamen | 25607358 | -0.25 | 0.13 | 6E-02 | 1E-01 | 0.29 |
| General health behaviors/outcomes | ||||||
| Cigarettes smoked per day | 20418890 | 0.58 | 0.22 | 9E-03 | 2E-02 | 0.05 |
| Body fat | 26833246 | 0.48 | 0.12 | 5E-05 | 2E-04 | 0.11 |
| Body mass index | 20935630 | 0.30 | 0.09 | 1E-03 | 3E-03 | 0.19 |
| Sleep duration | 27494321 | -0.18 | 0.11 | 9E-02 | 2E-01 | 0.05 |
| Age of smoking initiation | 20418890 | -0.64 | 0.25 | 1E-02 | 2E-02 | 0.05 |
| Parent’s age at death | ||||||
| Parent’s age at death | 27015805 | -0.20 | 0.16 | 2E-01 | 3E-01 | 0.03 |
| Reproduction | ||||||
| Number of children ever born | 27798627 | 0.30 | 0.11 | 5E-03 | 2E-02 | 0.02 |
Bold rows indicate correlates with statistical significance after multiple testing correction
PMID PubMed ID, rG Genetic Correlation, SE Standard Error, p P-value
q False Discovery Rate Adjusted P-values, h2 SNP heritability
Sensitivity analyses
Total psychiatric problems measured with CBCL and SDQ genetically correlated with rG = 0.84 (SE = 0.31, p = 0.008). The Rutter questionnaire correlated more modestly with both CBCL (rG = 0.43, SE = 0.39, p = 0.073) and SDQ (rG = 0.63, SE = 0.29, p = 0.030). The genetic correlations of total psychiatric problems assessed both before or after age 12 with schizophrenia and bipolar disorder were small and consistent with a null effect irrespective of age group (S6 Table).
Discussion
The current study reports the first GWAS examining global psychopathology in children. Two genetic loci were genome-wide significant in the total sample. Additionally, we found support for the involvement of gene SBF2 in the development of psychopathology. The genetic effects underlying global psychopathology were shared with common psychiatric disorders (ADHD, anxiety, depression, insomnia), but not with less common and on average more severe ones (schizophrenia, bipolar disorder, autism, eating disorders).
The two genome-wide significant variants are one SNP (rs10767094) and one InDel (rs202005905). To the best of our knowledge these variants have not been associated with psychiatric traits before. It is unclear, how exactly these variants or tagged causal variants may affect general psychopathology, as functional annotation for these loci is sparse. The modest imputation quality possibly affected study results as both variants failed quality control in most cohorts. Measurement error of the genotypes could explain the relatively high estimates heterogeneity. An important next step would therefore be to replicate these SNPs using direct genotyping or denser arrays.
While just not genome-wide significant, the evidence for an involvement of SBF2 with the lead SNP rs72854494 in total psychiatric problems is more convincing. This locus has been implicated in neuroticism based on two GWAS. In a GWAS of neuroticism [47] rs1557341, located in SBF2, showed genome-wide significance. In a second larger independent GWAS of 449,484 participants, SBF2 showed a genome-wide significant effect for both neuroticism and worry in gene-based tests [48]. Furthermore, according to TWAS hub, the predicted gene products of SBF2 correlate with neuroticism based on several GWAS. Neuroticism describes a disposition to experience negative emotions and a higher stress reactivity. It robustly and substantially associates with general psychopathology in children, [8, 49] adolescence, [50] and adults [51] (between r = 0.13 and r = 0.81). A twin study suggested that this correlation arises partly due to shared genetic causes [52] and in this GWAS the genetic correlations between total psychiatric problems and neuroticism were substantial as well, similar to the phenotypic association (rG = 0.41). These results suggest that SBF2 pleiotropically affects neuroticism and psychopathology, but the mechanisms would need to be explored further. Neuroticism has been hypothesized to contribute strongly to general psychopathology, [53] thus it may mediate the effect of genetic variants on total psychiatric problems, but both phenotypes may also be independently affected. In regards to biology, human and mice studies points towards abnormal myelination as one of the consequences of SBF2 alterations [54, 55]. We recently reported an association between lower global white matter integrity and higher levels of general psychopathology in school-aged children [56]. Thus, one may speculate that SBF2 affects psychiatric problems via white matter development.
We additionally tested, whether genetic variants associated with total psychiatric problems were associated with gene expression in the brain. Association with gene expression in the limbic system of the brain showed the most support, but did not survive multiple testing correction. The findings are thus compatible with the possibility of a chance finding, but strong theoretical support for a major role of the limbic system exists. The limbic system includes evolutionary preserved regions responsible for emotion regulation and motivation, [57] which were previously implicated in affective disorders, ADHD and OCD, [58, 59] and are a potential intervention target [60].
In this study we observed 5% SNP heritability, which is similar to the LD score estimated SNP heritability of continuously measured ADHD, [4] depression, [47] and anxiety symptoms [46] in population based cohorts. The total psychiatric problem scores were based on various instruments, which all included items for common psychiatric internalizing, attention, and externalizing symptoms. Therefore, it is not surprising that common psychiatric symptoms and disorders such as ADHD and depression shared 36% or more of the genetic variation with the total psychiatric problem score. The extent to which the questionnaires used in this study covered other less common problems, such as psychotic, bipolar or autistic symptoms varied greatly by instrument. This may explain the low genetic correlation between total psychiatric problem scores with these disorders. Also, these disorders are less prevalent in the general population and thus may be reflected less in total problem scores. Furthermore, age of onset for schizophrenia and bipolar disorder is typically in late adolescence and early adulthood [61–63]. We did not find support for the notion that total psychiatric problems assessed in at later ages would genetically correlate more strongly with thought disorders than those assessed at younger ages. For autism spectrum disorder, the age of onset is early, but the prevalence in the cohorts was low. Thus, the total psychiatric problem score covered broad symptomatology but was not representative of severe psychiatric disorders with lower prevalence rates or emergence at later ages. The differential genetic correlations with common and relatively rare disorders suggests a continuum of genetic effects varying from very specific variants, variants which underlie either common or less common disorders, to variants which underlie most psychiatric problems. The presence of these universal variants is supported by genetic correlations between common and less common disorders, such as ADHD and schizophrenia [2, 10]. The latter set of variants may be better detected with measures of global psychopathology in older children, when thought disorders such as schizophrenia and bipolar disorder occur.
In addition to the genetic correlations with different psychiatric disorders, total psychiatric problems genetically correlated with various psychological and health-related phenotypes, namely intelligence, educational attainment, wellbeing, smoking, body fat and number of children in adulthood. This observation suggests that children genetically predisposed to higher or lower total psychiatric problems are also at risk for or protected against various outcomes related to general development and health. How this correlation arises cannot be inferred from our data, as both vertical and horizontal pleiotropy is plausible. Genetic factors may impact psychiatric problems, which may then lead to adverse health and educational outcomes. However, the reverse is also plausible: genetically determined factors could impact non-psychiatric problems, which then give rise to psychiatric problems. Finally, genetic variants could independently increase the vulnerability to develop problems in many different areas of life. The observed genetic correlations are most likely the result of a combination of these three mechanisms and further research is necessary to examine the causal pathways in detail.
A limitation of this study is the heterogeneity in the measures of psychopathology. On the one hand, using different assessment methods is an advantage, since any associations that are detected will likely be more generalizable. On the other hand, it might limit the ability to detect less robustly associated variants. However, our genetic correlation analyses showed highly consistent effects between cohorts utilizing the CBCL and SDQ questionnaires, which contributed the majority of study participants (65%). Considering that these correlations represent agreement across independent cohorts with numerous methodological and population differences, a genetic correlation above 0.8 provides very strong support for jointly analyzing total psychiatric problems scores assessed by either CBCL or SDQ. The total psychiatric problems assessed by the Rutter questionnaire had a lower genetic correlation with the other assessment methods. However, the Rutter sub-group meta-analysis was only based on three cohorts, representing only 20% of the total study population, which makes it difficult to distinguish instrument effects from other cohort-specific characteristics. Overall, the genetic correlation analyses suggest limited heterogeneity between the majority of cohorts. We thus argue that the lack of power to identify more loci probably stemmed from insufficient sample size, measurement error and the relatively low number of children with severe psychiatric problems in the general population.
Another limitation is that the study sample was not sufficient to consider potential sex interactions. The prevalence of child psychiatric problems differs by gender, with school-age boys typically showing more externalizing problems and girls more internalizing problems. Total scores tend to be less different on average, with boys showing only slightly higher scores [64]. Gene-sex interactions tend to be small for psychiatric disorders and require very high sample sizes to be robustly detected [65]. Future studies with higher sample sizes are therefore needed to identify sex-interactions in the context of total psychiatric problems.
Finally, as in any other GWAS study, the extent to which the found associations can be interpreted causally is difficult. Due to linkage disequilibrium it is unclear whether the two top variants have causal influence on psychopathology or are a marker for other causal variants. The same is true for the association of SBF2 with total psychiatric problems. However, the association of predicted SBF2 gene products with neuroticism and psychiatric problems, as well as the influence on myelination in an experimental mouse model, suggest a causal role.
In conclusion, this GWAS of total psychiatric problem scores suggests that common genetic variants exist that are associated simultaneously with internalizing, externalizing, attention and other psychiatric problems in childhood. The pleiotropy was not restricted to psychiatric phenotypes, but also included intelligence, educational attainment, wellbeing, smoking, body fat and number of children in adulthood. Interestingly, we did not find shared genetic effects with autism, schizophrenia and bipolar disorder. Two novel loci were genome-wide significant, though, the low sample size and modest imputation quality necessitate replication before firm conclusions can be drawn whether they influence total psychiatric problems. Furthermore, we found evidence that the gene SBF2, which was previously known to be associated with neuroticism, is also implicated in general psychopathology in children. Our results merit further investigation for confirmation and exploration of potential causal mechanisms.
Supporting information
(PDF)
(XLSX)
(XLSX)
(PDF)
(PDF)
(PDF)
(PDF)
(PNG)
Note: SNP only available in subset of cohorts, due to insufficient imputation quality in most studies.
(PNG)
Note: SNP only available in subset of cohorts, due to insufficient imputation quality in most studies.
(PNG)
(PNG)
Quantile-quantile plot of observed -log 10 p values vs expected -log 10 p values assuming chance findings in gene based analysis. Diagonal line indicates a p value distribution compatible with chance finding. Upward deviations indicate p values more significant than expected.
(PNG)
Manhattan plot of -log 10 p values vs SNP position for gene based analysis. Genes above the red horizontal line indicate genome-wide significant findings.
(PNG)
Acknowledgments
1958 British Birth Cohort
This work made use of data and samples generated by the 1958 Birth Cohort (NCDS), which is managed by the Centre for Longitudinal Studies at the UCL Institute of Education, funded by the Economic and Social Research Council (grant number ES/M001660/1). Access to these resources was enabled via the 58READIE Project funded by Wellcome Trust and Medical Research Council (grant numbers WT095219MA and G1001799). A full list of the financial, institutional and personal contributions to the development of the 1958 Birth Cohort Biomedical resource is available at http://www2.le.ac.uk/projects/birthcohort/1958bc/about/ contributors-funders. The Medical Research Council funded the 2002–2004 clinical follow-up of the 1958 birth cohort (grant G0000934). Genotyping was undertaken as part of the Wellcome Trust Case-Control Consortium (WTCCC) under Wellcome Trust award 076113, and a full list of the investigators who contributed to the generation of the data is available at www.wtccc.org.uk. This research used resources provided by the Type 1 Diabetes Genetics Consortium, a collaborative clinical study sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Allergy and Infectious diseases, National Human Genome Research Institute, National Institute of Child Health and Human Development, and Juvenile Diabetes Research Foundation International (JDRF) and supported by U01DK062418. The research was supported by the National Institute for Health Research Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London.
ALSPAC
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. The UK Medical Research Council and Wellcome (Grant ref: 102215/2/13/2) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors and they will serve as guarantors for the contents of this paper. GWAS data was generated by Sample Logistics and Genotyping Facilities at Wellcome Sanger Institute and LabCorp (Laboratory Corporation of America) using support from 23andMe.
BREATHE
The research leading to these results has received funding from the European Research Council under the ERC Grant Agreement number 268479 –the BREATHE project. We are acknowledged with all the children and their families participating into the BREATHE project for their altruism.
SA and NVT are funded by Juan de la Cierva Programme (Ministry of Science, Innovation and Universities—Spanish State Research Agency, ref. IJCI-2017-34068, and ref. FJC-2018-038085-I).
CADD
AGW is supported by NIAAA grant 3T32AA7464-38 S1 (PI:Paula Hoffman); Data collection and genotyping funded by DA05131,DA011015, & DA012845
CATSS
The CATSS is supported by the Swedish Council for Working Life and Social Research, the Swedish Research Council, Systembolaget, the National Board of Forensic Medicine, the Swedish Prison and Probation Service, Bank of Sweden Tercentenary Foundation, the Söderström-Königska foundation, and the Karolinska Institutet Center of Neurodevelopmental Disorders (KIND).
COPSAC
We express our deepest gratitude to the children and families of the COPSAC cohort study for all their support and commitment. We acknowledge and appreciate the unique efforts of the COPSAC research team. All funding received by COPSAC is listed on www.copsac.com. The Lundbeck Foundation (Grant no R16-A1694); The Ministry of Health (Grant no 903516); Danish Council for Strategic Research (Grant no 0603-00280B) and The Capital Region Research Foundation have provided core support to the COPSAC research center.
FiNNTWIN12
FinnTwin12 Study is conducted by the University of Helsinki in close collaboration with Indiana University. Data collection was funded by the National Institute of Alcohol Abuse and Alcoholism and by the Academy of Finland. The present analysis was supported by the Academy of Finland (grants 265240 & 312073)
GenR
The Generation R Study is conducted by the Erasmus Medical Center in close collaboration with the Erasmus University Rotterdam, Faculty of Social Sciences, the Municipal Health Service Rotterdam area, and the Stichting Trombosedienst and Artsenlaboratorium Rijnmond (STAR), Rotterdam. We gratefully acknowledge the contribution of general practitioners, hospitals, midwives and pharmacies in Rotterdam. The Generation R Study is made possible by financial support from: Erasmus Medical Center, Rotterdam, and the Netherlands Organization for Health Research and Development (ZonMw). A. Neumann and H. Tiemeier are supported by a grant of the Dutch Ministry of Education, Culture, and Science and the Netherlands Organization for Scientific Research (NWO grant No. 024.001.003, Consortium on Individual Development). A. Neumann is also supported by a Canadian Institutes of Health Research team grant. The work of H. Tiemeier is further supported by a European Union’s Horizon 2020 research and innovation program (Contract grant number: 633595, DynaHealth) and a NWO-VICI grant (NWO-ZonMW: 016.VICI.170.200). We would like to thank Anis Abuseiris, Karol Estrada, Dr. Tobias A. Knoch, and Rob de Graaf as well as their institutions Biophysical Genomics, Erasmus MC Rotterdam, The Netherlands, and especially the national German MediGRID and Services@MediGRID part of the German D-Grid, both funded by the German Bundesministerium fuer Forschung und Technology under grants #01 AK 803 A-H and # 01 IG 07015 G for access to their grid resources.
Gini-Lisa
The authors thank all families for participation in the studies and the LISAplus and GINIplus study teams for their excellent work.
Glaku
The study has been supported by Academy of Finland, University of Helsinki, Finnish Foundation for Pediatric Research, Sigrid Juselius Foundation, Jalmari and Rauha Ahokas Foundation, Signe and Ane Gyllenberg Foundation, Yrjo Jahnsson Foundation, Juho Vainio Foundation, Emil Aaltonen Foundation, and Ministry of Education and Culture, Finland. The 352 samples were genotyped at the Genotyping and Sequencing Core Facility of the Estonian Genome Centre, University of Tartu.
INMA
The INMA project was funded by grants from Instituto de Salud Carlos III (Red INMA G03/176 and CB06/02/0041). The INMA-Sabadell cohort received funding from Instituto de Salud Carlos III (FIS-FEDER: PI041436 and PI081151), Generalitat de Catalunya-CIRIT 1999SGR 00241, and EU sixth framework project NEWGENERIS FP6-2003-Food-3-A-016320. The INMA-Valencia cohort received funding from UE (FP7-ENV-2011 cod 282957 and HEALTH.2010.2.4.5–1), and from Instituto de Salud Carlos III (FIS-FEDER: 03/1615, 04/1509, 04/1112, 04/1931, 05/1079, 05/1052, 06/1213, 07/0314, 09/02647, 11/0178, 11/01007, 11/02591, 11/02038, 13/1944, 13/2032, 14/00891, 14/01687). The authors also would particularly like to thank all the participants of INMA project for their generous collaboration.
NV-T is funded by a pre-doctoral grant from the Agència de Gestió d’Ajuts Universitaris i de Recerca (2017 FI_B 00636) Generalitat de Catalunya–Fons Social Europeu.
SA is supported by a Sara Borrell grant from the Instituto de Salud Carlos III (CD14/00214).
MUSP
The authors thank MUSP participants, the MUSP Research Team, the MUSP data collection teams, the Mater Misericordiae Hospital and the Schools of Social Science, Public Health, and Medicine, at The University of Queensland for their support; and, the National Health and Medical Research Council (NHMRC).
NFBC1986
Sigrid Juselius Foundation and the Strategic Research Funding from the University of Oulu. NFBC1966 and 1986 have received financial support from the Academy of Finland (project grants 104781, 120315, 129269, 1114194, 24300796, Center of Excellence in Complex Disease Genetics and SALVE), University Hospital Oulu, Biocenter, University of Oulu, Finland (75617), NIHM (MH063706, Smalley and Jarvelin), Juselius Foundation, NHLBI grant 5R01HL087679-02 through the STAMPEED program (1RL1MH083268-01), NIH/NIMH (5R01MH63706:02), the European Commission (EURO-BLCS, Framework 5 award QLG1-CT-2000-01643), ENGAGE project and grant agreement HEALTH-F4-2007-201413, EU FP7 EurHEALTHAgeing -277849, the Medical Research Council, UK (G0500539, G0600705, G1002319, PrevMetSyn/SALVE) and the MRC, Centenary Early Career Award. The program is currently being funded by the H2020 DynaHEALTH action (grant agreement 633595) and academy of Finland EGEA-project (285547). The DNA extractions, sample quality controls, biobank up-keeping and aliquotting was performed in the National Public Health Institute, Biomedicum Helsinki, Finland and supported financially by the Academy of Finland and Biocentrum Helsinki. We thank the late Professor Paula Rantakallio (launch of NFBCs), and Ms Outi Tornwall and Ms Minttu Jussila (DNA biobanking). The authors would like to acknowledge the contribution of the late Academian of Science Leena Peltonen.
NTR
NTR warmly thanks all participating twin families. We acknowledge funding from multiple sources, including The Netherlands Organization for Scientific Research (NWO 480-15-001/674): Netherlands Twin Registry Repository: researching the interplay between genome and environment; Consortium on Individual Development (CID; NWO grant number 024.001.003), Genetic influences on stability and change in psychopathology from childhood to young adulthood (ZonMW 912-10-020), Biobanking and Biomolecular Resources Research Infrastructure (BBMRI–NL, 184.021.007); the Avera Institute, Sioux Falls, South Dakota (USA) and Grand Opportunity grants 1RC2 MH089951 and 1RC2 MH089995).
RAINE
This study was supported by the National Health and Medical Research Council of Australia [grant numbers 572613 and 40398] and the Canadian Institutes of Health Research [grant number MOP-82893]. The authors are grateful to the Raine Study participants and their families, and to the Raine Study research staff for cohort coordination and data collection. The authors gratefully acknowledge the NH&MRC for their long term funding to the study over the last 25 years and also the following institutes for providing funding for Core Management of the Raine Study: The University of Western Australia (UWA), Curtin University, the Raine Medical Research Foundation, the UWA Faculty of Medicine, Dentistry and Health Sciences, the Telethon Kids Institute, the Women’s and Infant’s Research Foundation (King Edward Memorial Hospital), Murdoch University, The University of Notre Dame (Australia), and Edith Cowan University. The authors gratefully acknowledge the assistance of the Western Australian DNA Bank (National Health and Medical Research Council of Australia National Enabling Facility). Andrew Whitehouse is funded by Senior Research Fellowship from the NHMRC (1077966). We would also like to acknowledge the Raine Study participants for their ongoing participation in the study, and the Raine Study Team for study co-ordination and data collection. This work was supported by resources provided by the Pawsey Supercomputing Centre with funding from the Australian Government and Government of Western Australia.
TCHAD
The TCHAD study is funded by the Swedish Council for Working Life and Social Research (project 2004–0383) and the Swedish Research Council (2004–1415).
TEDS
We gratefully acknowledge the ongoing contribution of the participants in TEDS and their families. TEDS is supported by UK Medical Research Council Program Grant MR/M021475/1 (and previously Grant G0901245) (to R.P.), with additional support from National Institutes of Health Grant AG046938. The research leading to these results has also received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013)ERC Grant Agreement 295366. R.P. is supported by Medical Research Council Professorship Award G19/2. KR is supported by the Sir Henry Wellcome Postdoctoral fellowship.
TRAILS
TRAILS (TRacking Adolescents’ Individual Lives Survey) is a collaborative project involving various departments of the University Medical Center and University of Groningen, the University of Utrecht, the Radboud Medical Center Nijmegen, and the Parnassia Group, all in the Netherlands. TRAILS has been financially supported by various grants from the Netherlands Organization for Scientific Research NWO (Medical Research Council program grant GB-MW 940-38-011; ZonMW Brainpower grant 100-001-004; ZonMw Risk Behavior and Dependence grants 60-60600-97-118; ZonMw Culture and Health grant 261-98-710; Social Sciences Council medium-sized investment grants GB-MaGW 480-01-006 and GB-MaGW 480-07-001; Social Sciences Council project grants GB-MaGW 452-04-314 and GB-MaGW 452-06-004; NWO large-sized investment grant 175.010.2003.005; NWO Longitudinal Survey and Panel Funding 481-08-013 and 481-11-001; NWO Vici 016.130.002 and 453-16-007/2735; NWO Gravitation 024.001.003), the Dutch Ministry of Justice (WODC), the European Science Foundation (EuroSTRESS project FP-006), the European Research Council (ERC-2017-STG-757364 en ERC-CoG-2015-681466), Biobanking and Biomolecular Resources Research Infrastructure BBMRI-NL (CP 32), the Gratama foundation, the Jan Dekker foundation, the participating universities, and Accare Centre for Child and Adolescent Psychiatry. Statistical analyses were carried out on the Genetic Cluster Computer (http://www.geneticcluster.org), which is financially supported by the Netherlands Scientific Organization (NWO 480-05-003) along with a supplement from the Dutch Brain Foundation. We are grateful to everyone who participated in this research or worked on this project to make it possible.
YFS
YFS has been financially supported by the Academy of Finland: grants 286284, 134309 (Eye), 126925, 121584, 124282, 129378 (Salve), 117787 (Gendi), and 41071 (Skidi); the Social Insurance Institution of Finland; Competitive State Research Financing of the Expert Responsibility area of Kuopio, Tampere and Turku University Hospitals (grant X51001); Juho Vainio Foundation; Paavo Nurmi Foundation; Finnish Foundation for Cardiovascular Research; Finnish Cultural Foundation; The Sigrid Juselius Foundation; Tampere Tuberculosis Foundation; Emil Aaltonen Foundation; Yrjö Jahnsson Foundation; Signe and Ane Gyllenberg Foundation; Diabetes Research Foundation of Finnish Diabetes Association; and EU Horizon 2020 (grant 755320 for TAXINOMISIS); European Research Council (grant 742927 for MULTIEPIGEN project); and The Wihuri Foundation. We thank the teams that collected data at all measurement time points; the persons who participated as both children and adults in these longitudinal studies; and biostatisticians Irina Lisinen, Johanna Ikonen, Noora Kartiosuo, Ville Aalto, and Jarno Kankaanranta for data management and statistical advice.
Data Availability
The full summary results can be found at https://doi.org/10.6084/m9.figshare.17170994.v1. Individual-level data cannot be shared publicly to protect participant privacy and to comply with EU law. Access to Generation R individual-level genotype and phenotype data can be requested by contacting Generation R data management (datamanagementgenr@erasmusmc.nl) and the corresponding author. For individual data access of other contributing studies, see supplementary information for contact information.
Funding Statement
A. Neumann and H. Tiemeier are supported by a grant of the Dutch Ministry of Education, Culture, and Science and the Netherlands Organization for Scientific Research (NWO grant No. 024.001.003, Consortium on Individual Development). The work of H. Tiemeier is further supported by a European Union’s Horizon 2020 research and innovation program (Contract grant number: 633595, DynaHealth) and a NWO-VICI grant (NWO-ZonMW: 016.VICI.170.200). https://www.nwo.nl/ The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Polderman TJC, Benyamin B, de Leeuw CA, Sullivan PF, van Bochoven A, Visscher PM, et al. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat Genet. 2015. May 18;47(7):702–9. doi: 10.1038/ng.3285 [DOI] [PubMed] [Google Scholar]
- 2.Lee SH, Ripke S, Neale BM, Faraone S V, Purcell SM, Perlis RH, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45(9):984–94. doi: 10.1038/ng.2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pappa I, Fedko IO, Mileva-Seitz VR, Hottenga J-J, Bakermans-Kranenburg MJ, Bartels M, et al. Single Nucleotide Polymorphism Heritability of Behavior Problems in Childhood: Genome-Wide Complex Trait Analysis. J Am Acad Child Adolesc Psychiatry [Internet]. 2015. Jun;54(9):737–44. doi: 10.1016/j.jaac.2015.06.004 [DOI] [PubMed] [Google Scholar]
- 4.Middeldorp CM, Hammerschlag AR, Ouwens KG, Groen-blokhuis MM, Greven CU, Pappa I, et al. A Genome-Wide Association Meta-Analysis of Attention-Deficit/Hyperactivity Disorder Symptoms in Population-Based Pediatric Cohorts. J Am Acad CHILD Adolesc PSYCHIATRY. 2016;55(10):896–905. doi: 10.1016/j.jaac.2016.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zarrella I, Russolillo LA, Caviglia G, Perrella R. Continuity and discontinuity between psychopathology of childhood and adulthood: A review on retrospective and prospective studies. Res Psychother Psychopathol Process Outcome. 2017;20(2):101–9. doi: 10.4081/ripppo.2017.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lahey BB, Van Hulle CA, Singh AL, Waldman ID, Rathouz PJ. Higher-order genetic and environmental structure of prevalent forms of child and adolescent psychopathology. Arch Gen Psychiatry. 2011. Feb 7;68(2):181–9. doi: 10.1001/archgenpsychiatry.2010.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spatola CAM, Fagnani C, Pesenti-Gritti P, Ogliari A, Stazi M-A, Battaglia M. A general population twin study of the CBCL/6-18 DSM-oriented scales. J Am Acad Child Adolesc Psychiatry. 2007. May;46(5):619–27. doi: 10.1097/CHI.0b013e3180335b12 [DOI] [PubMed] [Google Scholar]
- 8.Neumann A, Pappa I, Lahey BB, Verhulst FC, Medina-Gomez C, Jaddoe VW, et al. Single Nucleotide Polymorphism Heritability of a General Psychopathology Factor in Children. J Am Acad Child Adolesc Psychiatry. 2016;55(12):1038–45. doi: 10.1016/j.jaac.2016.09.498 [DOI] [PubMed] [Google Scholar]
- 9.Pettersson E, Anckarsäter H, Gillberg C, Lichtenstein P. Different neurodevelopmental symptoms have a common genetic etiology. J Child Psychol Psychiatry. 2013. Dec;54(12):1356–65. doi: 10.1111/jcpp.12113 [DOI] [PubMed] [Google Scholar]
- 10.Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, Duncan L, et al. Analysis of shared heritability in common disorders of the brain. Science. 2018;360(6395):eaap8757. doi: 10.1126/science.aap8757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solovieff N, Cotsapas C, Lee PH, Purcell SM, Smoller JW. Pleiotropy in complex traits: challenges and strategies. Nat Rev Genet. 2013. Jul;14(7):483–95. doi: 10.1038/nrg3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plana-Ripoll O, Pedersen CB, Holtz Y, Benros ME, Dalsgaard S, De Jonge P, et al. Exploring Comorbidity Within Mental Disorders among a Danish National Population. JAMA Psychiatry. 2019;76(3):259–70. doi: 10.1001/jamapsychiatry.2018.3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alnæs D, Kaufmann T, Doan NT, Córdova-Palomera A, Wang Y, Bettella F, et al. Association of Heritable Cognitive Ability and Psychopathology With White Matter Properties in Children and Adolescents. JAMA Psychiatry [Internet]. 2018;75(3):287–95. doi: 10.1001/jamapsychiatry.2017.4277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee PH, Anttila V, Won H, Feng YCA, Rosenthal J, Zhu Z, et al. Genomic Relationships, Novel Loci, and Pleiotropic Mechanisms across Eight Psychiatric Disorders. Cell. 2019;179:1469–82. doi: 10.1016/j.cell.2019.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brikell I, Larsson H, Lu Y, Pettersson E, Chen Q, Kuja-Halkola R, et al. The contribution of common genetic risk variants for ADHD to a general factor of childhood psychopathology. Mol Psychiatry. 2018;1–13. doi: 10.1038/s41380-018-0109-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allegrini AG, Cheesman R, Rimfeld K, Selzam S, Pingault J, Eley TC, et al. The p factor: genetic analyses support a general dimension of psychopathology in childhood and adolescence. J Child Psychol Psychiatry. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Autism Spectrum Disorders Working Group of The Psychiatric Genomics. Meta-analysis of GWAS of over 16,000 individuals with autism spectrum disorder highlights a novel locus at 10q24.32 and a significant overlap with schizophrenia. Mol Autism. 2017;8(1):21. doi: 10.1186/s13229-017-0137-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benke KS, Nivard MG, Velders FP, Walters RK, Pappa I, Scheet P a, et al. A genome-wide association meta-analysis of preschool internalizing problems. J Am Acad Child Adolesc Psychiatry. 2014. Jun;53(6):667–676.e7. doi: 10.1016/j.jaac.2013.12.028 [DOI] [PubMed] [Google Scholar]
- 19.Pappa I, St Pourcain B, Benke K, Cavadino A, Hakulinen C, Nivard MG, et al. A genome-wide approach to children’s aggressive behavior: The EAGLE consortium. Am J Med Genet Part B Neuropsychiatr Genet. 2016;171(5):562–72. doi: 10.1002/ajmg.b.32333 [DOI] [PubMed] [Google Scholar]
- 20.Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet. 2019;51(1):63–75. doi: 10.1038/s41588-018-0269-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galesloot TE, van Steen K, Kiemeney L a LM, Janss LL, Vermeulen SH. A comparison of multivariate genome-wide association methods. PLoS One [Internet]. 2014. Jan;9(4):e95923. doi: 10.1371/journal.pone.0095923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Thompson WK, Schork AJ, Holland D, Chen C, Bettella F, et al. Leveraging Genomic Annotations and Pleiotropic Enrichment for Improved Replication Rates in Schizophrenia GWAS. PLOS Genet. 2016;12(1):1–22. doi: 10.1371/journal.pgen.1005803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shevlin M, McElroy E, Murphy J. Homotypic and heterotypic psychopathological continuity: a child cohort study. Soc Psychiatry Psychiatr Epidemiol. 2017;52(9):1135–45. doi: 10.1007/s00127-017-1396-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms and Profiles. University of Vermont. Burlington, VT; 2001. [Google Scholar]
- 25.Goodman R. The Strengths and Difficulties Questionnaire: a research note. J Child Psychol Psychiatry. 1997. Jul 1;38(5):581–6. http://doi.wiley.com/10.1111/j.1469-7610.1997.tb01545.x [DOI] [PubMed] [Google Scholar]
- 26.Pulkkinen L, Kaprio J, Rose RJ. Peers, teachers and parents as assessors of the behavioural and emotional problems of twins and their adjustment: the Multidimensional Peer Nomination Inventory. Twin Res. 1999. Aug 21;2(4):274–85. doi: 10.1375/136905299320565762 [DOI] [PubMed] [Google Scholar]
- 27.Rutter M. A CHILDREN’S BEHAVIOUR QUESTIONNAIRE FOR COMPLETION BY TEACHERS: PRELIMINARY FINDINGS. J Child Psychol Psychiatry. 1967. May 1;8(1):1–11. http://doi.wiley.com/10.1111/j.1469-7610.1967.tb02175.x [DOI] [PubMed] [Google Scholar]
- 28.Hansson SL, Röjvall AS, Rastam M, Gillberg C, Gillberg C, Anckarsäter H. Psychiatric telephone interview with parents for screening of childhood autism—Tics, attention-deficit hyperactivity disorder and other comorbidities (A-TAC): Preliminary reliability and validity. Vol. 187, British Journal of Psychiatry. Cambridge University Press; 2005. p. 262–7. doi: 10.1192/bjp.187.3.262 [DOI] [PubMed] [Google Scholar]
- 29.Wells E. Behavioral patterns of children in school. Vitality Heal Stat. 1980;77:113. [Google Scholar]
- 30.The 1000 Genomes Project Consortium. A global reference for human genetic variation. Nature [Internet]. 2015. Oct 1;526(7571):68–74. doi: 10.1038/nature15393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas Winkler. EasyQC [Internet]. [cited 2017 Dec 21]. Available from: www.genepi-regensburg.de/easyqc
- 32.Winkler TW, Day FR, Croteau-Chonka DC, Wood AR, Locke AE, Mägi R, et al. Quality control and conduct of genome-wide association meta-analyses. Nat Protoc. 2014;9(5):1192–212. doi: 10.1038/nprot.2014.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Der Most PJ, Vaez A, Prins BP, Munoz ML, Snieder H, Alizadeh BZ, et al. QCGWAS: A flexible R package for automated quality control of genome-wide association results. Bioinformatics. 2014;30(8):1185–6. doi: 10.1093/bioinformatics/btt745 [DOI] [PubMed] [Google Scholar]
- 34.R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria; 2016. Available from: https://www.r-project.org/
- 35.Han B, Eskin E. Random-effects model aimed at discovering associations in meta-analysis of genome-wide association studies. Am J Hum Genet. 2011;88(5):586–98. doi: 10.1016/j.ajhg.2011.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Visscher PM, Wray NR, Zhang Q, Sklar P, McCarthy MI, Brown MA, et al. 10 Years of GWAS Discovery: Biology, Function, and Translation. Am J Hum Genet. 2017. Jul 6;101(1):5–22. doi: 10.1016/j.ajhg.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanabe K, Taskesen E, van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat Commun [Internet]. 2017;8(1):1826. doi: 10.1038/s41467-017-01261-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaunt TR, Shihab HA, Hemani G, Min JL, Woodward G, Lyttleton O, et al. Systematic identification of genetic influences on methylation across the human life course. Genome Biol. 2016;17:61. doi: 10.1186/s13059-016-0926-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Leeuw CA, Mooij JM, Heskes T, Posthuma D. MAGMA: Generalized Gene-Set Analysis of GWAS Data. PLoS Comput Biol. 2015;11(4):1–19. doi: 10.1371/journal.pcbi.1004219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lonsdale J, Thomas J, Salvatore M, Phillips R, Lo E, Shad S, et al. The genotype-tissue expression (GTEx) project. Nat Genet. 2013;45(6):580–5. doi: 10.1038/ng.2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gusev A, Ko A, Shi H, Bhatia G, Chung W, Penninx BWJH, et al. Integrative approaches for large-scale transcriptome-wide association studies. Nat Genet. 2016;48(3):245–52. doi: 10.1038/ng.3506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gusev A. TWAS Hub [Internet]. [cited 2019 Jun 30]. Available from: http://twas-hub.org/
- 43.Mancuso N, Shi H, Goddard P, Kichaev G, Gusev A, Pasaniuc B. Integrating Gene Expression with Summary Association Statistics to Identify Genes Associated with 30 Complex Traits. Am J Hum Genet. 2017;100(3):473–87. doi: 10.1016/j.ajhg.2017.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bulik-Sullivan BK, Loh P-R, Finucane HK, Ripke S, Yang J, Patterson N, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015. Feb 2;47(3):291–5. doi: 10.1038/ng.3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng J, Erzurumluoglu AM, Elsworth BL, Kemp JP, Howe L, Haycock PC, et al. LD Hub: A centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics. 2017;33(2):272–9. doi: 10.1093/bioinformatics/btw613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Otowa T, Hek K, Lee M, Byrne EM, Mirza SS, Nivard MG, et al. Meta-analysis of genome-wide association studies of anxiety disorders. Mol Psychiatry [Internet]. 2016. Jan 12;21:1391. doi: 10.1038/mp.2015.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okbay A, Baselmans BML, De Neve JE, Turley P, Nivard MG, Fontana MA, et al. Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nat Genet [Internet]. 2016. Jun 18;48(6):624–33. doi: 10.1038/ng.3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagel M, Jansen PR, Stringer S, Watanabe K, Leeuw CA De, Bryois J, et al. Meta-analysis of genome-wide association studies for neuroticism in 449,484 individuals identifies novel genetic loci and pathways. Nat Genet. 2018;50(July):920–7. doi: 10.1038/s41588-018-0151-7 [DOI] [PubMed] [Google Scholar]
- 49.Olino TM, Dougherty LR, Bufferd SJ, Carlson G a., Klein DN. Testing Models of Psychopathology in Preschool-aged Children Using a Structured Interview-based Assessment. J Abnorm Child Psychol. 2014;1201–11. doi: 10.1007/s10802-014-9865-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brandes CM, Herzhoff K, Smack AJ, Tackett JL. The p Factor and the n Factor: Associations Between the General Factors of Psychopathology and Neuroticism in Children. Clin Psychol Sci. 2019;7(6):1266–84. [Google Scholar]
- 51.Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, et al. The p Factor: One General Psychopathology Factor in the Structure of Psychiatric Disorders? Clin Psychol Sci. 2014. Aug 14;2(2):119–37. doi: 10.1177/2167702613497473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tackett JL, Lahey BB, van Hulle C, Waldman I, Krueger RF, Rathouz PJ. Common genetic influences on negative emotionality and a general psychopathology factor in childhood and adolescence. J Abnorm Psychol. 2013. Nov;122(4):1142–53. doi: 10.1037/a0034151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Widiger TA, Sellbom M, Chmielewski M, Clark LA, DeYoung CG, Kotov R, et al. Personality in a Hierarchical Model of Psychopathology. Clin Psychol Sci. 2019;7(1):77–92. [Google Scholar]
- 54.Senderek J, Bergmann C, Weber S, Ketelsen UP, Schorle H, Rudnik-Schöneborn S, et al. Mutation of the SBF2 gene, encoding a novel member of the myotubularin family, in Charcot-Marie-Tooth neuropathy tye 4B2/11p15. Hum Mol Genet. 2003;12(3):349–56. doi: 10.1093/hmg/ddg030 [DOI] [PubMed] [Google Scholar]
- 55.Tersar K, Boentert M, Berger P, Bonneick S, Wessig C, Toyka K V., et al. Mtmr13/Sbf2-deficient mice: An animal model for CMT4B2. Hum Mol Genet. 2007;16(24):2991–3001. doi: 10.1093/hmg/ddm257 [DOI] [PubMed] [Google Scholar]
- 56.Neumann A, Muetzel RL, Lahey BB, Bakermans-Kranenburg MJ, van IJzendoorn MH, Jaddoe VW, et al. White Matter Microstructure and the General Psychopathology Factor in Children. J Am Acad Child Adolesc Psychiatry. 2020. doi: 10.1016/j.jaac.2019.12.006 [DOI] [PubMed] [Google Scholar]
- 57.Morgane PJ, Galler JR, Mokler DJ. A review of systems and networks of the limbic forebrain/limbic midbrain. Prog Neurobiol. 2005;75(2):143–60. doi: 10.1016/j.pneurobio.2005.01.001 [DOI] [PubMed] [Google Scholar]
- 58.Bora E, Fornito A, Pantelis C, Yücel M. Gray matter abnormalities in Major Depressive Disorder: A meta-analysis of voxel based morphometry studies. J Affect Disord. 2011; doi: 10.1016/j.jad.2011.03.049 [DOI] [PubMed] [Google Scholar]
- 59.Norman LJ, Carlisi C, Lukito S, Hart H, Mataix-Cols D, Radua J, et al. Structural and Functional Brain Abnormalities in Attention-Deficit/Hyperactivity Disorder and Obsessive-Compulsive Disorder: A Comparative Meta-analysis. JAMA Psychiatry. 2017;73(8):815–25. [DOI] [PubMed] [Google Scholar]
- 60.Buchheim A, Viviani R, Kessler H, Kächele H, Cierpka M, Roth G, et al. Changes in Prefrontal-Limbic Function in Major Depression after 15 Months of Long-Term Psychotherapy. Bruce A, editor. PLoS One. 2012. Mar 28;7(3):e33745. doi: 10.1371/journal.pone.0033745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Post RM, Altshuler LL, Kupka R, McElroy SL, Frye MA, Rowe M, et al. Age of onset of bipolar disorder: Combined effect of childhood adversity and familial loading of psychiatric disorders. J Psychiatr Res. 2016;81:63–70. doi: 10.1016/j.jpsychires.2016.06.008 [DOI] [PubMed] [Google Scholar]
- 62.McGrath JJ, Saha S, Al-Hamzawi AO, Alonso J, Andrade L, Borges G, et al. Age of onset and lifetime projected risk of psychotic experiences: Cross-national data from the world mental health survey. Schizophr Bull. 2016;42(4):933–41. doi: 10.1093/schbul/sbw011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pedersen CB, Mors O, Bertelsen A, LindumWaltoft B, Agerbo E, McGrath JJ, et al. A comprehensive nationwide study of the incidence rate and lifetime risk for treated mental disorders. JAMA Psychiatry. 2014;71(5):573–81. doi: 10.1001/jamapsychiatry.2014.16 [DOI] [PubMed] [Google Scholar]
- 64.Rescorla L, Achenbach T, Ivanova MY, Dumenci L, Almqvist F, Bilenberg N, et al. Behavioral and emotional problems reported by parents of children ages 6 to 16 in 31 societies. J Emot Behav Disord. 2007;15(3):130–42. [Google Scholar]
- 65.Martin J, Khramtsova EA, Goleva SB, Blokland GAM, Traglia M, Walters RK, et al. Examining Sex-Differentiated Genetic Effects Across Neuropsychiatric and Behavioral Traits. Biol Psychiatry. 2021;89(12):1127–37. doi: 10.1016/j.biopsych.2020.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(XLSX)
(XLSX)
(PDF)
(PDF)
(PDF)
(PDF)
(PNG)
Note: SNP only available in subset of cohorts, due to insufficient imputation quality in most studies.
(PNG)
Note: SNP only available in subset of cohorts, due to insufficient imputation quality in most studies.
(PNG)
(PNG)
Quantile-quantile plot of observed -log 10 p values vs expected -log 10 p values assuming chance findings in gene based analysis. Diagonal line indicates a p value distribution compatible with chance finding. Upward deviations indicate p values more significant than expected.
(PNG)
Manhattan plot of -log 10 p values vs SNP position for gene based analysis. Genes above the red horizontal line indicate genome-wide significant findings.
(PNG)
Data Availability Statement
The full summary results can be found at https://doi.org/10.6084/m9.figshare.17170994.v1. Individual-level data cannot be shared publicly to protect participant privacy and to comply with EU law. Access to Generation R individual-level genotype and phenotype data can be requested by contacting Generation R data management (datamanagementgenr@erasmusmc.nl) and the corresponding author. For individual data access of other contributing studies, see supplementary information for contact information.