Abstract
Using rabbit polyclonal antibodies, we have shown that the Dcm cytosine methylase of Escherichia coli is maintained at a constant level during cell growth, while Vsr endonuclease levels are growth phase dependent. Decreased production of Vsr relative to Dcm during the log phase may contribute substantially to the mutability of 5-methylcytosine.
Many organisms, although by no means all, methylate selected cytosines in their genomes. Such methylation is important, particularly in mammalian cells, where it is required for normal development (5). However, the tendency of 5-methylcytosine (5-MeC) to deaminate to thymine adds a significant mutational burden to the cell. Despite the fact that organisms which methylate their genomes have DNA repair systems dedicated to correcting deamination-induced T/G mismatches (6, 14), C-to-T transitions at methylated cytosines are among the most prevalent spontaneous mutations (1, 11). Escherichia coli K-12 is a case in point. This bacterium has a single DNA (cytosine-5) methyltransferase (Dcm), a product of the dcm gene, which methylates the second C of the sequence CCWGG (W = A or T). T/G mismatches which result from deamination of the methylated C are corrected to CG base pairs by a process known as very-short-patch (VSP) repair (reviewed in reference 7). The process is initiated by a single-stranded endonuclease (Vsr) which cleaves 5′ of the mismatched T (4). Removal of several bases 3′ of the nick and their resynthesis by DNA polymerase I complete the repair process. However, despite VSP repair, 5-MeCs remain hot spots for mutation in E. coli (1).
It has been shown that inefficient VSP repair in replicating cells is a major contributor to mutation at 5-MeC in E. coli (8). Recently, we proposed that the inefficiency is a deliberate strategy for mutation avoidance (9). This counterintuitive hypothesis is based on our finding that artificially increased production of Vsr results in very high levels of mutation at non-CCWGG sequences (3). The types of mutation which occur (transitions and frameshifts), the frequency with which they occur, and the fact that their frequency is reduced by addition of plasmids containing mutH or mutL (9) all suggest that Vsr-stimulated mutation is caused by interference with mismatch repair. If this is indeed the case, then Vsr production may have to be maintained at low levels in dividing cells to ensure optimal correction of DNA replication errors. Unfortunately, this strategy could also result in suboptimal VSP repair and a concomitant increase in C-to-T mutations at 5-MeC.
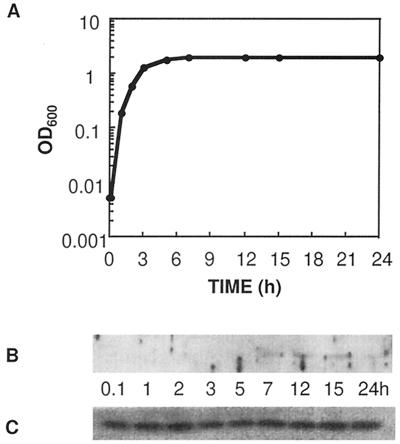
We have now made a rabbit polyclonal antibody to Vsr and used it to measure production of the protein during the growth of a culture of E. coli. CSH142 cells [ara (gpt-lac)5 thi] were grown in Luria-Bertani (LB) medium (10), and samples were taken at various times throughout the log phase and stationary phase, as shown in Fig. 1A. Cell extracts containing equal amounts of protein were electrophoresed on sodium dodecyl sulfate-polyacrylamide gels, and the separated proteins were transferred to nylon membranes for Western analysis (12). Antigen-antibody complexes were detected with a chemiluminescent substrate, following the procedure outlined by the supplier (NEN Life Science Products). Figure 1B shows that Vsr is undetectable during the log phase, appearing only as the cells enter the stationary phase.
FIG. 1.
Production of Vsr and Dcm in growing and stationary-phase E. coli cells. Cultures were initiated from a 1:100 dilution of an overnight, saturated culture. Culture growth was measured by optical density at 600 nm (OD600) (A). Samples were taken from the culture at the times shown, and protein extracts were subjected to Western analysis with antibodies to Vsr (B) or to Dcm (C).
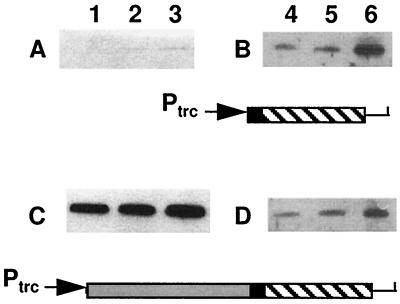
The 5′ end of vsr overlaps the 3′ end of dcm in a +1 reading frame (13), and the genes are apparently cotranscribed from a promoter 5′ of dcm (2). If the stationary-phase-dependent regulation of Vsr production were transcriptional, one would expect Dcm production to follow the same pattern as Vsr. Figure 1C shows the results of Western analysis with a polyclonal antibody that we made specific for Dcm. Unlike Vsr, the cellular levels of Dcm are independent of growth phase, strongly suggesting that regulation of Vsr production is posttranscriptional. To confirm this, we used a plasmid, pKK-DV (9), in which dcm and vsr are expressed from a plasmid-borne promoter (trc). Transcription of the operon is constitutive since the strain is lacl. pKK-DV transformants, grown in LB medium plus ampicillin (100 μg/ml), were collected in the early log, late log, and stationary phases and subjected to Western analysis. Production of Dcm from the plasmid remained independent of growth phase (Fig. 2C); production of Vsr remained dependent on growth phase (Fig. 2D).
FIG. 2.
Growth phase-dependent production of Vsr is independent of the dcm promoter and of operon structure. Cells were transformed with pKK-V (A and B) or pKK-DV (C and D); maps of the corresponding plasmid inserts are shown (shaded bar, dcm; black bar, overlap between dcm and vsr; hatched bar, vsr). Cultures were grown in LB medium plus ampicillin from a 1:100 dilution of a saturated overnight culture. Samples were taken in the early log (lanes 1 and 4), late log (lanes 2 and 5), and stationary (lanes 3 and 6) phases, and protein extracts were subjected to Western analysis. The top half of each blot was treated with antibodies to Dcm (A and C), and the bottom half was treated with antibodies to Vsr (B and D). The small amount of Dcm visible in Fig. 2A is the product of the chromosomal dcm gene.
Vsr disappears very rapidly when stationary-phase cells are diluted into fresh medium (Fig. 1B), suggesting that the protein is actively degraded as cells prepare to reenter log phase. The subsequent slow buildup of the protein could be due to inefficient translation, occasioned by the fact that the vsr ribosome binding site (RBS) is within the 3′ end of the dcm coding region (13). To determine whether this is the case, we measured Vsr production in cells transformed with pKK-V (3). In this plasmid, vsr is transcribed directly from the trc promoter and translated from a plasmid-borne RBS. Although pKK-V transformants produce higher levels of Vsr than pKK-DV transformants in the stationary phase (compare lanes 6 in Fig. 2B and D), the pattern of reduced Vsr expression during the log phase is maintained (Fig. 2B).
We do not yet know what mechanism controls growth phase-dependent production of Vsr. However, the fact that the dcm promoter is not required and that the operon arrangement of dcm and vsr does not contribute significantly suggests that a large part of the regulation is posttranslational. The rapidity with which the protein disappears when cells leave the stationary phase suggests active degradation. The targeted proteolysis of the E. coli sigma factor, ςS, during the log phase and its stabilization during the stationary phase provide an attractive model for this form of regulation (15).
Based on our previous observation that high levels of Vsr are mutagenic (3), we had hypothesized that levels of the Vsr endonuclease are tightly controlled in growing cells (9). In particular, we suggested that mutagenesis could be avoided by keeping levels of Vsr low during periods of DNA replication in order to avoid interference with mismatch repair. The results presented in this paper show that, as predicted, the amount of Vsr is very low in growing cells and only increases as cells enter the stationary phase.
It has always been assumed that the operon arrangement of dcm and vsr serves to minimize mutation by coordinating production of the methylase and the repair endonuclease (2, 13). Clearly, this is not the case. Instead, limited amounts of Vsr are available in the log phase to repair deamination damage. This could be a major contributor to 5-MeC mutability in E. coli.
Acknowledgments
We thank Danielle Couture (Lady Davis Institute, SMBD Jewish General Hospital, Montréal, Québec, Canada) for invaluable help with antibody production.
This work was supported by grants to C.G.C. from the National Cancer Institute of Canada (with funds from the Canadian Cancer Society), the Natural Sciences and Engineering Research Council of Canada, and the Medical Research Council of Canada.
REFERENCES
- 1.Coulondre C, Miller J H, Farabaugh P J, Gilbert W. Molecular basis of base substitution hotspots in Escherichia coli. Nature. 1978;274:775–780. doi: 10.1038/274775a0. [DOI] [PubMed] [Google Scholar]
- 2.Dar M E, Bhagwat A S. Mechanism of expression of DNA repair gene vsr, an Escherichia coli gene that overlaps the DNA cytosine methylase gene, dcm. Mol Microbiol. 1993;9:823–833. doi: 10.1111/j.1365-2958.1993.tb01741.x. [DOI] [PubMed] [Google Scholar]
- 3.Doiron K M J, Viau S, Koutroumanis M, Cupples C G. Overexpression of vsr in Escherichia coli is mutagenic. J Bacteriol. 1996;178:4294–4296. doi: 10.1128/jb.178.14.4294-4296.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hennecke F, Kolmar H, Bründl K, Fritz H-J. The vsr gene product of E. coli K-12 is a strand- and sequence-specific DNA mismatch endonuclease. Nature. 1991;353:776–778. doi: 10.1038/353776a0. [DOI] [PubMed] [Google Scholar]
- 5.Li E, Bestor T H, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 6.Lieb M. Spontaneous mutation at 5-methylcytosine hotspot is prevented by very short patch (VSP) mismatch repair. Genetics. 1991;128:23–27. doi: 10.1093/genetics/128.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lieb M, Bhagwat A S. Very short patch repair: reducing the cost of cytosine methylation. Mol Microbiol. 1996;20:467–473. doi: 10.1046/j.1365-2958.1996.5291066.x. [DOI] [PubMed] [Google Scholar]
- 8.Lieb M, Rehmat S. 5-Methylcytosine is not a mutation hotspot in nondividing Escherichia coli. Proc Natl Acad Sci USA. 1997;94:940–945. doi: 10.1073/pnas.94.3.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macintyre G, Doiron K M J, Cupples C G. The Vsr endonuclease of Escherichia coli: an efficient DNA repair enzyme and a potent mutagen. J Bacteriol. 1997;179:6048–6052. doi: 10.1128/jb.179.19.6048-6052.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller J H. A short course in bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 11.Rideout W M, III, Coetzee G A, Olumi A F, Jones P A. 5-Methylcytosine as an endogenous mutagen in the human LDL receptor and p53 genes. Science. 1990;249:1288–1290. doi: 10.1126/science.1697983. [DOI] [PubMed] [Google Scholar]
- 12.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 13.Sohail A, Lieb M, Dar M, Bhagwat A S. A gene required for very short patch repair in Escherichia coli is adjacent to the DNA cytosine methylase gene. J Bacteriol. 1990;172:4214–4221. doi: 10.1128/jb.172.8.4214-4221.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiebauer K, Jiricny J. In vitro correction of G. T mispairs to G. C pairs in nuclear extracts from human cells. Nature. 1989;339:234–236. doi: 10.1038/339234a0. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Y, Gottesman S. Regulation of proteolysis of the stationary-phase sigma factor RpoS. J Bacteriol. 1998;180:1154–1158. doi: 10.1128/jb.180.5.1154-1158.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]