Abstract
To determine whether solute transport across yeast membranes was facilitated, we measured the water and solute permeations of vacuole-derived and late secretory vesicles in Saccharomyces cerevisiae; all permeations were consistent with passive diffusive flow. We also overexpressed Fps1p, the putative glycerol facilitator in S. cerevisiae, in secretory vesicles but observed no effect on water, glycerol, formamide, or urea permeations. However, spheroplasts prepared from the strain overexpressing Fps1p showed enhanced glycerol uptake, suggesting that Fps1p becomes active only upon insertion in the plasma membrane.
Yeasts regulate their osmolality through a variety of mechanisms in response to environmental osmotic changes. First, intracellular water may be transported from the vacuole to the cytoplasm to compensate for increases in osmolytes, and mutants defective for this process are salt sensitive (15). Second, the production of internal osmolytes such as glycerol and trehalose may be regulated (10). Third, ion flux across the plasma membrane might be controlled by a mechanosensitive or voltage-dependent channel (1, 3, 4, 8, 9). Finally, several reports indicate that yeasts may regulate their osmolality by varying their membrane lipid composition, which in turn can influence the ability of water to permeate (14, 21, 24).
Yet another possibility is that yeasts control their internal osmolality by expressing aquaporin (AQP) water channels that facilitate rapid water or solute transport across either the plasma or vacuolar membranes. The AQPs are a family of proteins that transport water and small nonelectrolytes (16, 18). AQPs typically contain six transmembrane-spanning segments and have been identified in mammals, plants, and prokaryotes. Four open reading frames (ORFs) that encode members of the AQP family also exist in the yeast genome. The first ORF, YPR192w (AQY1), when obtained from environmental yeast strains, has been shown to facilitate water transport when expressed in Xenopus oocytes (5); AQY1 isolated from laboratory yeast strains, however, contains a mutation that prevents this activity.
A second potential homologue would be encoded by two overlapping reading frames, YLL052c and YLL053c, whose products resemble the N and C termini of AQPs, respectively. The introduction of a single base would fuse the two ORFs to generate a single, full-length AQP homologue. Although the presence of these separate, overlapping reading frames has been verified in a number of strains, site-directed mutations that restore a single reading frame result in functional water channel activity (13). Thus, it remains unclear whether a functional AQP derived from YLL052c and YLL053c is normally expressed.
The product of a third gene encoding an AQP homologue in yeast, FPS1, resembles AQPs in the transmembrane region but has extended N- and C-terminal ends (25). The internal, 280-amino-acid sequence of the protein encoded by FPS1 (Fps1p) is ∼30% identical to the Escherichia coli glycerol facilitator protein (GlpF), and strains lacking FPS1 display aberrant glycerol transport activity and glycerol retention in response to hypo-osmotic shock (17). Nonetheless, whether Fps1p is a bona fide glycerol transporter and whether it facilitates the transport of another substrate have not been demonstrated.
The product of a fourth gene, YFL054c, shows similarity to E. coli GlpF and to the Salmonella typhimurium propanediol diffusion facilitator and is about 35% identical to Fps1p for a stretch of 84 amino acids. It has not been studied although it shows more similarity to the products of both human AQP1 and yeast AQY1 than does Fps1p.
To investigate if water transport in yeast is facilitated by AQPs, we measured water and solute permeations across distinct membranes from Saccharomyces cerevisiae. To perform the transport assays, the desired membrane-enclosed vesicles were preloaded with the concentration-dependent fluorescent dye carboxyfluorescein (CF) and buffer of a defined osmotic strength (7, 14). When the vesicles were rapidly mixed with a hyperosmotic buffer, water exited the vesicle, resulting in vesicle shrinkage and a decrease in the yield of CF fluorescence. Results were obtained on a stopped-flow fluorimeter. The presence of water channels greatly facilitates the rate at which water exits and lowers the activation energy (Ea) for transport. The transport of other solutes (e.g., glycerol) can also be measured if a gradient for the desired solute is established between the buffer in the preloaded vesicles and in the mixing solution (7, 14).
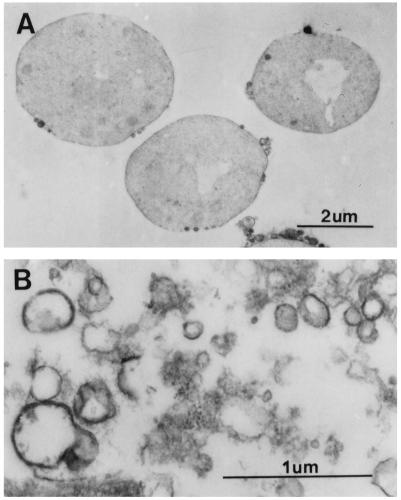
Because the vacuole and cell membrane play a vital role in regulating the cellular osmolyte balance, we prepared vacuole-derived vesicles that are reported to contain an active H+-ATPase and are sealed (20). The integrity of the vacuolar vesicles was verified by three methods. First, an electron micrograph showed that a significant fraction of the membranes appeared as closed vesicles with diameters ranging from 0.2 to 0.5 μm (Fig. 1B). Second, a H+-ATPase-dependent acidification of the vesicles was established by following the rate of acridine orange fluorescence quenching (17a). And third, spontaneous CF efflux from the vesicles was not apparent when examined by fluorometry (data not shown).
FIG. 1.
Electron micrograph of yeast spheroplasts (A) and vacuole-derived membranes (B). Spheroplasts and vesicles were fixed in 2% glutaraldehyde–0.5% osmium tetroxide, dehydrated with increasing concentrations of ethanol, and embedded in Epon-Araldite. Samples were stained with 1% uranyl acetate and Reynolds lead citrate and viewed and photographed with a Philips 300 electron microscope at 60 keV.
The solute permeability of the plasma membrane was assessed by using post-Golgi, plasma membrane-targeted vesicles from the sec6-4 mutant (19); sec6 vesicles contain the proteins destined to constitute the cell membrane and form stable, sealed vesicles (as compared to plasma membrane vesicles) and have been used to biophysically characterize active, human AQPs in yeast (7, 11, 12). Using the CF fluorescence assay in which water transport can be measured on the millisecond time scale (7, 14), we found that neither the vacuolar nor sec6 vesicular membranes exhibit high water permeability (Table 1). All have an Ea for water transport consistent with passive diffusion across the lipid bilayer (Table 2 and Fig. 2) (14). These results suggest that water transport across the yeast plasma membrane and vacuolar membrane is not AQP mediated.
TABLE 1.
Solute permeabilities of S. cerevisiae membranes
| Solute | Permeability (cm/s) ofa:
|
||
|---|---|---|---|
| Vacuoles | sec6 vesicles | sec6 vesicles (FPS1) | |
| Water | (1.3 ± 0.9) × 10−3 | (2.2 ± 0.4) × 10−3 | (2.9 ± 0.5) × 10−3 |
| Formamide | (1.8 ± 0.5) × 10−5 | (3.3 ± 0.7) × 10−6 | (3.4 ± 0.7) × 10−6 |
| Urea | (1.2 ± 0.9) × 10−6 | (2.5 ± 1.1) × 10−6 | (2.0 ± 0.2) × 10−6 |
| Glycerol | (1.3 ± 0.6) × 10−6 | (1.1 ± 0.1) × 10−6 | (1.7 ± 0.4) × 10−6 |
TABLE 2.
Eas for proteins overexpressed in secretory vesicles
FIG. 2.
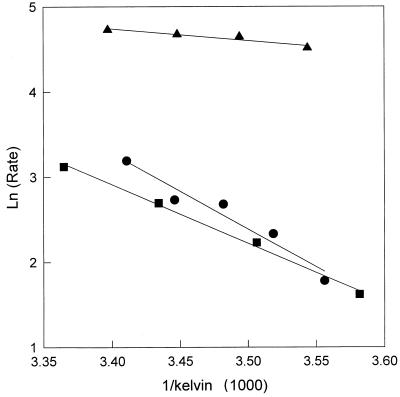
Eas for water transport in vesicles prepared from sec6 S. cerevisiae. The natural log of the rate of secretory vesicle shrinkage (measured by the change in CF fluorescence) is plotted versus the reciprocal of temperature (in degrees kelvin) multiplied by 1,000. Eas were calculated from the slope of the linear regression. Closed squares, control vesicles; closed circles, Fps1p-containing vesicles; closed triangles, AQP1-containing vesicles.
By measuring the change in CF fluorescence in a stopped-flow fluorimeter, we also discovered that nonelectrolytic solutes such as formamide, urea, and glycerol passively diffuse across the sec6 and vacuole-derived membranes (Table 1) (compare values to those in reference 14). As a positive control for these experiments, we expressed human AQP1 in yeast under the control of a galactose-inducible promotor and observed facilitated water transport across the membranes of sec6-derived plasma membrane-targeted vesicles (Fig. 2 and Table 2), as observed previously (7). The Ea for water transport across sec6 vesicles harboring AQP1 was 4.6 kcal/mol, as compared to an Ea of 13.2 kcal/mol in vesicles lacking AQP1 (Fig. 2 and Table 2).
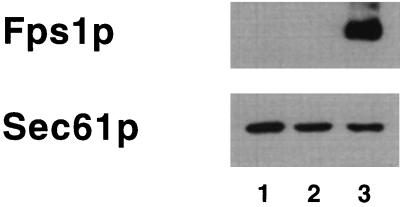
To determine the solute specificity of the putative glycerol transporter Fps1p (17, 23, 25), we introduced a multicopy vector containing the FPS1 gene into S. cerevisiae and prepared sec6 vesicles; vesicles were also prepared from a sec6 strain containing the vector but lacking the FPS1 insert. The identical FPS1 overexpression system was used previously to show that Fps1p facilitates glycerol transport across the yeast plasma membrane (17). Using an antibody against a peptide fragment of the Fps1 protein, we observed that secretory vesicles contain amounts of Fps1p that are undetectable unless the protein is overexpressed (Fig. 3). The Eas for water transport in sec6 vesicles prepared from the strain containing only the vector and those from strains harboring overexpressed Fps1p were similar (13.2 ± 1.2 and 17.2 ± 1.2 kcal/mol, respectively) (see Table 2), indicating that water diffusion was again passive (Fig. 2). Surprisingly, overexpression of Fps1p failed to increase glycerol permeation in sec6 vesicles (Table 1), suggesting either that Fps1p does not facilitate glycerol transport on the millisecond time scale or that it may be inactive in the sec6 vesicles. It is possible that Fps1p function only becomes evident upon insertion into the plasma membrane.
FIG. 3.
Overexpression of Fps1p in S. cerevisiae sec6 vesicles. Yeast endoplasmic reticulum (ER)-derived microsomes (lane 1) and sec6 vesicles prepared from cells containing a multicopy vector either lacking FPS1 (lane 2) or containing FPS1 (lane 3) were immunoblotted by using an antipeptide antibody prepared against amino acid residues 173 to 183 (HLSRRRSRSRA) and 161 to 168 (KNADDAHT) of Fps1p and antiserum prepared against Sec61p (an ER marker protein [23]). Quantitative immunoblotting indicated that sec6 vesicles are approximately twofold more depleted by Sec61p than are ER membranes.
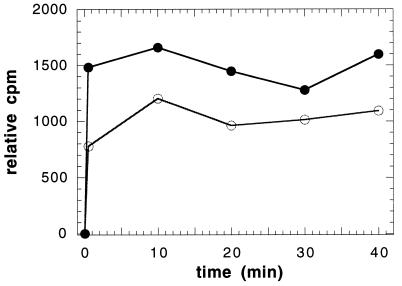
To address this hypothesis, we prepared spheroplasts from the control and Fps1p-overexpressing strains grown to late log phase (23) by enzymatic digestion of the cell wall (2). Following digestion, the spheroplasts were isolated by centrifugation through buffer containing 20 mM HEPES (pH 7.4), 0.8 M sucrose, and 1.5% Ficoll (Cushion 1) and were prepared for electron microscopy analysis (see Fig. 1A) and glycerol uptake studies. [3H]glycerol transport into spheroplasts was measured as described (17) except that the spheroplasts were collected at the indicated time points by recentrifugation in a microcentrifuge for 10 s (16,000 × g) through Cushion 1. The presence of osmotic support, which was required to prevent spheroplast lysis, precluded our ability to reisolate the spheroplasts by vacuum filtration as performed previously (17). The amount of incorporated glycerol in the spheroplast pellet was obtained by liquid scintillation counting. As observed in Fig. 4, we discovered that spheroplasts prepared from cells containing the Fps1p overexpression plasmid sequestered glycerol faster and to a greater extent. We conclude from these results that Fps1p is active in the yeast plasma membrane and may have to assemble with other proteins in the plasma membrane to become active. The more rapid rate of glycerol sequestration in spheroplasts (Fig. 4) as compared to intact cells (17) suggests that the cell wall may impede glycerol transport.
FIG. 4.
[3H]glycerol uptake (expressed as counts per minute, 103) was measured at 26°C in yeast spheroplasts containing the FPS1 overexpression plasmid (closed circles) or the same plasmid lacking the FPS1 insert (open circles). An immunoblot analysis was performed as described in the legend to Fig. 3 on each sample to verify that Fps1p was overexpressed (data not shown). Light scattering of the different preparations ensured that equal amounts of spheroplasts were used in these experiments. The background (0 min), obtained by mixing spheroplasts with [3H]glycerol and immediately processing the sample (see text for details), was subtracted from each data set.
In sum, our results indicate that the putative water channels in laboratory strains of S. cerevisiae are either not functional, as suggested by others (5), or are absent from the sec6 vesicles and vacuole membranes.
Since we have only examined membranes from logarithmically growing cells, it seems likely that these genes may be expressed and/or become active under other conditions. Thus, we noted with interest that AQY1 (YPR192w) contributes to the average temporal profile for the early-induced gene class in the transcriptional program when S. cerevisiae is sporulated in nitrogen-deficient medium; YFL054c shows a similar profile (6). YLL052c-YLL053c, on the other hand, is apparently repressed by the initial nitrogen starvation (6). These results indicate that functional AQPs may be important for sporulation. The assessment of AQP function in yeasts growing under special conditions is a topic of continuing research in our laboratories.
Acknowledgments
This work was supported by grants DK43955-7 (to M.L.Z. and J.L.B.) and GM29713 (to E.W.J.) from the National Institutes of Health. L.A.C. was supported by a National Research Service Award from the National Institutes of Health.
We are indebted to Joe Suhan for the electron microscopy performed in this study.
REFERENCES
- 1.Andre B. An overview of membrane transport proteins in yeast. Yeast. 1995;11:1575–1611. doi: 10.1002/yea.320111605. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons; 1995. [Google Scholar]
- 3.Bertl A, Slayman C L, Gradmann D. Gating and conductance in an outward rectifying K+ channel from the plasma membrane of Saccharomyces cerevisiae. J Membr Biol. 1993;132:183–199. doi: 10.1007/BF00235737. [DOI] [PubMed] [Google Scholar]
- 4.Bertl A, Gradmann D, Slayman C L. Calcium- and voltage-dependent ion channels in Saccharomyces cerevisiae. Philos Trans R Soc Lond B Biol Sci. 1992;338:63–72. doi: 10.1098/rstb.1992.0129. [DOI] [PubMed] [Google Scholar]
- 5.Bonhivers M, Carbrey J M, Gould S J, Agre P. Aquaporins in Saccharomyces. J Biol Chem. 1998;273:27565–27572. doi: 10.1074/jbc.273.42.27565. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, DeRisi J, Eisen M, Mullholland J, Botstein D, Brown P O, Herskowitz I. The transcriptional program of sporulation in budding yeast. Science. 1998;282:699–705. doi: 10.1126/science.282.5389.699. [DOI] [PubMed] [Google Scholar]
- 7.Coury L A, Mathai J C, Prasad G V, Brodsky J L, Agre P, Zeidel M L. Reconstitution of water channel function of aquaporins 1 and 2 by expression in yeast secretory vesicles. Am J Physiol. 1998;274:F34–F42. doi: 10.1152/ajprenal.1998.274.1.F34. [DOI] [PubMed] [Google Scholar]
- 8.Gustin M C, Martinac B, Saimi Y, Culbertson M R, Kung C. Ion channels in yeast. Science. 1986;233:1195–1197. doi: 10.1126/science.2426783. [DOI] [PubMed] [Google Scholar]
- 9.Gustin M C, Zhou X-L, Martinac B, Kung C. A mechanosensitive ion channel in the yeast plasma membrane. Science. 1988;242:762–765. doi: 10.1126/science.2460920. [DOI] [PubMed] [Google Scholar]
- 10.Hazell B W, Kletsas S, Nevalainen H, Attfield P V. Involvement of CIF1 (GGS1/TPS1) in osmotic stress response in Saccharomyces cerevisiae. FEBS Lett. 1997;414:353–358. doi: 10.1016/s0014-5793(97)01033-8. [DOI] [PubMed] [Google Scholar]
- 11.Lagrèe V, Pellerin I, Hubert J-F, Tacnet F, Le Cahèrec F, Roudiers N, Thomas D, Gouranton J, Deschamps S. A yeast recombinant aquaporin mutant that is not expressed or mistargeted in Xenopus oocyte can be functionally analyzed in reconstituted proteoliposomes. J Biol Chem. 1998;20:12422–12426. doi: 10.1074/jbc.273.20.12422. [DOI] [PubMed] [Google Scholar]
- 12.Laize V, Rousselet G, Verbavatz J M, Berthounaud V, Gobin R, Roudier N, Abrami L, Ripoche P, Tacnet F. Functional expression of the human CHIP28 water channel in a yeast secretory mutant. FEBS Lett. 1995;373:269–274. doi: 10.1016/0014-5793(95)01060-r. [DOI] [PubMed] [Google Scholar]
- 13.Laize V, Roudier N, Rousselet G, Ripoche P, Tacnet F. Molecular cloning and characterization of two water channels from the yeast S. cerevisiae. FASEB J. 1998;12:A422. (abstract). [Google Scholar]
- 14.Lande M B, Donovan J M, Zeidel M L. The relationship between membrane fluidity and permeabilities to water, solutes, ammonia, and protons. J Gen Physiol. 1995;106:67–84. doi: 10.1085/jgp.106.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Latterich M, Watson M D. Isolation and characterization of osmosensitive vacuolar mutants of Saccharomyces cerevisiae. Mol Microbiol. 1991;5:2417–2426. doi: 10.1111/j.1365-2958.1991.tb02087.x. [DOI] [PubMed] [Google Scholar]
- 16.Lee M D, King L S, Agre P. The aquaporin family of water channels in clinical medicine. Medicine. 1997;76:141–156. doi: 10.1097/00005792-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Luyton K, Albertyn J, Skibbe W F, Prior B A, Ramos J, Thevelein J M, Hohmann S. Fps1, a yeast member of the MIP family of channel proteins, is a facilitator for glycerol uptake and efflux and is inactive under osmotic stress. EMBO J. 1995;14:1360–1371. doi: 10.1002/j.1460-2075.1995.tb07122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Manolson M F, Proteau D, Preston R A, Stenbit A, Roberts B T, Hoyt M A, Preus D, Mulholland J, Botstein D, Jones E W. The VPH1 gene encodes a 95-kDa integral membrane polypeptide required for in vivo assembly and activity of the yeast vacuolar H+-ATPase. J Biol Chem. 1992;267:14294–14303. [PubMed] [Google Scholar]
- 18.Park J H, Saier M H., Jr Phylogenetic characterization of the MIP family of transmembrane channel proteins. J Membr Biol. 1995;153:171–180. doi: 10.1007/s002329900120. [DOI] [PubMed] [Google Scholar]
- 19.Potenza M, Bowser R, Muller H, Novick P. SEC6 encodes an 85 kD soluble protein required for exocytosis in yeast. Yeast. 1992;8:549–558. doi: 10.1002/yea.320080706. [DOI] [PubMed] [Google Scholar]
- 20.Roberts C J, Raymond C K, Yamashiro C T, Stevens T H. Methods for studying the yeast vacuole. Methods Enzymol. 1991;194:644–659. doi: 10.1016/0076-6879(91)94047-g. [DOI] [PubMed] [Google Scholar]
- 21.Sharma S C, Raj D, Forouzandeh M, Bansal M P. Salt-induced changes in lipid composition and ethanol tolerance in Saccharomyces cerevisiae. Appl Biochem Biotechnol. 1996;56:189–195. doi: 10.1007/BF02786949. [DOI] [PubMed] [Google Scholar]
- 22.Stirling C J, Rothblatt J, Hosobuchi M, Deshaies R, Schekman R. Protein translocation mutants defective in the insertion of integral membrane proteins into the endoplasmic reticulum. Mol Biol Cell. 1992;3:129–142. doi: 10.1091/mbc.3.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutherland F C W, Lages F, Lucas C, Luyten K, Albertyn J, Hohmann S, Prior B A, Kilian S G. Characteristics of Fps1p-dependent and -independent glycerol transport in Saccharomyces cerevisiae. J Bacteriol. 1997;179:7790–7795. doi: 10.1128/jb.179.24.7790-7795.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swan T M, Watson K. Membrane fatty acid composition and membrane fluidity as parameters of stress tolerance in yeast. Can J Microbiol. 1997;43:70–77. doi: 10.1139/m97-010. [DOI] [PubMed] [Google Scholar]
- 25.Van Aelst L, Hohmann S, Zimmerman F K, Jans A W H, Thevelein J M. A yeast homologue of the bovine lens fibre MIP gene family complements the growth defect of a Saccharomyces cerevisiae mutant on fermentable sugars but not its defect in glucose-induced RAS-mediated cAMP signalling. EMBO J. 1991;10:2095–2104. doi: 10.1002/j.1460-2075.1991.tb07742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]