Abstract
Infantile nephropathic cystinosis, due to impaired transport of cystine out of lysosomes, occurs with an incidence of 1 in 100-200,000 live births. It is characterized by renal Fanconi syndrome in the first year of life and glomerular dysfunction progression to end-stage kidney disease by approximately 10 years of age. Treatment with oral cysteamine therapy helps preserve glomerular function, but affected individuals eventually require kidney replacement therapy. This is because glomerular damage had already occurred by the time a child is diagnosed with cystinosis, typically in the second year of life. We performed a retrospective multicenter study to investigate the impact of initiating cysteamine treatment within the first 2 months of life in some infants and comparing two different levels of adherence in patients diagnosed at the typical age. We collected 3983 data points from 55 patients born between 1997 and 2020; 52 patients with 1592 data points could be further evaluated. These data were first analyzed by dividing the patient cohort into three groups: (i) standard treatment start with good adherence, (ii) standard treatment start with less good adherence, and (iii) early treatment start. At every age, mean estimated glomerular filtration rate (eGFR) was higher in early-treated patients than in later-treated patients. Second, a generalized additive mixed model (GAMM) was applied showing that patients with initiation of treatment before 2 months of age are expected to have a 34 ml/min/1.73 m2 higher eGFR than patients with later treatment start while controlling for adherence and patients’ age. These data strongly suggest that oral cysteamine treatment initiated within 2 months of birth preserves kidney function in infantile nephropathic cystinosis and provide evidence of the utility of newborn screening for this disease.
Keywords: cystinosis, cysteamine, glomerular function
1. INTRODUCTION
Nephropathic cystinosis (OMIM 219800) is a rare lysosomal storage disease caused by biallelic mutations of the CTNS gene on chromosome 17p13.2[1,2]. CTNS encodes the lysosomal membrane protein cystinosin, which transports the disulfide amino acid cystine out of lysosomes[3,4]. When cystinosin is deficient, free, nonprotein cystine accumulates in lysosomes, resulting in crystal formation and cell damage in several tissues and organs. The severity of the disease depends on the extent of cystine storage and the remaining cystine-transporting capacity. Infantile nephropathic cystinosis is the most common and most severe form of the disease, although there are less severe phenotypes[1].
Nephropathic cystinosis is a multisystemic disease, but the kidney is the first organ to be severely affected. Patients are usually diagnosed between 12 and 24 months of age with failure to thrive, polydipsia, polyuria, and rickets due to severe disruption of proximal tubular reabsorptive function, i.e., De-Toni-Debre-Fanconi syndrome. This requires oral replacement of electrolytes and minerals. In the natural history of cystinosis, kidney glomerular damage leads to dialysis and/or kidney transplantation by approximately 10 years of age [5,6]. The disease does not recur in transplanted organs1.
The kidney tubule damage of cystinosis requires replacement of potassium, sodium, bicarbonate, phosphate, calcium, and water lost in the urine due to the Fanconi Syndrome[6]. Some children receive recombinant human growth hormone and others require thyroid hormone supplementation[7]. Additional complications include corneal crystal formation[8] other ophthalmic involvement[9], and damage to muscles[10–12], brain [13,14], and other organs[15,16].
The course of cystinosis was significantly improved by the use of oral cysteamine (beta-mercaptamine) to deplete cells of lysosomal cystine [17–19]. This weak base permeates lysosomes and participates in a disulfide interchange reaction with cystine to produce cysteine and cysteine-cysteamine mixed disulfide, both of which can exit the lysosome without the defective cystinosin protein[20]. Oral cysteamine therapy, monitored by measurement of leukocyte cystine levels[1], allows patients to survive into adulthood and prevents the late complications of the disease[18,19,21,22]. In addition, topical cysteamine eyedrops can dissolve the corneal cystine crystals that develop by 16 months of age[8,23,24]. Preservation of kidney glomerular function depends upon the level of adherence to cysteamine treatment[25], which is adversely influenced by the drug’s foul taste and smell.
Cysteamine treatment generally begins at the time of diagnosis in the second year of life, but some glomerular damage has already occurred by then. This situation could be ameliorated by diagnosing patients shortly after birth, employing molecular genetics-based newborn screening, as demonstrated for cystinosis in Germany[26]. Standard mass spectrometry-based methods for newborn screening cannot detect the increased cystine content of cystinosis leukocytes.
Having evaluated 55 cystinosis patients longitudinally over 23 years, we now describe the effects of early initiation of cysteamine treatment as well as adherence to a strict cystine-depleting regimen, on glomerular function.
2. METHODS
2.1. Patients
A total of 55 cystinosis patients from Germany, Austria, and Switzerland contributed data between 1997 and 2020. The diagnosis of cystinosis was based upon an elevated leukocytic cystine level and typical clinical presentation. Since October 2012, an interdisciplinary cystinosis clinic examined both pediatric and adult cystinosis patients once a year[27]. Longitudinal data included age, height, weight, cysteamine dosage, leukocyte cystine levels, and kidney data such as serum creatinine levels, point in time of initiation of renal replacement therapy, and the type of renal replacement therapy. Additional data points were provided by interim visits to local medical centers; retrospective data from prior to October 2012 were obtained from medical records. Note that some of the data of 29 patients, including date of birth, start of cysteamine treatment, date of last visit before 2017, ESKD status, and genotype, have been reported[28].
2.2. Data exclusion
A total of 3983 points of measurements were obtained on 55 cystinosis patients. We then excluded measurements collected at or after dialysis or transplantation as well as measurements collected before the start of cysteamine treatment or within 4 weeks of starting treatment. We also excluded measurements that had both cystine levels and eGFR values missing; this led to the exclusion of three patients. Since the main statistical model includes imputed cystine levels, we also excluded measurements with missing cystine levels if the next and the last measurements of non-missing cystine levels were more than 4 years apart. These exclusions left us with 1592 measurements from 52 patients.
2.3. Determination of leukocyte cystine and eGFR
Cystine levels were determined almost exclusively in the metabolic laboratory in Muenster[29], where the cystine measurement was carried out by the method described by Spackman et al[30]. eGFRcr was calculated based on age- and sex-specific formulas[31,32].
2.4. Adherence score
Rules for determining a patient’s score for adherence to cysteamine therapy based on their cystine levels are in Supplementary Materials. In general, an adherence score of ≥2.0 corresponds to leukocyte cystine levels being on average below 1 nmol cystine/mg protein.
2.5. Statistical analysis
In the first part of the analysis, we divided the patient cohort into three groups. Group 1 consists of patients who started cysteamine treatment after two months of age and complied well, i.e., had a mean adherence score of ≥ 2. Group 2 consists of patients who also started treatment after two months of age but complied less well, i.e., had a mean adherence score of < 2. Group 3 consists of patients who started treatment before 2 months of age; their mean adherence scores were ≥ 2. We estimated the relation between eGFRcr and age using a generalized additive mixed model (GAMM, implemented in the R package mgcv[33]) for each group separately. Random intercepts were used to account for correlation within patients. The effect of age was modeled by splines to allow for a nonlinear effect. Measurements with missing eGFRcr values were removed.
To statistically test the effect of treatment start and adherence on eGFRcr, we first performed two Mann–Whitney U tests comparing the mean eGFRcr values of Groups 1 and 2 vs. Group 3. We then fitted another GAMM with eGFRcr as outcome, not using the group assignments but the cystine levels and the dichotomized age (≥ 2 months vs. < 2 months) at treatment start as separate covariates. Details on the model can be found in the Supplementary Materials. Statistical analysis was performed using the statistical software R version 4.1.0[34]; the code used to generate the results is also provided in the Supplementary Information.
2.6. Study approval
The study was approved by the ethics committee of the Bavarian Medical Association (internal No: 2015-030).
3. RESULTS
3.1. Patient characteristics
Fifty-two individuals (23 females; 29 males) contributed a total of 1592 data points between February 1, 2000, and December 14, 2020 (Table 1). Six of 52 patients (11.5%) received oral cysteamine prior to 2 months of age (mean, 0.7 months); five of them had a sibling previously diagnosed with cystinosis and one was diagnosed through a neonatal screening project[26]. These 6 patients, whose longitudinal renal glomerular and tubular function is reported in an accompanying article (REF), comprised Group 3 and ranged in age from 1.2 to 17.2 years (mean, 8.2 years) at time of the analysis of their eGFR. The remaining 46 patients (comprising Groups 1 and 2) began cystine-depleting treatment at an average age of 1.8 ± 1.2 (SD) years; they ranged in age from 1.6 to 22.8 years at time of their most recent evaluation (mean, 12.4 years). Of the remaining 46 patients, 11 (24%) had kidney failure with replacement therapy, and 10 (22%) were homozygous for the common 57-kb deletion in CTNS. In the early-treated group, none of 6 patients had kidney failure with replacement therapy, and 3 of 6 (50%) were homozygous for the 57-kb CTNS deletion. The mean number of measurements in the early-treated group (26.7 per patient) and in the later-treated group (31.1 per patient) was comparable.
Table 1.
Patient characteristics.
| Overall (n=52) | Treatment start at ≥2 months of age (n=46) | Treatment start at <2 months of age (n=6) | |
|---|---|---|---|
| Sex (N, %) | |||
| Female | 23 (44.2) | 20 (43.5) | 3 (50.0) |
| Male | 29 (55.8) | 26 (56.5) | 3 (50.0) |
| Age at diagnosis (years) | |||
| Mean (SD) | 1.45 (1.05) | 1.63 (0.97) | 0.04 (0.03) |
| Median (Min, Max) | 1.21 (0, 4.71) | 1.42 (0.42, 4.71) | 0.04 (0, 0.06) |
| Age at treatment start (years) | |||
| Mean (SD) | 1.59 (1.25) | 1.78 (1.19) | 0.06 (0.04) |
| Median (Min, Max) | 1.20 (0.02, 6.48) | 1.42 (0.44, 6.48) | 0.05 (0.02, 0.14) |
| Age at last visit (years) | |||
| Mean (SD) | 11.9 (6.37) | 12.4 (6.22) | 8.2 (6.87) |
| Median (Min, Max) | 10.1 (1.2, 22.8) | 10.5 (1.6, 22.8) | 6.62 (1.2, 17.2) |
| Kidney replacement therapy (N, %) | |||
| No | 41 (78.8) | 35 (76.1) | 6 (100) |
| Yes | 11 (21.2) | 11 (23.9) | 0 (0) |
| 57-kb deletion (N, %) | |||
| Heterozygous | 16 (30.8) | 16 (34.8) | 0 (0) |
| Homozygous | 13 (25.0) | 10 (21.7) | 3 (50.0) |
| Neither | 13 (25.0) | 10 (21.7) | 3 (50.0) |
| Unknown | 10 (19.2) | 10 (21.7) | 0 (0) |
| Number of measurements | |||
| Mean (SD) | 30.6 (20.4) | 31.1 (20.5) | 26.7 (21.0) |
| Median (Min, Max) | 26.5 (3, 101) | 26.5 (3, 101) | 26.0 (5, 55) |
3.2. Association of eGFRcr with treatment start and cystine depletion
Comparing eGFRcr across treatment groups.
Table 2 provides eGFRcr data for Group 1 (standard age at start of treatment, good adherence), Group 2 (standard age at start of treatment, less good adherence), and Group 3 (early start of treatment). Low leukocyte cystine levels reflect good adherence with oral cysteamine treatment. There were on average more eGFRcr values than cystine values available per patient (Table 2). Normal cystine levels are < 0.1 nmol/mg of protein; values for untreated cystinosis patients range from 1.5 to 10 nmol cystine/mg of protein. Mean pre-treatment cystine levels were 2.6, 3.0, and 2.6 nmol/mg protein for Groups 1, 2, and 3, respectively, consistent with a diagnosis of infantile nephropathic cystinosis. The target range for treated patients is < 0.5 nmol/mg protein so that, overall, the achieved levels of cystine depletion were quite good. Group 3, in particular, had a mean leukocyte cystine level of 0.48 nmol/mg of protein, similar to that of Group 1 (0.53 nmol/mg of protein).
Table 2.
Age at last visit, mean leukocyte cystine levels, and estimated GFRcr at last visita
| Overall (n=52 patients; N=1592 measurements) | Group 1 (n=34 patients; N=1073 measurements) | Group 2 (n=12 patients; N=359 measurements) | Group 3 (n=6 patients; N=160 measurements) | |
|---|---|---|---|---|
| Age at last visit (years) | ||||
| Mean (SD) | 11.9 (6.4) | 12.0 (6.1) | 13.4 (6.7) | 8.2 (6.9) |
| Median (Min, Max) | 10.1 (1.2 ,22.8) | 10.0 (1.6, 22.8) | 16.3 (2.0, 21.9) | 6.6 (1.2, 17.2) |
| Mean leukocyte cystine (nmol/mg of protein) | ||||
| Mean (SD) | 0.62 (0.25) | 0.53 (0.14) | 0.97 (0.25) | 0.48 (0.14) |
| Median (Min, Max) | 0.59 (0.28, 1.49) | 0.53 (0.28, 0.83) | 0.93 (0.67, 1.49) | 0.48 (030), 0.66) |
| Number of cystine values per patient | ||||
| Mean (SD) | 20.6 (13.9) | 21.6 (14.4) | 18.7 (13.9) | 19.2 (13.0) |
| Median (Min, Max) | 17.5 (1, 59) | 17.5 (1, 59) | 18.5 (2, 38) | 19.0 (4, 35) |
| eGFRcr at last visit (mL/min/1.73 m2) | ||||
| Mean (SD) | 87.9 (35.0) | 88.8 (35.0) | 67.6 (20.2) | 123 (32.0) |
| Median (Min, Max) | 82.3 (26, 196) | 82.3 (26, 196) | 65.8 (32, 92) | 124 (78, 159) |
| Number of eGFRcr values per patient | ||||
| Mean (SD) | 23.0 (17.5) | 23.2 (17.4) | 25.2 (19.7) | 17.7 (15.6) |
| Median (Min, Max) | 19.0 (1, 89) | 19.0 (4, 89) | 23.0 (1, 57) | 17.0 (2, 39) |
Each patient contributed more than one value. The values were averaged within each patient.
Mean eGFRcr was significantly greater for the early-treated patients than for those treated after 2 months of age, regardless of the adherence level (Table 2); the p values were 0.021 comparing Group 3 to Group 1 and 0.001 comparing Group 3 to Group 2. However, these data have to be interpreted with caution, because the mean age at last visit of Group 3 (8.2 years) was lower than the mean age of Group 1 (12 years) or Group 2 (13.4 years).
eGFR as a function of age for the three treatment groups.
Figure 1 shows the individual eGFRcr values for each visit of the 52 patients as a function of patients’ ages. The lines represent the expected eGFRcr with 95% pointwise confidence intervals as a function of age for Groups 1, 2, and 3 (estimated using GAMMs). For each year of age, the early-treated patients (Group 3) had on average the highest eGFRcr, followed by the patients with good adherence and standard age at start of treatment (Group 1). Cystinosis patients not treated with cysteamine are known to exhibit a continuous decline in eGFR[19]. Note that the small number of early-treated patients (Group 3) is reflected by the wide confidence intervals.
Figure 1: eGFRcr values as a function of age for different groups of cysteamine-treated cystinosis patients.
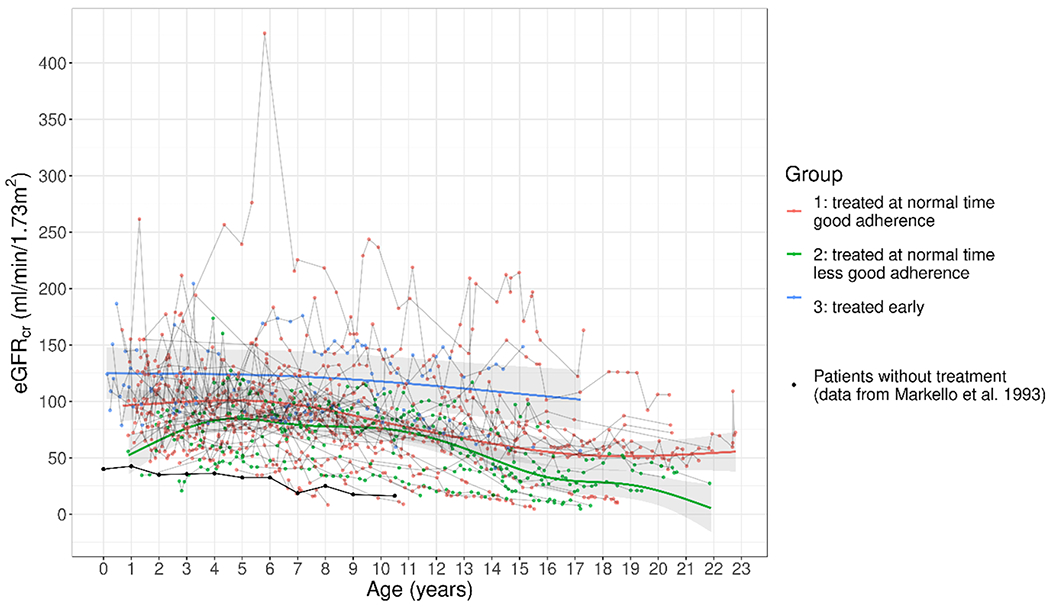
Red line: Group 1, initiation of treatment at standard age, good adherence. Green line: Group 2, initiation of treatment at standard age, less good adherence. Blue line: Group 3, initiation of treatment before 2 months of age. Black line: No cysteamine treatment (19).
eGFRcr as a function of age, cystine levels and treatment start.
These data were further analyzed using another generalized additive mixed model (GAMM) with the covariates age at start of treatment, leukocyte cystine level, and age (Table 3). The intercept of 82.1 ml/min/1.73 m2 refers to the expected eGFRcr of an approximately 10 year-old patient who is treated after 2 months of age and who has a (hypothetical) cystine level of 0 nmol/mg of protein. For early treated patients, mean eGFRcr increased significantly by 34.2 ml/min/1.73 m2 (p = 0.015) compared to patients with the same cystine level and age in whom treatment was started after 2 months of age. Regarding leukocyte cystine levels, there was a significant decrease of 4.91 ml/min/1.73 m2in eGFRcr if cystine increased by 1 nmol/mg of protein given that age and treatment start remain the same (p = 0.011). For a statistical analysis of the association of age and eGFRcr as well as the estimated eGFR values for different values of age, cystine, and treatment start, see Supplementary Materials (Fig. S1 and Table S1).
Table 3:
Effect estimates resulting from the generalized additive mixed model with covariates treatment start, leukocyte cystine level, and age.
| A. parametric coefficients | Estimate | Std. Error | p-value |
|---|---|---|---|
| Intercept | 82.05 | 4.86 | < 0.0001 |
| Treatment start: < 2 months | 34.23 | 14.05 | 0.015 |
| Cystine | −4.91 | 1.94 | 0.011 |
| B. smooth terms | edf | Ref.df | p-value |
| Age | 5.49 | 5.49 | < 0.0001 |
Note that this model assumes that the effect of age on eGFRcr is the same for both early and later diagnosed patients, which means that the information provided by the data points of the later treated patients is also shared with the early treated patients that have few data points for higher ages. Nevertheless, the model takes into account the uncertainty that is generated by the limited number of early treated patients.
4. DISCUSSION
Oral cysteamine therapy has proven effective in allowing for a normal growth rate and for substantial preservation of renal glomerular function in nephropathic cystinosis[18,19]. A study of 365 cystinosis patients showed that children born in the 1970s had renal function lasting 11.8 years compared with 12.9 years for those born in the 1980s and 16.6 years for those born in the 1990s[28]. The preservation of glomerular function by cysteamine is considered to be directly related to how early in life the treatment began[35,36] and to how closely patients adhered to optimal dosing. The initiation of cysteamine treatment generally does not occur until the second year of life because that is when cystinosis is usually diagnosed; glomerular damage by that time has already ordained that kidney replacement therapy will eventually be required.
We sought to quantify the benefits of early and compliant cysteamine therapy with respect to glomerular function. To accomplish this, we examined 23 years of data involving 1592 evaluations of 52 patients, including 6 patients who received oral cysteamine prior to 2 months of age. All 52 patients had typical infantile nephropathic cystinosis, as demonstrated by their age at diagnosis (mean, 1.5 years; range, 0-4.7 years; Table 1) and their untreated leukocyte cystine levels; the means for each patient ranged from 0.93 to 9.2 nmol/mg protein.
Several findings emerged from this investigation. First, there was a remarkably beneficial effect of cysteamine for the cohort as a whole. Although the patients were, on average, observed until the age of 12 years, when virtually all nephropathic cystinosis patients not treated with cysteamine have lost their kidney function, only 21% had kidney failure with replacement therapy (Table 1). The mean eGFRcr of our entire cohort was 87.9 ml/min/1.73 m2 (Table 2). Second, better adherence with cysteamine treatment and lower leukocyte cystine levels were associated with better preservation of eGFRcr. Specifically, the 34 patients with 1073 measurements in Group 1, with a mean leukocyte cystine level of 0.53 nmol/mg protein, had a mean eGFRcr of 89 ml/min/1.73 m2, while the 12 patients with 359 measurements in Group 2, with a mean leukocyte cystine level of 0.97 nmol/mg protein, had a mean eGFRcr of 68 ml/min/1.73 m2 (p=0.04) (Table 2). This is confirmed by Figure 1, where the estimated eGFRcr differs between Group 1 and 2 for every age.
Third, the data suggest that initiation of cysteamine therapy prior to 2 months of age has an even greater beneficial effect on eGFRcr. The six patients in Group 3, with 160 measurements and mean leukocyte cystine of 0.48 nmol/mg protein, had a mean eGFRcr of 123 ml/min/1.73 m2 (Table 2) and had on average a higher eGFRcr than later treated patients at every age (Figure 1). It has to be noted, however, that the size of this group was limited (n=6 patients).
The findings regarding the effect of age at treatment start and adherence are consistent with a statistical analysis using a GAMM model that took into account age at the initiation of cysteamine therapy, levels of cystine depletion, and patient age. The effect estimates derived from this analysis indicated that initiation of cysteamine before 2 months of age was associated with an increase of 34.2 ml/min/1.73 m2 in eGFRcr and a reduction of leukocyte cystine by 1 nmol/mg protein was associated with an increase of 4.9 ml/min/1.73 m2 ceteris paribus (Table 3). The effect of age on eGFRcr varied with age at which it was assessed (Fig. S1).
5. CONCLUSION
This study strongly bolsters previous evidence[19,25] that the better the adherence with oral cysteamine therapy, as reflected by an appropriate reduction in leukocyte cystine levels, the greater the preservation of renal glomerular function (Table 3, Table S1, Fig. S1). Even more compelling, however, is the apparent impact of very early (i.e., within the first two months of life) treatment of cystinosis with cysteamine. It is possible that neonates diagnosed and treated from birth might avoid kidney replacement therapy altogether. This implies that, by the usual age of initiation of cysteamine treatment (1-2 years), substantial glomerular damage has occurred, emphasizing the importance of newborn screening for early diagnosis of cystinosis[26].
Supplementary Material
ACKNOWLEDGEMENT
We thank Mrs. Holla for her technical support.
FUNDING
This study was supported by the Cystinosis Foundation Germany and, in part, by the Intramural Research Program of the National Human Genome Research Institute.
DISCLOSURE:
CN, ALB, KP, PS, SW, EW, AB, HB, BH, MZ, MK, KM, MB, MB, MG, UJ-K, GK, DM-B, KM, DM, LP, MP, BS, UT, WAG, KH declared no competing interests. JO has received consulting fees. JO, DH received payment or honoraria for lectures or presentations from Recordati GmbH and Chiesi GmbH, BA-R from Kyowa Kirin and Chiesi GmbH. KA received payement advisory board fee from Chiesi GmbH. BT is a consultant of Chiesi GmbH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DATA AVAILABILITY STATEMENT
All values that were calculated can be found in the Supplementary tables. The raw clinical data as well as laboratory values and anamnestic data are documented in the patient files non-anonymized with the treating physicians in Rosenheim and cannot be shared freely. The R-code used can be found in the Supplementary Information.
References
- [1].Gahl WA, Thoene JG, Schneider JA, Cystinosis, The New England journal of medicine 347 (2002) 111–121. 10.1056/NEJMra020552. [DOI] [PubMed] [Google Scholar]
- [2].Town M, Jean G, Cherqui S, Attard M, Forestier L, Whitmore SA, Callen DF, Gribouval O, Broyer M, Bates GP, van’t Hoff W, Antignac C, A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis, Nature genetics 18 (1998) 319–324. 10.1038/ng0498-319. [DOI] [PubMed] [Google Scholar]
- [3].Gahl WA, Bashan N, Tietze F, Bernardini I, Schulman JD, Cystine transport is defective in isolated leukocyte lysosomes from patients with cystinosis, Science (New York, N.Y.) 217 (1982) 1263–1265. 10.1126/science.7112129. [DOI] [PubMed] [Google Scholar]
- [4].Gahl WA, Tietze F, Bashan N, Steinherz R, Schulman JD, Defective cystine exodus from isolated lysosome-rich fractions of cystinotic leucocytes, The Journal of biological chemistry 257 (1982) 9570–9575. [PubMed] [Google Scholar]
- [5].Manz F, Gretz N, Progression of chronic renal failure in a historical group of patients with nephropathic cystinosis. European Collaborative Study on Cystinosis, Pediatric nephrology (Berlin, Germany) 8 (1994) 466–471. 10.1007/BF00856532. [DOI] [PubMed] [Google Scholar]
- [6].Gahl WA, Cystinosis coming of age, Adv Pediatr 33 (1986) 95–126. [PubMed] [Google Scholar]
- [7].Kimonis VE, Troendle J, Rose SR, Yang ML, Markello TC, Gahl WA, Effects of early cysteamine therapy on thyroid function and growth in nephropathic cystinosis, The Journal of clinical endocrinology and metabolism 80 (1995) 3257–3261. 10.1210/jcem.80.11.7593434. [DOI] [PubMed] [Google Scholar]
- [8].Gahl WA, Kuehl EM, Iwata F, Lindblad A, L Kaiser-Kupfer M, Corneal crystals in nephropathic cystinosis: natural history and treatment with cysteamine eyedrops, Molecular genetics and metabolism 71 (2000) 100–120. 10.1006/mgme.2000.3062. [DOI] [PubMed] [Google Scholar]
- [9].Kaiser-Kupfer MI, Caruso RC, Minkler DS, Gahl WA, Long-term ocular manifestations in nephropathic cystinosis, Archives of ophthalmology (Chicago, Ill. 1960) 104 (1986) 706–711. 10.1001/archopht.1986.01050170096030. [DOI] [PubMed] [Google Scholar]
- [10].Gahl WA, Dalakas MC, Chamas L, Chen KT, Pezeshkpour GH, Kuwabara T, Davis SL, Chesney RW, Fink J, Hutchison HT, Myopathy and cystine storage in muscles in a patient with nephropathic cystinosis, The New England journal of medicine 319 (1988) 1461–1464. 10.1056/NEJM198812013192206. [DOI] [PubMed] [Google Scholar]
- [11].Sonies BC, Ekman EF, Andersson HC, Adamson MD, Kaler SG, Markello TC, Gahl WA, Swallowing dysfunction in nephropathic cystinosis, The New England journal of medicine 323 (1990) 565–570. 10.1056/NEJM199008303230903. [DOI] [PubMed] [Google Scholar]
- [12].Anikster Y, Lacbawan F, Brantly M, Gochuico BL, Avila NA, Travis W, Gahl WA, Pulmonary dysfunction in adults with nephropathic cystinosis, Chest 119 (2001) 394–401. 10.1378/chest.119.2.394. [DOI] [PubMed] [Google Scholar]
- [13].Dogulu CF, Tsilou E, Rubin B, Fitzgibbon EJ, L Kaiser-Kupper M, Rennert OM, Gahl WA, Idiopathic intracranial hypertension in cystinosis, The Journal of pediatrics 145 (2004) 673–678. 10.1016/j.jpeds.2004.06.080. [DOI] [PubMed] [Google Scholar]
- [14].Fink JK, Brouwers P, Barton N, Malekzadeh MH, Sato S, Hill S, Cohen WE, Fivush B, Gahl WA, Neurologic complications in long-standing nephropathic cystinosis, Archives of neurology 46 (1989) 543–548. 10.1001/archneur.1989.00520410077027. [DOI] [PubMed] [Google Scholar]
- [15].Theodoropoulos DS, Krasnewich D, Kaiser-Kupfer MI, Gahl WA, Classic nephropathic cystinosis as an adult disease, Jama 270 (1993) 2200–2204. [PubMed] [Google Scholar]
- [16].Nesterova G, Gahl W, Nephropathic cystinosis: late complications of a multisystemic disease, Pediatric nephrology (Berlin, Germany) 23 (2008) 863–878. 10.1007/s00467-007-0650-8. [DOI] [PubMed] [Google Scholar]
- [17].Thoene JG, Oshima RG, Crawhall JC, Olson DL, Schneider JA, Cystinosis. Intracellular cystine depletion by aminothiols in vitro and in vivo, The Journal of clinical investigation 58 (1976) 180–189. 10.1172/jcil08448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gahl WA, Reed GF, Thoene JG, Schulman JD, Rizzo WB, Jonas AJ, Denman DW, Schlesselman JJ, Corden BJ, Schneider JA, Cysteamine therapy for children with nephropathic cystinosis, The New England journal of medicine 316 (1987) 971–977. 10.1056/nejml98704163161602. [DOI] [PubMed] [Google Scholar]
- [19].Markello TC, Bernardini IM, Gahl WA, Improved renal function in children with cystinosis treated with cysteamine, The New England journal of medicine 328 (1993) 1157–1162. 10.1056/NEJM199304223281604. [DOI] [PubMed] [Google Scholar]
- [20].Gahl WA, Tietze F, Butler JD, Schulman JD, Cysteamine depletes cystinotic leucocyte granular fractions of cystine by the mechanism of disulphide interchange, The Biochemical journal 228 (1985) 545–550. 10.1042/bj2280545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gahl WA, Thoene JG, Schneider JA, O’Regan S, Kaiser-Kupfer MI, Kuwabara T, Cystinosis: progress in a prototypic disease, Annals of internal medicine 109 (1988) 557–569. [DOI] [PubMed] [Google Scholar]
- [22].Gahl WA, Balog JZ, Kleta R, Nephropathic cystinosis in adults: natural history and effects of oral cysteamine therapy, Annals of internal medicine 147 (2007) 242–250. [DOI] [PubMed] [Google Scholar]
- [23].Kaiser-Kupfer MI, Fujikawa L, Kuwabara T, Jain S, Gahl WA, Removal of corneal crystals by topical cysteamine in nephropathic cystinosis, The New England journal of medicine 316 (1987) 775–779. 10.1056/NEJM198703263161304. [DOI] [PubMed] [Google Scholar]
- [24].Kaiser-Kupfer MI, Gazzo MA, Datiles MB, Caruso RC, Kuehl EM, Gahl WA, A randomized placebo-controlled trial of cysteamine eye drops in nephropathic cystinosis, Archives of ophthalmology (Chicago, 111. 1960) 108 (1990) 689–693. 10.1001/archopht.1990.01070070075038. [DOI] [PubMed] [Google Scholar]
- [25].Nesterova G, Williams C, Bernardini I, Gahl WA, Cystinosis: renal glomerular and renal tubular function in relation to compliance with cystine-depleting therapy, Pediatric nephrology (Berlin, Germany) 30 (2015) 945–951. 10.1007/s00467-014-3018-x. [DOI] [PubMed] [Google Scholar]
- [26].Hohenfellner K, Bergmann C, Fleige T, Janzen N, Burggraf S, Olgemöller B, Gahl WA, Czibere L, Froschauer S, Roschinger W, Vill K, Harms E, Nennstiel U, Molecular based newborn screening in Germany: Follow-up for cystinosis, Molecular genetics and metabolism reports 21 (2019) 100514. 10.1016/j.ymgmr.2019.100514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hohenfellner K, Deerberg-Wittram J, Coordinated, cost-effective care for rare disease: the cystinosis outpatient consultation program at RoMed, NEJM Catalyst Innovations in Care Delivery 1 (2020). [Google Scholar]
- [28].Emma F, Hoff WV, Hohenfellner K, Topaloglu R, Greco M, Ariceta G, Bettini C, Bockenhauer D, Veys K, Pape L, Hulton S, Collin S, Ozaltin F, Servais A, Deschênes G, Novo R, Bertholet-Thomas A, Oh J, Cornelissen E, Janssen M, Haffner D, Ravàa L, Antignac C, Devuyst O, Niaudet P, Levtchenko E, An international cohort study spanning five decades assessed outcomes of nephropathic cystinosis, Kidney international (2021). 10.1016/j.kint.2021.06.019. [DOI] [PubMed] [Google Scholar]
- [29].Linden S, Klank S, Harms E, Grüneberg M, Park JH, Marquardt T, Cystinosis: Therapy adherence and metabolic monitoring in patients treated with immediate-release cysteamine, Molecular genetics and metabolism reports 24 (2020) 100620. 10.1016/j.ymgmr.2020.100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Spackman DH, Stein WH, Moore S, Automatic recording apparatus for use in chromatography of amino acids, Analytical chemistry 30 (1958) 1190–1206. [PubMed] [Google Scholar]
- [31].Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL, New equations to estimate GFR in children with CKD, Journal of the American Society of Nephrology 20 (2009) 629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro III AF, Feldman HI, Kusek JW, Eggers P, van Lente F, Greene T, A new equation to estimate glomerular filtration rate, Annals of internal medicine 150 (2009) 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wood SN, Stable and efficient multiple smoothing parameter estimation for generalized additive models, Journal of the American Statistical Association 99 (2004) 673–686. [Google Scholar]
- [34].R.C. Team, R: a Language and environment for statistical computing.(Version 4.0)[Computer software; ]; 2021, 2021. [Google Scholar]
- [35].Reznik VM, Adamson M, Adelman RD, Murphy JL, Gahl WA, Clark KF, Schneider JA, Treatment of cystinosis with cysteamine from early infancy, The Journal of pediatrics 119 (1991)491–493. 10.1016/s0022-3476(05)82072-4. [DOI] [PubMed] [Google Scholar]
- [36].Kleta R, Bernardini I, Ueda M, Varade WS, Phornphutkul C, Krasnewich D, Gahl WA, Long-term follow-up of well-treated nephropathic cystinosis patients, The Journal of pediatrics 145 (2004) 555–560. 10.1016/j.jpeds.2004.03.056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All values that were calculated can be found in the Supplementary tables. The raw clinical data as well as laboratory values and anamnestic data are documented in the patient files non-anonymized with the treating physicians in Rosenheim and cannot be shared freely. The R-code used can be found in the Supplementary Information.


