Abstract
Healthy immune responses require efficient protection without excessive inflammation. Recent discoveries on the degree of fucosylation of a human N-linked glycan at a conserved site in the immunoglobulin IgG-Fc domain might add an additional regulatory layer to adaptive humoral immunity. Specifically, afucosylation of IgG-Fc enhances the interaction of IgG with FcγRIII and thereby its activity. Although plasma IgG is generally fucosylated, afucosylated IgG is raised in responses to enveloped viruses and Plasmodium falciparum proteins expressed on infected erythrocytes, as well as during alloimmune responses. Moreover, while afucosylation can exacerbate some infectious diseases (e.g., COVID-19), it also correlates with traits of protective immunity against malaria and HIV-1. Herein we discuss the implications of IgG afucosylation for health and disease, as well as for vaccination.
Keywords: glycosylation, fucosylation, galactosylation, humoral immunity, Fc-receptors, complement, IgG antibodies
Significance
IgG is normally fully fucosylated, and afucosylated IgG is only raised against antigens associated with human cells, thus introducing a mechanism for fine-tuning IgG potency through increased affinity for FcγRIII. Unveiling the molecular mechanisms underlying the formation of afucosylated IgG can enhance our understanding of infectious/non-infectious diseases and improved vaccine design.
Beyond quantity: when fucosylation of IgG matters
In response to a wide range of pathogens, antibodies are deposited on target surfaces, allowing recognition of their constant region (the Fc domain; see Glossary) by other components of the immune system such as the first molecule initiating the classical complement pathway (C1q) as well as cellular antibody receptors (Fc receptors) (Figure 1A) [1].
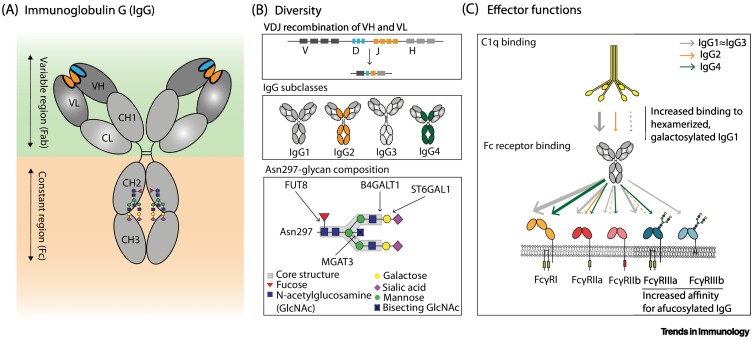
Figure 1.
Diversity and effector functions of human IgG.
(A) Schematic representation of IgG with variable (Fab) and constant (Fc) regions in the green and orange panels, respectively. The localization of the conserved Asn297 glycan in the Fc domain (CH2) is indicated [2]. (B) Antibody diversity arises during B cell development when rearrangement of variable, diversity, and joining gene regions [V(D)J] results in a diverse repertoire of antigen-specificities (upper panel) [2]. Upon B cell activation, B cells undergo isotype switching allowing them to produce antibodies of the IgG class, which is further subdivided into IgG1, IgG2, IgG3 and IgG4 subclasses (middle panel) [2]. The variable composition of the Asn297 glycan also contributes to IgG diversity, and different glycosyltransferases are responsible for enzymatic addition of residues to the core structure (GlcNAc4Man3) [30]. These modifications include the addition of galactose (B4GALT1), sialic acid (ST6GALT), bisecting GlcNAc (MGAT3), and core fucose (FUT8) (lower panel) [30]. (C) The diverse repertoire of IgG shapes effector functions, including the interaction of IgG-Fc with C1q, to activate the complement system, and FcγRs, to elicit antibody-dependent cellular cytotoxicity and phagocytosis [antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP)] [2]. The affinities of the different IgG subclasses for effector molecules are indicated by the individual color and thickness of the arrows [5]. Only the Asn162 glycan present in FcγRIIIa is shown as a biantennary glycan, although all FcγRs contain multiple N-linked glycans and also some of high-mannose structures. Abbreviations: CH1–3, immunoglobulin heavy-chain constant regions 1–3; CL, light-chain constant region; VH, heavy-chain variable region; VL, light-chain variable region.
Extensive antibody diversity is created during B cell development in the bone marrow when immunoglobulin variable gene segments rearrange and produce a unique variable domain in each B cell precursor [1] (Figure 1B). Further diversity is created upon antigen recognition and activation of mature B cells by somatic hypermutation and isotype class-switch recombination of gene segments encoding the constant region of the immunoglobulin [1,2]. This diverges the effector functions from the initial IgM-mediated to specialized isotype-specific functions, a process largely governed by the local cytokine environment [3,4]. IgG can activate both complement and bind to IgG-Fc receptors (FcγRs) and thereby shape immune responses [2,5,6] (Figure 1C).
Recent evidence underlines how the monosaccharide composition of a highly conserved N-linked glycan at position 297 in the heavy chain of human IgG plays a central role in fine-tuning both complement and FcγR-mediated effector functions [7]. Although elevated galactosylation of the IgG-Fc glycan stimulates C1q binding and downstream complement activity [8,9], only the degree of fucosylation has shown strong effects on FcγR binding and activation in biochemical and cellular assays and in vivo models, as described in detail later [8,10., 11., 12.]. Although the increased FcγRIII affinity of IgG lacking core fucose (afucosylation) has been known for decades [10,11], it has remained a biochemical curiosity because IgG is almost exclusively fucosylated, therefore questioning the importance of afucosylated IgGs in human immunity, and limiting the interest to mainly biotechnical and therapeutic exploitation of this phenomenon in cancer [12,13]. However, a growing body of evidence highlights that some antigen-specific IgG responses can be predominantly afucosylated, including responses to alloantigens, enveloped viruses, and Plasmodium falciparum proteins, which can be either beneficial or detrimental depending on the setting [14., 15., 16., 17., 18., 19., 20., 21., 22., 23., 24., 25., 26., 27., 28.]. In this article we focus on the biological and clinical significance of antibody glycosylation in humans, including fucosylation, that affect FcγR-mediated effector functions; we allude to a possible regulatory mechanism of afucosylated antibody responses by introducing a novel evolutionary hypothesis which might help to explain why self membrane-bound antigens are a necessity for afucosylated IgG responses to occur. Lastly, we examine the possibility of exploiting IgG afucosylation for putative vaccine development.
N-linked IgG glycosylation and Fc effector functions
N-linked glycan structures are not directly encoded by the genome but are constructed through enzymatic pathways. Following initial assembly on a lipid anchor at the cytoplasmic site of the endoplasmic reticulum (ER) membrane, glycans are flipped to the luminal side for further extension to form N-glycan precursors (reviewed in [29]). Glycan precursors are cotranslationally attached to proteins at accessible consensus motifs (NxS/T; x being any amino acid except proline) by oligosaccharyltransferase complexes and are further modified by a coordinated series of glycosyltransferases and glycosidases as the protein moves from the endoplasmic reticulum (ER) to the Golgi apparatus [29].
The N-linked glycosylation motif at Asn297 in IgG is highly conserved [7]. The IgG-Fc glycan consists of an N-acetylglucosamine (GlcNAc) and a mannose biantennary core structure. These can be modified by addition of a fucose residue to the primary GlcNAc, a bisecting GlcNAc to the core structure, galactose residues to the antennary GlcNAcs, and sialic acid to galactose residues [30] (Figure 1B). These modifications allow the formation of up to 36 different glycans that are readily detectable in human plasma [13]. In addition to the conserved Fc N-glycosylation site, 10–20% of IgGs also possess N-glycosylation sites in Fab regions, which are formed during affinity maturation, and influence the affinity of antibodies for antigens [31].
Residues proximal to the hinge region in the CH2 domain of IgG contain different but overlapping binding sites for C1q and FcγRs [32]. Although interactions between IgG and C1q require an avidity-docking platform of Fc:Fc-oligomerized IgG, optimally forming hexamers for binding of C1q and subsequent complement activation [33], FcγR activation requires less or differently structured clustering of IgG on effector cells [34]. Humans have several activating FcγRs (FcγRI, FcγRIIa, FcγRIIc, FcγRIIIa, and FcγRIIIb) and at least one inhibitory receptor (FcγRIIb) [35]. The integrated signal from these receptor–IgG-Fc interactions directs the type and strength of the effector functions, such as antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP), in natural killer (NK) cells and various myeloid cells which express a selection of these receptors [36]. The Fc glycan is essential for IgG effector function as it maintains the structure of the Fc domain required for both FcγR and C1q docking [37,38]; indeed, removal of the IgG-Fc glycan abrogates Fc effector functions [39., 40., 41.]. Furthermore, the exact composition of the IgG-Fc glycan affects its interaction with C1q and FcγRs, and thereby shapes IgG effector activity, as described later [8].
In summary, the N-linked glycan of IgG-Fc is required for IgG effector functions and its composition fine-tunes Fc effector functions.
Fc fucosylation
In recent decades studies have demonstrated that omitting the core fucose of the Fc glycan enhances FcγRIII activity, as evidenced from the improved ADCC capacity of afucosylated IgG compared with fucosylated IgG against multiple cellular targets, using human peripheral blood mononuclear cells (PBMCs) or NK cells as effector cells [10,11]. Because of this, afucosylated IgG is currently being exploited to increase the efficacy of therapeutic antibodies [12].
A unique glycan at Asn162 in FcγRIIIa and FcγRIIIb [42,43] collides with the core fucose and limits the accessibility of the FcγRIII-glycan which needs to be accommodated within the conformational space of the IgG-Fc, thereby leading to suboptimal IgG binding [44., 45., 46.]. As a consequence, afucosylated IgG has an up to 40-fold increased affinity compared with fucosylated IgG, as evidenced from surface plasmon resonance (SPR) experiments, and this explains the enhanced ADCC activity [11,45,47., 48., 49.] (Figure 2 ). The functional effect on NK cell ADCC in many cases has even exceeded the aforementioned 40-fold affinity increase, most likely due to the increased avidity of the interaction [10,11]. The higher affinity of afucosylated IgG for FcγRIIIb correlates with enhanced phagocytosis of human platelets and bacteria by FcγRIIIb+ neutrophils, but also reduces ADCC of human cancer cells by neutrophils [14,50,51]. The latter is presumably because less IgG is available for FcγRIIa activation, which receptor-blocking experiments have shown to be essential for neutrophil-mediated ADCC [50,51]. Afucosylation of IgG thus functions as a binary switch which increases the potency of IgG by enhanced affinity to FcγRIII.
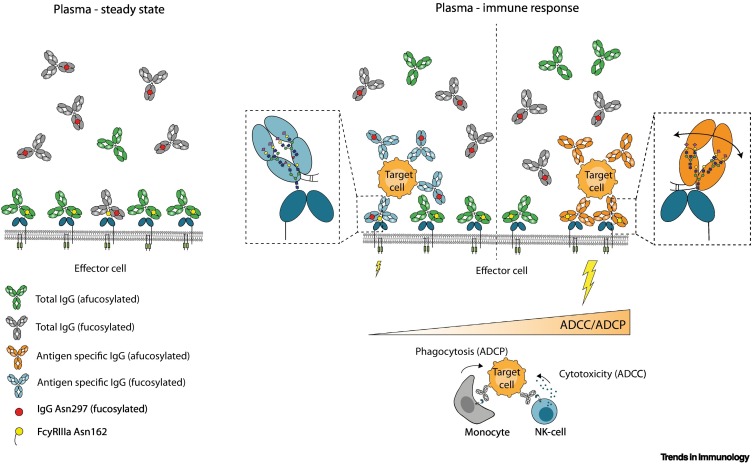
Figure 2.
Afucosylation of antigen-specific IgG can affect steady-state FcγRIII occupancy and effector activities in humans.
Under steady-state conditions (left panel), most FcγRIII are occupied by afucosylated IgGs with random specificities (depicted in green, ~6% of total plasma IgG) as their affinity is higher than their fucosylated counterparts (depicted in gray, ~94% of total plasma IgG) [48,58]. During effector responses (right panel), fucosylated IgG (in blue) displaces an aspecific afucosylated IgG already occupying FcγRIII less efficiently, due to differences in affinities [48]. By contrast, afucosylated IgG response (in orange) will displace an aspecific afucosylated IgG more easily, resulting in strong antibody-dependent cellular cytotoxicity and phagocytosis (ADCC/ADCP) activity [48]. Abbreviations: ADCC, antibody-dependent cellular cytotoxicity; ADCP, antibody-dependent cellular phagocytosis; NK, natural killer.
Fc galactosylation, sialylation, and bisection
Although no effect was initially found on NK cell-mediated ADCC activity against various cell lines by Fc galactosylation [10,52], recent SPR and chromatography data demonstrate that Fc galactosylation doubles the affinity of afucosylated IgG for FcγRIIIa, whereas it has less effect on fucosylated IgG [8,53,54]. Moreover, studies emphasize that galactosylation enhances the hexamerization potential of IgG; indeed, increased C1q binding and complement activation have been demonstrated on multiple solid-phase assays and cellular targets with human complement [8,9,55,56]. Although difficult to isolate from galactosylation, Fc sialylation appears to marginally enhance C1q binding and complement activation [8,56,57], whereas only negligible effects of FcγR and C1q binding have been reported that are attributable to the bisecting GlcNAc [8].
Changes in IgG glycosylation with aging
Plasma IgG-Fc glycans of human adults are ~94% fucosylated, 40% galactosylated, 8% bisected, and 4% sialylated (where both the degrees of galactosylation and sialylation refer to antenna occupation of the IgG-Fc glycan) [58]. Fc galactosylation and sialylation decrease with age in both males and females, the latter exhibiting the most prominent reduction during menopause, but a transient increase occurs during pregnancy, suggesting hormonal regulation of these traits [58,59].
By contrast, the degree of bisecting GlcNAc and Fc fucosylation is largely unchanged throughout the human lifespan [58]. Newborns harbor almost exclusively maternal IgG, and hence IgG glycosylation of neonates is virtually identical to that of the mother [60,61], although minor differences have been reported, possibly caused by methodological differences [62]. As the infant’s own IgG displaces the maternal IgG, IgG-Fc fucosylation of the infant reaches almost 100% 1 month after birth [61]. The fucosylation degree gradually decreases to 94% at ~20 years of age and only decreases marginally thereafter [58,61]. As explained later, this decrease of fucosylation with age is possibly due to the accumulation of afucosylated IgG acquired during immune responses against pathogens that stimulate the formation of afucosylated IgG [27]. The degree of bisecting GlcNAc generally correlates negatively with the degree of fucosylation, suggesting that bisection may sterically inhibit fucosylation [61].
Thus, Fc glycosylation of IgG changes throughout life. Although the galactosylation and sialylation of IgG may be partly hormonally regulated, the changes in IgG-Fc fucosylation likely arise from the accumulation of antigen-specific IgG responses with decreased Fc fucosylation.
Afucosylated IgG responses in alloimmunity and infectious diseases
Despite the low abundance and relatively stable concentrations of afucosylated IgG in plasma, multiple studies of antigen-specific IgG, all using affinity-purified IgG and mass spectrometry analysis, recently highlighted the clinical importance of some antigen-specific afucosylated IgG responses, which were also verified by FcγRIIIa-dependent serological methods in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) responses [14., 15., 16., 17., 18., 19., 20., 21., 22., 23., 24., 25., 26., 27., 28.,63., 64., 65.]. These afucosylated IgG responses include those directed towards alloantigens (Table 1 ), which can contribute to alloimmune-mediated diseases (Box 1 ), against enveloped viruses, and against P. falciparum proteins expressed on red blood cells (RBCs), all responses in which the degree of afucosylation can influence disease severity [14., 15., 16., 17., 18., 19., 20., 21., 22., 23., 24., 25., 26., 27., 28.,63., 64., 65.].
Table 1.
Characteristics of afucosylated IgG responses in humans
| Antigen | Degree of maximum afucosylation | Interindividual spread | Stability of response | Consequences for disease | Vaccine responsea | Refs | |
|---|---|---|---|---|---|---|---|
| Alloimmune diseases | |||||||
| Pregnancy | HPA-1a | ↑↑↑ | High | Stable for up to 7 years post-delivery | Correlates with increased disease severity in FNAIT | N/A | [14,15,21] |
| HPA-3a, HPA-5b | ↑↑↑ | Unknown | Unknown | Unknown | N/A | [21] | |
| RhD | ↑↑↑ | High | Stable for up to 9 years post-delivery | Associated with a high risk of HDFN | N/A | [22., 23., 24.] | |
| K | ↑↑↑ | High | Unknown | Associated with severe fetal anemia | N/A | [24,25] | |
| Transplantation | HLA | ↑↑↑ | High | Unknown | Associated with antibody-mediated graft rejection | N/A | [20] |
| Enveloped viruses | |||||||
| HIV | Envelope protein | ↑ | Low | Stable for at least 3 years | Feature of HIV elite controllers | Unknown | [26,27] |
| Dengue virus | Envelope protein | ↑↑ | Intermediate | Unknown | Associated with disease progression | Unknown | [28] |
| Cytomegalovirus | Antigen mixture | ↑↑↑ | High | Stable for up to a decade | Unknown | Unknown | [27] |
| Hepatitis B virus | HBsAg | ↑ | Intermediate | Unknown | Unknown | Soluble HBsAg protein subunit vaccine induces fucosylated HBsAg-specific IgG, unlike natural infection | [27] |
| Measles virus (morbillivirus measles virus) |
Antigen mixture | ↑ | Low | Stable for multiple decades | Unknown | Live attenuated viruses induce afucosylated antigen-specific IgG, similarly to natural infection | [27] |
| Mumps virus (mumps orthorubulavirus) | Antigen mixture | ↑↑ | Low | Stable for multiple decades | Unknown | Live attenuated viruses induce afucosylated antigen-specific IgG, similarly to natural infection | [27] |
| SARS-CoV-2 | Spike protein | ↑↑ | Intermediate | Transient afucosylation after seroconversion; long-term unknown | Correlates with systemic immunopathology | mRNA vaccine induces afucosylated spike-specific IgG, similarly to natural infection | [16,17,27,63,64] |
| Respiratory syncytial virus | Antigen mixture | Low | Unknown | Correlates with NK cell activity | Unknown | [19] | |
| Intracellular parasites | |||||||
| Plasmodium falciparum | VAR2CSA | ↑↑↑ | High | Present for at least a decade; decreases with antigen exposure | Protection acquired in a parity-dependent manner correlates with decreasing Fc fucosylation levels with increasing exposure | Soluble VAR2CSA protein subunit vaccine induces fucosylated antigen-specific IgG, unlike natural infection | [18] |
| VAR6 | ↑↑↑ | Low | Present for at least a decade | Protection acquired during childhood. Fc fucosylation most likely decreases with antigen exposure, similarly to VAR2CSA | Unknown | [18] | |
N/A, not applicable.
Box 1. Alloantigens and alloimmune-mediated diseases.
Alloimmune responses are elicited by incompatible pregnancies, blood transfusions, and transplantations, whereby human, but foreign, antigens (alloantigens) are introduced [94]. The most relevant alloantibodies in such responses are those against red blood cell (RBC) antigens, including RhD and K, human platelet antigen (HPA) such as HPA-1a, and human leukocyte antigens (HLA) [94].
Alloimmunization occurs naturally during pregnancy when paternally inherited alloantigens on fetal cells induce maternal immune responses [94]. Only IgG alloantibodies cross the placenta by neonatal Fc receptor-mediated transcytosis irrespective of Fc glycosylation, and are clinically relevant for RBC or platelet alloimmunization in pregnancy [60,94]. These alloantibodies display a strong degree of afucosylation in some instances, which is deemed to be clinically important because of the enhanced affinity of afucosylated IgG for FcγRIII expressed on immune cells; in addition, such afucosylation adds prognostic value not solely provided by antibody titers because it significantly influences the quality of the immune responses and therefore of disease severity [14,22,67,69].
Alt-text: Box 1
Fetal and neonatal alloimmune thrombocytopenia
Fetal and neonatal alloimmune thrombocytopenia (FNAIT) is caused by alloantibodies against fetal human platelet antigens (HPAs) induced by maternal exposure to fetal cells carrying a paternally derived incompatible alloantigen [66]. More than 80% of FNAIT cases are estimated to result from HPA-1a incompatibility [66]. HPA-1a-specific alloantibodies can be found in ~0.2% of pregnancies in the Caucasian population, and these result in thrombocytopenia (<150 × 109 thrombocytes/l) in ~50% of cases with HPA-1a-specific alloantibodies [66]. Severe FNAIT is much more rare (~1–2% of HPA-1a-immunized cases) and is characterized by internal bleeding, especially intracranial hemorrhage (ICH), often resulting in lifelong disabilities or death [66]. Fc fucosylation of HPA-1a-specific IgG shows great variation, from virtually 100% down to ~10% [14,15,21]. Decreased Fc fucosylation causes enhanced phagocytosis of platelets in vitro by FcγRIIIa+ monocytes and FcγRIIIb+ neutrophils, both likely contributing to enhanced platelet phagocytosis in the fetal liver and spleen [22,45]. In agreement, antibody titers and platelet counts do not strictly correlate with ICH incidence, whereas HPA-1a-specific IgG-Fc fucosylation correlates positively with neonatal platelet counts and negatively with disease severity [14,15,67]. Furthermore, Fc fucosylation was found to be the strongest predictor of bleeding severity, followed by elevated galactosylation and antibody titers [15]. As HPA-1a-specific IgG afucosylation is stable during pregnancy and in subsequent pregnancies for at least a decade, afucosylation could potentially identify pregnancies at risk [14,15].
Hemolytic disease of the fetus and newborn
Similarly to immune responses against platelets, IgG afucosylation has also been reported in cases where these antibodies are directed against alloantigens on RBCs, causing hemolytic disease of the fetus and newborn (HDFN) [22]. Most cases with severe HDFN arise in pregnant RhD-negative women immunized by alloantigen from a RhD-positive child (Box 1). The maternal RhD-specific afucosylated IgG subsequently targets and destroys fetal RBCs, resulting in anemia with risk of fetal death [68]. Of note, disease severity is more reliably predicted by a monocyte- or NK cell-driven ADCC test than by antibody titers alone, highlighting the importance of the interaction between RhD-specific IgG and FcγRs in contributing to severe disease [48,69,70]. Decreased RhD-specific IgG-Fc fucosylation (reduced to ~10%, a degree which is similar to that of HPA-1a) [22], is associated with significantly lowered neonatal hemoglobin concentrations relative to the normal range. Presumably, the enhanced removal of fetal RBCs by afucosylated RhD-specific IgG occurs through FcγRIIIa+ monocytes/macrophages and NK cells in the spleen and liver [22]. Concordant with the risk of severe disease, afucosylation is also observed in IgG responses to the similarly clinically relevant blood group K, but is rarely reported for Rhc- and RhE-specific IgG; this is in agreement with rarity of severe cases caused by these antibodies [24].
Transplantation
HLA-specific IgG, formed either during pregnancy, transfusion, or after transplantation, can in some cases exhibit afucosylation [20]. For instance, in kidney transplant recipients, preliminary data suggest that afucosylation appears to be associated with IgG-mediated rejection of donor organs, although this requires validation [20]. As discussed later, the nature of HLA as an antigen suggests that afucosylated IgG might be a general and key feature in antibody-mediated graft rejection, although this remains conjectural and warrants further studies.
Enveloped viruses: HIV-1 and dengue virus infections
In addition to alloantibodies, responses to some enveloped viruses have been reported to elicit varying degrees of afucosylated IgG [26., 27., 28.]. For instance, HIV-1-specific IgGs that target the viral envelope have shown afucosylation (~80%) [26,27], an occurrence which has been deemed to be more prominent in HIV-1-infected elite controllers compared with non-elite controllers. For the former, strong NK cell-mediated ADCC responses against HIV-1-infected human CD4+ T cells correlated with viral inhibition in vitro [26], suggesting that afucosylation of HIV-1-specific IgGs is associated with protection.
In contrast to HIV-1, decreased Fc fucosylation of IgG directed against antigens of the viral envelope of dengue virus is associated with disease escalation [28]. Moreover, non-neutralizing antibodies from heterotypic dengue virus serotypes are considered to be risk factors for developing dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) because they enhance viral uptake, replication, and the production of proinflammatory cytokines, mediated by FcγRs expressed on immune cells, referred to as antibody-dependent enhancement (ADE) [28,71]. In agreement with the increased affinity of afucosylated IgGs for FcγRIII and the fact that ADE often relies on FcγR, the higher degree of afucosylation of antigen-specific IgGs is associated with DHF and DSS, as evidenced by the elevated concentrations of afucosylated dengue-specific IgG in patients with DHF/DSS, thus suggesting a pathogenic role of afucosylated non-neutralizing IgGs in these diseases [28,72].
Enveloped viruses: cytomegalovirus, hepatitis B virus, respiratory syncytial virus, measles virus, and mumps virus infections
Other enveloped viruses also elicit afucosylated IgG immune responses [27]. Although the extent of afucosylated IgGs that are generated against respiratory syncytial virus (RSV) and hepatitis B virus (HBV) are minor, they vary (<80% fucosylation) and correlate with NK cell activation, stimulated by immobilized RSV-specific IgG [19,27]. The extent of afucosylated IgGs against measles (morbillivirus measles virus) and mumps virus (mumps orthorubulavirus) are similar to those elicited by dengue virus and HIV-1, whereas cytomegalovirus (CMV) stimulates the strongest afucosylated IgG responses of all viruses studied so far, with fucosylation levels resembling those of RhD- and HPA-1a-specific IgGs, and these can remain stable for at least a decade [14,15,23,27]. Moreover, the responses to measles and mumps viruses that have been examined several decades after the primary response also reveal stable Fc fucosylation profiles for these antibodies. However, the clinical implications of stably afucosylated IgG for protection and disease progression during these viral infections remain unknown [27].
Enveloped virus: SARS-CoV-2 infections
The pathogenesis of coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2, is highly variable, ranging from asymptomatic to severe respiratory failure, and fatal outcomes are linked to excessive immune activation [73,74]. Afucosylated IgGs targeting the viral membrane spike protein have been reported, particularly in hospitalized patients [16,17,27,63]. The most prominent afucosylation (reduced to ~60%) coincided with seroconversion and admission to the intensive care unit (ICU), after which fucosylation can increase to >90% within weeks following recovery of individuals who did not succumb to the first inflammatory wave [27]. The disease progression in individuals with afucosylated IgG might be explained by increased binding of viral immune complexes to FcγRIII-bearing cells, leading to potent activation of the latter, and which could have potentially led to excessive release of proinflammatory cytokines associated with severe COVID-19. In partial agreement with this hypothesis, in vitro stimulation of monocyte-derived alveolar type macrophages from healthy donors with afucosylated IgG immune complexes, in the presence of a Toll-like receptor (TLR) stimulus, has been reported to enhance FcγRIIa- and FcγRIIIa-dependent secretion of proinflammatory cytokines [17,27].
Moreover, recent data suggest that CD16+ monocytes are a primary target for the uptake and sterile RNA replication of IgG-opsonized SARS-CoV-2 through FcγRIIIa, thereby triggering inflammasome activation and pyroptosis, and supporting the idea that afucosylated IgGs fuel strong proinflammatory responses [75,76]. In addition, interleukin (IL)-6 and C-reactive protein plasma concentrations have been reported to be elevated in COVID-19 ICU patients, but not in individuals with mild symptoms, and the former inversely correlate with Fc fucosylation of spike-specific IgG [27]. This suggests that afucosylated IgG early in the response might cause excessive FcγRIIIa stimulation of cells such as alveolar macrophages, presumably contributing to systemic immunopathology – that might partly resolve as the degree of Fc fucosylation increases, a phenomenon which remains to be directly validated [27]. Indeed, as studies of responses against other viruses so far have all been carried out after the primary response has ceased [26., 27., 28.], it remains unclear whether transient afucosylation, as observed for SARS-CoV-2, is a general phenomenon.
Plasmodium falciparum parasite infections
Immune responses towards the protozoan parasite P. falciparum, that is responsible for most malaria cases in sub-Saharan Africa, also show markedly reduced fucosylation of IgG against parasite antigens expressed on infected RBCs [18]. These proteins, members of the P. falciparum erythrocyte membrane protein-1 (PfEMP1) family, are required for adhesion of infected RBCs to host receptors on endothelium and tissues, and are therefore necessary for parasite survival [77]. PfEMP1 antigen VAR6-specific IgG has shown marked afucosylation, whereas IgG against the merozoite protein glutamate-rich protein (GLURP), which is not displayed on host RBCs, has not [18]. In contrast to alloimmune responses and antiviral responses, IgG specific for the pregnancy-restricted PfEMP1 VAR2CSA displays exposure-dependent Fc fucosylation because the fucosylation decreases with increasing parity – a proxy for possible exposures to VAR2CSA in endemic areas [18]. This might offer a putative molecular mechanism to explain the correlation between parity number and reduced risk of pregnancy-associated malaria [78]. Of note, the afucosylation in the responses to PfEMP1s is more prominent than that observed in IgGs against enveloped viruses (~25% vs ~85% fucosylation, respectively) [18]. As the degree of fucosylation of PfEMP1-specific IgG correlates with features known to associate with protection (parity), it is tempting to assign decreased fucosylation as a protective trait, although further validation is needed [18,78].
In summary, alloimmune responses against RBCs and platelets, as well as responses against enveloped viruses and specific P. falciparum antigens, can elicit afucosylated IgGs, and these appear to be of clinical importance, although the underlying mechanisms remain to be investigated. Of note, the gradual decrease of Fc fucosylation during adolescence coincides with the accumulation of immunity to enveloped viruses and other intracellular pathogens, suggesting that these antigens form a unique category that stimulates B cells to produce afucosylated IgGs, which are otherwise rare.
The importance of antigen context for Fc fucosylation
Most immune responses induce fully fucosylated IgG, in agreement with plasma IgG findings of ~94% IgG fucosylation [58]. This includes responses against bacterial polysaccharides (and protein-conjugated vaccines thereof), inactivated influenza virus, tetanus toxoid vaccine (Clostridium tetani), and citrullinated proteins [79., 80., 81., 82.] (Figure 3A, Key figure). Conversely, afucosylated IgG responses share a common feature in that the antigen is localized in the host cell membrane [14,18,20,22,26., 27., 28.]. For instance, in FNAIT, HDFN, and P. falciparum infections, the antigens localize to platelet- or RBC membranes (Figure 3B). Similarly, during infections with enveloped viruses, viral antigens are exposed on the host membrane after fusion of the viral envelope during invasion and when virions are assembled for viral budding [83] (Figure 3C). Based on the similarity of IgG-Fc fucosylation in these responses, we recently proposed that antigen recognition by B cells in the context of a host membrane activates a so far unknown receptor–ligand pairs which could be necessary for the induction of afucosylated IgG [27] (Figure 3). However, although membrane sensing is simple and plausible (Box 2 and see Figure I in Box 2), it is insufficient per se – as illustrated by the large interindividual and interantigen variation of IgG afucosylation, suggesting that more factors are involved [14,18,20,22,26., 27., 28.].
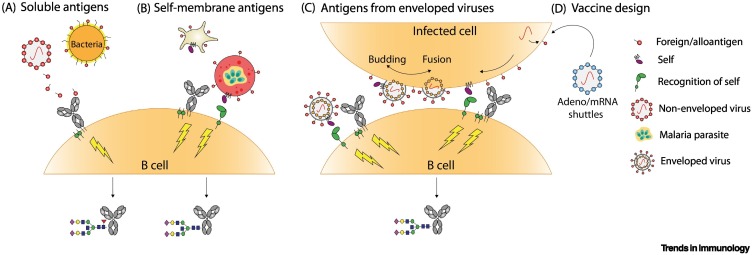
Figure 3.
Key Figure. Hypothetical model illustrating the possible role of antigen context-induced alteration of immune signaling and the production of afucosylated IgG in humans.
(A) Soluble antigens, derived from non-enveloped viruses or bacteria, signal through the B cell receptor (BCR) resulting in B cell activation and the production of fucosylated antigen-specific IgG [79., 80., 81., 82.]. (B) For alloantigens on platelets and red blood cells (RBCs) (not shown), and Plasmodium falciparum proteins expressed on infected RBCs, we propose that antigen recognition by the BCR is accompanied by a so far unknown receptor–ligand pair that provides a signal for 'self'. Signaling through these complexes is required for the induction of afucosylated IgG [14,18,20,22]. (C) Similarly, responses towards enveloped viral infections resemble responses described in (B) for alloantigens and P. falciparum proteins, as these antigens are also self-membrane-bound [27]. Self-recognition might either occur from the virion (left) if segments of the host cell membrane are included in the viral envelope, or (right) from virus-infected cells as the viral envelope fuses with the host membrane [83], both resulting in the production of afucosylated antigen-specific IgG [26., 27., 28.]. (D) The potency of afucosylated IgG might be harnessed in vaccine design by mimicking natural antigen display as depicted in (B) and (C). Using mRNA templates or viral shuttles for transcription in host cells, foreign antigens might be expressed in the context of a self-membrane which could induce afucosylated IgG [64]. Abbreviation: adeno, adenovirus.
Box 2. Receptor–ligand pairs involved in regulating B cell activation and differentiation.
Fine-tuning of B cell responses is provided by a vast array of regulatory receptor–ligand pairs [4] (Figure IA–D). Upon antigen engagement, B cell receptors (BCRs) cluster on the membrane and initiate signaling pathways that are essential for B cell survival, proliferation, and differentiation [95]. In addition, B cell-specific receptors such as CD19 and CD21, MHC class II, and CD40 are important surface molecules for B cell activation [4]. Furthermore, T helper cells and dendritic cells modulate the B cell response by secreting various cytokines including IL-4, interferon-γ, and transforming growth factor-β which regulate B cell differentiation and antibody features such as isotype switching [4]. Other cytokines such as IL-21 and IL-17, as well as co-stimulation through Toll-like receptors (TLRs), also play a vital role in B cell activation and shaping the antibody response [4,96,97]. During antigen binding by BCRs in the context of human B cell membranes, receptor–ligand pairs, including known 'self-markers' such as CD47 or sialoside ligands, and their receptors SIRP-γ and CD22, respectively, might serve as putative checkpoints for the expression of afucosylated IgGs, given that self-membrane association appears to be a commonality of antigens that can stimulate afucosylated IgGs [27,98., 99., 100.] (Figure IE,F).
Alt-text: Box 2
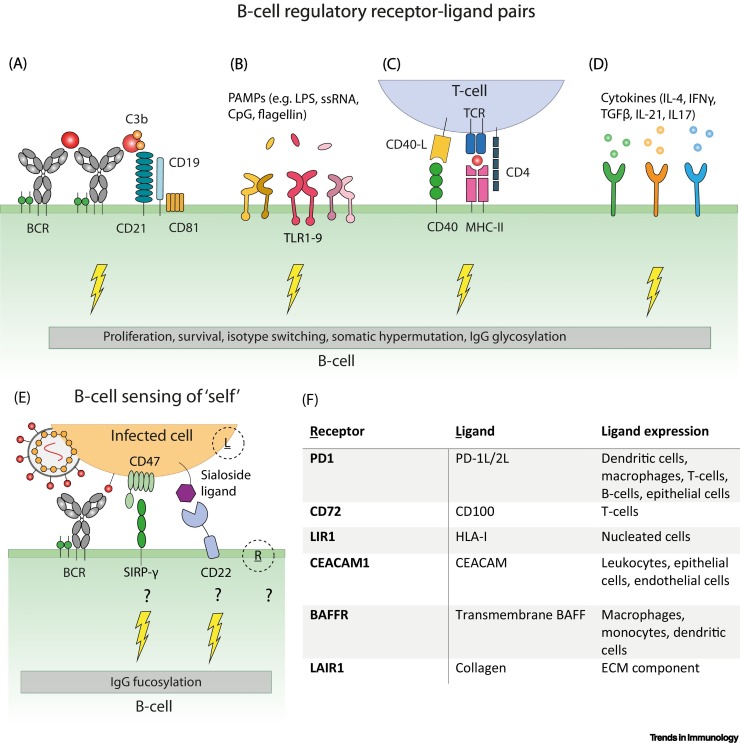
Figure I.
Immunoregulatory receptor–ligand pairs involved in fine-tuning the B cell response in humans.
(A–D) Recognition of antigen by B cell receptors (BCRs) results in BCR clustering and crosslinking with the CD19–CD21 coreceptor complex thereby increasing BCR mediated signaling [4,95]. In addition, signaling via Toll-like receptors (TLRs), CD40–CD40L, and cytokine receptors is important for B cell differentiation, isotype switching, and IgG glycosylation [4]. (E) A similar signaling axis might be involved in regulating IgG fucosylation through sensing of ‘self’ (e.g., CD47 or sialoside ligands sensed by B cells) [98,99] (F), but other receptors and ligands, depicted in (E) as R and L, respectively, might also be involved depending on the infected cell type, thus driving the switch to producing afucosylated IgG [100]. Abbreviations: CpG, CpG-rich DNA; ECM, extracellular matrix; IL, interleukin; LPS, lipopolysaccharide; PAMP, pathogen-associated molecular pattern; ssRNA, single-stranded RNA; TCR, T cell receptor; TGF, transforming growth factor.
Genome-wide association studies have implied that the transcription regulator IKAROS might account for small differences in IgG fucosylation in B cells, whereas the transcription factor HNF-1α might control the expression of fucosyltransferases in hepatic cells, suggesting that protein fucosylation is likely controlled on the transcriptomic level [84,85]. Furthermore, during HIV-1 infections, peripheral B cells from elite controllers, as well as plasma cells during acute natural and vaccination responses against SARS-CoV-2, have been reported to exhibit decreased expression of α1,6-fucosyltransferase FUT8 [16,26,64]. As FUT8 is responsible for core fucosylation in B cells, and because the degree of antigen-specific IgG fucosylation correlates with FUT8 expression, FUT8 is a tempting candidate for studying IgG-Fc fucosylation given that no clear molecular mechanism for this process has been described [16,26,64].
The current literature on antigen-specific human IgG responses therefore underlines the importance of antigen presentation in the context of a self-membrane as being necessary for the production of afucosylated IgGs which are potent inducers of FcγRIII-mediated cytotoxicity.
The common ancestry of afucosylated responses
A major difference between alloimmune responses and pathogen-initiated responses is the lack of pattern recognition and the ensuing inflammation. Of note, the inflammation induced by enveloped viruses suggests a possible inverse relationship with the degree of afucosylation. This is exemplified by responses to CMV and HIV-1, which are latent viruses that cause low inflammation and a stable low degree of Fc fucosylation [86]. Moreover, in severe SARS-CoV2 responses, the initial low degree of Fc fucosylation upon seroconversion increases while the plasma concentrations of proinflammatory cytokines are at their highest [27,64]. High antibody titers are insufficient to induce strong cytotoxic responses at low antigen density [48], and antibodies with strong effector functions are thus probably necessary to clear specific infections. Speculatively, a transient afucosylated response might be advantageous against viruses where efficient killing of infected cells is an immediate defense strategy following seroconversion, thereby quenching viral replication. However, although afucosylated IgG might be important for eliminating some pathogens, it can also cause detrimental inflammation, as observed in highly proliferative SARS-CoV-2 infections in immunologically naïve individuals [17,27].
Moreover, Plasmodium species are known to have exerted a strong selection pressure on human evolution [48,87., 88., 89.]. As these pathogens infect RBCs, which are devoid of antigen-presenting MHC molecules, protective immunity mainly relies on antibody-mediated effector functions. The possible beneficial value of generating afucosylated antibodies in P. falciparum-specific responses might in theory resonate with some of the reasons why afucosylated maternal alloantibodies can also be raised during pregnancies, and may have been tolerated during evolution, albeit at the cost of increased fetal morbidity and mortality [14,22]. However, alternative and/or complementary explanations cannot be ruled out.
Concluding remarks
We posit that the question of how to prevent or induce afucosylated IgG responses might never have been more relevant. This knowledge could potentially allow us to tailor vaccines by obtaining a desired Fc fucosylation antibody response against, for example, P. falciparum, thus imitating the natural protective response (see Outstanding questions).
Outstanding questions.
What are the precise molecular mechanisms leading to afucosylated IgG? Understanding these might allow fine-tuning of vaccine designs against diseases in which IgG-Fc fucosylation is important. It might also be possible to intervene in the formation of afucosylated IgG responses when these are undesirable.
Can responses that are naturally steered towards the formation of afucosylated IgG be further afucosylated by vaccination in settings in which this might be beneficial (e.g., HIV-1 and P. falciparum infections)? This might serve as an alternative means to combat diseases for which prophylactic vaccination has proved to be difficult.
Would immunization with cell-surface antigens by using engineered RBCs, mRNA vaccines, viral vectors, engineered constructs, or vesicles increase vaccine efficiency against difficult targets by generating afucosylated IgG? This might potentially lead to new therapeutic approaches for diseases that currently lack efficient treatments and/or vaccines.
Does the dynamics of afucosylation in the initial phase of an immune response differ based on the target, or do these responses initially follow a similar trajectory?
Can ongoing immune responses, which are likely to raise pathogenic afucosylated IgG (e.g., in conditions such as COVID-19 and dengue fever), be prevented therapeutically? By halting the formation of afucosylated IgG in settings in which these might be pathogenic, favorable disease outcomes might ensue.
Alt-text: Outstanding questions
Protein subunit vaccination stimulates highly fucosylated IgG responses, as reported for HBV surface antigen (HBsAg) [27] and P. falciparum VAR2CSA subunit vaccines [18], whereas the natural counterparts on HBV and on P. falciparum-infected RBCs, respectively, induce afucosylated IgG [18,27]. Furthermore, vaccination with live attenuated mumps and measles viruses can induce a degree of IgG afucosylation similar to that of natural infections [27]. Although these data support the concept that self-membrane-associated antigen display is necessary to induce afucosylated IgGs [27], they also suggest possible approaches to induce or avoid IgG afucosylation in different contexts.
For instance, in response to the COVID-19 pandemic, many parallel vaccine approaches were taken, but, of the vaccine platforms so far approved, the majority provided mRNA templates for transcription of the membrane-bound SARS-CoV-2 spike in host cells, thus imitating natural infection [27,90] (Figure 3D). In accordance with our hypothesized model describing how only self-membrane-bound antigens induce afucosylated IgG, naïve mRNA SARS-CoV-2 vaccinees responded with transiently afucosylated spike-specific IgGs that increased within the first weeks following seroconversion, and with kinetics similar to those of natural SARS-CoV2 infections, potentially suggesting that shared signaling pathways are used in these contexts [64]. Whether adenoviral COVID-19 vaccines behave similarly remains unknown. However, the current data on afucosylated antigen-specific IgGs as presented earlier suggest that the induction of long-lived afucosylated IgGs might require approaches different from those based on mRNA vaccines, such as using engineered RBCs or specific local cytokine environments to induce stably afucosylated IgGs [14,18,22,23,27].
Future research on afucosylated IgG responses is challenged by the lack of animal models which would be essential for expanding our understanding of the mechanisms behind such immune responses. The Asn162 glycan of human FcγRIII is conserved in the orthologous mouse receptor (FcγRIV), and also provides discrimination between fucosylated and afucosylated mouse IgG [42], even across species; indeed, afucosylated human IgG has demonstrated improved FcγRIV-mediated antitumor activity, relative to fucosylated IgG, in B16 melanoma mouse models [91]. However, afucosylated IgG is almost completely absent in inbred as well as outbred mice, which possibly excludes the mouse as a model organism for studying afucosylated IgG responses [92,93]. Furthermore, the complete mechanism(s) behind afucosylated IgG responses is probably complex, involving multiple immune cells that can be differentially regulated across species. Therefore, a robust in vivo model to study afucosylated IgG responses remains a challenge and multiple obstacles need to be addressed.
In conclusion, a better understanding of the balance and regulation of afucosylated responses is a fascinating area of future research which holds potential for advancing our knowledge of humoral immunity in infectious and non-infectious diseases, as well as in improved vaccine design aiming to mimic the appropriate antigen display and environment.
Acknowledgments
Acknowledgments
J.J.O. and M.D.L. were supported by Landsteiner Stichting for Bloodtranfusion Research (LSBR) grants 1908 and 1721, respectively, awarded to G.V.
Declaration of interests
The authors declare no conflicts of interests.
Glossary
- Affinity maturation
the process by which B cells increase their affinity for cognate antigen through random mutations of the variable region.
- Alloantigens/alloimmunization
immune response towards antigen from the same species towards a protein (alloantibody) other than that of the responding individual.
- Antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP)
Fc effector functions induced upon engagement of Fc receptors on immune cells by opsonized targets resulting in cytotoxicity or phagocytosis of targets, respectively.
- Antibody-dependent enhancement (ADE)
the phenomenon where antibody binding enhances viral entry and/or replication.
- Avidity-docking platform
a platform created by multiple target-bound IgGs that increases the overall binding strength between IgG-opsonized cells and FcγR-expressing effector cells, beyond those of individual IgG–FcγR pairs, as a result of the multivalency of the interaction. This also applies for C1q binding.
- Bisecting GlcNAc
a N-acetylglucosamine (GlcNAc) extension on the N-glycan core structure between the two N-glycan branches.
- Classical complement pathway
the pathway of the complement cascade that is optimally activated upon the formation of IgG hexamers on target surfaces that allow binding of the recognition molecule C1q.
- Class-switch recombination
a genetic rearrangement of the constant gene segments of antibodies in B cells that leads to antibody class-switching.
- Dengue hemorrhagic fever (DHF)
a rare complication of dengue virus infection characterized by high fever and damage to lymph and blood vessels, leading to severe bleeding and organ failure.
- Dengue shock syndrome (DSS)
a life-threatening complication in dengue disease defined as hypotension and narrow pulse pressure.
- Elite controllers
individuals infected with HIV-1 who maintain undetectable viral loads in the absence of therapy.
- Fab regions
fragment antigen binding, the variable domain of an antibody that is responsible for antigen binding.
- Fc domain
fragment crystallizable, the constant domain of an antibody that is responsible for binding to effector molecules of the immune system.
- Fetal and neonatal alloimmune thrombocytopenia (FNAIT)
a bleeding disorder during pregnancy caused by maternal alloantibodies that target paternally inherited antigens on the platelets of the fetus/newborn.
- Fucosylation
the addition of a fucose residue to a glycan/sugar branch.
- Galactosylation
the addition of a galactose residue to a glycan/sugar branch.
- Glycosidases
enzymes that catalyze the hydrolysis of glycosidic bonds during the processing or catabolism of oligosaccharides.
- Glycosylation
a post-translational modification in which sugar branches are attached to other macromolecules including proteins.
- Glycosyltransferases
enzymes that catalyze the transfer of monosaccharides to carbohydrates during the synthesis of oligosaccharides by creating a glycosidic bond.
- Hemolytic disease of the fetus and newborn (HDFN)
a bleeding disorder that can occur when maternal alloantibodies against red blood cells (RBCs) cross the placenta and mediate the destruction of cells expressing the targeted antigen.
- N-linked glycans
oligosaccharides attached to asparagine (Asn, N) residues at a consensus motif N-x-S/T where x is any amino acid except proline.
- Non-neutralizing antibodies
antibodies that bind to pathogens without disturbing receptor–ligand interactions necessary for infection; they can (potentially) have protective or disease-enhancing effects through Fc-mediated effector functions.
- Pyroptosis
inflammatory lytic programmed cell death, often triggered by intracellular pathogens, that culminates in activation of caspases and the inflammasome leading to proinflammatory cytokine release and cellular lysis.
- RhD and K
alloantigens on RBCs, also known as blood group systems.
- Sialoside ligands
ligands with sialic acid-containing carbohydrates.
- Sialylation
the addition of a sialic acid residue to a glycan/sugar branch.
- Somatic hypermutation
hypermutation in the variable gene regions of antibodies in fully developed B cells contributing to affinity maturation of the antibody produced by the cell.
- Surface plasmon resonance (SPR)
a label-free analytical technique for measuring binding kinetics and affinity of molecular interactions.
- Toll-like receptors (TLRs)
immune receptors expressed by a wide range of immune cells that sense pathogen-associated molecular patterns.
References
- 1.Schroeder H.W., Cavacini L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010;125:S41–S52. doi: 10.1016/j.jaci.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vidarsson G., et al. IgG subclasses and allotypes: from structure to effector functions. Front. Immunol. 2014;5:520. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tesfaye D.Y., et al. Targeting conventional dendritic cells to fine-tune antibody responses. Front. Immunol. 2019;10:1529. doi: 10.3389/fimmu.2019.01529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pone E.J., et al. Toll-like receptors and B-cell receptors synergize to induce immunoglobulin class-switch DNA recombination: relevance to microbial antibody responses. Crit. Rev. Immunol. 2010;30:1–29. doi: 10.1615/critrevimmunol.v30.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruhns P., et al. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716–3725. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 6.de Taeye S.W., et al. FcγR binding and ADCC activity of human IgG allotypes. Front. Immunol. 2020;11:740. doi: 10.3389/fimmu.2020.00740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold J.N., et al. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu. Rev. Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 8.Dekkers G., et al. Decoding the human immunoglobulin G-glycan repertoire reveals a spectrum of Fc-receptor- and complement-mediated-effector activities. Front. Immunol. 2017;8:877. doi: 10.3389/fimmu.2017.00877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peschke B., et al. Fc-galactosylation of human immunoglobulin gamma isotypes improves C1q binding and enhances complement-dependent cytotoxicity. Front. Immunol. 2017;8:646. doi: 10.3389/fimmu.2017.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shinkawa T., et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 2003;278:3466–3473. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- 11.Shields R.L., et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 2002;277:26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 12.Pereira N.A., et al. The 'less-is-more' in therapeutic antibodies: afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. MAbs. 2018;10:693–711. doi: 10.1080/19420862.2018.1466767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wuhrer M., et al. Glycosylation profiling of immunoglobulin G (IgG) subclasses from human serum. Proteomics. 2007;7:4070–4081. doi: 10.1002/pmic.200700289. [DOI] [PubMed] [Google Scholar]
- 14.Kapur R., et al. A prominent lack of IgG1-Fc fucosylation of platelet alloantibodies in pregnancy. Blood. 2014;123:471–480. doi: 10.1182/blood-2013-09-527978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sonneveld M.E., et al. Glycosylation pattern of anti-platelet IgG is stable during pregnancy and predicts clinical outcome in alloimmune thrombocytopenia. Br. J. Haematol. 2016;174:310–320. doi: 10.1111/bjh.14053. [DOI] [PubMed] [Google Scholar]
- 16.Chakraborty S., et al. Early non-neutralizing, afucosylated antibody responses are associated with COVID-19 severity. Sci. Transl. Med. 2022;14 doi: 10.1126/scitranslmed.abm7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoepel W., et al. High titers and low fucosylation of early human anti–SARS-CoV-2 IgG promote inflammation by alveolar macrophages. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abf8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsen M.D., et al. Afucosylated Plasmodium falciparum-specific IgG is induced by infection but not by subunit vaccination. Nat. Commun. 2021;12:5838. doi: 10.1038/s41467-021-26118-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Erp E.A., et al. Natural killer cell activation by respiratory syncytial virus-specific antibodies is decreased in infants with severe respiratory infections and correlates with Fc-glycosylation. Clin. Transl. Immunol. 2020;9 doi: 10.1002/cti2.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bharadwaj P., et al. Afucosylation of HLA-specific IgG1 as a potential predictor of antibody pathogenicity in kidney transplantation. MedRxiv. 2022 doi: 10.1101/2022.03.09.22272152. Published online March 12, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wuhrer M., et al. Regulated glycosylation patterns of IgG during alloimmune responses against human platelet antigens. J. Proteome Res. 2009;8:450–456. doi: 10.1021/pr800651j. [DOI] [PubMed] [Google Scholar]
- 22.Kapur R., et al. Low anti-RhD IgG-Fc-fucosylation in pregnancy: a new variable predicting severity in haemolytic disease of the fetus and newborn. Br. J. Haematol. 2014;166:936–945. doi: 10.1111/bjh.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapur R., et al. Prophylactic anti-D preparations display variable decreases in Fc-fucosylation of anti-D. Transfusion. 2015;55:553–562. doi: 10.1111/trf.12880. [DOI] [PubMed] [Google Scholar]
- 24.Sonneveld M.E., et al. Antigen specificity determines anti-red blood cell IgG-Fc alloantibody glycosylation and thereby severity of haemolytic disease of the fetus and newborn. Br. J. Haematol. 2017;176:651–660. doi: 10.1111/bjh.14438. [DOI] [PubMed] [Google Scholar]
- 25.Sonneveld M.E., et al. Fc-glycosylation in human IgG1 and IgG3 is similar for both total and anti-red-blood cell anti-K antibodies. Front. Immunol. 2018;9:31. doi: 10.3389/fimmu.2018.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ackerman M.E., et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J. Clin. Invest. 2013;123:2183–2192. doi: 10.1172/JCI65708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsen M.D., et al. Afucosylated IgG characterizes enveloped viral responses and correlates with COVID-19 severity. Science. 2021;371 doi: 10.1126/science.abc8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang T.T., et al. IgG antibodies to dengue enhanced for FcγRIIIA binding determine disease severity. Science. 2017;355:395–398. doi: 10.1126/science.aai8128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schjoldager K.T., et al. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell Biol. 2020;21:729–749. doi: 10.1038/s41580-020-00294-x. [DOI] [PubMed] [Google Scholar]
- 30.Jennewein M.F., Alter G. The immunoregulatory roles of antibody glycosylation. Trends Immunol. 2017;38:358–372. doi: 10.1016/j.it.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 31.van de Bovenkamp F.S., et al. Adaptive antibody diversification through N-linked glycosylation of the immunoglobulin variable region. Proc. Natl. Acad. Sci. U. S. A. 2018;115:1901–1906. doi: 10.1073/pnas.1711720115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Taeye S.W., et al. The ligands for human IgG and their effector functions. Antibodies. 2019;8:30. doi: 10.3390/antib8020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diebolder C.A., et al. Complement is activated by IgG hexamers assembled at the cell surface. Science. 2014;343:1260–1263. doi: 10.1126/science.1248943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vidarsson G., van de Winkel J.G.J. Fc receptor and complement receptor-mediated phagocytosis in host defence. Curr. Opin. Infect. Dis. 1998;11:271–278. doi: 10.1097/00001432-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Nimmerjahn F., Ravetch J.V. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 36.Getahun A., Cambier J.C. Of ITIMs, ITAMs, and ITAMis: revisiting immunoglobulin Fc receptor signaling. Immunol. Rev. 2015;268:66–73. doi: 10.1111/imr.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krapp S., et al. Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. J. Mol. Biol. 2003;325:979–989. doi: 10.1016/s0022-2836(02)01250-0. [DOI] [PubMed] [Google Scholar]
- 38.Ganesh Subedi A.P., et al. The structural role of antibody N-glycosylation in receptor interactions. Structure. 2015;23:1573–1583. doi: 10.1016/j.str.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang G., et al. Molecular basis of assembly and activation of complement component C1 in complex with immunoglobulin G1 and antigen. Mol. Cell. 2016;63:135–145. doi: 10.1016/j.molcel.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 40.Lux A., et al. Impact of immune complex size and glycosylation on IgG binding to human FcγRs. J. Immunol. 2013;190:4315–4323. doi: 10.4049/jimmunol.1200501. [DOI] [PubMed] [Google Scholar]
- 41.Jung S.T., et al. Bypassing glycosylation: engineering aglycosylated full-length IgG antibodies for human therapy. Curr. Opin. Biotechnol. 2011;22:858–867. doi: 10.1016/j.copbio.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Dekkers G., et al. Conserved FcγR-glycan discriminates between fucosylated and afucosylated IgG in humans and mice. Mol. Immunol. 2018;94:54–60. doi: 10.1016/j.molimm.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Ferrara C., et al. The carbohydrate at FcγRIIIa Asn-162: an element required for high affinity binding to non-fucosylated IgG glycoforms. J. Biol. Chem. 2006;281:5032–5036. doi: 10.1074/jbc.M510171200. [DOI] [PubMed] [Google Scholar]
- 44.Sakae Y., et al. Conformational effects of N-glycan core fucosylation of immunoglobulin G Fc region on its interaction with Fcγ receptor IIIa. Sci. Rep. 2017;7:13780. doi: 10.1038/s41598-017-13845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrara C., et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc. Natl. Acad. Sci. U. S. A. 2011;108:12669–12674. doi: 10.1073/pnas.1108455108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Falconer D.J., et al. Antibody fucosylation lowers FcγRIIIa/CD16a affinity by limiting the conformations sampled by the N162-glycan. ACS Chem. Biol. 2018;13:2179. doi: 10.1021/acschembio.8b00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niwa R., et al. IgG subclass-independent improvement of antibody-dependent cellular cytotoxicity by fucose removal from Asn297-linked oligosaccharides. J. Immunol. Methods. 2005;306:151–160. doi: 10.1016/j.jim.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 48.Temming A.R.R., et al. Functional attributes of antibodies, effector cells, and target cells affecting NK cell-mediated antibody-dependent cellular cytotoxicity. J. Immunol. 2019;203:3126–3135. doi: 10.4049/jimmunol.1900985. [DOI] [PubMed] [Google Scholar]
- 49.Karampatzakis A., et al. Antibody afucosylation augments CD16-mediated serial killing and IFNγ secretion by human natural killer cells. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.641521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Treffers L.W., et al. FcγRIIIb restricts antibody-dependent destruction of cancer cells by human neutrophils. Front. Immunol. 2019;10:3124. doi: 10.3389/fimmu.2018.03124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peipp M., et al. Antibody fucosylation differentially impacts cytotoxicity mediated by NK and PMN effector cells. Blood. 2008;112:2390–2399. doi: 10.1182/blood-2008-03-144600. [DOI] [PubMed] [Google Scholar]
- 52.Raju T.S. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr. Opin. Immunol. 2008;20:471–478. doi: 10.1016/j.coi.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 53.Thomann M., et al. In vitro glycoengineering of IgG1 and its effect on Fc receptor binding and ADCC activity. PLoS One. 2015;10 doi: 10.1371/journal.pone.0134949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kiyoshi M., et al. Assessing the heterogeneity of the Fc-glycan of a therapeutic antibody using an engineered FcγReceptor IIIa-immobilized column. Sci. Rep. 2018;8:3955. doi: 10.1038/s41598-018-22199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wei B., et al. Fc galactosylation follows consecutive reaction kinetics and enhances immunoglobulin G hexamerization for complement activation. MAbs. 2021;13:1893427. doi: 10.1080/19420862.2021.1893427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Osch T.L.J., et al. Fc galactosylation promotes hexamerization of human IgG1, leading to enhanced classical complement activation. J. Immunol. 2021;207:1545–1554. doi: 10.4049/jimmunol.2100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quast I., et al. Sialylation of IgG Fc domain impairs complement-dependent cytotoxicity. J. Clin. Invest. 2015;125:4160–4170. doi: 10.1172/JCI82695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baković M.P., et al. High-throughput IgG Fc N-glycosylation profiling by mass spectrometry of glycopeptides. J. Proteome Res. 2013;12:821–831. doi: 10.1021/pr300887z. [DOI] [PubMed] [Google Scholar]
- 59.van de Geijn F.E., et al. Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: results from a large prospective cohort study. Arthritis Res. Ther. 2009;11:R193. doi: 10.1186/ar2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Einarsdottir H.K., et al. Comparison of the Fc glycosylation of fetal and maternal immunoglobulin G. Glycoconj. J. 2013;30:147–157. doi: 10.1007/s10719-012-9381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Haan N., et al. Changes in healthy human IgG Fc-glycosylation after birth and during early childhood. J. Proteome Res. 2016;15:1853–1861. doi: 10.1021/acs.jproteome.6b00038. [DOI] [PubMed] [Google Scholar]
- 62.Jennewein M.F., et al. Fc glycan-mediated regulation of placental antibody transfer. Cell. 2019;178:202–215. doi: 10.1016/j.cell.2019.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chakraborty S., et al. Proinflammatory IgG Fc structures in patients with severe COVID-19. Nat. Immunol. 2021;22:67–73. doi: 10.1038/s41590-020-00828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Coillie J., et al. The BNT162b2 mRNA SARS-CoV-2 vaccine induces transient afucosylated IgG1 in naive but not antigen-experienced vaccinees. BioRxiv. 2022 doi: 10.1101/2022.02.14.480353. Published online February 15, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Šuštić T., et al. Immunoassay for quantification of antigen-specific IgG fucosylation. EBioMedicine. 2022;81 doi: 10.1016/j.ebiom.2022.104109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kamphuis M.M., et al. Screening in pregnancy for fetal or neonatal alloimmune thrombocytopenia: systematic review. BJOG. 2010;117:1335–1343. doi: 10.1111/j.1471-0528.2010.02657.x. [DOI] [PubMed] [Google Scholar]
- 67.Tiller H., et al. Current perspectives on fetal and neonatal alloimmune thrombocytopenia – increasing clinical concerns and new treatment opportunities. Int. J. Women's Health. 2017;9:223. doi: 10.2147/IJWH.S90753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Haas M., et al. Haemolytic disease of the fetus and newborn. Vox Sang. 2015;109:99–113. doi: 10.1111/vox.12265. [DOI] [PubMed] [Google Scholar]
- 69.Oepkes D., et al. Clinical value of an antibody-dependent cell-mediated cytotoxicity assay in the management of Rh D alloimmunization. Am. J. Obstet. Gynecol. 2001;184:1015–1020. doi: 10.1067/mob.2001.112970. [DOI] [PubMed] [Google Scholar]
- 70.Hadley A.G. Laboratory assays for predicting the severity of haemolytic disease of the fetus and newborn. Transpl. Immunol. 2002;10:191–198. doi: 10.1016/s0966-3274(02)00065-5. [DOI] [PubMed] [Google Scholar]
- 71.Guzman M.G., et al. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: An historical perspective and role of antibody-dependent enhancement of infection. Arch. Virol. 2013;158:1445–1459. doi: 10.1007/s00705-013-1645-3. [DOI] [PubMed] [Google Scholar]
- 72.Thulin N.K., et al. Maternal anti-dengue IgG fucosylation predicts susceptibility to dengue disease in infants. Cell Rep. 2020;31 doi: 10.1016/j.celrep.2020.107642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang C., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu B., et al. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021;19:141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sefik E., et al. Inflammasome activation in infected macrophages drives COVID-19 pathology. Nature. 2022;606:585–593. doi: 10.1038/s41586-022-04802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Junqueira C., et al. FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature. 2022;606:576–584. doi: 10.1038/s41586-022-04702-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jensen A.R., et al. Cerebral Plasmodium falciparum malaria: the role of PfEMP1 in its pathogenesis and immunity, and PfEMP1-based vaccines to prevent it. Immunol. Rev. 2020;293:230–252. doi: 10.1111/imr.12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salanti A., et al. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J. Exp. Med. 2004;200:1197–1203. doi: 10.1084/jem.20041579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vestrheim A.C., et al. A pilot study showing differences in glycosylation patterns of IgG subclasses induced by pneumococcal, meningococcal, and two types of influenza vaccines. Immun. Inflamm. Dis. 2014;2:76. doi: 10.1002/iid3.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Selman M.H.J., et al. Changes in antigen-specific IgG1 Fc N-glycosylation upon influenza and tetanus vaccination. Mol. Cell. Proteomics. 2012;11 doi: 10.1074/mcp.M111.014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang T.T., et al. Anti-HA glycoforms drive B cell affinity selection and determine influenza vaccine efficacy. Cell. 2015;162:160–169. doi: 10.1016/j.cell.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rombouts Y., et al. Anti-citrullinated protein antibodies acquire a pro-inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Ann. Rheum. Dis. 2015;74:234–241. doi: 10.1136/annrheumdis-2013-203565. [DOI] [PubMed] [Google Scholar]
- 83.Takahashi T., Suzuki T. Function of membrane rafts in viral lifecycles and host cellular response. Biochem. Res. Int. 2011;2011 doi: 10.1155/2011/245090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Klarić L., et al. Glycosylation of immunoglobulin G is regulated by a large network of genes pleiotropic with inflammatory diseases. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aax0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lauc G., et al. Genomics meets glycomics – the first GWAS study of human N-glycome identifies HNF1A as a master regulator of plasma protein fucosylation. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Christensen-Quick A., et al. Cytomegalovirus and HIV persistence: pouring gas on the fire. AIDS Res. Hum. Retrovir. 2017;33:S23–S30. doi: 10.1089/aid.2017.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miller L.H., et al. Interaction between cytochalasin B-treated malarial parasites and erythrocytes attachment and junction formation. J. Exp. Med. 1979;149:172–184. doi: 10.1084/jem.149.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barnwell J.W., et al. In vitro evaluation of the role of the Duffy blood group in erythrocyte invasion by Plasmodium vivax. J. Exp. Med. 1989;169:1795–1802. doi: 10.1084/jem.169.5.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kwiatkowski D.P. How malaria has affected the human genome and what human genetics can teach us about malaria. Am. J. Hum. Genet. 2005;77:171–192. doi: 10.1086/432519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grigoryan L., Pulendran B. The immunology of SARS-CoV-2 infections and vaccines. Semin. Immunol. 2020;50 doi: 10.1016/j.smim.2020.101422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Braster R., et al. Afucosylated IgG targets FcγRIV for enhanced tumor therapy in mice. Cancers (Basel) 2021;13:2372. doi: 10.3390/cancers13102372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Haan N., et al. The N-glycosylation of mouse immunoglobulin G (IgG)-fragment crystallizable differs between IgG subclasses and Strains. Front. Immunol. 2017;8:31. doi: 10.3389/fimmu.2017.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Krištić J., et al. Profiling and genetic control of the murine immunoglobulin G glycome. Nat. Chem. Biol. 2018;14:516–524. doi: 10.1038/s41589-018-0034-3. [DOI] [PubMed] [Google Scholar]
- 94.Sonneveld M.E., et al. The elements steering pathogenesis in IgG-mediated alloimmune diseases. J. Clin. Immunol. 2016;36:76–81. doi: 10.1007/s10875-016-0253-x. [DOI] [PubMed] [Google Scholar]
- 95.Kwak K., et al. B cell signaling in context. Nat. Immunol. 2019;20:963–969. doi: 10.1038/s41590-019-0427-9. [DOI] [PubMed] [Google Scholar]
- 96.Wang J., et al. Fc-glycosylation of IgG1 is modulated by B-cell stimuli. Mol. Cell. Proteomics. 2011;10:04655. doi: 10.1074/mcp.M110.004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cao Y., et al. Cytokines in the immune microenvironment change the glycosylation of IgG by regulating intracellular glycosyltransferases. Front. Immunol. 2022;12:6022. doi: 10.3389/fimmu.2021.724379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Paulson J.C., et al. Siglecs as sensors of self in innate and adaptive immune responses. Ann. N. Y. Acad. Sci. 2012;1253:37–48. doi: 10.1111/j.1749-6632.2011.06362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brooke G., et al. Human lymphocytes interact directly with CD47 through a novel member of the signal regulatory protein (SIRP) family. J. Immunol. 2004;173:2562–2570. doi: 10.4049/jimmunol.173.4.2562. [DOI] [PubMed] [Google Scholar]
- 100.Rubin S.J.S., et al. B cell checkpoints in autoimmune rheumatic diseases. Nat. Rev. Rheumatol. 2019;15:303–315. doi: 10.1038/s41584-019-0211-0. [DOI] [PubMed] [Google Scholar]