Abstract
Background:
Increasing evidence demonstrates the benefits of palliative care among individuals with Parkinson’s disease and related disorders (PDRD), but the critical components that contribute to therapeutic effects are not well understood.
Aim:
To determine the specific items most responsive to a palliative care intervention in PDRD and identify key correlates of improvement in patient and care partner outcomes.
Design:
The main trial was a pragmatic comparative effectiveness trial of outpatient integrated palliative care compared to standard care among participants with PDRD (NCT02533921), showing significantly higher patient QOL at 6 months and lower care partner burden at 12 months. We used longitudinal regression models to analyze changes in subdomains of patient QOL and care partner burden and Spearman correlations to evaluate key correlates of change scores in patient and care partner outcomes.
Setting/Participants:
We performed a secondary analysis of data from 210 patients and 175 care partners.
Results:
Compared to controls, patients in the intervention reported greater improvement in perceptions of the “self as a whole” at 6 months (coeff=0.22, p<0.05) and care partners reported greater reduction in stress, anger, and loss of control at 12 months (coeff =−.40, −0.25, −0.31, p<0.05). Positive change in numerous patient non-motor symptoms and grief correlated with improved patient QOL, reduced patient anxiety, and increased care partner spirituality. Alleviation of care partner anxiety and depression correlated with reduced care partner burden.
Conclusions:
Specific benefits of an integrated palliative approach in PDRD include improvement in patient holistic self-impressions, care partner self-efficacy, and non-motor symptoms.
Keywords: Parkinson’s disease, palliative care, clinical trial
Introduction
There are a growing number of clinical trials investigating palliative care interventions, many of which show positive results for patient-reported outcomes(1–6) and decreased health care utilization(7). The potential mechanisms by which a palliative care approach could provide benefit over current standards include thorough symptom management, advance care planning, care partner support, spiritual care, and psychosocial support.(8–15) However, the primary components of palliative care that drive its benefits are poorly understood.
There is increasing recognition of the unmet and potentially unique outpatient palliative care needs of people with diagnoses other than cancer.(16, 17) Among serious illnesses, neurodegenerative conditions pose particular challenges for both patients and care partners due to factors related to physical symptoms, cognitive impairment, neuropsychiatric disturbances, and care partner burden.(18–20) Individuals with Parkinson’s disease report symptom burden that is equivalent to those with cancer and express many potential palliative needs(10), but these are often unmet under current models of care(12). At least two models of integrated palliative care for Parkinson’s disease and related disorders (PDRD) have been shown to improve patient and care partner outcomes,(3, 4) but the critical components and key drivers of improvement are not well understood.
We present here a secondary analysis of data from a randomized comparative effectiveness trial of a palliative care intervention in PDRD which reported significantly higher patient quality of life (QOL) at 6 months and lower care partner burden at 12 months. Our aim was to determine the outcomes that were most responsive to our outpatient palliative care intervention and identify key mechanisms of improvement in QOL for patients and care partners. To do so, we 1) evaluated which items on QOL, care partner burden, and symptom burden scales had the most significant change with the intervention, 2) examined correlates of global impression of change from patient and care partner perspectives, care partner burden, and patient QOL and 3) evaluated the impact of advance directive completion on patient and care partner outcomes. We hypothesized that a detailed exploration of the impact of the intervention could increase our understanding of the key therapeutic components.
Methods
Study Design
The trial was a nonblinded, randomized, pragmatic comparative effectiveness trial of outpatient integrated palliative care compared to standard care. Full details of the study design are published elsewhere.(21) Enrolled participants were randomized using a 1:1 ratio and stratified by site, presence of a care partner, and the presence of dementia. If randomized to standard care, the patient continued to receive care from their primary care physician and primary neurologist. The intervention arm consisted of standard care plus an outpatient palliative care interdisciplinary team consisting of an interdisciplinary team including a neurologist, nurse, social worker, chaplain, and board-certified palliative medicine physician. Visits were standardized using checklists for each palliative care team member and conducted every 3 months. Patient and care partner outcomes were assessed every 3 months for a total of 12 months. An external advisory council to the parent clinical trial with patient and care partner representatives reviewed study protocols, assisted with recruitment, and contributed to the interpretation of results.
Standard Protocols, Registrations, and Participant Consents
The study protocol was approved by the institutional review boards of all 3 sites and posted on ClinicalTrials.gov (NCT02533921). Written or verbal consent was obtained from all care partners and patients (or legally authorized representatives if they lacked capacity).
Participants
Between November 1, 2015 and September 30, 2017, we enrolled patients with Parkinson’s Disease and Related Disorders (PDRD) and their care partners (when available) from three academic medical centers (University of Alberta in Edmonton, Alberta, Canada; University of Colorado, Aurora, CO, USA; and University of California, San Francisco, CA, USA). Participants were referred from community neurologist, regional support organizations, clinical trial websites, and academic medical centers. Eligibility criteria included English language fluency, a probable diagnosis of PDRD (Parkinson’s disease, multiple system atrophy, corticobasal degeneration, Lewy body dementia, or progressive supranuclear palsy), and the presence of moderate to high palliative care needs based on the Palliative Care Needs Assessment Tool modified for PD (NAT-PD).(22) Exclusion criteria included urgent palliative care needs, inability to commit to study protocols, comorbid illnesses requiring palliative care, or baseline utilization of palliative care. Care partners were identified by patients as the person who helps the most with managing PDRD outside of the clinic. For patients with dementia, care partners self-identified at the time of screening.
Measures
We assessed patient QOL using the 13-item Quality of Life in Alzheimer’s Disease (QoL-AD) scale.(23) This tool asks patients to rate items from poor to excellent to yield a total score ranging from 13 to 52, where 52 represents the best QOL. The QoL-AD encompasses many domains that are relevant to patient with PDRD(12), including feelings about physical health, energy, mood, activities, functional ability, relationships, and their self as a whole. This tool is validated(24) and responsive to change(25) in patients with dementia. Care partner burden was ascertained with the Zarit Burden Interview,(26) which has been validated(27) and frequently used(28, 29) in PD. The range of this 12-item assessment is 0 to 48, with scores over 20 indicating high care partner burden. Participants and care partners also rated their clinical global impression of change on a scale ranging from −3 (worse) to 3 (improved), with 0 representing no change.
The Edmonton Symptom Assessment Scale for PD (ESAS-PD) is an extended version of the original ESAS with additional items to assess underrecognized non-motor symptoms in PD.(10, 30) Previous studies found that the scale captures changes in symptoms following treatment.(10) The ESAS-PD asks patients to rate fourteen symptoms on a 1–10 Likert scale: pain, tiredness, nausea, depression, anxiety, drowsiness, anorexia, well-being, shortness of breath, stiffness, constipation, dysphagia, confusion, and other. We added similarly structured questions about apathy and hallucinations given their relevance to patients with PDRD, calculating both the previously validated and an extended summary score. We designated an item score of 4 as a cut-off point between mild and moderate to severe symptoms.
Spirituality was assessed by the three factor model of the Functional Assessment of Chronic Illness Therapy-Spiritual Well-Being (FACIT-Sp-12)(31), which is well validated in elderly populations.(32) Participants and care partners rated feelings of grief on the Prolonged Grief inventory (PG-12), which is rated on a scale from 0–44 (0 indicates minimal symptoms and 44 indicates maximal symptoms).(33) Patient and care partner mood was evaluated with the 14-item Hospital Anxiety and Depression scale (score range for each subscale 0–21, with 21 indicating the highest levels of anxiety or depression).(34)
Statistical Analysis
Descriptive statistics were calculated for all variables using counts and percentages for categorical data, means and standard deviations for continuous variables. To evaluate baseline differences, we used t-tests for continuous variables and chi squared tests for categorical variables. We used longitudinal regression models to analyze changes and treatment differences at 6 months for patient QOL and 12 months for care partner burden, as these were the time points with largest effect sizes in the initial trial.(21) Changes in the ESAS-PD were evaluated at 6 and 12 months as both time points had significant effect sizes in the initial trial. In the intervention arm, we used Spearman correlations (the non-parametric alternative to Pearson’s correlation coefficients, reported as ρ) to evaluate relationships between individual symptoms, spirituality, mood, grief, and advance directive completion and change scores in patient QOL, caregiver burden, and global impressions of change. Given our aims were to explore potential factors predictive of benefit and to describe patterns of results, we did not control for multiple comparisons. SAS 9.4 was used for all statistical analyses.
Results
Clinical and Demographic Features of Study Participants
Participant characteristics are summarized in Table 1.
Table 1:
Baseline characteristics of study participants by treatment group.
| Variable | Usual Care (Patient N = 104 Care partner N = 88) | Usual Care Plus Palliative Care (Patient N=106, Care partner N = 87) | p value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Patient Age (years) | 70.7 | 8.0 | 69.5 | 8.3 | 0.293 |
| Disease Duration (months) | 114.3 | 79.2 | 116.5 | 83.7 | 0.851 |
| Care partner Age (years) | 66.4 | 11.1 | 65.7 | 11.7 | 0.697 |
| Care partner Duration (Months) | 66.3 | 50.5 | 70.7 | 73.2 | 0.655 |
| MOCA Score (Baseline) | 23.7 | 5.1 | 24.0 | 4.8 | 0.667 |
| Palliative Performance Scale (Baseline) | 66.2 | 12.1 | 65.5 | 13.7 | 0.679 |
| N | % | N | % | ||
| Site: | 0.967 | ||||
| UCD | 37 | 35.6 | 36 | 34.0 | |
| UCSF | 34 | 32.7 | 36 | 34.0 | |
| UAlberta | 33 | 31.7 | 34 | 32.1 | |
| Patient Sex (Male) | 70 | 67.3 | 65 | 61.3 | 0.365 |
| Hoehn and Yahr ≥ 3 (Baseline) | 36 | 36.0 | 53 | 51.0 | 0.031 |
| Dementia Present | 30 | 28.9 | 30 | 28.3 | 0.930 |
| Non-standard PD: | 12 | 11.5 | 13 | 12.3 | 0.871 |
| Multiple Systems Atrophy (MSA) | 3 | 2.9 | 4 | 3.8 | 1.000 |
| Corticobasal Degeneration (CBD) | 0 | 0.0 | 1 | 0.9 | 1.000 |
| Progressive Supranuclear Palsy (PSP) | 7 | 6.7 | 6 | 5.7 | 0.748 |
| Lewy Body Dementia (LBD) | 2 | 1.9 | 3 | 2.8 | 1.00 |
| Care partner Present | 88 | 84.62 | 87 | 82.1 | 0.622 |
| Care partner Shares Household | 82 | 93.2 | 77 | 88.5 | 0.283 |
| Patient Race: | |||||
| Native American | 1 | 1.0 | 0 | 0.0 | 0.495 |
| Asian | 4 | 3.9 | 2 | 1.9 | 0.443 |
| African American | 2 | 1.9 | 1 | 0.9 | 0.620 |
| Pacific Islander | 0 | 0.0 | 0 | 0.0 | NA |
| Caucasian | 93 | 89.4 | 100 | 94.3 | 0.192 |
| Other | 1 | 1.0 | 3 | 2.8 | 0.622 |
| Mixed | 2 | 1.9 | 0 | 0.0 | 0.244 |
| No Response | 1 | 1.0 | 0 | 0.0 | 0.495 |
| Patient Ethnicity: Hispanic | 3 | 2.9 | 3 | 2.8 | 1.000 |
Change in Subdomains of Patient QOL and Care Partner Burden
At six months, patients in the intervention arm were more likely to show improvements in their feeling about their self as a whole, a subdomain of the patient QOL-AD scale, compared to standard of care (Table 2). At 12 months, care partner feelings of stress, anger, and loss of control as measured by the ZBI were more likely to improve in the intervention arm compared to standard of care (Table 3).
Table 2:
Results of longitudinal regression model of change for individual items comprising the QOL-AD in the intervention arm compared to standard of care at 6 months, in order from largest to smallest coefficient.
| Patient QOL Individual Items | Change in Item Score | |
|---|---|---|
| Coefficient (95% CI) | p value | |
| Summary | 1.50 (0.17, 2.83) | 0.0272 |
| Self as a Whole | 0.22 (0.02, 0.42) | 0.0313 |
| Energy | 0.18 (−0.01, 0.36) | 0.0575 |
| Memory | 0.16 (−0.03, 0.35) | 0.1105 |
| Family | 0.15 (−0.05, 0.35) | 0.1297 |
| Life as a Whole | 0.15 (−0.03, 0.33) | 0.1040 |
| Mood | 0.13 (−0.08, 0.33) | 0.2236 |
| Ability to do Chores | 0.13 (−0.09, 0.34) | 0.2616 |
| Living Situation | 0.12 (−0.07, 0.31) | 0.2248 |
| Money | 0.11 (−0.08, 0.30) | 0.2421 |
| Marriage/Relationships | 0.11 (−0.09, 0.30) | 0.2780 |
| Ability to Do Things for Fun | 0.10 (−0.11, 0.31) | 0.3314 |
| Friends | −0.01 (−0.19, 0.18) | 0.9552 |
| Physical health | −0.01 (−0.21, 0.19) | 0.9202 |
Table 3:
Results of longitudinal regression model of change for individual items on the Zarit Burden Interview (ZBI) in the intervention arm compared to standard of care at 12 months, in order from largest to smallest coefficient.
| ZBI Item | Change in Item Score | |
|---|---|---|
| Coefficient (95% CI) | p value | |
| Overall burden | −2.27 (−4.11, −0.44) | 0.0155 |
| Stressed | −0.40 (−0.67, −0.14) | 0.0026 |
| Lost control | −0.31 (−0.59, −0.04) | 0.0244 |
| Should be doing more | −0.26 (−0.53, 0.01) | 0.0578 |
| Angry | −0.25 (−0.46, −0.04) | 0.0215 |
| Time for yourself | −0.25 (−0.50, 0.00) | 0.0510 |
| Uncertainty | −0.23 (−0.49, 0.03) | 0.0845 |
| Effect on relationships | 0.19 (−0.05, 0.43) | 0.1184 |
| Social life effects | −0.17 (−0.45, 0.10) | 0.2161 |
| Personal health | −0.15 (−0.41, 0.12) | 0.2681 |
| Strained | 0.05 (−0.20, 0.29) | 0.7202 |
| Should be doing better | −0.03 (−0.21, 0.29) | 0.7670 |
| Not enough privacy | −0.02 (−0.28, 0.24) | 0.8859 |
Correlates of Change in Patient QOL
In the intervention group, improvement in anxiety, well-being, shortness of breath, stiffness, confusion, hallucinations, “other,” and total symptom burden were significantly correlated with improved patient QOL at 6 months (Table 4). Increases in patient spiritual well-being, decreased patient anxiety, decreased patient depression, and decreased grief over the course of the study also correlated significantly with increases in patient QOL.
Table 4:
Correlations between changes in patient and care partner items (ESAS-PD, patient FACIT-Sp-12, care partner FACIT-Sp-12, patient HADS, care partner HADS, and patient PG-12) and overall scores for patient QOL (QOL-AD) at 6 months, caregiver burden (ZBI) at 12 months, patient global impression of improvement at 12 months, and care partner global impression of change at 12 months.
| Item Change Scores | Patient QOL (QOL-AD) Change Score (6 months) |
Caregiver Burden (ZBI) Change Score (12 months) |
Patient Global impression of improvement Change Score (12 months) |
Care partner Global impression of improvement Change Score (12 months) |
|---|---|---|---|---|
| ρ | ρ | ρ | ρ | |
| Pain | 0.02 | 0.04 | −0.18 | −0.19 |
| Tiredness | −0.16 | 0.05 | −0.14 | −0.05 |
| Nausea | −0.15 | 0.13 | −0.02 | −0.06 |
| Depression | −0.18 | 0.11 | −0.19 | −0.03 |
| Anxiety | −0.24 | 0.25 | −0.20 | −0.20 |
| Drowsiness | −0.18 | 0.12 | −0.13 | 0.00 |
| Appetite | −0.03 | 0.13 | −0.18 | −0.16 |
| Wellbeing | −0.43 | 0.02 | −0.34 | −0.26 |
| Shortness of Breath | −0.24 | 0.04 | −0.06 | 0.14 |
| Other | −0.41 | 0.28 | −0.39 | |
| Stiffness | −0.27 | 0.10 | −0.16 | −0.05 |
| Constipation | −0.12 | 0.15 | 0.08 | 0.06 |
| Dysphagia | −0.09 | 0.08 | −0.07 | 0.04 |
| Confusion | −0.27 | 0.10 | −0.12 | −0.19 |
| Amotivation | −0.10 | 0.16 | −0.27 | −0.24 |
| Hallucinations | −0.23 | 0.09 | −0.04 | 0.06 |
| ESAS Total (12 point) | −0.39 | 0.22 | −0.30 | −0.22 |
| ESAS-Total (14 point) | −0.39 | 0.22 | −0.29 | −0.21 |
| Patient FACIT-Sp | 0.40 | −0.07 | 0.26 | 0.16 |
| Care partner FACIT-Sp | −0.10 | −0.27 | 0.12 | 0.13 |
| Patient HADS-Anxiety | −0.40 | 0.22 | −0.39 | −0.34 |
| Patient HADS-depression | −0.56 | 0.00 | −0.36 | −0.33 |
| Care partner HADS-Anxiety | −0.12 | 0.43 | −0.02 | 0.00 |
| Care partner HADS-Depression | 0.05 | 0.41 | −0.12 | −0.19 |
| PG-12 | −0.46 | 0.11 | −0.40 | −0.23 |
| Completion of advance directives | 0.06 | 0.13 | −0.14 | −0.13 |
ρ =Spearman correlation coefficients
Light gradation=p<0.05
Dark gradation=p<0.0001
Correlates of Change in Care Partner Burden
In the intervention group, decreases in levels of patient anxiety significantly correlated with decreased care partner burden (Table 4). Decreases in patient and care partner anxiety and depression also correlated with decreased care partner burden over the course of the study. Increased care partner spirituality over the course of the study correlated with decreased care partner burden.
Change in Individual Non-motor Symptoms
At 6 months, patients experienced significant improvement in symptoms of drowsiness, constipation, confusion, and apathy in the intervention arm compared to standard care (insert Figure 1A). At 12 months, there was significant improvement in tiredness, nausea, drowsiness, well-being, shortness of breath, constipation, and apathy in the intervention arm compared to standard of care (insert Figure 1B).
Figure 1:
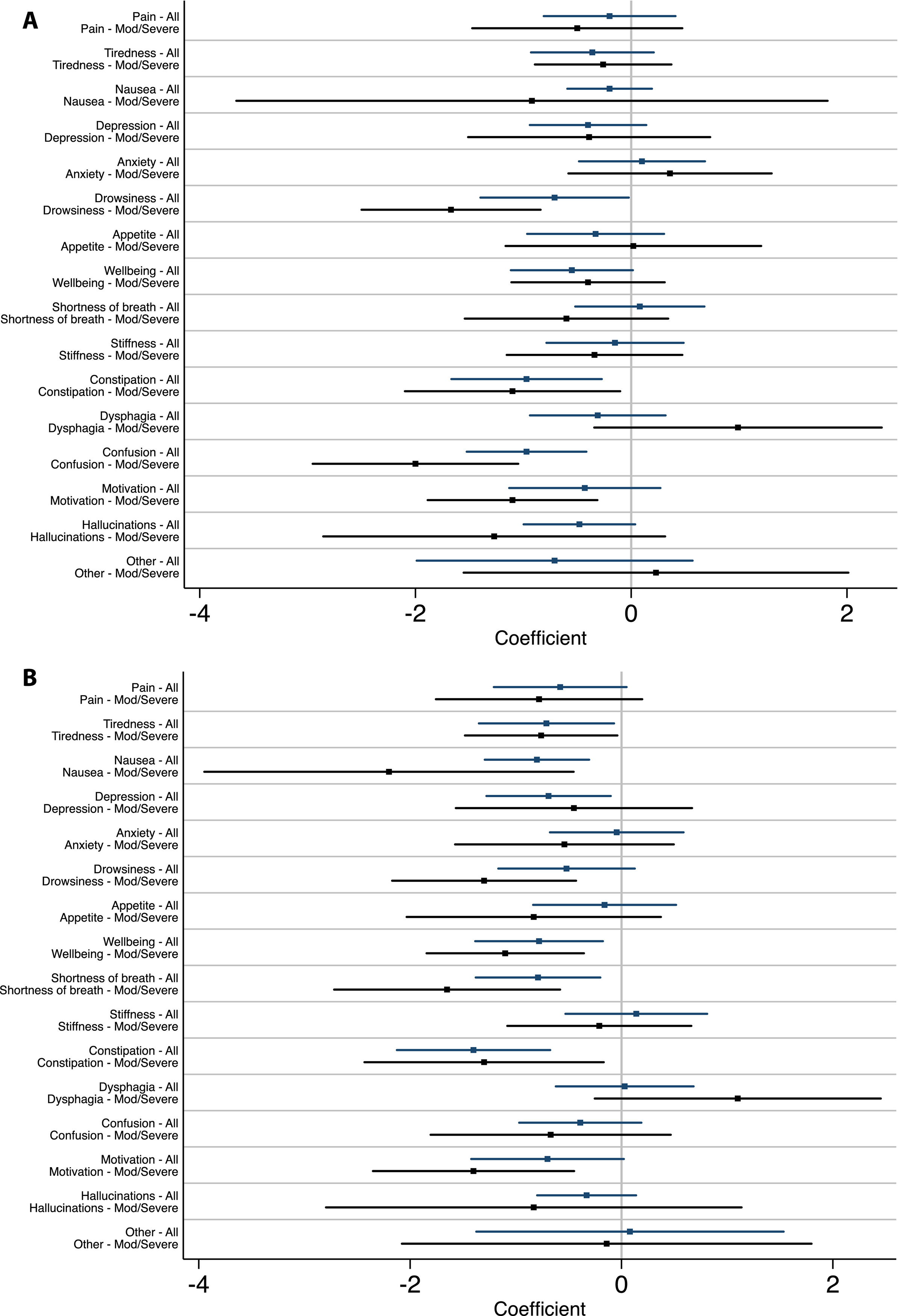
Results of longitudinal regression model of change in individual symptoms as measured by the ESAS-PD in the intervention arm compared to standard of care at 6 months (A) and 12 months (B).
Correlates of Patient and Care partner Global Impressions of Change
In the intervention group, correlates of Patient Global Impression of Improvement included improved well-being, total symptom burden, patient anxiety, and patient depression. Care partner global impressions of change were similarly correlated with overall well-being, as well as patient anxiety and depression. Table 4 summarizes these results.
Correlates of Completion of Advance directives
Completion of advance directives was correlated with a small improvement in care partner burden at 12 months in the overall cohort (ρ=0.32, p=0.02) but this was no longer significant when restricted to the intervention group (Table 4).
Discussion
In the parent randomized controlled trial, participants in the palliative care intervention arm had significantly higher patient QOL at 6 months, lower patient symptom burden at 6 and 12 months, and significantly decreased care partner burden at 12 months.(21) We present here a secondary analysis that further investigates the individual components and drivers of this improvement. At an item level, we found that patients’ perception of “self as a whole,” and care partner stress, anger, and feelings of loss of control were significantly improved in the intervention arm compared to standard of care. At 12 months, individual patient symptoms of tiredness, nausea, drowsiness, well-being, shortness of breath, constipation, and apathy were significantly improved in the intervention group compared to standard of care. Improvement in a sense of well-being correlated with three out of the four outcome measures (patient QOL, patient global impressions of improvement, and care partner global impressions of improvement) in the intervention arm, indicating that this may be an important driver of change. Mood symptoms, spiritual well-being, and grief additionally emerged as important correlates of improvement in both patient- and care partner-related global outcomes. Completion of advance directives had a small but significant correlation with improved care partner burden over 12 months in the overall cohort, but this was no longer significant when restricted to the intervention arm.
Of the QOL domains, patients’ feeling about their “self as a whole” was the most responsive to the intervention compared to standard of care. This item was added to the original QOL-AD measure to ascertain the patient’s global self-impression(35) and this subdomain typically correlates with the overall QOL-AD score(23). We suspect this improvement was related to our interdisciplinary and holistic approach to the patient as a person,(36) as well our observations that eroded self-worth and identity is an underrecognized source of distress for patients with PDRD. An improved self-impression may also be related to alleviation of grief noted in the intervention arm compared to standard of care. The finding that several individual components of symptom burden correlated with improved QOL suggests that improved management of medical symptoms is one means by which palliative care improves patient-centered outcomes. This finding is supported by another study showing symptom response as measured by the ESAS-PD in a longitudinal cohort of patients with PD receiving palliative care.(10) Our result that improved patient and care partner mood symptoms, grief, and spirituality are associated with higher patient QOL further highlights the likely multimodal impact of the palliative care intervention. Our study builds on prior cross-sectional(37–39) and longitudinal(40) investigations demonstrating a relationship between mood symptoms and patient QOL in PDRD, further indicating that alleviation of mood symptoms may be a key benefit of the palliative intervention. Patient and caregiver impressions of change correlated with several non-motor and mood symptoms. This may be a useful additional outcome measure to consider in future trials as patient QOL is not always responsive to change in palliative care interventions for non-cancer illness.(16)
We found that care partners experienced substantial improvement across a variety of factors relevant to caregiving ability at 12 months in the intervention group and these improvements correlate with patient and care partner mood symptoms, which confirms previous investigations in non-randomized samples and demonstrates that this relationship holds up over time.(41) Our result that improvement in patient anxiety correlates with improved care partner burden highlights treatable non-motor symptoms as an area of focus relevant for both patient and care partner well-being. In other investigations, depression has been shown to improve with palliative care interventions in patients with cancer despite no difference in new antidepressant prescriptions.(1) Patient symptom burden and mood symptoms are likely significant contributors to care partner burden, in addition to level of disability, care partner affective state, and social support.(41–43) Our observation of improvement in care partner perceptions on the quality of their caregiving in the intervention arm indicates that palliative models addressing care partner perceptions of self-efficacy may serve as one means for addressing their burden. The finding that patient advanced care planning correlates with a small but significant decrease in care partner burden in the overall sample also confirms prior qualitative reports that advanced care planning improves the care partner experience in PDRD.(44) However, this benefit was not clearly a correlate of change in caregiver burden in the intervention arm, either due to decreased power or because advanced care planning was not a main driver of improvement in caregiver burden in the intervention. Overall, these findings support that palliative care models improve outcomes for care partners both directly and indirectly by assisting patients and advanced care planning in any context is likely to confer caregiver benefit.
Our finding that many individual symptoms were responsive to the intervention and that the magnitude of response for most symptoms increased with time highlights the efficacy of this palliative intervention in symptom management despite the progressive nature of PDRD. This result adds to the only other published clinical trial evaluating a palliative care intervention in PDRD and other neurologic conditions, which found improvements in levels of pain, dyspnea, sleep symptoms, and bowel symptoms.(3) Whereas constipation and nausea have several efficacious treatments in PDRD, there are fewer evidence-based treatments available for drowsiness and apathy.(8) However, these symptoms improved significantly with the intervention and suggest aspects of the palliative care approach can influence symptoms considered refractory to pharmacologic intervention, perhaps through individualized medical management and psychosocial support around coping. Palliative care is one method to address the often unmet needs surrounding non-motor symptoms in PDRD.(45)
This study also highlights the impact of palliative care on spirituality and feelings of grief in patients, which in turn is a key correlate of improvement in patient-reported outcomes in this trial. Grief is a commonly reported feeling surrounding the diagnosis of Parkinson’s disease(12) and this is the first study to our knowledge that shows how addressing grief correlates with improvement in patient QOL and patient global impressions of change over time. We also highlight the importance of patient spirituality in driving improvement in QOL and patient impression of change as well as care partner spirituality in decreasing care partner burden. Spirituality has long been considered an essential component of the interdisciplinary palliative care approach by incorporating spiritual counselors such as chaplains into the interdisciplinary team(46) and our study elucidates its specific effects. The role of spirituality in palliative care encompasses multiple components, including providing another source of social support as well as tools for coping with disease and managing grief.(12, 47) Addressing spiritual distress is an important component of caring for individuals with PDRD, but this is not incorporated into neurology training and remains a largely unmet need.
Strengths of this study include randomized controlled design, broad inclusion criteria, and breadth of patient and care partner measures. The interpretation of our findings is limited by its exploratory nature, including testing for multiple hypotheses, item analyses, and correlations. We may underestimate the effect of the intervention as patients with urgent palliative needs were excluded from the trial. It remains unclear if these findings are generalizable to patients outside of academic settings.
These results highlight the multimodal effects of an interdisciplinary approach to palliative care for people with PDRD and their care partners. Key aspects of the intervention include a focus on patient symptom burden, mood, grief, and spirituality, which were associated with improvements in both patient and care partner outcomes. Palliative care appears to help care partners both directly and indirectly via assisting the patient, with an emphasis on care partner self-efficacy and a greater focus on mood symptoms emerging as specific strategies for alleviation of burden. The possible drivers of improvement identified by this analysis align with the areas of focus of palliative care and support extension of these approaches to more patients and care partners affected by PDRD. Further understanding the specific effects of palliative care that lead to positive change is a critical step in designing future interventions and educational programs for neurologists, primary care physicians, and others that care for individuals with PDRD to achieve similar benefits.
Key Message:
We performed a secondary analysis of a randomized controlled trial of palliative care for PDRD. Compared to standard of care, there was improvement in patient perceptions of their “self as a whole” and care partner stress, anger and feelings of loss of control at 12 months.
Disclosures and Acknowledgments
We would like to acknowledge the patients and care partners who participated in this trial. This work would not have been possible without the funders and participation of clinicians at all three enrolling sites.
Study funding:
NIH/NINR R01NR016037, NIH/NIA T32AG044296
Funding:
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by grant IHS-1408–20134 from the Patient-Centered Outcomes Research Institute, NIH/NIA T32AG044296. The funding sources was not involves in study design, collection of data, writing the report, or decision to submit the article for publication.
Footnotes
Conflicts of interest: The authors declare that there is no conflict of interest. All authors have signed the conflict of interest form.
Statistical Analysis:
Statistical analysis was primarily conducted by Stefan Sillau (associated with the Department of Neurology at University of Colorado, Denver).
Disclaimer: All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors, or Methodology Committee.
Research Ethics and Patient Consents: The study protocol was approved by the institutional review boards of all 3 medical centers and posted on ClinicalTrials.gov (NCT02533921).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Citations
- 1.Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–42. [DOI] [PubMed] [Google Scholar]
- 2.Follwell M, Burman D, Le LW, Wakimoto K, Seccareccia D, Bryson J, et al. Phase II study of an outpatient palliative care intervention in patients with metastatic cancer. J Clin Oncol. 2009;27(2):206–13. [DOI] [PubMed] [Google Scholar]
- 3.Veronese S, Gallo G, Valle A, Cugno C, Chiò A, Calvo A, et al. Specialist palliative care improves the quality of life in advanced neurodegenerative disorders: NE-PAL, a pilot randomised controlled study. BMJ Support Palliat Care. 2017;7(2):164–72. [DOI] [PubMed] [Google Scholar]
- 4.Kluger BM, Miyasaki J, Katz M, Galifianakis N, Hall K, Pantilat S, et al. Comparison of Integrated Outpatient Palliative Care With Standard Care in Patients With Parkinson Disease and Related Disorders: A Randomized Clinical Trial. JAMA Neurol. 2020;77(5):551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanks GW, Robbins M, Sharp D, Forbes K, Done K, Peters TJ, et al. The imPaCT study: a randomised controlled trial to evaluate a hospital palliative care team. Br J Cancer. 2002;87(7):733–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grudzen CR, Richardson LD, Johnson PN, Hu M, Wang B, Ortiz JM, et al. Emergency Department-Initiated Palliative Care in Advanced Cancer: A Randomized Clinical Trial. JAMA Oncol. 2016;2(5):591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lustbader D, Mudra M, Romano C, Lukoski E, Chang A, Mittelberger J, et al. The Impact of a Home-Based Palliative Care Program in an Accountable Care Organization. J Palliat Med. 2017;20(1):23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernal-Pacheco O, Limotai N, Go CL, Fernandez HH. Nonmotor manifestations in Parkinson disease. Neurologist. 2012;18(1):1–16. [DOI] [PubMed] [Google Scholar]
- 9.Shulman LM, Taback RL, Rabinstein AA, Weiner WJ. Non-recognition of depression and other non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord. 2002;8(3):193–7. [DOI] [PubMed] [Google Scholar]
- 10.Miyasaki JM, Long J, Mancini D, Moro E, Fox SH, Lang AE, et al. Palliative care for advanced Parkinson disease: an interdisciplinary clinic and new scale, the ESAS-PD. Parkinsonism Relat Disord. 2012;18 Suppl 3:S6–9. [DOI] [PubMed] [Google Scholar]
- 11.Kluger BM, Shattuck J, Berk J, Sebring K, Jones W, Brunetti F, et al. Defining Palliative Care Needs in Parkinson’s Disease. Mov Disord Clin Pract. 2019;6(2):125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boersma I, Jones J, Carter J, Bekelman D, Miyasaki J, Kutner J, et al. Parkinson disease patients’ perspectives on palliative care needs: What are they telling us? Neurol Clin Pract. 2016;6(3):209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boersma I, Jones J, Coughlan C, Carter J, Bekelman D, Miyasaki J, et al. Palliative Care and Parkinson’s Disease: Caregiver Perspectives. J Palliat Med. 2017;20(9):930–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goy ER, Carter JH, Ganzini L. Needs and experiences of caregivers for family members dying with Parkinson disease. J Palliat Care. 2008;24(2):69–75. [PubMed] [Google Scholar]
- 15.Lanoix M Palliative care and Parkinson’s disease: managing the chronic-palliative interface. Chronic Illn. 2009;5(1):46–55. [DOI] [PubMed] [Google Scholar]
- 16.Quinn KL, Shurrab M, Gitau K, Kavalieratos D, Isenberg SR, Stall NM, et al. Association of Receipt of Palliative Care Interventions With Health Care Use, Quality of Life, and Symptom Burden Among Adults With Chronic Noncancer Illness: A Systematic Review and Meta-analysis. Jama. 2020;324(14):1439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocker G, Downar J, Morrison RS. Palliative care for chronic illness: driving change. Cmaj. 2016;188(17–18):E493–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudlicka A, Clare L, Hindle JV. Quality of life, health status and caregiver burden in Parkinson’s disease: relationship to executive functioning. Int J Geriatr Psych. 2014;29(1):68–76. [DOI] [PubMed] [Google Scholar]
- 19.Martínez-Martín P, Forjaz MJ, Frades-Payo B, Rusinol AB, Fernández-García JM, Benito-León J, et al. Caregiver burden in Parkinson’s disease. Movement Disord. 2007;22(7):924–31. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Martin P, Rodriguez-Blazquez C, Forjaz MJ, Frades-Payo B, Agüera-Ortiz L, Weintraub D, et al. Neuropsychiatric symptoms and caregiver’s burden in Parkinson’s disease. Parkinsonism Relat D. 2015;21(6):629–34. [DOI] [PubMed] [Google Scholar]
- 21.Kluger BM, Katz M, Galifianakis N, Pantilat SZ, Kutner JS, Sillau S, et al. Does outpatient palliative care improve patient-centered outcomes in Parkinson’s disease: Rationale, design, and implementation of a pragmatic comparative effectiveness trial. Contemp Clin Trials. 2019;79:28–36. [DOI] [PubMed] [Google Scholar]
- 22.Waller A, Girgis A, Currow D, Lecathelinais C. Development of the palliative care needs assessment tool (PC-NAT) for use by multi-disciplinary health professionals. Palliat Med. 2008;22(8):956–64. [DOI] [PubMed] [Google Scholar]
- 23.Thorgrimsen L, Selwood A, Spector A, Royan L, de Madariaga Lopez M, Woods RT, et al. Whose quality of life is it anyway? The validity and reliability of the Quality of Life-Alzheimer’s Disease (QoL-AD) scale. Alzheimer Dis Assoc Disord. 2003;17(4):201–8. [DOI] [PubMed] [Google Scholar]
- 24.Logsdon RG, Gibbons LE, McCurry SM, Teri L. Assessing quality of life in older adults with cognitive impairment. Psychosom Med. 2002;64(3):510–9. [DOI] [PubMed] [Google Scholar]
- 25.Caramelli P, Laks J, Palmini AL, Nitrini R, Chaves ML, Forlenza OV, et al. Effects of galantamine and galantamine combined with nimodipine on cognitive speed and quality of life in mixed dementia: a 24-week, randomized, placebo-controlled exploratory trial (the REMIX study). Arq Neuropsiquiatr. 2014;72(6):411–7. [DOI] [PubMed] [Google Scholar]
- 26.Bédard M, Molloy DW, Squire L, Dubois S, Lever JA, O’Donnell M. The Zarit Burden Interview: a new short version and screening version. Gerontologist. 2001;41(5):652–7. [DOI] [PubMed] [Google Scholar]
- 27.Hagell P, Alvariza A, Westergren A, Årestedt K. Assessment of Burden Among Family Caregivers of People With Parkinson’s Disease Using the Zarit Burden Interview. J Pain Symptom Manage. 2017;53(2):272–8. [DOI] [PubMed] [Google Scholar]
- 28.Bhimani R Understanding the Burden on Caregivers of People with Parkinson’s: A Scoping Review of the Literature. Rehabil Res Pract. 2014;2014:718527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosley PE, Moodie R, Dissanayaka N. Caregiver Burden in Parkinson Disease: A Critical Review of Recent Literature. J Geriatr Psychiatry Neurol. 2017;30(5):235–52. [DOI] [PubMed] [Google Scholar]
- 30.Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7(2):6–9. [PubMed] [Google Scholar]
- 31.Canada AL, Murphy PE, Fitchett G, Peterman AH, Schover LR. A 3-factor model for the FACIT-Sp. Psychooncology. 2008;17(9):908–16. [DOI] [PubMed] [Google Scholar]
- 32.Stéfanie Monod EL, Etienne Rochat, Brenda Spencer, Laurence Seematter-Bagnoud, Anne-Sylvie Martin-Durussel, Christophe Büla. Validity of the FACIT-Sp to Assess Spiritual Well-Being in Elderly Patients. Psychology. 2015;06:1311–22. [Google Scholar]
- 33.Prigerson HGM PK Prolonged Grief Disorder Inventory (PG–12). Unpublished instrument. 2008. [Google Scholar]
- 34.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. [DOI] [PubMed] [Google Scholar]
- 35.Logsdon RG, Gibbons LE, McCurry SM, Teri L. Quality of Life in Alzheimer’s Disease: Patient and Caregiver Reports. Journal of Mental Health and Aging. 1999;5:21–32. [Google Scholar]
- 36.Organization WH. WHO definition of palliative care. : World Health Organization website.; [Available from: https://www.who.int/cancer/palliative/definition/en/. [Google Scholar]
- 37.Su W, Liu H, Jiang Y, Li S, Jin Y, Yan C, et al. Correlation between depression and quality of life in patients with Parkinson’s disease. Clin Neurol Neurosurg. 2021;202:106523. [DOI] [PubMed] [Google Scholar]
- 38.Sławek J, Derejko M, Lass P. Factors affecting the quality of life of patients with idiopathic Parkinson’s disease--a cross-sectional study in an outpatient clinic attendees. Parkinsonism Relat Disord. 2005;11(7):465–8. [DOI] [PubMed] [Google Scholar]
- 39.Trang I, Katz M, Galifianakis N, Fairclough D, Sillau SH, Miyasaki J, et al. Predictors of general and health-related quality of life in Parkinson’s disease and related disorders including caregiver perspectives. Parkinsonism Relat Disord. 2020;77:5–10. [DOI] [PubMed] [Google Scholar]
- 40.Jones JD, Marsiske M, Okun MS, Bowers D. Latent growth-curve analysis reveals that worsening Parkinson’s disease quality of life is driven by depression. Neuropsychology. 2015;29(4):603–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez-Martin P, Arroyo S, Rojo-Abuin JM, Rodriguez-Blazquez C, Frades B, de Pedro Cuesta J. Burden, perceived health status, and mood among caregivers of Parkinson’s disease patients. Mov Disord. 2008;23(12):1673–80. [DOI] [PubMed] [Google Scholar]
- 42.Edwards NE, Scheetz PS. Predictors of burden for caregivers of patients with Parkinson’s disease. J Neurosci Nurs. 2002;34(4):184–90. [DOI] [PubMed] [Google Scholar]
- 43.Schrag A, Hovris A, Morley D, Quinn N, Jahanshahi M. Caregiver-burden in parkinson’s disease is closely associated with psychiatric symptoms, falls, and disability. Parkinsonism Relat Disord. 2006;12(1):35–41. [DOI] [PubMed] [Google Scholar]
- 44.Armstrong MJ, Alliance S, Taylor A, Corsentino P, Galvin JE. End-of-life experiences in dementia with Lewy bodies: Qualitative interviews with former caregivers. PLoS One. 2019;14(5):e0217039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfeiffer RF. Non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord. 2016;22 Suppl 1:S119–22. [DOI] [PubMed] [Google Scholar]
- 46.Richardson P Spirituality, religion and palliative care. Ann Palliat Med. 2014;3(3):150–9. [DOI] [PubMed] [Google Scholar]
- 47.Peterman AH, Fitchett G, Brady MJ, Hernandez L, Cella D. Measuring spiritual well-being in people with cancer: the functional assessment of chronic illness therapy--Spiritual Well-being Scale (FACIT-Sp). Ann Behav Med. 2002;24(1):49–58. [DOI] [PubMed] [Google Scholar]


