Abstract
Objectives
The potential benefit of convalescent plasma (CP) therapy for coronavirus disease 2019 (COVID-19) is highest when administered early after symptom onset. Our objective was to determine the effectiveness of CP therapy in improving the disease course of COVID-19 among high-risk outpatients.
Methods
A multicentre, double-blind randomized trial was conducted comparing 300 mL of CP with non-CP. Patients were ≥50 years, were symptomatic for <8 days, had confirmed RT-PCR or antigen test result for COVID-19 and had at least one risk factor for severe COVID-19. The primary endpoint was the highest score on a 5-point ordinal scale ranging from fully recovered (score = 1) or not (score = 2) on day 7, over hospital admission (score = 3), intensive care unit admission (score = 4) and death (score = 5) in the 28 days following randomization. Secondary endpoints were hospital admission, symptom duration and viral RNA excretion.
Results
After the enrolment of 421 patients and the transfusion in 416 patients, recruitment was discontinued when the countrywide vaccination uptake in those aged >50 years was 80%. Patients had a median age of 60 years, symptoms for 5 days, and 207 of 416 patients received CP therapy. During the 28 day follow-up, 28 patients were hospitalized and two died. The OR for an improved disease severity score with CP was 0.86 (95% credible interval, 0.59–1.22). The OR was 0.58 (95% CI, 0.33–1.02) for patients with ≤5 days of symptoms. The hazard ratio for hospital admission was 0.61 (95% CI, 0.28–1.34). No difference was found in viral RNA excretion or in the duration of symptoms.
Conclusions
In patients with early COVID-19, CP therapy did not improve the 5-point disease severity score.
Keywords: Antibodies, Convalescent plasma, COVID-19, Outpatients, Therapy
Introduction
Older patients and patients with medical comorbidities are at an increased risk of hospitalization or death because of coronavirus disease 2019 (COVID-19). Over the last year, several antiviral therapies in the form of virus-neutralizing monoclonal antibodies or as direct-acting antiviral drugs have been shown to reduce the risk of hospital admission when administered in the first days after disease onset [[1], [2], [3], [4], [5]].
However, escape by new variants of concern (VOC) from neutralization by monoclonal antibodies has been observed for every single new VOC. Most recently, the Omicron BA.1 variant was shown to be completely resistant to casirivimab/imdevimab and much less susceptible to tixagevimab/cilgavimab [6], and although sotrovimab retained most of its activity against BA.1, this is no longer the case against BA.2 [7].
An alternative source of severe acute respiratory syndrome virus 2 (SARS-CoV-2) antibodies can be found in convalescent plasma (CP) from patients recovered from COVID-19 and more recently also from the plasma of fully vaccinated people. CP has the advantage of being polyclonal and is less likely to be completely inactive against a new VOC. Just like monoclonal antibodies, the potential use of CP as a treatment option for COVID-19 lies in its use among high-risk patients early after symptom onset [8]. Herein, we report the results of the CoV-Early study—a randomized clinical trial designed to evaluate the effectiveness of CP in high-risk outpatients with COVID-19 with less than 8 days of symptoms.
Methods
Study design
The CoV-Early study (clinical registry number: NCT04589949) was a phase 3, multicentre, randomized, double-blind, placebo-controlled trial conducted in the Netherlands The study was funded by ZonMw (grant-number 10430062010001) and supported by Sanquin (Dutch blood supply), which provided the plasma. A complete list of participating sites is available in our online protocol. This study was approved by the medical ethical review board of the Erasmus MC (METC-2020-0682).
Randomization and intervention
All patients were randomized 1:1 CP or regular plasma (non-CP) as placebo. Randomization was performed using a web-based system, and only one person from the transfusion laboratory was unblinded. Details regarding the CP and control plasma and the neutralizing antibody assessment are provided in the Supplementary material.
Patients
The inclusion criteria were a nasopharyngeal RT-PCR or a Conformité Européenne (CE)-antigen test that confirmed the SARS-CoV-2 infection, ≤7 days of symptoms at the time of screening (maximum of eight on the day of transfusion) and not admitted to the hospital. In addition, patients needed to be at an increased risk of severe disease, which we defined as one of the following: (a) patients aged ≥70 years, (b) patients aged ≥50 years with an additional comorbidity, and (c) patients aged ≥18 years who were severely immunocompromised (criteria available in the full protocol) [9,10]. Patients were excluded when their life expectancy was <28 days, they were unable to provide informed consent, COVID-19 symptoms were already improving, they had a documented IgA deficiency or they had a previous history of transfusion-related acute lung injury. All study participants provided written informed consent. Details on the recruitment and referral process are available in the Supplementary material.
Procedures and outcomes
After consent, patients received CP or non-CP therapy at one of the study sites and left the hospital 1 hour after the infusion. Patients were contacted to evaluate their illness severity on days 7, 14 and 28. This was assessed using a 5-point ordinal disease severity scale: (a) a score of 1 indicated that the patient had fully recovered within 7 days, (b) a score of 2 indicated continued symptoms on day 7, (c) a score of 3 indicated admitted to hospital but no invasive ventilation, (d) a score of 4 indicated admitted to hospital and invasive ventilation needed, and (e) a score of 5 indicated death. Serum was collected at baseline to determine the antibody status preceding the transfusion.
Nasopharyngeal swab and serum were collected on days 1, 3, 7, 14 and 28 in a subgroup of patients able and willing to attend extra visits at two dedicated study sites. A SARS-CoV-2 RT-PCR test was performed on these swabs to determine the cyclic threshold (Ct) value of the E-gene to evaluate the rate of viral decay for both study arms (Cobas 6800 [Roche]). Antibody titres in binding antibody units/mL were determined on serum using an IgG anti-spike SARS-CoV-2 test (Trimeric Spike antibody test, Liaison, DiaSorin).
The primary outcome was the improvement on the 5-point ordinal disease severity scale. Improvement was determined using the highest score in 28 days. Secondary outcomes were hospital admission and symptom duration. The exploratory endpoints were the treatment modifying effect of age, clinical frailty using the clinical frailty score version 2.0, symptom duration before inclusion and the height of the antibody titres in CP on the disease severity scale [11]. Additional exploratory endpoints were the difference in the probability of a positive PCR in 28 days after inclusion, the difference in Ct value in 28 days and the difference in antibody levels in 28 days between CP and non-CP therapy. We also explored the difference in the length of hospital stay in the two groups. Details of the Bayesian statistical analysis are available in the Supplementary material.
Results
Recruitment
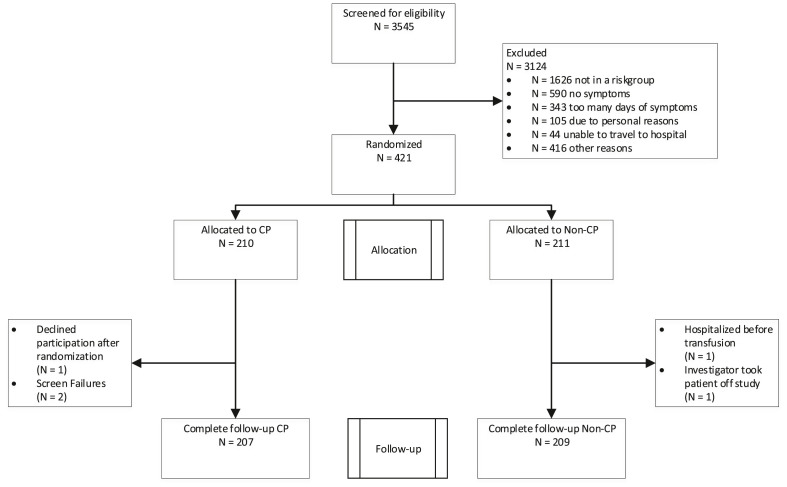
Between November 2020 and July 2021 (when the D614G and alpha B.1.1.7 variant were dominant) 3545 patients with COVID-19 were contacted and screened. The reasons for a patient being excluded from participation are summarized in Fig. 1 . A total of 421 outpatients were randomized, of whom 416 were transfused and included in the intention-to-treat population and none were lost to follow-up; 207 were included in the CP group and 209 in the non-CP group. During study monitoring, we observed that five of the 416 patients had received incorrect treatment (4 received non-CP instead of CP and one received CP instead of non-CP). These patients were included in the intention-to-treat analysis and analysed according to the study arm they had been randomized to. None of these five patients were hospitalized during follow-up. Based on the recommendation by the Data Safety Monitoring Board (DSMB), the study was terminated on 13 July 2021 before the planned sample size of 690 was reached. More details are available in the Supplementary material.
Fig. 1.
Consolidated Standards of Reporting Trials flow diagram. CP, convalescent plasma.
Baseline characteristics
The patients had a median age of 60 years (interquartile range [IQR], 55–65 years), median of 5 days of symptoms before inclusion (IQR, 4–6 days), median of one comorbidity (IQR, 1–2) and median frailty score of 2 (IQR, 1–2) that was measured in 174 patients only. Moreover, 93 (22.4%) patients were female. The median oxygen saturation at baseline (immediately before transfusion) without supplementary oxygen was 97% (IQR, 96–98%). All but 30 participants (7.9%) were SARS-CoV-2 IgG antibody negative at baseline. In addition, 12 (2.9%) patients had been fully vaccinated, and 21 (5.0%) patients had received one vaccination. The baseline characteristics of the patients in the CP and non-CP groups were comparable (Table 1 ). CP had a median neutralization level of 386 IU/mL (IQR, 271–707 IU/mL).
Table 1.
Baseline characteristics
| Characteristic | Total (N = 416) | CP (n = 207) | Non-CP (n = 209) |
|---|---|---|---|
| Male sex, n (%) | 323 (77.6%) | 164 (79.2%) | 159 (76.1%) |
| Age (y), median (IQR) | 60 (55–65) | 59 (55–65) | 60 (54–66) |
| Oxygen saturation, median (IQR)a | 97 (96–98) | 98 (96–98) | 97 (96–98) |
| BMI (kg/m2), median (IQR) | 27.4 (24.6–30.7) | 27.4 (24.5–30.8) | 27.5 (24.8–30.6) |
| No. of comorbidities, median (IQR)b | 1 (1–2) | 1 (1–2) | 1 (1–2) |
| Obesity, n (%) | 46 (11.1%) | 21 (10.2%) | 25 (12.0%) |
| Cardiac and/or pulmonary disease, n (%) | 140 (33.7%) | 72 (34.8%) | 68 (32.5%) |
| Neurological disease, n (%) | 22 (5.3%) | 15 (7.2%) | 7 (3.3%) |
| Diabetes, n (%) | 29 (7.0%) | 8 (3.9%) | 21 (10.0%) |
| Chronic kidney disease, n (%) | 20 (4.8%) | 11 (5.3%) | 9 (4.3%) |
| Rheumatic disease, n (%) | 21 (5.0%) | 11 (5.3%) | 10 (4.8%) |
| Severe immunodeficiency, n (%) | 15 (3.6%) | 5 (2.4%) | 10 (4.8%) |
| Cancer, n (%) | 14 (3.4%) | 7 (3.4%) | 7 (3.3%) |
| Chronic liver disease, n (%) | 3 (0.7%) | 1 (0.5%) | 2 (1.0%) |
| HIV, n (%) | 1 (0.2%) | 0 (0) | 0 (0.5%) |
| Days since first symptoms, median (IQR) | 5 (4–6) | 5 (4–6) | 5 (4–7) |
| Positive antibody status at baseline, n (%)c | 30 (7.9%) | 17 (8.8%) | 13 (7.1%) |
| Fully vaccinated at baseline, n (%) | 12 (2.9%) | 6 (2.9%) | 6 (2.9%) |
| Only one vaccination at baseline, n (%) | 21 (5.0%) | 8 (3.9%) | 13 (6.2%) |
CP, convalescent plasma; IQR, interquartile range.
Baseline oxygen saturation without supplementary oxygen.
Obesity, cardiac disease, lung disease, neurological disease, diabetes, chronic renal failure, cancer and/or liver disease. Please see the Supplementary file for additional details of the comorbidities.
The anti-S, Liaison IgG test (Diasorin) was available for 381 of the 416 patients at baseline.
Primary endpoints
Table 2 shows the distribution of the 5-point disease severity scale. The estimated common OR for the highest disease status in the 28 days after randomization was 0.86 (95% credible interval, 0.59–1.22) for patients treated with CP. Hospital admission was required for 10 (4.8%) patients receiving CP therapy versus 18 (8.6%) patients in the non-CP arm, with an adjusted hazard ratio of 0.61 (95% CI, 0.28–1.34). Death occurred in 1 (0.5%) patient treated with CP and in 1 (0.5%) patient treated with placebo. No difference was found in the median duration of symptoms between patients in the CP and non-CP groups (13 vs. 12 days; p = 0.99) (Fig. S1).
Table 2.
Distribution of the outcome of the patients in the 28 days after inclusion across the 5-point disease severity scale
| Worst disease severity score | Total |
CP |
Non-CP |
|---|---|---|---|
| (n = 416) | (n = 207) | (n = 209) | |
| Recovered, n (%)a | 112 (26.9%) | 59 (28.5%) | 53 (25.4%) |
| Continued symptoms, n (%)b | 274 (65.9%) | 137 (66.2%) | 137 (65.6%) |
| Admitted to hospital but no invasive ventilation, n (%) | 27 (6.5%) | 10 (4.8%) | 17 (8.1%) |
| Admitted to hospital and invasive ventilation, n (%) | 1 (0.2%) | 0 (0%) | 1 (0.5%) |
| Death, n (%) | 2 (0.5%) | 1 (0.5%) | 1 (0.5%) |
CP, convalescent plasma.
Recovered with no symptoms within 7 days after inclusion.
Continued symptoms attributable to COVID-19 at day 7.
Subgroup and laboratory analyses
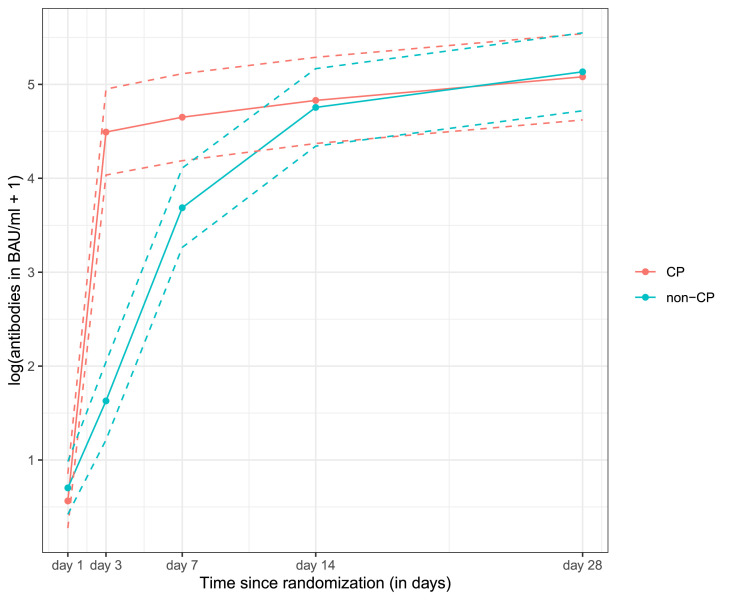
No significant treatment modifying effect of age, clinical frailty, symptom duration or height of antibody titres in CP was found, as all these interaction terms had p of >0.1 (Figs S2, S3) (Table S1). Table S2 shows the distribution of the 5-point disease severity scale in patients in the non-CP group across these subgroups. Moreover, 85 patients were followed with nasopharyngeal SARS-CoV-2 RT-PCR testing over time. No difference in the probability of having SARS-CoV-2 detected by RT-PCR over time was observed between CP and placebo (p = 0.51) (Fig. S4). Similarly, there was no difference in the evolution of Ct values over time between the two groups (p = 0.35) (Fig. S5). The antibody levels of CP rose faster than that of the placebo after transfusion (p < 0.0001) (Fig. 2 ), but this effect was short-lived. Figures S6 and S7 show the individual antibody titres and Ct values. The median duration of hospital stay was 6 days (IQR, 4.5–9 days) in the patients treated with CP and was 4 days (IQR, 3.5–10.5 days) in the control group (p = 0.56, post hoc analysis).
Fig. 2.
Antibody titres over time between patients in the CP and non-CP groups using a mixed effects model. The line represents fitted log (antibodies in BAU/mL + 1), and the dotted line represents 95% CIs. Eighty-five patients had a day 1 measurement, 82 patients had a day 3 measurement, 79 patients had a day 7 measurement, 83 patients had a day 14 measurement and 82 patients had a day 28 measurement. BAU, binding antibody unit; CP, convalescent plasma.
Discussion
In this randomized trial, we evaluated whether 300 mL of CP improved the outcome of COVID-19 in outpatients at risk of severe disease. The outcome was evaluated using a 5-point ordinal disease severity scale, which included speed of recovery, hospital admission, intensive care unit admission and survival during a 28-day follow-up period. Overall, CP therapy did not significantly prevent a more severe disease course. The OR (0.58) was numerically lower in the subgroup of patients with ≤5 days of symptoms. The duration of symptoms and time to become PCR negative was not reduced. Hospital admission was decreased from 8.6% to 4.8%, but the 95% CI of the OR was wide (0.28–1.34).
This is the fifth clinical trial on an early CP therapy for COVID-19. Some of the previous studies recruited different populations, which makes the comparison across studies difficult. Libster et al. observed a beneficial effect of CP but focused on patients aged 75 or older with ≤72 hours of symptoms [12]. The small sample size and the specific patient population preclude definite conclusions. The second trial by Korley et al. recruited patients presenting with COVID-19 at the emergency room [13]. These patients were probably in a later inflammatory stage of the disease at a time when the benefit of CP could be low. Indeed, a large number of hospital admissions in this trial occurred on the day of study enrolment and consequently diluted any potential treatment effect. In a third randomized trial by Alemany et al., CP did not reduce the risk of hospital admission in patients aged 50 or older [14]. Similar to our study, this trial was terminated early because of the rapid SARS-CoV-2 vaccination uptake in Spain and was, therefore, underpowered. In the most recent and largest trial on CP for outpatients with COVID-19, Sullivan et al. observed a significant reduction in hospital admission (from 6.3% to 2.9%) with CP [15]. The benefit of CP was limited to the patients with ≤5 days of symptoms at the time of transfusion. The non-significant reduction in hospital admission from 8.6% to 4.8% that we observed is in agreement with these findings.
Our study has several strengths. First, we found that >90% of patients were tested negative at baseline for SARS-CoV-2 antibody. This demonstrates that we were able to identify patients with early disease and in whom clinical benefit from CP could be expected. Second, as the CP donors were selected with a whole SARS-CoV-2 plaque reduction neutralization test (PRNT)50, we were certain that the CP we used contained functional antibodies. This is also demonstrated by the significant difference in antibody levels during follow-up between the CP and non-CP groups.
Our study also has limitations. Despite that patients were selected based on their age and having at least one comorbidity, the hospital admission rate in the control arm was 8.6%, rather than the 20% that we had anticipated in the sample size calculation. This illustrates that even in an unvaccinated population and while using data provided by the Dutch Center for Disease Control and Prevention, Rijksinstituut voor Volksgezondheid en Milieu (RIVM) on risk factors for hospital admission, identifying patients at risk for a poor outcome is challenging.
Second, the study was discontinued after 421 rather than the planned 690 inclusions. This decision was made after a meeting with the DSMB at the time when the vaccination uptake in people aged ≥50 years had reached 80% and monoclonal antibodies had become available as outpatient treatment in the Netherlands. This made further recruitment futile because the vaccination rate lowered the hospital admission rate far below our anticipated hospitalization rate. Also, randomizing high-risk patients to a placebo when monoclonal antibodies are available was ethically not acceptable. In conclusion, our study was insufficiently powered to detect the relatively large effect size that we anticipated reflected by the wide credible and confidence intervals. Therefore, a meta-analysis of individual patient data from all available studies on CP for outpatients with early COVID-19 is needed and will help get a more precise estimate of the actual effect size. The ongoing Cochrane meta-analysis of CP for COVID-19 will be looking at studies on outpatients with COVID-19 in their next update to fill this knowledge gap [16].
Third, we acknowledge that the much lower hospital admission rates observed with the currently circulating Omicron VOC in an almost fully vaccinated population of which many have also recovered from COVID-19 will make the magnitude of any potential benefit from CP smaller.
Fourth, a selection of the perfect placebo can be complicated because each potential placebo has advantages and disadvantages (regulatory and practical). First, the obvious advantage of plasma as a control intervention is its identical physical characteristics and, therefore, perfect masking of the patient. Furthermore, by using plasma as the control intervention, the effect of the SARS-CoV-2–neutralizing antibodies on top of any other theoretical effect that plasma may have on COVID-19 can be studied. However, much higher immunoglobulin doses are typically used when this immune modulating effect is pursued; thus, we considered this effect unlikely. Furthermore, any additional impact of plasma on the disease course would then be present in both study arms.
Finally, it should be emphasized that at the time the study was designed, we were uncertain about the minimum dose of antibodies required to result in a clinically relevant antiviral effect. In fact, this remains an important knowledge gap, and based on the currently available evidence, it is unlikely that the dose of 250–300 mL from a single donor with a minimum neutralization titre of 1:160 that we used in this trial is the optimal dose [17]. Fortunately, the collection of plasma with substantially high virus-neutralizing antibody titres has become less challenging. We recently tested the BA.2 virus neutralization properties of plasma from 103 donors who had been fully vaccinated and received a booster dose with an mRNA vaccine. Twenty donors (19.4%) had a PRNT50 titre of 1:10 000 or higher against Omicron BA.2. Consequently, treatment with CP containing much higher doses of virus-neutralizing antibodies than those used in the current study has become possible. With the BA.1, BA.2 and now also BA.4/5 variants escaping neutralization by many of the currently available monoclonal antibodies, the interest in CP as a treatment for COVID-19 will likely increase again. Because the protection from vaccination against Omicron VOC is often suboptimal in the immunocompromised patients; the morbidity of COVID-19 in these patients continues to be substantial despite the lower pathogenicity of the Omicron [[18], [19], [20]]. As a result, CP could become a valuable part of the anti–COVID-19 armamentarium for selected patients. In our opinion, the focus of future studies on the clinical efficacy of CP should therefore be on the immunocompromised patient. Indeed, it is very unfortunate that 2 years into the pandemic, data from large randomized trials on the value of CP in immunocompromised patients are still lacking completely [21].
In conclusion, in high-risk outpatients with COVID-19, treatment with CP in the first week after symptom onset did not improve the overall disease severity. The reduced hospital admission rate that we observed is in line with data from Sullivan et al. and should be the subject of an individual patient data meta-analysis of the currently available trials on CP for outpatients with early COVID-19.
Transparency declaration
This study was supported by a research grant from ZonMw, the Netherlands (10430062010001). Sanquin Blood Supply provided convalescent plasma free of charge for study sites in the Netherlands. The authors declare that they have no conflicts of interest.
Author contributions
A.G., C.J., L.Z., Pv.W., Jd.H., F.K., Ev.L.-S., R.S., J.L., D.P., L.K., G.G., H.G., and S.B. contributed to the acquisition of the data. B.J.A.R., A.G., and G.P. have full access to the data, and B.J.A.R. is the guarantor for the data. A.G., G.P., Nv.G., and B.R. contributed to the analysis and interpretation of the data. A.G., C.J., G.P., Nv.G., F.S., C.Ev.S., B.H., M.K., C.G., J.J.Z., C.R., and B.R. contributed to the conceptualization and intellectual input of the protocol and manuscript. J.J.Z., C.R., and B.R. contributed equally as last authors. All authors contributed to the final approval of the version to be submitted.
Acknowledgements
The authors thank the following: the Data Safety Monitoring Board (DSMB) members for CoV-Early study (Prof. P.M. Bossuyt, Dr. J.L. Nouwen, Prof, M.C. de Vries); all Public Health Service contact tracers who informed potential study candidates; all infectiologists at Erasmus MC who worked even harder during the COVID-19 times to let the study team focus on the COVID-19 trials (Adam Anas, Hannelore Bax, Mariana de Mendonça-Melo, Els van Nood, Jan Nouwen, Karin Schurink, Lennert Slobbe, Dorine de Vries-Sluijs and Annelies Verbon) and the emergency department physicians and colleagues from the department of internal medicine who referred patients and facilitated patient recruitment; the medical students who contacted the patients (Romée Land, Eva Pruijt, Silje Taal, Liselotte Jeletich, Willem Sebrechts, Femke de Vries and Tia Rijlaarsdam); the many research assistants, research nurses and other participants who helped in facilitating the study (Maartje Wagemaker, Ayten Karisli, René van Engen, Marita Tjauw Joe Kim – Amadmoestar, Diane Struik, Denise Heida-Peters, Sabine Harinck, Marjo van der Poel, Greetje van Asselt, Danielle Orij – Westerhof, Marloes Romeijn, Marlies Bouterse, Pepita de Vries, Dewi Dubbelaar, Cynthia Oud, Jo Anne den Ouden, Milly Haverkort); Stichting Hemato-Oncologie voor Volwassenen Nederland (HOVON) for building the electronic case report form (eCRF) and providing real-time support with the eCRF on very short notice (Henk Hofwegen, Ronnie van der Holt, Mirjam Stomp, Marleen Luten and Monique Steijart); all the persons involved in the blinding and distribution of plasma products at the sites; and last but certainly not least, all patients who participated in the trial, the thousands of plasma donors and the COVID-19 test centres that informed patients across the Netherlands about the study.
Editor: J. Rodriguez-Baño
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2022.08.005.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Dougan M., Nirula A., Azizad M., Mocherla B., Gottlieb R.L., Chen P., et al. Bamlanivimab plus etesevimab in mild or moderate Covid-19. N Engl J Med. 2021;385:1382–1392. doi: 10.1056/NEJMoa2102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinreich D.M., Sivapalasingam S., Norton T., Ali S., Gao H., Bhore R., et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with covid-19. N Engl J Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A., Gonzalez-Rojas Y., Juarez E., Crespo Casal M., Moya J., Falci D.R., et al. Early treatment for covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941–1950. doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- 4.Jayk Bernal A., Gomes da Silva M.M., Musungaie D.B., Kovalchuk E., Gonzalez A., Delos Reyes V., et al. Molnupiravir for oral treatment of covid-19 in nonhospitalized patients. N Engl J Med. 2022;386:509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammond J., Leister-Tebbe H., Gardner A., Abreu P., Bao W., Wisemandle W., et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386:1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.VanBlargan L.A., Errico J.M., Halfmann P.J., Zost S.J., Crowe J.E., Purcell L.A., et al. An infectious SARS-CoV-2 B.1.1.529 omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat Med. 2022;28:490–495. doi: 10.1038/s41591-021-01678-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takashita E., Kinoshita N., Yamayoshi S., Sakai-Tagawa Y., Fujisaki S., Ito M., et al. Efficacy of antiviral agents against the SARS-CoV-2 omicron subvariant BA.2. N Engl J Med. 2022;386:1475–1477. doi: 10.1056/NEJMc2201933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gharbharan A., Jordans C.C.E., GeurtsvanKessel C., den Hollander J.G., Karim F., Mollema F.P.N., et al. Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection. Nat Commun. 2021;12:3189. doi: 10.1038/s41467-021-23469-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’Donnell L., Chernyak Y., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rockwood K., Theou O. Using the clinical frailty scale in allocating scarce health care resources. Can Geriatr J. 2020;23:210–215. doi: 10.5770/cgj.23.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Libster R., Pérez Marc G., Wappner D., Coviello S., Bianchi A., Braem V., et al. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med. 2021;384:610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korley F.K., Durkalski-Mauldin V., Yeatts S.D., Schulman K., Davenport R.D., Dumont L.J., et al. Early convalescent plasma for high-risk outpatients with Covid-19. N Engl J Med. 2021;385:1951–1960. doi: 10.1056/NEJMoa2103784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alemany A., Millat-Martinez P., Corbacho-Monné M., Malchair P., Ouchi D., Ruiz-Comellas A., et al. High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: a randomised, placebo-controlled trial. Lancet Respir Med. 2022;10:278–288. doi: 10.1016/S2213-2600(21)00545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sullivan D.J., Gebo K.A., Shoham S., Bloch E.M., Lau B., Shenoy A.G., et al. Early outpatient treatment for Covid-19 with convalescent plasma. N Engl J Med. 2022;386:1700–1711. doi: 10.1056/NEJMoa2119657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piechotta V., Iannizzi C., Chai K.L., Valk S.J., Kimber C., Dorando E., et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev. 2021;5:CD013600. doi: 10.1002/14651858.CD013600.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rijnders B.J.A., Huygens S., Mitjà O. Evidence-based dosing of convalescent plasma for COVID-19 in future trials. Clin Microbiol Infect. 2022;28:667–671. doi: 10.1016/j.cmi.2022.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malahe S.R.K., Hoek R.A.S., Dalm V.A.S.H., Broers A.E.C., den Hoed C.M., Manintveld O.C., et al. Clinical characteristics and outcome of immunocompromised patients with COVID-19 caused by the Omicron variant: a prospective observational study. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar D., Hu Q., Samson R., Ferreira V.H., Hall V.G., Ierullo M., et al. Neutralization against Omicron variant in transplant recipients after three doses of mRNA vaccine. Am J Transplant. 2022;22:2089–2093. doi: 10.1111/ajt.17020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huygens S., Munnink B.O., Gharbharan A., Koopmans M., Rijnders B. Sotrovimab resistance and viral persistence after treatment of immunocompromised patients infected with the SARS-CoV-2 Omicron variant. Clin Inf Dis. 2022 doi: 10.1093/cid/ciac601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huygens S., Hofsink Q., Nijhof I.S., Goorhuis A., Kater A.P., te Boekhorst P.A.W., et al. Hyperimmune globulin for severely immunocompromised patients hospitalized with COVID-19: a randomized, controlled trial. J Inf Dis. 2022 doi: 10.1093/infdis/jiac334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.