Abstract
Background
Viral- and host-targeted traditional Chinese medicine (TCM) formulae NRICM101 and NRICM102 were administered to hospitalized patients with COVID-19 during the mid-2021 outbreak in Taiwan. We report the outcomes by measuring the risks of intubation or admission to intensive care unit (ICU) for patients requiring no oxygen support, and death for those requiring oxygen therapy.
Methods
This multicenter retrospective study retrieved data of 840 patients admitted to 9 hospitals between May 1 and July 26, 2021. After propensity score matching, 302 patients (151 received NRICM101 and 151 did not) and 246 patients (123 received NRICM102 and 123 did not) were included in the analysis to assess relative risks.
Results
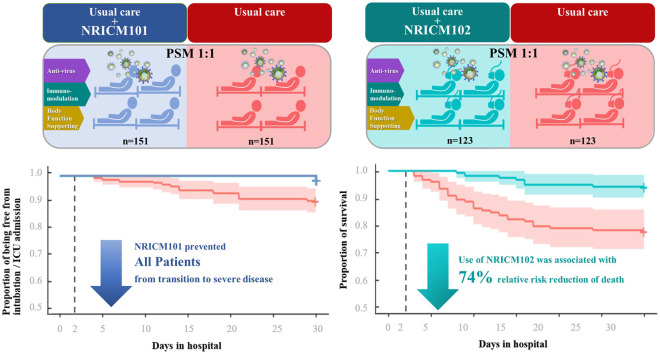
During the 30-day observation period, no endpoint occurred in the patients receiving NRICM101 plus usual care while 14 (9.27%) in the group receiving only usual care were intubated or admitted to ICU. The numbers of deceased patients were 7 (5.69%) in the group receiving NRICM102 plus usual care and 27 (21.95%) in the usual care group. No patients receiving NRICM101 transitioned to a more severe status; NRICM102 users were 74.07% less likely to die than non-users (relative risk= 25.93%, 95% confidence interval 11.73%-57.29%).
Conclusion
NRICM101 and NRICM102 were significantly associated with a lower risk of intubation/ICU admission or death among patients with mild-to-severe COVID-19. This study provides real-world evidence of adopting broad-spectrum oral therapeutics and shortening the gap between outbreak and effective response. It offers a new vision in our preparation for future pandemics.
Keywords: COVID-19, NRICM101, NRICM102, Propensity score, Traditional Chinese Medicine
Graphical Abstract
1. Background
Coronavirus disease 2019 (COVID-19) has devastated the world population and health care systems since 2020, and the accelerated emergence of infectious diseases is expected. Understanding the necessity to develop effective therapeutic approaches to tackle ongoing and future outbreaks, the World Health Organization has recently called for action to enhance the contribution of traditional Chinese medicine (TCM) in managing global pandemics on the grounds of mounting evidence [1], [2]. In Taiwan, the “Traditional Chinese Medicine Clinical Guideline for COVID-19″ (TCM-CG) suggested integrated treatment of mild-to-moderate and severe-to-critical cases with TCM formulae NRICM101 and NRICM102, respectively, both of which consist of herbs classified as dietary supplements in the United States. While NRICM101 was granted an Emergency Use Authorization in Taiwan and used in more than 50 countries worldwide and NRICM102 was administered during a serious outbreak in mid-2021, a robust evaluation is needed to provide more clinical evidence of these herbal preparations with different treatment priorities in different stages of COVID-19 illness (Supplementary file 1).
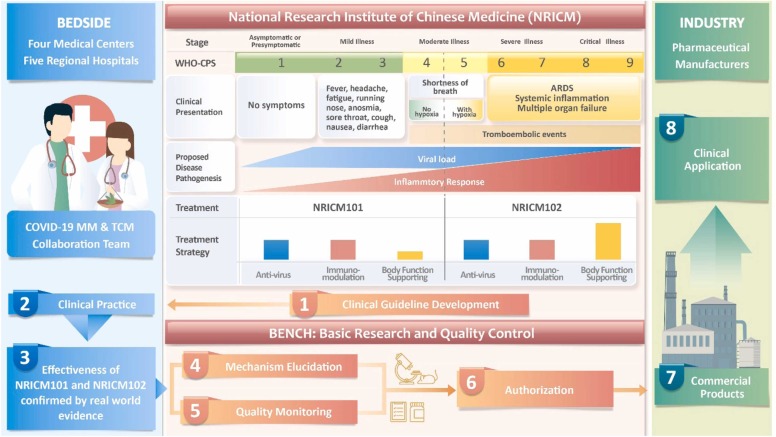
The first report of NRICM101 was based on the results of a bed-to-bench study, where real-world evidence derived from integrated TCM and modern medicine (MM) was strengthened by confirmative laboratory findings of the antiviral, anti-inflammatory and immunoregulatory effects of NRICM101 [3]. However, randomized control trials (RCTs) were not viable as Taiwan managed to halt COVID-19 transmission with nonpharmacological interventions until May 2021 [4], when a sudden surge in COVID-19 cases overwhelmed the local health system. Given the limitation of traditional RCTs in delivering timely evidence necessary for clinical decisions regarding the use of novel therapies [5], [6], health care providers, scientists and pharmaceutical companies leveraged the earlier TCM-MM cotreatment experience by providing the patients with NRICM101 and NRICM102, in addition to usual care ( Fig. 1).
Fig. 1.
Flowchart of Bedside-Bench-Industry Model for Traditional Chinese Medicine. This research model incorporated professionals in modern and traditional Chinese medical, academic and industrial fields. Soon after the COVID-19 pandemic outbreak in December 2019, the National Research Institute of Chinese Medicine (NRICM) developed the Traditional Chinese Medicine Clinical Guideline for COVID-19 (TCM-CG) based on the experience treating SARS patients and understanding the manifestations and pathogenesis of COVID-19. The formulae NRICM101 & NRICM102 are suggested to treat COVID-19 patients who present with mild-to-moderate (corresponding to WHO Clinical Progression Scale, WHO-CPS 1–4) and severe-to-critical respiratory illness, respectively. NRICM101 & NRICM102 are composed of herbs with three main effects but with different dose ratios: (1) antivirus, (2) immune modulation, and (3) body function support. The COVID-19 MM & TCM Collaboration Team adopted the TCM-CG to treat COVID-19 patients and confirmed the effectiveness of NRICM101 & NRICM102. NRICM elucidated the multi-targeting mechanism and established quality control profiles of NRICM101 & NRICM102. Commercialized products of NRICM101 are being manufactured by GMP pharmaceutical companies and used clinically.
In the study, we examined the association between TCM (NRICM101 & NRICM102) use and progression to severe COVID-19 or death in 9 hospitals caring for a substantial number of patients during the 2021 outbreak. We hypothesized that TCM use would be associated with a lower risk of an endpoint of intubation or intensive care unit (ICU) admission, or death in propensity score analysis. The objective was to demonstrate the applicability of the real-world TCM-MM model in the rapid development of effective therapeutics to build our capacities for pandemic preparedness.
2. Methods
2.1. Study design and assessed variables
In this multicenter retrospective study, we obtained data from all admitted patients who had a COVID-19-related admission with diagnosis confirmed by a positive nasopharyngeal or oropharyngeal real-time polymerase chain reaction test for SARS-CoV-2 from May 1 to July 26, 2021. Patient data were extracted from the hospital information system (HIS) of each participating hospital. An HIS is an integrated comprehensive information system to manage the medical, administrative, financial, and legal information of a hospital's operation. The data included all outpatient and inpatient demographics, visit and admission information, diagnoses, medications, orders and prescriptions, procedures, vital signs, care provider notes, laboratory results and radiology reports. We extracted and analyzed the data, and we obtained research ethics approval from each participating hospital. Extracted data included the following:
-
(1)
Demographics: age, sex, body height, body weight.
-
(2)
Vital signs on presentation: heart rate, temperature, respiratory rate, blood pressure.
-
(3)
Laboratory tests: Complete blood cell count and differential count, aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), creatinine, C-reactive protein (CRP), and lactate dehydrogenase (LDH).
-
(4)
Diagnostic categories: chronic lung disease (including asthma, chronic obstructive pulmonary disease (COPD) and chronic bronchitis), cancer, chronic kidney disease, hypertension, and diabetes.
-
(5)
Medication: remdesivir, dexamethasone, hydroxychloroquine, azithromycin, tocilizumab, sarilumab, lopinavir, darunavir; and antibiotics (with the exclusion of azithromycin as it is categorized separately)
-
(6)
Admission to the intensive care unit.
-
(7)
Use of supplement oxygen, intubation and invasive mechanical ventilation.
-
(8)
Death records.
2.2. Stratification
We stratified all patients by whether they received oxygen therapy at admission. For the group without oxygen therapy (corresponding to World Health Organization - Clinical Progression Scale, WHO-CPS 1–4), the patients were defined as receiving NRICM101 if they were initially prescribed NRICM101; for the group with oxygen therapy WHO-CPS 5-7, the patients were defined as receiving NRICM102. To avoid overestimation of TCM effectiveness, patients who received TCM for less than 2 days were excluded.
2.3. Propensity score matching
To help account for the nonrandomized treatment administration of NRICM101 and NRICM102, we used propensity score methods to reduce the effects of confounding. In the propensity score-matched analysis, the nearest neighbor method was applied to create a matched control sample that included demographic factors, clinical factors, laboratory tests, and medications. Multiple imputation was used to account for missing data on body mass index (BMI), systolic pressure, respiratory rate, temperature, ALT, AST, creatinine, CRP, LDH, lymphocytes and neutrophils count. To prevent intubated cases from being unselected by matching, we used two-stage propensity score-matching. In the first stage, we matched intubation/ICU admission and intubation/ICU admission-free groups. In the second stage, we performed the propensity score matching with the remaining intubation/ICU admission-free cases. Similar procedure was carried out with death as the endpoint.
2.3.1. Time-to-event test and comparing the proportion of Intubation/ICU Admission or death
After propensity-scored matching, the data of the variables in NRICM101 plus usual care and usual care were not independent and had similar distribution. Therefore, simple comparisons of two matched samples were appropriate. The marginal cox regression, McNemar’s test and log-rank test were employed to explore the association between TCM and non-TCM use. All statistical analyses were performed with the use of R software, version 3.6.1 (R Project for Statistical Computing). In addition, we have also evaluated the impact of potential unmeasured confounders, such as use of corticosteroids, that may have similar association with the intervention and the risk of outcome by calculating an e-value.
3. Results
We conducted this retrospective study in collaboration with 4 medical centers and 5 regional hospitals in northern and central Taiwan. In these 9 hospitals, physicians prescribed NRICM101 or NRICM102 according to whether oxygen was required when the COVID-19 patient requested or agreed to receive TCM treatment in addition to usual care. Both formulae were administered orally three times daily. For the group without oxygen therapy, the primary endpoint was the subsequent need for intubation or ICU admission to the, and for the group with oxygen therapy, the primary endpoint was death.
3.1. Characteristics of the cohort
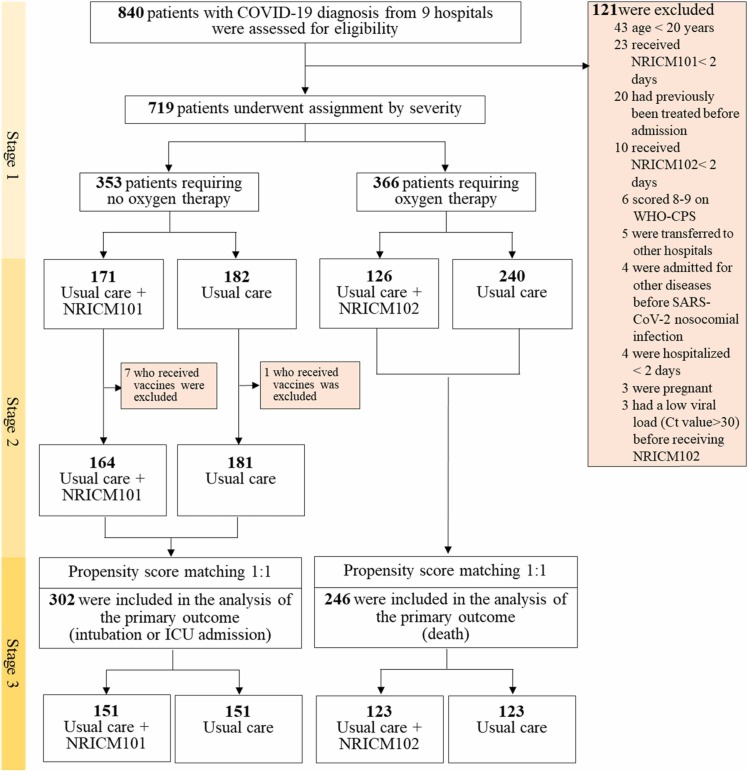
Overall, 840 patients with COVID-19 admitted to 9 hospitals between May 1 and July 26, 2021 were observed for 30 days. Among them, 121 patients were excluded from the base cohort because they were under 20 years of age, previously treated elsewhere, admitted for other diseases, critically ill, or hospitalized for less than 2 days. The base cohort of 719 patients was initially divided into the mild-to-moderate group of 353 cases who required no oxygen therapy (non-oxygen group) and the severe-to-critical group of 366 cases requiring oxygen therapy (oxygen group), with the former receiving NRICM101 or not and the latter receiving NRICM102 or not. After further excluding those who were vaccinated from the non-oxygen group (not for the oxygen group because vaccination was no longer a factor at this stage), propensity score matching was performed to attenuate the differences between usual care and usual care plus adjunctive treatment. Finally, 302 patients who did (151) and did not (151) receive NRICM101 as well as 246 patients who did (123) and did not (123) receive NRICM102 were included in the analysis ( Fig. 2).
Fig. 2.
Enrollment and Study Design.
In the unmatched non-oxygen group, the mean ( ± SD) age was 50.41 ( ± 16.48) years, 171 (49.6%) were male, 85 (24.6%) had at least one preexisting condition and received prescription medications, and the majority 174 (50.4%) scored 4 on the WHO-CPS. The most common medication used in usual care was dexamethasone/glucocorticoid (20.6%), in addition to antibiotics. Among the patients who received NRICM101 (median duration of treatment 7 days), the first dose was administered after a median of 2 days from admission. The distribution of the patients’ characteristics by NRICM101 exposure is displayed in Table 1, both in the unmatched and propensity score-matched samples.
Table 1.
Characteristics and Clinical Outcomes of Patients Receiving or Not Receiving NRICM101, before and after Propensity Score Matching.
| Characteristics | All Patients (N = 345) |
Unmatched Patients |
Propensity Score-Matched Patients |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRICM101 +Usual Care (N = 164) |
Usual Care (N = 181) |
NRICM101 +Usual Care (N = 151) |
Usual Care (N = 151) |
|||||||||
| Age (mean ± SD) | 50.41 ± 16.48 | 49.70 ± 15.52 | 51.05 ± 17.32 | 49.43 ± 15.55 | 49.25 ± 16.95 | |||||||
| Age60 — no. (%) | 114 | (33.0) | 50 | (30.3) | 64 | (32.6) | 46 | (30.5) | 41 | (29.1) | ||
| BMI — no. (%) | ||||||||||||
| BMI | 291 | (84.3) | 145 | (88.4) | 146 | (80.7) | 143 | (94.7) | 141 | (93.4) | ||
| BMI | 25 | (7.2) | 11 | (6.7) | 14 | (7.7) | 8 | (5.3) | 10 | (6.6) | ||
| BMI missing | 29 | (8.4) | 8 | (4.9) | 21 | (11.6) | – | – | ||||
| Male sex — no. (%) | 171 | (49.6) | 87 | (53.0) | 84 | (46.4) | 80 | (53.0) | 81 | (53.6) | ||
| Smoking — no. (%) | 58 | (16.8) | 27 | (16.5) | 31 | (17.1) | 25 | (16.6) | 26 | (17.2) | ||
| Drinking — no. (%) | 49 | (14.2) | 28 | (17.1) | 21 | (11.6) | 25 | (16.6) | 20 | (13.2) | ||
| Comorbidity — no. (%) | ||||||||||||
| Chronic heart disease | 85 | (24.6) | 40 | (24.4) | 45 | (24.9) | 33 | (21.9) | 39 | (25.8) | ||
| Diabetes | 39 | (11.3) | 16 | (9.8) | 23 | (12.7) | 15 | (9.9) | 14 | (9.3) | ||
| Hyperlipidemia | 25 | (7.2) | 12 | (7.3) | 13 | (7.2) | 12 | (7.9) | 10 | (6.6) | ||
| Thyroid disease | 8 | (2.3) | 4 | (2.4) | 4 | (2.2) | 4 | (2.6) | 1 | (0.7) | ||
| Chronic liver disease | 13 | (3.8) | 7 | (4.3) | 6 | (3.3) | 7 | (4.6) | 5 | (3.3) | ||
| Chronic kidney disease | 12 | (3.5) | 5 | (3.0) | 7 | (3.9) | 5 | (3.3) | 7 | (4.6) | ||
| Chronic pulmonary disease | 10 | (2.9) | 4 | (2.4) | 6 | (3.3) | 4 | (2.6) | 2 | (1.3) | ||
| Cancer | 15 | (4.3) | 7 | (4.3) | 8 | (4.4) | 7 | (4.6) | 8 | (5.3) | ||
| Stroke | 4 | (1.2) | 4 | (2.4) | 0 | (0.0) | 4 | (2.6) | 0 | (0.0) | ||
| Others | 58 | (16.8) | 28 | (17.1) | 32 | (16.6) | 23 | (15.2) | 22 | (14.6) | ||
| Medications | ||||||||||||
| Remdesivir | 11 | (3.2) | 8 | (4.9) | 3 | (1.7) | 8 | (5.3) | 2 | (1.3) | ||
| Dexamethasone/Glucocorticoid | 71 | (20.6) | 37 | (22.6) | 34 | (18.8) | 35 | (23.2) | 25 | (16.6) | ||
| Hydroxychloroquine | 2 | (0.6) | 2 | (1.2) | 0 | (0.0) | 3 | (2.0) | 0 | (0.0) | ||
| Azithromycin | 14 | (4.1) | 4 | (2.4) | 11 | (5.5) | 3 | (2.0) | 8 | (5.3) | ||
| Other antibiotic agent | 100 | (29.0) | 40 | (24.2) | 60 | (33.1) | 36 | (23.8) | 46 | (30.5) | ||
| Tocilizumab/Sarilumab | 2 | (0.6) | 0 | (0.0) | 2 | (1.1) | 0 | (0.0) | 2 | (1.3) | ||
| Lopinavir/Darunavir | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 1 | (0.7) | 0 | (0.0) | ||
| WHO Clinical Progression Scale score | ||||||||||||
| 1 | 25 | (7.2) | 7 | (4.3) | 18 | (9.9) | 6 | (4.0) | 15 | (9.9) | ||
| 2 | 139 | (40.3) | 75 | (45.7) | 64 | (35.4) | 71 | (47.0) | 56 | (37.1) | ||
| 3 | 7 | (2.0) | 4 | (2.4) | 3 | (1.7) | 3 | (2.0) | 3 | (2.0) | ||
| 4 | 174 | (50.4) | 78 | (47.6) | 96 | (53.0) | 71 | (47.0) | 77 | (51.0) | ||
| Initial vital signs — mean (SD) | ||||||||||||
| Systolic blood pressure — mmHg | 131.62 | (18.63) | 131.87 | (17.83) | 131.39 | (19.38) | 131.94 | (17.62) | 128.81 | (17.61) | ||
| Respiratory Rate — breaths/min | 18.35 | (1.86) | 18.17 | (1.32) | 18.51 | (2.23) | 18.19 | (1.29) | 18.40 | (2.32) | ||
| Temperature —℃ | 37.34 | (1.01) | 37.35 | (0.99) | 37.32 | (1.03) | 37.37 | (1.01) | 37.31 | (1.06) | ||
| AST — U/L | 34.98 | (41.08) | 34.70 | (35.41) | 35.24 | (45.79) | 35.1 | 0(34.19) | 36.10 | (46.07) | ||
| ALT — U/L | 31.20 | (28.51) | 33.31 | (30.57) | 29.24 | (26.41) | 34.28 | (30.77) | 30.41 | (27.3) | ||
| Creatinine — mg/dL | 1.06 | (1.45) | 0.95 | (0.97) | 1.16 | (1.77) | 0.96 | (0.95) | 1.29 | (2.07) | ||
| CRP — mg/dL | 2.36 | (3.83) | 2.11 | (3.31) | 2.59 | (4.25) | 1.94 | (3.19) | 2.71 | (4.39) | ||
| LDH — U/L | 254.84 | (193.95) | 243.54 | (114.59) | 263.54 | (238.09) | 225.32 | (96.7) | 260.07 | (252.03) | ||
| Lymphocytes — % | 23.57 | (10.62) | 23.00 | (10.70) | 24.10 | (10.56) | 23.11 | (11.12) | 23.21 | (10.2) | ||
| Neutrophils — % | 66.39 | (12.76) | 66.65 | (13.22) | 66.14 | (12.35) | 66.70 | (13.52) | 67.08 | (12.01) | ||
| Intervention | ||||||||||||
| No. of days from admission to start NRICM101 (Median (IQR)) | – | – | 2 | (4) | – | 2 | (4) | – | ||||
| No. of days receiving NRICM101 (Median (IQR)) | – | – | 7 | (6) | – | 8 | (6) | – | ||||
| Outcome | ||||||||||||
| Intubation or ICU admission - no(%) | 14 | (4.06) | 0 | (0.0) | 14 | (7.73) | 0 | (0.0) | 14 | (9.27) | ||
ALT: alanine aminotransferase; AST: aspartate aminotransferase; BMI: body mass index; BUN: blood urea nitrogen; CRP: creatinine, C-reactive protein; IC: intensive care unit; LDH: lactate dehydrogenase; SD: standard deviation; WHO: World Health Organization.
In the unmatched oxygen group, the mean ( ± SD) age was 63.30 ( ± 14.16) years, 201 (54.9%) were male, 191 (52%) had at least one preexisting condition and received prescription medications, the majority 291 (79.5%) scored 5 on the WHO-CPS, and all had very high lactate dehydrogenase (LDH) levels. In addition to antibiotics, the most common medications used in usual care were dexamethasone/glucocorticoid 263 (71.9%), followed by remdesivir 79 (21.6%) and tocilizumab/sarilumab 65 (17.8%). Among those who received NRICM102 (median duration of treatment 9 days), the first dose was administered after a median of 2 days from admission. The unmatched and propensity score-matched distribution of the patients’ characteristics by NRICM102 exposure is displayed in Table 2.
Table 2.
Characteristics and Clinical Outcomes of Patients Receiving or Not Receiving NRICM102, before and after Propensity Score Matching.
| Characteristics | All Patients (N = 366) |
Unmatched Patients |
Propensity Score-Matched Patients |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRICM102 +Usual Care (N = 126) |
Usual Care (N = 240) |
NRICM102 +Usual Care (N = 123) |
Usual Care (N = 123) |
||||||||||
| Age (mean ± SD) | 63.30 ± 14.16 | 61.55 ± 14.54 | 64.22 ± 13.90 | 62.03 ± 14.29 | 62.84 ± 15.40 | ||||||||
| Age60 — no. (%) | 236 | (64.5) | 76 | (60.3) | 160 | (66.7) | 76 | (61.8) | 73 | (59.3) | |||
| BMI — no. (%) | |||||||||||||
| BMI | 276 | (75.4) | 95 | (75.4) | 181 | (75.4) | 102 | (82.9) | 100 | (81.3) | |||
| BMI | 55 | (15.0) | 23 | (18.3) | 32 | (13.4) | 21 | (17.1) | 23 | (18.7) | |||
| BMI missing | 35 | (9.6) | 8 | (6.3) | 27 | (11.2) | – | – | |||||
| Male sex — no. (%) | 201 | (54.9) | 65 | (51.6) | 136 | (56.7) | 63 | (51.2) | 68 | (55.3) | |||
| Smoking — no. (%) | 67 | (18.3) | 21 | (16.7) | 46 | (19.2) | 21 | (17.1) | 19 | (15.4) | |||
| Drinking — no. (%) | 46 | (12.6) | 22 | (17.5) | 24 | (10.0) | 19 | (15.4) | 16 | (13.0) | |||
| Comorbidity — no. (%) | |||||||||||||
| Chronic heart disease | 191 | (52.0) | 64 | (50.8) | 127 | (52.9) | 63 | (51.2) | 70 | (56.9) | |||
| Diabetes | 103 | (28.1) | 33 | (26.2) | 70 | (29.2) | 33 | (26.8) | 29 | (23.6) | |||
| Hyperlipidemia | 39 | (10.7) | 16 | (12.7) | 23 | (9.6) | 17 | (13.8) | 12 | (9.8) | |||
| Thyroid disease | 8 | (2.2) | 4 | (3.2) | 4 | (1.7) | 4 | (3.3) | 1 | (0.8) | |||
| Chronic liver disease | 12 | (3.3) | 4 | (3.2) | 8 | (3.3) | 4 | (3.3) | 6 | (4.9) | |||
| Chronic kidney disease | 21 | (5.7) | 7 | (5.6) | 14 | (5.8) | 7 | (5.7) | 7 | (5.7) | |||
| Chronic pulmonary disease | 25 | (6.8) | 8 | (6.4) | 17 | (7.1) | 8 | (6.5) | 10 | (8.1) | |||
| Cancer | 16 | (4.0) | 7 | (5.6) | 9 | (3.8) | 8 | (6.5) | 6 | (4.9) | |||
| Stroke | 21 | (5.7) | 8 | (6.4) | 13 | (5.4) | 8 | (6.5) | 9 | (7.3) | |||
| Others | 4 | (1.1) | 1 | (0.8) | 3 | (1.3) | 1 | (0.8) | 3 | (2.4) | |||
| Medications | |||||||||||||
| Remdesivir | 79 | (21.6) | 23 | (18.3) | 56 | (23.3) | 23 | (18.7) | 20 | (16.3) | |||
| Dexamethasone/Glucocorticoid | 263 | (71.9) | 83 | (65.9) | 180 | (75.0) | 82 | (66.7) | 84 | (68.3) | |||
| Hydroxychloroquine | 1 | (0.3) | 1 | (0.8) | 0 | (0.0) | 1 | (0.8) | 0 | (0.0) | |||
| Azithromycin | 16 | (4.4) | 9 | (7.1) | 7 | (2.9) | 9 | (7.3) | 4 | (3.3) | |||
| Other antibiotic agent | 155 | (42.3) | 49 | (38.9) | 106 | (44.2) | 47 | (38.2) | 44 | (35.8) | |||
| Tocilizumab/Sarilumab | 65 | (17.8) | 10 | (7.9) | 55 | (22.9) | 10 | (8.1) | 25 | (20.3) | |||
| Lopinavir/Darunavir | 1 | (0.3) | 1 | (0.8) | 0 | (0.0) | 1 | (0.8) | 0 | (0.0) | |||
| WHO Clinical Progression Scale score | |||||||||||||
| 5 | 291 | (79.5) | 111 | (88.1) | 180 | (75.0) | 108 | (87.8) | 109 | (88.6) | |||
| 6 | 48 | (13.1) | 12 | (9.5) | 36 | (15.0) | 12 | (9.8) | 10 | (8.1) | |||
| 7 | 27 | (7.4) | 3 | (2.4) | 24 | (10.0) | 3 | (2.4) | 4 | (3.3) | |||
| Initial vital signs —mean (SD) | |||||||||||||
| Systolic blood pressure — mmHg | 132.33 | (20.77) | 133.52 | (21.54) | 131.72 | (20.38) | 133.76 | (21.61) | 132.54 | (21.22) | |||
| Respiratory Rate — breaths/min | 20.62 | (5.00) | 20.35 | (3.36) | 20.76 | (5.67) | 20.32 | (3.39) | 20.30 | (4.14) | |||
| Temperature —℃ | 37.30 | (0.98) | 37.44 | (1.00) | 37.23 | (0.96) | 37.46 | (1.00) | 37.36 | (1.10) | |||
| AST — U/L | 47.12 | (33.45) | 43.55 | (26.26) | 49.06 | (36.69) | 43.55 | (26.05) | 48.13 | (28.51) | |||
| ALT — U/L | 36.60 | (25.46) | 34.05 | (22.32) | 37.9 | (26.87) | 33.45 | (21.39) | 34.41 | (22.68) | |||
| Creatinine — mg/dL | 1.26 | (1.47) | 1.22 | (1.61) | 1.29 | (1.4) | 1.21 | (1.61) | 1.23 | 1.18) | |||
| CRP — mg/dL | 7.72 | (6.45) | 6.93 | (6.49) | 8.21 | (6.4) | 6.79 | (6.39) | 7.74 | (6.45) | |||
| LDH — U/L | 416.48 | (224.22) | 425.9 | (200.85) | 412.54 | (233.69) | 413.89 | (193.98) | 392.32 | (192.53) | |||
| Lymphocytes — % | 16.63 | (9.31) | 18.65 | (9.96) | 15.58 | (8,78) | 18.71 | (10.25) | 16.13 | (8.95) | |||
| Neutrophils — % | 77.60 | (41.82) | 72.77 | (13.30) | 80.31 | (51.15) | 72.98 | (13.45) | 75.40 | (11.66) | |||
| Intervention | |||||||||||||
| No. of days from admission to start NRICM102 (Median (IQR)) | 2 | (4) | 2 | (4) | – | 2 | (4) | – | |||||
| No. of days of receiving NRICM102 (Median (IQR)) | 9 | (8) | 9 | (8) | – | 9 | (8) | – | |||||
| Outcome | |||||||||||||
| Death — no. (%) | 49 | (13.39) | 7 | (5.56) | 42 | (17.50) | 7 | (5.69) | 27 | (21.95) | |||
ALT: alanine aminotransferase; AST: aspartate aminotransferase; BMI: body mass index; BUN: blood urea nitrogen; CRP: creatinine, C-reactive protein; LDH: lactate dehydrogenase; SD: standard deviation; WHO: World Health Organization.
3.2. Primary outcome
During the 30-day observation period, 14 patients (4.06%) in the non-oxygen group had a primary endpoint event (intubation or ICU admission), and 49 patients (13.39%) in the oxygen group died. No patient receiving NRICM101 plus usual care experienced the endpoint, while 14 (9.27%) in the group receiving only usual care were intubated or admitted to ICU. The numbers of deceased patients were 7 (5.69%) in the group receiving NRICM102 plus usual care and 27 (21.95%) in the group receiving usual care.
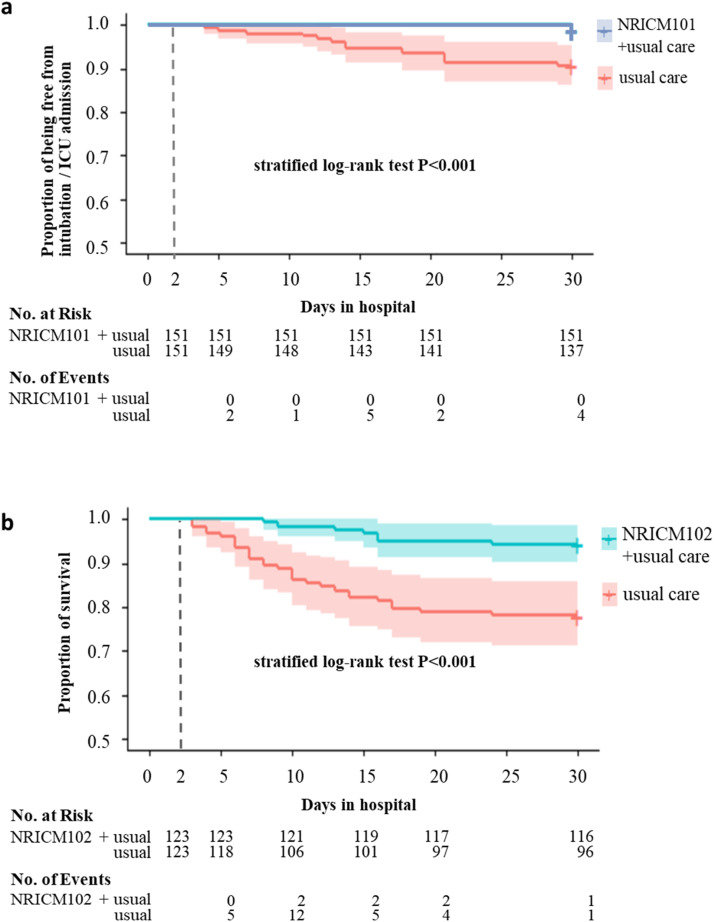
We present a seriously underestimated relative risk 15.8% (95% confidence interval [CI], 3.6%−68.3%) for unmatched data and 14.3% (95% CI, 3.3%−71.8%) for matched data when we set 2 censored cases as intubation or ICU admission. Additionally, the results of marginal Cox regression and log-rank tests for days of without intubation or transfer to ICU after matching indicated a significant association between NRICM101 use and usual care (hazard ratio, 13.58%; 95% CI, 3.40%−54.21%). Patients who did not receive NRICM102 were more likely to have experienced a primary endpoint event than were patients who did (relative risk, 40.80%; 95% CI, 20.54%−81.12%) in the unmatched data analysis. The results of marginal Cox regression, McNemar’s test and log-rank tests after propensity score matching indicated a significant association between NRICM102 use and death (relative risk, 25.93%; 95% CI, 11.73%−57.29%; hazard ratio, 23.17%; 95% CI, 10.36%−51.82%) ( Table 3, Fig. 3). Regarding the impact of potential confounders, the e-value was 7.1756 which is bigger than the RR of corticosteroids [7]. Hence, the treatment effect of TCM was robust.
Table 3.
Associations between TCM Use and the Endpoint.
| Analysis | NRICM101 |
NRICM102 |
|---|---|---|
| Intubation or ICU Admission |
Death | |
| No. of events/no. of patients at risk (%) | ||
| TCM + Usual Care | 0/164 (0.00) | 7/126 (5.56) |
| Usual Care | 14/181 (7.73) | 42/240 (17.50) |
| Relative Risk (95% CI) | --&^ | 40.80% (20.54%-81.12%)^ |
| Propensity score analyses - with matching | ||
| TCM + Usual Care (%) | 0/151 (0.00) | 7/123 (5.69) |
| Usual Care (%) | 14/151 (9.27) | 27/123 (21.95) |
| Relative Risk (95% CI) | --&* | 25.93% (11.73%−57.29%)* |
| Hazard Ratio (95% CI) | --$# | 23.17% (10.36–51.82%)# |
& Seriously underestimated relative risk (95% CI) = 15.8% (3.6%−68.3%) for unmatched data and 14.3% (3.3%−71.8%) for matched data when we included 2 censored cases as the endpoint.
^ The chi-square test was used for unmatched data (p value = 0.002 for death and p value = 0.006 when we set 2 censored cases as intubation or ICU admission).
* McNemar’s test compared the proportion of intubation or ICU admission (p value = 0.003) and death (p value < 0.001) for matched data. The power of McNemar’s test being larger than 0.852 for NRICM101 and 0.929 for NRICM102 indicates that the significance of both is not due to chance.
$ Seriously underestimated hazard ratio= 13.58% (3.40–54.21%) when we set 2 censored cases as the endpoint by the marginal Cox model.
# Hazard ratio by marginal Cox regression and p value < 0.001 by stratified log-rank test for both NRICM101 and NRICM102.
Fig. 3.
Survival Curves according to TCM Use. a, Kaplan-Meier estimates of by NRICM101 or not after propensity score 1:1 matching. And “+ ” indicates that patients not intubated or admitted to ICU were censored as of day 30 following hospital admission. b, Kaplan-Meier estimates of survival by NRICM102 or not after propensity score 1:1 matching. And “+ ” indicates that patients still alive were censored as of day 30 following hospital admission.
4. Discussion
In this study involving hospitalized patients with mild to severe COVID-19, patients requiring oxygen therapy were older and had higher percentages of underlying health conditions, such as obesity (BMI≥30), chronic heart disease and diabetes, than those requiring no oxygen. These characteristics are known risk factors reported in previous studies [8], [9], [10]. NRICM101 & NRICM102 plus usual care was associated with a reduced risk of clinical deterioration leading to intubation/ICU admission or death. While it was not possible to launch a randomized clinical trial due to constraining conditions in Taiwan, we used propensity score methods to reduce the effects of confounding factors in the analysis. Multiple analyses concluded that NRICM101 & NRICM102 plus usual care was effective, and the consistency of the results was reassuring. Our findings provide clinical evidence in support of the guideline (TCM-CG) and the previous study which suggest use of NRICM101 and NRICM102 in patients with COVID-19 [3]. Moreover, the outcomes exhibited the ability of TCM to handle the disease at different stages during its progression.
Approximately 10–15% of people infected with SARS-CoV-2 experience moderate or severe COVID-19 and require hospitalization and oxygen support, and 3–5% are admitted to ICU [11]. Drugs recommended by international bodies for severe-to-critical cases included dexamethasone, tocilizumab and remdesivir, which were the three most prescribed medications in our data. While later investigations indicated that tocilizumab and remdesivir were unassociated with improved survival [12], [13], the incidence of death among patients receiving dexamethasone (29.3% for those receiving invasive mechanical ventilation and 23.3% for those receiving oxygen without invasive mechanical ventilation) [14] appears close to that of the usual care group (21.95%) but still much higher than that of the NRICM102 plus usual care group (5.69%) in the present study.
The use of mixtures of interacting medicinal plants to reach multiple pharmacological targets and produce clinical efficacy was proposed long ago [15], NRICM101 & NRICM102 represent an example of multicomponent herbal therapeutics with active compounds that work as broad-spectrum agents. They target the host-cell pathway essential for viral infection and replication [3] on the one hand and the host response by providing support to maintain body functions on the other, as suggested by Fedson [16], [17], in treating viral diseases (Fig. 1). This strategy might offer a partial explanation for the relatively high effectiveness of NRICM101 & NRICM102 compared to single target agents. Although the subjects in this study were infected with the alpha variant, additional laboratory assays confirmed that both formulae had an inhibitory effect against 5 major variants, including delta and omicron strains, which addressed the concerns over the evolution of the virus. Inhibitory activities of TCM against the ACE2-Spike interaction is provided in the Supplementary file 2.
Understanding the vital role of vaccines in reducing the threat posed by SARS-CoV-2 variants [18], we excluded patients who were vaccinated from the analysis to rule out the effect of vaccines and more realistically simulate a real-world scenario. In the face of emerging infectious diseases such as COVID-19, a prompt deployment of specifically targeted vaccines or new drugs is unlikely, as it requires a lengthy process of development and testing and often faces the issue of partial efficacy as the virus mutates. Nevertheless, it is relatively viable to develop broad-spectrum therapies based on traditional medicine for a fast response to deal with an outbreak crisis before vaccines become available.
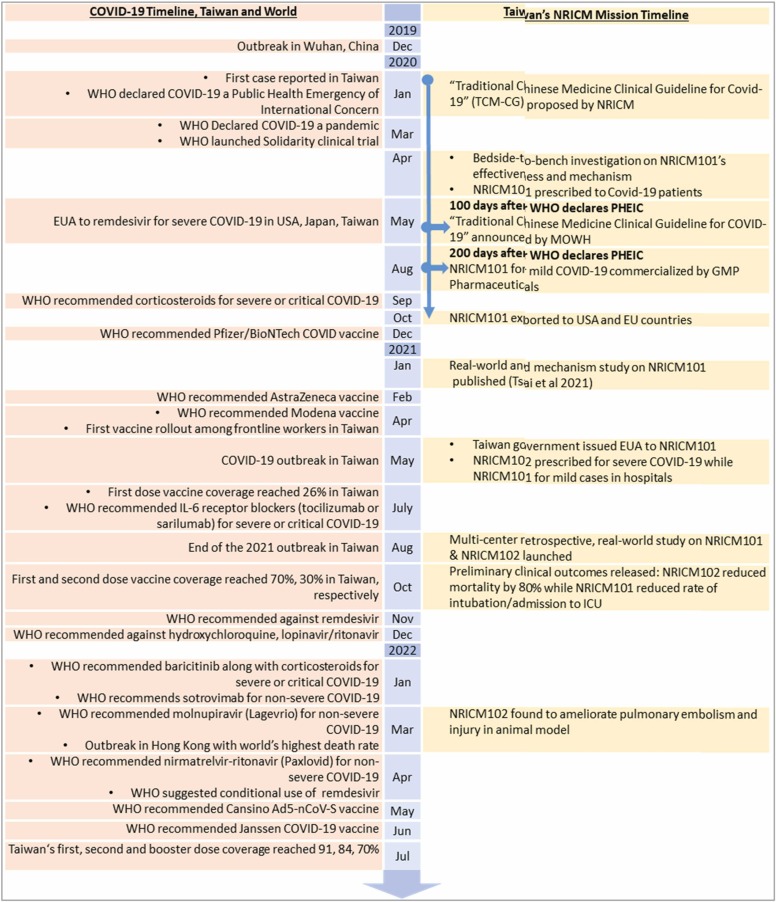
Traditional medicine is practiced in many societies and offers a rich reservoir of safe-to-use drug candidates and experiences in dealing with endemics over a long period of time [15], [19]. Learning from the past yields a higher chance of success, as is the case with NRICM101, which appeared in the “Traditional Chinese Medicine Clinical Guideline for COVID-19″ 100 days after the WHO declared COVID-19 a Public Health Emergency of International Concern (PHEIC) and was commercialized in another 100 days ( Fig. 4). The development and application of NRICM101 and NRICM102 also followed the recommendations of the scientific community by ensuring validation, characterization and standardization of multicomponent herbal therapeutics to facilitate acceptance into mainstream medicine [15]. The high performance liquid chromatography (HPLC) profiles of different batches of NRICM101 and NRICM102 prepared by pharmaceuticals and pharmacies in hospitals are provided in the Supplementary file 3.
Fig. 4.
Covid-19 Timeline & Course of TCM Development and Application.
Other notable examples, including Lianhua Qingwen, Huoxiang Zhengqi [20], Jing Si Herbal Tea [21], and Jing Guan Fang [22] in East Asia, Ashwagandha (Withania somnifera) and Giloy (Tinospora cordifolia) in South Asia [23], and propolis in South America [24], have demonstrated potential in combating COVID-19 with different levels of evidence. Building collaborative networks that transcend the boundary of traditional medicine by incorporating modern and traditional medicine practitioners, bench scientists, and industry in societies where traditional medicine is culturally embedded may facilitate the development of evidence-based, commercially viable options for larger populations during epidemic outbreaks in a timely manner [25], [26].
This study has several limitations. First, the observational study has limited ability to make causal inferences given inherent known and unknown confounders. We used propensity score methods to adjust for known confounders. Second, our study recruited patients in Taiwan, which limited the applicability to the populations in other geographic regions. Third, we were unable to show information on adverse effects as no TCM-associated events was reported. Additionally, pharmacovigilance mechanisms, including the National Adverse Drug Reaction (ADR) reporting system, National Health Insurance Administration and periodic safety update reports by pharmaceutical companies, have not documented the occurrence of serious adverse events after the use of NRICM101 or NRICM102 in Taiwan and overseas. Fourth, this study excluded pregnant women, children and other vulnerable groups because of few case numbers and ethics review regulations. We acknowledge that our results should be interpreted with caution and that further efficacy research is warranted.
5. Conclusion
NRICM101 and NRICM102 were significantly associated with a lower risk of intubation/ICU admission or death among patients with mild-to-severe COVID-19. This study provides real-world evidence of adopting broad-spectrum oral therapeutics and shortening the gap between outbreak and effective response. It offers a new vision in our preparation for future pandemics.
Ethics approval and consent to participate
Institutional Review Board (IRB) approval for data extraction and analysis was obtained from each participating hospital: Shin Kong Wu Ho-Su Memorial Hospital (20211005 R), Tri-Service General Hospital (C202005067), Taipei Hospital (TH-IRB-0021–0026), Chang-Hua Hospital (CS1–21132), Taoyuan General Hospital (TYGH110051), Feng Yuan Hospital (10023), Taichung Veterans General Hospital (SE21380A), Chang Bing Show Chwan Memorial Hospital (1100902), and Chung Shan Medical University Hospital (CS2–21126). The IRB waived the requirement for patient informed consent as this was a non-interventional study utilizing routinely collected data for secondary research purposes.
Funding
The Ministry of Health and Welfare, Taiwan supported the study (NRICM-110T88, MOHW110-NRICM-B-325–000400, MOHW111-NRICM-M-325–122400). The funder has no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
CRediT authorship contribution statement
Yi-Chang Su: Supervision, Conceptualization. Li-Hsiang Wang, Chia-Ching Liaw, Wen-Chi Wei, Keng-Chang Tsai, Wen-Fei Chiou, Yi-Chang Su: TCM development and quality control. Sheng-Mou Hou, Chih-Hung Wang, Shun-Ping Cheng, Kung-Yen Tseng, Yi-Chia Huang, Chien-Jung Lin, Chi-Kuei Lin, Tsung-Lung Tsai, Chen-Shien Lin, Ming-Huei Cheng, Tieng-Siong Fong, Chia-I Tsai, Yu-Wen Lu, Jung-Chih Lin, Yi-Wen Huang, Wei-Chen Hsu, and Hsien-Hwa Kuo: Patient care and clinical data acquisition. Ming-Yung Lee, Shen-Ming Lee and Yu-Hwei Tseng: Data curation, Sunny Jui-Shan Lin, Yi-Chia Huang, Chi-Kuei Lin, Chen-Shien Lin, Tieng-Siong Fong, Wei-Chen Hsu, Ming-Huei Cheng, Chia-I Tsai, Yu-Wen Lu and Jung-Chih Lin: Data abstraction, Yu-Hwei Tseng and Sunny Jui-Shan Lin: Writing – original draft preparation. Jaung-Geng Lin, Yuh-Chiang Shen and Wen-Fei Chiou: Supervision and Reviewing.
Acknowledgements
We acknowledge the contribution and sacrifice of the care providers and personnel in the participating hospitals (Supplementary file 4 provides a full list of the COVID-19 MM & TCM Collaboration Team), and the suffering of the patients and their families through the COVID-19 pandemic.
Declarations
none.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.phrs.2022.106412.
Appendix A. Supplementary material
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Data Availability
Data will be made available on request.
References
- 1.Meganck R.M., Baric R.S. Developing therapeutic approaches for twenty-first-century emerging infectious viral diseases. Nat. Med. 2021;27(3):401–410. doi: 10.1038/s41591-021-01282-0. [DOI] [PubMed] [Google Scholar]
- 2.WHO. WHO expert meeting on evaluation of traditional Chinese Medicine in the treatment of COVID-19. 2022. https://www.who.int/publications/m/item/who-expert-meeting-on-evaluation-of-traditional-chinese-medicine-in-the-treatment-of-covid-19. Accessed June 21, 2022.
- 3.Tsai K.-C., Huang Y.-C., Liaw C.-C., et al. A traditional Chinese medicine formula NRICM101 to target COVID-19 through multiple pathways: a bedside-to-bench study. Biomed. Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.111037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S.-C. Taiwan’s experience in fighting COVID-19. Nat. Immunol. 2021;22(4):393–394. doi: 10.1038/s41590-021-00908-2. [DOI] [PubMed] [Google Scholar]
- 5.Corrigan-Curay J., Sacks L., Woodcock J. Real-world evidence and real-world data for evaluating drug safety and effectiveness. JAMA. 2018;320(9):867–868. doi: 10.1001/jama.2018.10136. [DOI] [PubMed] [Google Scholar]
- 6.Sherman R.E., Anderson S.A., Dal Pan G.J., et al. Real-world evidence—what is it and what can it tell us? Mass Med. Soc. 2016;375(23):2293–2297. doi: 10.1056/NEJMsb1609216. [DOI] [PubMed] [Google Scholar]
- 7.RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19. New Engl. J. Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mikami T., Miyashita H., Yamada T., et al. Risk factors for mortality in patients with COVID-19 in New York City. J. Gen. Intern Med. 2021;36(1):17–26. doi: 10.1007/s11606-020-05983-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverio A., Di Maio M., Citro R., et al. Cardiovascular risk factors and mortality in hospitalized patients with COVID-19: systematic review and meta-analysis of 45 studies and 18,300 patients. BMC Cardiovasc. Disord. 2021;21(1):23. doi: 10.1186/s12872-020-01816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tharaux P.-L., Pialoux G., Pavot A., et al. Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomized controlled trial. Lancet Respir. Med. 2021;9(3):295–304. doi: 10.1016/S2213-2600(20)30556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohl M.E., Miller D.R., Lund B.C., et al. Association of remdesivir treatment with survival and length of hospital stay among US veterans hospitalized with COVID-19. JAMA Netw. Open. 2021;4(7) doi: 10.1001/jamanetworkopen.2021.14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salama C., Han J., Yau L., et al. Tocilizumab in patients hospitalized with COVID-19 pneumonia. New Engl. J. Med. 2020;384(1):20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wootton D. Dexamethasone in hospitalized patients with COVID-19. New Engl. J. Med Overseas Ed. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt B.M., Ribnicky D.M., Lipsky P.E., Raskin I. Revisiting the ancient concept of botanical therapeutics. Nat. Chem. Biol. 2007;3(7):360–366. doi: 10.1038/nchembio0707-360. [DOI] [PubMed] [Google Scholar]
- 16.Fedson D.S. Clinician-initiated research on treating the host response to pandemic influenza. Hum. Vaccin Immunother. 2018;14(3):790–795. doi: 10.1080/21645515.2017.1378292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fedson D.S. Treating the host response to emerging virus diseases: lessons learned from sepsis, pneumonia, influenza and Ebola. Ann. Transl. Med. 2016;4(21):421. doi: 10.21037/atm.2016.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tregoning J.S., Flight K.E., Higham S.L., Wang Z., Pierce B.F. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021;21(10) doi: 10.1038/s41577-021-00592-1. 626-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paudyal V., Sun S., Hussain R., Abutaleb M.H., Hedima E.W. Complementary and alternative medicines use in COVID-19: a global perspective on practice, policy and research. Res Soc. Adm. Pharm. 2022;18(3):2524–2528. doi: 10.1016/j.sapharm.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao M., Tian J., Zhou Y., et al. Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: a randomized controlled trial. Pharmacol. Res. 2020;161 doi: 10.1016/j.phrs.2020.105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsieh P.-C., Chao Y.-C., Tsai K.-W., et al. Efficacy and safety of complementary therapy With Jing Si Herbal Tea in patients with mild-to-moderate COVID-19: a prospective cohort study. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.832321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ping Y.-H., Yeh H., Chu L.-W., et al. The traditional chinese medicine formula Jing Guan Fang for preventing SARS-CoV-2 infection: from clinical observation to basic research. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.744439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shree P., Mishra P., Selvaraj C., et al. Targeting COVID-19 (SARS-CoV-2) main protease through active phytochemicals of ayurvedic medicinal plants–Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy) and Ocimum sanctum (Tulsi)–a molecular docking study. J. Biomol. Struct. Dyn. 2022;40(1):190–203. doi: 10.1080/07391102.2020.1810778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berretta A.A., Silveira M.A.D., Capcha J.M.C., De Jong D. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease: Running title: Propolis against SARS-CoV-2 infection and COVID-19. Biomed. Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao X., Zheng X., Fan T.-P., Li Z., Zhang Y., Zheng J. A novel drug discovery strategy inspired by traditional medicine philosophies. Science. 2015;347(6219):S38–S40. [Google Scholar]
- 26.Tseng Y.-H., Chang F.-R. Bringing scientific methods to traditional medicine. J. Formos. Med Assoc. 2019;118:1574–1575. doi: 10.1016/j.jfma.2019.08.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Data Availability Statement
Data will be made available on request.