Abstract
Severe acute respiratory disease coronavirus 2 (SARS-COV-2) first emerged in Wuhan, China, in December 2019 and has caused a global pandemic of a scale unprecedented in the modern era. People infected with SARS-CoV-2 can be asymptomatic, moderate symptomatic or develop severe COVID-19. Other than the typical acute respiratory distress syndrome (ARDS), patients with moderate or severe COVID-19 also develop a distinctive systemic coagulopathy, known as COVID-19-associated coagulopathy (CAC), which is different from sepsis-related forms of disseminated intravascular coagulation (DIC). Endotheliopathy or endotheliitis are other unique features of CAC. The endothelial cell perturbation can further increase the risk of thrombotic events in COVID-19 patients. In this review, we will summarize the current knowledge on COVID-19 coagulopathy and the possible mechanisms for the condition. We also discuss the results of clinical trials testing methods for mitigating thrombosis events in COVID-19 patients.
Keywords: COVID-19, Coagulopathy, CAC, Endotheliitis, NETs
1. Clinical and lab findings related to COVID-19 coagulopathy
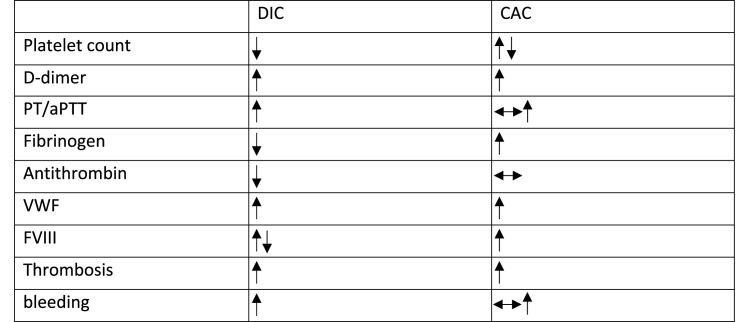
One of the early evaluations of thrombotic events in COVID-19 patients admitted to the intensive care unit (ICU) showed that the incidence of thrombotic events was 31%, of which venous thromboembolic events (VTEs) were the most common, accounting for some 27% of events [1]. This evaluation is consistent with a more recent meta-analysis of VTEs in hospitalized COVID-19 patients [2]. It is also noteworthy to mention that the thrombotic events developed in most COVID-19 patients showed distinct features compared to the well-known DIC, and hence it was named CAC (Table 1 ) [3].
Table 1.
Similarities and differences between CAC and DIC.
Studies from different groups suggested that the level of D-dimer, a fibrin (ogen) degradation product, is dramatically elevated in COVID-19 patients with thrombotic events [[4], [5], [6]]. D-dimer is widely used as a biomarker for diagnosis of thrombotic disorders. Although multiple studies have shown that D-dimer level on hospital admission predicts disease severity, some reports, such as Vincent et al., suggest that, unless DIC is suspected, repeated measurement of D-dimer level has little value [3].
Other important characteristics of CAC include significantly increased circulating levels of Von Willebrand factor (VWF) and factor VIII, as well as increased activity of VWF [7]. The elevated levels of coagulation factors in CAC do not cause prolongation of activated partial thromboplastin time (aPTT) or prothrombin time (PT). This is in contrast to DIC, in which the PT is prolonged [8].
Another important finding related to CAC is endotheliopathy or endotheliitis, indicating the direct infection of endothelial cells by the virus. Indeed, biopsies from patients showed the presence of viral particles in endothelial cells within different organs [9]. Endotheliitis increases the risk of thrombotic events in patients with COVID-19 pneumonia, and autopsy studies have confirmed the presence of macrovascular and microvascular thrombi in the lungs and other organs of non-survivors of COVID-19 [[9], [10], [11]].
Although platelets play an important role in thrombosis, many patients with COVID-19 have only slightly lowered platelet count (between 100 x 10^9 and 150 x 10^9/L). Severe thrombocytopenia is rarely seen, only occurring in less than 5% of patients [12]. Importantly, thrombocytopenia associated with a higher risk of severe COVID disease [13].
2. Possible mechanisms of COVID-19 coagulopathy
2.1. Platelets
Since platelets are pivotal for thrombus formation, their role in SARS-CoV-2 mediated coagulopathy has been widely-studied. Evidence has shown that traces of SARS-CoV-2 mRNA are detectable in isolated platelets by RT-qPCR, and virions have been visualized within platelet sections by electron microscopy [[14], [15], [16]]. In contrast, Bury et al. couldn't detect SARS-CoV-2 mRNA in either platelets or serum samples, despite the fact that some patients had high viral loads [17]. They concluded that SARS-CoV-2 could only occasionally enter into platelets and this was not considered clinically significant.
Although whether or not platelets can interact with and internalize SARS-CoV-2 is still controversial, several receptors on platelets have been suggested to mediate the binding and internalization of SARS-CoV-2; however, published data on this is controversial. Angiotensin converting enzyme-2 (ACE2) is the major receptor for RBD (receptor binding domain) of SARS-CoV-2 spike protein [18], and its expression on platelets was only recently explored. Manne et al. [19] couldn't detect platelet ACE2 mRNA or ACE2 protein using RNA-seq, RT-PCR, and western blotting. These findings were confirmed by Zaid et al. [14] using qRT-PCR analysis. However, Zhang et al. [15] could detect robust expression of ACE2 in both human and mouse platelets using RT-PCR and western blotting. The possibility of those different results could be due to the way platelets were prepared, as suggested by Campbell et al. [16].
Another plausible receptor group is the RGD (Arg-Gly-Asp) binding integrins, particularly platelet expressing integrin αIIbβ3. Early studies noted the presence of an RGD motif in the RBD of SARS-CoV-2 spike glycoprotein, leading to the hypothesis that RGD binding integrins may be involved in SARS-CoV-2 infection [20,21]. However, a recent molecular dynamics microscale simulation did not support this hypothesis [22]. Unlike the RGD motifs of known integrin ligands, usually located in a flexible loop (so-called RGD finger), the SARS2-S RGD motif resides in a rigid α-helical structure, leaving the sidechain orientation unfavorable for integrin binding.
Another potential receptor for SARS-CoV-2 on platelets is CD147 (basigin). Direct interaction between CD147 and SARS-CoV-2 spike protein has been reported using a range of different techniques [23]. Another paper from the same group suggests that CD147 is a universal receptor for different strains of SARS-CoV-2 [24]. However, Shilts et al. [25] couldn't detect the interaction between overexpressed CD147 and recombinant spike protein using flow cytometry. Similarly, ELISA and avidity-based binding assays failed to detect their interaction. Moreover, Ragotte et al. [26] also failed to detect the interaction using both size exclusion chromatography and SPR.
2.2. Endotheliitis
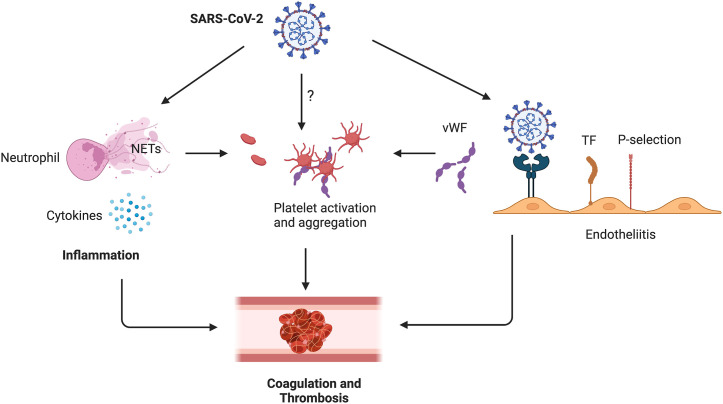
Endothelial cells play a pivotal role in inflammation-induced coagulation in COVID-19 (Fig. 1 ). The pro-inflammatory response to COVID-19 results in the release of pro-inflammatory cytokines, such as cytokines interleukin-1 beta (IL1β), interleukin-6 (IL6), and TNF, all of which contribute to endotheliitis in COVID-19 patients [27,28]. IL6 can further promote the release of pro-inflammatory cytokines by endothelial cells, which enhances cytokine secretion [29]. IL-1β and TNF can activate glucuronidases, which can degrade the glycocalyx, increase the deposition of hyaluronic acid in the extracellular matrix, and promote fluid retention via upregulation of expression of hyaluronic acid synthase 2 [29]. Since ACE2 receptors are also expressed by endothelial cells, binding of SARS-CoV-2 to ACE2 on endothelial cells impairs the activity of ACE2 [30] and results in endothelial cell activation [31,32]. All of these events can lead to the disruption of vascular integrity and endothelial cell death, thereby causing exposure of the thrombogenic basement membrane and the activation of the clotting cascade [33]. Further, endothelial cell activation can lead to elevated plasma VWF antigen and down-regulation of the protein disintegrin and metalloproteinase with thrombospondin motifs 13 (ADAMTS13), which results in an abnormal ratio of VWF to ADAMTS-13 [34]. Endothelial cell activation can also increase the level of plasminogen activator inhibitor 1 (PAI-1), inhibiting tissue plasminogen activator (tPA), thereby reducing fibrinolysis [35,36]. The resulting hypofibrinolytic state can increase fibrin deposition in alveolar tissues and the microvasculature, leading to ARDS [37].
Fig. 1.
Formation of CAC in COVID-19 patients. SARS-CoV-2 infection will cause neutrophil activation and NET release, resulting in pathological prothrombotic environment. Pro-inflammatory cytokines can activate neutrophils and endothelial cells, which will further stimulate thrombosis. SARS-CoV-2 might be able to interact with platelets directly, leading to platelet activation and thrombosis. SARS-CoV-2 binding to ACE2 on endothelial cells causes endotheliitis, shifting the vascular equilibrium towards a procoagulant state.
2.3. Neutrophil extracellular traps (NETs)
Another important factor contributing to coagulation in COVID-19 is the elevated NETs released by circulatory or infiltrating neutrophils in COVID-19 patients. Although NETs are important for preventing pathogen invasion, activated neutrophils and NETs can also contribute to a pathological prothrombotic environment (Fig. 1) [38,39]. NETs can stimulate DVT via a third thrombus scaffold acting with elevated fibrin and VWF [40]. Additionally, cell-free DNA from NETs have been implicated in activation of the coagulation pathway [41,42]. Moreover, the expression of tissue factor in NETs and neutrophil-derived microparticles can directly activate the coagulation pathway [[43], [44], [45]].
Several mechanisms potentially contribute to COVID-19 induced NET formation. Both in vivo and in vitro studies have shown that SARS-CoV-2 can directly interact with healthy neutrophils and induce NET release through ACE2, virus replication, and PAD-4 signaling [46,47]. Pro-inflammatory cytokines such as IL-8 and IL-1β can also induce NET release in tissues and intravascular neutrophils [48,49]. Platelet activation is another factor triggering NET release through toll-like receptor 4 (TLR4), platelet factor 4 (PF4), and extracellular vesicle-dependent processes [50,51]. The direct interaction between activated platelets and neutrophils could also enhance NET formation and thromboinflammation in COVID-19 patients. Indeed, previous studies have shown that platelet surface proteins, such as P-selectin, TLR4, ICAM-2, platelet glycoprotein GPIb, CD40L, could bind to their receptors on the neutrophil, promoting NET release [[51], [52], [53], [54]].
3. Clinical trials related to COVID-19 coagulopathy so far
In order to prevent or treat coagulopathy in COVID-19 patients, several drugs have been tested in clinical trials (Table 2 ). Here, we summarized the clinical trial results and the efficacy of tested drugs in anticoagulation.
Table 2.
Anti-COVID-19 drugs in clinical trials targeting coagulopathy.
| Intervention | Trial No. | Patient status | Cohort size | Significant or not of primary outcome | Reference No. |
|---|---|---|---|---|---|
| Prophylactic heparin | N/A | Hospitalized | 4297 | Yes | [55] |
| NCT04662684 | Discharged at high risk of VT | 320 | Yes | [64] | |
| Therapeutic-dose heparin | NCT04372589, NCT04505774, NCT02735707, NCT04359277 | Noncritically ill | 2219 | Yes | [56] |
| NCT02735707, NCT04505774, NCT04359277, NCT04372589 | Critically ill | 1098 | No | [57] | |
| NCT04362085 | Moderately ill with increased D-dimer level | 465 | No | [59] | |
| NCT04394377 | Hospitalized with increased D-dimer level | 615 | No | [60] | |
| REBEC RBR-949z6v | Requiring mechanical ventilation | 20 | Yes | [61] | |
| Therapeutic or intermediate-dose heparin | NCT04401293 | Hospitalized with increased D-dimer level | 257 | No | [58] |
| Intermediate or standard-dose heparin | N/A | Critically ill | 562 | No | [62] |
| NCT04360824 | ICU and/or had coagulopathy | 176 | No | [63] | |
| Aspirin | NCT04381936 | Hospitalized | 14,892 | No | [65] |
| Aspirin or therapeutic dose heparin | NCT04498273 | outpatients | 700 | No | [66] |
| Aspirin or P2Y12 inhibitor | NCT02735707 | Critically ill | 1557 | No | [67] |
| Therapeutic dose heparin with or without P2Y12 inhibitor | NCT04505774 | Non-critically ill | 562 | No | [68] |
| TCZ | N/A | Patients with COVID-19 | 11 | No | [71] |
| CTRI/2020/05/025,369 | Hospitalized | 180 | No | [73] | |
| NCT04356937 | Moderately ill | 243 | No | [74] | |
| NCT04372186 | Hospitalized without mechanical ventilation | 389 | No | [75] | |
| NCT04403685 | Severely or critically ill | 129 | No | [76] | |
| N/A | 42 | Yes | [80] | ||
| ChiCTR2000029765 | Patients confirmed by PCR | 65 | Yes | [77] | |
| NCT04317092 | 1221 | Yes | [79] | ||
| Methylprednisolone with or without TCZ | N/A | Hospitalized | 76 | No | [72] |
| TCZ or sarilumab | NCT02735707 | Critically ill | 895 | Yes | [78] |
| Subcutaneous TCZ | IRCT20150303021315N17 | Severely and critically ill | 126 | Yes | [81] |
| Low-dose TCZ | NCT04331795 | Hospitalized | 32 | Yes | [82] |
| Colchicine | NCT04322682 | PCR confirmed | 4488 | No | [84] |
| N/A | Suspects in the community | 2755 | No | [85] | |
| NCT04381936 | Hospitalized | 11,340 | No | [86] | |
| NCT04326790 | 105 | Yes | [87] | ||
| NCT04350320 | 103 | No | [88] | ||
| NCT04328480 | 1279 | No | [91] | ||
| IRCT20190810044500N5 | Moderately to severely ill | 153 | Yes | [89] | |
| RBR- 8jyhxh | 72 | Yes | [92] | ||
| NCT04367168 | Severely ill | 116 | No | [90] | |
| Dexamethasone | NCT04381936 | Hospitalized | 6425 | Yes | [93] |
| NCT04327401 | Moderately to severely ill | 299 | Yes | [94] | |
| High vs standard dose dexamethasone | NCT04509973 | Severe hypoxemia | 1000 | No | [95] |
| NCT04509973 | 1000 | Yes | [96] | ||
| Low vs medium vs high dose dexamethasone | IRCT20100228003449N31 | Moderately to severely ill | 133 | No | [97] |
| High vs low dose dexamethasone | NCT04395105 | ARDS | 98 | Yes | [98] |
| Systemic corticosteroids with or without TCZ | NCT04381936 | Hypoxia and systemic inflammation | 4116 | Yes | [99] |
| Systemic corticosteroids with or without baricitinib | NCT04421027 | Hospitalized | 1525 | No | [100] |
| Inhaled budesonide | NCT04416399 | Mild | 146 | Yes | [101] |
| ISRCTN86534580 | At higher risk of complications | 2530 | Yes | [102] | |
| Inhaled ciclesonide | NCT04377711 | Nonhospitalized | 413 | No | [103] |
| Inhaled and intranasal ciclesonide | NCT04435795 | Outpatients | 203 | No | [104] |
N/A, not applicable; VT, venous thromboembolism.
3.1. Heparin-related clinical trials
An observational clinical trial evaluated whether early initiation of prophylactic anticoagulation compared with no anticoagulation was associated with decreased risk of death in hospitalized patients with COVID-19 [55]. This large trial found that prophylactic heparin treatment reduced 30-day mortality and didn't increase serious bleeding events.
Several clinical trials have evaluated the efficacy of therapeutic-dose anticoagulation in hospitalized patients. The large ATTACC, ACTIV-4a, and REMAP-CAP multiplatform trial is designed to evaluate the effects of therapeutic-dose anticoagulation with unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH) in non-critically ill and critically ill patients with COVID-19, respectively [56,57]. For non-critically ill patients, therapeutic-dose heparin improved organ support-free days but didn't improve mortality or length of hospitalization. For critically ill patients, therapeutic-dose heparin did not improve the primary outcome. The HEP-COVID clinical trial tested the efficacy of therapeutic-dose LMWH vs standard prophylactic, or intermediate-dose LMWH in high-risk patients hospitalized with COVID-19 [58]. Their results showed that therapeutic-dose LMWH reduced VTE, arterial thromboembolism (ATE), and death in non-ICU patients with highly elevated levels of D-dimer. The RAPID randomized clinical trial also investigated the efficacy of therapeutic dose heparin (LMWH or UFH) in adult COVID-19 patients with elevated D-dimer levels [59]. Although there was no significant improvement of the primary outcome, therapeutic dose heparin was found to significantly reduce mortality. The ACTION clinical trial evaluated whether therapeutic anticoagulation can improve clinical outcomes in hospitalized patients displaying an elevated level of D-dimer [60]. Compared with prophylactic anticoagulation, therapeutic dose of rivaroxaban did not improve clinical outcomes. The HESACOVID studied whether therapeutic enoxaparin can improve gas exchange and increase the mechanical ventilation-free days in severely ill COVID-19 patients [61]. Although therapeutic enoxaparin intervention resulted in improved primary outcome and increased mechanical ventilation-free days, due to the small sample size, this investigation cannot accurately assess the difference in mortality between these two groups.
Two clinical trials also examined the effects of intermediate-dose heparin treatment in COVID-19 patients. The INSPIRATION clinical trial evaluated the efficacy of intermediate-dose enoxaparin in COVID-19 patients admitted to ICU [62]. Trial results suggest that intermediate-dose, compared with standard-dose, prophylactic enoxaparin had no benefit in ICU patients. Another smaller trial also found that intermediate-dose enoxaparin did not prevent mortality and thrombosis in adult patients admitted to ICU and/or with laboratory test evidence of coagulopathy [63].
3.2. Clinical trials after patient discharge
The MICHELLE trial examined the efficacy of rivaroxaban for post-discharge thromboprophylaxis in 320 patients hospitalized with COVID-19 and at increased risk for venous thromboembolism [64]. Extended post-discharge thromboprophylaxis with rivaroxaban 10 mg/day for 35 days significantly improved primary clinical outcomes without increasing bleeding events.
4. Clinical trials targeting platelets
The large RECOVERY trial examined whether standard care plus 150 mg aspirin could reduce 28-day mortality in patients hospitalized with COVID-19 [65]. Their results suggested that aspirin intervention didn't improve the primary outcome. The ACTIV-4B outpatient thrombosis prevention trial examined the benefits of aspirin and therapeutic-dose apixaban in symptomatic but clinically stable outpatients with COVID-19 [66]. The study was terminated after enrolment of 9% of participants since treatment with aspirin or apixaban did not improve the composite clinical outcome. The large REMAP-CAP trial tested whether aspirin or P2Y12 inhibitor could improve outcomes in critically ill patients with COVID-19 [67]. Their results showed that treatment with aspirin or P2Y12 inhibitor didn't improve the organ support-free days within 21 days. Another randomized clinical trial investigated the addition of P2Y12 inhibitor to anticoagulant therapy in non-critically ill patients hospitalized for COVID-19 [68]. Compared to therapeutic dose of heparin alone, addition of P2Y12 inhibitor didn't improve organ support-free days within 21 days of hospitalization.
4.1. Clinical trials related to anti-inflammation
4.1.1. Tocilizumab
Tocilizumab (TCZ) is a humanized monoclonal antibody which blocks the IL-6 signaling pathway through competitive inhibition of IL-6 receptor (IL-6R). Several clinical trials have evaluated its therapeutic effect and its effect on the cytokine storm in COVID-19 patients. Most of the clinical trials did not show improvement in COVID-19 patients with TCZ intervention [[69], [70], [71], [72], [73], [74], [75], [76]]. It is noticeable that in one clinical trial significantly increased IL-6, α-defensin, a pro-thrombotic peptide, and D-dimers were observed after TCZ intervention [69]. Another clinical trial examined the serum profile of 12 cytokines from COVID-19 patients before and after TCZ administration [70]. Their result indicated that except IL-6, the concentration of IL-1β, −2, −4, −10, −12p70, -18 and sIL-6R were all unexpected increased after TCZ treatment, suggesting that some inflammatory pathways escape IL-6R blockade and were even amplified by treatment. However, several clinical trials indicated that TCZ could be beneficial for COVID-19 patients [[77], [78], [79], [80], [81], [82]]. The major improvement after TCZ administration was for hypoxia in moderate, severe or critically ill patients with COVID-19 [[77], [78], [79], [80], [81]]. Two clinical trials also showed that TCZ, if used at the early stages of respiratory failure, could improve clinical parameters and reduce the risk of death for severe or critically ill patients [80,81].
4.1.2. Colchicine
Colchicine is an alkaloidal anti-inflammatory compound and can bind to free tubulin dimers and prevent microtubule polymerization, which is considered to be important in inhibiting coronavirus infection [83]. The COLCORONA and PRINCIPLE trials were two large scale clinical trials to examine the efficacy of colchicine in non-hospitalized patients [84,85]. The COLCORONA trial found only subtle improvement in patients whose diagnosis was confirmed by a positive PCR result. Similarly, the PRINCIPAL trial didn't find any benefits for patients treated with colchicine. The RECOVERY, COLCOVID, and GRECCO-19 trials investigated the effect of colchicine in hospitalized patients [[86], [87], [88]]. There were no statistically significant differences between the colchicine and usual care patients in both RECOVERY and COLCOVID trials. Although the GRECCO-19 trial suggested that colchicine intervention could reduce clinical deterioration, the interpretation of this result should be viewed with caution as the study was open-label and both the sample size and number of clinical events were relatively small. Several small, randomized trials have also evaluated colchicine in hospitalized patients [[89], [90], [91], [92]]. Some of those trials suggested benefits of intervention with colchicine, which could reduce oxygen supplement and levels of certain inflammatory markers. However, the results were again difficult to interpret due to small sample size and open-label design.
4.1.3. Corticosteroids
Several clinical trials have investigated the efficacy and dosage of corticosteroids for treatment of COVID-19. The RECOVERY trial reported that dexamethasone was beneficial in hospitalized patients who were mechanically ventilated or who required supplemental oxygen at enrolment [93]. However, there was no benefit in patients who did not require supplemental oxygen at enrolment of dexamethasone. Although the CoDEX clinical trial terminated early, the results suggested that dexamethasone was beneficial in hospitalized patients [94]. The COVID STEROID 2 trial investigated the use of dexamethasone with 6 mg or 12 mg once a day for up to 10 days in hospitalized patients with severe hypoxemia [95]. A higher dose of dexamethasone did not result in better primary outcomes compared with low dose. However, a pre-planned secondary Bayesian analysis of the COVID STEROID 2 trial found a higher probability of benefit and a lower probability of harm for the high dose compared with low dose [96]. Another two small randomized trials also examined the dosage of dexamethasone in COVID-19 patients. Data from one trial suggested that a lower dose of dexamethasone (8 mg once daily) resulted in a shorter time to clinical improvement, a lower frequency of adverse events, and lower mortality rate compared to intermediate or high dose of dexamethasone treatment [97]. However, the second small randomized trial did not find any difference in primary outcome [98]. Moreover, several clinical trials also investigated the efficacy of systemic corticosteroid in combination with other immunomodulators, such as TCZ [99,78] and baricitinib [100]. Their results showed benefits in certain subsets of hospitalized patients, especially those with early critical illness or with signs of systemic inflammation. Inhaled corticosteroids, because of their direct anti-inflammatory effects on the lungs, have also been tested for treatment of COVID-19. Although inhaled budesonide [101,102] and ciclesondie [103,104] have shown some benefits to patients with COVID-19, these findings should be interpreted with caution due to the small sample size and open-label design.
5. Conclusion
Patients with moderate or severe COVID-19 developed a distinctive systemic coagulopathy, known as CAC. In addition to classical coagulation pathways, the acute inflammatory response caused endotheliitis and NETs also contributed to CAC. Based on the current literature and clinical trial data, there is evidence that several procedures are beneficial in management of coagulopathy in COVID-19 patients. For non-hospitalized patients without evidence of VTE, anticoagulants or anti-platelet therapy should be avoided. VTE prophylaxis should not be used in patients after hospital discharge. In hospitalized COVID patients, LMWH or UFH, rather than oral anticoagulants, is recommended for treatment. For adult patients who require low-flow oxygen, but not ICU care, therapeutic doses of heparin seem to be clinically beneficial. For adult patients who require ICU care, prophylactic doses of heparin can be used for treatment but intermediate or therapeutic doses of heparin should be avoided. Of the drugs designed to mitigate inflammation responses, colchicine should be avoided for treatment of both non-hospitalized and hospitalized patients. Dexamethasone can be applied to non-hospitalized patients for an underlining condition and to hospitalized patients who require supplemental oxygen. Finally, data supports the cautious use of TCZ or sarilumab in hospitalized patients who need oxygen supplementation.
Declaration of competing interest
Authors declare that they have no competing interests.
Practice points.
-
•
In hospitalized COVID patients, LMWH or UFH is preferred for treatment.
-
•
Therapeutic does of heparin is beneficial to non-ICU patients but not ICU patients.
-
•
Dexamethasone, TCZ or sarilumab can be applied to patients under certain coditions.
Research agenda.
-
•
Investigate the causes of CAC in COVID-19 patients.
-
•
Investigate the clinical trials targeting coagulopathy in COVID-19 patients.
References
- 1.Klok F.A., Kruip M.J.H.A., Meer NJM van der, Arbous M.S., Gommers D.A.M.P.J., Kant K.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:56. doi: 10.1016/j.thromres.2020.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiménez D., García-Sanchez A., Rali P., Muriel A., Bikdeli B., Ruiz-Artacho P., et al. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Chest. 2021;159:1182–1196. doi: 10.1016/j.chest.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincent J.L., Levi M., Hunt B.J. Prevention and management of thrombosis in hospitalised patients with COVID-19 pneumonia. Lancet Respir Med. 2022;10:214–220. doi: 10.1016/S2213-2600(21)00455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemostasis. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Léonard-Lorant I., Delabranche X., Séverac F., Helms J., Pauzet C., Collange O., et al. Acute pulmonary embolism in patients with COVID-19 at CT angiography and relationship to d-dimer levels. Radiology. 2020;296:E189–E191. doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iba T., Levy J.H., Connors J.M., Warkentin T.E., Thachil J., Levi M. The unique characteristics of COVID-19 coagulopathy. Crit Care. 2020;24:4–11. doi: 10.1186/s13054-020-03077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/nejmoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Remmelink M., De Mendonça R., D'Haene N., De Clercq S., Verocq C., Lebrun L., et al. Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Crit Care. 2020;24:1–10. doi: 10.1186/s13054-020-03218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsen J.B., Pasalic L., Hvas A.-M. Platelets in coronavirus disease 2019. Semin Thromb Hemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaid Y., Puhm F., Allaeys I., Naya A., Oudghiri M., Khalki L., et al. Platelets can associate with SARS-CoV-2 RNA and are hyperactivated in COVID-19. Circ Res. 2020 doi: 10.1161/CIRCRESAHA.120.317703. 1404–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang S., Liu Y., Wang X., Yang L., Li H., Wang Y., et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol. 2020;13:1–22. doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell R.A., Boilard E., Rondina M.T. Is there a role for the ACE2 receptor in SARS-CoV-2 interactions with platelets? J Thromb Haemostasis. 2021;19:46–50. doi: 10.1111/jth.15156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bury L., Camilloni B., Castronari R., Piselli E., Malvestiti M., Borghi M., et al. Search for SARS-CoV-2 RNA in platelets from COVID-19 patients. Platelets. 2021;32:284–287. doi: 10.1080/09537104.2020.1859104. [DOI] [PubMed] [Google Scholar]
- 18.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 19.Kanth Manne B., Denorme F., Middleton E.A., Portier I., Rowley J.W., Stubben C., et al. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136:1317–1329. doi: 10.1182/blood.2020007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sigrist C.J.A., Bridge A., Mercier P Le. 2020. A potential role for integrins in host cell entry by SARS-CoV-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makowski L., Olson-Sidford W., Weisel J.W. Biological and clinical consequences of integrin binding via a rogue rgd motif in the sars cov-2 spike protein. Viruses. 2021;13 doi: 10.3390/v13020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Othman H., Messaoud H Ben, Khamessi O., Ben-Mabrouk H., Ghedira K., Bharuthram A., et al. SARS-CoV-2 spike protein unlikely to bind to integrins via the arg-gly-asp (RGD) motif of the receptor binding domain: evidence from structural analysis and microscale Accelerated molecular dynamics. Front Mol Biosci. 2022;9:1–11. doi: 10.3389/fmolb.2022.834857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang K., Chen W., Zhang Z., Deng Y., Lian J.Q., Du P., et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Targeted Ther. 2020;5:1–10. doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geng J., Chen L., Yuan Y., Wang K., Wang Y., Qin C., et al. CD147 antibody specifically and effectively inhibits infection and cytokine storm of SARS-CoV-2 and its variants delta, alpha, beta, and gamma. Signal Transduct Targeted Ther. 2021;6 doi: 10.1038/s41392-021-00760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shilts J., Crozier T.W.M., Greenwood E.J.D., Lehner P.J., Wright G.J. No evidence for basigin/CD147 as a direct SARS-CoV-2 spike binding receptor. Sci Rep. 2021;11:1–10. doi: 10.1038/s41598-020-80464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ragotte R.J., Pulido D., Donnellan F.R., Hill M.L., Gorini G., Davies H., et al. Human basigin (CD147) does not directly interact with SARS-CoV-2 spike glycoprotein. mSphere. 2021;6:1–11. doi: 10.1128/msphere.00647-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pons S., Fodil S., Azoulay E., Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care. 2020;24:4–11. doi: 10.1186/s13054-020-03062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levi M., Thachil J. Coronavirus disease 2019 coagulopathy: disseminated intravascular coagulation and thrombotic microangiopathy-either, neither, or both. Semin Thromb Hemost. 2020;46:781–784. doi: 10.1055/s-0040-1712156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perico L., Benigni A., Casiraghi F., Ng L.F.P., Renia L., Remuzzi G. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat Rev Nephrol. 2021;17:46–64. doi: 10.1038/s41581-020-00357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw R.J., Bradbury C., Abrams S.T., Wang G., Toh C.H. COVID-19 and immunothrombosis: emerging understanding and clinical management. Br J Haematol. 2021;194:518–529. doi: 10.1111/bjh.17664. [DOI] [PubMed] [Google Scholar]
- 33.Nachman R.L., Rafii S. Platelets, petechiae, and preservation of the vascular wall. N Engl J Med. 2008;359:1261–1270. doi: 10.1056/NEJMRA0800887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward S.E., Fogarty H., Karampini E., Lavin M., Schneppenheim S., Dittmer R., et al. ADAMTS13 regulation of VWF multimer distribution in severe COVID-19. J Thromb Haemostasis. 2021;19:1914–1921. doi: 10.1111/jth.15409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramanathan K., Antognini D., Combes A., Paden M., Zakhary B., Ogino M., et al. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020;19–21 doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henry B.M., Vikse J., Benoit S., Favaloro E.J., Lippi G. Hyperinflammation and derangement of renin-angiotensin-aldosterone system in COVID-19: a novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clin Chim Acta. 2020;507:167–173. doi: 10.1016/j.cca.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whyte C.S., Morrow G.B., Mitchell J.L., Chowdary P., Mutch N.J. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. J Thromb Haemostasis. 2020;18:1548–1555. doi: 10.1111/jth.14872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinod K., Wagner D.D. Thrombosis: tangled up in NETs. Blood. 2014;123:2768–2776. doi: 10.1182/blood-2013-10-463646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kapoor Sargam, Aman Opneja, Ln The role of neutrophils in thrombosis. Thromb Res. 2018;170 doi: 10.1016/j.thromres.2018.08.005. The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuchs T a, Brill A., Wagner D.D. NET impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2012;32:1777–1783. doi: 10.1161/ATVBAHA.111.242859.NET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Busch M.H., Timmermans S.A.M.E.G., Nagy M., Visser M., Huckriede J., Aendekerk J.P., et al. Neutrophils and contact activation of coagulation as potential drivers of COVID-19. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.050656. 1787–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becker R.C. COVID-19 update: covid-19-associated coagulopathy. J Thromb Thrombolysis. 2020;50:54–67. doi: 10.1007/s11239-020-02134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y., Luo L., Braun O., Westman J., Madhi R., Herwald H., et al. Neutrophil extracellular trap-microparticle complexes enhance thrombin generation via the intrinsic pathway of coagulation in mice. Sci Rep. 2018;8:1–14. doi: 10.1038/s41598-018-22156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skendros P., Mitsios A., Chrysanthopoulou A., Mastellos D.C., Metallidis S., Rafailidis P., et al. Complement and tissue factor–enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J Clin Invest. 2020;130:6151–6157. doi: 10.1172/JCI141374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fletcher-sandersjöö A., Bellander B. Is COVID-19 associated thrombosis caused by overactivation of the complement cascade? A literature review. Thromb Res. 2020 doi: 10.1016/j.thromres.2020.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veras F., Pontelli M., Silva C., Toller-Kawahisa J., Lima M de, Nascimento D., et al. SARS-CoV-2 triggered neutrophil extracellular traps (NETs) mediate COVID-19 pathology. J Exp Med. 2020:217. doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arcanjo A., Logullo J., Menezes C.C.B., de Souza Carvalho Giangiarulo T.C., dos Reis M.C., de Castro G.M.M., et al. The emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID-19) Sci Rep. 2020;10:1–11. doi: 10.1038/s41598-020-76781-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yaqinuddin A., Kashir J. Novel therapeutic targets for SARS-CoV-2-induced acute lung injury: targeting a potential IL-1β/neutrophil extracellular traps feedback loop. Med Hypotheses. 2020:143. doi: 10.1016/j.mehy.2020.109906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park J.H., Lee H.K. Re-Analysis of single cell transcriptome reveals that the NR3C1-CXCL8-neutrophil Axis determines the severity of COVID-19. Front Immunol. 2020;11:1–9. doi: 10.3389/fimmu.2020.02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sung P.S., Hsieh S.L. C-type lectins and extracellular vesicles in virus-induced NETosis. J Biomed Sci. 2021;28:1–12. doi: 10.1186/s12929-021-00741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clark S.R., Ma A.C., Tavener S.A., McDonald B., Goodarzi Z., Kelly M.M., et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 52.Pitchford S., Pan D., Welch H.C.E. Platelets in neutrophil recruitment to sites of inflammation. Curr Opin Hematol. 2017;24:23–31. doi: 10.1097/MOH.0000000000000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Evangelista V., Manarini S., Rotondo S., Martelli N., Polischuk R., McGregor J.L., et al. Platelet/polymorphonuclear leukocyte interaction in dynamic conditions: evidence of adhesion cascade and cross talk between P-selectin and the β2 integrin CD11b/CD18. Blood. 1996;88:4183–4194. doi: 10.1182/blood.v88.11.4183.4183. [DOI] [PubMed] [Google Scholar]
- 54.Zarbock A., Müller H., Kuwano Y., Ley K. PSGL-1-dependent myeloid leukocyte activation. J Leukoc Biol. 2009;86:1119–1124. doi: 10.1189/jlb.0209117. [DOI] [PubMed] [Google Scholar]
- 55.Rentsch C.T., Beckman J.A., Tomlinson L., Gellad W.F., Alcorn C., Kidwai-Khan F., et al. Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: cohort study. BMJ. 2021;372 doi: 10.1136/bmj.n311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.The ATTACC, ACTIV-4a and R-CI. Therapeutic anticoagulation with heparin in noncritically ill patients with covid-19. N Engl J Med. 2021;385:790–802. doi: 10.1056/nejmoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.The REMAP-CAP, ACTIV-4a and AI. Therapeutic anticoagulation with heparin in critically ill patients with covid-19. N Engl J Med. 2021;385:777–789. doi: 10.1056/nejmoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spyropoulos A.C., Goldin M., Giannis D., Diab W., Wang J., Khanijo S., et al. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial. JAMA Intern Med. 2021;181:1612–1620. doi: 10.1001/jamainternmed.2021.6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sholzberg M., Tang G.H., Rahhal H., Alhamzah M., Kreuziger L.B., Áinle F.N., et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ. 2021;375 doi: 10.1136/bmj.n2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lopes R.D., de Barros e Silva P.G.M., Furtado R.H.M., Macedo A.V.S., Bronhara B., Damiani L.P., et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397:2253–2263. doi: 10.1016/S0140-6736(21)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lemos A.C.B., Santo Da do E., Salvetti M.C., Gilio R.N., Agra L.B., Pazin-Filho A., et al. Therapeutic versus prophylactic anticoagulation for severe COVID-19: a randomized phase II clinical trial (HESACOVID) Thromb Res. 2020;14(4):293. doi: 10.1016/j.thromres.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bikdeli B., Talasaz A.H., Rashidi F., Bakhshandeh H., Rafiee F., Rezaeifar P., et al. Intermediate-dose versus standard-dose prophylactic anticoagulation in patients with COVID-19 admitted to the intensive care unit: 90-day results from the INSPIRATION randomized trial. Thromb Haemostasis. 2021;122:131–141. doi: 10.1055/a-1485-2372. [DOI] [PubMed] [Google Scholar]
- 63.Perepu U.S., Chambers I., Wahab A., Ten Eyck P., Wu C., Dayal S., et al. Standard prophylactic versus intermediate dose enoxaparin in adults with severe COVID-19: a multi-center, open-label, randomized controlled trial. J Thromb Haemostasis. 2021;19:2225–2234. doi: 10.1111/jth.15450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramacciotti E., Leandro Barile Agati, Calderaro D., Aguiar V.C.R., Spyropoulos A.C., Oliveira CCC de, et al. Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 (MICHELLE): an open-label, multicentre, randomised, controlled trial Eduardo. Lancet. 2022:19–21. doi: 10.1016/S0140-6736(21)02392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Horby P.W., Pessoa-Amorim G., Staplin N., Emberson J.R., Campbell M., Spata E., et al. Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2022;399:143–151. doi: 10.1016/S0140-6736(21)01825-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Connors J.M., Brooks M.M., Sciurba F.C., Krishnan J.A., Bledsoe J.R., Kindzelski A., et al. Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19: the ACTIV-4B randomized clinical trial. JAMA, J Am Med Assoc. 2021;326:1703–1712. doi: 10.1001/jama.2021.17272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bradbury C.A., Lawler P.R., Stanworth S.J., McVerry B.J., McQuilten Z., Higgins A.M., et al. Effect of antiplatelet therapy on survival and organ support-free days in critically ill patients with COVID-19: a randomized clinical trial. JAMA, J Am Med Assoc. 2022;327:1247–1259. doi: 10.1001/jama.2022.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berger J.S., Kornblith L.Z., Gong M.N., Reynolds H.R., Cushman M., Cheng Y., et al. Effect of P2Y12 inhibitors on survival free of organ support among non-critically ill hospitalized patients with COVID-19: a randomized clinical trial. JAMA, J Am Med Assoc. 2022;327:227–236. doi: 10.1001/jama.2021.23605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abdeen S., Abu-Fanne R., Bdeir K., Maraga E., Higazi M., Cines D.B., et al. Divergent impacts of tocilizumab and colchicine in COVID-19-associated coagulopathy: the role of alpha-defensins. Br J Haematol. 2022;196:923–927. doi: 10.1111/bjh.17885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ponthieux F., Dauby N., Maillart E., Fils J.F., Smet J., Claus M., et al. Tocilizumab-induced unexpected increase of several inflammatory cytokines in critically ill COVID-19 patients: the anti-inflammatory side of IL-6. Viral Immunol. 2022;35:60–70. doi: 10.1089/vim.2021.0111. [DOI] [PubMed] [Google Scholar]
- 71.Morgan C.E., Rimland C.A., Bell G.J., Kim M.K., Hedrick T., Marx A., et al. Rapid analysis of local data to inform off-label tocilizumab use early in the COVID-19 pandemic. Healthcare. 2021 doi: 10.1016/j.hjdsi.2021.100581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hamed D.M., Belhoul K.M., Al Maazmi N.A., Ghayoor F., Moin M., Al Suwaidi M., et al. Intravenous methylprednisolone with or without tocilizumab in patients with severe COVID-19 pneumonia requiring oxygen support: a prospective comparison. J Infect Public Health. 2021;14:985–989. doi: 10.1016/j.jiph.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Soin A.S., Kumar K., Choudhary N.S., Sharma P., Mehta Y., Kataria S., et al. Tocilizumab plus standard care versus standard care in patients in India with moderate to severe COVID-19-associated cytokine release syndrome (COVINTOC): an open-label, multicentre, randomised, controlled, phase 3 trial. Lancet Respir Med. 2021;9:511–521. doi: 10.1016/S2213-2600(21)00081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stone J.H., Frigault M.J., Serling-Boyd N.J., Fernandes A.D., Harvey L., Foulkes A.S., et al. Efficacy of tocilizumab in patients hospitalized with covid-19. N Engl J Med. 2020;383:2333–2344. doi: 10.1056/nejmoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salama C., Han J., Yau L., Reiss W.G., Kramer B., Neidhart J.D., et al. Tocilizumab in patients hospitalized with covid-19 pneumonia. N Engl J Med. 2021;384:20–30. doi: 10.1056/nejmoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Veiga V.C., Prats J.A.G.G., Farias D.L.C., Rosa R.G., Dourado L.K., Zampieri F.G., et al. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ. 2021:372. doi: 10.1136/bmj.n84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang D., Fu B., Peng Z., Yang D., Han M., Li M., et al. Tocilizumab in patients with moderate or severe COVID-19: a randomized, controlled, open-label, multicenter trial. Front Med. 2021;15:486–494. doi: 10.1007/s11684-020-0824-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gordon A.C., Mouncey P.R., Ai-Beidh F., Rowan K.M., Nichol A.D., Arabi Y.M., et al. Interleukin-6 receptor antagonists in critically ill patients with covid-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/nejmoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perrone F., Piccirillo M.C., Ascierto P.A., Salvarani C., Parrella R., Marata A.M., et al. Tocilizumab for patients with COVID-19 pneumonia. The single-arm TOCIVID-19 prospective trial. J Transl Med. 2020;18:1–11. doi: 10.1186/s12967-020-02573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dastan F., Saffaei A., Haseli S., Marjani M., Moniri A., Abtahian Z. Promising effects of tocilizumab in COVID-19: a non-controlled, prospective clinical trial. Int Immunopharm. 2020 doi: 10.1016/j.intimp.2020.106869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Malekzadeh R., Abedini A., Mohsenpour B., Sharifipour E., Ghasemian R., Javad-Mousavi S.A., et al. Subcutaneous tocilizumab in adults with severe and critical COVID-19: a prospective open-label uncontrolled multicenter trial. Int Immunopharm. 2020;89 doi: 10.1016/j.intimp.2020.107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Strohbehn G.W., Heiss B.L., Rouhani S.J., Trujillo J.A., Yu J., Kacew A.J., et al. COVIDOSE: a phase II clinical trial of low-dose tocilizumab in the treatment of noncritical COVID-19 pneumonia. Clin Pharmacol Ther. 2021;109:688–696. doi: 10.1002/cpt.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nunez J., Fellous A., Francon J., Lennon A.M. Competitive inhibition of colchicine binding to tubulin by microtubule-associated proteins. Proc Natl Acad Sci U S A. 1979;76:86–90. doi: 10.1073/pnas.76.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tardif J.C., Bouabdallaoui N., L'Allier P.L., Gaudet D., Shah B., Pillinger M.H., et al. Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir Med. 2021;9:924–932. doi: 10.1016/S2213-2600(21)00222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dorward J., Yu L.-M., Hayward G., Saville B.R., Gbinigie O., Van Hecke O., et al. Colchicine for COVID-19 in the community (PRINCIPLE): a randomised, controlled, adaptive platform trial. Br J Gen Pract. 2022 doi: 10.3399/bjgp.2022.0083. BJGP.2022.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Horby P.W., Campbell M., Spata E., Emberson J.R., Staplin N., Pessoa-Amorim G., et al. Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet Respir Med. 2021;9:1419–1426. doi: 10.1016/S2213-2600(21)00435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Deftereos S.G., Giannopoulos G., Vrachatis D.A., Siasos G.D., Giotaki S.G., Gargalianos P., et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial. JAMA Netw Open. 2020;3:1–14. doi: 10.1001/jamanetworkopen.2020.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pascual-Figal D.A., Roura-Piloto A.E., Moral-Escudero E., Bernal E., Albendín-Iglesias H., Pérez-Martínez M.T., et al. Colchicine in recently hospitalized patients with COVID-19: a randomized controlled trial (COL-COVID) Int J Gen Med. 2021;14:5517–5526. doi: 10.2147/IJGM.S329810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pourdowlat G., Saghafi F., Mozafari A., Sahebnasagh A., Abedini A., Nabi Meybodi M., et al. Efficacy and safety of colchicine treatment in patients with COVID-19: a prospective, multicenter, randomized clinical trial. Phyther Res. 2022;36:891–898. doi: 10.1002/ptr.7319. [DOI] [PubMed] [Google Scholar]
- 90.Absalón-Aguilar A., Rull-Gabayet M., Pérez-Fragoso A., Mejía-Domínguez N.R., Núñez-Álvarez C., Kershenobich-Stalnikowitz D., et al. Colchicine is safe though ineffective in the treatment of severe COVID-19: a randomized clinical trial (COLCHIVID) J Gen Intern Med. 2022;37:4–14. doi: 10.1007/s11606-021-07203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Diaz R., Orlandini A., Castellana N., Caccavo A., Corral P., Corral G., et al. Effect of colchicine vs usual care alone on intubation and 28-day mortality in patients hospitalized with COVID-19: a randomized clinical trial. JAMA Netw Open. 2021;4:1–12. doi: 10.1001/jamanetworkopen.2021.41328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lopes M.I., Bonjorno L.P., Giannini M.C., Amaral N.B., Menezes P.I., Dib S.M., et al. Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial. RMD Open. 2021;7:1–8. doi: 10.1136/rmdopen-2020-001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., et al. Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/nejmoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tomazini B.M., Maia I.S., Cavalcanti A.B., Berwanger O., Rosa R.G., Veiga V.C., et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA, J Am Med Assoc. 2020;324:1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Munch M.W., Myatra S.N., Vijayaraghavan B.K.T., Saseedharan S., Benfield T., Wahlin R.R., et al. Effect of 12 mg vs 6 mg of dexamethasone on the number of days alive without life support in adults with COVID-19 and severe hypoxemia: the COVID STEROID 2 randomized trial. JAMA, J Am Med Assoc. 2021;326:1807–1817. doi: 10.1001/jama.2021.18295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Granholm A., Munch M.W., Myatra S.N., Vijayaraghavan B.K.T., Cronhjort M., Wahlin R.R., et al. Dexamethasone 12 mg versus 6 mg for patients with COVID-19 and severe hypoxaemia: a pre-planned, secondary Bayesian analysis of the COVID STEROID 2 trial. Intensive Care Med. 2022;48:45–55. doi: 10.1007/s00134-021-06573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Toroghi N., Abbasian L., Nourian A., Davoudi-Monfared E., Khalili H., Hasannezhad M., et al. Comparing efficacy and safety of different doses of dexamethasone in the treatment of COVID-19: a three-arm randomized clinical trial. Pharmacol Rep. 2022;74:229–240. doi: 10.1007/s43440-021-00341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maskin L.P., Bonelli I., Olarte G.L., Palizas F., Velo A.E., Lurbet M.F., et al. High- versus low-dose dexamethasone for the treatment of COVID-19-related acute respiratory distress syndrome: a multicenter, randomized open-label clinical trial. J Intensive Care Med. 2022;37:491–499. doi: 10.1177/08850666211066799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Horby P.W., Campbell M., Staplin N., Spata E., Emberson J.R., Pessoa-Amorim G., et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marconi V.C., Ramanan A.V., de Bono S., Kartman C.E., Krishnan V., Liao R., et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. 2021;9:1407–1418. doi: 10.1016/S2213-2600(21)00331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ramakrishnan S., Jr., Dvn, Langford B., Mahdi M., Jeffers H., Mwasuku C., et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a Phase 2, open-label, randomised controlled trial. Lancet Respir Med. 2021:19–21. doi: 10.1016/S2213-2600(21)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu L.M., Bafadhel M., Dorward J., Hayward G., Saville B.R., Gbinigie O., et al. Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;398:843–855. doi: 10.1016/S0140-6736(21)01744-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Clemency B.M., Varughese R., Gonzalez-Rojas Y., Morse C.G., Phipatanakul W., Koster D.J., et al. Efficacy of inhaled ciclesonide for outpatient treatment of adolescents and adults with symptomatic COVID-19: a randomized clinical trial. JAMA Intern Med. 2022;182:42–49. doi: 10.1001/jamainternmed.2021.6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ezer N., Belga S., Daneman N., Chan A., Smith B.M., Daniels S.A., et al. Inhaled and intranasal ciclesonide for the treatment of covid-19 in adult outpatients: CONTAIN phase II randomised controlled trial. BMJ. 2021;375:1–8. doi: 10.1136/bmj-2021-068060. [DOI] [PMC free article] [PubMed] [Google Scholar]