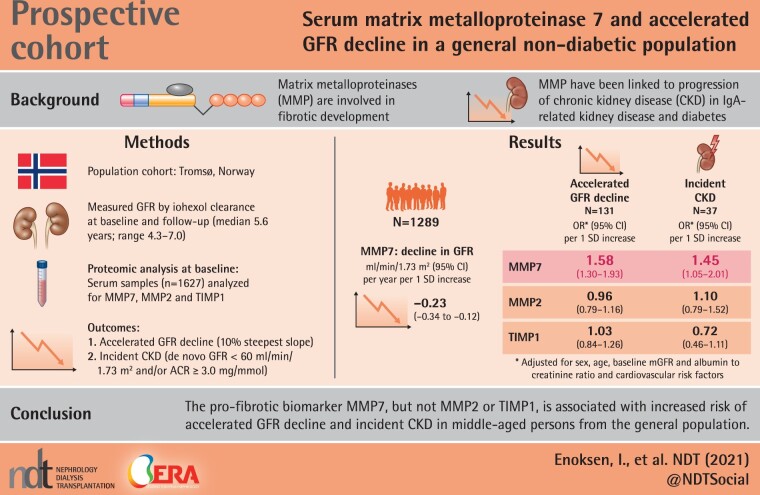
Abstract
Background
Age-related reduction of glomerular filtration rate (GFR) is a major contributor to the global chronic kidney disease (CKD) epidemic. We investigated whether baseline serum levels of the pro-fibrotic matrix metalloproteinase 2 (MMP2), MMP7 and their inhibitor, tissue inhibitor of metalloproteinase 1 (TIMP1), which mediates fibrosis development in aging animals, were associated with GFR decline in a general non-diabetic population.
Methods
In the Renal Iohexol Clearance Survey, we measured GFR using iohexol clearance in 1627 subjects aged 50–64 years without self-reported diabetes, kidney or cardiovascular disease. After a median of 5.6 years, 1324 had follow-up GFR measurements. Using linear mixed models and logistic regression analyses, we evaluated the association of MMP7, MMP2 and TIMP1 with the mean GFR decline rate, risk of accelerated GFR decline (defined as subjects with the 10% steepest GFR slopes: ≥1.8 mL/min/1.73 m2/year) and incident CKD [GFR <60 mL/min/1.73 m2 and/or urinary albumin to creatinine ratio (ACR) ≥3.0 mg/mmol].
Results
Higher MMP7 levels (per standard deviation increase of MMP7) were associated with steeper GFR decline rates [−0.23 mL/min/1.73 m2/year (95% confidence interval −0.34 to −0.12)] and increased risk of accelerated GFR decline and incident CKD [odds ratios 1.58 (1.30–1.93) and 1.45 (1.05–2.01), respectively, in a model adjusted for age, sex, baseline GFR, ACR and cardiovascular risk factors]. MMP2 and TIMP1 showed no association with GFR decline or incident CKD.
Conclusions
The pro-fibrotic biomarker MMP7, but not MMP2 or TIMP1, is associated with increased risk of accelerated GFR decline and incident CKD in middle-aged persons from the general population.
Keywords: accelerated GFR decline, epidemiology, fibrosis, incident CKD, MMP7
Graphical Abstract
Graphical Abstract.
Key Learning Points
What is already known about this subject?
Matrix metalloproteinases (MMPs) and their inhibitors are involved in fibrotic development and have been linked to progression of chronic kidney disease (CKD) in populations with immunoglobulin A-related kidney disease and diabetes.
What impact this may have on practice or policy?
It is unknown whether blood levels of MMP7, MMP2 and tissue inhibitor of metalloproteinase 1 (TIMP1) are linked to loss of kidney function in a general population.
What this study adds?
In middle-aged persons from the general non-diabetic population, we investigated whether serum levels of MMP7, MMP2 and TIMP1 were related to future loss of kidney function assessed by accurate measurements of the glomerular filtration rate (GFR).
MMP7 was associated with a more rapid loss of the kidney function (GFR) and new-onset CKD, particularly in subjects with hypertension.
This study suggests that MMP7 may be a promising biomarker for early kidney function loss and CKD development.
More research is needed to determine if MMP7 may be a useful biomarker for detection of persons at high risk of kidney function loss and new-onset CKD.
This study may inspire more research on MMP7 as a potential treatment target for halting fibrosis development in the kidney and could lead to new insight into the pathophysiology behind CKD development and kidney function loss in the ageing kidney
INTRODUCTION
Chronic kidney disease (CKD) affects 30–40% of elderly persons and is associated with increased morbidity and mortality [1]. The death rate by CKD has increased by ∼40% worldwide since 1990 [2], and the need for renal replacement therapy in Norway has doubled [3], mainly due to an aging population [2]. Age-related glomerular filtration rate (GFR) decline is a major driving force behind the increasing prevalence of CKD [1]. GFR declines with age even in healthy persons [4], and the decline rate differs significantly between people regardless of risk factors, such as diabetes [5], indicating the potential for preventive measures.
The underlying mechanisms leading to accelerated age-related GFR decline in some persons are unknown. Although interstitial fibrosis (IF) is a key component of the pathophysiology leading to CKD and CKD progression, it is unknown whether increased IF also contributes to accelerated age-related GFR decline. The proportion of healthy kidney donors with IF (defined as >5% IF on renal biopsy) has been shown to increase with age, from 1.4% at the age of 18–29 years to 27% at 70–77 years, but there was no effect modification between nephrosclerosis score (including degree of IF) and the decline in GFR with age in this cross-sectional study [6]. However, in another study, the percentage of IF predicted progression of CKD in patients who underwent a radical nephrectomy for a tumour [7]. Only a few longitudinal studies of the general population have investigated the association of fibrotic biomarkers with GFR loss, with inconsistent results [8–10].
Matrix metalloproteinases (MMPs) play a central role in interstitial remodelling. MMP2 and MMP7 contribute to the development of renal fibrosis through conversion of the epithelium to a fibroblastic or myofibroblastic phenotype [epithelial–mesenchymal transition (EMT)] [11–13]. It has been suggested that MMP7 may serve as a non-invasive biomarker for renal fibrosis and that MMP7 could be a potential treatment target to reduce fibrotic development [14]. However, whether increased levels of circulating MMPs are associated with accelerated GFR decline in the general population has not been investigated.
In the population-based Renal Iohexol Clearance Survey (RENIS), we investigated whether serum MMP2, MMP7 and one of their inhibitors, tissue inhibitor of metalloproteinase 1 (TIMP1), were associated with accelerated decline in measured GFR (mGFR) or incident CKD in middle-aged persons without diabetes or pre-existing CKD.
MATERIALS AND METHODS
Study population
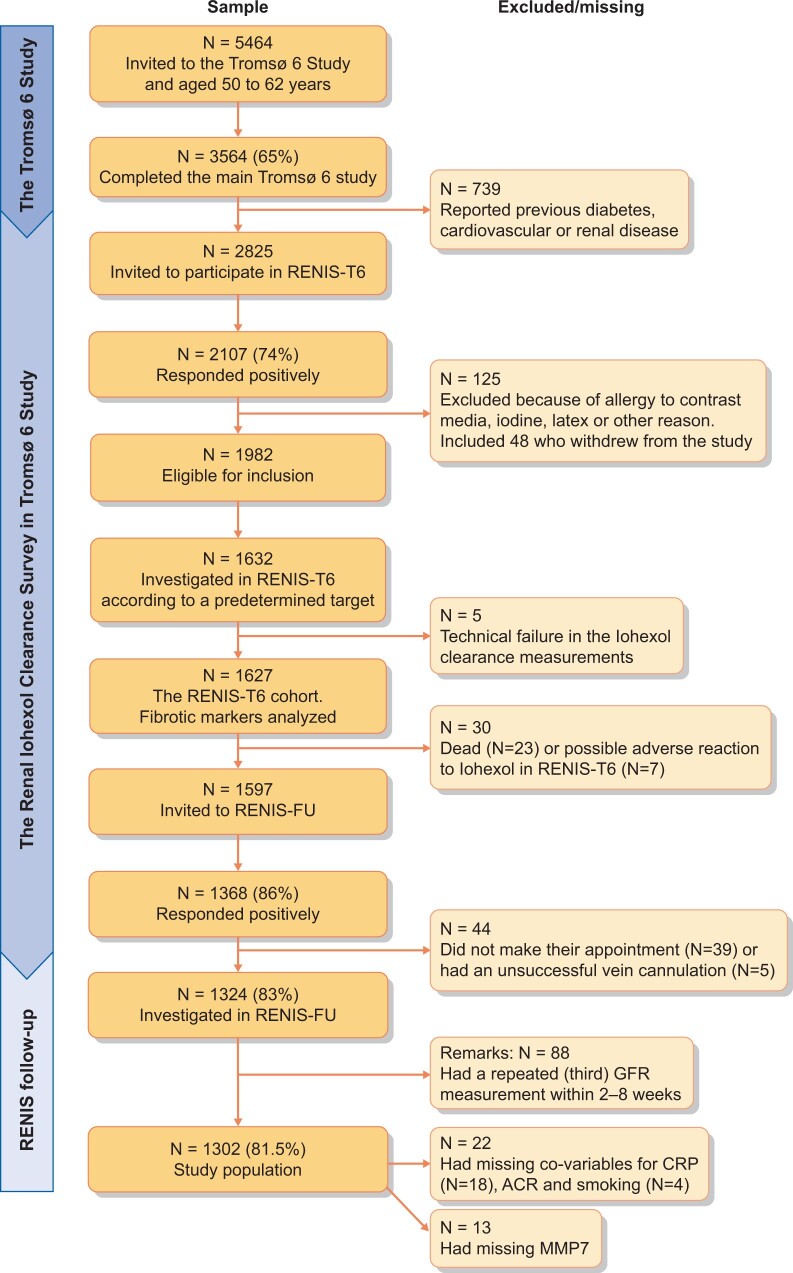
Participants were recruited from the general population as part of The Tromsø Study: Tromsø 6 (T6) [15], a population survey in the municipality of Tromsø, Northern Norway. In total, 2825 persons between 50 and 64 years of age without self-reported kidney disease, cardiovascular disease (CVD) or diabetes were invited to the RENIS-T6 (Figure 1). Of these, 2107 (74%) persons responded positively, and we randomly included 1627 participants according to a predetermined target study size. After a median of 5.6 years (range 4.3–7.0), 1324 (81%) persons participated in the RENIS follow-up (FU), and 88 were randomly selected to have a second FU GFR measurement within 2–8 weeks. Repeated measurements in this subsample enabled us to calculate both the intra-individual variation in GFR measurements and the GFR slope for each individual using a linear mixed model with random intercept and slope [16].
FIGURE 1.
Flowchart: an overview of the RENIS cohort. Number (N) of invited participants, response rate (%) and number of participants excluded/missing due to different reasons.
Of the 1324 participants at FU (Figure 1), 25 had baseline GFR <60 mL/min/1.73 m2, and 30 had urinary albumin to creatinine ratio (ACR) ≥3.0 mg/mmol and were excluded in incident CKD analysis. Furthermore, baseline data on smoking and/or ACR were missing for four individuals and C-reactive protein (CRP) was missing for 18 individuals. After calculating the annual GFR slope for each individual who had a FU measurement of GFR, data were available for 1302 individuals in the accelerated GFR decline analysis.
All participants provided written informed consent. The Regional Committee for Medical and Health Research Ethics approved the study, which was conducted in accordance with the Helsinki Declaration. The design and methods of the Tromsø study [15] and RENIS study [17] can be found elsewhere.
Iohexol clearance
GFR was measured at baseline and FU using single sample plasma clearance of iohexol (Omnipaque, 300 mgI/mL; Amersham Health, London, UK) in both surveys [18]. Details are given in Supplementary material. Briefly, 5 mL of iohexol from a syringe was injected into a Teflon catheter in an antecubital vein and flushed with 30 mL isotonic saline. The time of blood sampling after injection of iohexol for each person was determined using Jacobsson’s method [19]. The method has been validated and shows substantial agreement with plasma iohexol clearance using multiple sampling methods [20, 21]. The intra-individual day-to-day variation of the GFR measurements in RENIS was 4.2% [95% confidence interval (CI) 3.4–4.9%] [16].
Proteomic analysis
Baseline serum samples (n = 1627) were analysed for MMP7, MMP2 and TIMP1 using the Luminex xMap multiplex technology (Bio-Plex 200 systems, Bio-Rad). All samples were analysed in duplicate as described in Supplementary method. Two internal controls were made to measure the coefficient of variation (CV) for the proteins. Intra-assay (variation between duplicates on the same plate) and inter-assay CV (plate-to-plate variation) for the controls for MMP7, MMP2 and TIMP1 were 3.1%, 5.0% and 3.3%, and 19.3%, 6.2% and 5.8%, respectively (Supplementary data, Table S1).
Other measurements
Blood samples were drawn between 08:00 and 10:00 h in the morning after an overnight fast and abstinence from tobacco. Three samples of first void morning spot urine were collected on consecutive days for calculation of ACR (mg/mmol) in unfrozen urine [15]. Data on medication use were collected. Blood pressure (BP) was measured three times with 1 min in between the measurements after the participants had rested for 5–10 min using an automated device (model UA 799; A&D, Tokyo, Japan). The mean value of the last two measurements was used in the analyses. Hypertension was defined as systolic BP (sBP) ≥140 mmHg, diastolic BP (dBP) ≥90 mmHg and/or the use of antihypertensive medication. Ever smoker was categorized as current or previous daily smoker and never smoker.
Estimated GFR (eGFR) used in sensitivity analyses, was determined using the CKD Epidemiology Collaboration (CKD-EPI) equations based on serum creatinine (eGFRcre) [22], cystatin C (eGFRcys) and the combined creatinine–cystatin equation (eGFRcrecys) [23].
Statistical analysis
Descriptive statistics are given as the means [standard deviations (SDs)] for normally distributed data, medians [interquartile range (IQR)] for skewed data or numbers with percentages.
The association of MMP7 with annual change in mGFR was calculated using mixed model linear regression analyses using random intercept and slopes for time.
Accelerated GFR decline between baseline and FU was defined as the 10% of subjects with the steepest GFR slope. The GFR slope for each subject was calculated using a linear mixed model as described above and adjusted for age, sex and CKD risk factors at baseline [body mass index (BMI), sBP, antihypertensive medication, fasting glucose, high-density lipoprotein (HDL) cholesterol, triglycerides, smoking and CRP]. This method was used to increase the precision of the slope estimates and to minimize confounding in the longitudinal analyses due to baseline associations between the protein biomarkers, CKD risk factors and GFR [24, 25].
In sensitivity analyses, we defined accelerated GFR decline as the 25% fastest decliners based on the GFR slope calculated as described above, and as an annual GFR loss of >3 mL/min/1.73 m2 or as twice the unadjusted cohort mean (calculated by the difference in GFR from baseline to FU divided by the observation time), methods that have been used in previous studies [8, 26].
The risk for accelerated GFR decline and incident CKD was evaluated using multivariable logistic regression analyses. Fully adjusted models were tested for effect modification by the following subgroups: baseline GFR, sex, smoking, age, BMI, ACR and hypertension, using two-way interaction terms.
Incident CKD was defined as new-onset GFR <60 mL/min/1.73 m2 and/or ACR ≥3.0 mg/mmol at FU [5]. Three alternative definitions were used in sensitivity analysis (Supplementary data, Table S2).
We adjusted for the following covariates in three separate models. Model 1: sex, age, baseline GFR and ACR; Model 2: Model 1 + BMI, sBP and fasting glucose; Model 3: as Model 2 + ever smoker and the use of antihypertensive medication. Non-linear associations between the fibrotic markers and accelerated GFR decline were investigated in generalized additive models with the mgcv package in R [27].
To evaluate the improvement in risk prediction in models with and without the fibrotic markers, we compared the area under the receiver-operating characteristics curve (AUC) for the nested logistic regression model using the likelihood ratio test [28]. We also assessed the net reclassification index (NRI) and relative integrated discrimination improvement (rIDI) [29], the continuous NRI was used because it overcomes the problem of using categories that do not naturally exist [30].
To evaluate the improvement in prediction of incident CKD we included the newly developed incident CKD risk prediction equation by Nelson et al. [31] as the base model.
Finally, internal validation was performed using bootstrapping (N = 1000) to all analyses.
All statistical analyses were performed with STATA version 16.1 (StataCorp, College Station, TX, USA) and R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org). A P-value of <0.05 was considered statistically significant. For more details on methods see Supplementary material.
RESULTS
Participant and MMP7 characteristics
The study population characteristics at baseline grouped by quartiles of MMP7 are presented in Table 1, and by quartiles of MMP2 and TIMP1 (Supplementary data, Tables S3 and S4). Participants with baseline MMP7 in the upper quartile were older, had higher BP and ACR, had lower GFR, and were more likely to be current or ever smokers and users of antihypertensive medication compared with the three lower quartiles.
Table 1.
Baseline study populationa characteristics according to quartile of MMP7
| Quartile of MMP7, pg/mL (IQR) |
P-value linear trend | ||||
|---|---|---|---|---|---|
| Characteristics | Quartile 1 (n = 322) | Quartile 2 (n = 322) | Quartile 3 (n = 322) | Quartile 4 (n = 323) | |
| (49–1436) | (1437–1900) | (1901–2347) | (2348–7463) | ||
| Sex (men) [n (%)] | 161 (50) | 168 (52) | 148 (46) | 165 (51) | 0.43 |
| Age (years) | 57.2 (53.5–61.0) | 58.0 (54.3–61.1) | 58.6 (55.0–61.3) | 60.5 (56.0–61.9) | <0.001 |
| Height (cm) | 171.0 (8.9) | 171.2 (8.5) | 170.4 (8.7) | 170.6 (8.3) | 0.22 |
| Weight (kg) | 78.3 (12.6) | 81.1 (13.9) | 79.0 (14.4) | 79.8 (14.5) | 0.84 |
| BMI (kg/m2) | 26.7 (3.4) | 27.6 (4.0) | 27.1 (4.0) | 27.3 (4.0) | 0.29 |
| mGFRiohexol (mL/min/1.73 m2) | 95.7 (13.2) | 95.7 (13.6) | 92.8 (14.2) | 91.1 (15.6) | <0.001 |
| eGFRcre | 97.1 (90.5–101.6) | 97.4 (92.0–101.4) | 96.9 (90.0–100.6) | 95.6 (87.1–100.5) | <0.001 |
| eGFRcys | 108.6 (10.5) | 107.3 (11.7) | 105.0 (11.3) | 102.0 (13.5) | <0.001 |
| eGFRcrecys | 105.4 (10.3) | 104.6 (10.5) | 102.6 (10.3) | 100.0 (12.6) | <0.001 |
| Urinary ACR (mg/mmol) | 0.20 (0.1–0.46) | 0.1 (0.1–0.37) | 0.27 (0.1–0.62) | 0.31 (0.1–0.66) | <0.001 |
| sBP (mmHg) | 125 (114–138) | 126 (116–138) | 129 (118–143) | 131 (119–142) | <0.001 |
| dBP (mmHg) | 82 (9) | 83 (9) | 83 (10) | 85 (10) | <0.001 |
| Antihypertensive medication [n (%)] | 38 (12) | 37 (11) | 48 (15) | 105 (33) | <0.001 |
| RAS inhibitors | 16 (5) | 25 (8) | 30 (9) | 61 (19) | <0.001 |
| Fasting blood glucose (mmol/L) | 5.3 (0.5) | 5.4 (0.5) | 5.4 (0.6) | 5.3 (0.5) | 0.83 |
| LDL cholesterol (mmol/L) | 3.6 (0.8) | 3.7 (0.9) | 3.7 (0.9) | 3.7 (0.8) | 0.08 |
| HDL cholesterol (mmol/L) | 1.6 (0.4) | 1.5 (0.4) | 1.5 (0.4) | 1.5 (0.4) | 0.39 |
| Triglycerides (mmol/L) | 1.0 (0.7–1.4) | 1.0 (0.7–1.4) | 1.0 (0.8–1.5) | 1.1 (0.8–1.5) | 0.03 |
| CRP (mg/L) | 1.02 (0.6–1.8) | 1.21 (0.66–2.13) | 1.17 (0.63–2.28) | 1.3 (0.72–2.8) | <0.001 |
| Daily smoker [n (%)] | 203 (63) | 216 (67) | 216 (67) | 236 (73) | <0.001 |
| Yes, currently | 45 (14) | 51 (16) | 59 (18) | 95 (29) | <0.001 |
| Yes, previously | 158 (49) | 165 (51) | 157 (49) | 141 (44) | 0.12 |
| Never | 119 (37) | 106 (33) | 106 (33) | 87 (27) | <0.001 |
| MMP7 (pg/mL) | 1192 (918–1345) | 1684 (1561–1805) | 2113 (1992–2233) | 2773 (2519–3186) | <0.001 |
| MMP2 (ng/mL) | 311.7 (49.8) | 309.8 (52.8) | 316.5 (51.7) | 323.7 (76.9) | 0.017 |
| TIMP1 (ng/mL) | 156.8 (24.0) | 160.7 (37.7) | 163.3 (25.4) | 166.3 (26.0) | <0.001 |
The data are presented as the means (SD) and median (IQR) for continuous variables, and proportions (%) for dichotomous variables.
Study population N = 1302, with 13 missing for MMP7 (N = 1289 for MMP7 analyses).
mGFRiohexol, mGFR with iohexol; RAS, renin–angiotensin system; LDL, low-density lipoprotein.
Almost all characteristics had a statistically significant change from baseline to FU, except for weight, triglycerides, BMI and ever smokers (Supplementary data, Table S5). These changes were not necessarily clinically significant.
MMP7 concentration showed a statistically significant weak correlation with baseline GFR (r −0.14, P < 0.001, Supplementary data, Figure S1), age (r 0.15, P < 0.001) and ACR (r 0.13, P < 0.001). MMP7 and MMP2 were not correlated, while TIMP1 had a weak correlation with both MMP7 (r 0.15, P < 0.001) and MMP2 (r 0.08, P = 0.001).
Accelerated GFR decline and incident CKD
The median annual change in GFR was −0.87 mL/min/1.73 m2/year (IQR −1.33 to −0.41). A negative rate of change signifies a decline in GFR. Higher baseline levels of MMP7 were associated with steeper GFR change rates calculated using linear mixed model [−0.23 mL/min/1.73 m2/year per SD increase in MMP7 in the fully adjusted model, P-value < 0.001 (Supplementary data, Table S6)]. The threshold to define accelerated GFR decline, using the 10th percentile of the distribution of the GFR slope, was −1.78 mL/min/1.73 m2/year (n = 131). Of the 1269 persons with GFR >60 mL/min/1.73 m2 and ACR <3.0 mg/mmol at baseline, 37 individuals had developed CKD at FU.
Higher levels of MMP7 were associated with an increased risk of accelerated GFR decline (n = 131) and incident CKD (n = 37) in crude and adjusted analyses (Table 2). Odds ratios (ORs) in the fully adjusted model were 1.58 (95% CI 1.30–1.93) and 1.62 (95% CI 1.19–2.19), respectively (only four variables were included in the fully adjusted model for incident CKD due to few endpoints). All the models were well-calibrated, judged by calibration plots (Supplementary data, Figures S2 and S3). There was a non-linear association between MMP7 and accelerated GFR decline (P = 0.010) in Model 3 of Table 2 as illustrated in Figure 2.
Table 2.
Multiple logistic regression analyses with ORs for accelerated GFR decline and incident CKD
| Outcome definition: | Accelerated declinea |
Incident CKDb |
||||
|---|---|---|---|---|---|---|
| Model | OR | 95% CI | P | OR | 95% CI | P |
| Crude: Protein only. | ||||||
| MMP7, per SD increase | 1.52 | 1.28–1.80 | <0.001 | 1.91 | 1.43–2.55 | <0.001 |
| MMP7, first quartile | Ref. | – | – | – | ||
| MMP7, second quartile | 0.59 | 0.33–1.07 | 0.08 | – | – | – |
| MMP7, third quartile | 0.90 | 0.52–1.53 | 0.69 | – | – | – |
| MMP7, fourth quartile | 1.77 | 1.10–2.84 | 0.019 | – | – | – |
| MMP7, first to third quartiles | Ref. | Ref. | ||||
| MMP7, fourth quartile | 2.11 | 1.45–3.07 | <0.001 | 3.00 | 1.55–5.78 | 0.001 |
| Model 1 | ||||||
| MMP7, per SD increase | 1.68 | 1.39–2.04 | <0.001* | 1.62 | 1.19–2.19 | 0.002** |
| MMP7, first quartile | Ref. | – | – | – | ||
| MMP7, second quartile | 0.56 | 0.30–1.02 | 0.06 | – | – | – |
| MMP7, third quartile | 0.84 | 0.48–1.47 | 0.54 | – | – | – |
| MMP7, fourth quartile | 1.74 | 1.05–2.87 | 0.031 | – | – | – |
| MMP7, first to third quartiles | Ref. | Ref. | ||||
| MMP7, fourth quartile | 2.18 | 1.46–3.25 | <0.001 | 2.35 | 1.19–4.64 | 0.01 |
| Model 2 | – | – | – | |||
| MMP7, per SD increase | 1.66 | 1.37–2.01 | <0.001* | – | – | – |
| MMP7, first quartile | Ref. | – | – | – | ||
| MMP7, second quartile | 0.56 | 0.30–1.03 | 0.06 | – | – | – |
| MMP7, third quartile | 0.82 | 0.47–1.45 | 0.54 | – | – | – |
| MMP7, fourth quartile | 1.72 | 1.04–2.85 | 0.031 | – | – | – |
| MMP7, first to third quartiles | Ref. | – | – | – | ||
| MMP7, fourth quartile | 2.17 | 1.45–3.24 | <0.001 | – | – | – |
| Model 3 | – | – | – | |||
| MMP7, per SD increase | 1.58 | 1.30–1.93 | <0.001* | – | – | – |
| MMP7, first quartile | Ref. | – | – | – | ||
| MMP7, second quartile | 0.56 | 030–1.04 | 0.07 | – | – | – |
| MMP7, third quartile | 0.81 | 0.46–1.42 | 0.46 | – | – | – |
| MMP7, fourth quartile | 1.52 | 0.90–2.56 | 0.11 | – | – | – |
| MMP7, first to third quartiles | Ref. | – | – | – | ||
| MMP7, fourth quartile | 1.93 | 1.27–2.92 | 0.002 | – | – | – |
Model 1: adjusted for: sex, age, baseline mGFR and ACR (mg/mmol).
Model 2: adjusted for: Model 1 + BMI, sBP and fasting glucose.
Model 3: adjusted for: Model 2 + ever smoker and BP medication.
Defined as 10% steepest decline (annual GFR decline rate >1.78 mL/min/1.73 m2 calculated using linear mixed model regression). n = 1289 in total and n = 131 with accelerated GFR decline.
Incident CKD, defined as new-onset GFR <60 mL/min/1.73 m2 and/or ACR ≥3.0 at FU (n = 1256 in total and n = 37 with incident CKD). Only four variables were included in the fully adjusted model (Model 1) due to few outcomes.
P = 0.008, P = 0.011 and P = 0.009 for the quadratic term of MMP7 in Models 1–3.
P = 0.23 for the quadratic term of MMP7 in Model 1.
FIGURE 2.
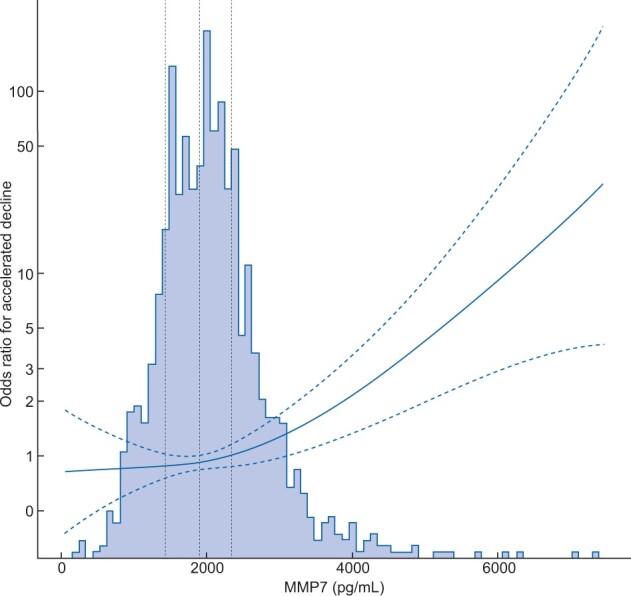
The non-linear association between MMP7 concentration and risk of accelerated GFR decline based on Model 3 in Table 2. Baseline MMP7 concentration is on the x-axis, the ORs for accelerated decline are on the y-axis and dotted lines indicating the change point for each quartile of MMP7 concentration. The dashed lines indicate 95% CIs. MMP7 concentrations are shown as picogram per millilitre.
Prediction of accelerated GFR decline improved after addition of MMP7 to a model with age, sex, baseline GFR and ACR and in the fully adjusted model. In the fully adjusted model, the AUC increased from 0.74 (95% CI 0.70–0.79) to 0.77 (95% CI 0.73–0.81) (P < 0.001) (Table 3). The improvement in rIDI was 0.25 (95% CI 0.08–0.41, P = 0.003) and the total NRI was 0.25 (95% CI 0.07–0.43) (Supplementary data, Table S7). The event NRI was −2% while the non-event NRI was 26%, indicating that the total NRI improvement is driven by a positive value for non-events, meaning that adding MMP7 to the model mostly down-classifies individuals correctly to the non-accelerated decline category (to a lower risk). For incident CKD based on mGFR, MMP7 improved the prediction beyond the recent CKD prediction model proposed by Nelson et al. [31], as AUC increased from 0.74 (95% CI 0.64–0.83) to 0.77 (95% CI 0.68–0.86) (P = 0.001) (Supplementary data, Table S8).
Table 3.
Prediction of accelerated GFR declinea before and after addition of MMP7 to the models
| Prediction model (AUC, IDI and NRI) | Model 1 | P | Model 2 | P | Model 3 | P |
|---|---|---|---|---|---|---|
| C-statistics without MMP7 (95% CI) | 0.717 (0.672–0.763) | – | 0.717 (0.672–0.763) | – | 0.743 (0.697–0.789) | – |
| C-statistics with MMP7 (95% CI) | 0.758 (0.717–0.799) | <0.001 | 0.757 (0.716–0.798) | <0.001 | 0.769 (0.726–0.812) | <0.001 |
Model 1: adjusted for age, sex and baseline GFR.
Model 2: adjusted for age, sex, baseline GFR and ACR (mg/mmol).
Model 3: as Model 2 and further adjusted for BMI, ever smoker, sBP, BP medication and fasting glucose. All the analysis includes 1285 participants.
Accelerated mGFR decline is defined as 10% steepest decline (RENIS mean annual decline rate >1.78 mL/min/1.73 m2, n = 131).
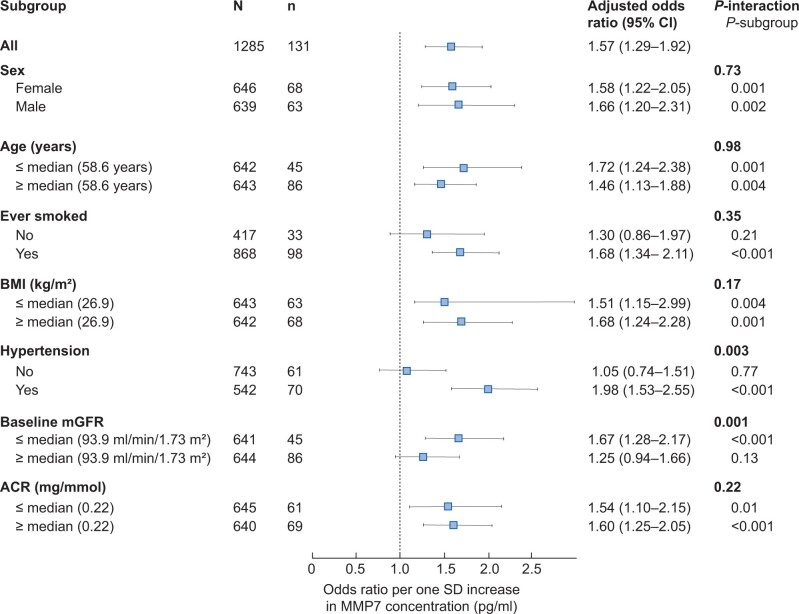
The associations with accelerated GFR decline were robust across subgroups (Figure 3). However, a statistically significant effect modification was found for baseline GFR and hypertension. The association of MMP7 with GFR decline was stronger for participants with baseline GFR below the median level (94 mL/min/1.73 m2, n = 641) and for those with hypertension (n = 542) (Figure 3).
FIGURE 3.
Interaction analysis (forest plot) of the adjusted ORs for accelerated decline by various groups per SD increase in MMP7 concentration. Adjusted for sex, age, baseline GFR, BMI, ever smoker, sBP, BP medication, ACR and fasting glucose. P-values for the interaction between MMP7 and the given variables are shown in addition to the P-values for the different subgroups.
Internal validation using bootstrapping (N = 1000) gave identical ORs but slightly wider 95% CIs and prediction analysis remained similar (Supplementary data, Table S9).
Neither MMP2 nor TIMP1 was associated with accelerated decline or incident CKD (Supplementary data, Table S10).
Sensitivity analyses
Analyses using three alternative definitions for accelerated decline [a GFR decline rate >3 mL/min/1.73 m2/year (n = 138), twice the cohort mean (>1.68 mL/min/1.73 m2/year, n = 409)], and the 25% steepest GFR slope (n = 325), revealed similar results (Supplementary data, Table S11). Similar results were also obtained after adjustment for current smoking instead of ever smoking (Supplementary data, Table S12), with three alternative definitions of incident CKD (Supplementary data, Table S2), and after additional adjustment for variables included in Models 2–3 (Table 2) in the incident CKD analyses.
Due to a high inter-assay CV of MMP7, we excluded 30 participants on one Luminex assay where all six standards in the standard curve deviated from expected concentrations by 7.5–28%. Accordingly, the MMP7 inter-assay CV for the total study population was reduced from 19.3% to 13.7%. The ORs for accelerated GFR decline and incident CKD increased slightly for the main analyses and remained similar in the sensitivity analysis (Supplementary data, Tables S13 and S14).
Analysis with eGFR based on the three CKD-EPI equations instead of mGFR revealed similar and statistically significant results in all analyses given in Table 2, although with lower ORs in most models (Supplementary data, Table S15).
DISCUSSION
In this middle-aged cohort from the general population, without self-reported kidney disease, diabetes or CVD, we found that higher baseline serum MMP7 concentrations were independently associated with an increased risk of accelerated GFR decline and incident CKD. MMP2 and TIMP1 showed no association with GFR decline or incident CKD.
We are not aware of previous studies of MMP7 and kidney outcomes in the general population. In patients with diabetes and kidney disease, higher levels of urinary MMP7 (uMMP7) have been linked to increased risk of end-stage renal disease and mortality [32]. In immunoglobulin A nephropathy, higher serum and uMMP7 levels predicted risk of renal failure and disease progression [33, 34]. Among patients with Type 2 diabetes with and without albuminuria, the risk of accelerated GFR decline (defined as ≥5 mL/min/1.73 m2/year) increased with increasing baseline levels of MMP7 [35]. Our study extends these findings by showing that serum MMP7 is also associated with accelerated GFR decline and incident CKD in a non-diabetic general population without pre-existing CKD or CVD.
We observed a baseline association between higher MMP7 and lower GFR, but it remains unclear whether elevated levels of serum MMP7 originate from a general fibrotic process in the body, from renal fibrosis as part of CKD development, from other conditions upregulating MMP7, or due to reduced renal elimination [14, 36, 37].
However, an increase in MMP7 expression was reported in renal biopsies of CKD patients compared with healthy controls, which was accompanied by increased uMMP7 and slightly increased serum MMP7. In addition, uMMP7 correlated closely with the fibrosis score of the biopsies [14]. Furthermore, healthy human and mouse kidneys do not express MMP7 [12, 38], but expression increases with CKD severity and is upregulated in renal fibrosis [12, 14, 38]. In addition to being a direct target of the pro-fibrotic Wnt/β-catenin pathway [13, 38], MMP7 is a causal mediator of renal fibrosis by activating β-catenin via a Wnt-independent pathway [14].
MMP7 has been proposed as a therapeutic target for halting CKD progression [14, 39, 40]. A direct role for MMP7 in renal fibrosis development was demonstrated in unilateral ureteral obstruction (UUO) animal models of renal fibrosis. Drug inhibition of MMP7 in vivo in these models using MMP inhibitor II showed reduced fibrotic development in MMP7-knockout mice compared with their wild-type counterparts [14]. Studies of the renal protective abilities of resveratrol using human kidney cell lines and UUO models found a reduction in EMT, kidney damage and fibrosis due to MMP7 inhibition [39]. Furthermore, sodium–glucose cotransporter 2 (SGLT2) inhibitors, which delay diabetic kidney disease, were recently studied using in silico modelling and plasma samples from a 2-year clinical trial. The results indicated that a reduction in MMP7 levels contributed to the renal protective abilities of SGLT2 inhibitors [40].
The mean GFR decline observed in the RENIS study population was mainly due to age-related GFR decline rather than specific causes of CKD, since these individuals were relatively healthy at inclusion. In rodent models, MMP7 kidney gene expression has been found to increase with age [12, 41]. One study found a 500-fold increase in MMP7 gene expression from young to old animals [12], and another study found an >8-fold increase in gene expression, which also correlated highly to fibrosis grade [41]. However, the increase could not be explained by ageing itself since these associations only were found in kidneys with fibrosis and dysfunction, and not in those without kidney impairment, suggesting that MMP7 plays a role in the pathogenesis of fibrosis and may represent a fibrotic marker.
In our study, those with hypertension or GFR <94 mL/min/1.73 m2 had a stronger association between MMP7 and accelerated GFR decline. Although these subgroup effects should be interpreted with caution, we speculate that MMP7 may improve the prediction of subjects with early kidney damage and increased risk of CKD. Similar to our results, another study of patients with CKD also found a stronger association between uMMP7 and GFR loss in those with eGFR <90 mL/min/1.73 m2 [14].
There is no consensus on the definition of accelerated GFR decline in population-based studies, and some have used an absolute or percentage change in GFR based on the baseline to FU assessment [8, 9, 26]. This method provides an imprecise estimate of GFR change that is more prone to misclassification compared with using the rate of GFR change. Therefore, in this study, accelerated GFR decline was defined as the top 10% of subjects with the steepest GFR slopes calculated by linear mixed models. A slope obtained by a linear mixed model was recently found to be more precise and closely associated with risk of end-stage renal disease compared with a slope based on linear regression, particularly in subjects with baseline GFR >60 mL/min/1.73 m2 [25]. The majority of patients in the RENIS study had only two GFR measurements and a subset had three GFRs, which is sufficient for using linear mixed models. However, for optimal calculation, three GFRs for a majority of participants would be preferable.
Adding MMP7 to conventional CKD risk factors improved the prediction of accelerated GFR decline assessed by AUC. The prediction increment was only modest; however, although several biomarkers for GFR decline have been proposed, very few improved the prediction of GFR decline or incident CKD over a conventional model using age, sex, baseline GFR and proteinuria/ACR [8, 42].
MMP7 also improved the prediction of incident CKD when added to a recently proposed CKD prediction model developed by Nelson et al. [31]. Although accelerated GFR decline and CKD have been associated with increased risk of end-stage kidney disease and mortality in high- and low-risk groups of the general population, it is questionable whether additional risk prediction afforded by MMP7 may be of clinical utility or that its use would be cost-effective. Nevertheless, our findings provide information regarding possible mechanisms that may contribute to accelerated GFR decline with age.
Previous studies have indicated that MMP2 and TIMP1 may be involved in renal fibrosis [11, 43, 44], but our findings do not support an important role for these biomarkers in age-related GFR decline in a general population without CKD, diabetes or CVD.
Potential explanations for the lack of associations with MMP2 and TIMP1 in our study may be that we investigated a relatively healthy baseline population without established kidney disease, compared with others who mostly investigated CKD patients, and low statistical power in the incident CKD analyses. TIMP1 has been found to increase later than MMPs during development of kidney failure [44]. In addition, another study indicated that a threshold level of MMP2 could be necessary to initiate fibrosis [45], and this level may not have been reached in our baseline sample. Our results may indicate a similar threshold level for MMP7, as we found a non-linear association with GFR decline, with increased risk primarily in the upper quartile of MMP7.
Although the intra-assay CV was low, the inter-assay CV for MMP7 was 19.3%, which is relatively high. In part, it may be due to low concentrations in one of our internal controls, which fell in the lower part of the 5-PL standard curve. However, any large inter-assay CV would most likely attenuate any true association between MMP7 and GFR decline. Indeed, the association of MMP7 and GFR decline was stronger in the main analysis after we had excluded subjects (n = 30) from the Luminex assay with some faults in the standard curve.
The main strengths of our study are that we used accurate GFR measurements in a prospective study of the general population. eGFR from creatinine or cystatin C is imprecise in the near-normal range of GFR [46] and is biased by muscle wasting and inflammation in aging individuals [47, 48]. Our findings persisted after adjustment for several covariates, and sensitivity analyses showed consistently increased risk across different outcome definitions for kidney function decline.
There are also several limitations. First, our sample consisted only of middle-aged persons without self-reported CVD and diabetes mainly of North European ancestry, and the results cannot be generalized to other ethnicities or age groups. Second, prolonged storage of serum samples before analysis and repeated freeze–thaw cycles can affect protein stability and concentration. However, MMP7 has been found to have excellent stability at temperatures below −75°C [49], although studies on TIMP1 and MMP2 are scarce and conflicting [49, 50]. Third, only baseline serum samples of the proteins of interest were analysed. Whether changes in protein concentration over time, or if additional urine samples could add increased predictive value, should be investigated in future studies. Fourth, analyses for incident CKD lack power due to few incident cases. Fifth, misclassification of incident CKD could have occurred because the CKD diagnosis was based on one GFR and urinary ACR measurement only. Finally, we cannot imply causality based on the associations observed since this was an observational study.
To conclude, our results indicate that the pro-fibrotic biomarker MMP7, but not MMP2 or TIMP1, is independently associated with GFR loss in persons without diabetes or pre-existing CKD. MMP7 improved the prediction of accelerated GFR decline and incident CKD in this population. Further research is needed to determine if MMP7 may be a useful biomarker for detection of persons at high risk of accelerated GFR decline and incident CKD.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to the staff at the Clinical Research Unit at the University Hospital of North Norway who made it possible to conduct this study and to Gro Bolstad at the Metabolic Laboratory of UiT The Arctic University of Norway who analysed baseline MMP7, MMP2 and TIMP1 levels. We thank all the participants in the RENIS cohort for their contributions to this investigation.
FUNDING
The study was funded by the Northern Norway Regional Health Authorities (SFP 1100-13) and a grant obtained from Boehringer Ingelheim (1235.104 IIS).
AUTHORS’ CONTRIBUTIONS
All authors meet the requirements for authorship.
CONFLICT OF INTEREST STATEMENT
None declared.
DATA AVAILABILITY STATEMENT
The data underlying this article cannot be shared publicly since this was not included in the research permission, due to ethical considerations and the privacy of individuals that participated in the study. The data can be shared on request as part of research collaboration.
Contributor Information
Inger T Enoksen, Metabolic and Renal Research Group, UiT, The Arctic University of Norway, Tromsø, Norway.
Dmitri Svistounov, Metabolic and Renal Research Group, UiT, The Arctic University of Norway, Tromsø, Norway.
Jon V Norvik, Metabolic and Renal Research Group, UiT, The Arctic University of Norway, Tromsø, Norway; Section of Nephrology, Clinic of Internal Medicine, University Hospital of North Norway, Tromsø, Norway.
Vidar T N Stefansson, Metabolic and Renal Research Group, UiT, The Arctic University of Norway, Tromsø, Norway.
Marit D Solbu, Metabolic and Renal Research Group, UiT, The Arctic University of Norway, Tromsø, Norway; Section of Nephrology, Clinic of Internal Medicine, University Hospital of North Norway, Tromsø, Norway.
Bjørn O Eriksen, Metabolic and Renal Research Group, UiT, The Arctic University of Norway, Tromsø, Norway; Section of Nephrology, Clinic of Internal Medicine, University Hospital of North Norway, Tromsø, Norway.
Toralf Melsom, Metabolic and Renal Research Group, UiT, The Arctic University of Norway, Tromsø, Norway; Section of Nephrology, Clinic of Internal Medicine, University Hospital of North Norway, Tromsø, Norway.
REFERENCES
- 1. Coresh J, Selvin E, Stevens LA. et al. Prevalence of chronic kidney disease in the united states. JAMA 2007; 298: 2038–2047 [DOI] [PubMed] [Google Scholar]
- 2. GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 385: 117–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reisæter AV, Aasberg A, Leivestad T.. Annual Report 2016. The NorwegianRenal Registry (Norsk Nyreregister). Norsk nyreregister.http://www.nephro.no/nnr/AARSM2016.pdf (15 August 2020, date last accessed)
- 4. Eriksen BO, Palsson R, Ebert N. et al. GFR in healthy aging: an individual participant data meta-analysis of iohexol clearance in European population-based cohorts. J Am Soc Nephrol 2020; 31: 1602–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. KDIGO. Chapter 1: Definition and classification of CKD. Kidney Int Suppl 2013; 3: 19–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rule AD, Amer H, Cornell LD. et al. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med 2010; 152: 561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Denic A, Elsherbiny H, Mullan AF. et al. Larger nephron size and nephrosclerosis predict progressive CKD and mortality after radical nephrectomy for tumor and independent of kidney function. J Am Soc Nephrol 2020; 31: 2642–2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O’Seaghdha CM, Hwang SJ, Ho JE. et al. Elevated galectin-3 precedes the development of CKD. J Am Soc Nephrol 2013; 24: 1470–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bansal N, Katz R, Seliger S. et al. Galectin-3 and soluble ST2 and kidney function decline in older adults: the cardiovascular health study (CHS). Am J Kidney Dis 2016; 67: 994–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lieb W, Song RJ, Xanthakis V. et al. Association of circulating tissue inhibitor of metalloproteinases-1 and procollagen type III aminoterminal peptide levels with incident heart failure and chronic kidney disease. J Am Heart Assoc 2019; 8: e011426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Du X, Shimizu A, Masuda Y. et al. Involvement of matrix metalloproteinase-2 in the development of renal interstitial fibrosis in mouse obstructive nephropathy. Lab Invest 2012; 92: 1149–1160 [DOI] [PubMed] [Google Scholar]
- 12. Oelusarz A, Nichols LA, Grunz-Borgmann EA. et al. Overexpression of MMP-7 increases collagen 1A2 in the aging kidney. Physiol Rep 2013; 1: e00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou D, Tan RJ, Zhou L. et al. Kidney tubular β-catenin signaling controls interstitial fibroblast fate via epithelial-mesenchymal communication. Sci Rep 2013; 3: 1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou D, Tian Y, Sun L. et al. Matrix metalloproteinase-7 is a urinary biomarker and pathogenic mediator of kidney fibrosis. J Am Soc Nephrol 2017; 28: 598–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eggen AE, Mathiesen EB, Wilsgaard T. et al. The sixth survey of the Tromso Study (Tromso 6) in 2007–08: collaborative research in the interface between clinical medicine and epidemiology: study objectives, design, data collection procedures, and attendance in a multipurpose population-based health survey. Scand J Public Health 2013; 41: 65–80 [DOI] [PubMed] [Google Scholar]
- 16. Eriksen BO, Stefansson VTN, Jenssen TG. et al. Elevated blood pressure is not associated with accelerated glomerular filtration rate decline in the general non-diabetic middle-aged population. Kidney Int 2016; 90: 404–410 [DOI] [PubMed] [Google Scholar]
- 17. Eriksen BO, Mathisen UD, Melsom T. et al. Cystatin C is not a better estimator of GFR than plasma creatinine in the general population. Kidney Int 2010; 78: 1305–1311 [DOI] [PubMed] [Google Scholar]
- 18. Delanaye P, Ebert N, Melsom T. et al. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 1: How to measure glomerular filtration rate with iohexol? Clin Kidney J 2016; 9: 682–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jacobsson L. A method for the calculation of renal clearance based on a single plasma sample. Clin Physiol 1983; 3: 297–305 [DOI] [PubMed] [Google Scholar]
- 20. Bird NJ, Peters C, Michell AR. et al. Comparison of GFR measurements assessed from single versus multiple samples. Am J Kidney Dis 2009; 54: 278–288 [DOI] [PubMed] [Google Scholar]
- 21. Eriksen BO, Schaeffner E, Melsom T. et al. Comparability of plasma iohexol clearance across population-based cohorts. Am J Kidney Dis 2020; 76: 54–62 [DOI] [PubMed] [Google Scholar]
- 22. Levey AS, Stevens LA, Schmid CH. et al. ; for the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inker LA, Schmid CH, Tighiouart H. et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367: 20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leffondre K, Boucquemont J, Tripepi G. et al. Analysis of risk factors associated with renal function trajectory over time: a comparison of different statistical approaches. Nephrol Dial Transplant 2015; 30: 1237–1243 [DOI] [PubMed] [Google Scholar]
- 25. Grams ME, Sang Y, Ballew SH. et al. Evaluating glomerular filtration rate slope as a surrogate end point for ESKD in clinical trials: an individual participant meta-analysis of observational data. J Am Soc Nephrol 2019; 30: 1746–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eriksen BO, Småbrekke S, Jenssen TG. et al. Office and ambulatory heart rate as predictors of age-related kidney function decline. Hypertension 2018; 72: 594–601 [DOI] [PubMed] [Google Scholar]
- 27. Wood SN. Generalized Additive Models. An Introduction with R. 2nd edn. Boca Raton, FL: CRC Press, 2017 [Google Scholar]
- 28. Kerr KF, Meisner A, Thiessen-Philbrook H. et al. Developing risk prediction models for kidney injury and assessing incremental value for novel biomarkers. Clin J Am Soc Nephrol 2014; 9: 1488–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pencina MJ, D’Agostino RB, Pencina KM. et al. Interpreting incremental value of markers added to risk prediction models. Am J Epidemiol 2012; 176: 473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leening MJG, Vedder MM, Witteman JCM. et al. Net reclassification improvement: computation, interpretation, and controversies. Ann Intern Med 2014; 160: 122–131 [DOI] [PubMed] [Google Scholar]
- 31. Nelson RG, Grams ME, Ballew SH. et al. ; CKD Prognosis Consortium. Development of risk prediction equations for incident chronic kidney disease. JAMA 2019; 322: 2104–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Afkarian M, Zelnick LR, Ruzinski J. et al. Urine matrix metalloproteinase-7 and risk of kidney disease progression and mortality in type 2 diabetes. J Diabetes Complications 2015; 29: 1024–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang J, Ren P, Wang Y. et al. Serum matrix metalloproteinase-7 level is associated with fibrosis and renal survival in patients with IgA nephropathy. Kidney Blood Press Res 2017; 42: 541–552 [DOI] [PubMed] [Google Scholar]
- 34. Yang X, Ou J, Zhang H. et al. Urinary matrix metalloproteinase 7 and prediction of IgA nephropathy progression. Am J Kidney Dis 2020; 75: 384–393 [DOI] [PubMed] [Google Scholar]
- 35. Ihara K, Skupien J, Kobayashi H. et al. Profibrotic circulating proteins and risk of early progressive renal decline in patients with type 2 diabetes with and without albuminuria. Diabetes Care 2020; 43: 2760–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zuo F, Kaminski N, Eugui E. et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci USA 2002; 99: 6292–6297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang CC, Chuang JH, Chou MH. et al. Matrilysin (MMP-7) is a major matrix metalloproteinase upregulated in biliary atresia-associated liver fibrosis. Mod Pathol 2005; 18: 941–950 [DOI] [PubMed] [Google Scholar]
- 38. He W, Tan RJ, Li Y. et al. Matrix metalloproteinase-7 as a surrogate marker predicts renal Wnt/β-catenin activity in CKD. J Am Soc Nephrol 2012; 23: 294–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xiao Z, Chen C, Meng T. et al. Resveratrol attenuates renal injury and fibrosis by inhibiting transforming growth factor-β pathway on matrix metalloproteinase 7. Exp Biol Med (Maywood) 2016; 241: 140–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heerspink HJL, Perco P, Mulder S. et al. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia 2019; 62: 1154–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marti HP, Fuscoe JC, Kwekel JC. et al. Metzincins and related genes in experimental renal ageing: towards a unifying fibrosis classifier across species. Nephrol Dial Transplant 2014; 29: 1177–1185 [DOI] [PubMed] [Google Scholar]
- 42. Nowak N, Skupien J, Niewczas MA. et al. Increased plasma Kidney Injury Molecule-1 suggests early progressive renal decline in non-proteinuric patients with Type 1 diabetes. Kidney Int 2016; 89: 459–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang X, Chen X, Hong Q. et al. TIMP-1 promotes age-related renal fibrosis through upregulating ICAM-1 in human TIMP-1 transgenic mice. J Gerontol A Biol Sci Med Sci 2006; 61: 1130–1143 [DOI] [PubMed] [Google Scholar]
- 44. Musiał K, Zwolińska D.. Matrix metalloproteinases (MMP-2,9) and their tissue inhibitors (TIMP-1,2) as novel markers of stress response and atherogenesis in children with chronic kidney disease (CKD) on conservative treatment. Cell Stress Chaperones 2011; 16: 97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tveitarås MK, Skogstrand T, Leh S. et al. Matrix metalloproteinase-2 knockout and heterozygote mice are protected from hydronephrosis and kidney fibrosis after unilateral ureteral obstruction. PLoS One 2015; 10: e0143390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Poggio ED, Wang X, Greene T. et al. Performance of the modification of diet in renal disease and cockcroft-gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol 2005; 16: 459–466 [DOI] [PubMed] [Google Scholar]
- 47. Schei J, Stefansson VTN, Mathisen UD. et al. Residual associations of inflammatory markers with eGFR after accounting for measured GFR in a community-based cohort without CKD. Clin J Am Soc Nephrol 2016; 11: 280–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. van Rijn MHC, Metzger M, Flamant M. et al. Performance of creatinine-based equations for estimating glomerular filtration rate changes over time. Nephrol Dial Transplant 2020; 35: 819–827 [DOI] [PubMed] [Google Scholar]
- 49. Kisand K, Kerna I, Kumm J. et al. Impact of cryopreservation on serum concentration of matrix metalloproteinases (MMP)-7, TIMP-1, vascular growth factors (VEGF) and VEGF-R2 in Biobank samples. Clin Chem Lab Med 2011; 49: 229–235 [DOI] [PubMed] [Google Scholar]
- 50. Rouy D, Ernens I, Jeanty C. et al. Plasma storage at −80°C does not protect matrix metalloproteinase-9 from degradation. Anal Biochem 2005; 338: 294–298 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly since this was not included in the research permission, due to ethical considerations and the privacy of individuals that participated in the study. The data can be shared on request as part of research collaboration.