ABSTRACT
Background
Itching (pruritus) is common in dialysis patients, but little is known about its impact on health-related quality of life (HRQOL), sleep problems and psychological symptoms. This study investigates the impact of itching in dialysis patients by looking into the persistence of itching, the effect of itching on the course of HRQOL and the combined effect of itching with sleep problems and with psychological symptoms on HRQOL.
Methods
Data were obtained from the RENINE/PROMs registry and included 2978 dialysis patients who completed patient-reported outcome measures between 2018 and 2020. Itching, sleep problems and psychological symptoms were assessed with the Dialysis Symptom Index (DSI) and HRQOL with the 12-item Short Form Health Survey. Effects of itching on HRQOL and interactions with sleep problems and psychological symptoms were investigated cross-sectionally and longitudinally using linear regression and linear mixed models.
Results
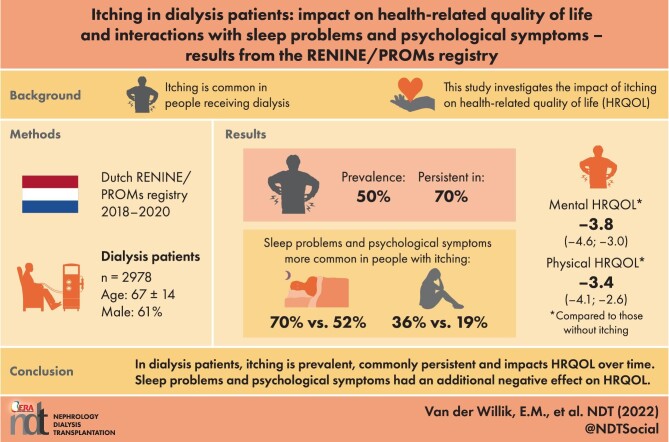
Half of the patients experienced itching and in 70% of them, itching was persistent. Itching was associated with a lower physical and mental HRQOL {−3.35 [95% confidence interval (CI) −4.12 to −2.59) and −3.79 [95% CI −4.56 to −3.03]}. HRQOL remained stable during 2 years and trajectories did not differ between patients with or without itching. Sleep problems (70% versus 52%) and psychological symptoms (36% versus 19%) were more common in patients with itching. These symptoms had an additional negative effect on HRQOL but did not interact with itching.
Conclusions
The persistence of itching, its impact on HRQOL over time and the additional effect on HRQOL of sleep problems and psychological symptoms emphasize the need for recognition and effective treatment of itching to reduce symptom burden and improve HRQOL.
Keywords: dialysis, health-related quality of life (HRQOL), pruritus, psychological symptoms, sleep problems
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What is already known about this subject?
Chronic kidney disease–associated pruritus, better known as itching, is a highly prevalent and distressing symptom in patients receiving dialysis treatment.
In cross-sectional studies, itching has been associated with a decreased health-related quality of life (HRQOL), psychological symptoms and sleep problems.
Longitudinal information about the impact of itching on HRQOL over time and the combined effect of itching with sleep problems and psychological symptoms is lacking.
What this study adds?
Itching was persistent in 70% of the dialysis patients.
Individuals with itching experienced a lower physical and mental HRQOL, which remained stable during 2 years of follow-up.
Sleep problems and psychological symptoms had an additional negative effect on HRQOL but did not interact with itching.
What impact this may have on practice or policy?
The study findings emphasize the need for recognition and effective treatment of itching to reduce symptom burden and improve HRQOL in dialysis patients.
These insights may help us to better understand and inform patients, which may enhance shared decision-making.
INTRODUCTION
Patients with end-stage kidney disease (ESKD) experience numerous physical and emotional disease-related symptoms, such as fatigue, muscle cramps, itching, sleep problems and depressive symptoms [1, 2]. The heavy symptom burden has a disruptive impact on individuals’ lives and has been shown to be associated with impaired health-related quality of life (HRQOL) in this population [3, 4].
A common and highly distressing symptom is chronic kidney disease–associated pruritus, better known as itching. Itching is experienced by both haemodialysis (HD) and peritoneal dialysis (PD) patients with a prevalence of ∼50% [1, 2, 5, 6]. Itching was found to be one of the 10 most burdensome symptoms experienced by dialysis patients [2] and is considered a main research priority by patients with ESKD, their caregivers and healthcare professionals [7]. The pathogenesis of itching in dialysis patients is not yet fully understood, but several factors seem to influence the occurrence or burden of itching, including abnormal calcium, phosphate and parathyroid hormone levels; opioid imbalance; peripheral neuropathy; dialysis efficiency and dry skin [5, 6]. Furthermore, itching has been associated with adverse clinical outcomes, such as hospitalization and mortality, and poor patient-reported outcomes, such as decreased HRQOL, psychological symptoms (e.g. depressive symptoms) and sleep problems [5, 6, 8, 9]. Large cohort studies have found that HRQOL, depressive symptoms and sleep quality were worse with more severe itching in dialysis patients [8, 9]; these associations suggest a causal effect of itching on HRQOL. However, information about the impact of itching on the course of HRQOL over time is lacking. Moreover, although itching is often accompanied by sleep problems and psychological symptoms, no literature is currently available about the extent to which the combinations of these symptoms affect patients’ physical and mental HRQOL.
Insight into the impact of itching on HRQOL and into the combined effect of itching with sleep problems and with psychological symptoms in the association with HRQOL could help us better understand patients’ outcomes and ultimately reduce symptom burden and increase HRQOL. Therefore, the aim of this study was to investigate the impact of itching in patients receiving dialysis treatment by looking into the persistence of itching over time, the relationship between itching and HRQOL and the combined effect of itching with sleep problems and psychological symptoms on HRQOL. These associations will be examined both cross-sectionally and longitudinally using data from routine Dutch dialysis care.
MATERIALS AND METHODS
Study design and population
Data were obtained from RENINE (Registratie Nierfunctievervanging Nederland: www.nefrovisie.nl/renine), the nationwide Dutch renal registry of patients receiving kidney replacement therapy. The registry collects information on demographics and clinical characteristics that are registered every 3 months. In addition, patient-reported outcome measures (PROMs) were introduced into routine dialysis care in September 2016 as part of a pilot study in 16 Dutch dialysis centres and have now been implemented nationally since November 2018 [2]. The PROMs were selected in collaboration with patients and experts [10] and include the 12-item Short Form Health Survey (SF-12) [11] to assess HRQOL and the Dialysis Symptom Index (DSI) [12] to assess symptom burden. PROMs invitations are distributed one to two times per year to all patients receiving dialysis treatment (i.e. total prevalent dialysis population). To ensure inclusion of all hospitals and a consistent follow-up period, data from 2018 to 2020 were used for this study. All dialysis patients who completed the PROMs at least once in this period were included in the analysis.
All patients included in RENINE gave consent to collect and use their data for scientific research purposes. Additionally, the current study protocol was reviewed and approved by the scientific committees of Nefrovisie and of the Clinical Epidemiology Department at Leiden University Medical Centre (LUMC). The study is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [13] with the extension of the Reporting of Studies Conducted Using Observational Routinely Collected Health Data (RECORD) statement [14].
Itching, sleep problems and psychological symptoms
The DSI is a 30-item kidney disease–specific questionnaire to assess physical and emotional symptom burden. Patients were asked to report the presence of 30 symptoms (yes/no) during the past week and, if present, the burden of each symptom on a 5-point Likert scale ranging from 1 ‘not at all’ to 5 ‘very much’ bothersome [12]. Two overall scores were calculated: the total number of symptoms present (0–30 symptoms) and the total symptom burden score (score range 0–150), which is the sum of burden on individual symptoms whereby missing items were assumed absent (i.e. burden score: 0) [2, 15].
The symptoms of interest in this study—itching, sleep problems and psychological symptoms—were assessed by means of the DSI. Itching was reported using a single item assessing whether itching was experienced in the past week and, if present, how bothersome this was. For the main analysis, patients were stratified based on the presence of itching (yes/no) at baseline (i.e. the patient's first PROM measurement). The burden score for itching ranges from 0 to 5, with higher scores indicating a higher burden.
Sleep problems were assessed using two symptoms, namely trouble falling asleep and trouble staying asleep. Sleep problems were defined as at least one of these two symptoms being present. The burden score of sleep problems ranges from 0 to 10, with higher scores indicating a higher burden.
The psychological cluster includes the following five symptoms: worrying, feeling nervous, feeling irritable, feeling sad and feeling anxious. Psychological symptoms were considered present when at least three of these five symptoms were experienced by the patient. The total burden score of psychological symptoms ranges from 0 to 25, with higher scores indicating a higher burden.
HRQOL
The SF-12 is a generic health questionnaire consisting of 12 questions assessing the following 8 domains of HRQOL: physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional and mental health. These domains contribute (in different proportions) to the scoring of a physical component summary (hereafter referred to as physical HRQOL) and a mental component summary (hereafter referred to as mental HRQOL). HRQOL scores range from 0 to 100, with higher scores indicating a better physical and mental HRQOL [11].
Population characteristics
Demographics and clinical characteristics were age, sex, primary kidney disease (PKD) according to European Renal Association codes [16], socio-economic status [SES; classified as low, middle and high using standard deviation (SD) scores based on zip code], dialysis modality [haemodialysis (HD) or peritoneal dialysis (PD)], number of dialysis sessions and hours per week in HD, time since dialysis initiation, kidney transplantation in the past (yes/no), residual glomerular filtration rate (rGFR, mL/min/1.73m2), dialysis adequacy [Kt/V: dialyser urea clearance (K) in mL/min by time (t) to urea distribution volume (V)] using single-pool Kt/V per dialysis session in HD patients and total Kt/V per week in PD patients, haemoglobin (mmol/L), ferritin (μg/L), transferrin saturation (%), albumin-adjusted calcium (mmol/L), phosphate (mmol/L) and parathyroid hormone (pmol/L).
Statistical analysis
The baseline was defined as the patient's first PROMs measurement. Baseline characteristics of the dialysis population, stratified for the experience of itching (yes/no), are presented as frequencies with percentages for categorical variables, as mean with SD for normally distributed continuous variables and as median with interquartile range (IQR) for skewed continuous variables. The prevalence and persistence of itching over time are shown graphically based on calendar time (prevalence of itching over time) and on patients’ follow-up time stratified for itching at baseline (persistence of itching over time).
The main analyses were performed both cross-sectionally and longitudinally, so that all patients and all PROMs measurements could be included in the analyses and to expand on existing (mainly cross-sectional) literature [5]. The cross-sectional analysis was performed at baseline and included all patients in the study population (N = 2978 patients). The longitudinal analysis included all PROMs measurements (n = 5042), with 1218 (40.9%) patients having multiple PROMs measurements. Of the individuals who had only one PROMs measurement (n = 1760), 1032 (58.6%) patients started with PROMs in 2020 and 322 (18.3%) patients died, which prevented follow-up data from being available. The main analyses were performed crude and adjusted for the following potential baseline confounders: age, sex, PKD, SES, dialysis modality, time since dialysis initiation and kidney transplantation in the past. The symptoms, HRQOL and potential confounding variables included in the main analyses had no or ˂2 % missing values.
Cross-sectionally, the association between the presence of itching and physical and mental HRQOL was investigated using linear regression analysis. Furthermore, in two separate linear regression models, a cross-product interaction term for itching (yes/no) and sleep problems (yes/no) and for itching and psychological symptoms (yes/no) was included to assess the interaction effects of these symptoms in the association with HRQOL.
The associations described above were also investigated longitudinally using linear mixed models. By using this statistical method, all measurements from all individuals could be included, as the model takes account of a varying number of follow-up measurements across individuals and even single measurements can be included in the estimation of the trajectory over time at the population level [17]. The presence of itching at baseline was included in the model as a fixed independent variable, time as a random variable and the continuous physical and mental HRQOL over time as a dependent variable. The interaction between time and itching was included, indicating the annual change in HRQOL for individuals with itching compared with individuals without itching.
Sensitivity analyses were conducted to assess the robustness of our main results. Both the cross-sectional and the longitudinal analyses were repeated using the continuous burden score of itching, sleep problems and psychological symptoms. The analyses were also performed with the symptoms classified based on low or high burden, e.g. no or mild itching (burden score: 0–2) versus moderate to severe itching (burden score: 3–5) and similar categories for sleep problems and psychological symptoms. Furthermore, the analyses were repeated comparing persistent itching (i.e. presence of itching reported both at baseline and at the first follow-up measurement) with no or non-persistent itching. Last, analyses were performed using 2019–2020 data, to only include measurements from the official start of the PROMs registry in November 2018.
All statistical analyses were performed using SPSS version 25.0 (IBM, Armonk, NY, USA).
RESULTS
Population characteristics
Table 1 presents the characteristics of all patients (N = 2978) who completed the PROMs at least once in 2018–2020, stratified for the presence of itching at baseline. Itching was present in approximately half of the patients and was more common in individuals receiving PD (59.4%) compared with HD (48.7%). (See also Supplementary data, part A for the population characteristics stratified by dialysis modality). Patients with itching were more often male, had a higher SES and more often had diabetes as the primary kidney disease compared with patients without itching. No differences were observed in calcium, phosphate and parathyroid hormone levels. The total symptom burden was higher in patients who experienced itching, with on average 14 symptoms with a median total burden score of 35 (IQR 23–51), compared with 8 symptoms with a median total burden score of 19 (IQR 10–32) in patients who did not experience itching. Patients with itching more often had dry skin compared with patients without itching (73% versus 43%, respectively). Sleep problems were experienced by 70% of the patients with itching and by 52% of the patients without itching. Psychological symptoms occurred in 36% of the patients with itching compared with 19% in patients without itching.
Table 1.
Characteristics of dialysis patients, stratified by presence of itching (yes/no) at baseline
| Characteristics | Total dialysis population (n = 2978) | Patients with itching [n = 1493 (50.1%)] | Patients without itching [n = 1485 (49.9%)] |
|---|---|---|---|
| Age (years), mean (SD) | 67.3 (14.1) | 67.4 (14.0) | 67.3 (14.2) |
| Sex (male), n (%) | 1827 (61.4) | 927 (62.1) | 900 (60.7) |
| SES, n (%) | |||
| Low | 1430 (48.4) | 711 (48.0) | 719 (48.8) |
| Middle | 907 (30.7) | 435 (29.4) | 472 (32.1) |
| High | 617 (20.9) | 336 (22.7) | 281 (19.1) |
| Primary kidney disease, n (%) | |||
| Glomerulonephritis/sclerosis | 333 (11.2) | 160 (10.7) | 173 (11.7) |
| Pyelonephritis | 140 (4.7) | 68 (4.6) | 72 (4.9) |
| Polycystic kidney disease | 171 (5.7) | 86 (5.8) | 85 (5.7) |
| Hypertension/renal vascular disease | 809 (27.2) | 411 (27.5) | 398 (26.8) |
| Diabetes mellitus type ½ | 601 (20.2) | 320 (21.4) | 281 (18.9) |
| Miscellaneous | 535 (18.0) | 257 (17.2) | 278 (18.7) |
| Unknown | 387 (13.0) | 191 (12.8) | 196 (13.2) |
| Dialysis modality, n (%) | |||
| HD | 2583 (87.9) | 1258 (85.6) | 1325 (90.1) |
| PD | 357 (12.1) | 212 (14.4) | 145 (9.9) |
| Dialysis sessions per week (HD), n (%) | |||
| <3 | 266 (12.8) | 125 (12.3) | 141 (13.2) |
| 3 | 1672 (80.3) | 817 (80.6) | 855 (80.1) |
| >3 | 144 (6.9) | 72 (7.1) | 72 (6.7) |
| Dialysis hours per week (HD), mean (SD) | 11.2 (4.3) | 11.1 (3.8) | 11.4 (4.8) |
| Time since dialysis initiation (months), median (IQR) | 15 (3–43) | 14 (3–41) | 17 (3–46) |
| Kidney transplantation in past (yes), n (%) | 327 (11.2) | 162 (11.1) | 165 (11.3) |
| rGFR (mL/min/1.73m2), median (IQR) | 4.7 (2.0–7.6) | 5.0 (2.0–8.0) | 4.5 (2.1–7.1) |
| Single-pool Kt/V in HD, mean (SD) | 1.47 (0.53) | 1.46 (0.54) | 1.48 (0.52) |
| Total Kt/V in PD, mean (SD) | 2.63 (1.06) | 2.70 (1.10) | 2.52 (0.99) |
| Haemoglobin (mmol/L), mean (SD) | 6.8 (0.9) | 6.8 (0.9) | 6.8 (0.9) |
| Ferritin (µg/L), median (IQR) | 318 (168–534) | 300 (153–517) | 330 (187–547) |
| Transferrin saturation (%), mean (SD) | 22.3 (10.5) | 21.8 (10.4) | 22.8 (10.6) |
| Calcium (mmol/L)a, mean (SD) | 2.31 (0.19) | 2.31 (0.19) | 2.31 (0.18) |
| Phosphate (mmol/L), mean (SD) | 1.59 (0.48) | 1.62 (0.49) | 1.57 (0.47) |
| Parathyroid hormone (pmol/L), median (IQR) | 30 (17–51) | 30 (17–52) | 30 (17–50) |
| Symptom burden | |||
| Total number of symptoms (0–30), mean (SD) | 11.0 (6.4) | 13.7 (6.2) | 8.3 (5.3) |
| Total symptom burden score (0–150), median (IQR) | 27 (14–42) | 35 (23–51) | 19 (10–32) |
| Dry skin (yes), n (%) | 1726 (58.0) | 1091 (73.1) | 635 (42.8) |
| Sleep problems, n (%) | |||
| Sleep problems (yes)b | 1816 (61.0) | 1044 (69.9) | 772 (52.0) |
| Trouble falling asleep (yes) | 1312 (44.1) | 798 (53.4) | 514 (34.6) |
| Trouble staying asleep (yes) | 1549 (52.0) | 907 (60.8) | 642 (43.2) |
| Psychological symptoms, n (%) | |||
| Psychological symptoms (yes)c | 820 (27.5) | 534 (35.8) | 286 (19.3) |
| Worrying (yes) | 1195 (40.1) | 708 (47.4) | 487 (32.8) |
| Feeling nervous (yes) | 816 (27.4) | 517 (34.6) | 299 (20.1) |
| Feeling irritable (yes) | 843 (28.3) | 558 (37.4) | 285 (19.2) |
| Feeling sad (yes) | 1088 (36.5) | 669 (44.8) | 419 (28.2) |
| Feeling anxious (yes) | 647 (21.7) | 425 (28.5) | 222 (14.9) |
Missing values: age, n = 3 (0.10%); sex, n = 2 (0.07%); SES, n = 24 (0.81%); primary kidney disease, n = 2 (0.07%); dialysis modality, n = 38 (1.29%); dialysis sessions per week (HD), n = 501 (19.4%); dialysis hours per week (HD), n = 505 (19.6%); time since dialysis initiation, n = 58 (1.99%); kidney transplantation in past, n = 58 (1.99%); residual GFR, n = 1977 (66.4%); single-pool Kt/V in HD, n = 647 (25.0%); total Kt/V in PD, n = 221 (61.9%); haemoglobin, n = 286 (9.60%); ferritin, n = 416 (14.0%); transferrin saturation, n = 993 (33.3%); calcium, n = 369 (12.4%); phosphate, n = 280 (9.40%); parathyroid hormone, n = 522 (17.5%).
Albumin-adjusted calcium.
Sleep problems are considered present if at least one of the two symptoms are experienced by the patient.
cPsychological symptoms are considered present if three of the five symptoms are experienced by the patient.
Prevalence and persistence of itching over time
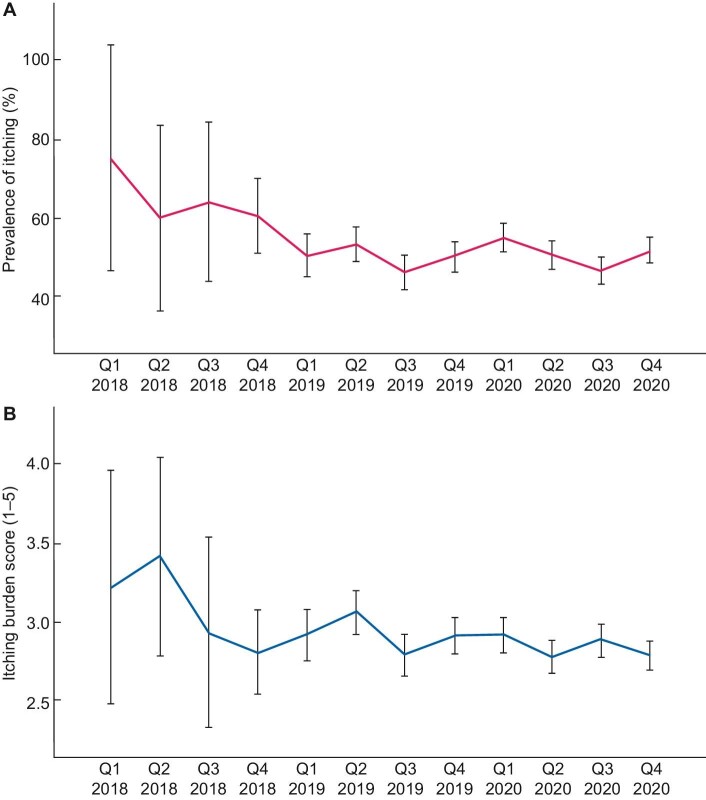
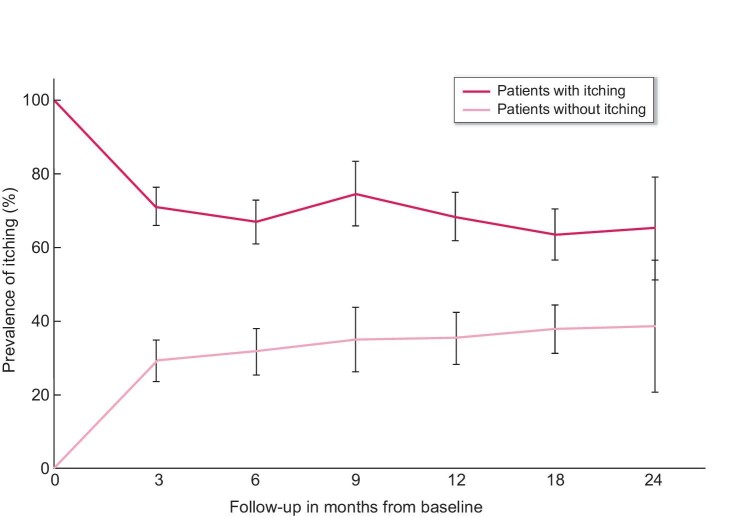
In total, 1218 patients have multiple PROMs measurements [median 2 (IQR 2–3)], with on average 6.7 months (SD 5.0) between baseline and the second PROMs measurement. Throughout the whole study period, the prevalence of itching was ∼50% with a moderate burden (mean burden scores between 2.8 and 3.4 on a 1–5 scale) (Figures 1A and B). No clear differences in prevalence or burden of itching could be detected between the yearly quartiles (i.e. no seasonal effects). Figure 2 shows that itching persisted over time in ∼70% of the patients who experienced itching at baseline. Of the patients without itching at baseline, 30–40% developed itching during follow-up. Sleep problems and psychological symptoms also persisted over time in the majority of the patients (see Supplementary data, part B).
FIGURE 1:
(A) Prevalence of itching (yes/no) over the study period (calendar time). Percentage of dialysis patients who experience itching (solid line) with 95% CI (bars) at each quartile in 2018–2020. (B) Burden of itching (scale: 1–5) over the study period (calendar time) in patients who experienced itching. Average itching burden score (solid line) with 95% CI (bars) in dialysis patients who experienced itching at each quartile in 2018–2020. Note that Q1–Q3 2018 includes a small number of patients (n = 12, n = 20, n = 25, respectively), as the PROMs registry officially started in November 2018. Some patients already participated in Q1–Q3 2018 for scientific research purposes.
FIGURE 2:
Persistence of itching during follow-up in patients with itching (black) and patients without itching (grey) at baseline. Black solid line (black bars) shows the percentage (95% CI) of dialysis patients in which itching is persistent during follow-up since baseline. Grey solid line (grey bars) shows the percentage (95% CI) of dialysis patients in which itching was newly developed during follow-up since baseline. Note that the average time between follow-up measurements was 6.7 months, meaning that the number of patients who contribute data fluctuates across the time points in the graph.
Association between itching and HRQOL at baseline
The mean physical and mental HRQOL scores in the total dialysis population were 35.8 (SD 10.4) and 48.1 (SD 10.4), respectively. Table 2 shows the cross-sectional effects of itching, combined with sleep problems and psychological symptoms, on physical and mental HRQOL. Patients with itching experienced a lower physical {−3.35 [95% confidence interval (CI) −4.12 to −2.59]; P < .001} and mental HRQOL [−3.79 (95% CI −4.56 to −3.03); P < .001] compared with patients without itching. Sleep problems and psychological symptoms had an additional negative effect on HRQOL. No interaction was observed between itching and sleep problems or psychological symptoms in the association with HRQOL. Table 3a shows the average physical and mental HRQOL in patients with itching, sleep problems or a combination of both. Table 3b shows the average physical and mental HRQOL in patients with itching, psychological symptoms or a combination of both.
Table 2.
Cross-sectional effects of the presence of itching (yes/no), combined with sleep problems and psychological symptoms, on physical and mental HRQOL
| Physical HRQOL | Mental HRQOL | |||
|---|---|---|---|---|
| Characteristics | Coefficient (95 %CI) | P-value | Coefficient (95 %CI) | P-value |
| Itching | ||||
| Model 1, unadjusted | −3.36 (−4.13 to −2.59) | <.001 | −3.82 (−4.58 to −3.06) | <.001 |
| Model 2, adjusteda | −3.35 (−4.12 to −2.59) | <.001 | −3.79 (−4.56 to −3.03) | <.001 |
| Itching and sleep problems (model 3b) | ||||
| Itching | −3.38 (−4.62 to −2.13) | <.001 | −2.38 (−3.61 to −1.15) | <.001 |
| Sleep problems | −3.85 (−4.92 to −2.78) | <.001 | −3.37 (−4.42 to −2.31) | <.001 |
| Itching * sleep problems | 1.00 (−0.58–2.58) | .214c | −1.18 (−2.74–0.38) | .139c |
| Itching and psychological symptoms (model 4b) | ||||
| Itching | −2.81 (−3.70 to −1.92) | <.001 | −2.35 (−3.15 to −1.56) | <.001 |
| Psychological symptoms | −3.51 (−4.87 to −2.15) | <.001 | −11.34 (−12.56 to −10.13) | <.001 |
| Itching * psychological symptoms | 0.03 (−1.72–1.79) | .971c | 1.01 (−0.56–2.58) | .208c |
Adjusted for age, sex, primary kidney disease, SES, dialysis modality, time since dialysis initiation and kidney transplantation in the past.
Models 3 and 4 build on model 2 and include the interaction with sleep problems and psychological symptoms, respectively.
P-value for interaction.
Table 3a.
Physical and mental HRQOL in patients with itching, sleep problems or a combination of both
| Physical HRQOL a | Mental HRQOL a | ||||
|---|---|---|---|---|---|
| Itching | Sleep problems | Mean | SD | Mean | SD |
| No | No | 39.44 | 2.23 | 51.89 | 1.79 |
| Yes | No | 36.01 | 2.03 | 49.68 | 1.69 |
| No | Yes | 35.58 | 2.21 | 48.47 | 1.83 |
| Yes | Yes | 33.25 | 2.22 | 44.89 | 1.81 |
Adjusted for age, sex, primary kidney disease, SES, dialysis modality, time since dialysis initiation and kidney transplantation in the past.
Table 3b.
Physical and mental HRQOL in patients with itching, psychological symptoms or a combination of both
| Physical HRQOL a | Mental HRQOL a | ||||
|---|---|---|---|---|---|
| Itching | Psychological symptoms | Mean | SD | Mean | SD |
| No | no | 38.12 | 2.15 | 52.34 | 1.46 |
| Yes | no | 35.33 | 2.03 | 50.02 | 1.40 |
| No | yes | 34.51 | 2.17 | 40.80 | 1.54 |
| Yes | yes | 31.75 | 2.19 | 39.46 | 1.47 |
Adjusted for age, sex, primary kidney disease, SES, dialysis modality, time since dialysis initiation and kidney transplantation in the past.
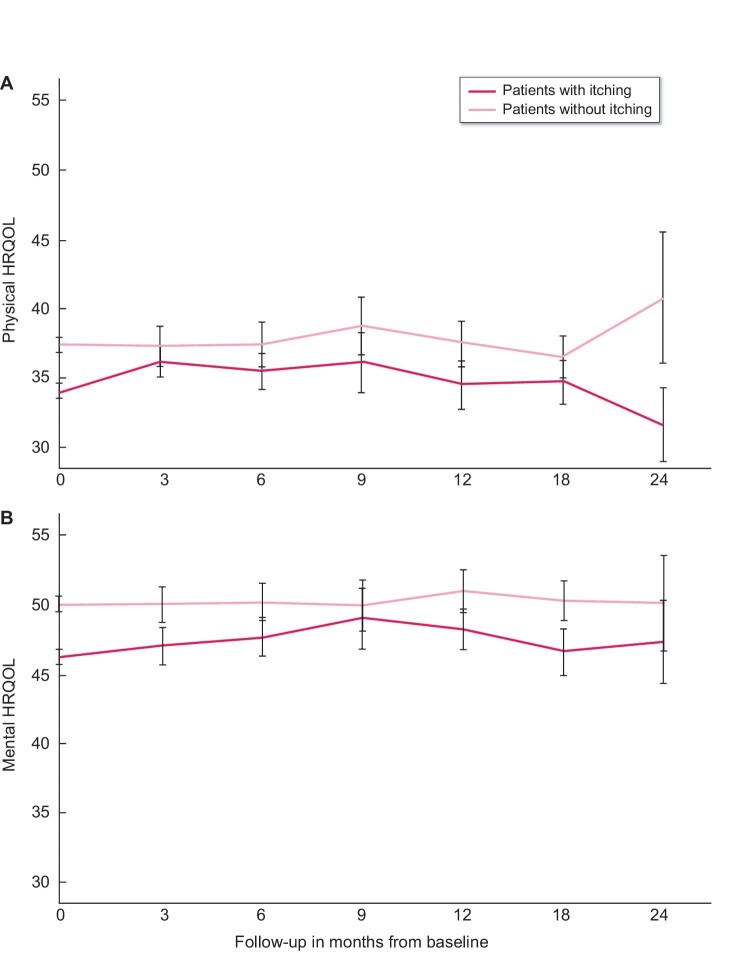
Association between itching and HRQOL over time
Figures 3A and B show the trajectories of physical and mental HRQOL during follow-up, stratified by itching at baseline. Findings from the longitudinal analyses using linear mixed models were similar to the cross-sectional analyses, showing that patients with itching experienced a lower physical and mental HRQOL compared with patients without itching [−3.12 (95% CI −3.86 to −2.38; P < .001) and −3.62 (95% CI −4.35 to −2.88; P < .001), respectively]. No changes in physical and mental HRQOL over time were observed in the total population throughout follow-up [annual change 0.01 (95% CI −0.68–0.70; P = .97) and −0.04 (95% CI −0.75–0.67; P = .91), respectively]. No differences in physical and mental HRQOL trajectories were observed between patients with and without itching [extra annual change in patients with itching 0.10 (95% CI −0.83–1.03; P = .83) and −0.07 (95% CI −1.04–0.90; P = .88), respectively]. Also longitudinally, in the association with physical and mental HRQOL, there was no significant interaction between itching and sleep problems (P = .52 and P = .22, respectively) or itching and psychological symptoms (P = .66 and P = .29, respectively).
FIGURE 3:
(A) Trajectory of physical HRQOL during follow-up in patients with itching (black) and patients without itching (grey) at baseline. Black solid line (black bars) shows the mean physical HRQOL (95% CI) over time in dialysis patients with itching at baseline. Grey solid line (grey bars) shows the mean physical HRQOL (95% CI) over time in dialysis patients without itching at baseline. (B) Trajectory of mental HRQOL during follow-up in patients with itching (black) and patients without itching (grey) at baseline. Black solid line (black bars) shows the mean mental HRQOL (95% CI) over time in dialysis patients with itching at baseline. Grey solid line (grey bars) shows the mean mental HRQOL (95% CI) over time in dialysis patients without itching at baseline. Note that the average time between follow-up measurements was 6.7 months, meaning that the number of patients who contribute data fluctuates across the time points in the graph.
A post hoc subgroup analysis showed an increase in physical and mental HRQOL when itching disappeared [+0.56 (P = .49) and +1.78 (P = .02), respectively] and a decrease when itching newly occurred [−0.44 (P = .61) and −0.68 (P = .38), respectively] between the patients’ first and second PROMs measurement (Supplementary data, part C).
Sensitivity analyses
All sensitivity analyses yielded results comparable to the main analyses, both cross-sectionally and longitudinally (Supplementary data, part D). Analyses using the continuous burden scores for symptoms showed that physical and mental HRQOL were −1.26 (95% CI −1.50 to −1.02; P < .001) and −1.42 (95% CI −1.65 to −1.18; P < .001) points lower, respectively, for each point increase in burden of itching. Using the continuous burden scores, the interaction between itching and psychological symptoms in the association with physical and mental HRQOL became statistically significant, although with a similarly small effect. Moderate to severe itching (prevalence 26.1%) compared with no or mild itching showed a larger decrease in physical and mental HRQOL [−4.20 (95% CI −5.07 to −3.33; P < .001) and −4.90 (95% CI −5.76 to −4.03; P < .001), respectively]. Comparing persistent itching with no or non-persistent itching showed comparable results. Restriction to 2019–2020 data yielded similar results.
DISCUSSION
This nationwide Dutch study investigated the impact of itching (pruritus) on HRQOL and interactions with sleep problems and psychological symptoms in patients receiving dialysis treatment. Half of the dialysis patients experienced itching and in 70% of them, itching was persistent over time. Individuals with itching experienced a lower physical and mental HRQOL. This is the first study showing that HRQOL remained stable during 2 years of follow-up and HRQOL trajectories did not differ between patients with or without itching. Furthermore, we found that sleep problems and psychological symptoms were more common in individuals who also experienced itching. These symptoms had an additional negative effect on physical and mental HRQOL but did not interact with itching (i.e. the combination of both symptoms did not result in a significantly lower or higher HRQOL than the sum of individual effects).
The high prevalence of itching and its persistence over time demonstrate that itching is a major problem in patients receiving dialysis treatment. Although the estimated prevalence varies between 20 and 90% across studies [5], for instance, due to differences in populations and in definitions of itching, it is clear that itching affects many dialysis patients’ lives, especially given that itching appeared to be persistent in many patients. In line with our results, two other studies found that itching was persistent for >1 year in 50–69% of the dialysis patients [18, 19]. The reason why itching was persistent in some patients and not in others remains unclear. According to one of the studies, differences could not be explained by whether or not the patients received treatment for their itching, as itching was often underestimated and left untreated [18]. Therefore, more research is needed to identify patients with persistent itching in order to treat them in a timely manner.
The impact of itching on patients’ lives is clearly reflected in the decreased HRQOL scores. In line with existing literature, physical and mental HRQOL were ∼3–4 points lower in patients who experienced itching compared with those without itching, and HRQOL scores decreased further with more severe itching [8, 9, 20–22]. Information regarding the relevance of this difference according to dialysis patients is lacking [23], but comparable differences in HRQOL have been considered important in other populations [24–26]. In addition to the existing literature, this study also investigated the impact of itching on the course of HRQOL and showed that the difference in HRQOL between individuals with and without itching persisted over time. A possible explanation for this result is that itching also persisted over time in the majority of the patients. However, in contrast to what might be expected based on previous research about the effect of itching on clinical outcomes over time (e.g. mortality and hospitalizations) [5, 8, 27, 28], this study showed no faster deterioration of HRQOL in patients with (persistent) itching during 2 years of follow-up. Future research should investigate these relationships using a longer follow-up period.
Findings from our study confirm that sleep problems and psychological symptoms often co-occur with itching [6, 8, 29–31]. Results from previous studies suggest that sleep problems and psychological symptoms may partly explain the effect of itching on HRQOL [5, 20, 30]. Our study does not contradict this suggestion, but it does show that sleep problems and psychological symptoms also independently affect HRQOL, in addition to the effect that itching has on HRQOL. Since these symptoms often co-occur in dialysis patients, many individuals have to deal with a substantially decreased HRQOL.
Our findings emphasize the importance of an effective treatment for itching in dialysis patients. Although unadjusted for potential confounding, findings from two observational studies suggest that HRQOL improves when itching disappears [18, 19]. A post hoc analysis of our data also showed an improved physical HRQOL (P < .05) and mental HRQOL (P = ns) when itching disappeared. Additionally, several treatment trials showed that a reduced itching intensity may result in improved HRQOL scores and sleep quality [5]. Furthermore, the literature suggests that better management of itching and HRQOL might even result in improved clinical outcomes, such as mortality and hospitalizations [5, 8, 27, 28]. The need for and potential benefits of a treatment for itching are thus evident. However, effective treatment of itching in dialysis patients appears challenging: some treatment options are available (e.g. prevention of hyperphosphatemia, adequate dialysis dose, ultraviolet B light therapy, gabapentin and several emollient creams), but seem to have limited efficacy or side effects [5, 6, 32].
With this study we aimed to provide insights into and awareness of the high prevalence and impact of itching, as this may still be underestimated [33, 34]. We believe that our research can contribute to existing knowledge due to the longitudinal design using national data from routine dialysis care. As PROMs are part of and used for routine care, patients are more likely to participate (compared with research purposes only), which enhances the generalizability of our results. On the downside, due to this design, the study mainly includes prevalent dialysis patients, which means that patients have been followed from different points in their trajectory (e.g. differences in time since start of dialysis). However, we do not believe this has affected the relationship between itching and HRQOL. Another limitation of this study is that no information was available on treatments that may have induced or reduced itching. It is therefore unclear how treatment may have affected the results (e.g. is the prevalence and persistence of itching this high despite treatment of itching?) or to what extent available treatment options may decrease the burden of itching in dialysis patients. Furthermore, additional knowledge about factors that may influence itching is needed and may be informative for treatment choices, for example, to tailor the dialysis schedule or nutritional advice. Taken together, current findings show that itching is a major problem in dialysis patients and call for further research to effectively identify and treat (persistent) itching to reduce symptom burden and improve HRQOL.
Of course, to reduce the burden of itching in dialysis patients, attention must be paid to itching on the individual patient level. However, the literature suggests that itching remains underreported and therefore undertreated due to a lack of knowledge and assessment during consultations [34]. We believe that the use of PROMs in routine dialysis care improves the reporting and prompts discussion of patients’ experiences and treatment options [2]. Current findings can be used to better inform patients and may enhance shared decision-making.
In conclusion, the high prevalence and persistence of itching, its impact on HRQOL over time and the additional effect on HRQOL of the often co-occurring sleep problems and psychological symptoms emphasize the need for recognition and effective treatment of itching to reduce symptom burden and improve HRQOL in dialysis patients. No individual prognoses can be derived from our study, but the findings may be used in shared decision-making. We hope that this study provided insights into and awareness of the major impact that itching can have, to enable early recognition and treatment of itching.
DATA AVAILABILITY STATEMENT
The data used for this research are available upon request. Contact information: Friedo W. Dekker (F.W.Dekker@lumc.nl).
Supplementary Material
ACKNOWLEDGEMENTS
The authors are grateful to all the patients, healthcare professionals and dialysis centres that participated in the registry of PROMs. The authors thank the Dutch nOcturnal and hoME dialysis Study To Improve Clinical Outcomes (DOMESTICO) study group for their collaboration in the collection of PROMs to relieve dialysis patients from unnecessary questionnaire burden. Finally, the authors gratefully acknowledge the Nefrovisie Foundation for facilitating the registry of PROMs and data management for this study.
Contributor Information
Esmee M van der Willik, Department of Clinical Epidemiology, Leiden University Medical Center, Leiden, The Netherlands.
Robin Lengton, Department of Clinical Epidemiology, Leiden University Medical Center, Leiden, The Netherlands.
Marc H Hemmelder, Department of Internal Medicine, Maastricht University Medical Center and CARIM School for Cardiovascular Diseases, Maastricht University, Maastricht, The Netherlands.
Ellen K Hoogeveen, Department of Clinical Epidemiology, Leiden University Medical Center, Leiden, The Netherlands; Department of Nephrology, Leiden University Medical Center, Leiden, The Netherlands; Department of Nephrology, Jeroen Bosch Hospital, Den Bosch, The Netherlands.
Hans A J Bart, Dutch Kidney Patients Association, Bussum, The Netherlands.
Frans J van Ittersum, Department of Nephrology, Amsterdam University Medical Center, Amsterdam, The Netherlands.
Marc A G J ten Dam, Nefrovisie Foundation, Utrecht, The Netherlands; Department of Internal Medicine, Canisius Wilhelmina Hospital, Nijmegen, The Netherlands.
Willem Jan W Bos, Department of Nephrology, Leiden University Medical Center, Leiden, The Netherlands; Department of Internal Medicine, St Antonius Hospital, Nieuwegein, The Netherlands.
Friedo W Dekker, Department of Clinical Epidemiology, Leiden University Medical Center, Leiden, The Netherlands.
Yvette Meuleman, Department of Clinical Epidemiology, Leiden University Medical Center, Leiden, The Netherlands.
FUNDING
This study was supported by unrestricted grants from Nierstichting Nederland (A1D1P04), Patiëntenfederatie Nederland, Zorgverzekeraars Nederland and Vifor Pharma International. The funding organizations had no role in the study design; collection, analysis and interpretation of the data; writing the report; or the decision to submit the report for publication.
AUTHORS’ CONTRIBUTIONS
E.v.d.W., M.H., F.D. and Y.M. designed the study. E.v.d.W., F.D. and Y.M. conducted the data analysis and drafted the manuscript. F.D. and Y.M. provided supervision and mentorship. All authors (E.v.d.W., R.L., M.H., E.H., H.B., F.v.I., M.t.D., W.J.B., F.D., Y.M.) assisted in the interpretation of results, provided important intellectual content and revised the final version of the manuscript. All authors provided final approval of the version to be published.
CONFLICT OF INTEREST STATEMENT
M.H. is a member of the advisory board of Vifor Pharma International. The results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Almutary H, Bonner A, Douglas C. Symptom burden in chronic kidney disease: a review of recent literature. J Ren Care 2013; 39: 140–150 [DOI] [PubMed] [Google Scholar]
- 2. van der Willik EM, Hemmelder MH, Bart HAJet al. Routinely measuring symptom burden and health-related quality of life in dialysis patients: first results from the Dutch registry of patient-reported outcome measures. Clin Kidney J 2021; 14: 1535–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Voskamp PWM, van Diepen M, Evans Met al. The impact of symptoms on health-related quality of life in elderly pre-dialysis patients: effect and importance in the EQUAL study. Nephrol Dial Transplant 2019; 34: 1707–1715 [DOI] [PubMed] [Google Scholar]
- 4. Raj R, Ahuja KD, Frandsen Met al. Symptoms and their recognition in adult haemodialysis patients: interactions with quality of life. Nephrology (Carlton) 2017; 22: 228–233 [DOI] [PubMed] [Google Scholar]
- 5. Shirazian S, Aina O, Park Yet al. Chronic kidney disease-associated pruritus: impact on quality of life and current management challenges. Int J Nephrol Renovasc Dis 2017; 10: 11–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Verduzco HA, Shirazian S. CKD-associated pruritus: new insights into diagnosis, pathogenesis, and management. Kidney Int Rep 2020; 5: 1387–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Manns B, Hemmelgarn B, Lillie Eet al. Setting research priorities for patients on or nearing dialysis. Clin J Am Soc Nephrol 2014; 9: 1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sukul N, Karaboyas A, Csomor PAet al. Self-reported pruritus and clinical, dialysis-related, and patient-reported outcomes in hemodialysis patients. Kidney Med 2021; 3: 42–53.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramakrishnan K, Bond TC, Claxton Aet al. Clinical characteristics and outcomes of end-stage renal disease patients with self-reported pruritus symptoms. Int J Nephrol Renovasc Dis 2013; 7: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van der Willik EM, Meuleman Y, Prantl Ket al. Patient-reported outcome measures: selection of a valid questionnaire for routine symptom assessment in patients with advanced chronic kidney disease—a four-phase mixed methods study. BMC Nephrol 2019; 20: 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ware JE Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996; 34:220–233 [DOI] [PubMed] [Google Scholar]
- 12. Weisbord SD, Fried LF, Arnold RMet al. Development of a symptom assessment instrument for chronic hemodialysis patients: the Dialysis Symptom Index. J Pain Symptom Manage 2004; 27: 226–240 [DOI] [PubMed] [Google Scholar]
- 13. von Elm E, Altman DG, Egger Met al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61: 344–349 [DOI] [PubMed] [Google Scholar]
- 14. Benchimol EI, Smeeth L, Guttmann Aet al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med 2015; 12: e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abdel-Kader K, Unruh ML, Weisbord SD. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol 2009; 4: 1057–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. ERA-EDTA Registry. ERA-EDTA Registry Annual Report 2019 . Amsterdam: Amsterdam UMC, location AMC, Department of Medical Informatics, 2021 [Google Scholar]
- 17. Janmaat CJ, van Diepen M, Tsonaka Ret al. Pitfalls of linear regression for estimating slopes over time and how to avoid them by using linear mixed-effects models. Nephrol Dial Transplant 2019; 34: 561–566 [DOI] [PubMed] [Google Scholar]
- 18. Plewig N, Ofenloch R, Mettang Tet al. The course of chronic itch in hemodialysis patients: results of a 4-year follow-up study of GEHIS (German Epidemiological Hemodialysis Itch Study). J Eur Acad Dermatol Venereol 2019; 33: 1429–1435 [DOI] [PubMed] [Google Scholar]
- 19. Mathur VS, Lindberg J, Germain Met al. A longitudinal study of uremic pruritus in hemodialysis patients. Clin J Am Soc Nephrol 2010; 5: 1410–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pisoni RL, Wikström B, Elder SJet al. Pruritus in haemodialysis patients: International results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 2006; 21: 3495–3505 [DOI] [PubMed] [Google Scholar]
- 21. Weiss M, Mettang T, Tschulena Uet al. Health-related quality of life in haemodialysis patients suffering from chronic itch: results from GEHIS (German Epidemiology Haemodialysis Itch Study). Qual Life Res 2016; 25: 3097–3106 [DOI] [PubMed] [Google Scholar]
- 22. Kimata N, Fuller DS, Saito Aet al. Pruritus in hemodialysis patients: results from the Japanese Dialysis Outcomes and Practice Patterns Study (JDOPPS). Hemodial Int 2014; 18: 657–667 [DOI] [PubMed] [Google Scholar]
- 23. van der Willik EM, Terwee CB, Bos WJWet al. Patient-reported outcome measures (PROMs): making sense of individual PROM scores and changes in PROM scores over time. Nephrology (Carlton) 2021; 26: 391–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clement ND, Weir D, Holland Jet al. Meaningful changes in the Short Form 12 physical and mental summary scores after total knee arthroplasty. Knee 2019; 26: 861–868 [DOI] [PubMed] [Google Scholar]
- 25. von der Lippe N, Waldum B, Brekke FBet al. From dialysis to transplantation: a 5-year longitudinal study on self-reported quality of life. BMC Nephrol 2014; 15: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ogura K, Yakoub MA, Christ ABet al. What are the minimum clinically important differences in SF-36 scores in patients with orthopaedic oncologic conditions? Clin Orthop Relat Res 2020; 478: 2148–2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ting SW, Fan PC, Lin YSet al. Uremic pruritus and long-term morbidities in the dialysis population. PLoS One 2020; 15: e0241088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Orasan OH, Muresan F, Mot Aet al. Hemodialysis patients with pruritus and insomnia have increased risk of death. Blood Purif 2020; 49: 419–425 [DOI] [PubMed] [Google Scholar]
- 29. Rehman IU, Chohan TA, Bukhsh Aet al. Impact of pruritus on sleep quality of hemodialysis patients: a systematic review and meta-analysis. Medicina (Kaunas) 2019; 55: 699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li J, Guo Q, Lin Jet al. Prevalence and associated factors of uraemic pruritus in continuous ambulatory peritoneal dialysis patients. Intern Med 2015; 54: 2827–2833 [DOI] [PubMed] [Google Scholar]
- 31. Zucker I, Yosipovitch G, David Met al. Prevalence and characterization of uremic pruritus in patients undergoing hemodialysis: uremic pruritus is still a major problem for patients with end-stage renal disease. J Am Acad Dermatol 2003; 49: 842–846 [DOI] [PubMed] [Google Scholar]
- 32. Simonsen E, Komenda P, Lerner Bet al. Treatment of uremic pruritus: a systematic review. Am J Kidney Dis 2017; 70: 638–655 [DOI] [PubMed] [Google Scholar]
- 33. Rayner HC, Larkina M, Wang Met al. International comparisons of prevalence, awareness, and treatment of pruritus in people on hemodialysis. Clin J Am Soc Nephrol 2017; 12: 2000–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aresi G, Rayner HC, Hassan Let al. Reasons for underreporting of uremic pruritus in people with chronic kidney disease: a qualitative study. J Pain Symptom Manage 2019; 58: 578–86.e2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used for this research are available upon request. Contact information: Friedo W. Dekker (F.W.Dekker@lumc.nl).