ABSTRACT
Background
Despite renin–angiotensin–aldosterone system blockade and immunosuppressive treatment, focal segmental glomerulosclerosis (FSGS) often progresses to kidney failure. The objective of this prespecified analysis of the dapagliflozin and prevention of adverse outcomes in chronic kidney disease trial (DAPA-CKD) was to assess efficacy and safety of dapagliflozin in a small subgroup of participants with FSGS confirmed by kidney biopsy.
Methods
In DAPA-CKD, patients with an estimated glomerular filtration rate (eGFR) 25–75 mL/min/1.73 m2 and urinary albumin:creatinine ratio (UACR) 200–5000 mg/g (22.6–565 mg/mol) were randomized to dapagliflozin 10 mg once daily or placebo as an adjunct to standard care and followed for median 2.4 years. The primary composite endpoint was sustained eGFR decline ≥50%, end-stage kidney disease, or kidney or cardiovascular death. The endpoint of interest for this analysis was eGFR slope (acute effects from baseline to Week 2 and chronic effects from Week 2 to end of treatment).
Results
Of 104 participants with biopsy-confirmed FSGS, 45 were randomized to dapagliflozin and 59 to placebo. Mean (standard deviation) age was 54.0 (14.3) years, mean eGFR 41.9 (11.5) mL/min/1.73 m2 and median (interquartile range) UACR 1248 (749–2211) mg/g. The primary outcome occurred in 4 (8.9%) and 7 (11.9%) participants randomized to dapagliflozin and placebo, respectively [hazard ratio 0.62, 95% confidence interval (95% CI) 0.17, 2.17]. Dapagliflozin led to a larger acute reduction (standard error) in eGFR compared with placebo (−4.5, 95% CI −5.9 to −3.1 versus −0.9, −2.1 to 0.4 mL/min/1.73 m2/2 weeks). Thereafter, mean rates of chronic eGFR decline with dapagliflozin and placebo were −1.9 (−3.0, −0.9) and −4.0 (−4.9, −3.0) mL/min/1.73 m2/year, respectively (difference 2.0, 95% CI 0.6 to 3.5, mL/min/1.73 m2/year). Adverse events leading to study drug discontinuation were similar in both groups; there were fewer serious adverse events with dapagliflozin.
Conclusions
Among DAPA-CKD participants with FSGS, dapagliflozin reduced the rate of chronic decline of eGFR compared with placebo, although this difference was not statistically significant.
Keywords: dapagliflozin, DAPA-CKD, eGFR slope, focal segmental glomerulosclerosis
KEY LEARNING POINTS.
What is already known about this subject?
Focal segmental glomerulosclerosis (FSGS) is a heterogeneous disease that often progresses to cause end-stage kidney disease.
There have been few treatment strategies proven to slow the progression of FSGS, and the clinical trials in FSGS have been challenging due to the requirement for ‘hard’ clinical outcomes to prove efficacy.
What this study adds?
In the dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) study, 104 patients with biopsy-confirmed FSGS (estimated glomerular filtration rate 25–75 mL/min/1.73 m2, urinary albumin:creatinine ratio [UACR] [UACR] 200–5000 mg/g) were randomized to dapagliflozin 10 mg or placebo. The primary outcome occurred numerically less frequently in the patients randomized to dapagliflozin versus placebo, although the total numbers of events were few. The rate of chronic eGFR decline (from Week 2 to end of treatment) was lower in patients with FSGS randomized to dapagliflozin compared with placebo. Adverse event rates leading to study drug discontinuation were similar between the groups.
What impact this may have on practice or policy?
Dapagliflozin offers an additional treatment option for patients with FSGS who meet the DAPA-CKD inclusion criteria.
INTRODUCTION
Focal segmental glomerulosclerosis (FSGS) is clinically characterized by proteinuria, hypertension and progressive loss of kidney function [1]. The incidence of FSGS has been estimated between 0.2 and 1.8/100 000 population/year [2]. FSGS is caused by a number of diseases and is characterized by the damage of podocytes [1]. The traditional classification is into primary FSGS and adaptive (or secondary) FSGS, the latter thought to result from excess nephron workload as occurs in obesity or following a reduction in the nephron number [1, 3]. This simplistic classification is becoming outdated as additional causes of FSGS, including genetic mutations, viral infections and medications, are recognized [4]. Treatment of FSGS depends on the aetiology and severity, with immunosuppression usually offered to patients with primary disease and supportive therapies including renin–angiotensin–aldosterone system (RAAS) blockade for those with the adaptive form [5–7]. There are no disease-specific therapies that have been approved by regulatory authorities, partly because no large-scale randomized clinical trials have demonstrated benefits on ‘hard outcomes’ such as a reduction in the risk of end-stage kidney disease (ESKD) with any therapeutic intervention. Use of a surrogate endpoint such as change in albuminuria or an intermediate endpoint such as change in estimated glomerular filtration rate (eGFR) slope has recently been advocated as a means to enhance feasibility of clinical trials in patients with specific and uncommon kidney diseases in whom traditional clinical endpoints rarely occur [8] and is an approach that has been adopted in ongoing trials in FSGS [9].
Dapagliflozin is a sodium-glucose cotransporter-2 (SGLT2) inhibitor that reduces glucose reabsorption in the proximal convoluted tubule of the kidney, thereby enhancing urinary glucose excretion [10]. Developed to improve glycaemic control in patients with diabetes, the SGLT2 inhibitors empagliflozin, canagliflozin, dapagliflozin and ertugliflozin slowed the rate of decline of eGFR and reduced albuminuria in cardiovascular safety trials involving participants with type 2 diabetes [11–14]. Clinical studies in type 1 and type 2 diabetes have shown early reversible reductions in eGFR and reductions in albuminuria following initiation of SGLT2 inhibitors, even in participants with good glycaemic control [15, 16], suggesting that SGLT2 inhibitors reduce intraglomerular pressure, which may in the long-term preserve kidney function. This same effect has also been observed in nondiabetic patients with proteinuric CKD [17], providing a rationale for the use of these agents as renoprotective therapies in patients with CKD due to causes other than diabetes.
The dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial demonstrated that dapagliflozin was superior to placebo in reducing the risk of major adverse kidney and cardiovascular events, as well as prolonging overall survival in a broad group of individuals with proteinuric CKD [18, 19] and in the subgroup of participants with a diagnosis of immunoglobulin A (IgA) nephropathy [20]. As previously reported, the DAPA-CKD study included 115 participants with a diagnosis of FSGS [21]. In this prespecified analysis, we investigated the effects of dapagliflozin in patients with FSGS confirmed by kidney biopsy.
MATERIALS AND METHODS
Trial design and study participants
DAPA-CKD was a multicentre, double-blind, placebo-controlled, randomized trial conducted at 386 study sites in 21 countries. The trial was designed to assess the effects of dapagliflozin on kidney and cardiovascular outcomes in patients with CKD, with or without type 2 diabetes, and was registered with ClinicalTrials.gov as NCT03036150. The trial was approved by Ethics Committees at each participating centre. All participants provided a written informed consent before the commencement of any study-specific procedure. An Independent Data Monitoring Committee provided oversight. The study protocol, statistical analysis plan and patient eligibility criteria have been previously published, as have manuscripts describing trial design [22], baseline characteristics [21], primary results [18] and results stratified by diabetes status [19], history of cardiovascular disease [23] and IgA nephropathy diagnosis [20].
Briefly, patients were eligible if they had an eGFR between 25 and ≤75 mL/min/1.73 m2 and urinary albumin:creatinine ratio (UACR) between 200 and ≤5000 mg/g (22.6 to ≤565.6 mg/mmol), and were receiving a stable dose of an angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB) for at least 4 weeks prior to enrolment into the trial, unless contraindicated. Exclusion criteria included patients with type 1 diabetes, those with polycystic kidney disease and those receiving immunotherapy for primary or secondary kidney disease within the previous 6 months prior to the trial enrolment [22].
Baseline categorization of cause of kidney disease
At the screening visit, investigators recorded the diagnosis of kidney disease and were asked to indicate whether this diagnosis was based on information obtained from a prior kidney biopsy. FSGS was included as a prespecified category among the participants with glomerulonephritis.
Randomization and study procedures
As described previously [22], the participants were randomly assigned to dapagliflozin 10 mg once-daily or matching placebo, in accordance with the sequestered, fixed randomization schedule, using balanced blocks to ensure an ∼1:1 ratio of the two regimens. Randomization was conducted using an interactive voice- or web-based system and stratified on the diagnosis of type 2 diabetes and UACR (≤1000 mg/g or >1000 mg/g). The study personnel (except the Independent Data Monitoring Committee) and the participants were blinded to the treatment allocation. Drug and placebo were identically packaged, with uniform tablet appearance, labelling and administration schedule. After randomization, the study visits occurred at 2 weeks, at 2, 4 and 8 months, and at 4-month intervals thereafter. At each visit, we collected blood and urine samples for laboratory assessment, recorded vital signs and gathered information on potential study endpoints, adverse events, concomitant therapies and study drug adherence. The study was stopped early because of overwhelming efficacy following a recommendation by the Independent Data Monitoring Committee.
Outcomes
The primary outcome of the trial was a composite endpoint of sustained ≥50% decline in eGFR (confirmed by a second serum creatinine after at least 28 days), onset of ESKD (defined as maintenance dialysis for at least 28 days, kidney transplantation, or eGFR <15 mL/min/1.73 m2 confirmed by a second measurement after at least 28 days), or death from a kidney or cardiovascular cause. Secondary outcomes included a kidney disease-specific composite outcome, which was similar to the primary outcome but excluding cardiovascular death, and all-cause mortality. An independent event adjudication committee assessed all clinical endpoints using these prespecified endpoint definitions. Because of the relatively small number of patients with FSGS, we replaced, post hoc, the ≥50% eGFR decline component for ≥40% eGFR decline in the primary outcome. A 40% eGFR decline is an accepted component of a composite kidney endpoint and occurs more frequently than 50% eGFR decline [24, 25]. For the current analysis we also examined changes in eGFR slope in the acute (baseline to Week 2), chronic (Week 2 to end of treatment) and total [baseline to end of treatment (median 2.4 years)] periods, as well as change in albuminuria.
Statistical analysis
We prespecified analyses of the effects of dapagliflozin on the primary and secondary efficacy endpoints in participants according to the aetiology of kidney disease, with the glomerulonephritis category further subcategorized by underlying cause, including FSGS. We included data from all randomized patients according to the intention-to-treat principle, but focused this analysis on those with biopsy-proven FSGS. Study data in tables and text are presented as mean ± standard deviation (SD) or ± standard error (SE) for slope data, or as median with 25th to 75th percentile range.
For event-driven analyses (which we anticipated would be underpowered), we fitted Cox proportional hazards regression models, stratified by type 2 diabetes and UACR (≤1000 and >1000) and adjusted for baseline eGFR to estimate the hazard ratio (HR) and 95% confidence intervals (CI) (dapagliflozin versus placebo) for the primary composite endpoint, secondary endpoints and prespecified exploratory endpoints.
The effect of dapagliflozin on the mean on-treatment eGFR slope was analysed by fitting a two-slope mixed effects linear spline model (with a knot at Week 2) to eGFR values, with random intercept and random slopes for treatment. The variance–covariance matrix was assumed to be unstructured, i.e. purely data dependent. The mean total slope was computed as a weighted combination of the acute and chronic slopes to reflect the mean rate of eGFR change to last on-treatment visit. We also visually presented the pattern of change in mean eGFR using a restricted maximum likelihood repeated measures approach. This analysis included the fixed effects of treatment, visit and the treatment-by-visit interaction, with visit treated as a categorical factor, as well as the continuous, fixed covariates of baseline eGFR and baseline eGFR-by-visit interaction. We conducted sensitivity analyses for all participants with FSGS as reported by the investigator, with or without a recorded confirmatory kidney biopsy.
A two-slope random effects model was used to assess the association between albuminuria at 14 days after randomization and longer-term effects of dapagliflozin on the chronic eGFR slope (Week 2 to end of treatment). This model included fixed effects for baseline eGFR, log-transformed UACR, change in UACR (at Day 14), type 2 diabetes status, treatment, age, sex, race, systolic blood pressure, HbA1c, haemoglobin, smoking status and cardiovascular disease history. The model also included a term for continuous time, a linear time-spline term combined with a three-way interaction between time-spline, treatment and change in UACR at Day 14. We used restricted maximum likelihood (REML) for estimation of statistical inference. The intercept and time were included as random effects and modelled using an unstructured variance–covariance matrix.
We performed all analyses using SAS version 9.4 (SAS Institute) or R version 4.0.2 (R-Foundation).
RESULTS
The trial included 115 participants with FSGS reported as the cause of kidney disease by the investigator, of whom 104 (90%) had undergone a kidney biopsy to substantiate this diagnosis. Of these 104 participants, 45 were randomized to dapagliflozin and 59 to placebo. Overall, the mean age was 54.0 years, 67.3% were male, 28.8% were Asian and 19.2% had type 2 diabetes. Mean eGFR (SD) was 41.9 (11.5) mL/min/1.73 m2 and median UACR (25th–75th percentile range) was 1248 mg/g (749–2211). Participants assigned to dapagliflozin or placebo had largely similar baseline characteristics, although there were some notable differences in the proportion with type 2 diabetes and in the racial distribution (Table 1).
Table 1.
Baseline characteristics in patients enrolled in DAPA-CKD with FSGS
| Characteristic | Dapagliflozin (N = 45) | Placebo (N = 59) | Total (N = 104) |
|---|---|---|---|
| Age (years) | 52.2 (14.2) | 55.4 (14.3) | 54.0 (14.3) |
| Sex, female, n (%) | 13 (28.9) | 21 (35.6) | 34 (32.7) |
| Race, n (%) | |||
| Asian | 8 (17.8) | 22 (37.3) | 30 (28.8) |
| Black or African American | 5 (11.1) | 2 (3.4) | 7 (6.7) |
| White | 28 (62.2) | 30 (50.8) | 58 (55.8) |
| Other | 4 (8.9) | 5 (8.5) | 9 (8.7) |
| Weight (kg) | 89.9 (18.2) | 81.7 (20.4) | 85.3 (19.8) |
| BMI (kg/m2) | 30.7 (6.5) | 28.7 (5.8) | 29.6 (6.1) |
| Blood pressure (mmHg) | |||
| Systolic | 127.0 (15.2) | 129.0 (14.7) | 128.2 (14.9) |
| Diastolic | 75.7 (9.8) | 76.1 (9.0) | 75.9 (9.3) |
| HbA1c, % | 5.7 (0.5) | 6.0 (1.1) | 5.9 (0.9) |
| eGFR (mL/min/1.73 m2) | 40.3 (10.6) | 43.2 (12.1) | 41.9 (11.5) |
| Haemoglobin (g/L) | 129.9 (16.1) | 133.3 (16.9) | 131.8 (16.6) |
| Serum potassium (mEq/L) | 4.6 (0.5) | 4.6 (0.4) | 4.6 (0.5) |
| Median UACR (interquartile range) | 997 (736–2290) | 1410 (769–2170) | 1248 (749–2211) |
| Type 2 diabetes, n (%) | 5 (11.1) | 15 (25.4) | 20 (19.2) |
| Heart failure, n (%) | 2 (4.4) | 0 (0) | 2 (1.9) |
| Prior medication, n (%) | |||
| ACEi | 17 (37.8) | 21 (35.6) | 38 (36.5) |
| ARB | 28 (62.2) | 36 (61.0) | 64 (61.5) |
| Diuretic | 19 (42.2) | 16 (27.1) | 35 (33.7) |
| Statin | 28 (62.2) | 45 (76.3) | 73 (70.2) |
Data are mean (SD) unless otherwise stated.
BMI, body mass index.
Effects of dapagliflozin on the primary composite and other endpoints
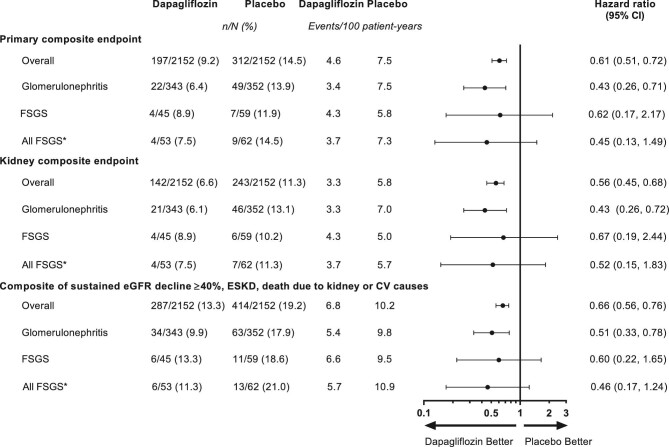
The primary composite endpoint occurred in 4 (8.9%) participants with FSGS in the dapagliflozin group and 7 (11.9%) participants with FSGS in the placebo group (HR 0.62, 95% CI 0.17, 2.17) (Figure 1). This finding was consistent with the overall DAPA-CKD population and the broad population with glomerulonephritis. All but one primary composite endpoint event occurred in patients with a baseline UACR >1000 mg/g. The effect of dapagliflozin among all participants with FSGS was consistent with the overall analyses (HR 0.45, 95% CI 0.13, 1.49; Figure 1). The secondary kidney-disease specific composite endpoint occurred in 4 (8.9%) participants in the dapagliflozin group and 6 (10.2%) participants in the placebo group (HR 0.67, 95% CI 0.19, 2.44). The post hoc composite outcome of a sustained ≥40% eGFR decline, ESKD or death from a kidney or cardiovascular cause occurred in 6 (13.3%) participants in the dapagliflozin group and 11 (18.6%) participants in the placebo group (HR 0.60, 95% CI 0.22, 1.65). Few patients reached ESKD during follow-up: 2 (4.4%) in the dapagliflozin group and 3 (5.1%) in the placebo group. During follow-up, two (3.4%) patients died in the placebo group and none in the dapagliflozin group.
Figure 1:
Forest plot for the primary composite endpoint, kidney-disease specific composite endpoint and a post hoc exploratory composite endpoint of ≥40% eGFR decline, onset of ESKD or death from kidney or cardiovascular causes in the overall DAPA-CKD population and in patients with glomerulonephritis and those with FSGS. *Includes those with biopsy-confirmed FSGS (n = 104) and those classed as FSGS but without biopsy confirmation (n = 11). Primary composite outcome, sustained ≥50% decline in eGFR, onset of ESKD, or death from a kidney or cardiovascular cause; kidney-specific composite outcome, sustained ≥50% decline in eGFR, onset of ESKD or death from a kidney cause.
Effects of dapagliflozin on continuous outcomes
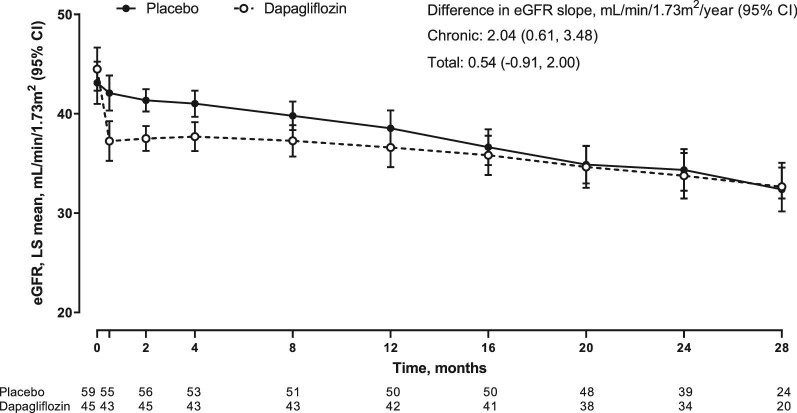
Between baseline and Week 2, and as expected, patients with FSGS randomized to dapagliflozin experienced a larger acute reduction in eGFR compared with placebo (−4.5, 95% CI −5.9, −3.1 versus −0.9, 95% CI −2.1, 0.4 mL/min/1.73 m2/2 weeks). Thereafter (chronic slope; Week 2 to end of treatment) the mean annual rates of eGFR decline with dapagliflozin and placebo were −1.9 (95% CI −3.0, −0.9) and −4.0 (95% CI −4.9, −3.0) mL/min/1.73 m2/year, respectively (difference 2.0, 95% CI 0.6, 3.5 mL/min/1.73 m2/year). The total slope, which combines the acute effect and chronic slope (baseline to end of treatment), was −3.7 (95% CI −4.8, −2.6) versus −4.2 (95% CI −5.2, −3.3) mL/min/1.73 m2/year in the dapagliflozin and placebo group respectively (difference 0.5, 95% CI −0.9, 2.0 mL/min/1.73 m2/year; Figure 2) (Supplementary data, Figure S1).
Figure 2:
eGFR trajectory over time in patients with focal segmental glomerulosclerosis.
At baseline, median UACR (25th–75th percentile range) in the dapagliflozin and placebo groups was 997 (736–2290) mg/g and 1410 (769–2170) mg/g, respectively. At Week 2, the geometric mean change from baseline in UACR was −26.1% (95% CI −35.2, −15.6) in the dapagliflozin group and −9.9% (95% CI −19.8, 1.1) in the placebo group, corresponding to a between-group difference of 19.7% (SE 9.0). This reduction in UACR persisted through to Month 12, although after 12 months UACR levels were similar between the two groups (Supplementary data, Figure S2).
Association between early UACR changes and chronic eGFR slope
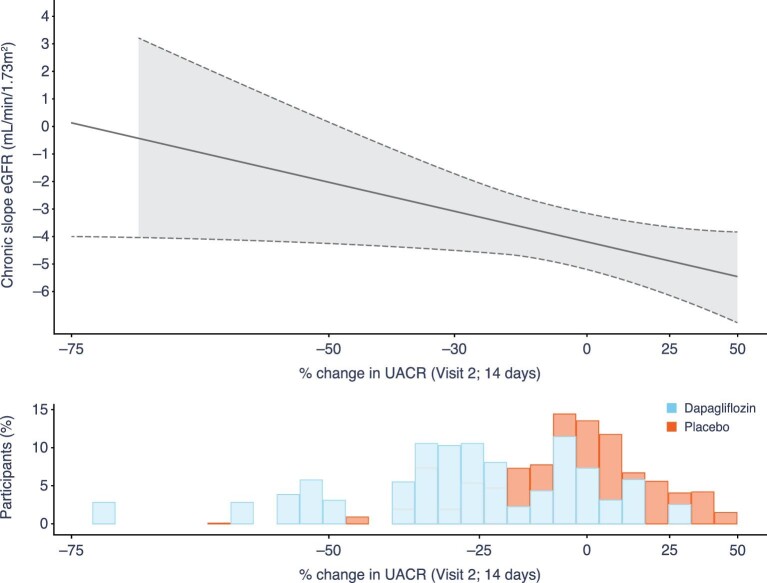
We observed a log-linear relationship between the early change in UACR at Week 2 and subsequent eGFR decline (chronic slope) with dapagliflozin treatment, such that each 10% reduction in UACR was associated with a subsequent average −0.67 (95% CI −0.93, −0.42) mL/min/1.73 m2/year lesser decline in eGFR (Figure 3) (Supplementary data, Figure S3).
Figure 3:
Relationship between percentage change in UACR from baseline to Week 2 and subsequent chronic eGFR slope* in patients with FSGS in the dapagliflozin and placebo groups combined. *Chronic eGFR slope is calculated from Week 2 to end of treatment and expressed as mL/min/1.73 m2/year. The solid line represents the chronic eGFR slope in the overall FSGS population at different values of acute UACR change. The shaded area represents the 95% CI. The distribution of % change in UACR from baseline to Week 2 in the dapagliflozin and placebo group is shown in the histogram, with the bars for the treatment groups superimposed on one another.
Effects of dapagliflozin on blood pressure
At baseline, mean systolic and diastolic blood pressure in the dapagliflozin and placebo groups were 127.0 and 129.0 mmHg, and 75.7 and 76.1 mmHg, respectively. During follow-up, there were no differences in blood pressure between treatment groups (Supplementary data, Figure S4).
Safety
Overall, adverse events leading to discontinuation of study drug were similar in the dapagliflozin and placebo groups. There were fewer serious adverse events with dapagliflozin versus placebo (Table 2). Of the participants developed major hypoglycaemia. There were no cases of diabetic ketoacidosis.
Table 2.
Safety outcomes in patients with FSGS
| n (%) | Dapagliflozin (N = 45) | Placebo (N = 58) |
|---|---|---|
| Adverse event leading to study drug discontinuation | 3 (6.7) | 3 (5.2) |
| Any serious adverse eventa | 9 (20.0) | 16 (27.6) |
Includes death.
DISCUSSION
The effect of dapagliflozin 10 mg, compared with placebo, was assessed in the DAPA-CKD trial in patients with CKD of various aetiologies, all of whom exhibited at least a modest degree of albuminuria, with nearly all on RAAS inhibitors. Investigator-reported causes of CKD were collected at the time of participant enrolment. After diabetic nephropathy (n = 2510), ischaemic/hypertensive nephropathy (n = 687) and IgA nephropathy (n = 270), FSGS (n = 115) comprised the fourth largest group with a single specific kidney disease [19, 21]. The diagnosis of FSGS was based on a kidney biopsy in 90% of the participants. In this prespecified analysis, we demonstrated that, among participants with biopsy-confirmed FSGS, dapagliflozin reduced the risk of the primary composite endpoint by 38% (95% CI 0.17, 2.17). Although the treatment effect did not approach statistical significance within the FSGS subgroup, the estimated effect was similar between FSGS patients and the full DAPA-CKD study population. Understanding that this analysis was limited by the small number of clinical events, we also examined eGFR slopes as an intermediate outcome (a surrogate for large decrements in kidney function or incidence of kidney failure) and demonstrated an attenuated chronic decline in eGFR among patients randomized to dapagliflozin (after the initial eGFR reduction at 2 weeks) of 2.0 mL/min/1.73 m2/year [with an overall slope difference of 0.5 mL/min/1.73 m2/year over the whole treatment period (median 2.4 years)]. The concordance between the significant change in rate of chronic eGFR decline and the trend to a reduction in kidney outcomes supports the use of eGFR slopes in clinical trials involving patients with FSGS in whom major adverse kidney events are rare [8].
Conducting clinical trials in FSGS has been challenging for multiple reasons including difficulties in recruiting sufficient patients. For example, the FONT (Novel Therapies for Resistant FSGS) Phase II clinical trial (NCT00814255, registration date: 22 December 2008) was designed to assess the efficacy of adalimumab and galactose compared with standard medical therapy, but recruited just 21 patients. A US National Institutes of Health-sponsored trial comparing cyclosporine to steroids and mycophenolate mofetil took 5 years to complete and included only 138 participants [26]. There is therefore a need for validated surrogate endpoints that allow efficacy assessment of established and new therapies, an approach that may appeal to regulators as well as to investigators and patients. A recent workshop supported by the US National Kidney Foundation concluded that changes in albuminuria and eGFR slope fulfil criteria for surrogacy for use as endpoints in clinical trials of chronic kidney disease, but that implementation required better understanding of conditions under which each surrogate was likely to perform well and to restrict the use of those settings [8]. Our data support the use of eGFR slopes as a surrogate marker in patients with a diagnosis of FSGS.
Remission of proteinuria has been used in past and ongoing clinical trials in patients with FSGS as a surrogate endpoint. Our data demonstrating that larger reductions in albuminuria correlate with less marked eGFR decline during subsequent follow-up support a potential role for albuminuria as a surrogate endpoint in future trials in patients with FSGS. These findings are in accord with a recent analysis from the FONT trial demonstrating that a 1-log unit reduction in UACR over 26 weeks was associated with an increase in eGFR of 3.9 mL/min/1.73 m2/year [27]. Traditionally remission in proteinuria (e.g. to urine protein:creatinine ratio <0.3 g/g) has also previously been used as an endpoint in trials in patients with FSGS [28]. Our data demonstrating a log-linear relationship between albuminuria change and eGFR support use albuminuria as a continuous measure without a requirement to achieve a minimal absolute threshold. A 25% reduction in albuminuria has been shown to provide high confidence that the intervention will infer clinical benefit [29]. The loss of any difference in UACR levels after 12 months in our analysis is likely to reflect the diminishing number of patients with FSGS who had data available for analysis as the trial progressed. Another important finding from our analysis was that all but one participant with a baseline UACR >1000 mg/g reached a clinical endpoint during the study, suggesting that these patients might be more informative in trials assessing progression to ESKD.
The ongoing EMPA-KIDNEY study (NCT03594110) assessing empagliflozin in patients with CKD, with and without diabetes, has enrolled ∼6600 participants. We anticipate that this trial has recruited patients with FSGS and may further contribute to our understanding of the treatment effect of SGLT2 inhibitors in this heterogeneous disease.
Recently updated international guidelines suggest using ACEi/ARBs as first-line supportive therapy in patients with glomerular disease, hypertension and proteinuria (>1 g/day) with up-titration depending on blood pressure [30]. Other general measures include lifestyle modifications, dietary sodium restriction and lipid management [30]. The guidelines also suggest a test-course of immunosuppressive therapy in patients with primary FSGS, starting with corticosteroids followed by calcineurin inhibitors for those who fail to achieve remission [30]. The guidelines recommend against using immunosuppression in those patients with FSGS of undetermined cause or secondary FSGS, leaving only supportive treatment options available. Given a lack of effective interventions, clinicians and patients are likely to welcome SGLT2 inhibitors as a new therapeutic approach that can be used as an adjunct to ACEi/ARB treatment.
Our findings in the FSGS subgroup of DAPA-CKD are consistent with the findings from other smaller, mechanistic trials of SGLT2 inhibitors in the patients without diabetes which suggest that dapagliflozin reduces intraglomerular pressure. In a cross-over study including 53 patients with proteinuric CKD without diabetes of whom 11 (21%) had FSGS, dapagliflozin 10 mg led to an acute but reversible reduction in measured GFR, reduced body weight and increased haematocrit, suggesting enhanced glycosuria and natriuresis [31]. These physiological changes are believed to preserve long-term kidney function in patients with and without type 2 diabetes, as was observed in the current study and was observed in those enrolled in DAPA-CKD with a diagnosis of IgA nephropathy [20]. While the mechanisms for kidney function preservation by SGLT2 inhibitors are not fully understood, other proposed pathways include suppression of inflammation and fibrosis, possibly through RAAS inhibition, and reduction in ischaemia in the kidney [32, 33].
Our findings have clinical implications for the management of patients with FSGS who share the clinical characteristics of the trial participants and who are already on RAAS blocking therapy. DAPA-CKD is the first event-driven trial of an SGLT2 inhibitor to include patients with CKD due to a range of underlying aetiologies, including patients with FSGS, and to demonstrate a beneficial effect on major adverse kidney events. Although, probably given our small sample size, we were unable to demonstrate a significant difference in the risk of the trial’s primary composite endpoint, the attenuated decline of eGFR in patients randomized to dapagliflozin is likely to translate into improvements in ‘clinically meaningful’ outcomes in a larger population over a longer treatment period. For example, if the annual rate of decline in eGFR were reduced from 4 to 2 mL/min/1.73 m2/year (slightly less than the benefit experienced by DAPA-CKD participants with FSGS), a patient at a starting eGFR of 43 mL/min/1.73 m2 (the mean eGFR of DAPA-CKD participants) would experience a roughly 8-year delay before reaching an eGFR of 10 mL/min/1.73 m2, a point at which many patients would require dialysis or kidney transplantation.
Dapagliflozin was well tolerated in the FSGS subpopulation, confirming its established safety profile. Clinicians will be reassured by the fact that there were no cases of diabetic ketoacidosis or hypoglycaemia in participants with FSGS receiving dapagliflozin.
With respect to limitations, the DAPA-CKD study was not specifically designed to test our hypothesis in patients with FSGS, and the relatively small sample size of this subgroup limited the precision of estimates of treatment effects on the study endpoints. However, the analysis presented herein was included in the original study design, without knowing a priori how many participants with FSGS would ultimately be enrolled. As a secondary analysis of the DAPA-CKD study, the participants in this analysis were not randomized into the treatment groups, which resulted in imbalances in characteristics between the groups; these imbalances should be considered when interpreting the results in this subgroup. A further point to note is that 10% of participants reported to have FSGS had not, according to the relevant investigator, undergone a kidney biopsy. The diagnosis of FSGS in these participants was likely to be based on the clinical acumen of the investigator and it is possible that some or all had another glomerular or kidney disease. These 11 patients did not alter our conclusions. We did not ask clinicians to sub-classify FSGS according to the likely underlying aetiology, so we do not know the proportion of patients with primary versus secondary disease; while the exclusion criteria in the DAPA-CKD study, such as previous use of immunosuppression within 6 months prior to enrolment, may mean that the majority of the FSGS patients have secondary disease, we are unable to provide further information as these specific data were not collected. Finally, we did not investigate the effects of dapagliflozin in patients with FSGS who had normal or near normal kidney function, or those with eGFR below 25 mL/min/1.73 m2. It should be noted that participants were not required to stop study drug based on any eGFR threshold.
CONCLUSION
In conclusion, this prespecified analysis of the DAPA-CKD study demonstrated that, when added to ACEi/ARB therapy, dapagliflozin reduced the rate of chronic eGFR decline over a median of 2.4 years treatment in patients with FSGS, in concordance with fewer primary endpoint events versus placebo, and had a favourable safety profile.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank all investigators, trial teams and patients for their participation in the trial. The authors would also like to acknowledge Nicola Truss, inScience Communications, London, UK, for assistance in editing and preparation of figures. This support was funded by AstraZeneca.
Contributor Information
David C Wheeler, Department of Renal Medicine, University College London, London, UK.
Niels Jongs, Department of Clinical Pharmacy and Pharmacology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Bergur V Stefansson, Late-Stage Development, Cardiovascular, Renal and Metabolism, Biopharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden.
Glenn M Chertow, Departments of Medicine and Epidemiology and Population Health, Stanford University School of Medicine, Stanford, CA, USA.
Tom Greene, Study Design and Biostatistics Center, University of Utah Health Sciences, Salt Lake City, UT, USA.
Fan Fan Hou, Division of Nephrology, Nanfang Hospital, Southern Medical University, National Clinical Research Center for Kidney Disease, Guangzhou, China.
Anna Maria Langkilde, Late-Stage Development, Cardiovascular, Renal and Metabolism, Biopharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden.
John J V McMurray, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK.
Peter Rossing, Steno Diabetes Center Copenhagen, Gentofte, Denmark; Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark.
Michal Nowicki, Department of Nephrology, Hypertension and Kidney Transplantation, Medical University of Łódź, Łódź, Poland.
István Wittmann, 2nd Department of Medicine and Nephrology-Diabetes Center, University of Pécs Medical School, Pécs, Hungary.
Ricardo Correa-Rotter, National Medical Science and Nutrition Institute Salvador Zubiran, Mexico City, Mexico.
C David Sjöström, Late-Stage Development, Cardiovascular, Renal and Metabolism, Biopharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden.
Robert D Toto, Department of Internal Medicine, UT Southwestern Medical Center, Dallas, TX, USA.
Hiddo J L Heerspink, Department of Clinical Pharmacy and Pharmacology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
FUNDING
The DAPA-CKD study was funded by AstraZeneca.
AUTHORS’ CONTRIBUTIONS
D.C.W. was involved in the study design, conduct of the study, data collection and interpretation, and wrote the first draft of the manuscript. H.J.L.H. was involved in the study design, data collection, conduct of the study, data analysis and interpretation and critical revision of all drafts of the manuscript. R.D.T., G.M.C., J.J.V.McM, T.G., F.F.H., P.R. and R.C.-R. are members of the executive committee of the DAPA-CKD study and were involved in the study design and data collection, analysis and interpretation. M.N. and I.W. were investigators for the study and were involved in data collection and interpretation. N.J. performed the data analyses. A.M.L., C.D.S. and B.V.S. were involved in the study design, conduct of the study, data collection and interpretation. D.C.W. and H.J.L.H. had full access to all data and had the final responsibility to submit for publication. All authors reviewed the manuscript drafts, provided approval of the final version for submission, and take the responsibility for the accuracy and integrity of the data.
CONFLICT OF INTEREST STATEMENT
D.C.W. provides ongoing consultancy services to AstraZeneca and has received honoraria and/or consultancy fees from Amgen, AstraZeneca, Boehringer Ingelheim, Bayer, GlaxoSmithKline, Janssen, Napp, Mundipharma, Medscape, Merck Sharp and Dohme, Pharmacosmos, Reata, Takeda, Tricida, Vifor Fresenius and Zydus. N.J. has nothing to declare. B.V.S., C.D.S. and A.M.L. are employees and stockholders of AstraZeneca. G.M.C. has received fees from AstraZeneca for the DAPA-CKD trial steering committee, and research grants from NIDDK and Amgen; he is on the board of directors for Satellite Healthcare, has received fees for advisory boards for Baxter, Cricket, DiaMedica and Reata, and holds stock options for Ardelyx, CloudCath, Durect, DxNow and Outset; has received fees from Akebia, Sanifit and Vertex for Trial steering committees and has received fees for DSMB service from Angion, Bayer and ReCor. T.G. has received grants for statistical consulting from AstraZeneca, CSL and Boehringer-Ingelheim; and has received personal fees from Janssen Pharmaceuticals, DURECT Corporation and Pfizer for statistical consulting. F.F.H. has received honoraria from AbbVie and AstraZeneca. J.J.V.McM. received payments to his employer, Glasgow University, for his work on clinical trials, consulting and other activities: Alnylam, Amgen, AstraZeneca, Bayer, BMS, Cardurion, Cytokinetics, GSK, Novartis, Pfizer and Theracos, and personal lecture fees from The Corpus, Abbott, Hickma, Sun Pharmaceuticals and Medsca. P.R. has received honoraria to Steno Diabetes Center Copenhagen for consultancy from AstraZeneca, Astellas, Bayer, Boehringer Ingelheim, Gilead, Novo Nordisk, Merck, Mundipharma, Sanofi and Vifor, and research support from Astra Zeneca and Novo Nordisk. M.N. has received honoraria from Boehringer Ingelheim, Swixx Biopharma, Roche, Sanofi Genzyme and Omeros. I.W. has received honoraria from AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Eli Lilly, Merck Sharp & Dohme and Sanofi. R.C.-R. has received honoraria from AbbVie, AstraZeneca, GlaxoSmithKline, Medtronic and Boehringer Ingelheim, has lectured for Amgen, Janssen, Takeda, AstraZeneca and Boehringer Ingelheim, and has received research support from GlaxoSmithKline, Novo Nordisk and AstraZeneca. R.D.T. is a consultant for AstraZeneca, Amgen, Bayer, Boehringer-Ingelheim, Medscape, Otsuka, Reata and Relypsa. H.J.L.H. is a consultant for AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, CSL Pharma, Gilead, Janssen, Merck, Mundi Pharma, Mitsubishi Tanabe, Novo Nordisk and Retrophin. He received research support from Abbvie, AstraZeneca, Boehringer Ingelheim and Janssen.
DATA AVAILABILITY STATEMENT
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
REFERENCES
- 1. Rosenberg AZ, Kopp JB.. Focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 2017; 12: 502–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McGrogan A, Franssen CF, de Vries CS.. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant 2011; 26: 414–430 [DOI] [PubMed] [Google Scholar]
- 3. Barisoni L, Schnaper HW, Kopp JB.. A proposed taxonomy for the podocytopathies: a reassessment of the primary nephrotic diseases. Clin J Am Soc Nephrol 2007; 2: 529–542 [DOI] [PubMed] [Google Scholar]
- 4. D'Agati VD, Kaskel FJ, Falk RJ.. Focal segmental glomerulosclerosis. N Engl J Med 2011; 365: 2398–2411 [DOI] [PubMed] [Google Scholar]
- 5. Rovin BH, Caster DJ, Cattran DCet al. . Management and treatment of glomerular diseases (part 2): conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int 2019; 95: 281–295 [DOI] [PubMed] [Google Scholar]
- 6. Liu Y, Shi Y, Ren Ret al. . Advanced therapeutics in focal and segmental glomerulosclerosis. Nephrology 2018; 23: 57–61 [DOI] [PubMed] [Google Scholar]
- 7. KDIGO Working Group . KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2012; 2: 139–274 [Google Scholar]
- 8. Levey AS, Gansevoort RT, Coresh Jet al. . Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the national kidney foundation in collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis 2020; 75: 84–104 [DOI] [PubMed] [Google Scholar]
- 9. Komers R, Diva U, Inrig JKet al. . Study design of the phase 3 sparsentan versus irbesartan (DUPLEX) study in patients with focal segmental glomerulosclerosis. Kidney Int Rep 2020; 5: 494–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vallon V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu Rev Med 2015; 66: 255–270 [DOI] [PubMed] [Google Scholar]
- 11. Wanner C, Inzucchi SE, Lachin JMet al. . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375: 323–334 [DOI] [PubMed] [Google Scholar]
- 12. Neal B, Perkovic V, Mahaffey KWet al. . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657 [DOI] [PubMed] [Google Scholar]
- 13. Mosenzon O, Wiviott SD, Cahn Aet al. . Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol 2019; 7: 606–617 [DOI] [PubMed] [Google Scholar]
- 14. Cherney DZI, Charbonnel B, Cosentino Fet al. . Effects of ertugliflozin on kidney composite outcomes, renal function and albuminuria in patients with type 2 diabetes mellitus: an analysis from the randomised VERTIS CV trial. Diabetologia 2021; 64: 1256–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cherney DZ, Perkins BA, Soleymanlou Net al. . Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014; 129: 587–597 [DOI] [PubMed] [Google Scholar]
- 16. Pollock C, Stefánsson B, Reyner Det al. . Albuminuria-lowering effect of dapagliflozin alone and in combination with saxagliptin and effect of dapagliflozin and saxagliptin on glycaemic control in patients with type 2 diabetes and chronic kidney disease (DELIGHT): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2019; 7: 429–441 [DOI] [PubMed] [Google Scholar]
- 17. Heerspink HJ, Perkins BA, Fitchett DHet al. . Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 2016; 134: 752–772 [DOI] [PubMed] [Google Scholar]
- 18. Heerspink HJL, Stefansson BV, Correa-Rotter Ret al. . Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383: 1436–1446 [DOI] [PubMed] [Google Scholar]
- 19. Wheeler DC, Stefansson BV, Jongs Net al. . Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and nondiabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol 2021; 9: 22–31 [DOI] [PubMed] [Google Scholar]
- 20. Wheeler DC, Toto RD, Stefansson BVet al. . A prespecified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int 2021; 100: 215–224 [DOI] [PubMed] [Google Scholar]
- 21. Wheeler DC, Stefansson BV, Batiushin Met al. . The dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial: baseline characteristics. Nephrol Dial Transplant 2020; 35: 1700–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heerspink HJL, Stefansson BV, Chertow GMet al. . Rationale and protocol of the dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) randomized controlled trial. Nephrol Dial Transplant 2020; 35: 274–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McMurray JJV, Wheeler DC, Stefansson BVet al. . Effect of dapagliflozin on clinical outcomes in patients with chronic kidney disease, with and without cardiovascular disease. Circulation 2021; 143: 438–448 [DOI] [PubMed] [Google Scholar]
- 24. Lambers Heerspink HJ, Weldegiorgis M, Inker LAet al. . Estimated GFR decline as a surrogate end point for kidney failure: a post hoc analysis from the reduction of end points in non-insulin-dependent diabetes with the angiotensin II antagonist losartan (RENAAL) study and irbesartan diabetic nephropathy trial (IDNT). Am J Kidney Dis 2014; 63: 244–250 [DOI] [PubMed] [Google Scholar]
- 25. Inker LA, Levey AS, Pandya Ket al. . Early change in proteinuria as a surrogate end point for kidney disease progression: an individual patient Meta-analysis. Am J Kidney Dis 2014; 64: 74–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gipson DS, Trachtman H, Kaskel FJet al. . Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney Int 2011; 80: 868–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Troost JP, Trachtman H, Spino Cet al. . Proteinuria reduction and kidney survival in focal segmental glomerulosclerosis. Am J Kidney Dis 2021; 77: 216–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Troost JP, Trachtman H, Nachman PHet al. . An outcomes-based definition of proteinuria remission in focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 2018; 13: 414–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heerspink HJL. Predicting individual treatment response in diabetes. Lancet Diabetes Endocrinol 2019; 7: 415–417 [DOI] [PubMed] [Google Scholar]
- 30. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Disease Work Group . KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int 2021; 100(4s): S1–S276 [DOI] [PubMed] [Google Scholar]
- 31. Cherney DZI, Dekkers CCJ, Barbour SJet al. . Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in nondiabetic patients with chronic kidney disease (DIAMOND): a randomised, double-blind, crossover trial. Lancet Diabetes Endocrinol 2020; 8: 582–593 [DOI] [PubMed] [Google Scholar]
- 32. Woods TC, Satou R, Miyata Ket al. . Canagliflozin prevents intrarenal angiotensinogen augmentation and mitigates kidney injury and hypertension in mouse model of type 2 diabetes mellitus. Am J Nephrol 2019; 49: 331–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Packer M. Mechanisms leading to differential hypoxia-inducible factor signaling in the diabetic kidney: modulation by SGLT2 inhibitors and hypoxia mimetics. Am J Kidney Dis 2021; 77: 280–286 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.