Abstract
Background
Targeting the MDM2-p53 interaction using AMG 232 is synergistic with MAPK inhibitors (MAPKi) in preclinical melanoma models. We postulated that AMG 232 plus MAPKi is safe and more effective than MAPKi alone in TP53-wild type, MAPKi-naïve metastatic melanoma.
Methods
Patients were treated with increasing (120 mg, 180 mg, 240 mg) oral doses of AMG 232 (seven-days-on, 15-days-off, 21-day cycle) plus dabrafenib (D) and trametinib (T) (Arm 1, BRAFV600-mutant) or T alone (Arm 2, BRAFV600-wild type). Patients were treated for seven days with AMG 232 alone before adding T±D. Safety and efficacy were assessed using CTCAE v4.0 and RECIST v1.1 criteria, respectively. Pharmacokinetic (PK) analysis was performed at baseline and steady-state levels for AMG 232.
Results
31 patients were enrolled. Ten and 21 patients were enrolled in Arm 1 and Arm 2, respectively. The most common AMG 232-related adverse events (AEs) were nausea (87%), diarrhea (77%), and fatigue (74%). Seven patients (23%) were withdrawn from the study due to AMG 232-related AEs. Three dose-limiting AEs occurred (Arm 1, 180 mg, nausea; Arm 2, 240 mg, grade 3 pulmonary embolism; Arm 2, 180 mg, grade 4 thrombocytopenia). AMG 232 PK exposures were not altered when AMG 232 was combined with T±D. Objective responses were seen in 8/10 (Arm 1) and 3/20 (Arm 2) evaluable patients. The median progression-free survival for Arm 1 and Arm 2 was 19.0 months-not reached and 2.8 months, respectively.
Conclusion
The maximum tolerated dose of AMG 232 for both arms was 120 mg. AMG 232 plus T±D exhibited a favorable PK profile. Although objective responses occurred in both arms, adding AMG 232 to T±D did not confer additional clinical benefit.
Supplementary information
The online version contains supplementary material available at 10.1007/s10637-022-01253-3.
Keywords: MDM2 inhibitors, BRAFV600E, Dabrafenib, Trametinib, Metastatic melanoma
Introduction
Despite the high rate of somatic mutations in cutaneous melanoma, only a small number of these mutations can be directly pharmacologically targeted. Treatment of BRAFV600-mutant metastatic melanoma (MM) with BRAF/MEK inhibitors has been associated with durable responses. However, many patients still die even if treated with immunotherapies [1]. Efforts to concurrently target pathways other than the mitogen-activated protein kinase (MAPK) pathway are usually toxic and have a marginal clinical benefit [2, 3] with the possible exception of programmed cell death protein 1 (PD1)/programmed death-ligand 1(PD-L1) pathway inhibitors [4]. Targeting the second most frequent somatic mutation in melanoma, NRASQ61, with single-agent MEK inhibitors was associated with marginal clinical benefit [5]. Therefore, there are no standard treatments specifically for patients with NRASQ61-mutant melanoma. MM was under-represented in the National Cancer Institute Molecular Analysis for Therapy Choice (NCI-MATCH). Moreover, of the screened patients, only a quarter was assigned to one of the available treatment arms [6, 7]. Targeting essential proteins that regulate cell death pathways and are intact/non-mutated is an alternative treatment strategy that may increase responses to existing targeted therapies in MM [8].
The murine double minute-2 (MDM2) proto-oncogene encodes a multimeric protein that regulates cellular stress responses [9]. The MDM2 gene infrequently undergoes genetic aberrations in MM [10, 11]. However, the MDM2 protein is frequently upregulated [11] (or activated) in melanoma secondary to the decreased or absent expression of MDM2 inhibitors, such as p14ARF. Among the >100 proteins that the MDM2 protein partners with, its physical interaction with p53 is the most well-studied [12]. Under physiologic conditions, MDM2 prevents p53 from entering the nucleus, inhibits its transactivation domain, and induces p53 ubiquitin-mediated degradation [13]. Various forms of cellular stress can affect interaction among p53 and several other partners, including MDM2, leading to p53 accumulation in the nucleus and activation of cell-cycle arrest, DNA repair, senescence, and programmed cell death programs [13]. In contrast with other cancers, the majority of melanomas express wild-type (WT) TP53. [14] Given that both MDM2 and TP53 genes are infrequently mutated in melanoma and that inhibition of MDM2 may activate p53’s tumor suppressor program, blocking the p53/MDM2 interaction may be an effective anticancer strategy for TP53-WT melanomas. [15]
AMG 232 was developed as a potent and selective piperidinone inhibitor of the MDM2-p53 protein interaction [16]. AMG 232 activates TP53 signaling and inhibits cancer cell proliferation in vitro. [16] When combined with inhibitors of either the phosphoinositide 3-kinase (PI3K) or the MAPK signaling pathway, AMG 232 demonstrated synergy in cell death across various TP53-WT melanoma cell lines [17]. Daily oral administration of AMG 232 in various TP53-WT cancer xenograft models showed dose-dependent antitumor activity with effective doses ranging between 9.1-78 mg/kg [16]. AMG 232 administered at 50 mg/kg dose demonstrated antitumor activity either alone or combined with dabrafenib and trametinib, especially in TP53-WT patient-derived melanoma xenografts [8]. This study (ClinicalTrials.gov NCT02110355) assessed the safety, tolerability, pharmacokinetics (PK), and maximum tolerated dose (MTD) of AMG 232 combined with dabrafenib-trametinib or trametinib alone in patients with MM with or without BRAFV600 mutations, respectively, and without prior treatment with BRAF or MEK inhibitors.
Patients and methods
Study design
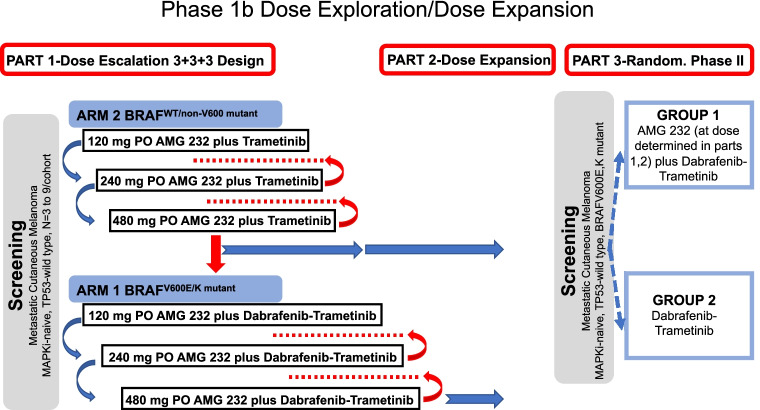
Supplementary Information includes major inclusion and exclusion criteria and details regarding the 3-part study design. Figure 1 summarizes the 3-part design. The Institutional Review Board of each participating institution approved the study. All study patients provided written informed consent before enrollment. Each participating institution used an analytically validated molecular test to confirm the non-mutated status of the entire TP53 gene (TP53-WT) and BRAFV600 mutational status in archival tumor tissues before study enrollment. Patients with a BRAFV600 mutation were enrolled in Arm 1, and patients with BRAFV600 wild-type status were enrolled in Arm 2.
Fig. 1.
Study design and treatment schema for AMG 232 study in patients with TP53-WT MM. Patient enrollment would start from Arm 2 (BRAFWT/non-V600mutant; AMG 232 in combination with trametinib 2 mg PO QD). AMG 232 (PO QD, 7-days-on, 15-days-off in a 21-day cycle) dose (120 mg, 240 mg, 480 mg) would escalate (blue arrows) until development of dose-limiting toxicity (DLT). Intermediate doses would be investigated and considered between the prespecified dose cohorts only if DLT, or MTD, developed in the immediately higher prespecified dose cohort (red arrows, red dots). The MTD from Arm 2 would be used to drive decisions for AMG 232 dosing in Arm 1 (BRAFV600-mutant). Part 2 would expand and refine established MTD dose for AMG 232 across more patients enrolled in each Arm. Part 3 would compare the efficacy of the dabrafenib-trametinib-AMG 232 combination with dabrafenib-trametinib alone in patients with BRAFV600-mutant melanoma as part of a randomized (1:1) phase II study. Only Part 1 was completed and therefore presented in this report
We only present data from Part 1 in which AMG 232 was dose-escalated (3+3+3) in Arm 1 and Arm 2. AMG 232 was administered orally at doses of 120, 180, and 240 mg once daily for seven days at every three-week treatment cycle in dose-escalating cohorts. Only for cycle 1, patients received AMG 232 as a single-agent during the first seven days. Trametinib, 2 mg once daily (both Arms), and dabrafenib, 150 mg twice daily (Arm 1 only), were subsequently started on day 8, cycle 1 (Fig. 1). Treatment was administered continuously in consecutive treatment cycles until disease progression, intolerability, or withdrawal of consent.
Safety and response assessment
Patients were reviewed for safety every three weeks, using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) toxicity criteria, v4.0. Supplementary Information includes definitions for the dose-limiting toxicity (DLT) period, non-hematologic DLTs, and other DLT definitions. We decided to establish separate MTDs for each of the treatment arms, because we postulated that they will be different, given that one arm was treated with three drugs (Arm 1, BRAFV600-mutant) and the other arm was treated with two drugs (Arm 2, non-BRAFV600-mutant). A patient was considered DLT-evaluable if he/she experienced a DLT or otherwise received ≥85% of AMG 232 over 28 days following the first dose. In the event of a DLT or other clinically significant AEs in any part of the study, treatment was withheld, and supportive therapy was administered as clinically indicated. If toxicity resolved to baseline or grade 1 in ≤14 days of stopping therapy, treatment could be restarted. Alternatively, if toxicity was attributed to AMG 232 and did not resolve in 14 days despite symptomatic management (e.g., antiemetics for nausea and vomiting), withdrawal from AMG 232 was recommended. Either trametinib and/or dabrafenib were dose-reduced if toxicity appeared related to any of the two drugs. A dose reduction below 75 mg twice daily for dabrafenib and 1 mg once daily for trametinib was not allowed. If trametinib was dose-reduced to <1 mg once daily, trametinib was permanently discontinued. In that case, however, the patient was allowed to continue dabrafenib and AMG 232. AMG 232 could be discontinued at the patient’s request or safety concerns. Response assessment by the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 was performed by local review four weeks and eight weeks after cycle 1, day 1, then every eight weeks after that using contrast-enhanced computerized tomography or magnetic resonance imaging of the chest, abdomen, and pelvis.
Pharmacokinetic assessments
We collected PK samples in cycle 1, day 7 (end of single-agent AMG 232 run-in phase; predose, 30 min, 1 hour, 2 hours, 4 hours, and 6 hours), day 8 (predose), and day 21 (predose, 30 min, 1, hours, 2 hours, 4 hours, and 6 hours), and in cycle 2, day 1 (predose), day 7 (predose, 30 min, 1 hour, 2 hours, 4 hours, and 6 hours), and day 8 (predose). Based on AMG 232 half-life estimates reported previously [18], AMG 232 drug levels were deemed steady-state on cycle 1, day 7, and cycle 2, day 7. Supplementary Information provides details about determining plasma concentrations and other PK parameter estimates for AMG 232, the AMG 232 glucuronide, trametinib, dabrafenib, and hydroxy- and desmethyl-dabrafenib metabolites.
Statistical analysis
We included patients who received ≥1 dose of AMG 232 and had ≥1 post-baseline efficacy assessment in the efficacy endpoint analysis. In addition, patients who received ≥1 dose of AMG 232 and completed 28 days after the initial AMG 232 dose or discontinued due to toxicity were evaluable for DLT. The sample size for part 1 was based on the 3+3+3 design [20]. Descriptive statistics were provided for demographics, treatment-emergent AEs, and efficacy and included means, medians, standard deviations, and ranges. In addition, we performed exploratory survival analyses using the Kaplan-Meier method for each Arm.
Results
Patients
Thirty-one patients (Arm 1, n=10; Arm 2, n=21) with BRAF and MEK inhibitor-naïve MM were enrolled in part 1 of the study between December 2014 and May 2017 across six academic institutions in the United States of America and Australia. Table 1 shows patient baseline characterstics. Most patients had cutaneous melanoma (n=19, 61%), were <65 years old (n=24, 77%), had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 (61%), and had received prior anticancer therapies (n=25, 81%). Of the previously treated patients, the majority (n=22, 88%) had received ≥2 systemic therapies, particularly PD1/PDL-1 inhibitor-based (n=21, 84%).
Table 1.
Baseline Demographics – Part 1 (n=31)
| Age (yrs; median, range) | 57 (24,79) |
|---|---|
| 18-64 | 24 (77) |
| ≥65 | 7 (23) |
| Sex (%) | |
| Male | 16 (52) |
| Female | 15 (48) |
| Melanoma Subtypes (%) | |
| Cutaneous | 19 (61) |
| Acral | 5 (16) |
| Mucosal | 2 (6) |
| Unknown Primary | 5 (16) |
| AJCC staging (v7) at Original Diagnosis | |
| IIIB | 1 (3) |
| IIIC | 4 (13) |
| M1a | 8 (26) |
| M1b | 3 (10) |
| M1c | 15 (48) |
| BRAFV600 mutation status | |
| Yes (Group 1, all BRAFV600E) | 10 (32) |
| No (Group 2) | 21 (68) |
| Prior Cancer Therapies (number of patients, %) | |
| No | 6 (19) |
| Yes | 25 (81) |
| Received PD1/PD-L1i only | 2 |
| Received ipilimumab only | 2 |
| Received PD1/PD-L1i + ipilimumab | 19 |
| Did not receive PD1/PD-L1i or ipilimumab | 2 |
| Cancer Treatment types (total) | 73 |
| PD1/PD-L1i | 27 (37) |
| Ipilimumab | 21 (29) |
| Non-PD1/PD-L1/CTLA4i | 2 (3) |
| HDIL-2 | 6 (8) |
| High-dose interferon-α2b | 2 (3) |
| Targeted therapies | 2 (3) |
| Chemotherapy alone | 7 (10) |
| Intralesional treatment | 3 (4) |
| Other | 3 (4) |
| Number of Patients with x Lines of Prior Therapy | |
| 1 prior treatment | 3 (10) |
| 2 prior treatments | 8 (26) |
| 3 prior treatments | 7 (23) |
| 4 prior treatments | 5 (16) |
| ≥5 prior treatments | 2 (6) |
| ECOG Performance Status at Screening | |
| 0 | 19 (61) |
| 1 | 10 (32) |
| 2 | 2 (6) |
PD1/PD-L1i, PD1/PD-L1 inhibitors (pembrolizumab, nivolumab, avelumab), AJCC American Joint Committee on Cancer, HDIL-2 high-dose bolus interleukin-2, IC immune checkpoint proteins
All patients received ≥1 dose of AMG 232; 22 (71%) patients were discontinued from study due to: death from progressive disease (n=17, 55%), decision by sponsor (n=1, 3%), protocol-specified criteria (n=2, 6.5%), and withdrawal of study consent (n=2, 6.5%). All had been discontinued from AMG 232 at the time of the final analysis. Specifically, the reasons for discontinuing AMG 232 were progressive disease (n=15, 48%), AEs (n=7, 23%), withdrawal by patient (n=7, 23%), death (n=1, 3%), and decision by sponsor (n=1, 3%). Reasons for discontinuing trametinib were progressive disease (n=14, 45%), AEs (n=6, 19%), withdrawal by patient (n=7, 23%), death (n=2, 65%), decision by sponsor (n=1, 3%), and non-compliance (n=1, 3%). Reasons for discontinuing dabrafenib for the 10 patients enrolled in Arm 1 were progressive disease (n=3, 33%), AEs (n=2, 20%), and withdrawal by patient (n=5, 50%).
Dose escalation
In Arm 2, the first arm where study accrual initiated, the first two AMG 232 doses evaluated in combination with trametinib were 120 mg (n=6) and 240 mg (n=6). While no DLTs occurred in the 120 mg dose cohort, a patient treated with 240 mg developed a grade 3 pulmonary embolism on day 13 from initiation of AMG 232. This AE was attributed to both AMG 232 and trametinib and was considered a DLT. We therefore treated 9 patients with the intermediate dose (180 mg). However, a patient treated with the intermediate AMG 232 dose developed grade 4 thrombocytopenia on day 27, after the first cycle. This AE was attributed to AMG 232, was resolved on day 55, and was considered a DLT. Based on the two DLTs, one in each of the 180 mg and 240 mg dose cohorts, as well as the high frequency of gastrointestinal toxicities causally attributed to AMG 232 (Table 2), the sponsor’s study team leadership and site clinical investigators determined that the MTD of the seven-days-on, 14-days-off AMG 232 schedule combined with trametinib was 120 mg once daily.
Table 2.
Summary of the most frequent treatment-related AEs. ‘Any drug’ implies AEs attributed to either dabrafenib (dabraf), trametinib (tram), or AMG 232
| Group 1 (BRAFV600E/K) | Group 2 (BRAFWT OR non-V600) | All Patients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMG 232 + Trametinib + Dabrafenib | AMG 232 + Trametinib | |||||||||||
| 120 mg | 180 mg | 120 mg | 180 mg | 240 mg | ||||||||
| N=4 (%) | N=6 (%) | N=6 (%) | N=9 (%) | N=6 (%) | N=31 (%) | |||||||
| Summary of AEs | ||||||||||||
| Grade 1 | 4 (100) | 6 (100) | 6 (100) | 9 (100) | 6 (100) | 31 (100) | ||||||
| Grade 2 | 4 (100) | 6 (100) | 6 (100) | 8 (89) | 6 (100) | 30 (97) | ||||||
| Grade 3 | 2 (50) | 4 (67) | 6 (100) | 7 (78) | 6 (100) | 25 (81) | ||||||
| Grade 4 | 0 | 0 | 1 (17) | 1 (11) | 0 | 2 (6) | ||||||
| Leading to AMG 232 d/c | 1 (25) | 1 (17) | 1 (17) | 2 (22) | 2 (33) | 7 (23) | ||||||
| Leading to tram d/c | 1 (25) | 1 (17) | 1 (17) | 1 (11) | 2 (33) | 6 (19) | ||||||
| Leading to dabraf d/c | 1 (25) | 1 (17) | N/A | 2 (6) | ||||||||
| Most frequent (>20% patients) AEs according to drug type | ||||||||||||
| Attributed to … (%) | Attributed to ... (%) | Attributed to ... (%) | Attributed to ... (%) | Attributed to … (%) | Attributed to … (%) | |||||||
| Any drug | AMG 232 only | Any drug | AMG 232 only | Any drug | AMG 232 only | Any drug | AMG 232 only | Any drug | AMG 232 only | Any drug | AMG 232 only | |
| Nausea | 4 (100) | 6 (100) | 6 (100) | 6 (67) | 5 (56) | 6 (100) | 28 (90) | 27 (87) | ||||
| Diarrhea | 4 (100) | 6 (100) | 5 (83) | 4 (67) | 6 (67) | 5 (56) | 6 (100) | 5 (83) | 27 (87) | 24 (77) | ||
| Fatigue | 4 (100) | 4 (67) | 5 (83) | 8 (89) | 6 (67) | 4 (67) | 25 (81) | 23 (74) | ||||
| Vomiting | 3 (75) | 5 (83) | 6 (100) | 6 (67) | 5 (56) | 2 (33) | 22 (71) | 21 (68) | ||||
| Decreased appetite | 2 (50) | 1 (25) | 5 (83) | 4 (67) | 3 (50) | 3 (33) | 2 (22) | 3 (50) | 16 (52) | 13 (42) | ||
| Headache | 2 (50) | 0 | 4 (67) | 1 (17) | 4 (67) | 2 (33) | 2 (22) | 0 | 3 (50) | 0 | 15 (48) | 3 (10) |
| Dermatitis acneiform | 1 (25) | 1 (17) | 0 | 3 (50) | 0 | 5 (56) | 1 (11) | 4 (67) | 1 (17) | 14 (45) | 3 (10) | |
| Peripheral Edema | 1 (25) | 0 | 2 (33) | 0 | 4 (67) | 1 (17) | 3 (33) | 0 | 3 (50) | 0 | 13 (42) | 1 (3) |
| Pruritus | 3 (75) | 0 | 2 (33) | 0 | 2 (33) | 0 | 2 (22) | 0 | 2 (33) | 0 | 11 (35) | 0 |
| Pyrexia | 2 (50) | 0 | 4 (67) | 1 (17) | 3 (50) | 1 (17) | 1 (11) | 0 | 1 (17) | 0 | 11 (35) | 2 (6) |
| Anemia | 1 (25) | 0 | 1 (17) | 3 (50) | 2 (33) | 1 (11) | 0 | 3 (50) | 2 (33) | 9 (29) | 5 (16) | |
| Dehydration | 1 (25) | 0 | 2 (33) | 0 | 3 (50) | 1 (17) | 2 (22) | 0 | 1 (17) | 0 | 9 (29) | 1 (3) |
| Hypotension | 2 (50) | 0 | 2 (33) | 1 (17) | 0 | 2 (22) | 0 | 2 (33) | 0 | 8 (26) | 1 (3) | |
| Cough | 1 (25) | 0 | 2 (33) | 0 | 2 (33) | 1 (11) | 0 | 2 (33) | 0 | 8 (26) | 2 (6) | |
| Chills | 1 (25) | 0 | 3 (50) | 2 (33) | 1 (17) | 0 | 2 (22) | 0 | 1 (17) | 0 | 8 (26) | 2 (6) |
| Hypertension | 2 (50) | 0 | 3 (50) | 2 (33) | 2 (33) | 0 | 1 (11) | 0 | 0 | 8 (26) | 2 (6) | |
| Thrombocytopenia | 0 | 1 (17) | 0 | 2 (33) | 1 (11) | 0 | 3 (50) | 1 (17) | 7 (23) | 3 (10) | ||
| Rash | 1 (25) | 0 | 1 (17) | 1 (17) | 0 | 3 (33) | 1 (11) | 1 (17) | 0 | 7 (23) | 2 (6) | |
| Abdominal pain | 1 (25) | 1 (17) | 0 | 0 | 2 (22) | 2 (33) | 6 (19) | 5 (16) | ||||
| Dysgeusia | 0 | 2 (33) | 0 | 1 (17) | 1 (11) | 2 (33) | 6 (19) | 4 (13) | ||||
| AST increased | 1 (25) | 0 | 3 (50) | 2 (33) | 1 (17) | 1 (11) | 0 | 0 | 6 (19) | 3 (10) | ||
| Dry mouth | 2 (50) | 0 | 1 (17) | 1 (17) | 0 | 1 (17) | 5 (16) | 3 (10) | ||||
| Neutropenia | 0 | 2 (33) | 1 (17) | 2 (33) | 1 (17) | 0 | 1 (17) | 5 (16) | 3 (10) | |||
| Dizziness | 1 (25) | 2 (33) | 1 (17) | 1 (17) | 0 | 0 | 1 (17) | 5 (16) | 3 (10) | |||
| Pulmonary embolism | 0 | 0 | 2 (33) | 0 | 1 (11) | 0 | 2 (33) | 5 (16) | 2 (6) | |||
| Lipase increased | 0 | 3 (50) | 2 (33) | 0 | 1 (11) | 1 (17) | 5 (16) | 4 (13) | ||||
| Malaise | 1 (25) | 1 (17) | 0 | 0 | 2 (33) | 4 (13) | 4 (13) | |||||
| Dyspepsia | 1 (25) | 2 (33) | 1 (17) | 0 | 0 | 1 (17) | 4 (13) | 3 (10) | ||||
| Seizure | 0 | 1 (17) | 0 | 0 | 1 (11) | 0 | 1 (17) | 0 | 3 (10) | 0 | ||
| Adrenal insufficiency | 0 | 0 | 0 | 1 (11) | 0 | 1 (17) | 0 | 2 (6) | 0 | |||
| Cellulitis | 0 | 0 | 1 (17) | 0 | 1 (11) | 0 | 0 | 2 (6) | 0 | |||
| Serious AEs | ||||||||||||
| Nausea | 0 | 1 (17) | 2 (33) | 1 (11) | 1 (17) | 5 (16) | ||||||
| Vomiting | 1 (25) | 1 (17) | 2 (33) | 1 (11) | 0 | 5 (16) | ||||||
| Pyrexia | 1 (25) | 2 (33) | 1 (17) | 1 (11) | 0 | 5 (16) | ||||||
| Pulmonary embolism | 0 | 0 | 2 (33) | 1 (11) | 2 (33) | 5 (16) | ||||||
| Diarrhea | 1 (25) | 0 | 1 (17) | 0 | 2 (33) | 4 (13) | ||||||
| Dehydration | 0 | 0 | 2 (33) | 1 (11) | 0 | 3 (10) | ||||||
| Seizure | 0 | 1 (17) | 0 | 1 (11) | 1 (17) | 3 (10) | ||||||
| Adrenal insufficiency | 0 | 0 | 0 | 1 (11) | 1 (17) | 2 (6) | ||||||
| Cellulitis | 0 | 0 | 1 (17) | 1 (11) | 0 | 2 (6) | ||||||
| Fatigue | 0 | 0 | 1 (17) | 0 | 0 | 1 (3) | ||||||
| Peripheral Edema | 0 | 1 (17) | 0 | 0 | 0 | 1 (3) | ||||||
| Anemia | 0 | 0 | 0 | 0 | 1 (17) | 1 (3) | ||||||
| Hypotension | 0 | 0 | 0 | 1 (11) | 0 | 1 (3) | ||||||
| Thrombocytopenia | 0 | 0 | 0 | 1 (11) | 0 | 1 (3) | ||||||
d/c, discontinue; AE, adverse events; N/A, non applicable; %, percentage of patients experiencing adverse event
Based on the experience from Arm 2, the AMG 232 doses evaluated in Arm 1 were 120 mg (n=4) and 180 mg (n=6) combined with trametinib and dabrafenib. There were no DLTs for the 120 mg dose cohort. However, one patient treated with 180 mg AMG 232 had <75% of AMG 232 during the DLT period due to protracted AMG 232-related grade 3 nausea that begun on day 11 and was considered a DLT. Based on the single DLT in the 180 mg dose cohort as well as the high frequency of gastrointestinal toxicities causally attributed to AMG 232 (Table 2), the sponsor’s study team leadership and site clinical investigators determined that the MTD of the seven-days-on, 14-days-off AMG 232 schedule combined with dabrafenib plus trametinib was 120 mg once daily.
Following accrual in the 180 mg dose cohort in Arm 1, the study was completed because the sponsor decided to cease further clinical development of AMG 232.
Safety and tolerability
A total of 614 treatment-emergent AEs occurred (Table 2). The most common (occurring in ≥20% of patients) AEs were nausea (90%), diarrhea (87%), fatigue (81%), vomiting (71%), decreased appetite (52%), headache (48%), acneiform dermatitis (45%), peripheral edema (42%), pruritus and pyrexia (35.5% each). 83 (13.5%) of the treatment-emergent AEs were considered serious (SAEs) and occurred in 24 (77%) patients. These were predominantly gastrointestinal symptoms, including nausea (16%), vomiting (16%), and diarrhea (13%). Diarrhea could be managed with dietary modifications (discontinue lactose-containing products, BRAT diet, and small-meal portions), hydration, and loperamide (up to a maximum daily dose of 16 mg). Nausea was managed with aprepitant, dexamethasone, and compazine. Table 2 shows a summary of the most frequent (≥20% of patients) treatment-emergent AEs and SAEs according to dose level and Arm.
240 treatment-emergent AEs (39%) were considered by the investigators to be attributable to AMG 232 and occurred in 30 subjects during the study. The most common AMG 232-related AEs (occurring in ≥10% of subjects) were nausea (87%), diarrhea (77%), fatigue (74%), vomiting (68%), decreased appetite (42%), thrombocytopenia (23%), abdominal pain and anemia (16% each); dysgeusia, increased lipase, and malaise (13% each). Most AMG 232-related AEs (26%) were of grade 1 or grade 2. Serious AMG 232-related AEs were reported in 8 patients; nausea (13%), diarrhea (10%), vomiting (10%), and pulmonary embolism (6.5%) were the most frequent. Withdrawals from AMG 232 due to AEs were reported for seven subjects (23%), such as grade 3 arterial injury; grade 2 and 3 nausea; grade 3 diarrhea, nausea and vomiting; grade 1 thrombocytopenia; grade 2 anemia and acneiform dermatitis; grade 5 (death from MM); grade 1 diarrhea; and grade 2 fatigue. Two patients in Arm 1 who had complete antitumor response stopped treatment due to preference; one patient did not tolerate AMG 232 due to gastrointestinal side effects (6-1012) and the other subject stopped all three drugs due to high frequency of doctor’s visits for the trial (6-1011).
Phamacokinetics
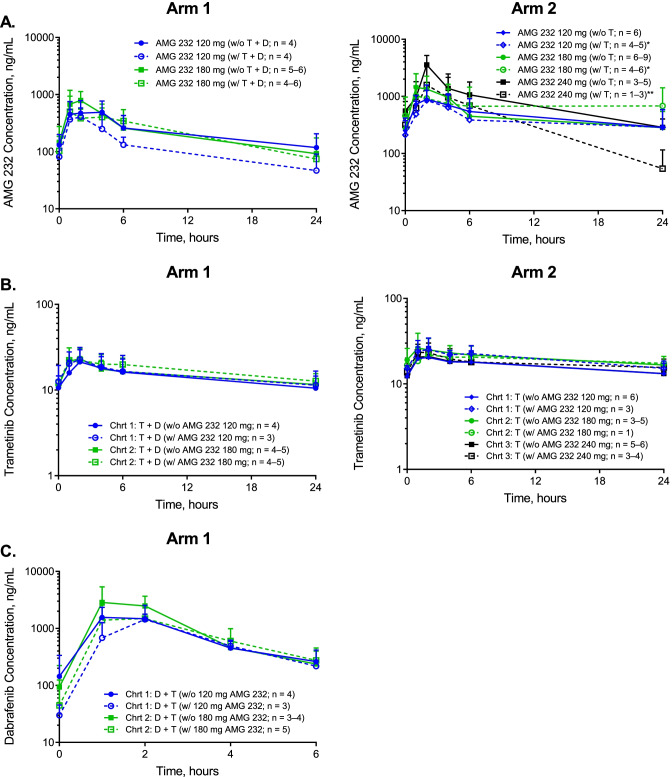
Figure 2a shows plasma PK profiles of AMG 232 at steady-state for both arms. Table 3 shows PK parameter estimates of AMG 232. As previously shown [19], following dosing on cycle 1, day 7, the last of a 7-day treatment of AMG 232 daily monotherapy, AMG 232 was absorbed rapidly (median tmax of 2.0 hours) across dose cohorts in both arms. In addition, AMG 232 plasma exposures, as assessed by Cmax and AUC24h, generally increased with increasing AMG 232 dose in both arms (doses from 120 to 180 mg in Arm 1 and 120 to 240 mg in Arm 2 dose ranges in Arms 1 and 2, respectively). In addition, in Arm 1, we observed 1.7- and 1.1-fold increases in the geometric mean Cmax and AUC24hr, respectively, for a 1.5-fold increase in dose. In Arm 2, we observed a 3.6- and 2.2-fold increase in the geometric mean Cmax and AUC24hr for a 2-fold increase in dose. Finally, mean terminal half-life (t1/2,z) and geometric mean apparent clearance (CL/F) across dose cohorts in both arms were 7.04 to 8.32 hours and 11.4 to 30.6 L/hour, respectively.
Fig. 2.
Plasma pharmacokinetics of AMG 232, trametinib, and dabrafenib at steady-state for combinations of AMG 232 with trametinib and dabrafenib (Arm 1) or trametinib (Arm 2) given orally in adult subjects with MM. In Arms 1 and 2, AMG 232 was administered QD on days 1–7 of each cycle with a 14-day treatment-free period on days 8–21. In addition, trametinib was dosed continuously QD starting on day 8 of cycle 1. In Arm 1 only, dabrafenib was dosed continuously BID starting on day 8 of cycle 1. A. Mean (+SD) plasma concentration-time profiles of AMG 232 following AMG 232 dosing on cycle 1, day 7, when administered alone or on cycle 2, day 7, when co-administered with trametinib and dabrafenib [Arm 1] or trametinib [Arm 2]. B. Mean (+SD) plasma concentration-time profiles of trametinib following dosing of trametinib and dabrafenib [Arm 1] or trametinib alone [Arm 2] on cycle 1, day 21, without co-administration of AMG 232 (at the end of the 14-day AMG 232 treatment-free period) or on cycle 2, day 7, when co-administered with AMG 232. C. Mean (+SD) plasma concentration-time profiles of dabrafenib following dosing of dabrafenib and trametinib in Arm 1 on cycle 1, day 21, without co-administration of AMG 232 (at the end of the 14-day AMG 232 treatment-free period) or on cycle 2, day 7, when co-administered with AMG 232. Abbreviations: BID, twice a day; Chrt, cohort; D, dabrafenib; QD, once daily; SD, standard deviation; T, trametinib
Table 3.
Descriptive statistics of AMG 232 plasma PK parameter estimates after oral administration of AMG 232 for 6 days at steady-state levels on cycle 1, day 7, without trametinib (and dabrafenib for Arm 1) and cycle 2, day 7, with trametinib (and dabrafenib for Arm 1) in subjects with metastatic melanoma
| Arm | Cohort | AMG 232 dose (mg) | tmax (hr) | Cmax (ng/mL) |
AUC24hr (hr•ng/mL) |
t1/2,z (hr) |
CL/F (L/hr) |
|---|---|---|---|---|---|---|---|
| Cycle 1, Day 7 (AMG 232 monotherapy; prior to co-administration with T + D [Arm 1] or T alone [Arm 2) | |||||||
| Arm 1: AMG 232 + T + D | 1 | 120 |
2.0 (1.0–4.0) n = 4 |
491 (59.4%) n = 4 |
5140 (59.4%) n = 4 |
7.04 (NR) n = 1 |
23.4 (53.6%) n = 4 |
| 2 | 180 |
2.0 (1.0–4.0) n = 6 |
847 (51.3%) n = 6 |
5890 (34.6%) n = 5 |
7.79 (0.718) n = 4 |
30.6 (37.5%) n = 5 |
|
| Arm 2: AMG 232 + T | 1 | 120 |
2.0 (2.0–4.0) n = 6 |
914 (37.4%) n = 6 |
9810 (61.4%) n = 6 |
8.32 (NR) n = 2 |
12.2 (69.7%) n = 6 |
| 2 | 180 |
2.0 (0.0–4.0) n = 7 |
1250 (62.2%) n = 7 |
10600 (71.7%) n = 7 |
7.54 (NR) n = 2 |
16.9 (115.6%) n = 7 |
|
| 3 | 240 |
2.0 (2.0–2.0) n = 4 |
3300 (46.5%) n = 4 |
21100 (28.3%) n = 3 |
8.12 n = 1 |
11.4 (24.7%) n = 3 |
|
| Cycle 2, Day 7 (AMG 232 with co-administration of T + D [Arm 1] or T alone [Arm 2]) | |||||||
| Arm 1: AMG 232 + T + D | 1 | 120 |
2.0 (2.0–4.0) n = 4 |
392 (43.3%)a n = 4 |
3140 (31.4%)a n = 4 |
5.86 (NR) n = 2 |
38.2 (32.5%) n = 4 |
| 2 | 180 |
3.0 (1.0–6.0) n = 6 |
647 (44.3%)a n = 6 |
4750 (38.7%)a n = 4 |
6.93 (NR) n = 2 |
37.9 (38.2%) n = 4 |
|
| Arm 2: AMG 232 + T | 1 | 120 |
3.0 (2.0–6.0) n = 4 |
630 (77.2%)a n = 4 |
6740 (62.9%)a n = 4 |
ND n = 0 |
17.8 (101.2%) n = 4 |
| 2 | 180 |
1.0 (1.0–6.0) n = 5 |
1010 (52.0%)a n = 5 |
9410 (107.0%)a n = 5 |
ND n = 0 |
19.1 (99.6%) n = 5 |
|
| 3 | 240 |
1.5 (1.0–2.0) n = 2 |
953 (NR)b n = 2 |
6280 (NR)b n = 2 |
5.30 (NR) n = 2 |
38.2 (NR) n = 2 |
|
Data are presented as geometric mean (CV%) except for tmax and t1/2,z, which were presented as median (minimum-maximum) and mean (SD), respectively. Number of subjects (n) are presented for each parameter
AUC24h area under the concentration-time curve from time 0 to 24 hrs post-dose, CL/F apparent drug clearance after extravascular administration, Cmax maximum observed plasma concentration, CV% percent coefficient of variation, D dabrafenib (150 mg twice daily dosing), hr hour, ND no data, NR not reported, SD standard deviation, T trametinib (2 mg once daily dosing), t1/2,z terminal half-life, tmax time to reach Cmax
a p>0.05 comparing AMG 232 Cmax and AUC24h values for subjects following cycle 1, day 7 (AMG 232 monotherapy), and cycle 2, day 7 (AMG 232 in combination with trametinib and dabrafenib or trametinib alone, Wilcoxon matched-pairs signed῏rank test)
b Wilcoxon test not performed (insufficient subjects to conduct test)
AMG 232 absorption remained rapid (median tmax of 1.0–3.0 hours) when AMG 232 was combined with dabrafenib plus trametinib or trametinib alone. When combined with trametinib and dabrafenib or trametinib alone, exposure to AMG 232 (Cmax and AUC24hr), increased dose-proportionally over the dose range of 120 to 180 mg. In Arm 2, increases in the geometric mean Cmax and AUC values were not observed between dose levels of 180 and 240 mg, although there were limited data available with only two patients treated at the 240 mg dose level.
Supplementary Information, Fig. 2b and c, Tables 4 and 5 summarize plasma PK profiles of AMG 232, trametinib (alone and in combination with dabrafenib), and dabrafenib at steady-state with and without co-administration of AMG 232. In summary, AMG 232 PK exposures were not significantly altered when AMG 232 was combined with trametinib, with and without dabrafenib. In addition, PK parameter estimates for both trametinib and dabrafenib were within range of those observed in previous studies following repeat dosing monotherapy for each drug.
Table 4.
Descriptive statistics of trametinib plasma pharmacokinetic parameter estimates after oral administration of trametinib (and dabrafenib for Arm 1) at steady-state levels on cycle 1, day 21, without AMG 232 and cycle 2, day 7, with AMG 232 in adult subjects with metastatic melanoma
| Arm | Cohort and AMG 232 dose given QD on days 1–7 of each cycle |
Cycle 1, Day 21 (without AMG 232 co-adminstered) |
Cycle 2, Day 7 (with AMG 232 co-administered) |
|||||
|---|---|---|---|---|---|---|---|---|
| tmax (hr) | Cmax (ng/mL) |
AUC24hr (hr•ng/mL) |
tmax (hr) | Cmax (ng/mL) |
AUC24hr (hr•ng/mL) |
|||
| Arm 1: AMG 232 + T + D |
Cohort 1: 120 mg |
2.0 (2.0–2.0) n = 4 |
20.1 (38.7%) n = 4 |
326 (38.6%) n = 4 |
2.0 (1.0–2.0) n = 3 |
22.4 (36.9%)a n = 3 |
338 (47.3%)a n = 3 |
|
|
Cohort 2: 180 mg |
2.0 (1.0–2.0) n = 5 |
24.1 (23.2%) n = 5 |
362 (17.2%) n = 5 |
2.0 (1.0–6.0) n = 5 |
25.9 (22.3%)a n = 5 |
402 (22.5%)a n = 4 |
||
| Arm 2: AMG 232 + T |
Cohort 1: 120 mg |
1.5 (0.0–4.0) n = 6 |
21.5 (33.5%) n = 6 |
370 (30.5%) n = 6 |
2.0 (2.0–4.0) n = 3 |
24.8 (33.7%)a n = 3 |
468 (23.5%)a n = 3 |
|
|
Cohort 2: 180 mg |
4.0 (1.0–6.0) n = 5 |
25.3 (31.1%) n = 5 |
471 (26.7%) n = 5 |
2.0 n = 1 |
21.8b n = 1 |
463b n = 1 |
||
|
Cohort 3: 240 mg |
2.0 (1.0–4.0) n = 6 |
22.1 (25.0%) n = 6 |
385 (22.9%) n = 6 |
2.0 (1.0–4.0) n = 4 |
24.3 (22.5%)a n = 4 |
351 (41.9%)a n = 4 |
||
Data are presented as geometric mean (CV%) except for tmax, which was presented as median (minimum-maximum). In addition, number of subjects (n) are presented for each parameter
AUC24h area under the concentration-time curve from time 0 to 24 hrs post-dose, Cmax maximum observed plasma concentration, CV% percent coefficient of variation, D dabrafenib (150 mg twice daily dosing), hr hour, QD once daily, T trametinib (2 mg QD), tmax time to reach Cmax.
a p > 0.05 comparing trametinib Cmax and AUC24h values for subjects following cycle 1 day 21 (without AMG 232) and cycle 2 day 7 (with AMG 232) (Wilcoxon matched-pairs signed῏rank test)
b Wilcoxon test not performed (insufficient subjects to conduct test)
Table 5.
Descriptive statistics of dabrafenib plasma pharmacokinetic parameter estimates after oral administration of trametinib (and dabrafenib for Arm 1) at steady-state levels on cycle 1, day 21, without AMG 232 and cycle 2, day 7, with AMG 232 in adult subjects with metastatic melanoma
| Arm | Cohort and AMG 232 dose given QD on days 1–7 of each cycle |
Cycle 1, Day 21 (T + D without AMG 232 co-adminstered) |
Cycle 2 Day 7 (T + D with AMG 232 co-administered) |
|||||
|---|---|---|---|---|---|---|---|---|
| tmax (hr) | Cmax (ng/mL) |
AUC6hr (hr•ng/mL) |
tmax (hr) | Cmax (ng/mL) |
AUC6hr (hr•ng/mL) |
|||
| Arm 1: AMG 232 + T + D |
Cohort 1: 120 mg |
1.0 (1.0–2.0) n = 4 |
1740 (20.9%) n = 4 |
4840 (28.0%) n = 4 |
2.0 (2.0–2.0) n = 3 |
1200 (76.3%)a n = 3 |
3640 (60.5%)a n = 3 |
|
|
Cohort 2: 180 mg |
1.0 (1.0–2.0) n = 4 |
3310 (52.2%) n = 4 |
7450 (46.8%) n = 4 |
2.0 (1.0–2.0) n = 5 |
1750 (40.5%)a n = 5 |
4910 (30.0%)a n = 5 |
||
Data are presented as geometric mean (CV%) except for tmax, which was presented as median (minimum-maximum). In addition, number of subjects (n) are presented for each parameter
AUC6h area under the concentration-time curve from time 0 to 6 hrs post-dose, Cmax maximum observed plasma concentration, CV% percent coefficient of variation, D dabrafenib (150 mg twice daily dosing), hr hour, ND no data, NR not reported, QD once daily, T trametinib (2 mg QD), tmax time to reach Cmax
a p > 0.05 comparing dabrafenib Cmax and AUC24h values for subjects following cycle 1 day 21 (without AMG 232) and cycle 2 day 7 (with AMG 232) (Wilcoxon matched-pairs signed-rank test)
Antitumor activity
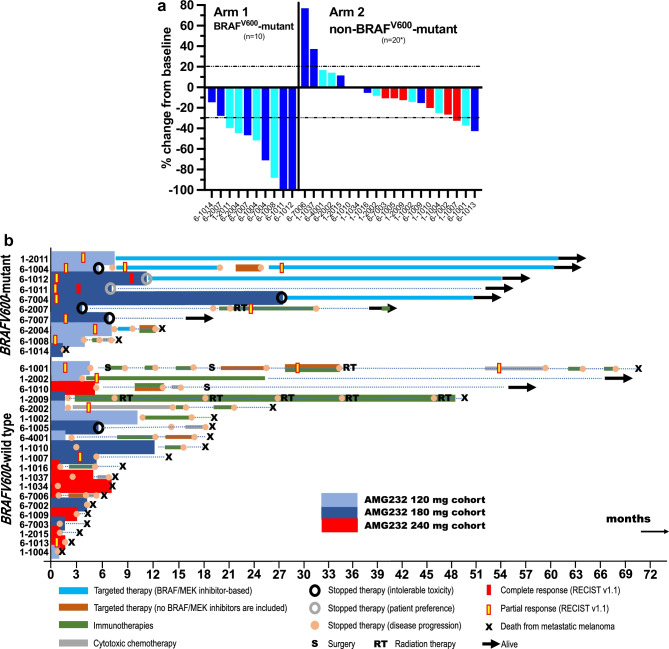
Figure 3a shows waterfall plots corresponding to the investigators’ assessment of best antitumor response (RECIST v1.1 criteria) from baseline to day 28 for the 30 evaluable patients. Figure 3b shows corresponding swimmer’s plots, including details about treatments they may have received following AMG 232 discontinuation. For example, patient 6-2005 was enrolled in Arm 2, cohort 3 (AMG 232 240 mg), received AMG 232 for only four days, and subsequently withdrew. Unfortunately, he died from metastatic melanoma 5.3 months after study enrollment.
Fig. 3.
Antitumor benefit of AMG 232 in combination with trametinib alone or trametinib plus dabrafenib in patients with unresectable stage III/IV melanoma (*evaluable patients n=30, see text for details). A. Waterfall plots corresponding to investigators’ assessment of objective tumor responses (RECIST v1.1 criteria) and according to each treatment arm. B. Swimmers’ plots of individual patients in each Arm 1
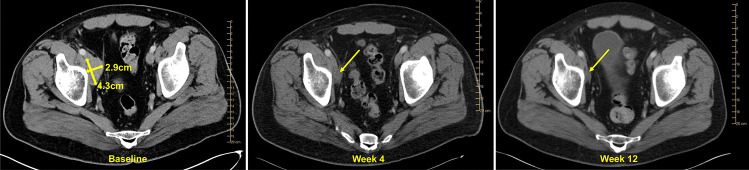
In Arm 1 (n=10), the overall objective response rate was 80% (two complete and six partial responses) (Fig. 3a). Six out of eight responders did not have stage IV melanoma. Both Arm 1 complete responders (6-1012 and 6-1011) were treated with 180 mg of AMG 232, had oligometastatic, M1a, disease. Interestingly, patient 6-1011 received dabrafenib-trametinib-AMG 232 for nearly seven months; he subsequently chose to be observed. Figure 4 shows patient 6-1011’s representative images from IV contrast CT scans at baseline, week 4, and week 8 while on AMG 232, dabrafenib, and trametinib treatment. After >4 ½ years of follow-up, he did not have any melanoma recurrence. Two other Arm 1 patients (6-2007, 6-7007) stopped AMG 232 for toxicity and entered radiographic surveillance; they did not progress for at least seven months. In Arm 2 (n=20), three patients had a partial response, one in each dose cohort. The overall objective response rate was 15% (3/20, Fig. 3a). All three responders subsequently progressed within six months of study initiation. Interestingly, two of the three responders had M1c disease and NRASQ61R mutations. Five patients (25%) remained free of recurrence for at least five months.
Fig. 4.
Complete antitumor response in the AMG 232, dabrafenib, trametinib study (Arm 1). Representative images were obtained from patient 6-1011’s CT scans with IV contrast at baseline, week 4, and week 8
Seven patients in Arm 1 were alive (70%) at the last follow-up (Fig. 3b). The median progression-free survival (PFS) was 19.0 months-not reached (range, 1.9 months-not reached), and the median overall survival (OS) for Arm 1 was 47.7 months (range, 2.3-62.3 months). Most responders in Arm 1 who developed toxicities from AMG 232 requiring discontinuation from the study elected to continue on BRAF/MEK inhibition and were alive at the last follow-up. Two of the three patients in Arm 1 who progressed (6-2007, 6-1008) had not previously received PD1/PD-L1 inhibitors; following progression from our study, they both received PD1 inhibitors and eventually progressed.
At the last follow-up, two patients in Arm 2 were alive (9.5%) (Fig. 3b). 18 patients died from MM and one patient died from pulmonary embolism not attributed to AMG 232. The median PFS (n=20, one non-evaluable) for Arm 2 was 2.8 months (0.9-14.1 months), and the median OS (n=21) was 8.7 months (range, 1.2-70-7 months). Four patients in Arm 2 survived for >2 years after study enrollment. Two of these patients had not previously received PD1 inhibitors. Patient 1-2002 (NRAS-mutant) developed a complete metabolic response to single-agent pembrolizumab and is free of disease. Patient 1-2009 (NRASQ61R-mutant) remained on single-agent pembrolizumab for an extended time while receiving radiation therapy to new lesions that would appear periodically. Patient 6-1010 (acral melanoma) had a single mutation (NRASQ61K) identified as part of the FoundationOne CDx assay following progression on AMG 232. He eventually became disease-free after a hip disarticulation surgery. The other two patients who had received PD1 inhibitors and ipilimumab prior to study enrollment (6-1001, 6-2002) developed antitumor responses to cytotoxic chemotherapy.
Discussion
Our study showed that AMG 232 could be safely combined with trametinib alone or with concurrent dabrafenib-trametinib. The MTD was defined as 120 mg once daily for seven days of a 21-day cycle in both arms because DLTs occurred at the 180 mg and 240 mg dose cohorts. Interestingly, the MTD in our study was higher than that in a recently reported phase 1b study of the AMG 232-trametinib combination in relapsed/refractory acute myeloid leukemia [19]. Although gastrointestinal toxicities were seen in both the melanoma and leukemia studies, more serious and frequent hematologic toxicities occurred in the leukemia study, which may possibly account for the lower MTD established in the latter.
Preclinical data have shown that AMG 232 affects the p53 pathway, as measured by the upregulation of p53’s downstream effectors, p21, and the circulating macrophage inhibitor cytokine-1 (MIC-1), at doses as low as 5 mg/kg. However, the effect of AMG 232 on the p53 pathway was more consistent and pronounced at doses ranging between 25-100 mg/kg [16]. By this dose-effect relationship, an AMG 232 dose of 50 mg/kg was selected to test the efficacy of AMG 232 alone and in combination with dabrafenib plus trametinib in melanoma patient-derived xenograft models. This AMG 232 dose corresponds to a human daily dose of 250 mg [8]. Evidence that the biologically effective AMG 232 dose in humans is at least 240 mg daily comes from the phase I study of single-agent AMG 232 in solid tumors or multiple myeloma; in this study, significant increases in serum MIC-1 protein were only seen when the AMG 232 daily dose was higher than 240 mg [18]. Although we did not measure serum MIC-1 protein, or other relevant biomarker in our study, we can extrapolate from the biomarker results of the phase I solid tumor or multiple myeloma study to suggest that our recommended phase 2 dose of AMG 232 combined with trametinib alone or trametinib plus dabrafenib at 120 mg may not be sufficiently high to yield a biological effect that is significantly higher than the antitumor effect seen with MEK inhibition alone in patients with non-BRAFV600-mutant or with combined BRAF/MEK inhibition in patients with BRAFV600-mutant melanoma. In support of this notion, the response rate and PFS that we observed in patients from Arm 2 were not significantly different from that seen in the early studies of single-agent trametinib in non-BRAFV600-mutant MM [22], or in the more recent study of single-agent binimetinib in patients with NRASQ61-mutant melanoma [5]. Furthermore, we observed higher antitumor responses in Arm 1 with the highest dose of AMG 232 tested (180 mg) and perhaps a higher rate of stable disease in Arm 2 patients who received the highest dose of AMG 232 (240 mg). Nevertheless, we caution that the number of patients treated was too small to draw any definite conclusions. Also, we did not observe a significant effect of AMG 232 on the PK profiles of trametinib and dabrafenib, and vice versa, suggesting that there was no overall significant and clinically relevant PK interaction among the three drugs. Finally, the observed steady-state AMG 232 exposures (AUC after seven days of daily dosing) at the MTD of 120 mg in Arms 1 and 2 of this study were in general lower than corresponding exposures for the putative biologically effective dose of 240 mg AMG 232, as discussed above, in patients with solid tumors or multiple myeloma. We, therefore, conclude that we were unable to escalate AMG 232 to dose levels that could translate to substantial clinical activity in our study.
In summary, we have conducted a clinical study in MM that targets a protein, p53, that is not frequently mutated in MM and plays a fundamental role in melanoma’s decisions for cell death versus survival. The combination of AMG 232 with the standard of care dose of trametinib or trametinib plus dabrafenib was generally well tolerated, especially at relatively lower AMG 232 doses. However, the inability to escalate AMG 232 to higher doses underlies the vital role of p53 in human physiology [23], and perhaps may account for the lack of significantly higher clinical activity compared to trametinib alone or dabrafenib plus trametinib. Our experience of low activity due to the inability to escalate experimental drugs to biologically effective levels due to toxicity when combined with MAPK pathway inhibitors is similar to that seen with other promising targets in melanoma [24, 25].
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the patients and their families who participated in this study and made the clinical evaluation of AMG 232 possible. We thank Gina Hashem MPH, PhD, and Laura Greene, PharmD, both Amgen employees, who provided assistance in data collection. The study was funded by Amgen, Inc., Thousand Oaks, CA, USA.
Abbreviations
- MM
metastatic melanoma
- MAPK
mitogen-activated protein kinase
- PD1
programmed cell death protein 1
- PD-L1
programmed death-ligand 1
- MDM2
the murine double minute-2 proto-oncogene
- WT
wild-type
- p53
the protein encoded by the TP53 gene
- PK
pharmacokinetics
- MTD
maximum tolerated dose
- NCI-CTCAE
the National Cancer Institute Common Terminology Criteria for Adverse Events
- DLT
dose-limiting toxicity
- AEs
adverse events
- RECIST
Response Evaluation Criteria in Solid Tumors
- ECOG
Eastern Cooperative Oncology Group
- SAEs
serious adverse events
- AUC24hr
area under the curve over 24 hours
- Cmax
peak plasma concentration
- tmax
time to reach Cmax
- t1/2,z
mean terminal half-life
- CL/F
oral clearance
- PFS
progression-free survival
- OS
overall survival
- MIC-1
macrophage inhibitory cytokine 1
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Drs. Stergios J Moschos, Shahneen Sandhu, Karl D. Lewis, Ryan J Sullivan, Igor Puzanov, Douglas B. Johnson, Haby A. Henary, Hansen Wong, Vijay V. Upreti, Georgina V. Long, and Keith T. Flaherty. The first draft of the manuscript was written by Dr. Stergios J Moschos and all authors commented on previous version of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Amgen, Inc., Thousand Oaks, CA, USA
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
The study was approved by the institutional review board of each participating academic institution. The study was performed in line with the principles of the Declaration of Helsinki.
Consent to participate
Written informed consent was obtained from all individual participants included in the study.
Consent for publication
Consent to publish has been received from all individual participants included in the study
Competing Interests
HAH, HW, VVU are employees of Amgen Inc, and may own stock/stock options in the company. DBJ has served on advisory boards or as a consultant for Bristol-Myers Squibb (BMS), Catalyst Biopharma, Iovance, Jansen, Mallinckrodt, Merck Sharp & Dohme (MSD), Mosaic ImmunoEngineering, Novartis, Oncosec, Pfizer, and Targovax, and has received research funding from BMS and Incyte. RJS has served as consultant and/or advisory boards for Asana Biosciences, AstraZeneca, BMS, Eisai, Iovance, MSD, Novartis, Oncosec, Pfizer, Replimune and has received research funding from Amgen and Merck. SS has served on advisory boards for BMS, Jansen, MSD, Novartis, and Astra Zeneca and has received research funding from Genentech, MSD, Merck Serono, Novartis and Amgen. GVL has served as consultant advisor or on advisory boards for Aduro Biotech, Agenus, Amgen, Array Biopharma, Boehringer Ingelheim, BMS, Evaxion, Hexel AG, Highlight Therapeutics, MSD, Novartis, OncoSec, Pierre Fabre, QBiotics, Regeneron, Specialised Therapeutics. KTF has served on the board of directors of Loxo Oncology, Clovis Oncology, Strata Oncology, Vivid Biosciences, Checkmate Pharmaceuticals, Kinnate Pharmaceuticals and Scorpion Therapeutics; Corporate Advisory Board of X4 Pharmaceuticals; has served on the advisory boards of PIC Therapeutics, Sanofi, Amgen, Asana, Adaptimmune, Aeglea, Shattuck Labs, Tolero, Apricity, Oncoceutics, Fog Pharma, Neon, Tvardi, xCures, Monopteros, Vibliome, and ALX Oncology; has served as a consultant to Elli Lilly and Company, Novartis, Genentech, BMS, MSD, Takeda, Verastem, Boston Biomedical, Pierre Fabre, Debiopharm; and has received research funding from Novartis and Sanofi.
Research involving human participants
Written informed consent was obtained from all individual participants included in the study.
Disclosure of potential conflicts of interest
see Competing interests in the Author Declarations Section.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Robert C, Grob JJ, Stroyakovskiy D, Karaszewska B, Hauschild A, Levchenko E, Chiarion-Sileni V, Schachter J, Garbe C, Bondarenko I, Gogas H, Mandala M, Haanen J, Lebbe C, Mackiewicz A, Rutkowski P, Nathan PD, Ribas A, Davies MA, Flaherty KT, Burgess P, Tan M, Gasal E, Voi M, Schadendorf D, Long GV. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N Engl J Med. 2019;381:626–636. doi: 10.1056/NEJMoa1904059. [DOI] [PubMed] [Google Scholar]
- 2.Yam C, Xu X, Davies MA, Gimotty PA, Morrissette JJD, Tetzlaff MT, Wani KM, Liu S, Deng W, Buckley M, Zhao J, Amaravadi RK, Haas NB, Kudchadkar RR, Pavlick AC, Sosman JA, Tawbi H, Walker L, Schuchter LM, Karakousis GC, Gangadhar TC. A Multicenter Phase I Study Evaluating Dual PI3K and BRAF Inhibition with PX-866 and Vemurafenib in Patients with Advanced BRAF V600-Mutant Solid Tumors. Clin Cancer Res. 2018;24:22–32. doi: 10.1158/1078-0432.CCR-17-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan RJ, Mehnert J, Tawbi H, Lawrence D, Flaherty K, Chen H, Takabe N, Davies M (2018) First in human, dose escalation trial of the combination of dabrafenib, trametinib, and navitoclax in patients with BRAF mutant solid tumors. AACR; Mol Cancer Ther 17(1 Suppl):abstract nr LB-B30
- 4.Gutzmer R, Stroyakovskiy D, Gogas H, Robert C, Lewis K, Protsenko S, Pereira RP, Eigentler T, Rutkowski P, Demidov L, Manikhas GM, Yan Y, Huang KC, Uyei A, McNally V, McArthur GA, Ascierto PA. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAF(V600) mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395:1835–1844. doi: 10.1016/S0140-6736(20)30934-X. [DOI] [PubMed] [Google Scholar]
- 5.Dummer R, Schadendorf D, Ascierto PA, Arance A, Dutriaux C, Di Giacomo AM, Rutkowski P, Del Vecchio M, Gutzmer R, Mandala M, Thomas L, Demidov L, Garbe C, Hogg D, Liszkay G, Queirolo P, Wasserman E, Ford J, Weill M, Sirulnik LA, Jehl V, Bozon V, Long GV, Flaherty K. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:435–445. doi: 10.1016/S1470-2045(17)30180-8. [DOI] [PubMed] [Google Scholar]
- 6.Flaherty KT, Gray R, Chen A, Li S, Patton D, Hamilton SR, Williams PM, Mitchell EP, Iafrate AJ, Sklar J, Harris LN, McShane LM, Rubinstein LV, Sims DJ, Routbort M, Coffey B, Fu T, Zwiebel JA, Little RF, Marinucci D, Catalano R, Magnan R, Kibbe W, Weil C, Tricoli JV, Alexander B, Kumar S, Schwartz GK, Meric-Bernstam F, Lih CJ, McCaskill-Stevens W, Caimi P, Takebe N, Datta V, Arteaga CL, Abrams JS, Comis R, O'Dwyer PJ, Conley BA, NCI-MATCH Team The Molecular Analysis for Therapy Choice (NCI-MATCH) Trial: Lessons for Genomic Trial Design. J Natl Cancer Inst. 2020;112:1021–1029. doi: 10.1093/jnci/djz245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flaherty KT, Gray RJ, Chen AP, Li S, McShane LM, Patton D, Hamilton SR, Williams PM, Iafrate AJ, Sklar J, Mitchell EP, Harris LN, Takebe N, Sims DJ, Coffey B, Fu T, Routbort M, Zwiebel JA, Rubinstein LV, Little RF, Arteaga CL, Comis R, Abrams JS, O'Dwyer PJ, Conley BA, NCI-MATCH Team Molecular Landscape and Actionable Alterations in a Genomically Guided Cancer Clinical Trial: National Cancer Institute Molecular Analysis for Therapy Choice (NCI-MATCH) J Clin Oncol. 2020;38:3883–3894. doi: 10.1200/JCO.19.03010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shattuck-Brandt RL, Chen SC, Murray E, Johnson CA, Crandall H, O'Neal JF, Al-Rohil RN, Nebhan CA, Bharti V, Dahlman KB, Ayers GD, Yan C, Kelley MC, Kauffmann RM, Hooks M, Grau A, Johnson DB, Vilgelm AE, Richmond A. Metastatic Melanoma Patient-Derived Xenografts Respond to MDM2 Inhibition as a Single Agent or in Combination with BRAF/MEK Inhibition. Clin Cancer Res. 2020;26:3803–3818. doi: 10.1158/1078-0432.CCR-19-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fahraeus R, Olivares-Illana V. MDM2's social network. Oncogene. 2014;33:4365–4376. doi: 10.1038/onc.2013.410. [DOI] [PubMed] [Google Scholar]
- 10.Ticha I, Hojny J, Michalkova R, Kodet O, Krkavcova E, Hajkova N, Nemejcova K, Bartu M, Jaksa R, Dura M, Kanwal M, Martinikova AS, Macurek L, Zemankova P, Kleibl Z, Dundr P. A comprehensive evaluation of pathogenic mutations in primary cutaneous melanomas, including the identification of novel loss-of-function variants. Sci Rep. 2019;9:17050. doi: 10.1038/s41598-019-53636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polsky D, Bastian BC, Hazan C, Melzer K, Pack J, Houghton A, Busam K, Cordon-Cardo C, Osman I. HDM2 protein overexpression, but not gene amplification, is related to tumorigenesis of cutaneous melanoma. Cancer Res. 2001;61:7642–7646. [PubMed] [Google Scholar]
- 12.Aubrey BJ, Strasser A, Kelly GL. Tumor-Suppressor Functions of the TP53 Pathway. Cold Spring Harb Perspect Med. 2016 doi: 10.1101/cshperspect.a026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collavin L, Lunardi A, Del Sal G. p53-family proteins and their regulators: hubs and spokes in tumor suppression. Cell Death Differ. 2010;17:901–911. doi: 10.1038/cdd.2010.35. [DOI] [PubMed] [Google Scholar]
- 14.ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium Pan-cancer analysis of whole genomes. Nature. 2020;578:82–93. doi: 10.1038/s41586-020-1969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang S, Zhao Y, Aguilar A, Bernard D, Yang CY. Targeting the MDM2-p53 Protein-Protein Interaction for New Cancer Therapy: Progress and Challenges. Cold Spring Harb Perspect Med. 2017 doi: 10.1101/cshperspect.a026245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canon J, Osgood T, Olson SH, Saiki AY, Robertson R, Yu D, Eksterowicz J, Ye Q, Jin L, Chen A, Zhou J, Cordover D, Kaufman S, Kendall R, Oliner JD, Coxon A, Radinsky R. The MDM2 Inhibitor AMG 232 Demonstrates Robust Antitumor Efficacy and Potentiates the Activity of p53-Inducing Cytotoxic Agents. Mol Cancer Ther. 2015;14:649–658. doi: 10.1158/1535-7163.MCT-14-0710. [DOI] [PubMed] [Google Scholar]
- 17.Saiki AY, Caenepeel S, Yu D, Lofgren JA, Osgood T, Robertson R, Canon J, Su C, Jones A, Zhao X, Deshpande C, Payton M, Ledell J, Hughes, PE, Oliner JD (2014) MDM2 antagonists synergize broadly and robustly with compounds targeting fundamental oncogenic signaling pathways. Oncotarget 5:2030-2043. 10.18632/oncotarget.1918 [DOI] [PMC free article] [PubMed]
- 18.Gluck WL, Gounder MM, Frank R, Eskens F, Blay JY, Cassier PA, Soria JC, Chawla S, de Weger V, Wagner AJ, Siegel D, De Vos F, Rasmussen E, Henary HA. Phase 1 study of the MDM2 inhibitor AMG 232 in patients with advanced P53 wild-type solid tumors or multiple myeloma. Invest New Drugs. 2020;38:831–843. doi: 10.1007/s10637-019-00840-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erba HP, Becker PS, Shami PJ, Grunwald MR, Flesher DL, Zhu M, Rasmussen E, Henary HA, Anderson AA, Wang ES. Phase 1b study of the MDM2 inhibitor AMG 232 with or without trametinib in relapsed/refractory acute myeloid leukemia. Blood Adv. 2019;3:1939–1949. doi: 10.1182/bloodadvances.2019030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamberg P, Ratain MJ, Lesaffre E, Verweij J. Dose-escalation models for combination phase I trials in oncology. Eur J Cancer. 2010;46:2870–2878. doi: 10.1016/j.ejca.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Infante JR, Fecher LA, Falchook GS, Nallapareddy S, Gordon MS, Becerra C, DeMarini DJ, Cox DS, Xu Y, Morris SR, Peddareddigari VG, Le NT, Hart L, Bendell JC, Eckhardt G, Kurzrock R, Flaherty K, Burris HA, 3rd, Messersmith WA. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:773–781. doi: 10.1016/S1470-2045(12)70270-X. [DOI] [PubMed] [Google Scholar]
- 22.Falchook GS, Lewis KD, Infante JR, Gordon MS, Vogelzang NJ, DeMarini DJ, Sun P, Moy C, Szabo SA, Roadcap LT, Peddareddigari VG, Lebowitz PF, Le NT, Burris HA, 3rd, Messersmith WA, O'Dwyer PJ, Kim KB, Flaherty K, Bendell JC, Gonzalez R, Kurzrock R, Fecher LA. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:782–789. doi: 10.1016/S1470-2045(12)70269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 24.Moschos SJ, Sullivan RJ, Hwu WJ, Ramanathan RK, Adjei AA, Fong PC, Shapira-Frommer R, Tawbi HA, Rubino J, Rush TS, 3rd, Zhang D, Miselis NR, Samatar AA, Chun P, Rubin EH, Schiller J, Long BJ, Dayananth P, Carr D, Kirschmeier P, Bishop WR, Deng Y, Cooper A, Shipps GW, Moreno BH, Robert L, Ribas A, Flaherty KT. Development of MK-8353, an orally administered ERK1/2 inhibitor, in patients with advanced solid tumors. JCI Insight. 2018 doi: 10.1172/jci.insight.92352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louveau B, Resche-Rigon M, Lesimple T, Da Meda L, Pracht M, Baroudjian B, Delyon J, Amini-Adle M, Dutriaux C, Reger de Moura C, Sadoux A, Jouenne F, Ghrieb Z, Vilquin P, Bouton D, Tibi A, Huguet S, Rezai K, Battistella M, Mourah S, Lebbe C. Phase I-II Open-Label Multicenter Study of Palbociclib + Vemurafenib in BRAF (V600MUT) Metastatic Melanoma Patients: Uncovering CHEK2 as a Major Response Mechanism. Clin Cancer Res. 2021;27:3876–3883. doi: 10.1158/1078-0432.CCR-20-4050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.