Abstract
Purpose
The relationships of postural stability with its three potential contributing factors, namely, muscle strength, tactile sensation, and proprioception, have not been clarified at different ages among older adults. Differences in the relationships may explain the increased incidence of falls among older adults 75 and older. This study compared the postural stability and its three factors between the older adults younger or older than 75 and investigated their age-specific relationships.
Methods
A total of 152 participants were recruited and divided into younger-old (65–74 years, n = 83) or older-old adults (≥75 years, n = 69) groups. Their Berg Balance Scale (BBS) and the Timed Up and Go (TUG) performance, muscle strength, tactile sensation, and proprioception were tested. The group differences and age-specific relationships of the performance with the three factors were examined.
Results
Compared to the younger-older adults, the older-old adults had lower BBS and higher TUG scores, weaker muscle strength, and worse proprioception. Muscle strength and proprioception were correlated with BBS and TUG among the younger-older adults. Only muscle strength but not proprioception among the older-old adults was correlated with BBS and TUG.
Conclusion
The older-old adults over 75 years have poorer postural stability, muscle strength, and proprioception compared to the younger-old adults aged 65–74 years. Proprioception provides information on postural stability among younger-old adults but not among older-old adults. Keeping proprioception from deteriorating with age could be a key to reducing falls in older-old adults.
Keywords: Falls, Muscle strength, Older adults, Postural stability, Proprioception, Tactile sensation
1. Introduction
With the increased longevity, it is becoming more important than ever to identify life transitions in the later years and to recognize the heterogeneity among older adults.1 Falls and fall-related injuries are a significant public health issue, the leading cause of substantial mortality and morbidity rates among older adults aged 65 and older,2 and the first cause of accidental death among older adults aged 75 and older.3
Postural stability impairment has been recognized as a major risk factor for falls among older adults.4 Performance-oriented functional mobility tests such as the Berg balance scale (BBS) and the Timed Up and Go test (TUG) are widely adopted in clinical practice as an objective quantification of fall risks to evaluate the postural stability deterioration among older adults. The BBS consists of 14 simple balancing-related tasks, ranging from standing up from sitting to standing on one foot. It is demonstrated to discriminate fallers from non-fallers successfully.5 The TUG is usually used to assess a person's functional mobility in the time taken to complete the task.6 Many falls occur when older adults walk or transfer (e.g., getting up from a chair),7 and the two tasks were involved in TUG.6 BBS and TUG are complementary in simulating high fall risk environments. The BBS assesses postural control during standing with little attention to balance during gait.5 In contrast, the TUG assesses gait balance with the older adults who can walk, but it only assesses one sequential task of walking and turning.6
Postural stability is achieved through complex interactions of multiple sensory, motor, and integrative systems.8 Muscle strength, tactile sensation, and proprioception are potential contributors to postural stability and fall prevention in daily life among older adults. The ability of muscles to generate adequate force is critical for maintaining balance.9 Hip and ankle strength is significant since the older adults at the risk of falling are more likely to use hip strategies than those with a low risk of falling, who use ankle strategies to maintain postural stability.10 Tactile sensation is an important source of information for stabilizing the center of mass and controlling body sway.11 Proprioception is critical for generating smooth and coordinated movements, maintaining normal body posture, and regulating balance.12 Knee and ankle joints are the main supporting joints of human lower limbs, and the input of proprioceptive information around them is crucial to maintaining postural control and joint stability.13
The rate of falls and their associated complications increases progressively with age and are about twice these figures for the elderly over 75 years of age.14 Compared to the elderly under 75, hip fractures are more common in falls among those aged 75 and above,14 which is a particularly serious consequence of falls, with the 1-year mortality rate following hip fracture being approximately 25%.15
Older adults have a functional decline in postural stability, muscle strength, tactile sensation, and proprioception, compared to the young.16, 17, 18, 19 These functional declines may contribute to the increase in falls among older adults. However, few studies divided the older adult population into different age groups to study the sustained decline effect of aging on these factors. In addition, controversies remain on whether muscle strength, tactile sensation, and proprioception are related to postural stability. It had been reported in some studies that impaired muscle strength, tactile sensation, and proprioception were correlated with postural stability.11,20,21 Other studies indicated no significant association of muscle strength, tactile sensation, and proprioception with postural stability.22, 23, 24 These contradictions may arise because the relationships between these factors and postural stability are different in different age groups. Such as, Muehlbauer and co-workers reported the nonsignificant correlations between muscle strength and postural stability among middle-aged adults,22 but Pijnappels and colleagues reported that muscle strength was associated with postural stability among older adults.20 However, few studies have divided the older adult population into different age groups to investigate which factor of decline affects postural stability among older adults.
Moreover, the rate of falls increases dramatically with age, especially among those aged 75 and older.14,18 However, the mechanism behind the observation has hardly been studied. Therefore, if we can clarify the above points, we can speculate which factors older adults of different ages rely on to maintain postural stability, and determine the mechanisms for the increase in falls among older adults 75 and older, so that we can choose targeted physical exercise to improve postural stability, develop precise fall prevention programs among older adults.
This study aimed to compare postural stability and its three potential factors, muscle strength, tactile sensation, and proprioception, between the older adults younger and older than 75, and investigate their age-specific relationships. It was hypothesized that 1. The older adults aged over 75 have less postural stability, weaker muscle strength, and impaired tactile sensation and proprioception, compared to the younger-older adults; 2. Muscle strength, tactile sensation, and proprioception were significantly correlated with postural stability in older adults over and under 75 years of age.
2. Material and methods
2.1. Participants
The participants were recruited by distributing flyers and providing presentations in the local communities and nursing homes. The inclusion criteria were as follows:1) age of 65 or older; 2) independently ambulatory without using assistive devices; 3) no global cognitive impairment defined by Mini-Mental State Exam (MMSE) score ≥24. The exclusion criteria included:1) Self-reported central nervous system dysfunction, visual defects, dizziness, vertigo, any other vestibular disorders, or psychological problems related to the fall, such as fear of falling, anxiety, or depression; 2) evidence of foot sole ulcers by direct assessment. A total of 152 participants were included after the qualification assessment and included in the final analysis. The older adults were identified as younger-old (65–74 years) and older-old (≥75 years) groups. No significant differences were detected in age, body mass, and height between the two groups. See details in Table 1. Independent t-tests showed that the two groups had significant differences in age (p < .001), body mass (p = .034), and height (p = .004). All participants signed approved written informed consent forms before participation. Human participation was approved by Institutional Review Boards in Shandong Sport University (19003) and was in accordance with the Declaration of Helsinki.
Table 1.
The baseline of the participants’ information.
| Group | Younger-old |
Older-old |
p |
|---|---|---|---|
| (n = 83, female = 44) | (n = 69, female = 44) | ||
| From community | 83 | 58 | -- |
| From nursing homes | 0 | 11 | -- |
| Age (years) | 68.0 ± 2.4 | 80.8 ± 4.4 | <.001 |
| Body mass (kg) | 65.6 ± 9.7 | 62.4 ± 8.9 | .034 |
| Height (cm) | 163.5 ± 6.8 | 160.2 ± 7.1 | .004 |
| BMI (kg/m2) | 24.5 ± 3.1 | 24.3 ± 3.2 | .733 |
Presented as mean ± standard deviation.
BMI: Body mass index. Bold: p < .05.
2.2. Protocol
The BBS, TUG, muscle strength, tactile sensation, and proprioceptive were measured for all of the eligible participants. The testers in this study were trained prior to the experiment and, each test was conducted by the same tester to ensure consistency. All the equipment used in this study is regularly maintained and in good condition. The equipment was operated in accordance with the manuals provided by their manufacturers. All the tests were conducted in the Lab of Biomechanics at Shandong Sport University, which has a quiet environment and is equipped with air conditioning to ensure consistent temperature and humidity during the tests.
2.3. BBS test
The BBS test consists of 14 simple daily functional activity tests (e.g., sit-to-stand conversion and turning 360°), which showed excellent inter-rater and intra-rater reliability (ICC value, 0.98–0.99).5 The researchers evaluated and scored the participants’ performance during the test. Each test was given (0–4) points, with a maximum score of 56 points. The BBS scores were tested only once, and the total score was recorded after all the tests were completed.
2.4. TUG test
The Timed Up and Go (TUG) test was used to quantify functional mobility, which showed good test-retest reliability and discrimination validity (ICC = 0.99).6 The TUG test collected the time the participants stood up from a standard armchair, walked a distance of 3 m, turned around, walked back to the chair, and sat down again. A total of 3 trials were conducted. The mean value of the trials was used for data analysis.
2.5. Muscle strength, tactile sensation, and proprioception tests
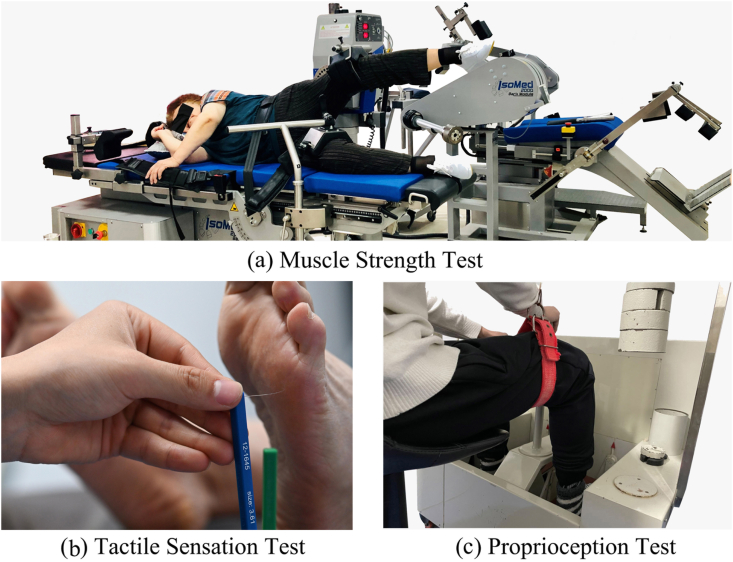
Details on muscle strength, tactile sensation, and proprioceptive tests were reported elsewhere.21 Briefly, the muscle strength of ankle plantarflexion/dorsiflexion and hip abduction was measured by the IsoMed 2000 strength testing system (D. & R. Ferstl GmbH, Hemau, Germany; Fig. 1a), which showed good test-retest reliability (ICC value, 0.77–0.98).25 During the ankle muscle strength test, the participant was in a supine position on the dynamometer bed with hips and knees in full extension. The waist and thigh of the test leg were constrained with straps. the participant's waist, thigh, and foot of the testing leg were fixed with Velcro straps to ensure ankle stability. During the hip muscle strength test, the participant was asked to lay on their side with the hip fully extended. They were then stabilized with belts strapped around the pelvis. The test leg was stabilized by straps with the knee fully extended, and the other leg was stabilized on the bed with the knee slightly flexed. A total of 3 trials from each direction were conducted. The tactile sensation was tested at the great toe, 1st and 5th metatarsal heads, arch, and heel with a set of Semmes-Weinstein monofilaments (North Coast Medical, Inc., Morgan Hill, CA, USA; Fig. 1b), which showed good test-retest reliability (ICC value, 0.83–0.86).26 The filaments were applied to the skin on the bases of the great toe, 1st and 5th metatarsals, arch, and heel in random order. These touches were performed for 1 s and with 2 repetitions. The minimum monofilament gauge determined the sensitivity threshold. A less sensitivity threshold indicates better plantar tactile sensation. The proprioception of ankle plantarflexion/dorsiflexion and knee adduction/abduction was measured using the proprioceptive testing device (Toshimi, Jinan, Shandong, China; Fig. 1c). Good test-retest reliability (ICC value, 0.737–0.935) for the device has been reported previously.27 A total of 5 trials were performed in each direction. The mean value of the trials was used for data analysis.
Fig. 1.
Test illustrations. a. The muscle strength test uses the IsoMed 2000 strength testing system. b. The tactile sensation test with Semmes–Weinstein monofilaments. c. The proprioception test uses a proprioception test device.
2.6. Statistics
The normality of data distribution was checked using Shapiro-Wilk tests. All outcome variables' mean and standard deviations were subjected to descriptive analysis. Independent t-tests (normality) or Mann-Whitney U tests (non-normality) were used to compare differences between aging groups. Cohen's d (normality) or η2 (non-normality) was used to evaluate the effect size of between-group differences.28,29 Pearson (normality) or Spearman (non-normality) correlations were used to determining BBS and TUG's relationship with each group's muscle strength, tactile sensation, and proprioception. Participants' height, body mass, and sex were adjusted as covariates. The thresholds for the correlation coefficient (r) were as follows: low (0.20–0.39), moderate (0.40–0.59), high (0.60–0.79), and very high (0.80–1.00).30 All analyses were performed in SPSS 20.0.
3. Results
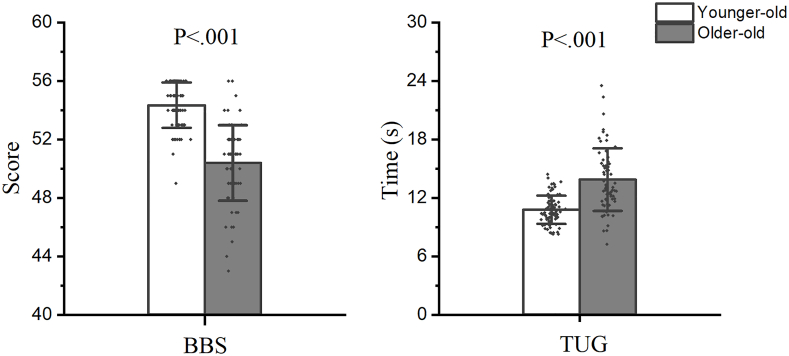
The Shapiro–Wilk test showed the non-normal distribution of BBS, tactile sensation, and proprioception variables. The descriptive characteristics of the BBS and TUG were showed in Fig. 2. Compared to the younger-older adults, the older-old adults had significantly lower BBS and higher TUG scores.
Fig. 2.
Descriptive characteristics of the Berg Balance Scale and the Timed Up and Go tests.
BBS = the Berg Balance Scale; TUG = the Timed Up and Go test.
The descriptive characteristics of muscle strength, tactile sensation, and proprioception are shown in Table 2. Compared to the younger-older adults, the older-old adults had less muscle strength of ankle plantar/dorsiflexion and hip abduction, and larger proprioception threshold of knee flexion/extension and ankle plantar/dorsiflexion. No significant differences were detected in tactile sensation.
Table 2.
Descriptive characteristics of the muscle strength, tactile sensation and proprioception.
| Younger-old | Older-old | p | Cohen’ d | η2 | ||
|---|---|---|---|---|---|---|
| Muscle strength (N∗m/kg) | Ankle plantarflexion | 0.4 ± 0.1 | 0.3 ± 0.1 | <.001 | 1.000 | – |
| Ankle dorsiflexion | 0.23 ± 0.08 | 0.18 ± 0.07 | <.001 | .665 | – | |
| Hip abduction | 0.5 ± 0.2 | 0.3 ± 0.2 | <.001 | .811 | – | |
| Tactile sensation (gauge) | Great toe | 4.2 ± 0.5 | 4.4 ± 0.7 | .184 | – | .010 |
| 1st Metatarsal | 4.3 ± 0.6 | 4.3 ± 0.5 | .079 | – | .016 | |
| 5th Metatarsal | 4.3 ± 0.4 | 4.4 ± 0.5 | .233 | – | .008 | |
| Arch | 4.4 ± 0.5 | 4.5 ± 0.6 | .989 | – | .000 | |
| Heel | 4.6 ± 0.5 | 4.7 ± 0.7 | .526 | – | .002 | |
| Proprioception (O) | Knee flexion | 2.6 ± 1.6 | 3.8 ± 2.4 | <.001 | – | .081 |
| Knee extension | 2.9 ± 2.3 | 3.9 ± 2.5 | .002 | – | .066 | |
| Ankle plantarflexion | 2.6 ± 2.1 | 5.3 ± 3.9 | <.001 | – | .179 | |
| Ankle dorsiflexion | 2.8 ± 2.6 | 5.4 ± 4.3 | <.001 | – | .141 | |
Data are presented as mean ± SD.
Bold: p < .05.
The thresholds for effect size (Cohen’ d) were as follows: <0.20, trivial; 0.21–0.50, small; 0.51–0.80, medium; >0.81, large. The thresholds for effect size (η2) were as follows: 0.01–0.059, small; 0.06–0.14, medium; >0.14, large.
The age-specific correlations of BBS and TUG with muscle strength, tactile sensation, and proprioception are shown in Table 3. Among the younger-older adults, the muscle strength of ankle plantarflexion and hip abduction was correlated with BBS and TUG. The proprioception of knee flexion and ankle plantar/dorsiflexion was correlated with BBS and TUG, and knee extension was correlated with TUG. Among the older-old adults, the muscle strength of hip abduction was correlated with BBS and TUG, and ankle plantar/dorsiflexion was correlated with TUG. None of the tactile sensations were related to BBS or TUG in both groups.
Table 3.
The age-specific correlations of the Berg Balance Scale and Timed Up and Go scores with muscle strength, tactile sensation, and proprioception.
4. Discussion
This present study compared the postural stability and its potential contributing factors, muscle strength, tactile sensation, and proprioception between the younger-old or older-old adults, and investigated their age-specific relationships. The outcomes partly support our hypotheses. We detected that among the elderly population, postural stability, muscle strength, and proprioception sensitivity continue to decline with age. And, postural stability was correlated with muscle strength and proprioception among the younger-old adults while correlated with muscle strength only among the older-old adults.
The results showed that the BBS and TUG scores were lower among the older-old adults. Worse postural stability observations and higher risk of falls among senior older adults are consistent with previous studies.31 Weaker muscle strength was detected among the older-old adults agreeing with muscle strength decline with aging,9,19 which lead to decreased ability to generate adequate force for maintaining balance.9 The non-difference in tactile sensation between the two groups is supported by one previous study,23 however, they are inconsistent with one another.32 Only one monofilament (5.07, 10 g) was used in that study,32 while six sizes were used in ours. Compared with the younger-old adults, the worse proprioception among older adults has been reported by several studies,12 indicating a decline in the dynamic response of muscle spindles and the atrophy of axons among the older-old adults, which would lead to defects in the processing and input of sensory information.33
The relationship between muscle strength and postural stability is consistent with some previous studies12,21; however, inconsistent with others. Melzer and co-workers reported that ankle muscle strength had no relationship with postural stability.34 Their study measured only static postural stability during quiet standing, which was different from ours. Although muscle strength decreased with aging, there were significant correlations between muscle strength and posture stability in both groups, indicating that muscle strength is still a significant contributor to postural stability among adults older than 75. Therefore, an exercise that enhances muscle strength may increase postural stability among older adults.
No relationships were detected between tactile sensation and postural stability in this study, which was consistent with a previous study,23 but differed from another one.11 In that study, the stability was measured by the anteroposterior torque variance during repeated calf vibrations while standing,11 which was quite different from our study. Tactile sensation is not the main source of information to maintain postural stability. It may be compensated by other sensory information, such as proprioception, vestibular, and vision.12,35,36 Since no differences in tactile sensation were detected between younger-old and older-old adults, and no relationships of tactile sensation with postural stability were confirmed in either group, it is reasonable to infer that tactile-enhancing approaches should not be included in fall prevention exercise prescriptions.
One interesting observation of this study was that proprioception was related to postural stability among the younger-old adults while not among the older-old adults. Our outcomes were inconsistent with one study, which argued that the relative contribution of proprioception to postural stability does not alter with age.37 The discrepancy could be attributed to the protocol differences in postural stability assessments. In a study conducted by Colledge and co-workers, postural stability was assessed by measuring postural sway when standing still,37 whereas assessed by measuring BBS and TUG in our study. Given that most of the falls occur when the body is moving rather than standing still,7 the BBS and TUG, which were tested during locomotion, may be better associated with falls. Proprioception is considered the most important sensory resource to maintain postural stability among older adults.12 Proprioceptive receptors send signals about the stationary position of a limb, along with the speed and direction of its movement, to the central nervous system to ensure a smooth and coordinated movement while maintaining postural stability.38 It is no surprise to detect a relationship between proprioception to postural stability among the younger-old group. However, our outcomes further indicated the proprioception among older-old adults could not provide sufficient information for postural stability. The disappearance of the relationship among the older-old adults can be inferred that proprioception deteriorated to a point where it could not provide any meaningful functional assistance to the postural stability of the older adults older than 75, which might reasonably explain the twofold risk of falls among this age group.14 Given the above inference and the confirmation of the proprioception decline among the older-old adults, it is reasonable to assume that the rehabilitation of proprioception could be a key to decreased falls among older adults, and exercises that enhance proprioception should be included in the exercise prescriptions for fall prevention among older adults aged 75 and older.
There are some limitations to this study. First, only the effects of muscle strength, tactile sensation, and proprioception on posture stability were studied in this study. Other factors, such as visual, vestibular, or cognitive functions, might also affect postural stability and falling. However, visual function contributed only about 10% to postural stability,35 the vestibular system is more involved in maintaining balance on an unstable platform,36 which was not applied in the present study. In contrast, the correlation of cognitive function to postural stability was usually detected among patients with stroke or Parkinson's,39,40 but rarely in healthy individuals. Second, eleven participants were recruited from nursing homes. However, only those who could complete all the tests were included in this study, so their fitness levels would be in the top tier and therefore probably not representative of the majority of older adults in nursing homes. Third, no history of falls was collected from participants in this study. Future studies may further explore the mechanism of falls among older adults by comparing the relationships of falls (or the number of falls) to sensations and strength between older adults with and without fall history.
It is concluded that compared to their younger counterparts aged 65–74 years, older-old adults aged over 75 years have less postural stability, weaker muscle strength, and impaired proprioception. Proprioception provides information on postural stability among younger-old adults but not among older-old adults. Keeping proprioception from deteriorating with age could be a key to reducing falls in old adults aged 75 and older.
Declaration of interest statement
No potential conflict of interest was reported by the authors.
Availability of data
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author contributions
Qi Wang: Writing - Data curation, original draft, Formal analysis.
Li Li: Writing, Writing - original draft, Formal analysis.
Min Mao: Data curation, Writing - original draft, Methodology.
Wei Sun: Data curation, Writing - review & editing, Methodology.
Cui Zhang: Data curation, Writing - review & editing, Methodology.
Dewei Mao: Project administration, Methodology, Writing-review & editing.
Qipeng Song: Conceptualization, Methodology, Project administration, Writing - review & editing, Funding acquisition.
Acknowledgments
This work was supported by the Shandong Young Innovative Talent Team of China (2019-183) and China National Natural Science Foundation (12102235). The authors would like to thank Huifen Zheng, Hengshuo Zhang, Xiao Gao, Xiu Hu, Fang Liu, and Teng Zhang, graduate students at Shandong Sport University, for participating in the experiment and data acquisition for this work.
References
- 1.Alterovitz S.S., Mendelsohn G.A. Relationship goals of middle-aged, young-old, and old-old Internet daters: an analysis of online personal ads. J Aging Stud. 2013;27(2):159–165. doi: 10.1016/j.jaging.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Cuevas-Trisan R. Balance problems and fall risks in the elderly. Clin Geriatr Med. 2019;35(2):173–183. doi: 10.1016/j.cger.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Meyer M., Constancias F., Vogel T., et al. Gait disorder among elderly people, psychomotor disadaptation syndrome: post-fall syndrome, risk factors and follow-up - a cohort study of 70 patients. Gerontology. 2021;67(1):17–24. doi: 10.1159/000511356. [DOI] [PubMed] [Google Scholar]
- 4.Preszner-Domjan A., Nagy E., Szíver E., et al. When does mechanical plantar stimulation promote sensory re-weighing: standing on a firm or compliant surface? Eur J Appl Physiol. 2012;112(8):2979–2987. doi: 10.1007/s00421-011-2277-5. [DOI] [PubMed] [Google Scholar]
- 5.Berg K. Measuring balance in the elderly: preliminary development of an instrument. Physiother Can. 1989;41(6):304–311. [Google Scholar]
- 6.Diane Podsiadlo B., Sandra Richardson M.D. The Timed ‘Up and Go’: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 7.Robinovitch S.N., Feldman F., Yang Y., et al. Video capture of the circumstances of falls in elderly people residing in long-term care: an observational study. Lancet. 2013;381(9860):47–54. doi: 10.1016/S0140-6736(12)61263-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menz H.B., Morris M.E., Lord S.R. Foot and ankle characteristics associated with impaired balance and functional ability in older people. J Gerontol A Biol Sci Med Sci. 2005;60(12):1546–1552. doi: 10.1093/gerona/60.12.1546. [DOI] [PubMed] [Google Scholar]
- 9.Aartolahti E., Lönnroos E., Hartikainen S., et al. Long-term strength and balance training in prevention of decline in muscle strength and mobility in older adults. Aging Clin Exp Res. 2020;32(1):59–66. doi: 10.1007/s40520-019-01155-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maki B.E., Edmondstone M.A., McIlroy W.E. Age-related differences in laterally directed compensatory stepping behavior. J Gerontol A Biol Sci Med Sci. 2000;55(5):M270–M277. doi: 10.1093/gerona/55.5.m270. [DOI] [PubMed] [Google Scholar]
- 11.Patel M., Magnusson M., Kristinsdottir E., et al. The contribution of mechanoreceptive sensation on stability and adaptation in the young and elderly. Eur J Appl Physiol. 2009;105(2):167–173. doi: 10.1007/s00421-008-0886-4. [DOI] [PubMed] [Google Scholar]
- 12.Henry M., Baudry S. Age-related changes in leg proprioception: implications for postural control. J Neurophysiol. 2019;122(2):525–538. doi: 10.1152/jn.00067.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lephart S.M., Pincivero D.M., Rozzi S.L. Proprioception of the ankle and knee. Sports Med. 1998;25(3):149–155. doi: 10.2165/00007256-199825030-00002. [DOI] [PubMed] [Google Scholar]
- 14.Rubenstein L.Z. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing. 2006;35(Suppl 2):ii37–ii41. doi: 10.1093/ageing/afl084. [DOI] [PubMed] [Google Scholar]
- 15.Elliott D.B., Foster R.J., Whitaker D., et al. Public Health Research. Analysis of lower limb movement to determine the effect of manipulating the appearance of stairs to improve safety: a linked series of laboratory-based, repeated measures studies. NIHR Journals Library Copyright © Queen’s Printer and Controller of HMSO. 2015 This work was produced by Elliott et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.; 2015. [PubMed] [Google Scholar]
- 16.Choy N.L., Brauer S., Nitz J. Changes in postural stability in women aged 20 to 80 years. J Gerontol A Biol Sci Med Sci. 2003;58(6):525–530. doi: 10.1093/gerona/58.6.m525. [DOI] [PubMed] [Google Scholar]
- 17.Relph N., Herrington L. The effects of knee direction, physical activity and age on knee joint position sense. Knee. 2016;23(3):393–398. doi: 10.1016/j.knee.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 18.Machado Á S., Bombach G.D., Duysens J., et al. Differences in foot sensitivity and plantar pressure between young adults and elderly. Arch Gerontol Geriatr. 2016;63:67–71. doi: 10.1016/j.archger.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Doherty T.J. The influence of aging and sex on skeletal muscle mass and strength. Curr Opin Clin Nutr Metab Care. 2001;4(6):503–508. doi: 10.1097/00075197-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Pijnappels M., van der Burg P.J., Reeves N.D., et al. Identification of elderly fallers by muscle strength measures. Eur J Appl Physiol. 2008;102(5):585–592. doi: 10.1007/s00421-007-0613-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song Q., Zhang X., Mao M., et al. Relationship of proprioception, cutaneous sensitivity, and muscle strength with the balance control among older adults. J Sport Health Sci. 2021;10(5):585–593. doi: 10.1016/j.jshs.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muehlbauer T., Gollhofer A., Granacher U. Relationship between measures of balance and strength in middle-aged adults. J Strength Condit Res. 2012;26(9):2401–2407. doi: 10.1519/JSC.0b013e31823f8c41. [DOI] [PubMed] [Google Scholar]
- 23.Unver B., Akbas E. Effects of plantar sensitivity on balance and mobility in community-dwelling older adults: a Turkish study. Australas J Ageing. 2018;37(4):288–292. doi: 10.1111/ajag.12558. [DOI] [PubMed] [Google Scholar]
- 24.Amin D.J., Herrington L.C. The relationship between ankle joint physiological characteristics and balance control during unilateral stance. Gait Posture. 2014;39(2):718–722. doi: 10.1016/j.gaitpost.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Gonosova Z., Linduska P., Bizovska L., et al. Reliability of Ankle⁻Foot complex isokinetic strength assessment using the isomed 2000 dynamometer. Medicina (Kaunas) 2018;54(3) doi: 10.3390/medicina54030043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins S., Visscher P., De Vet H.C., et al. Reliability of the Semmes Weinstein Monofilaments to measure coetaneous sensibility in the feet of healthy subjects. Disabil Rehabil. 2010;32(24):2019–2027. doi: 10.3109/09638281003797406. [DOI] [PubMed] [Google Scholar]
- 27.Sun W., Song Q., Yu B., et al. Test-retest reliability of a new device for assessing ankle joint threshold to detect passive movement in healthy adults. J Sports Sci. 2015;33(16):1667–1674. doi: 10.1080/02640414.2014.1003589. [DOI] [PubMed] [Google Scholar]
- 28.Cohen J. Lawrence Erlbaum Associates; NJ: 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 29.Fritz C.O., Morris P.E., Richler J.J. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen. 2012;141(1):2–18. doi: 10.1037/a0024338. [DOI] [PubMed] [Google Scholar]
- 30.Malgady R.G., Krebs D.B. Understanding correlation coefficients and regression. Phys Ther. 1986;66(1):110. doi: 10.1093/ptj/66.1.110. 112, 114 passim. [DOI] [PubMed] [Google Scholar]
- 31.Vervoort D., Vuillerme N., Kosse N., et al. Multivariate analyses and classification of inertial sensor data to identify aging effects on the timed-up-and-go test. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0155984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cruz-Almeida Y., Black M.L., Christou E.A., et al. Site-specific differences in the association between plantar tactile perception and mobility function in older adults. Front Aging Neurosci. 2014;6:68. doi: 10.3389/fnagi.2014.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferlinc A., Fabiani E., Velnar T., et al. The importance and role of proprioception in the elderly: a short review. Mater Sociomed. 2019;31(3):219–221. doi: 10.5455/msm.2019.31.219-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melzer I., Benjuya N., Kaplanski J., et al. Association between ankle muscle strength and limit of stability in older adults. Age Ageing. 2009;38(1):119–123. doi: 10.1093/ageing/afn249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peterka R.J. Sensorimotor integration in human postural control. J Neurophysiol. 2002;88(3):1097–1118. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- 36.Lacour M., Borel L. Vestibular control of posture and gait. Arch Ital Biol. 1993;131(2-3):81–104. [PubMed] [Google Scholar]
- 37.Colledge N.R., Cantley P., Peaston I., et al. Ageing and balance: the measurement of spontaneous sway by posturography. Gerontology. 1994;40(5):273–278. doi: 10.1159/000213596. [DOI] [PubMed] [Google Scholar]
- 38.Sadeghi H., Hakim M.N., Hamid T.A., et al. The effect of exergaming on knee proprioception in older men: a randomized controlled trial. Arch Gerontol Geriatr. 2017;69:144–150. doi: 10.1016/j.archger.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Yu H.X., Wang Z.X., Liu C.B., et al. Effect of cognitive function on balance and posture control after stroke. Neural Plast. 2021;2021 doi: 10.1155/2021/6636999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris R., Martini D.N., Smulders K., et al. Cognitive associations with comprehensive gait and static balance measures in Parkinson's disease. Park Relat Disord. 2019;69:104–110. doi: 10.1016/j.parkreldis.2019.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.