Abstract
Ablative therapies have recently emerged as an alternate to the gold-standard liver resection in treatment of malignant liver tumors. These modalities include radiofrequency ablation and microwave ablation which utilize thermocoagulative energy and microwave energy, respectively, to create a complete necrosis zone encircling the lesion. In this review, we aimed to show different perspectives of these treatment methods including indications, outcomes, surgical techniques, and perioperative management.
BACKGROUND
Thermal liver tumor ablation is an alternative treatment option for patients with unresectable malignant liver tumors. Although surgical resection remains the "gold standard" for primary or secondary liver malignancies, many patients are not candidates for liver resection secondary to tumor extent prohibiting an adequate functional liver remnant or comorbidities precluding undergoing major liver resection. Thermal tumor ablation has gained popularity as an alternative locoregional therapy for malignant liver tumors secondary to its high efficacy and low morbidity.
The primary end point of thermal liver tumor ablation is achieving complete necrosis of the liver tumor, including a surrounding ablation margin. The most frequently used ablation technologies are radiofrequency ablation (RFA), a longstanding thermoablative modality that causes tumor destruction via thermocoagulative necrosis secondary to ionic excitement [1], and microwave ablation (MWA), which causes tumor destruction through an oscillating microwave field. This has a theoretical advantage over RFA including a larger, producing a more homogenous tissue heating zone, deeper tissue penetration, and a less pronounced heat-sink effect from surrounding vessels [2].
Indications and Outcomes
Indications for thermal liver tumor ablation currently include the presence of unresectable liver lesions, significant medical comorbidities precluding a major liver resection, or a small (< 3 cm) solitary lesion which would otherwise necessitate a major liver resection [3]. It may also be considered as a downstaging debulking option in select patients with colorectal or neuroendocrine liver metastases. It is important that the total ablation volume does not cover more than 20% of total liver volume in order not to cause liver failure. Thermal tumor ablation can also be used in combination with major lobectomy, as a parenchymal preserving strategy to clear the liver remnant from a limited tumor burden.
Colorectal Cancer Liver Metastases
In the United States, colorectal cancer metastases are among the most frequent types of malignant liver tumor treated. Among all tumor types, colorectal cancer is the most challenging tumor type in terms of gaining local tumor control owing to the frequency of a multifocal or extensive disease burden. There have been multiple nonrandomized retrospective and prospective studies on the results of ablation in patients with colorectal cancer liver metastases demonstrating disease-free survival ranging from 6 to 26 months and overall survival ranging from 24 to 57 months [[4], [5], [6]]. Local recurrence rates have been reported to range from 4% to 48%; however, there is a wide variance of ablation technology and approach used in these studies [[7], [8], [9]]. Additionally, oncologic outcomes of selected patients with small solitary colorectal metastasis have been shown to be similar between ablation and resection, with the added benefit of a significantly shorter operative time and shorter length of stay, which have led to a significantly less total cost for initial ablative therapy compared to liver resection in this selected patient population [10,11].
Neuroendocrine Tumor Liver Metastases
Approximately 5%–57% of patients with neuroendocrine tumors develop liver metastases, which can cause both hormonal and pressure symptoms. Current hormonal therapies aimed at symptom palliation only offer limited oncological effects, with only 3% of patients demonstrating tumor remission. Liver guided therapies such as resection, ablation, or locoregional therapies which aim to decrease the tumor burden by debulking and improve hormonal and pressure symptoms are often indicated. However, due to the frequent prevalence of widespread liver disease, complete oncological resection is not usually possible. Furthermore, neuroendocrine liver metastases have demonstrated a better response to ablation compared to other tumors, with local recurrence rates as low as 5%–6% per lesion [12]. Given the indolent nature of neuroendocrine tumors, this disease is characterized by a long overall survival, with 10-year survival ranging from 35% to 70%, and liver recurrence rate up to 84%. Liver tumor ablation is an effective modality for repeated intervention to achieve hormonal relief and a favorable quality of life [13].
Noncolorectal Non-neuroendocrine Liver Metastases
Patients with noncolorectal non-neuroendocrine liver metastases comprise a distinct cohort. In these patients, the disease tends to me more systemic, and the role of resection may be limited. Selected patients with limited liver-dominant involvement may be candidates for ablation as well.
Contraindications
Contraindications for consideration of ablative therapies include significant medical comorbidities precluding the ability to undergo general anesthesia, laparoscopic or open surgery, or uncorrectable coagulopathy. Tumor-related contraindications include significant diffuse metastatic disease of the liver or untreatable extrahepatic disease, which precludes a favorable oncological outcome, or factors inhibiting safe ablation, such as a tumor abutting the hilum or right or left hepatic ducts. Lastly, biliary dilatation is also a contraindication for an ablation procedure due to the risk of thermal injury, which could lead to biliary complications.
ABLATION TECHNOLOGIES
Radiofrequency Ablation
RFA uses an alternating electrical current at a frequency of 400 MHz to generate thermal energy. A generator applies a high-frequency alternating electrical current, causing ionic excitement transmitted through an applicator tip to the tissue. This heats a spherical volume of tissue in the area of the applicator tip leading to thermocoagulative necrosis. The width of the ablation zone can be adjusted by controlling the energy output, as well as time of ablation according to set algorithms.
There are multiple systems available which can produce up to 5-cm ablation zones with a single stick that use either temperature or impedance regulation, with or without saline infusion. Although RFA has been well recognized since its introduction in the early 1990s and accepted as an option by early 2000s as an effective treatment option for patients with unresectable disease, it has some limitations [14]. The ablation process may be lengthy and may take up to 25–30 minutes when creating larger 5-cm treatment zones. It is also susceptible to the heat-sink effect, causing difficulties to treat lesions in close proximity to large vessels. Furthermore, except for initial improvements resulting in more powerful (200 W versus 50 W) generators that could be used to achieve up to a 5-cm ablation zone with a single deployment, technology has not advanced significantly over the recent years. Additionally, high local recurrence rates up to 20%–40% have been reported in various series [15] (Fig 1).
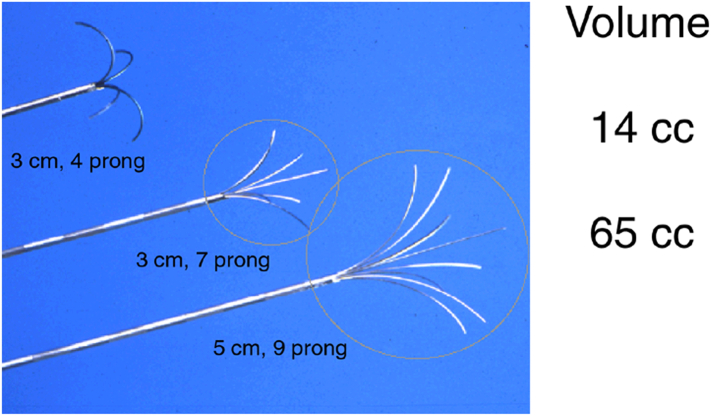
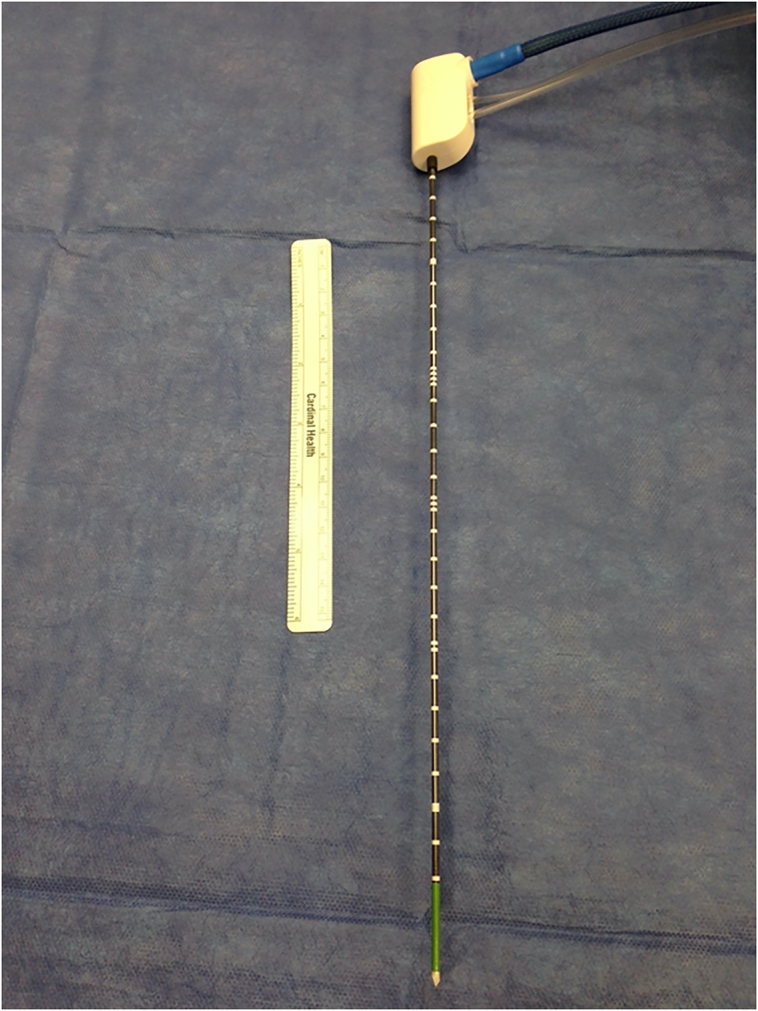
Fig 1.
Representative RFA catheters of different sizes and configuration.
Microwave Ablation
MWA was introduced as an improvement upon existing RFA technology used in thermal ablation of liver tumors, given the reported failure rates up to 40%. Tumor destruction occurs in MWA actively through an oscillating microwave field independent of tumor tissue conductivity. This produces higher tissue temperatures of 160°–180° [16] compared to RFA which causes tumor destruction through ionic excitement conducting through the tumor, reaching maximum tissue temperatures of 100°. MWA also is characterized by a more rapid rise in tissue temperature, leading to more efficient ablation times; more homogenous tissue heating zones with deeper tissue penetration; and a less pronounced heat-sink effect since energy transmission is not affected by coagulative tissue changes during ablation or the “heat sink” cooling effect from nearby blood vessels and structures, both leading to an inadequate ablation zone during traditional RFA.
The initial reports of MWA results were conducted utilizing a 915-MHz microwave generator. This earlier system has been criticized for producing a more ellipsoid ablative zone, requiring more than one probe to achieve a spherical ablative zone. Advances in surgical technology have since led to the development of 2.45 GHz systems with a saline cooled antenna capable of producing spherical ablation zones [17] (Fig 2).
Fig 2.
A representative microwave ablation catheter. Note the antenna design that uses a straight needle, in contrast to the design of RFA catheters.
TECHNIQUE
Approach
Initial reports of thermal liver tumor ablation focused on the percutaneous approach [14,18]. However, the main limitations of this approach are a greater difficulty to target peripheral lesions, as well as an increased risk of injury to organs adjacent to the liver. Although the open approach overcomes these limitations and allows for concurrent abdominal staging, it carries the morbidities of open surgery. The laparoscopic approach enables a minimally invasive approach for inspection of the abdominal cavity for peritoneal metastases and tumor ablation. Laparoscopically, tumor targeting is facilitated by the upward movement of the diaphragm and laparoscopic ultrasonography which allows for the highly sensitive staging of the liver. It has been demonstrated that in up to 20% of patients undergoing ablation of liver tumors, laparoscopic ultrasound demonstrates at least 1 additional tumor compared to preoperative triphasic computed tomographic (CT) scans [19]. Furthermore, although there are no randomized studies in the literature, meta-analyses suggest a better control rate with surgical (open or laparoscopic) rather than percutaneous ablation.
Access and Setup
Laparoscopic liver tumor ablation is performed under general endotracheal anesthesia. Two 12-mm laparoscopic ports are placed in the right upper quadrant; one will be used for the laparoscope and the other for the ultrasound transducer. We use an optical access trocar to enter the abdominal cavity under direct visualization, given that a significant number of these patients have had prior abdominal surgeries. Routinely, the falciform, triangular, or coronary ligaments do not need to be divided, but if there are any perihepatic adhesions from prior abdominal surgeries, these are divided using laparoscopic scissors or the ultrasonic scalpel to allow for comprehensive sonographic evaluation of the liver. It is important to identify any adherent viscera that may be in proximity to the planned ablation zones and preemptively mobilize them away from the liver. For lesions encroaching upon the gallbladder fossa, the gallbladder should also be resected prior to ablation to avoid thermal injury to the wall of the gallbladder leading to delayed bile leakage.
We first perform a diagnostic laparoscopy to inspect the diaphragm, abdominal wall, omentum, viscera, and pelvic cavity for the presence of metastases not evident on preoperative imaging studies. For most tumor types, the finding of limited amounts of extrahepatic disease is not a contraindication to proceed with ablation, but widespread disease is.
Ultrasound and Biopsy
Using a high-frequency laparoscopic transducer, a complete ultrasonographic evaluation of the liver is completed. Routinely, two laparoscopic monitors are used: one with the laparoscopic imagery and the other ultrasonographic imagery. This allows us to coordinate the movements of the US transducer using the laparoscopic image. We use a 10-mm rigid, linear, side-viewing transducer. Scanning the liver is performed in 1 of 2 ways: geographic or systematic. In the geographic technique, the liver parenchyma is swept analogous to "lawn-moving" to cover all segments. The transducer is placed at the dome and translocated across the liver from the falciform ligament to the right lateral edge with lateral movements. The transducer needs to have good contact with the liver surface (contact scanning), and this requires some downward compression with the distal end of the probe on the liver. After sweep scan of each path by lateral movement, the probe is pulled back around 3 cm to sweep the next "row" of parenchyma. If a suspicious lesion is encountered, the transducer can be held fixed over that area and rotated along its axis medially and laterally to characterize the lesion in more detail. The rigid transducer can be used to lift the liver up and scan from underneath as well. This is especially useful to image the deep segments of six and seven. In the systematic method, the scanning is started by finding the main portal vein in the junction of the fourth and fifth segments and tracing the peripheral branches to the Couinaud segments. The tracing of the three hepatic veins from their confluence with the inferior vena cava also helps to determine the exact anatomic localization of a lesion. The transducer is again moved by lateral movement.
Contact scanning is generally adequate if good contact is maintained between the ultrasound transducer and the surface of the liver. There is no need to fill the subdiaphragmatic space with saline, except for the visualization of superficial lesions at the dome. If the curvature at the dome does not allow for adequate contact between the transducer and the liver parenchyma, the use of saline as a stand-off medium can be helpful to characterize the superficial lesions at these locations.
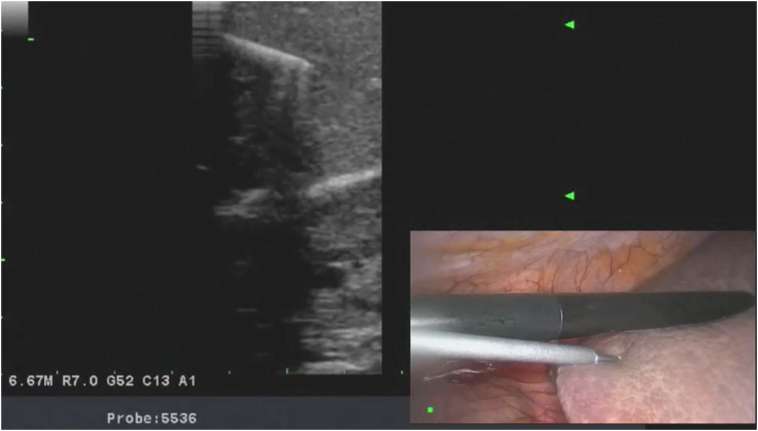
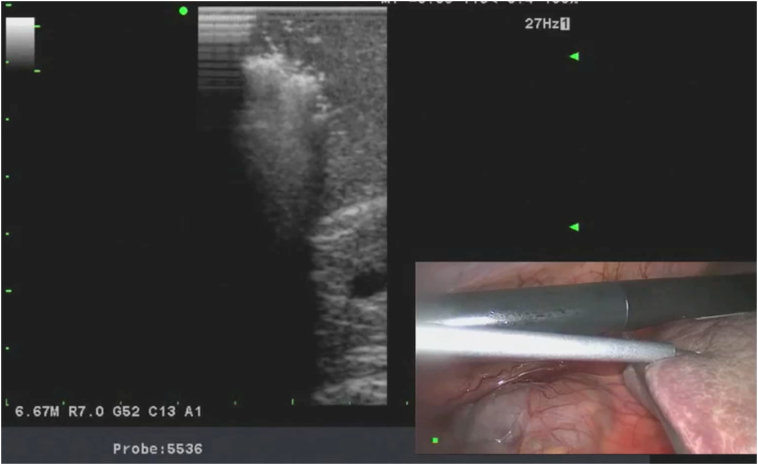
Once the liver ultrasound is completed and the lesions are localized, core biopsy is performed using an 18G spring-loaded biopsy gun. The lesion is centered under the transducer, and the biopsy needle is inserted percutaneously into the liver tissue, under direct view, using a free-hand technique. The needle is inserted in parallel and within the plane of the transducer to visualize the needle as it traverses the tissues. The distal 2 cm of the needle is echogenic. If the tip is not visualized, the transducer plane is changed with subtle pronation and supination, and accordingly, the angle of the needle is adjusted. If the needle is not parallel to the ultrasound plane, only the tip of the needle can be seen on the ultrasound screen, making orientation and direction of the needle more difficult. Once the tumor is targeted with the biopsy needle, the tip of the needle is positioned at the edge of the tumor and the trigger is fired taking into account that the needle has a 2-cm throw-in length. A 45° laparoscope is used for targeting the needle to able to look down at the ultrasound transducer and the needle, further helping with coordination. Upon retrieval of the needle, if there is any oozing from the liver capsule, the entry site is coagulated with the tip of a laparoscopic instrument. It is important to guide the biopsy needle with ultrasound to avoid major vessels and bile ducts in the trajectory of the needle (Fig 3, Fig 4, Fig 5).
Fig 3.
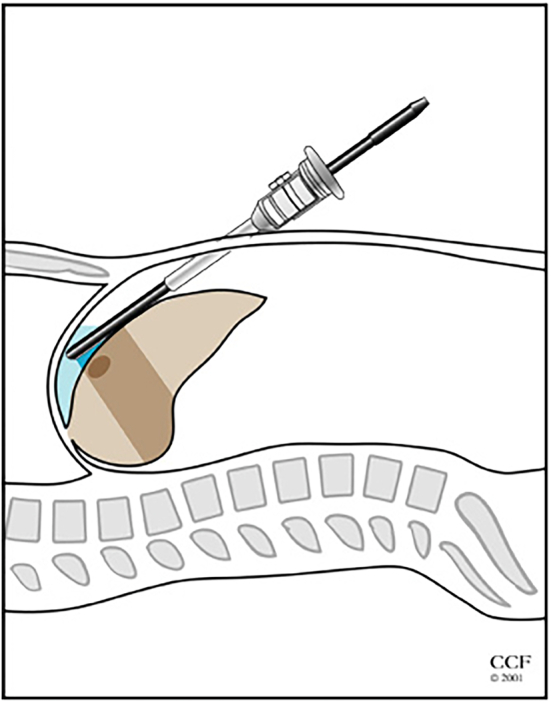
Illustration showing the use of laparoscopic ultrasound to identify liver tumors.
Fig 4.
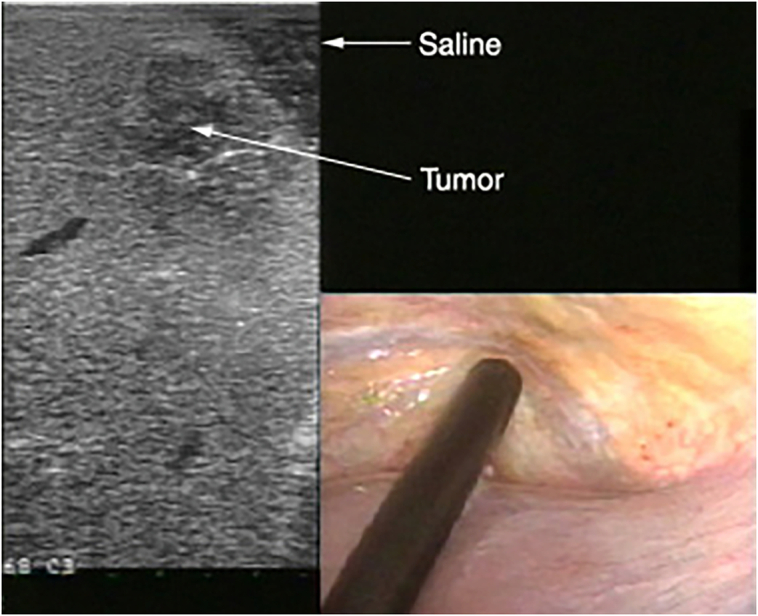
Use of stand-off technique to visualize tumors located at the periphery of the liver.
Fig 5.
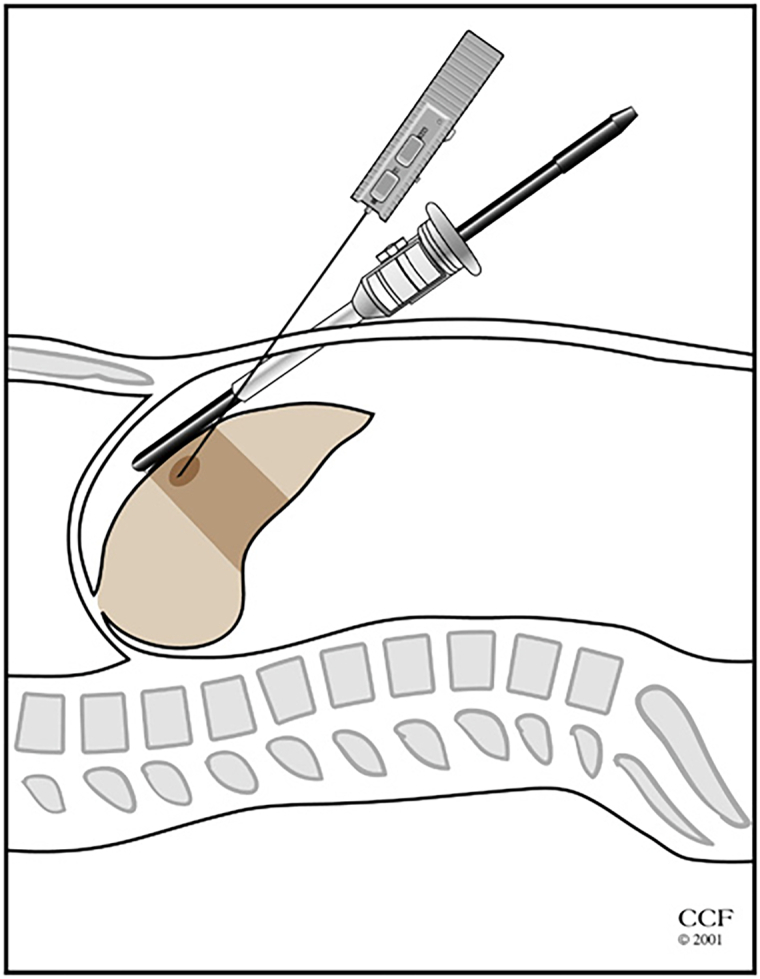
Illustration showing the performance of laparoscopic ultrasound-guided biopsy procedure.
Ablation
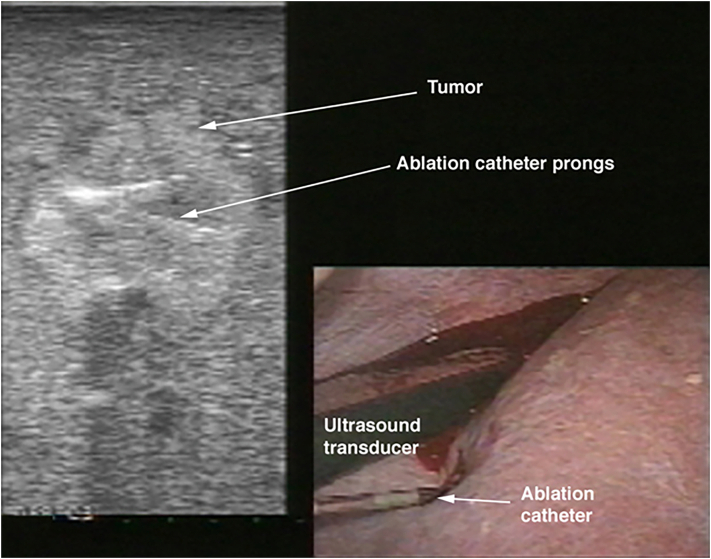
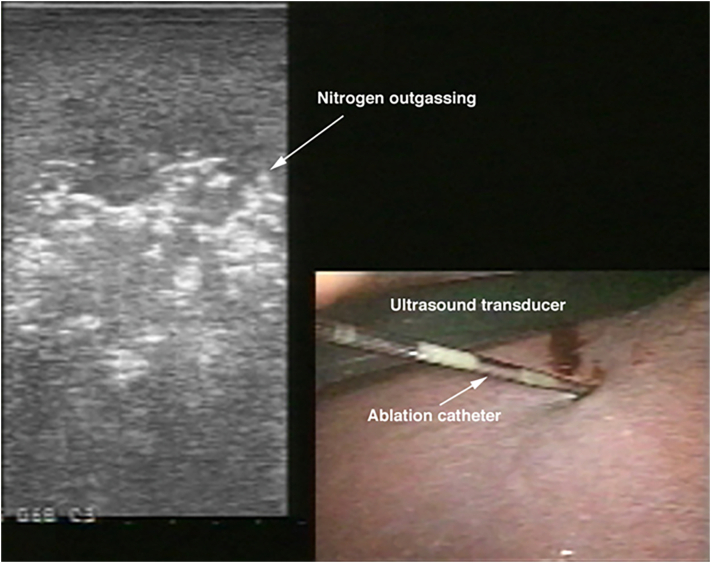
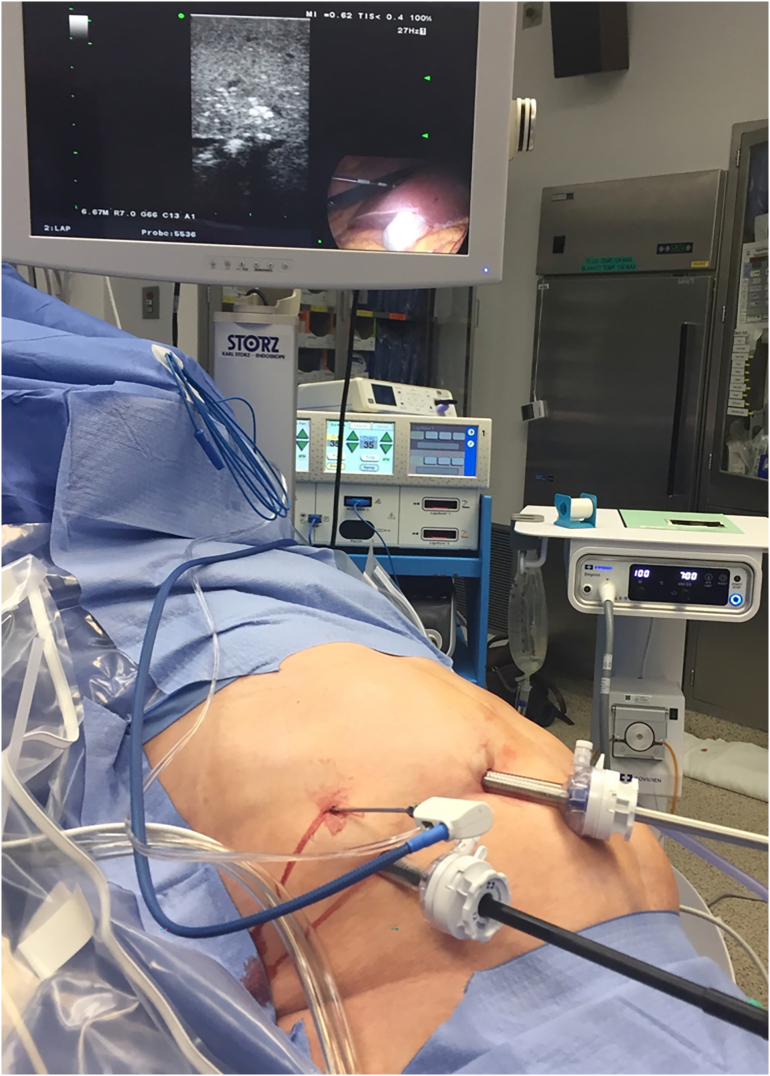
Next, the ablation catheter is introduced. Depending on its stiffness, the catheter may be inserted directly into the abdominal cavity either using a stab puncture or via a separate 3 mm trocar. Once the ablation catheter is placed into the tumor, then the generator is activated to deliver either radiofrequency or microwave energy to the tissue to create an ablation zone. This is performed using different algorithms based on the type of ablation device used. We advocate aiming for a 1-cm ablation margin. Real-time assessment of the ablative changes in the liver tissues is performed using ultrasonography. This is demonstrated as outgassing of dissolved nitrogen into the heating tissues. As the tissues are heated, the solubility of dissolved nitrogen decreases, resulting in microbubble formation within the tissue. This appears as an echogenic blush that enlarges to encompass the zone of ablation. In tumors that demonstrate tumor flow preablation, color flow Doppler is repeated postablation to demonstrate the absence of blood flow within the ablated zones (Fig 6, Fig 7, Fig 8, Fig 9, Fig 10, Fig 11).
Fig 6.
Intraoperative photo showing the placement of RFA catheter into the tumors under laparoscopic ultrasound guidance.
Fig 7.
Intraoperative photo showing performance of RFA procedure under laparoscopic ultrasound guidance.
Fig 8.
Intraoperative photo showing the insertion of a microwave needle into a colorectal liver metastasis under laparoscopic ultrasound guidance.
Fig 9.
Intraoperative photo showing performance of microwave ablation procedure under laparoscopic ultrasound guidance.
Fig 10.
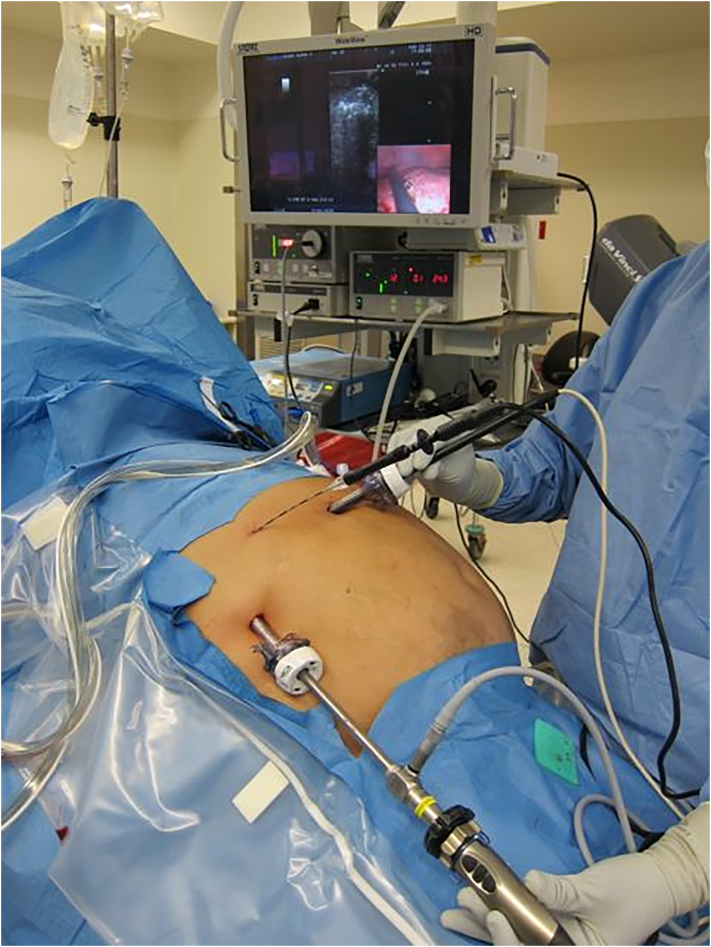
Intraoperative photo demonstrating operative setup for a laparoscopic RFA procedure.
Fig 11.
Intraoperative photo demonstrating operative setup for a laparoscopic microwave ablation procedure.
Bleeding from the needle track is rarely a problem on withdrawal of the needle. Nevertheless, the needle tract may be coagulated with 100 W of power during withdrawal of the needle.
POSTOPERATIVE CARE AND COMPLICATIONS
Patients not undergoing a concomitant surgical procedure are routinely discharged within 24 hours, usually without any narcotic requirements. Laboratory studies including a CBC, renal panel, and liver function panel are routinely obtained on postoperative day 1.
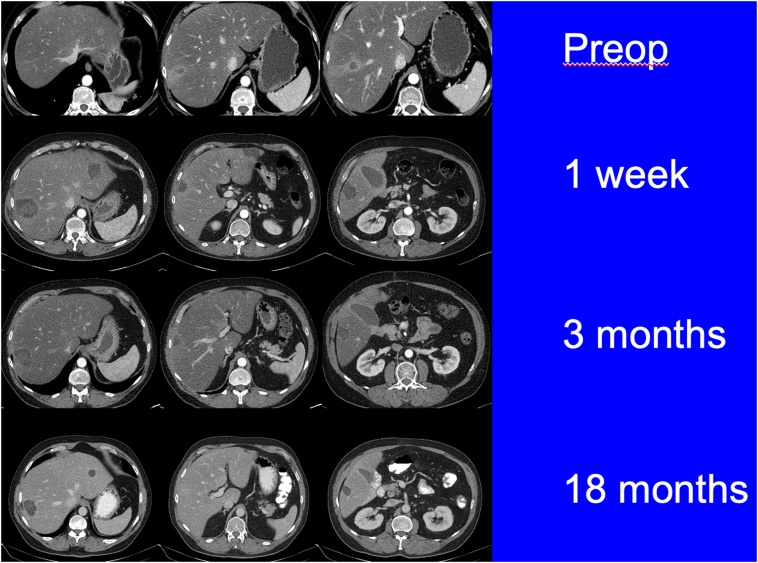
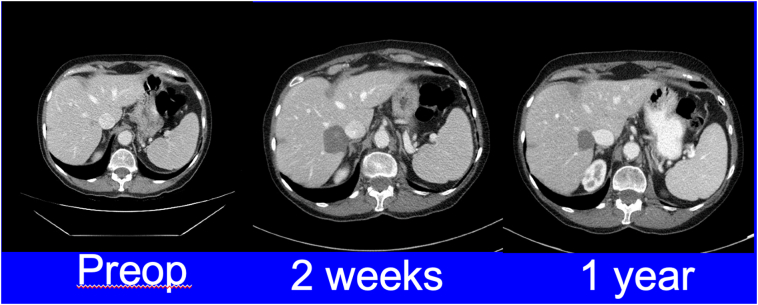
All patients are followed by a standardized surveillance imaging protocol, which includes cross-sectional imaging with either triphasic CT or magnetic resonance imaging. The initial postablative scan is completed 1–2 weeks following liver tumor ablation to confirm adequate ablation zones. This is followed by cross-sectional imaging every 3 months for 1 year and then every 6 months for 2 years (Fig 12, Fig 13).
Fig 12.
CT scans of a patient with neuroendocrine live metastases who underwent microwave ablation of multiple bilobar lesions. Follow-up imaging did not demonstrate any local recurrence.
Fig 13.
CT images of an elderly patient with a solitary tumor in liver segment 7. After discussing treatment options including liver resection, patient opted for a laparoscopic microwave ablation. Follow-up imaging demonstrated a successful outcome with no evidence of local recurrence.
OTHER ABLATION TECHNOLOGIES
Irreversible electroporation utilizes targeted delivery of millisecond electrical pulses to induce permeabilization of cell membranes through nanoscale defects. When energy increases to a certain level, irreversible apoptosis occurs. Cell death occurs without injury to extracellular matrix. This technology uses multiple antennas to create the electrical field. There may be a utility for liver tumors located close to critical structures, such as portal vein or bile ducts.
In conclusion, liver tumor thermal ablation remains an important tool in the armamentarium of the hepatobiliary surgeon for treatment of liver tumors in a safe and effective manner. The role of the liver tumor thermal ablation will continue to evolve as newly emerging technologies are introduced expanding the indications of liver tumor ablation and improving the local treatment failure rates.
Author Contribution
Dr Mohammed Elshamy: Investigation, Writing – original draft.
Dr Onuralp Ergun: Writing – review & editing, Visualization.
Dr Eren Berber: Writing – review & editing, Supervision, Visualization, Project administration.
Conflict of Interest
Dr Eren Berber is a consultant to Ethicon, Aesculap, and Medtronic. He has received honoraria for consulting work. Other authors report no conflict of interest.
Funding Source
There was no funding for any of the authors in this study.
Ethical Statement
Authors assure that the manuscript "Ablation Technologies" is the authors' original work and was created in a truthful and complete manner. All sources have been properly disclosed. Since this work is a review article, no institutional review board approval is included.
Footnotes
Special Issue on HPB Surgical Ultrasound; Edited by Dr. Ellen Hagopian
References
- 1.Lounsberry W., Goldschmidt V., Linke C.A., Walder H.J., Chrzan D. The early histologic changes following electrocoagulation. J Urol. 1961;86:321–329. doi: 10.1016/S0022-5347(17)65169-3. [DOI] [PubMed] [Google Scholar]
- 2.McGahan J.P., Brock J.M., Tesluk H., Gu W.Z., Schneider P., Browning P.D. Hepatic ablation with use of radio-frequency electrocautery in the animal model. J Vasc Interv Radiol. 1992;3(2):291–297. doi: 10.1016/s1051-0443(92)72028-4. [DOI] [PubMed] [Google Scholar]
- 3.Aliyev S., Agcaoglu O., Aksoy E., Taskin H.E., Vogt D., Fung J., et al. Efficacy of laparoscopic radiofrequency ablation for the treatment of patients with small solitary colorectal liver metastasis. Surgery. 2013;154(3):556–562. doi: 10.1016/j.surg.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Siperstein A.E., Berber E., Ballem N., Parikh R.T. Survival after radiofrequency ablation of colorectal liver metastases: 10-year experience. Ann Surg. 2007;246(4):559–567. doi: 10.1097/SLA.0b013e318155a7b6. [DOI] [PubMed] [Google Scholar]
- 5.Berber E., Siperstein A. Perioperative outcome after laparoscopic radiofrequency ablation of liver tumors: an analysis of 521 cases. Surg Endosc. 2007;21(4):613–618. doi: 10.1007/s00464-006-9139-y. [DOI] [PubMed] [Google Scholar]
- 6.Ballem N., Berber E., Pitt T., Siperstein A. Laparoscopic radiofrequency ablation of unresectable hepatocellular carcinoma: long-term follow-up. HPB (Oxford) 2008;10(5):315–320. doi: 10.1080/13651820802247102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berber E., Siperstein A. Local recurrence after laparoscopic radiofrequency ablation of liver tumors: an analysis of 1032 tumors. Ann Surg Oncol. 2008;15(10):2757–2764. doi: 10.1245/s10434-008-0043-7. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi H., Berber E. Role of thermal ablation in the management of colorectal liver metastasis. Hepatobiliary Surg Nutr. 2020;9(1):49–58. doi: 10.21037/hbsn.2019.06.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi H., Akyuz M., Aksoy E., Karabulut K., Berber E. Local recurrence after laparoscopic radiofrequency ablation of malignant liver tumors: results of a contemporary series. J Surg Oncol. 2017;115(7):830–834. doi: 10.1002/jso.24599. [DOI] [PubMed] [Google Scholar]
- 10.Berber E., Tsinberg M., Tellioglu G., Simpfendorfer C.H., Siperstein A.E. Resection versus laparoscopic radiofrequency thermal ablation of solitary colorectal liver metastasis. J Gastrointest Surg. 2008;12(11):1967–1972. doi: 10.1007/s11605-008-0622-8. [DOI] [PubMed] [Google Scholar]
- 11.Karabulut K., Aucejo F., Akyildiz H.Y., Siperstein A., Berber E. Resection and radiofrequency ablation in the treatment of hepatocellular carcinoma: a single-center experience. Surg Endosc. 2012;26(4):990–997. doi: 10.1007/s00464-011-1983-8. [DOI] [PubMed] [Google Scholar]
- 12.Akyildiz H.Y., Mitchell J., Milas M., Siperstein A., Berber E. Laparoscopic radiofrequency thermal ablation of neuroendocrine hepatic metastases: long-term follow-up. Surgery. 2010;148(6):1288–1293. doi: 10.1016/j.surg.2010.09.014. [discussion 93] [DOI] [PubMed] [Google Scholar]
- 13.Kose E., Kahramangil B., Aydin H., Donmez M., Takahashi H., Aucejo F., et al. Outcomes of laparoscopic tumor ablation for neuroendocrine liver metastases: a 20-year experience. Surg Endosc. 2020;34(1):249–256. doi: 10.1007/s00464-019-06759-1. [DOI] [PubMed] [Google Scholar]
- 14.Rossi S., Fornari F., Buscarini L. Percutaneous ultrasound-guided radiofrequency electrocautery for the treatment of small hepatocellular carcinoma. J Intervent Radiol. 1993;8(3):97–103. [Google Scholar]
- 15.Agcaoglu O., Aliyev S., Karabulut K., El-Gazzaz G., Aucejo F., Pelley R., et al. Complementary use of resection and radiofrequency ablation for the treatment of colorectal liver metastases: an analysis of 395 patients. World J Surg. 2013;37(6):1333–1339. doi: 10.1007/s00268-013-1981-1. [DOI] [PubMed] [Google Scholar]
- 16.Erten O., Li P., Gokceimam M., Akbulut S., Berber E. Impact of ablation algorithm versus tumor-dependent parameters on local control after microwave ablation of malignant liver tumors. J Surg Oncol. 2021;123(1):179–186. doi: 10.1002/jso.26237. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi H., Kahramangil B., Berber E. Local recurrence after microwave thermosphere ablation of malignant liver tumors: results of a surgical series. Surgery. 2018;163(4):709–713. doi: 10.1016/j.surg.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 18.JP McGahan, Schneider P., Brock J.M., Tesluk H., editors. Seminars in interventional radiology. Copyright© 1993 by Thieme Medical Publishers, Inc; 1993. Treatment of liver tumors by percutaneous radiofrequency electrocautery. [Google Scholar]
- 19.Foroutani A., Garland A.M., Berber E., String A., Engle K., Ryan T.L., et al. Laparoscopic ultrasound vs triphasic computed tomography for detecting liver tumors. Arch Surg. 2000;135(8):933–938. doi: 10.1001/archsurg.135.8.933. [DOI] [PubMed] [Google Scholar]