Abstract
Leishmaniasis (visceral and cutaneous), Chagas disease and human African trypanosomiasis cause substantial death and morbidity, particularly in low- and middle-income countries. Although the situation has improved for human African trypanosomiasis, there remains an urgent need for new medicines to treat leishmaniasis and Chagas disease; the clinical development pipeline is particularly sparse for Chagas disease. In this Review, we describe recent advances in our understanding of the biology of the causative pathogens, particularly from the drug discovery perspective, and we explore the progress that has been made in the development of new drug candidates and the identification of promising molecular targets. We also explore the challenges in developing new clinical candidates and discuss potential solutions to overcome such hurdles.
Subject terms: Drug discovery, Parasitic infection
In this Review, Gilbert and colleagues discuss recent progress in drug discovery for kinetoplastid diseases and how an improved understanding of parasite biology affects the drug discovery process
Introduction
Kinetoplastids are protozoans that are characteristically defined as having a kinetoplast, which is a dense network of concatenated DNA within the mitochondrion1. Trypanosomatids form an order within the kinetoplastid class, and encompass several species that cause parasitic infections in humans (Table 1). Human African trypanosomiasis (HAT; also known as sleeping sickness) is caused by Trypanosoma brucei gambiense and Trypanosoma brucei rhodesiense2,3. This disease has two phases, a peripheral phase with generally mild symptoms, and a second phase when the parasites enter the brain, which can lead to neurological symptoms and death without treatment. Leishmaniasis is caused by various Leishmania species, and the term refers to several different diseases4: visceral leishmaniasis (also known as kala azar), a systemic infection that is almost invariably fatal without treatment, is caused by Leishmania donovani and Leishmania infantum; cutaneous leishmaniasis, which gives rise to skin lesions that heal very slowly, is caused by multiple species and subspecies of Leishmania; mucocutaneous leishmaniasis, which involves the destruction of the nasal and buccal cavities, is caused by multiple species and subspecies of Leishmania; and post-kala-azar dermal leishmaniasis (PKDL), which is a rash that can appear sometime after a patient has recovered from visceral leishmaniasis. Chagas disease5,6 is caused by Trypanosoma cruzi, and is characterized by an initial acute infection, which has vague symptoms and is often not diagnosed at this stage. Patients remain asymptomatic for many years, and it is very difficult to detect the parasites. A subset of patients will progress to symptomatic disease, which usually manifests itself as heart or digestive tract disease. Chronic disease pathogenesis is complex and thought to result from complex interplay between the immune system and the parasites7. Leishmaniasis, Chagas disease and HAT are primarily transmitted by various insect vectors, but Chagas disease can also be transmitted congenitally, through contaminated blood and through contaminated food and drinks.
Table 1.
Kinetoplastid diseases in humans
| Disease | Causative pathogen | Major symptoms | Vector | Endemic regions | Prevalence | Disability-adjusted life years | Years of life lost (estimate for 2019) | Years lived with disability (estimate for 2019) | Deaths per year120 | Treatments |
|---|---|---|---|---|---|---|---|---|---|---|
| Human African trypanosomiasis | Trypanosoma brucei gambiense, Trypanosoma brucei rhodesiense | 1st stage: fever, headache, enlarged lymph nodes, joint pain, itching. 2nd stage: confusion, sensory, coordination and sleep cycle disturbance | Tsetse fly | Sub-Saharan Africa | 3,800 | 83,000 | 82,000 | 1,000 | 1,400 | Pentamidine (stage 1, T. b. gambiense); suramin (stage 1, T. b. rhodiense); nifurtimox–eflornithine combination therapy, fexinidazole (acoziborole in late-stage clinical trials), melarsoprol (stage 2, T. b. gambiense); melarsoprol (stage 2, T. b. rhodesiense) |
| Chagas disease | Trypanosoma cruzi | Acute phase: asymptomatic or mild; fever, occasionally swelling at site of inoculation. Chronic phase: cardiac and digestive disorders, heart failure | Reduviid bug | South America. Now spread through migration to large parts of the world | 6,500,000 | 280,000 | 217,000 | 58,000 | 9,500 | Benznidazole, nifurtimox |
| Visceral leishmaniasisa | Leishmania donovani, Leishmania infantum | Fever, weight loss, enlargement of the spleen and liver, anaemia | Sandfly | Key foci: India, East Africa, Brazil | 8,600 | 400,000 | 403,000 | 600 | 5,700 | Amphotericin B, miltefosine, antimonials, paromomycin |
| Cutaneous and mucocutaneous leishmaniasisb | Leishmania tropica, Leishmania aethiopica, Leishmania major, L. infantum, Leishmania mexicana, Leishmania amazonensis, Leishmania braziliensis, Leishmania guyanensis | Cutaneous leishmaniasis: skin lesions, mainly ulcers. Mucocutaneous leishmaniasis: destruction of mucous membranes of nose, mouth and throat | Sandfly | Key foci: Middle East, Mediterranean basin, Latin America | 4,600,000 | 290,000 | NA | 293,000 | NA | Antimonials, miltefosine, amphotericin B |
This table is based on data from the Global Burden of Disease study. Data are rounded. Obtaining these epidemiological data is very challenging owing to lack of diagnosis of patients and reporting. NA, not applicable. aPost-kala-azar dermal leishmaniasis, a complication following cure from visceral leishmaniasis is not included in this table. bCutaneous and mucocutaneous leishmaniasis are combined in this table. Some species cause both diseases. Mucocutaneous leishmaniasis is found in South America, whereas cutaneous leishmaniasis is found across large parts of the tropical and subtropical world.
Recently, there has been substantial progress in the development of new therapeutics for HAT, with one new drug registered8 and another in advanced, late-stage clinical trials9. There is a promising pipeline developing for visceral leishmaniasis, with a number of new compounds with novel modes of action in early clinical development. However, the drug discovery and development pipelines for Chagas disease, cutaneous leishmaniasis, mucocutaneous leishmaniasis and PKDL are poor, and there remains an urgent need for new drugs to treat them10. New drugs will be required for leishmaniasis and Chagas disease to achieve the WHO road map for neglected tropical diseases, which aims to fulfil the United Nations Sustainable Development Goals11.
Growing evidence suggests the existence of ‘persister’ forms in T. cruzi12,13 and Leishmania spp.13,14. These transiently dormant stages of the parasites have serious implications for the development of therapies capable of curing kinetoplastid diseases. Other challenges include patients with no symptoms (asymptomatic patients), who are very difficult to identify and may act as reservoirs of infection or may experience clinical symptoms in the future (Chagas disease). In addition, animals may act as reservoirs of infection. Recently, a range of new tools have been developed that are beginning to have a notable impact on drug discovery for kinetoplastid diseases. These include new tools to facilitate studies of drug mechanisms of action, new tools that enable genetic manipulation of parasites, and refined cellular and animal models of infection that more closely mimic the human diseases.
In this Review, we discuss recent progress in drug discovery for kinetoplastid diseases and how an improved understanding of parasite biology affects the drug discovery process.
Improved understanding of parasite biology
Unique features of parasite biology have been reviewed previously1,3–6. In this section, we discuss some recent insights that may have the potential to affect drug discovery for these pathogens.
Persisters
Persister forms are commonly identified in infectious diseases. Initially identified in bacteria15, their role in antibiotic resistance has been studied extensively in recent years16. They are phenotypically defined as a transient subpopulation that is less susceptible to drug treatment (Fig. 1). This recalcitrance is either due to differing degrees of metabolic quiescence, which makes them less susceptible to drugs that target processes found in actively dividing and growing cells, or due to other physiological changes that reduce drug effect, rather than genetically encoded resistance17. If the cell is not dividing, or is dividing only very slowly, drugs that target DNA replication and cell division may not be effective. There is also indication of changes in regulation of transporters in some circumstances, which may affect drug levels in the pathogen, by reducing either uptake or efflux. However, the precise molecular mechanisms that define persisters are not well understood. Persister forms are not limited to bacteria, and have been identified in eukaryotic protozoan parasites such as Plasmodium vivax (hypnozoites)18, and Toxoplasma gondii (bradyzoites)19, and they have been indicated to have a key role in disease persistence. Recently it has become apparent that persister forms also occur in trypanosomatids. Although understanding of persisters in kinetoplastids is in its infancy, this emerging knowledge needs to be considered in drug discovery efforts. The small number of persisters in the parasite population and the potential interference of reagents used for their study20 pose substantial challenges for the development of persister-specific drug discovery assays (see later).
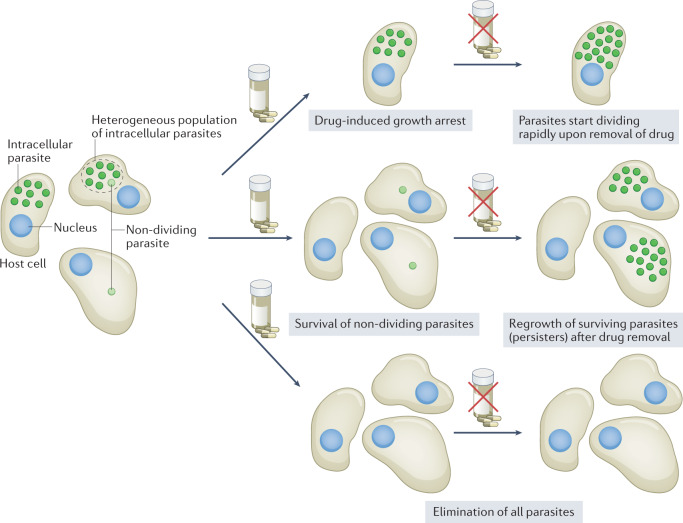
Fig. 1. Potential effects of parasite persisters on drug treatment.
Persisters are phenotypically defined as a transient subpopulation that is less susceptible to drug treatment, possibly owing to slow growth and changed metabolism. Following drug treatment, multiple outcomes have been observed. Some compounds induce reversible growth arrest (for example, GNF7686 (ref.25), which is active against T. cruzi), and parasites start dividing rapidly upon removal of the drug (top panel). Other compounds kill most of the intracellular parasites, with only a very small number surviving treatment (persisters; middle panel). The surviving parasites have been shown to be in a state of spontaneous and reversible growth arrest12, which is likely a key factor in their ability to survive drug treatment. The ideal outcome of drug treatment is that all parasites are killed, which can be achieved by treating parasites for extended durations and/or with high concentrations of drugs23,27, or potentially with compounds that target mechanisms essential for the survival of persisters (bottom panel).
In Chagas disease, both long-term persistence of parasites in patients and frequent failure of standard therapies (benznidazole and nifurtimox)21,22 may at least partially be explained by a recently described small subpopulation of T. cruzi parasites that survives drug treatments that kill most of the parasite population12,23,24. Some persisters occur spontaneously, both in vitro and in vivo, and, as for other pathogens, the T. cruzi persister phenotype seems to be associated with quiescence12. Whether they represent a discrete life stage or are simply the slowest replicating parasites in a distribution of normal amastigotes remains to be understood. Mouse model data indicate that population-level replication rates in chronically infected animals are lower than in acute stage animals20, which may contribute to the lower efficacy of treatments in patients who are in the chronic phase of the disease. Like many other single-cell organisms, T. cruzi regulates its growth rate in response to stresses such as nutrient limitation, inhibition of key energy pathways and drug treatment, but the specific pathways regulating this in intracellular T. cruzi have not yet been defined25.
For Chagas disease, the drug discovery community aims to achieve full cure (that is, every single parasite in the patient is killed), as long-term low parasite burden is a hallmark of chronic disease and eventually leads to symptomatic disease in about 30% of individuals. Eliminating persister parasites is therefore likely to be critical. Broadly, two approaches can be envisioned. One approach is to explore extended treatment durations. This option relies on the natural dynamics of persister parasites, with all persisters eventually becoming susceptible to drug treatment. The challenges with this approach are that the dynamics of spontaneous persister state entry and exit are not understood, and the potential existence of drug-induced persistence. Long-term intermittent treatment may overcome these challenges; proof of concept for this approach in mice using benznidazole has been demonstrated26,27. Researchers found that a higher dose (2.5–5 times the conventional dose) of benznidazole gave a more sustained lowering of parasite numbers. The higher dose was more effective than the conventional dose in clearing the persister state. They investigated intermittent (weekly) testing, and found that the duration (30 weeks) was important in obtaining parasitological cure, even at the higher doses. The study authors suggest that this is due to extending treatment beyond the time period when the parasites remain ‘dormant’ or as ‘persisters’. The lower doses did not lead to clearance of the persister forms at 30 weeks. They conclude that both the higher dose and the extended treatment regimens are required for cure. It remains to be determined whether this high-dose intermittent treatment could be an approach to treat humans. However, a pilot clinical study indicates that intermittent benznidazole treatment was not more effective than standard daily treatment26. Nevertheless, this approach may provide a reduction in adverse effects, and longer intermittent dosing schemes may offer higher efficacy. The major drawback from a public health perspective is that shorter treatments are preferred over longer ones. A more direct approach that may enable the treatment duration to be shortened is through identification of compounds that kill persister parasites. Although persister parasites may resist treatment owing to slower metabolism, they are likely to still maintain pathways essential for their survival. Increased understanding of persister biology as well as large-scale phenotypic screens to identify compounds that target persisters are key for this approach to be successful. As an extension of this approach, combination therapy may overcome the challenges presented by the persister population, in a similar way as applied in the tuberculosis field28.
Evidence for persister forms also exists in leishmaniasis. PKDL occurs in up to 15% of patients with apparently cured visceral leishmaniasis on the Indian subcontinent, and a similar relapse condition exists for cutaneous leishmaniasis (leishmaniasis recidivans). These conditions may result from persister forms in leishmaniasis13,29,30. Laboratory experiments support this and confirm the existence of slow-dividing and potentially quiescent parasites in in vitro cell-based systems as well as in animal models14,31,32.
Parasite distribution
Understanding where the parasite is distributed within the host provides insight into host–parasite interactions and the pathology in the host. In addition, from a drug discovery perspective, this can inform the required distribution of a therapeutic to different organs and tissues. Care has to be taken, as there may be differences in compound distribution among different hosts.
African trypanosomes can circulate in the host bloodstream and invade the central nervous system (CNS), causing the potentially fatal neurological disorders associated with sleeping sickness. Data from humans, other natural hosts and experimental animal models indicate that these parasites are also found in interstitial spaces and at other extravascular sites33–35, including adipose tissue36, skin37,38, (cardiac) muscle, eyes, lungs, lymph, endocrine glands and reproductive organs39. Invasion of these tissues has been linked to weight loss, dermatological symptoms, heart disease, ocular symptoms and respiratory symptoms. Complicating this picture further, tissue distribution may change during an infection, and could differ between species and subspecies. This extensive tissue invasion was initially observed about 100 years ago35, but the clinical significance has not perhaps been fully taken into account. Recent research in mouse models reveals that adhesion molecules can drive tissue tropism40, and parasites that occupy adipose tissue, for example, can adapt their metabolism to local conditions36, which may in turn have an impact on drug susceptibility. Some sites may be immune privileged or may be sources of relapse infections after chemotherapy. Potentially further challenging elimination efforts, parasite reservoirs found in human skin in asymptomatic cases can contribute to transmission38. In this regard, the development of a portable and non-invasive human skin test could be particularly useful41.
In their mammalian host, Leishmania parasites live and multiply intracellularly in phagocytic cells within phagolysosomes. Different species and subspecies of Leishmania cause different symptoms and different diseases (Table 1). In cutaneous leishmaniasis, the parasites infect macrophages resident in the skin. Cutaneous leishmaniasis occurs in three different forms: localized cutaneous leishmaniasis, diffuse cutaneous leishmaniasis and mucocutaneous leishmaniasis. Localized cutaneous leishmaniasis is the most common form, characterized by skin lesions and ulcers on exposed parts of the body, leaving permanent scars. In diffuse cutaneous leishmaniasis, parasites spread through the skin and cause numerous non-ulcerated nodules, and in mucocutaneous leishmaniasis, after the initial skin lesion has healed, the disease spreads to the mucous membranes of the nose, mouth and throat. Subsequently, the mucosal ulcers cause destruction of the nasal septum, lips and palate, leading to extensive facial disfiguring42. In all forms of cutaneous leishmaniasis, the Leishmania amastigotes reside in the phagolysosome of macrophages within the dermal layer of the skin at the borders of the lesion close to the inflammatory cells43. When the host cell is full of parasites, it bursts, and the released amastigotes will infect neighbouring macrophages in the skin. However, in visceral leishmaniasis, the released amastigotes are spread by the blood circulation and infect cells of the mononuclear phagocyte system (reticuloendothelial system) of the liver, spleen, bone marrow, lymph nodes and intestine44,45. With the introduction of bioluminescent mouse models of visceral leishmaniasis, it has become easier to track infection; this could potentially be used longitudinally to track the location of parasites as the disease develops. For example, in a recent study46 it was reported that the thymus, a lymphatic organ, seems to be infected from the beginning until the late phases of the infection.
Cutaneous leishmaniasis and visceral leishmaniasis clearly pose differing drug distributional challenges, with good dermal distribution through to more extensive tissue distribution requirements, respectively. This aside, delivering compounds across multiple membrane barriers with a substantial pH barrier to reach the intracellular amastigotes in the phagolysosomes of predominantly macrophages remains a fundamental prerequisite for the treatment of both cutaneous leishmaniasis and visceral leishmaniasis.
In cell culture systems T. cruzi propagates in essentially any nucleated mammalian cell. This promiscuity is reflected in the wide tissue distribution seen in patients and animal models. In acute disease, parasites can be readily detected in patient blood47, and autopsy reports indicate widespread infection of internal organs48. A similar pattern is observed in mouse models, where parasites are found in essentially all tissues49. Chronic disease is characterized by low parasite levels and frequently no detectable parasitaemia (that is, no detectable parasites in blood). Human autopsy studies have detected parasites in the heart and other organs, such as liver and kidney, as well as smooth muscle and adipose tissue49. Mouse models of chronic Chagas disease replicate this, with widespread distribution of parasites. Interestingly, live animal studies using bioluminescence to detect parasites in real time reveal a complex and spatio-temporally dynamic picture, with parasites detected in multiple organs and tissues (Fig. 2). In these studies, the gastrointestinal tract, in particular the stomach and colon, were identified as the main sites of parasite persistence50. The wide-ranging tissue distribution of T. cruzi poses a substantial challenge for drug discovery efforts as it is far from straightforward to develop compounds that achieve the extensive tissue distribution required to reach all parasites.
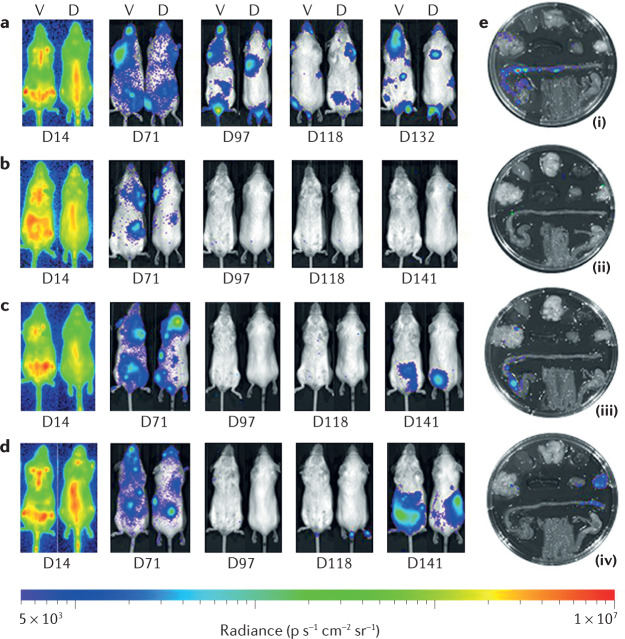
Fig. 2. Efficacy studies.
Whole-body images of mice (ventral (V) and dorsal (D)) infected with bioluminescent Trypanosoma cruzi, taken with an IVIS system. a | Untreated mice. b | Mice treated with benznidazole (100 mg kg−1) from day 74 (D74) to D93, followed by immunosuppression with cyclophosphamide (200 mg kg−1; D113, D118 and D128). c | Mice treated with posaconazole (20 mg kg−1), formulated in Noxafil, dosed with the same procedure as in panel b, including immunosuppression. d | As per panel c, except posaconazole was formulated in HPMC-SV. e | Ex vivo imaging of the mouse organs, following euthanasia at the end of the procedure. Adapted from ref.121, CC BY 3.0 (https://creativecommons.org/licenses/by/3.0/).
Compounds and targets
New chemical entities in development
There has been great progress in drug discovery and development for kinetoplastid diseases since we reviewed this area in 2017 (ref.1). In particular, fexinidazole has been registered for the treatment of HAT caused by T. b. gambiense (2021) and is in clinical trials for treatment of HAT caused by T. b. rhodesiense (NCT03974178); the oxaborole, acoziborole, is in late-stage clinical trials for treatment of HAT (NCT05256017); and a number of new chemical entities have been developed and have progressed into phase I clinical development for treatment of visceral leishmaniasis (see below). This marks a major step forward, although there is still a long way to go with the compounds for treatment of visceral leishmaniasis, and there is little in the pipeline for treatment of Chagas disease. Most compounds in later stages of drug discovery and clinical development are being developed by or co-developed with the Drugs for Neglected Diseases initiative (DNDi). Of particular note is the development of new chemical entities (Fig. 3). There are several reasons for the marked improvement in this situation. First, there has been substantial investment in drug discovery through organizations such as DNDi, Wellcome and the Global Health Innovative Technology Fund. Second, more integrated, industry-standard approaches to discovery have been adopted, with several pharmaceutical companies (notably GlaxoSmithKline, Novartis, Eisai, Takeda and Daichii Sankyo) now involved in discovery and development, along with a substantial number of academic groups. This has attracted expertise in key areas such as pharmacokinetics and safety pharmacology. Third, there has been great progress in understanding disease biology and developing assays, both cellular and animal, that are likely to be predictive of human disease. All of the compounds in the development pipeline were discovered through phenotypic screening processes. Importantly, methods are being developed to facilitate the identification of the molecular targets that these compounds inhibit (Box 1).
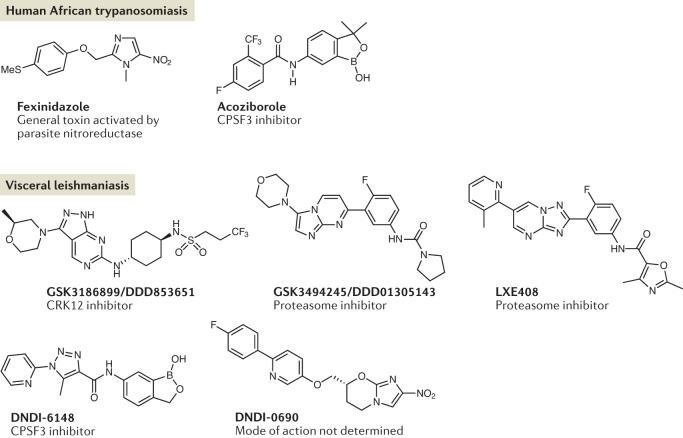
Fig. 3. New anti-trypanosomatid drugs and targets.
There has been great progress in drug discovery and development for kinetoplastid diseases, and shown are some examples of new chemical entities in development, including their mode of action if known. Fexinidazole, which is a general toxin, has been registered for the treatment of human African trypanosomiasis caused by Trypanosoma brucei gambiense and is in clinical trials for treatment of human African trypanosomiasis caused by Trypanosoma brucei rhodesiense. Acoziborole is an orally active oxaborole, now in phase IIb/III clinical studies. This compound has been shown to inhibit T. brucei cleavage and polyadenylation specificity factor 3 (CPSF3), which is an endonuclease involved in mRNA maturation. The vast majority of drug discovery related to leishmaniasis has been focused on visceral leishmaniasis, and less on cutaneous leishmaniasis, mucocutaneous leishmaniasis or post-kala-azar dermal leishmaniasis. This includes GSK3186899/DDD853651, which has been shown to inhibit cdc2-related kinase 12 (CRK12), as well as two compounds that have been shown to be proteasome inhibitors, one from the University of Dundee–GlaxoSmithKline collaboration (GSK3494245/DDD01305143) and one from Novartis (LXE408). These compounds inhibit the ‘chymotrypsin’-like active site on the proteasome β5 subunit. Other compounds in clinical development include the oxaborole DNDI-6148, which, like acoziborole, targets CPSF3, and the nitroimidazole DNDI-0690, the mechanism of action of which is yet to be determined.
Fexinidazole, a compound rediscovered by DNDi, is an orally active drug against both the peripheral nervous system stage and the CNS stage of HAT. It has now been registered for treatment of HAT caused by T. b. gambiense, and is in advanced clinical trials for treatment of HAT caused by T. b. rhodesiense8. Fexinidazole is activated by a parasite nitroreductase to a reactive intermediate, which then is likely to act against multiple targets within the parasite51. Acoziborole is an orally active oxaborole, active against both the peripheral nervous system stage and the CNS stage of HAT and now in phase IIb/III clinical studies. This compound has been shown to inhibit T. brucei cleavage and polyadenylation specificity factor 3 (CPSF3), which is an endonuclease involved in mRNA maturation52.
The vast majority of drug discovery for leishmaniasis has been focused on visceral leishmaniasis, and less on cutaneous leishmaniasis, mucocutaneous leishmaniasis or PKDL. Several new treatments are in clinical development, including combinations of existing drugs, to shorten treatment duration, improve safety and reduce the risk of drug resistance (for example, miltefosine–paromomycin; NCT03129646). There are a number of new chemical entities in clinical development (Fig. 3). These compounds were all identified through phenotypic screening, followed by successful target deconvolution for most of them. These new chemical entities include GSK3186899/DDD853651, which has been shown to inhibit cdc2-related kinase 12 (CRK12)53, as well as two compounds that have been shown to be proteasome inhibitors, one from the University of Dundee–GlaxoSmithKline collaboration (GSK3494245/DDD01305143)54,55 and one from Novartis (LXE408)56. These compounds inhibit the ‘chymotrypsin’-like active site on the proteasome β5 subunit. Their binding site includes a selectivity pocket that is found in kinetoplastid enzymes but not in the corresponding human orthologue54,55. Other compounds in clinical development include the oxaborole DNDI-6148 (ref.57), which, like acoziborole, targets CPSF3, and the nitroimidazole DNDI-0690 (refs.58,59), the mechanism of action of which is yet to be determined. Having five compounds in clinical development for treatment of visceral leishmaniasis is a substantial step towards the delivery of new and better treatments for the disease and is testament to the concerted efforts of multiple groups in this field. Although very little direct drug discovery has been conducted for cutaneous leishmaniasis, it seems that many compounds active against visceral leishmaniasis have the potential to be repurposed for treatment of cutaneous leishmaniasis59. However, given the different tissue distribution of cutaneous leishmaniasis and mucocutaneous leishmaniasis compared with visceral leishmaniasis, compounds that might kill the parasites causing visceral leishmaniasis may not have appropriate pharmacokinetics for treating cutaneous leishmaniasis, and further work is needed to develop a robust target candidate profile for cutaneous leishmaniasis.
The situation with Chagas disease has proved far more challenging. Various new benznidazole regimens are being investigated. There is a high rate of treatment discontinuation with the current treatments owing to side effects. The recent small-scale BENDITA study60 (NCT03378661) looked at both different lengths of treatment and different doses of benznidazole. The findings suggested that it may be possible to reduce the length of treatment, which would be a notable boost for treatment of Chagas disease. Similar responses were seen after 6 months in patients who received a shortened (2-week) regimen of benznidazole compared with the standard treatment for 8 weeks60, and other studies61 (NCT03191162 and NCT04897516) are following up on the results. Fexinidazole is also undergoing clinical trials for the treatment of Chagas disease (NCT03587766). The oxaborole DNDI-6148 has also been nominated as a clinical candidate for the treatment of Chagas disease and is in phase I clinical trials (EudraCT 2018-004023-37). Its mode of action in T. cruzi has yet to be reported. Besides those, there are no further compounds reported in clinical trials or good laboratory practice toxicology studies. As described elsewhere in this Review, there have been great challenges in finding molecules that can achieve radical cure.
A major advance in recent years has been to establish the molecular targets of the compounds in clinical development (Box 1). This has given rise to some highly validated drug targets. The question arises as to whether the limited drug discovery effort should be more focused on developing new chemotypes (backup series) for these highly validated targets or on new targets. As attrition in clinical development is high, the backup strategy with highly validated targets clearly has merits; however, these targets are not fully validated until we obtain clinical evidence in humans. For this reason, and to develop future combination treatments, it remains vital to develop new candidates with differentiated modes of action.
Box 1 New genetic methods: mechanism of action determination.
Determining the mechanism of action and molecular targets of compounds identified via phenotypic screening can be vital. The information that such studies provide can facilitate the medicinal chemistry optimization of compounds, facilitate improved compound selectivity, aid selection of appropriate compounds for combination studies and allow the emergence of target-driven resistance in the clinic to be monitored. Determining the molecular targets of active compounds can be challenging. However, through implementation of various novel approaches to drug target identification, great strides are now being made52–54,122–124. The ability to advance the development of drugs through knowledge of their mechanisms of action is proving transformative and can ultimately lead to a more diverse drug discovery portfolio. Focused mechanism-of-action studies have provided information that enables prioritization of compound series acting on the same molecular targets and removal of compound series with undesirable modes of action65,125.
Genetic tools and technologies, applied to the trypanosomatids, have had substantial impacts on efforts to characterize protein function and to identify drug resistance mechanisms and drug targets in recent years. To increase throughput, ‘loss-of-function’, ‘gain-of-function’ and tagging approaches have all been scaled up, to a genomic scale in several cases. Indeed, high-throughput approaches have been parallelized, whereby millions of parasites, each with a specific single protein depleted or overexpressed, can be screened in one experiment. These latter approaches have been applied to drug mode of action and drug resistance studies.
RNA interference loss-of-function screening has been used for these mechanism-of-action studies for several years126, and has more recently facilitated identification of the proteasome as the target of a new preclinical candidate in Trypanosoma brucei and Leishmania spp.54 and, for a separate chemical series, facilitated identification of divalent metal chelation as the mechanism underpinning toxicity125; the latter compounds were deprioritized as anti-leishmanial leads as a result. This approach also revealed a novel African trypanosome-specific prodrug metabolism and potential resistance mechanism for a candidate veterinary benzoxaborole127.
Overexpression gain-of-function screening has emerged more recently and is more likely to yield direct drug-target identification. For example, T. brucei cleavage and polyadenylation specificity factor 3 (CPSF3) was identified as the target of both clinical and veterinary trypanocidal benzoxaboroles52, including acoziborole, currently in phase II/III clinical trials for treatment of sleeping sickness. In addition, the approach was used to validate Leishmania N-myristoyltransferase as a target70. A kinase overexpression screen was also used to identify the kinetoplastid kinetochore protein KKT10 (also known as CLK1) as a promising target of a potent amidobenzimidazole with potential to treat all three human trypanosomal diseases124, whereas the cosmid sequencing approach continues to reveal genes linked to drug resistance in Leishmania128.
Genome editing is also increasingly affecting drug discovery efforts against trypanosomatids129. CRISPR–Cas9-based approaches have revolutionized biotechnology by enabling RNA-programmed targeting of specific chromosomal loci. Precision editing of drug targets, for example, facilitates the generation of drug-resistant strains and provides insight into structure–activity relationships. Quantitative measures of drug resistance can also be important in determining whether specific mutations are likely to have a detrimental impact in a clinical setting. Cas9-based editing has been applied to T. brucei CPSF3, providing insights into selective anti-trypanosomal action52, and to the Trypanosoma cruzi proteasome, revealing cross-resistance between arylsufonamides and distinct anti-leishmanials130. Oligonucleotide targeting, which is Cas9 independent, has also now been used to edit priority drug targets in trypanosomatids131. In recent years, compounds that inhibit the promising chemical, genetically and often pharmacologically validated targets detailed above have progressed into and through clinical development. New target-based screening programmes have also been initiated in parallel. Further insights relating to these drug targets should facilitate surveillance for drug resistance, understanding of toxicity, rational combination design and rational back-up planning.
Structural information
Recent developments in protein structure prediction and determination are having an impact on drug discovery. DeepMind Technologies has used artificial intelligence in the form of AlphaFold to predict structures for thousands of proteins from the parasitic trypanosomatids62. Emerging protein structural information, combined with more robust validation of drug targets, has the potential to refocus drug discovery efforts more towards target-based approaches, as opposed to just phenotypic approaches.
One of the most striking examples of the use of structural information is cryogenic electron microscopy of the proteasome inhibitors for Leishmania species. The University of Dundee–GlaxoSmithKline54 and Novartis63 groups both obtained high-resolution cryogenic electron microscopy images of their proteasome inhibitors bound to proteasomes from Leishmania tarentolae. Analysis of these structures55 has revealed that these compounds bind at the interface of the β4 and β5 subunits and inhibit the chymotrypsin-like activity. The inhibitors bind close to the key threonine residue in the active site. The inhibitors also bind into a small hydrophobic pocket, which is not found in the human orthologues, explaining the selectivity for the parasite proteasome. These cryogenic electron microscopy structures have the potential to be used for design purposes. Use of structural information has the potential to greatly facilitate medicinal chemistry optimization of compounds and to provide new insights. For example, the structure of the L. donovani ribosome with paromomycin highlighted interactions that could aid in the development of new drugs64.
Key challenges and solutions
There are several key challenges in drug discovery for kinetoplastid diseases, which we discuss below.
There remains a severe lack of robustly validated molecular targets that can be exploited for anti-kinetoplastid drug discovery. This situation is slowly improving, owing to substantial improvements in determining the mechanism of action of phenotypic hits (Box 1). However, it has been shown that substantial numbers of phenotypically active compounds interact with the same small number of molecular targets, such as CYP51 (ref.65), cytochrome b66 and the proteasome54,56. Furthermore, some of these targets will not progress to clinical development, as they are very unlikely to be effective as defined by current target product profiles. Of particular note is CYP51 in Chagas disease, as clinical trials with posaconazole and fosravuconazole revealed very high levels of relapse67,68. This has resulted in very limited drug discovery portfolios, and severely limits the scope for development of combination therapies, as there are too few drugs and candidates. The lack of novel drug targets and the repeated ‘rediscovery’ of known targets is probably due to a combination of the limited chemical space that is being screened, the permissive nature of some active sites66,69 and the set-up of the assays used for screening, which may be particularly sensitized to inhibition of these targets. To address the issue of compounds acting at known targets, it is important to evaluate novel compound series against these targets at an early stage. This is particularly important for undesirable targets such as CYP51, for which an assay has been developed that can be used early on in a screening cascade65. Persister parasites pose an additional challenge, as discovering the likely limited number of suitable targets for these forms will be challenging, owing to the difficulties in isolating persisters, the lack of understanding of their biology and the likelihood that many cellular processes are either of very low turnover or suspended. Evaluation of compound series in washout assays is most likely a way of identifying compounds that are active against persisters, which will need to be followed up by mechanism-of-action studies23.
There are some common issues for all drug discovery programmes, including toxicology, physiologically relevant assays, animal models predictive of human disease and understanding pharmacokinetic–pharmacodynamic issues10. It is important to understand these challenges in the context of anti-kinetoplastid drug discovery, and it is essential to develop compounds that have a very high safety profile. Most of the individuals who have been infected with a kinetoplastid live in either rural or economically deprived areas that lack sophisticated health care facilities and trained staff. In particular, for Chagas disease, where patients may not be experiencing any symptoms and may never become ill, new drugs need to be highly selective and have no or minimal adverse effects. This is very important both from an ethical viewpoint and for patient adherence.
There are several ways to mitigate the risk of potential toxicity. One of the most common risks is inhibition of a human orthologue of the parasite target. However, it is often possible to obtain highly selective inhibitors for the pathogen target compared with the human orthologue, even if there is close similarity between them, as exemplified by the proteasome54,56, CPSF3 (ref.52) or N-myristoyltransferases70,71. Thus, potential drug targets in pathogens causing infectious diseases should not be disregarded simply because they have a human orthologue. However, this selectivity must be monitored from a very early stage in a drug discovery programme. Use of protein structural information on the binding of inhibitors can greatly facilitate development of selective inhibitors, even if there is high similarity between the parasite protein and its human orthologue.
Another cause of adverse effects is off-target effects. Most drugs interact with multiple proteins, and such interactions are difficult to predict, but several mitigation approaches are available, including counter-screens against mammalian cell lines, human profiling panels of enzymes and receptors and in vivo toxicity studies. There are particular assays for common toxicities, such as screening against particular ion channels (for example, human ether à go-go-related gene (hERG; also known as KCNH2)) to test for cardiotoxicity, the Ames assay72,73 to test for potential genotoxicity and assays to test for mitochondrial toxicity. Some chemical functional groups are known to be reactive or potentially toxic. Either these functional groups can be removed from lead compound series or these risks should be assessed early on with appropriate assays. For example, some, but not all, aromatic nitro compounds and anilines are known to be genotoxic. Therefore, if a compound series contains one of these functionalities, it is important to include the Ames assay in a screening cascade from an early stage or try to remove this functionality altogether. Other approaches to predict off-target activity being developed include artificial intelligence, which may be used to highlight risks at earlier stages during the drug discovery process.
A key challenge is to develop cell-based and in vivo models that are predictive of the response in human disease, in particular when the diseases are not fully understood. Many of the more-high-throughput primary assays lack the detailed physiological relevance1,10. Various approaches to improve relevance are being explored: replacement of tissue-culture cell lines with more-relevant complex cell systems (for example, primary human host cells74,75, stem cell-derived host cells76 and explant models74,75); improved assay set-up (many viability assays cannot distinguish between cytostatic and cytocidal compounds as starting pathogen density is below the detection limit, and methods that overcome this have been reported77,78); and alternative assay platforms to detect activity against key rare populations such as persisters (for example, washout outgrowth assays23). Often the more relevant models are challenged by limited throughput and/or long duration. It is therefore necessary to develop appropriate screening cascades that combine high-throughput primary assays with more physiologically relevant secondary assays so that compounds of interest are identified in the most efficient manner (Fig. 4).
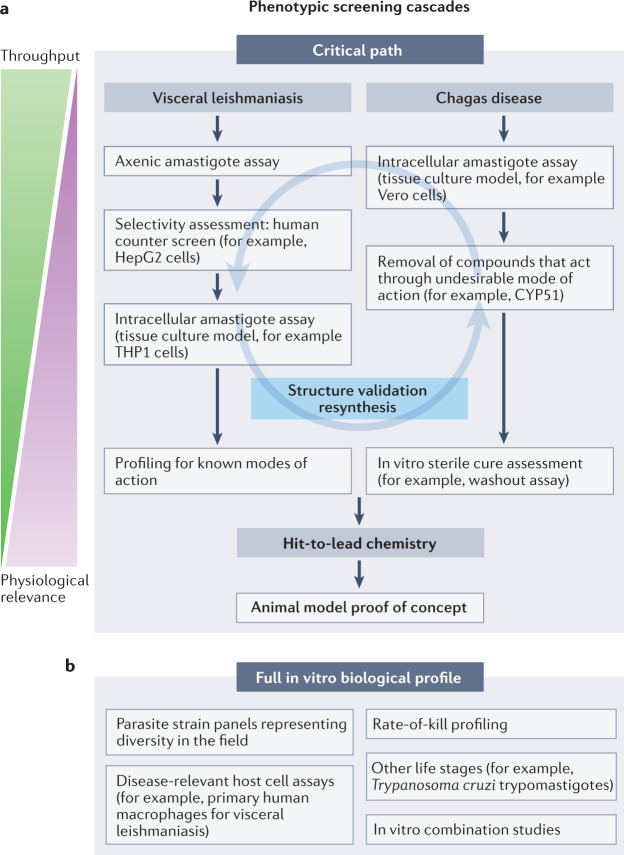
Fig. 4. Representative screening cascades for drugs to treat visceral leishmaniasis and Chagas disease.
a | Key assays for a phenotypic hit discovery programme are shown. For visceral leishmaniasis, the main purpose is to quickly identify compounds that are active in intracellular models, as such compounds have a high chance of demonstrating proof of concept in animal models (provided they have appropriate pharmacokinetics). High-throughput screening for visceral leishmaniasis is typically performed with axenically grown parasites, in particular axenic amastigotes. Initial screening can also be conducted in intracellular models, if high-throughput assays are available, or for smaller compound libraries. Following axenic assays, compounds that are non-selective regarding human cells are removed, and compounds of interest progress to intracellular assays. At this point, any compounds with suitable activity should be validated through structure and purity determination and/or resynthesis. Confirmed active compounds are next subjected to analysis of known modes of action, in particular to identify compounds that have a mode of action that is already being tested in the clinic. For Chagas disease, the cascade needs to quickly remove compounds with undesirable modes of action and identify compounds that can achieve complete cure. Hits are usually identified in high-throughput intracellular systems, as there are no suitable axenic models. Typically, a large fraction of hits act through undesirable modes of action such as CYP51, and screening to remove these is done early in the cascade. Remaining compounds of interest should at this stage be validated for structure and purity. For Chagas disease, it is thought that compounds that can kill all parasites have the highest chance of success in the clinic. To assess ability to achieve sterile cure, compound washout and parasite outgrowth assays are applied. For both diseases, validated hits next progress to hit-to-lead chemistry, with the main aim to achieve proof-of-concept efficacy in a suitable animal model of disease. b | For key compounds in each series, a full biological profile should be determined. This includes determination of potency against multiple relevant strains and host cells, determination of the rate of kill of compounds and profiling against different life stages. In addition, to understand potential for future combination treatments, key series can be profiled in combination experiments.
Animal models of disease do not always replicate the course of infection and disease pathology found in humans. It is important to understand these differences, which will enable the tailoring of models to better predict the efficacy of new compounds in humans. A key case in point is animal models for Chagas disease1 (Box 2). It is important that the learnings from clinical trials of new chemical entities are fed back into the development of both cellular and animal models of disease to ensure that they are relevant to disease. The interpretation of animal models is also important in the context of vaccine development (see later), as there are differences between the animal and the human immune systems (typically the mouse is the pharmacodynamic model), and there may also be differences in the distribution and physiological context of the parasites between animal models and humans.
Related to the development of predictive animal models of infection is understanding pharmacokinetic–pharmacodynamic relationships. Determining what the efficacy drivers are in terms of pharmacokinetics (specifically the blood and tissue compound exposure profile in relation to its in vitro antiparasitic activity) has not proved trivial for visceral leishmaniasis and Chagas disease. Studies have addressed the pharmacokinetics and pharmacodynamics in recent years for visceral leishmaniasis79,80, but for Chagas disease, in particular, there remains poor understanding of the distribution of parasites in the host, which makes developing compounds with appropriate distribution properties challenging. For cutaneous leishmaniasis, variable pharmacokinetic profiles are needed for drug efficacy with local accumulation within a single simple lesion versus high systemic exposure and distribution to all skin sites for diffuse cutaneous leishmaniasis and PKDL. With the need to permeate biological barriers and reach infected dermal macrophages crucial for therapeutic efficacy, more research investigating skin pharmacokinetics in animal models of cutaneous leishmaniasis is needed81–83. With no impact on infectivity and thus pharmacodynamic readout, integrated blood sampling in efficacy studies is actively encouraged to aid our understanding of pharmacokinetics and pharmacodynamics.
Resistance to drugs is a major issue with antimicrobials, hampering treatment of HIV-1 infection, malaria and tuberculosis, and drug resistance also presents a problem to treat kinetoplastid diseases. Resistance has been found in T. b. gambiense to melarsoprol, which was previously used to treat HAT84, mediated via mutation or loss of aquaglyceroporin 2 (refs.85–88) In the case of visceral leishmaniasis, resistance against antimonials has emerged through mutations of aquaglyceroporin 1, again involved in drug uptake89. In the state of Bihar, India, resistance to antimonials has been linked to high levels of arsenicals found in drinking water90. Moreover, resistance against miltefosine (which is used to treat visceral leishmaniasis) possibly emerged through reduced drug uptake91. While there is no room for complacency, and the situation must continue to be monitored, resistance to drugs that are used to treat kinetoplastid diseases is not as severe as with some other infectious diseases. There are several reasons for this: the causative organisms are diploid, the rates of transmission from people being treated to other people are lower (in particular for the zoonotic diseases) and parasite burdens can be low, particularly during chronic infection. The use of combination therapies should further mitigate against the emergence of drug-resistant parasites. Knowledge of the molecular targets of drugs (Box 1) is very important in monitoring resistance emergence as one of the common mechanisms underlying resistance emergence is single-nucleotide polymorphisms affecting the primary target. Monitoring those, if they occur, during or after clinical trials is important for understanding the risks of resistance emergence. It must be noted that many of the currently used drugs for kinetoplastid diseases are reactive, while many of the newer-generation compounds under development have specific enzymatic targets, so it will be important to monitor the clinical development of resistance to these newer drugs in case there is a higher rate of resistance.
Many of the current treatments exhibit low efficacy, long treatment duration and adverse effects. These issues can potentially be overcome through combination treatment, either with current treatments or with new chemical entities. However, developing drug combinations is complex. In vitro combination effects can be assessed at multiple levels (potency, efficacy and rate-of-kill interactions), but it is not always clear whether in vitro experiments translate well to the in vivo situation. In vivo it is thought to be important to match the partners’ pharmacokinetics, to maintain equivalent drug exposure and avoid treatment failure. This is difficult to achieve owing to the multifactorial nature of each compound’s pharmacokinetics, and this is challenging to model, owing to differences in pharmacokinetics across species. Furthermore, the limited number of validated drug targets and the small number of compounds going into clinical development limits the choice of potential combination partners. Nevertheless, combination therapies have been successfully developed in multiple infectious disease areas, such as HAT, malaria and tuberculosis.
Parasitic diseases cause asymptomatic cases, which has multiple implications for the long-term health of patients, disease elimination due to reservoirs of infection, diagnostics, how asymptomatic cases are detected or diagnosed, and for treatment, with what and how. Parasites have a range of mechanisms by which they evade the human immune system, enabling them to establish chronic infections, some of which are asymptomatic92.
HAT caused by T. b. gambiense can manifest itself as long periods of latency before symptoms develop. However, more recently a subpopulation of seropositive patients with no detectable parasitaemia has been reported; at least some individuals in this population seem to not develop clinical symptoms over an extended period, or to clear infection93,94. It has been hypothesized that parasites in these patients reside in the skin37,94. The impact of asymptomatic cases as an infection reservoir is not fully understood, but they are thought to contribute to transmission92,93. Detecting and treating this asymptomatic population is likely to be important in elimination of disease. The recent development of safer treatment options for HAT means that this may now be feasible, although a practical means to identify these asymptomatic patients is required.
In general, most people infected with L. donovani or L. infantum (which cause visceral leishmaniasis) are asymptomatic, and only some infected individuals develop clinical disease. Whether a patient remains asymptomatic or develops active visceral leishmaniasis depends on multiple factors. Diagnosis of asymptomatic visceral leishmaniasis is not always straightforward, as patients can be seronegative, and PCR results can be problematic owing to very low levels of parasites in the blood. However, there is a cellular stimulation assay, followed by detection of chemokines and cytokines, that seems to be sensitive in these cases. The importance of asymptomatic patients as a reservoir of infection and the levels of transmission from asymptomatic patients are poorly understood92.
Chagas disease is characterized by prolonged periods of asymptomatic infection. The initial acute phase either has mild, nonspecific symptoms or is asymptomatic and is followed by an often long asymptomatic chronic phase (the indeterminate phase), with very low levels of parasites circulating in the bloodstream. Only a subset of patients in the indeterminate phase will develop symptomatic disease, at which point it may be too late to treat them with antiparasitic agents21. Key issues associated with asymptomatic Chagas disease include the following: patients are often not identified until disease has advanced too far; treatment adherence can be poor as patients do not feel ill, in particular when treatments are long and have side effects; and demonstrating cure in clinical trials is difficult as the inability to detect parasites in the bloodstream is not evidence of cure.
Animal reservoirs of infection are not fully understood, but probably have an underestimated impact on these diseases. HAT caused by T. b. rhodesiense is a zoonosis, with the main reservoir being both wild and domesticated animals and livestock, but the extent to which the animal reservoir contributes to human infections is unclear95,96. A recent review indicated that multiple mammalian species can be infected with Leishmania spp. and act as a notable reservoir of infection97. Visceral leishmaniasis caused by L. infantum is thought to be primarily a zoonotic disease, with the main reservoir being dogs98. L. donovani has been found in various agricultural animals and dogs on the Indian subcontinent99. Cutaneous leishmaniasis is caused by different species and subspecies of Leishmania, which have a variety of different reservoirs, including rodents, sloths and armadillos98. In the case of T. cruzi, infection is widespread among domestic and wild animals100,101. T. cruzi, as well as a number of other trypanosomes, has also been found in bats102. In cases where the main or a substantial reservoir is in animal populations, eradication as a strategy to tackle disease will not be possible.
Another critical challenge is the clinical development of both new chemical entities and new combinations or formulations of existing drugs. It is challenging to identify sites that are suitable for clinical trials and then to set them up with appropriate facilities and personnel, as many of the patients live in remote rural areas. Some of these areas are also in politically unstable places or suffer from conflict. There is also the question of funding these trials, as there are no strong economic drivers to develop new medicines for these diseases. Clinical trials are costly and require sustained funding. All of these factors mean that there is a limit to the number of compounds that can progress through to the clinical development stage, even when promising molecules are identified. Furthermore, as in all disease areas, there will be a high attrition rate during clinical trials, leading to disappointment in many cases. It is estimated that for anti-infectives only 19–25% of compounds that are advanced into clinical trials progress through to registration103,104. Therefore, a much larger number of compounds need to be advanced into clinical trials than are required, particularly considering the aim of developing combination therapies. Fortunately, there are some initiatives in place to support the clinical development process; in particular, DNDi is very active in coordinating and supporting clinical development of compounds as are the European and Developing Countries Clinical Trials Partnership and the Global Health Innovative Technology Fund. However, it is imperative that clinical trial sites and funding are in place to enable clinical development of much needed new therapies for these diseases.
Box 2 Animal models.
Animal models of infection are important in the selection of molecules to progress to human clinical trials. It is important to develop animal models that are predictive of efficacy in humans. An example for the importance of this has been the failure of the CYP51 inhibitors posaconazole and ravuconazole (the E1224 prodrug) to clear parasites as effectively as benznidazole in clinical trials for treatment of Chagas disease67,68,132.This has driven the establishment of mouse models of disease (both acute and chronic) that can distinguish between posaconazole-type compounds, for which there is a very high level of relapse in humans, and benznidazole, which has clinical efficacy133.
In human African trypanosomiasis, it is important to be able to treat stage 2 infection, when the parasite has invaded the central nervous system. The classic model uses GVR35 mice assessed over 180 days134,135. The length of this assessment has a very detrimental effect on timelines for drug discovery. Recent bioluminescent imaging procedures have reduced this to a 90-day experiment and have also reduced the number of animals required136.
Both mice and hamsters are used as animal models of disease for visceral leishmaniasis. The Syrian golden hamster is highly susceptible to infection with visceralizing Leishmania species (Leishmania donovani and Leishmania infantum) and is considered the best experimental model to study visceral leishmaniasis because it reproduces the clinicopathological features of human disease137. However, because it is challenging to sample blood from hamsters for integrated assessment of pharmacokinetics and pharmacodynamics, mouse models are generally preferred as a disease model in a drug discovery setting. In mice, during the first few weeks of infection, the parasites multiply rapidly in the liver; however, 4 weeks later, the mice develop an effective immune response, clear the liver parasites and become resistant to reinfection. Although pathology in the liver is limited, the parasites persist in the spleen, and the infection progresses for a longer period. Drug treatment in mice therefore usually assesses the ability of the compound to clear liver parasites only, and not those in the spleen or bone marrow as this takes several weeks to develop to a robustly detectable level. As compound levels may vary between different tissues, there is thus a risk of poor translation of pharmacokinetics and pharmacodynamics from mice to humans. The development of a bioluminescent mouse model of visceral leishmaniasis for drug screening has overcome this potential weakness in drug efficacy assessment in mice, enabling investigation of drug efficacy for liver, spleen and bone marrow parasites within 2 weeks of infection138. A bioluminescent mouse model for systematic screening of vaccines for visceral leishmaniasis is also now available139.
In vivo models of cutaneous leishmaniasis for the evaluation of drug efficacy are mainly mouse based with the Leishmania major BALB/c mouse model, which is the most commonly used140. Skin lesions develop rapidly (within 2–4 weeks), an advantage for drug screening. Because humans often self-cure cutaneous leishmaniasis, whereas BALB/c mice are incapable of self-curing, lack of clinical relevance is the main limitation of the model. Alternative rodent models such as C57/bl6 mice or golden hamsters do self-cure, but disease onset is much slower. Drug efficacy is determined by reduction in lesion size compared with that in vehicle-dosed controls. With inflammation being an important factor in lesion size and a confounding factor for drug efficacy, quantification of parasite load is also considered for therapeutic effect.
For Chagas disease, a number of different animal models have been developed (reviewed in ref.141). Mouse models that use bioluminescence to monitor the infection142 are widely used (Fig. 2). The advantage of bioluminescence is that it can be used to monitor where the infection occurs longitudinally and also identifies only live parasites. Following treatment, the mice are left for some time to see if relapse occurs, and then this is followed by several rounds of immunosuppression to allow replication of small numbers of any remaining parasites. This could potentially include ‘persister’ cells, although this needs experimental confirmation. It is also possible to perform ex vivo imaging on individual tissues, which is effective in pinpointing reservoirs of infection. Researchers developed both an acute model and a chronic model. In the acute stage of infection, there is a very high level of infection, with parasites found throughout the body, reaching a maximum at around 14 days. The innate immune system then reduces the level of infection, and in the chronic phase (reached at around 50–60 days), parasites are found mainly in the colon, skin and stomach, although some parasites are also found in other tissues, and this is also dependent on the species of mouse used. In general, lower doses of clinical compounds such as benznidazole are required to achieve efficacy in the chronic model compared with the acute model, which could be a reflection of the lower parasite burden. These models are effective in distinguishing between compounds known to be clinically active, such as benznidazole, and those for which relapse in the clinic is an issue, such as posaconazole.
One of the arguments against using mice as a Chagas disease model in drug discovery is the differing immune response in mice versus humans towards Trypanosoma cruzi infection. Recently, a mouse model of Chagas disease was developed, which was genetically modified to remove α-1,3-galactosyltransferase (α-GalT)143. Mice naturally express the α-Gal epitope and therefore do not produce anti-α-Gal antibodies, such that the anti-α-Gal immune response, a critical factor for protection against T. cruzi infection in humans, is absent. Infection of these mice with T. cruzi led to an increased immune response in the heart tissue, and thus offers an interesting possibility for testing novel effective therapeutics at different stages of infection to explore the optimal window for when to commence treatment to reduce or prevent cardiac damage.
Although animal models of infectious disease prove very valuable in drug discovery, there are key differences between animal models and human infection. These include differences in disease pathology and immune response to pathogens among species and differences in pharmacokinetics of compounds in different species. However, genetically modified mice are becoming important tools in understanding and addressing these differences and to aid selection of compounds most likely to have clinical efficacy in humans. For progress of compounds to any of these mouse models of infectious disease, a level of drug optimization for a mouse is required, so that the pharmacokinetic characteristics in mice will deliver the desired efficacious outcome following dosing, be that oral or parenterally for proof of concept. To overcome this and also potentially to provide better translational pharmacokinetics and pharmacodynamics, there is a strong argument for better exploring the use of recently developed mouse models, humanized for key drug-metabolizing enzymes, as disease models for any of the kinetoplastid diseases. Provided the humanized mouse model infection characteristics are consistent with those in current wild type mouse models, humanized models in which the mouse cytochromes P450 and promotors have been removed and replaced with human cytochromes P450 and promotors offer potential substantial advantages over the current models, especially in terms of circumventing differences in drug metabolism144. Various knockout mice are also being used to understand the effect of various components of the immune system on disease pathology and progression145,146.
Other approaches
Vaccines
Development of vaccines for kinetoplastid infections has proven very challenging. T. brucei subspp. have a variable surface glycoprotein that constantly changes, meaning the human immune system is constantly ‘catching up’, and T. cruzi and Leishmania spp. are predominantly intracellular parasites, remaining mostly hidden from the human immune system.
Recently, a potential vaccine antigen that induces protective immunity against the animal pathogen Trypanosoma vivax was identified. The study authors selected potential candidate proteins, predicted to be exposed at the cell surface or secreted, using a genetic approach. Following expression of 39 potential antigens that were used to inoculate mice before challenge with T. vivax, they identified IFX, which is found at the flagellum. The IFX-based vaccine was shown to provide protective immunity in a mouse model of T. vivax infection105. Unfortunately, when this vaccine was evaluated in a goat (a natural host of T. vivax) infected with T. vivax, there was no protection against infection, despite production of high levels of antibody to IFX106. Therefore, there seem to be other factors that are important in the immune response to T. vivax in goats. This illustrates the difficulties in extrapolating data from one animal species to another, particularly when developing vaccines, as subtle differences between immune systems can have important consequences that affect vaccine efficacy.
Although there are knowledge gaps that must be addressed before effective Leishmania spp. vaccines are provided for humans or for companion animals, there have been notable examples of recent progress in this area107. Prophylactic vaccines, therapeutic vaccines and vaccines for use in combination with host-directed immunochemotherapy are all under active development. For example, the ChAd63-KH (KMP-11/HASPB) adenovirus-based vaccine, for use against visceral leishmaniasis and PKDL, proved to be safe and to elicit a robust CD8+ T cell response in healthy volunteers in a phase I trial108. An effective, yet historically less safe, approach involves ‘leishmanization’; that is, vaccination with live Leishmania major. A safer alternative to leishmanization could be achieved using live but genetically attenuated Leishmania spp. Indeed, a recent study using a CRISPR–Cas9-edited L. major vaccine shows promise in experimental models109 and suggests that these attenuated, transgene-free vaccines can now progress to human trials. There are additional challenges associated with delivering a live attenuated vaccine in resource-poor settings. More-sophisticated vaccine delivery systems are also under development, using, for example, dissolvable microneedle skin patches that combined with the LiHyp1 vaccine induced protective immunity in BALB/c mice110. The LetiFend vaccine, which is based on recombinant protein Q and used against canine leishmaniasis caused by L. infantum, was also shown to be safe and effective, reducing confirmed cases by 72% in dogs111.
There has been a rapid recent development of vaccine technology, such as mRNA vaccines; it is unknown whether any of this may have applicability to kinetoplastids. Controlled human infection models have been very powerful in accelerating clinical trials, notably in malaria. Recent work has characterized a potential challenge agent, a clinical L. major strain, which may facilitate the development of a human challenge model of cutaneous leishmaniasis112. If this strain is successfully translated from mice into humans, it has the potential to be useful for vaccine development and possibly drug development. However, such models are not likely to be viable for the other kinetoplastid diseases, owing to the uncertainty in rescue treatment for these lethal diseases.
Host-directed therapies
The interactions between the host and the parasite are complex — the kinetoplastid parasites evade the human immune system, while also obtaining sufficient nutrients for growth and replication, and also for onward transmission to other hosts. There is growing interest in developing agents that target host–pathogen interactions in infectious diseases113–116. Tackling these host–pathogen interactions is still in its infancy, as understanding of these interactions remains limited. There is also the risk that targeting the host (for example, modulating the immune or inflammatory systems) could have unintended consequences, such as increasing the vulnerability of the host to infection by the parasite in question or other pathogens. However, there is potential in this area, likely to be in combination with compounds acting directly on the parasite. Furthermore, it is thought that compounds that target host–parasite interactions are less likely to lead to the emergence of drug resistance113,116.
Various methods are being used to identify host targets for treating infectious diseases, including transcriptomic and proteomic analysis of host cells as well as gain-of-function and loss-of-function studies using cDNA or small interfering RNA, respectively114. One approach to target the host involves the modulation of the immune response through interfering with signalling pathways (for example, kinase inhibitors or G protein-coupled receptors) that affect processes such as activation of NF-κB (a transcription factor that stimulates the immune response) or cytokine signalling (interleukins, interferons or TNF). Many of the approaches for disrupting host–pathogen interactions are expensive (for example, relying on antibodies and recombinant proteins) and outside the target product profiles for kinetoplastid diseases, although some approaches may be more feasible, such as nutritional products or repurposing of commonly used small-molecule drugs for non-communicable diseases. One particularly promising immune stimulator is CpG oligonucleotide D35, which increases the efficacy of short-course, low-dose antimony treatment in a macaque model of cutaneous leishmaniasis117 and may also function as an anti-leishmanial vaccine adjuvant110.CpG oligonucleotide D35 progressed to a phase I clinical trial in 2021 (ref.118). Immunomodulators may also be combined with chemotherapy to tackle cutaneous leishmaniasis. For example, treatment with antimony was associated with an increased, albeit not statistically significant, cure rate when combined with use of a TLR7 agonist in a clinical trial in Peru119.
Leishmania spp. and T. cruzi are predominantly intracellular parasites. In some cases the immune system may contribute to pathology, and controlling the immune response may be beneficial. In the case of Chagas disease, the damage to the heart and colon is thought to result from an inflammatory response of the host, so approaches to dampen this response may have a role in treatment during chronic infection. Similarly, overactivation of the immune system (through overproduction of TNF) is thought to be responsible for the extensive tissue damage seen in mucocutaneous leishmaniasis113. T. brucei subspp. are extracellular pathogens but have a variable surface glycoprotein that constantly changes. At initial stages of infection, stimulation of the immune system may be suitable, but during later stages, when the parasites have infected the CNS, inflammation causes damage, and hence anti-inflammatory treatment may be appropriate113.
Conclusions
Over the past few years substantial progress has been made in understanding kinetoplastid disease biology. This knowledge has enabled the development of both in vitro assays and in vivo assays that can identify compounds with realistic potential to treat kinetoplastid diseases in humans. However, there is still much to learn about these complex parasites and the diseases that they cause, especially in regard to T. cruzi, the causative agent of Chagas disease. Parasite persistence and tissue distribution perhaps represent the biggest challenges to successfully treat Chagas disease. In the past, these factors were not considered, and thus there has been little or no progress in filling the drug pipeline for Chagas disease. It remains to be seen how our increasing understanding of parasite and disease biology will affect drug discovery for Chagas disease, but the knowledge gained to date is certainly having an impact on the approaches being used for discovery.
Despite concerted efforts to determine the mechanism of action of phenotypically active compounds, there are still few robustly validated molecular targets in kinetoplastid parasites, with a small subset of known targets identified repeatedly through cell-based screening. Thus, there is a pressing need for novel, exploitable drug targets to be identified. Great progress has been made in developing drugs and identifying new drug candidates for HAT. In the case of visceral leishmaniasis, it is encouraging to see a number of new chemical entities entering phase I studies. However, there is no room for complacency, bearing in mind the high attrition rate of compounds in clinical development owing to issues including efficacy and toxicity. In the case of cutaneous leishmaniasis, mucocutaneous leishmaniasis, PKDL and Chagas disease, drug discovery pipelines are sparse and there remains an urgent need for new drugs. Alternative approaches, such as interfering with host–pathogen interactions and vaccines, are also being explored. Outstanding issues that will need to be addressed to ensure the long-term control and ultimate eradication of kinetoplastid diseases include identification and treatment of asymptomatic patients. Therefore, much work remains to fully understand kinetoplastid diseases and to develop effective drugs to treat these infections.
Acknowledgements
The authors acknowledge the support of Wellcome for their work in kinetoplastid biology and drug discovery projects over many years (grant numbers 100476, 105021, 092340, 203134, 204672, 218448 and 223608).
Author contributions
The authors contributed equally to all aspects of the article.
Peer review
Peer review information
Nature Reviews Microbiology thanks Harry de Koning; Jeremy Mottram, who co-reviewed with Nathaniel Jones; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
AlphaFold: https://alphafold.ebi.ac.uk
Drugs for Neglected Diseases initiative: https://dndi.org/research-development
European and Developing Countries Clinical Trials Partnership: https://www.edctp.org
Global Burden of Disease study: http://ghdx.healthdata.org/gbd-results-tool
Global Health Innovative Technology Fund: https://www.ghitfund.org
Change history
9/13/2022
A Correction to this paper has been published: 10.1038/s41579-022-00797-8
References
- 1.Field MC, et al. Anti-trypanosomatid drug discovery: an ongoing challenge and a continuing need. Nat. Rev. Microbiol. 2017;15:217–231. doi: 10.1038/nrmicro.2016.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ponte-Sucre A. An overview of Trypanosoma brucei infections: an intense host-parasite interaction. Front. Microbiol. 2016;7:2126–2126. doi: 10.3389/fmicb.2016.02126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Büscher P, Cecchi G, Jamonneau V, Priotto G. Human African trypanosomiasis. Lancet. 2017;390:2397–2409. doi: 10.1016/S0140-6736(17)31510-6. [DOI] [PubMed] [Google Scholar]
- 4.Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet. 2018;392:951–970. doi: 10.1016/S0140-6736(18)31204-2. [DOI] [PubMed] [Google Scholar]
- 5.Echeverria LE, Morillo CA. American trypanosomiasis (Chagas disease) Infect. Dis. Clin. North. Am. 2019;33:119–134. doi: 10.1016/j.idc.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Pérez-Molina JA, Molina I. Chagas disease. Lancet. 2018;391:82–94. doi: 10.1016/S0140-6736(17)31612-4. [DOI] [PubMed] [Google Scholar]
- 7.Bonney KM, Luthringer DJ, Kim SA, Garg NJ, Engman DM. Pathology and pathogenesis of Chagas heart disease. Annu. Rev. Pathol. 2019;14:421–447. doi: 10.1146/annurev-pathol-020117-043711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kande Betu Ku Mesu V, et al. Oral fexinidazole for stage 1 or early stage 2 African Trypanosoma brucei gambiense trypanosomiasis: a prospective, multicentre, open-label, cohort study. Lancet Glob. Health. 2021;9:e999–e1008. doi: 10.1016/S2214-109X(21)00208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickie EA, et al. New drugs for human African trypanosomiasis: a twenty first century success story. Trop. Med. Infect. Dis. 2020;5:29. doi: 10.3390/tropicalmed5010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Rycker M, Baragana B, Duce SL, Gilbert IH. Challenges and recent progress in drug discovery for tropical diseases. Nature. 2018;559:498–506. doi: 10.1038/s41586-018-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021–2030 (WHO, 2020).
- 12.Sanchez-Valdez FJ, Padilla A, Wang W, Orr D, Tarleton RL. Spontaneous dormancy protects Trypanosoma cruzi during extended drug exposure. eLife. 2018;7:e34039. doi: 10.7554/eLife.34039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrett MP, Kyle DE, Sibley LD, Radke JB, Tarleton RL. Protozoan persister-like cells and drug treatment failure. Nat. Rev. Microbiol. 2019;17:607–620. doi: 10.1038/s41579-019-0238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandell MA, Beverley SM. Continual renewal and replication of persistent Leishmania major parasites in concomitantly immune hosts. Proc. Natl Acad. Sci. USA. 2017;114:E801–E810. doi: 10.1073/pnas.1619265114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bigger JW. Treatment of staphylococcal infections with penicillin by intermittent sterilisation. Lancet. 1944;244:497–500. doi: 10.1016/S0140-6736(00)74210-3. [DOI] [Google Scholar]
- 16.Fisher RA, Gollan B, Helaine S. Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 2017;15:453–464. doi: 10.1038/nrmicro.2017.42. [DOI] [PubMed] [Google Scholar]
- 17.Balaban NQ, et al. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Microbiol. 2019;17:441–448. doi: 10.1038/s41579-019-0196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voorberg-van der Wel A, Kocken CHM, Zeeman AM. Modeling relapsing malaria: emerging technologies to study parasite-host interactions in the liver. Front. Cell Infect. Microbiol. 2020;10:606033. doi: 10.3389/fcimb.2020.606033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerutti A, Blanchard N, Besteiro S. The bradyzoite: a key developmental stage for the persistence and pathogenesis of toxoplasmosis. Pathogens. 2020;9:234. doi: 10.3390/pathogens9030234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ward AI, Olmo F, Atherton RL, Taylor MC, Kelly JM. Trypanosoma cruzi amastigotes that persist in the colon during chronic stage murine infections have a reduced replication rate. Open Biol. 2020;10:200261. doi: 10.1098/rsob.200261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morillo CA, et al. Randomized trial of benznidazole for chronic Chagas’ cardiomyopathy. N. Engl. J. Med. 2015;373:1295–1306. doi: 10.1056/NEJMoa1507574. [DOI] [PubMed] [Google Scholar]
- 22.Díaz-Bello Z, et al. Ten-year follow-up of the largest oral Chagas disease outbreak. Laboratory biomarkers of infection as indicators of therapeutic failure. Acta Trop. 2021;222:106034. doi: 10.1016/j.actatropica.2021.106034. [DOI] [PubMed] [Google Scholar]
- 23.MacLean LM, et al. Development of Trypanosoma cruzi in vitro assays to identify compounds suitable for progression in Chagas’ disease drug discovery. PLoS Negl. Trop. Dis. 2018;12:e0006612. doi: 10.1371/journal.pntd.0006612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward AI, et al. In vivo analysis of Trypanosoma cruzi persistence foci at single-cell resolution. mBio. 2020;11:e01242-20. doi: 10.1128/mBio.01242-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dumoulin PC, Burleigh BA. Stress-induced proliferation and cell cycle plasticity of intracellular Trypanosoma cruzi amastigotes. mBio. 2018;9:e00673-18. doi: 10.1128/mBio.00673-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Álvarez MG, et al. New scheme of intermittent benznidazole administration in patients chronically infected with Trypanosoma cruzi: clinical, parasitological, and serological assessment after three years of follow-up. Antimicrob. Agents Chemother. 2020;64:e00439-20. doi: 10.1128/AAC.00439-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bustamante JM, et al. A modified drug regimen clears active and dormant trypanosomes in mouse models of Chagas disease. Sci. Transl Med. 2020;12:eabb7656. doi: 10.1126/scitranslmed.abb7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandal S, Njikan S, Kumar A, Early JV, Parish T. The relevance of persisters in tuberculosis drug discovery. Microbiology. 2019;165:492–499. doi: 10.1099/mic.0.000760. [DOI] [PubMed] [Google Scholar]
- 29.Goyal V, et al. Long-term incidence of relapse and post-kala-azar dermal leishmaniasis after three different visceral leishmaniasis treatment regimens in Bihar, India. PLoS Negl. Trop. Dis. 2020;14:e0008429. doi: 10.1371/journal.pntd.0008429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gitari JW, et al. Leishmaniasis recidivans by Leishmania tropica in Central Rift Valley region in Kenya. Int. J. Infect. Dis. 2018;74:109–116. doi: 10.1016/j.ijid.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Kloehn J, Saunders EC, O’Callaghan S, Dagley MJ, McConville MJ. Characterization of metabolically quiescent Leishmania parasites in murine lesions using heavy water labeling. PLoS Pathog. 2015;11:e1004683. doi: 10.1371/journal.ppat.1004683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tegazzini D, et al. A replicative in vitro assay for drug discovery against Leishmania donovani. Antimicrob. Agents Chemother. 2016;60:3524–3532. doi: 10.1128/AAC.01781-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolbach SB, Binger CAL. A contribution to the parasitology of trypanosomiasis. J. Med. Res. 1912;27:83–107. [PMC free article] [PubMed] [Google Scholar]
- 34.Peruzzi, M. in Final Report of the League of Nations International Commission on Human Trypanosomiasis 245–328 (League of Nations International Commission, 1928).
- 35.Ikede BO, Losos GJ. Pathology of the disease in sheep produced experimentally by Trypanosoma brucei. Vet. Pathol. 1972;9:278–289. doi: 10.1177/030098587200900408. [DOI] [PubMed] [Google Scholar]
- 36.Trindade S, et al. Trypanosoma brucei parasites occupy and functionally adapt to the adipose tissue in mice. Cell Host Microbe. 2016;19:837–848. doi: 10.1016/j.chom.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Capewell P, et al. The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes. eLife. 2016;5:e17716. doi: 10.7554/eLife.17716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Camara M, et al. Extravascular dermal trypanosomes in suspected and confirmed cases of gambiense human African trypanosomiasis. Clin. Infect. Dis. 2021;73:12–20. doi: 10.1093/cid/ciaa897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crilly NP, Mugnier MR. Thinking outside the blood: perspectives on tissue-resident Trypanosoma brucei. PLoS Pathog. 2021;17:e1009866. doi: 10.1371/journal.ppat.1009866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Niz M, et al. Organotypic endothelial adhesion molecules are key for Trypanosoma brucei tropism and virulence. Cell Rep. 2021;36:109741. doi: 10.1016/j.celrep.2021.109741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Girard A, et al. Raman spectroscopic analysis of skin as a diagnostic tool for human African trypanosomiasis. PLoS Pathog. 2021;17:e1010060. doi: 10.1371/journal.ppat.1010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reithinger R, et al. Cutaneous leishmaniasis. Lancet Infect. Dis. 2007;7:581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 43.Caridha D, et al. Route map for the discovery and pre-clinical development of new drugs and treatments for cutaneous leishmaniasis. Int. J. Parasitol. Drugs Drug Resist. 2019;11:106–117. doi: 10.1016/j.ijpddr.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steverding D. The history of leishmaniasis. Parasit. Vectors. 2017;10:82. doi: 10.1186/s13071-017-2028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meleney HE. The histopathology of kala-azar in the hamster, monkey and man. Am. J. Pathol. 1925;1:147–168. [PMC free article] [PubMed] [Google Scholar]
- 46.Domínguez-Asenjo B, et al. Bioluminescent imaging identifies thymus, as overlooked colonized organ, in a chronic model of Leishmania donovani mouse visceral leishmaniasis. ACS Infect. Dis. 2021;7:871–883. doi: 10.1021/acsinfecdis.0c00864. [DOI] [PubMed] [Google Scholar]
- 47.Feilij H, Muller L, Cappa SMG. Direct micromethod for diagnosis of acute and congenital Chagas’ disease. J. Clin. Microbiol. 1983;18:327–330. doi: 10.1128/jcm.18.2.327-330.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silva AE, et al. Acute Chagas’ disease in postrenal transplant and treatment with benzonidazole. Ann. Diagn. Pathol. 2010;14:199–203. doi: 10.1016/j.anndiagpath.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 49.Lewis MD, Kelly JM. Putting infection dynamics at the heart of Chagas disease. Trends Parasitol. 2016;32:899–911. doi: 10.1016/j.pt.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewis MD, et al. Bioluminescence imaging of chronic Trypanosoma cruzi infections reveals tissue-specific parasite dynamics and heart disease in the absence of locally persistent infection. Cell. Microbiol. 2014;16:1285–1300. doi: 10.1111/cmi.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wittlin S, Mäser P. From magic bullet to magic bomb: reductive bioactivation of antiparasitic agents. ACS Infect. Dis. 2021;7:2777–2786. doi: 10.1021/acsinfecdis.1c00118. [DOI] [PubMed] [Google Scholar]
- 52.Wall RJ, et al. Clinical and veterinary trypanocidal benzoxaboroles target CPSF3. Proc. Natl Acad. Sci. USA. 2018;115:9616–9621. doi: 10.1073/pnas.1807915115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wyllie S, et al. Cyclin-dependent kinase 12 is a drug target for visceral leishmaniasis. Nature. 2018;560:192–197. doi: 10.1038/s41586-018-0356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wyllie S, et al. Preclinical candidate for the treatment of visceral leishmaniasis that acts through proteasome inhibition. Proc. Natl Acad. Sci. USA. 2019;116:9318–9323. doi: 10.1073/pnas.1820175116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas M, et al. Scaffold-hopping strategy on a series of proteasome inhibitors led to a preclinical candidate for the treatment of visceral leishmaniasis. J. Med. Chem. 2021;64:5905–5930. doi: 10.1021/acs.jmedchem.1c00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khare S, et al. Proteasome inhibition for treatment of leishmaniasis, Chagas disease and sleeping sickness. Nature. 2016;537:229–233. doi: 10.1038/nature19339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mowbray CE, et al. DNDI-6148: a novel benzoxaborole preclinical candidate for the treatment of visceral leishmaniasis. J. Med. Chem. 2021;64:16159–16176. doi: 10.1021/acs.jmedchem.1c01437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van den Kerkhof M, et al. In vitro and in vivo pharmacodynamics of three novel antileishmanial lead series. Int. J. Parasitol. Drugs Drug Resist. 2018;8:81–86. doi: 10.1016/j.ijpddr.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wijnant GJ, et al. Pharmacokinetics and pharmacodynamics of the nitroimidazole DNDI-0690 in mouse models of cutaneous leishmaniasis. Antimicrob. Agents Chemother. 2019;63:e00829-19. doi: 10.1128/AAC.00829-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Torrico F, et al. New regimens of benznidazole monotherapy and in combination with fosravuconazole for treatment of Chagas disease (BENDITA): a phase 2, double-blind, randomised trial. Lancet Infect. Dis. 2021;21:1129–1140. doi: 10.1016/S1473-3099(20)30844-6. [DOI] [PubMed] [Google Scholar]
- 61.Molina-Morant D, et al. Efficacy and safety assessment of different dosage of benznidazol for the treatment of Chagas disease in chronic phase in adults (MULTIBENZ study): study protocol for a multicenter randomized phase II non-inferiority clinical trial. Trials. 2020;21:328. doi: 10.1186/s13063-020-4226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jumper J, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nagle A, et al. Discovery and characterization of clinical candidate LXE408 as a kinetoplastid-selective proteasome inhibitor for the treatment of leishmaniases. J. Med. Chem. 2020;63:10773–10781. doi: 10.1021/acs.jmedchem.0c00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shalev-Benami M, et al. Atomic resolution snapshot of Leishmania ribosome inhibition by the aminoglycoside paromomycin. Nat. Commun. 2017;8:1589. doi: 10.1038/s41467-017-01664-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Riley J, et al. Development of a fluorescence-based Trypanosoma cruzi CYP51 inhibition assay for effective compound triaging in drug discovery programmes for Chagas disease. PLoS Negl. Trop. Dis. 2015;9:e0004014. doi: 10.1371/journal.pntd.0004014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wall RJ, et al. The Qi site of cytochrome b is a promiscuous drug target in Trypanosoma cruzi and Leishmania donovani. ACS Infect. Dis. 2020;6:515–528. doi: 10.1021/acsinfecdis.9b00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Molina I, et al. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N. Engl. J. Med. 2014;370:1899–1908. doi: 10.1056/NEJMoa1313122. [DOI] [PubMed] [Google Scholar]
- 68.Torrico F, et al. Treatment of adult chronic indeterminate Chagas disease with benznidazole and three E1224 dosing regimens: a proof-of-concept, randomised, placebo-controlled trial. Lancet Infect. Dis. 2018;18:419–430. doi: 10.1016/S1473-3099(17)30538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gunatilleke SS, et al. Diverse inhibitor chemotypes targeting Trypanosoma cruzi CYP51. PLoS Negl. Trop. Dis. 2012;6:e1736. doi: 10.1371/journal.pntd.0001736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Corpas-Lopez V, et al. Pharmacological validation of N-myristoyltransferase as a drug target in Leishmania donovani. ACS Infect. Dis. 2019;5:111–122. doi: 10.1021/acsinfecdis.8b00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brand S, et al. Discovery of a novel class of orally active trypanocidal N-myristoyltransferase inhibitors. J. Med. Chem. 2012;55:140–152. doi: 10.1021/jm201091t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ames BN, Lee FD, Durston WE. An improved bacterial test system for the detection and classification of mutagens and carcinogens. Proc. Natl Acad. Sci. USA. 1973;70:782–786. doi: 10.1073/pnas.70.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ames BN, McCann J, Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat. Res. 1975;31:347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- 74.Peniche AG, et al. Development of an ex vivo lymph node explant model for identification of novel molecules active against Leishmania major. Antimicrob. Agents Chemother. 2014;58:78–87. doi: 10.1128/AAC.00887-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Domínguez-Asenjo B, et al. Ex vivo phenotypic screening of two small repurposing drug collections identifies nifuratel as a potential new treatment against visceral and cutaneous leishmaniasis. ACS Infect. Dis. 2021;7:2390–2401. doi: 10.1021/acsinfecdis.1c00139. [DOI] [PubMed] [Google Scholar]
- 76.da Silva Lara L, et al. Trypanosoma cruzi infection of human induced pluripotent stem cell-derived cardiomyocytes: an in vitro model for drug screening for Chagas disease. Microbes Infect. 2018;20:312–316. doi: 10.1016/j.micinf.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 77.De Rycker M, et al. A static-cidal assay for Trypanosoma brucei to aid hit prioritisation for progression into drug discovery programmes. PLoS Negl. Trop. Dis. 2012;6:e1932. doi: 10.1371/journal.pntd.0001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nuhs A, et al. Development and validation of a novel Leishmania donovani screening cascade for high-throughput screening using a novel axenic assay with high predictivity of leishmanicidal intracellular activity. PLoS Negl. Trop. Dis. 2015;9:e0004094. doi: 10.1371/journal.pntd.0004094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiménez-Antón MD, et al. Pharmacokinetics and disposition of miltefosine in healthy mice and hamsters experimentally infected with Leishmania infantum. Eur. J. Pharm. Sci. 2018;121:281–286. doi: 10.1016/j.ejps.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 80.Voak AA, Harris A, Qaiser Z, Croft SL, Seifert K. Pharmacodynamics and biodistribution of single-dose liposomal amphotericin B at different stages of experimental visceral leishmaniasis. Antimicrob. Agents Chemother. 2017;61:e00497-17. doi: 10.1128/AAC.00497-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van Bocxlaer K, et al. Topical treatment for cutaneous leishmaniasis: dermato-pharmacokinetic lead optimization of benzoxaboroles. Antimicrob. Agents Chemother. 2018;62:e02419-17. doi: 10.1128/AAC.02419-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Van Bocxlaer K, Yardley V, Murdan S, Croft SL. Drug permeation and barrier damage in Leishmania-infected mouse skin. J. Antimicrob. Chemother. 2016;71:1578–1585. doi: 10.1093/jac/dkw012. [DOI] [PubMed] [Google Scholar]
- 83.Van Bocxlaer K, Yardley V, Murdan S, Croft SL. Topical formulations of miltefosine for cutaneous leishmaniasis in a BALB/c mouse model. J. Pharm. Pharmacol. 2016;68:862–872. doi: 10.1111/jphp.12548. [DOI] [PubMed] [Google Scholar]
- 84.Fairlamb AH, Horn D. Melarsoprol resistance in African trypanosomiasis. Trends Parasitol. 2018;34:481–492. doi: 10.1016/j.pt.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 85.Carter NS, Fairlamb AH. Arsenical-resistant trypanosomes lack an unusual adenosine transporter. Nature. 1993;361:173–176. doi: 10.1038/361173a0. [DOI] [PubMed] [Google Scholar]
- 86.Kasozi KI, MacLeod ET, Ntulume I, Welburn SC. An update on African trypanocide pharmaceutics and resistance. Front. Vet. Sci. 2022;9:828111. doi: 10.3389/fvets.2022.828111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baker N, et al. Aquaglyceroporin 2 controls susceptibility to melarsoprol and pentamidine in African trypanosomes. Proc. Natl Acad. Sci. USA. 2012;109:10996–11001. doi: 10.1073/pnas.1202885109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Graf FE, et al. Chimerization at the AQP2-AQP3 locus is the genetic basis of melarsoprol-pentamidine cross-resistance in clinical Trypanosoma brucei gambiense isolates. Int. J. Parasitol. Drugs Drug Resist. 2015;5:65–68. doi: 10.1016/j.ijpddr.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Potvin JE, Leprohon P, Queffeulou M, Sundar S, Ouellette M. Mutations in an aquaglyceroporin as a proven marker of antimony clinical resistance in the parasite Leishmania donovani. Clin. Infect. Dis. 2021;72:e526–e532. doi: 10.1093/cid/ciaa1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Perry MR, Wyllie S, Raab A, Feldmann J, Fairlamb AH. Chronic exposure to arsenic in drinking water can lead to resistance to antimonial drugs in a mouse model of visceral leishmaniasis. Proc. Natl Acad. Sci. USA. 2013;110:19932–19937. doi: 10.1073/pnas.1311535110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gamarro, F., Sánchez-Cañete, M. P. & Castanys, S. in Drug Resistance in Leishmania Parasites: Consequences, Molecular Mechanisms and Possible Treatments (eds Ponte-Sucre, A., Diaz, E. & Padrón-Nieves, M.) 351–379 (Springer, 2013).
- 92.Alvar J, et al. Implications of asymptomatic infection for the natural history of selected parasitic tropical diseases. Semin. Immunopathol. 2020;42:231–246. doi: 10.1007/s00281-020-00796-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Capewell P, et al. Resolving the apparent transmission paradox of African sleeping sickness. PLoS Biol. 2019;17:e3000105. doi: 10.1371/journal.pbio.3000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.De Niz M, et al. Intravital imaging of host-parasite interactions in skin and adipose tissues. Cell Microbiol. 2019;21:e13023. doi: 10.1111/cmi.13023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Franco JR, Simarro PP, Diarra A, Jannin JG. Epidemiology of human African trypanosomiasis. Clin. Epidemiol. 2014;6:257–275. doi: 10.2147/CLEP.S39728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Franco JR, et al. Monitoring the elimination of human African trypanosomiasis at continental and country level: update to 2018. PLoS Negl. Trop. Dis. 2020;14:e0008261. doi: 10.1371/journal.pntd.0008261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Azami-Conesa I, Gomez-Munoz MT, Martinez-Diaz RA. A systematic review (1990–2021) of wild animals infected with zoonotic Leishmania. Microorganisms. 2021;9:1101. doi: 10.3390/microorganisms9051101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gramiccia M, Gradoni L. The current status of zoonotic leishmaniases and approaches to disease control. Int. J. Parasitol. 2005;35:1169–1180. doi: 10.1016/j.ijpara.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 99.Kushwaha AK, et al. Domestic mammals as reservoirs for Leishmania donovani on the Indian subcontinent: possibility and consequences on elimination. Transbound. Emerg. Dis. 2021 doi: 10.1111/tbed.14061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rodríguez-Monguí E, Cantillo-Barraza O, Prieto-Alvarado FE, Cucunubá ZM. Heterogeneity of Trypanosoma cruzi infection rates in vectors and animal reservoirs in Colombia: a systematic review and meta-analysis. Parasit. Vectors. 2019;12:308. doi: 10.1186/s13071-019-3541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jansen AM, Xavier SC, Roque ALR. Trypanosoma cruzi transmission in the wild and its most important reservoir hosts in Brazil. Parasit. Vectors. 2018;11:502. doi: 10.1186/s13071-018-3067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Austen JM, Barbosa AD. Diversity and epidemiology of bat trypanosomes: a one health perspective. Pathogens. 2021;10:1148. doi: 10.3390/pathogens10091148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mullard A. Parsing clinical success rates. Nat. Rev. Drug Discov. 2016;15:447. doi: 10.1038/nrd.2016.136. [DOI] [PubMed] [Google Scholar]
- 104.Wong CH, Siah KW, Lo AW. Estimation of clinical trial success rates and related parameters. Biostatistics. 2019;20:273–286. doi: 10.1093/biostatistics/kxx069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Autheman D, et al. An invariant Trypanosoma vivax vaccine antigen induces protective immunity. Nature. 2021;595:96–100. doi: 10.1038/s41586-021-03597-x. [DOI] [PubMed] [Google Scholar]
- 106.Romero-Ramirez, A. I. Antigen discovery in Trypanosoma vivax. Thesis, Univ. Liverpool https://livrepository.liverpool.ac.uk/3088027/1/201193495_Feb2020.pdf (2020).
- 107.Kaye PM, et al. Overcoming roadblocks in the development of vaccines for leishmaniasis. Expert Rev. Vaccines. 2021;20:1419–1430. doi: 10.1080/14760584.2021.1990043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Osman M, et al. A third generation vaccine for human visceral leishmaniasis and post kala azar dermal leishmaniasis: first-in-human trial of ChAd63-KH. PLoS Negl. Trop. Dis. 2017;11:e0005527. doi: 10.1371/journal.pntd.0005527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang WW, et al. A second generation leishmanization vaccine with a markerless attenuated Leishmania major strain using CRISPR gene editing. Nat. Commun. 2020;11:3461. doi: 10.1038/s41467-020-17154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lanza JS, et al. A TLR9-adjuvanted vaccine formulated into dissolvable microneedle patches or cationic liposomes protects against leishmaniasis after skin or subcutaneous immunization. Int. J. Pharm. 2020;586:119390. doi: 10.1016/j.ijpharm.2020.119390. [DOI] [PubMed] [Google Scholar]
- 111.Fernández Cotrina J, et al. A large-scale field randomized trial demonstrates safety and efficacy of the vaccine LetiFend® against canine leishmaniosis. Vaccine. 2018;36:1972–1982. doi: 10.1016/j.vaccine.2018.02.111. [DOI] [PubMed] [Google Scholar]
- 112.Ashwin H, et al. Characterization of a new Leishmania major strain for use in a controlled human infection model. Nat. Commun. 2021;12:215. doi: 10.1038/s41467-020-20569-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zumla A, et al. Host-directed therapies for infectious diseases: current status, recent progress, and future prospects. Lancet Infect. Dis. 2016;16:e47–e63. doi: 10.1016/S1473-3099(16)00078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Varikuti S, et al. Host-directed drug therapies for neglected tropical diseases caused by protozoan parasites. Front. Microbiol. 2018;9:2655. doi: 10.3389/fmicb.2018.02655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rao SPS, et al. Drug discovery for kinetoplastid diseases: future directions. ACS Infect. Dis. 2019;5:152–157. doi: 10.1021/acsinfecdis.8b00298. [DOI] [PubMed] [Google Scholar]
- 116.Lamotte S, Späth GF, Rachidi N, Prina E. The enemy within: targeting host-parasite interaction for antileishmanial drug discovery. PLoS Negl. Trop. Dis. 2017;11:e0005480. doi: 10.1371/journal.pntd.0005480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thacker SG, et al. CpG ODN D35 improves the response to abbreviated low-dose pentavalent antimonial treatment in non-human primate model of cutaneous leishmaniasis. PLoS Negl. Trop. Dis. 2020;14:e0008050. doi: 10.1371/journal.pntd.0008050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Drugs for Neglected Diseases initiative. CpG-D35 for cutaneous leishmaniasis. DNDi https://dndi.org/research-development/portfolio/cpg-d35/ (2022).
- 119.Miranda-Verastegui C, et al. First-line therapy for human cutaneous leishmaniasis in Peru using the TLR7 agonist imiquimod in combination with pentavalent antimony. PLoS Negl. Trop. Dis. 2009;3:e491. doi: 10.1371/journal.pntd.0000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mitra AK, Mawson AR. Neglected tropical diseases: epidemiology and global burden. Trop. Med. Infect. Dis. 2017;2:36. doi: 10.3390/tropicalmed2030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Francisco AF, et al. Limited ability of posaconazole to cure both acute and chronic Trypanosoma cruzi infections revealed by highly sensitive in vivo imaging. Antimicrob. Agents Chemother. 2015;59:4653–4661. doi: 10.1128/AAC.00520-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Paradela LS, et al. Multiple unbiased approaches identify oxidosqualene cyclase as the molecular target of a promising anti-leishmanial. Cell Chem. Biol. 2021;28:711–721.e8. doi: 10.1016/j.chembiol.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wyllie S, et al. Activation of bicyclic nitro-drugs by a novel nitroreductase (NTR2) in. Leishmania. PLoS Pathog. 2016;12:e1005971. doi: 10.1371/journal.ppat.1005971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Saldivia M, et al. Targeting the trypanosome kinetochore with CLK1 protein kinase inhibitors. Nat. Microbiol. 2020;5:1207–1216. doi: 10.1038/s41564-020-0745-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wall RJ, et al. Antitrypanosomal 8-hydroxy-naphthyridines are chelators of divalent transition metals. Antimicrob. Agents Chemother. 2018;62:e00235-18. doi: 10.1128/AAC.00235-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Horn D. Genome-scale RNAi screens in African trypanosomes. Trends Parasitol. 2022;38:160–173. doi: 10.1016/j.pt.2021.09.002. [DOI] [PubMed] [Google Scholar]
- 127.Giordani F, et al. Veterinary trypanocidal benzoxaboroles are peptidase-activated prodrugs. PLoS Pathog. 2020;16:e1008932. doi: 10.1371/journal.ppat.1008932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fernandez-Prada C, et al. High-throughput Cos-Seq screen with intracellular Leishmania infantum for the discovery of novel drug-resistance mechanisms. Int. J. Parasitol. Drugs Drug Resist. 2018;8:165–173. doi: 10.1016/j.ijpddr.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jones NG, Catta-Preta CMC, Lima A, Mottram JC. Genetically validated drug targets in Leishmania: current knowledge and future prospects. ACS Infect. Dis. 2018;4:467–477. doi: 10.1021/acsinfecdis.7b00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lima M, et al. Identification of a proteasome-targeting arylsulfonamide with potential for the treatment of Chagas’ disease. Antimicrob. Agents Chemother. 2021;66:e0153521. doi: 10.1128/AAC.01535-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Altmann S, et al. Oligo targeting for profiling drug resistance mutations in the parasitic trypanosomatids. Nucleic Acids Res. 2022 doi: 10.1093/nar/gkac319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Morillo CA, et al. Benznidazole and posaconazole in eliminating parasites in asymptomatic T. cruzi carriers: the STOP-CHAGAS trial. J. Am. Coll. Cardiol. 2017;69:939–947. doi: 10.1016/j.jacc.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 133.Chatelain E, Scandale I. Animal models of Chagas disease and their translational value to drug development. Expert Opin. Drug Discov. 2020;15:1381–1402. doi: 10.1080/17460441.2020.1806233. [DOI] [PubMed] [Google Scholar]
- 134.Kennedy PG, et al. A substance P antagonist, RP-67,580, ameliorates a mouse meningoencephalitic response to Trypanosoma brucei brucei. Proc. Natl Acad. Sci. USA. 1997;94:4167–4170. doi: 10.1073/pnas.94.8.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jennings FW, et al. Human African trypanosomiasis: potential therapeutic benefits of an alternative suramin and melarsoprol regimen. Parasitol. Int. 2002;51:381–388. doi: 10.1016/S1383-5769(02)00044-2. [DOI] [PubMed] [Google Scholar]
- 136.Burrell-Saward H, Rodgers J, Bradley B, Croft SL, Ward TH. A sensitive and reproducible in vivo imaging mouse model for evaluation of drugs against late-stage human African trypanosomiasis. J. Antimicrob. Chemother. 2015;70:510–517. doi: 10.1093/jac/dku393. [DOI] [PubMed] [Google Scholar]
- 137.Loría-Cervera EN, Andrade-Narváez FJ. Animal models for the study of leishmaniasis immunology. Rev. Inst. Med. Trop. Sao Paulo. 2014;56:1–11. doi: 10.1590/S0036-46652014000100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mendes Costa D, Cecílio P, Santarém N, Cordeiro-da-Silva A, Tavares J. Murine infection with bioluminescent Leishmania infantum axenic amastigotes applied to drug discovery. Sci. Rep. 2019;9:18989. doi: 10.1038/s41598-019-55474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ong HB, Clare S, Roberts AJ, Wilson ME, Wright GJ. Establishment, optimisation and quantitation of a bioluminescent murine infection model of visceral leishmaniasis for systematic vaccine screening. Sci. Rep. 2020;10:4689. doi: 10.1038/s41598-020-61662-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mears ER, Modabber F, Don R, Johnson GE. A review: the current in vivo models for the discovery and utility of new anti-leishmanial drugs targeting cutaneous leishmaniasis. PLoS Negl. Trop. Dis. 2015;9:e0003889. doi: 10.1371/journal.pntd.0003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chatelain E, Konar N. Translational challenges of animal models in Chagas disease drug development: a review. Drug Des. Devel. Ther. 2015;9:4807–4823. doi: 10.2147/DDDT.S90208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Francisco AF, et al. Challenges in Chagas disease drug development. Molecules. 2020;25:2799. doi: 10.3390/molecules25122799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ayala EV, et al. C57BL/6 α-1,3-galactosyltransferase knockout mouse as an animal model for experimental Chagas disease. ACS Infect. Dis. 2020;6:1807–1815. doi: 10.1021/acsinfecdis.0c00061. [DOI] [PubMed] [Google Scholar]
- 144.Henderson CJ, et al. An extensively humanized mouse model to predict pathways of drug disposition and drug/drug interactions, and to facilitate design of clinical trials. Drug Metab. Dispos. 2019;47:601–615. doi: 10.1124/dmd.119.086397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Magez S, Caljon G. Mouse models for pathogenic African trypanosomes: unravelling the immunology of host-parasite-vector interactions. Parasite Immunol. 2011;33:423–429. doi: 10.1111/j.1365-3024.2011.01293.x. [DOI] [PubMed] [Google Scholar]
- 146.Antoine-Moussiaux N, Magez S, Desmecht D. Contributions of experimental mouse models to the understanding of African trypanosomiasis. Trends Parasitol. 2008;24:411–418. doi: 10.1016/j.pt.2008.05.010. [DOI] [PubMed] [Google Scholar]