Abstract
Background
The introduction of human epidermal growth factor receptor 2 (HER2)-targeted treatment options, including dual HER2 blockade, has improved the prognosis for patients with HER2-positive breast cancer (BC) substantially. However, most of these treatments are administered via the intravenous (IV) route, which can present many challenges, such as long infusion and observation times, issues associated with repeated IV access, and increased strain on time and resources of medical centers and healthcare professionals. A fixed-dose combination of pertuzumab and trastuzumab for subcutaneous (SC) injection (pertuzumab, trastuzumab, and hyaluronidase-zzxf (PHESGO®, F. Hoffmann-La Roche Ltd, Basel, Switzerland; PH FDC SC)) has been approved for use alongside chemotherapy for early-stage and metastatic HER2-positive BC.
Objectives
This systematic literature review was performed to identify evidence relating to time/resource use and resulting cost differences between SC and IV administration of oncology biologics in a hospital setting, and, ultimately, to inform economic modeling and associated health technology assessment of PH FDC SC.
Methods
Electronic databases (Embase, MEDLINE, and EconLit) were searched on 9 April 2020. Additional hand searches were performed to identify publications not captured in the electronic database search. Publication screening and data extraction (study characteristics, participants, interventions, costs, and time/resource use) were carried out per the standard Cochrane review methodology. The quality of economic evidence of cost analyses was assessed using the 36-item checklist of the National Institute for Health and Care Excellence Single Technology Appraisal Specification for submission of evidence (January 2015).
Results
The database search identified 2,740 records, of which 237 underwent full text screening. Full text screening, prioritization of publications about patients with a cancer diagnosis, and the addition of four citations identified during the hand search resulted in 72 final included publications, relating to 71 unique studies. This included 40 publications that described the time/resource use and/or costs associated with SC versus IV trastuzumab administration for the treatment of HER2-positive BC, and 28 publications that described time/resource use and/or costs associated with rituximab SC versus IV administration for the treatment of non-Hodgkin’s lymphoma/follicular lymphoma and diffuse large B-cell lymphoma. The majority of publications showed substantial time savings for preparation and administration of SC versus IV therapy, and cost savings associated with reductions in healthcare professional time and resource use for SC administration.
Limitations
There was a lack of consensus between publications regarding time and cost measurements. In addition, the search was limited to publications related to anticancer drugs; the majority of the studies included were performed in European countries.
Conclusions and implications
This review indicated a substantial body of evidence showing time/resource and cost savings of SC versus IV administration of oncology biologics in a hospital setting, which can be used to inform economic evaluations of PH FDC SC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s41669-022-00361-3.
Key Points for Decision Makers
| Most of the publications identified in this systematic review showed time/resource and cost savings associated with subcutaneous versus intravenous administration of anticancer biologics in a hospital setting. |
| This evidence can provide relevant inputs for economic evaluations of the fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection (pertuzumab, trastuzumab, and hyaluronidase-zzxf (PHESGO®, F. Hoffmann-La Roche Ltd; PH FDC SC)). |
Introduction
Breast cancer (BC) is the most prevalent form of invasive cancer among women, with over 2.2 million cases and almost 700,000 deaths worldwide in 2020 [1, 2]. Approximately 20% of BC cases are human epidermal growth factor receptor 2 (HER2)-positive, a subtype defined by amplification of the HER2 oncogene and overexpression of the HER2 transmembrane receptor protein on the surface of tumor cells. HER2 interacts with other HER family proteins as part of signal transduction pathways, mediating cell growth, survival, and differentiation [3]. HER2-positive BC is associated with poor prognosis, arising from increased tumor aggressiveness, higher rates of recurrence, and increased mortality [3, 4].
Trastuzumab (Herceptin®, F. Hoffmann-La Roche Ltd, Basel, Switzerland), the first approved HER2-targeted monoclonal antibody, transformed the treatment and prognosis of patients with HER2-positive BC in both the early and the metastatic settings [5–15]. This has led to the development of dual anti-HER2 blockade with pertuzumab plus trastuzumab (PERJETA® and Herceptin®, F. Hoffmann-La Roche Ltd; standard of care in first-line HER2-positive metastatic BC (MBC) and high-risk early BC (EBC)) [13–21] and the anti-HER2 antibody-drug conjugate ado-trastuzumab emtansine (Kadcyla®, F. Hoffmann-La Roche Ltd; used in second-line HER2-positive MBC and in EBC for the treatment of residual invasive disease following neoadjuvant therapy and surgery) [13–16, 22–24]. These treatment options have improved the prognosis for patients with HER2-positive BC substantially.
However, intravenous (IV) administration of anticancer biologics can present multiple challenges for many patients, including long infusion and observation times, the need for repeated, invasive IV access (sometimes over long periods of time in cases where there is evidence of a treatment response), and the potential risks associated with indwelling venous access (e.g., catheter-associated pain/discomfort, thrombosis, or risk of systemic infections) [25–28]. Moreover, the increasing use of IV administered agents in oncology has placed a strain on medical centers and healthcare professionals (HCPs) with respect to the time and resources required to prepare and administer infusions [25, 29].
A subcutaneous (SC) formulation has previously been developed for trastuzumab (Herceptin® SC or Herceptin HylectaTM, F. Hoffmann-La Roche Ltd) [11, 30]. The HannaH study (NCT00950300) compared the pharmacokinetics, efficacy, and safety of SC trastuzumab with IV trastuzumab. SC trastuzumab was shown to be non-inferior to IV, for both co-primary endpoints (serum trough concentration at pre-dose cycle 8 and pathologic complete response rates), demonstrating that the SC formulation is a valid treatment alternative to IV [31–34]. Further to this, the safety and efficacy profiles for SC trastuzumab in combination with IV pertuzumab and docetaxel as a first-line treatment for patients with HER2-positive MBC in the MetaPHER study (NCT02402712) was found to be consistent with those observed for IV trastuzumab in combination with IV pertuzumab and docetaxel in the CLEOPATRA study (NCT00567190) [21, 35–38].
The PrefHer study (NCT01401166), in which patients with EBC were randomized to receive four cycles of SC trastuzumab followed by four cycles of IV trastuzumab, or vice versa, demonstrated a strong patient preference and increased HCP satisfaction with SC over IV administration [39, 40]. These results were also confirmed in the metastatic setting in the MetaspHer study (NCT01810393) [41]. The approval of a fixed-dose combination of pertuzumab and trastuzumab for SC injection (pertuzumab, trastuzumab, and hyaluronidase-zzxf (PHESGO®, F. Hoffmann-La Roche Ltd; PH FDC SC)) [42] presents an opportunity for an option that is preferred by patients and can potentially provide time-saving benefits to patients and HCPs versus IV administration, according to patient and HCP questionnaires in the PHranceSCa study (NCT03674112) [43].
This systematic literature review (SLR) was performed to identify evidence relating to differences in time/resource use and the resulting cost differences between SC and IV administration (but not differences in the drug costs themselves). The rationale for performing the SLR was as preliminary work that will ultimately inform economic modeling and associated health technology assessment of PH FDC SC. The most analogous evidence was likely to be data relating to the time/resource use and cost differences for SC versus IV administration of trastuzumab for the treatment of BC, or of rituximab (Rituxan® or MabThera®, F. Hoffmann-La Roche Ltd) for treatment of non-Hodgkin’s lymphoma (NHL)/follicular lymphoma (FL) and diffuse large B-cell lymphoma (DLBCL). Thus, the SLR initially sought to identify cost analyses as well as time-and-motion analyses for any indication where patients’ treatment requires IV or SC administration in a hospital setting, and was then restricted to oncology biologics.
Methods
A systematic search was conducted via the Ovid platform (Wolters Kluwer, Alphen aan den Rijn, Netherlands) on 9 April 2020 using a predefined search strategy within the Embase (1980–present), MEDLINE (1946–present), and EconLit (1961–present) electronic databases. The database search strings identified all relevant studies (full papers or abstracts from any conferences) indexed in Embase, and were modified for performing searches in MEDLINE and EconLit to account for differences in syntax and thesaurus headings. Searches included terms for free text and Medical Subject Heading (MeSH) terms. The search strategies used and details of any additional hand searches that were carried out to identify publications not captured in the electronic database search are provided in the Online Supplemental Material, Resource 1. Details on the study eligibility criteria are presented in Table 1.
Table 1.
Eligibility criteria for the systematic literature review
| Description | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | Any patients receiving treatment in a hospital settinga | Patients treated exclusively at home |
| Intervention/comparator | Studies comparing any IV- versus SC-administered interventions | Studies not comparing IV- versus SC-administered interventions |
| Outcomes |
Costs and time/resource use Direct medical costs: Port versus PICC versus CVC costs Direct non-medical costs: Transportation Childcare costs Additional caregiver costs Indirect/societal costs: Productivity losses Absenteeism Presenteeism Withdrawal from labor force Estimates of time/resource use including: Hospitalization and length of stay Pharmacist time Nurse time Drug wastage Cost drivers Time-and-motion outcomes including: Patient waiting time Drug preparation time Administration time Monitoring/observation time Nurse set-up time AE management time |
Clinical outcomes |
| Study design/setting |
Cost and time/resource use studies: Any studies reporting original cost and/or time/resource use data |
Systematic literature reviewsb Studies based on animal models Preclinical and biologic studies Narrative reviews, editorials, opinions |
| Language of publication | Not restricted | NA |
| Date of publication |
Full publications: 2012c to present Conference abstracts: 2017 to presentd |
Full publications prior to 2012 Conference abstracts prior to 2017 |
| Countries | Not restricted | NA |
AE adverse event, CVC central venous catheter, IV intravenous, NA not applicable, PICC peripherally inserted central catheter, SC subcutaneous, SLR systematic literature review
aAs a result of the deliberately inclusive eligibility criteria originally designed for this SLR, a larger than anticipated number of potentially eligible studies were identified after the completion of first pass screening. A decision was taken to deprioritize any study that did not focus on a population of patients with a cancer diagnosis
bRelevant systematic literature reviews were reference checked before being excluded
cYear of approval of SC trastuzumab
dConference abstracts that were superseded by a full publication were excluded unless the abstract reported some unique data
The SLR followed the standard Cochrane review methodology [44] and included double screenings by two independent reviewers. Relevant data from included publications were extracted by a reviewer and verified by a second independent reviewer; any disputes were resolved through discussion. The types of data to be collected were predefined and included: study country, study design, industry sponsor, inclusion/exclusion criteria, target population, study aims, data source, intervention, study limitations, and conclusions. Cost and time/resource use outcomes were also captured and stratified by disease and route of administration. Quality assessments of the studies in the included publications were conducted by a single analyst and verified by a second analyst or project lead. The quality of economic evidence reported in the included cost analysis publications were assessed using the 36-item checklist of the National Institute for Health and Care Excellence Single Technology Appraisal Specification for manufacturer/sponsor submission of evidence (January 2015), adapted from Drummond and Jefferson [45]. The methodologic limitations of publications reporting on time/resource use and costs were assessed based on a model described by Drummond et al. [46] and adapted to cost of illness by Molinier et al. [47].
Results
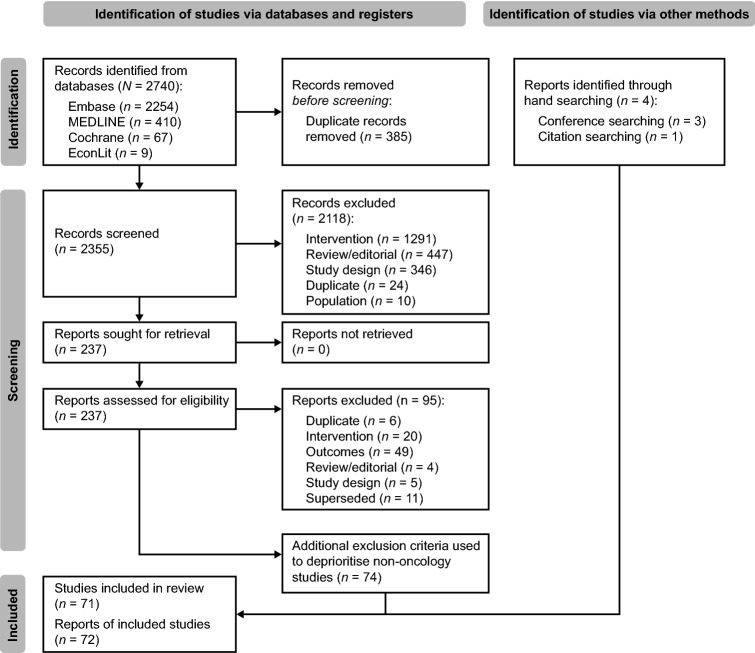
This search identified 2,740 records, of which 237 underwent full-text screening. Ninety-five publications were excluded during full-text screening, leaving 142 potentially eligible publications, a higher number than anticipated due to broad eligibility criteria. Prioritization was therefore given to publications of patients with a cancer diagnosis, as noted in Table 1, given the target population for PH FDC SC, resulting in exclusion of 74 non-oncology publications. Hand searching identified a further four citations that met the revised eligibility criteria, resulting in 72 final included publications, relating to 71 unique studies. The PRISMA diagram is presented in Fig. 1.
Fig. 1.
PRISMA diagram: Study flow of included and excluded publications. PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Characteristics of Included Studies
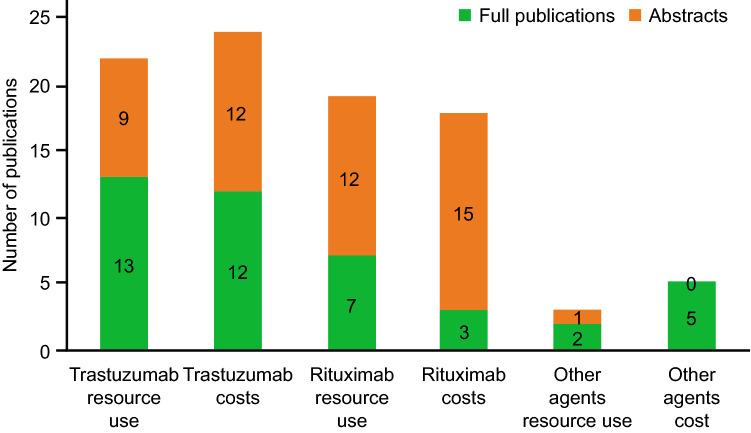
Table 2 summarizes the characteristics of all included studies. In total, 40 publications were identified that described the time/resource use and/or costs associated with SC versus IV trastuzumab administration for the treatment of HER2-positive BC. Of these, 22 publications [25, 48–68] (13 full papers [25, 51–55, 59, 62–67] and nine abstracts [48–50, 56–58, 61, 68]) reported time/resource use for administration of SC versus IV trastuzumab (Fig. 2). This included two publications related to PrefHer, a multinational study conducted in eight countries (Canada, France, Switzerland, Denmark, Italy, Russia, Spain, and Turkey), 18 publications that reported studies that were conducted in at least 12 individual European countries (the country was not stated in one of the publications) [48–54, 57–61, 63–68], one publication that reported a study in Hong Kong [56], and one that reported a study in New Zealand [62]. A total of 24 publications reported on the costs of SC versus IV trastuzumab administration. Of these, 19 reported data for at least 13 individual European countries (the country was not stated in one of the publications) [48–51, 53, 54, 57, 59–61, 63–65, 67]. The other five were in Canada, Chile, Singapore, Hong Kong, and New Zealand [56, 62, 69–71]. Budget impacts of introducing SC trastuzumab were reported by five publications (Arabia, Ecuador, Canada, Brazil, Spain) [69, 72–75]. Six other publications described costs related to SC trastuzumab: three compared SC trastuzumab with an IV trastuzumab biosimilar [76–78], two described cost minimization analyses for SC versus IV administration [79, 80], and one reported on cost savings for the administration of the SC route over 18 months compared with a combination of SC and IV [81].
Table 2.
Summary of all publications included in this systematic literature review
| Publication | Study country | Study design and aim | Data source | Patient population and intervention | Cost outcomes identified | Time/resource-use outcomes identified |
|---|---|---|---|---|---|---|
| Trastuzumab—full publications | ||||||
| De Cock 2016 [25] | Canada, France, Switzerland, Denmark, Italy, Russia, Spain, Turkey | Prospective, observational time-and-motion study (PrefHer; NCT01401166) to quantify patient chair time and active HCP time associated with SC and IV trastuzumaba | Multiple centers across different countries |
HER2-positive BC (NR) 1) SC trastuzumab (single-use injection device or handheld syringe) 2) IV trastuzumab |
– |
Treatment room time, 17.9 versus 9.8–11.2 min, Preparation, 13.9 versus 5.0–7.6 min Total time, 31.8 versus 14.8–18.8 min, i.e. 13–17 min shorter Chair time, 55–57 min shorter; 77.8 versus 20.9–22.6 min, p<0.0001 |
| Farolfi 2017 [51] | Italy | Retrospective cohort study to compare the time/resource utilization of SC and IV trastuzumab and to conduct an economic evaluationa |
Time/resource utilization retrieved from Institutional medical record database (Log80); unit costs for healthcare professionals retrieved from the Italian National Contract |
EBC (n = 114) 1) SC trastuzumab 2) IV trastuzumab |
Direct costs, €13,655 versus €14,154/patient/year €14,233 versus €14,273/patient/year (including indirect costs) Preparation, €92.60 versus €57.50/patient/year Day hospital cost, €575.82 versus €61.51/patient/year |
Preparation, 14.1 versus 10.7 min Administration, 90 versus 5 min (loading dose); 30 versus 5 min (maintenance dose) |
| Hedayati 2019 [54] | Sweden | Retrospective study to estimate the economic efficiency of SC trastuzumab by assessing the economic benefits of actual SC-driven process changes at one single Swedish healthcare institutiona | Karolinska University Hospital |
HER2-positive BC (n = 178) 1) IV trastuzumab 2) SC trastuzumab |
Savings for cohort over 1 year: Avoiding surgery to implant catheters, €419,012 Preparation time, €167,087 Consumables, €17,389 Direct cost saving, €603,488 (for cohort over 1 year) |
Administration, 90 versus 10 min (first session; 30 versus 10 min (subsequent sessions) |
| Jackisch 2015 [55] | Canada, Denmark, France, Russia, Spain, Switzerland | Prospective study to provide an overview of the study data on SC trastuzumab and summarizes and evaluates the experience of 7 German centers over 18 months of administering SC trastuzumab in routine clinical practicea | NR |
HER2-positive BC (n = 415) 1) IV trastuzumab 2) SC trastuzumab |
– |
Active HCP time: Denmark 7.2 versus 4.9 min France, 9 versus 5.7 Canada, 11.8 versus 6.3 Russia, 9.9 versus 5.2 Spain, 8.2 versus 4.0 Switzerland, 10.5 versus 7.2 Chair time: Denmark, 57 versus 24 min France, 85 versus 27 Canada, 67 versus 24 Russia, 47 versus 13 Spain, 100 versus 20 Switzerland, 133 versus 38 |
| Lopez-Vivanco 2017 [59] | Spain | Prospective observational study to describe HCP and patient time and related costs associated with IV and SC trastuzumab in patients with HER2-positive early BCa | NR |
HER2-positive BC (n = 10) 1) IV trastuzumab 2) SC trastuzumab |
Preparation and administration, €12.76 versus €6.01 Consumables, €8.64 versus €2.39 Costs per 18 cycles: HPC, €230 versus €108 Consumables, €155 versus €43 Drug costs, €29,046 versus €28,301 Direct costs, €29,432 versus €28,452 (for 18 cycles) €29,635 versus €28,503 (for 18 cycles including indirect costs) Lost productivity, €204 versus 51 (for 18 cycles) |
Active HCP time, 27.2 versus 13.2 min [Nursing time, 21.8 versus 11.2 min Pharmacist time, 4.2 versus 1.2 min Nursing assistant time, 1.1 versus 0.8 min] Chair time, 101 versus 20 min Treatment room time. 120 versus 30 min Hospital time, 205 versus 115 min |
| North 2015 [62] | New Zealand | Noninterventional, descriptive study to determine medical time/resource utilization associated with administration of SC trastuzumab injection via handheld syringe versus IV trastuzumab infusion in patients with HER2-positive BC in New Zealanda | Auckland City Hospital (IV trastuzumab) and Tauranga Hospital (SC and IV trastuzumab) |
HER2-positive BC (n = 18) 1) IV trastuzumab 2) SC trastuzumab |
Total, NZD 76.94 For administration and preparation, NZD 61,67 [Chair time, NZD 40.03 HCP nurse time, NZD 4.59 Total pharmacist time, NZD 17.05] Consumables, NZD 15.27 |
Pharmacist time, 20.5 versus 0 min Administration, 37.6 versus 5.7 min HCP (nurse) time, 13.0 versus 6.9 min Chair time, 47.4 versus 10.5 min |
| O’Brien 2019 [63] | Ireland | Prospective observational study to analyze which route of trastuzumab administration, for the treatment of HER2-positive BC, was more cost-effective and time-saving in relation to active HCP timea | Two large acute Irish University teaching hospitals within the south/southwest |
HER2-positive BC (NR) 1) IV trastuzumab 2) SC trastuzumab |
Consumables, €56.28 versus €25.91 Preparation and administration time, €44.93 versus €9 Direct costs, for 17-cycle treatment €36,619 versus €35,010 €36,863 versus €35,077 (including indirect costs) Societal costs, for 17-cycle treatment €243.74 versus €67.15 |
HCP time, 74.7 versus 15.4 min |
| Olofsson 2016 [64] | Sweden | Observational study to estimate the societal value of trastuzumab administered through SC injection compared to IV infusionb | Five oncology clinics |
HER2-positive BC (n = 195) 1) IV trastuzumab 2) SC trastuzumab |
Direct costs, First visit, €2695 versus €1938 €2976 versus €2079 (including indirect costs) Subsequent visits, €2033 versus €1933 €2099 versus €1983 (including indirect costs) First visit: Consumables, €53 versus €1 Nurse time, €22 versus €13 Drug costs, €2616 versus €1920 Subsequent visits: Consumables, €53 versus €1 Nurse time, €15 versus €8 Drug costs, €1962 versus €1920 First visit: Lost productivity, €94 versus €16 Lost leisure time, €187 versus €125 (includes patient and accompanying kin) Subsequent visits Lost productivity, €26 versus €16 Lost leisure time, €40 versus €34 |
HCP time, 44 versus 26 min (first treatment) 30 versus 16 (subsequent treatment), p < 0.01 for both Hospital time: 414 versus 313 min (first treatment), p < 0.05; 90 versus 67 min, p < 0.01 (subsequent treatment) |
| Olsen 2018 [65] | Denmark | Study design NR. To estimate the costs of administration of IV and SC trastuzumab treatmenta | Seven departments located at regional hospitals and five at university hospitals |
HER2-positive BC (NR) 1) IV trastuzumab 2) SC trastuzumab |
Non-drug costs excluding patient’s time: 1st cycle, €171 versus €60 4+ cycle, €103 versus €52 Non-drug costs including patient’s time: 1st cycle, €290 versus €153 4+ cycle, €112 versus €55 |
HCP time, 92 versus 30 min (first cycle), subsequent therapy, 56 versus 30 min Direct contact, 111 versus 13 min (first cycle), 51 versus 13 min (subsequent cycle) Observation time, same for first cycle; second cycle, 61 versus 93 min |
| Tjalma 2018 [67] | Belgium | Observational, non–interventional, prospective, monocentric time, motion and cost assessment study to determine and compare the time and costs of SC versus IV trastuzumab administration in patients with HER2-positive BCa | LEAN day care oncology unit of the Antwerp University Hospital |
HER2-positive BC (n = 130) 1) SC trastuzumab 2) IV trastuzumab |
Administration, €224.48 versus €10.60 HCP time, €37.4 versus €7.9 Consumables, €23.60 versus €2.70 Drug wastage, €139.00 versus 0 Overall saving per administration excluding drug costs, €212.93 |
Total time in center, 173 versus 51 min HCP time, 68 versus 14 min Chair time, 137 versus 10.6 min |
| Rojas 2020 [71] | Chile | Cost-minimization analysis to compare the direct and indirect medical and non-medical costs associated with SC trastuzumab and IV trastuzumab in patients with HER2+ early BC in a private health center in Chileb | Nuestra Señora de la Esperanza Cancer Center, Red de Salud UC-Christus network |
HER2-positive BC (n = 100) 1) IV trastuzumab 2) SC trastuzumab |
Direct costs, per treatment (18 doses) including indirect costs, US$83,309 versus US$77,068 Per treatment (18 doses): Preparation, US$78,076 versus US$73,225 Administration, US$3485 versus US$2005 Aes, US$1574 versus US$1715 Societal costs, per treatment (18 doses) Indirect costs, US$173 versus US$1216 |
– |
| De La Vega Zamorano 2017 [81] | Spain | Retrospective observational study to evaluate and quantify the economica impact of SC presentation of trastuzumab | Hospital de la Ribera. Alzira. Valencia |
HER2-positive EBC or MBC (n = 28) 1) SC trastuzumab 2) IV trastuzumab |
||
| Lazaro Cebas 2017 [82] | Spain | Cross-sectional questionnaire-based study to investigate patient satisfaction and preferences regarding SC versus IV trastuzumab and to evaluate the financial impact derived from the use of the SC formulationa | Oncology outpatient hospital (Hospital Universitario 12 de Octubre, Madrid) |
HER2-positive BC (n = 76) 1) IV trastuzumab 2) SC trastuzumab |
Direct costs, annual saving, €35,332 | – |
| Mylonas 2017 [86] | Greece | Economic analysis to conduct an economic evaluation comparing SC trastuzumab with IV trastuzumab, in the treatment of patients with HER2-positive early and metastatic BC (EBC-MBC), in the Greek health care settinga | NR |
HER2-positive BC (NR) 1) IV trastuzumab 2) SC trastuzumab |
Direct costs, €23,118 versus €21,870 per therapy Administration, €266.94 versus €64.41 CVAD, €289.80 versus 0 Overhead, €249.94 versus €67.19 Drug cost, €22,311 versus €21,738 |
– |
| Trastuzumab—abstracts | ||||||
| Andrade 2013 [48] | Portugal | Study design NR. To determine the costs associated with the preparation and administration of HER2-positive BC treatment with IV trastuzumab and to estimate the difference compared with SC trastuzumaba | Five public and two private hospitals; official sources or price tables provided by manufacturer |
HER2-positive BC (mean: n = 12 patients/week) 1) IV trastuzumab 2) SC trastuzumab |
€43.22 versus €3.18 | – |
| Blein 2018 [49] | France | Observational study to evaluate the organizational and economic impacts generated by the administration of SC versus IV trastuzumaba | Nine healthcare facilities |
HER2-positive BC (n = 411) 1) SC trastuzumab 2) IV trastuzumab |
Consumables, €11.07 lower |
Preparation, 12 min shorter Administration, 107 min shorter |
| De Cock 2014 [50] | Russia | Prospective, time-and-motion study to quantify HCP time and patient chair time related to trastuzumab treatment to estimate potential time and cost savings with a transition from IV to SCa | Three centers |
HER2-positive BC (NR) 1) IV trastuzumab 2) SC trastuzumab |
HCP time, 1175 roubles saved for 18 sessions Chair time, 6314 roubles saved for 18 sessions |
HCP time, 38.7 versus 20.1 min, 18.6 min shorter Chair time, 67.1 versus 7.6 min, 59.5 min shorter |
| Lee 2018 [56] | Hong Kong | Cost-minimization analysis to investigate the cost differences between IV and SC trastuzumab in Hong Kong by applying the medical time/resources utilization data in other countriesa | NR |
HER2-positive BC (NR) 1) IV trastuzumab 2) SC trastuzumab |
Saving for 18-cycle treatment: HCP time costs, HKD 4416 Drug costs: HKD 73,720 |
Nursing time, 0.18 FTE per week saved Pharmacist time, 0.14 FTE saved per week |
| Lewis 2017 [57] | UK | Retrospective study to evaluate the impact of SC trastuzumab on out-patient BC services at the Royal United Hospital, Bath and to determine the necessity for prolonged observation after its administrationa | Royal United Hospital, Bath |
BC (NR) 1) SC trastuzumab 2) IV trastuzumab |
Saving for given year: Consumables, £2220 Drug costs £94,327 |
HCP time, 15 min saved Chair time, 38 min saved |
| Lopez 2017 [58] | NR | Retrospective study to evaluate the impact on drug costs, patient chair time and safety profile of switching from IV to SC trastuzumaba | NR |
HER2-positive BC (n = 74) 1) IV trastuzumab 2) SC trastuzumab |
– | Chair time, 6-fold reduction over 1 year |
| Nestorovska 2015 [60] | Republic of Macedonia | Cost-minimization analysis to compare the total cost of SC trastuzumab versus IV trastuzumab for HER2-positive BC patients from the Republic of Macedoniaa | Data from prior prospective time-motion study; unit costs obtained utilizing official (government and hospital pharmacy) publicly available data |
EBC (n = 169) 1) SC trastuzumab 2) IV trastuzumab |
Direct costs, €30,500 versus €30,102 per treatment course Non-drug costs, €196 versus €4.20 |
Preparation and administration, 47 min saved |
| Nierenberger 2017 [61] | France | Time-motion study to compare times (preparation, nurse and medical) and costs of IV trastuzumab and SC trastuzumaba | Hospital |
HER2 positive BC (NR) 1) SC trastuzumab 2) IV trastuzumab |
Consumables, 655 versus €240 Nurse time, 15.7 versus 7.0 min |
Preparation, 8.3 versus 2 min (first dose); 6.5 versus 2 min (subsequent doses) |
| Coombes 2019 [69] | Canada | Cost-minimization analysis to estimate the incremental costs/savings associated with the use of SC trastuzumab for the treatment of HER2-positive BC if reimbursed with the same provincial funding criteria as IV trastuzumab in Ontario, Canadaa | NR |
HER2-positive BC (NR) 1) IV trastuzumab 2) SC trastuzumab |
Savings of Can$11,943 per treatment course BIM savings Year 1, Can$15.8 million Year 2, Can$19.4 million Year 3, Can$23.0 million |
– |
| Ali 2017 [72] | Kingdom of Saudi Arabia | Budget impact model to estimate financial impact of introducing fixed dose SC trastuzumab for treatment of HER2-positive EBC in KSAa | Clinical and cost inputs were obtained through discussion with medical oncologists and hematologists; outputs from budget impact model |
HER2-positive EBC (NR) 1) SC trastuzumab 2) IV trastuzumab |
BIM, total savings over 3 years: 7.2–9.4% and 12.4–16.6% (assuming 25% and 50% reductions in non-drug costs) | – |
| Kashiura 2018 [73] | Brazil | Economic analysis to estimate the budgetary impact of SC trastuzumab, compared with IV trastuzumab, in the Brazilian Private Healthcare System, to treat early and metastatic HER-2 positive BCa | Data from National Regulatory Agency for Private Health Insurance and Plans; data from Survey performed with 28 HMOs |
HER2 positive BC (n = 31,909) 1) SC trastuzumab 2) IV trastuzumab |
BIM savings over 5 years, 948.2 million BRL | – |
| Poquet-Jornet 2018 [74] | Spain | Economic analysis to assess the hospital budget impact of only SC trastuzumab against IV + SC trastuzumab for patients with BCa | RWD from Hospital Marina Salud de Denia and electronic records |
HER2-positive BC (n = 58) 1) IV trastuzumab 2) SC trastuzumab |
BIM, current scenario (66% receiving iv), €466,480 All patients receiving IV, €393,654 |
– |
| Calvache 2017 [75] | Ecuador | Economic analysis to conduct an economic analysis for decision making between IV and SC trastuzumab for the treatment of HER2-positive BC in Ecuadora | NR |
HER2-positive BC (n = 515) 1) IV Trastuzumab 2) SC Trastuzumab |
BIM, IV, year 1, US$15,711,000 IV, year 2, US$15,199,000 SC, year 1/2, US$14,842,000 Saving over 5 years, US$2,296,000 |
– |
| Agirrezabal 2018 [76] | Italy | Cost-saving study to analyze the per-patient costs and potential savings of KANJINTI® compared with originator Herceptin® and other trastuzumab biosimilars (IV) in HER2-positive EBC and MBC and MGC in Italya | Official, public drug prices |
HER2-positive BC and gastric cancer (NR) 1) KANJINTI® IV 2) Herceptin® IV 3) Herceptin® SC |
– | Preparation and administration, 79 versus 18 min |
| D’Arpino 2019 [77] | Italy | Study design NR. To compare the total medical costs for a hospital of treatment with subcutaneously injected Herceptin® and intravenously infused KANJINTI®a | A single hospital institution |
HER2-positive EBC or MBC and metastatic gastric cancer (NR) 1) Herceptin® SC 2) KANJINTI® IV |
||
| Todorovic 2017 [79] | Montenegro | Economic analysis to compare the total cost of SC trastuzumab versus IV trastuzumab (IV-TRA) for HER2-positive patients with BC at the Oncology Department at Clinical Center of Montenegroa | Oncology Department at Clinical Center of Montenegro |
HER2-positive BC (n = 55) 1) IV trastuzumab 2) SC trastuzumab |
||
| Villarreal-Garza 2019 [80] | Mexico | Economic analysis to estimate the cost savings of the use of SC trastuzumab compared with IV trastuzumab according to patient weight, and calculate the infusion time of SC trastuzumab in a tertiary healthcare facility at TecSalud from Feb 2018–Jan 2019a | Tertiary healthcare facility at TecSalud |
HER2-positive BC (NR) 1) SC trastuzumab 2) IV trastuzumab |
||
| Kulikov 2015 [83] | Russia | Economic analysis to determine the preferable treatment scheme for BC from the pharmacoeconomic perspective by the comparison of SC and IV administrationa | NR |
BC (NR) 1) IV trastuzumab 2) SC trastuzumab |
Direct costs, €25,016 versus €21,863 Saving of €3153 per treatment course |
– |
| Martin 2017 [84] | Panama | Cost minimization study to carry out a cost minimization study considering the cost of the treatment, the supplies and the time utilized by Pharmacy and Nursing to prepare and administer it (IV, SC trastuzumab)a | Instituto Oncológico Nacional |
NR (NR) 1) IV trastuzumab 2) SC trastuzumab |
Drug costs, 17% saving Nursing supplies, 87% saving Pharmacy supplies, 47% saving Nursing time costs, 91% saving Pharmacy time costs, 69% saving Direct costs, US$5985/patient/year (18-cycles) |
– |
| Mitchell 2019 [85] | UK | Economic analysis to compare and determine the experience of patients receiving all of their trastuzumab treatment via the IV route versus the SC routea | The Christie NHS Foundation Trust |
HER2-positive BC (n = 116) 1) SC trastuzumab 2) IV trastuzumab |
– |
Nursing time, £105 versus £26 Preparation, £78 versus £14 Drug cost, £1500 versus £1223 Direct costs, £3629 versus 1263 per administration (including insertion of port for IV administration) |
| Trastuzumab/rituximab—full publications | ||||||
| Favier 2018 [52] | France | Observational study to evaluate the medical and economic consequences of switching to SC trastuzumab and SC rituximaba | 36 day hospitals |
Patients with cancer (NR) 1) IV trastuzumab/rituximab 2) SC trastuzumab/rituximab |
– |
Chair time trastuzumab, reduced by 56% Chair time rituximab, reduced by 74% |
| Franken 2018 [53] | The Netherlands | Observational noninterventional micro costing study to investigate time/resource use for hospitals and patients and compared healthcare and societal costs for IV and SC administration of trastuzumab and rituximabb | NR |
HER2-positive EBC or MBC or NHL (n = 126) 1) SC trastuzumab/rituximab 2) IV trastuzumab/rituximab |
Trastuzumab Direct costs, €1856 versus €1763 Total direct costs excluding drug costs, €118 versus €50 [Preparation: Active HCP time, €9.02 versus €2.94 Consumables, €5.19 versus €1.83 Administration: Active HCP time cost, €20.27 versus €17.40 Consumables, €8.92 versus €0.46] Societal costs, €48.60 versus €26.39 Rituximab Drug costs, €2000 versus 1828 Administration, €146 versus €76 [Day care unit costs, €96 versus €38 Consumables, €12 saving HCP time, €9 saving] Societal cost, €26 saving |
Preparation, 17.1 versus 8.4 min Administration, 97.4 versus 6.6 min Active HCP time, 44.5 versus 29.7 min |
| Cicchetti 2018 [105] | Italy | Economic analysis to provide a multidimensional assessment of the impact of SC rituximab and trastuzumab compared with IV, providing a particular focus on expected social and economic benefits for the patientb | NR |
Patients with cancer (NR) 1) IV trastuzumab/rituximab 2) SC trastuzumab/rituximab |
Direct costs, €4050 saving per patient per year | – |
| Trastuzumab/rituximab—abstracts | ||||||
| Vangheluwe 2018 [68] | France | Cost analysis study to evaluate the realized and potential medico-economic benefits in OCUs induced by the use of SC rituximab and SC trastuzumaba | Data obtained from chemotherapy prescription software, Medical Information System 2016, patient satisfaction surveys and interviews with hematologists, oncologists, nurses and pharmacists |
Patients with cancer (NR) 1) IV trastuzumab/rituximab 2) SC trastuzumab/rituximab |
– |
Preparation, 8.9 min saved HCP, 6.8 min saved Chair time, 3 versus 2 hours |
| Ghosh 2018 [70] | Singapore | Cost-minimization analysis to analyze cost-differences between SC versus IV trastuzumab for EBC and MBC in Singaporea | NR |
EBC or MBC (NR) 1) SC trastuzumab/rituximab 2) IV trastuzumab/rituximab |
Non-drug savings, US$22,449 versus US$4036, 17% of total savings for 26 cycles | – |
| Jang 2018 [78] | UK | Economic analysis to assess the budget impact of adopting IV biosimilar rituximab and IV biosimilar trastuzumab compared with SC and IV originators from the perspective of the UK National Health Servicec | Drug acquisition and administration costs obtained from national tariffs |
Rheumatoid arthritis, chronic lymphocytic leukemia, NHL, granulomatosis with polyangiitis and microscopic polyangiitis, BC and MGC (NR) 1) SC trastuzumab/rituximab 2) IV trastuzumab/rituximab |
||
| Rituximab—full publications | ||||||
| Ponzetti 2016 [66] | Italy | Systematic survey to analyze the time/resource and cost implications from different perspectives (patient, medical staff) in the real worlda | Nineteen centers across six regions: Emilia Romagna, Lazio, Liguria, Piemont, Toscana, Umbria |
NHL and BC (NR) 1) Rituximab SC 2) Rituximab IV |
– |
Patients time, 4.81 versus 1.45 h, 200 min saving HCP preparation time: 23 versus 10 min |
| De Cock 2016b [88] | Austria, Brazil, France, Italy, Russia, Slovenia, Spain, UK | Prospective time-and-motion study (MabCute; NCT01461928) to quantify active HCP time as well as chair time associated with rituximab IV and SC administrations in patients with iNHL, and to estimate the potential time savings with a conversion from rituximab IV to SC, for a single administration session and for the first year of treatmenta | Eight countries and 30 day oncology units |
iNHL (NR) 1) Rituximab SC 2) Rituximab IV |
– |
Patient time, treatment room time, 281.8 versus 95.9 min Active HCP time, 35.0 versus 23.7 min Treatment room time, 12.2–40.6 min versus 7.8–19.9 min Preparation time, 3.7–38.9 min versus 1.6–33.3 min; pooled data, 35.0 versus 23.7 min 262.1 versus 67.3 min Chair time, infusion duration, 180.9 versus 8.3 min Increase in time for first infusion, 83.1 min |
| Lugtenburg 2017 [89] | The Netherlands, Turkey, Spain, Switzerland, Germany | Randomized, open-label study to examine the efficacy and safety of rituximab SC versus the IV formulation as part of a R-CHOP regimen. Patient satisfaction with treatment was also assesseda | 151 centers |
DLBCL (n = 576) 1) R-CHOP with rituximab SC 2) R-CHOP with rituximab IV |
– | 2.6–3.0 h versus 6 min (infusion duration) |
| Mihajlovic 2017 [90] | The Netherlands | Prospective, observational, bottom-up micro costing study to identify and compare all direct costs of intravenous and subcutaneous rituximab given to patients with diffuse large B-cell lymphoma in the Netherlandsa | Isala Clinics, Medical Center Leeuwarden (MCL), AZC Dordrecht, MCAl-kmaar, OMC Sittard-Geleen, Maasstad Hospital |
DLBCL (NR) 1) Rituximab SC 2) Rituximab IV |
Total direct cost saving €265.17 (€2176.77 versus €1911.09) Drug cost saving €85.34 Labour cost saving, €1.24 |
Nursing: 16.5 versus 13.7 min Pharmacy: 4.0 versus 3.7 min, 16 s shorter for SC |
| Fargier 2018 [91] | France | Observational cross-sectional to evaluate the economic impact of using SC rituximab as maintenance therapy compared with usual patient care for follicular lymphoma, and to investigate HRQoL, patients’ and nurses’ perceptions and preferences, in the context of the French health servicea | Three teaching hospitals in Lyon, Nantes, and Tours |
Follicular lymphoma (n = 73) 1) Rituximab IV 2) Rituximab SC |
Direct costs, €1897.40 versus €1788.10/cycle Cost difference of €109.20 Rituximab €1863 versus €1777 Pre-medication, €1.10 versus €0.20 Consumables, €21 versus €0.51 Active HCP time, €21.90 versus €9.90 |
Pharmacist, 9.1 versus 3.5 min Nurse, 23.7 versus 11.8 min |
| Rule 2014 [92] | UK | Prospective, observational, time-and-motion study to investigate the staff time and costs associated with administration of SC and IV rituximaba | Plymouth, London, Oxford |
NHL (n = 700) 1) Rituximab SC 2) Rituximab IV |
Mean staff cost savings: £115.17 (£146.43 versus £31.26) £575.85 per course (5 sessions per year) |
Treatment room time, 264 versus 70 min Hospital time, 304 versus 110 min Total HCP, 223 versus 48.5 min, saving 174.8 min per infusion Chair time, 239 versus 46 min, 193.1 min saving per infusion |
| Rituximab—abstracts | ||||||
| Ben Lakhal 2019 [93] | Tunisia | Cost-minimization analysis and budget impact model to evaluate cost and time/resource use impact associated with the administration of rituximab SC and IV administrations on the Tunisian health system over 3 yearsa | Hospital Aziza Othmana |
B NHL (NR) 1) Rituximab SC 2) Rituximab IV |
TND 545 versus TND 330 per patient |
Annual saving of nursing time, 22.13 days Chair time annual saving, 193.5 days |
| Chansung 2018 [94] | Thailand | Randomized controlled trial to evaluate preference, satisfaction of patients, efficacy and economic burden between rituximab IV versus rituximab SCb | NR |
DLBCL and FL (n = 30) 1) Rituximab SC 2) Rituximab IV |
Productivity loss THB 291.28 versus 8.66 |
Infusion time, 212.4 versus 6.3 min |
| Delgado-Sánchez 2019 [95] | Spain | Retrospective study to compare the direct costs associated with the use of IV and SC rituximab, not only considering the drug price but also the costs of pharmacy handling, place occupation and administration in the day care unit. To determine whether the results could be modified with the availability of the new IV biosimilar of rituximab and develop an exploratory analysis in a nonlymphoma groupa | Son Espases University Hospital, the third-level reference hospital of Balearic Islands (database of the Pharmacy Department, Oncosafety®-AVIDA software) |
Adult patients with lymphoma (n = 105) 1) Rituximab IV 2) Rituximab SC |
Direct cost, €1955.94 versus €1460.01, saving of €496 (€1729 for IV biosimilar) Drug costs, €1458 versus €1335 Preparation, €4.49 versus €2.24 per cycle Day care unit cost, €493 versus €123 |
HCP time, day care unit time, 4 versus 1 h (standard practice times) |
| Di Rocco 2017 [96] | Italy | Prospective study to evaluate the costs of the two different formulations of rituximab (IV versus SC) combined with CHOP and the efficacy in terms of complete response rates and toxicitya | Department of Cellular Biotechnologies and Haematology, University of Rome Sapienza |
DLBCL (n = 71) 1) Rituximab SC 2) Rituximab IV |
Direct costs, €472,227 versus €449,870 for 35 and 36 patients, respectively | Chair time, 240 versus 135 min |
| Fisher 2017 [97] | USA | Prospective, observational, time-and-motion study to understanding the patient perspective regarding rituximab administration to make decisions between intravenous (IV) and SC methods of administrationa | NR |
FL, DLBCL and CLL patients (n = 40) 1) Rituximab SC 2) Rituximab IV |
– |
Time at infusion center, 319 min Time in infusion chair, 212 min |
| Irwin 2017 [98] | UK | Observational study to investigate cost savings and reduction in chair times for patients treated with chemotherapy regimens containing SC rituximab therapy compared to IV rituximab therapy in a single center in the UKa | University Hospital of Wales |
NHL and DLBCL (NR) 1) Rituximab SC 2) Rituximab IV |
– |
Rituximab maintenance: 150 versus 11 min (R-CHOP: 260 versus 130 min R-CVP: 135 versus 50 min) |
| Lebas 2018 [99] | France | Retrospective study to review the issues introduced by a switch from Rituximab (Mabthera®) to its biosimilar product and quantify what cost savings can be expecteda | Alpes Leman Hospital |
Patients who had been provided Rituximab (Mabthera) (n = 52) 1) Mabthera IV/SC 2) Truxima IV/SC |
Annual hospital saving €49,372 |
Annual total hospital infusion time saving 101 h |
| McBride 2018b [100] (linked with McBride 2018a [102]) | USA | Economic model to perform a time and cost simulation of reference IV, SC, and biosimilar IV rituximab from the US payer perspectived | Simulation study |
NHL (NR) 1) Rituximab SC 2) Rituximab IV-S 3) Rituximab IV-R90 |
Direct incremental costs: US$4261 higher if BSA, 1.62 m2 US$27 higher if BSA 1.85 m2 US$4,208 saving if BSA 2.1 m2 |
Chair time, administration time saving 2 h 9 min–2 h 23 min per infusion versus standard IV infusion (depending on BSA) |
| McBride 2018c [101] | USA | Economic model to conduct a time and cost simulation of RITUX single agent maintenance therapy for FL comparing SC RITUX, rRITUX, and bRITUX from the U.S. payer perspective following initiation therapyd | Simulation study |
FL (NR) 1) Rituximab SC 2) Rituximab IV-S 3) Rituximab IV-R90 |
Direct costs savings over 2 years: US$1120–19,331 depending on BSA 1.62–2.1 m2 | Chair time, administration time saving 2 h 10 min–2 h 24 min per infusion (depending on BSA) |
| McBride 2018a [102] (linked with McBride 2018b [100]) | USA | Economic model to perform a time and cost simulation of SC RITUX, rRITUX and bRITUX from the US payer perspectived | Simulation study |
NHL (NR) 1) Rituximab SC 2) Rituximab IV-S 3) Rituximab IV-R90 |
Direct costs savings of US$104–4012 depending on BSA (1.62–2.1 m2) | Chair time, administration time saving 2 h 10 min–2 h 24 min per infusion (depending on BSA) |
| Nikolov 2017 [103] | Macedonia | Cost-minimization analysis to identify and compare the total costs of subcutaneous versus intravenous administration of rituximab for the treatment of NHL patients in the Republic of Macedoniaa | Stem Cell Transplantation Unit, Outpatient Clinic, Clinical Department, University Clinic of Hematology, Skopje |
NHL (n = 220) 1) Rituximab SC 2) Rituximab IV |
Total direct cost saving, €75 (€1621 versus 1546) Savings: €12.86 for HCP time (€14.62 versus €1.76) €8.9 for chair occupying cost (€10.10 versus €1.20) |
Chair time, 6 h 12 min versus 10 min |
| Tomarchio 2017 [104] | Italy | Economic analysis to evaluate, in patients with DLBCL and FL, the economic and social impact of subcutaneous rituximab administrationa | NR |
DLBCL and FL (n = 40) 1) Rituximab IV 2) Rituximab SC |
Total direct cost saving: €274.49 Drug cost saving €156.27 per patient |
Patient time, total saving per eight-cycle course 17.5 h Nursing: 144 versus 111 min Pharmacy, 40 versus 19 min |
| Chansung 2018b [106] | Thailand | Retrospective study of rituximab SC and rituximab IV to investigate whether adopting rituximab SC would yield cost saving in payers’ perspectived | CHI of Thailand; HER |
DLBCL (n = 1011) 1) Rituximab SC 2) Rituximab IV |
THB 562 saving per cycle | – |
| Annibali 2017 [107] | Italy | Cross-sectional study to evaluate in DLBCL and FL the economic and social impact of SC Rituximaba | NR |
DLBCL (n = 45 patients; n = 45 caregivers) 1) Rituximab SC 2) Rituximab IV |
€274.49/patient saving | – |
| Gomes 2017 [108] | Brazil | Economic analysis to compare the total cost of rituximab IV versus SC in both indications approved by ANVISA for rituximab SC: FL first line and maintenance and DLBCL first linea | Hospital Geral de Curitiba |
FL and DLBCL patients (NR) 1) Rituximab SC 2) Rituximab IV |
Total savings: FL: 12,091.66 BRL DLBCL: 4454.82 BRL |
– |
| Kashiura 2018b [109] | Brazil | Economic analysis to estimate the budgetary impact of the introduction of subcutaneous rituximab, compared with intravenous rituximab, in the Brazilian Private Healthcare System, to treat diffuse large B-cell and follicular CD-20+ patients with NHLa | Data from National Regulatory Agency for Private Health Insurance and Plans; data from Survey performed with 28 HMOs |
NHL (n = 5783) 1) Rituximab SC 2) Rituximab IV |
BIM, savings over 5 years, 344.7 million BRL for large health maintenance organization, (0.4 million beneficiaries) | – |
| Rauf 2017 [110] | Kingdom of Saudi Arabia | Economic analysis to investigate the economic implications of using rituximab SC compared to IV formulation in KSA for NHLa | Clinical and cost inputs were obtained through discussion with medical oncologists and hematologists; other data came from budget impact model |
NHL (NR) 1) Mabthera IV 2) Mabthera SC |
BIM, total budget reduction Year 1: 1.6–4.4% Year 2: 3.3–8.8% Year 3: 4.9–13.2% |
– |
| Stewart 2018 [111] | Canada | Economic analysis to estimate the effect (on systemic therapy suite time and on the costs of drug acquisition and administration) of implementing SC rituximab in the initial chemoimmunotherapy for FL and DLBCL over 3 years in the Canadian marketa | Time-and-motion data from UK based study; key input parameters of the model were based on literature and chart review data; data outputs from authors’ model |
FL and DLBCL (NR) 1) Rituximab SC 2) Rituximab IV |
BIM, over 3 years: 128,715 systemic therapy suite hours saved Approximately Can$40 million saved in drug and administration costs |
– |
| Other agents—full publications | ||||||
| Cristino 2017 [112] | Czech Republic | Cost analysis to assess denosumab versus zoledronic acid in the prevention of SREs in adults with bone metastases from prostate cancer, BC, and OST (excluding hematological malignancies) from a national payer perspective in the Czech Republicd | Unit cost inputs for the model taken from multiple sources |
Prostate cancer, BC and patients with other solid tumors (NR) 1) Denosumab SC 2) Zoledronic acid IV |
Denosumab SC, CZK186.20 Zoledronic acid IV, CZK409.17 |
– |
| Li 2019 [113] | China | Randomized controlled trial to treat Chinese patients with malignant melanoma using a high dose of subcutaneous IFN-α or continuous intravenous IL-2 for 4 months. The secondary end point of the trial was to compare the efficacy and safety of IFN-α therapy with IL-2 therapy and to do the research that Chinese guidelines are inadequate when it comes to using of IFN-α as a cancer treatmenta | Cancer Hospital of China Medical University |
Malignant melanoma (n = 250) 1) INF-α SC 2) Continuous IL-2 IV |
Total direct cost/patient IFN-α SC, ¥105,345 IL-2 IV, ¥95,656; p < 0.0001 |
– |
| Raje 2018 [114] | USA | Cost analysis to estimate the incremental cost ratio and the net monetary benefit of denosumab versus zoledronic acid in patients with MM, from the perspectives of both society and payersd | Unit costs for the model taken from multiple sources |
Multiple myeloma (NR) 1) Denosumab SC 2) Zoledronic acid IV |
Denosumab SC administration, US$42.18 Zoledronic acid IV, US$184.17 US$ 2017 |
– |
| Stopeck 2012 [115] | USA | Cost analysis to assess denosumab relative to zoledronic acid for the prevention of SREs among patients with bone metastases secondary to castration-resistant prostate cancer, BC, or non-small cell lung cancer, based on a lifetime Markov cohort model from a US managed care perspective. Authors aimed to obtain incremental cost ratios including cost per quality-adjusted life year gained and cost per SRE avoidede | Unit costs for the model taken from multiple source |
Castration-resistant prostate cancer, BC, and non-small-cell lung cancer, and bone metastases (NR) 1) Denosumab SC 2) Zoledronic acid IV |
Denosumab SC administration, US$35.42 Zoledronic acid IV administration, US$154.64 US$ 2011 |
– |
| Zhang 2018 [116] | Australia, Belgium, Brazil, Canada, Czech Republic, Germany, Hungary, Denmark, Italy, Spain, France, Great Britain, Greece, Israel, Japan, Korea, Mexico, The Netherlands, Turkey, Ukraine, Poland, Russia, Sweden, Taiwan, USA | Cost analysis of DVd, Vd (both including SC bortezomib) and DRd (all IV) in patients with RRMM using data from two phase 3 trials (CASTOR and POLLUX)a | Multiple centers across different countries |
RRMM (n = 569 [POLLUX]; n = 498 [CASTOR]) 1) Lenalidomide and dexamethasone 2) Lenalidomide, dexamethasone and daratumumab OR 1) Bortezomib and dexamethasone 2) Bortezomib, dexamethasone and daratumumab |
Cost per administration: DRd: $134 DVd: $203 Vd: $69 |
|
| Body 2017 [117] | Belgium, Germany, Italy | Observational time-and-motion study to estimate total task time and total active healthcare professional time for predefined tasks associated with denosumab and zoledronic acid monotherapy administration. Secondary objectives included estimating patient time in the DOU, time in the treatment unit, and time in the treatment chair or on the examination tablea | Ten day-oncology units across Belgium, Germany, and Italy |
Bone metastases secondary to a solid tumor (n = 189) 1) Denosumab SC 2) Zoledronic acid IV |
– |
Zoledronic acid IV versus denosumab SC Total time per administration, 44.2 versus 8.4 min Administration time, 25.1 versus 1.5 min Active HCP time, 12.2 versus 6.9 min Chair time, 44.7 versus 7.3 min Treatment room time, 46.7 versus 12.3 min Patient time in hospital, 103 versus 69 min |
| Despiau 2017 [118] | France | Observational study to assess the consequences of this new route of administration on the organization of a day hospital in real conditions, at the oncology day hospital of the Toulouse University Cancer Institute Oncopolea | Institut universitaire du cancer Toulouse–Oncopole |
Patients with cancer (n = 48) 1) SC 2) IV |
– |
Duration of outpatient stay, 1 h shorter for SC versus IV administration Reflects 82% decrease in treatment duration Waiting times before treatment did not differ |
| Other agents—abstracts | ||||||
| Mateos 2019 [119] | Sweden, Poland, Czech Republic, Ukraine, Canada, UK, Japan, USA | Randomized, open-label, non-inferiority, phase 3 study. Study aim NRa | NR |
RRMM (n = 522) 1) Daratumumab SC 2) Daratumumab IV |
– |
Median duration for administration: IV: 421/255/205 min for the first/second/subsequent infusions SC: 5 min |
BIM budget impact model, BRL Brazilian real, BSA body surface area, CVAD, central venous access device; CHI, Center Office of Healthcare Information; CZK, Czech Republic Koruna; DLBCL, diffuse large B-cell lymphoma; DRd, daratumumab; DVd, bortezomib, daratumumab and dexamethasone; FL, follicular lymphoma; h hour, FTE full-time equivalents, HCP healthcare professional, HKD Hong Kong dollar, IFN-α interferon-alpha, IL-2 interleukin-2, IV intravenous, MBC metastatic breast cancer, MGC metastatic gastric cancer, min minute, NHL non-Hodgkin’s lymphoma, NHS National Health Service, NR not reported, NZD New Zealand dollar, R-CHOP rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone, R-CVP, rituximab, cyclophosphamide, vincristine sulfate and prednisone, RCT z controlled trial, SC subcutaneous, SLR systematic literature review, THB Thai Bhat
aThe perspective of this study was not explicitly stated in the publication
bThis study was conducted from the societal perspective
cThis study was conducted from the perspective of the UK NHS
dThis study was conducted from the payer perspective
eThis study was conducted from the US managed care perspective
Fig. 2.
Numbers of publications reporting time/resource use and costs for each agent/indication
A total of 28 publications were identified that described time/resource use and/or costs associated with rituximab SC versus IV administration for the treatment of NHL/FL or DLBCL. Nineteen of these publications reported on time/resource use, 11 of which also described related costs. There were an additional seven publications that reported only on costs, to give 18 publications with cost-related analyses. The remaining three publications described the likely budget impact of introducing the rituximab SC formulation for the treatment of NHL or DLBCL, and provided limited evidence relating to the comparative costs of rituximab SC and administrations.
Quality Assessment Results
A quality assessment of all full publications was conducted. Overall, the studies were considered to be of adequate quality. However, due to the wide range of study designs and the paucity of studies reporting individual outcomes, it was not feasible to categorize the studies according to risk. Although the study designs of the economic evaluations were generally well described, reporting of data collection methods and of analysis and interpretation of the results was inconsistent between studies. For example, time horizons of costs and benefits, discount rates, and sensitivity analyses were only discussed in a small proportion of the publications.
Time/Resource Use With IV Versus SC Administration of Trastuzumab
Of the 22 publications reporting data regarding time required, or the difference in time required, for administration of SC versus IV trastuzumab for the treatment of BC, 16 reported data either from time-and-motion studies or studies where the time for each specific procedure was directly measured. Some reported single-center studies, while others reported studies involving up to 16 centers. Two publications reported studies that estimated time based on information provided by drug preparation/administration software [51, 68]; one publication reported a study that estimated time from a survey of HCPs [66], and three publications did not report the manner in which time was estimated [52, 56, 58].
HCP time includes drug preparation and administration times, and may be reported according to specific roles (e.g., pharmacists, nurses, nursing assistants), or as an average of the HCP times. Variation in the description of the elements involved in preparation and administration of trastuzumab may limit comparison between publications. For example, one time-and-motion study publication [63] described the measured time for each step involved in preparation and administration, including involvement of the pharmacist, staff nurse, and clinical nurse specialist, and then provided the average HCP time required for administration based on this. Another publication [53] only reported active HCP times for preparation and administration, obtained from detailed case reports and stopwatch time measurements for all nurse activities for a subgroup of observed cases.
Preparation Time
Preparation time for trastuzumab was reported in seven publications, including two where only the difference in preparation time between SC and IV was reported. Within these, preparation time was directly measured [25, 49, 53, 62] or estimated from software records [51, 68] or HCP questionnaires [66]. HCP estimates were consistent with publications from studies in which time was measured directly. Preparation of IV trastuzumab for administration was reported to require 14–21 min, compared with 0–11 min for SC trastuzumab. The time difference between SC and IV was 3–14 min per preparation [25, 49, 51, 53, 62, 66, 68]. An additional publication reported preparation time for the loading dose to be 8 versus 2 min, for IV and SC trastuzumab, respectively. Nursing time was reported as 16 versus 7 min, and was deemed likely to relate to preparation rather than administration of the dose, giving a total time of 24 versus 9 min [61].
Administration Time
Administration time for trastuzumab was reported in five publications, of which four directly measured time [49, 53, 54, 62]; one publication reported estimated time from drug delivery software [51], which was found to be consistent with the other four publications. Administration times of 90 and 30 min were reported for IV trastuzumab loading and subsequent doses, respectively, in two of the publications [51, 54]. A further two publications reported times of 38 and 97 min for IV trastuzumab administration [53, 62]. In contrast, the reported times for SC trastuzumab administration ranged from 5 to 10 min, with no difference between loading and subsequent doses. The differences in administration time between IV and SC were 80–85 (loading dose) and 20–25 min (subsequent doses) [51, 54], and 32–107 min in the three publications in which loading/subsequent doses were not specified [49, 53, 62]. Additionally, two publications reported time savings of 47 min [60] and 61 min [48] with SC trastuzumab, for combined preparation and administration times.
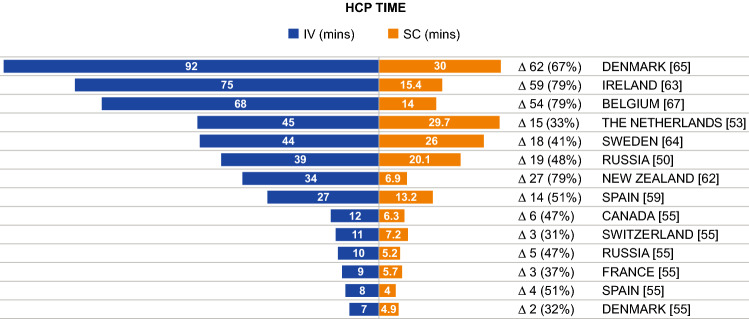
Active Healthcare Professional (HCP) Time
Active HCP time, and time savings with SC versus IV trastuzumab, were reported in nine publications and are shown in Fig. 3. Based on direct measurements, active HCP time was 13–92 min (IV) versus 7–30 min (SC); a difference of 6–62 min [50, 53, 59, 62–65, 67]. Two publications reported a longer administration time for the loading dose of IV trastuzumab (92 and 44 min); here, the time differences between the loading dose of IV and SC were 62 and 18 min [64, 65]. One publication reported only on the time difference (15 min) between IV and SC [57]. One other publication reported only the difference in HCP time (7 min), which was estimated based on drug preparation software [68]. One report of active HCP time included a range of 7–12 min (IV) versus 4–7 min (SC) [55]; however, it is unclear what was included within the time, and why the HCP times in this report were shorter than those of other publications. Active time differences were also reported for different HCPs and were all in favor of SC versus IV administration: differences in nursing times were 6.1 min [62] and 10.6 min [59]; pharmacist and nursing assistant time differences were 3.0 min and 0.3 min, respectively [59]; and one publication reported time savings of 0.18 full-time equivalents (FTE) and 0.14 FTE with SC for nurses and pharmacists, respectively [56].
Fig. 3.
HCP time as reported across included studies. HCP healthcare professional, IV intravenous, mins min, SC subcutaneous. Δ, difference in HCP time between IV and SC administration presented in min and as a percentage of total time
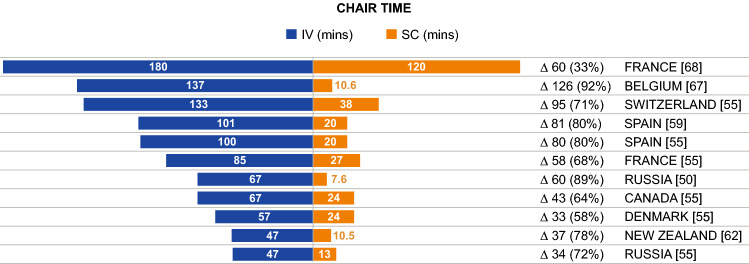
Patient Chair/Infusion Time
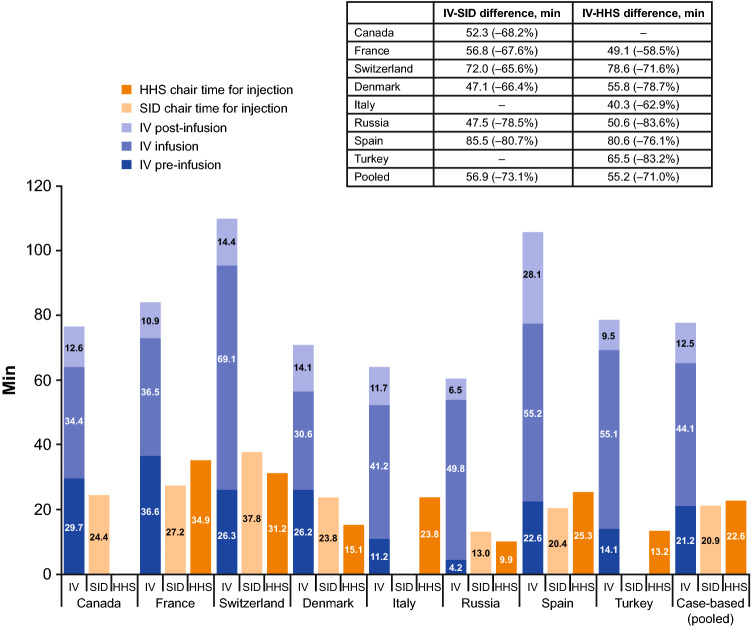
Chair time, where defined, was described consistently as the period between entry and exit from the infusion chair; however, how the time was determined varied between publications, with some reporting studies that measured the time directly [25, 59, 62, 67] and others reporting studies that estimated time from chemotherapy prescription software and HCP interviews [57, 68]. Differences in chair time for trastuzumab administration were reported in 10 publications [25, 50, 52, 55, 57–59, 62, 67, 68], of which seven reported actual chair times and differences obtained through direct measurement; one publication estimated the chair time based on drug delivery software [68] and was consistent with the other seven publications. Chair time was 47–180 min (IV) versus 8–120 min (SC); the difference in time was 33–126 min (Fig. 4). One publication reported a sixfold decrease in chair time with SC administration [58], with another describing a 56% reduction [52]. The PrefHer time-and-motion study reported chair time differences from nine countries (Fig. 5), ranging from a difference of 47.1 min (Denmark) to 85.5 min (Spain) for administration with the single-use injection device and 40.3 min (Italy) to 80.6 min (Spain) for administration with a hand-held syringe [25]. Total time spent at the hospital was also reported, ranging from 3–7 h (IV) to 1–5 h (SC), with a difference of 1.5–2 h [59, 64, 67]. One publication reported the difference for subsequent doses (following the loading dose) to be lower (23-min difference: 90 min IV vs. 67 min SC) [64].
Fig. 4.
Patient chair time reported across included studies. IV intravenous, mins min, SC subcutaneous. Δ, difference in chair time between IV and SC administration presented in min and as a percentage of total time
Fig. 5.
Differences in chair time for IV and SC administration for countries included in the PrefHer time-and-motion study. HHS hand-held syringe, IV intravenous, SC subcutaneous, SID single-use injection device.
Source: De Cock et al 2016 [25]
Costs Associated with IV Versus SC Administration of Trastuzumab
Costs for IV versus SC administration of trastuzumab were gathered from 24 publications with sufficient level of detail to show how the costs were defined. Costs were reported per administration, per treatment course, per patient per year, or for a particular cohort. Of these 24 publications, 18 reported costs based on data from time-and-motion studies or studies in which the time for specific procedures was directly measured; one based estimates on information provided by drug preparation/administration software [51], one estimated time from a survey of HCPs [70], and four did not report how time was estimated [56, 69, 82, 83]. Twelve of the publications were full publications, with the other 12 being congress abstracts with limited detail (Fig. 2).
Thirteen publications covering ten countries reported total direct medical costs for IV versus SC administration [51, 53, 54, 59, 60, 63, 64, 69, 82–86], which included non-drug costs for preparation, administration, HCP time and consumables, and savings related to reduced drug wastage or administered dose. All but four publications [51, 69, 82, 83] indicated that time assessments were made directly. Cost savings for SC compared with IV administration were reported in all but one publication, which reported data from a study conducted in Italy that used time estimates from drug delivery software rather than direct time assessments [51]. In this publication, total costs were numerically greater for SC administration; however, the difference was not statistically significant and was likely related to differences in drug acquisition costs as preparation and day hospital costs were shown to be significantly lower for SC administration [51].
In seven of the publications, direct costs for SC administration were approximately 1.3–6% lower than those for IV administration. One publication from a Russian study reported a 12.6% decrease [83] and one from the UK reported a 2.8-fold decrease [85] in direct costs with SC versus IV administration. Twelve publications provided information on costs directly related to active HCP time, preparation and/or administration time, patients’ time, or chair time [50, 53, 54, 56, 59, 62–65, 67, 84, 85]. All reported reduced time-related costs with SC versus IV administration.
In the five publications reporting total costs including indirect costs, indirect costs were lower for patients who received SC versus IV trastuzumab [51, 59, 63, 64, 71]. SC administration costs were also lower for consumables [49, 53, 54, 57, 59, 61–64, 67], drug wastage [67], use of central venous access devices [86], overheads [87], nursing and pharmacy supplies [84], and avoiding catheter implantation surgeries [54] compared with administration costs for IV in all publications that reported such information. One publication reported non-drug costs for the first and for subsequent cycles of therapy; reported costs were higher for the first cycle of therapy for both SC and IV administration [65]. One publication reported the costs for management of adverse events, which were slightly higher for SC versus IV delivery (US$1574 vs. US$1715 for 18 cycles) [71].
In addition to the 24 publications reporting cost-related data, five further publications were identified that reported on the budget impact of introducing SC trastuzumab; all reported cost savings across varying time periods [69, 72–75].
Time/Resource Use with IV Versus SC Administration of Rituximab
Nineteen publications (seven full publications [52, 66, 88–92] and 12 abstracts [93–104]) reported data on time required, or differences in time required, for IV versus SC rituximab for the treatment of lymphoma (NHL/FL or DLBCL in most publications) (Fig. 2). Of these, 12 reported data from time-and-motion studies or studies in which the time for specific procedures was directly measured, two estimated time from a survey of HCPs [66, 93], and five did not report how time was estimated [52, 89, 101].
Preparation Time
Preparation time or pharmacist time per infusion of rituximab was reported in five publications and ranged from 4–40 min (IV) to 2–20 min (SC) [66, 88, 90, 91, 104]. One of these publications reported a study that collected relevant data using a survey [66]; however, data were consistent with those estimated from direct measurement. In all publications, preparation/pharmacist time was shorter for SC administration, with time savings of 5.6–21 min (in one publication there was a marginal difference of 0.3 min (4.0 vs. 3.7 min) [90]).
Active HCP Time
Differences in HCP time per infusion of rituximab were reported in five publications and directly measured [88, 90–92, 104]. HCP times were 17–35 min (IV) compared with 12–24 min (SC) in three publications, whereas two publications reported longer times of 144 and 223 min (IV) versus 111 and 49 min (SC). All five publications found that SC administration was associated with a time saving. A further publication reported annual savings of nurse time to be 22 days for a single center in Tunisia [93] and one reported a time saving of 3 h in the day-care unit per infusion [95].
Patient Chair/Infusion Time
Chair time/infusion time for rituximab was reported in ten publications [88, 89, 92, 94, 96, 98, 100–103] and one further publication reported the time for IV administration [97]. For four of these publications, the method of time assessment was not reported; the remaining publications used direct time measurements. Chair time/infusion time was considerably shorter for SC versus IV administration in all publications, ranging from 150–262 min (IV; one publication reported a time of 6 h and 12 min (372 min)) to 6–11 min (46 and 135 min in two publications) for SC administration. Time saved with SC administration ranged from 1 h 45 min to 3 h 26 min (a saving of 6 h in one publication with a particularly long time for IV administration). A 74% reduction in chair time with SC administration was reported in another publication [52], and annual time savings per center of 101 h [99] and 193.5 days (administration time estimated using a survey) [93] were also reported. Time spent in the treatment room was directly measured and ranged from 264–321 min (IV) to 70–105 min (SC). Time savings with SC administration were ~200 min [66, 88, 92, 97]. One publication reported a time saving of 17.5 h per eight-cycle course of treatment [104].
Costs Associated with IV Versus SC Administration of Rituximab
Costs for management of patients with NHL/FL or DLBCL receiving IV versus SC administration of rituximab were gathered from 18 publications. Of these, 11 reported costs based on direct assessment of HCP time, one estimated time from a survey of HCPs [93], and six did not report how time was estimated [100–102, 105–107]. Only three of the publications were full papers; the rest were congress abstracts (Fig. 2). Three simulation analysis study publications conducted in the USA report cost savings for SC versus IV administration incrementally according to patient body surface area [100–102], which is used to calculate the IV dose (the SC dose is fixed). One publication reporting costs for rituximab maintenance therapy for FL over 2 years [101] and one publication reporting costs of rituximab as part of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone therapy (R-CHOP) for patients with NHL [102] reported higher cost savings for patients with higher body surface area (BSA). The other publication, which also reported costs of rituximab as part of R-CHOP therapy for NHL, reported the highest cost savings for patients in the highest and lowest BSA categories, respectively [100]. However, the reason for this discrepancy is unclear. Reductions in direct medical costs for SC versus IV administration were reported in all nine of the publications reporting European studies, all but one [107] of which estimated time savings based on direct measurements, and in four additional publications of studies from Tunisia (time savings estimated by HCP survey) [93], Thailand [94, 106], and Brazil [108]. In the European publications, non-drug-related cost savings included savings related to HCP time [53, 91, 92, 103], consumables [53, 91], and day-care unit costs [53, 95]. One of the publications from Thailand, which reported societal costs, reported a reduction in productivity loss with SC versus IV administration [94]. A further three congress abstracts describing the budget impact of introducing SC rituximab in Brazil, Saudi Arabia, and Canada were identified, all of which reported cost savings at 1, 2, 3, or 5 years [109–111].
Other Publications
In addition to the publications regarding trastuzumab and rituximab, eight other publications were identified that report relative time or cost information for IV versus SC administration (Table 2) [112–119]:
Three of four cost analysis publications were considered less relevant as they reported time and cost of supportive therapies (SC-administered denosumab vs. IV-administered zoledronic acid) rather than treatment with a targeted oncology drug [112, 114, 115]. The remaining cost analysis publication reported higher administration costs for the regimens containing SC bortezomib, daratumumab, and dexamethasone than for those containing daratumumab, lenalidomide, and dexamethasone (all IV) in patients with relapsed/refractory multiple myeloma from two Phase III clinical trials [116].
One publication from a randomized controlled trial at a single Chinese hospital reported significantly (p < 0.0001) lower direct costs per patient for high-dose SC interferon-alpha compared with continuous IV interleukin-2 administration in patients with malignant myeloma [113].
Three publications reported directly measured time assessments for various oncology therapies; all of these publications demonstrated reduced administration time for therapies administered by the SC route versus the IV route [117–119].
Discussion
Interpretation of Results
Global increases in yearly cancer rates have resulted in increased numbers of IV infusions of chemotherapies and anticancer biologics. This represents a growing burden for medical centers and HCPs, and has led to a shortage of chair time for patients with cancer. The substantially shorter administration times of therapies administered subcutaneously has the potential to offer several advantages over IV administration, including shorter treatment times, a reduction in healthcare resource use, increased convenience for patients, and greater patient preference [25, 39, 40, 120].
In this SLR, we identified 72 publications reporting on the time/resource use and/or costs associated with IV versus SC administration of oncology biologics in a hospital setting or on the budget impact of introducing an SC formulation. The majority of reported publications were of studies conducted in single countries or even single centers; all studies were published between 2012 and 2020.
Overall, the results were largely consistent in demonstrating the time savings associated with preparation and administration of SC therapies, across both oncology biologics and other supportive therapies. Moreover, reductions were seen in the HCP time and resource use (including non-drug consumables and drug wastage) required for SC versus IV therapy administration. Patient hospital time was also shorter with SC versus IV administration, and additional cost savings may be achieved at the society level due to a reduction in the loss of productivity and leisure time associated with patients attending the hospital for treatment. However, these improvements in patient productivity are likely to be greater in patients receiving maintenance therapy than those receiving SC-administered oncology biologics in combination with chemotherapy, due to the increased patient chair time required for chemotherapy administration. Cost savings due to reduced production and leisure time loss for SC versus IV trastuzumab across five Swedish oncology clinics were €78 and €62, respectively, for first-time patients and €10 and €6, respectively, for subsequent patients [64]. Similarly, a study conducted in six hospitals in the Netherlands reported lower societal costs (travel expenses and costs related to informal care and loss of productivity) for SC versus IV administration for both trastuzumab (cost saving of €22) and rituximab (cost saving of €28) [53].
There was some variation in times reported for IV and SC preparation and administration of trastuzumab, which may reflect differences in time estimate methodologies, definitions of time periods, and clinical practice/hospital setup between the different participating centers. Notably, the multinational PrefHer time-and-motion study reported time differences between countries [25], despite presumably using similar definitions for each time period across the different centers involved in the study. Similar variations were also seen with studies of rituximab [88]; however, a consistent trend in favor of SC administration was observed across all publications. During the COVID-19 pandemic, urgent cancer referrals and chemotherapy attendances declined by up to 84.3% and 63.4%, respectively, which might have resulted in increased mortality rates in patients with cancer and multimorbidity [121]. The time savings of SC administration have the potential to help increase throughput of patients now that cancer services have resumed. In addition to this, decreased hospital time for patients with cancer may help to reduce the risk of COVID-19 infection and the associated high probability of mortality in these patients [122].
As expected, there were also variations in costs for IV and SC administration between publications that could be compared based on use of the same currency. However, six European publications reported similar percentage savings in direct costs and most publications showed a trend for cost saving with SC versus IV administration of trastuzumab. Two of the rituximab publications that showed cost differences for the SC and IV formulations incrementally based on BSA reported that the largest cost savings occurred for patients with higher BSA.
The findings of this SLR are consistent with other published SLRs that have reported on the time, resource, and cost savings associated with SC administration versus IV, for both oncology and non-oncology biologics [123–125]. However, cost reductions associated with time savings for HCPs may be difficult to measure and achieve in clinical practice [126]. Therefore, methods of improving the transferability of time-related cost savings to the clinic should be investigated.
The purpose of this SLR was to ultimately inform economic modeling and associated health technology assessment of PH FDC SC. Recommendations for durations of post-administration surveillance for SC and IV trastuzumab are identical, with 6 h of observation recommended after the first dose and 2 h of observation for all subsequent doses [42, 127–129]. Within the context of this SLR, observation times for SC and IV cancel out (as they are the same). The efficacy and safety profiles of SC and IV trastuzumab were also assumed to be comparable in this study. However, real-world evidence suggests that target levels of trastuzumab may not be reached with the first SC administration in patients with a high body weight and, although cardiotoxicity risk does not appear to be increased in patients with low body weight, Phase III trials have reported higher rates of adverse events with SC vs. IV administration [130].
It is important to note that the focus of this SLR was on administration in the hospital setting only. However, similar to the benefits provided by other SC-administered oncology biologics [131–133], PH FDC SC is expected to offer advantages with regard to reduced time/resource utilization, improved patient quality of life, and the potential to be used in the future in a flexible care setting [43]. There is considerable evidence demonstrating that PH FDC SC is well suited to at-home administration by a HCP [134]. Providing at-home treatment requires planning, training, careful patient selection and technology to link patients, caregivers, and specialists in oncology clinics, as well as innovative methods for treatment delivery (e.g., mobile care units) [134]. A US expanded access study (NCT04395508) investigating at-home administration of PH FDC SC by a home health nursing provider is ongoing. This study focuses on patients with HER2-positive breast cancer previously treated with IV pertuzumab plus trastuzumab and chemotherapy and currently receiving or due to receive maintenance therapy with pertuzumab plus trastuzumab alone.
Strengths and Limitations
One strength of this SLR is that it identified studies with a range of designs, although some were described only as economic analyses with no additional clearly defined design details included. The inclusion of both clinical trials and studies conducted in the real-world setting suggests that the reported time and cost savings may be translated to SC versus IV oncology biologics administered during standard clinical practice. The publications included in this SLR also reported several different methodologies for time assessments; however, results from the two studies that reported time estimates from drug delivery software or based on HCP surveys were largely consistent with those from studies that used direct measurements from time-and-motion-type methodologies.
Although the majority of the trastuzumab and rituximab studies identified were performed in European countries, full-text publications were identified for studies conducted in Canada, Chile, China, New Zealand, and the USA, and abstracts identified for studies conducted in Ecuador, Hong Kong, Japan, Mexico, Panama, Singapore, Thailand, Tunisia, and Saudi Arabia. Despite the potentially limited generalizability of the conclusions due to country-specific differences in approaches to healthcare, the consistency of the evidence supporting SC-related time and cost savings compared with IV administration presented in this SLR suggest that the findings are likely to be applicable across different healthcare systems and countries.
Due to the large number of potentially relevant publications comparing SC with IV administration, the decision was made to focus the literature search on anticancer drugs. Although this pragmatic approach could lead to relevant data/insights being missed, the oncology publications included in this SLR can offer a chance of providing valuable insights into the impact of the route of administration on treatment costs.
The focus of this SLR was to identify potential time differences associated with SC versus IV administration, as well as differences in non-time-related cost elements such as non-drug consumables. As a result, the drug costs of the treatments being administered were not considered. However, it is important to acknowledge the need for decision makers to take a holistic approach to healthcare resource utilization, which accounts for both drug and non-drug costs, in order to ensure optimal management of resources.
In the future, more studies should address the economic benefits for different institutions of patients switching from IV to SC oncology biologics [54], rather than simply comparing different patient populations treated via either IV or SC administration. Furthermore, as the current economic assessments have mainly been performed in developed countries and there are concerns about the transferability of SC versus IV benefits to less developed countries [126], more studies should be conducted in regions such as Eastern Europe or Latin America to assess the applicability of our findings there.
Conclusion
This SLR indicates that there is a substantial body of evidence demonstrating oncology biologics administered by the SC route in a hospital setting to be associated with important time and resource use savings versus IV administration. The identified evidence provides valuable inputs for economic evaluations of PH FDC SC, or for other SC oncology treatments.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Support for third-party medical-writing assistance for this manuscript, furnished by Katie Wilson, PhD, and Brian Law, PhD, of Health Interactions, was provided by F. Hoffmann-La Roche Ltd.
Declarations
Funding
This research was supported by F. Hoffmann-La Roche Ltd.
Conflict of interest
All authors received research funding support in the form of third-party medical writing support from F. Hoffmann-La Roche Ltd. CM is an employee of Clarivate, which was commissioned to perform this systematic review by F. Hoffmann-La Roche Ltd, and received travel expenses from F. Hoffmann-La Roche Ltd to attend a project scoping meeting. MTO and SN are employees of Clarivate, which was commissioned to perform this systematic review by F. Hoffmann-La Roche Ltd. MJG and FM are employees of and hold shares in F. Hoffmann-La Roche Ltd.
Ethics approval
Not applicable. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The data supporting the findings of this study are available within the article or its supplementary materials.
Code availability
Not applicable.
Author contributions
All authors were involved in the concept and design of this systematic literature review (SLR). The protocol for this SLR was designed by CM, MTO, and SN, and was reviewed and approved by F. Hoffmann-La Roche Ltd. CM and MTO conducted the publication screening, data extraction, and quality assessments. SN was involved in quality control and offered strategic advice during all aspects of the project, including publication screening, data extraction, and quality assessments. CM and SN prepared the SLR report. All authors reviewed and critically revised the manuscript, approved the final version of the manuscript, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- 1.Ruiz-Fernández MD, Hernández-Padilla JM, Ortiz-Amo R, Fernández-Sola C, Fernández-Medina IM, Granero-Molina J. Predictor factors of perceived health in family caregivers of people diagnosed with mild or moderate Alzheimer's disease. Int J Environ Res Public Health. 2019;16(19):3762. doi: 10.3390/ijerph16193762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Cancer Observatory. Estimated number of deaths in 2020, worldwide, females, all ages. 2021. https://gco.iarc.fr/today/online-analysis-pie?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=total&sex=2&cancer=39&type=1&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=7&group_cancer=1&include_nmsc=1&include_nmsc_other=1&half_pie=0&donut=0. Accessed Dec 2021.
- 3.Patel A, Unni N, Peng Y. The changing paradigm for the treatment of HER2-positive breast cancer. Cancers (Basel). 2020;12(8):2081. doi: 10.3390/cancers12082081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ménard S, Fortis S, Castiglioni F, Agresti R, Balsari A. HER2 as a prognostic factor in breast cancer. Oncology. 2001;61(Suppl 2):67–72. doi: 10.1159/000055404. [DOI] [PubMed] [Google Scholar]
- 5.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 6.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 7.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 8.Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: The M77001 study group. J Clin Oncol. 2005;23(19):4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 9.Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet (London, England). 2010;375(9712):377–384. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 10.Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roche Registration Ltd. Herceptin® (trastuzumab). Summary of Product Characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/herceptin-epar-product-information_en.pdf. Accessed 1 June 2022.
- 12.Genentech Inc. HERCEPTIN® (trastuzumab). Prescribing Information. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/103792s5345lbl.pdf. Accessed 1 June 2022.
- 13.National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Breast Cancer. Version 1.2022. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed Dec 2021.
- 14.Arbeitsgemeinschaft Gynäkologische Onkologie (German Gynecological Oncology Group A. Guidelines of the AGO Breast Committee. 2022. https://www.ago-online.de/en/leitlinien-empfehlungen/leitlinien-empfehlungen/kommission-mamma. Accessed June 2022.
- 15.Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, André F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31(12):1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 17.Roche Registration Ltd. PERJETA® (pertuzumab). Summary of Product Characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/perjeta-epar-product-information_en.pdf. Accessed 1 June 2022.
- 18.Genentech Inc. PERJETA® (pertuzumab). Prescribing Information. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125409s124lbl.pdf. Accessed 1 June 2022.
- 19.Gianni L, Pienkowski T, Im YH, Tseng LM, Liu MC, Lluch A, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17(6):791–800. doi: 10.1016/S1470-2045(16)00163-7. [DOI] [PubMed] [Google Scholar]
- 20.von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377(2):122–131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swain SM, Miles D, Kim SB, Im YH, Im SA, Semiglazov V, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:519–530. doi: 10.1016/S1470-2045(19)30863-0. [DOI] [PubMed] [Google Scholar]
- 22.Roche Registration Ltd. Kadcyla® (trastuzumab emtansine). Summary of Product Characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/kadcyla-epar-product-information_en.pdf. Accessed June 2022.
- 23.Genentech Inc. KADCYLA® (ado-trastuzumab emtansine). Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125427s108lbl.pdf. Accessed June 2022.
- 24.von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 25.De Cock E, Pivot X, Hauser N, Verma S, Kritikou P, Millar D, et al. A time and motion study of subcutaneous versus intravenous trastuzumab in patients with HER2-positive early breast cancer. Cancer Med. 2016;5(3):389–397. doi: 10.1002/cam4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fallowfield L, Osborne S, Langridge C, Monson K, Kilkerr J, Jenkins V. Implications of subcutaneous or intravenous delivery of trastuzumab; further insight from patient interviews in the PrefHer study. Breast. 2015;24(2):166–170. doi: 10.1016/j.breast.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Jackisch C, Müller V, Maintz C, Hell S, Ataseven B. Subcutaneous administration of monoclonal antibodies in oncology. Geburtshilfe Frauenheilkd. 2014;74(4):343–349. doi: 10.1055/s-0034-1368173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shivakumar SP, Anderson DR, Couban S. Catheter-associated thrombosis in patients with malignancy. J Clin Oncol. 2009;27(29):4858–4864. doi: 10.1200/JCO.2009.22.6126. [DOI] [PubMed] [Google Scholar]
- 29.De Cock E, Semiglazov V, Lopez-Vivanco G, Verma S, Pivot X, Gligorov J, et al. Time savings with trastuzumab subcutaneous vs. intravenous administration: a time and motion study. In: Poster presentation at the 13th St Gallen International Breast Cancer Conference; 13–16 March 2013. Abstract XXX; St Gallen.
- 30.Genentech Inc. Herceptin Hylecta™ (trastuzumab and hyaluronidase-oysk). Prescribing Information. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761106s000lbl.pdf. Accessed Dec 2021.
- 31.Ismael G, Hegg R, Muehlbauer S, Heinzmann D, Lum B, Kim SB, et al. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I-III breast cancer (HannaH study): a phase 3, open-label, multicentre randomised trial. Lancet Oncol. 2012;13(9):869–878. doi: 10.1016/S1470-2045(12)70329-7. [DOI] [PubMed] [Google Scholar]
- 32.Jackisch C, Kim SB, Semiglazov V, Melichar B, Pivot X, Hillenbach C, et al. Subcutaneous versus intravenous formulation of trastuzumab for HER2-positive early breast cancer: updated results from the phase III HannaH study. Ann Oncol. 2015;26(2):320–325. doi: 10.1093/annonc/mdu524. [DOI] [PubMed] [Google Scholar]
- 33.Jackisch C, Stroyakovskiy D, Pivot X, Ahn JS, Melichar B, Chen SC, et al. Efficacy and safety of subcutaneous or intravenous trastuzumab in patients with HER2-positive early breast cancer after 5 years’ treatment-free follow-up: final analysis from the phase III, open-label, randomized HannaH study. Cancer Res. 2017;78(4 Suppl):Abstract PD3-11 (and associated poster presentation).
- 34.Jackisch C, Stroyakovskiy D, Pivot X, Ahn JS, Melichar B, Chen S-C, et al. Subcutaneous vs intravenous trastuzumab for patients with ERBB2-positive early breast cancer: Final analysis of the HannaH phase 3 randomized clinical trial. JAMA Oncol. 2019;5(5):e190339. doi: 10.1001/jamaoncol.2019.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kümmel S, Tondini CA, Abraham J, Nowecki Z, Itrych B, Hitre E, et al. Subcutaneous trastuzumab with pertuzumab and docetaxel in HER2-positive metastatic breast cancer: Final analysis of MetaPHER, a phase IIIb single-arm safety study. Breast Can Res Treat. 2021;187(2):467–476. doi: 10.1007/s10549-021-06145-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swain SM, Kim S-B, Cortés J, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14(6):461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pivot X, Gligorov J, Müller V, Barrett-Lee P, Verma S, Knoop A, et al. Preference for subcutaneous or intravenous administration of trastuzumab in patients with HER2-positive early breast cancer (PrefHer): an open-label randomised study. Lancet Oncol. 2013;14(10):962–970. doi: 10.1016/S1470-2045(13)70383-8. [DOI] [PubMed] [Google Scholar]
- 40.Pivot X, Gligorov J, Müller V, Curigliano G, Knoop A, Verma S, et al. Patients' preferences for subcutaneous trastuzumab versus conventional intravenous infusion for the adjuvant treatment of HER2-positive early breast cancer: final analysis of 488 patients in the international, randomized, two-cohort PrefHer study. Ann Oncol. 2014;25(10):1979–1987. doi: 10.1093/annonc/mdu364. [DOI] [PubMed] [Google Scholar]
- 41.Pivot X, Spano JP, Espie M, Cottu P, Jouannaud C, Pottier V, et al. Patients' preference of trastuzumab administration (subcutaneous versus intravenous) in HER2-positive metastatic breast cancer: results of the randomised MetaspHer study. Eur J Cancer. 2017;82:230–236. doi: 10.1016/j.ejca.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 42.Genentech Inc. PHESGO (pertuzumab, trastuzumab, and hyaluronidase-zzxf). Prescribing Information. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761170s000lbl.pdf. Accessed 1 June 2022.
- 43.O'Shaughnessy J, Sousa S, Cruz J, Fallowfield L, Auvinen P, Pulido C, et al. Preference for the fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection in patients with HER2-positive early breast cancer (PHranceSCa): a randomised, open-label phase II study. Eur J Cancer. 2021;152:223–232. doi: 10.1016/j.ejca.2021.03.047. [DOI] [PubMed] [Google Scholar]
- 44.Higgins J, Thomas J. Cochrane handbook for systematic reviews of interventions. Version 6.2. 2021. https://training.cochrane.org/handbook/current. Accessed Dec 2021.
- 45.Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ. 1996;313(7052):275–283. doi: 10.1136/bmj.313.7052.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drummond MF, Sculpher MJ, Torrance GW, O'Brien BJ, Stoddart GL. Methods for the exonomic evaluation of health care programmes. 3. Oxford: Oxford University Press; 2005. [Google Scholar]
- 47.Molinier L, Bauvin E, Combescure C, Castelli C, Rebillard X, Soulié M, et al. Methodological considerations in cost of prostate cancer studies: a systematic review. Value Health. 2008;11(5):878–885. doi: 10.1111/j.1524-4733.2008.00327.x. [DOI] [PubMed] [Google Scholar]
- 48.Andrade S, Santos A. Hospital resources consumption associated with trastuzumab treatment in breast cancer in Portugal. Value Health. 2013;16(7):Abstract PCN147.
- 49.Blein C, bernard Marty C, Priou V, Borg MC, mouret-Reynier M, Lebozec G, et al. A multicentric evaluation of consumables and transports cost of breast cancer patient’s treated by trastuzumab according to the administration form (IV versus SC). Value Health. 2018;21(Suppl 3):Abstract PCN95.
- 50.De Cock E, Pan YI, Tao S, Baidin P. Time savings with transtuzumab subcutaneous (SC) injection verse trastuzumab intravenous (IV) infusion: A time and motion study in 3 Russian centers. Value Health. 2014;17(7):Abstract PCN221. [DOI] [PubMed]
- 51.Farolfi A, Silimbani P, Gallegati D, Petracci E, Schirone A, Altini M, et al. Resource utilization and cost saving analysis of subcutaneous versus intravenous trastuzumab in early breast cancer patients. Oncotarget. 2017;8(46):81343–81349. doi: 10.18632/oncotarget.18527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Favier M, Le Goc-Sager F, Vincent-Cantini I, Launay V, Giroux EA, Lievremont K, et al. Medico-economic benefits of subcutaneous formulations of trastuzumab and rituximab in day hospitalisation (SCuBA Study) Bull Cancer. 2018;105(10):862–872. doi: 10.1016/j.bulcan.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 53.Franken MG, Kanters TA, Coenen JL, de Jong P, Koene HR, Lugtenburg PJ, et al. Potential cost savings owing to the route of administration of oncology drugs: a microcosting study of intravenous and subcutaneous administration of trastuzumab and rituximab in the Netherlands. Anticancer Drugs. 2018;29(8):791–801. doi: 10.1097/CAD.0000000000000648. [DOI] [PubMed] [Google Scholar]
- 54.Hedayati E, Fracheboud L, Srikant V, Greber D, Wallberg S, Linder SC. Economic benefits of subcutaneous trastuzumab administration: a single institutional study from Karolinska University Hospital in Sweden. PLoS ONE. 2019;14(2):e0211783. doi: 10.1371/journal.pone.0211783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jackisch C, Müller V, Dall P, Neumeister R, Park-Simon TW, Ruf-Dördelmann A, et al. Subcutaneous trastuzumab for HER2-positive breast cancer—evidence and practical experience in 7 German centers. Geburtshilfe Frauenheilkd. 2015;75(6):566–573. doi: 10.1055/s-0035-1546172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee VW, Cheng F. Cost-minimization analysis of trastuzumab intravenous versus trastuzumab subcutaneous regimen for breast cancer management in Hong Kong. Value Health. 2018;21(Suppl 2):Abstract PCN57. [DOI] [PubMed]
- 57.Lewis P, Jones H, Skelley K, Simpson R, Beresford M. Switching to subcutaneous trastuzumab administration: quantifying the benefits. Clin Oncol. 2017;29(6):Abstract E101.
- 58.López MA, Samanes MS, Tena IP, Alonso EF, Turlan VC, Sanchez MC, et al. Switching from intravenous to subcutaneous formulation of TRASTUZUMAB: Costs and safety. Eur J Hosp Pharm. 2017;24(Suppl 1):Abstract CP-127.
- 59.Lopez-Vivanco G, Salvador J, Diez R, López D, De Salas-Cansado M, Navarro B, et al. Cost minimization analysis of treatment with intravenous or subcutaneous trastuzumab in patients with HER2-positive breast cancer in Spain. Clin Transl Oncol. 2017;19(12):1454–1461. doi: 10.1007/s12094-017-1684-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nestorovska A, Naumoska Z, Grozdanova A, Stoleski D, Ivanovska A, Risteski M, et al. Subcutaneous vs intravenous administration of trastuzumab in HER2+ breast cancer patients: a Macedonian cost-minimization analysis. Value Health. 2015;18(7):Abstract PCN188.
- 61.Nierenberger A, Gessier F, Forges F, Simoens X. Subcutaneous trastuzumab versus intravenous trastuzumab: an impact study. Eur J Hosp Pharm. 2017;24(Suppl 1):Abstract PP-034.
- 62.North RT, Harvey VJ, Cox LC, Ryan SN. Medical resource utilization for administration of trastuzumab in a New Zealand oncology outpatient setting: a time and motion study. Clinicoecon Outcomes Res. 2015;7:423–430. doi: 10.2147/CEOR.S85599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O'Brien GL, O'Mahony C, Cooke K, Kinneally A, Sinnott SJ, Walshe V, et al. Cost minimization analysis of intravenous or subcutaneous trastuzumab treatment in patients with HER2-positive breast cancer in Ireland. Clin Breast Cancer. 2019;19(3):e440–e451. doi: 10.1016/j.clbc.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 64.Olofsson S, Norrlid H, Karlsson E, Wilking U, Ragnarson TG. Societal cost of subcutaneous and intravenous trastuzumab for HER2-positive breast cancer—an observational study prospectively recording resource utilization in a Swedish healthcare setting. Breast. 2016;29:140–146. doi: 10.1016/j.breast.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 65.Olsen J, Jensen KF, Olesen DS, Knoop A. Costs of subcutaneous and intravenous administration of trastuzumab for patients with HER2-positive breast cancer. J Comp Eff Res. 2018;7(5):411–419. doi: 10.2217/cer-2017-0048. [DOI] [PubMed] [Google Scholar]
- 66.Ponzetti C, Canciani M, Farina M, Era S, Walzer S. Potential resource and cost saving analysis of subcutaneous versus intravenous administration for rituximab in non-Hodgkin's lymphoma and for trastuzumab in breast cancer in 17 Italian hospitals based on a systematic survey. Clinicoecon Outcomes Res. 2016;8:227–233. doi: 10.2147/CEOR.S97319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tjalma WAA, Van den Mooter T, Mertens T, Bastiaens V, Huizing MT, Papadimitriou K. Subcutaneous trastuzumab (Herceptin) versus intravenous trastuzumab for the treatment of patients with HER2-positive breast cancer: a time, motion and cost assessment study in a lean operating day care oncology unit. Eur J Obstet Gynecol Reprod Biol. 2018;221:46–51. doi: 10.1016/j.ejogrb.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 68.Vangheluwe E, Giraud J, Pingaud C. Medico-economic impacts of use of subcutaneous formulations of rituximab and trastuzumab in outpatient care units. Eur J Oncol Pharm. 2018;1(3S):Abstract 191.
- 69.Coombes M, Yin L, Liu I, Shek N, Rusu F, Mukherjee S. Subcutaneous trastuzumab for the treatment of HER2+ breast cancer in Canada: a cost-minimization study. Cancer Res. 2020;80(4 Suppl):Abstract P6-13-02.
- 70.Ghosh W, Lim S, Wong A. Cost-minimisation analysis of subcutaneous versus intravenous trastuzumab for the treatment of early breast cancer and metastatic breast cancer in Singapore. Value Health. 2018;21:Abstract PCN55.
- 71.Rojas L, Muñiz S, Medina L, Peña J, Acevedo F, Pinto MP, et al. Cost-minimization analysis of subcutaneous versus intravenous trastuzumab administration in Chilean patients with HER2-positive early breast cancer. PLoS ONE. 2020;15(2):e0227961. doi: 10.1371/journal.pone.0227961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.A N, Ali A, Al-Tweigeri T, Zekri J, Awad N, Safwat M, et al. Financial impact of introducing SC trastuzumab (Herceptin) versus currently used IV trastuzumab (Herceptin) on the budgets of different hospitals across Kingdom of Saudi Arabia (KSA). Value Health. 2017;20(9):Abstract PCN60.
- 73.Kashiura D, Souza PV, Garrido SD, Nardi E, Alves M. Budget impact model of subcutaneous trastuzumab compared with intravenous trastuzumab on the treatment of HER-2 positive breast cancer in the brazilian private healthcare system. Value Health. 2018;21(Suppl 3):Abstract PCN75.
- 74.Poquet-Jornet JE, Carrera Hueso J, Crespo C, Cuesta-Grueso C, Ramón Barrios MA, Gasent-Blesa JM, et al. Budget impact analysis with use of subcutaneus trastuzumab. Value Health. 2018;21(Suppl 3):Abstract PCN80.
- 75.Calvache J, Briceno V. Budget impact analysis of the use of trastuzumab SC in the treatment of HER2 positive in public health institutions of Ecuador. Value Health. 2017;20.
- 76.Agirrezabal I, Gaikwad I, Cirillo L, Lothgren M. Predicted treatment costs and savings per patient of Kanjinti® (trastuzumab biosimilar) vs. subcutaneous (SC) and intravenous (IV) Herceptin® and other trastuzumab biosimilars in italy. Value Health. 2018;21(Suppl 3):Abstract PCN103.
- 77.D'Arpino A, Savoia M, Cirillo L, Despiégel N, Haffemayer B, Giannopoulou A, et al. Comparative cost analysis of subcutaneous trastuzumab originator (Herceptin®) vs intravenous trastuzumab biosimilar (Kanjinti) from a hospital perspective in Italy. Value Health. 2019;22(Suppl 3):Abstract PCN72.
- 78.Jang Y, Byrne A, Toron F, Yoon S. Budget impact analysis of intravenous biosimilars compared with intravenous originators and subcutaneous products. Value Health. 2018;21(Suppl 3):Abstract PMU26.
- 79.Todorovic V, Durutovic I, Ivanovska A, Zajmovic A. Subcutaneous vs intravenous administration of trastuzumab in HER2+ breast cancer patients: a montenegrin cost-minimization analysis. Value Health. 2017;20(9):Abstract PCN172.
- 80.Villarreal-Garza C, O-Maldonado CDl, Díaz-Pérez H, Mesa-Chavez F, García-García M, Cardona-Huerta S, et al. Cost and time savings of subcutaneous trastuzumab (SC-T) in a public health system in Mexico. J Clin Oncol. 2019;37(15 Suppl):Abstract e18387.
- 81.De La Vega ZI, Celia A, Manuel P, Agustín S, Miguel C. Economic impact of the introduction of subcutaneous trastuzumab in the pharmacotherapeutic guide. Eur J Clin Pharm. 2017;19(3):213–215. [Google Scholar]
- 82.Lazaro Cebas A, Cortijo Cascajares S, Pablos Bravo S, Del Puy Goyache Goni M, Gonzalez Monterrubio G, Perez Cardenas MD, et al. Subcutaneous versus intravenous administration of trastuzumab: preference of HER2+ breast cancer patients and financial impact of its use. J BUON. 2017;22(2):334-9. [PubMed]
- 83.Kulikov A, Rybchenko Y. Pharmacoeconomic evaluation of the use of trastuzumab for subcutaneous administration compared to intravenous dosage form in the treatment of breast cancer. Value Health. 2015;18(7):Abstract PCN189.
- 84.Martin C, Alcedo J, Araúz E. Comparative analysis of costs between subcutaneous formulation of trastuzumab versus intravenous formulation from the perspective of the Instituto Oncológico Nacional of Panamá from January to December 2016. Cancer Res. 2018;78(4 Suppl):Abstract P4-12-3.
- 85.Mitchell H, Morrissey D. Intravenous versus subcutaneous trastuzumab: an economic and patient perspective. Br J Nurs. 2019;28(10):S15–S20. doi: 10.12968/bjon.2019.28.10.S15. [DOI] [PubMed] [Google Scholar]
- 86.Mylonas C, Skroumpelos A, Fountzilas G, Maniadakis N. Cost minimization analysis of Herceptin subcutaneous versus herceptin intravenous treatment for patients with HER2+ breast cancer in Greece. J Cancer Policy. 2017;13:11–17. doi: 10.1016/j.jcpo.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 87.Mylonas C, Kourlaba G, Fountzilas G, Skroumpelos A, Maniadakis N. Cost-minimization analysis of trastuzumab intravenous versus trastuzumab subcutaneous for the treatment of patients with HER2+ early breast cancer and metastatic breast cancer in Greece. Value Health. 2014;17(7):A640–A641. doi: 10.1016/j.jval.2014.08.2310. [DOI] [PubMed] [Google Scholar]
- 88.De Cock E, Kritikou P, Sandoval M, Tao S, Wiesner C, Carella AM, et al. Time savings with rituximab subcutaneous injection versus rituximab intravenous infusion: a time and motion study in eight countries. PLoS ONE. 2016;11(6):e0157957. doi: 10.1371/journal.pone.0157957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lugtenburg P, Avivi I, Berenschot H, Ilhan O, Marolleau JP, Nagler A, et al. Efficacy and safety of subcutaneous and intravenous rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in first-line diffuse large B-cell lymphoma: the randomized MabEase study. Haematologica. 2017;102(11):1913–1922. doi: 10.3324/haematol.2017.173583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mihajlovic J, Bax P, van Breugel E, Blommestein HM, Hoogendoorn M, Hospes W, et al. Microcosting study of rituximab subcutaneous injection versus intravenous infusion. Clin Ther. 2017;39(6):1221–1232. doi: 10.1016/j.clinthera.2017.05.342. [DOI] [PubMed] [Google Scholar]
- 91.Fargier E, Ranchon F, Huot L, Guerre P, Safar V, Dony A, et al. SMABcare study: subcutaneous monoclonal antibody in cancer care: cost-consequence analysis of subcutaneous rituximab in patients with follicular lymphoma. Ann Hematol. 2018;97(1):123–131. doi: 10.1007/s00277-017-3147-y. [DOI] [PubMed] [Google Scholar]
- 92.Rule S, Collins GP, Samanta K. Subcutaneous vs intravenous rituximab in patients with non-Hodgkin lymphoma: a time and motion study in the United Kingdom. J Med Econ. 2014;17(7):459–468. doi: 10.3111/13696998.2014.914033. [DOI] [PubMed] [Google Scholar]
- 93.Lakhal RB, Kacem K, Raoudha M, Jabeur D, Denguir MS, Bouattour H, et al. Cost-minimization and budget impact analysis of mabthera subcutaneous formulation in non-Hodgkin’s lymphoma (NHL): a case study from Tunisia. HemaSphere. 2019;3:Abstract PB2294.
- 94.Chansung K, Chuncharunee S, Khuhapinant A, Bunworasate U, Norasetthada L, Prayongratana K. Preference of rituximab subcutaneous (R-SC) in Thai patients with diffused large b-cell lymphoma (DLBCL) and follicular lymphoma (FL): a Thai context. Value Health. 2018;21(Suppl 2):Abstract PCN67.
- 95.Delgado Sánchez O, Gutiérrez A, do Pazo F, Ginés J, Martorell C, Boyeras B, et al. Comparative cost analysis of intravenous and subcutaneous administration of rituximab in lymphoma patients. Clinicoecon Outcomes Res. 2019;11:695-701. [DOI] [PMC free article] [PubMed]
- 96.Di Rocco A, Scerbo G, Ansuinelli M, al e. Efficacy, safety and cost analysis of subcutaneous vs intravenous rituximab in patients with diffuse large b-cell lymphoma treated with RCHOP. Haematologica. 2017;102:108.
- 97.Fisher MD, Wallick C, Miller PJ, Walker MS, Lash S, Dawson KL, et al. How time spent on rituximab infusion impacts patient satisfaction, stress, employment, and caregiver burden. Blood. 2017;130(Suppl 1):Abstract 5666.
- 98.Irwin S, Rowntree C, Cosh H, Bloodworth C. Positive benefits of changing from intravenous rituximab administration to subcutaneous administration: a single centre experience. British Journal of Haematology. 2017;176:Abstract E1151.
- 99.Lebas E, Guillotel R, Evrard J, Kugarajah R, Lassiaz C, Diakhate C. Time and money: the issues of setting up a rituximab biosimilar in hospital. Eur J Oncol Pharm. 2018;1:Abstract 197.
- 100.McBride A, Balu S, Campbell K, MacDonald K, Abraham I. Economic modeling for the US of intravenous vs subcutaneous rituximab in non-Hodgkin’s lymphoma treated with R-CHOP. Value Health. 2018;21(Suppl 3):Abstract PCN93.
- 101.McBride A, Balu S, Campbell K, MacDonald K, Abraham I. Economic modeling for the U.S. of intravenous versus subcutaneous rituximab as single-agent maintenance therapy in follicular lymphoma. J Manag Care Spec Pharm. 2018;24(10A Suppl):Abstract C29.
- 102.McBride A, Balu S, Campbell K, MacDonald K, Abraham I. Subcutaneous versus intravenous rituximab in non-Hodgkin lymphoma treated with R-CHOP: Economic modeling for the US. Blood. 2018;132(Suppl 1):Abstract 4776.
- 103.Nikolov O, Sterjev Z, Dimovski A, Kapedanovska-Nestorovska A, Naumovska Z, Grozdanova A, et al. Cost-minimization analysis of rituximab subcutaneous formulation versus intravenous administration of rituximab for the treatment of non-hodgkin’s lymphoma in the Republic of Macedonia. Haematologica. 2017;102:Abstract E1471.
- 104.Tomarchio V, Surano MA, Tafuri MA, Becilli M, Sarlo C, Berti P, et al. Management, economic and social impact of sub-cutaneous rituximab administration in lymphoproliferative malignancies. Haematologica. 2017;102(Suppl 1):Abstract P736.
- 105.Cicchetti A, Coretti S, Mascia D, Mazzanti N, Refolo P, Rolli FR, et al. Assessing social and economic impact of subcutaneous mAbs in oncology. Glob Reg Health Technol Assess. 2018;5(1):1–9. [Google Scholar]
- 106.Chansung K, Sirijerachai C, Teawtrakul N. Cost minimization study between intravenous rituximab (r-iv) vs subcutaneous rituximab (R-SC) in Thai patients with diffused large b-cell lymphoma (DLBCL): a simulation of electronic health record (E-HR) and evidence synthesis. Value Health. 2018;21(Suppl 2):Abstract PSY9.
- 107.Annibali O, Tomarchio V, Becilli M. Management, economic and social impact of sub-cutaneous rituximab administration in diffuse large b cell lymphoma (DLBCL) and follicular lymphoma. Haematologica. 2017;102:111. [Google Scholar]
- 108.Gomes G, Ho R, Rufino C, Alves M. Budget impact analysis of rituximab IV versus SC from public Brazilian hospital. Value Health. 2017;20(5):Abstract PCN63.
- 109.Kashiura D, Souza PV, Sa AB, Santos BR, Nardi E, Alves M. Budget impact model of subcutaneous rituximab compared with intravenous rituximab on the treatment of CD-20 positive non-Hodgkin lymphoma in the Brazilian private healthcare system. Value Health. 2018;21:Abstract PCN67.
- 110.Rauf M, Dada R, Awad N, Safwat M, Narang A, Goyal R. To determine the financial imapct of introducing SC rituximab (MABTHERA) vs. currently used IV rituximab (MABTHERA) on the budgets of different hospitals across the Kingdom of Saudi Arabia (KSA). Value Health. 2017;20(9):Abstract PCN69.
- 111.Stewart DA, Boudreault JS, Maturi B, Boras D, Foley R. Evaluation of subcutaneous rituximab administration on Canadian systemic therapy suites. Curr Oncol. 2018;25(5):300–306. doi: 10.3747/co.25.4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cristino J, Finek J, Jandova P, Kolek M, Pásztor B, Giannopoulou C, et al. Cost-effectiveness of denosumab versus zoledronic acid for preventing skeletal-related events in the Czech Republic. J Med Econ. 2017;20(8):799–812. doi: 10.1080/13696998.2017.1328423. [DOI] [PubMed] [Google Scholar]
- 113.Li S, Wu X, Chen P, Pei Y, Zheng K, Wang W, et al. Interferon-alpha versus interleukin-2 in Chinese patients with malignant melanoma: a randomized, controlled, trial. Anticancer Drugs. 2019;30(4):402–409. doi: 10.1097/CAD.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Raje N, Roodman GD, Willenbacher W, Shimizu K, García-Sanz R, Terpos E, et al. A cost-effectiveness analysis of denosumab for the prevention of skeletal-related events in patients with multiple myeloma in the United States of America. J Med Econ. 2018;21(5):525–536. doi: 10.1080/13696998.2018.1445634. [DOI] [PubMed] [Google Scholar]
- 115.Stopeck A, Rader M, Henry D, Danese M, Halperin M, Cong Z, et al. Cost-effectiveness of denosumab vs zoledronic acid for prevention of skeletal-related events in patients with solid tumors and bone metastases in the United States. J Med Econ. 2012;15(4):712–723. doi: 10.3111/13696998.2012.675380. [DOI] [PubMed] [Google Scholar]
- 116.Zhang TT, Wang S, Wan N, Zhang L, Zhang Z, Jiang J. Cost-effectiveness of daratumumab-based triplet therapies in patients with relapsed or refractory multiple myeloma. Clin Ther. 2018;40(7):1122–1139. doi: 10.1016/j.clinthera.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 117.Body JJ, Gatta F, De Cock E, Tao S, Kritikou P, Wimberger P, et al. An observational time and motion study of denosumab subcutaneous injection and zoledronic acid intravenous infusion in patients with metastatic bone disease: Results from three European countries. Support Care Cancer. 2017;25(9):2823–2832. doi: 10.1007/s00520-017-3697-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Despiau F, Zagala Y, Delord JP, Montastruc M, Lacaze JL, Ferrand R, et al. Observational study of outpatient unit duration of stay depending on the route of administration (intravenous vs subcutaneous) for a targeted therapy. Bull Cancer. 2017;104(10):869–874. doi: 10.1016/j.bulcan.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 119.Mateos MV, Nahi H, Legiec W. Efficacy and safety of the randomized, open-label, non-inferiority, phase 3 study of subcutaneous (SC) versus intravenous (IV) daratumumab (DARA) administration in patients (pts) with relapsed or refractory multiple myeloma (RRMM): COLUMBA. J Clin Oncol. 2019;37(15 Suppl):Abstract 8005.
- 120.Wynne C, Harvey V, Schwabe C, Waaka D, McIntyre C, Bittner B. Comparison of subcutaneous and intravenous administration of trastuzumab: a phase I/Ib trial in healthy male volunteers and patients with HER2-positive breast cancer. J Clin Pharmacol. 2013;53(2):192–201. doi: 10.1177/0091270012436560. [DOI] [PubMed] [Google Scholar]
- 121.Lai AG, Pasea L, Banerjee A, Hall G, Denaxas S, Chang WH, et al. Estimated impact of the COVID-19 pandemic on cancer services and excess 1-year mortality in people with cancer and multimorbidity: near real-time data on cancer care, cancer deaths and a population-based cohort study. BMJ Open. 2020;10:e043828. doi: 10.1136/bmjopen-2020-043828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tagliamento M, Agostinetto E, Bruzzone M, Ceppi M, Saini KS, de Azambuja E, et al. Mortality in adult patients with solid or hematological malignancies and SARS-CoV-2 infection with a specific focus on lung and breast cancers: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2021;163:103365. doi: 10.1016/j.critrevonc.2021.103365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Anderson KC, Landgren O, Arend RC, Chou J, Jacobs IA. Humanistic and economic impact of subcutaneous versus intravenous administration of oncology biologics. Future Oncol. 2019;15(28):3267–3281. doi: 10.2217/fon-2019-0368. [DOI] [PubMed] [Google Scholar]
- 124.Tetteh E, Morris S. Systematic review of drug administration costs and implications for biopharmaceutical manufacturing. Appl Health Econ Health Policy. 2013;11(5):445–456. doi: 10.1007/s40258-013-0045-x. [DOI] [PubMed] [Google Scholar]
- 125.Bittner B, Richter W, Schmidt J. Subcutaneous administration of biotherapeutics: an overview of current challenges and opportunities. BioDrugs. 2018;32(5):425–440. doi: 10.1007/s40259-018-0295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Inotai A, Agh T, Karpenko AW, Zemplenyi A, Kalo Z. Behind the subcutaneous trastuzumab hype: evaluation of benefits and their transferability to Central Eastern European countries. Expert Rev Pharmacoecon Outcomes Res. 2019;19(2):105–113. doi: 10.1080/14737167.2019.1554437. [DOI] [PubMed] [Google Scholar]
- 127.Roche Registration Ltd. Herceptin® (trastuzumab). Summary of Product Characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/herceptin-epar-product-information_en.pdf. Accessed Sept 2021.
- 128.Genentech Inc. Herceptin® (trastuzumab). Prescribing Information. 2021. https://www.gene.com/download/pdf/herceptin_prescribing.pdf. Accessed Sept 2021.
- 129.Roche Registration GmbH. PHESGO® (pertuzumab and trastuzumab). Summary of Product Characteristics. 2022. https://www.ema.europa.eu/documents/product-information/phesgo-epar-product-information_en.pdf. Accessed 1 June 2022.
- 130.Kolberg HK, Jackisch C, Hurvitz SA, Winstone J, Barham H, Hanes V, et al. Is weight-based IV dosing of trastuzumab preferable to SC fixed-dose in some patients? A systematic scoping review. Breast. 2021;57. [DOI] [PMC free article] [PubMed]
- 131.Wright JM, Jones GB. Developing the subcutaneous drug delivery route. Med Res Archiv. 2017;5(12).
- 132.Anderson KC, Landgren O, Arend RC, Chou J, Jacobs IA. Humanistic and economic impact of subcutaneous versus intravenous administration of oncology biologics. Fut Oncol. 2019;15(28):3267–3281. doi: 10.2217/fon-2019-0368. [DOI] [PubMed] [Google Scholar]
- 133.Altini M, Gentili N, Balzi W, Musuraca G, Maltoni R, Masini C, et al. The challenge of sustainability in healthcare systems: economic and organizational impact of subcutaneous formulations for rituximab and trastuzumab in onco-hematology. Expert Rev Pharmacoeconomics Outcomes Res. 2020:1–7. [DOI] [PubMed]
- 134.Wardley A, Canon JL, Elsten L, Pena Murillo C, Badovinac Crnjevic T, Fredriksson J, et al. Flexible care in breast cancer. ESMO Open. 2021;6(1):100007. doi: 10.1016/j.esmoop.2020.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.