Abstract
The aim of this real-life, big data population-based study was to evaluate differences in symptomatic presentation of children infected with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) between the third and fourth waves of the pandemic in Israel, dominated by the Alpha and Delta variants, respectively. Our cohort included all children and adolescents, members of the second-largest Health Maintenance Organization in Israel that had positive real-time polymerase chain reaction (RT-PCR) test during the third and fourth waves of the pandemic (December 1, 2020, to April 30, 2021, and June 1, 2021, to October 10, 2021, respectively). A total of 32,485 and 44,130 children and adolescents in the third and fourth waves were included in the final analysis. The rate of children with symptomatic disease among patients with documented SARS-CoV-2 infection was higher in the fourth wave compared to the third wave (49.9% vs. 37.5%). The most commonly reported symptom and the only symptom that substantially differed between waves was fever, with 33% of SARS-CoV-2 infected children in the fourth wave vs. 13.6% in the third wave. Preschool children had the lowest prevalence of febrile illness compared to other age groups.
Conclusion: Children and adolescents infected during the fourth wave of the pandemic in Israel, a Delta-dominant period, had a significantly higher rate of symptomatic febrile illness than the Alpha-dominant period. This phenomenon occurred across all age groups.
|
What is Known: • There are differences in COVID-19 severity among adults and children during different waves of the pandemic. • There is a paucity of data regarding symptomatic characteristics in children in large-scale cohorts aside from hospital settings. | |
|
What is New: • In a time period dominated by the Delta variant, there were substantially more children with symptomatic disease and febrile illness compared to a period dominated by the alpha variant. • Preschool children had the lowest rate of febrile illness among all age groups. |
Supplementary Information
The online version contains supplementary material available at 10.1007/s00431-022-04531-7.
Keywords: Coronavirus, Fever, Variants, Symptoms
Introduction
The novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) virus has caused over 250 million confirmed cases of coronavirus disease 2019 (COVID-19) globally and more than 5 million deaths as of November 2021 [1]. Although children and adolescents are more susceptible to certain infectious diseases due to their immature immune system [2], they had a less severe presentation of COVID-19 with significantly lower morbidity and mortality rates than adults [3–5].
Pediatric hospitalization rates and outcomes were the focus of early research on COVID-19 [6]. However, as most children are diagnosed and followed in the community, there is a paucity of data regarding symptoms in children with mild disease.
Children infected with COVID-19 present with a broad spectrum of non-specific symptoms [7], and a substantial amount of them (about 25%) were considered asymptomatic [3], a presentation more prominent among younger children [8].
Israel, like other countries, underwent a few surges during the COVID-19 pandemic since the initial occurrence in March 2020, with a probable different dominant variant at each period [9]. In the third and fourth waves, which account for most COVID-19 cases in Israel, the Alpha (B.1.1.7) [10] and the Delta (B.1.617.2) [11] were the most dominant variants (Supplemental Fig. 1S) [12]. Few reports to date investigated the differences in the manifestations attributed to different variants in the pediatric population, and these have found that the variant might influence the clinical characteristics [13] and disease severity [14] of COVID-19 among children.
The present population-based study aimed to explore the clinical characteristics of SARS-CoV-2 infection reported in children and adolescents treated in ambulatory settings during different periods dominated by the Alpha and the Delta variants.
Methods
Data sources
In this study, we used data originating from Maccabi Healthcare Services (MHS). MHS is the second-largest Health Maintenance Organization (HMO) in Israel, accounting for 26.7% of the Israeli population. Membership in an HMO is compulsory in Israel under the National Health Insurance Law of 1995, according to which all Israeli citizens must join one of the four official HMOs.
MHS has a centralized computerized database that contains extensive longitudinal data of a stable population of over 2.5 million individuals (of which 32% are children aged 0–16), with less than 1% annual turnover. The database contains demographic data, ambulatory and hospital diagnoses, anthropometric measurements, medication dispensed, and comprehensive laboratory data from a single central lab.
Study design and population
A retrospective cross-sectional study was conducted. The study included children and adolescents under the age of 16 with SARS-CoV-2 infection confirmed by a positive real-time polymerase chain reaction (RT-PCR) test during the third and fourth waves of the pandemic for whom a symptoms questionnaire was available (see below). In Israel, PCR tests are readily available and offered for free, and the tests are obtained from nasopharyngeal swabs using nationally approved SARS-CoV-2 PCR testing kits [15, 16].
Children were considered to have contracted SARS-CoV-2 in the third and the fourth wave if they tested positive between December 1, 2020, and April 30, 2021, and between June 1, 2021, and October 10, 2021 (end of analysis), respectively. As different variants characterized Israel’s third and fourth waves, we did not include individuals infected during May 2021 to minimize the possible overlap between the variants. Patients with previously positive SARS-CoV-2 PCR tests and patients who received the COVID-19 vaccine were excluded.
Data extraction and definition of the study variables
Symptoms data
According to the MHS workflow during the pandemic, upon a positive PCR test, a member’s pediatrician or family physician was notified and obligated to initiate a phone call visit to assess the child’s health status and whether the child has any symptoms. Correspondingly, in the electronic medical record, the physician filled in a structured symptom questionnaire (during the following 48 h in most of the cases). If the patient presented with symptoms that were not included in the structured symptoms list, the physician could add them as free text. The physician remained in contact with the patient family, and in case of symptoms appearance, the questionnaire was updated (occurred in approximately 10% of the cases). During data collection, our team tagged the free text information into structured binary categories and later assembled these categories into four groups as detailed below:
Respiratory symptoms: cough, runny nose, sore throat, shortness of breath, ear pain, and hoarseness
Gastrointestinal symptoms: abdominal pain, vomiting, diarrhea, nausea, loss of appetite
Neurology symptoms: disturbances of smell or taste, dizziness, headache
General and other symptoms: fever, chills, fatigue, myalgia, conjunctivitis, ocular pain, chest pain, restlessness, weakness
Age groups
We categorized age into categorical variables using the following cutoffs: 0–1 years old (infants), 1–3 years old (toddlers), 3–6 years old (preschoolers), 6–12 years old (middle childhood), and 12–16 years old (adolescents) to measure the magnitude of the difference in symptoms across the different age groups.
Covariates
Individual-level data of the study population included demographics, namely, age, sex, and socioeconomic status (SES), measured on a scale from 1 (lowest) to 10.
Statistical analysis
We performed two separate analyses: the first included the whole cohort, both symptomatic and asymptomatic SARS-CoV-2 infected patients, and the second comprised solely of the fraction of patients who had at least one symptom, defined as COVID-19 patients.
We summarized the patients’ characteristics as mean ± standard deviation for continuous data and as percentages for categorical data. We then compared the proportion of symptoms between the two waves. All outcomes were reported in the overall population and the sub-group by age. Linear regression models with quadratic, cubic, and linear terms were evaluated using the R2 value to determine the best fit functional form between age and prevalence of fever.
Due to the large population size, p values were accompanied by standardized mean differences (the difference between the two groups’ means divided by the pooled SD), in which a standardized mean difference greater than 0.2 was considered meaningful.
Statistical analyses were performed using SPSS statistical software version 27 (IBM).
Ethics declaration
The study was approved by the MHS (Maccabi Healthcare Services) Institutional Review Board (IRB).
Results
Primary analysis: all infected individuals
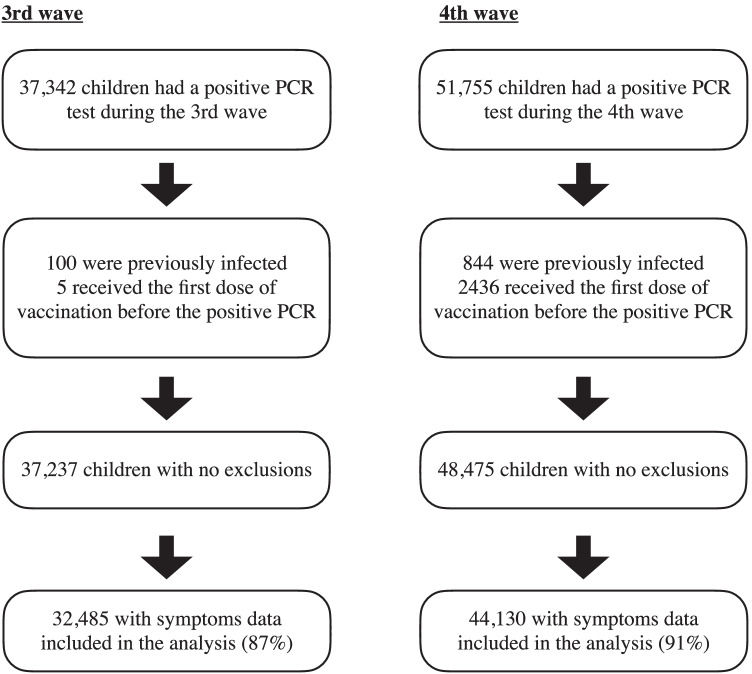
A total of 32,485 and 44,130 children were eligible for the analysis in the third and fourth waves, respectively. Figure 1 reveals a flowchart of the exclusion criteria. The mean age of the children with SARS-CoV-2 infection was 8.54 ± 4.55 and 7.86 ± 3.84 years old, respectively (SMD = 0.16). Gender proportion was similar between the two waves.
Fig. 1.
Flowchart of the children infected with SARS-CoV-2 included in the study cohort. Polymerase chain reaction (PCR). Period of the third wave: December 1, 2020–April 30, 2021. Period of the fourth wave: June 1, 2021–October 10 (the end of analysis)
Demographics and rate of symptoms during both the third and fourth waves are presented in Table 1. We found a significant difference in the proportion of symptomatic children infected with SARS-CoV-2 between the third and the fourth waves with 37.5% and 49.9% of the children with SARS-CoV-2 infection that had at least one reported symptom, respectively (SMD = 0.25). Fever was the most prevalent symptom in both waves and was 2.4-fold more prevalent in the fourth wave than the third one (SMD = 0.47). The differences between the two waves’ respiratory, gastrointestinal, and neurology symptoms were negligible. Hospitalization due to COVID-19 was rare and similar between the waves with 84 (0.3%) and 96 (0.2%) cases in the third and fourth waves, respectively.
Table 1.
Demographic and symptoms proportion by wave in the overall cohort
| 3rd wave | 4th wave | SMD | |
|---|---|---|---|
| Age, mean (SD), years | 8.54 ± 4.55 | 7.86 ± 3.84 | 0.16 |
| Gender, % male | 51.8 | 51.2 | 0.013 |
| Socioeconomic status, median (IQR) | 5 (4–7) | 6 (4–7) | 0.282 |
| Symptoms | |||
| Any symptom no. (%) | 12,198 (37.5) | 22,007 (49.9) | 0.25 |
| General symptoms, n (%) | 6671 (20.5) | 16,680 (37.8) | 0.387 |
| Fever | 4409 (13.6) | 14,552 (33.0) | 0.472 |
| Weakness | 2426 (7.5) | 3938 (8.9) | 0.053 |
| Myalgia | 1489 (4.6) | 2259 (5.1) | 0.025 |
| Restlessness | 108 (0.3) | 69 (0.2) | 0.036 |
| Conjunctivitis | 46 (0.1) | 155 (0.4) | 0.042 |
| Chest pain | 16 (0.0) | 25 (0.1) | 0.003 |
| Ocular pain | 41 (0.1) | 90 (0.2) | 0.019 |
| Fatigue | 60 (0.2) | 96 (0.2) | 0.007 |
| Chills | 34 (0.1) | 23 (0.1) | 0.019 |
| Neurology symptoms, n (%) | 3377 (10.4) | 5306 (12.0) | 0.052 |
| Headache | 2366 (7.3) | 4095 (9.3) | 0.072 |
| Disturbances in smell and taste | 1152 (3.5) | 1418 (3.2) | 0.018 |
| Dizziness | 56 (0.2) | 68 (0.2) | 0.005 |
| Gastrointestinal symptoms, n (%) | 1217 (3.7) | 2120 (4.8) | 0.052 |
| Diarrhea | 698 (2.1) | 962 (2.2) | 0.002 |
| Abdominal pain | 349 (1.1) | 685 (1.6) | 0.042 |
| Vomiting | 121 (0.4) | 437 (1.0) | 0.075 |
| Loss of appetite | 90 (0.3) | 105 (0.2) | 0.008 |
| Nausea | 42 (0.1) | 129 (0.3) | 0.036 |
| Respiratory symptoms, n (%) | 6605 (20.3) | 9269 (21.0) | 0.017 |
| Cough | 4499 (13.8) | 6438 (14.6) | 0.021 |
| Runny nose | 1698 (5.2) | 2589 (5.9) | 0.028 |
| Sore throat | 1106 (3.4) | 1219 (2.8) | 0.037 |
| Breath shortness | 272 (0.8) | 318 (0.7) | 0.013 |
| Hoarseness | 30 (0.1) | 36 (0.1) | 0.004 |
| Earache | 35 (0.1) | 46 (0.1) | 0.001 |
| Hospitalization | 84 (0.3) | 96 (0.2) | 0.04 |
Standardized mean difference (SMD) is the difference between the groups’ means divided by the pooled SD
IQR interquartile range, SD standard deviation, CNS central nervous system
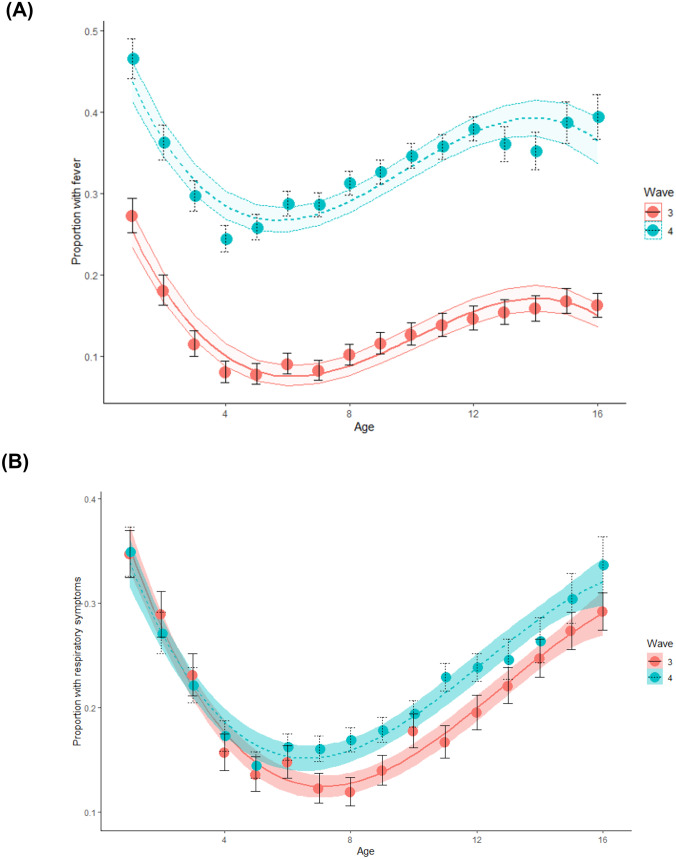
The proportion of symptoms by age is summarized in Table 2. Across all age groups, the proportion of general symptoms was more prevalent, ranging from 1.5 to 2.6-folded prevalence in the fourth wave compared to the third. Among the components of the general symptoms, fever was significantly more frequent (1.7–3.8-fold more prevalent) in the fourth wave than the third wave across all age groups (Fig. 2).
Table 2.
Symptoms proportion stratified by wave and age group in the entire cohort
| 0–1 years | 1–3 years | 3–6 years | 6–12 years | > 12 years | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wave | 3 | 4 | SMD | 3 | 4 | SMD | 3 | 4 | SMD | 3 | 4 | SMD | 3 | 4 | SMD |
| n | 1696 | 1585 | 3339 | 4102 | 5339 | 8957 | 12,983 | 23,346 | 9128 | 6140 | |||||
| General and other symptoms, n (%) | 557 (32.8) | 784 (49.5) | 0.34 | 607 (18.2) | 1431 (34.9) | 0.38 | 581 (10.9) | 2595 (29.0) | 0.46 | 2277 (17.5) | 8954 (38.4) | 0.47 | 2649 (29.0) | 2916 (47.5) | 0.38 |
| Respiratory symptoms, n (%) | 589 (34.7) | 553 (34.9) | < 0.01 | 867 (26) | 1000 (24.4) | 0.03 | 783 (14.7) | 1424 (15.9) | 0.03 | 1995 (15.4) | 4562 (19.5) | 0.11 | 2371 (26) | 1730 (28.2) | 0.05 |
| Gastrointestinal symptoms, n (%) | 144 (8.5) | 130 (8.2) | 0.01 | 205 (6.1) | 263 (6.4) | 0.01 | 167 (3.1) | 367 (4.1) | 0.05 | 429 (3.3) | 1126 (4.8) | 0.07 | 272 (3.0) | 234 (3.8) | 0.04 |
| Neurology symptoms, n (%) | 3 (0.2) | 4 (0.3) | 0.01 | 14 (0.4) | 32 (0.8) | 0.04 | 102 (1.9) | 353 (3.9) | 0.12 | 1410 (10.9) | 3481 (14.9) | 0.12 | 1848 (20.2) | 1436 (23.4) | 0.07 |
SMD (standardized mean difference) is the difference between the groups’ means divided by the pooled SD
Fig. 2.
(A) Rate of febrile illness by age in the third and fourth waves. (B) Rate of respiratory systems by age among symptomatic children in the third and fourth wave
During both waves, infants had the highest rate of febrile illness with a decrease in the rate until the age of 6 years (preschool children) and a steady increase in older children and adolescents, creating a J-like curve (Fig. 2).
Secondary analysis: only symptomatic individuals
There were 12,198 and 22,007 children with at least one symptom in the third and fourth waves. Those children composed the COVID-19 patients’ cohort. There was a significant difference in the proportions of general symptoms between the two waves reported in 54.6% and 75.7% (SMD = 0.45) among COVID-19 patients in the third and fourth wave, respectively. Fever was the most dominant symptom and the only component with a significant difference. In the third and fourth waves, it was reported in 36.1% and 66.1% (SMD = 0. 62) among COVID-19 patients, respectively. We also found a reduced frequency of respiratory symptoms in the fourth wave compared with the third (42.1% vs. 54.1% respectively, SMD = 0.24), which was more prominent in younger ages (up to 6 years) presented in Fig. 2b. The proportion of gastrointestinal and neurology symptoms was similar between the two waves. The demographic, clinical characteristics, and difference in symptoms according to age groups are presented in Supplemental digital content (SDC) Tables 3 and 4.
Table 3.
Demographic and symptoms proportion by wave in symptomatic patients
| 3rd Wave | 4th Wave | SMD | |
|---|---|---|---|
| No. of patients | 12,198 | 22,007 | |
| Age, mean (SD), years | 9.26 ± 4.89 | 8.29 ± 4.00 | 0.17 |
| Gender, % male | 49.6 | 49.6 | < 0.01 |
| Socioeconomic status, median (IQR) | 5 (4–7) | 6 (4–7) | 0.317 |
| General symptoms, n (%) | 6671 (54.6) | 16,680 (75.7) | 0.45 |
| Fever | 4409 (36.1) | 14,552 (66.1) | 0.62 |
| Weakness | 2426 (19.8) | 3938 (17.8) | 0.05 |
| Myalgia | 1424 (11.6) | 2122 (9.64) | 0.06 |
| Restlessness | 108 (0.88) | 69 (0.31) | 0.07 |
| Conjunctivitis | 46 (0.37) | 155 (0.70) | 0.04 |
| Chest pain | 16 (0.13) | 25 (0.11) | 0.005 |
| Ocular pain | 41 (0.33) | 90 (0.40) | 0.01 |
| Fatigue | 60 (0.49) | 96 (0.43) | 0.008 |
| Chills | 34 (0.27) | 23 (0.10) | 0.03 |
| Neurology symptoms, n (%) | 3377 (27.6) | 5306 (24.1) | 0.08 |
| Headache | 2366 (19.3) | 4095 (18.6) | 0.01 |
| Disturbances in smell and taste | 1152 (9.44) | 1418 (6.44) | 0.11 |
| Dizziness | 56 (0.45) | 68 (0.30) | 0.02 |
| Gastrointestinal symptoms, n (%) | 1217 (9.97) | 2120 (9.63) | 0.01 |
| Diarrhea | 698 (5.72) | 962 (4.37) | 0.06 |
| Abdominal pain | 349 (2.86) | 685 (3.11) | 0.01 |
| Vomiting | 121 (0.99) | 437 (1.98) | 0.08 |
| Loss of appetite | 90 (0.73) | 105 (0.47) | 0.03 |
| Nausea | 42 (0.34) | 129 (0.58) | 0.03 |
| Respiratory symptoms, n (%) | 6605 (54.1) | 9269 (42.1) | 0.24 |
| Cough | 4499 (36.8) | 6438 (29.2) | 0.16 |
| Runny nose | 1698 (13.9) | 2589 (11.7) | 0.06 |
| Sore throat | 1106 (9.06) | 1219 (5.53) | 0.13 |
| Breath shortness | 272 (2.22) | 318 (1.44) | 0.05 |
| Hoarseness | 30 (0.24) | 36 (0.16) | 0.01 |
| Earache | 35 (0.28) | 46 (0.20) | 0.01 |
IQR interquartile range, SD standard deviation; SMD (standardized mean difference) is the difference between the groups’ means divided by the pooled SD
Table 4.
Symptoms proportion stratified by wave and age group in the symptomatic patients
| 0–1 years | 1–3 years | 3–6 years | 6–12 years | 12–16 years | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wave | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | |||||
| n | 897 | 999 | SMD | 1231 | 1908 | SMD | 1262 | 3457 | SMD | 4231 | 11,816 | SMD | 4557 | 3827 | SMD |
| General and other symptoms, n (%) | 557 (62.0) | 784 (78.4) | 0.36 | 581 (46.0) | 1431 (75.0) | 0.54 | 581 (46.0) | 2595 (75.0) | 0.62 | 2277 (53.8) | 8954 (75.7) | 0.47 | 2649 (57.8) | 2916 (76.1) | 0.39 |
| Respiratory symptoms, n (%) | 589 (65.6) | 553 (55.3) | 0.21 | 867 (70.4) | 1000 (52.4) | 0.37 | 783 (62.0) | 2371 (47.1) | 0.42 | 1995 (47.1) | 4562 (38.6) | 0.17 | 2371 (51.8) | 1730 (45.2) | 0.13 |
| Gastrointestinal symptoms, n (%) | 144 (16.0) | 130 (13.0) | 0.08 | 205 (16.6) | 263 (13.7) | 0.08 | 167 (13.2) | 367 (10.6) | 0.08 | 429 (10.1) | 1126 (9.5) | 0.02 | 272 (5.9) | 234 (6.1) | < 0.01 |
| Neurology symptoms, n (%) | 3 (0.3) | 4 (0.4) | 0.01 | 14 (1.1) | 32 (1.6) | 0.04 | 102 (8.0) | 353 (10.2) | 0.07 | 1410 (33.3) | 3481 (29.4) | 0.08 | 1848 (40.3) | 1436 (37.5) | 0.05 |
SMD (standardized mean difference) is the difference between the groups' means divided by the pooled SD
Discussion
Our study represents one of the largest pediatric cohorts to date describing symptoms of SARS-CoV-2 infected patients in ambulatory settings. The study describes changes in the rate of symptoms in different time frames where different variants were dominant. Specifically, it demonstrates that fever was the most common symptom with different rates in different age groups and significantly more dominant during the fourth wave in Israel that was dominated by the Delta variant. The rate of febrile illness during the fourth wave was 33% among SARS-CoV-2 infected patients and 66% among COVID-19 patients (symptomatic patients).
Differences in symptoms during differing pandemic periods were described in smaller cohorts. Chua et al. described the clinical characteristics and transmission patterns among children and youths with COVID-19 in Hong Kong in 2020 [13]. Their study found significant differences in the clinical presentations across the three waves of outbreaks (all before the Delta variant became dominant). The number of symptomatic children declined in the second and third waves compared to the first, but absolute numbers were low, and screening patterns might have changed over time. Somekh et al. described the increased spreading effectiveness of the Alpha variant in children compared to the first two waves of the pandemic in Israel; however, they did not report symptoms [14].
The difference in febrile illness among different age groups is intriguing and enhances some of the assumptions of why children have less severe diseases than adults [5]. Preschool children had the lowest rate of febrile illness. Preschool children are post their toddler years where they were exposed to numerous viral illnesses that might enhance their innate and adapted immune response to coronaviruses in general.
Aydillo et al. [17] followed patients hospitalized with COVID-19 and looked for preexisting immunity for seasonal coronavirus. They found evidence for preexisting immunity that affects the humoral response to SARS-CoV-2, the effect was in vitro, and they concluded that whether these antibodies contribute to protection or disease is unclear. Anderson et al. [18] demonstrated that human serum samples before and after the onset of the COVID-19 pandemic with antibodies against common seasonal human coronaviruses are cross-reactive against SARS-CoV-2 but do not confer cross-protection against infection or hospitalization. Similarly, Renk et al. [19] found that seasonal coronavirus antibody responses were not associated with a lower likelihood of seroconversion following SARS-CoV-2 exposure. In contrast, Gouma et al. [20] suggest that recent seasonal coronavirus infections potentially limit the duration of symptoms following SARS-CoV-2 infections through mechanisms that do not involve cross-reactive antibodies and postulate that cellular immune responses are involved. Therefore, in our opinion, cellular and innate immunity but not humoral response to seasonal coronavirus are involved in the mechanism of protection against symptomatic illness. This assumption is strengthened by our findings of the steady increase of febrile illness as children advance in age after the age of 6 years. This population of preschool children should be the focus of further assessments in order to look for the complex mechanism behind this phenomenon. Interestingly, infants had the highest level of febrile response. This finding is in line with descriptions of higher morbidity in infants compared to older children [21].
The proportion of fever and other symptoms in children was described in a few studies. The largest was an international network cohort described by Duarte-Salles et al. [6]. They described fever as the most common symptom ranging from 4.8 to 26.4% in different countries. This study took place before the emergence of the Delta variant. In another study from the USA, Parcha et al. described 12,306 COVID-19 patients. They have shown, similarly to our study, that fever was the most common symptom. In another cohort from the beginning of the pandemic in the USA (data collection ended May 2020), Stokes et al. [22] described fever as the most common symptom in children. Recently, Molteni et al. [23] showed similar results to our findings with an increasing rate of general symptoms and fever with the Delta variant compared to the Alpha variant in children in the UK. Their data was based on self-report by parents rather than physician questionnaires.
The strength of our study relates to the structure of the healthcare system in Israel, which offers extensive medical care to the general public at no cost with countrywide geographical coverage, allowing excellent access to care; MHS is the second-largest health maintenance organization in Israel with a diverse population that represents the Israeli population [24]. Specifically, during the COVID-19 pandemic, PCR tests were offered at no cost for symptomatic patients and individuals exposed to verified SARS-CoV-2 patients [25], allowing us to present the full spectrum of the disease in children from asymptomatic patients to patients with a variety of symptoms.
The limitation of the study includes the type of interaction between the physician and the patient’s parents, which was based mainly on a telephone interview and not on office appointments. In addition, the questionnaire was filled once in most of the cases; post-positive PCR results, limiting the opportunity to capture symptoms occurring later in the disease course, therefore, might underestimate that the actual rate of symptomatic infections PCR results in the time period of the study did not undergo genomic sequencing; nevertheless, monitoring by the ministry of health showed that the third and fourth waves were dominated by the Alpha and Delta wave, respectively [10, 11]. This study was limited to children because symptoms comparison in adults between waves could not be made during the same time period as most Israeli population was already vaccinated during the fourth wave.
Conclusion
In conclusion, there was a significant difference in the rate of symptomatic febrile illness in children infected with SARS-CoV-2, along with differences in respiratory symptoms between times where the Alpha and Delta variants were dominant. These findings imply that different variants have different virulence and clinical presentations. Given the rapidly evolving pandemic, the clinical relevance of emerging variants is important to public health. Therefore, ongoing surveillance of symptoms in large cohorts is crucial to provide meaningful and accurate information to clinicians and policymakers.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1: The proportion of total number of sequences over time, that falls into defined variant groups, the Alpha variant in red and the Delta variant in green. Adapted from: Hodcroft EB (2022) CoVariants: SARS-CoV-2 Mutations and Variants of Interest. https://covariants.org/ (PDF 80 KB)
Abbreviations
- COVID-19
Coronavirus disease 2019
- HMO
Health Maintenance Organization
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus-2
Authors’ contributions
Acquisition of data: ABT, RL, SG, GP. Analysis and interpretation of data: All authors. Drafting of the manuscript: ABT, RL. Critical revision of the manuscript for important intellectual content: SG, GC, GP, MMR, TP. Study supervision: TP. All authors had full access to all of the data and approved the final version of this manuscript. All authors take responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Funding
No funding interest to declare.
Availability of data and material
N/A.
Code availability
N/A.
Declarations
Ethics approval
The study was approved by the MHS (Maccabi Healthcare Services) Institutional Review Board (IRB).
Consent to participate
N/A.
Consent for publication
All authors approved the latest version of the manuscript.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Amir Ben-Tov and Roni Lotan contributed equally to this work.
Contributor Information
Amir Ben-Tov, Email: amir.bentov@gmail.com.
Roni Lotan, Email: lotanronnie@gmail.com.
Sivan Gazit, Email: gazit_s@mac.org.il.
Gabriel Chodick, Email: hodik_g@mac.org.il.
Galit Perez, Email: perez_g@mac.org.il.
Miri Mizrahi-Reuveni, Email: mizrahi_mi@mac.org.il.
Tal Patalon, Email: patalon_t@mac.org.il.
References
- 1.Hopkins J (2020) COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE). Coronavirus Resource Center
- 2.Simon AK, Hollander GA, Mcmichael A. Evolution of the immune system in humans from infancy to old age. Proc Royal Soc B: Biol Sci. 2015;282:20143085. doi: 10.1098/rspb.2014.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li B, Zhang S, Zhang R, Chen X, Wang Y, Zhu C. Epidemiological and clinical characteristics of COVID-19 in children: a systematic review and meta-analysis. Front Pediatr. 2020;8:591132. doi: 10.3389/fped.2020.591132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey LC, Razzaghi H, Burrows EK, Bunnell HT, Camacho PEF, Christakis DA, Eckrich D, Kitzmiller M, Lin SM, Magnusen BC, Newland J, Pajor NM, Ranade D, Rao S, Sofela O, Zahner J, Bruno C, Forrest CB. Assessment of 135 794 pediatric patients tested for severe acute respiratory syndrome coronavirus 2 across the United States. JAMA Pediatr. 2021;175:176. doi: 10.1001/jamapediatrics.2020.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimmermann P, Curtis N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch Dis Child. 2021;106:429–439. doi: 10.1136/archdischild-2020-320338. [DOI] [PubMed] [Google Scholar]
- 6.Duarte-Salles T, Vizcaya D, Pistillo A, Casajust P, Sena AG, Lai LYH, Prats-Uribe A, et al. Thirty-day outcomes of children and adolescents with COVID-19: an international experience. Pediatrics. 2021;148:e2020042929. doi: 10.1542/peds.2020-042929. [DOI] [PubMed] [Google Scholar]
- 7.Parcha V, Booker KS, Kalra R, Kuranz S, Berra L, Arora G, Arora P (2021) A retrospective cohort study of 12,306 pediatric COVID-19 patients in the United States. Sci Rep 11 [DOI] [PMC free article] [PubMed]
- 8.Katayama Y, Zha L, Kitamura T, Hirayama A, Takeuchi T, Tanaka K, Komukai S, Shimazu T, Sobue T. Characteristics and outcomes of pediatric COVID-19 patients in Osaka, Japan. Int J Environ Res Public Health. 2021;18:5911. doi: 10.3390/ijerph18115911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muhsen K, Na'Aminh W, Lapidot Y, Goren S, Amir Y, Perlman S, Green MS, Chodick G, Cohen D. A nationwide analysis of population group differences in the COVID-19 epidemic in Israel, February 2020–February 2021. The Lancet Regional Health - Europe. 2021;7:100130. doi: 10.1016/j.lanepe.2021.100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munitz A, Yechezkel M, Dickstein Y, Yamin D, Gerlic M (2021) BNT162b2 vaccination effectively prevents the rapid rise of SARS-CoV-2 variant B.1.1.7 in high-risk populations in Israel. Cell Rep Med 2:100264 [DOI] [PMC free article] [PubMed]
- 11.Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman L, Haas EJ, Milo R, Alroy-Preis S, Ash N, Huppert A (2021) Waning immunity after the BNT162b2 vaccine in Israel. New Eng J Med [DOI] [PMC free article] [PubMed]
- 12.Hodcroft EB (2022) CoVariants: SARS-CoV-2 mutations and variants of interest. https://covariants.org/
- 13.Chua GT, Wong JSC, Lam I, Ho PPK, Chan WH, Yau FYS, Rosa Duque JS, et al. Clinical characteristics and transmission of COVID-19 in children and youths during 3 waves of outbreaks in Hong Kong. JAMA Netw Open. 2021;4:e218824. doi: 10.1001/jamanetworkopen.2021.8824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Somekh I, Stein M, Karakis I, Simões EAF, Somekh E. Characteristics of SARS-CoV-2 infections in Israeli children during the circulation of different SARS-CoV-2 variants. JAMA Netw Open. 2021;4:e2124343. doi: 10.1001/jamanetworkopen.2021.24343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chodick G, Tene L, Rotem RS, Patalon T, Gazit S, Ben-Tov A, Weil C, Goldshtein I, Twig G, Cohen D, Muhsen K (2021) The effectiveness of the two-dose BNT162b2 vaccine: Analysis of real-world data. Clin Infect Dis [DOI] [PMC free article] [PubMed]
- 16.Mizrahi B, Shilo S, Rossman H, Kalkstein N, Marcus K, Barer Y, Keshet A, Shamir-Stein NA, Shalev V, Zohar AE, Chodick G, Segal E (2020) Longitudinal symptom dynamics of COVID-19 infection. Nat Commun 11 [DOI] [PMC free article] [PubMed]
- 17.Aydillo T, Rombauts A, Stadlbauer D, Aslam S, Abelenda-Alonso G, Escalera A, Amanat F, Jiang K, Krammer F, Carratala J, García-Sastre A (2021) Immunological imprinting of the antibody response in COVID-19 patients. Nat Commun 12 [DOI] [PMC free article] [PubMed]
- 18.Anderson EM, Goodwin EC, Verma A, Arevalo CP, Bolton MJ, Weirick ME, Gouma S, et al. Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection. Cell. 2021;184:1858–1864.e1810. doi: 10.1016/j.cell.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Renk H, Dulovic A, Seidel A, Becker M, Fabricius D, Zernickel M, Junker D et al (2022) Robust and durable serological response following pediatric SARS-CoV-2 infection. Nat Commun 13 [DOI] [PMC free article] [PubMed]
- 20.Gouma S, Weirick ME, Bolton MJ, Arevalo CP, Goodwin EC, Anderson EM, Mcallister CM, Christensen SR, Dunbar D, Fiore D, Brock A, Weaver J, Millar J, Derohannessian S, Unit TUCP, Frank I, R ader DJ, Wherry EJ, Hensley SE (2021) Health care worker seromonitoring reveals complex relationships between common coronavirus antibodies and COVID-19 symptom duration. JCI Insight 6 [DOI] [PMC free article] [PubMed]
- 21.Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, Tong S. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145:e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 22.Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, Felix SEB, Tie Y, Fullerton KE. Coronavirus disease 2019 case surveillance—United States, January 22–May 30, 2020. Morb Mortal Wkly Rep. 2020;69:759. doi: 10.15585/mmwr.mm6924e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molteni E, Sudre CH, Canas LS, Bhopal SS, Hughes RC, Chen L, Deng J, Murray B, Kerfoot E, Antonelli M, Graham M, Kläser K, May A, Hu C, Pujol JC, Wolf J, Hammers A, Spector TD, Ourselin S, Modat M, Steves CJ, Absoud M, Duncan EL (2021) Illness characteristics of COVID-19 in children infected with the SARS-CoV-2 Delta variant. medRxiv:2021.2010.2006.21264467
- 24.Clarfield AM, Manor O, Nun GB, Shvarts S, Azzam ZS, Afek A, Basis F, Israeli A. Health and health care in Israel: an introduction. The Lancet. 2017;389:2503–2513. doi: 10.1016/S0140-6736(17)30636-0. [DOI] [PubMed] [Google Scholar]
- 25.Mizrahi B, Lotan R, Kalkstein N, Peretz A, Perez G, Ben-Tov A, Chodick G, Gazit S, Patalon T (2021) Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Nat Commun 12 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1: The proportion of total number of sequences over time, that falls into defined variant groups, the Alpha variant in red and the Delta variant in green. Adapted from: Hodcroft EB (2022) CoVariants: SARS-CoV-2 Mutations and Variants of Interest. https://covariants.org/ (PDF 80 KB)
Data Availability Statement
N/A.
N/A.