Abstract
Background:
Present study assessed whether Sinopharm, AstraZeneca, Sputnik V, and Covaxin’s vaccinated women reveal a distinct incidence of menstruation disturbances, hirsutism, and metrorrhagia.
Materials and Methods:
Data collection was performed from June to August 2021, and 427 women working in seven selected hospitals in Tehran were studied in this descriptive-analytical cross-sectional study. All of these women had received one or both doses of the vaccines with one of the assessed vaccines. Required data was collected via questionnaire and imported to SPSS 16 for further assessment and analysis. Fisher’s Exact Test and Chi-Squared test were main statistical tests used to understand whether any significant relation exists or not.
Results:
The participant’s mean age and body mass index (BMI) were 29.78 ± 10.55 and 23.27 ± 3.82, respectively. Three hundred ninety-five cases (92.4%) had received both doses of the vaccines. Also, 154 cases (36.1%) had a history of COVID-19. A total of 38 cases (8.8%) of menstruation disturbances, 20 cases (4.6%) of metrorrhagia, and 7 cases (1.6%) of hirsutism were reported after receiving the vaccines. There was a significant difference among the vaccinated groups with the vaccines as mentioned earlier in terms of menstruation disturbances (hypermenorrhea, dysmenorrhea, Amenorrhea) (P=0.01). The highest and the lowest incidence of menstruation disturbances were recorded in the group vaccinated with Covaxin (17.6%) and Sputnik V (5%), respectively. There was also no significant difference amongst the vaccinated groups with the four vaccines regarding the incidence of metrorrhagia and hirsutism (P=0.10 and P=0.12, respectively). There was no significant relationship between all three complications incidence with the previous infection concerning all vaccines (coefficient=0.46, 1.27, -0.15 respectively for menstruation disturbances, metrorrhagia, and, hirsutism).
Conclusion:
Seemingly, Covaxin revealed the most side effects in terms of menstruation disturbances. As a result, professionals must carry out several studies with reasonable samples to recommend the vaccine to those women confidently
Keywords: Side Effects, Menstrual Cycle, Hirsutism, Metrorrhagia, COVID-19 Vaccines
Introduction
COVID-19 is regarded as the most challenging pandemic disease, engaging global health and the economy. Since its outbreak in Wuhan, China, various initiatives, measures, and tools have been devised to control the pandemic. For instance, mask shields (1, 2), traffic and lockdown restrictions (3, 4), and social distancing (5, 6) are clear examples of disease control measures.
Interestingly, with the development of the vaccines in 2020, general vaccination became the most important method of controlling COVID-19, which, in turn, could reduce the pandemic prevalence and mortality rate (7, 8). Of course, general vaccination would be coupled with further studies in this regard (9). The most common complications of vaccination include fever, fatigue, headache (10), restlessness, injection site pain, and joint pain (11). On the other hand, researchers have always sought to scrutinize these vaccines' effects on particular groups, including women, such as their hormones, menstrual status, metrorrhagia, and hirsutism (14-12).
Menstruation is overshadowed with the following factors (15) as: sleep (16), stress (17, 18), nutrition (19, 20), occupation and its hazards (21), age (22), race and environment (26-23). Notwithstanding the relatively limited studies, this work offers valuable insights and compares the side effects of four types of COVID-19 vaccines (i.e., Sinopharm, AstraZeneca, Sputnik V, and Covaxin) in women in terms of menstruation disturbances, hirsutism, and metrorrhagia.
Materials and Methods
This study is part of a large-scale undertaken to investigate the side effects resulting from Sinopharm, AstraZeneca, SputnikV, and Covaxin vaccines among the female participants regarding menstruation disturbances, hirsutism, and metrorrhagia. A descriptive-analytical cross-sectional study was performed in 427 female participants working as part of a medical care team in seven selected hospitals in Tehran, Iran.
All subjects were enrolled in the study between June and August 2021. Of course, they had already received one of the four vaccines, Sinopharm, AstraZeneca, SputnikV, and Covaxin (92.4% two doses, 7.6% one dose), and at least more than twenty days had passed since they received the first dose. All participants have chosen their vaccine deliberately from available vaccine types named previously. Also, they had no history of menstrual irregularities (such as hypermenorrhea-dysmenorrhea), hirsutism, and metrorrhagia before vaccination. It should be noted that all the above-mentioned staff were regarded as our study sample (N=n). Data were collected through a modified questionnaire.
The design of the questionnaires was based on the valid documents of the Center for Disease Control and Prevention (CDC) and World Health Organization (WHO) which was thoroughly reviewed by five gynecologists, and the necessary corrections were made. To confirm the research tool's validity, the questions were checked with the content validity index (CVI), and then questions with CVI below 0.7 were removed, while the questions with CVI between 0.7 and 0.79 were reviewed. Finally, the CVI of the tool was reviewed and calculated again. To ensure the reliability of the research tool, Cronbach’s alpha coefficient (27) was also evaluated, and the questionnaire's reliability was substantiated (α=0.86).
The designed questionnaire encompassed four open-ended questions, seven two-choice questions, and two multiple-choice questions. Those questions covered demographic information such as age, sex, height, weight, marital status, type of vaccine received, number of doses of vaccine received, and questions about the underlying disease (such as hypertension, hyperthyroidism or hypothyroidism, kidney, heart, lung, skin disease, and diabetes), and history of COVID-19 disease before receiving the vaccine. Also Three questions pertained to possible vaccine side effects in women. these questions included questions about menstruation disturbances (hypermenorrheadysmenorrhea-amenorrhea), metrorrhagia, and hirsutism after vaccination (IR.AJAUMS.REC.1400.163).
In receiving written informed consent from the participants, researchers referred to the selected medical centers and administered the questionnaire among the women on the care-treatment team of the seven mentioned centers. Inclusion criteria consist of these conditions: i. Being employed in 7 chosen centers, ii. Receiving one or both doses of vaccine with one of the four selected types of vaccine, iii. Being vaccinated at least twenty days earlier, and iv. Having no history of menstruation disturbances, hirsutism, and metrorrhagia. Besides, our exclusion criterion was an incomplete questionnaire. All participants were asked on the study’s objectives and assured that all information would remain confidential. The research tool was distributed among participants. Having collected all the questionnaires, the data was analyzed using SPSS 16, International Business Machines Corporation (IBM), NY, USA.
Having matched the questionnaires and the software data, possible significant relationships between the study variables were determined using the Chi-Squared and Fisher’s Exact Test with a significance level of 0.05. A multivariate analysis was done by logistic regression to determine the effect of demographic variables on menstruation disturbances, metrorrhagia, and hirsutism. Also, the crosstab method was applied to assess the effect of COVID-19 history on the aforementioned side effects. Central statistical and dispersion indices such as mean and standard deviation were also analyzed.
Results
According to the results, 427 women working in seven selected hospitals would complete the questionnaires (mean age: 29.78 ± 10.55). As for body mass index (BMI) index, 274 (64.1%) were normal and 102 (23.8%) were overweight. Also, 363 (86.9%) did not report any underlying disease; hypothyroidism or hyperthyroidism, hypertension, and a history of allergies were the most commonly reported diseases, respectively. Additionally, 157 (36.1%) reported a history of COVID-19 with a positive PCR test. Also, 203 (46.6%) had received Sinopharm vaccine, 116 (26.6%) AstraZeneca vaccine, 80 (18.8%) SputnikV vaccine and 34 (8%) Covaxin vaccine (Table 1).
Table 1.
Demographic characteristics of participants
|
| ||||
|---|---|---|---|---|
| Vaccines | Sputnik | Covaxin | AstraZeneca | Sinopharm |
| Variables | n (%) | n (%) | n (%) | n (%) |
|
| ||||
| Marital status | ||||
| Single | 20 (25) | 15 (40) | 34 (31.1) | 147 (73.4) |
| Married | 60 (75) | 19 (60) | 78 (68.9) | 54 (26.6) |
| Number of received dose | ||||
| One | 6 (7.3) | 1 (2.9) | 22 (19.8) | 3 (1.5) |
| Two | 74 (92.7) | 33 (97.1) | 90 (80.2) | 198 (98.5) |
| History of underlying disease | ||||
| No disease | 65 (82) | 26 (79.8) | 95 (84.7) | 175 (87.5) |
| Hypertension | 1 (1.2) | 1 (2.9) | 2 (1.8) | 3 (1.5) |
| Hypo-or hyperthyroidism | 2 (2.4) | 1 (2.9) | 3 (2.7) | 3 (1.5) |
| Allergy | 2 (2.4) | 1 (2.9) | 2 (1.8) | 2 (1) |
| Neurological disease | 1 (1.2) | 0 (0) | 0 (0) | 1 (0.5) |
| Kidney disease | 1 (1.2) | 0 (0) | 0 (0) | 2 (1) |
| Lung disease | 0 (0) | 0 (0) | 1 (0.9) | 0 (0) |
| Diabetes | 1 (1.2) | 0 (0) | 2 (1.8) | 3 (1.5) |
| Skin disease | 1 (1.2) | 1 (2.9) | 1 (0.9) | 4 (2) |
| Heart disease | 1 (1.2) | 0 (0) | 2 (1.8) | 1 (0.5) |
| Liver disease | 0 (0) | 0 (0) | 1 (0.9) | 2 (1) |
| Addiction | 2 (2.4) | 1 (2.9) | 1 (0.9) | 3 (1.5) |
| Others | 3 (3.6) | 1 (2.9) | 2 (1.8) | 2 (1) |
| History of allergy to influenza vaccine | 12 (5.6) | 3 (9.4) | 6 (1.9) | 7 (0.8) |
| Previous COVID-19 Infection | 24 (29.3) | 8 (23.5) | 38 (34.5) | 84 (42) |
| Age (Y) | ||||
| <20 | 0 (0) | 0 (0) | 1 (0.5) | 24 (12) |
| 20-29 | 23 (29.4) | 14 (40) | 25 (22.9) | 131 (65.8) |
| 30-39 | 27 (32.4) | 9 (26.7) | 33 (29.7) | 22 (11.4) |
| 40-49 | 23 (29.4) | 2 (6.7) | 42 (37.5) | 15 (7.6) |
| >=50 | 7 (8.8) | 9 (26.7) | 11 (9.4) | 5 (3.3) |
| BMI (kg/m2) | ||||
| Underweight (<18.5) | 5 (6.3) | 3 (6.5) | 2 (1.6) | 25 (12.5) |
| Normal (18.5-25) | 42 (53.1) | 21 (70) | 68 (60.7) | 123 (61.4) |
| Overweight (25-29.9) | 25 (31.3) | 8 (29) | 33 (29.5) | 40 (20.1) |
| Obese (>30) | 8 (9.4) | 2 (4.5) | 10 (8.2) | 13 (6) |
|
| ||||
BMI; Body mass index.
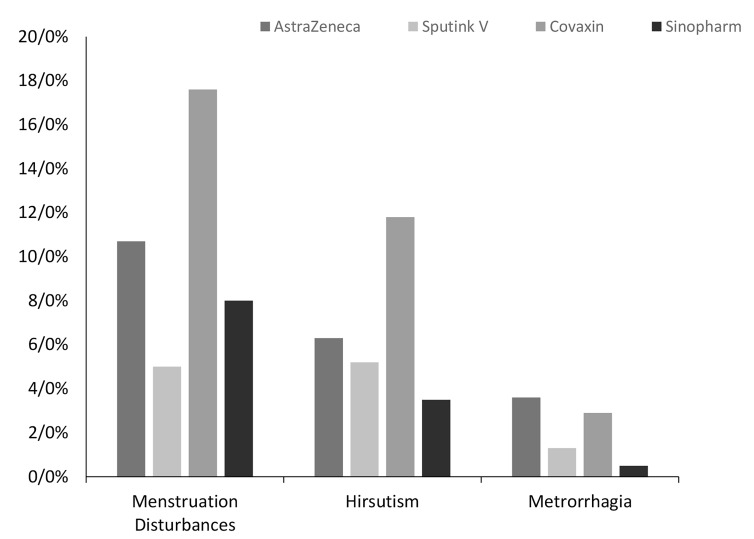
The study showed that 8% of women’s Sinopharm vaccine, 10.7% for AstraZeneca vaccine, 5% receiving SputnikV vaccine, and 17.6% of women receiving Covaxin reported menstrual irregularities (hypermenorrhea-dysmenorrheamenorrhea). The difference between the effects of these four types of vaccines on menstruation via the Chi-Squared test showed a significant difference among all of them (P=0.01). For this reason, we compared the four types of vaccines in terms of menstruation disturbances in pairs (6 cases) and, interestingly, only a significant difference was observed between Covaxin and SputnikV vaccines in terms of menstruation disturbances (P=0.02).
Based on the responses and the type of vaccine analyses, it was shown that the group vaccinated with Bharat Biotech vaccine (n=4, 11.8%) and SputnikV vaccine (2.5%, n=2) experienced the highest and lowest metrorrhagia incidence, respectively. The two groups of women vaccinated with the AstraZeneca vaccine (6.3%, n=7) and Sinopharm (3.5%, n=7) were placed in the second and third ranks. It should be noted that Fisher’s Exact Test did not indicate a significant difference in the incidence of metrorrhagia (P=0.12).
The participants developed hirsutism were assigned in the following groups in a descending order: (0.5%, n=1) Sinopharm vaccination group (1.3%, n=1) SputnikV vaccination group (2.9%, n=1) Covaxin vaccination group, and AstraZeneca vaccine (3.6%, n=4) (Fig .1). However, Fisher’s exact test did not show any significant difference in this connection (P=0.10, Table 2).
Fig 1.
Comparison of 4 types of vaccines in terms of women's menstruation disturbances, metrorrhagia and hirsutism.
Table 2.
Frequency of three complications of menstruation disturbances, metrorrhagia, hirsutism among participants receiving the four vaccines
|
| ||||||
|---|---|---|---|---|---|---|
| Disorder type | AstraZenec | Sputnik-V | Covaxin | Sinopharm | P value* | |
|
| ||||||
| Menstruation disturbances | Unreported | 100 (89.3) | 76 (95) | 28 (82.4) | 185 (92) | 0.01 |
| Reported | 12 (10.7) | 4 (5) | 6 (17.6) | 16 (8) | ||
| Metrorrhagia | Unreported | 105 (93.7) | 78 (97.5) | 30 (88.2) | 194 (96.5) | 0.12 |
| Reported | 7 (6.3) | 2 (2.5) | 4 (11.8) | 7 (3.5) | ||
| Hirsutism | Unreported | 108 (96.4) | 79 (98.8) | 33 (97.1) | 200 (99.5) | 0.10 |
| Reported | 4 (3.6) | 1 (1.3) | 1 (2.9) | 1 (0.5) | ||
|
| ||||||
Data are presented as n (%). P values were obtained from Fisher's exact test.
Furthermore; a multivariate analysis by logistic regression has been done to assess possible effect of demographic variables on developed side effects (i.e., menstruation disturbances, metrorrhagia, and hirsutism). There was no significant relationship between the incidence of mentioned side effects and demographic variables (Table 3).
Table 3.
Multivariate analysis of menstruation disorder, hirsutism, and metrorrhagia
|
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Disorder | Variables | Groups | Coefficient | SE | OR | 95% CI for OR | P value | |
| Lower | Upper | |||||||
|
| ||||||||
| Menstruation disturbances | Age (Y) | 0.014 | .027 | 1.014 | 0.962 | 1.068 | 0.603 | |
| BMI (kg/m2)(reference: normal) | Thin | -0.614 | 1.079 | .541 | 0.065 | 4.486 | 0.569 | |
| Overweight | -0.021 | 0.527 | .979 | 0.349 | 2.751 | 0.968 | ||
| Obese | 0.543 | 0.757 | 1.721 | 0.390 | 7.586 | 0.473 | ||
| Covid-19 morbidity history | Yes | 0.468 | 0.425 | 1.597 | 0.694 | 3.674 | 0.270 | |
| Marital status | Married | -0.008 | 0.526 | 0.992 | 0.354 | 2.782 | 0.989 | |
| Vaccine type (reference: Sinopharm) | AstraZeneca | -0.419 | 0.826 | 0.658 | 0.130 | 3.318 | 0.612 | |
| Covaxin | 1.083 | 0.772 | 2.954 | 0.651 | 13.412 | 0.161 | ||
| Sputnik-V | 0.048 | 0.580 | 1.049 | 0.336 | 3.271 | 0.935 | ||
| Constant | -2.978 | 0.738 | 0.051 | 0.000 | ||||
| Metrorrhagia | Age (Y) | 0.027 | 0.037 | 1.027 | 0.955 | 1.105 | 0.469 | |
| BMI (kg/m2) (reference: normal) | Thin | -17.614 | 7903.849 | 0.000 | 0.000 | 0.998 | ||
| Overweight | -0.548 | 0.843 | 0.578 | 0.111 | 3.015 | 0.522 | ||
| Obese | 0.624 | 0.975 | 1.867 | 0.276 | 12.625 | 0.522 | ||
| Covid-19 morbidity history | Yes | 1.274 | 0.661 | 3.574 | 0.979 | 13.044 | 0.054 | |
| Marital Status | Married | 0.266 | 0.718 | 1.304 | 0.319 | 5.331 | 0.711 | |
| Vaccine type (reference: Sinopharm) | AstraZeneca | -18.253 | 6839.732 | 0.000 | 0.000 | 0.998 | ||
| Covaxin | 1.395 | 0.991 | 4.034 | 0.578 | 28.147 | 0.159 | ||
| Sputnik-V | -0.047 | 0.806 | 0.954 | 0.197 | 4.629 | 0.954 | ||
| Constant | -4.654 | 1.154 | 0.010 | 0.000 | ||||
| Hirsutism | Age (Y) | 0.024 | 0.049 | 1.024 | 0.930 | 1.127 | 0.630 | |
| BMI (kg/m2) (reference: normal) | Thin | -16.042 | 8076.7 | 0.000 | 0.000 | 0.998 | ||
| Overweight | -0.462 | 1.179 | 0.630 | 0.063 | 6.348 | 0.695 | ||
| Obese | 0.972 | 1.299 | 2.642 | 0.207 | 33.729 | 0.455 | ||
| Covid-19 morbidity history | Yes | -0.152 | 0.918 | 0.859 | 0.142 | 5.188 | 0.868 | |
| Marital status | Married | -0.753 | 0.923 | 0.471 | 0.077 | 2.878 | 0.415 | |
| Vaccine type (reference: Sinopharm) | AstraZeneca | -15.9 | 7000.3 | 0.000 | 0.000 | 0.998 | ||
| Covaxin | 2.575 | 1.549 | 13.127 | 0.630 | 273.412 | 0.097 | ||
| Sputnik-V | 2.455 | 1.263 | 11.646 | 0.980 | 138.429 | 0.052 | ||
| Constant | -5.447 | 1.649 | 0.004 | 0.001 | ||||
|
| ||||||||
SE; Standard error, OR; Odds ratio, CI; Confidence intervals, and BMI; BMI; Body mass index. P values obtained by logistic regression analysis.
Overally, regardless of the vaccine, 38 (8.8%) reported menstruation disturbances, 20 (4.6%) metrorrhagia, and 7 (1.6%) hirsutism. Also, 43 (10%) had experienced at least one of the three afor-mentioned complications. Besides, 19 women reported simultaneous onset of two or three complications as follows: three reported onsets of all three complications (2 in the Covaxin group and 1 in AstraZeneca, respectively), 16 reported the onset of menstrual irregularities and metrorrhagia (5 AstraZeneca, 2 SputnikV, 3 Covaxin and 6 Sinopharm). However; 24 others reported only one of three complications, and 19 (5 AstraZeneca, 2 SputnikV, 2 Covaxin, and 10 Sinopharm) referred to menstruation disturbances. Additionally, four participants (2 AstraZeneca, 1 SputnikV We, 1 Covaxin) reported hirsutism, and 1 (1 Sinopharm) reported metrorrhagia.
Furthermore, the present study considered the relationship between pre-vaccination with COVID-19 infection and the onset of three side effects - i.e., Changes in menstruation - the incidence of metrorrhagia, and hirsutism. We compared those affected with COVID-19 and those who reported no history in this regard before vaccination. However; there was no significant difference between people with and without a history of COVID-19 in any of the three complications incidence of menstruation disturbances, hirsutism, and metrorrhagia concerning all 4 vaccines (Table 4).
Table 4.
Frequency of three complications: menstruation disturbances, hirsutism, and metrorrhagia in two groups with and without a history of COVID-19
|
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Vaccine type | Sputnik-V | Covaxin | AstraZeneca | Sinopjarm | |||||
| Side effect | Positive COVID-19 history | Negative COVID-19 history | Positive COVID-19 history | Negative COVID-19 history | Positive COVID-19 history | Negative COVID-19 history | Positive COVID-19 history | Negative COVID-19 history | |
|
| |||||||||
| Menstruation disturbance | Yes | 3 (13) | 1 (1.8) | 3 (37.5) | 3 (11.5) | 4 (10.3) | 8 (11) | 8 (9.3) | 8 (7) |
| No | 20 (87) | 56 (98.2) | 5 (88.5) | 23 (88.5) | 35 (89.7) | 65 (89) | 78 (90.7) | 107 (93) | |
| Total | 23 (100) | 57 (100) | 8 (100) | 26 (100) | 39 (100) | 73 (100) | 86 (100) | 115 (100) | |
| P value* | P=0.06 | P=0.12 | P=0.59 | P=0.60 | |||||
| Metrorrhagia | Yes | 1 (4.3) | 1 (1.8) | 2 (25) | 2 (7.7) | 3 (7.7) | 4 (5.5) | 5 (5.8) | 2 (1.7) |
| No | 22 (95.7) | 56 (98.2) | 6 (75) | 24 (92.3) | 36 (92.3) | 69 (94.5) | 81 (94.2) | 113 (98.3) | |
| Total | 23 (100) | 57 (100) | 8 (100) | 26 (100) | 39 (100) | 73 (100) | 86 (100) | 115 (100) | |
| P value* | P=0.49 | P=0.22 | P=0.69 | P=0.14 | |||||
| Hirsutism | Yes | 1 (4.3) | 0 (0) | 0 (0) | 1 (3.8) | 2 (5.1) | 2 (2.7) | 0 (0) | 1 (0.9) |
| No | 22 (95.7) | 57 (100) | 8 (100) | 25 (96.2) | 37 (94.9) | 71 (97.3) | 86 (100) | 114 (99.1) | |
| Total | 23 (100) | 57 (100) | 8 (100) | 26 (100) | 39 (100) | 73 (100) | 86 (100) | 115 (100) | |
| P value* | P=0.28 | P=0.76 | P=0.60 | P=0.57 | |||||
|
| |||||||||
Data are presented as N (%). P values were obtained from Fisher's exact test.
Discussion
The primary purpose of the present study was to compare the side effects of 4 types of covid -19 vaccines i.e., Sinopharm, AstraZeneca, SputnikV, and Covaxin in women in terms of change in menstruation, hirsutism, and metrorrhagia. The result of the studies showed that the amount of change in menstruation in vaccinated groups with four AstraZeneca vaccines, SputnikV, Covaxin, and Sinopharm, was significantly different. The most commonly vaccine-induced change in menstruation was reported for Covaxin (17.6%), while its lowest change was recorded in SputnikV (5%). Also, accordingly, AstraZeneca (10.7%) and Sinopharm (8%) vaccines ranked second and third.
Two complications of hirsutism and metrorrhagia were also examined. The results indicated that all types of vaccines in this study did not differ significantly regarding both complications. The most common complication (in terms of number) was menstruation disturbances (38 cases (8.8%), followed by metrorrhagia, 20 cases (4.6%), and finally, 7 cases (1.6%) of hirsutism.
Only a limited number of similar studies examined the effect of COVID-19 vaccines on gynecological disorders such as menstruation, hirsutism, and metrorrhagia, and, therefore, more research is needed. As a result, we believe our study is novel. Literature has been mostly restricted to limited comparisons of the incidence of blood clots following the injection of Pfizer-Moderna (26) and AstraZeneca vaccines. Previous research has suggested that thrombocytopenia may be a factor in heavy menstrual bleeding (27) or metrorrhagia (28). Studies have also shown that produced antibodies may invade platelets and be a precursor to idiopathic purpura (ITP) thrombocytopenia (29).
According to the Mathioudakis study, pre-vaccination infection with COVID-19 was associated with an increased incidence of side effects following receiving the vaccine by 1.08 (30). However, according to the present study, COVID-19 and the occurrence of three side effects of menstruation disturbances-hirsutism and metrorrhagia were evaluated, and the results did not indicate a significant difference in the incidence of these three complications between the two groups with history and without a history of COVID-19.
Late studies have indicated that coagulopathy disorders commonly emerge as complications of SARS-Cov-2 disease and its long-term effects (31). The Aforementioned side effect may appear in the form of Venous Thromboembolism (VTE), Disseminated Intravascular Coagulation (DIC) (32). In addition, coagulopathy disorders may cause metrorrhagia (33). However; our study indicated no significant relation between past COVID-19 history and metrorrhagia.
In this study, a total of 427 Iranian women were studied. As a result, the racial difference factor was ineffective in this experiment (21, 23). The participants were in the same age group and had the same place of employment and job proximity (19) (all engaged in health services), knowing that workplace stress is one of the factors affecting the menstrual cycle (15, 16). Their body mass index was also approximately the same. Needless to say that fitness and weight are factors that also affect the menstrual cycle (17, 18).
Using a questionnaire is a limitation of this study. Further studies are crucial, and more specific and sensitive methods should be used. Due to the funding limitation of this study, a direct diagnosis of menstruation disturbances was not possible. Because of the cross-sectional methodology, it is not possible to conclude any causal inference, and only the prevalence of menstruation disturbances can be assessed directly (34). Also, it is not possible to evaluate the long-term relationship between vaccines and menstruation disturbances. Also Sampling strategy can be disputable. Choosing all women among health workers can be a selection bias that does not represent all women in the population. We would attempt to match the sample group as similar as possible to minimize the effect of other compelling factors. Other strengths of this study include the high statistical population and its minimal missing data. This study was conducted in a hospital setting amongst medical staff members. Most of the participants showcased mastery over general health knowledge who were even working professionally in this field. Therefore, they presented more accurate and complete information about their health status (35, 36). However, the collected data through a questionnaire potentially represent errors or biases like nonresponse bias or recall bias common in cross-section studies (34). It was also possible that some respondents did not answer the questions honestly (the complications mentioned above are deemed taboo in some groups and cultures (37). However; efforts were made to minimize these errors by choosing the right time between the vaccine injection and filling out the questionnaire and selecting a knowledgeable group.
Nonetheless, due to the vaccines’ novelty and the ongoing licensing process in various countries, more research is required to determine their effect on the menstrual cycle (38). One of the most up-to-date studies discusses the effect of COVID-19 on women's pregnancy (39) which underpins the significance of our study.
Some vaccines-based experimental groups were not significant due to the limited number of injectable doses and hence there is a potential for being bias due to the small sample size. Also, due to the urgent necessity to inject these vaccines to prevent the further spread of COVID-19 and reduce its risk, it is not possible to test these vaccines in a clinical trial, and such studies are minimal. Consequently, the effects of these vaccines can be studied in more precise ways in the future. Due to the ever-growing concerns in women about the incidence of the complications mentioned above and also the strong possibility of injecting, a dose or booster doses of the vaccines in the future (40), is recommended that more research be implemented on this issue.
It is also recommended that group studies and clinical trials be put on the agenda to understand the effects of these vaccines better. Additionally, injection of these vaccines in pregnant women and those prone to sex hormone disorders, as well as those women undergoing infertility treatment, should be performed with extra caution.
Conclusion
This study indicated no association between COVID-19 history and assessed feminine side effects (i.e.menstruation disturbances, metrorrhagia, and hirsutism). Also, a significant difference between vaccines regarding menstruation disturbances was found. This side effect was more frequent in Covaxin recipients than in other groups. It should be noted that further study is needed to evaluate the hypothesis.
Acknowledgements
There is no financial support and conflict of interest in this study.
Authors’ Contributions
I.N., A.A., M.Y.Z.; Designed the study. A.A., F.K., M.N.; Contributed to data collection and creation of data resources. F.K., A.A.; Checked and verified the dataset and prepared it for analysis. M.H.K.-G.; Did the statistical analysis. A.A., S.S.B., M.H.K.-G., M.N., F.K.; Wrote the manuscript. F.K., M.N., M.H.K.-G., I.N.; Reviewed and edited the manuscript. I.N., F.K., M.Y.Z.; Supervised the work. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
References
- 1.Clapham HE, Cook AR. Face masks help control transmission of COVID-19. Lancet Digit Health. 2021;3(3):e136–e137. doi: 10.1016/S2589-7500(21)00003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yadav AK. Impact of lockdown to control over Novel Coronavirus and COVID-19 in India. J Family Med Prim Care. 2020;9(10):5142–5147. doi: 10.4103/jfmpc.jfmpc_692_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang X, Shao X, Xing L, Hu Y, Sin DD, Zhang X. The impact of lockdown timing on COVID-19 transmission across US counties. EClinicalMedicine. 2021;38:101035–101035. doi: 10.1016/j.eclinm.2021.101035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saki M, Ghanbari MK, Behzadifar M, Imani-Nasab MH, Behzadifar M, Azari S, et al. The impact of the social distancing policy on COVID-19 incidence cases and deaths in iran from february 2020 to january 2021: Insights from an interrupted time series analysis. Yale J Biol Med. 2021;94(1):13–21. [PMC free article] [PubMed] [Google Scholar]
- 5.Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021;21(10):626–636. doi: 10.1038/s41577-021-00592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshikawa T. Implementing vaccination policies based upon scientific evidence in Japan. Vaccine. 2021;39(38):5447–5450. doi: 10.1016/j.vaccine.2021.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han X, Xu P, Ye Q. Analysis of COVID-19 vaccines: Types, thoughts, and application. J Clin Lab Anal. 2021;35(9):e23937–e23937. doi: 10.1002/jcla.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abu-Hammad O, Alduraidi H, Abu-Hammad S, Alnazzawi A, Babkair H, Abu-Hammad A, et al. Side effects reported by jordanian healthcare workers who received COVID-19 vaccines. Vaccines (Basel) 2021;9(6):577–577. doi: 10.3390/vaccines9060577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jayadevan R, Shenoy RS, Anithadevi T. Survey of symptoms following COVID-19 vaccination in India.medRxiv. medRxiv; 2021. [Google Scholar]
- 10.Stevenson WJ. An aspect of salk vaccination in adult women. Med J Aust. 1958;2(10):325–326. doi: 10.5694/j.1326-5377.1958.tb58387.x. [DOI] [PubMed] [Google Scholar]
- 11.Hunter PR. Thrombosis after covid-19 vaccination. BMJ. 2021;373:n958–n958. doi: 10.1136/bmj.n958. [DOI] [PubMed] [Google Scholar]
- 12.Bell G, Montgomery D, Jarrett P. An unusual chronic cutaneous vaccine reaction; localised hypertrichosis, papular eczema and a subcutaneous nodule. Aust J Dermatol. 2016;57(4):324–326. doi: 10.1111/ajd.12449. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Gold EB, Lasley BL, Johnson WO. Factors affecting menstrual cycle characteristics. Am J Epidemiol. 2004;160(2):131–140. doi: 10.1093/aje/kwh188. [DOI] [PubMed] [Google Scholar]
- 14.Baker FC, Driver HS. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007;8(6):613–622. doi: 10.1016/j.sleep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Edozien LC. Mind over matter: psychological factors and the menstrual cycle. Curr Opin Obstet Gynecol. 2006;18(4):452–456. doi: 10.1097/01.gco.0000233942.67049.ad. [DOI] [PubMed] [Google Scholar]
- 16.Xiao E, Ferin M. Stress-related disturbances of the menstrual cycle. Ann Med. 1997;29(3):215–219. doi: 10.3109/07853899708999339. [DOI] [PubMed] [Google Scholar]
- 17.Onieva-Zafra MD, Fernández-Martínez E, Abreu-Sánchez A, Iglesias-López MT, García-Padilla FM, Pedregal-González M, et al. Relationship between diet, menstrual pain and other menstrual characteristics among spanish students. Nutrients. 2020;12(6):1759–1759. doi: 10.3390/nu12061759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reavey JJ, Walker C, Murray AA, Brito-Mutunayagam S, Sweeney S, Nicol M, et al. Obesity is associated with heavy menstruation that may be due to delayed endometrial repair. J Endocrinol. 2021;249(2):71–82. doi: 10.1530/JOE-20-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinberg CR. The influence of occupational activity on the menstrual cycle and fecundability. Epidemiology. 1994;5(1):14–18. doi: 10.1097/00001648-199407000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Batista MC, Cartledge TP, Zellmer AW, Merino MJ, Axiotis C, Bremner WJ, et al. Effects of aging on menstrual cycle hormones and endometrial maturation. Fertil Steril. 1995;64(3):492–499. [PubMed] [Google Scholar]
- 21.Braithwaite D, Moore DH, Lustig RH, Epel ES, Ong KK, Rehkopf DH, et al. Socioeconomic status in relation to early menarche among black and white girls. Cancer Causes Control. 2009;20(5):713–720. doi: 10.1007/s10552-008-9284-9. [DOI] [PubMed] [Google Scholar]
- 22.James-Todd T, Tehranifar P, Rich-Edwards J, Titievsky L, Terry MB. The impact of socioeconomic status across early life on age at menarche among a racially diverse population of girls. Ann Epidemiol. 2010;20(11):836–842. doi: 10.1016/j.annepidem.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tehranifar P, Reynolds D, Flom J, Fulton L, Liao Y, Kudadjie-Gyamfi E, et al. Reproductive and menstrual factors and mammographic density in African American, Caribbean, and white women. Cancer Causes Control. 2011;22(4):599–610. doi: 10.1007/s10552-011-9733-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia C, Lee PC, Rosetta L. Impact of social environment on variation in menstrual cycle length in captive female olive baboons (Papio anubis) Reproduction. 2008;135(1):89–97. doi: 10.1530/REP-06-0320. [DOI] [PubMed] [Google Scholar]
- 25.Tavakol M, Dennick R. Making sense of Cronbach's alpha. Int J Med Educ. 2011;2:53–55. doi: 10.5116/ijme.4dfb.8dfd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee EJ, Cines DB, Gernsheimer T, Kessler C, Michel M, Tarantino MD, et al. Thrombocytopenia following Pfizer and Moderna SARSCoV- 2 vaccination. Am J Hematol. 2021;96(5):534–537. doi: 10.1002/ajh.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajpurkar M, O'Brien SH, Haamid FW, Cooper DL, Gunawardena S, Chitlur M. Heavy menstrual bleeding as a common presenting symptom of rare platelet disorders: illustrative case examples. J Pediatr Adolesc Gynecol. 2016;29(6):537–541. doi: 10.1016/j.jpag.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Díaz-Quijano FA, Villar-Centeno LA, Martínez-Vega RA. Complications associated to severe thrombocytopenia in patients with dengue. Rev Med Chil. 2006;134(2):167–173. [PubMed] [Google Scholar]
- 29.Perricone C, Ceccarelli F, Nesher G, Borella E, Odeh Q, Conti F, et al. Immune thrombocytopenic purpura (ITP) associated with vaccinations: a review of reported cases. Immunol Res. 2014;60(2-3):226–235. doi: 10.1007/s12026-014-8597-x. [DOI] [PubMed] [Google Scholar]
- 30.Mathioudakis AG, Ghrew M, Ustianowski A, Ahmad S, Borrow R, Papavasileiou LP, et al. Self-reported real-world safety and reactogenicity of covid-19 vaccines: a vaccine recipient survey. Life (Basel) 2021;11(3):249–249. doi: 10.3390/life11030249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382(17):e38–e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Acanfora D, Acanfora C, Ciccone MM, Scicchitano P, Bortone AS, Uguccioni M, et al. The cross-talk between thrombosis and inflammatory storm in acute and long-COVID-19: therapeutic targets and clinical cases. Viruses. 2021;13(10):1904–1904. doi: 10.3390/v13101904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitaker L, Critchley HO. Abnormal uterine bleeding. Best Pract Res Clin Obstet Gynaecol. 2016;34:54–65. doi: 10.1016/j.bpobgyn.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X, Cheng Z. Cross-sectional studies: strengths, weaknesses, and recommendations. Chest. 2020;158(1S):S65–S71. doi: 10.1016/j.chest.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Silles MA. The causal effect of education on health: evidence from the United Kingdom. Econ Educ Rev. 2009;28(1):122–128. [Google Scholar]
- 36.Simonds SK. Health education as social policy. Health Educ. 1974;2(1_suppl):1–10. [Google Scholar]
- 37.McHugh MC. In: The palgrave handbook of critical menstruation studies. Bobel C, Winkler IT, Fahs B, Hasson KA, Kissling EA, Roberts TA, editors. Vol. 32. Singapore: Palgrave Macmillan; 2020. Menstrual Shame: Exploring the role of 'menstrual moaning'; pp. 409–422. [Google Scholar]
- 38.Hernández AF, Calina D, Poulas K, Docea AO, Tsatsakis AM. Safety of COVID-19 vaccines administered in the EU: Should we be concerned? Toxicol Rep. 2021;8:871–879. doi: 10.1016/j.toxrep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bentov Y, Beharier O, Moav-Zafrir A, Kabessa M, Godin M, Greenfield CS, et al. Ovarian follicular function is not altered by SARS-Cov-2 infection or BNT162b2 mRNA Covid-19 vaccination. Hum Reprod. 2021;36(9):2506–2513. doi: 10.1093/humrep/deab182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N Engl J Med. 2021;385(15):1393–1400. doi: 10.1056/NEJMoa2114255. Comparison of Side Effects of Covid-19 Vaccines. [DOI] [PMC free article] [PubMed] [Google Scholar]