Abstract
Background and Objectives
Human mesenchymal stem cells (MSCs) are emerging as a treatment for atopic dermatitis (AD), a chronic inflammatory skin disorder that affects a large number of people across the world. Treatment of AD using human umbilical cord blood-derived MSCs (hUCB-MSCs) has recently been studied. However, the mechanism underlying their effect needs to be studied continuously. Thus, the objective of this study was to investigate the immunomodulatory effect of epidermal growth factor (EGF) secreted by hUCB-MSCs on AD.
Methods and Results
To explore the mechanism involved in the therapeutic effect of MSCs for AD, a secretome array was performed using culture medium of hUCB-MSCs. Among the list of genes common for epithelium development and skin diseases, we focused on the function of EGF. To elucidate the effect of EGF secreted by hUCB-MSCs, EGF was downregulated in hUCB-MSCs using EGF-targeting small interfering RNA. These cells were then co-cultured with keratinocytes, Th2 cells, and mast cells. Depletion of EGF disrupted immunomodulatory effects of hUCB-MSCs on these AD-related inflammatory cells. In a Dermatophagoides farinae-induced AD mouse model, subcutaneous injection of hUCB-MSCs ameliorated gross scoring, histopathologic damage, and mast cell infiltration. It also significantly reduced levels of inflammatory cytokines including interleukin (IL)-4, tumor necrosis factor (TNF)-α, thymus and activation-regulated chemokine (TARC), and IL-22, as well as IgE levels. These therapeutic effects were significantly attenuated at all evaluation points in mice injected with EGF-depleted hUCB-MSCs.
Conclusions
EGF secreted by hUCB-MSCs can improve AD by regulating inflammatory responses of keratinocytes, Th2 cells, and mast cells.
Keywords: Atopic dermatitis, Epidermal growth factor, Umbilical cord blood-derived mesenchymal stem cells, HaCaT, Mast cells, TARC
Introduction
Atopic dermatitis (AD) is an inflammatory skin disease that usually occurs in infancy or early childhood. Patients with AD show dry, itchy, and inflamed skin. They often develop other atopic symptoms, including allergic rhinoconjunctivitis and allergic asthma. Most patients continue to have problems after reaching adulthood (1, 2).
Keratinocytes of AD patients express many chemokines including CCL27, thymus and activation-regulated chemokine (TARC, also known as CCL17), and CCL22/macrophage-derived chemokine that can introduce dendritic cells (DCs), T cells, and other leukocytes into the skin to enhance inflammation (3-5). These chemokines can cause migration and infiltration of Th2 cells into inflammatory sites, resulting in a Th2 immune response through CCR4 (6). Immunological pathologies of AD include the following: 1) increased production of chemokines such as thymic stromal lymphopoietin (TSLP), regulated upon activation, normal T cell expressed and presumably secreted (RANTES), and TARC; 2) increased number of Th2 cells compared to Th1 cells; 3) increased production of cytokines (e.g., interleukin (IL)-1β, IL-4, and tumor necrosis factor (TNF)-α); and 4) increased production of IgE. A new therapeutic strategy for AD could involve targeting Th2 cells, keratinocytes, B cells, and inflammatory cells related to skin immune response.
Mesenchymal stem cells (MSCs) can differentiate into mesenchymal lineages and affect the immune system by regulating immune cells including T cells, B cells, DCs, and natural killer cells (2). Recent studies have shown that MSCs may overcome hurdles in the treatment of inflammatory and autoimmune diseases such as arthritis, inflammatory bowel disease, and AD (7-9). In addition, MSCs can secrete various immune modulators including hepatocyte growth factor, transforming growth factor (TGF)-β, IL-10, and IL-6 (10). These secreted cytokines can inhibit the proliferation of CD8+ T cells, CD4+ cells, and natural killer cells as well as the maturation of DCs, which are highly activated in AD. Thus, these cytokines have important functions in reducing allergic response and chronic inflammation in AD.
MSCs can secrete numerous immune modulators and growth factors. Among them, epidermal growth factor (EGF) is a potential therapeutic target for epidermis restoration and immunosuppression in AD. The EGF family consists of many mediators, including heparin-binding EGF, TGF-α, amphiregulin, and epiregulin. These mediators are involved in tissue-specific differentiation and proliferation homeostasis (11). In addition, the EGF receptor (EGFR) signaling pathway is crucial for skin development and homeostasis (12). Several studies, including a study using a genome-wide approach and a histopathologic study, have reported that EGFR expression is lower in lesional skin of AD patients than in healthy controls (13). Furthermore, recent studies have reported that the EGFR pathway not only plays a crucial role as a signal transducer, but also plays an important role in inflammatory reactions of skin (12-15). In accordance with findings regarding EGFR expression, serum level of EGF is also significantly downregulated in AD patients (16). EGF plays an essential role in the dermal wound-healing process and stabilization of mast cell degranulation (14). Additionally, EGF treatment can reduce epithelial thickness, cutaneous inflammation, and serum IgE level in allergen-induced AD skin (17). Although treatment of AD using human umbilical cord blood-derived MSCs (hUCB-MSCs) has been studied, it is unclear whether growth factors secreted by these cells can facilitate skin recovery. Moreover, the mechanism by which EGF secreted by MSCs improves AD symptoms remains unclear. Therefore, the objective of this study was to determine the role of EGF in therapeutic effects of hUCB-MSCs on AD.
Materials and Methods
Cell culture
hUCB-MSCs were provided by Kangstem Biotech GMP Center (South Korea). All experiments described below were conducted with hUCB-MSCs at passage 5. hUCB-MSCs were maintained with KSB-3 Basal medium (Kangstem Biotech, South Korea) supplemented with 10% fetal bovine serum (FBS; Gibco, USA) and 100 μg/ml primocin (In-vitrogen, USA). HaCaT (Addexbio, USA), human dermal fibroblast (HDF; Cell Applications, USA), and HEK293FT (ATCC, USA) cell lines were maintained with Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS and 100 μg/ml primocin (Invitrogen). Human LAD2 cells were cultured with StemPro-34 medium (Invitrogen) containing 100 ng/ml recombinant human stem cell factor/c-kit ligand (R&D Systems, USA) and 2 mM L-glutamine (Sigma, USA). All cells were maintained at 37℃ in a 5% CO2 incubator. Recombinant human EGF (rhEGF), human interferon (IFN)-γ, and human TNF-α were purchased from PeproTech (NJ, USA).
Antibody array assay and analysis
For secreted protein analysis, cell culture supernatants were collected from two lots of hUCB-MSCs. Cells (1×106/well) were cultured in 6-well plates in 2 ml KSB-3 Basal medium for 48 h. Supernatants were analyzed by E-biogen, Inc. (Korea) using a Human Antibody Array L-1000 (Cat# AAH-BLG-1000-4; RayBiotech, USA) according to the manufacturer’s instructions. Gene ontology (GO) term and Medical Subject Headings (MeSH) enrichment analysis were performed with R packages clusterprofiler and meshes (18).
Enzyme-linked immunosorbent assay (ELISA)
hUCB-MSCs (2×106) were suspended in 0.6 ml DMEM containing 10% FBS and 100 μg/ml primocin, transferred to 24-well plate in duplicates, and cultured for 24 h. Thereafter, the culture medium was collected and centrifuged at 500 g for 5 min. The concentration of EGF was measured using a Human EGF Quantikine ELISA Kit (R&D Systems) according to the manufacturer’s instructions. In addition, 1×105 HaCaT cells/well were co-cultured with 5×105 hUCB-MSCs/transwell (0.4 μm polyethylene terephthalate membrane; Falcon, USA) in 24-well plates and stimulated with TNF-α/IFN-γ (each at 10 ng/ml) for 24 h. The concentration of TARC was measured using a Human TARC Quantikine ELISA Kit (R&D Systems) according to the manufacturer’s instructions.
Scratch assay
Migration of HaCaT cells was examined using a scratch wound-healing assay. All cells (2×105/well) were maintained as a monolayer in DMEM containing 10% FBS in 6-well plates until they reached 80∼90% confluency. A wound was generated using a sterile P1000 micropipette tip. HaCaT cells were washed with phosphate-buffered saline and co-cultured with 2×106 hUCB-MSCs for 48 h in a transwell insert (19). Wound widths in three fields were examined at 10× magnification using a phase-contrast microscope (Ti-U; Nikon, Japan). Wound closure area was measured using ImageJ 1.41 software (NIH, USA). Cell migration was quantitatively analyzed by determining the average wound area in three fields.
Quantitative real-time polymerase chain reaction (qRT-PCR)
A total of 2×105 HaCaT cells/well were co-cultured with 2×106 hUCB-MSCs cells/transwell in 6-well plates and stimulated with TNF-α/IFN-γ (each at 10 ng/ml) for 24 h. Total RNA was extracted from HaCaT cells using TRIzol reagent (Invitrogen) according to the manufacturer’s inst-ructions. A total of 1 μg purified total RNA was converted into cDNA using a cDNA synthesis kit (Bioneer, South Korea). All cDNA samples were stored at −20℃. cDNA was analyzed by qRT-PCR using PowerUpTM SYBR Green Master Mix (Applied Biosystems, CA, USA) and appropriate primers (Supplementary Table S1). qRT-PCR was performed using an ABI 7700 sequence detection system (Applied Biosystems). mRNA expression level was normalized against the level of GAPDH as an internal control. Relative expression was calculated using the comparative CT method (2−ΔΔCt).
Small interfering RNA (siRNA) transfection
All transfections were performed using Lipofectamine 3000 (Invitrogen) according to the manufacturer’s instruc-tions. hUCB-MSCs (1×106/well) were seeded into 6-well plates in KSB-3 medium containing 10% FBS without antibiotics at one day before transfection at 60∼70% con-fluency. On the day of transfection, 200 pmol of control siRNA (siCTL; Santa Cruz Biotechnology, USA) or human EGF-targeting siRNA (siEGF; Santa Cruz Biotech-nology) was mixed with 10 μl Lipofectamine 3000 in 1 ml Opti-MEM medium (Invitrogen). The mixture was incubated at room temperature for 5 min and then added to hUCB-MSCs in fresh medium without serum. The cell culture medium was replaced with fresh medium at 24 h after transfection.
Mast cell degranulation
For IgE-mediated mast cell degranulation, hUCB-MSCs were cultured in 24-well plates for 24 h. Thereafter, LAD2 cells were cultured in the presence of hUCB-MSCs, sensitized with 100 ng/ml human IgE (Millipore, MA, USA) for 24 h, and then challenged with 6 μg/ml anti-IgE for 1 h. Degranulation of LAD2 cells was stopped on ice. Conditioned media were harvested, transferred to a 96-well plate in triplicate, and mixed with substrate solution (p-nitrophenyl-N-acetyl-β-D-glucosaminide, pH 4.5) at a 1:1 ratio. The mixture was incubated in a shaking incubator at 37℃ for 2 h. An equal volume of 0.2 M glycine (pH 10.7) was then added. Released β-hexosa-minidase was quantified by measuring absorbance at 410 nm using a microplate reader.
Th2 cell isolation and polarization
Naïve CD4+ T lymphocytes were isolated from peripheral blood mononuclear cells (PBMCs) using a Naïve CD4+ T cell Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. Isolated CD4+ cells were treated with 50 ng/ml of anti-CD3/28 antibody and IL-2 beads to induce prolife-ration. To polarize Th2 cells, the medium was supplemented with 25 ng/ml IL-6, 50 μg/ml anti-IFN-γ, and 25 ng/ml IL-4. Th2 cells were further cultured for 5 days in the presence of hUCB-MSCs. Polarized Th2 cells were analyzed by flow cytometry to detect intracellular or surface markers. Thereafter, Th2 cells were fixed and incubated with a FITC-conjugated anti-CD4 antibody for surface marker staining. Intracellular marker staining was performed using an intracellular staining buffer set (BD Biosciences, San Jose, USA). Cells were then incubated with a PE-conjugated anti-IL-4 antibody. Signals were detected with a FACSCalibur flow cytometer and quantified using Cell Quest software (BD Bioscience).
Animals
Seven-week-old male NC/Nga mice were purchased from Central Laboratory Animal, Inc. (Seoul, Korea). Mice were acclimatized at a temperature of 22℃±2℃ and a humidity of 55%±5% in an air-conditioned conventional area with a 12 h/12 h light/dark cycle. Three mice were placed in each cage and fed ad libitum. All in vivo experimental procedures were approved by Seoul National University (Approval No. SNU-190925-3-1).
Induction of AD in NC/Nga mice
AD-like symptoms were induced in 8-week-old male NC/Nga mice using Dermatophagoides farinae (Df) extract (Biostir, Inc., Hiroshima, Japan). After one week of acclimation, mice were divided into four groups (n=3 for the negative control group and n=5 for each of other groups). Df extract was applied to shaved dorsal skin and ears twice per week for three weeks. The skin barrier was disrupted by applying 200 μl of 4% sodium dodecyl sulfate to the shaved dorsal skin and each ear at 3∼4 h before topical administration of 100 mg Df extract. On the 3rd day after the last induction (day 21), the following experiments were performed: 1) 2×106 siEGF transfected hUCB-MSCs with anti-human EGF IgG were injected subcutaneously into AD-induced mice; 2) 2×106 scrambled siRNA transfected hUCB-MSCs with control IgG were injected subcutaneously into AD-induced mice; and 3) an identical volume of PBS was injected subcutaneously into AD-induced mice.
Measurement of dermatitis severity and ear thickness
The severity of dermatitis was assessed by calculating the sum of individual scores (0, no symptoms; 1, mild; 2, moderate; and 3, severe) of dryness/scarring, erythema/hemorrhage, edema, and erosion three times per week for four weeks. Assessment was performed by two investigators who were blinded to the experiment. Ear thickness was measured three times per week using Digimatic Calipers (Mitutoyo Corporation, Kanagawa, Japan). Images of clinical symptoms were acquired using a digital camera (DSC-RX100 III; Sony, Inc., Tokyo, Japan) for four weeks.
Statistical analysis
All data were analyzed using GraphPad Prism version 5 (GraphPad Software) and expressed as mean±standard error of the mean (SEM). Clinical severity and ear thickness in the AD mouse model were analyzed by a two-way analysis of variance (ANOVA) with a post hoc test. Statistical comparisons were performed using GraphPad Prism version 5 (T-test). Statistical significance was considered at p<0.05.
Results
Secretome anlaysis of hUCB-MSCs
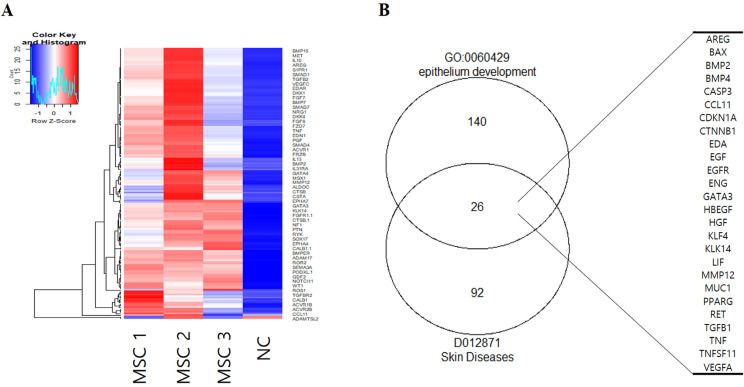
To screen for AD-related therapeutic factors, we performed an antibody array using the cell culture supernatant of hUCB-MSCs with basal medium as a negative control (NC, basal medium). Atopic dermatitis is a typical skin disease. Thus, we performed a heatmap analysis of epithelium development related protein using GO term (Fig. 1A). GO term and Medical Subject Headings (MeSH) enrichment analyses were performed for epithelium development and skin diseases using R packages clusterProfiler and meshes (18). As a result, we found a total of 26 proteins expressed in both epithelium development and skin diseases (Fig. 1B). Among these proteins, the EGFR pathway not only acts as a crucial signal transducer, but also plays an important role in inflammatory reactions in skin biology (12, 15, 20). EGF is also as a potential therapeutic target to recover a damaged epithelium (21, 22). Accordingly, we investigated the role of EGF known to be closely related to skin diseases in the therapeutic efficacy of hUCB-MSCs for atopic dermatitis.
Fig. 1.
Array analysis for secreted proteins from hUCB-MSCs. (A) Heatmap of epithelium development-related proteins by GO term (GO:0060429). (B) Venn diagram of skin diseases and epithelium development-related proteins by GO term and MeSH. NC: negative control (media control), MSC: mesenchymal stem cells.
hUCB-MSC secrets functional concentration of EGF
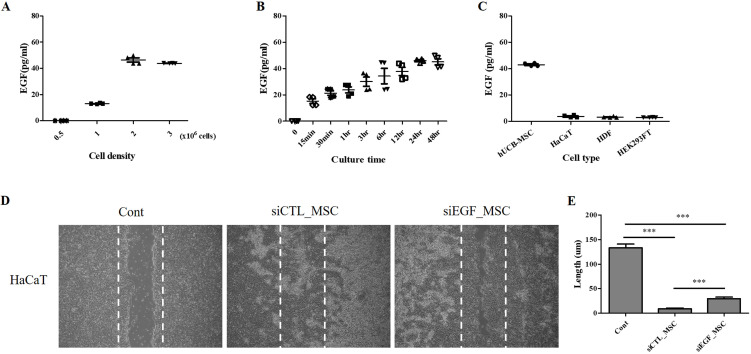
To investigate whether hUCB-MSCs could secrete EGF, we measured EGF secretion according to cell density using an ELISA. The concentration of EGF in cell culture medium was higher than 40 pg/ml when the cell density was 1×106 cells/cm2 or greater (Fig. 2A). We further measured EGF secretion according to cell culture duration. Secretion of EGF was the highest at 24 h after cell seeding (Fig. 2B). hUCB-MSCs secreted significantly higher concentrations (>10-fold higher) of EGF than other cell types (Fig. 2C). EGF is known to be involved in skin development and regeneration (23, 24). Thus, we investigated whether hUCB-MSCs could facilitate keratinocyte migration and wound-healing process by secreting EGF. To evaluate scratch wound healing, HaCaT cells were co-cultured with hUCB-MSCs for 48 h (19). The wound area was significantly decreased following co-culture with hUCB-MSCs. The wound was completely closed after co-culture with hUCB-MSCs for 48 h. This effect was attenuated when EGF was depleted in hUCB-MSCs using siEGF (Fig. 2D∼E). These results indicate that hUCB-MSCs can enhance migration of keratinocytes and that EGF secreted by hUCB-MSCs can accelerate wound healing for AD treatment.
Fig. 2.
Quantitative analysis of EGF secretion and effect of hUCB-MSCs on migration of HaCaT cells. (A) Measurement of EGF secretion according to density of hUCB-MSCs (n=4). (B) Measurement of EGF secretion according to culture duration of hUCB-MSCs (n=4). (C) HaCaT cells, HDFs, and HEK293FT cells secrete low levels of EGF, whereas hUCB-MSCs secrete a high level of EGF (n=4). (D) HaCaT cells were cultured until they reached 90% confluency and then scratched with a sterile pipette tip. Representative microscopic images of scratched cells after co-culture with hUCB-MSCs for 48 h are shown. (E)The length of the wound was expressed as mean±SEM of three independent experiments. Cont: positive control, MSC: mesenchymal stem cells, EGF: epidermal growth factor. *p<0.05, **p<0.01, and ***p<0.001.
EGF secreted by hUCB-MSCs inhibits expression of proinflammatory cytokines and suppresses degranulation of stimulated mast cells
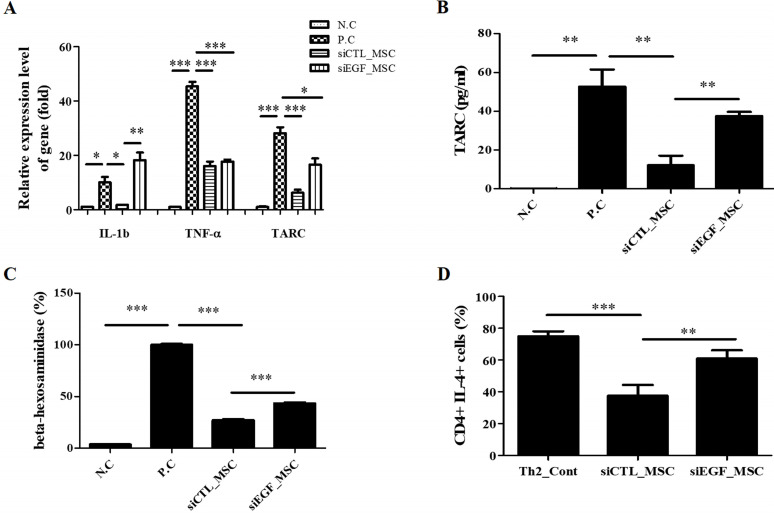
To investigate anti-inflammatory effects of hUCB-MSCs on human keratinocytes, we measured the production of proinflammatory cytokines in HaCaT cells upon TNF-α/IFN-γ stimulation. We first investigated changes in mRNA expression levels of proinflammatory factors IL-1β, TNF-α, and TARC. HaCaT cells were co-cultured with hUCB-MSCs and stimulated with TNF-α/IFN-γ for 24 h. qRT-PCR analysis showed that TNF-α/IFN-γ treatment significantly increased mRNA expression levels of these proinflammatory factors in HaCaT cells. However, such effect was attenuated by co-culture with hUCB-MSCs. Silencing of EGF attenuated the inhibitory effect of hUCB-MSCs on mRNA expression of proinflammatory factors in HaCaT cells (Fig. 3A). Among proinflammatory factors, TARC is a well-known representative biomarker of AD (5, 25). Thus, secretion level of TARC in HaCaT cells co-cultured with hUCB-MSCs was investigated using an ELISA. TNF-α/IFN-γ treatment increased secretion of TARC by HaCaT cells. Secretion of TARC by HaCaT cells was significantly decreased upon co-culture with hUCB-MSCs. This effect was attenuated when EGF was depleted in hUCB-MSCs using siEGF (Fig. 3B). We further measured secretion of β-hexosaminidase by LAD2 cells to investigate IgE-mediated mast cell degranulation. Secretion of β-hexosaminidase was increased in stimulated LAD2 cells. It was suppressed by co-culture with hUCB-MSCs. However, this effect was attenuated when EGF was depleted in hUCB-MSCs using siEGF (Fig. 3C). These results suggest that hUCB-MSCs can inhibit the expression of proinflammatory factors including TARC in keratinocytes in an inflammatory environment and suppress degranulation of stimulated mast cells, with secreted EGF being crucial for these effects.
Fig. 3.
Effects of hUCB-MSCs in an immune-stimulated environment. (A) HaCaT cells were co-cultured with hUCB-MSCs transfected with siCTL or siEGF under inflammatory conditions. Co-culture with hUCB-MSCs reduced mRNA levels of inflammatory cytokines (n=3). (B) Inhibitory effects of hUCB-MSCs on TARC secretion by TNF-α/IFN-γ-stimulated HaCaT cells (n=4). (C) hUCB-MSCs transfected with siCTL or siEGF were co-cultured with LAD2 cells for 48 h (n=3). (D) Th2 cells were co-cultured with hUCB-MSCs transfected with siCTL or siEGF under maturation conditions. Co-culture with hUCB-MSCs inhibited maturation of CD4/IL4+ Th2 cells (n=4). NC: negative control, PC: positive control, MSC: mesenchymal stem cells. *p<0.05, **p<0.01, and ***p<0.001.
EGF secreted by hUCB-MSCs inhibits Th2 cell maturation
Keratinocytes in AD can produce a high level of TARC, which has a major impact on maturation of Th2 cells (25, 26). This suggests that EGF can regulate maturation of Th2 cells by inhibiting TARC expression. Thus, we investigated the direct effect of EGF secreted by hUCB-MSCs on Th2 cell maturation. Immature Th2 cells were isolated from human PBMCs and co-cultured with hUCB-MSCs that had been transfected with siCTL or siEGF under maturation conditions. Maturation of CD4/IL4+ Th2 cells were inhibited by co-culture with hUCB-MSCs. However, these effects were attenuated by silencing of EGF expression in hUCB-MSCs (Fig. 2D). These results suggest that EGF secreted by hUCB-MSCs can directly suppress Th2 cell maturation.
EGF secreted by hUCB-MSCs improves pathological phenotypes of AD
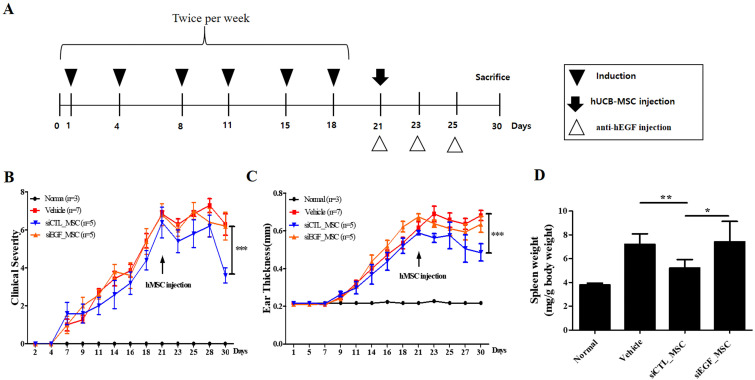
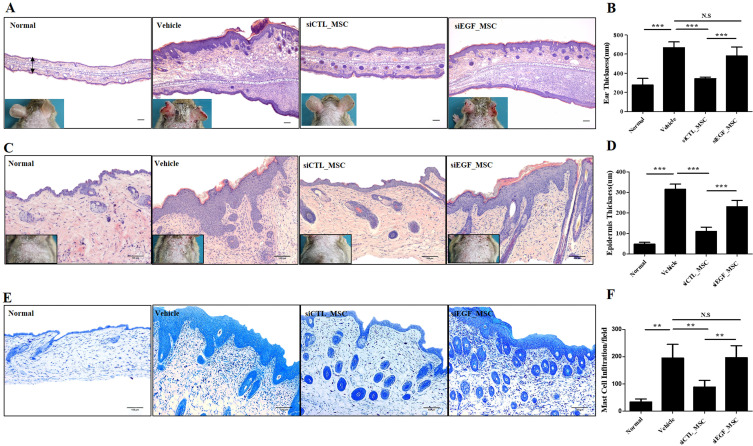
We demonstrated that EGF secreted by hUCB-MSCs was involved in the regulation of diverse inflammatory cells associated with the pathology of AD in vitro. To confirm the therapeutic effect of EGF secreted by hUCB-MSCs in vivo, we investigated effects of EGF-silenced hUCB-MSCs in mice with Df-induced AD. We applied Df to the shaved dorsal skin including surfaces of ears for 3 weeks to induce AD-like lesions in mice. Mice were injected with vehicle, siCTL-transfected hUCB-MSCs, or siEGF-transfected hUCB-MSCs on day 21. Silencing of EGF was maintained for four days. Therefore, a human anti-EGF antibody was further injected (Fig. 4A). Mice injected with siCTL-transfected hUCB-MSCs had significantly lower clinical severity score index, ear thickness, and spleen weight than control mice. However, therapeutic effects of siEGF-transfected hUCB-MSCs were similar to those of the vehicle control (Fig. 4B∼D). Histological analysis showed that epidermal thicknesses of skin lesions and ear thicknesses were reduced in mice injected with siCTL-transfected hUCB-MSCs. However, these effects were attenuated by silencing EGF in hUCB-MSCs (Fig. 5A∼D). Toluidine blue staining was also performed to determine the degree of infiltration of inflammatory leukocytes and mast cells in skin tissues. The disappearance of cells from the basal layer of the epidermis was remarkable in mice administered with the vehicle and siEGF-transfected hUCB-MSCs. The number of infiltrated mast cells was significantly higher in these mice than in mice administered with siCTL-transfected hUCB-MSCs (Fig. 5E and 5F).
Fig. 4.
EGF secreted by hUCB-MSCs suppresses Df-induced AD in NC/Nga mice. (A) Schedule of sensitization of AD and administration of hUCB-MSCs. (B) Dermatitis severity score. Five symptoms (dryness, itching, erythema, edema, and excoriation) were each scored on a scale between 0 and 3 (0, none; 1, mild; 2, moderate; and 3, severe). (C) Ear thickness. (D) Spleen weight (n=3 for normal group, n=5 for other groups). Results are expressed as mean±SEM. *p<0.05, **p<0.01, and ***p<0.001.
Fig. 5.
Histopathological analysis of Df-induced AD mice following administration of hUCB-MSCs. Mice with Df-induced AD were administered vehicle, siCTL-transfected hUCB-MSCs, or siEGF-transfected hUCB-MSCs and sacrificed. Hematoxylin-and-eosin staining was performed to determine (A) the ear thickness and (C) epidermal thickness. (B) Ear thickness and (D) epidermal thickness was measured. (E) Skin lesions on the back were stained with toluidine blue. (F) The number of mast cells in toluidine blue-stained sections was counted. Results are expressed as mean±SEM (n=3 for normal group, n=5 for other groups). *p<0.05, **p<0.01, and ***p<0.001, scale bar 100 μm.
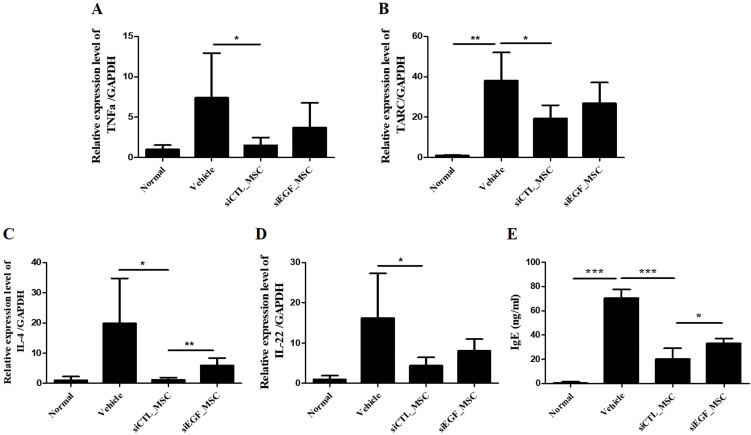
To investigate inflammatory cytokines affected by EGF, we measured mRNA expression levels of crucial inflammatory cytokines in ears of mice by qRT-PCR (Fig. 6A∼D). IL-4 is a representative proinflammatory cytokine secreted by Th2 cells. It can induce the differentiation of mast cells and eosinophils. TARC is a biomarker of AD. It can cause infiltration of CD4+ T cells at lesion sites. TNF-α and IL-22 are inflammatory cytokines that can induce inflammatory mediators, thus exacerbating AD. Administration of siCTL-transfected hUCB-MSCs significantly reduced expression levels of IL-4, TNF-α, TARC, and IL-22 in ears of mice. However, these effects were attenuated by silencing EGF in hUCB-MSCs. In addition, serum IgE level was decreased in mice injected with siCTL-transfected hUCB-MSCs. However, this effect was attenuated by silencing EGF in hUCB-MSCs (Fig. 6E).
Fig. 6.
mRNA expression levels of IL-4, TNF-α, TARC, and IL-22 in skin lesions and the serum IgE level of mice with Df-induced AD following administration of hUCB-MSCs. Mice with Df-induced AD were administered vehicle, siCTL-transfected hUCB-MSCs, or siEGF-transfected hUCB-MSCs and sacrificed. mRNA levels of (A) IL-4, (B) TNF-α, (C) TARC, and (D) IL-22 were determined by qRT-PCR. (E) Serum IgE level was determined by an ELISA. Results are expressed as mean±SEM (n=3 for normal group, n=5 for other groups). *p<0.05, **p<0.01, and ***p<0.001.
Discussion
MSCs can elicit therapeutic effects on various immune-related diseases including AD due to their immunomodulatory ability (2). On the other hand, EGF can help restore the skin barrier in AD (21, 27). However, the effect of EGF secreted by MSCs on immune-related diseases including AD has not been clearly determined yet. Thus, this study investigated immunomodulatory effects of EGF secreted by hUCB-MSCs on AD for the first time. To confirm that hUCB-MSCs could secrete EGF, we first measured the amount of EGF secreted by these cells in the absence of supplemental growth factors. Secretion of EGF by hUCB-MSCs increased in a time- and cell density-dependent manner. In addition, hUCB-MSCs secreted significantly more EGF than HaCaT cells, HDFs, and HEK293FT cells. EGFR signaling in MSCs can enhance cell migration, proliferation, and angiogenic effects (28). According to previous reports, secretion of EGF can be promoted by autocrine and EGFR signaling (29). The present study confirmed that hUCB-MSCs secreted EGF even in the absence of a specific stimulating signal, suggesting that EGF secretion might be promoted via autocrine effects under our culture condition.
We have previously reported that conditioned medium of hUCB-MSCs contains various growth factors to promote the migration of HDFs and synthesis of extracellular matrix proteins (19, 30). Although roles of EGF in proliferation, regeneration, and wound healing of skin cells are well-known (31-34), whether EGF secreted by MSCs itself can elicit regenerative effects has not been determined clearly. The present study found that EGF at concentrations secreted by hUCB-MSCs in the absence of any additional stimulatory factor was functional and that it was a key factor in the wound-healing effect of hUCB-MSCs.
To investigate the immunomodulatory effect of EGF secreted by hUCB-MSCs, hUCB-MSCs in which EGF had been depleted using siEGF were co-cultured with responder cells. Immunomodulatory effects were then inves-tigated. Our analyses revealed that EGF secreted by MSCs was important for effectively controlling inflammatory cells and associated cells to trigger an allergic reaction in AD, including Th2 cells, mast cells, and keratinocytes.
Kim et al. (21) have reported that topical administration of EGF can suppress immune responses and protect the skin barrier of NC/Nga mice with dinitrochloroben-zene-induced AD. They suggested that EGF could improve AD symptoms, consistent with findings of the present study. Moreover, several reports have suggested that EGF can regulate innate immunity by affecting TLR expression, especially in keratinocytes (12, 27). Shibata et al. (35) have reported that EGF can regulate TNF-α-induced TARC in canine keratinocytes. For the first time, the present study revealed that EGF secreted by hUCB-MSCs could directly regulate the differentiation of Th2 cells, degranulation of mast cells (LAD2 cells), and TARC expression in human keratinocytes (HaCaT cells) via in vitro co-culture experiments.
An imbalance between Th1 and Th2 cells is a major pathogenic mechanism in AD. Biologics targeting the Th2 cell-derived cytokines IL-4 and IL-13 can effectively improve AD symptoms (36-38). We have previously confirmed that administration of hUCB-MSCs can significantly downregulate cytokine IL-4 in AD model mice (39). However, this effect is abrogated when secretion of TGF-β by hUCB-MSCs is downregulated. Kim et al. (21) have reported that various inflammatory cytokines, including IL-4 and IL-13 whose levels are increased in AD, are downregulated upon topical treatment of EGF in dinitrochlorobenzene-induced AD model mice. However, they did not elucidate whether these effects were induced indirectly by recovery of the skin barrier or directly by modulation of specific immune cells. Interestingly, the present study demonstrated that hUCB-MSCs suppressed differentiation and maturation of Th2 cells and that these effects were significantly attenuated by depletion of EGF in hUCB-MSCs. This suggests that hUCB-MSCs can ameliorate AD symptoms by regulating Th2 cells and that EGF is a key factor in Th2 cell regulation.
Inhibition of mast cell degranulation by EGF in AD has not been well studied yet. However, it has been reportedly that EGF can stabilize mast cell degranulation and histamine secretion in a gastric damage model and protect gastric mucosa (14). We confirmed that EGF secreted by hUCB-MSCs also regulated degranulation and infiltration of mast cells in allergic skin lesions. Our previous studies have confirmed that TGF-β and prostaglandin E2 secreted by MSCs are involved in differentiation, maturation, degranulation, and activation of mast cells (39). The present study demonstrates that EGF is another key molecule in the regulation of mast cell activity by hUCB-MSCs.
The immunomodulatory function of EGF secreted by hUCB-MSCs observed in vitro was confirmed in a Df-induced AD mouse model. Improvements in gross lesions, spleen weight, histologic damage, serum IgE level, and levels of inflammatory cytokines observed upon injection of hUCB-MSCs were significantly attenuated when EGF expression was downregulated in these cells. This study clearly demonstrates that EGF secreted by hUCB-MSCs is important for improving inflammatory reaction in AD. Moreover, EGF secreted by hUCB-MSCs effectively controlled differentiation/maturation of Th2 cells and activities of mast cells and keratinocytes as well as regeneration of the skin barrier in AD model mice. Several studies have reported that EGF can improve symptoms of AD (21, 27). However, this is the first study to confirm that EGF secreted by MSCs plays a key role in ameliorating AD symptoms. Serum IgE level, IL-22 level, and spleen weight were significantly improved in AD model mice injected with hUCB-MSCs. However, these effects were attenuated when EGF was depleted in hUCB-MSCs. These results suggest that EGF secreted by hUCB-MSCs can affect immune cells such as Th2 cells and B cells. Additional studies are needed to further elucidate these effects and the underlying mechanism.
Taken together, our findings reveal that EGF secreted by hUCB-MSCs plays a key role not only in promoting skin regeneration, but also in effectively controlling Th2 cells, mast cell degranulation, and keratinocyte-derived cytokines known to induce AD symptoms. It is important to clarify the mechanisms underlying the actions of stem cells, although such taks is often difficult. Elucidation of these mechanisms provides a scientific basis for cell therapy products. It is also important for quality control during commercial manufacturing. Novel findings presented in this study highlight the potential of hUCB-MSCs for treating AD and can be utilized to predict efficacy. Moreover, considering that MSCs can secret EGF with an immunomodulatory ability regardless of their origin (40), this study indicates that enhancement of EGF secretion by MSCs derived from various sources is one way to increase the therapeutic efficacy of these cells in AD.
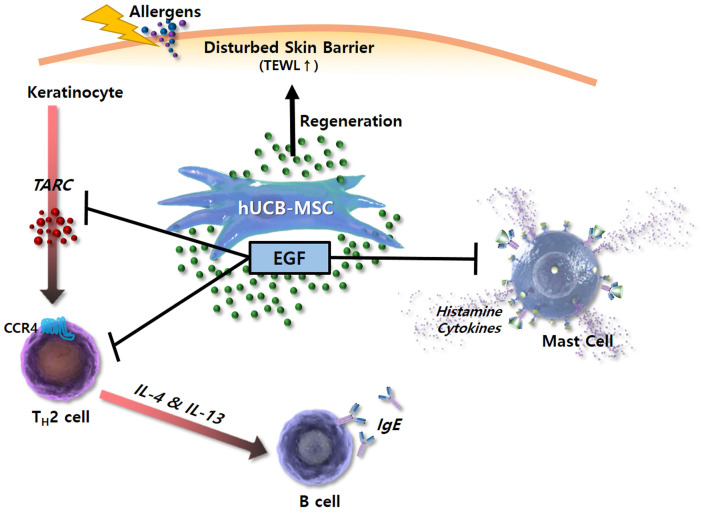
In conclusion, this study demonstrates that hUCB-MSCs can ameliorate AD symptoms by secreting EGF. EGF is involved in the regeneration of epidermis and regulation of cellular immune responses, including TARC secretion by keratinocytes, degranulation of mast cells, IgE secretion by B cells, and polarization of Th2 cells (Fig. 7). To develop stem cell therapeutics, the mechanisms underlying their effects must be clarified and efficacy markers must be developed. This study provides the scientific rationale for the therapeutic use of hUCB-MSCs in allergic diseases including AD.
Fig. 7.
Schematic diagram showing broad immunomodulatory properties of hUCB-MSCs in AD. EGF secreted by hUCB-MSCs inhibits functions of Th2 cells, B cells, and mast cells but promotes keratinocyte regeneration with TARC suppression. Through this mechanism, hUCB-MSCs improve skin barrier function, thereby increasing skin moisture and reduce TEWL. TEWL: Transepidermal water loss.
Table 1.
List of abbreviation
| Abbreviation | Explanation |
|---|---|
| MSCs | Mesenchymal stem cells |
| AD | Atopic dermatitis |
| hUCB-MSCs | Human umbilical cord blood-derived MSCs |
| EGF | Epidermal growth factor |
| EGFR | Epidermal growth factor receptor |
| IL | Interleukin |
| TNF | Tumor necrosis factor |
| TARC | Thymus and activation-regulated chemokine |
| DCs | Dendritic cells |
| TGF | Transforming growth factor |
| IFN | Interferon |
| CCR4 | CC chemokine receptor 4 |
| IgE | Immunoglobulin E |
| FBS | Fetal bovine serum |
| DMEM | Dulbecco’s modified Eagle’s medium |
| rhEGF | Recombinant human EGF |
| Df | Dermatophagoides farinae |
| GO | Gene ontology |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| siRNA | Small interfering RNA |
| PBMCs | Peripheral blood mononuclear cells |
| NC | Negative control |
| PC | Positive control |
| TEWL | Transepidermal water loss |
Supplementary Materials
Supplementary data including one table can be found with this article online at https://doi.org/10.15283/ijsc21173.
Acknowledgments
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Potential Conflict of Interest
The authors have no conflicting financial interest.
Ethics Approval
This study was approved by the Institutional Review Board (IRB) of the Public Institutional Bioethics Committee designated by the MOHW (Approval No. P01-201605-BS-02). All in vivo experimental procedures were approved by Seoul National University (Approval No. SNU-190925-3-1).
Author Contributions
Conception and design, Supervising all experimental testing, Data analysis and interpretation, Manuscript writing: SL, KSK.
Conception and design, Data analysis and interpretation, Manuscript writing: NJ.
Collection and assembly of data, Data analysis and interpretation, Manuscript writing: THK.
Conception and design, Data analysis and interpretation: YY.
Collection and assembly of data: HP, EL, SMY, SYB.
All authors read and approved the final manuscript.
References
- 1.Bantz SK, Zhu Z, Zheng T. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. J Clin Cell Immunol. 2014;5:202. doi: 10.4172/2155-9899.1000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daltro SRT, Meira CS, Santos IP, Ribeiro Dos Santos R, Soares MBP. Mesenchymal stem cells and atopic dermatitis: a review. Front Cell Dev Biol. 2020;8:326. doi: 10.3389/fcell.2020.00326.fd999ad7be19495680b604708ba660bc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biedermann T, Skabytska Y, Kaesler S, Volz T. Regulation of T cell immunity in atopic dermatitis by microbes: the Yin and Yang of cutaneous inflammation. Front Immunol. 2015;6:353. doi: 10.3389/fimmu.2015.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kakinuma T, Nakamura K, Wakugawa M, Mitsui H, Tada Y, Saeki H, Torii H, Asahina A, Onai N, Matsushima K, Tamaki K. Thymus and activation-regulated chemokine in atopic dermatitis: serum thymus and activation-regulated chemokine level is closely related with disease activity. J Allergy Clin Immunol. 2001;107:535–541. doi: 10.1067/mai.2001.113237. [DOI] [PubMed] [Google Scholar]
- 5.Vestergaard C, Bang K, Gesser B, Yoneyama H, Matsushima K, Larsen CG. A Th2 chemokine, TARC, produced by keratinocytes may recruit CLA+CCR4+ lymphocytes into lesional atopic dermatitis skin. J Invest Dermatol. 2000;115:640–646. doi: 10.1046/j.1523-1747.2000.00115.x. [DOI] [PubMed] [Google Scholar]
- 6.Ritto D, Tanasawet S, Singkhorn S, Klaypradit W, Hutamekalin P, Tipmanee V, Sukketsiri W. Astaxanthin induces migration in human skin keratinocytes via Rac1 activation and RhoA inhibition. Nutr Res Pract. 2017;11:275–280. doi: 10.4162/nrp.2017.11.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sah SK, Agrahari G, Nguyen CT, Kim YS, Kang KS, Kim TY. Enhanced therapeutic effects of human mesenchymal stem cells transduced with superoxide dismutase 3 in a murine atopic dermatitis-like skin inflammation model. Allergy. 2018;73:2364–2376. doi: 10.1111/all.13594. [DOI] [PubMed] [Google Scholar]
- 8.Shigemoto-Kuroda T, Oh JY, Kim DK, Jeong HJ, Park SY, Lee HJ, Park JW, Kim TW, An SY, Prockop DJ, Lee RH. MSC-derived extracellular vesicles attenuate immune responses in two autoimmune murine models: type 1 diabetes and uveoretinitis. Stem Cell Reports. 2017;8:1214–1225. doi: 10.1016/j.stemcr.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ocansey DKW, Qiu W, Wang J, Yan Y, Qian H, Zhang X, Xu W, Mao F. The achievements and challenges of mesenchymal stem cell-based therapy in inflammatory bowel disease and its associated colorectal cancer. Stem Cells Int. 2020;2020:7819824. doi: 10.1155/2020/7819824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss ARR, Dahlke MH. Immunomodulation by mesenchymal stem cells (MSCs): mechanisms of action of living, apoptotic, and dead MSCs. Front Immunol. 2019;10:1191. doi: 10.3389/fimmu.2019.01191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puccinelli TJ, Bertics PJ, Masters KS. Regulation of keratinocyte signaling and function via changes in epidermal growth factor presentation. Acta Biomater. 2010;6:3415–3425. doi: 10.1016/j.actbio.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pastore S, Mascia F, Mariani V, Girolomoni G. The epidermal growth factor receptor system in skin repair and inflammation. J Invest Dermatol. 2008;128:1365–1374. doi: 10.1038/sj.jid.5701184. [DOI] [PubMed] [Google Scholar]
- 13.Sääf A, Pivarcsi A, Winge MC, Wahlgren CF, Homey B, Nordenskjöld M, Tengvall-Linder M, Bradley M. Characterization of EGFR and ErbB2 expression in atopic dermatitis patients. Arch Dermatol Res. 2012;304:773–780. doi: 10.1007/s00403-012-1242-4. [DOI] [PubMed] [Google Scholar]
- 14.Erkasap S, Erkasap N, Aral E, Koken T, Kahraman A, Aydin Y, Yilmaz S, Ates E. Mast cell stabilizator and antioxidant effects of epidermal growth factor (EGF) on gastric mucosal injury induced by ethanol in rats. Chin J Physiol. 2005;48:1–6. Erratum in: Chin J Physiol 2005;48:114. [PubMed] [Google Scholar]
- 15.Jost M, Kari C, Rodeck U. The EGF receptor - an essential regulator of multiple epidermal functions. Eur J Dermatol. 2000;10:505–510. [PubMed] [Google Scholar]
- 16.Jacob M, Bin Khalaf D, Alhissi S, Arnout R, Alsaud B, Al-Mousa H, Lopata AL, Alazami AM, Dasouki M, Abdel Rahman AM. Quantitative profiling of cytokines and chemokines in DOCK8-deficient and atopic dermatitis patients. Allergy. 2019;74:370–379. doi: 10.1111/all.13610. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, Xiao C, Gibson AM, Bass SA, Khurana Hershey GK. EGFR signaling blunts allergen-induced IL-6 production and Th17 responses in the skin and attenuates development and relapse of atopic dermatitis. J Immunol. 2014;192:859–866. doi: 10.4049/jimmunol.1301062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu G. Using meshes for MeSH term enrichment and semantic analyses. Bioinformatics. 2018;34:3766–3767. doi: 10.1093/bioinformatics/bty410. [DOI] [PubMed] [Google Scholar]
- 19.Saheli M, Bayat M, Ganji R, Hendudari F, Kheirjou R, Pakzad M, Najar B, Piryaei A. Human mesenchymal stem cells-conditioned medium improves diabetic wound healing mainly through modulating fibroblast behaviors. Arch Dermatol Res. 2020;312:325–336. doi: 10.1007/s00403-019-02016-6. [DOI] [PubMed] [Google Scholar]
- 20.Sääf AM, Tengvall-Linder M, Chang HY, Adler AS, Wahlgren CF, Scheynius A, Nordenskjöld M, Bradley M. Global expression profiling in atopic eczema reveals reciprocal expression of inflammatory and lipid genes. PLoS One. 2008;3:e4017. doi: 10.1371/journal.pone.0004017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YJ, Choi MJ, Bak DH, Lee BC, Ko EJ, Ahn GR, Ahn SW, Kim MJ, Na J, Kim BJ. Topical administration of EGF suppresses immune response and protects skin barrier in DNCB-induced atopic dermatitis in NC/Nga mice. Sci Rep. 2018;8:11895. doi: 10.1038/s41598-018-30404-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Troyer KL, Luetteke NC, Saxon ML, Qiu TH, Xian CJ, Lee DC. Growth retardation, duodenal lesions, and aberrant ileum architecture in triple null mice lacking EGF, amphiregulin, and TGF-alpha. Gastroenterology. 2001;121:68–78. doi: 10.1053/gast.2001.25478. [DOI] [PubMed] [Google Scholar]
- 23.Mao Y, Ma J, Xia Y, Xie X. The overexpression of epidermal growth factor (EGF) in HaCaT cells promotes the proliferation, migration, invasion and transdifferentiation to epidermal stem cell immunophenotyping of adipose-derived stem cells (ADSCs) Int J Stem Cells. 2020;13:93–103. doi: 10.15283/ijsc18146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia Y, You XE, Chen H, Yan YJ, He YC, Ding SZ. Epidermal growth factor promotes mesenchymal stem cell-mediated wound healing and hair follicle regeneration. Int J Clin Exp Pathol. 2017;10:7390–7400. [PMC free article] [PubMed] [Google Scholar]
- 25.Lin L, Nonoyama S, Oshiba A, Kabasawa Y, Mizutani S. TARC and MDC are produced by CD40 activated human B cells and are elevated in the sera of infantile atopic dermatitis patients. J Med Dent Sci. 2003;50:27–33. [PubMed] [Google Scholar]
- 26.Vestergaard C, Deleuran M, Gesser B, Larsen CG. Thymus- and activation-regulated chemokine (TARC/CCL17) induces a Th2-dominated inflammatory reaction on intradermal injection in mice. Exp Dermatol. 2004;13:265–271. doi: 10.1111/j.0906-6705.2004.00149.x. [DOI] [PubMed] [Google Scholar]
- 27.Choi SY, Lee YJ, Kim JM, Kang HJ, Cho SH, Chang SE. Epidermal growth factor relieves inflammatory signals in Staphylococcus aureus-treated human epidermal keratinocytes and atopic dermatitis-like skin lesions in Nc/Nga mice. Biomed Res Int. 2018;2018:9439182. doi: 10.1155/2018/9439182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Luca A, Gallo M, Aldinucci D, Ribatti D, Lamura L, D'Alessio A, De Filippi R, Pinto A, Normanno N. Role of the EGFR ligand/receptor system in the secretion of angiogenic factors in mesenchymal stem cells. J Cell Physiol. 2011;226:2131–2138. doi: 10.1002/jcp.22548. [DOI] [PubMed] [Google Scholar]
- 29.Maheshwari G, Wiley HS, Lauffenburger DA. Autocrine epidermal growth factor signaling stimulates directionally persistent mammary epithelial cell migration. J Cell Biol. 2001;155:1123–1128. doi: 10.1083/jcb.200109060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim YJ, Seo DH, Lee SH, Lee SH, An GH, Ahn HJ, Kwon D, Seo KW, Kang KS. Conditioned media from human umbilical cord blood-derived mesenchymal stem cells stimulate rejuvenation function in human skin. Biochem Biophys Rep. 2018;16:96–102. doi: 10.1016/j.bbrep.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yaish P, Gazit A, Gilon C, Levitzki A. Blocking of EGF-dependent cell proliferation by EGF receptor kinase inhibitors. Science. 1988;242:933–935. doi: 10.1126/science.3263702. [DOI] [PubMed] [Google Scholar]
- 32.Heo JS, Lee YJ, Han HJ. EGF stimulates proliferation of mouse embryonic stem cells: involvement of Ca2+ influx and p44/42 MAPKs. Am J Physiol Cell Physiol. 2006;290:C123–C133. doi: 10.1152/ajpcell.00142.2005. [DOI] [PubMed] [Google Scholar]
- 33.Wan J, Ramachandran R, Goldman D. HB-EGF is necessary and sufficient for Muller glia dedifferentiation and retina regeneration. Dev Cell. 2012;22:334–347. doi: 10.1016/j.devcel.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Ye X, Qi J, Fan R, Gao X, Wu Y, Zhou L, Tong A, Guo G. EGF and curcumin co-encapsulated nanoparticle/hydrogel system as potent skin regeneration agent. Int J Nanomedicine. 2016;11:3993–4009. doi: 10.2147/IJN.S104350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shibata S, Maeda S, Kondo N, Chimura N, Inoue A, Fukata T. Identification of the signaling pathway of TNF-α-induced CCL17/TARC transcription in a canine keratinocyte cell line. Vet Immunol Immunopathol. 2011;139:90–98. doi: 10.1016/j.vetimm.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Choi JH, Kim HG, Jin SW, Han EH, Khanal T, Do MT, Hwang YP, Choi JM, Chun SS, Chung YC, Jeong TC, Jeong HG. Topical application of Pleurotus eryngii extracts inhibits 2,4-dinitrochlorobenzene-induced atopic dermatitis in NC/Nga mice by the regulation of Th1/Th2 balance. Food Chem Toxicol. 2013;53:38–45. doi: 10.1016/j.fct.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 37.Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13:425–437. doi: 10.1080/1744666X.2017.1298443. [DOI] [PubMed] [Google Scholar]
- 38.Jeong NH, Yang EJ, Jin M, Lee JY, Choi YA, Park PH, Lee SR, Kim SU, Shin TY, Kwon TK, Jang YH, Song KS, Kim SH. Esculetin from Fraxinus rhynchophylla attenuates atopic skin inflammation by inhibiting the expression of inflammatory cytokines. Int Immunopharmacol. 2018;59:209–216. doi: 10.1016/j.intimp.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Park HH, Lee S, Yu Y, Yoo SM, Baek SY, Jung N, Seo KW, Kang KS. TGF-β secreted by human umbilical cord blood-derived mesenchymal stem cells ameliorates atopic dermatitis by inhibiting secretion of TNF-α and IgE. Stem Cells. 2020;38:904–916. doi: 10.1002/stem.3183. [DOI] [PubMed] [Google Scholar]
- 40.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886.6b6f2edcba8b4e85983c024338f231bd [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.