Abstract
Background
Postoperative radiotherapy (RT) is known to play an important role in the treatment of hepatocellular carcinomas (HCCs), but the specific role of intraoperative electron radiotherapy (IOERT) in HCCs remains unclear. The aim of this study was to investigate the safety and efficacy of IOERT in centrally located HCCs treated with narrow-margin (<1 cm) hepatectomy.
Methods
This was a single-center, phase 2, prospective non-randomized controlled study, including 268 patients with centrally located HCCs who underwent narrow-margin hepatectomy. The patients were subsequently allocated to the IOERT group (n=59) or to the control group (n=65). The primary outcome of the study was to compare recurrence-free survival (RFS) between the IOERT group and the control group, and the secondary outcome was to compare overall survival (OS) rate between the two groups.
Results
Of 268 patients enrolled, a total of 124 were included in the study: 59 in IOERT group, 65 in control group. The 1-, 2-, 3-year RFS rates were 79.3%, 62.1% and 45.8% for patients in the IOERT group, and 47.6%, 28.6%, and 22.9% for patients in the control group, respectively (P=0.025). The 1-, 2-, and 3-year OS rates were 100.0%, 94.9%, and 83.7% for patients in the IOERT group, and 92.3%, 87.5%, and 79.4% for patients in the control group, respectively (P=0.314). Subgroup analysis of MVI (+) patients revealed that RFS and OS are significantly prolonged in the IOERT subgroup as compared to the control, whereas there was no significant difference of RFS and OS between the two groups in MVI (−) patients.
Conclusions
IOERT for centrally located HCCs with concurrent narrow-margin hepatectomy was feasible and safe. Statistically better RFS rate was observed in the IOERT group compared to the control group. Subgroup analysis revealed that IOERT was more beneficial for postoperative survival of HCC patients with MVI.
Trial Registration
ChiCTR-TRC-12002802; www.who.int/ictrp.
Keywords: Hepatocellular carcinoma (HCC), intraoperative electron radiotherapy (IOERT), microvascular invasion (MVI), relapse survival, overall survival (OS)
Introduction
Liver cancer is the sixth most common cancers in the world (1). Hepatocellular carcinoma (HCC) accounts for 75% to 85% of primary liver cancer, and is the third leading cause of cancer deaths worldwide (1,2). The geographic distribution of HCC differs significantly, with 80% cases occurring in developing countries such as Far East and South Asia, where there is greater prevalence of viral hepatitis (3). More than half of new HCC cases are reported in China every year (4). Surgical resection is the standard curative treatment in selected patients with reported 5-year survival rates of 60%. However, at 5-year the tumor recurrence rate is as high as 70% (2,5). For patients unsuitable for hepatic resection, interesting techniques such as iron chelating therapy with deferasirox and deferoxamine have recently been explored in basic experimental studies in order to deprive cancer cells of the essential iron required for proliferation (6-8). However, owing to its toxicity and low efficiency in vivo, limited clinical research has been reported on iron chelation therapy (6,7).
Centrally located HCC is defined as carcinoma adjoining hepatic hilum, less than 1 cm from major vascular structures (including the inferior vena cava, main portal branches, and main trunks of the hepatic veins), usually located in Couinaud segments Ⅰ, Ⅳ, Ⅴ, Ⅷ, or at the junction of the central segments (9). Since 2006, our team has performed safe surgical resection for over 500 patients with centrally located HCC (9,10). These resections were performed with <1 cm narrow or null margins (if the tumor is closely adhered to the major vascular structures). However, few patients relapsed shortly post narrow-margin resection. Such narrow margins tend to result in microscopic residual lesions, that can diffuse through intrahepatic vessels causing relapses (11-13). Thus, exploring effective adjuvant therapies for patients undergoing narrow margin hepatectomy to reduce recurrence is crucial, and a research hotspot (14).
Adjuvant therapies such as radiotherapy (RT) has shown to reduce recurrence in HCC patients (15). The safety and efficacy of RT is widely accepted, and is generally used in HCC patients exhibiting either unresectable HCC, small HCC, HCC with portal or hepatic vein tumor thrombus, or distant metastasis of HCC (16-20). However, a major limitation of conventionally used RT technology is difficulty in targeting effective dose to the tumor site alone rather than whole liver (21). Thus, numerous other techniques, such as three-dimensional conformal RT, intensity modulated radiation therapy (IMRT), and stereotactic body radiation therapy (SBRT) techniques have been developed, facilitating the delivery of high-dose radiation to the tumor site, while protecting the non-tumor segment of the liver and surrounding healthy tissues (22,23). More recently, proton beam therapy (PBT) has also been explored for the treatment of HCCs, to potentially reduce radiation related hepatotoxicity, allowing tumor dose escalation (24). However, these techniques are either costly, time-consuming, complex to deliver, or exhibit adverse effects such as fatigue, swelling and nausea (25,26). Thus, alternative techniques that overcome these challenges need to be explored.
Intraoperative electron radiotherapy (IOERT) is one such adjuvant RT that is delivered to the tumor bed that are exposed during surgery. The most important feature of IOERT is its precision and nominal exposure to the neighboring healthy tissues (27). The effectiveness of one single IOERT dose is 2–3 times higher than the same dose given by conventional fractionation (28). IOERT has reported to reduce recurrence and improve overall survival (OS) in various types of tumors such as breast, rectal and renal cancers (28-31). However, to the best of our knowledge, the efficacy of IOERT on HCC has not been reported till date. In the present study, we aim to evaluate the safety and efficacy of IOERT for centrally located HCC with concurrent narrow-margin hepatectomy. We present the following article in accordance with the TREND reporting checklist (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-223/rc).
Methods
Study design and patients
This prospective, single center study was conducted at the Cancer Hospital, Chinese Academy of Medical Sciences (CAMS) between December 2012 and January 2019. The clinical trial was registered in December 2012 in Chinese clinical trial register (ChiCTR-TRC-12002802; www.who.int/ictrp), but was formally launched in March 2015 due to technology testing and debugging of the equipment.
The inclusion criteria considered for the study were centrally located HCC, complete removal of tumor by preoperative evaluation, Barcelona Clinic Liver Cancer (BCLC) A or B stage, Child-Pugh liver function class A, Eastern Cooperative Oncology Group Performance Status of 0 or 1. Exclusion was determined by intraoperative or postoperative evaluation. Patients were excluded if resection margin was ≥1 cm, palliative resection with residual tumor was performed, either received preoperative RT, or were recommended postoperative RT after multi-disciplinary team (MDT) evaluation, exhibited simultaneous malignant tumor/diseases, non-HCC or BCLC C stage was confirmed by postoperative pathology.
Patients were fully informed about the study conditions. The patients were divided into two groups based on decision that was made by doctors and patients both sides jointly, mimics real-world scenario and cohort study, hepatectomy followed by IOERT (IOERT group), or only hepatectomy (control group). The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (No. NCC2013S-005) and was conducted in compliance with principles of Good Clinical Practice and informed consent was taken from all individual participants (ChiCTR-TRC-12002802; www.who.int/ictrp).
Sample size determination
Power analysis and sample size (PASS) software was used to estimate the sample size of 119 patients, with recurrence-free survival (RFS) as the main endpoint. Based on the RFS rates reported by previous studies (9,10,32), the 2-year RFS rate in the IOERT vs. control group was assumed to reach 68% vs. 45%, respectively in the present study, with a two-tailed α value of 0.05, test efficiency (power, i.e., 1-β) of 0.9 and the dropout rate of 5%. The ratio of cases between the two group was 1:1 where the patients were non-randomly enrolled to the IOERT group or control group. Each patient was followed up for at least 2 years.
Hepatectomy and IOERT procedure
After confirming the absence of extrahepatic metastasis of HCC, intraoperative ultrasonography was performed to define the tumor location accurately and determine the range of resection according to tumor location, size, distance to major vascular structures and degree of hepatic cirrhosis. Operation methods included anatomical resection such as standard liver segment resection, combined liver segments resection and hemi-hepatectomy, and non-anatomical resection. Tumors adherent to major vascular structures were resected carefully from the vascular surface using null margin resection. After tumor removal, the specimen was examined to measure resection margins.
For patients in the IOERT group, rapid pathological examinations were performed to confirm their specimens as HCCs or other hepatic malignant tumors. The treatment plan was determined and executed by the same team of radio-oncologists and surgeons. Parameters considered for the treatment plan included the target volume, location, distance to major vascular structures, the range of tumor bed, possible sites of microscopic residual disease, and radiation dosage with an appropriately sized applicator according to the tumor size. The target volume was defined as tumor bed plus 1.0 cm margin and the median treatment depth was 1.0 cm (range, 0.5–1.5 cm). A 0.5 or 1.0 cm thick bolus was used to adjust the depth of treatment, with several sheets of lead protecting the surrounding tissues. IOERT was performed by the mobile intraoperative electron linear accelerator (Mobetron, IntraOp Medical Corporation, Sunnyvale, CA, USA) (Figure S1). Median IOERT dose was 15 Gy (range, 15–17 Gy), prescribed to the 90% isodose line. The energy was 6 MeV (90.62%) or 9 MeV (9.38%) and RT lasted for about 3 minutes.
Study outcomes
The post-operative outcome included evaluating the acute toxicity of IOERT. Radiation-induced liver disease (RILD) was evaluated after 4 months from surgery. Classic RILD was defined as either a ≥2-fold increase in anicteric elevation of alkaline phosphatase level and nonmalignant ascites, and non-classic RILD was defined as ≥5-fold increase in the normal upper limit or the pre-treatment level of transaminases (33). Primary outcome of the study was RFS, defined from the date of surgery until the date when HCC recurrence was first diagnosed. The secondary outcome was OS, recorded between the date of surgery until the death of patients.
Follow-up
All enrolled patients were followed up for 2 years, once every 2–3 months, post 1–2 months of surgery, and thereafter once every 4 to 6 months until either the death of the patient, or the study cut-off date (i.e., December 2020). After 2 years, patients who were lost to follow-up, and those who did not exhibit an endpoint event (recurrence or death) at the end of the clinical trial were censored at the date of their last observation. Levels of serum alpha-fetoprotein (AFP), routine blood tests, liver function tests, abdominal contrast-enhanced magnetic resonance imaging (MRI) or computed tomography (CT), and chest X-rays were performed during follow-up. Recurrence of HCC was diagnosed based on elevated AFP levels, development of extrahepatic metastasis, and typical morphological changes observed in MRI or CT scan (such as arterial enhancement and portal delayed/washed out) (34).
Treatment for recurrence
The treatment strategy employed for recurrent HCC was based on the characteristics of tumor, liver function, general condition and choice of the patient, along with advice from the multidisciplinary team. For nodular recurrence, local or regional curative treatment such as reoperation-hepatectomy, radiofrequency ablation (RFA) and SBRT were executed. Alternatively, for diffuse recurrences, systemic palliative treatments such as transhepatic arterial chemoembolization (TACE), molecular targeted therapy and chemotherapy were performed.
Subgroup analysis
Subgroup analysis was performed to further evaluate the factors affecting RFS and OS in patients in the IOERT group and the control group. Since the independent prognostic factor associated with both RFS and OS was found to be MVI, patients were sub-grouped into MVI (+) or MVI (−).
Statistical analysis
The experimental data was statistically analyzed using IBM SPSS software, version 26.0. Students t-test was used to compare continuous variables consistent with normal distribution and were expressed as means with standard deviation (SD) while rank-sum tests to compare continuous variables consistent with non-normal and were expressed as median with interquartile range (IQR). Chi-square tests or Fischer’s exact tests were used to compare categorical variables. RFS and OS were evaluated by Kaplan-Meier method, and compared using the stratified log-rank test. Univariate analysis was used to evaluate the factors affecting RFS and OS, and multivariate cox proportional hazard regression analysis was used to understand the independent prognostic factors associated with RFS and OS. Statistical significance was defined as P<0.05.
Results
Patients
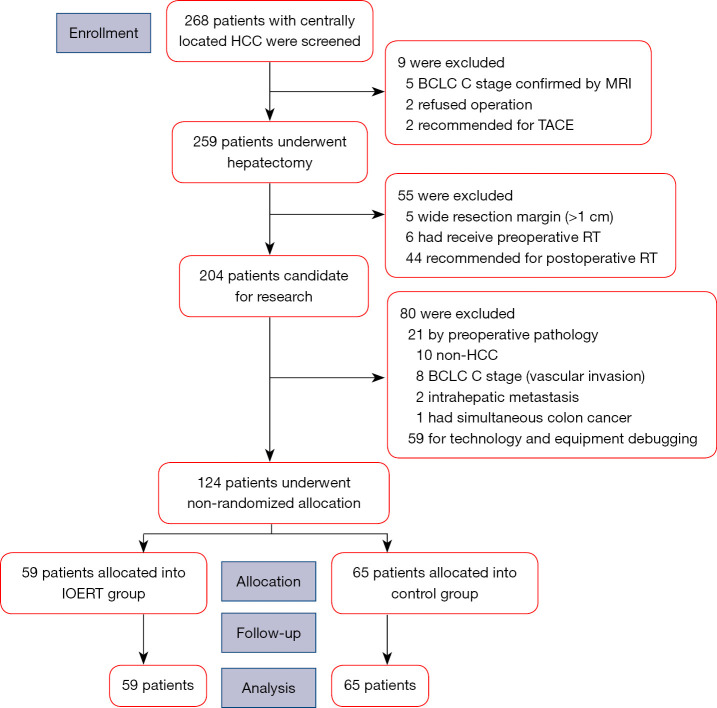
A total of 268 patients with centrally located HCCs were initially enrolled, of which 9 patients were excluded before operation as they were unsuitable for surgery. Among the 259 patients that received hepatectomy, 135 patients were excluded and finally 124 patients were considered for the study. Figure 1 represents the flowchart for selection of patients. Of the 124 patients, 59 patients were allocated to the IOERT group (hepatectomy followed by IOERT) and 65 patients were allocated to the control group (only hepatectomy).
Figure 1.
Patient disposition. HCC, hepatocellular carcinoma; BCLC, Barcelona Clinic Liver Cancer; MRI, magnetic resonance imaging; TACE, transhepatic arterial chemoembolization; RT, radiotherapy; IOERT, intraoperative electron radiotherapy.
Patients in both groups had similar baseline demographic characteristics with no significant difference with respect to age, gender, body mass index (BMI), viral hepatitis (HBV and/or HCV), pre/postoperative antiviral therapy, cirrhosis, alcohol intake, preoperative serum AFP, BCLC stage, tumor size, differentiation, satellite nodules, liver capsule invasion, and microvascular invasion (MVI) (Table 1).
Table 1. Baseline characteristics of the patients.
| Variables | IOERT group (n=59) | Control group (n=65) | P value |
|---|---|---|---|
| Age (year, mean ± SD) | 54.47±10.12 | 56.92±9.68 | 0.171 |
| Gender (male/female, n) | 52/7 | 59/6 | 0.633 |
| BMI, (kg/m2, mean ± SD) | 24.60±3.35 | 25.16±3.56 | 0.367 |
| Viral hepatitis (n) | 0.626 | ||
| Nil | 2 | 6 | |
| Hepatitis B virus | 53 | 55 | |
| Hepatitis C virus | 2 | 2 | |
| Hepatitis B + hepatitis C virus | 2 | 2 | |
| Preoperative antiviral therapy (yes/no, n) | 18/41 | 18/47 | 0.730 |
| Postoperative antiviral therapy (yes/no, n) | 48/11 | 52/13 | 0.849 |
| Cirrhotic liver (yes/no, n) | 56/3 | 57/8 | 0.158 |
| Alcohol intake (yes/no, n) | 26/33 | 29/36 | 0.951 |
| Alpha-fetoprotein (n) | 0.417 | ||
| ≤7 ng/mL | 22 | 26 | |
| 7–400 ng/mL | 27 | 23 | |
| ≥400 ng/mL | 10 | 16 | |
| BCLC (stage A/B, n) | 58/1 | 61/4 | 0.368 |
| Tumor size (cm, median ± IQR) | 4.57±2.24 | 4.84±2.64 | 0.554 |
| Tumor size (>5/≤5 cm, n) | 20/39 | 27/38 | 0.381 |
| Differentiation (well/moderate/poor, n) | 1/38/20 | 7/40/18 | 0.112 |
| Presence of satellite nodules (yes/no, n) | 4/55 | 8/57 | 0.298 |
| Liver capsule invasion (yes/no, n) | 21/38 | 30/35 | 0.233 |
| Microvascular invasion (yes/no, n) | 29/30 | 21/44 | 0.056 |
Continuous variables consistent with normal distribution were expressed as mean ± SD and compared by Student’s t-test. Continuous variables consistent with non-normal distribution were expressed as median ± IQR and compared by rank-sum test. Categorical variables were compared by chi-square test or Fisher’s exact test, as appropriate. IOERT, intraoperative electron radiotherapy; SD, standard deviation; BMI, body mass index; BCLC, Barcelona Clinic Liver Cancer; IQR, interquartile range.
Surgical variables and postoperative outcomes
No significant difference was observed between the two groups in operative methods, ischemia time, intraoperative bleeding, and transfusion. However, a significant difference was observed in null-margin resection (P=0.001), operative time (P<0.001) and type of blood occlusion (P=0.003) between the two groups (Table 2).
Table 2. Operative variables and postoperative outcomes.
| Variables | IOERT group (n=59) | Control group (n=65) | P value |
|---|---|---|---|
| Operative method (anatomical/non-anatomical, n) | 18/41 | 21/44 | 0.829 |
| Null surgical margin (yes/no, n) | 47/12 | 34/31 | 0.001 |
| Operative time (min, mean ± SD) | 297.71±78.85 | 237.37±88.31 | <0.001 |
| Type of blood occlusion (nil/pringle/SDRVO, n) | 11/3/45 | 16/16/33 | 0.003 |
| Warm ischemia time {min, median [IQR]} | 13 [10–15] | 12 [2.5–15] | 0.143 |
| Intraoperative bleeding {mL, median [IQR]} | 300 [200–600] | 300 [100–500] | 0.261 |
| Intraoperative blood transfusion (yes/no, n) | 13/46 | 12/53 | 0.620 |
| Postoperative blood transfusion (yes/no, n) | 8/51 | 12/53 | 0.459 |
| Postoperative complication | |||
| Bile leakage (yes/no, n) | 3/56 | 3/62 | 1.000 |
| Bleeding (yes/no, n) | 5/54 | 4/61 | 0.735 |
| Impaired wound healing (yes/no, n) | 3/56 | 3/62 | 1.000 |
| Delayed gastric emptying (yes/no, n) | 5/54 | 1/64 | 0.101 |
| Intestinal obstruction (yes/no, n) | 0/59 | 2/63 | 0.497 |
| Infection (yes/no, n) | 4/55 | 3/62 | 0.708 |
| Renal dysfunction (yes/no, n) | 2/57 | 0/65 | 0.224 |
| Transient liver impairment (yes/no, n) | 0/59 | 1/64 | 1.000 |
| Postoperative hospitalization (days, mean ± SD) | 8.75±3.96 | 9.15±3.64 | 0.551 |
| KPS score when discharged (mean ± SD) | 65.34±12.03 | 68.77±13.44 | 0.138 |
| 30-day operative mortality (yes/no, n) | 0/59 | 0/65 | – |
| RILD (yes/no, n) | 0/59 | 0/65 | – |
Continuous variables consistent with normal distribution were expressed as mean ± SD and compared by Student’s t-test. Continuous variables consistent with non-normal distribution were expressed as median ± IQR and compared by rank-sum test. Categorical variables were compared by chi-square test or Fisher’s exact test, as appropriate. Child-Pugh C status on postoperative day 7. IOERT, intraoperative electron radiotherapy; SD, standard deviation; SDRVO, selective and dynamic region-specific vascular occlusion; IQR, interquartile range; KPS, Karnofsky performance status; RILD, radiation-induced liver disease.
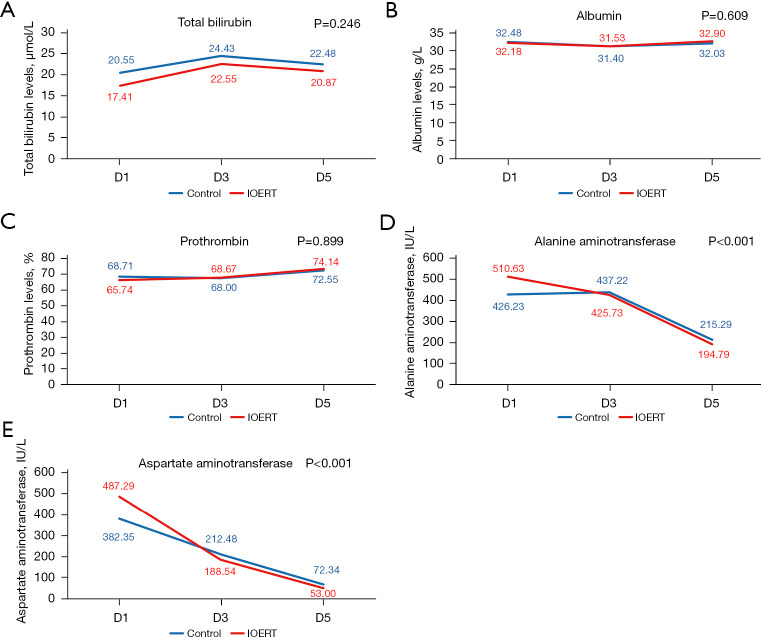
Acute toxicity of IOERT was evaluated in terms of postoperative hospitalization, liver function, and postoperative complications. No significant difference was observed between the IOERT group and the control group in any of these parameters (Table 2). The two groups showed no significant difference in terms of complications like biliary leakage, bleeding, impaired wound healing, delayed gastric emptying, intestinal obstruction, infection, renal dysfunction, and transient liver impairment (Child-Pugh C status on postoperative day 7). Total bilirubin levels were found to be maximum on postoperative day 3 and subsequently decreased on day 5, while albumin and plasma thromboplastin antecedent (PTa) levels remained stable on post-operative day 3, followed by an increase on day 5. The trend observed for ALB, TBIL and PTa levels were statistically similar in both groups (Figure 2A-2C). Further, aspartate aminotransferase/alanine aminotransferase (AST/ALT) levels peaked on day 1, and were significantly higher (P<0.001) in the IOERT group as compared to the control group. This was followed by a gradual decline with a similar trend on days 3 and 5 (Figure 2D,2E).
Figure 2.
Liver function tests post hepatectomy in IOERT vs. control groups on days 1, 3 and 5. (A) Total bilirubin levels; (B) albumin levels; (C) prothrombin levels; (D) alanine aminotransferase; (E) aspartate aminotransferase. IOERT, intraoperative electron radiotherapy; d1, day 1; d2, day 2; d3, day 3.
Factors effecting RFS and OS
From the univariate analysis, RFS was found to be associated with MVI (P=0.024), tumor size (P=0.016), satellite nodules (P=0.039), envelope invasion (P=0.023) and IOERT (P=0.034). Factors influencing OS were preoperative AFP level (P=0.022), tumor size (P=0.002), and MVI (P<0.001). According to the multivariate Cox proportional hazard regression analysis, IOERT (HR =0.528, 95% CI: 0.319–0.874, P=0.013), MVI (HR =2.063, 95% CI: 1.261–3.375, P=0.004) and envelope invasion (HR =1.712, 95% CI: 1.056–2.778, P=0.029) were found to be independent prognostic factors associated with RFS. MVI (HR =6.718, 95% CI: 2.499–18.057, P<0.001) was the only independent prognostic factors associated with OS (Table 3).
Table 3. Univariate and multivariate Cox analysis of RFS and OS.
| Variable | Cox | ||||
|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | ||||
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| RFS | |||||
| Age | 1.005 (0.982–1.029) | 0.654 | |||
| Gender | 1.251 (0.540–2.896) | 0.601 | |||
| Operative method | 1.031 (0.619–1.718) | 0.906 | |||
| Null surgical margin | 0.765 (0.467–1.252) | 0.286 | |||
| Intraoperative bleeding | 1.000 (0.999–1.000) | 0.264 | |||
| IOERT | 0.588 (0.360–0.961) | 0.034 | 0.528 (0.319–0.874) | 0.013 | |
| Preoperative AFP level | 1.115 (0.813–1.529) | 0.500 | |||
| Tumor size | 1.127 (1.022–1.242) | 0.016 | |||
| Number of tumors | 1.715 (0.622–4.732) | 0.298 | |||
| Differentiation | 0.720 (0.468–1.108) | 0.135 | |||
| MVI | 1.738 (1.075–2.809) | 0.024 | 2.063 (1.261–3.375) | 0.004 | |
| Satellite nodules | 2.098 (1.038–4.244) | 0.039 | |||
| Envelope invasion | 1.744 (1.079–2.819) | 0.023 | 1.712 (1.056–2.778) | 0.029 | |
| OS | |||||
| Age | 1.005 (0.965–1.046) | 0.813 | |||
| Gender | 0.616 (0.211–1.801) | 0.376 | |||
| Operative method | 1.057 (0.455–2.452) | 0.898 | |||
| Null surgical margin | 0.636 (0.289–1.403) | 0.263 | |||
| Intraoperative bleeding | 1.000 (0.999–1.001) | 0.970 | |||
| IOERT | 0.662 (0.296–1.485) | 0.317 | |||
| Preoperative AFP level | 1.847 (1.094–3.117) | 0.022 | |||
| Tumor size | 1.242 (1.082–1.425) | 0.002 | |||
| Number of tumors | 1.910 (0.448–8.131) | 0.382 | |||
| Differentiation | 0.696 (0.338–1.431) | 0.324 | |||
| MVI | 6.718 (2.499–18.057) | <0.001 | 6.718 (2.499–18.057) | <0.001 | |
| Satellite nodules | 2.490 (0.930–6.671) | 0.069 | |||
| Envelope invasion | 1.349 (0.615–2.960) | 0.455 | |||
RFS, recurrence-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; IOERT, intraoperative electron radiotherapy; AFP, alpha-fetoprotein; MVI, microvascular invasion.
Survival analysis
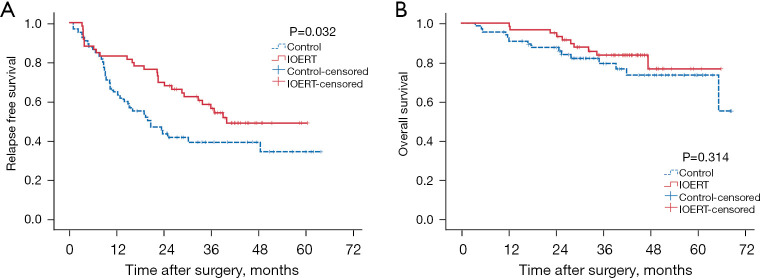
Recurrence was observed in 28 patients in the IOERT group and 39 patients in the control group. The cumulative 1-, 2-, 3-year RFS rates of all 124 patients were 73.4%, 56.0% and 46.9%, respectively while the cumulative 1-, 2-, 3-year OS rates were 92.3%, 87.5% and 79.4%, respectively. Overall, 10 and 15 patients of IOERT group and control group, respectively died. The median RFS duration for the IOERT group was 39.90 as compared to 20.59 months in the control group. The 1-, 2-, 3-year RFS rates were 79.3%, 62.1% and 45.8% for patients in the IOERT group, and 47.6%, 28.6%, and 22.9% for patients in the control group, respectively. The IOERT group showed a significantly longer RFS rate (P=0.032) compared to the control group, (Figure 3A). The median duration of OS was not reached in either group. The 1-, 2-, and 3-year OS rates were 100.0%, 94.9%, and 83.7% for patients in the IOERT group, and 92.3%, 87.5%, and 79.4% for patients in the control group, respectively. The IOERT and the control groups exhibited statistically similar OS (P=0.314; Figure 3B).
Figure 3.
Survival curves in IOERT and control groups. (A) Relapse free survival curve; (B) overall survival curve. IOERT, intraoperative electron radiotherapy.
Recurrence pattern
Recurrence was observed in 67 (IOERT group: 28 and control group: 39) out of 124 patients. The incidence of intrahepatic and extrahepatic recurrence was 22 and 6 in IOERT group, 30 and 9 in control group, respectively, with no significant difference between the two groups (P=0.873). For patients with intrahepatic recurrence, the incidence of marginal and non-marginal recurrences was 4 and 18 in IOERT group, 7 and 23 in control group, respectively, and no significant difference was observed between the two groups (P=0.741). The incidence of nodular and diffuse recurrence was 13 and 9 in IOERT group, 21 and 9 in control group, respectively, with no significant difference between the two groups (P=0.414). For patients with extrahepatic recurrence, the incidence of limited and disseminated recurrence was 3 and 3 in IOERT group, 2 and 7 in control group, respectively (P=0.329; Table 4).
Table 4. Pattern of recurrence in the IOERT and control groups.
| Recurrence pattern | IOERT group | Control group | P value |
|---|---|---|---|
| Location (for all) | 28 | 39 | 0.873 |
| Intrahepatic | 22 | 30 | |
| Extrahepatic | 6 | 9 | |
| Location (intrahepatic) | 22 | 30 | 0.741 |
| Margin | 4 | 7 | |
| Non-margin | 18 | 23 | |
| Growth pattern (intrahepatic) | 22 | 30 | 0.414 |
| Nodular | 13 | 21 | |
| Diffuse | 9 | 9 | |
| Growth pattern (extrahepatic) | 6 | 9 | 0.329 |
| Limited | 3 | 2 | |
| Disseminated | 3 | 7 |
IOERT, intraoperative electron radiotherapy.
Survival analysis of subgroup patients
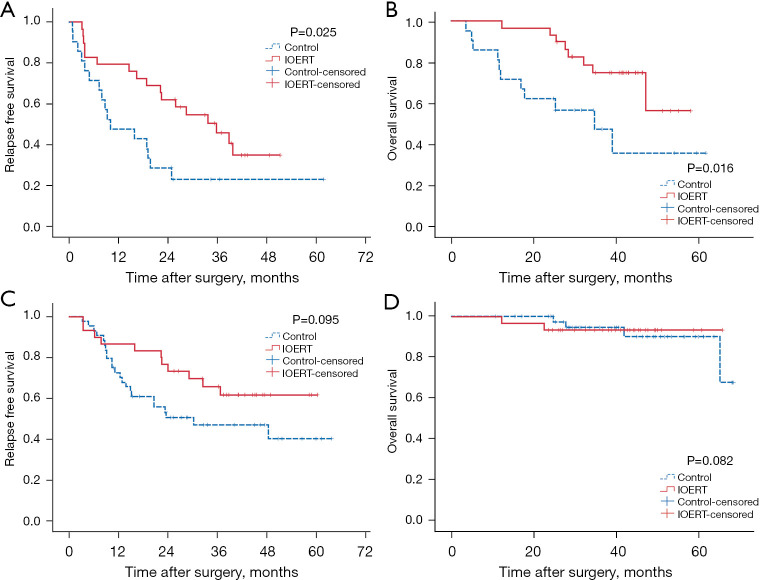
Since the independent prognostic factor associated with both RFS and OS was MVI, patients were sub-grouped into MVI (+; n=50) or MVI (−; n=70). In the MVI (+) subgroup, median RFS time was 22.33 months with 1-, 2-, and 3-year cumulative RFS rates of 66.0%, 48.0%, and 35.6%, respectively. Among them, the 1-, 2-, and 3-year RFS rates were 79.3%, 62.1% and 45.8%, respectively, in the IOERT group, and 47.6%, 28.6% and 22.9% in the control group, respectively with statistically significant difference (P=0.025; Figure 4A). The median OS time in MVI (+) subgroup was not reached; the cumulative 1-, 2-, and 3-year OS rates were 90.0%, 82.0%, and 63.5%, respectively. Of which, the 1-, 2-, and 3-year OS rates in the IOERT group were 100.0%, 96.6%, and 74.6%, respectively, and 76.2%, 61.9%, and 46.9%, respectively, in the control group. This difference was found to be statistically significant (P=0.016; Figure 4B).
Figure 4.
Survival curves in IOERT and control groups stratified by MVI (+/−). (A) Relapse free survival in MVI (+) subgroup; (B) overall survival in MVI (+) subgroup; (C) relapse free survival in MVI (−) subgroup; (D) overall survival in MVI (−) subgroup. IOERT, intraoperative electron radiotherapy; MVI, microvascular invasion.
In the MVI (−) subgroup, the median RFS time was 48.46 months. The cumulative 1-, 2-, and 3-year RFS rates for the MVI (−) subgroup were 78.4%, 61.5%, and 54.7%, respectively, while the 1-, 2-, and 3-year RFS rates were 86.7%, 76.7% and 65.8%, respectively, in the IOERT group, and 72.7%, 50.7% and 47.0% in the control group, respectively. This difference was not found to be statistically significant (P=0.095) (Figure 4C). Median OS time for MVI (−) subgroup was not reached; the cumulative 1-, 2-, and 3-year OS rates were 100.0%, 97.2%, and 94.1%, respectively. Among them, the 1-, 2-, and 3-year OS rates in the IOERT group were 100.0%, 93.3%, and 93.3%, and in the control group were 100.0%, 100.0%, and 94.6%, respectively. This difference was not found to be statistically significant (P=0.082; Figure 4D).
Discussion
Postoperative recurrence is the most important risk factor associated with the prognosis of patients with HCC (5,35). According to previous reports, narrow surgical margins result in poorer prognosis of centrally located HCC after resection as compared to peripheral HCC (10,12) which is related to narrow surgical margin. Adjuvant methods such as RT, IMRT, and PBT have shown to improve survival outcomes in patients with HCC undergoing narrow margin hepatectomy (9,24,32).
However, as compared to these methods, IOERT treatment has numerous advantages such as direct visualization of the target volume which eliminates the risk of missing out residual lesions, and protection of surrounding healthy tissues from damage by application of lead sheets to shield non-tumor liver tissues. From an oncological perspective, a single large dose of irradiation immediately after hepatectomy avoids the progression of microscopic residual lesions, enhances biological effect by exhibiting beneficial effects on the tumor microenvironment with more convenience and cost-effectiveness (36-38).
In the present study, the IOERT group had an increased operation time (65–75 min) as compared to the control group (P<0.001), due to the IOERT process. This operation time could be shortened by optimization of the IOERT process and formulating operating specifications in order to minimize the planning time. Acute toxicity of IOERT was mainly manifested in the side-effects caused by irradiation delivered to the tissues exposed directly. In this regard, König et al. reported delayed wound healing, wound infection and breast edema as the main complications of IOERT in breast cancer (29). On the contrary, in the current study the postoperative complications in the IOERT group were not statistically different to those in the control group. IOERT treated patients with such complications gradually recovered with conservative and non-secondary surgical treatments. Furthermore, RILD was not observed in patients in the intervention group, demonstrating the safety of IOERT. These findings are consistent with studies where no significant complications were found in patients with locally advanced rectal cancer and cervical metastasis who were treated with and without IOERT (28,39,40).
The incidence of RFS and OS in our study was found to be associated with various factors including tumor size, satellite nodules, envelope invasion, and AFP levels. The association of these factors with poor prognosis of HCC is well reported in literature (41-43). In this study, the RFS rate was found to improve significantly in the IOERT group as compared to the control group. This is in accordance with reports demonstrating the use of IOERT to improve RFS rates in patients with head and neck cancer and colorectal cancer (44,45). In the present study, no statistically significant difference was observed in the cumulative 3-year OS rates in the IOERT group verses control group. In agreement with these findings, a randomized phase-III study also reported no significant difference (P=0.258) with regards to OS rates between the IOERT group versus control group in patients suffering from locally advanced rectal cancer (46).
Subgroup analysis of MVI (+) patients revealed that both RFS and OS are significantly prolonged in the IOERT subgroup as compared to the control, whereas there was no significant difference of RFS and OS between the two groups in MVI (−) patients. These findings are consistent with previous studies reported indicating that the survival benefit of IOERT may be derived from patients with MVI (47,48). Multiple studies have confirmed that MVI is associated with significantly poor prognosis in HCC patients after curative hepatectomy (49-52). MVI (+) patients exhibit cluster of tumor cells distanced at –1 cm from the tumor capsule, which usually requires to be cut at a margin of >1–2 cm to avoid microscopic residual lesions (53). We further speculated that the presence of residual microscopic lesions or minimal residual disease (MRD) might be one of the main causes of recurrence in MVI (+) patients (54). Such microscopic lesions are severely damaged by a single large dose of IOERT irradiation, thus significantly prolonging the RFS and OS time in MVI (+). Likewise, the absence of residual microscopic lesions in MVI (−) patients possibly explain why no significant improvements were observed in the RFS and OS time in MVI (−) patients. In this aspect, Oertel et al. reported the ability of IOERT to compensate for microscopic tumor residue (55).
However, the presence of MVI can only be confirmed by postoperative pathology examinations, and cannot be diagnosed pre/intraoperatively by pathological methods. Currently, many preoperative parameters have illustrated the relationship between MVI and HCC (56,57), and few studies have reported multi-factor prediction models for MVI (58,59). Such prediction models can further assist in patient selection for IOERT treatment.
Although this was a prospective study, a few limitations are worth noting. First, this was a single-center non-randomized study, which may render potential sampling bias. Also, due to the low number of deaths in both groups, the clinical interpretation of statistical results should be accepted with caution. Nevertheless, this study provides original data of IOERT treatment of HCC, offering rationale to develop more in-depth studies with multi-center, randomized, controlled clinical trials with larger patient cohorts and follow-up timelines.
Conclusions
Treatment with IOERT at a dose of 15–17 Gy in centrally located HCC concurrent with narrow-margin hepatectomy was found to be technically feasible and safe. The RFS rates of patients in the IOERT group were significantly improved compared to those in the control group. Furthermore, subgroup analysis revealed that IOERT was more beneficial for postoperative survival of HCC patients with MVI.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by Beijing Municipal Science & Technology Commission (No. Z131107002213166), the Beijing Hope Run Special Fund of Cancer Foundation of China (No. LC2018A15) and the PUMC Fund of the Funds for the Central Universities (No. 3332018193).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (No. NCC2013S-005) and was conducted in compliance with principles of Good Clinical Practice and informed consent was taken from all individual participants (ChiCTR-TRC-12002802; www.who.int/ictrp).
Footnotes
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-223/rc
Data Sharing Statement: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-223/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-223/coif). The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2.Wang W, Guo Y, Zhong J, et al. The clinical significance of microvascular invasion in the surgical planning and postoperative sequential treatment in hepatocellular carcinoma. Sci Rep 2021;11:2415. 10.1038/s41598-021-82058-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lodato F, Mazzella G, Festi D, et al. Hepatocellular carcinoma prevention: a worldwide emergence between the opulence of developed countries and the economic constraints of developing nations. World J Gastroenterol 2006;12:7239-49. 10.3748/wjg.v12.i45.7239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen W, Sun K, Zheng R, et al. Cancer incidence and mortality in China, 2014. Chin J Cancer Res 2018;30:1-12. 10.21147/j.issn.1000-9604.2018.01.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishizawa T, Hasegawa K, Aoki T, et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology 2008;134:1908-16. 10.1053/j.gastro.2008.02.091 [DOI] [PubMed] [Google Scholar]
- 6.Saeki I, Yamamoto N, Yamasaki T, et al. Effects of an oral iron chelator, deferasirox, on advanced hepatocellular carcinoma. WJG 2016;22:8967. 10.3748/wjg.v22.i40.8967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ba Q, Hao M, Huang H, et al. Iron Deprivation Suppresses Hepatocellular Carcinoma Growth in Experimental Studies. Clin Cancer Res 2011;17:7625-33. 10.1158/1078-0432.CCR-10-3099 [DOI] [PubMed] [Google Scholar]
- 8.Min L. The Safety and Efficacy of Deferoxamine Combined With Conventional Transarterial Chemoembolization in Patients With Unresectable Hepatocellular Carcinoma. clinicaltrials.gov; 2019 Feb [cited 2021 Aug 25]. Report No.: NCT03652467. Available online: https://clinicaltrials.gov/ct2/show/NCT03652467
- 9.Yu W, Wang W, Rong W, et al. Adjuvant radiotherapy in centrally located hepatocellular carcinomas after hepatectomy with narrow margin (<1 cm): a prospective randomized study. J Am Coll Surg 2014;218:381-92. 10.1016/j.jamcollsurg.2013.11.030 [DOI] [PubMed] [Google Scholar]
- 10.Yu W, Rong W, Wang L, et al. R1 hepatectomy with exposure of tumor surface for centrally located hepatocellular carcinoma. World J Surg 2014;38:1777-85. 10.1007/s00268-013-2429-3 [DOI] [PubMed] [Google Scholar]
- 11.Shi M, Guo RP, Lin XJ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg 2007;245:36-43. 10.1097/01.sla.0000231758.07868.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008;47:97-104. 10.1002/hep.21966 [DOI] [PubMed] [Google Scholar]
- 13.Lim KC, Chow PK, Allen JC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg 2011;254:108-13. 10.1097/SLA.0b013e31821ad884 [DOI] [PubMed] [Google Scholar]
- 14.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol 2008;48:S20-37. 10.1016/j.jhep.2008.01.022 [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Wang W, Yao X, et al. Postoperative adjuvant radiotherapy is associated with improved survival in hepatocellular carcinoma with microvascular invasion. Oncotarget 2017;8:79971-81. 10.18632/oncotarget.20402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben-Josef E, Normolle D, Ensminger WD, et al. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol 2005;23:8739-47. 10.1200/JCO.2005.01.5354 [DOI] [PubMed] [Google Scholar]
- 17.Mornex F, Girard N, Beziat C, et al. Feasibility and efficacy of high-dose three-dimensional-conformal radiotherapy in cirrhotic patients with small-size hepatocellular carcinoma non-eligible for curative therapies--mature results of the French Phase II RTF-1 trial. Int J Radiat Oncol Biol Phys 2006;66:1152-8. 10.1016/j.ijrobp.2006.06.015 [DOI] [PubMed] [Google Scholar]
- 18.Yu JI, Park HC. Radiotherapy as valid modality for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol 2016;22:6851-63. 10.3748/wjg.v22.i30.6851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He J, Zeng ZC, Tang ZY, et al. Clinical features and prognostic factors in patients with bone metastases from hepatocellular carcinoma receiving external beam radiotherapy. Cancer 2009;115:2710-20. 10.1002/cncr.24300 [DOI] [PubMed] [Google Scholar]
- 20.Kim K, Chie EK, Kim W, et al. Absence of symptom and intact liver function are positive prognosticators for patients undergoing radiotherapy for lymph node metastasis from hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2010;78:729-34. 10.1016/j.ijrobp.2009.08.047 [DOI] [PubMed] [Google Scholar]
- 21.Feng M, Ben-Josef E. Radiation therapy for hepatocellular carcinoma. Semin Radiat Oncol 2011;21:271-7. 10.1016/j.semradonc.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 22.Hawkins MA, Dawson LA. Radiation therapy for hepatocellular carcinoma: from palliation to cure. Cancer 2006;106:1653-63. 10.1002/cncr.21811 [DOI] [PubMed] [Google Scholar]
- 23.Klein J, Dawson LA. Hepatocellular carcinoma radiation therapy: review of evidence and future opportunities. Int J Radiat Oncol Biol Phys 2013;87:22-32. 10.1016/j.ijrobp.2012.08.043 [DOI] [PubMed] [Google Scholar]
- 24.Yeung RH, Chapman TR, Bowen SR, et al. Proton beam therapy for hepatocellular carcinoma. Expert Rev Anticancer Ther 2017;17:911-24. 10.1080/14737140.2017.1368392 [DOI] [PubMed] [Google Scholar]
- 25.Cheung K. Intensity modulated radiotherapy: advantages, limitations and future developments. Biomed Imaging Interv J 2006;2:e19. 10.2349/biij.2.1.e19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tipton KN, Sullivan N, Bruening W, et al. Stereotactic Body Radiation Therapy [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2011 May. Report No.: 10(11)-EHC058-EF.
- 27.Paunesku T, Woloschak GE. Future Directions of Intraoperative Radiation Therapy: A Brief Review. Front Oncol 2017;7:300. 10.3389/fonc.2017.00300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alberda WJ, Verhoef C, Nuyttens JJ, et al. Intraoperative radiation therapy reduces local recurrence rates in patients with microscopically involved circumferential resection margins after resection of locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2014;88:1032-40. 10.1016/j.ijrobp.2014.01.014 [DOI] [PubMed] [Google Scholar]
- 29.König L, Lang K, Heil J, et al. Acute Toxicity and Early Oncological Outcomes After Intraoperative Electron Radiotherapy (IOERT) as Boost Followed by Whole Breast Irradiation in 157 Early Stage Breast Cancer Patients-First Clinical Results From a Single Center. Front Oncol 2019;9:384. 10.3389/fonc.2019.00384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paly JJ, Hallemeier CL, Biggs PJ, et al. Outcomes in a multi-institutional cohort of patients treated with intraoperative radiation therapy for advanced or recurrent renal cell carcinoma. Int J Radiat Oncol Biol Phys 2014;88:618-23. 10.1016/j.ijrobp.2013.11.207 [DOI] [PubMed] [Google Scholar]
- 31.Roeder F, Alldinger I, Uhl M, et al. Intraoperative Electron Radiation Therapy in Retroperitoneal Sarcoma. Int J Radiat Oncol Biol Phys 2018;100:516-27. 10.1016/j.ijrobp.2017.10.034 [DOI] [PubMed] [Google Scholar]
- 32.Wang WH, Wang Z, Wu JX, et al. Survival benefit with IMRT following narrow-margin hepatectomy in patients with hepatocellular carcinoma close to major vessels. Liver Int 2015;35:2603-10. 10.1111/liv.12857 [DOI] [PubMed] [Google Scholar]
- 33.Koay EJ, Owen D, Das P. Radiation-Induced Liver Disease and Modern Radiotherapy. Semin Radiat Oncol 2018;28:321-31. 10.1016/j.semradonc.2018.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hori M, Murakami T, Kim T, et al. CT Scan and MRI in the Differentiation of Liver Tumors. Dig Dis 2004;22:39-55. 10.1159/000078734 [DOI] [PubMed] [Google Scholar]
- 35.Villanueva A. Hepatocellular Carcinoma. N Engl J Med 2019;380:1450-62. 10.1056/NEJMra1713263 [DOI] [PubMed] [Google Scholar]
- 36.Harris EER, Small W, Jr. Intraoperative Radiotherapy for Breast Cancer. Front Oncol 2017;7:317. 10.3389/fonc.2017.00317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaidya JS, Wenz F, Bulsara M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 2014;383:603-13. 10.1016/S0140-6736(13)61950-9 [DOI] [PubMed] [Google Scholar]
- 38.Vaidya JS, Baldassarre G, Massarut S. Beneficial effects of intraoperative radiotherapy on tumor microenvironment could improve outcomes (Int J Radiat Oncol Biol Phys 2008;72:1575-1581). Int J Radiat Oncol Biol Phys 2009;74:976; author reply 976-7. 10.1016/j.ijrobp.2009.02.041 [DOI] [PubMed] [Google Scholar]
- 39.Potemin S, Kübler J, Uvarov I, et al. Intraoperative radiotherapy as an immediate adjuvant treatment of rectal cancer due to limited access to external-beam radiotherapy. Radiat Oncol 2020;15:11. 10.1186/s13014-020-1458-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeidan YH, Yeh A, Weed D, et al. Intraoperative radiation therapy for advanced cervical metastasis: a single institution experience. Radiat Oncol 2011;6:72. 10.1186/1748-717X-6-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hennenfent KL, Girvan AC, Chaudhry A, et al. Overall survival (OS) in patients (pts) with advanced hepatocellular carcinoma (HCC) and elevated alpha-fetoprotein (AFP): A real-world retrospective study. J Clin Oncol 2017;35:e15658. 10.1200/JCO.2017.35.15_suppl.e15658 [DOI] [Google Scholar]
- 42.Cai MY, Wang FW, Li CP, et al. Prognostic factors affecting postoperative survival of patients with solitary small hepatocellular carcinoma. Chin J Cancer 2016;35:80. 10.1186/s40880-016-0143-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnaoutakis DJ, Mavros MN, Shen F, et al. Recurrence patterns and prognostic factors in patients with hepatocellular carcinoma in noncirrhotic liver: a multi-institutional analysis. Ann Surg Oncol 2014;21:147-54. 10.1245/s10434-013-3211-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hilal L, Al Feghali KA, Ramia P, et al. Intraoperative Radiation Therapy: A Promising Treatment Modality in Head and Neck Cancer. Front Oncol 2017;7:148. 10.3389/fonc.2017.00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dresen RC, Gosens MJ, Martijn H, et al. Radical resection after IORT-containing multimodality treatment is the most important determinant for outcome in patients treated for locally recurrent rectal cancer. Ann Surg Oncol 2008;15:1937-47. 10.1245/s10434-008-9896-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dubois JB, Bussieres E, Richaud P, et al. Intra-operative radiotherapy of rectal cancer: results of the French multi-institutional randomized study. Radiother Oncol 2011;98:298-303. 10.1016/j.radonc.2011.01.017 [DOI] [PubMed] [Google Scholar]
- 47.Wang L, Chen B, Li Z, et al. Optimal postoperative adjuvant treatment strategy for HBV-related hepatocellular carcinoma with microvascular invasion: a propensity score analysis. Onco Targets Ther 2019;12:1237-47. 10.2147/OTT.S179247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang L, Wang W, Rong W, et al. Postoperative adjuvant treatment strategy for hepatocellular carcinoma with microvascular invasion: a non-randomized interventional clinical study. BMC Cancer 2020;20:614. 10.1186/s12885-020-07087-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen J, Wen J, Li C, et al. The prognostic value of microvascular invasion in early-intermediate stage hepatocellular carcinoma: a propensity score matching analysis. BMC Cancer 2018;18:278. 10.1186/s12885-018-4196-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roayaie S, Blume IN, Thung SN, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology 2009;137:850-5. 10.1053/j.gastro.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Du M, Chen L, Zhao J, et al. Microvascular invasion (MVI) is a poorer prognostic predictor for small hepatocellular carcinoma. BMC Cancer 2014;14:38. 10.1186/1471-2407-14-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang C, Zhu XD, Ji Y, et al. Microvascular invasion has limited clinical values in hepatocellular carcinoma patients at Barcelona Clinic Liver Cancer (BCLC) stages 0 or B. BMC Cancer 2017;17:58. 10.1186/s12885-017-3050-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cong WM, Bu H, Chen J, et al. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol 2016;22:9279-87. 10.3748/wjg.v22.i42.9279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou KQ, Sun YF, Cheng JW, et al. Effect of surgical margin on recurrence based on preoperative circulating tumor cell status in hepatocellular carcinoma. EBioMedicine 2020;62:103107. 10.1016/j.ebiom.2020.103107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oertel S, Niethammer AG, Krempien R, et al. Combination of external-beam radiotherapy with intraoperative electron-beam therapy is effective in incompletely resected pediatric malignancies. Int J Radiat Oncol Biol Phys 2006;64:235-41. 10.1016/j.ijrobp.2005.06.038 [DOI] [PubMed] [Google Scholar]
- 56.Sumie S, Nakashima O, Okuda K, et al. The significance of classifying microvascular invasion in patients with hepatocellular carcinoma. Ann Surg Oncol 2014;21:1002-9. 10.1245/s10434-013-3376-9 [DOI] [PubMed] [Google Scholar]
- 57.Goh BK, Chow PK, Teo JY, et al. Number of nodules, Child-Pugh status, margin positivity, and microvascular invasion, but not tumor size, are prognostic factors of survival after liver resection for multifocal hepatocellular carcinoma. J Gastrointest Surg 2014;18:1477-85. 10.1007/s11605-014-2542-0 [DOI] [PubMed] [Google Scholar]
- 58.Lin S, Ye F, Rong W, et al. Nomogram to Assist in Surgical Plan for Hepatocellular Carcinoma: a Prediction Model for Microvascular Invasion. J Gastrointest Surg 2019;23:2372-82. 10.1007/s11605-019-04140-0 [DOI] [PubMed] [Google Scholar]
- 59.Liu M, Wang L, Zhu H, et al. A Preoperative Measurement of Serum MicroRNA-125b May Predict the Presence of Microvascular Invasion in Hepatocellular Carcinomas Patients. Transl Oncol 2016;9:167-72. 10.1016/j.tranon.2016.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as