Abstract
Background
Reactivation of viruses such as Epstein-Barr virus (EBV) and cytomegalovirus (CMV) are common in critically ill patients and have been described in patients with severe COVID-19. However, it is unclear whether these reactivations are associated with increased mortality and whether targeted treatments are beneficial.
Methods
In a retrospective single-center cohort study, patients with severe COVID-19 treated on our intensive care unit (ICU) were screened for EBV and CMV reactivation as detected by polymerase chain reaction. If present, patient characteristics, temporal connections to severe acute respiratory syndrome coronavirus 2 diagnosis and corticosteroid use, the use of targeted treatments as well as the course of disease and outcome were analyzed. As control group, non-COVID-19 patients with sepsis, treated within the same time period on our ICU, served as control group to compare incidences of viral reactivation.
Results
In 19 (16%) of 117 patients with severe COVID-19 treated on our ICU EBV reactivations were identified, comparable 18 (14%) of 126 in the non-COVID-19 control group (P = .672). Similarly, in 11 (9%) of 117 patients CMV reactivations were identified, comparable to the 16 (13%) of 126 in the non-COVID-19 sepsis patients (P = .296). The majority of EBV (58%) and CMV reactivations (55%) were detected in patients under systemic corticosteroid treatment. 7 (37%) of 19 patients with EBV reactivation survived the ICU stay, 2 (29%) of 7 patients with rituximab treatment and 5 (42%) of 12 patients without treatment (P = .568). Five (50%) of 10 patients with CMV reactivation survived the ICU stay, 5 (83%) of 6 patients with ganciclovir treatment and 0 of 4 patients without treatment (P = .048). Follow-up analysis in these patients showed that the initiation of treatment lead to decrease in viral load.
Conclusion
Critically ill patients with COVID-19 are at a high risk for EBV and CMV reactivations. Whether these reactivations are a cause of hyperinflammation and require targeted treatment remains uncertain. However, in patients with clinical deterioration or signs of hyperinflammation targeted treatment might be beneficial and warrants further studying.
Keywords: intensive care unit, COVID-19, EBV, CMV, SARS-CoV-2, ARDS, hyperinflammation
Background
Reactivation of Herpes viruses (HV) as Epstein-Barr virus (EBV) and cytomegalovirus (CMV) are common in critically ill patients treated on the intensive care unit (ICU) and have been associated with higher morbidity and mortality.1-4 Most patients who develop HV reactivation have an underlying hematologic disorder or are treated with immunosuppressive agents. However, up to 77% of otherwise immunocompetent but critically ill patients develop HV reactivation. The use of corticosteroids during the ICU stay has been described as an independent risk factor for an HV reactivation in immunocompetent patients.2,5
Reactivations of HV have also been observed in individuals with severe coronavirus disease 2019 (COVID-19).6–9 Hyperinflammatory states resembling secondary hemophagocytic lymphohistiocytosis were described in severe COVID-19, and the role of viral reactivation as a potential trigger has been discussed.10 EBV viremia appears to be correlated with COVID-19 severity, a longer ICU stay, increased interleukin-6 levels as well as reduced CD8+ T and NK cell counts.8,10 However, there is an ongoing discussion whether HV reactivations are consequence of the patient's critical condition or independent triggers for hyperinflammation—and if so, whether targeted treatment is required.11
In addition to the controversial results regarding the impact of HV reactivations on the morbidity and mortality of ICU patients, there is also only very limited data available regarding the treatment of HV reactivations in immunocompetent patients on the ICU. Recently, a double-blinded, randomized trial did not show any benefit for a preemptive treatment with ganciclovir in mechanically ventilated patients with CMV reactivations.12
Due to these controversies regarding clinical meaning, therapeutic approach and a possible COVID-19-associated hyperinflammation triggering HV reactivations, the aim of the present study is to descriptively analyze the interrelationships between EBV and CMV reactivation, corticosteroid treatment, and targeted treatments with respect to the clinical course of disease and outcome in patients with severe COVID-19. Therefore, we report a retrospective single-center cohort study of clinical and laboratory data from individuals treated on the medical ICU of an academic tertiary care center in Germany.
Methods
Prior to the start of the analysis, approval was obtained by the local ethics committee of the Faculty of Medicine of the University of Cologne (approval number: 20–11729). Given the noninterventional retrospective nature of the study, no informed consent had to be obtained from the included patients.
We retrospectively reviewed charts of all patients with severe COVID-19 treated in our ICU between March 2020 and March 2021. COVID-19 was confirmed by positive real-time polymerase chain reaction (RT-PCR) for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)*** in respiratory tract samples in all patients.
Quantification of EBV and CMV in whole blood was performed using RT-PCR. EBV and CMV reactivation were defined as the detection of DNA levels higher than 1000 IU/mL in real-time quantitative PCR from peripheral blood according to previous publications.2,13
All patients with COVID-19 and EBV or CMV reactivation were included in this analysis. Patient's characteristics, course of disease and outcome, as well as laboratory parameters at the time of maximum DNA levels for EBV or CMV over the course of the ICU stay were extracted and summarized (Supplemental Table 1).
Table 1.
Patient Characteristics, Temporal Connection to SARS-CoV-2 Diagnosis and Corticosteroid Treatment as Well as Clinical Outcome in ICU COVID-19 Patients With Epstein-Barr Virus (EBV) and Cytomegalovirus (CMV) Reactivations With and Without Targeted Treatment (Rituximab or Ganciclovir, Respectively).
| EBV reactivation | CMV reactivation | |||
| Percentage of reactivation from entire patient population | 19/117 (16%) | 11/117 (9%) | ||
| Age (median, range) | 60 (16-80) | 51 (16-80) | ||
| Sex (% male) | 17/19 (89%) | 9/11 (82%) | ||
| SARS-CoV2 positive at time of reactivation | 12/19 (63%) | 4/10 (40%) | ||
| Days from first positive SARS-CoV2 PCR to reactivation (median, range) | 17 (0-43) | 33 (3-82) | ||
| Reactivation while receiving steroid treatment | 11/19 (58%) | 6/11 (55%) | ||
| Reactivation after receiving steroid treatment | 6/19 (32%) | 3/11 (27%) | ||
| Patients with immunosuppression | 8/19 (42%) | 3/11 (27%) | ||
| Maximum viral copies in IU/mL (median, range) | 7160 (1030-4 090 000) | 4440 (1030-36 900) | ||
| ICU survival | 7/19 (37%) | 6/11 (55%) | ||
Abbreviations: ICU, intensive care unit; PCR, polymerase chain reaction.
For better comparability, patients with sepsis, who were tested negative for SARS-CoV-2 and treated within the same time period in the reporting ICU, served as control group and their patient charts were reviewed for EBV or CMV reactivations. We further subdivided the patients into immunosuppressed patients (defined as hematological disease, EBV-induced malignancy, having received immunosuppressive therapies in the last 3 months or having immunodeficiency syndromes) and patients who received corticosteroids during their ICU stay. Death and ICU discharge were used as events for the time to event analysis, respectively.
Statistical analyses were performed using Microsoft Excel (Version 16.48, Microsoft) and SPSS (Version 27.0.1.0, IBM). Numbers and proportions were noted for categorical variables; medians and ranges were calculated for continuous variables. The Shapiro-Wilk test was used to test if the data are normally distributed. Fisher exact test and Pearson χ2 test as well as Spearman correlation coefficients were used for analysis of categorical and continuous variables, respectively. Statistical significance was set to 2-sided P < .05.
Results
Incidence of HV reactivation
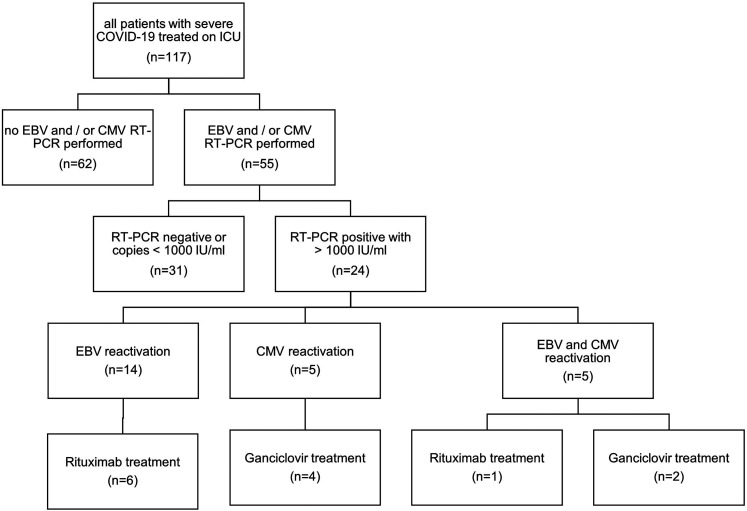
Testing for EBV and CMV reactivations was performed in 55 of all 117 COVID-19 patients, who were treated during the analyzed time period on the reporting ICU. Testing was performed in patients without clinical improvement, having persisting fever >2 days and/or persisting laboratory signs of hyperinflammation in the absence of alternative explanations such as pathogens in bronchoalveolar lavages, blood, or urine cultures. Twenty-four (21%) of these 117 patients tested positive for either EBV or CMV reactivation (Figure 1).
Figure 1.
Flowchart of Epstein-Barr virus (EBV) and cytomegalovirus (CMV) diagnostics and treatment in patients with severe COVID-19 treated on intensive care unit (ICU).
In 19 (16%) of all 117 patients EBV reactivations were identified. The majority of patients had EBV reactivation while receiving corticosteroid treatment, mostly consisting of dexamethasone (6 mg/d) as part of the current COVID-19 treatment protocol.14
In 11 (9%) of 117 patients CMV reactivations were identified. Five (55%) of these patients had CMV reactivation while receiving corticosteroid treatment. Six of the patients were diagnosed with both EBV and CMV reactivation.
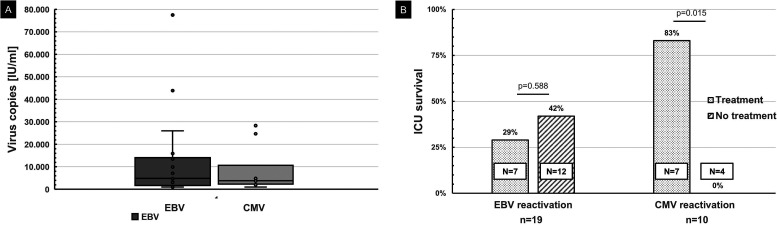
The distribution of the maximum virus loads of EBV and CMV reactivations, respectively, are illustrated in Figure 2A. Patient characteristics, temporal connection to SARS-CoV-2 diagnosis and corticosteroid treatment as well as clinical outcomes are listed in Table 1. Supplemental Table 1 shows the individual patient data of the cohort.
Figure 2.
A, Box-whisker-plot of Epstein-Barr virus (EBV) and cytomegalovirus (CMV) reactivations in patients with severe COVID-19 treated on intensive care unit (ICU). Center lines show the medians; box limits indicate the 25th and 75th percentiles; whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles, outliers are represented by dots. One patient with an EBV reactivation 4.08 million IU/mL was excluded for better illustration. B, ICU survival of patients with EBV or CMV reactivations with and without rituximab or ganciclovir treatment, respectively.
Treatment and Outcome
Treatment with rituximab or ganciclovir was performed in patients with clinical deterioration, defined as need for increase in vasopressor dose or increase in ventilation pressure or fraction of inspired oxygen.
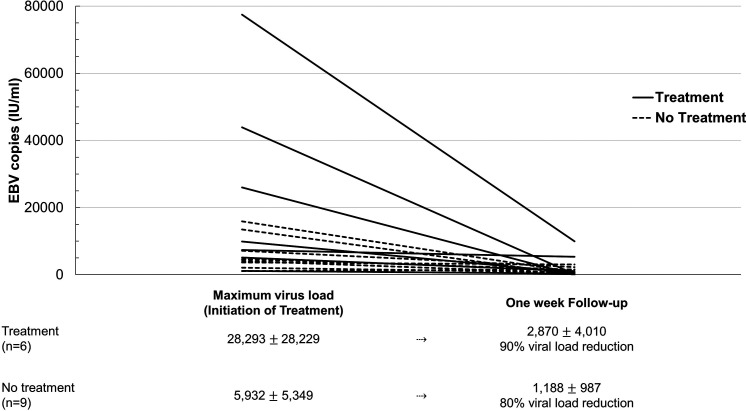
Seven (37%) of all 19 patients with EBV reactivation survived the ICU stay, compared to 50% of COVID-19 patients without virus reactivation (P = .351). Treatment with rituximab was administered in seven of 19 (32%) patients with a median copy number of 26,000 IU/mL. Two (29%) of the 7 patients with EBV reactivation who received rituximab survived the ICU stay, 5 (42%) of the 12 patients with EBV reactivation who did not receive rituximab survived the ICU stay (P = .568) (Table 1, Figure 2B). The percentage of viral load reduction compared to maximum virus copies in patients with and without rituximab treatment is illustrated in Figure 3.
Figure 3.
Epstein-Barr virus (EBV) load in IU/mL at time of maximum virus copies (in the treatment group at time of treatment) and at one-week follow-up for all available patients. The number of patients included and percentage share of viral load reduction are listed below the graph.
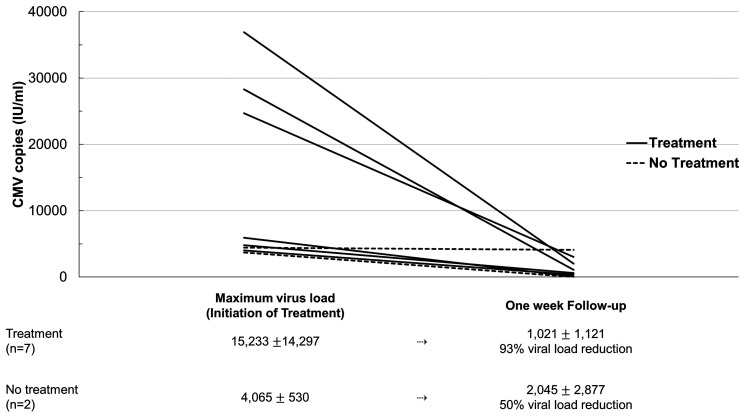
Six (55%) of all 11 patients with CMV reactivation survived the ICU stay, compared to 46% survival in patients without CMV reactivation. Treatment with ganciclovir was administered in 7 (64%) of 11 patients with a median copy number of 5,930 IU/mL. Six (86%) of the 7 patients with CMV reactivation who received ganciclovir survived the ICU stay. None of the 4 patients with CMV reactivation who did not receive ganciclovir survived the ICU stay (P = .015) (Table 1, Figure 2B). The percentage of viral load reduction compared to maximum virus copies in patients with and without ganciclovir treatment is illustrated in Figure 4.
Figure 4.
Cytomegalovirus (CMV) load in IU/mL at time of maximum virus copies (in the treatment group at time of treatment) and at one-week follow-up for all available patients. The number of patients included and percentage share of viral load reduction are listed below the graph.
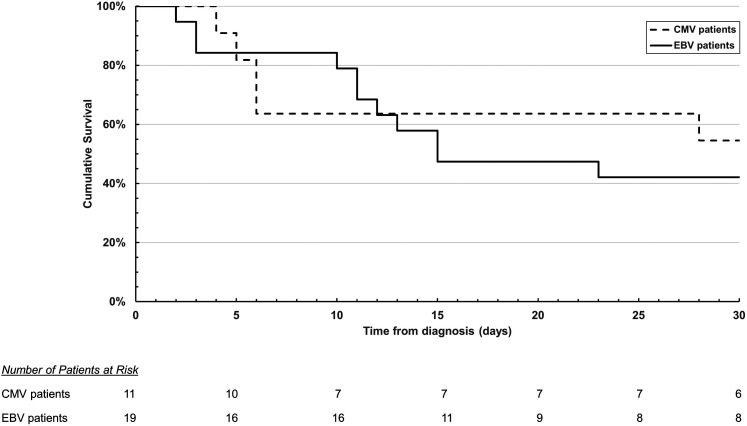
As time to event analysis, the cumulative survival of ICU COVID-19 patients with EBV and CMV reactivations is displayed as Kaplan-Meier curve in Figure 5. Additionally, time from viral reactivation to ICU discharge or time from viral reactivation to death is illustrated in Table 1.
Figure 5.
Kaplan-Meier survival curve of intensive care unit (ICU) COVID-19 patients with Epstein-Barr virus (EBV) and cytomegalovirus (CMV) reactivations.
Laboratory Values
Twenty-one (88%) of 24 patients with EBV or CMV reactivation had a persisting fever. Inflammatory laboratory parameters varied distinctly between patients (Supplemental Table 1). However, no correlations between viremia and C-reactive protein, ferritin, interleukin-6, and neutrophil-to-lymphocyte ratio were identified for both EBV and CMV reactivations. In addition, no correlations between viremia and the transaminases as well as bilirubin were detected.
Control Group
A total of 126 SARS-CoV-2 negative patients were treated for sepsis on the reporting ICU in the analyzed time period between March 2020 and March 2021 and were therefore included as control group. Thirty-four (27%) of these 126 patients tested positive for either EBV or CMV reactivation during their ICU stay. Eighteen (14%) of 126 patients tested positive for EBV reactivation. Fifteen (83%) of these 18 patients were immunosuppressed; 7 (47%) of these 15 immunosuppressed patients were additionally treated with corticosteroids during their ICU stay. Two patients with EBV reactivation were neither immunosuppressed nor underwent treatment with corticosteroids.
Sixteen (13%) of the 126 patients in the control group tested positive for CMV reactivation; 14 (88%) of these 16 patients were immunosuppressed and 5 (36%) of these 14 patients also received corticosteroids during their ICU stay. One of these 16 patients was neither immunosuppressed nor received corticosteroids. One patient who tested positive for both EBV and CMV reactivation was not immunosuppressed but was treated with corticosteroids.
Discussion
The present descriptive analysis indicates that reactivation of EBV and CMV with more than 1000 IU/mL are highly prevalent in critically ill patients with COVID-19. These findings are in line with previously presented data.7,8,10
Although EBV reactivations occurred earlier after SARS-CoV-2 diagnosis and some patients still received dexamethasone as part of their COVID-19 treatment at the time of EBV reactivation, CMV reactivations were diagnosed later after a median of 30 days from SARS-CoV-2 diagnosis. At this time point, corticosteroids were administered only in case of persistent hyperinflammation and not as part of the initial COVID-19 treatment.
However, the majority of EBV (58%) and CMV reactivations (55%) in our study were detected in patients under high-dose systemic corticosteroid treatment. This finding is in agreement with previous findings in critically ill, non-COVID patients, where the use of high-dose corticosteroid treatment has been reported as a risk factor for HV reactivation.2,5 Nonetheless, whether corticosteroids play a substantial role in EBV and CMV reactivation due to their immunosuppressive effects or if HV reactivations are triggered by the critical medical condition itself, as other studies reported,1–3 is still up to debate.
The findings of our control group show that the percentage of critically ill patients with HV reactivation is similar (21% vs 27%) in SARS-CoV-2 positive and negative patients. This suggests that HV reactivation is triggered by the critical condition and rather not specifically by SARS-CoV-2. However, larger studies are needed to exclude SARS-CoV-2 infection as an independent risk factor.
Although the number of immunosuppressed patients both in the SARS-CoV-2 and the control group were high, our results show similar or lower rates of HV reactivation than previous studies.7,9
There is also controversy whether EBV and CMV reactivations in patients with severe COVID-19 represent independent factors that trigger hyperinflammation requiring treatment or whether reactivations of these viruses are solely epiphenomena of the critical medical condition.
In most patients, viral reactivations were considered as epiphenomena and follow-up tests were performed regularly until viremia resolved. No correlations between viremia and inflammatory laboratory parameters were identified. Treatment with rituximab (used in 32% of all patients with EBV reactivations) or ganciclovir (used in 60% of all patients with CMV reactivations) was only initiated in a subset of patients, showing evidence of severe hyperinflammation and/or critical clinical deterioration defined as an increase in vasopressor dose or increase in ventilation pressure or fraction of inspired oxygen. Follow-up analysis in these patients showed that the initiation of treatment lead to decrease in viral load. Interestingly, 5 of the patients who were treated with ganciclovir survived the ICU stay, while none of the patients with CMV reactivation without ganciclovir treatment survived. Nonetheless given the limited patient number in our analysis and especially considering the results of the recently published double-blind, controlled, randomized trial, which showed no benefit for a preemptive treatment with ganciclovir in critically ill patients,12 a treatment recommendation cannot be derived. No such difference in the ICU survival was observed in the patients with EBV reactivation with regard to rituximab treatment.
This analysis has several limitations, in particular the retrospective design, the limited patient number, the lack of histological and autopsy specimen, as well as the selection bias due to analyzing only patients with signs of hyperinflammation or clinical deterioration.
However, the illustrated cases shed light on EBV and CMV reactivations in COVID-19 patients with respect to systemic corticosteroid treatment and provide insights for larger studies on monitoring and potentially treating viral reactivations in COVID-19 patients. A larger, multicentric, prospective trial would be needed to systematically analyze HV reactivations in COVID-19 patients and evaluate the necessity for targeted treatment.
Conclusion
Critically ill patients with COVID-19 are at high risk for both EBV and CMV reactivations. Whether viral reactivations are a pathologic cause of hyperinflammation in need of treatment or epiphenomena related to the severity of COVID-19 or the treatment with systemic corticosteroids remains uncertain. However, in patients with clinical deterioration or signs of hyperinflammation, targeted treatment for viral reactivation might be beneficial and warrants further studying.
Supplemental Material
Supplemental material, sj-docx-1-jicm-10.1177_08850666211053990 for Reactivation of EBV and CMV in Severe COVID-19—Epiphenomena or Trigger of Hyperinflammation in Need of Treatment? A Large Case Series of Critically ill Patients by Jan-Hendrik Naendrup, Jorge Garcia Borrega, Dennis Alexander Eichenauer, Alexander Shimabukuro-Vornhagen, Matthias Kochanek and Boris Böll in Journal of Intensive Care Medicine
Footnotes
Author Contributions: JHN, JGB, MK and BB conceived the study. JHN and JGB conducted the chart reviews of patient. JHN, JGB, MK and BB analyzedand interpreted the data. JHN and JGB wrote the first draft of the manuscript. DEA, ASV, MK and BB critically revised the manuscript. All theauthors reviewed the final draft of the manuscript and agreed on submitting it to the Journal of Intensive Care Medicine.
Authors’ Note: Jan-Hendrik Naendrup, Jorge Garcia Borrega, Matthias Kochanek and Boris Böll contributed equally. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: BB received scientific grants and honoraria from Novartis, Kite/Gilead, Miltenyi, Roche and Janssen & Janssen outside of the submitted work. JHN, JGB, DAE, ASV and MK declare that they have no competing interests.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Garcia Borrega Jorge https://orcid.org/0000-0003-1326-1560
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Libert N, Bigaillon C, Chargari Cet al. et al. Epstein-Barr virus reactivation in critically ill immunocompetent patients. Biomed J. 2015;38(1):70–76. doi: 10.4103/2319-4170.132905 [DOI] [PubMed] [Google Scholar]
- 2.Ong DSY, Bonten MJM, Spitoni Cet al. Epidemiology of multiple herpes viremia in previously immunocompetent patients with septic shock. Clin Infect Dis. 2017;64(9):1204-1210. doi: 10.1093/cid/cix120 [DOI] [PubMed] [Google Scholar]
- 3.Walton AH, Muenzer JT, Rasche Det al. Reactivation of multiple viruses in patients with sepsis. PLoS One. 2014;9(6):1-13. doi: 10.1371/journal.pone.0098819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Textoris J, Mallet F. Immunosuppression and herpes viral reactivation in intensive care unit patients: one size does not fit all. Crit Care. 2017;21(1):1-2. doi: 10.1186/s13054-017-1803-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiche L, Forel J-M, Roch Aet al. Active cytomegalovirus infection is common in mechanically ventilated medical intensive care unit patients*. Crit Care Med. 2009;37(6):1850-1857. doi: 10.1186/s13613-020-00793-2 [DOI] [PubMed] [Google Scholar]
- 6.García-Martínez FJ, Moreno-Artero E, Jahnke S. SARS-CoV-2 and EBV coinfection. Med Clín. 2020;155(7):319-320. doi: 10.1016/j.medcle.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonnet A, Engelmann I, Moreau A-Set al. High incidence of Epstein-Barr virus, cytomegalovirus, and human-herpes virus-6 reactivations in critically ill patients with COVID-19. Infect Dis Now. 2021;51(3):296-299. doi: 10.1016/j.idnow.2021.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paolucci S, Cassaniti I, Novazzi F, et al. EBV DNA increase in COVID-19 patients with impaired lymphocyte subpopulation count. Int J Infect Dis. 2021;104:315-319. doi: 10.1016/j.ijid.2020.12.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saade A, Moratelli G, Azoulay E, Darmon M. Herpesvirus reactivation during severe COVID-19 and high rate of immune defect. Infect Dis Now. 2021;S2666-S9919(21):000468-1. doi: 10.1016/j.idnow.2021.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehner GF, Klein SJ, Zoller H, Peer A, Bellmann R, Joannidis M. Correlation of interleukin-6 with Epstein-Barr virus levels in COVID-19. Crit Care. 2020;24(1):657. doi: 10.1186/s13054-020-03384-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Limaye AP, Boeckh M. CMV In critically ill patients: pathogen or bystander? Rev Med Virol. 2010;20(6):372-379. doi: 10.1002/rmv.664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papazian L, Jaber S, Hraiech Set al. Preemptive ganciclovir for mechanically ventilated patients with cytomegalovirus reactivation. Ann Intensive Care. 2021;11(1):33. doi: 10.1186/s13613-020-00793-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanakry J, Ambinder R. The biology and clinical utility of EBV monitoring in blood. Curr Top Microbiol Immunol. 2015;391:475-499. doi: 10.1007/978-3-319-22834-1_17 [DOI] [PubMed] [Google Scholar]
- 14.Group TRC. Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021;384(8):693-704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jicm-10.1177_08850666211053990 for Reactivation of EBV and CMV in Severe COVID-19—Epiphenomena or Trigger of Hyperinflammation in Need of Treatment? A Large Case Series of Critically ill Patients by Jan-Hendrik Naendrup, Jorge Garcia Borrega, Dennis Alexander Eichenauer, Alexander Shimabukuro-Vornhagen, Matthias Kochanek and Boris Böll in Journal of Intensive Care Medicine