Abstract
Nephropathic apolipoprotein L1 (APOL1) risk alleles (G1/G2) have been associated with focal segmental glomerulosclerosis, HIV-associated nephropathy, Systemic lupus erythematosus (SLE)-associated collapsing glomerulopathy and other glomerulonephritides. These alleles confer protection from Trypanosoma brucei infections which are enriched in sub-Saharan African populations. We present a young woman with obesity, hypertension, subnephrotic range proteinuria who was found to have obesity-related glomerulopathy on kidney biopsy while harbouring two high-risk APOL1 alleles (G1/G2). Given the potential effects on lipid metabolism and their association with obesity, the presence of APOL1 risk alleles may impact cardiovascular health in addition to renal disease in these patients.
Keywords: Renal system, Pathology, Metabolic disorders
Background
The term obesity-related glomerulopathy (ORG) was first coined in 2001 by D’Agati and colleagues when the incidence of this entity was found to have increased 10-fold from 1986 to 1990 to 1996–2000.1 Morphologically, the renal tissue is found to have glomerulomegaly with or without focal segmental glomerulosclerosis (FSGS). Clinically, patients with ORG had a lower incidence of nephrotic range proteinuria and nephrotic syndrome, higher serum albumin, lower serum cholesterol and less oedema. We describe a case report of a young woman with obesity, hypertension (HTN) and ORG discovered on renal biopsy who was later found to harbour the apolipoprotein L1 (APOL-1) high-risk alleles (G1/G2). We discuss how these alleles may affect not only her renal outcome but also her cardiovascular health.
Case presentation
We present a young woman in her 30s with a history of HTN for 7 years, hyperlipidaemia and obesity (body mass index (BMI) 32 kg/m2) who was referred to the nephrology clinic for the evaluation of proteinuria and lower extremity oedema. Of note, the patient’s obstetric history reveals G7P3225 with a third pregnancy that was complicated by superimposed pre-eclampsia at age 25, which led to the diagnosis of chronic HTN in the post-partum period. An evaluation for secondary causes of HTN including hyperaldosteronism, pheochromocytoma and hyperthyroidism was unrevealing. The patient also denied a history of premature birth or low birth weight for herself, although noted frequent urinary tract infections as a child but was never further evaluated. Her family history was significant for her mother who developed HTN in the mid-20s, her maternal grandmother who experienced a cerebrovascular accident at the age of 40 and her maternal grandfather who died of end-stage kidney disease (ESKD) of unknown aetiology. Her medications included atorvastatin 10 mg daily and nifedipine 60 mg daily. Physical examination revealed a blood pressure of 150/100 mm Hg, clear lungs, normal cardiac and abdominal examinations, but bilateral 2+ lower extremity pitting oedema. Infectious workups including HIV, hepatitis B and hepatitis C were non-reactive. Comprehensive autoimmune workups including Anti-nuclear antibody (ANA), C3, C4 and antibodies for ds-DNA, Smith, SCL-70, Jo 1, centromere, histone, anti–Sjögren's-syndrome-related antigen A autoantibodies(SSA), anti-Sjögren’s syndrome type B (SSB), ribonucleoprotein, myeloperoxidase and proteinase-3 were negative. The remaining laboratory studies are listed in table 1. Given the subnephrotic range proteinuria with negative serological testing, a kidney biopsy was performed.
Table 1.
Laboratory parameters
| Laboratory variable | Laboratory value (normal range if applicable) |
| Blood urea nitrogen (BUN) | 10 (5–26 mg/dL) |
| Creatinine | 1.1 (0.6–1.2 mg/dL) |
| e*stimated glomerular filtration rate (eGFR) | 65 (82–137 mL/min/1.73 m2) |
| Albumin | 4.4 (3.5–5.5 g/dL) |
| Total cholesterol | 239 (120–220 mg/dL) |
| Low-density lipoprotein (LDL) | 140 (70–160 mg/dL) |
| High-density lipoprotein (HDL) | 68 (36–77 mg/dL) |
| Triglycerides | 155 (35–135 mg/dL) |
| Urinalysis | Clear, negative glucose, >300 protein, trace leucocyte esterase, negative nitrite, pH 5.5, specific gravity 1.025, red blood cells 31, no blood, bacteria 1+, white blood cells 15 hpf |
| Urine microscopy | No cells or casts |
| Urine protein/creatinine ratio | 2368 mg/g |
Renal biopsy report.
*eGFR calculated by the CKD-EPI formula.30
On light microscopy, 1 out of 12 glomeruli was globally sclerotic, 1 showed segmental wrinkling of basement membranes, while the remaining 10 were mildly enlarged and normocellular (figure 1A). The glomerular basement membranes appeared normal in thickness and contour. Tubular atrophy and interstitial fibrosis affected 5% of the cortex and were accompanied by patchy chronic interstitial inflammation comprised of plasma cells, lymphocytes and mononuclear leucocytes (figure 1B). Immunofluorescence staining was negative for IgG, IgM, IgA, C1, C3, kappa and lambda (figure not shown). On electron microscopy, glomerular basement membranes showed segmental thickening (figure 1C). No immune-type electron-dense deposits or endothelial cell tubuloreticular inclusions were seen. Podocytes demonstrated 5% foot process effacement. The final diagnoses were mild focal global glomerulosclerosis with glomerulomegaly with mild tubular atrophy and interstitial fibrosis. The findings of glomerulomegaly indicated glomerular hypertension/hyperfiltration, which may be related to obesity, HTN and/or reduced nephron number.
Figure 1.
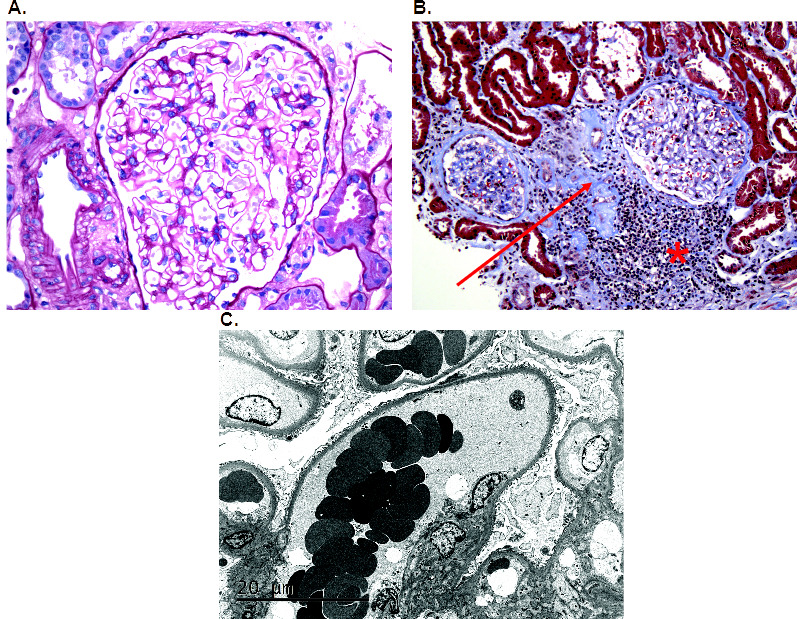
(A) A representative glomerulus that is enlarged and normocellular. The glomerular basement membranes appeared normal in thickness and contour (H&E, ×40). (B) The cortex demonstrated 5% tubular atrophy and interstitial fibrosis (red arrow) which was accompanied by patchy chronic interstitial inflammation comprised of plasma cells, lymphocytes and mononuclear leucocytes (red asterisk) (H&E, ×20). (C) By electron microscopy, glomerular basement membranes showed segmental thickening with 5% foot process effacement. No immune-type electron-dense deposits or endothelial cell tubuloreticular inclusions were seen (electron micrograph, ×3000).
Differential diagnosis
Glomerulomegaly related with either obesity, HTN and/or reduced nephron number.
Treatment
We encouraged healthy weight loss and a referral to a weight management clinic. The patient was maintained on losartan and atorvastatin was increased to 40 mg daily. Her blood pressure remained below 140/90 mm Hg on clinic follow-up.
Outcome and follow-up
Four months post-renal biopsy, the patient’s urine protein-creatinine ratio decreased to 1618 mg/g (from 2368 mg/g before biopsy). Her Low-density lipoprotein (LDL) remained above target at 96 mg/dL. We increased losartan to 100 mg daily to minimise proteinuria and to target blood pressure to be below 130/80 mm Hg, and increased atorvastatin to 20 mg daily for a more aggressive lipid-lowering effect.
Discussion
Apolipoprotein L1 (APOL1) is one of the six members of apolipoprotein L, encoded in chromosome 22.2 APOL1 is an High-density lipoprotein (HDL)-associated protein that was described in several organs, including the pancreas, lung, liver, spleen and prostate.3–5 In the kidneys, APOL1 is localised to podocytes and proximal tubules.6
The evolution of APOL1 variants, G1 (S342G and I384M mutations) and G2 (deletion of amino acids N388 and Y389), confer protections against Trypanosoma brucei infections which cause sleeping sickness in sub-Saharan Africa.7 The HDL-associated APOL1 is taken up by trypanosomes through the endocytic pathway to the lysosome, leading to pore formation, chloride influx, osmotic swelling and eventual lysis of trypanosomes.8 Through natural selection, these alleles are commonly identified in Black Americans but rare in European Americans.9 Approximately one-third of Black Americans possess one high-risk APOL1 allele and 15% have two of these alleles. While the APOL1 G1 and G2 alleles protect against trypanosomiasis, they increase the risk of various kidney diseases by mechanisms that remain currently unknown.
The APOL1 gene follows a recessive genetic model. Therefore, possession of two high-risk alleles leads to an increased rate of Chronic kidney disease (CKD) progression and ESRD compared with zero or one copy.10 These risk alleles are also associated with increased risks of primary FSGS,11 HIV-associated nephropathy,11 lupus nephritis,12 SLE-associated collapsing glomerulopathy,13 PLA2R-associated membranous glomerulopathy,14 non-diabetic CKD15 and hypertension-associated ESKD.9
Obesity is an independent risk factor for the development of CKD after adjusting for diabetes and HTN.16 ORG is characterised by variable proteinuria although usually subnephrotic, with the absence of oedema and hypoalbuminemia, even in cases with massive proteinuria.17 Pathologically, there is glomerulomegaly with or without FSGS lesions, with less extensive foot process effacement as compared with idiopathic FSGS.1 Single-nephron hyperfiltration is observed in early stages of CKD in patients with ORG compared with obese and non-obese controls.18 Glomerulomegaly is thought to be a compensatory response to single-nephron hyperfiltration.18 The hyperfiltration leads to increased proximal tubular sodium reabsorption.19 This leads to a decrease in the solute delivery to the macula densa, leading to afferent arteriolar dilation and glomerular hyperfiltration.20 Additionally, adipose tissues have their intrinsic renin-angiotensin-aldosterone (RAAS) system, independent of the kidneys.21 The overactivation of the RAAS system associated with obesity also increases sodium and water reabsorption at the proximal tubules, further potentiating glomerular hyperfiltration. This increases tensile stress in the glomerular capillaries, leading to expansion of the glomerular basement membrane and hypertrophy of the overlying podocytes.22 Eventually, these stressed podocytes undergo detachment and cell death.22 The long-term outcome of ORG is ESRD.1 23 Patients with ORG have low glomerular density and a reduced number of functioning nephrons, which are implicated in CKD progression in this population.18 The mainstay treatment for ORG includes weight loss and the use of renin-angiotensin blockers.17
Not all obese patients develop ORG, a cross-sectional study of renal biopsies of morbidly obese patients revealed that only 12% of biopsies develop this entity,24 begging the question of which factors are contributory. With our emerging understanding of APOL1 risk alleles, obesity may serve as a ‘second hit’’ to develop ORG. Moreover, APOL1 risk alleles may be associated with increased cardiovascular and metabolic derangements. A higher prevalence of obesity, high LDL cholesterol, uncontrolled HTN and left ventricular hypertrophy was found in African American children with two high-risk APOL1 alleles and FSGS as compared with those with zero or one risk variant, despite treatment with antihypertensives and adjustment for indicators of socioeconomic status.25 Both the Jackson Health Study and the Women’s Health Initiative found that adult participants with two high-risk APOL1 alleles had an increased cardiovascular disease risk after correcting for traditional risk factors and CKD as compared with those without the high-risk alleles.26 High-risk variants were associated with BMI and obesity in an additive manner in a genotype-phenotype association study.27 Since APOL1 is an important component of HDL 3 particles that attenuate LDL oxidation, it may play a role in reverse cholesterol transport. APOL1 risk alleles, on the other hand, have been found to impair reverse cholesterol transport through decreased expression of cholesterol efflux transporters.28 Thus, APOL1 risk variants might promote foam cell formation and the growth of a necrotic core with increased plaque instability. Indeed, patients with two high-risk alleles have demonstrated larger necrotic cores with greater plaque areas that are enriched for APOL1.29 These epidemiological and histopathological studies pose an intriguing link between high-risk APOL1 alleles and metabolic/cardiovascular abnormalities which need to be further studied.
To our knowledge, this is the first case showing the association between APOL1 risk alleles and ORG. Given the substantial morbidity and mortality from the combination of cardiovascular disease and CKD, knowledge of APOL1 risk allele status may help to better stratify cardiovascular and renal risks to help guide clinical care. In particular, aggressive control of hyperlipidaemia should be emphasised in these patients, in addition to weight loss and the use of renin-angiotensin blockers.
Patient’s perspective.
I did not realize that my body weight can affect the kidneys. I will try my best to lose weight and eat a healthy diet for a better outcome.
Learning points.
Obesity may act as a ‘second hit’ in patients with two APOL-1 risk alleles to develop obesity-related glomerulomegaly (ORG).
The presence of APOL-1 risk alleles is associated with metabolic and cardiovascular abnormalities.
The treatment of ORG in the setting of two APOL-1 risk alleles may need to include aggressive lipid control in addition to weight loss and renin-angiotensin blockade.
Understanding the mechanism of APOL-1 G1/G2 variants in cardiovascular and renal disease pathogenesis is paramount to unlocking potential treatment strategies to prevent cardiac and renal failure.
Footnotes
Contributors: Dr RIV acquired, interpreted the data, wrote and edited the manuscript. Dr NSHLS acquired, interpreted the data, wrote and edited the manuscript. Dr MBS acquired, interpreted the data, significantly edited the paper. Dr BJ acquired, interpreted the data and significantly edited and revised the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Kambham N, Markowitz GS, Valeri AM, et al. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int 2001;59:1498–509. 10.1046/j.1523-1755.2001.0590041498.x [DOI] [PubMed] [Google Scholar]
- 2.Page NM, Butlin DJ, Lomthaisong K, et al. The human apolipoprotein L gene cluster: identification, classification, and sites of distribution. Genomics 2001;74:71–8. 10.1006/geno.2001.6534 [DOI] [PubMed] [Google Scholar]
- 3.Duchateau PN, Pullinger CR, Orellana RE, et al. Apolipoprotein L, a new human high density lipoprotein apolipoprotein expressed by the pancreas. Identification, cloning, characterization, and plasma distribution of apolipoprotein L. J Biol Chem 1997;272:25576–82. 10.1074/jbc.272.41.25576 [DOI] [PubMed] [Google Scholar]
- 4.Duchateau PN, Pullinger CR, Cho MH, et al. Apolipoprotein L gene family: tissue-specific expression, splicing, promoter regions; discovery of a new gene. J Lipid Res 2001;42:620–30. 10.1016/S0022-2275(20)31171-8 [DOI] [PubMed] [Google Scholar]
- 5.Shukha K, Mueller JL, Chung RT, et al. Most ApoL1 is secreted by the liver. J Am Soc Nephrol 2017;28:1079–83. 10.1681/ASN.2016040441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madhavan SM, O'Toole JF, Konieczkowski M, et al. APOL1 localization in normal kidney and nondiabetic kidney disease. J Am Soc Nephrol 2011;22:2119–28. 10.1681/ASN.2011010069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Limou S, Nelson GW, Kopp JB, et al. APOL1 kidney risk alleles: population genetics and disease associations. Adv Chronic Kidney Dis 2014;21:426–33. 10.1053/j.ackd.2014.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pérez-Morga D, Vanhollebeke B, Paturiaux-Hanocq F, et al. Apolipoprotein L-I promotes trypanosome lysis by forming pores in lysosomal membranes. Science 2005;309:469–72. 10.1126/science.1114566 [DOI] [PubMed] [Google Scholar]
- 9.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 2010;329:841–5. 10.1126/science.1193032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parsa A, Kao WHL, Xie D, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 2013;369:2183–96. 10.1056/NEJMoa1310345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 2011;22:2129–37. 10.1681/ASN.2011040388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freedman BI, Langefeld CD, Andringa KK, et al. End-stage renal disease in African Americans with lupus nephritis is associated with APOL1. Arthritis Rheumatol 2014;66:390–6. 10.1002/art.38220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsen CP, Beggs ML, Saeed M, et al. Apolipoprotein L1 risk variants associate with systemic lupus erythematosus-associated collapsing glomerulopathy. J Am Soc Nephrol 2013;24:722–5. 10.1681/ASN.2012121180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsen CP, Beggs ML, Walker PD, et al. Histopathologic effect of APOL1 risk alleles in PLA2R-associated membranous glomerulopathy. Am J Kidney Dis 2014;64:161–3. 10.1053/j.ajkd.2014.02.024 [DOI] [PubMed] [Google Scholar]
- 15.Ulasi II, Tzur S, Wasser WG, Shemer R, et al. High population frequencies of APOL1 risk variants are associated with increased prevalence of non-diabetic chronic kidney disease in the Igbo people from south-eastern Nigeria. Nephron Clin Pract 2013;123:123–8. 10.1159/000353223 [DOI] [PubMed] [Google Scholar]
- 16.Hsu C-yuan, McCulloch CE, Iribarren C, et al. Body mass index and risk for end-stage renal disease. Ann Intern Med 2006;144:21–8. 10.7326/0003-4819-144-1-200601030-00006 [DOI] [PubMed] [Google Scholar]
- 17.Praga M, Morales E. The fatty kidney: obesity and renal disease. Nephron 2017;136:273–6. 10.1159/000447674 [DOI] [PubMed] [Google Scholar]
- 18.Okabayashi Y, Tsuboi N, Sasaki T, et al. Single-nephron GFR in patients with obesity-related glomerulopathy. Kidney Int Rep 2020;5:1218–27. 10.1016/j.ekir.2020.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chagnac A, Herman M, Zingerman B, et al. Obesity-Induced glomerular hyperfiltration: its involvement in the pathogenesis of tubular sodium reabsorption. Nephrol Dial Transplant 2008;23:3946–52. 10.1093/ndt/gfn379 [DOI] [PubMed] [Google Scholar]
- 20.Tsuboi N, Okabayashi Y, Shimizu A, et al. The renal pathology of obesity. Kidney Int Rep 2017;2:251–60. 10.1016/j.ekir.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlsson C, Lindell K, Ottosson M, et al. Human adipose tissue expresses angiotensinogen and enzymes required for its conversion to angiotensin II. J Clin Endocrinol Metab 1998;83:3925–9. 10.1210/jc.83.11.3925 [DOI] [PubMed] [Google Scholar]
- 22.Chagnac A, Zingerman B, Rozen-Zvi B, et al. Consequences of glomerular hyperfiltration: the role of physical forces in the pathogenesis of chronic kidney disease in diabetes and obesity. Nephron 2019;143:38–42. 10.1159/000499486 [DOI] [PubMed] [Google Scholar]
- 23.Tsuboi N, Koike K, Hirano K, et al. Clinical features and long-term renal outcomes of Japanese patients with obesity-related glomerulopathy. Clin Exp Nephrol 2013;17:379–85. 10.1007/s10157-012-0719-y [DOI] [PubMed] [Google Scholar]
- 24.Choung H-YG, Bomback AS, Stokes MB, et al. The spectrum of kidney biopsy findings in patients with morbid obesity. Kidney Int 2019;95:647–54. 10.1016/j.kint.2018.11.026 [DOI] [PubMed] [Google Scholar]
- 25.Woroniecki RP, Ng DK, Limou S, et al. Renal and cardiovascular morbidities associated with APOL1 Status among african-american and non-African-American children with focal segmental glomerulosclerosis. Front Pediatr 2016;4:122. 10.3389/fped.2016.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito K, Bick AG, Flannick J, et al. Increased burden of cardiovascular disease in carriers of APOL1 genetic variants. Circ Res 2014;114:845–50. 10.1161/CIRCRESAHA.114.302347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nadkarni GN, Fei K, Galarneau G, et al. APOL1 renal risk variants are associated with obesity and body composition in African ancestry adults: an observational genotype-phenotype association study. Medicine 2021;100:e27785. 10.1097/MD.0000000000027785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryu J-H, Ge M, Merscher S, et al. APOL1 renal risk variants promote cholesterol accumulation in tissues and cultured macrophages from APOL1 transgenic mice. PLoS One 2019;14:e0211559. 10.1371/journal.pone.0211559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cornelissen A, Fuller DT, Fernandez R, et al. APOL1 genetic variants are associated with increased risk of coronary atherosclerotic plaque rupture in the black population. Arterioscler Thromb Vasc Biol 2021;41:2201–14. 10.1161/ATVBAHA.120.315788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]


