Abstract
A man in early 40s met with an accident with a complex pelvic fracture and extraperitoneal bladder injury and posterior urethral disruption 16 years ago. He additionally had left lumbar spinal segment mixed nerve injury, resulting in a foot drop. He underwent laparotomy and a diverting cystostomy at the time with a primary perineal urethroplasty a year later. He later developed pseudoarthrodesis of the hip joint, and poorly compliant bladder with complete block at bulbar urethra. A redo anastomotic urethroplasty was performed, hyperreflexive neurogenic bladder was managed with intravesical botox injections and underwent a hip replacement. Having defaulted botox injections, he developed a vesico-acetabulo-cutaneous fistula and the hip prosthesis was explanted. Later he underwent a ileal cystoplasty and a revision hip replacement. Ten years later, he presented with a recurrent fistula due to poor compliance with clean intermittent catheterisation. A challenging exploration with fistula excision was done with a primary bladder repair.
Keywords: Hip prosthesis implantation, Urology
Background
Vesico-acetabular-cutaneous fistula (VACF) is a rare entity, the few cases reported have predominantly occurred following hip arthroplasty.1–5 The present unique case is a recurrent VACF, recurring 10 years following corrective surgery in a patient with post-traumatic neurogenic bladder with hip arthroplasty. We discuss the etiopathogenesis and management of this vexing problem.
Case presentation
A man in his early 40s met with road traffic accident 16 years ago. He was primarily managed for abdominal trauma, fracture pelvis, extraperitoneal bladder injury and posterior urethral disruption with an exploratory laparotomy and diverting cystostomy at a trauma centre (figure 1). After recuperation, he underwent a primary perineal anastomotic urethroplasty 1 year later at a district hospital. He was unable to void at catheter removal and continued to have urinary diversion in the form of a suprapubic cystostomy.
Figure 1.
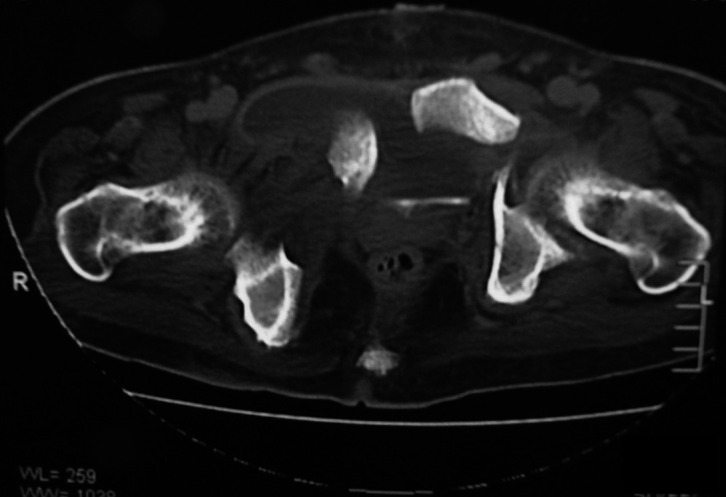
CT scan showing left acetabular fracture and pubic diastasis with bony fragments causing bladder injury.
He was re-evaluated after 6 months at a tertiary hospital, which revealed a malunited fracture pelvis involving left superior and inferior pubic rami, pseudoarthrodesis of the left hip joint, pubic diastasis and a shear deformity of the left sacro-iliac (SI) joint (figure 2A). He had a suprapubic cystostomy in situ which was associated with intense catheter sensation. Neurological assessment revealed a normal anal tone; sensory deficit which was restricted to medial aspect of left foot (L4–5 dermatome); deep tendon motor reflexes at the knee were brisk, however a left foot drop was noted. This suggested a limited mixed upper and lower motor neuron type L4–5 spinal segment injury. This could be attributed to the shear injury involving the left SI joint. Urethrocystogram performed was associated with painful bladder filling. It revealed a small bladder with a pseudodiverticulum arising from the right anterior portion of the bladder, herniating through the distracted pubic symphysis, a smaller pseudodiverticulum was noted in left lateral aspect, a competent bladder neck and complete block at proximal bulbar urethra (figure 2B).
Figure 2.
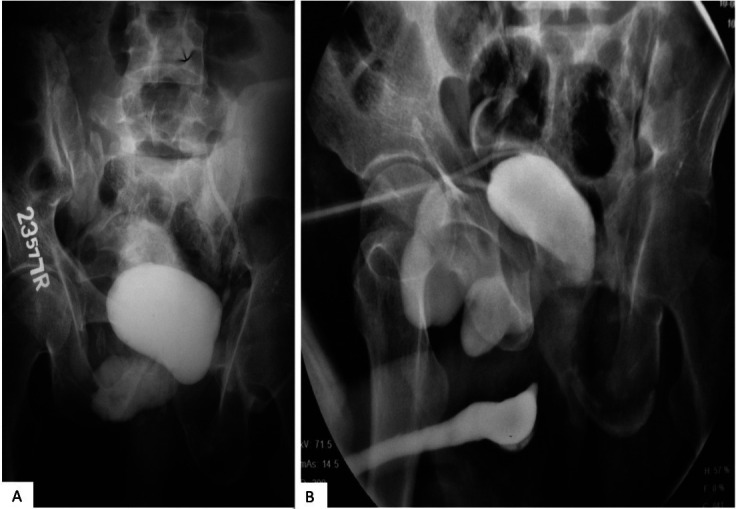
Malunited fracture pelvis involving left superior and inferior pubic rami, pseudoarthrodesis of the left hip joint, pubic diastasis and a shear deformity of the left SI joint (A) and urethrocystogram showing small bladder with a pseudodiverticulum arising from the right anterior portion of the bladder, and a competent bladder neck with complete block at proximal bulbar urethra (B). SI, sacroiliac.
He underwent a successful revision anastomotic urethroplasty 2 months later. A urodynamics done thereafter confirmed a small capacity, hyper-reflexive neurogenic bladder. The detrusor hyperreflexia and low compliance was treated with intradetrusor botulinum toxin (300 IU) injection. Following this, the patient was alleviated of bladder pain and was able to void with a good stream to completion. He then underwent a successful left total hip replacement (THR) 3 months later. A year later, he defaulted his third Botox injection and presented with fluid drainage from the left hip healed suture line. High fluid creatinine confirmed urine, as the drainage fluid from the hip wound. A contrast-enhanced CT cystogram delineated left VACF (figure 3). A per urethral foleys was inserted, which promptly dried up the wound. The infected left hip prosthesis was explanted.
Figure 3.
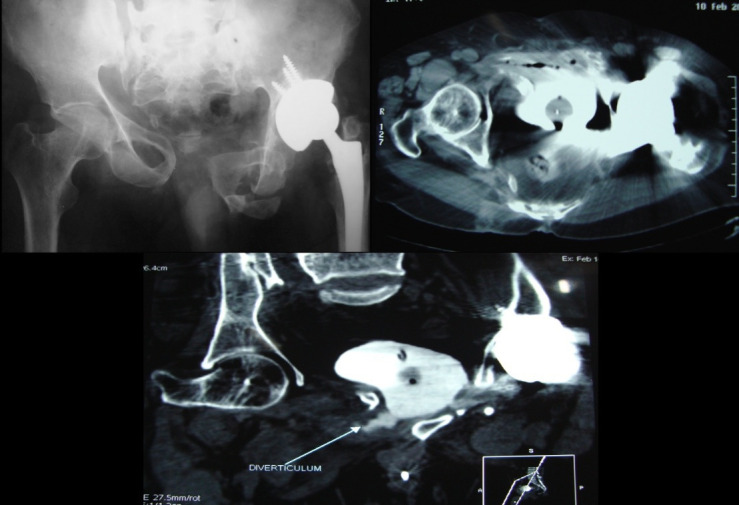
Contrast-enhanced CT cystogram delineating left vesico-acetabular-cutaneous fistula.
A clam ileal cystoplasty was performed after radiologically confirming that the fistula had healed; dense adhesions/near frozen pelvis precluded fistula excision at the time. The patient instituted regular clean intermittent catheterisation (CIC). A revision left THR was performed 6 months after the cystoplasty. The patient developed an incisional hernia in the lower midline scar and underwent a mesh hernioplasty 2 years later. He was back at his desk job, performing CIC regularly.
He presented to us 10 years postcystoplasty after a hiatus of follow-up of 5 years. He had become erratic with CIC and was resorting to voiding with abdominal straining. While voiding with considerable straining he felt a ‘give’ and promptly noted a gush off urine leak from the left hip scar site. The patient self-catheterised himself which stopped the leakage. Examination revealed the mouth of the fistula at the cranial aspect of a healed left hip scar (figure 4).
Figure 4.
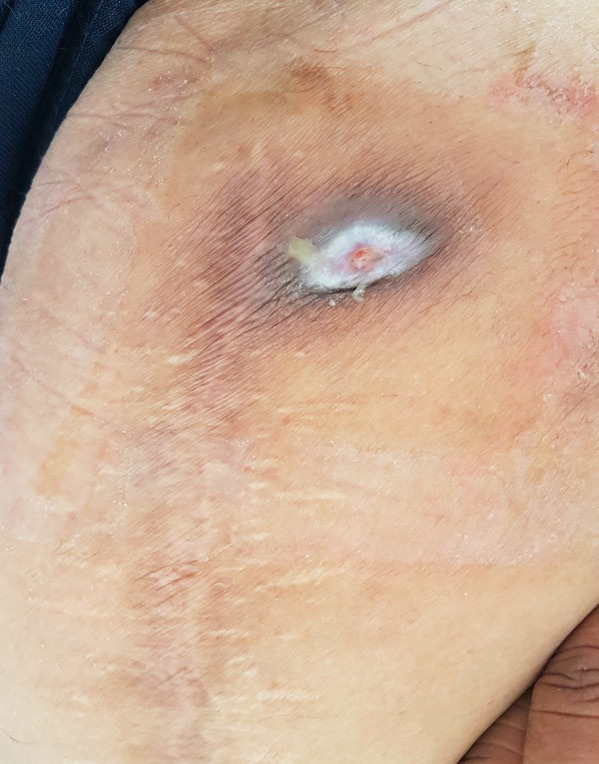
Mouth of the fistula at the cranial aspect of a healed left hip scar.
Investigations
Blood investigations were unremarkable; urine microscopy revealed elevated White blood cells (WBCs), proteinuria and mucus. Urine culture was sterile. Secretions from the fistula site cultured Staphylococcus epidermidis. MRI of the abdomen and pelvis confirmed site of fistula at the previous site, from the left small pseudodiverticula, tracking to the acetabular implant head (figure 5). The functional bladder capacity was measured to be 275 mL.
Figure 5.
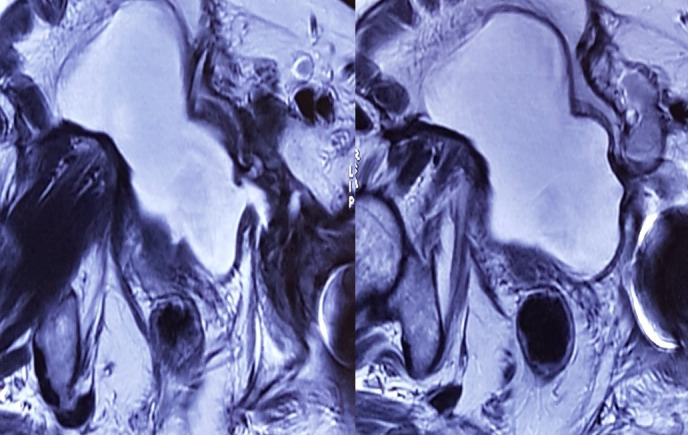
MRI of the abdomen and pelvis confirming site of fistula, from the left small pseudodiverticula, tracking to the acetabular implant head.
Treatment
Given the complexity of the case in view of prior abdominal surgeries, the mesh in the midline and patient’s proclivity to non-adherence of instructions (CIC protocol) in the long term, he and the family were counselled for a permanent supravesical diversion. However, he was adamant in his demand for an attempt at correction of the fistula closure and resolute adherence to CIC.
With a consent for conversion to a supravesical diversion, in the event of formidable adhesions, a left Gibson incision was used to perform a transvesical approach for fistula excision. The augmented bladder was entered through the augmented portion of the bladder (the ileal patch) as it was deemed the least encumbered by adhesions. The internal mouth of the fistula was identified (figure 6). The portion of the bladder bearing the fistula was densely adherent to the obturator fossa area, therefore, the entire portion—measuring 2 cm2 area was excluded from the bladder (excision by exclusion of the entire tract). The lateral aspect of the bladder was primarily repaired from within the bladder with 2/0 Vicryl after interposing an omental patch between the bladder and excluded fistula tract interposition. The bladder was closed after placing a suprapubic catheter (SPC) in addition to the per urethral catheter. He had an uneventful recovery. A cystogram was performed at 3 weeks postoperatively to confirm healing, then the SPC was removed, CIC was resumed after another week.
Figure 6.
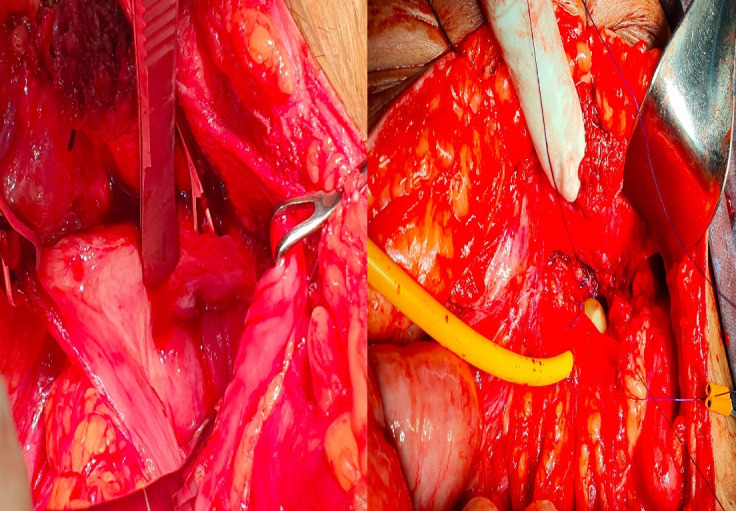
Intraoperative pictures revealing the fistula site and the dense adhesions.
Outcome and follow-up
At 1-month follow-up, he had resumed his desk job. At 1-year follow-up, there is no recurrence of fistula, with the CIC protocol strictly being adhered to. He does have pain in the left hip with intermittent infection of the hip and is planned for an explantation of left hip prosthesis by the orthopaedic team, to be later considered for a re-do hip implant.
Discussion
Most vesical fistulae occur between the urinary bladder and the female urogenital tract or the enteric system. They are commonly iatrogenic, postinflammatory (tuberculosis, Crohn’s disease, ulcerative colitis and schistosomiasis), postradiation due to tissue failure, postpenetrating trauma or due to infiltrating malignant disease. Vesicocutaneous fistulae can occur due to non-healing suprapubic tracts especially in neurogenic bladders or associated distal urethral strictures. It has also been reported due to chronically infected stone bearing bladder diverticulae.6
VACF is rarely reported in literature, predominantly occurring post-THR, due to mechanical injury, misplaced screw placement leading to chronic prosthetic infection3–5 or displacement of cement.7 Wang and Xu reported a vesico cutaneous (hip) fistula formation secondary to inadequately treated gluteal abscess leading to repeated infections which led to cystitis glandularis.8 A trapped bladder postfracture of acetabulum has also been reported as an aetiology for a vesicocutaneous hip fistula.9 Urinary fistula formation in patients following hip arthroplasty are reported to occur due to intrapelvic cement (methylmethacrilate) spilling, loosening and dislocation of the prosthesis and infection.6
In this unique case, there was a spontaneously healed extraperitoneal bladder injury in the vicinity of the replaced left femoro-acetabular prosthesis. Polymethylmethacrylate (PMMA) cement used to fuse the acetabular bracket caused, an expected thermochemical reaction, causing the scarred extraperitoneal bladder pseudodiverticulum to weld closer.
Ordinarily, this may not have posed a problem if the bladder had been a normal low-pressure system without an outflow resistance. However, the additional detrusor hyper-reflexia, secondary to the neurogenic bladder caused by the partial lumbar 4–5 segmental injury, leading to a high-pressure bladder. This caused the scar site to give way and cause final communication with the neo-acetabular joint cavity, which then leaked though the skin implant scar site.
In the first instance, the defaulting on the Botox, caused recurrence of a high intrabladder pressure, possibly causing the weakened bladder pseudodiverticulum to give way. The reduction in bladder pressures with the ileal cystoplasty prevented the pseudodiverticulum from venting the pressure over the decade. The ileal patch provides for increasing bladder capacity, and improves compliance, promoting low pressure urine storage, safe guarding the upper urinary tracts and thus reduces the intravesical pressure. However, this low-pressure bladder needs better emptying, while maintaining a low pressure.
Non-compliance with CIC for bladder emptying and using high-pressure abdominal voiding instead, led to the same scar site to give way in the second instance, a decade later.
Complications in the present case occurred due to a combination of an untreated extraperitoneal bladder injury, leading to a pseudodiverticulum, in a high-pressure neurogenic bladder with associated hip replacement, compounded by the poor compliance by the patient in performing CIC. This case also highlights the challenges in lower urinary tract rehabilitation following complex pelvic injury.
Urinarycutaneous fistula repairs in patients with neurogenic bladders are a challenge to manage. Patients who undergo surgical repair of their fistula are likely to require repeat repairs with eventual need for a permanent urinary diversion.10 11 Raup et al described decubitus ulcers, wound infections or abscess formation, condom catheter complications, traumatic catheterisation and pelvic trauma as the causes of urinary cutaneous fistulae. In their study, majority eventually required permanent surgical or suprapubic tube urinary diversion (81%), of these 53% required suprapubic tube, 23% were diverted with an ileal conduit (23%), 18% with a conduit catheter and 6% with a perineal urethrostomy.10
Primary outcomes between cystorrhaphy and conservatively managed patients have been previously described.12 13 Patients of extraperitoneal bladder rupture managed conservatively who undergo open non-urological pelvic or abdominal procedures without having simultaneous cystorrhaphy are at significantly higher risk of developing major urological complications as compared with those who do undergo cystorrhaphy. American urological association (AUA) urotrauma guidelines recommend performing cystorrhaphy on patients undergoing open repair and internal fixation, although we believe these guidelines should include a recommendation to proceed with repair in the setting of exploratory laparotomy as well, urinary cutaneous fistula following extraperitoneal bladder rupture represent a rare, but morbid, complication of non-operative management.
Patient’s perspective.
This journey has been such an ordeal and is difficult to describe in words. Having met with an accident 16 years ago and having suffered physically, emotionally and financially, all I can say is it’s due to the blessings of the almighty that I can go about doing my daily activities today and earn a living. In this journey, I would like to thank my doctors and caretakers who have time and again proved their competence and tried to help me in every possible way. Certain things could have been avoided due to my own negligence, however it was my state of mind which made me act as such. I am wiser now and promise to follow all my doctor’s instructions and would regularly follow-up to prevent any unforeseen complications in the future. A big thank you to my family and the team of doctors.
Learning points.
This case highlights the challenges in lower urinary tract rehabilitation following complex pelvic injury.
It reiterates the need to repair large extraperitoneal bladder injuries.
It also exemplifies the need to impress on the patient continued need for CIC in neurogenic bladders and strongly avoiding straining to micturate.
Most vesico-acetabulo-cutaneous fistulae occurred posthip arthroplasty due to penetrating mechanical injury or chronic prosthetic infection or displacement of the PMMA cement.
Footnotes
Twitter: @sunnygoel
Contributors: PW: Manuscript preparation, editing, final approval. SG: Data collection and refinement. HST: Manuscript editing and images. FZ: Intellectual content and manuscript editing.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Tripp BM, Tanzer M, Laplante MP, et al. Vesico-acetabular fistula. J Urol 1995;153:1910–1. 10.1016/S0022-5347(01)67350-6 [DOI] [PubMed] [Google Scholar]
- 2.Schneider HJ, Mufti GR. Vesico-acetabular fistula after total hip replacement. Br J Urol 1993;71:754. 10.1111/j.1464-410X.1993.tb16083.x [DOI] [PubMed] [Google Scholar]
- 3.Jones ALC, Acher P, Cynk M. Vesico-acetabular cutaneous fistula: a delayed complication of hip surgery. Urology 2011;78:323–4. 10.1016/j.urology.2010.06.007 [DOI] [PubMed] [Google Scholar]
- 4.Russell RD, Incavo SJ, Mineo MT, et al. Vesicoacetabular fistula in a chronically infected total hip arthroplasty. J Arthroplasty 2010;25:659.e9–659.e12. 10.1016/j.arth.2009.04.017 [DOI] [PubMed] [Google Scholar]
- 5.Vishwanath J, Ng YP, Teo YS, et al. Vesico-hip fistula from bladder puncture with subsequent infected total hip arthroplasty. J Arthroplasty 2007;22:939–41. 10.1016/j.arth.2006.08.008 [DOI] [PubMed] [Google Scholar]
- 6.Kishore TA, Bhat S, John PR. Vesicocutaneous fistula arising from a bladder diverticulum. Indian J Med Sci 2005;59:265–7. 10.4103/0019-5359.16302 [DOI] [PubMed] [Google Scholar]
- 7.Gallmetzer J, Gozzi C, Herms A. Vesicocutaneous fistula 23 years after hip arthroplasty. A case report. Urol Int 1999;62:180–2. 10.1159/000030387 [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Xu Y. Vesico-cutaneous fistula to the hip: a case report and review of the literature. J Postgrad Med 2013;59:220–2. 10.4103/0022-3859.118043 [DOI] [PubMed] [Google Scholar]
- 9.Tolkach Y, Gadjiev N, Korol V, et al. Vesico-Acetabular fistula and urolithiasis in the hip joint cavity due to persistent bladder entrapment after acetabular fracture. Korean J Urol 2011;52:221–4. 10.4111/kju.2011.52.3.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raup VT, Eswara JR, Weese JR, et al. Urinary-cutaneous fistulae in patients with neurogenic bladder. Urology 2015;86:1222–7. 10.1016/j.urology.2015.07.057 [DOI] [PubMed] [Google Scholar]
- 11.Johnsen NV, Sosland R, Kaufman MR, et al. Urinary-cutaneous fistulae following conservative management of extraperitoneal bladder ruptures. Urology 2017;109:195–200. 10.1016/j.urology.2017.06.034 [DOI] [PubMed] [Google Scholar]
- 12.Johnsen NV, Young JB, Reynolds WS, et al. Evaluating the role of operative repair of extraperitoneal bladder rupture following blunt pelvic trauma. J Urol 2016;195:661–5. 10.1016/j.juro.2015.08.081 [DOI] [PubMed] [Google Scholar]
- 13.Gousse AE, Pareek K, Lavernia CJ, et al. Omental interposition for repair of a vesico-acetabular fistula. J Urol 2001;166:2313. 10.1016/S0022-5347(05)65566-8 [DOI] [PubMed] [Google Scholar]


