Abstract
Objective
Histone deacetylase 4 (HDAC4) modulates immunity, inflammation, and osteoblast differentiation to engage in rheumatoid arthritis (RA) etiology. This study aimed to evaluate the HDAC4 longitudinal change and its relationship with clinical features and outcomes in RA patients.
Methods
Eighty‐three RA patients were enrolled. Their serum HDAC4 level was detected by ELISA at baseline (W0), week (W) 4, W12, and W24 after treatment. RA patients were divided into response or non‐response, low disease activity (LDA) or non‐LDA, remission or non‐remission patients according to their treatment outcomes at W24. Meanwhile, serum HDAC4 was detected by ELISA in 20 osteoarthritis patients and 20 healthy controls (HCs).
Results
HDAC4 level was reduced in RA patients compared with HCs (p < 0.001) and osteoarthritis patients (p = 0.009). HDAC4 was negatively related to some of the disease activity indexes such as C‐reactive protein (p = 0.003), tender joint count (p = 0.025), and disease activity score based on 28 joints (p = 0.013) in RA patients; it was also negatively correlated with TNF‐α (p = 0.003), IL‐6 (p = 0.022), and IL‐17A (p = 0.015). However, the HDAC4 level was not related to different treatment histories or current initiating treatment regimens (all p < 0.05). After treatment, HDAC4 was gradually elevated along with the time (p < 0.001). Interestingly, HDAC4 level at W12 (p = 0.041) and W24 (p = 0.012) was higher in response patients versus non‐response patients, and its level at W24 was higher in LDA patients versus non‐LDA patients (p = 0.019), and in remission patients versus non‐remission patients (p = 0.039).
Conclusion
HDAC4 gradually increases during treatment and its elevation estimates good treatment outcomes in RA patients.
Keywords: disease features, HDAC4, rheumatoid arthritis, treatment remission, treatment response
After the enrollment of 83 RA patients, their serum histone deacetylase 4 (HDAC4) level was detected by ELISA at baseline (W0), week (W) 4, W12, and W24 after treatment. Meanwhile, serum HDAC4 was detected by ELISA in 20 osteoarthritis patients and 20 healthy controls (HCs). HDAC4 level was reduced in RA patients compared with HCs (p < 0.001) and osteoarthritis patients (p = 0.009). In RA patients, HDAC4 was negatively related to C‐reactive protein (p = 0.003), tender joint count (p = 0.025), and disease activity score based on 28 joints (p = 0.013); it was also negatively correlated with TNF‐α (p = 0.003), IL‐6 (p = 0.022), and IL‐17A (p = 0.015). After treatment, HDAC4 was gradually elevated along with the time (p < 0.001). HDAC4 level at W12 (p = 0.041) and W24 (p = 0.012) was correlated with treatment response. Hence, HDAC4 gradually increases during treatment and its elevation estimates good treatment outcomes in RA patients.

1. INTRODUCTION
Rheumatoid arthritis (RA), one of the top prevalent autoimmune and inflammatory diseases, affects nearly 1% population, especially elder women over the world. 1 , 2 Aberrant autoantibody, elevated systemic inflammation, symmetrical polyarthritis, and bone erosion are the main manifestations of RA. 3 , 4 Apart from them, RA patients also suffer from other complications, including cardiovascular injury, interstitial lung disease, neurological abnormalities, etc., 5 , 6 , 7 which make RA treatment even more challenging. Although novel drugs and treatment strategies are developed, there exists a nonnegligible proportion of RA patients who fail to respond or easily flare. 8 , 9 Therefore, potential marker exploration for RA supervision and treatment outcome prediction is valid for personalized medicine. 10
Histone deacetylase 4 (HDAC4), an essential member of histone deacetylase (HDAC), is initially observed to modify chondrogenesis, osteoblast differentiation, neural survival, etc. 11 Recently, HDAC4 is also honored to regulate immunity and inflammation, so as to be closely involved in the pathogenesis of immune/inflammation‐related diseases. 12 , 13 , 14 , 15 In the aspect of RA, HDAC4 attenuates the growth and inflammation of RA fibroblast‐like synoviocytes (RA‐FLS) 16 ; it also exhibits a lower level in RA synovium and postpones RA progression and inflammation via AKT/mTOR pathway. 17 However, the value of its circulating level measurement in RA patients' supervision and treatment outcome prediction is not reported.
Hence, the current study aimed to assess the change in serum HDAC4 level during treatment and its relation to disease inflammation, activity, and clinical outcomes in RA patients.
2. METHODS
2.1. Subjects
From February 2019 to April 2021, 83 active RA patients were sequentially recruited in this prospective, observational study. The recruitment criteria for RA patients were as follows: (i) diagnosis of RA per European League Against Rheumatism classification criteria 18 ; (ii) older than 18 years old; (iii) disease activity score of 28 joint counts (erythrocyte sedimentation rate) (DAS28 score [ESR]) over 3.2; (iv) willing to comply with the study protocol and provide serum samples. The exclusion criteria were as follows: (i) presented as infections; (ii) complicated with hematological malignancy or cancer; (iii) during pregnancy or breastfeeding. Besides, 20 osteoarthritis (OA) patients were recruited as disease controls. The enrollment criteria for OA patients were as follows: (i) confirmed as OA by X‐ray examination; (ii) had matched age and gender to RA patients; (iii) voluntary for serum sample collection; (iv) without infections; (v) without malignant hematological diseases and solid tumors; (vi) non‐pregnancy and non‐lactating. Twenty healthy subjects were also included in the study as healthy controls (HCs). The inclusion criteria for HCs were as follows: (i) without any abnormalities in physical examinations; (ii) with matched age and gender to RA patients; (iii) willing to provide serum. The study was permitted by the Ethics Committee. Each subject signed the informed consent.
2.2. Data collection and sample examination
Clinical characteristics of RA patients were documented, including demographics, disease characteristics, and treatment history. Serum samples were obtained from RA patients before current therapy (W0, N = 83), 4 weeks (W4, n = 80), 12 weeks (W12, n = 75), and 24 weeks (W24, n = 66) after treatment initiation, as well as from OA patients (N = 20) and HCs (N = 20) after inclusion. The serum levels of HDAC4, tumor necrosis factor α (TNF‐α), interleukin (IL)‐6, and IL‐17A were analyzed by enzyme‐linked immunosorbent assay (ELISA) using commercial Human ELISA Kits per instructions. The kits used in the study were purchased from Shanghai Enzyme‐linked Biotechnology Co., Ltd. (for HDAC4), and Bio‐Techne China Co., Ltd. (for TNF‐α, IL‐6, and IL‐17A).
2.3. Treatment and assessment
Rheumatoid arthritis patients initiated treatment (combination of disease‐modifying antirheumatic drugs [DMARDs], or biologics with/without DMARDs) for 24 weeks based on actual disease status. During treatment, RA patients were closely followed up. Clinical response, clinical low disease activity (LDA), and clinical remission were evaluated at W4, W12, and W24 according to the RA clinical assessment criteria. 19 , 20 Clinical response was defined as a decline of DAS28 score (ESR) > 1.2; clinical LDA was defined as DAS28 score (ESR) ≤ 3.2; clinical remission was defined as DAS28 score (ESR) ≤ 2.6. The missing data were processed using the last observation carried forward (LOCF) mode for analysis.
2.4. Statistics
Clinical data were analyzed using SPSS V.24.0 (IBM Corp.). Graphs were made using GraphPad Prism V.6.01 (GraphPad Software Inc.). Kruskal–Wallis H rank‐sum test and Wilcoxon rank‐sum test were used for comparison analysis. A post hoc comparison was carried out using the Bonferroni test. Receiver operating characteristic (ROC) curves were presented to evaluate the ability of circ‐PVT1 in distinguishing different patients. Spearman's rank correlation test was used for association analysis. Friedman's test was used to examine the change of HDAC4 over time. p < 0.05 was considered significant.
3. RESULTS
3.1. RA patients' characteristics and treatment information
The age of enrolled RA patients was 56.7 ± 9.5 years, and there were 16.9% of males and 83.1% of females. They had a disease duration of 3.6 (interquartile range [IQR]: 1.6–5.9) years. Meanwhile, the ESR, CRP, and DAS28 score (ESR) was 34.6 (IQR: 22.6–45.4) mm/h, 21.2 (IQR: 12.5–41.4) mg/L, 5.1 ± 0.7, respectively (Table 1). In the aspect of treatment information, 94.4%, 84.3%, 86.7%, and 27.7% of patients had a history of NSAID, GC, DMARD, and biologics, respectively (Table 2). Besides, 72.3% of patients initiated DMARD combination treatment, whereas the other 27.7% of patients initiated biologics with/without DMARD treatment.
TABLE 1.
Clinical characteristics of RA patients
| Items | RA patients (N = 83) |
|---|---|
| Demographics | |
| Age (years), mean ± SD | 56.7 ± 9.5 |
| Gender, no. (%) | |
| Male | 14 (16.9) |
| Female | 69 (83.1) |
| BMI (kg/m2), mean ± SD | 22.8 ± 2.6 |
| Disease characteristics | |
| Disease duration (years), median (IQR) | 3.6 (1.6–5.9) |
| RF positive, no. (%) | |
| No | 15 (18.1) |
| Yes | 68 (81.9) |
| ACPA positive, no. (%) | |
| No | 25 (30.1) |
| Yes | 58 (69.9) |
| Tender joint count, median (IQR) | 7.0 (5.0–9.0) |
| Swollen joint count, median (IQR) | 6.0 (4.0–8.0) |
| ESR (mm/h), median (IQR) | 34.6 (22.6–45.4) |
| CRP (mg/L), median (IQR) | 21.2 (12.5–41.4) |
| DAS28 score (ESR), mean ± SD | 5.1 ± 0.7 |
| HAQ‐DI score, mean ± SD | 1.1 ± 0.3 |
Abbreviations: ACPA, anti‐cyclic citrullinated peptide antibody; BMI, body mass index; CRP, C‐reactive protein; DAS28, disease activity score of 28 joint counts; ESR, erythrocyte sedimentation rate; HAQ‐DI, health assessment questionnaire disability index; IQR, interquartile range; RA, rheumatoid arthritis; RF, rheumatoid factor; SD, standard deviation.
TABLE 2.
Treatment of RA patients
| Items | RA patients (N = 83) |
|---|---|
| Treatment history | |
| History of NSAID, no. (%) | |
| No | 5 (6.0) |
| Yes | 78 (94.0) |
| History of GC, no. (%) | |
| No | 13 (15.7) |
| Yes | 70 (84.3) |
| History of DMARDs, no. (%) | |
| No | 11 (13.3) |
| Yes | 72 (86.7) |
| History of biologics, no. (%) | |
| No | 60 (72.3) |
| Yes | 23 (27.7) |
| Current treatment regimen | |
| DMARD combination, No. (%) | |
| No | 23 (27.7) |
| Yes | 60 (72.3) |
| Biologics with/without DMARDs, No. (%) | |
| No | 60 (72.3) |
| Yes | 23 (27.7) |
Abbreviations: DMARDs, disease‐modifying antirheumatic drugs; GC, glucocorticoid; NSAID, non‐steroidal anti‐inflammatory drug; RA, rheumatoid arthritis.
3.2. Decreased HDAC4 level in RA patients
HDAC4 level was 26.7 (IQR: 18.2–47.2) pg/ml, 38.5 (IQR: 29.8–73.1) pg/ml, and 75.1 (IQR: 50.6–120.9) pg/ml in RA patients, OA patients, and HCs, respectively. Three‐group comparison analysis disclosed the lowest HDAC4 level in RA patients (p < 0.001) (Figure 1). Then, post hoc comparisons revealed that HDAC4 level was lower in RA patients compared to OA patients (p = 0.009) and HCs (p < 0.001). Besides, HDAC4 could well distinguish the RA patients from OA patients and HCs (Figure S1A,B).
FIGURE 1.
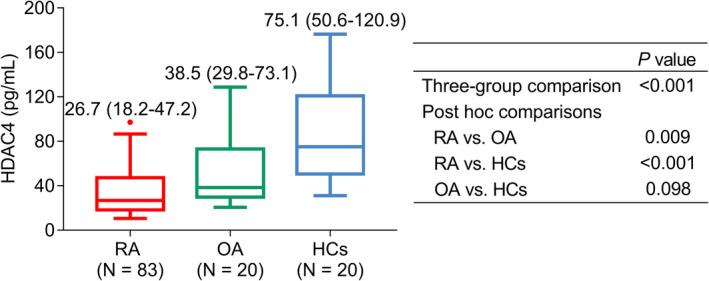
Comparison of HDAC4 level among RA patients, OA patients, and HCs
3.3. HDAC4 level related to inflammation and activity in RA patients
HDAC4 level negatively correlated with tender joint count (p = 0.025), CRP (p = 0.003), and DAS28 score (ESR) (p = 0.013) (Figure 2A,D,E), but it was not associated with swollen joint count (p = 0.376), ESR (p = 0.059), or HAQ‐DI score (p = 0.210) (Figure 2B,C,F) in RA patients. Besides, HDAC4 was negatively linked with TNF‐α (p = 0.003) (Figure 3A), IL‐6 (p = 0.022) (Figure 3B), and IL‐17A (p = 0.015) (Figure 3C).
FIGURE 2.
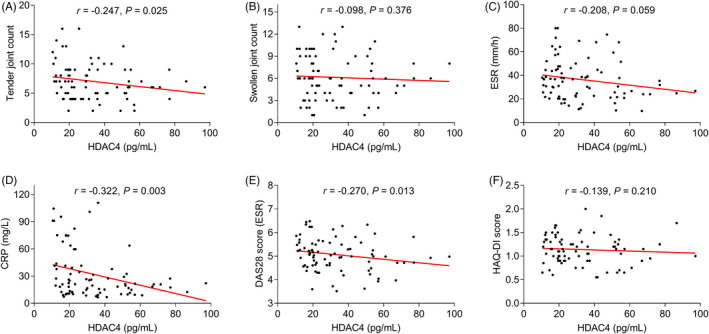
Correlation of HDAC4 level with disease activity indexes. Correlation of HDAC4 level with tender joint count (A), swollen joint count (B), ESR (C), CRP (D), DAS28 score (ESR) (E), and HAQ‐DI score (F) in RA patients
FIGURE 3.
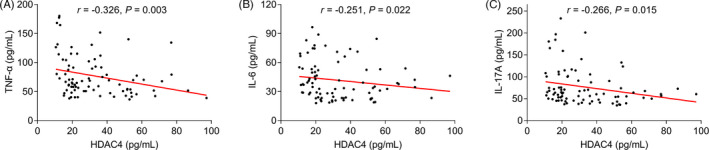
Correlation of HDAC4 level with inflammatory cytokines. Correlation of HDAC4 level with TNF‐α (A), IL‐6 (B), and IL‐17A (C) in RA patients
3.4. HDAC4 level was not related to treatment in RA patients
HDAC4 level was not linked with a history of NSAID (p = 0.503), history of GC (p = 0.880), history of DMARDs (p = 0.722), or history of biologics (p = 0.445) (Table 3). Meanwhile, the HDAC4 level was not related to the current DMARD combination treatment (p = 0.171) or current biologics with/without DMARD treatment (p = 0.171).
TABLE 3.
Correlation of HDAC4 with treatment in RA patients
| Characteristics | HDAC4 (pg/ml), median (IQR) | p Value |
|---|---|---|
| History of NSAID | ||
| No | 41.8 (21.8–49.5) | 0.503 |
| Yes | 24.5 (18.2–45.3) | |
| History of GC | ||
| No | 27.8 (18.2–44.5) | 0.880 |
| Yes | 26.2 (18.2–49.8) | |
| History of DMARDs | ||
| No | 27.8 (16.2–41.8) | 0.722 |
| Yes | 26.2 (18.2–50.4) | |
| History of biologics | ||
| No | 23.3 (18.1–41.6) | 0.445 |
| Yes | 29.5 (19.3–53.4) | |
| Current treatment‐DMARD combination | ||
| No | 21.4 (16.4–37.3) | 0.171 |
| Yes | 29.5 (19.4–51.0) | |
| Current treatment‐biologics with/without DMARDs | ||
| No | 29.5 (19.4–51.0) | 0.171 |
| Yes | 21.4 (16.4–37.3) | |
Abbreviations: DMARDs, disease‐modifying antirheumatic drugs; GC, glucocorticoid; HDAC4, histone deacetylase 4; IQR, interquartile range; NSAID, non‐steroidal anti‐inflammatory drug; RA, rheumatoid arthritis.
3.5. HDAC4 increased during treatment and related to treatment outcomes in RA patients
HDAC4 level was 24.5 (IQR: 18.2–48.1) pg/ml, 31.1 (IQR: 19.0–53.8) pg/ml, 35.8 (IQR: 25.4–63.8), and 43.6 (IQR: 29.9–81.5) pg/ml at W0 (baseline), W4, W12, and W24 in RA patients, respectively, which showed an increased tread during treatment (p < 0.001) (Figure 4).
FIGURE 4.
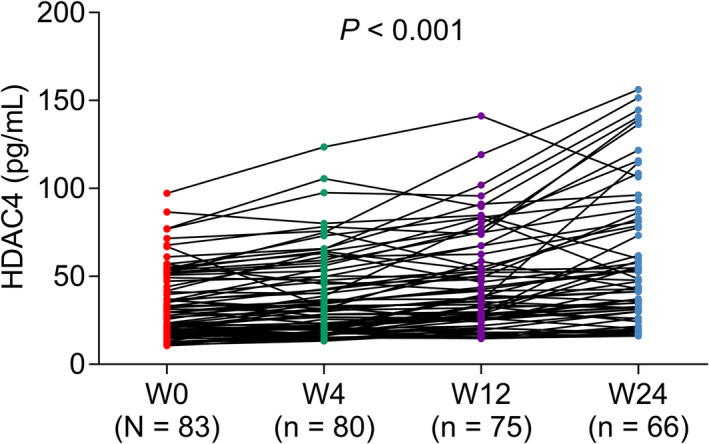
Change of HDAC4 level during treatment
After treatment, clinical response was 15.7%, 38.6%, and 53.0% at W4, W12, and W24, respectively; Meanwhile, LDA was 9.6%, 28.9%, and 34.9% at W4, W12, and W24, respectively (Table 4). Besides, clinical remission was 2.4%, 12.0%, and 26.5% at W4, W12, and W24, respectively.
TABLE 4.
Rates of clinical response, LDA, and remission in RA patients
| Rates | W4 | W12 | W24 |
|---|---|---|---|
| Clinical response, % | 15.7 | 38.6 | 53.0 |
| Clinical LDA, % | 9.6 | 28.9 | 34.9 |
| Clinical remission, % | 2.4 | 12.0 | 26.5 |
Abbreviations: LDA, low disease activity; RA, rheumatoid arthritis; W12, at 12 weeks after treatment initiation; W24, at 24 weeks after treatment initiation; W4, at 4 weeks after treatment initiation.
It was inspiringly discovered that HDAC4 level at W0 (p = 0.655) and W4 (p = 0.670) was similar, but its level at W12 (p = 0.041) and W24 (p = 0.012) was increased in patients with clinical response compared to those without clinical response (Figure 5A). Meanwhile, the HDAC4 level at W24 was also higher in patients with LDA compared to those without LDA (p = 0.019) (Figure 5B) and was higher in patients with clinical remission compared to those without clinical remission (p = 0.039) (Figure 5C). No matter whether patients with or without treatment history, their HDAC4 all showed an increasing trend during the current treatment (p < 0.05) (Figure S2A,B). Besides, no matter whether patients with or without current biologic treatment, their HDAC4 all showed an increasing trend during the current treatment (p < 0.001) (Figure S3A,B).
FIGURE 5.
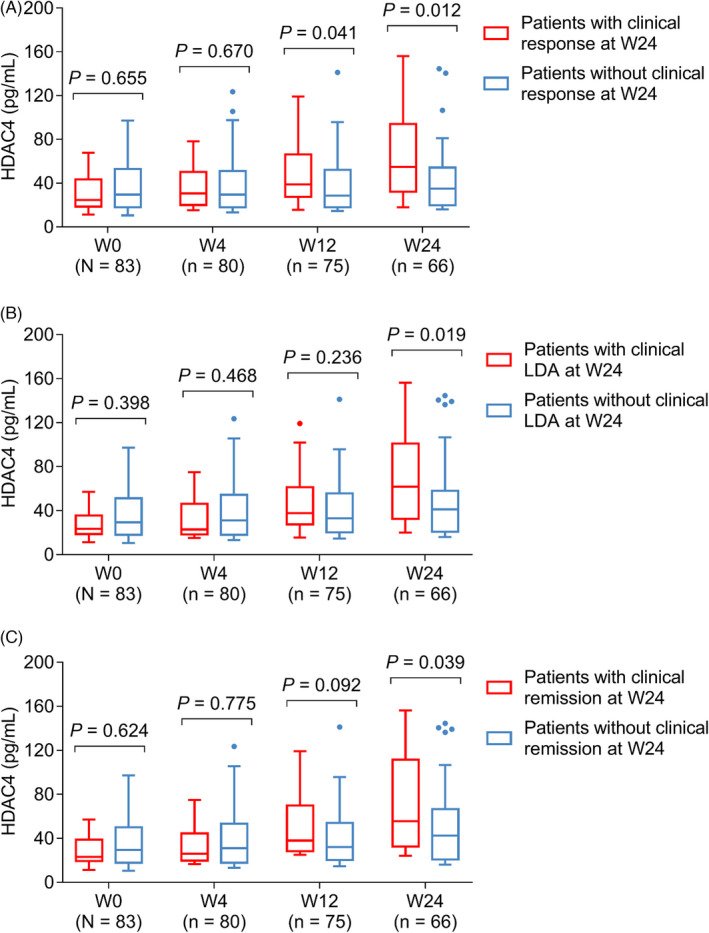
Relation of HDAC4 level during treatment with clinical outcomes. Comparison of HDAC4 levels at W0, W4, W12, and W24 between response patients versus non‐response patients (A), between LDA patients versus non‐LDA patients (B), and between remission patients versus non‐remission patients (C)
4. DISCUSSION
Previously, the clinical role of HDAC4 has been widely determined in patients with neurodegenerative diseases (including Huntington's disease and amyotrophic lateral sclerosis). 21 , 22 , 23 Recently, with a deep understanding of HDAC4 in regulating inflammation, its clinical value in autoimmune diseases has been preliminary explored. 24 , 25 , 26 For instance, one study shows that HDAC4 is dysregulated in Graves' ophthalmopathy patients compared with the healthy population. 24 Another study discloses that the HDAC4 locates in the differentially methylated regions (vs. health population) of progressive multiple sclerosis patients. 25 In this study, it was shown that the HDAC4 was downregulated in RA patients compared with HCs which was partially in line with a previous study. 27 This phenomenon could be explained as follows: HDAC4 acted as an anti‐inflammatory role in the inflammatory process by inhibiting the NF‐κB activation, while the inflammation was aggravated in RA patients, therefore, the HDAC4 was downregulated in RA patients. 28 , 29 Apart from that whether the HDAC dysregulation causes the occurrence of RA or RA leading to the dysregulation of HDAC4 is not clear now. Therefore, a further study should be carried out to explore this issue.
In recent decades, the HDAC4 is participated in regulating the inflammatory process. For instance, the inhibition of HDAC4 in rheumatoid fibroblast‐like synoviocytes could promote inflammation. 27 Another study exhibits that the silence of HDAC4 in macrophages could induce hepatic and adipose tissue inflammation in diet‐induced non‐alcoholic steatohepatitis. 30 However, the correlation of HDAC4 with inflammation in RA patients has never been discovered. In the present study, it was revealed that HDAC4 was negatively correlated with inflammatory status (indicated by the CRP, TNF‐α, IL‐6, and IL‐17A), which could be explained as: (i) HDAC4 inhibited the inflammation via various signaling pathways and biological processes, such as NF‐κB pathway and autophagy, therefore, HDAC4 was negatively related to the inflammatory status 28 , 31 ; (ii) The downregulation of HDAC4 promoted the T helper 17 response, which further increased the IL‐17 level, therefore, HDAC4 was negatively correlated with IL‐17A. 32 Apart from the findings mentioned above, the negative correlation between HDAC4 with disease severity (reflected by the DAS28 score and tender joint counts) was also observed in this study, which might be explained that the HDAC4 was negatively associated with the inflammatory status, which was involved in the evaluation of DAS28 score (the higher inflammatory status indicated the higher DAS28 score), therefore, HDAC4 was negatively related to the disease severity.
Another interesting finding in this study was that the HDAC4 was increased in RA patients after treatment, besides, higher HDAC4 at W12 and W24 was correlated with the treatment response (at W24). These phenomena could be explained as follows: (i) As mentioned above, HDAC4 negatively correlated with the inflammatory status, and the inflammation of RA patients would be relieved after the treatment, therefore, the HDAC4 was increased in RA patients after the treatment 28 , 31 ; (ii) The inflammatory status of RA patients with a higher HDAC4 level after treatment was better relieved compared with those with a lower HDAC4 level, which meant that those RA patients with a higher HDAC4 level after treatment were easier to achieve the treatment response. Therefore, higher HDAC4 after treatment was associated with the treatment response.
Despite the innovation of this study, some limitations were still non‐neglectable: (i) The methylation level of HDAC4 was also dysregulated in the inflammation‐related diseases, which was not detected in this study, therefore, a further study was needed; (ii) The small sample size was the main limitation; (iii) Clinical role of HDAC4 in other autoimmune diseases, such as ankylosing spondylitis and psoriasis could also determine; (iv) The detailed mechanism of HDAC4 in regulating the pathogenesis of RA could be explored in the further study.
In conclusion, HDAC4 gradually increases during treatment and its elevation estimates good treatment outcomes in RA patients.
CONFLICT OF INTEREST
None.
CONSENT TO PARTICIPATE
Each subject signed informed consent.
Supporting information
Figure S1
Figure S2
Figure S3
Mou X, Jin Y, Jin D, Guan J, Zhang Q. Serum HDAC4 level in rheumatoid arthritis: Longitudinal change during treatment and correlation with clinical outcomes. J Clin Lab Anal. 2022;36:e24594. doi: 10.1002/jcla.24594
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Daikh DI. Rheumatoid arthritis: evolving recognition of a common disease. Best Pract Res Clin Rheumatol. 2022;36(1):101740. [DOI] [PubMed] [Google Scholar]
- 2. van der Woude D, van der Helm‐van Mil AHM. Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2018;32(2):174‐187. [DOI] [PubMed] [Google Scholar]
- 3. Cush JJ. Rheumatoid arthritis: early diagnosis and treatment. Rheum Dis Clin North Am. 2022;48(2):537‐547. [DOI] [PubMed] [Google Scholar]
- 4. Deane KD, Holers VM. Rheumatoid arthritis pathogenesis, prediction, and prevention: an emerging paradigm shift. Arthritis Rheumatol. 2021;73(2):181‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park E, Griffin J, Bathon JM. Myocardial dysfunction and heart failure in rheumatoid arthritis. Arthritis Rheumatol. 2022;74(2):184‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Akiyama M, Kaneko Y. Pathogenesis, clinical features, and treatment strategy for rheumatoid arthritis‐associated interstitial lung disease. Autoimmun Rev. 2022;21(5):103056. [DOI] [PubMed] [Google Scholar]
- 7. Kim JW, Suh CH. Systemic manifestations and complications in patients with rheumatoid arthritis. J Clin Med. 2020;9(6):2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2021;73(7):924‐939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Han J, Geng Y, Deng X, Zhang Z. Risk factors of flare in rheumatoid arthritis patients with both clinical and ultrasonographic remission: a retrospective study from China. Clin Rheumatol. 2017;36(8):1721‐1727. [DOI] [PubMed] [Google Scholar]
- 10. Heutz J, de Jong PHP. Possibilities for personalised medicine in rheumatoid arthritis: hype or hope. RMD Open. 2021;7(3):e001653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Z, Qin G, Zhao TC. HDAC4: mechanism of regulation and biological functions. Epigenomics. 2014;6(1):139‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pei XF, Cao LL, Huang F, et al. Role of miR‐22 in intestinal mucosa tissues and peripheral blood CD4+ T cells of inflammatory bowel disease. Pathol Res Pract. 2018;214(8):1095‐1104. [DOI] [PubMed] [Google Scholar]
- 13. Yang Q, Tang J, Pei R, et al. Host HDAC4 regulates the antiviral response by inhibiting the phosphorylation of IRF3. J Mol Cell Biol. 2019;11(2):158‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu M, Huang Z, Huang W, et al. microRNA‐124‐3p attenuates myocardial injury in sepsis via modulating SP1/HDAC4/HIF‐1alpha axis. Cell Death Discov. 2022;8(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kang H, Park YK, Lee JY. Inhibition of alcohol‐induced inflammation and oxidative stress by astaxanthin is mediated by its opposite actions in the regulation of sirtuin 1 and histone deacetylase 4 in macrophages. Biochim Biophys Acta Mol Cell Biol Lipids. 2021;1866(1):158838. [DOI] [PubMed] [Google Scholar]
- 16. Hao J, Chen Y, Yu Y. Circular RNA circ_0008360 inhibits the proliferation, migration, and inflammation and promotes apoptosis of fibroblast‐like synoviocytes by regulating miR‐135b‐5p/HDAC4 axis in rheumatoid arthritis. Inflammation. 2022;45(1):196‐211. [DOI] [PubMed] [Google Scholar]
- 17. Peng T, Ji D, Jiang Y. Long non‐coding RNA GAS5 suppresses rheumatoid arthritis progression via miR‐128‐3p/HDAC4 axis. Mol Cell Biochem. 2021;476(6):2491‐2501. [DOI] [PubMed] [Google Scholar]
- 18. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569‐2581. [DOI] [PubMed] [Google Scholar]
- 19. van Gestel AM, Prevoo ML, Van't Hof MA, Van Rijswijk MH, van De Putte LB, Van Riel PL. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum. 1996;39(1):34‐40. [DOI] [PubMed] [Google Scholar]
- 20. Fleischmann R, Pangan AL, Song IH, et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double‐blind, randomized controlled trial. Arthritis Rheumatol. 2019;71(11):1788‐1800. [DOI] [PubMed] [Google Scholar]
- 21. Quinti L, Chopra V, Rotili D, et al. Evaluation of histone deacetylases as drug targets in Huntington's disease models. Study of HDACs in brain tissues from R6/2 and CAG140 knock‐in HD mouse models and human patients and in a neuronal HD cell model. PLoS Curr. 2010;2:RRN1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arosio A, Sala G, Rodriguez‐Menendez V, et al. MEF2D and MEF2C pathways disruption in sporadic and familial ALS patients. Mol Cell Neurosci. 2016;74:10‐17. [DOI] [PubMed] [Google Scholar]
- 23. Pegoraro V, Marozzo R, Angelini C. MicroRNAs and HDAC4 protein expression in the skeletal muscle of ALS patients. Clin Neuropathol. 2020;39(3):105‐114. [DOI] [PubMed] [Google Scholar]
- 24. Ekronarongchai S, Palaga T, Saonanon P, et al. Histone deacetylase 4 controls extracellular matrix production in orbital fibroblasts from Graves' ophthalmopathy patients. Thyroid. 2021;31(10):1566‐1576. [DOI] [PubMed] [Google Scholar]
- 25. Maltby VE, Lea RA, Burnard S, et al. Epigenetic differences at the HTR2A locus in progressive multiple sclerosis patients. Sci Rep. 2020;10(1):22217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo S, Zhu Q, Jiang T, et al. Genome‐wide DNA methylation patterns in CD4+ T cells from Chinese Han patients with rheumatoid arthritis. Mod Rheumatol. 2017;27(3):441‐447. [DOI] [PubMed] [Google Scholar]
- 27. Chang L, Kan L. Mesenchymal stem cell‐originated exosomal circular RNA circFBXW7 attenuates cell proliferation, migration and inflammation of fibroblast‐like synoviocytes by targeting miR‐216a‐3p/HDAC4 in rheumatoid arthritis. J Inflamm Res. 2021;14:6157‐6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang Q, Tang J, Xu C, et al. Histone deacetylase 4 inhibits NF‐kappaB activation by facilitating IkappaBalpha sumoylation. J Mol Cell Biol. 2020;12(12):933‐945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu Z, Chen D. MiR‐129‐5p inactivates NF‐kappaB pathway to block rheumatoid arthritis development via targeting BRD4. J Healthc Eng. 2022;2022:8330659. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30. Kang H, Lee Y, Kim MB, et al. The loss of histone deacetylase 4 in macrophages exacerbates hepatic and adipose tissue inflammation in male but not in female mice with diet‐induced non‐alcoholic steatohepatitis. J Pathol. 2021;255(3):319‐329. [DOI] [PubMed] [Google Scholar]
- 31. Yang D, Xiao C, Long F, et al. HDAC4 regulates vascular inflammation via activation of autophagy. Cardiovasc Res. 2018;114(7):1016‐1028. [DOI] [PubMed] [Google Scholar]
- 32. Lu W, You R, Yuan X, et al. The microRNA miR‐22 inhibits the histone deacetylase HDAC4 to promote T(H)17 cell‐dependent emphysema. Nat Immunol. 2015;16(11):1185‐1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Figure S3
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


