Abstract
Background
Pentavalent antimonials (Sb(V)) such as meglumine antimoniate (Glucantime®) and sodium stibogluconate (Pentostam®) are used as first‐line treatments for leishmaniasis, either alone or in combination with second‐line drugs such as amphotericin B (Amp B), miltefosine (MIL), methotrexate (MTX), or cryotherapy. Therapeutic aspects of these drugs are now challenged because of clinical resistance worldwide.
Methods
We reviewedthe recent original studies were assessed by searching in electronic databases such as Scopus, Pubmed, Embase, and Web of Science.
Results
Studies on molecular biomarkers involved in drug resistance are essential for monitoring the disease. We reviewed genes and mechanisms of resistance to leishmaniasis, and the geographical distribution of these biomarkers in each country has also been thoroughly investigated.
Conclusion
Due to the emergence of resistant genes mainly in anthroponotic Leishmania species such as L. donovani and L. tropica, as the causative agents of ACL and AVL, respectively, selection of an appropriate treatment modality is essential. Physicians should be aware of the presence of such resistance for the selection of proper treatment modalities in endemic countries.
Keywords: drug resistance, gene markers, global distribution, leishmaniasis
Geographical distribution of resistant biomarkers gene based on studies carried out on clinical‐resistant isolate.
1. INTRODUCTION
The protozoan parasites belonging to the genus Leishmania are pathogenic agents of a complex and non‐contagious disease, leishmaniasis. 1 , 2 Different clinical manifestations are present for this tropical disease ranging from benign self‐healing cutaneous (CL) and mucocutaneous (MCL) to a deadly visceral (VL) leishmaniasis. 3 The major species to cause CL in the Old World consist of Leishmania major (L. major) and Leishmania tropica (L. tropica). 4 Over 70% of the global CL cases occur in Algeria, Afghanistan, Colombia, Iran, Syria, Ethiopia, North Sudan, Costa Rica, Brazil, and Peru. 5 Chemotherapy is a crucial measure to control leishmaniasis. 3 Current treatments are based on pentavalent antimonials (SbV) such as meglumine antimoniate (Glucantime®) and sodium stibogluconate (Pentostam®) as the first‐line drugs alone or combined with second‐choice drugs including amphotericin B (Amp B), miltefosine (MIL), methotrexate (MTX), or cryotherapy. However, the toxic adverse effects of these drugs and difficulty with distribution make these options less than ideal. Unfortunately, therapeutic aspects of these drugs are now challenged because of clinical resistance in many parts of the world. Resistance to these drugs has become a serious problem in the treatment of leishmaniasis in some endemic areas.
Studies on molecular biomarkers involved in drug resistance are essential for monitoring the disease. 6 This phenomenon is probably an interaction between efflux, uptake, sequestration, mutation, or downregulation of an uptake system controlled by Leishmania genes. 7 , 8 The response rate to anti‐Leishmania drugs varies between species and strains of Leishmania. However, this function's molecular and biochemical mechanisms are unknown. 9 The drug resistance mechanisms have often been studied in laboratory‐generated strains or field‐resistant strains obtained from patients in endemic regions, suggesting the involvement of different pathways. Due to the increasing rate of drug‐resistant leishmaniasis cases to control the disease globally, identifying genes in each species and country is highly vital. This article aimed to review genes and mechanisms underlying resistance to leishmaniasis. All studies conducted so far have been considered in this review. Also, the spatial distribution of these biomarkers in each country has thoroughly been investigated.
2. DRUG RESISTANCE GENE MARKERS
2.1. Aquaglyceroporin (AQP1)
AQP1 are channel proteins that pass through the water, glycerol, and other uncharged molecules such as Sb (III) across the membranes (Figure 1). AQP1 helps the cell afford the osmotic pressure. 9 Sb (V) is a prodrug that is reduced within the human and parasite into the toxic trivalent form (Sb (III)). 10 Sb (III) enters cells by AQP1 that is energy‐independent. 11 In in vitro studies, downregulation of AQP1 and high levels of trypanothione (T[SH]2) 12 have been evidenced. 13 Some studies propose that deletion of the AQP1 allele demonstrated to cause an increase in resistance to Sb (V) may be a mechanism resulting in downregulation of an uptake system. 13 Recent studies have proposed that the neutral Sb (OH)3 species serve as the substrate for AQP1 and transport within the parasite cell. The differential concentrations of Sb (V) and Sb (III) in Leishmania are evidence that Sb (V) uses a different way of entry. 11
FIGURE 1.
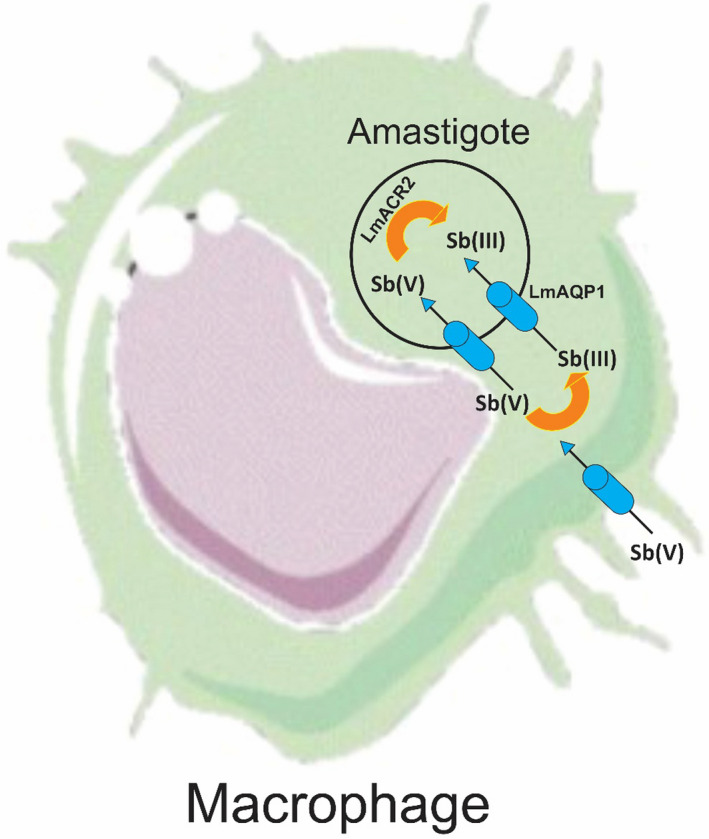
Schematic models of drug delivery in antimony resistant and sensitive Leishmania. AQP1, aquaglyceroporin 1; MRPA, multidrug resistance‐associated protein A; EP, efflux pump; EC, the extracellular concentration of Sb; IC, the intracellular concentration of Sb; VC, the vacuolar concentration of Sb; T, rate constant for passive transport; Tma, transport rate constant by MRPA or ATe; Te, rate constant of active efflux; Ts, rate constant for secretion
2.2. ATP‐binding cassette (ABC) transporters
Sb(III) conjugate with (T[SH]2) or glutathione(GSH), and this complex has packaged within vesicles or exited from the parasite by ABC transporters. 14 LABCI4 belongs to the ABCI subfamily, which increases the efflux of thiols and Sb(III), thereby producing resistance to antimonials in L. major. This transporter is in both the plasma membrane and mitochondria in Leishmania. LABCI4 is a pump capable of distinguishing thiol‐conjugated metals. 15 The ABCC3 transporter localized in vesicular membranes near the flagellar pocket was known in trivalent arsenate (As (III)) and Sb (III) Leishmania‐resistant isolates, and studies were proved that they offer the capability to transport thiol‐conjugated metals. It has also been demonstrated that the MRPA‐enriched vesicles possibly cooperate in a secretion pathway that reduces antimony concentration. It is also significant that either increased efflux or decreased influx of Sb (III) has been studied in Leishmania‐resistant mutants overexpressing MRPA. 16 There was also no link between MRPA expression in the parasite and the degree of antimony intracellular concentration. 16 The other studies have shown that the overexpression of Pgp‐like and MRP1‐like proteins was illustrated in both of the antimony‐resistant isolates of L. donovani, and overexpression was illustrated in both Sb (V)‐resistant isolates of L. donovani and the plasma membrane of macrophages (MQ). This parasite effluxes the drug, reducing concentration Sb (III) in intracellular and parasite survival. On the other hand, efflux pump overexpression was not shown in antimony‐sensitive Leishmania infected MQ. 17 This document proves the vesicle‐mediated cross‐talking between Leishmania and host cells. 18
ABCG transporters even have been related to drug resistance. 19 LiABCG6 is located at the Leishmania plasma membrane. This half‐transporter confers resistance to the sitamaquine and miltefosine when overexpressed by reducing intracellular drug concentration and short‐chain fluorescent phospholipid analogs of phosphatidylethanolamine, phosphatidylserine, and phosphatidylcholine. As a whole, these results indicated that LiABCG6 could be implicated in drug resistance and phospholipid trafficking. 19 LABCG4 transporter has been preoccupied with phosphatidylcholine transport and confers resistance to MIL. 20 LABCG2 is in vesicles that connect with the plasma membrane throughout exocytosis. Overexpressing the LABCG2 transporter gene in resistant isolates showed a decreased Sb (III) concentration due to increasing drug existence. Also, LABCG2 was capable of exporting thiols with Sb (III).
Leishmania LABCG2 transporter creates resistance to antimony drugs by exocytosis through flagellar pocket and packaging metal‐thiol in vesicles. 21 When LABCI4 was overexpressed in L. major confer resistance to antimonial drugs, As(III), and metal ions Cd (II). 15 LABCI4 is localized in both plasma membranes and mitochondria of the Leishmania and forms dimers to efflux the thiol‐conjugated metals through a thiol‐X‐pump. 15 MIL is used to treat these diseases effluxes through ABC transporter and P4 ATPase. P4 ATPase by the cdc50 protein transfers MIL from the outer to the inner cell and extrudes from the parasite by the ABC transporter protein, the energy‐dependent mechanism. The principal genes in the Leishmania amplify the portions of a gene that encodes P4 ATPase and ABC transporter and participate in resistance to MIL. 22 pentamidine resistance protein 1 (PRP1) is another ABC transporter that is produced resistance to pentamidine (PTD) in L. infantum. 23
P‐glycoproteins (Pgps) are also the ABC transporters. 24 They extrude drugs from the parasites 25 and tumor cells, 26 thus offering a multidrug‐resistant (MDR). Pgps contain two domains, the transmembrane domain (TMD) participated in medicine efflux, and a cytosolic nucleotide‐binding domain (NBD) involved in hydrolysis and ATP binding. 27 Some sesquiterpenes and flavonoids are effective against Leishmania MDR phenotype. 28 The flavonoids join the NBD, interact with the TMD, 28 and reverse the L. tropica resistance phenotype. 29 Also, some sesquiterpenes efficiently defeat the Leishmania MDR phenotype by increasing drug accumulation. 30 Overexpression of the LtrMDR1 leads to the weakness in drug internalization and production of the resistance to MIL in Leishmania. 30 The data showed that L. donovani mitogen‐activated protein kinase 1 (LdMAPK1) regulates the expression of the Pgps reversely. The reduced activity in the Pgps pump with an increase in Ld‐MAPK1 expression may cause an increased concentration of antimony in the Leishmania, producing it more sensitive to this drug. 31 Overexpression of PgpA has been studied in resistant isolates of L. infantum. The transfection of this gene demonstrates antimony resistance upon amastigotes and promastigotes of L. infantum. 32 The recent data have shown that the expression level of the PgpA gene in resistant L. major strains was 5‐fold higher than in sensitive strains. Therefore, overexpression of this gene can create resistance isolates. 33
2.3. Protein 14‐3‐3
This protein is in all eukaryotes, from mammals to plants, and more than 100 binding partners have been known so far. The targets of protein 14‐3‐3 are in all subcellular sections, and their functions are varied. They include biosynthetic enzymes, transcription factors, cytoskeletal proteins, apoptosis, signaling molecules, and tumor suppressors. 34 Protein 14‐3‐3 is capable of joining phosphorylated proteins participating in the apoptosis pathway. This protein is overexpressed in resistance Leishmania isolates. 35
2.4. Protein 299 (P299)
This gene encodes a 299 kDa polypeptide that displays no similarities to other proteins or functional motifs. Recent experiments propose that in L. infantum this gene is part of a 44 kbp duplicated loci on CHR29 and CHR08 chromosomes. Overexpression of this gene in L. infantum confers protection against Sb(III) but also against miltefosine. 36
2.5. Histone
Histones exist in nuclei in eukaryote cells which are alkaline proteins that pack the DNA into structural units named nucleosomes. They are the major protein of chromatin, acting as gene expression regulation. 37 Various histone genes from kinetoplastids have been identified. The sequences of the genes coding for histoneH1, H2A, H2B, H3, and H4 have been characterized in Leishmania species. 38 In resistant isolates of L. donovani, H1, H2A, and H4 were overexpressed, stating they play a role in drug resistance. 39 Overexpression of H4 was shown in L. major and L. infantum resistance antimony. 35
2.6. Leishmania‐activated C kinase gene (LACK1)
This protein is very stable in Leishmania species and expressed in amastigote and promastigote forms. 40 These proteins took part in RNA processing, signal transduction (ST), and cell cycle regulation. 41 Recently, it has been studied that it locates in the cytosol, and the temperature variation between the insect and the mammalian host persuades it to secretion. It joins and enhancement plasminogen activation in in vivo and participates in the invasiveness of Leishmania. 42 The LACK is the T‐cell epitope and induces the immune response and production of T‐helper 1 cell; therefore, several studies have demonstrated that the LACK gene is the target for the candidate vaccine. 43 , 44 LACK is essential for the infectivity and viability of Leishmania in the MQ. 41 LACK is required to develop an incision in BALB/c mice. 45 According to the different expressions of this antigen in sensitive and resistant isolates, this gene is the primary biomarker contributing to drug resistance. 6
2.7. Ubiquitin
This protein is the heat shock protein with critical roles in cellular functions such as endocytosis, degradation of defective proteins, apoptosis, and DNA repair. 46 One of its critical roles in protein decomposition by the ubiquitin‐proteasome pathway, which is protecting cells from abnormal proteins. 47 Ubiquitin through the ubiquitin‐proteasome pathway, ubiquitin binds to lysine residues of the target proteins, resulting in the decomposition of the ubiquitin‐tagged protein via the 26S proteasome. 48 Overexpression of this gene in L. tropica resistant clinical isolate could decompose oxidized proteins and protect Leishmania from oxidative stress related to drugs. 49
2.8. Amino acid permease (AAP3)
Various amino acid permease has been studied in kinetoplastids. 50 It is an arginine transporter that locates in the surface membrane of the parasite. 51 Arginine is the starter of polyamine biosynthesis 52 that is transported within Leishmania by AAP3. 51 Ornithine results from the breakdown of arginine by the arginase enzyme that takes part in the synthesis of T(SH)2 and polyamine. 52 The T(SH)2 is a mainly reduced thiol of Leishmania species and had a significant role in detoxifying antimonial components. 12 Additionally, the increased T(SH)2 in antimony resistance Leishmania isolates has been studied. 53 It was observed that high expression of the AAP3 gene in clinical antimony‐resistant isolates of L. tropica contributes to increasing the T(SH)2 and, as a result, detoxification of antimonial drugs. 49
2.9. Phosphoglycerate kinase (PGK)
Leishmania has two PGK genes: PGKB and PGKC. PGKB code the cytosolic, and PGKC codes the glycosomal isoforms of the enzyme. 54 In amastigote and promastigote stages, PGKB and PGKC transcripts and proteins are expressed at a ratio of 4:1. 55 PGK is the key enzyme of the glycolysis pathway and plays a role in ATP production. 56 Increasing glycolysis enzymes in the antimony‐resistant Leishmania isolates proposed requiring more energy to protect from oxidative stress. Also, overexpression of PGK increases the pyruvate that extrudes peroxides and participates in decreasing oxidative stress. 57
2.10. Mitogen‐activated protein kinase (MAPK)
MAPKs are major regulators of ST that act in parasite virulence via intracellular proliferation, stress response, 58 flagellar morphogenesis, and apoptosis. 59 Recent studies have evidenced that Sb(III) stimulates apoptosis by inducing the MAPK signaling cascade and activation of oxygen production. 60 It is overexpression in the sensitive clinical isolates and downregulated in L. donovani antimony‐resistant isolates and proposes that MAPK1 depends on the cell death pathway, which stimulates the cell death pathway and antimonial drugs. 61 Also, compared with sensitive L. tropica isolates, all transcription of this gene was reduced in clinical resistant isolates. 54
2.11. Protein tyrosine phosphatase (PTP)
PTP is the regulator of post‐translational participation in important functions in cells, such as cell death. PTPs were classified into three groups in kinetoplastids; (1) classical PTP, (2) cell division cycle 25 phosphatase, and (3) low molecular weight phosphatase. 62 PTPs have a major function in amastigote survival and virulence in the human host. 63 It has been shown that the function of the PTPs stops by the Sb (v). This inhibition is associated with activation of the MAPK pathway eventuated in apoptosis. Also, this enzyme as a virulence factor could enhance Leishmania survival in humans. 63 It was demonstrated that in L. tropica resistant clinical isolate, upregulation of this enzyme participates with downregulation of MAPK, suggesting that overexpression of PTP induces apoptosis in resistance isolates. 54
2.12. Pteridine reductase 1 (PTR1)
PTR1 is an NADPH‐dependent reductase that contributes to the salvage of pteridines that are necessary to develop the growth of Leishmania. 64 PTR1 catalyzes the reduction in biopterin and folate into their active forms, tetrahydrobiopterin, and tetrahydrofolate, respectively, which act as co‐factors. 65 , 66 Decreased pteridines in parasites lead to reduced intracellular survival. 67 Another study with L. major lines demonstrated that this enzyme participates in resistance parasites against MQ oxidative stress. 64 Also, as Leishmania is auxotrophic for pteridines, a disordering of their salvage pathway is a therapeutic strategy. The mechanisms of resistance to antimonial drugs in L. braziliensis and methotrexate in L. major and L. infantum have been studied. 68
2.13. Tryparedoxin peroxidase (TXNPx)
TXN belongs to the thioredoxin oxidoreductase superfamily and has a WCPPC motif neighbor the catalytic pocket. 69 TXNI and TXNII are two isoforms of the TXN, where TXNI is localized in the cytosol, and TXNII is localized in mitochondria. They both have a central core of 5 stranded b sheets restricted by 4 a‐helices. In mammals, it performs an equal act to glutathione peroxidase. It is a member of the 2‐cysteine peroxiredoxin family, and various isoforms of TXNPx have been studied, located in the mitochondria and cytosol. 70 A major role of the cytosolic TXNPx (cTXNPx) in Leishmania is decreasing the balance of cytosolic tryparedoxin (cTXN) made from trypanothione, unlike the other eukaryotes that apply GSH. 71 TXN and TXNPx are conserved in Leishmania species. 72 Their roles are defensive against oxidative stress, chemical reduction in organic hydroperoxides (ROOH), and hydrogen peroxide (H2O2) into alcohol and water, respectively. They also have a critical role in DNA replication, DNA biosynthesis, and ROS regulation. Mitochondrial isoform of TXN displacements electrons to the universal minicircle sequence binding protein (UMSBP), transcription factor, and a monothiol glutaredoxin by peroxidase. TXN‐TXNPx pair led to a redox state for the UMSBP and contributed to the starting of replication of kDNA. 73 , 74 TXN knockout studies in L. infantum 75 showed the necessity of this gene in these parasites' antioxidant metabolism and survival. In L. donovani it is identified that cTXN protein cooperates with cTXNPx to catalyze the reduction in ROOH or H2O2 into alcohol or water, respectively, implying its critical role under oxidative stress situations. Also, in Amp B resistant clinical isolates of L. donovani the cytosolic tryparedoxin level was upregulated demonstrating its role in drug resistance. 76
2.14. Kinetoplastid membrane (KMP11)
KMP‐11 is localized in Subcellular in Leishmania has proposed that may be is localized to the flagella and flagellar pocket. 77 , 78 It was associated with the basal flagellar body, which acts in cytokinesis. 79 , 80 It is amphipathic, represents membrane‐active properties, and increased lipid bilayer pressure. 81 , 82 Decreasing in KMP‐11 expression changes the activity of the transporter, such as the AQP1 8 or with putative efflux systems 83 with increased function for pumping Sb(III) out of the Leishmania species. In various independent studies, in isolation of Sb (III) resistant L. infantum cell line, it is demonstrated that the reduction in this protein but the mRNA levels have not changed. These data propose that in this resistant isolate the stability of it may be agreed to result in an enhanced turnover rate of KMP 11. Change in the post‐translational modifications of this protein in resistant isolates may speed up the degradation of this protein. Also, other studies have shown N‐terminal acetylation 84 and arginine methylation 82 of KMP‐11 that have been signified in regulating protein stability. 85 Proteomic screen data have demonstrated downregulation of its expression in Sb (III) resistant isolates in the amastigote stage. These data have marked a differentially expressed of this protein in the resistant isolate. The expression of the KMP‐11 was reduced in the drug‐resistant mutant. 86
2.15. Gamma glutamylcysteine synthase (GSH1)
Sensitivity in Leishmania to Sb (V) varies according to intrinsic cellular metabolism, intracellular thiol levels, or membrane compounds. Thiols are decreasing factors in the conversion of Sb (V) to Sb (III), which was occurring in the presence of thiols. 87 The Sb (III) mechanisms associated with its affinity toward biomolecule consisting of sulfhydryl, including proteins, enzymes, and thiols. Sb (III) conjugate with the intracellular GSH from 1:3 and trypanothione from 1:1 and formed Sb‐thiol species. 88 Other proteins such as thiols, TryR, and zinc‐finger protein are molecular targets of Sb (III). These molecules bind to the Sb (III) by Cys. Sb (III) disturbs the thiol metabolism by preventing TryR and stimulating the efflux of intracellular T(SH)2 and GSH and from parasite cells. 89 This function produces oxidative stresses that participate in cell death. Sb is the complex of trypanothione or GSH with Sb (III) excreted from the cell or packaging into vesicles by ATP‐binding cassette (ABC) transporters. 14 Resistance isolates of L. killicki and L. infantum represented synergistic gene overexpression of GSH1 and TRPER, and in L. infantum overexpression of GSH1 and MRPA in resistance, isolate has been studied. 35
2.16. Trypanothione reductase (TryR)
TRYR maintains an intracellular reducing environment by producing the reduced trypanothione in trypanosomatids and replacing GHS in these protozoans. TRYR gene in Leishmania is vital because attempts to delete both alleles of this gene have been unsuccessful, 90 stating that this protein is necessary for Leishmania survival, and reduced activity of this protein is associated with reduced survival in MQ. 90 This enzyme does not exist in mammals and can be an important drug target in Leishmania. 91
2.17. Calcineurin
Calcineurin is a protein phosphatase dependent on Ca2+ and calmodulin and set up by calcium and contributes to various cellular functions, including apoptosis pathway and cell survival. 41 Calcineurin is a necessary enzyme in cells for many signal transduction pathways. 92 Recent studies showed the adaptation's roles under different temperature changes and salt levels. 93 Calcineurin with heat shock proteins and other molecules generates suitable virulence and thermotolerance in L. major. 94 Although calcineurin is involved in surviving of cells, some data proposed that under different statuses, it could play a damaging function, such as the start of the apoptosis pathway in many organisms by the specific concentration of cytosolic reactive oxygen species (ROS), 95 , 96 downregulation of calcineurin have a reverse effect on apoptosis in Leishmania species and induced apoptosis in lymphocytes. 97 The function of this enzyme is related to Ca2+ concentrations cytoplasm. A study showed that elevated intracellular Ca2+ levels in cardiac cells induced cellular apoptosis by activating some transcriptional factors and calcineurin. 98 Also, other studies demonstrated the implication of increased intracellular Ca2+ concentrations in parasite death. 99 Antimony components stimulate the generation of oxidative agents, for example, hydrogen peroxide (H2O2) or nitric oxide (NO), that have leishmanicidal effects. 100 It is documented that oxidative stress is responsible for increasing Ca2+ and calcineurin activation resulting in apoptosis in the Leishmania parasite. Recent studies proved it as a drug resistance biomarker gene in L. infantum that downregulation of this biomarker prevents apoptosis and increases the survival rate of Leishmania by Ref. [92].
2.18. Leucine‐rich repeats (LRRs)
LinJ34.0570 gene in L. infantum encodes a protein with 621 amino acids and contains 26 amino acid repeats enriched in leucine and a conserved cysteine. This belongs to the superfamily of LRR proteins. This protein also exists in L. tarentolae and L. major (LmjF34.0550), respectively, with 84% and 95% homology. No putative transmembrane domains and signal peptides were identified for LinJ34.0570. 101 LRRs are a general motif and exist in various proteins, and they produce a structural framework for interactions of the proteins. 102 In L. major, more than 100 proteins, including promastigote surface antigen protein 2 (PSA2), have LRR repeats. Leishmania species that overexpressed this LRR protein were resistant to Sb (III) as axenic amastigotes and Sb (V) as intracellular parasites. 102
2.19. LiMT and LiRos3 transporter genes
A common characteristic in promastigotes of MIL‐resistant Leishmania isolates is a reduced MIL concentration that is caused either by a lack in the transport of MIL by inactivation of the L. donovani MIL transporter (LdMT) 103 and/or by its beta‐subunit LdRos3 104 or by an enhanced efflux mediated through the overexpression of ABC transporter proteins. LdMT is a P‐type ATPase gene by functional survival of the MIL‐resistant line. LdMT is a member of the aminophospholipid translocase subfamily and locates in the plasma membrane of the Leishmania. 103 These findings confirmed the prominent role of the LiMT/LiRos3 in resistance MIL L. donovani and L. infantum isolates. 105
2.20. ARM58 and ARM56, HSP23
ARM56, ARM58, and HSP23 are in chromosome 34 at the telomeric end. A recent study has shown that overexpression of these genes produced antimony resistance to amastigotes. The ARM58 gene produces a 58‐kDa protein that has four domains in the Leishmania, which confers Sb (V) resistance to amastigotes and Sb (III) resistance to promastigotes. 106 For the function of this protein, the first and the second domains are essential. The third domain is significant for generation Sb (III) resistance and transmembrane. 107 Studies have shown that the HSP23 (the small 23‐kDa heat shock protein) can also cause resistance to Sb (III) in vitro. 108 All three genes can generate antimony resistance to intracellular L. donovani amastigotes when overexpressed. ARM58 and ARM56 (ARM58rel) are secreted via exosomes. 106
2.21. Serine/threonine phosphatase protein (phosphatase 2C‐like proteins)
Studies on trypanosomatids phosphatases are signifying essential post‐translational modifications, 109 differentiation, 110 and drug resistance. 111 Serine and Threonine (Ser/Thr) residues in eukaryotes are phosphorylated in many proteins. Ser/Thr phosphatases have three families: (1) phosphoprotein phosphatases (PPPs), (2) aspartate‐based phosphatases, and (3) metallo‐dependent protein phosphatases (PPM). 112 Protein phosphatase 5 genes (PP5) membering of the PPP family that is distinct from other members of this family because of its N‐terminal the catalytic domain domains which contain tetratricopeptide repeat (TPR) that are important in autoinhibition and protein–protein interactions. 113 The catalytic domain of PP5 is similar to the catalytic domains of PP2A, 2B/calcineurin, and protein phosphatase 1 (PP1). 114 The role of protein PP2A has been proven in the mechanism of the effect of MTX in mammalian cells. 115 Thus MTX has likely comparable mechanisms of effect and resistance in mammalian cells, and Leishmania, 116 the three phosphatase‐related genes, emerged as biomarker resistance. LinJ.34.2310 and LinJ.34.2320 in WT L. infantum are phosphatase2C‐like and LinJ.12.0610. LinJ.12.0610 is a serine/threonine phosphatase protein that has a conserved protein PP2A domain and two EF‐hand motifs in a fused C‐terminal domain 117 that may relate to the recognized role of Sb (III) as a protein phosphatase inhibitor. 118 Treatment with anticancer drugs produces ROS, which can inactivate PP2A in mammalian cells. 119 Antimonial drugs such as Sb (III) are elevated ROS in Leishmania, 120 overexpression of LinJ.12.0610 allows the parasite to tolerate ROS generated on exposure to Sb (III). 23
2.22. Iron superoxide dismutase‐A
It has been proved that Leishmania has an antioxidant protection system for detoxifying ROS 121 and reactive nitrogen species. 122 The metalloenzyme superoxide dismutase (SOD) is a central part of the antioxidant protection system in various protozoa of various protozoa antioxidant protection systems. It eliminates other superoxide radicals by generating them into hydrogen peroxide and oxygen. 123 Cu/Mn/ZnSOD is present in eukaryotes, but FeSODs have been identified in protozoans. 123 FeSOD is absent in humans and can be a good target for the treatment of leishmaniasis. 73 FeSOD‐A and FeSOD‐B are the FeSOD species, demonstrated in L. infantum/chagasi, L. donovani, and L. tropica. 124 Recent studies showed the high activity of superoxide dismutase in Sb (III) resistant L. infantum and L. braziliensis in in vitro conditions and L. donovani in clinical isolates. 125
2.23. Folate transporter 1 (FT1)
The pathway of folate biosynthesis is used to make many medicines. Folates are made of a pterin that combines glutamic acid and para‐aminobenzoic acid. Resistance to MTX generated by various genes also decreased the concentration of the drug in the Leishmania. 126 Studies have shown that the reduction in MTX in Leishmania also reduces folate uptake proposing that the expression of a joint folate/MTX transporter is highly downregulated in MTX‐resistant isolates of Leishmania. Folate transport regulates the growth stage of the Leishmania in both the logarithmic and stationary phases. Recent studies presented that folate transporter 1 (FT1) is a member of the BT1 family responsible for the affinity of folate and MTX transporter in Leishmania. Variation of the expression of this gene‐modified antifolate sensitivity. This protein was localized in the plasma membrane. 127 Recent data showed that an FT1 disrupted in L. infantum MTX‐resistant mutant corresponds to the leading folate transporter in the parasite. It was proved as the major folate transporter through gene targeting studies. Variation of the FT1 gene expression changed the sensitivity of L. infantum to the MTX. 128
2.24. HSP83
The Leishmania HSP83 is similar to the mammalian HSP90. HSP90 was recognized to be an inverted controller of the mitochondria‐dependent apoptosis pathway. 129 HSP90 is associated with Bcl‐2 and inhibited mitochondrial apoptotic cascades. 130 The collaboration of HSP90 in programmed cell death (PCD) confirms the role of HSP83 in drug‐induced PCD in Leishmania. HSP83 interacts with other proteins in Leishmania to reversely regulate the mitochondrial apoptotic pathway. 131 In antimony resistance, clinical isolates of L. donovani were shown the overexpression of HSP83, and its role was proved in antimony resistance by gene targeting in sensitive L. donovani parasite. 132 In recent study demonstrated that the overexpression of this gene was elevated in four out of the ten resistant isolates.
Also, there was a slight correlation between the antimony susceptibility and HSP83 gene expression, demonstrating that this gene is not the only cause for resistance in clinical isolates. The resistant clinical isolate presented resistance to other medicines, including MIL and Amp B. proteomic studies have demonstrated various proteins differentially expressed, proposing that PCD is changed in the resistant isolates. Actually, drug‐induced PCD has changed the markers of apoptosis in the Sb (V) resistant isolate. The HSP83 and the SKCRP14.1 demonstrated two proteins to be involved in the drug‐induced PCD. HSP83 enhanced resistance and decreased drug‐mediated PCD by intervention with the mitochondrial membrane potential, also SKCRP14.1 initiated PCD but protected against MIL‐induced PCD. This finding demonstrated the role of PCD in drug sensitivity or resistance in the Leishmania species. 131
2.25. Small kinetoplastid calpain‐related protein (SKCRP14.1)
This protein belongs to the family of calcium‐dependent cysteine proteases. 133 This new protein was downregulated within the clinical isolate of L. donovani from India, and overexpression of this gene in the parasite resensitized the parasite to antimonial drugs through induced PCD. SKCRP14.1 overexpression in the existence of Sb (III) only quantitatively. 131 High expression of SKCRP14.1 increased the antimonial susceptibility in L. donovani but, interestingly, caused an increased resistance to MIL. Considering these resistance phenotypes, high expression of SKCRP14.1 caused increased protection versus MIL‐induced PCD. Therefore, a change in SKCRP14.1 expression had contradictory effects on sensitivity to antimonials and MIL. 131 SKCRP14.1 and HSP83 were demonstrated to be closely related to the drug‐induced PCD phenotype. SKCRP14.1 elevated antimonial‐induced PCD but protected clearly into MIL‐induced PCD, whiles HSP83 increased the drug resistance and reduced drug‐induced PCD activation via participating with the mitochondrial membrane potential. 131
2.26. LmACR2
Sb (V) must be diminished to Sb (III) to make this medication dynamic. MQ catches Sb (V), and a portion of it is reduced to Sb (III), which is then transported into the amastigotes by AQP1. The other bit of this medication decreased to Sb (III) by LmACR2 and TDR1. 134 The two pathways of drug activity would be related to the expression of their relevant components in both MQ and Leishmania. This had been varied in different species of Leishmania. LmACR2, in L. major, is the first known as metalloid reductase with a physiological function in activating the drug. 134 Transfection of the LmACR2 gene in L. infantum enhanced the susceptibility to Pentostam in intracellular amastigotes (Figure 2). These findings suggest that this gene is responsible for reducing the pentavalent antimonial compounds in pentostam® to the active Sb (III) in Leishmania. 134
FIGURE 2.
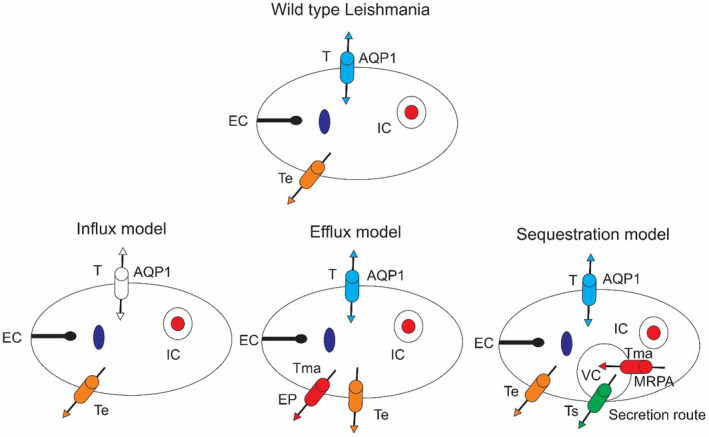
Model of Pentostam action in MQ and amastigotes of Leishmania
2.27. Thiol‐dependent reductase I (TDR1)
This protein is an enzyme detected in Leishmania species involved in deglutathionylation and activation of Sb (V) used in the treatment of leishmaniosis. 135 In Leishmania spp, TDR1 is involved in redox regulation and promoting sensitivity to the antimonial prodrugs glucantime and pentostam, known as the first‐line treatment of Leishmaniosis. 136 The therapeutic function of these drugs is to decrease the pentavalent species to trivalent species that are toxic. This procedure happens gradually under in vitro conditions within sight of glutathione (GSH) or the T(SH)2, particularly at low pH as found in the parasitophorous vacuole in which Leishmania dwells intracellularly in MQs. 137 TDR1, within sight of GSH, catalyzes the decrease of Sb (V) in vitro condition and thus could actuate the antimonial prodrugs. 138 This enzyme is more abundant in amastigote form and the amastigotes are intensely more sensitive to Sb (V) than promastigotes. 139 This enzyme is a member of the glutathione‐S‐transferase (GST) superfamily 138 which involves biological events such as signaling processes, stress response, and xenobiotic detoxification. 140
2.28. Heat shock protein70 (hsp70)
Conserved proteins of this class are molecular chaperones playing the leading role in maintaining cellular homeostasis approximately in all known organisms. T. cruzi, T. brucei, and L. major known as the Tritryps are human parasites. These parasites change their morphology in the life cycle of humans and insects. Hsp70s make these changes in different hosts and conditions also remaining viable and infective. 141 The hsp70 is part of a cellular network, which is frequently involved in protein folding processes and molecular chaperoning. 141 In Leishmania antimony‐resistant isolates, Hsp70 has been detected to be upregulated at mRNA, 142 and protein 143 levels; this does not directly produce resistance, but it enhances the metal tolerance in the Leishmania. So it allows the cell to create resistance mechanisms. 142 Mutation in the hsp70 gene of L. braziliensis could modulate the failure of antimonial treatment in patients. 144
3. GEOGRAPHICAL DISTRIBUTION
Table 1 provides information on studies on resistance biomarkers gene in Leishmania species, based on a study including laboratory studies and clinical‐resistant isolates.
TABLE 1.
Drug resistance gene markers in leishmaniasis
| Gene | Leishmania species | Type of isolate | Country (reference) |
|---|---|---|---|
| AQP1 | L. major | CRI | Iran 145 |
| L. tropica | CRI | Iran 54 | |
| L. panamensis | LRM | USA 9 | |
| L. infantum | CRI | Tunisia, Algeria 35 | |
| L. donovani | CRI | India 146 | |
| L. guyanensis | LRM | Brazil 147 | |
| L. braziliensis | LRM | Brazil 148 | |
| ABCI4 | L. major | LRM | Spain 15 |
| ABCC3 | L. infantum | LRM | Canada 149 |
| L. donovani | CRI | India 17 | |
| ABCG | L. donovani | LRM | India 150 |
| L. infantum | LRM | Spain 19 | |
| L. major | LRM | Spain 21 | |
| Pgps | L. tropica | LRM | Spain 29 |
| L. donovani | CRI | India 146 | |
| Pgp A | L. infantum | LRM | Canada 151 |
| L. major | LRM | Iran 33 | |
| L. guyanensis | LRM | Brazil 152 | |
| Protein 14‐3‐3 | L. major | CRI | Algeria 35 |
| L. infantum | CRI | Algeria 35 | |
| P299 | L. major | CRI | Algeria 35 |
| L. infantum | CRI | Algeria 35 | |
| Histon 4 | L. major | CRI | Algeria, Tunisia 35 |
| L. infantum | CRI | Algeria, Tunisia 35 | |
| L. donovani | CRI | India 39 | |
| Histon H2A | L. donovani | CRI | India 153 |
| Histon H1 | L. donovani | CRI | India 39 |
| LACK1 | L. tropica | CRI | Iran 6 |
| Ubiquitin | L. tropica | CRI | Iran 49 |
| AAP3 | L. tropica | CRI | Iran 49 |
| L. donovani | LRM | Spain 166 | |
| PGK | L. tropica | CRI | Iran 54 |
| MAPK | L. tropica | CRI | Iran 54 |
| L. donovani | CRI | India 61 | |
| PTP | L. tropica | CRI | Iran 54 |
| PTR1 | L. major | LRM | Canada 154 |
| L. braziliensis | LRM | Brazil 68 | |
| L. infantum | LRM | Canada 116 | |
| TXNPx | L. infantum | CRI | Algeria, Tunisia 35 |
| L. donovani | CRI | India 76 | |
| L. braziliensis | LRM | Brazil 155 | |
| L. major | LRM | United Kingdom 156 | |
| KMP11 | L. infantum | CRI | France 35 |
| Hsp70 | L. braziliensis | CRI | Brazil 144 |
| L. donovani | CRI | India 157 | |
| L. infantum | LRM | Brazil 158 | |
| GSH1 | L. guyanensis | CRI | Brazil 159 |
| L. infantum | CRI | Tunisia 35 | |
| L. donovani | CRI | United Kingdom 160 | |
| TryR | L. major | LRM | Canada 91 |
| L. donovani | CRI | India 161 | |
| Calcineurin | L. infantum | CRI | Iran 92 |
| LRRs | L. infantum | LRM | Canada 102 |
| L. donovani | CRI | India 162 | |
| LiMT and LiRos3 | L. infantum | CRI | France 105 |
| L. donovani | LRM | Spain 103 | |
| ARM58, ARM56, and HSP23 | L. infantum | LRM | Germany 106 |
| L. donovani | LRM | Germany 106 | |
| L. braziliensis | CRI | Peru 163 | |
| Serine/threonine phosphatase protein | L. infantum | LRM | Canada 23 |
| Iron superoxide dismutase A | L. infantum | LRM | Brazil 125 |
| L. braziliensis | LRM | Brazil 125 | |
| L. donovani | CRI | India 164 | |
| Folate transporter 1 | L. infantum | LRM | Canada 128 |
| L. major | LRM | Canada 165 | |
| HSP83 | L. donovani | CRI | India 132 |
| L. infantum | LRM | Brazil 158 | |
| L. braziliensis | LRM | Brazil 158 | |
| SKCRP14.1 | L. donovani | CRI | India 131 |
| LmACR2 | L. infantum | LRM | Canada 134 |
| L. donovani | LRM | Canada 134 | |
| TDR1 | L. major | LRM | United Kingdom 138 |
Abbreviations: CRI, clinical‐resistant isolate; LRM, laboratory‐resistant mutant.
In Figures 3 and 4, the geographic distribution of biomarkers is shown separately according to the study types, which are fully explained in the discussion section. These maps are drawn by ArcGIS v 10.1 software.
FIGURE 3.
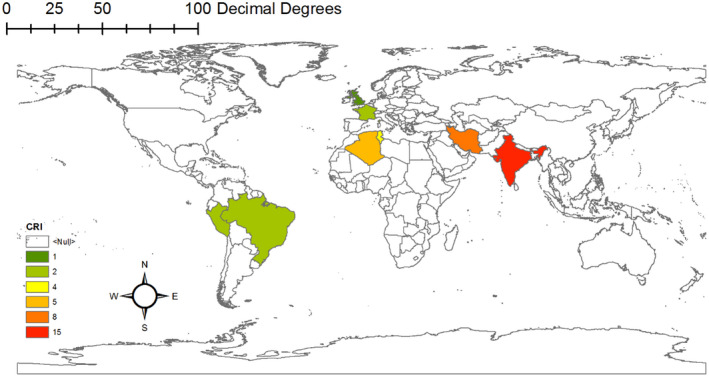
Geographical distribution of resistant biomarkers gene based on studies carried out on clinical‐resistant isolate
FIGURE 4.
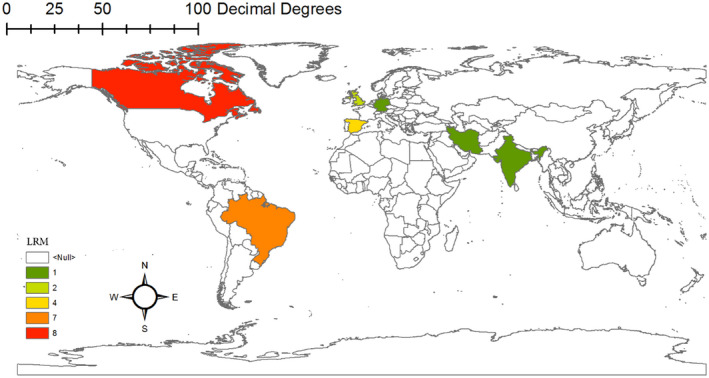
Geographical distribution of resistant biomarkers gene based on studies carried out on laboratory‐resistant mutant
4. DISCUSSION
There are numerous studies about the molecular biomarkers of drug resistance in leishmaniasis. In this article, we have tried to collect these biomarkers. Several of these investigations used resistant isolates taken from patients, while others used isolates resistant to drug treatment in the laboratory. In addition, in this article, we have determined that these biomarkers have been reported in patients in various countries.
The downregulation of the AQP1 gene has been reported from resistant isolates such as L. major in Iran, 145 L. tropica in Iran, 54 L. infantum in Algeria, and Tunisia, 35 L. donovani in India 146 from patients. However, this gene has been considered a resistance marker only in laboratory conditions in L. panamensis (USA), 9 L. guyanensis (Brazil), 147 L. braziliensis (Brazil). 148
The ABCI4 gene was studied in L. major and introduced as a drug resistance biomarker. 15
ABCC3 gene has been investigated in resistant isolates of human specimens and is known as a resistance biomarker in L. donovani in India. 17 The resistance of this gene has been reported in a laboratory model in L. infantum in Canada. 149
The potential for resistance to the ABCG gene has been studied only in vitro. In L. donovani in India 150 and L. major 21 and L. infantum in Spain 19 have been examined.
According to studies conducted in laboratory conditions in Spain, the Pgps gene can regulate drug accumulation and reverse the resistance phenotype of L. tropica. 29 In India, a study on resistant specimens of L. donovani showed this gene as a molecular marker of resistance. 146
All research on the PgpA gene has been conducted in laboratory conditions. These researches were carried out on L. infantum, L. major, and L. guyanensis in Canada, 151 Iran, 33 and Brazil, 152 respectively.
Changes in the expression of the protein of 14–3‐3, P299 genes have been recorded on samples taken from patients in Algeria. These resistance genes have been observed in L. major and L. infantum in this country. 35
Research on Histon 4, Histon H2A, and Histon H1 genes showed that they were used as resistance markers in human resistance specimens. Histon 4 in Algeria and Tunisia has been studied on L. major and L. infantum 35 and in India on L. donovani. 39 Histon H2A, 153 Histon H1 39 genes have been investigated only in L. donovani in India.
The information obtained from four genes (LACK1, Ubiquitin, AAP3and PGK) have been obtained from studies conducted on patients who were resistant to treatment in Iran. These resistant isolates were L. tropica. 6 , 49 One study was done on the AAP3 gene in in vitro conditions in Spain.
Change in expression of the MAPK gene that leads to resistance to treatment has been observed in patients in Iran and India. Resistant strains of L. tropica and L. donovani have been reported in Iran and India, respectively. 54 , 61
The PTP gene as a biomarker of resistance is isolated from patients only in Iran. These isolates are related to L. tropica. 54
All studies on the PTR1 gene have been performed on drug resistance in Leishmania species in vitro conditions. These studies have been conducted on L. major 154 and L. infantum 116 and L. braziliensis 68 in Canada and Brazil, respectively.
Studies were conducted on the TXNPx gene in resistant strains isolated from patients in Algiers and Tunisia 35 on L. infantum in India and L. donovani. 76 Laboratory studies have been conducted on L. major and L. braziliansis in Brazil 155 and the United Kingdom, respectively. 156 Research on the KMP11 gene has been conducted as a biomarker of resistance only in a patient from France. 35
Studies have been performed on the Hsp70 gene in human specimens on L. braziliansis 144 and L. donovani in Brazil and India, 157 respectively. A study on L. infantum in Brazil showed that drug resistance had been established due to this gene in vitro model. 158
There are three types of research on the GSH1 gene in resistant human isolates. The studies were performed on L. guyanensis, L. infantum and L. donovani in Brazil, 159 Tunisia, 35 and the United Kingdom, 160 respectively.
Two investigations have been published on TryR gene. A study was carried out on L. donovani on a drug‐resistant specimen from a patient in India. 161 Another study was conducted on L. major in Canada in in vitro condition. 91
Only a study on Calcineurin gene has been published on L. infantum of the human‐resistant specimen in Iran. 92
The study of the LRRs gene was carried out on L. donovani in resistant specimens from India 162 and on L. infantum in Canada in a laboratory model. 102
For LiMT and LiRos3 genes, two studies have been performed on L. infantum and L. donovani. The study has been done on these genes in L. infantum in human‐resistant samples in France. 105 Another study reported on L. donovani in in vitro in Spain. 103
Studies on ARM58, ARM56, and HSP23 genes have been conducted on L. infantum and L. donovani in in vitro conditions in Germany. 106 A survey of L. braziliansis has been published on resistant human specimens from Peru. 163
In the serine/threonine phosphatase gene, only one study was done in Canada in vitro. 23
Information about the iron superoxide dismutase A gene is available on L. infantum and L. braziliensis base laboratory studies, 125 which have been registered in Brazil, and a study on L. donovani from the clinical sample in India. 164
Available information on the folate transporter 1 gene is based on the experimental study on L. infantum 128 and L. major 165 from Canada.
HSP83 gene has been investigated as a biomarker of resistance in human specimens on L. donovani in India. 132 In Brazil, research has been done on L. infantum and L. braziliensis in laboratory conditions. 158
Information about the SKCRP14.1 gene has been obtained from a study conducted on human‐resistant samples of L. donovani 131 in India.
A study on the LmACR2 gene has been carried out on L. infantum and L. donovani in laboratory conditions in Canada. 134
The ability to create drug resistance by TDR1 gene in the laboratory has been studied on L. major in the United Kingdom. 138
5. CONCLUSION
According to current research, biomarkers of drug resistance are consistent with each country and the studied species are as follows: in Iran: AQP1 (L. major, L. tropica), LACK1 (L. tropica), ubiquitin (L. tropica), AAP3 (L. tropica), PGK (L. tropica), MAPK (L. tropica), PTP (L. tropica), Calcineurin (L. infantum), in Tunisia: AQP1 (L. infantum), Histon 4 (L. major, L. infantum), TRPER (L. infantum), GSH1 (L. infantum); in Algeria: AQP1 (L. infantum), Protein 14–3‐3 (L. major, L. infantum), P299 (L. major, L. infantum), Histon 4 (L. major, L. infantum), TRPER (L. infantum), in India: AQP1 (L. donovani), ABCC3 (L. donovani), Pgps (L. donovani), Histon 4 (L. donovani), Histon H2A, Histon H1 (L. donovani), MAPK (L. donovani), TRPER (L. donovani), Hsp70 (L. donovani), Hsp70 (L. donovani), TryR (L. donovani), LRRs (L. donovani), Iron superoxide dismutase A (L. donovani), HSP83 (L. donovani), SKCRP14.1 (L. donovani), in France: KMP11 (L. infantum), LiMT and LiRos3 (L. infantum), in Brazil: Hsp70 (L. braziliensis), GSH1 (L. guyanensis), in the UK: GSH1(L. donovani), in Peru: ARM58, ARM56, and HSP23 (L. braziliensis).
The significance of these findings is determined in two areas: (1) biomarkers should be considered in the diagnosis of resistant species in each country or region. (2) What biomarkers should be studied in drug studies in each country to find and use an appropriate drug to treat resistant species?
RECOMMENDATIONS
Due to the emergence of resistant genes mainly in anthroponotic Leishmania species such as L. donovani and L. tropica, as the causative agents of ACL and AVL, respectively, selection of an appropriate treatment modality is essential.
The control of leishmaniasis is complicated as there is no efficacious vaccine available. Controlling vectors and reservoir hosts is practical because of numerous species implicated in the life cycle.
At present, combination therapy would be a proper choice for selecting a treatment modality, more importantly among the first‐line agents along with the second‐choice drugs or cryotherapy. The use of two anti‐leishmanial simultaneously, especially when the drugs have different mechanisms of action, has the development of resistance to either of the components.
Physicians should be aware of the presence of such resistance for the selection of proper treatment modalities in endemic countries.
CONFLICT OF INTEREST
The authors confirm that this article's content has no conflicts of interest.
ACKNOWLEDGMENTS
The authors wish to thank the Leishmaniasis Research Center, Kerman University of Medical Sciences, Kerman, Iran.
Salari S, Bamorovat M, Sharifi I, Almani PGN. Global distribution of treatment resistance gene markers for leishmaniasis. J Clin Lab Anal. 2022;36:e24599. doi: 10.1002/jcla.24599
Contributor Information
Iraj Sharifi, Email: iraj.sharifi@yahoo.com.
Pooya Ghasemi Nejad Almani, Email: pooyaalmani@yahoo.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Chakravarty J, Sundar S. Drug resistance in leishmaniasis. J Glob Infect Dis. 2010;2(2):167‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Almani PG, Sharifi I, Kazemi B, et al. The role of GlcNAc‐PI‐de‐N‐acetylase gene by gene knockout through homologous recombination and its consequences on survival, growth and infectivity of Leishmania major in in vitro and in vivo conditions. Acta Trop. 2016;154:63‐72. [DOI] [PubMed] [Google Scholar]
- 3. Moreira DS, Monte Neto RL, Andrade JM, et al. Molecular characterization of the MRPA transporter and antimony uptake in four New World Leishmania spp. susceptible and resistant to antimony. Int J Parasitol Drugs Drug Resist. 2013;5(3):143‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paz CDS, Keita S, Sethi A. Cutaneous leishmaniasis in Mali. Dermatol Clin. 2011;29(1):75‐78. [DOI] [PubMed] [Google Scholar]
- 5. Cantacessi C, Dantas‐Torres F, Nolan MJ, Otranto D. The past, present, and future of Leishmania genomics and transcriptomics. Trends Parasitol. 2015;31(3):100‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hajjaran H, Kazemi‐Rad E, Mohebali M, et al. Expression analysis of activated protein kinase C gene (LACK1) in antimony sensitive and resistant Leishmania tropica clinical isolates using real‐time RT‐PCR. Int J Dermatol. 2016;55(9):1020‐1026. [DOI] [PubMed] [Google Scholar]
- 7. Aflatoonian MR, Sharifi I, Aflatoonian B, et al. Associated‐risk determinants for anthroponotic cutaneous leishmaniasis treated with meglumine antimoniate: a cohort study in Iran. PLoS Negl Trop Dis. 2019;13(6):e0007423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gourbal BSN, Bhattacharjee H, Legare D, et al. Drug uptake and modulation of drug resistance in Leishmania by an aquaglyceroporin. J Biol Chem. 2004;279(30):31010‐301017. [DOI] [PubMed] [Google Scholar]
- 9. Mandal G, Mandal S, Sharma M, et al. Species‐specific antimonial sensitivity in Leishmania is driven by post‐transcriptional regulation of AQP1. PLoS Negl Trop Dis. 2015;9(2):e0003500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hansen C, Hansen EW, Hansen HR, Gammelgaard B, Stürup S. Reduction of Sb(V) in a human macrophage cell line measured by HPLC‐ICP‐MS. Biol Trace Elem Res. 2011;144(1–3):234‐243. [DOI] [PubMed] [Google Scholar]
- 11. Brochu CWJ, Roy G, Messier N, Wang XY, Saravia NG, Ouellette M. Antimony uptake systems in the protozoan parasite Leishmania and accumulation differences in antimony‐resistant parasites. Antimicrob Agents Chemother. 2003;47(10):3073‐3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haimeur AGC, Pilote S, Mukhopadhyay R, Rosen BP, Poulin R, Ouellette M. Elevated levels of polyamines and trypanothione resulting from overexpression of the ornithine decarboxylase gene in arsenite‐resistant Leishmania. Mol Microbiol. 1999;34(4):726‐735. [DOI] [PubMed] [Google Scholar]
- 13. Marquis NGB, Rosen BP, Mukhopadhyay R, Ouellette M. Modulation in aquaglyceroporin AQP1 gene transcript levels in drug‐resistant Leishmania. Mol Microbiol. 2005;57(6):1690‐1699. [DOI] [PubMed] [Google Scholar]
- 14. Légaré DRD, Mukhopadhyay R, Stierhof YD, et al. The Leishmania ATP‐binding cassette protein PGPA is an intracellular metal‐thiol transporter ATPase. J Biol Chem. 2001;276(28):26301‐26307. [DOI] [PubMed] [Google Scholar]
- 15. Manzano JIG‐HR, Castanys S, Gamarro F. A new ABC half‐transporter in Leishmania major is involved in resistance to antimony. Antimicrob Agents Chemother. 2013;57(8):3719‐3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Callahan HLRW, Rainey PM, Beverley SM. The PGPA gene of Leishmania major mediates antimony (SbIII) resistance by decreasing influx and not by increasing efflux. Mol Biochem Parasitol. 1994;68(1):145‐149. [DOI] [PubMed] [Google Scholar]
- 17. Mookerjee Basu JMA, Banerjee R, Saha M, et al. Inhibition of ABC transporters abolishes antimony resistance in Leishmania infection. Antimicrob Agents Chemother. 2008;52(3):1080‐1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ghosh J, Bose M, Roy S, Bhattacharyya SN. Leishmania donovani targets Dicer1 to downregulate miR‐122, lower serum cholesterol, and facilitate murine liver infection. Cell Host Microbe. 2013;13(3):277‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Castanys‐Muñoz E, Pérez‐Victoria JM, Gamarro F, Castanys S. Characterization of an ABCG‐like transporter from the protozoan parasite Leishmania with a role in drug resistance and transbilayer lipid movement. Antimicrob Agents Chemother. 2008;52(10):3573‐3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Castanys‐Munoz E, Alder‐Baerens N, Pomorski T, Gamarro F, Castanys S. A novel ATP‐binding cassette transporter from Leishmania is involved in transport of phosphatidylcholine analogues and resistance to alkyl‐phospholipids. Mol Microbiol. 2007;64(5):1141‐1153. [DOI] [PubMed] [Google Scholar]
- 21. Perea A, Manzano JI, Castanys S, Gamarro F. The LABCG2 transporter from the protozoan parasite Leishmania is involved in antimony resistance. Antimicrob Agents Chemother. 2016;60(6):3489‐3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singh S, Mandlik V. Structure based investigation on the binding interaction of transport proteins in leishmaniasis: insights from molecular simulation. Mol Biosyst. 2015;11(5):1251‐1259. [DOI] [PubMed] [Google Scholar]
- 23. Gazanion E, Fernandez‐Prada CA‐O, Papadopoulou B, Leprohon P, Ouellette M. Cos‐Seq for high‐throughput identification of drug target and resistance mechanisms in the protozoan parasite Leishmania. Proc Natl Acad Sci U S A. 2016;113(21):E3012‐E3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67‐113. [DOI] [PubMed] [Google Scholar]
- 25. Pérez‐Victoria JMP‐TA, Torres C, Gamarro F, Castanys S. ABC transporters in the protozoan parasite Leishmania. Int Microbiol. 2001;4(3):159‐166. [DOI] [PubMed] [Google Scholar]
- 26. Ambudkar SVDS, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361‐398. [DOI] [PubMed] [Google Scholar]
- 27. Di Pietro ADG, Conseil G, Steinfels E, et al. P‐glycoprotein‐mediated resistance to chemotherapy in cancer cells: using recombinant cytosolic domains to establish structure‐function relationships. Braz J Med Biol Res. 1999;32(8):925‐939. [DOI] [PubMed] [Google Scholar]
- 28. Pérez‐Victoria JMCM, Conseil G, Dayan G, et al. Correlation between the affinity of flavonoids binding to the cytosolic site of Leishmania tropica multidrug transporter and their efficiency to revert parasite resistance to daunomycin. Biochemistry. 1999;38(6):1736‐1743. [DOI] [PubMed] [Google Scholar]
- 29. Pérez‐Victoria JMP‐VF, Conseil G, Maitrejean M, et al. High‐affinity binding of silybin derivatives to the nucleotide‐binding domain of a Leishmania tropica P‐glycoprotein‐like transporter and chemosensitization of a multidrug‐resistant parasite to daunomycin. Antimicrob Agents Chemother. 2001;45(2):439‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pérez‐Victoria JM, Pérez‐Victoria FJ, Parodi‐Talice A, et al. Alkyl‐lysophospholipid resistance in multidrug‐resistant Leishmania tropica and chemosensitization by a novel P‐glycoprotein‐like transporter modulator. Antimicrob Agents Chemother. 2001;45(9):2468‐2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garg M, Goyal N. MAPK1 of Leishmania donovani modulates antimony susceptibility by downregulating P‐glycoprotein efflux pumps. Antimicrob Agents Chemother. 2015;59(7):3853‐3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ashutosh SS, Goyal N. Molecular mechanisms of antimony resistance in Leishmania. J Med Microbiol. 2007;56(Pt 2):143‐153. [DOI] [PubMed] [Google Scholar]
- 33. Soleimanifard S, Arjmand R, Saberi S, et al. P‐glycoprotein a gene expression in glucantime‐resistant and sensitive Leishmania major (MRHO/IR/75/ER). Iran J Parasitol. 2014;9(3):423‐428. [PMC free article] [PubMed] [Google Scholar]
- 34. Dougherty MK, Morrison DK. Unlocking the code of 14‐3‐3. J Cell Sci. 2004;117(pt 10):1875‐1884. [DOI] [PubMed] [Google Scholar]
- 35. Jeddi F, Mary C, Aoun K, et al. Heterogeneity of molecular resistance patterns in antimony‐resistant field isolates of Leishmania species from the western Mediterranean area. Antimicrob Agents Chemother. 2014;58(8):4866‐4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Choudhury KZD, Kube M, Reinhardt R, Clos J. Identification of a Leishmania infantum gene mediating resistance to miltefosine and SbIII. Int J Parasitol Drug Resist. 2008;38(12):1411‐1423. [DOI] [PubMed] [Google Scholar]
- 37. Cox M, Nelson DL, Lehninger AL. Lehninger Principles of Biochemistry. W.H. Freeman; 2005. [Google Scholar]
- 38. Espinoza ITG, Hellman U, Galanti N. Histone H1 and core histones in Leishmania and Crithidia: comparison with Trypanosoma. Exp Cell Res. 1996;224(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 39. Kumar DSR, Bhandari V, Kulshrestha A, Negi NS, Salotra P. Biomarkers of antimony resistance: need for expression analysis of multiple genes to distinguish resistance phenotype in clinical isolates of Leishmania donovani. Parasitol Res. 2012;111(1):223‐230. [DOI] [PubMed] [Google Scholar]
- 40. Mougneau E, Altare F, Wakil AE, et al. Expression cloning of a protective Leishmania antigen. Science. 1995;268(5210):563‐566. [DOI] [PubMed] [Google Scholar]
- 41. Kelly BLSD, Locksley RM. Leishmania major LACK antigen is required for efficient vertebrate parasitization. J Exp Med. 2003;198(11):1689‐1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gómez‐Arreaza A, Acosta H, Barros‐Álvarez X, Concepción JL, Albericio F, Avilan L. Leishmania mexicana: LACK (Leishmania homolog of receptors for activated C‐kinase) is a plasminogen binding protein. Exp Parasitol. 2011;127(4):752‐761. [DOI] [PubMed] [Google Scholar]
- 43. Maillard I, Launois P, Himmelrich H, et al. Functional plasticity of the LACK‐reactive Vbeta4‐Valpha8 CD4(+) T cells normally producing the early IL‐4 instructing Th2 cell development and susceptibility to Leishmania major in BALB/c mice. Eur J Immunol. 2001;31(4):1288‐1296. [PubMed] [Google Scholar]
- 44. Tapia E, Pérez‐Jiménez E, López‐Fuertes L, Gonzalo R, Gherardi MM, Esteban M. The combination of DNA vectors expressing IL‐12 + IL‐18 elicits high protective immune response against cutaneous leishmaniasis after priming with DNA‐p36/LACK and the cytokines, followed by a booster with a vaccinia virus recombinant expressing p36/LACK. Microbes Infect. 2003;5(2):73‐84. [DOI] [PubMed] [Google Scholar]
- 45. Launois P, Maillard I, Pingel S, et al. IL‐4 rapidly produced by V beta 4 V alpha 8 CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity. 1997;6(5):541‐549. [DOI] [PubMed] [Google Scholar]
- 46. Welchman RL, Gordon C, Mayer RJ. Ubiquitin and ubiquitin‐like proteins as multifunctional signals. Nat Rev Mol Cell Biol. 2005;6(8):599‐609. [DOI] [PubMed] [Google Scholar]
- 47. Sherman MY, Goldberg AL. Cellular defenses against unfolded proteins: a cell biologist thinks about neurodegenerative diseases. Neuron. 2001;29(1):15‐32. [DOI] [PubMed] [Google Scholar]
- 48. Ciechanover A. Intracellular protein degradation: from a vague idea through the lysosome and the ubiquitin‐proteasome system and onto human diseases and drug targeting. Bioorg Med Chem. 2013;21(12):3400‐3410. [DOI] [PubMed] [Google Scholar]
- 49. Kazemi‐Rad E, Mohebali M, Khadem‐Erfan MB, et al. Overexpression of ubiquitin and amino acid permease genes in association with antimony resistance in Leishmania tropica field isolates. Korean J Parasitol. 2013;51(4):413‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Akerman M, Shaked‐Mishan P, Mazareb S, Volpin H, Zilberstein D. Novel motifs in amino acid permease genes from Leishmania. Biochem Biophys Res Commun. 2004;325(1):353‐366. [DOI] [PubMed] [Google Scholar]
- 51. Shaked‐Mishan P, Suter‐Grotemeyer M, Yoel‐Almagor T, Holland N, Zilberstein D, Rentsch D. A novel high‐affinity arginine transporter from the human parasitic protozoan Leishmania donovani. Mol Microbiol. 2006;60(1):30‐38. [DOI] [PubMed] [Google Scholar]
- 52. Colotti G, Ilari A. Polyamine metabolism in Leishmania: from arginine to trypanothione. Amino Acids. 2011;40(2):269‐285. [DOI] [PubMed] [Google Scholar]
- 53. Mukhopadhyay R, Dey S, Xu N, et al. Trypanothione overproduction and resistance to antimonials and arsenicals in Leishmania. Proc Natl Acad Sci U S A. 1996;93(19):10383‐10387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kazemi‐Rad E, Mohebali M, Khadem‐Erfan MB, et al. Identification of antimony resistance markers in Leishmania tropica field isolates through a cDNA‐AFLP approach. Exp Parasitol. 2013;135(2):344‐349. [DOI] [PubMed] [Google Scholar]
- 55. Adjé CA, Opperdoes FR, Michels PA. Organization, sequence and stage‐specific expression of the phosphoglycerate kinase genes of Leishmania mexicana mexicana. Mol Biochem Parasitol. 1997;90(1):155‐168. [DOI] [PubMed] [Google Scholar]
- 56. Opperdoes FR, Coombs GH. Metabolism of Leishmania: proven and predicted. Trends Parasitol. 2007;23(4):149‐158. [DOI] [PubMed] [Google Scholar]
- 57. Biyani N, Singh AK, Mandal S, Chawla B, Madhubala R. Differential expression of proteins in antimony‐susceptible and ‐resistant isolates of Leishmania donovani. Mol Biochem Parasitol. 2011;179(2):91‐99. [DOI] [PubMed] [Google Scholar]
- 58. Schaeffer HJ, Weber MJ. Mitogen‐activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19(4):2435‐2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bengs F, Scholz A, Kuhn D, Wiese M. LmxMPK9, a mitogen‐activated protein kinase homologue affects flagellar length in Leishmania mexicana. Mol Microbiol. 2005;55(5):1606‐1615. [DOI] [PubMed] [Google Scholar]
- 60. Mann KK, Davison K, Colombo M, et al. Antimony trioxide‐induced apoptosis is dependent on SEK1/JNK signaling. Toxicol Lett. 2006;160(2):158‐170. [DOI] [PubMed] [Google Scholar]
- 61. Ashutosh GM, Sundar S, Duncan R, Nakhasi HL, Goyal N. Downregulation of mitogen‐activated protein kinase 1 of Leishmania donovani field isolates is associated with antimony resistance. Antimicrob Agents Chemother. 2012;56(1):518‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Andreeva AV, Kutuzov MA. Protozoan protein tyrosine phosphatases. Int J Parasitol Drug Resist. 2008;38(11):1279‐1295. [DOI] [PubMed] [Google Scholar]
- 63. Nascimento M, Zhang WW, Ghosh A, et al. Identification and characterization of a protein‐tyrosine phosphatase in Leishmania: involvement in virulence. J Biol Chem. 2006;281(47):36257‐26268. [DOI] [PubMed] [Google Scholar]
- 64. Nare B, Garraway LA, Vickers TJ, Beverley SM. PTR1‐dependent synthesis of tetrahydrobiopterin contributes to oxidant susceptibility in the trypanosomatid protozoan parasite Leishmania major. Curr Genet. 2009;55(3):287‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nare B, Hardy LW, Beverley SM. The roles of pteridine reductase 1 and dihydrofolate reductase‐thymidylate synthase in pteridine metabolism in the protozoan parasite Leishmania major. J Biol Chem. 1997;272(21):13883‐13891. [DOI] [PubMed] [Google Scholar]
- 66. Sezavar M, Sharifi I, Ghasemi Nejad Almani P, et al. The potential therapeutic role of PTR1 gene in non‐healing anthroponotic cutaneous leishmaniasis due to Leishmania tropica. J Clin Lab Anal. 2021;35(3):e23670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Moreira W, Leblanc É, Ouellette M. The role of reduced pterins in resistance to reactive oxygen and nitrogen intermediates in the protozoan parasite Leishmania. Free Radic Biol Med. 2009;46(3):367‐375. [DOI] [PubMed] [Google Scholar]
- 68. de Souza Moreira D, Ferreira RF, Murta SM. Molecular characterization and functional analysis of pteridine reductase in wild‐type and antimony‐resistant Leishmania lines. Exp Parasitol. 2016;160:60‐66. [DOI] [PubMed] [Google Scholar]
- 69. Lüdemann H, Dormeyer M, Sticherling C, Stallmann D, Follmann H, Krauth‐Siegel RL. Trypanosoma brucei tryparedoxin, a thioredoxin‐like protein in African trypanosomes. FEBS Lett. 1998;431(3):381‐385. [DOI] [PubMed] [Google Scholar]
- 70. Montemartini M, Nogoceke E, Gommel DU, et al. Tryparedoxin and tryparedoxin peroxidase. Biofactors. 2000;11(1–2):71‐72. [DOI] [PubMed] [Google Scholar]
- 71. Castro H, Romao S, Gadelha FR, Tomás AM. Leishmania infantum: provision of reducing equivalents to the mitochondrial tryparedoxin/tryparedoxin peroxidase system. Exp Parasitol. 2008;120(4):421‐423. [DOI] [PubMed] [Google Scholar]
- 72. Castro H, Budde H, Flohé L, et al. Specificity and kinetics of a mitochondrial peroxiredoxin of Leishmania infantum. Free Radic Biol Med. 2002;33(11):1563‐1574. [DOI] [PubMed] [Google Scholar]
- 73. Turrens JF. Oxidative stress and antioxidant defenses: a target for the treatment of diseases caused by parasitic protozoa. Mol Aspects Med. 2004;25(1–2):211‐220. [DOI] [PubMed] [Google Scholar]
- 74. Brindisi M, Brogi S, Relitti N, et al. Structure‐based discovery of the first non‐covalent inhibitors of Leishmania major tryparedoxin peroxidase by high throughput docking. Sci Rep. 2015;5:9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Romao S, Castro H, Sousa C, Carvalho S, Tomás AM. The cytosolic tryparedoxin of Leishmania infantum is essential for parasite survival. Int J Parasitol. 2009;39(6):703‐711. [DOI] [PubMed] [Google Scholar]
- 76. Suman SS, Equbal A, Zaidi A, et al. Up‐regulation of cytosolic tryparedoxin in Amp B resistant isolates of Leishmania donovani and its interaction with cytosolic tryparedoxin peroxidase. Biochimie. 2016;121:312‐325. [DOI] [PubMed] [Google Scholar]
- 77. Salari S, Sharifi I, Bamorovat M, Almani PG. The immunity of the recombinant prokaryotic and eukaryotic subunit vaccines against cutaneous leishmaniasis. Microb Pathog. 2021;153:104807. [DOI] [PubMed] [Google Scholar]
- 78. Berberich C, Machado G, Morales G, Carrillo G, Jiménez‐Ruiz A, Alonso C. The expression of the Leishmania infantum KMP‐11 protein is developmentally regulated and stage specific. Biochim Biophys Acta. 1998;1442(2–3):230‐237. [DOI] [PubMed] [Google Scholar]
- 79. Li Z, Wang CC. KMP‐11, a basal body and flagellar protein, is required for cell division in Trypanosoma brucei. Eukaryot Cell. 2008;7(11):1941‐1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Salari S, Sharifi I, Keyhani AR, Ghasemi Nejad Almani P. Evaluation of a new live recombinant vaccine against cutaneous leishmaniasis in BALB/c mice. Parasit Vectors. 2020;13(1):415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fuertes MA, Berberich C, Lozano RM, Gimenez‐Gallego G, Alonso C. Folding stability of the kinetoplastid membrane protein‐11 (KMP‐11) from Leishmania infantum. Eur J Biochem. 1999;260(2):559‐567. [DOI] [PubMed] [Google Scholar]
- 82. Jardim A, Funk V, Caprioli RM, Olafson RW. Isolation and structural characterization of the Leishmania donovani kinetoplastid membrane protein‐11, a major immunoreactive membrane glycoprotein. Biochem J. 1995;305(pt 1):307‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dey S, Ouellette M, Lightbody J, Papadopoulou B, Rosen BP. An ATP‐dependent As(III)‐glutathione transport system in membrane vesicles of Leishmania tarentolae. Proc Natl Acad Sci U S A. 1996;93(5):2192‐2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rosenzweig D, Smith D, Myler PJ, Olafson RW, Zilberstein D. Post‐translational modification of cellular proteins during Leishmania donovani differentiation. Proteomics. 2008;8(9):1843‐1850. [DOI] [PubMed] [Google Scholar]
- 85. Polevoda B, Sherman F. Nalpha ‐terminal acetylation of eukaryotic proteins. J Biol Chem. 2000;275(47):36479‐36482. [DOI] [PubMed] [Google Scholar]
- 86. El Fadili K, Drummelsmith J, Roy G, Jardim A, Ouellette M. Down regulation of KMP‐11 in Leishmania infantum axenic antimony resistant amastigotes as revealed by a proteomic screen. Exp Parasitol. 2009;123(1):51‐57. [DOI] [PubMed] [Google Scholar]
- 87. Fairlamb AH, Cerami A. Metabolism and functions of trypanothione in the Kinetoplastida. Annu Rev Microbiol. 1992;46:695‐729. [DOI] [PubMed] [Google Scholar]
- 88. Yan S, Li F, Ding K, Sun H. Reduction of pentavalent antimony by trypanothione and formation of a binary and ternary complex of antimony(III) and trypanothione. J Biol Inorg Chem. 2003;8(6):689‐697. [DOI] [PubMed] [Google Scholar]
- 89. Wyllie S, Cunningham ML, Fairlamb AH. Dual action of antimonial drugs on thiol redox metabolism in the human pathogen Leishmania donovani. J Biol Chem. 2004;279(38):39925‐39932. [DOI] [PubMed] [Google Scholar]
- 90. Dumas C, Ouellette M, Tovar J, et al. Disruption of the trypanothione reductase gene of Leishmania decreases its ability to survive oxidative stress in macrophages. EMBO J. 1997;16(10):2590‐2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Drummelsmith J, Brochu V, Girard I, Messier N, Ouellette M. Proteome mapping of the protozoan parasite Leishmania and application to the study of drug targets and resistance mechanisms. Mol Cell Proteomics. 2003;2(3):146‐155. [DOI] [PubMed] [Google Scholar]
- 92. Bagher Khadem Erfan M, Mohebali M, Kazemi‐Rad E, et al. Downregulation of calcineurin gene is associated with glucantime([R]) resiatance in Leishmania infantum. Iran J Parasitol. 2013;8(3):359‐366. [PMC free article] [PubMed] [Google Scholar]
- 93. Blankenship JR, Wormley FL, Boyce MK, et al. Calcineurin is essential for Candida albicans survival in serum and virulence. Eukaryot Cell. 2003;2(3):422‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Naderer T, Dandash O, McConville MJ. Calcineurin is required for Leishmania major stress response pathways and for virulence in the mammalian host. Mol Microbiol. 2011;80(2):471‐780. [DOI] [PubMed] [Google Scholar]
- 95. Aramburu J, Heitman J, Crabtree GR. Calcineurin: a central controller of signalling in eukaryotes. EMBO Rep. 2004;5(4):343‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev. 2000;80(4):1483‐1521. [DOI] [PubMed] [Google Scholar]
- 97. Dhein J, Walczak H, Bäumler C, Debatin KM, Krammer PH. Autocrine T‐cell suicide mediated by APO‐1/(Fas/CD95). Nature. 1995;373(6513):438‐441. [DOI] [PubMed] [Google Scholar]
- 98. Bishopric NH, Andreka P, Slepak T, Webster KA. Molecular mechanisms of apoptosis in the cardiac myocyte. Curr Opin Pharmacol. 2001;1(2):141‐150. [DOI] [PubMed] [Google Scholar]
- 99. Lindoso JA, Cotrim PC, Goto H. Apoptosis of Leishmania (Leishmania) chagasi amastigotes in hamsters infected with visceral leishmaniasis. Int J Parasitol. 2004;34(1):1‐4. [DOI] [PubMed] [Google Scholar]
- 100. Sereno D, Holzmuller P, Mangot I, Cuny G, Ouaissi A, Lemesre JL. Antimonial‐mediated DNA fragmentation in Leishmania infantum amastigotes. Antimicrob Agents Chemother. 2001;45(7):2064‐2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Williamson MP. The structure and function of proline‐rich regions in proteins. Biochem J. 1994;297(Pt 2):249‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Genest PA, Haimeur A, Legare D, et al. A protein of the leucine‐rich repeats (LRRs) superfamily is implicated in antimony resistance in Leishmania infantum amastigotes. Mol Biochem Parasitol. 2008;158(1):95‐99. [DOI] [PubMed] [Google Scholar]
- 103. Perez‐Victoria FJ, Gamarro F, Ouellette M, Castanys S. Functional cloning of the miltefosine transporter. A novel P‐type phospholipid translocase from Leishmania involved in drug resistance. J Biol Chem. 2003;278(50):49965‐49971. [DOI] [PubMed] [Google Scholar]
- 104. Perez‐Victoria FJ, Sanchez‐Canete MP, Castanys S, Gamarro F. Phospholipid translocation and miltefosine potency require both L. donovani miltefosine transporter and the new protein LdRos3 in Leishmania parasites. J Biol Chem. 2006;281(33):23766‐23775. [DOI] [PubMed] [Google Scholar]
- 105. Mondelaers A, Sanchez‐Canete MP, Hendrickx S, et al. Genomic and molecular characterization of miltefosine resistance in Leishmania infantum strains with either natural or acquired resistance through experimental selection of intracellular amastigotes. PLoS One. 2016;11(4):e0154101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Tejera Nevado P, Bifeld E, Hohn K, Clos J. A telomeric cluster of antimony resistance genes on chromosome 34 of Leishmania infantum. Antimicrob Agents Chemother. 2016;60(9):5262‐5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Schafer C, Tejera Nevado P, Zander D, Clos J. Reduced antimony accumulation in ARM58‐overexpressing Leishmania infantum. Antimicrob Agents Chemother. 2014;58(3):1565‐1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hombach A, Ommen G, MacDonald A, Clos J. A small heat shock protein is essential for thermotolerance and intracellular survival of Leishmania donovani. J Cell Sci. 2014;127(Pt 21):4762‐4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Morales MA, Watanabe R, Dacher M, et al. Phosphoproteome dynamics reveal heat‐shock protein complexes specific to the Leishmania donovani infectious stage. Proc Natl Acad Sci U S A. 2010;107(18):8381‐8386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Szoor B, Dyer NA, Ruberto I, Acosta‐Serrano A, Matthews KR. Independent pathways can transduce the life‐cycle differentiation signal in Trypanosoma brucei. PLoS Pathog. 2013;9(10):e1003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bhandari V, Sundar S, Dujardin JC, Salotra P. Elucidation of cellular mechanisms involved in experimental paromomycin resistance in Leishmania donovani. Antimicrob Agents Chemother. 2014;58(5):2580‐2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Szoor B. Trypanosomatid protein phosphatases. Mol Biochem Parasitol. 2010;173(2):53‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Borthwick EB, Zeke T, Prescott AR, Cohen PT. Nuclear localization of protein phosphatase 5 is dependent on the carboxy‐terminal region. FEBS Lett. 2001;491(3):279‐284. [DOI] [PubMed] [Google Scholar]
- 114. Chinkers M. Protein phosphatase 5 in signal transduction. Trends Endocrinol Metab. 2001;12(1):28‐32. [DOI] [PubMed] [Google Scholar]
- 115. Fernandez‐Perez MP, Montenegro MF, Saez‐Ayala M, et al. Suppression of antifolate resistance by targeting the myosin Va trafficking pathway in melanoma. Neoplasia. 2013;15(7):826‐839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ubeda JM, Legare D, Raymond F, et al. Modulation of gene expression in drug resistant Leishmania is associated with gene amplification, gene deletion and chromosome aneuploidy. Genome Biol. 2008;9(7):R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Mills E, Price HP, Johner A, Emerson JE, Smith DF. Kinetoplastid PPEF phosphatases: dual acylated proteins expressed in the endomembrane system of Leishmania. Mol Biochem Parasitol. 2007;152(1):22‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Pathak MK, Yi T. Sodium stibogluconate is a potent inhibitor of protein tyrosine phosphatases and augments cytokine responses in hemopoietic cell lines. J Immunol. 2001;167(6):3391‐3397. [DOI] [PubMed] [Google Scholar]
- 119. Nakahata S, Morishita K. PP2A inactivation by ROS accumulation. Blood. 2014;124(14):2163‐2165. [DOI] [PubMed] [Google Scholar]
- 120. Moreira W, Leprohon P, Ouellette M. Tolerance to drug‐induced cell death favours the acquisition of multidrug resistance in Leishmania. Cell Death Dis. 2011;2:e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Wilson ME, Andersen KA, Britigan BE. Response of Leishmania chagasi promastigotes to oxidant stress. Infect Immun. 1994;62(11):5133‐5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Bhattacharyya S, Ghosh S, Dasgupta B, Mazumder D, Roy S, Majumdar S. Chemokine‐induced leishmanicidal activity in murine macrophages via the generation of nitric oxide. J Infect Dis. 2002;185(12):1704‐1708. [DOI] [PubMed] [Google Scholar]
- 123. Bannister JV, Bannister WH, Rotilio G. Aspects of the structure, function, and applications of superoxide dismutase. CRC Crit Rev Biochem. 1987;22(2):111‐180. [DOI] [PubMed] [Google Scholar]
- 124. Getachew F, Gedamu L. Leishmania donovani iron superoxide dismutase A is targeted to the mitochondria by its N‐terminal positively charged amino acids. Mol Biochem Parasitol. 2007;154(1):62‐69. [DOI] [PubMed] [Google Scholar]
- 125. Tessarollo NG, Andrade JM, Moreira DS, Murta SM. Functional analysis of iron superoxide dismutase‐A in wild‐type and antimony‐resistant Leishmania braziliensis and Leishmania infantum lines. Parasitol Int. 2015;64(2):125‐129. [DOI] [PubMed] [Google Scholar]
- 126. Papadopoulou B, Roy G, Ouellette M. Frequent amplification of a short chain dehydrogenase gene as part of circular and linear amplicons in methotrexate resistant Leishmania. Nucleic Acids Res. 1993;21(18):4305‐4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cunningham ML, Beverley SM. Pteridine salvage throughout the Leishmania infectious cycle: implications for antifolate chemotherapy. Mol Biochem Parasitol. 2001;113(2):199‐213. [DOI] [PubMed] [Google Scholar]
- 128. Richard D, Leprohon P, Drummelsmith J, Ouellette M. Growth phase regulation of the main folate transporter of Leishmania infantum and its role in methotrexate resistance. J Biol Chem. 2004;279(52):54494‐54501. [DOI] [PubMed] [Google Scholar]
- 129. Thakur CP, Kanyok TP, Pandey AK, et al. A prospective randomized, comparative, open‐label trial of the safety and efficacy of paromomycin (aminosidine) plus sodium stibogluconate versus sodium stibogluconate alone for the treatment of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 2000;94(4):429‐431. [DOI] [PubMed] [Google Scholar]
- 130. Cohen‐Saidon C, Carmi I, Keren A, Razin E. Antiapoptotic function of Bcl‐2 in mast cells is dependent on its association with heat shock protein 90beta. Blood. 2006;107(4):1413‐1420. [DOI] [PubMed] [Google Scholar]
- 131. Vergnes B, Gourbal B, Girard I, Sundar S, Drummelsmith J, Ouellette M. A proteomics screen implicates HSP83 and a small kinetoplastid calpain‐related protein in drug resistance in Leishmania donovani clinical field isolates by modulating drug‐induced programmed cell death. Mol Cell Proteomics. 2007;6(1):88‐101. [DOI] [PubMed] [Google Scholar]
- 132. Kumar A, Sisodia B, Misra P, Sundar S, Shasany AK, Dube A. Proteome mapping of overexpressed membrane‐enriched and cytosolic proteins in sodium antimony gluconate (SAG) resistant clinical isolate of Leishmania donovani. Br J Clin Pharmacol. 2010;70(4):609‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ersfeld K, Barraclough H, Gull K. Evolutionary relationships and protein domain architecture in an expanded calpain superfamily in kinetoplastid parasites. J Mol Evol. 2005;61(6):742‐757. [DOI] [PubMed] [Google Scholar]
- 134. Zhou Y, Messier N, Ouellette M, Rosen BP, Mukhopadhyay R. Leishmania major LmACR2 is a pentavalent antimony reductase that confers sensitivity to the drug pentostam. J Biol Chem. 2004;279(36):37445‐37451. [DOI] [PubMed] [Google Scholar]
- 135. Fyfe PK, Westrop GD, Silva AM, Coombs GH, Hunter WN. Leishmania TDR1 structure, a unique trimeric glutathione transferase capable of deglutathionylation and antimonial prodrug activation. Proc Natl Acad Sci U S A. 2012;109(29):11693‐11698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Olliaro PL, Guerin PJ, Gerstl S, Haaskjold AA, Rottingen JA, Sundar S. Treatment options for visceral leishmaniasis: a systematic review of clinical studies done in India, 1980‐2004. Lancet Infect Dis. 2005;5(12):763‐774. [DOI] [PubMed] [Google Scholar]
- 137. Ferreira Cdos S, Martins PS, Demicheli C, Brochu C, Ouellette M, Frezard F. Thiol‐induced reduction of antimony(V) into antimony(III): a comparative study with trypanothione, cysteinyl‐glycine, cysteine and glutathione. Biometals. 2003;16(3):441‐446. [DOI] [PubMed] [Google Scholar]
- 138. Denton H, McGregor JC, Coombs GH. Reduction of anti‐leishmanial pentavalent antimonial drugs by a parasite‐specific thiol‐dependent reductase, TDR1. Biochem J. 2004;381(Pt 2):405‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Shaked‐Mishan P, Ulrich N, Ephros M, Zilberstein D. Novel intracellular SbV reducing activity correlates with antimony susceptibility in Leishmania donovani. J Biol Chem. 2001;276(6):3971‐3976. [DOI] [PubMed] [Google Scholar]
- 140. Tew KD, Townsend DM. Regulatory functions of glutathione S‐transferase P1‐1 unrelated to detoxification. Drug Metab Rev. 2011;43(2):179‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Louw CA, Ludewig MH, Mayer J, Blatch GL. The Hsp70 chaperones of the Tritryps are characterized by unusual features and novel members. Parasitol Int. 2010;59(4):497‐505. [DOI] [PubMed] [Google Scholar]
- 142. Brochu C, Haimeur A, Ouellette M. The heat shock protein HSP70 and heat shock cognate protein HSC70 contribute to antimony tolerance in the protozoan parasite leishmania. Cell Stress Chaperones. 2004;9(3):294‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Walker J, Gongora R, Vasquez JJ, et al. Discovery of factors linked to antimony resistance in Leishmania panamensis through differential proteome analysis. Mol Biochem Parasitol. 2012;183(2):166‐176. [DOI] [PubMed] [Google Scholar]
- 144. Torres DC, Ribeiro‐Alves M, Romero GA, Davila AM, Cupolillo E. Assessment of drug resistance related genes as candidate markers for treatment outcome prediction of cutaneous leishmaniasis in Brazil. Acta Trop. 2013;126(2):132‐141. [DOI] [PubMed] [Google Scholar]
- 145. Eslami G, Zarchi MV, Moradi A, et al. Aquaglyceroporin1 gene expression in antimony resistance and susceptible Leishmania major isolates. J Vector Borne Dis. 2016;53(4):370‐374. [PubMed] [Google Scholar]
- 146. Rai S, Bhaskar GSK, Nath Dwivedi U, Sundar S, Goyal N. Role of efflux pumps and intracellular thiols in natural antimony resistant isolates of Leishmania donovani. PLoS One. 2013;8(9):e74862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Monte‐Neto R, Laffitte MC, Leprohon P, Reis P, Frezard F, Ouellette M. Intrachromosomal amplification, locus deletion and point mutation in the aquaglyceroporin AQP1 gene in antimony resistant Leishmania (Viannia) guyanensis. PLoS Negl Trop Dis. 2015;9(2):e0003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Andrade JM, Baba EH, Machado‐de‐Avila RA, et al. Silver and nitrate oppositely modulate antimony susceptibility through aquaglyceroporin 1 in Leishmania (Viannia) species. Antimicrob Agents Chemother. 2016;60(8):4482‐4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Leprohon P, Legare D, Girard I, Papadopoulou B, Ouellette M. Modulation of Leishmania ABC protein gene expression through life stages and among drug‐resistant parasites. Eukaryot Cell. 2006;5(10):1713‐1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. BoseDasgupta S, Ganguly A, Roy A, Mukherjee T, Majumder HK. A novel ATP‐binding cassette transporter, ABCG6 is involved in chemoresistance of Leishmania. Mol Biochem Parasitol. 2008;158(2):176‐188. [DOI] [PubMed] [Google Scholar]
- 151. El Fadili K, Messier N, Leprohon P, et al. Role of the ABC transporter MRPA (PGPA) in antimony resistance in Leishmania infantum axenic and intracellular amastigotes. Antimicrob Agents Chemother. 2005;49(5):1988‐1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Anacleto C, Abdo MC, Ferreira AV, et al. Structural and functional analysis of an amplification containing a PGPA gene in a glucantime‐resistant Leishmania (Viannia) guyanensis cell line. Parasitol Res. 2003;90(2):110‐118. [DOI] [PubMed] [Google Scholar]
- 153. Singh R, Kumar D, Duncan RC, Nakhasi HL, Salotra P. Overexpression of histone H2A modulates drug susceptibility in Leishmania parasites. Int J Antimicrob Agents. 2010;36(1):50‐57. [DOI] [PubMed] [Google Scholar]
- 154. Guimond C, Trudel N, Brochu C, et al. Modulation of gene expression in Leishmania drug resistant mutants as determined by targeted DNA microarrays. Nucleic Acids Res. 2003;31(20):5886‐5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Andrade JM, Murta SM. Functional analysis of cytosolic tryparedoxin peroxidase in antimony‐resistant and ‐susceptible Leishmania braziliensis and Leishmania infantum lines. Parasit Vectors. 2014;7:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Wyllie S, Vickers TJ, Fairlamb AH. Roles of trypanothione S‐transferase and tryparedoxin peroxidase in resistance to antimonials. Antimicrob Agents Chemother. 2008;52(4):1359‐1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Maharjan M, Madhubala R. Heat shock protein 70 (HSP70) expression in antimony susceptible/resistant clinical isolates of Leishmania donovani. Nepal J Biotechnol. 2015;3(1):7. [Google Scholar]
- 158. Matrangolo FS, Liarte DB, Andrade LC, et al. Comparative proteomic analysis of antimony‐resistant and ‐susceptible Leishmania braziliensis and Leishmania infantum chagasi lines. Mol Biochem Parasitol. 2013;190(2):63‐75. [DOI] [PubMed] [Google Scholar]
- 159. Torres DC, Adaui V, Ribeiro‐Alves M, et al. Targeted gene expression profiling in Leishmania braziliensis and Leishmania guyanensis parasites isolated from Brazilian patients with different antimonial treatment outcomes. Infect Genet Evol. 2010;10(6):727‐733. [DOI] [PubMed] [Google Scholar]
- 160. Carter KC, Hutchison S, Henriquez FL, et al. Resistance of Leishmania donovani to sodium stibogluconate is related to the expression of host and parasite gamma‐glutamylcysteine synthetase. Antimicrob Agents Chemother. 2006;50(1):88‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ghosh AK, Saini S, Das S, et al. Glucose‐6‐phosphate dehydrogenase and Trypanothione reductase interaction protects Leishmania donovani from metalloid mediated oxidative stress. Free Radic Biol Med. 2017;106:10‐23. [DOI] [PubMed] [Google Scholar]
- 162. Das S, Shah P, Tandon R, et al. Over‐expression of cysteine leucine rich protein is related to sag resistance in clinical isolates of Leishmania donovani. PLoS Negl Trop Dis. 2015;9(8):e0003992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Nuhs A, Schafer C, Zander D, et al. A novel marker, ARM58, confers antimony resistance to Leishmania spp. Int J Parasitol Drugs Drug Resist. 2014;4(1):37‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Das M, Saudagar P, Sundar S, Dubey VK. Miltefosine‐unresponsive Leishmania donovani has a greater ability than miltefosine‐responsive L. donovani to resist reactive oxygen species. FEBS J. 2013;280(19):4807‐4815. [DOI] [PubMed] [Google Scholar]
- 165. Drummelsmith J, Girard I, Trudel N, Ouellette M. Differential protein expression analysis of Leishmania major reveals novel roles for methionine adenosyltransferase and S‐adenosylmethionine in methotrexate resistance. J Biol Chem. 2004;279(32):33273‐33280. [DOI] [PubMed] [Google Scholar]
- 166. Vacchina P, Norris‐Mullins B, Abengozar MA, et al. Genomic appraisal of the multifactorial basis for in vitro acquisition of miltefosine resistance in Leishmania donovani. Antimicrob Agents Chemother. 2016;60(7):4089‐4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


