Single-cell RNA sequencing of porcine ileal lymphocytes reveals similarities to human cells and discovery of porcine intestinal innate lymphoid cells.
Abstract
Lymphocytes can heavily influence intestinal health, but resolving intestinal lymphocyte function is challenging as the intestine contains a vastly heterogeneous mixture of cells. Pigs are an advantageous biomedical model, but deeper understanding of intestinal lymphocytes is warranted to improve model utility. Twenty-six cell types were identified in the porcine ileum by single-cell RNA sequencing and further compared with cells in human and murine ileum. Though general consensus of cell subsets across species was revealed, some porcine-specific lymphocyte subsets were identified. Differential tissue dissection and in situ analyses conferred spatial context, revealing similar locations of lymphocyte subsets in Peyer’s patches and epithelium in pig-to-human comparisons. Like humans, activated and effector lymphocytes were abundant in the ileum but not periphery of pigs, suggesting tissue-specific and/or activation-associated gene expression. Gene signatures for peripheral and ileal innate lymphoid cells newly discovered in pigs were defined and highlighted similarities to human innate lymphoid cells. Overall, we reveal novel lymphocyte subsets in pigs and highlight utility of pigs for intestinal research applications.
Introduction
The intestine is a selectively permeable barrier that absorbs nutrients while simultaneously limiting entry of potentially harmful external organisms and compounds. Thus, the intestinal immune system continuously deciphers between innocuous and dangerous stimuli. Coordination of immune responses is crucial for maintaining intestinal homeostasis; dysregulation of even a small number of cells can negatively impact intestinal health, as evidenced in nonpathogenic inflammatory conditions such as celiac disease, Crohn’s disease, and ulcerative colitis (reviewed by Mowat and Agace [2014], Caio et al [2019], Caminero and Pinto-Sanchez [2020], and Caruso et al [2020]). Intestinal lymphocytes include B cells, T cells, and innate lymphoid cells (ILCs). The importance of lymphocytes in promoting intestinal homeostasis is well-documented in cases of intestinal dysfunction in individuals naturally lacking at least some lymphocyte populations (reviewed by Agarwal and Cunningham-Rundles [2019]) or experimental models where lymphocytes are depleted or immune pathways disrupted (Kühn et al, 1993; Mombaerts et al, 1993; Sadlack et al, 1993; Strober & Ehrhardt, 1993; Gärdby & Lycke, 2000; Laroux et al, 2004; Hepworth et al, 2015; Wang et al, 2017). Lymphocytes can be directed to provide protective adaptive immunity through mucosal vaccination strategies (reviewed by Li et al [2020] and Lavelle and Ward [2021]), whereas immune protection against a broad range of microorganisms may be achieved through nonconventional innate memory in some lymphocytes (reviewed by Wang et al [2019b]). Resultingly, there is pan-disciplinary interest in promoting health through modulation of intestinal lymphocytes, but decoding the complexity and function of these cells is an ongoing challenge. The intestine is a site of vast immune cellular diversity difficult to holistically characterize, yet defining heterogeneity within the cellular landscape of intestinal immune cells, such as lymphocytes, is one initial step to be taken toward better understanding intestinal immune dynamics and resulting effects on health.
Pigs (Sus scrofa) are a promising biomedical model and major global food source, yet the porcine intestinal immune cell landscape is poorly defined relative to humans and rodent models. Deeper exploration of the porcine intestinal immune system, particularly intestinal lymphocytes, will enhance utility of pigs as a well-defined and highly comparable biomedical model for gut health and/or disease (reviewed by Gonzalez et al [2015], Roura et al [2016], Ziegler et al [2016], and Käser [2021]) as pigs have greater physiologic and genetic similarities to humans than rodent models and are less expensive and more easily obtained than nonhuman primates (reviewed by Swindle et al [2011], Gün and Kues [2014], and Kobayashi et al [2018]). Enhanced characterization of porcine intestinal lymphocytes will also provide insight into promoting gut health and associated overall pig health to ultimately decrease disease susceptibility and strengthen pork as a major global food source. Though previous work has described porcine lymphocytes at the protein level (reviewed by Piriou-Guzylack and Salmon [2008]), annotations are confined by a limited toolbox of available porcine protein-specific immunoreagents (reviewed by Entrican et al [2020]). Thus, definitions of porcine lymphocytes lack cellular resolution comparable to that of humans. This is particularly true for B cells, as a pan-B cell–specific extracellular protein marker is not available (reviewed by Piriou-Guzylack and Salmon [2008] and Sinkora and Butler [2009]), and ILCs, for which only natural killer (NK) cells have been identified (reviewed by Gerner et al [2009]). Approaches to resolve the porcine immune cell landscape at the transcriptional level have also been employed; however, traditional bulk RNA sequencing (RNA-seq) or microarray approaches fail to provide cellular resolution needed to decode such a complex cellular community (Herrera-Uribe et al, 2021), especially when immunoreagents for sorting of cells into more homogenous populations are lacking. Numerous studies have assessed transcriptional dynamics in the porcine intestinal tract but did not attempt to deconvolute cells into specific populations, a critical step in understanding functions of specific cells (Wang et al, 2008, 2019a; Freeman et al, 2012; Mach et al, 2014; Zhu et al, 2014; Inoue et al, 2015; Tan et al, 2017; Maroilley et al, 2018; Beiki et al, 2019; Meng et al, 2020; Summers et al, 2020; Jin et al, 2021; Pan et al, 2021). Some bulk RNA-seq studies have sorted porcine immune cells into specific populations based on cell surface markers but primarily focused on studying cells from the periphery and non-intestinal tissues (Auray et al, 2016, 2020; Foissac et al, 2019; Herrera-Uribe et al, 2021; Kim et al, 2021). Consequently, it remains to be determined whether existing data adequately portray the transcriptional heterogeneity of intestinal immune cells or if novelties exist in the context of the porcine intestine.
Single-cell RNA-seq (scRNA-seq) has been used to describe porcine immune cell transcriptomes at granularity unparalleled by bulk RNA-seq or microarray approaches, including in peripheral blood (Herrera-Uribe et al, 2021), lung (Zhang et al, 2021b), skin (Han et al, 2022), brain (Zhu et al, 2021), and embryos (Ramos-Ibeas et al, 2019; Kong et al, 2020; Liu et al, 2021). In addition, epithelial cells in the porcine intestine were recently queried via scRNA-seq, and results provide new insight into biological development and epithelial cell functions (Meng et al, 2021). However, high-resolution transcriptomic analysis of porcine intestinal immune cells remains to be completed. We therefore used scRNA-seq to provide the first high-resolution, global transcriptomic profiles of porcine intestinal lymphocytes. Interrogation was focused to the ileum, the most distal segment of the small intestine, which contains a unique combination of not only lymphocytes residing in the lamina propria and epithelium but also lymphocytes found in association with gut-associated lymphoid tissue (GALT) called Peyer’s patches. Peyer’s patches are major sites of immune induction not highly prevalent in other intestinal segments (Keren et al, 1978; Fujihashi et al, 2001; Mora et al, 2003; Kwa et al, 2006; Kiriya et al, 2007; Nagai et al, 2007; Bonnardel et al, 2015). In pigs, ileal Peyer’s patches present as a continuous longitudinal strip along the length of the distal small intestine (Binns & Licence, 1985; Rothkötter, 2009) and are more easily identified and obtained compared with Peyer’s patches in humans and rodents, the species in which scRNA-seq approaches have been mostly employed. Consequently, pigs are an ideal candidate for studying Peyer’s patches because of easier gross identification and isolation for further study, but comparability of cells in porcine versus human Peyer’s patches need be determined.
By performing scRNA-seq on porcine ileal-derived cells, we documented and showcased previously undescribed levels of cellular heterogeneity for multiple populations of lymphocytes and some non-lymphocytes. Profiling of porcine ileal cells was completed with multiple approaches, including cross-location and cross-species analyses. Data were compared with an annotated reference scRNA-seq dataset of porcine PBMCs (Herrera-Uribe et al, 2021) to reveal transcriptional differences between porcine intestinal-derived cells and circulating counterparts. Comparison to human and murine ileum reference datasets (Xu et al, 2019; Elmentaite et al, 2020) unveiled similarities and differences for cells of the same intestinal location across species. We further recognized cells associated specifically with Peyer’s patches or the epithelium/lamina propria and confirmed findings by in situ and ex vivo detection using available canonical cell markers with locational context to further infer potential cell functions. Previously undescribed lymphocyte populations in pigs, particularly intestinal ILCs, were identified and characterized. We further leveraged our single-cell gene expression profiles to develop new cell marker combinations with currently available immunoreagents to label novel populations. ILC locational context within the ileum was determined, and transcriptional distinctions from circulating NK cells were denoted. Collectively, the data serve as a transcriptomic atlas of the porcine intestinal immune landscape resolved at the highest level of resolution (i.e., single-cell) to date and may be used to further decode cellular phenotype and function within the intestinal tract. To address research questions outside of the scope of this work, data are also available for interactive, online query (see Data Availability section).
Results
Experimental overview
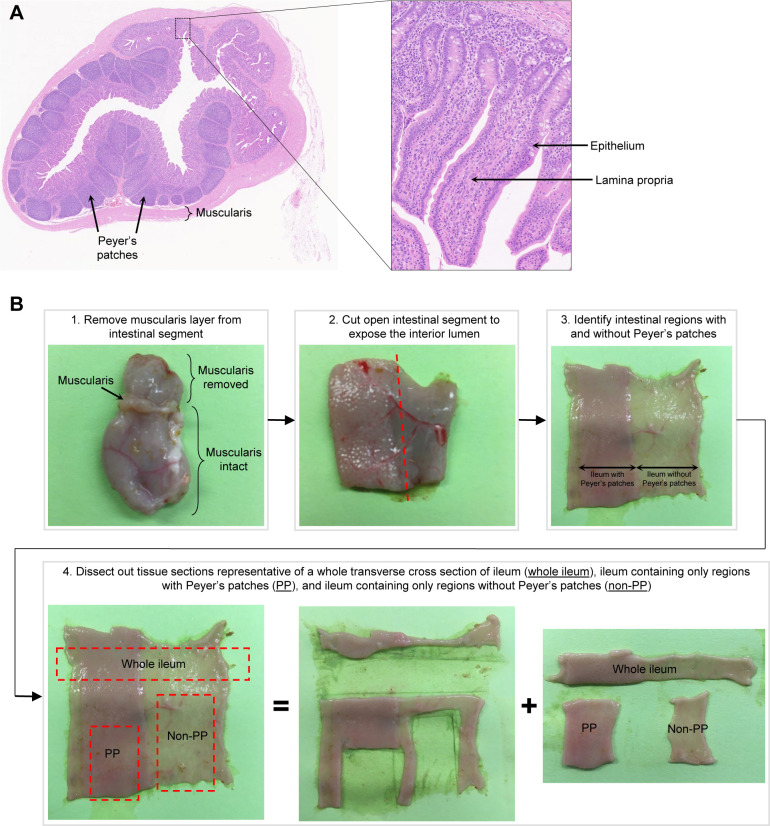
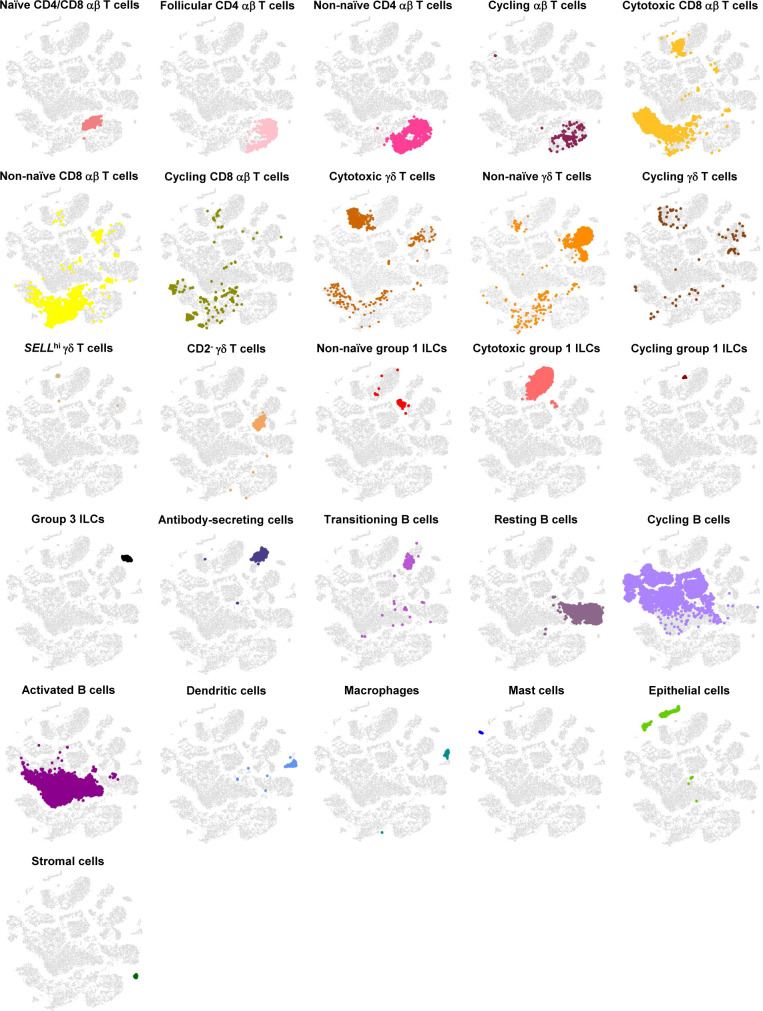
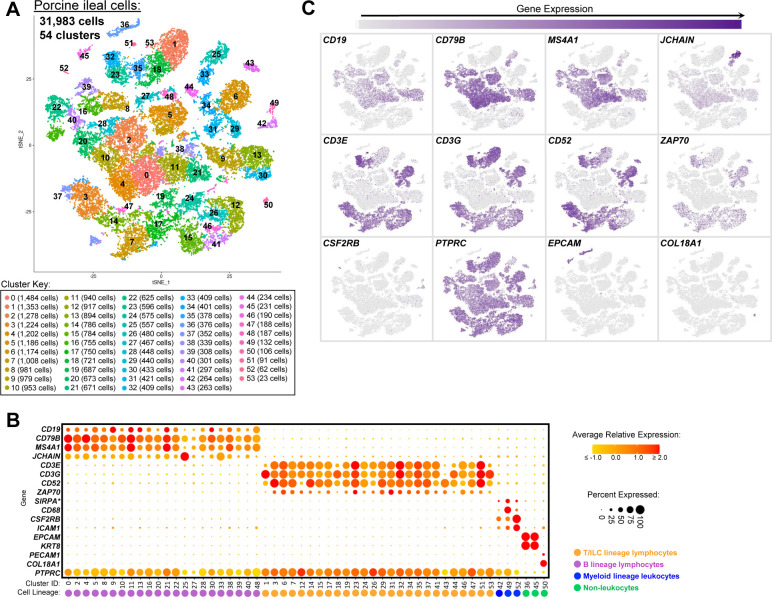
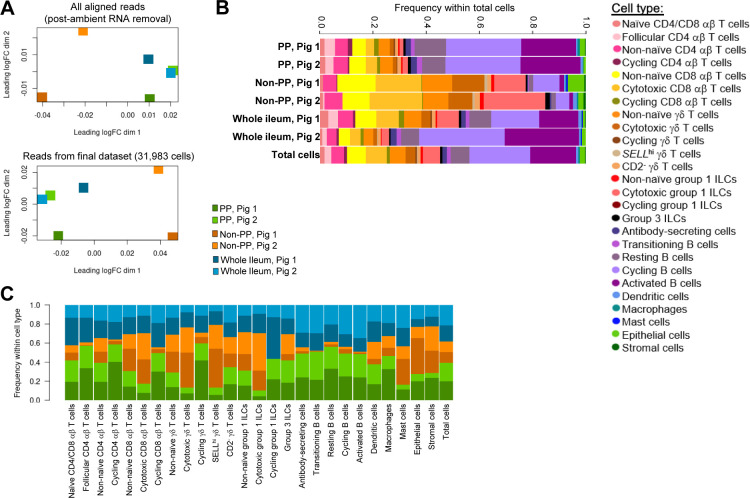
From each of two pigs, the distal ileum was grossly dissected into three distinct sections for cell isolation: (1) ileal tissue containing only regions with Peyer’s patches (PP), (2) ileal tissue excluding regions with Peyer’s patches (non-PP), and (3) a complete cross section of ileal tissue containing both regions (whole ileum; Figs 1A and S1A and B). For each region, a single-cell suspension of combined epithelium, lamina propria, Peyer’s patches (if present), and submucosa was retrieved, enriched for viable lymphocytes, and submitted for scRNA-seq, as described in the Materials and Methods section. Sequencing and further processing/quality control of scRNA-seq data are fully described in the Materials and Methods section and are shown in Fig S2A–E and Table S1. Our final dataset contained 31,983 total cells from six ileal samples (Fig 1B). Cells were classified into four cell lineages and further annotated as 26 cell types (Figs 1C and D and S3) using a multi-method annotation approach described fully in the Materials and Methods section and shown in Figs S4–S10 and Tables S2–S7. Cell type annotations were based on biological interpretation of genes encoding for both phenotypic and functional markers.
Figure 1. Experimental overview and annotation of cells recovered from scRNA-seq of the porcine ileum.

(A) Ileal samples collected from two 7-wk-old pigs for scRNA-seq. Left: representative image of tissue collection site from the ileum of the distal small intestine within the porcine gastrointestinal tract. Right: representative images of tissue dissections from transverse cross sections of the ileum. Dissections from each pig included a cross section of the whole ileum including areas with and without Peyer’s patches (whole ileum), the ileum containing only regions with Peyer’s patches (PP), and the ileum containing only regions without Peyer’s patches (non-PP), resulting in a total of six samples processed for scRNA-seq. (A) Two-dimensional t-SNE visualization of 31,983 cells isolated from porcine ileal samples described in (A), subjected to scRNA-seq, and included in the final dataset following data processing and quality filtering. Each point represents a single cell. (B, C, D) Plots show which sample cells are derived from (B) and cell lineage (C) or cell type (D) annotations. In (B), cells in individual panels are derived from a specified sample. In (C, D), the color of a cell indicates cell lineage (C) or cell type (D) annotation. (B, C, D) The number of cells belonging to each sample (B), cell lineage (C), and cell type (D) are listed next to corresponding panels. Abbreviations: ILC, innate lymphoid cell; PP, Peyer’s patch; scRNA-seq, single-cell RNA sequencing; tSNE, t-distributed stochastic neighbor embedding.
Figure S1. Histology and dissection of the porcine ileum.
(A) Transverse cross section of the ileum collected from the distal small intestine of a 7-wk-old pig used for scRNA-seq as shown in Fig 1A and stained with hemotoxylin (purple) and eosin (pink). Histological structures corresponding to tissue muscularis, Peyer’s patches, epithelium, and lamina propria are indicated. (B) Representative images of tissue dissections performed on the ileum to obtain a whole transverse cross section of the ileum (whole ileum), the ileum containing only regions with Peyer’s patches (PP), and the ileum containing only regions without Peyer’s patches (non-PP). Images shown in (B) were from a 9-wk-old pig and were not from animals used for scRNA-seq. Abbreviations: PP, Peyer’s patch; scRNA-seq, single-cell RNA sequencing.
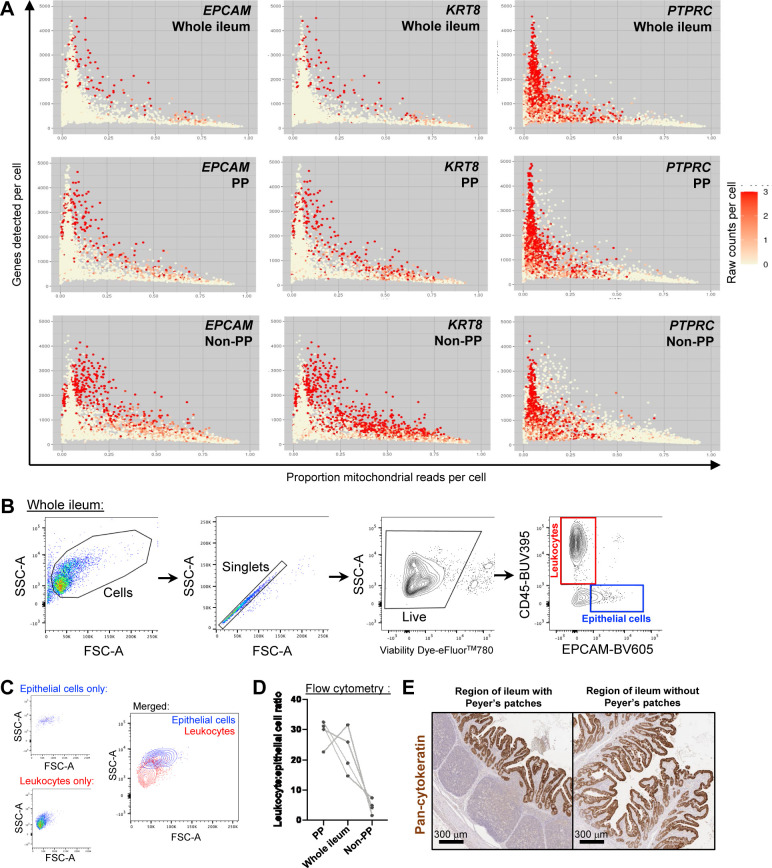
Figure S2. Enrichment of poor-quality epithelial cells in the ileum without Peyer’s patches.
(A) Plots of two quality control metrics (genes detected per cell [y-axis] and proportion mitochondrial reads per cell [x-axis]) used to identify and filter out poor quality cells from scRNA-seq data. Each point represents a single cell. Point fill color corresponds to raw gene counts for epithelial genes EPCAM (left) and KRT8 (center) and pan-leukocyte gene PTPRC (right). Plots are shown from the whole ileum (top), PP (middle), and non-PP (bottom) samples collected from one 7-wk-old pig used for scRNA-seq. (B) Flow cytometry gating strategy to identify leukocytes (CD45+) and epithelial cells (EPCAM+) from total live cells isolated from the porcine ileum. Gating is shown for a whole-ileum sample (containing both regions with and without Peyer’s patches). (B, C) Overlay of gated leukocytes and epithelial cells from (B) onto original forward- and side-scatter coordinates to infer parameters of cell size and complexity, respectively, that are consistent with leukocytes and epithelial cells. Ratio of the number of leukocytes to the number of epithelial cells (y-axis) identified by flow cytometry gating shown in (B). Cells were isolated from three types of ileal dissections (x-axis); samples derived from different ileal dissections of the same pig are connected with a gray line. (E) IHC staining for epithelial pan-cytokeratin protein (brown) in a region of the ileum with Peyer’s patches (left) or without Peyer’s patches (right). Flow cytometry and IHC experiments were not performed on animals used for scRNA-seq. (B, C, D) Flow cytometry experiments shown in (B, C, D) were conducted using four 6-wk-old pigs. (E) IHC staining in (E) was completed on a 5-wk-old pig. Abbreviations: FSC-A, forward scatter area; FSC-H, forward scatter height; IHC, immunohistochemistry; PP, Peyer’s patch; scRNA-seq, single-cell RNA sequencing; SSC-A, side scatter area.
Figure S3. Overlay of cell type annotations onto t-SNE visualization of cells from porcine-ileum scRNA-seq data.
Overlay of 26 annotated cell types onto two-dimensional t-SNE visualization of 31,983 cells recovered from the ileum of two 7-wk-old pigs via scRNA-seq. Each point represents a single cell. Cell type is indicated in a respective panel by one of 26 colors corresponding to cell types shown in Fig 1D, whereas all other cells not corresponding to a specified cell type are shown in light gray. Abbreviations: ILC, innate lymphoid cell; scRNA-seq, single-cell RNA sequencing; t-SNE, t-distributed stochastic neighbor embedding.
Figure S4. Cell lineage annotation of cells from porcine-ileum scRNA-seq data.
(A) Two-dimensional t-SNE visualization of 31,983 cells recovered from the porcine ileum via scRNA-seq. Each point represents a single cell; color of a point corresponds to one of 54 cell clusters a cell belonged to, with more transcriptionally similar cells belonging to the same cluster. The number of cells belonging to each cluster is listed in the cluster key. (B) Gene expression patterns of selected canonical genes (y-axis) across cell clusters shown in (A) (x-axis). Within the plot, size of a dot corresponds to the percentage of cells expressing a gene within a cell cluster; color of a dot corresponds to average expression level of a gene for those cells expressing it within a cell cluster relative to all other cells in the dataset shown in (A). Below cluster ID on the x-axis, the color of a circle corresponds to cell lineage annotation given to each cluster. (C) Expression of a subset of canonical genes from (B) overlaid onto two-dimensional t-SNE visualization coordinates of cells shown in (A). Color of a point corresponds to expression level of a specified gene within a cell relative to all other cells in the dataset shown in (A). scRNA-seq data shown in (A, B, C) were derived from the ileum of two 7-wk-old pigs. *Ensembl identifiers found in gene annotation were converted to gene symbols; refer to methods section “Gene name modifications” for more details Abbreviations: ILC, innate lymphoid cell; scRNA-seq, single-cell RNA sequencing; t-SNE, t-distributed stochastic neighbor embedding.
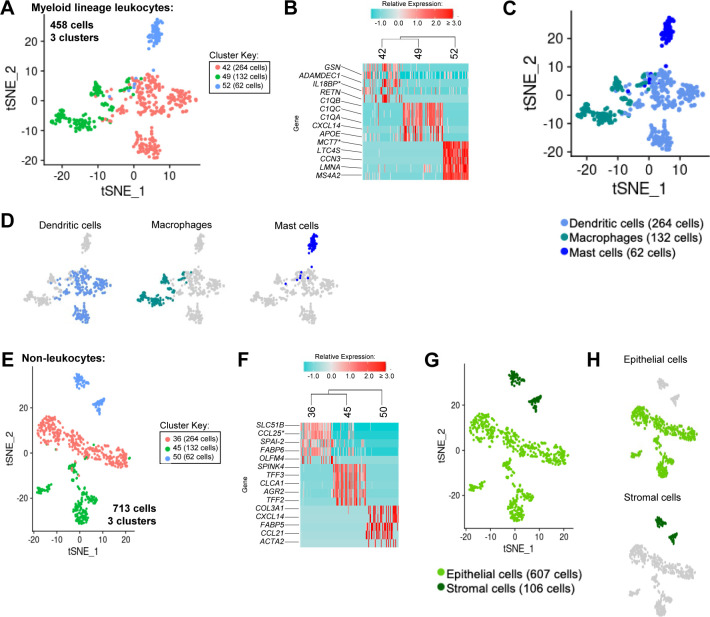
Figure S10. Annotation of non-lymphocytes from porcine-ileum scRNA-seq data.
(A) Two-dimensional t-SNE visualization of 458 cells recovered from the porcine ileum via scRNA-seq and classified as myeloid lineage leukocytes in Figs 1C and S4B. Each point represents a single cell; color of a point corresponds to one of three cell clusters a cell belongs to, with more transcriptionally similar cells belonging to the same cell cluster. The number of cells belonging to each cell cluster is listed in the cluster key. (B) Heatmap of top differentially expressed genes within each cell cluster shown in (A). Up to five differentially expressed genes with the highest positive logFC values were selected for each cell cluster. Genes were differentially expressed in a specified cell cluster relative to the average of all other cells in the dataset shown in (A). Gene expression profiles from up to 100 cells of each cell cluster are shown in the heatmap, with each column representing a single cell. Selected gene names are shown on the y-axis, and cell cluster IDs are shown on the x-axis. Hierarchical relationships of cell clusters are shown using a phylogenetic tree at the top of the heatmap. (C) Myeloid lineage leukocyte annotations established from cell clusters in (B) overlaid onto two-dimensional t-SNE visualization coordinates of cells shown in (A). Color of a point corresponds to cell type annotation. The number of cells belonging to each cell type is listed in the color key below. (D) Overlay of individual cell types onto two-dimensional t-SNE visualization shown in (C). Cell type is indicated in a respective panel by one of three colors corresponding to cell types shown in (C), whereas all other cells not corresponding to a specified cell type are shown in light gray. (E) Two-dimensional t-SNE visualization of 713 cells recovered from the porcine ileum via scRNA-seq and classified as non-leukocytes in Figs 1C and S4B. Each point represents a single cell; color of a point corresponds to one of three cell clusters a cell belonged to, with more transcriptionally similar cells belonging to the same cell cluster. The number of cells belonging to each cell cluster is listed in the cluster key. (F) Heatmap of top differentially expressed genes within each cell cluster shown in (E). Up to five differentially expressed genes with the highest positive logFC values were selected for each cell cluster. Genes were differentially expressed in a specified cell cluster relative to the average of all other cells in the dataset shown in (E). Gene expression profiles from up to 100 cells of each cell cluster are shown in the heatmap, with each column representing a single cell. Gene names are shown on the y-axis, and cell cluster IDs are shown on the x-axis. Hierarchical relationships of cell clusters are shown using a phylogenetic tree at the top of the heatmap. (G) Non-leukocyte annotations established from cell clusters in (F) overlaid onto two-dimensional t-SNE visualization coordinates of cells shown in (E). Color of a point corresponds to cell type annotation. The number of cells belonging to each cell type is listed in the color key below. (H) Overlay of individual cell types onto two-dimensional t-SNE visualization shown in (G). Cell type is indicated in a respective panel by one of three colors corresponding to cell types shown in (G), whereas all other cells not corresponding to a specified cell type are shown in light gray. scRNA-seq data shown in (A, B, C, D, E, F, G, H) were derived from the ileum of two 7-wk-old pigs. *Ensembl identifiers found in gene annotation were converted to gene symbols; refer to methods section “Gene name modifications” for more details. Abbreviations: logFC, log fold-change; scRNA-seq, single-cell RNA sequencing; t-SNE, t-distributed stochastic neighbor embedding.
Table S1 Quality control metrics for scRNA-seq samples. (12.7KB, xlsx)
Table S8 Differential gene expression and biological process enrichment results in porcine ileal T/ILCs. (646.1KB, xlsx)
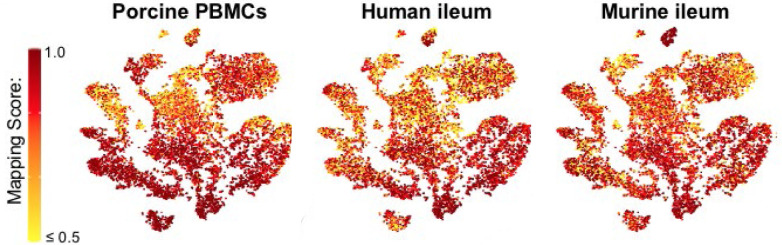
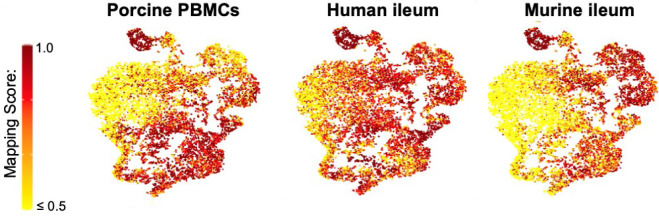
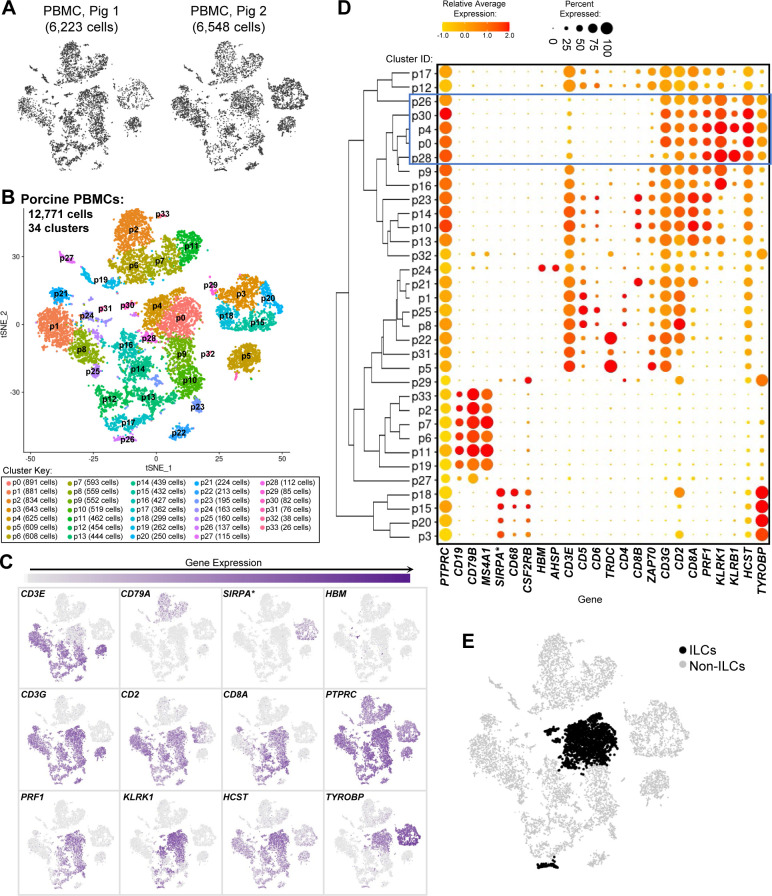
Annotated porcine ileal cells were next treated as query data for comparison to existing scRNA-seq datasets (as described in the Materials and Methods section, “Reference-based label transfer and mapping”) to provide greater insight into annotated cell identities. Because a comparable porcine intestinal scRNA-seq dataset was not available, scRNA-seq reference data of healthy porcine PBMCs (Herrera-Uribe et al, 2021), human ileum (Elmentaite et al, 2020), and murine ileum (Xu et al, 2019) were used to provide intraspecies/inter-tissue and interspecies/intra-tissue comparisons. Degree of similarity between query and reference cells was determined by calculating mapping scores via reference-based cell mapping (Fig S11). Transfer of cell labels from reference onto query single cells provided prediction probabilities to cell types described in each reference dataset (Fig S12). Gene expression profiles, enrichment of biological processes, and reference-based mapping and cell type prediction results for lymphocytes are presented in the next two results sections.
Figure S11. Mapping scores of porcine ileal cells to reference scRNA-seq datasets.
Mapping scores from mapping of porcine-ileum scRNA-seq query data to reference scRNA-seq datasets of porcine PBMCs (left), human ileum (center), and murine ileum (right) overlaid onto two-dimensional t-SNE visualization of porcine-ileum scRNA-seq data shown in Fig 1C and D. Each point represents a single cell; the color of each point indicates mapping score to a corresponding reference dataset. Higher mapping scores indicate better representation of a cell from the porcine ileum in a specified reference dataset. Query scRNA-seq data were derived from the ileum of two 7-wk-old pigs. Abbreviations: PBMC, peripheral blood mononuclear cell; scRNA-seq, single-cell RNA sequencing; t-SNE, t-distributed stochastic neighbor embedding.
Figure S12. Prediction scores of porcine ileal cells to annotated cell types in reference scRNA-seq datasets.
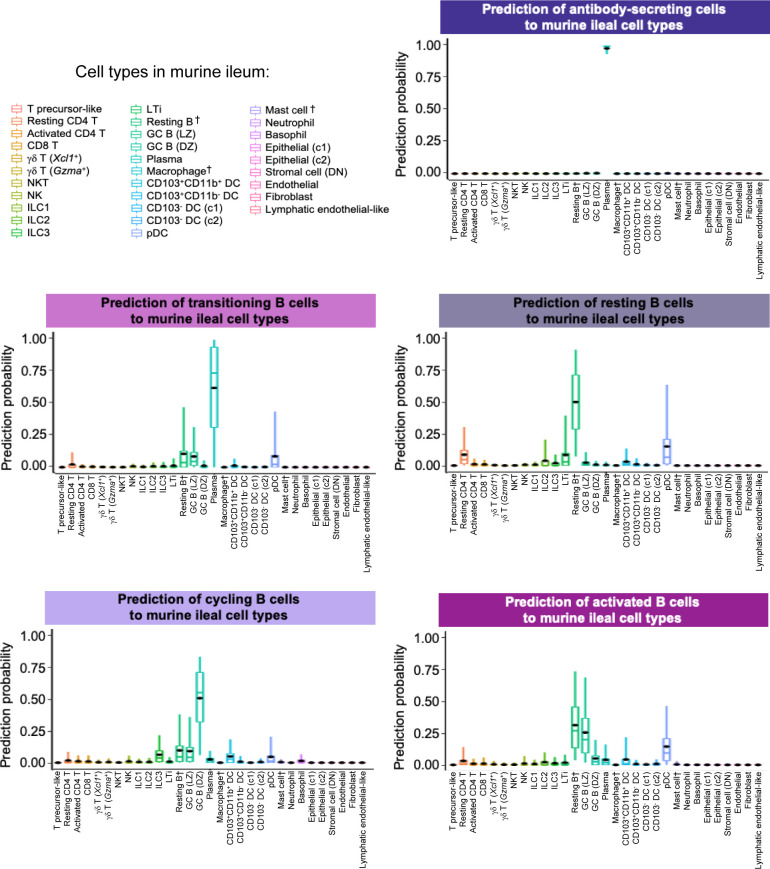
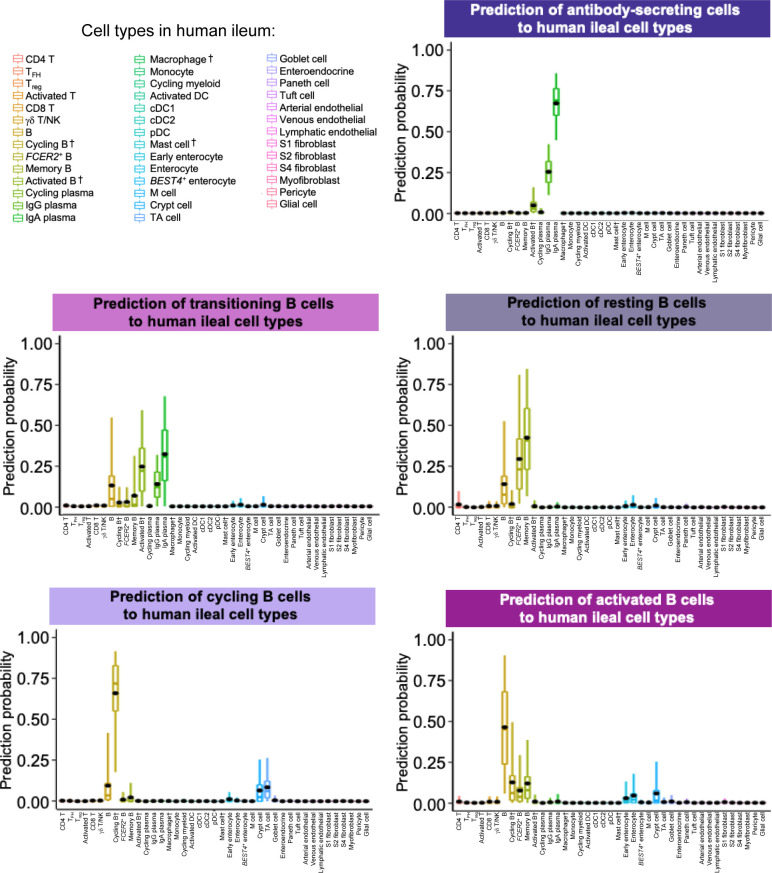
(A, B, C) Prediction probabilities for porcine-ileum scRNA-seq query data from label transfer of annotated cell types in reference scRNA-seq datasets of (A) porcine PBMCs, (B) human ileum, and (C) murine ileum overlaid onto two-dimensional t-SNE visualization of porcine-ileum scRNA-seq data shown in Fig 1C and D. Each point represents a single cell; the color of each point indicates prediction probability to a corresponding cell type annotation from a specified reference dataset. Cell lineage of each annotated reference cell type is indicated by a circle next to each respective annotated cell type name. Within each of (A, B, C), cumulative prediction probabilities for each cell across all annotated reference cell types are equal to one. Query scRNA-seq data were derived from the ileum of two 7-wk-old pigs. † Identical cell type annotations were given to cells in both the porcine ileum and a reference scRNA-seq dataset. Cell type annotations were given to each dataset by independent rationales, and identical annotations do not necessarily indicate identical cell types were recovered from both porcine-ileum and reference data. Abbreviations: ASC, antibody-secreting cell; c1, cluster 1; c2, cluster 2; cDC, convnentional dendritic cell; DC, dendritic cell; DN, double-negative; DZ, dark zone; GC, germinal center; ILC, innate lymphoid cell; LTi, lymphoid tissue inducer; LZ, light zone; PBMC, peripheral blood mononuclear cell; pDC, plasmacytoid dendritic cell; NK, natural killer; NKT, natural killer T; scRNA-seq, single-cell RNA sequencing; t-SNE, t-distributed stochastic neighbor embedding; TA, transit amplifying; TFH, T follicular helper; Treg, T regulatory.
The main purpose of this work was to deeply characterize porcine ileal lymphocytes, and most of the cells across all six ileal samples were annotated as belonging to B (50.25%) or T/ILC (46.09%) lymphocyte lineages. However, some non-lymphocytes were also identified, including myeloid lineage leukocytes (1.43%) and non-leukocytes (2.23%). Myeloid lineage leukocytes were composed of DCs (264 cells), macrophages (132 cells), and mast cells (62 cells). Identified non-leukocytes included epithelial (607 cells) and stromal (106 cells) cells. Because characterization of non-lymphocytes was not our primary intent for this work, non-leukocytes are not discussed further, but data are available for deeper inquiry (see Fig S10 and Tables S6 and S7 and our Data Availability section).
Defining the porcine ileal immune landscape: T cells and ILCs
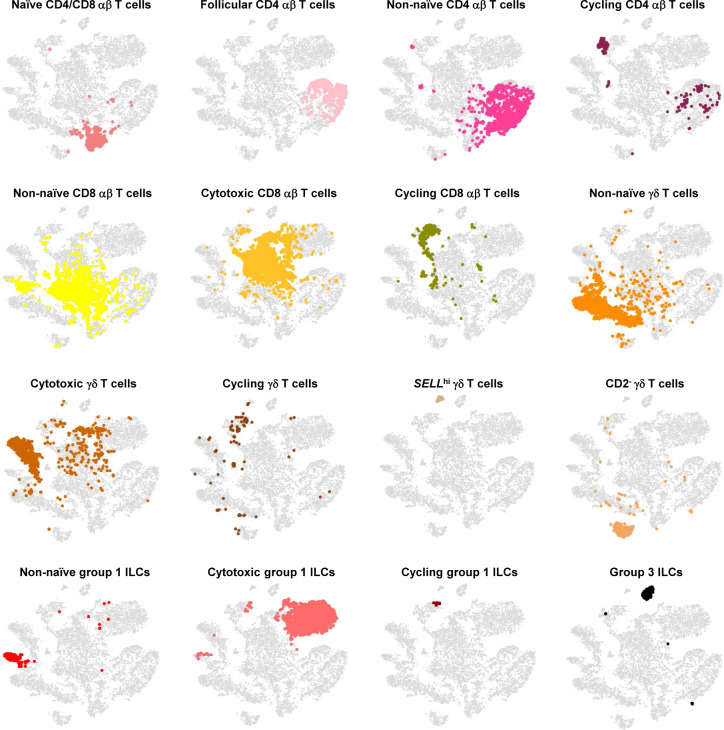
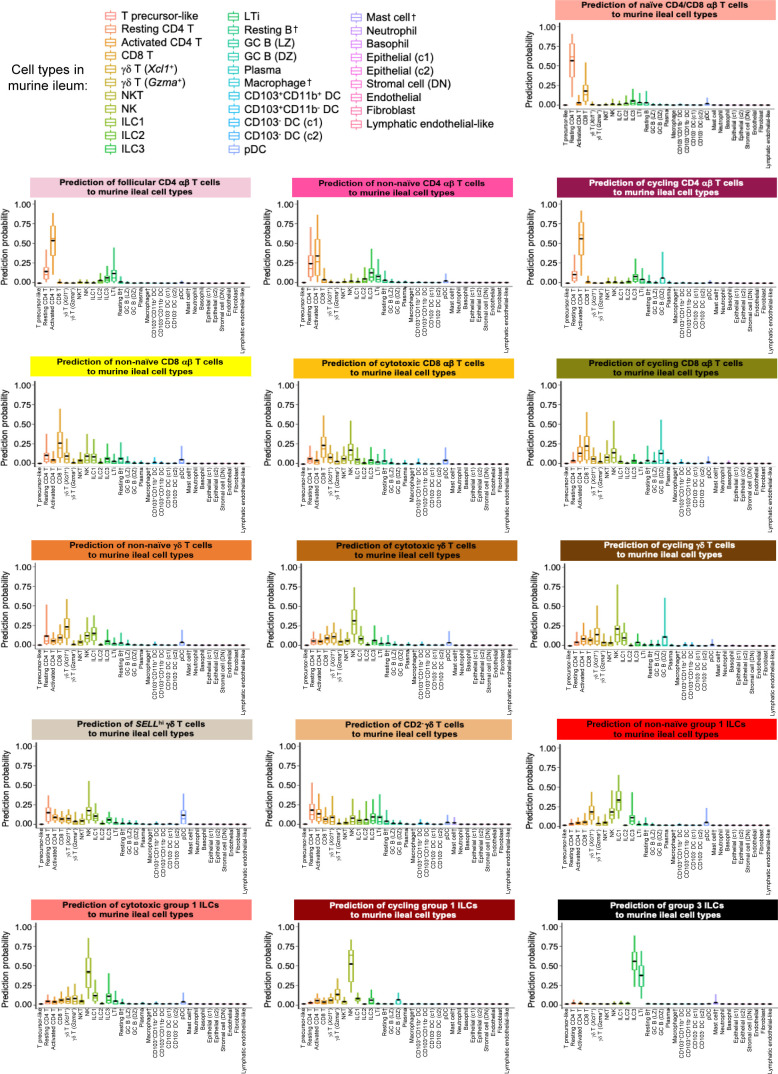
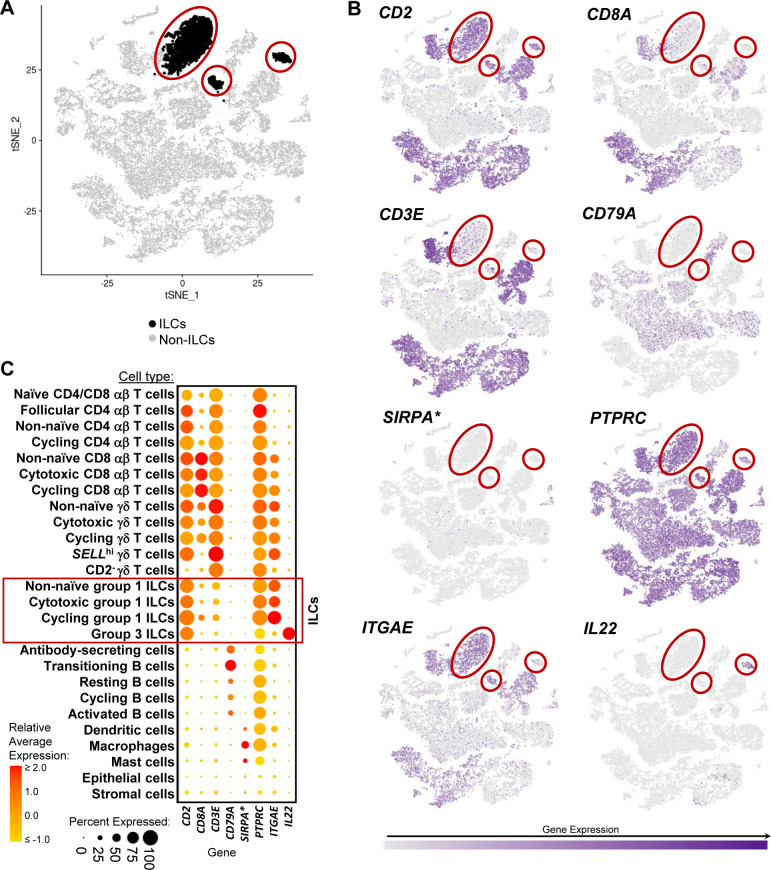
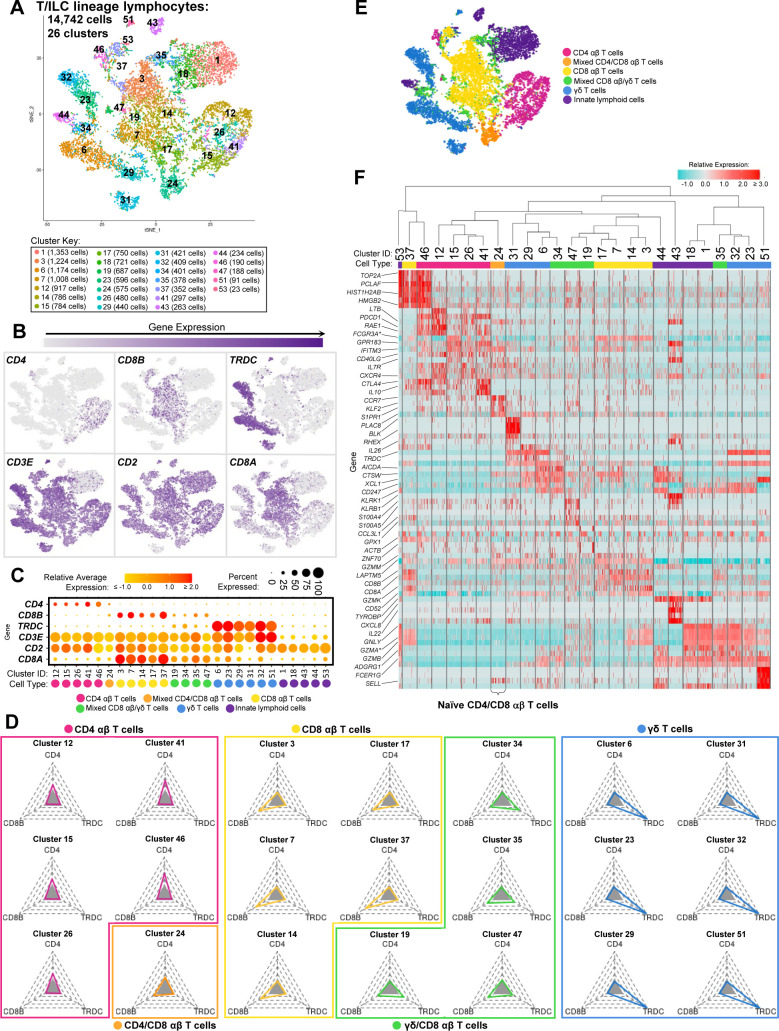
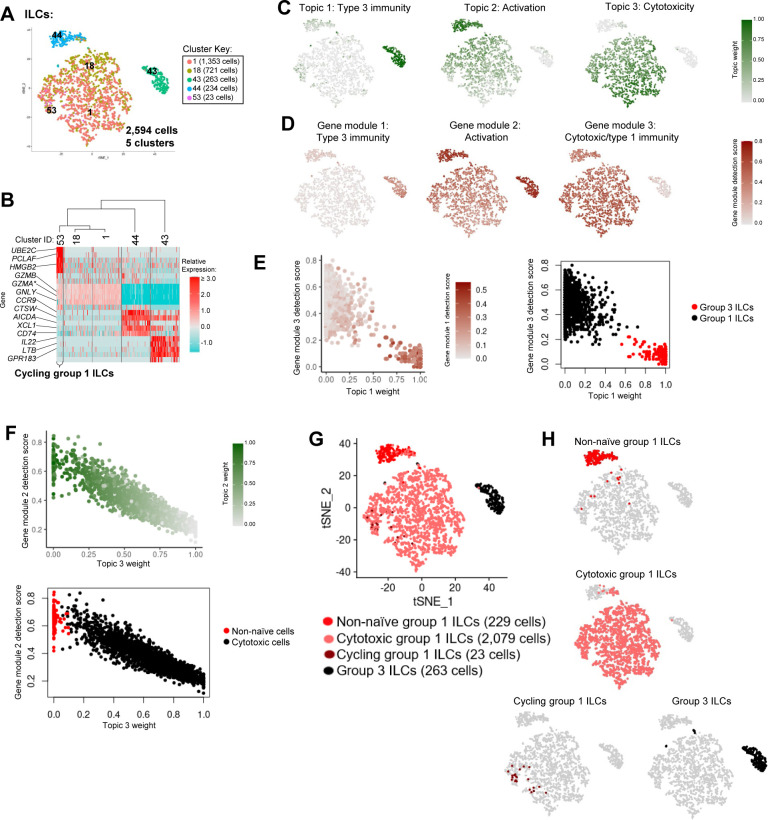
Similar to scRNA-seq results described elsewhere (Zhao et al, 2020; Elmentaite et al, 2021; Guo et al, 2021; Herrera-Uribe et al, 2021; Patel et al, 2021), T cells and ILCs were so transcriptionally similar to one another that they were annotated into a single cell lineage and further resolved into 16 cell types (Figs 2A and S13). T cells were identified by expression of the porcine pan-T cell marker CD3E (reviewed by Piriou-Guzylack and Salmon [2008] and Gerner et al [2009]) and included subsets of CD4 αβ, CD8 αβ, and γδ T cells expressing CD4, CD8B, and TRDC, respectively. ILCs largely lacked CD3E and included subsets of group 1 and group 3 ILCs based on expression of genes associated with type 1 or type 3 immunity, respectively (described in subsequent cell type descriptions below; Fig 2B). By hierarchical analysis, T/ILC types were more closely related by inferred function (e.g., cell cycling, activation, and cytotoxicity) rather than traditional T/ILC phenotypes (e.g., CD4 αβ T cells, CD8 αβ T cells, γδ T cells, group 1 ILCs, group 3 ILCs; Fig 2B), which are classically defined based on expression of a series of cell surface markers.
Figure 2. scRNA-seq profiles of T/ILC lineage lymphocytes in the porcine ileum.
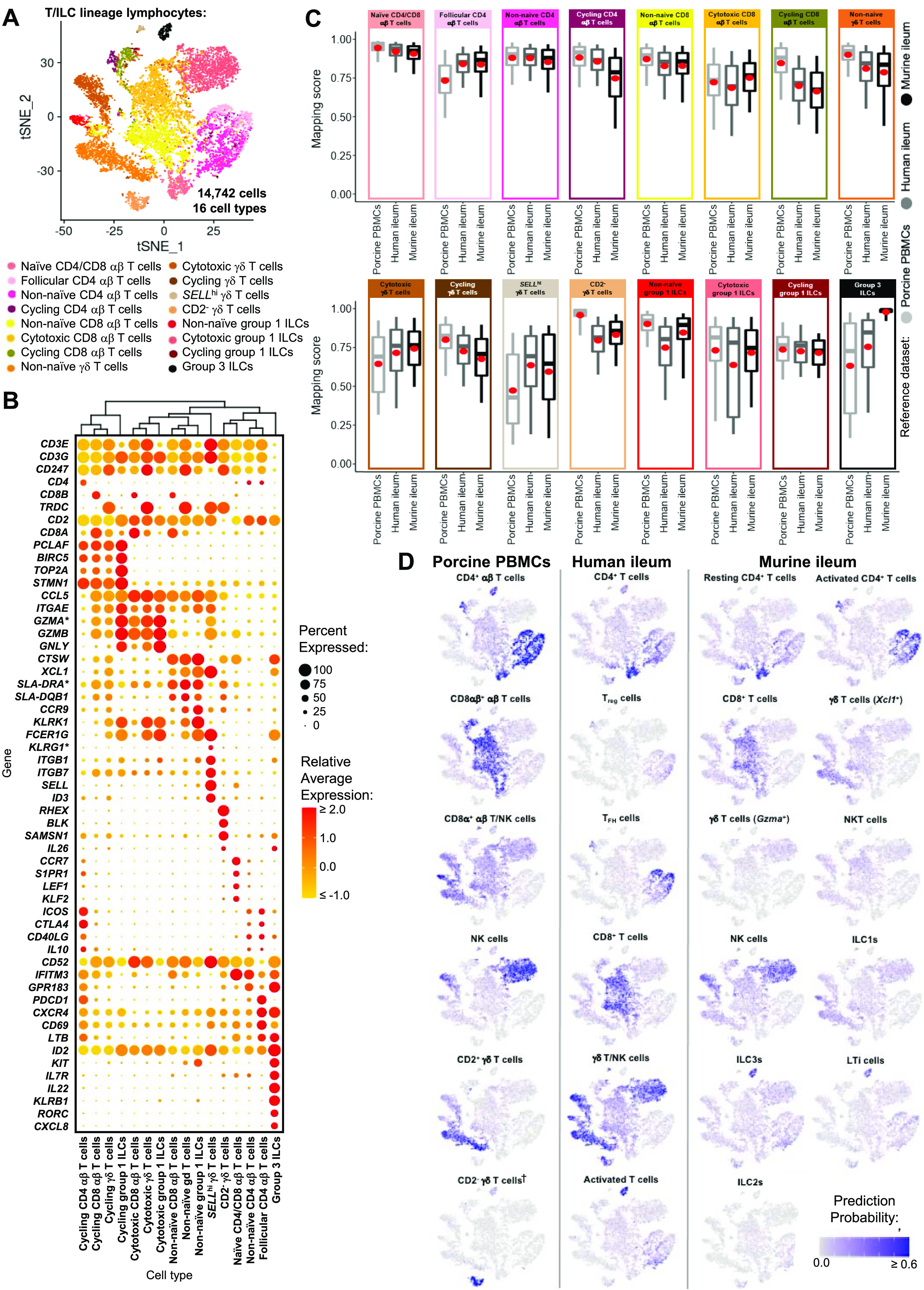
(A) Two-dimensional t-SNE visualization of 14,742 cells recovered from the porcine ileum via scRNA-seq that were classified as T/ILC lineage lymphocytes in Figs 1C and S4B and further annotated into 16 cell types in Figs 1D and S5–S8. Each point represents a single cell; the color of each point indicates cell types shown in Fig 1D. (B) Hierarchical relationship of T/ILC lineage lymphocyte cell types from the porcine ileum shown in a dendrogram (upper) and expression patterns of selected genes within each cell type shown in a dot plot (lower). In the dot plot, selected genes are listed on the y-axis, and cell types are listed on the x-axis. Within the dot plot, size of a dot corresponds to the percentage of cells expressing a gene within an annotated cell type; color of a dot corresponds to average expression level of a gene for those cells expressing it within a cell type relative to all other cells in the dataset shown in (A). (C) Box plots of the distribution of mapping scores for T/ILC lineage lymphocyte cell types from the porcine ileum mapped to each reference scRNA-seq dataset. Results for a single cell type are located within a single box, with color of the box corresponding to colors used for cell types in (A). The color of each box in a plot corresponds to the reference dataset porcine ileal cells were mapped to, including porcine PBMCs (light gray), human ileum (medium gray), and murine ileum (black). Boxes span the interquartile range (IQR) of the data (25th and 75th percentiles), with the median (50th percentile) indicated by a horizontal line. Whiskers span the 5th and 95th percentiles of the data. A red dot represents the data mean. (D) Prediction probabilities for porcine-ileum scRNA-seq query data from label transfer of selected annotated T/ILC types in reference scRNA-seq datasets of porcine PBMCs (left), human ileum (middle), and murine ileum (right) overlaid onto two-dimensional t-SNE visualization shown in (A). Each point represents a single cell; the color of each point indicates prediction probability to a corresponding cell type from reference data, as indicated directly above each t-SNE plot. A higher prediction probability indicates higher similarity to a specified annotated cell type in a reference scRNA-seq dataset. scRNA-seq data shown in (A, B, C, D) were derived from the ileum of two 7-wk-old pigs. *Ensembl identifiers found in gene annotation were converted to gene symbols; refer to methods section “Gene name modifications” for more details. † Identical cell type annotations were given to cells in both the porcine ileum and a reference scRNA-seq dataset. Cell type annotations were given to each dataset by independent rationales, and identical annotations do not necessarily indicate identical cell types were recovered from both porcine-ileum and reference data. Abbreviations: ILC, innate lymphoid cell; IQR, interquartile range; LTi, lymphoid tissue inducer; NK, natural killer; NKT, natural killer T; PBMC, peripheral blood mononuclear cell; scRNA-seq, single-cell RNA sequencing; t-SNE, t-distributed stochastic neighbor embedding; TFH, T follicular helper; Treg, T regulatory.
Figure S13. Overlay of T/ILC annotations onto t-SNE visualization of cells from porcine-ileum scRNA-seq data.
Overlay of 16 annotated T/ILC types onto two-dimensional t-SNE visualization of 14,742 cells recovered from the ileum of two 7-wk-old pigs via scRNA-seq and classified as T/ILC lineage lymphocytes in Figs 1C and S4B. Each point represents a single cell. Cell type is indicated in a respective panel by one of 16 colors corresponding to cell types shown in Fig 2A, whereas all other cells not corresponding to a specified cell type are shown in light gray. Abbreviations: ILC, innate lymphoid cell; scRNA-seq, single-cell RNA sequencing; t-SNE, t-distributed stochastic neighbor embedding.
Cycling T cells and ILCs
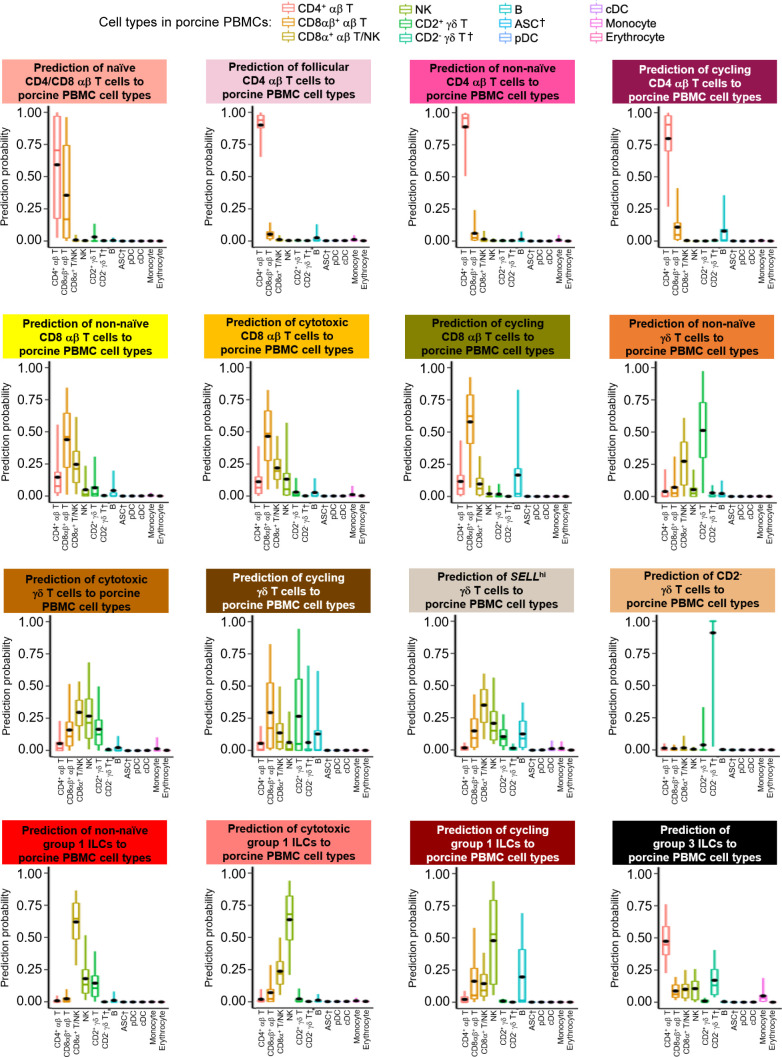
One hierarchical grouping of T/ILC types in Fig 2B was composed of all cycling T/ILCs, including cycling CD4 αβ T, CD8 αβ T, γδ T, and group 1 ILCs. All cycling cells had significantly increased expression of genes associated with replication/division (e.g., PCLAF, BIRC5, TOP2A, STMN1; Dabydeen et al, 2019; Giotti et al, 2019) and enrichment of related biological processes (e.g., establishment of mitotic spindle orientation [GO:0000132], regulation of mitotic centrosome separation [GO:0046602], DNA duplex unwinding [GO:0032508]) relative to other T/ILC types (Fig 2B and Table S8). Cycling T/ILCs had highest average mapping scores to reference porcine PBMCs (≥0.737), followed by human ileum (≥0.699) and murine ileum (≥0.664; Figs 2C and S14). Though predictions of many cycling T/ILC types were to similarly annotated T/ILCs in reference datasets (e.g., porcine ileal cycling CD4 αβ T cells having highest average predictions to reference CD4 αβ T cell types; Figs 2D and S15–S17), several cycling T/ILC types had high prediction to B cells in porcine PBMCs or cycling B cells in the human ileum (Figs S15 and S16). For instance, cycling CD8 αβ T, γδ T, and group 1 ILCs all had first or second highest average prediction probabilities to cycling B cells in human ileum (Fig S16), indicating cycling T/ILCs share transcriptional similarities to cycling B cells in the human ileum, likely because of shared replication/division-specific gene expression as opposed to shared expression of genes involved in lymphocyte lineage-specific immune functions of the cell.
Figure S14. Overlay of mapping scores onto t-SNE reduction of T/ILC lineage lymphocytes from porcine-ileum scRNA-seq data.
Mapping scores from mapping of porcine ileum scRNA-seq query data to reference scRNA-seq datasets of porcine PBMCs (left), human ileum (center), and murine ileum (right). Mapping scores are the same as shown in Fig S11 but are now shown only for T/ILC lineage lymphocytes overlaid onto two-dimensional t-SNE visualization of porcine-ileum scRNA-seq data shown in Fig 2A. Each point represents a single cell; the color of each point indicates mapping score to a corresponding reference dataset. Higher mapping scores indicate better representation of a cell from the porcine ileum in a specified reference dataset. Query scRNA-seq data were derived from the ileum of two 7-wk-old pigs. Abbreviations: ILC, innate lymphoid cell; PBMC, peripheral blood mononuclear cell; scRNA-seq, single-cell RNA sequencing; t-SNE, t-distributed stochastic neighbor embedding.
Figure S15. Prediction of porcine ileal T/ILC lineage lymphocytes to annotated cell types in porcine PBMCs.
Box plots of the distribution of prediction probabilities (y-axes) for T/ILC lineage lymphocyte cell types from the porcine ileum (represented by individual box plots) with labels transferred to annotated cell types of a porcine PBMC scRNA-seq reference dataset (x-axes and box plot color). Boxes span the interquartile range (IQR) of the data (25th and 75th percentiles), with the median (50th percentile) indicated by a horizontal line. Whiskers span the 5th and 95th percentiles of the data. A red dot represents the data mean. Query scRNA-seq data were derived from the ileum of two 7-wk-old pigs. † Identical cell type annotations were given to cells in both the porcine ileum and a reference scRNA-seq dataset. Cell type annotations were given to each dataset by independent rationales, and identical annotations do not necessarily indicate identical cell types were recovered from both porcine-ileum and reference data. Abbreviations: ASC, antibody-secreting cell; cDC, conventional dendritic cell; ILC, innate lymphoid cell; IQR, interquartile range; NK, natural killer; PBMC, peripheral blood mononuclear cell; pDC, plasmacytoid dendritic cell; scRNA-seq, single-cell RNA sequencing.
Figure S17. Prediction of porcine ileal T/ILC lineage lymphocytes to annotated cell types in the murine ileum.
Box plots of the distribution of prediction probabilities (y-axes) for T/ILC lineage lymphocyte cell types from the porcine ileum (represented by individual box plots) with labels transferred to annotated cell types of a murine-ileum scRNA-seq reference dataset (x-axes and box plot color). Boxes span the interquartile range (IQR) of the data (25th and 75th percentiles), with the median (50th percentile) indicated by a horizontal line. Whiskers span the 5th and 95th percentiles of the data. A red dot represents the data mean. Query scRNA-seq data were derived from the ileum of two 7-wk-old pigs. † Identical cell type annotations were given to cells in both porcine ileum and a reference scRNA-seq dataset. Cell type annotations were given to each dataset by independent rationales, and identical annotations do not necessarily indicate identical cell types were recovered from both porcine-ileum and reference data. Abbreviations: c1, cluster 1; c2, cluster 2; DC, dendritic cell; DN, double-negative; DZ, dark zone; GC, germinal center; ILC, innate lymphoid cell; IQR, interquartile range; LTi, lymphoid tissue inducer; LZ, light zone; pDC, plasmacytoid dendritic cell; NK, natural killer; NKT, natural killer T; scRNA-seq, single-cell RNA sequencing; TA, transit amplifying; TFH, T follicular helper; Treg, T regulatory.
Figure S16. Prediction of porcine ileal T/ILC lineage lymphocytes to annotated cell types in the human ileum.
Box plots of the distribution of prediction probabilities (y-axes) for T/ILC lineage lymphocyte cell types from the porcine ileum (represented by individual box plots) with labels transferred to annotated cell types of a human ileum scRNA-seq reference dataset (x-axes and box plot color). Boxes span the interquartile range (IQR) of the data (25th and 75th percentiles), with the median (50th percentile) indicated by a horizontal line. Whiskers span the 5th and 95th percentiles of the data. A red dot represents the data mean. Query scRNA-seq data were derived from the ileum of two 7-wk-old pigs. † Identical cell type annotations were given to cells in both the porcine ileum and a reference scRNA-seq dataset. Cell type annotations were given to each dataset by independent rationales, and identical annotations do not necessarily indicate identical cell types were recovered from both porcine-ileum and reference data. Abbreviations: cDC, convnentional dendritic cell; DC, dendritic cell; ILC, innate lymphoid cell; IQR, interquartile range; pDC, plasmacytoid dendritic cell; NK, natural killer; scRNA-seq, single-cell RNA sequencing; TA, transit amplifying; TFH, T follicular helper; Treg, T regulatory.
Cytotoxic T cells and ILCs
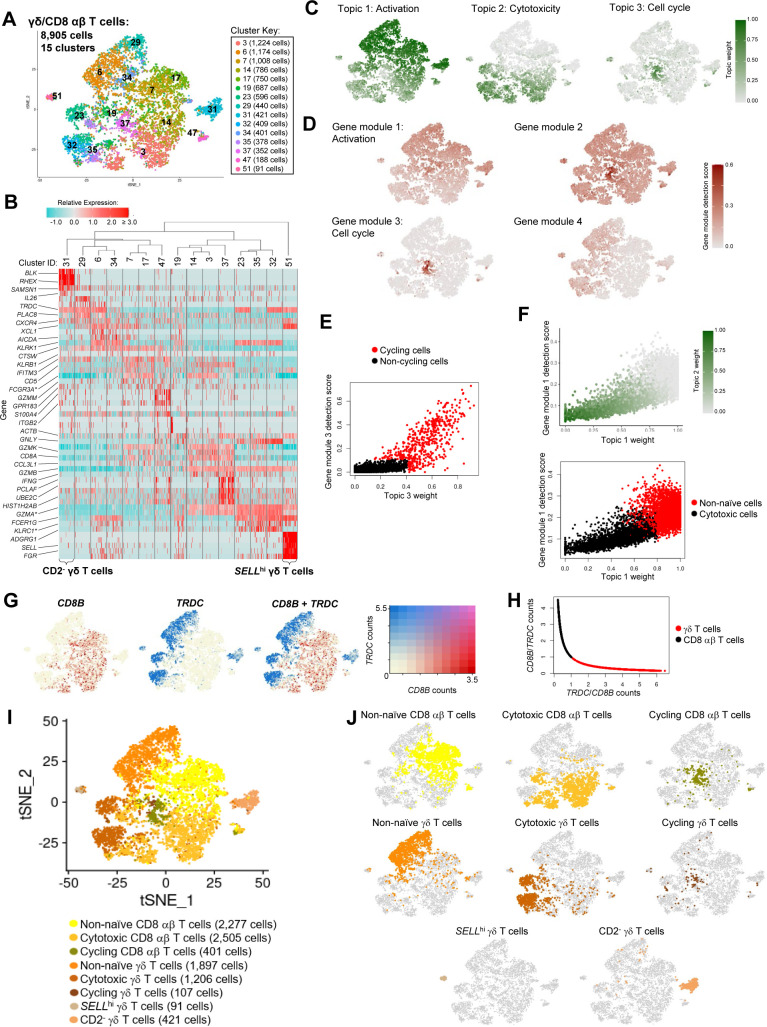
Cytotoxic CD8 αβ T, γδ T, and group 1 ILCs were most closely related to one another and had significantly elevated expression of genes encoding for cytotoxic molecules, including GZMA* (*Ensembl identifiers found in gene annotation were converted to gene symbols; refer to the Materials and Methods section “Gene name modifications” for more details), GZMB, and GNLY (Hidalgo et al, 2008), relative to other T/ILC types (Fig 2B and Table S8). The biological process leukocyte mediated cytotoxicity (GO:0001909) was enriched in cytotoxic CD8 αβ T cells, whereas regulation of natural killer cell–mediated cytotoxicity (GO:0042269) was enriched in cytotoxic γδ T and group 1 ILCs (Table S8). Cytotoxic cell types had some of the lowest average mapping scores to reference porcine PBMCs (range of means 0.645–0.732), indicating dissimilarity between cytotoxic ileal cells from any cells in circulation (Figs 2C and S14). Though cytotoxic cell types had lower mapping scores to porcine PBMCs, cytotoxic CD8 αβ T cells and group 1 ILCs still had the highest prediction to comparable cell types in porcine peripheral blood: CD8 αβ+ αβ T cells and NK cells, respectively (Figs 2D and S15). Ileal cytotoxic γδ T cells had highest average prediction to innate-like CD8α+ αβ T cells and NK cells from porcine peripheral blood rather than to peripheral CD2+ γδ T cells, further supporting poor representation of ileal cytotoxic γδ T cells by porcine peripheral γδ T cells and suggesting greater similarities to other peripheral innate or innate-like T/ILC types instead (Figs 2D and S15). A similar pattern was observed in murine ileum, where porcine ileal cytotoxic γδ T cells had the highest prediction to reference NK cells rather than Gzma+ γδ T cells, suggesting again that porcine ileal cytotoxic γδ T cells had greater similarity to other innate/innate-like T/ILC types rather than γδ T cells (Figs 2D and S17).
Non-naive γδ T cells, CD8 αβ T cells, and group 1 ILCs
Non-naive γδ T, CD8 αβ T, and group 1 ILCs also formed a hierarchical grouping closely related to cytotoxic cell counterparts in Fig 2B. In contrast to cytotoxic T/ILCs, non-naive γδ T, CD8 αβ T, and group 1 ILCs had lower expression of genes encoding cytotoxic molecules (e.g., GZMA*, GZMB, and GNLY) but significantly elevated expression of other genes indicative of previous or recent cell activation, including CTSW, XCL1, SLA-DRA*, SLA-DQB1, and CCR9 (Fig 2B and Table S8; Kelner et al, 1994; Boismenu et al, 1996; Svensson et al, 2002; Uehara et al, 2002; Iwata et al, 2004; Ondr & Pham, 2004; Gerner et al, 2009; Stoeckle et al, 2009). Non-naive γδ T, CD8 αβ T, and group 1 ILCs were all enriched for the biological processes positive regulation of T cell differentiation (GO:0045582) and positive regulation of T cell–mediated immunity (GO:0002711), further supporting a non-naive cell state (Table S8). Non-naive γδ T, CD8 αβ T, and group 1 ILCs had higher average mapping scores to all reference datasets (range of means 0.872–0.902) than did corresponding cytotoxic T/ILCs, indicating better representation by reference data (Figs 2C and S14). Unlike cytotoxic γδ T cells, non-naive γδ T cells had the highest prediction to γδ T cell types in reference datasets, including CD2+ γδ T cells (porcine PBMCs), γδ T/NK cells (human ileum), and Xcl1+ γδ T cells (murine ileum), suggesting greater similarity of porcine ileal non-naive γδ T cells to reference γδ Τ cell populations than observed for porcine ileal cytotoxic γδ T cells (Figs 2D and S15–S17). Non-naive CD8 αβ T cells had highest average predictions to CD8 αβ T cell types in reference datasets, as did non-naive group 1 ILCs to reference ILC types (Figs 2D and S15–S17). Though both non-naive and cytotoxic group 1 ILCs had the highest prediction to reference group 1 ILC types, activated group 1 ILCs had the highest prediction to porcine peripheral CD8α+ αβ T/NK cells and murine ileal ILC1s. In contrast, cytotoxic group 1 ILCs had the highest prediction to NK cells in the same reference datasets, delineating transcriptional distinctions between cytotoxic and non-naive group 1 ILCs that correspond better to different reference cell types (Figs 2D, S15, and S17).
SELLhi γδ T cells
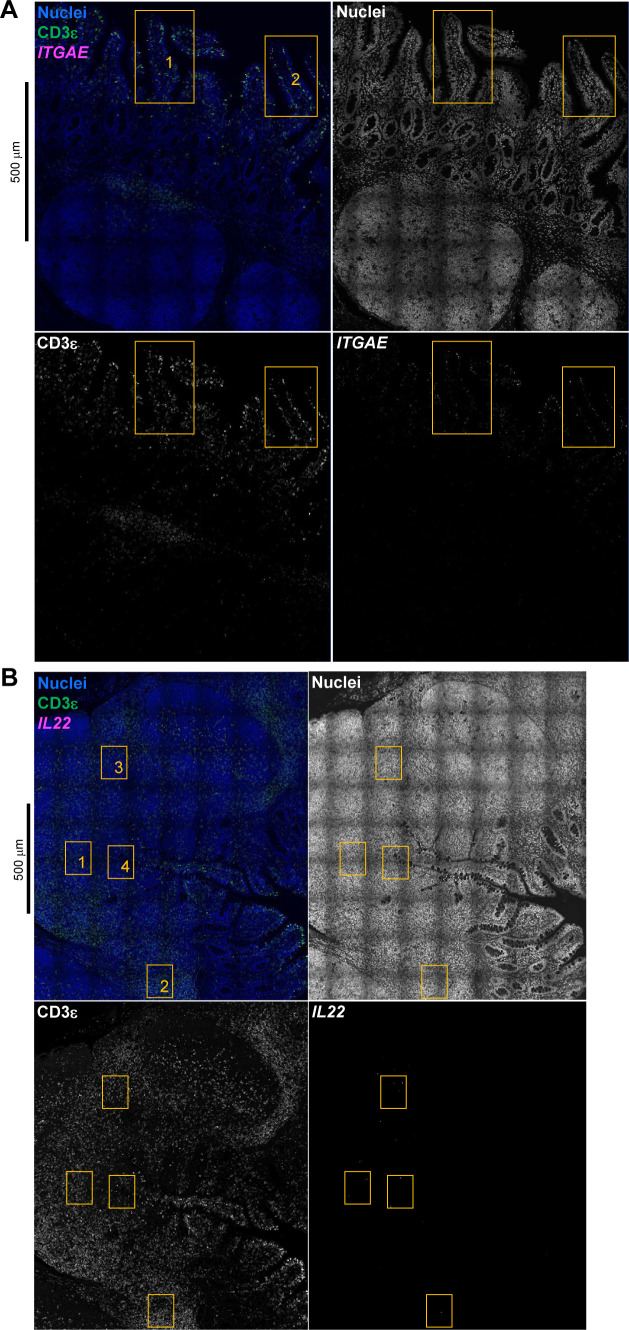
SELLhi γδ T cells were a minor fraction of porcine ileal γδ T cells (91 cells total) that shared a node with cytotoxic and non-naive T/ILCs expressing effector/activation molecules, including CCL5 and ITGAE (Fig 2B; Ling et al, 2007; Szabo et al, 2019a). SELLhi γδ T cells nearly ubiquitously expressed SELL (encoding CD62L) and genes related to cytotoxicity (e.g., GZMA* and GZMB) but also some genes expressed by non-naive T/ILCs, such as XCL1 (Fig 2B). SELLhi γδ T cells expressed genes encoding innate receptors, including FCER1G and KLRG1*, but lacked expression of others, such as KLRK1 (Fig 2B). Moreover, SELLhi γδ T cells had significantly higher expression of genes encoding for adhesion molecules (e.g., SELL, ITGB1, and ITGB7) and the transcriptional regulator and γδ T cell fate determinator, ID3 (Fig 2B and Table S8; Lauritsen et al, 2009). Five of the top eight enriched biological processes for SELLhi γδ T cells (as determined by smallest P-values) included actin filament depolymerization (GO:0030042), positive regulation of actin filament polymerization (GO:003038), establishment or maintenance of cell polarity (GO:0007163), integrin-mediated signaling pathway (GO:0007229), and natural killer cell activation (GO:0030101), indicating a highly activated state potentially related to cell receptor engagement/signaling (Table S8). SELLhi γδ T cells had the lowest average mapping scores of all cell types in comparison to each reference dataset (range of means 0.473–0.636; Figs 2C and S14), suggesting they were unique to the porcine ileum.
CD2− γδ T cells
CD2− γδ T cells (characterized as TRDC-expressing cells that lacked CD2 expression; Fig 2B) are present in pigs but absent from humans and mice (Stepanova & Sinkora, 2013). Correspondingly, CD2− γδ T cells had higher average mapping scores to porcine PBMCs (0.959) than to ileal cells from human (0.796) or mouse (0.832; Figs 2C and S14). Several lines of work support CD2− γδ T cells as a cell lineage separate from CD2+ γδ T cells (Sinkora et al, 2005, 2007; Stepanova & Sinkora, 2013; Sedlak et al, 2014; Rodríguez-Gómez et al, 2019; Hammer et al, 2020), whereas others have suggested CD2− γδ T cells are naive cells in pigs (Stepanova & Sinkora, 2012; Talker et al, 2013; Käser, 2021). We found that CD2− γδ T cells were distantly related from all other annotated γδ T cells (all considered CD2+ γδ T cells; Fig 2B), which could suggest that CD2− γδ T cells are a distinct cell lineage from CD2+ γδ T cells. Contrarily, CD2− γδ T cells were most closely related to naive CD4/CD8 αβ T cells in the porcine ileum by hierarchical clustering (Fig 2B), which could suggest CD2− γδ T cells are naive cells. Regardless of whether CD2− γδ T cells represent a distinct cell lineage or naive cells, CD2− γδ T cells had the highest average mapping scores to porcine PBMCs of all T/ILC types, indicating CD2− γδ T cells to be the porcine ileal T/ILC type most similar to cells in the porcine periphery. Besides lacking CD2 expression, ileal CD2− γδ T cells had significantly elevated expression of RHEX, BLK, SAMSN1, and IL26 (Fig 2B and Table S8), which were also highly expressed by CD2− γδ T cells in the porcine periphery (Herrera-Uribe et al, 2021). CD2− γδ T cells were predicted most similar to corresponding CD2− γδ T cells in porcine peripheral blood and to γδ T/NK cells in human ileum, whereas in murine ileum, predictions were lowly distributed across multiple T/ILC subsets (Figs 2D and S15–S17). Thus, CD2− γδ T cells can be found in both the ileum and periphery of pigs but do not have close counterparts in the human or murine ileum.
Naive CD4/CD8 αβ T cells
Naive CD4 and CD8 αβ T cells had significantly higher expression of genes related to cell circulation and a naive T cell phenotype, including CCR7, S1PR1, LEF1, and KLF2 (Fig 2B and Table S8; Willinger et al, 2006; Sebzda et al, 2008; Skon et al, 2013; Cano-Gamez et al, 2020; Shan et al, 2021). Of all T/ILCs, naive CD4/CD8 αβ T cells had the second-highest average mapping scores to porcine PBMCs (0.944; Figs 2C and S14), indicating naive CD4 and CD8 αβ T cells to be the porcine ileal T/ILC type second-best represented by cells in the porcine periphery, trailing only behind CD2− γδ T cells. High mapping scores to human and murine ileum (means 0.921 and 0.917, respectively) were also noted, indicating good representation of naive CD4 and CD8 αβ T cells in the ileum of both human and mouse. Porcine ileal naive CD4/CD8 αβ T cells had the highest prediction to corresponding populations in reference datasets, including CD4 and CD8 αβ T cell populations derived from porcine PBMCs or human ileum and resting CD4 and CD8 T cells derived from murine ileum (Figs 2D and S15–S17).
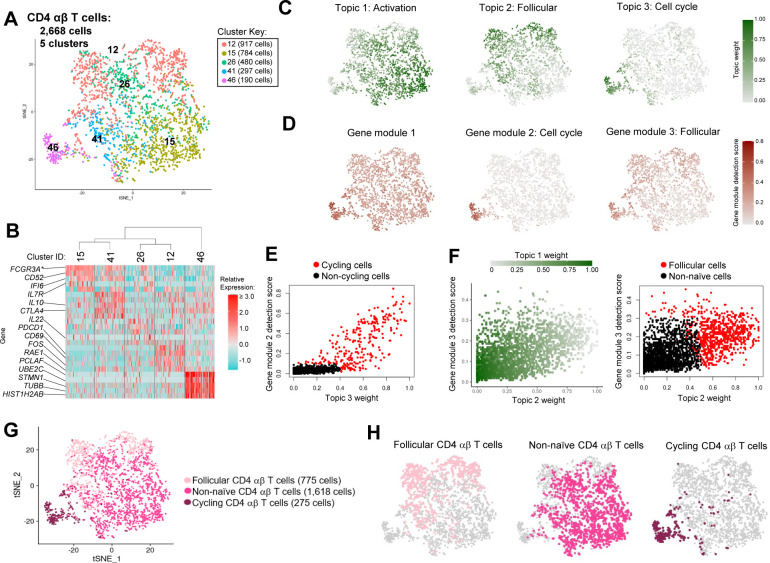
Non-naive and follicular CD4 αβ T cells
Remaining non-naive/non-cycling CD4 αβ T cells in the porcine ileum included follicular and non-naive CD4 αβ T cells, which were most closely related to one another (Fig 2B). Non-naive CD4 αβ T cells did not share elevated expression of several genes highly expressed by other non-naive T/ILC types (e.g., CCL5, ITGAE, CTSW, XCL1, SLA-DRA*, SLA-DQB1, and CCR9) but instead had significantly elevated expression of genes associated with CD4 αβ T cell activation (e.g., ICOS, CTLA4, and CD40LG; Jaiswal et al, 1996; Linsley & Golstein, 1996; Hutloff et al, 1999; Miragaia et al, 2019; Cano-Gamez et al, 2020), which were also elevated in follicular CD4 αβ T cells (Fig 2B and Table S8). However, non-naive CD4 αβ T cells had higher expression of activation-associated genes IFITM3 and GPR183 (Clottu et al, 2017; Bedford et al, 2019; Szabo et al, 2019a) relative to follicular CD4 αβ T cells. Follicular CD4 αβ T cells were characterized by higher expression of PDCD1, CXCR4, CD69, and LTB, all genes highly expressed by follicle-associated T cells (Schaerli et al, 2000; Haynes et al, 2007; Shi et al, 2018), such as T follicular helper (TFH) or T follicular regulatory (TFR) cells (Fig 2B). The top two enriched biological processes in follicular CD4 αβ T cells (smallest P-values) were related to B cell activation/humoral immunity, including humoral immune response mediated by circulating immunoglobulin (GO:0002455) and plasma cell differentiation (GO:0002317; Table S8). Follicular CD4 αβ T cells had lower mapping scores to porcine PBMCs (mean 0.733) than did non-naive CD4 αβ T cells (mean 0.880; Figs 2C and S15), indicating greater dissimilarity of follicular CD4 αβ T cells than non-naive CD4 αβ T cells to circulating cells in pigs. Porcine follicular CD4 αβ T cells had the highest prediction to TFH cells in human ileum and activated CD4 T cells in murine ileum (Figs 2D, S16, and S17), further supporting an activated role associated with follicular helper/regulatory functions. Non-naive CD4 αβ T cells were largely predicted as activated CD4 T cells in murine ileum and more so as CD4 T than TFH in human ileum (Figs 2D, S16, and S17), supporting an activated cell state.
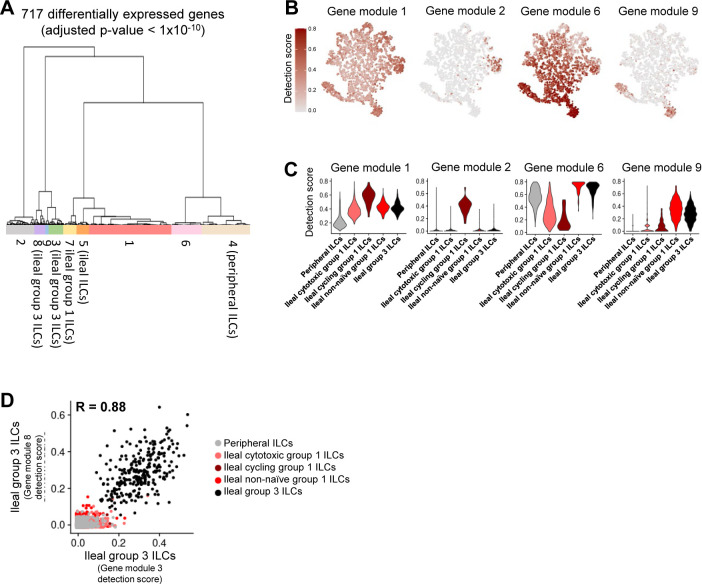
Group 3 ILCs
Group 3 ILCs expressed many genes characteristic of type 3 immunity, including IL22, RORC, and CXCL8 (Schaerli et al, 2000; Haynes et al, 2007; Shi et al, 2018; Qi et al, 2021) and were more closely related to non-cycling CD4 αβ and naive T cell subsets than to any type of group 1 ILC (Fig 2B). Though ILCs largely lacked expression of pan-T cell marker CD3E, group 1 ILCs still expressed other CD3 complex-associated genes, such as CD3G and CD247 (encoding CD3γ and CD3ζ, respectively). In contrast, group 3 ILCs largely lacked expression of all aforementioned CD3 subunit-encoding genes and also had significantly higher expression of classical ILC gene markers, including KIT, ID2, IL7R, and KLRB1 (Fig 2B and Table S8; Yokota et al, 1999; Yoshida et al, 1999; Boos et al, 2007; Satoh-Takayama et al, 2010; Spits et al, 2013), though these markers are already known to be variably expressed by intestinal group 1 ILCs based on species and regional location (Robinette et al, 2015; Simoni et al, 2017; Simoni & Newell, 2017; Van Acker et al, 2017; Meininger et al, 2020). Group 3 ILCs mapped best to cells of murine ileum (mean mapping score 0.979) and were predicted most similar to corresponding group 3 ILC populations of ILC3s or lymphoid tissue inducer (LTi) cells (Figs 2C and D and S17). In contrast, group 3 ILCs did not have as close a counterpart in porcine PBMCs or human ileum, as indicated by lower average mapping scores (0.632 and 0.754, respectively) and prediction most similar to CD4 αβ T cells or activated T cells, respectively (Figs 2C and D, S15, and S16).
Defining the porcine ileal immune landscape: B cells and antibody-secreting cells (ASCs)
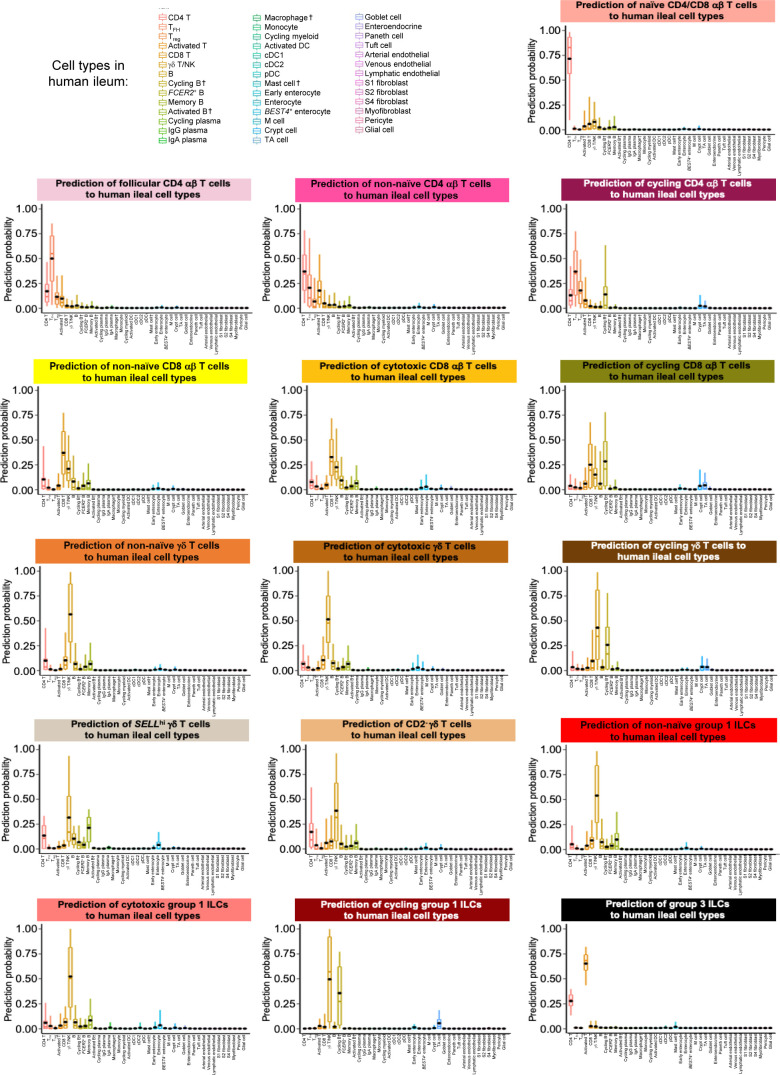
B lineage lymphocytes were annotated as ASCs, B cells transitioning into ASCs (referred to as transitioning B cells), and three additional populations of B cells, including resting, cycling, and activated B cells (Figs 3A and S9F).
Figure 3. scRNA-seq profiles of B lineage lymphocytes in the porcine ileum.
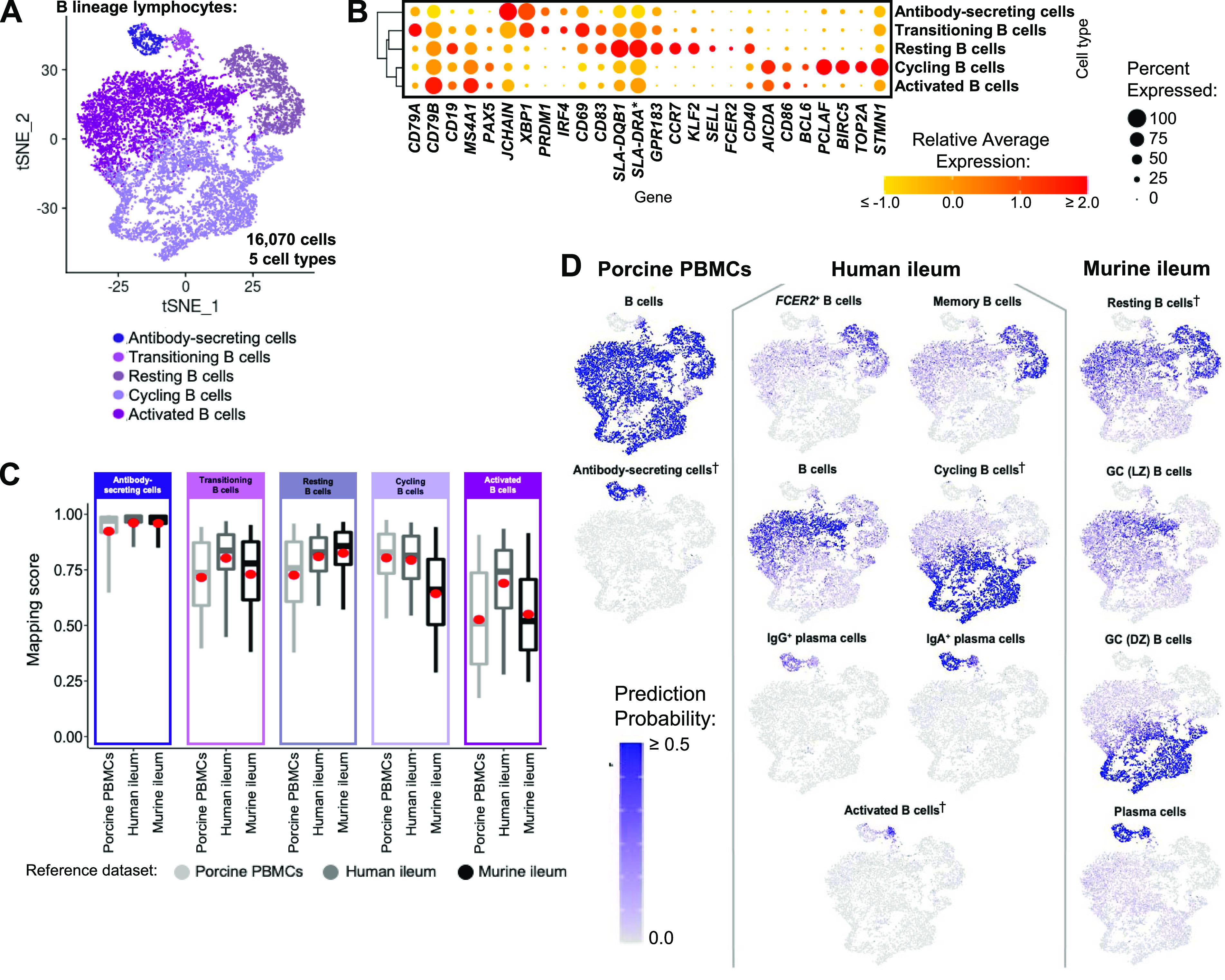
(A) Two-dimensional t-SNE visualization of 16,070 cells recovered from the porcine ileum via scRNA-seq that were classified as B lineage lymphocytes in Figs 1C and S4B and further annotated into five cell types in Figs 1D and S9. Each point represents a single cell; the color of each point indicates cell types shown in Fig 1D. (B) Hierarchical relationship of B lineage lymphocyte cell types from the porcine ileum shown in a dendrogram (left), and expression patterns of selected genes within each cell type shown in a dot plot (right). In the dot plot, selected genes are listed on the x-axis, and cell types are listed on the y-axis. Within the dot plot, size of a dot corresponds to the percentage of cells expressing a gene within an annotated cell type; color of a dot corresponds to average expression level of a gene for those cells expressing it within a cell type relative to all other cells in the dataset shown in (A). (C) Box plots of the distribution of mapping scores for B lineage lymphocyte cell types from the porcine ileum mapped to each reference scRNA-seq dataset. Results for a single cell type are located within a single box, with color of the box corresponding to colors used for cell types in (A). The color of each box in a plot corresponds to the reference dataset porcine ileal cells were mapped to, including porcine PBMCs (light gray), human ileum (medium gray), and murine ileum (black). Boxes span the interquartile range (IQR) of the data (25th and 75th percentiles), with the median (50th percentile) indicated by a horizontal line. Whiskers span the 5th and 95th percentiles of the data. A red dot represents the data mean. (D) Prediction probabilities for porcine-ileum scRNA-seq query data from label transfer of selected annotated B/antibody-secreting cell types in reference scRNA-seq datasets of porcine PBMCs (left), human ileum (middle), and murine ileum (right) overlaid onto two-dimensional t-SNE visualization shown in (A). Each point represents a single cell; the color of each point indicates prediction probability to a corresponding cell type from reference data, as indicated directly above each t-SNE plot. A higher prediction probability indicates higher similarity to a specified annotated cell type in a reference scRNA-seq dataset. scRNA-seq data shown in (A, B, C, D) were derived from ileum of two 7-wk-old pigs. *Ensembl identifiers found in gene annotation were converted to gene symbols; refer to methods section “Gene name modifications” for more details. † Identical cell type annotations were given to cells in both porcine ileum and a reference scRNA-seq dataset. Cell type annotations were given to each dataset by independent rationales, and identical annotations do not necessarily indicate identical cell types were recovered from both porcine-ileum and reference data. Abbreviations: DZ, dark zone; GC, germinal center; IQR, interquartile range; LZ, light zone; PBMC, peripheral blood mononuclear cell; scRNA-seq, single-cell RNA sequencing; t-SNE, t-distributed stochastic neighbor embedding.
Figure S9. Annotation of B lineage lymphocytes from porcine-ileum scRNA-seq data.
(A) Two-dimensional t-SNE visualization of 16,070 cells recovered from the porcine ileum via scRNA-seq and classified as B lineage lymphocytes in Figs 1C and S4B. Each point represents a single cell; color of a point corresponds to one of 22 cell clusters a cell belongs to, with more transcriptionally similar cells belonging to the same cell cluster. The number of cells belonging to each cell cluster is listed in the cluster key. (B) Heatmap of top differentially expressed genes within each cell cluster shown in (A). Up to five differentially expressed genes with the highest positive logFC values were selected for each cell cluster. Genes were differentially expressed in a specified cell cluster relative to the average of all other cells in the dataset shown in (A). Gene expression profiles from up to 100 cells of each cell cluster are shown in the heatmap, with each column representing a single cell. Selected gene names are shown on the y-axis, and cell cluster IDs are shown on the x-axis. Hierarchical relationships of clusters are shown using a phylogenetic tree at the top of the heatmap. At the bottom of the heatmap, cluster 33 was annotated as transitioning B cells, cluster 25 as antibody-secreting cells, and clusters 9, 13, and 30 as resting B cells. (C) Topic weights from topic modeling of cells shown in (A) overlaid onto two-dimensional t-SNE visualization coordinates. Color of a point corresponds to proportional weighting of a topic within a cell, where total weighting across all topics in each cell is equal to one. (D) Gene module detection scores from multidimensional differential gene expression analysis of cells shown in (A) overlaid onto two-dimensional t-SNE visualization coordinates. Color of a point corresponds to detection score for a gene module within a cell. (E) Scatter plots of gene module 3 detection scores (y-axis) versus topic 2 weights (x-axis) for all cells shown in (A), excluding resting B cells (clusters 9, 13, 20), transitioning B cells (cluster 33), and antibody-secreting cells (cluster 25). Each point represents a single cell. Cells with gene module 3 detection scores >0.06 and/or topic 2 weights >0.32 are shown in red and annotated as cycling cells. Remaining cells are shown in black and annotated as activated cells. (F) B lineage lymphocyte annotations established in (B, C, D, E) overlaid onto two-dimensional t-SNE visualization coordinates of cells shown in (A). Cell type is indicated in a respective panel by one of five colors corresponding to annotated cell types, whereas all other cells not corresponding to a specified cell type are shown in light gray. scRNA-seq data shown in (A, B, C, D, E, F) were derived from the ileum of two 7-wk-old pigs. *Ensembl identifiers found in gene annotation were converted to gene symbols; refer to methods section “Gene name modifications” for more details. Abbreviations: logFC, log fold-change; scRNA-seq, single-cell RNA sequencing; t-SNE, t-distributed stochastic neighbor embedding.
ASCs
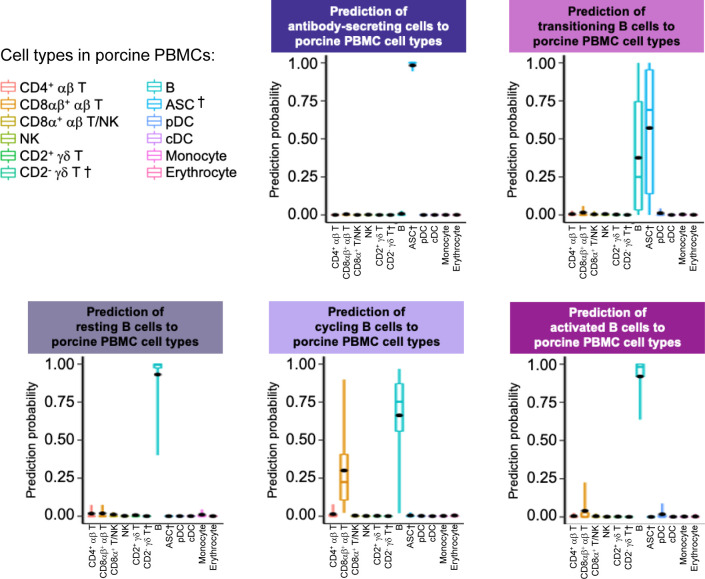
ASCs were most distantly related from other B cell types by hierarchical clustering and had significantly lower expression of several canonical B cell genes, including CD19, CD79A, CD79B, MS4A1, and PAX5 (Fig 3B and Table S9; Herrera-Uribe et al, 2021; Lee et al, 2021). Genes known to be expressed by porcine peripheral ASCs (e.g., JCHAIN, XBP1, IRF4, and PRDM1; Herrera-Uribe et al, 2021) had elevated expression in ileal ASCs as well. The top two enriched biological processes in ASCs relative to other B cells were related to B cell activation (positive regulation of B cell activation [GO:0050871]) and protein production, such as required for producing antibodies (positive regulation of protein exit from the endoplasmic reticulum [GO:0070863]; Table S9). ASCs were well-represented by all reference datasets, as indicated by high mapping scores (means ≥ 0.927; Figs 3C and S18) and were almost unanimously predicted as ASC/plasma cell types from all reference datasets (Figs 3D and S19–S21).
Figure S18. Overlay of mapping scores onto t-SNE reduction of B lineage lymphocytes from porcine-ileum scRNA-seq data.
Mapping scores from mapping of porcine-ileum scRNA-seq query data to reference scRNA-seq datasets of porcine PBMCs (left), human ileum (center), and murine ileum (right). Mapping scores are the same as shown in Fig S11 but are now shown only for B lineage lymphocytes overlaid onto two-dimensional t-SNE visualization of porcine-ileum scRNA-seq data shown in Fig 3A. Each point represents a single cell; the color of each point indicates mapping score to a corresponding reference dataset. Higher mapping scores indicate better representation of a cell from the porcine ileum in a specified reference dataset. Query scRNA-seq data were derived from the ileum of two 7-wk-old pigs. Abbreviations: PBMC, peripheral blood mononuclear cell; scRNA-seq, single-cell RNA sequencing; t-SNE, t-distributed stochastic neighbor embedding.
Figure S19. Prediction of porcine ileal B lineage lymphocytes to annotated cell types in porcine PBMCs.
Box plots of the distribution of prediction probabilities (y-axes) for B lineage lymphocyte cell types from the porcine ileum (represented by individual box plots) with labels transferred to annotated cell types of a porcine PBMC scRNA-seq reference dataset (x-axes and box plot color). Boxes span the interquartile range (IQR) of the data (25th and 75th percentiles), with the median (50th percentile) indicated by a horizontal line. Whiskers span the 5th and 95th percentiles of the data. A red dot represents the data mean. Query scRNA-seq data were derived from the ileum of two 7-wk-old pigs. † Identical cell type annotations were given to cells in both the porcine ileum and a reference scRNA-seq dataset. Cell type annotations were given to each dataset by independent rationales, and identical annotations do not necessarily indicate identical cell types were recovered from both porcine-ileum and reference data. Abbreviations: ASC, antibody-secreting cell; cDC, conventional dendritic cell; IQR, interquartile range; NK, natural killer; PBMC, peripheral blood mononuclear cell; pDC, plasmacytoid dendritic cell; scRNA-seq, single-cell RNA sequencing.
Figure S21. Prediction of porcine ileal B lineage lymphocytes to annotated cell types in the murine ileum.
Box plots of the distribution of prediction probabilities (y-axes) for B lineage lymphocyte cell types from the porcine ileum (represented by individual box plots) with labels transferred to annotated cell types of a murine-ileum scRNA-seq reference dataset (x-axes and box plot color). Boxes span the interquartile range (IQR) of the data (25th and 75th percentiles), with the median (50th percentile) indicated by a horizontal line. Whiskers span the 5th and 95th percentiles of the data. A red dot represents the data mean. Query scRNA-seq data were derived from the ileum of two 7-wk-old pigs. † Identical cell type annotations were given to cells in both the porcine ileum and a reference scRNA-seq dataset. Cell type annotations were given to each dataset by independent rationales, and identical annotations do not necessarily indicate identical cell types were recovered from both porcine-ileum and reference data. Abbreviations: c1, cluster 1; c2, cluster 2; DC, dendritic cell; DN, double-negative; DZ, dark zone; GC, germinal center; ILC, innate lymphoid cell; IQR, interquartile range; LTi, lymphoid tissue inducer; LZ, light zone; pDC, plasmacytoid dendritic cell; NK, natural killer; NKT, natural killer T; scRNA-seq, single-cell RNA sequencing; TA, transit amplifying; TFH, T follicular helper; Treg, T regulatory.
Table S9 Differential gene expression and biological process enrichment results in porcine ileal B/ASCs. (435.6KB, xlsx)
Transitioning B cells
Similar to ASCs, transitioning B cells had high expression of genes characteristic of porcine ASCs, including JCHAIN, XBP1, IRF4, and PRDM1, and were enriched for biological processes supporting antibody production, including the top three enriched processes of posttranslational protein targeting to endoplasmic reticulum membrane (GO:0006620), protein N-linked glycosylation (GO:0006487), and glycoprotein catabolic process (GO:0006516; Fig 3B and Table S9). Porcine ileal ASCs had highest average prediction to ASC/plasma cell types in reference datasets; however, prediction scores to ASC/plasma cell types for transitioning B cells were lower than those observed in ASCs, and transitioning B cells also had high prediction to activated B cells in human ileum (Figs 3D and S19–S21). Transitioning B cells had lower average mapping scores to all reference datasets than did ASCs (means ≥ 0.718; Figs 3C and S18), indicating poorer representation by the reference data. In contrast to ASCs, transitioning B cells had greater expression of canonical B cell genes (e.g., CD19, CD79A, CD79B, MS4A1, and PAX5) and higher expression of several markers of early B cell activation, including CD69, CD83, SLA-DQB1, and SLA-DRA* (Fig 3B; Van der Stede et al, 2005; Breloer et al, 2007; Prazma et al, 2007; Ashouri & Weiss, 2017; Rahe & Murtaugh, 2017), supporting functional inference that cells were a subset of more recently activated B cells transitioning to produce and secrete antibody.
Resting B cells
Remaining B cell types (resting, cycling, and activated B cells) all had greater expression of B cell canonical genes (e.g., CD19, CD79A, CD79B, MS4A1, and PAX5) than did ASCs and lesser expression of aforementioned genes expressed by both ASCs and transitioning B cells (e.g., JCHAIN, XBP1, IRF4, and PRDM1; Fig 3B). Resting B cells were most closely related to transitioning B cells in porcine ileum but, unlike remaining cycling/activated B cell subsets, lacked expression of several genes associated with activation and/or germinal centers, including AICDA, BCL6, and CD86 (Engel et al, 1994; Allman et al, 1996; Muramatsu et al, 1999; Lee et al, 2021), indicating cells in a resting state (Fig 3B). Resting B cells had increased expression of genes characteristic of cell circulation and naive/memory B cells, including KLF2, SELL (CD62L), CCR7, FCER2 (CD23), and CD40 (Fig 3B; Waldschmidt et al, 1988; Förster et al, 1999; Bhattacharya et al, 2007; Winkelmann et al, 2011; Rahe & Murtaugh, 2017; Zhang et al, 2021a; Lee et al, 2021); however, it remained indiscriminate as to whether resting B cells were naive, memory, or a combination of both as many of the same genes are expressed by both naive and memory B cell subsets. In comparison to reference datasets, resting B cells were mostly predicted as memory B cell types (memory or FCER2+ B cells) in human ileum and as resting B cells in murine ileum (Figs 3D, S20, and S21).
Figure S20. Prediction of porcine ileal B lineage lymphocytes to annotated cell types in the human ileum.
Box plots of the distribution of prediction probabilities (y-axes) for B lineage lymphocyte cell types from the porcine ileum (represented by individual box plots) with labels transferred to annotated cell types of a human-ileum scRNA-seq reference dataset (x-axes and box plot color). Boxes span the interquartile range (IQR) of the data (25th and 75th percentiles), with the median (50th percentile) indicated by a horizontal line. Whiskers span the 5th and 95th percentiles of the data. A red dot represents the data mean. Query scRNA-seq data were derived from the ileum of two 7-wk-old pigs. † Identical cell type annotations were given to cells in both the porcine ileum and a reference scRNA-seq dataset. Cell type annotations were given to each dataset by independent rationales, and identical annotations do not necessarily indicate identical cell types were recovered from both porcine-ileum and reference data. Abbreviations: cDC, convnentional dendritic cell; DC, dendritic cell; IQR, interquartile range; pDC, plasmacytoid dendritic cell; NK, natural killer; scRNA-seq, single-cell RNA sequencing; TA, transit amplifying; TFH, T follicular helper; Treg, T regulatory.
Activated and cycling B cells
The remaining two B cell types in porcine ileum included cycling and activated B cells, which were most closely related to one another in Fig 3B. Both cell types had high expression of genes related to B cell activation and/or germinal center–associated responses (e.g., AICDA, BCL6, and CD86; Ye et al, 1997; Muramatsu et al, 1999; Victora et al, 2010), but cycling B cells also had characteristics of cellular replication/division, including elevated expression of PCLAF, BIRC5, TOP2A, and STMN1 (Dabydeen et al, 2019; Giotti et al, 2019) and enrichment of biological processes such as nucleosome organization (GO:0034728), centriole–centriole cohesion (GO:0010457), and mitotic spindle organization (GO:0007052; Fig 3B and Table S9). Porcine ileal cycling B cells had the highest prediction to cycling B cells in human ileum and germinal center dark zone (GC DZ) B cells in murine ileum, whereas activated B cells instead had highest the prediction to cells labeled as B cells in the human ileum and germinal center light zone (GC LZ) or resting B cells in murine ileum (Figs 3D, S20, and S21). A subset of cycling B cells had higher prediction scores to porcine peripheral T/ILC lineage lymphocytes and were more specifically predicted to be CD8αβ+ αβ T cells (Fig S19). Of all B/ASC types, porcine ileal activated B cells had the lowest average mapping scores to all reference datasets (range of means 0.530–0.692), suggesting lack of a similar cell population in porcine circulation and human or murine ileum (Figs 3C and S18).
B lineage and cycling lymphocytes enriched in the ileum containing Peyer’s patches
Because Peyer’s patches are niches of GALT with specialized cellular functions different from those performed by cells in the lamina propria or epithelium, we assessed the impact of inclusion versus exclusion of Peyer’s patches on cellular compositions recovered from the porcine ileum. As already shown in Figs 1A and S1B, ileal tissue was dissected into sections with Peyer’s patches (PP), without Peyer’s patches (non-PP), and a whole cross section of ileum (whole ileum) for cell isolation and scRNA-seq. At pseudo-bulk RNA-seq rather than scRNA-seq resolution, overall gene expression profiles of PP and the whole ileum were distinct from non-PP samples both before and after data quality control/filtering (Fig S22A). Analysis at single-cell resolution revealed similar results, whereby cell type proportions and overall cell numbers in whole ileum samples more closely resembled PP than non-PP samples (Figs 4A and S22B and C). At the cell lineage level, whole ileum and PP samples were composed primarily of B lineage lymphocytes (59.12% and 63.89%, respectively), followed by T/ILC lineage lymphocytes (38.13% and 33.17%, respectively; Fig 4B). In contrast, most cells from non-PP samples were T/ILC lineage lymphocytes (82.07%), and only 11.45% were B lineage lymphocytes (Fig 4B).
Figure S22. Comparison of sample types from scRNA-seq of the porcine ileum.
(A) Multidimensional scaling (MDS) plot of pseudobulk samples from six porcine ileal samples subjected to scRNA-seq. Pseudobulk samples are comprised of the cumulative gene counts from all reads/cells of each sample before quality control filtering (top) and in the final filtered dataset (bottom). (B) Stacked bar plot of annotated cell type frequencies (x-axis) within each porcine-ileal sample and total cells (y-axis) subjected to scRNA-seq. Bar size is indicative of total frequency (1) within each sample and is not indicative of the number of cells in each sample. (C) Stacked bar plot of sample frequencies (y-axis) within each annotated porcine ileal cell type and total cells (x-axis) recovered via scRNA-seq. Bar size is indicative of total frequency (1) within each cell type and is not indicative of the number of cells in each cell type. scRNA-seq data shown in (A, B, C) were derived from the ileum of two 7-wk-old pigs. Abbreviations: dim, dimension; ILC, innate lymphoid cell; logFC, log fold-change; MDS, multidimensional scaling; PP, Peyer’s patch; scRNA-seq, single-cell RNA sequencing.
Figure 4. Compositional differences in lymphocytes from the ileum with or without Peyer’s patches.
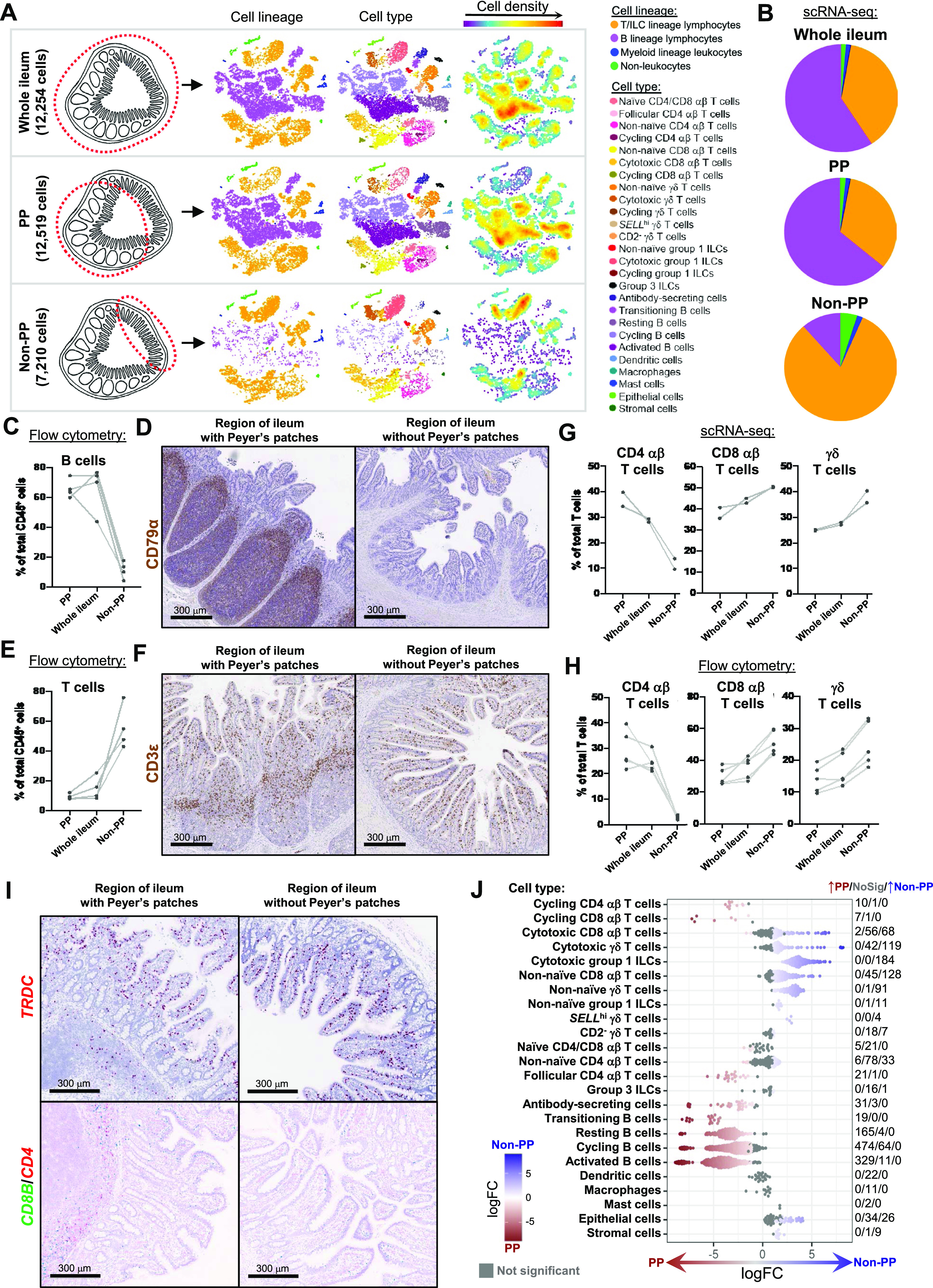
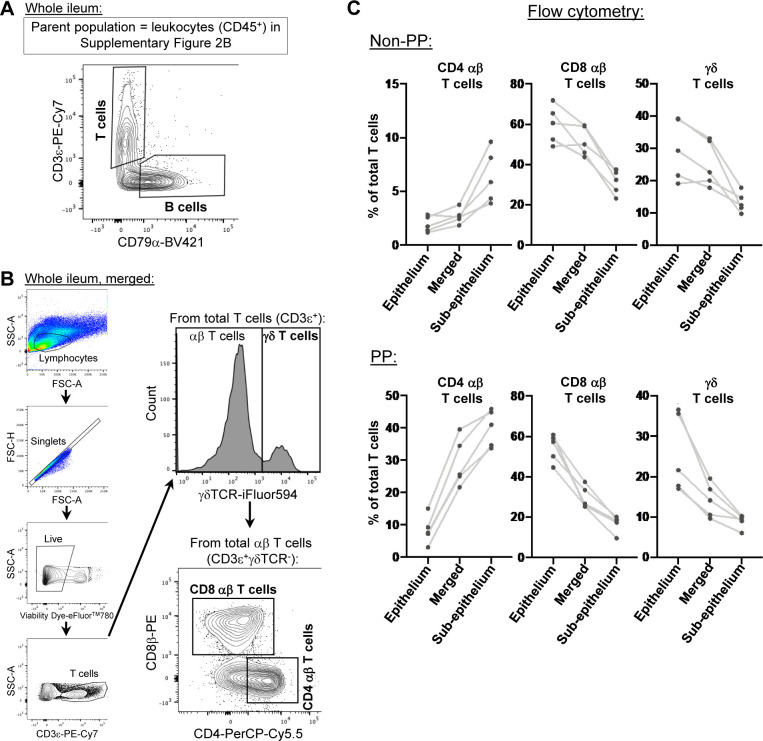
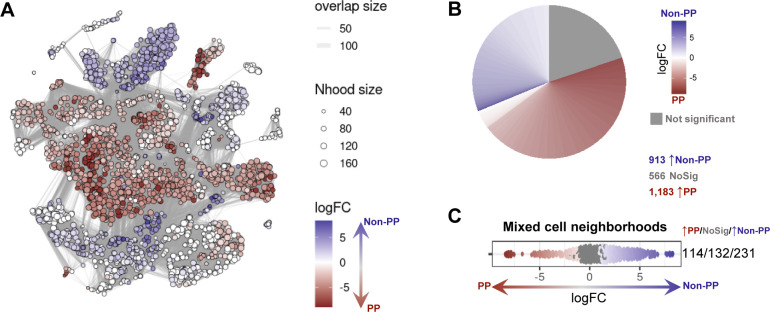
(A) Cell compositions of scRNA-seq data from the whole ileum (top), PP (middle), and non-PP (bottom) samples. Cells from each sample type (depicted on the far left) were combined from a total of two animals and overlaid onto t-SNE coordinates originally presented in Fig 1B–D. The total numbers of cells derived from the total of two animals for each sample type are listed on the far left. On the t-SNE plots, each point represents a single cell; the color of each point corresponds to cell lineage (left t-SNE), cell type (center t-SNE), or cell density (right t-SNE). (B) Pie charts showing proportions of cells from each annotated cell lineage within total cells derived from each sample type in (A). The color of a pie slice indicates cell lineage. The total area of each pie chart is not proportional to the total number of cells derived from each sample type. Proportions were calculated from total cells derived from two pigs for each sample type. (C) Plot of the percentage of B cells (CD79α+) within total leukocytes (CD45+; y-axis) from samples of the whole ileum, PP, and non-PP (x-axis), as assessed by flow cytometry gating shown in Fig S23A. Measurements from different sample types derived from the same animal are connected by a light gray line. (D) IHC staining for B cell CD79α protein (brown) in a region of the ileum with Peyer’s patches (left) or without Peyer’s patches (right). (E) Plot of the percentage of T cells (CD3ε+) within total leukocytes (CD45+; y-axis) from samples of the whole ileum, PP, and non-PP (x-axis), as assessed by flow cytometry gating shown in Fig S23A. Measurements from different sample types derived from the same animal are connected by a light gray line. (F) IHC staining for T cell CD3ε protein (brown) in a region of the ileum with Peyer’s patches (left) or without Peyer’s patches (right). (G) Plot of the percentage of CD4 αβ T cells (left), CD8 αβ T cells (center), or γδ T cells (right) within total T cells (y-axis) of the porcine-ileum scRNA-seq dataset. Percentages from samples of the whole ileum, PP, and non-PP are shown on the x-axis. CD4 αβ T cells included cells annotated as follicular CD4 αβ T cells, non-naive CD4 αβ T cells, or cycling CD4 αβ T cells and cells annotated as naive CD4/CD8 αβ T cells with prediction probability to porcine PBMC CD4+ αβ T cells > prediction probability to porcine PBMC CD8 αβ+ αβ T cells. CD8 αβ T cells included cells annotated as non-naive CD8 αβ T cells, cytotoxic CD8 αβ T cells, or cycling CD8 αβ T cells and cells annotated as naive CD4/CD8 αβ T cells with prediction probability to porcine PBMC CD8αβ+ αβ T cells > prediction probability to porcine PBMC CD4+ αβ T cells. γδ T cells included cells annotated as non-naive γδ T cells, cytotoxic γδ T cells, cycling γδ T cells, SELLhi γδ T cells, and CD2− γδ T cells. Measurements from different sample types derived from the same animal are connected by a light gray line. (H) Plot of the percentage of CD4 αβ T cells (γδTCR−CD4+; left), CD8 αβ T cells (γδTCR−CD8β+; center), or γδ T cells (γδTCR+; right) within total T cells (CD3ε+; y-axis) from samples of the whole ileum, PP, and non-PP (x-axis), as assessed by flow cytometry gating shown in Fig S23B. Measurements from different sample types derived from the same animal are connected by a light gray line. (I) RNA ISH staining for TRDC (top, red), CD8B (bottom, green), or CD4 (bottom, red) transcripts in regions of the ileum with Peyer’s patches (left) or regions of the ileum without Peyer’s patches (right). (J) Differential abundance analysis of cell types from porcine-ileum scRNA-seq PP versus non-PP samples. Annotated cell types are listed on the y-axis. Each point represents an individual cell neighborhood, where a neighborhood was assigned as a specific cell type if >70% of cells within the neighborhood belonged to the specified cell type annotation. Cell neighborhoods with <70% of cells belonging to a single cell type are not shown. Gray points indicate cell neighborhoods that were not significantly more abundant in a specific sample type. Non-gray points indicate cell neighborhoods exhibiting differential abundance (P < 0.1), and red/blue fill of differentially abundant points corresponds to the magnitude and direction of logFC (also corresponding to values listed on the x-axis). Red indicates increased abundance in PP samples, whereas blue indicates increased abundance in non-PP samples. On the far right, counts of cell neighborhoods with increased abundance in PP samples/no differential abundance/increased abundance in non-PP samples are shown for each cell type. Cycling γδ T cells and cycling group 1 ILCs are not shown on the y-axis because of no cell neighborhoods being assigned to these cell types. scRNA-seq data shown in (A, B, G, J) were derived from the ileum of two 7-wk-old pigs. (I) Images shown in (I) were also taken from a 7-wk-old pig used for ileum scRNA-seq. Flow cytometry and IHC experiments were not performed on animals used for scRNA-seq. Flow cytometry experiments shown in (C, E) were conducted using four 6-wk-old pigs. Flow cytometry data shown in (H) was performed using five 9-wk-old pigs. IHC staining in (D) and (F) was completed on 6-wk-old pigs. Abbreviations: IHC, immunohistochemistry; ILC, innate lymphoid cell; ISH, in situ hybridization; logFC, log fold-change; NoSig, no significance; PBMC, peripheral blood mononuclear cell; PP, Peyer’s patch; scRNA-seq, single-cell RNA sequencing; t-SNE, t-distributed stochastic neighbor embedding; TCR, T cell receptor.
The presence and abundance of selected lymphocyte populations in different ileal regions were further validated ex vivo and in situ. Flow cytometry was used to assess B cell abundance via intracellular CD79α protein expression. Larger proportions of CD45+ leukocytes were CD79α+ in PP and whole ileum samples when compared with non-PP samples (Figs 4C and S23A). Immunohistochemistry (IHC) labeling revealed CD79α protein primarily in follicular areas of Peyer’s patches but largely absent in lamina propria and epithelium (Fig 4D), indicating minimal CD79α detected in regions representative of non-PP samples. Because a dependable marker has not yet been established to identify ILCs in pigs, CD3ε protein staining was performed to label only T cells. By flow cytometry, higher percentages of CD3ε+ cells were detected within total CD45+ leukocyte populations of non-PP samples compared with PP or whole ileum samples (Figs 4E and S23A). By IHC, CD3ε protein staining was abundant in lamina propria, epithelium, and T cell areas of Peyer’s patches (Fig 4F), indicating CD3ε was present in regions representative of all ileal sections (PP, non-PP, and whole ileum). Collectively, ex vivo and in situ staining for CD79α and CD3ε protein supported scRNA-seq observations: B cells comprised a larger proportion of cells in PP and whole ileum samples, whereas T cells comprised a larger proportion of cells in non-PP samples. These results are informative in deciding sample preparation for inclusion of cells relevant to biological questions under investigation.
Figure S23. Validation of scRNA-seq lymphocyte compositions via flow cytometry.
(A) Flow cytometry gating strategy used to identify percentages of T cells (CD3ε+) and B cells (CD79α+) from total viable CD45+ leukocytes within porcine ileal samples. Gating is shown for the same whole-ileum sample (containing both regions with and without Peyer’s patches) shown in Fig S2B, starting from the parent population of cells captured and gated as leukocytes in Fig S2B. (B) Flow cytometry gating strategy used to identify percentages of CD4 αβ T cells (γδTCR−CD4+), CD8 αβ T cells (γδTCR−CD8β+), or γδ T cells (γδTCR+) within total viable CD3ε+ T cells of porcine ileal samples. Gating is shown for a whole-ileum sample (containing both regions with and without Peyer’s patches). (C) Plot of the percentage of CD4 αβ T cells (γδTCR−CD4+; left), CD8 αβ T cells (γδTCR−CD8β+; center), or γδ T cells (γδTCR+; right) within total T cells (CD3ε+; y-axis) from PP (upper) and non-PP (lower) samples. Within each sample, cells were collected from epithelial, subepithelial, and merged (containing epithelial and subepithelial cell fractions; same as shown in Fig 4H) cell fractions (x-axes) and assessed by the flow cytometry gating strategy in (B). Measurements from different cell fractions derived from the same animal are connected by a light gray line. Flow cytometry experiments were not performed on animals used for scRNA-seq and were instead performed on four 6-wk-old pigs in (A) and five 9-wk-old pigs in (B, C). Abbreviations: FSC-A, forward scatter area; FSC-H, forward scatter height; PP, Peyer’s patch; scRNA-seq, single-cell RNA sequencing; SSC-A, side scatter area; TCR, T cell receptor.
We further validated proportions of CD4 αβ, CD8 αβ, and γδ T cells in various regions of the ileum using flow cytometry and RNA in situ hybridization (ISH). T cells recovered via scRNA-seq were regrouped into CD4 αβ, CD8 αβ, and γδ T cells, and percentages of each subset within total T cells were calculated for each ileal scRNA-seq sample. Analysis revealed (1) increased proportions of CD4 αβ T cells in PP versus non-PP samples; (2) increased proportions of CD8 αβ T cells and γδ T cells in non-PP versus PP samples; and (3) intermediate proportions of all three T cell subsets in the whole ileum compared with PP and non-PP samples (Fig 4G). By flow cytometry, T cell proportions mirrored patterns obtained from scRNA-seq (Figs 4H and S23B). RNA ISH staining in regions of the ileum without Peyer’s patches (Fig 4I, right) revealed TRDC (γδ T cells) and CD8B (CD8 αβ T cells) transcripts were primarily expressed within the epithelial layer, whereas CD4 (CD4 αβ T cells) was expressed primarily within the lamina propria, supporting the conclusion that most γδ and CD8 αβ T cells were intraepithelial, and most CD4 αβ T cells resided in the lamina propria. Localization of CD4, CD8B, and TRDC did not change in epithelium and lamina propria adjacent to Peyer’s patches. However, all three transcripts were also expressed by cells in the T cell zones of Peyer’s patches, which were removed from non-PP samples (Fig 4I, left). Flow cytometry staining of epithelium-enriched, subepithelium-enriched, and merged cell fractions from PP and non-PP ileal samples was performed to validate ISH findings. The results revealed epithelium-enriched fractions from both PP and non-PP samples had higher percentages of γδ and CD8 αβ T cells and lower percentages of CD4 αβ T cells compared with subepithelium-enriched cell fractions (Fig S23C). In all, ex vivo and in situ staining to identify location of CD4 αβ, CD8 αβ, and γδ T cells mirrored results from scRNA-seq and provided further locational context of T cells in ileal epithelium, lamina propria, and Peyer’s patches.
PP and non-PP samples were dissected by complete inclusion or exclusion of Peyer’s patches, respectively, and could thus be directly compared to identify annotated cell types enriched in the presence versus absence of Peyer’s patches. To identify cell type enrichment, cell neighborhoods (conglomerates of cells located near each other in the multidimensional space of the dataset) were identified from cells of PP and non-PP samples, and differential abundance analysis was performed on cell neighborhoods (Figs 4J and S24). Though T/ILC lineage lymphocytes comprised a greater proportion of total cells in non-PP than PP samples (Fig 4B), several T/ILC types were more abundant in PP samples, including cycling CD4 αβ T cells, cycling CD8 αβ T cells, and follicular CD4 αβ T cells, with at least 87.5% of cell neighborhoods significantly more abundant in PP samples for each cell type (Fig 4J). In contrast, cytotoxic and non-naive γδ T, CD8 αβ T, and group 1 ILCs, along with SELLhi γδ T cells, had most of the cell neighborhoods (>50% for each cell type) significantly enriched in non-PP samples (Fig 4J). Remaining T/ILC types had no or lower percentages of differentially abundant cell neighborhoods: 94.1% of group 3 ILC cell neighborhoods were not significantly differentially abundant; CD2− γδ T cells had 28.0% of cell neighborhoods significantly increased in non-PP samples; naive CD4/CD8 αβ T cells had 19.2% of cell neighborhoods significantly increased in PP samples; and non-naive CD4 αβ T cells had 5.1% and 28.2% of cell neighborhoods significantly enriched in PP and non-PP samples, respectively (Fig 4J). Cycling γδ T and group 1 ILCs did not have any cell neighborhoods recovered for the analysis; however, no cycling group 1 ILCs were recovered from non-PP samples, and cycling γδ T cells derived from PP samples outnumbered those derived from non-PP samples by sixteen-to-three (Fig S22C). Similar to results evaluating compositions at the cell lineage level, all cell types from the B lymphocyte lineage (ASCs, transitioning B, resting B, cycling B, and activated B) had at least 88.1% of cell neighborhoods significantly more abundant in PP samples, indicating enrichment within Peyer’s patches (Fig 4J). Collectively, differential abundance analysis indicated that B cells, ASCs, cycling T/ILCs, and follicular CD4 αβ T cells were more abundant in PP samples, likely because of association of these cell types with functions of ileal Peyer’s patches and germinal center responses. Cytotoxic and non-naive subsets of γδ T, CD8 αβ T, and group 1 ILCs were more abundant in non-PP samples, indicating location and functions within the epithelium and/or lamina propria rather than in Peyer’s patches of the ileum.
Figure S24. Differential abundance analysis of the porcine ileum with versus without Peyer’s patches.
(A) Cell neighborhoods identified by differential abundance analysis. Only cells derived from PP and non-PP samples were included in differential abundance analysis but were overlaid back onto their original t-SNE coordinates of the full dataset that included whole-ileum samples, shown in Fig 1C and D. Size of a circle indicates the number of cells in a neighborhood (Nhood size); color of a circle indicates magnitude of logFC in abundance in non-PP (blue) versus PP (red) samples; width of lines between cell neighborhoods indicates the number of overlapping cells found in each of two neighborhoods (overlap size). (B) Pie chart of differential abundance results for cell neighborhoods shown in (A). Gray indicates the proportion of cell neighborhood that was not differentially abundant, whereas cell neighborhoods with significantly increased abundance (P < 0.01) in non-PP or PP samples are shown in blue and red, respectively. The logFC magnitude of differential abundance is also shown by red or blue shading. (C) Plot similar to that shown for differential abundance analysis in Fig 5J but for all mixed cell neighborhoods that were not assigned as a specific cell type because of having <70% of cells belonging to a single cell type annotation. Each point represents an individual cell neighborhood. Gray points indicate cell neighborhoods that were not significantly more abundant in a specific sample type. Non-gray points indicate cell neighborhoods exhibiting differential abundance (P < 0.1). Red/blue fill of differentially abundant points corresponds to the magnitude and direction of logFC. Red indicates increased abundance in PP samples, whereas blue indicates increased abundance in non-PP samples. On the far right, counts of cell neighborhoods with increased abundance in PP samples/no differential abundance/increased abundance in non-PP samples are shown for each cell type. scRNA-seq data shown in (A, B, C) were derived from the ileum of two 7-wk-old pigs. Abbreviations: logFC, log fold-change; Nhood, neighborhood; NoPP, Peyer’s patch; No Sig, no significance; t-SNE, t-distributed stochastic neighbor embedding.
Differential location of group 1 ILCs and group 3 ILCs in the porcine ileum indicated via ex vivo and in situ detection
To date, NK cells are the only porcine non–B/non–T lymphocyte subset identified and are typically identified as CD3ε−CD8α+ lymphocytes using ex vivo flow cytometry assessment (reviewed by Gerner et al [2009]). NK cell frequency in the porcine intestine has been assessed, but very few NK cells are detected in the porcine ileum (Sinkora et al, 2011; Annamalai et al, 2015, 2019; Potockova et al, 2015; Wasowicz et al, 2018). In contrast, we identified 2,594 cells (8.1% of total cells) as ILCs in porcine ileum via scRNA-seq, and few ILCs expressed CD8A (Figs 2B and S25). We therefore used scRNA-seq data to inform an approach to identify ILCs (not just CD3ε−CD8α+ NK cells) by flow cytometry.
Figure S25. Gene expression profiles of group 1 and group 3 ILCs in the porcine ileum.
(A) Overlay onto two-dimensional t-SNE visualization shown in Fig 1C and D of cells annotated as ILCs (activated group 1 ILCs, cytotoxic group 1 ILCs, cycling group 1 ILCs, or group 3 ILCs; shown in black) in porcine-ileum scRNA-seq data. All cells not annotated as ILCs (non-ILCs) are shown in light gray. Each point represents a single cell. (B) Expression of a subset of canonical genes used to identify group 1 and group 3 ILCs in porcine-ileum scRNA-seq data, overlaid onto two-dimensional t-SNE visualization coordinates of cells shown in (A). Color of a point corresponds to the expression level of a specified gene within a cell relative to all other cells in the dataset shown in (A). Regions on the t-SNE plot with concentrations of ILCs are indicated by red circles. (C) Gene expression patterns of canonical genes shown in (B) (x-axis) across annotated cell types in porcine-ileum scRNA-seq data (x-axis). Within the plot, size of a dot corresponds to the percentage of cells expressing a gene within an annotated cell type; color of a dot corresponds to average expression level of a gene for those cells expressing it within an annotated cell type relative to all other cells in the dataset shown in (A). scRNA-seq data shown in (A, B, C) were derived from the ileum of two 7-wk-old pigs. *Ensembl identifiers found in gene annotation were converted to gene symbols; refer to methods section “Gene name modifications” for more details. Abbreviations: ILC, innate lymphoid cell; scRNA-seq, singe-cell RNA sequencing; t-SNE, t-distributed stochastic neighbor embedding.
Further query of ILC single-cell gene expression profiles revealed ILCs expressed CD2 but lacked gene expression of canonical T, B, and myeloid lineage leukocyte markers (CD3E, CD79A, and SIRPA* [encoding CD172α], respectively; Figs 2B and S25). Because porcine-specific immunoreagents are commercially available for CD2, CD3ε, CD79α, and CD172α, we identified cells corresponding to an ILC phenotype from the porcine ileum via flow cytometry as CD2+Lin− cells (Lin− = CD3ε−CD79α−CD172α−; Fig 5A). Nearly all CD2+Lin− cells had forward- and side-scatter properties indicative of lymphocytes (Fig 5B). Most ILCs also expressed PTPRC (encoding CD45, considered to be a pan-leukocyte marker in pigs [reviewed by Piriou-Guzylack and Salmon (2008)]) in our scRNA-seq data. However, fewer group 3 ILCs expressed PTPRC (67.68%; Fig S25), and previous work in mice indicates CD45 expression may be lost by ILC3s, rendering it an inconsistent ILC marker (Xu et al, 2019). Congruently, the majority (≥83.1%) but not all CD2+Lin− cells in the porcine ileum were CD45+ (Fig 5C).
Figure 5. Ex vivo and in situ identification of ILCs in the porcine ileum.
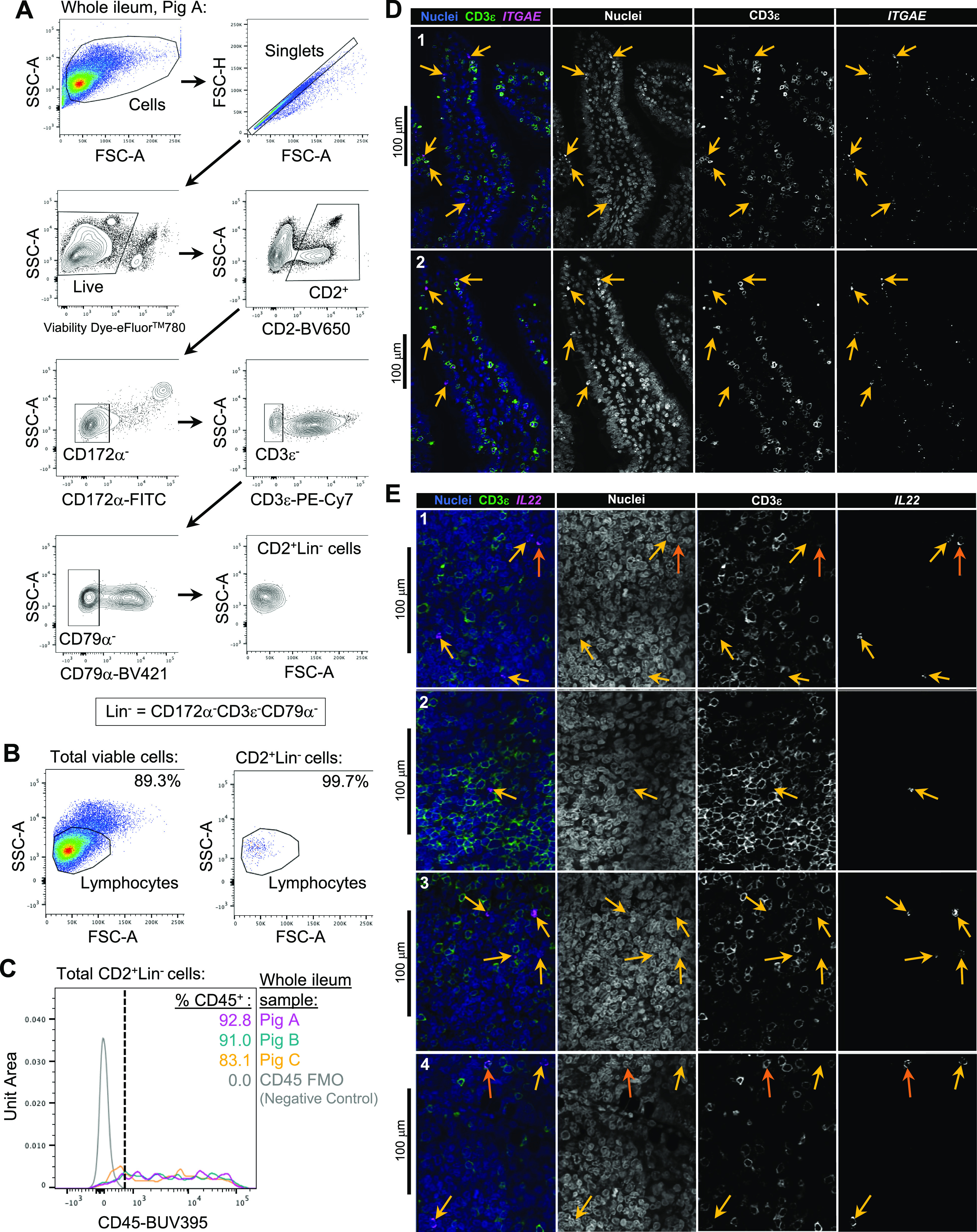
(A) Flow cytometry gating strategy used to identify CD2+Lin− (Lin− = CD172α−CD3ε−CD79α−) within total viable cells of porcine ileal samples. (C) Gating is shown for a whole-ileum sample (containing both regions with and without Peyer’s patches) for pig A (corresponding to pig IDs in (C)). (B) Flow cytometry forward- and side-scatter plots of total viable cells (left) and CD2+Lin− cells (right) within a sample of the porcine whole ileum shown in (A). A gate identifying cells with scatter profiles consistent with lymphocytes is shown, with percentages of cells within the lymphocyte gate listed in the top right of each plot. (C) Histogram of the percentage of CD45+ cells within CD2+Lin− cells identified from samples of the porcine ileum using the flow cytometry gating strategy shown in (A). A fluorescence-minus-one (FMO) sample lacking α-CD45 antibody staining was used as a negative control. (D) Dual fluorescent staining of CD3ε protein and ITGAE RNA in villi (epithelium + lamina propria) of the porcine ileum. Left column: overlay of all stains, including CD3ε protein (green), ITGAE RNA (magenta), and nuclei (DAPI staining; blue). Additional columns show individual stain overlays in white, including (from left to right) nuclei, CD3ε protein, and ITGAE RNA. Panels of two separate villi are shown in each row. Panels were selected from larger stitched images as shown in Fig S26A. Yellow arrows indicate location of ITGAE+CD3ε− cells. (E) Dual fluorescent staining of CD3ε protein and IL22 RNA in lamina propria/GALT of the porcine ileum. Left column: overlay of all stains, including CD3ε protein (green), IL22 RNA (magenta), and nuclei (DAPI staining; blue). Additional columns show individual stain overlays in white, including (from left to right) nuclei, CD3ε protein, and IL22 RNA. Panels of four separate tissue locations are shown in each row. Panels were selected from larger stitched images as shown in Fig S26B. Yellow arrows indicate location of IL22+CD3ε− cells. Orange arrows indicate location of IL22+CD3ε+ cells. Flow cytometry experiments shown in (A, B, C) were conducted using three 6-wk-old pigs. Dual IF/ISH experiments shown in (D, E) were conducted using a 7-wk-old pig used for ileum scRNA-seq. Abbreviations: FMO, fluorescence-minus-one; FSC-A, forward scatter area; FSC-H, forward scatter height; GALT, gut-associated lymphoid tissue; IF, immunofluorescence; ISH, in situ hybridization; ILC, innate lymphoid cell; scRNA-seq, single-cell RNA sequencing; SSC-A, side scatter area.
To assess spatial context of ILCs in tissue and cautiously infer cell function based on location, we developed in situ methods for detection of porcine group 1 and group 3 ILCs in the ileum. From initial scRNA-seq analysis, activated, cytotoxic, and cycling group 1 ILCs all had high expression of ITGAE, encoding the integrin α subunit of CD103 that is highly expressed by intraepithelial lymphocytes in the intestine of other species (Figs 2B and S25B and C; Kilshaw & Murant, 1990; Cepek et al, 1994; Mayassi & Jabri, 2018; Olivares-Villagómez & Van Kaer, 2018). Correspondingly, ITGAE was primarily detected in the epithelial layer of the porcine ileum by in situ analysis (Fig S26A). ITGAE was also strongly expressed by some T cells in our scRNA-seq dataset (Figs 2B and S25B and C); thus, we additionally assessed protein expression of CD3ε to decipher intraepithelial T cells (ITGAE+CD3ε+) from intraepithelial group 1 ILCs (ITGAE+CD3ε−). Dual ITGAE/CD3ε in situ labeling revealed most of the ITGAE+ intraepithelial cells were CD3ε+ T cells; however, ITGAE+CD3ε− intraepithelial cells were also noted in the porcine ileum (Figs 5D and S26A). Though ITGAE+CD3ε− intraepithelial cells could also be intraepithelial DCs, we found few DCs expressed ITGAE (Fig S25C), and ITGAE+CD3ε− cells could also be found located in the apical epithelium that was in closest proximity to the lumen, whereas intraepithelial DCs reside only in the basement membrane (Farache et al, 2013). Thus, most of the ITGAE+CD3ε− intraepithelial cells were assumed to be intraepithelial group 1 ILCs (Figs 5D and S26A).
Figure S26. Microscopy images of in situ ILC detection.
(A, B) Confocal images for in situ detection of group 1 ILCs (A) and group 3 ILCs (B) in the porcine ileum. Individual image frames were acquired at 60× magnification and stitched together. In both (A) and (B), the upper left image shows overlay of all stains together, including nuclei (DAPI staining; blue), CD3ε protein (green), and RNA for ITGAE (A) or IL22 (B) shown in magenta. The upper right image shows only nuclei staining (white); the lower left image shows only CD3ε protein staining (white); the lower right image shows only ITGAE (A) or IL22 (B) RNA staining (white). All images in (A) and all images in (B) show the same captured frames. Yellow boxes indicate tissue areas shown at higher magnification in Fig 5D and E. Number of a box in the upper left panels corresponds to the frame number shown in Fig 5D and E. Dual IF/ISH experiments shown in (A, B) were conducted using a 7-wk-old pig used for ileum scRNA-seq. Abbreviations: IF, immunofluorescence; ILC, innate lymphoid cell; ISH, in situ hybridization.
Group 3 ILCs in the porcine ileum had high expression of IL22 (Figs 2B and S25B and C), and IL22 was detected in situ primarily in the lamina propria and T cell zones of Peyer’s patches in porcine ileum (Fig S26B). Furthermore, most but not all IL22+ cells detected in situ lacked CD3ε expression, indicating that most of the IL22-expressing cells were not T cells (Figs 5E and S26B). Therefore, we identified group 3 ILCs as IL22+CD3ε− cells, though the in situ staining combination also identified a minority of IL22+CD3ε+ (presumably TH22) cells. Collectively, query of gene expression for ILCs at single-cell resolution allowed us to develop new reagent panels for ex vivo and in situ analysis of ILCs in the porcine ileum that expanded on previous panels catering to only NK cell identification.
Transcriptional distinctions between porcine ileal and circulating ILCs
Porcine NK cells were not representative of porcine ileal ILCs; therefore, ileal ILCs required different markers for ex vivo and in situ detection (Figs 5, S25, and S26). As porcine peripheral NK cells are readily characterized, we wanted to further explore transcriptional distinctions between porcine ileal ILCs and porcine peripheral ILCs (i.e., NK cells) to infer potential cell functions and identify additional targets for ex vivo and in situ detection. PBMCs from the same two pigs were processed for scRNA-seq in parallel to ileal samples, and peripheral ILCs were identified by the same criteria used to identify ILCs in the ileum (refer to the Materials and Methods section for full details). Porcine peripheral ILC gene expression profiles were largely concordant with NK cells described in our previous work (Fig S27A–E and Table S10; Herrera-Uribe et al, 2021). A new merged ILC scRNA-seq dataset containing only single cells annotated as ILCs from both PBMCs and ileal samples was generated (Fig 6A). Within the merged ILC dataset, 41.3% of detected cell neighborhoods were differentially abundant based on ileal versus peripheral cell origin (Fig 6B and C), suggesting differences between at least some ileal and peripheral ILCs may exist and meriting further exploration.
Figure S27. Identification of peripheral ILCs from porcine PBMC scRNA-seq data.
(A) Two-dimensional t-SNE visualization of porcine PBMCs subjected to scRNA-seq and included in a final dataset following data processing and quality filtering. Each point represents a single cell. Plots show whether cells were derived from pig 1 (6,223 cells; left) or pig 2 (6,548 cells; right). (B) Two-dimensional t-SNE visualization of 12,771 porcine PBMCs (combined cells from pig 1 and pig 2 shown in (A)). Each point represents a single cell; color of a point corresponds to one of 35 cell clusters a cell belongs to, with more transcriptionally similar cells belonging to the same cell cluster. The number of cells belonging to each cell cluster is listed in the cluster key. (C) Expression of a subset of canonical genes used to identify ILCs overlaid onto two-dimensional t-SNE visualization coordinates of cells shown in (B). Color of a point corresponds to expression level of a specified gene within a cell relative to all other cells in the dataset shown in (A). (D) Hierarchical relationship of cell clusters in porcine PBMCs shown in a dendrogram (left) and dot plot showing gene expression patterns within each cell cluster shown in (B) (right). In the dot plot, gene expression patterns of canonical genes used to identify ILCs (x-axis) across cell clusters shown in (B) are on the y-axis. Within the plot, size of a dot corresponds to the percentage of cells expressing a gene within a cell cluster; color of a dot corresponds to average expression level of a gene for those cells expressing it within a cell cluster relative to all other cells in the dataset shown in (B). A blue box is drawn around clusters identified as ILCs. (E) Overlay onto two-dimensional t-SNE visualization shown in (B) of cells annotated as ILCs (black; cell clusters p0, p4, p26, p28, and p30) in (D). All cells not annotated as ILCs (non-ILCs) are shown in light gray. Each point represents a single cell. scRNA-seq data shown in (A, B, C, D, E) were derived from PBMCs of two 7-wk-old pigs. Ileum and PBMC samples for scRNA-seq were collected from the same two pigs and processed in parallel. *Ensembl identifiers found in gene annotation were converted to gene symbols; refer to methods section “Gene name modifications” for more details. Abbreviations: ILC, innate lymphoid cell; PBMC, peripheral blood mononuclear cell; scRNA-seq, single-cell RNA sequencing; t-SNE, t-distributed stochastic neighbor embedding.
Figure 6. Peripheral ILCs are transcriptionally distinct from ileal ILCs.
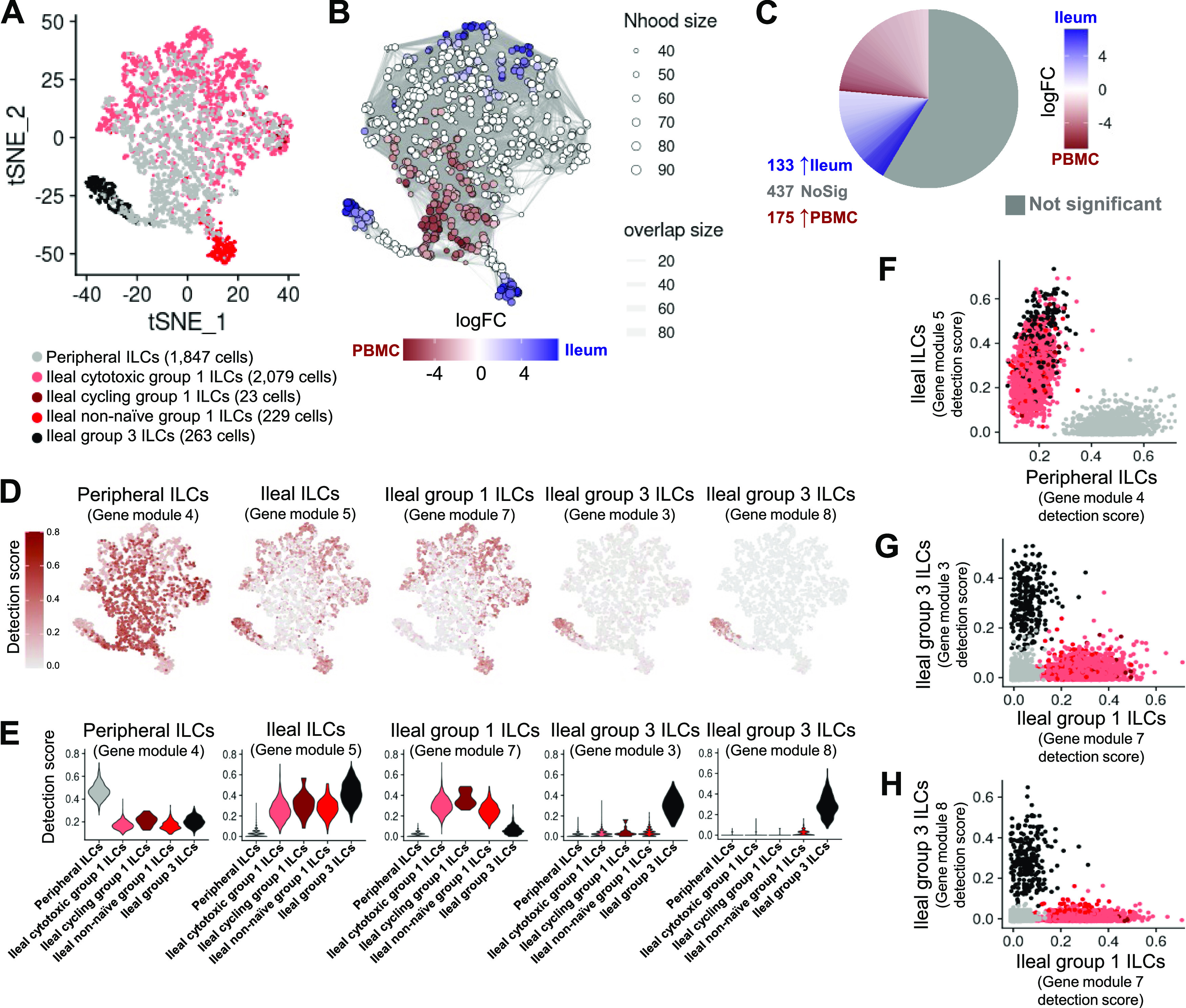
(A) Two-dimensional t-SNE visualization of 4,441 cells recovered from the porcine ileum (2,594 cells) and porcine PBMCs (1,847 cells) via scRNA-seq and classified as ILCs in Figs S5C–F and S27D and E. Each point represents a single cell; color of a point corresponds to one of five ILC annotations. The number of cells belonging to each cell type is listed in the color key below. Derivation from the ileum or PBMC scRNA-seq samples for annotated ILC types is indicated in the annotation name. (B) Cell neighborhoods identified by differential abundance analysis between cells derived from the ileum and PBMCs as shown in (A), overlaid onto t-SNE coordinates also shown in (A). Size of a circle indicates the number of cells in a neighborhood (Nhood size); color of a circle indicates magnitude of logFC in abundance in the ileum (blue) versus PBMCs (red); width of lines between cell neighborhoods indicates the number of overlapping cells found in each of two neighborhoods (overlap size). (C) Pie chart of differential abundance results for cell neighborhoods shown in (B). Gray indicates the proportion of cell neighborhoods that were not differentially abundant, whereas cell neighborhoods with significantly increased abundance (P < 0.01) in the ileum or PBMC samples are shown in blue and red, respectively. The logFC magnitude of differential abundance is also shown by red or blue shading. (D) Selected gene module detection scores from multidimensional differential gene expression analysis of cells shown in (A) overlaid onto two-dimensional t-SNE visualization coordinates. Color of a point corresponds to detection score for a gene module within a cell. (E) Violin plots summarizing gene module detections scores shown in (D) (y-axis) across annotated ILC types shown in (A) (x-axis). (F) Scatter plot of gene module 5 detection scores (y-axis) versus gene module 4 detection scores (x-axis) for all cells shown in (A). Each point represents a single cell; color of a point corresponds to cell type annotations shown in (A). (G) Scatter plot of gene module 3 detection scores (y-axis) versus gene module 7 detection scores (x-axis) for all cells shown in (A). Each point represents a single cell; color of a point corresponds to cell type annotations shown in (A). (H) Scatter plot of gene module 8 detection scores (y-axis) versus gene module 7 detection scores (x-axis) for all cells shown in (A). Each point represents a single cell; color of a point corresponds to cell type annotations shown in (A). scRNA-seq data shown in (A, B, C, D, E, F, G, H) were derived from the ileum and PBMCs of two 7-wk-old pigs. Ileum and PBMC samples for scRNA-seq were collected from the same two pigs and processed in parallel. Abbreviations: ILC, innate lymphoid cell; logFC, log fold-change; Nhood, neighborhood; No Sig, no significance; PBMC, peripheral blood mononuclear cell; scRNA-seq, single-cell RNA sequencing; t-SNE, t-distributed stochastic neighbor embedding.
Multidimensional differential gene expression (DGE) was performed to identify differentially expressed genes (DEGs) independent of cell type annotations and sample origin of cells (Table S10). Hierarchical clustering of DEGs with the lowest P-values defined nine gene modules with varying patterns of detection (Figs 6D and E and S28A–C). Gene module 4 had highest detection in peripheral ILCs, whereas module 5 had higher detection across all four types of ileal ILCs (Fig 6D and E), allowing us to easily segregate ILCs by peripheral or ileal origin based on gene module 4 versus 5 detection scores (Fig 6F). Gene module 7 had highest detection in ileal group 1 ILCs (activated, cytotoxic, and cycling), whereas gene modules 3 and 8 detection scores correlated positively with one another and were higher in ileal group 3 ILCs (Figs 6D and E and S28D). Collectively, the differences noted allowed for segregation between ileal group 1 and group 3 ILCs on the basis of detection for gene module 7 and 3/8, respectively (Fig 6G and H). Therefore, gene module 4 was considered as highly specific to peripheral ILCs, gene module 5 to all ileal ILCs, gene module 7 to ileal group 1 ILCs, and gene modules 3 and 8 to ileal group 3 ILCs. Further refinement focused on genes with the highest specificity for respectively assigned ILC subsets from gene modules in Fig 6D and E, yielding core gene signatures for peripheral ILCs, all ileal ILCs, ileal group 1 ILCs, and ileal group 3 ILCs (Fig 7A–D).
Figure S28. Gene module detection in ILCs from porcine-ileum and PBMC scRNA-seq data.
(A) Dendrogram of the top differentially expressed genes (P < 1 × 10−10) recovered through multidimensional differential gene expression analysis. Based on the dendrogram, genes were grouped into nine gene modules, as indicated at the bottom of the dendrogram. (B) Selected gene module detection scores from multidimensional differential gene expression analysis of cells shown in Fig 6A overlaid onto two-dimensional t-SNE visualization coordinates. Color of a point corresponds to detection score for a gene module within a cell. (C) Violin plots summarizing gene module detections scores shown in (B) (y-axis) across annotated ILC types shown in Fig 6A (x-axis). (D) Scatter plot of gene module 8 detection scores (y-axis) versus gene module 3 detection scores (x-axis) for all cells shown in Fig 6A. Each point represents a single cell; color of a point corresponds to cell type annotations shown in Fig 6A. Correlation value (R) is shown at the top of the plot. scRNA-seq data shown in (A, B, C, D) were derived from the ileum and PBMCs of two 7-wk-old pigs. Ileum and PBMC samples for scRNA-seq were collected from the same two pigs and processed in parallel. Abbreviations: ILC, innate lymphoid cell; PBMC, peripheral blood mononuclear cell; scRNA-seq, single-cell RNA sequencing; t-SNE, t-distributed stochastic neighbor embedding.
Figure 7. Core gene signatures of ileal and peripheral ILCs.
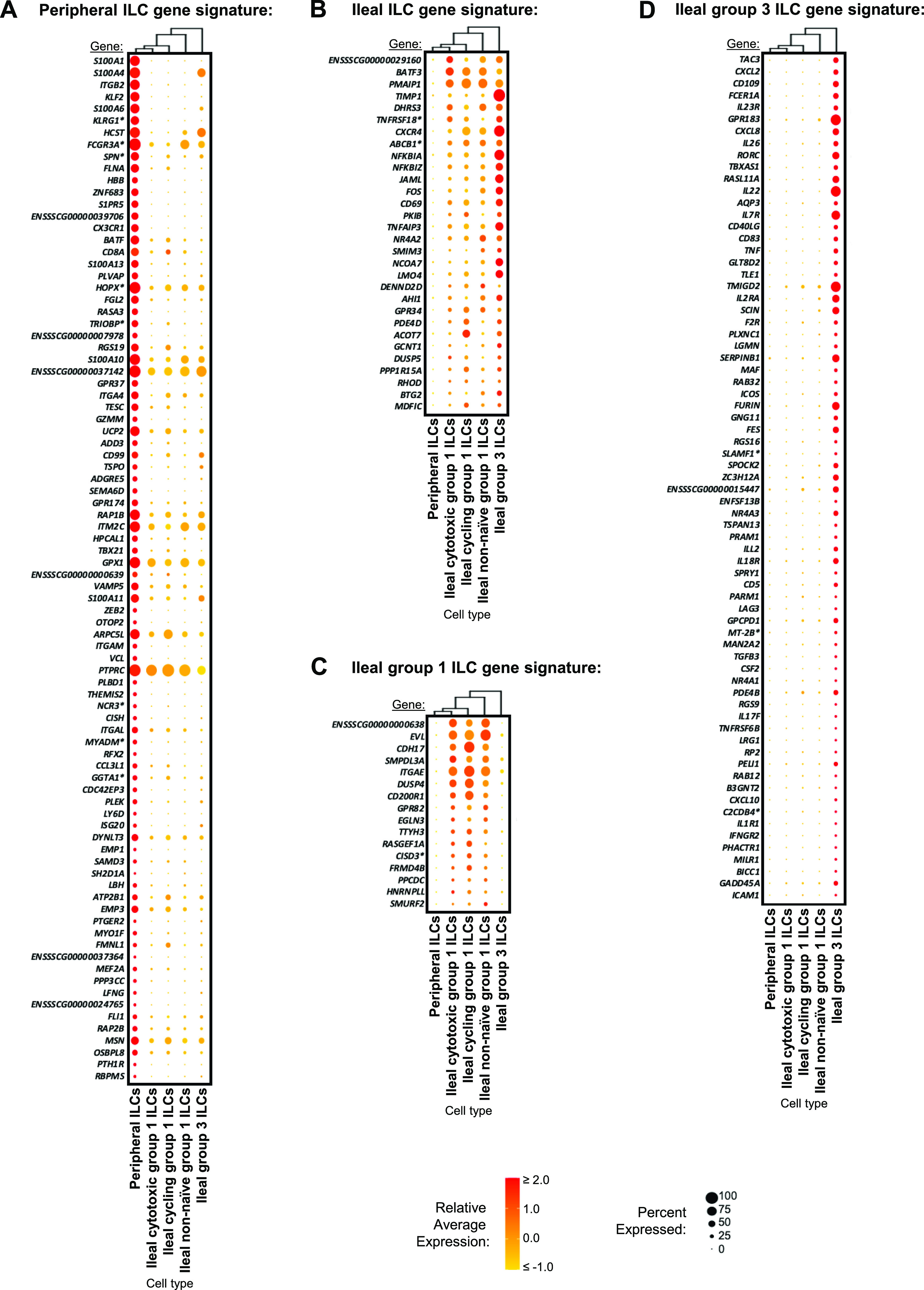
(A, B, C, D) Dot plots showing expression patterns within each annotated ILC type for selected genes from gene module 4 used to create a peripheral ILC gene signature (A), gene module 5 used to create an ileal ILC gene signature (B), gene module 7 used to create an ileal group 1 ILC gene signature (C), and gene modules 3 and 8 used to create an ileal group 3 ILC gene signature (D). Genes are shown on the y-axis, and annotated ILC types from the porcine ileum and PBMCs are shown on the x-axis. Within the dot plot, size of a dot corresponds to the percentage of cells expressing a gene within an annotated cell type; color of a dot corresponds to average expression level of a gene for those cells expressing it within a cell type relative to all other cells in the dataset shown in Fig 6A. Hierarchical relationships of annotated ILC types are shown with a dendrogram on the top of each dot plot. scRNA-seq data shown in (A, B, C, D) were derived from the ileum and PBMCs of two 7-wk-old pigs. Ileum and PBMC samples for scRNA-seq were collected from the same two pigs and processed in parallel. *Ensembl identifiers found in gene annotation were converted to gene symbols; refer to methods section “Gene name modifications” for more details. Abbreviations: ILC, innate lymphoid cell; PBMC, peripheral blood mononuclear cell; scRNA-seq, single-cell RNA sequencing.
An 86-gene signature derived from gene module 4 was identified for peripheral ILCs (Fig 7A) and included several genes recognized as canonical markers for porcine NK cells (CD8A, FCGR3A*, ITGAM, NCR3*, and HCST; Denyer et al, 2006; Piriou-Guzylack & Salmon, 2008; Gerner et al, 2009; Toka et al, 2009; Mair et al, 2013) or recognized as canonical NK cell markers across other species (KLRG1*, CD99, TBX21, and GZMM; Townsend et al, 2004; Huntington et al, 2007; Knox et al, 2014; Robinette et al, 2015; Crinier et al, 2018). FCGR3A* and CD8A encode for CD16 and CD8α, respectively, which are the two primary markers used to identify porcine NK cells at the protein level (reviewed by Piriou-Guzylack and Salmon [2008] and Gerner et al [2009]). Thus, inclusion of FCGR3A* and CD8A in a gene signature for peripheral ILCs concordant with NK cell descriptions suggests current porcine NK cell identifiers are insufficient for pan-ILC identification in pigs. Additional peripheral ILC signature genes encoded for integrins (ITGB2, ITGA4, ITGAM, and ITGAL) and other molecules associated with cell receptors/signaling (FGR, PTPRC, CX3CR1, and S1PR5), consistent with enrichment of biological processes including integrin-mediated signaling pathway (GO:0007229), leukocyte cell–cell adhesion (GO:0007159), and receptor clustering (GO:0043113) in gene module 4.
Thirty genes derived from gene module 5 comprised an ileal ILC gene signature identifying both group 1 and group 3 ILCs in the porcine ileum (Fig 7B). The gene signature for ileal ILCs included CD69, encoding for the cell surface receptor CD69 used to identify tissue resident and recently activated cells (Shiow et al, 2006; Ashouri & Weiss, 2017; Szabo et al, 2019b). Enriched biological pathways for module 5 included those associated with cell metabolism, including positive regulation of macromolecule metabolic process (GO:0010604), positive regulation of cellular protein catabolic process (GO:1903364), and long-chain fatty-acid metabolic process (GO:0001676), suggesting that ileal ILCs were in a highly active metabolic state.
A gene signature for ileal group 1 ILCs included 16 genes derived from gene module 7, as shown in Fig 7C. Similar to gene module 5, gene module 7 was enriched for metabolic processes, including catabolic processes such as nucleoside phosphate catabolic process (GO:1901292) and biosynthetic processes such as purine-containing compound biosynthetic process (GO:0072522), suggesting an active metabolic state defining group 1 ILCs in the ileum. Notably, ITGAE, which we used to identify intraepithelial group 1 ILCs in Fig 5D, was found in the ileal group 1 ILC gene signature, further supporting its use as an effective group 1 ILC marker in conjunction with other key markers in the porcine ileum.
Group 3 ILCs had a core gene signature of 71 genes derived from gene modules 3 and 8 (Fig 7D). Many of the signature genes were associated with a type 3 immune response and largely overlapped with DEGs identified for ileal group 3 ILCs relative to all other ileal T/ILCs and relative to all other ileal ILCs (Tables S4 and S8). Similarly, enriched biological processes for gene modules 3/8 also had high overlap with enriched biological processes found from DEGs in Tables S4 and S8. Notably, IL22, used as an in situ marker for group 3 ILCs in Fig 5E, was detected in the ileal group 3 ILC gene signature, supporting its use as an effective group 3 ILC marker in conjunction with other key markers, not only relative to other cells in ileum but also relative to peripheral ILCs.
Discussion
Understanding intestinal lymphocyte identity and function to cumulatively promote intestinal health outcomes in pigs has implications for biomedical research, animal health, and global food security. Phenotyping of porcine lymphocytes based on protein expression of a handful of cell surface markers has provided limited information about the biological dynamics of intestinal lymphocytes. Our work provides greater biological insight through further characterization of ileal lymphocytes into inferred functional subsets at transcriptional resolution not previously achieved in pigs, and we also include cross-species/cross-tissue comparisons and spatial context of specific lymphocyte types in different regions of the ileum. In addition to discoveries described herein, the data serves as a resource to be further explored and dissected for information about the heterogeneous landscape of lymphocytes in the porcine ileum.
Pigs readily serve as a biomedical model, with several innovations already applied to study intestinal lymphocytes. For example, germ-free pigs are used to study the dependence of intestinal lymphocyte development on microbial colonization (reviewed by Butler et al [2009], Sinkora and Butler [2009, 2016], and Pabst [2020]). Germ-free pigs can be colonized with human microbiomes to generate microbially humanized pigs for understanding the impact of the microbiota on local cellular responses associated with mucosal vaccination strategies and subsequent protection (Kumar et al, 2018; Miyazaki et al, 2018). Moreover, pigs serve as models for studying the impact of stress, nutrition, and infectious disease on intestinal physiology and immune status (reviewed by Gonzalez et al [2015], Roura et al [2016], Ziegler et al [2016], Moeser et al [2017], Hryhorowicz et al [2020], and Käser [2021]). Bolstering lymphocyte-mediated intestinal immunity is important for both humans and pigs, but mechanistic approaches rely heavily on first understanding key lymphocytes in the porcine intestine and recognizing shared annotation with humans. To help overcome these obstacles, we identified and described biological implications of lymphocytes in the porcine ileum in comparison to both human and murine ileum. Collectively, our data indicate a general consensus of annotated lymphocyte types across species; however, a few notable differences were described. Therefore, our cross-species comparisons serve as a foundation for deeper resolved exploration of pigs as models for human medicine. We further explore parallels between pigs and humans as it relates to intestinal lymphocytes throughout the rest of the discussion. However, we also note that some interspecies differences might be attributed to technical variables, such as differences in tissue collection, cell isolation, sequencing, or data interpretation.
A caveat of scRNA-seq is loss of cellular spatial context when tissues are dissociated, which we partially overcame by differentially dissecting the ileum into sections with or without Peyer’s patches before cell isolation. Cycling T/ILCs, follicular CD4 αβ T cells, B cells, and ASCs were enriched in Peyer’s patches, suggesting induction of germinal center antibody responses characteristic of GALT. Data support findings from human research, showing an enrichment of cycling cells, CD4 αβ T cells, and B cells in Peyer’s patches (Fujihashi et al, 2008; Junker et al, 2009) and thus strengthening the rationale for applicability of pigs to study Peyer’s patch–associated immune induction in biomedical research. Non-cycling γδ T, CD8 αβ T, and group 1 ILCs were enriched in the absence of Peyer’s patches and detected in situ primarily within the ileal epithelium, suggesting these three cell types mostly comprise the intraepithelial lymphocyte (IEL) community in the porcine ileum, similar to humans (reviewed by Lutter et al [2018], Mayassi and Jabri [2018], and Olivares-Villagómez and Van Kaer [2018]). Though porcine intraepithelial group 1 ILCs have not been studied in detail, we have conducted research investigating compositional changes of intraepithelial T lymphocytes (T-IELs) in the porcine intestine after weaning (Wiarda et al, 2020). Cross-species parallels were noted in our previous work, including a predominance of CD8+ αβ over γδ T-IELs in the small intestine of pigs and humans (Jarry et al, 1990; Lundqvist et al, 1995; Olivares-Villagómez & Van Kaer, 2018; Wiarda et al, 2020). In contrast, γδ T-IELs are more frequent than CD8+ αβ T-IELs in the murine small intestine (Guy-Grand et al, 1991; Hoytema van Konijnenburg et al, 2017). Thus, our previous work gives initial support of pigs for IEL-centric biomedical research, and scRNA-seq of γδ T, CD8 αβ T, and group 1 ILCs presented herein may help to build on this application. We caution that lamina propria versus epithelial location of single cells cannot be definitively concluded solely from our scRNA-seq data. However, T cells studied via scRNA-seq from the lamina propria and epithelium of human ileum had highly overlapping transcriptional profiles (Jaeger et al, 2021), and the same may be true for pigs.
Non-naive T and B cells in the porcine ileum were transcriptionally different from comparable cells in blood, as indicated by lower average mapping scores to porcine PBMCs. Transcriptional differences between ileal- and blood-derived non-naive T/B cells likely occurred because of inherently activated and/or differentiating states of ileal lymphocytes because of exposure to luminal contents, including the microbiota and microbial-derived metabolites. Our supposition is reinforced by work in germ-free pigs, where small-intestinal non-naive T and B cell abundances are reduced in the absence of microbial exposure (Rothkötter & Pabst, 1989; Rothkötter et al, 1994; Barman et al, 1996; Haverson et al, 2007; Sinkora et al, 2011; Potockova et al, 2015). Differences in T/B cells from mucosal tissues versus blood are also observed in humans, where non-naive lymphocytes increase in abundance, and tissue-specific gene signatures for lymphocyte activation, tissue residency, and/or effector functions are observed for tonsillar B cells (Glass et al, 2020; King et al, 2021), lung-derived T cells (Szabo et al, 2019a), and intestinal T cells (Venema et al, 2019) compared with similar lymphocyte subsets in the periphery. Collectively, the aforementioned studies and our own work suggest mucosal sites (including the ileum) are locations for congregation of non-naive T/B cells transcriptionally distinct from T/B cells found in the periphery, likely due in large part to ongoing immune stimulation via microbial exposure and other context-dependent signals at mucosal surfaces. Though transcriptional reprogramming of T cells and ILCs upon recruitment into the intestinal tract likely contributes to transcriptional distinctions between some T cells and ILCs found in blood and intestine, other intestinal T cells and ILCs are likely unique to the intestine. For example, thymic precursors of some intestinal resident T cells are recruited directly to the intestine where they further mature as tissue resident cells (Poussier & Julium, 1994; Poussier et al, 1992; McDonald et al, 2014). Therefore, findings ultimately support two potential scenarios for transcriptional distinctions between non-naive ileal and peripheral T cells and ILCs: (1) the existence of distinct cell populations exclusive to either circulation or the intestine and (2) transcriptional reprogramming of T cells and ILCs upon recruitment into the intestinal tract.
Similar to intestinal T and B cells, ILCs in the porcine intestine are likely heavily influenced by the microbiota; however, little is known about the influence of microbial colonization on porcine intestinal ILCs because of lack of methods to clearly label and identify intestinal ILCs in pigs. We established gene expression profiles and locational context of group 1 and group 3 ILCs in the porcine ileum to cautiously make functional inferences about ILCs in the porcine intestine. Our findings support comparable roles of porcine ileal ILC functions to intestinal ILCs in humans and mice, such as epithelial patrolling behaviors of intraepithelial group 1 ILCs (Fuchs et al, 2013; Talayero et al, 2016; Van Acker et al, 2017) and contribution of lamina propria and GALT-associated group 3 ILCs in immune defense, regulation of the commensal microbiota, GALT development, tissue homeostasis, and antibody production (Satoh-Takayama et al, 2008; Tsuji et al, 2008; Sonnenberg et al, 2012; Kruglov et al, 2013; Guo et al, 2014; Mortha et al, 2014; von Burg et al, 2014; Aparicio-Domingo et al, 2015). In mice, intestinal ILC1s proportionally contract and convert to a less polarized ILC3-like regulatory profile in germ-free compared with specific pathogen-free animals, suggesting ILC1 recruitment, differentiation, and/or function may be dampened without microbial stimulation (Gury-BenAri et al, 2016), and it remains plausible that similar phenomena occur in pigs. In support, porcine ileal ILCs gene signatures appeared to be heavily influenced by tissue-specific cell activation when compared with peripheral ILCs, and microbiota-derived signals likely supply tissue-specific transcriptional imprinting. In humans, ILCs from the tonsil, lung, and colon have tissue-specific gene expression indicative of tissue residency, cell activation, and modified metabolism compared with peripheral ILCs (Mazzurana et al, 2021), again supporting the contribution of external stimuli in driving activation state. Therefore, our data and newly developed methods for ILC detection should prove useful for further investigation, including defining the impact of the microbiota or intestinal infection on intestinal ILC function in pigs. We could not identify ileal group 2 ILCs in the porcine ileum; however, it is possible that group 2 ILCs were not found because of low abundances occurring under steady state conditions. In humans, intestinal group 2 ILCs are similarly rare under steady state conditions but may increase in abundance under conditions that facilitate their recruitment and/or expansion in the intestine, such as with parasitic infection of cancer (Meininger et al, 2020; Qi et al, 2021), and it remains possible that similar phenomena may occur in pigs. In contrast, group 2 ILCs comprise approximately one-quarter of ILCs in the lamina propria of the murine small intestine (Dutton et al, 2017). Thus, predominance of group 1 and group 3 ILCs in the pig ileum provides initial indication that pigs may have intestinal ILC populations more similar in composition to humans than that of mice under steady state.
Direct comparison of porcine ileal ILCs and peripheral NK cells brings into question the current methods for identification of non-T/non-B lymphocytes in pigs, as key markers used to identify NK cells (the only non–T/non–B lymphocyte subset currently identified in pigs) were found in gene signatures for peripheral but not ileal porcine ILCs. Ileal group 3 ILCs were easily distinguishable from peripheral NK cells, whereas ileal group 1 ILCs shared more similarities to peripheral NK cells. It remains undetermined whether ileal group 1 ILCs are ILC1s or NK cells, as detected differences between ileal group 1 ILCs and circulating NK cells in our data could indicate (1) identification of ILC1s present in the ileum but largely absent from periphery, (2) tissue-specific differences in ileal-derived NK cells that do not fit current phenotypic descriptions used to identify NK cells in pigs, or (3) a combination of both. Unfortunately, differentiation between ILC1s and NK cells remains complicated even in better characterized humans and mice because of species-to-species and tissue-to-tissue peculiarities within what can still be considered relatively recently discovered immune cell subsets (Robinette et al, 2015; Simoni et al, 2017; Simoni & Newell, 2017; Van Acker et al, 2017; Meininger et al, 2020). Regardless, we stress the importance of focusing on cell function over technical phenotype classification, as the functional role of cells in the context of immune protection and immunopathology is ultimately what contributes to biological outcome. To this point, gene signatures obtained for ileal and peripheral ILCs may further be used for identifying biomarkers for detection of specific ILC subsets and understanding their biological functions.
To our knowledge, our findings encompass the first single-cell descriptions of the immune cell transcriptomic landscape in the porcine intestine, with primary focus on the study of lymphocytes in the ileum, including T cells, ILCs, B cells, and ASCs. In addition, non-lymphocyte cell types in the intestine play important functional roles in intestinal health but fell outside the scope of the current study. The diverse spectrum of biological states for cells captured via scRNA-seq is difficult to holistically describe, and we only scratch the surface of the biological information and complexities contained within our scRNA-seq data. Although our data may serve as a starting point for understanding the roles of specific immune cells in a specific biological scenario, such understandings are best fine-tuned using the most appropriate species, tissue, and biological scenario of interest. Consequently, our data serve as an exciting starting point for query to seed future research questions.
Materials and Methods
Animals and sample collection
All animal experiments were performed according to procedures approved by the Institutional Animal Care and Use Committee (IACUC) at Iowa State University or the National Animal Disease Center, Agricultural Research Service (ARS), United States Department of Agriculture (USDA). Mixed-breed and mixed-gender pigs were obtained from commercial nursery settings for all experiments and assays. All pigs were weaned at ∼3 wk of age. For scRNA-seq, samples were collected from one female (pig 1) and one male (pig 2) at ∼7 wk of age. Postmortem intestinal tissues collected to complete ex vivo and in situ assays were from control animals in unrelated studies to reduce the overall number of animals used. The ages of animals are listed within respective figure captions and ranged from ∼5–9 wk of age. Humane euthanasia was carried out immediately preceding tissue collections.
Cell isolations
Ileum for scRNA-seq
For ileal cell isolations, reagents were equilibrated to RT, and samples were stored at RT between steps. Immediately after humane euthanasia, ileal tissue was collected and stored in stabilization buffer (2 mM EDTA [AM9260G; Invitrogen], 2 mM L-glutamine [25-030; Gibco], and 0.5% BSA [A9418; Sigma-Aldrich] in HBSS [14175; Gibco]) for transport back to the lab.
In the lab, the exterior muscularis layer was peeled off, and tissues were cut open longitudinally to expose the lumen. Tissues were gently rinsed with PBS, pH 7.2 (made in-house), to remove intestinal contents and carefully dissected into regions of interest (PP, non-PP, and whole ileum), as shown in Fig S1B. Dissected tissues were weighed out to between 1 and 1.5 g per sample for further use.
Tissues were sequentially transferred to and incubated in the following solutions in a shaking incubator (MaxQ 4000; Thermo Fisher Scientific) at 37°C, 200 rpm: 20 min in 30 ml mucus dissociation solution (5 mM dithiothretol [DTT; 15508; Invitrogen] and 2% heat-inactivated FCS [A38401; Gibco] in HBSS); 25 min in 30 ml epithelial removal solution (5 mM EDTA and 2% FCS in HBSS), repeated with fresh solution for a total of three times; and 10 min in 20 ml wash solution (10 mM Hepes [BP299; Fisher BioReagents] in HBSS). Epithelial removal and wash solutions were retained for processing of epithelial cells, whereas mucus dissociation solution was discarded.
After incubation in wash solution, tissues were minced and placed into gentleMACS C tubes (130-093-237; Miltenyi) containing 15 ml enzyme digestion solution (10 mM Hepes, 0.2 U/ml Liberase TM [5401127001; Roche], and 30 μg/ml DNase I [D5025; Sigma-Aldrich] in HBSS). Tissue dissociation was carried out using the intestine C-tube protocol on a gentleMACS Octo Dissociator (130-095-937; Miltenyi) followed by incubation at 37°C, 200 rpm for 45 min and another round of mechanical dissociation using the gentleMACs intestine C-tube protocol. A total of 10 ml stabilization buffer was added to each C-tube, and contents were strained through sterile nonwoven surgical gauze sponges (GZNW44; Starryshine), followed by a 100-μm nylon mesh cell strainer (352360; BD Falcon).
Although tissues were incubating in enzyme digestion solution, epithelial cells were collected by passing epithelial removal and wash solutions through a 100-μm nylon mesh cell strainer, centrifuging at 450g for 8 min RT, and resuspending in supplemented HBSS (2 mM L-glutamine and 2% FCS in HBSS).
Cell fractions from epithelial isolation and tissue digestion for each sample were combined, centrifuged at 450g for 8 min RT, and resuspended in 24 ml of RT 70% Percoll (1.088 g/ml at 22°C; 17-0891-01; GE Healthcare Life Sciences). Aliquots of 8 ml cell/Percoll suspension were overlayed with 4 ml HBSS and centrifuged at 400g for 30 min RT, with slow acceleration and centrifuge break turned off. The density interphase layer of cells was collected, washed with supplemented HBSS, centrifuged at 450g for 8 min RT, and resuspended again in supplemented HBSS. Quantity and viability of cells was assessed by the Muse Count & Viability Assay Kit (MCH100102; Luminex) using a Muse Cell Analyzer (0500-3115; Luminex).
To further enrich for live cells, cell suspensions were passed through another 100-μm nylon filter, centrifuged at 300g for 10 min RT, and processed using a Dead Cell Removal Kit (130-090-101; Miltenyi). Cells were resuspended in 100 μl kit microbeads per 107 total cells, incubated for 15 min, and divided into four equal parts per sample that were each rinsed with 1 ml 1× Binding Buffer. A total of four separate LS columns (130-042-401; Miltenyi) were used for the divided samples to facilitate magnetic sorting using a MultiMACS Cell24 Separator Plus (130-098-637; Miltenyi). LS columns were prerinsed with 1× Binding Buffer before applying cells. Columns were rinsed with an additional 3 ml of 1× Binding Buffer twice to facilitate cell pass through. The negative cell pass through was collected, centrifuged 300g for 10 min RT, resuspended in supplemented HBSS, centrifuged again, and resuspended in a final volume of supplemented HBSS. Muse count and viability was again assessed and deemed adequate for scRNA-seq (>84% live cells per sample). Samples were transported to the sequencing facility (∼15 min transport), and cell viability was reassessed using a Countess II Automated Cell Counter (Thermo Fisher Scientific). Viabilities from the Countess readings were deemed adequate to proceed with partitioning (>76% live cells per sample) for scRNA-seq.
PBMCs for scRNA-seq
PBMCs were isolated using Cell Preparation Tubes (CPT; 362782; BD Biosciences) according to manufacturer’s recommendations.
Ileum for flow cytometry
Ileal cells collected for flow cytometry experiments were isolated as described for scRNA-seq ileal cells above, with the exception that further enrichment of viable lymphocytes by Percoll density gradient centrifugation and magnetic dead cell removal were not performed.
Droplet-based scRNA-seq
Single-cell suspensions from the ileum and PBMCs were prepared for scRNA-seq by partitioning and preparing libraries for a target of 10,000 cells per sample according to the manufacturer’s protocol for Chromium Single Cell 3′ v2 Chemistry (10X Genomics CG00052). Samples were multiplexed, had equal proportions of cDNA from each sample pooled, and were run across multiple lanes of an Illumina HiSeq 3000 with 2 × 100 paired-end sequencing as previously described (Herrera-Uribe et al, 2021). Raw data were deposited in .fastq file format for both forward and reverse strands following image analysis, base calling, and demultiplexing.
scRNA-seq analysis of ileum data
Initial data processing
Initial processing of scRNA-seq data included read alignment/gene quantification, ambient RNA removal, gene/cell filtering, doublet removal, normalization, integration, and dimensionality reduction identical to as previously described (Herrera-Uribe et al, 2021) and as briefly outlined below.
Read alignment, mapping, and gene quantification were carried out with the shell data package, CellRanger v4.0.0 (10X Genomics), and the Sus scrofa 11.1 reference genome with 11.1.97 annotation file obtained from Ensembl (Cunningham et al, 2019) and modified as previously described (Herrera-Uribe et al, 2021). Ambient RNA removal was performed using the auto-estimation method from the R package, SoupX v1.4.5 (Young & Behjati, 2020).
Genes without any reads detected in the total of all sequencing reads across all cells and samples were removed from the dataset. Cells with >12.5% of total reads attributed to mitochondrial genes, <550 total genes detected, or <1,250 total unique molecular identifiers (UMIs) detected were removed from the dataset (Table S1).
The Python package Scrublet v0.1 (Wolock et al, 2019) was used to remove highly probable neotypic doublets from the remaining dataset using a doublet rate of 0.07. Cells with corresponding doublet probability scores >0.25 were removed from our dataset (Table S1).
Normalization, integration, and dimensionality reduction were performed using the R package, Seurat v3.2.2 (Butler et al, 2018; Stuart et al, 2019). SCT-normalized data were used to perform anchor-based sample integration with default parameters. Principle component analysis (PCA) was performed to identify the first 100 principle components (PCs) of the data, and a “significant” number of PCs to use for further analyses was determined as the smaller value of (1) the highest PC that had >0.1% change in variation between consecutive PCs or (2) the smallest PC that represented >90% of the cumulative variation and <5% of variation associated with a single PC. t-distributed stochastic neighbor embedding (t-SNE) coordinates were generated for visualization using the significant number of PCs. Log-normalized and scaled data were also calculated for the RNA assay of the Seurat object.
Throughout the workflow, intermediate modified count matrices were generated and converted back to 10X format using the function write10XCounts() from the R package, DropletUtils v1.8.0 (Griffiths et al, 2018; Lun et al, 2019).
Quality check of ileal sample types
Disparities in the percentage of cells removed between different ileal sample types were noted in Table S1, with smaller percentages of cells passing all cell filtering steps from non-PP (47.24% and 54.21%) compared with PP (75.31% and 77.13%) and whole ileum (71.56% and 77.40%) samples. Further investigation was performed to ensure disparities were because of differences in sample cell compositions and not differences in the quality of the same cell types between samples. Our query revealed many poor quality cells (>12.5% mitochondrial reads, <550 genes, <1,250 UMIs) that were more abundant in non-PP samples expressed genes characteristic of epithelial cells (EPCAM, KRT8) rather than immune cells (PTPRC [encoding pan-leukocyte marker CD45]; Fig S2A). Results were further validated by assessing the ratio of leukocytes (CD45+) to epithelial cells (EPCAM+) by flow cytometry (see flow cytometry methods) in independently collected ileal samples (Fig S2B and C). Non-PP samples had smaller leukocyte:epithelial cell ratios than did PP or whole-ileum samples (Fig S2D), indicating a higher occurrence of epithelial cells in non-PP samples. Similar observations were made via IHC, showing that a larger proportion of epithelial cells (stained by pan-cytokeratin protein expression; see IHC methods) were present in regions of the ileum lacking Peyer’s patches (Fig S2E). Thus, smaller percentages of cells passing all cell filtering steps from non-PP samples were largely attributed to a higher occurrence of poor quality epithelial cells originally present in these samples.
Cell clustering
The Seurat function FindNeighbors() was used to construct a shared nearest neighbor (SNN) graph, specifying to use the significant number of PCs calculated during data dimensionality reduction outlined above. The Seurat function FindClusters() using the significant number of PCs was used to identify clusters with clustering resolutions at 0.5 intervals between 0 and 5. Clustering at different resolutions was compared using the function clustree() from the R package clustree v0.4.3 (Zappia & Oshlack, 2018). A clustering resolution of 3.0 was selected for all downstream work.
Hierarchical clustering
Hierarchical clustering was performed with the Seurat function BuildClusterTree(), specifying to use only the number of significant PCs calculated during data dimensionality reduction outlined above for a respective dataset. Clustering dendrograms were visualized using the function PlotClusterTree().
Generating data subsets
Some analyses were conducted on only a subset of cells from the original ileum scRNA-seq dataset. To partition out only cells of interest into smaller datasets, we used the Seurat function subset() to specify which cells to allocate into smaller datasets. Genes with cumulative zero expression in the new data subsets, scaled data, and dimensionality reduction dimensions were removed using the function DietSeurat(). Data in the RNA assay were rescaled; the first 100 PCs were recalculated; the number of significant PCs was redetermined; and t-SNE dimensions were recalculated using methods described above. For some smaller data subsets, integration anchors could not be calculated with default parameter k.filter = 200 for the function FindIntegrationAnchors(), and the k.filter parameter was adjusted to the largest multiple of five at which integration anchors could still be calculated for the dataset.
Cell cluster/annotation-based DGE analysis
DGE analyses were performed using functions of the Seurat package and normalized gene counts from the RNA assay. DEGs were calculated for each cluster/cell type relative to the average gene expression across an entire dataset using FindAllMarkers(). To be considered differentially expressed (DE), a gene was expressed in >10% of cells in one of the populations being compared, had a |logFC| >0.25, and had a corrected P-value <0.05.
Multidimensional DGE analysis
Cell cluster-type-independent, multidimensional DGE analysis was performed with the R package singleCellHaystack v0.3.3 (Vandenbon & Diez, 2020). From log-normalized counts stored in the RNA assay of our Seurat object, median expression levels were calculated for each gene across the entire dataset, followed by determining if expression of each gene was above or below the median expression level within each cell. From this information, DGE was calculated using the function haystack_highD(), specifying to use the previously determined number of significant PCs to define the multidimensional space. A gene was considered DE if it had an adjusted P-value <0.05.
Gene modules were created by first selecting only DEGs with adjusted P-values <1 × 10−10, followed by hierarchical clustering of selected genes with the function hclust_haystack_highD(). The number of gene modules to split the gene dendrogram into (k) was specified between k = 3 to k = 10 and executed with the function cutree(). Detection scores of each gene module within each cell were calculated with the function plot_gene_set_haystack(). The final value of k was selected by examining each model and selecting the one producing the most interpretable and biologically relevant gene modules.
Topic modeling
Topic models were fit with the fastTopics package v0.5-54 (Dey et al, 2017). Multiple topic models were fit for each cell type subset (K = 3 to K = 10). The final value of K was selected by examining each model and selecting the one that produced the most interpretable and biologically relevant structure. Genes which were enriched in each topic were determined using the diff_count_analysis() function.
Biological process enrichment analysis
After identification of DEGs with increased expression in cell clusters, topics, or gene modules, gene sets were subjected to Gene Ontology (GO) term enrichment analysis. GO terms were mapped to Ensembl gene IDs using the biomaRt v2.48.3 (Durinck et al, 2009) R package. Only genes, and therefore GO terms, detected in our data were included in the background set which the enriched sets were compared against. A new GO term background set was created for each data subset. Genes and GO terms which were not detected in each data subset were removed from the respective background list. The R package topGO v2.44.0 (Alexa et al, 2006) was used to carry out GO term enrichment analysis using the “elimination” algorithm and the “Fisher” test statistic. GO terms with P-values <0.05 were considered enriched in the gene set in question relative to the appropriate background GO term set.
Ileal cell lineage annotations
Ileal cells were grouped into 54 clusters as described in Cell clustering methods (Fig S4A). Query of cell lineage canonical gene expression within clusters was used to group cells into four major cell lineages (Figs 1C and S4B and C).
Ileal cell type annotations
Ileal cells were further annotated into 26 cell types (Figs 1D and S3) with a hybrid, multi-method approach using (1) cell clustering and accompanying cluster-based DGE and hierarchical analyses (discrete cell/non-discrete gene classifications); (2) cell cluster-independent multidimensional DGE analyses and gene module detection (non-discrete cell/discrete gene classifications); and/or (3) grade of membership/topic modeling (non-discrete cell/non-discrete gene classifications) as described below.
T/ILC lineage lymphocyte annotations
Cells assigned as T/ILC lineage lymphocytes (Figs 1C and S4B and C) were extracted to form a subset of data for further query of T/ILC identities (as described in Generating data subsets methods; Fig S5A). T cell clusters were identified by expression of porcine pan-T cell marker CD3E (expressed by >75% of cells in a cluster; Fig S5B and C; reviewed by Piriou-Guzylack and Salmon [2008] and Gerner et al [2009]). Clusters were instead identified as ILCs if most of the cells did not express CD3E but still largely expressed CD2, similar to previously described porcine NK cells (Fig S5B and C; Herrera-Uribe et al, 2021). T cell clusters were classified as CD4 αβ T cells, CD8 αβ T cells, or γδ T cells if >10% of cells in a cluster expressed CD4, CD8B, or TRDC, respectively (Fig S5D and E). Mixed CD4/CD8 αβ and γδ/CD8 αβ T cell clusters were identified if >10% of cells expressed CD4 and CD8B or CD8B and TRDC, respectively (Fig S5D and E). Mixed T cell clusters appeared to be mixtures of cells expressing either CD4, CD8B, or TRDC rather than cells co-expressing any two of these markers (Fig S5B).
Figure S5. Annotation of T/ILC lineage lymphocytes from porcine-ileum scRNA-seq data.
(A) Two-dimensional t-SNE visualization of 14,742 cells recovered from the porcine ileum via scRNA-seq and classified as T/ILC lineage lymphocytes in Figs 1C and S4B. Each point represents a single cell; color of a point corresponds to one of 26 cell clusters a cell belonged to, with more transcriptionally similar cells belonging to the same cell cluster. The number of cells belonging to each cell cluster is listed in the cluster key. (B) Gene expression of selected canonical genes overlaid onto two-dimensional t-SNE visualization coordinates of cells shown in (A). Color of a point corresponds to expression level of a specified gene within a cell relative to all other cells in the dataset shown in (A). (C) Gene expression patterns of selected canonical genes (y-axis) across cell clusters shown in (A) (x-axis). Within the plot, size of a dot corresponds to the percentage of cells expressing a gene within a cell cluster. Color of a dot corresponds to average expression level of a gene for those cells expressing it within a cell cluster relative to other cells in the dataset shown in (A). Below cluster ID on the x-axis, the color of a circle corresponds to a further T/ILC classification given to each cell cluster. (D) Radial plots showing the percentage of cells expressing CD4, CD8B, or TRDC within each respective cell cluster shown in (A). Shaded gray triangles at the center of each plot indicate the minimum limit of positive detection (10%), whereas the outer limits of each plot are equivalent to 100%. T/ILC classification given to each cluster based on the percentage of cells positive for each gene are shown by plot color and outline. Two-dimensional t-SNE visualization of cells shown in (A), where color of a point now corresponds to T/ILC classification given to a cell cluster in (C, D). (F) Heatmap of top differentially expressed genes within each cell cluster shown in (A). Up to five differentially expressed genes with the highest positive logFC values were selected for each cell cluster. Genes were differentially expressed in a specified cluster relative to the average of all other cells in the dataset shown in (A). Gene expression profiles from up to 100 cells of each cluster are shown in the heatmap. Each column represents a single cell. Selected gene names are shown on the y-axis, and cell cluster IDs are shown on the x-axis. (C, D, E) T/ILC classification given to each cluster in (C, D, E) is indicated below each cell cluster ID on the x-axis. Hierarchical relationships of cell clusters are shown using a phylogenetic tree at the top of the heatmap. scRNA-seq data shown in (A, B, C, D, E, F) were derived from the ileum of two 7-wk-old pigs. *Ensembl identifiers found in gene annotation were converted to gene symbols; refer to methods section “Gene name modifications” for more details. Abbreviations: ILC, innate lymphoid cell; logFC, log fold-change; scRNA-seq, single-cell RNA sequencing; t-SNE, t-distributed stochastic neighbor embedding.
Naive αβ T cell annotation
Further cell cluster-based DGE analysis of all T/ILC lineage lymphocytes revealed a unique gene expression signature characteristic of circulating and/or naive T cells in the only mixed CD4/CD8 αβ T cell cluster, cluster 24 (Fig S5F). Thus, cluster 24 cells were given the designation of naive CD4/CD8 αβ T cells.
CD4 αβ T cell annotations
Cells identified as CD4 αβ T cells (Fig S5) were extracted to create a subset of data (as described in Generating data subsets methods). From this data subset, previous cell cluster assignments (as described in Cell clustering methods), topic modeling with weighted membership to three topics (as described in Topic modeling methods), and multidimensional DGE analysis with detection of three gene modules (as described in Multidimensional DGE analysis methods) were assessed to identify DEGs and associated enriched biological processes by each method (Fig S6A–D and Table S2). Further identification of CD4 αβ T cell populations is shown in Fig S6E–H.
Figure S6. Annotation of CD4 αβ T cells from porcine-ileum scRNA-seq data.
(A) Two-dimensional t-SNE visualization of 2,668 cells recovered from the porcine ileum via scRNA-seq and classified as CD4 αβ T cells in Fig S5C–F. Each point represents a single cell; color of a point corresponds to one of five cell clusters a cell belonged to, with more transcriptionally similar cells belonging to the same cell cluster. The number of cells belonging to each cell cluster is listed in the cluster key. (B) Heatmap of top differentially expressed genes within each cell cluster shown in (A). Up to five differentially expressed genes with the highest positive logFC values were selected for each cell cluster. Genes were differentially expressed in a specified cluster relative to the average of all other cells in the dataset shown in (A). Gene expression profiles from up to 100 cells of each cell cluster are shown in the heatmap, with each column representing a single cell. Selected gene names are shown on the y-axis, and cell cluster IDs are shown on the x-axis. Hierarchical relationships of cell clusters are shown using a phylogenetic tree at the top of the heatmap. (C) Topic weights from topic modeling of cells shown in (A) overlaid onto two-dimensional t-SNE visualization coordinates. Color of a point corresponds to proportional weighting of a topic within a cell, where total weighting across all topics in each cell is equal to one. (D) Gene module detection scores from multidimensional differential gene expression analysis of cells shown in (A) overlaid onto two-dimensional t-SNE visualization coordinates. Color of a point corresponds to detection score for a gene module within a cell. (E) Scatter plot of gene module 2 detection scores (y-axis) versus topic 3 weights (x-axis) for all cells shown in (A). Each point represents a single cell. Cells with a gene module 2 detection score >0.1 and/or topic 3 weight >0.4 are shown in red and were annotated as cycling cells. Remaining cells are shown in black and were classified as non-cycling cells. (F) Scatter plots of gene module 3 detection scores (y-axis) versus topic 2 weights (x-axis) for all non-cycling cells shown in (E). Each point represents a single cell. Left: point fill corresponds to topic 1 weights. Right: cells with a gene module 3 detection score >0.3 and/or topic 2 weight > topic 1 weight are shown in red and annotated as follicular cells. Remaining cells are shown in black and annotated as activated cells. (G) CD4 αβ T cell annotations established in (B, C, D, E, F) overlaid onto two-dimensional t-SNE visualization coordinates of cells shown in (A). Color of a point corresponds to cell type annotation. The number of cells belonging to each cell type is listed in the key on the right. (H) Overlay of individual cell types onto two-dimensional t-SNE visualization shown in (G). Cell type is indicated in a respective panel by one of three colors corresponding to cell types shown in (G), whereas all other cells not corresponding to a specified cell type are shown in light gray. scRNA-seq data shown in (A, B, C, D, E, F, G, H) were derived from the ileum of two 7-wk-old pigs. *Ensembl identifiers found in gene annotation were converted to gene symbols; refer to methods section “Gene name modifications” for more details. Abbreviations: logFC, log fold-change; scRNA-seq, single-cell RNA sequencing; t-SNE, t-distributed stochastic neighbor embedding.
γδ/CD8 αβ T cell annotations
Cells identified as γδ T cells, CD8 αβ T cells, or a mixture of γδ/CD8 αβ T cells (Fig S5) were extracted to create a subset of data (as described in Generating data subsets methods). From this data subset, previous cell cluster assignments (as described in Cell clustering methods), topic modeling with weighted membership to three topics (as described in Topic modeling methods), and multidimensional DGE analysis with detection of four gene modules (as described in Multidimensional DGE analysis methods) were assessed to identify DEGs and associated enriched biological processes by each method (Fig S7A–D and Table S3). Further identification of γδ and CD8 αβ T cell types is shown in Fig S7E–J.
Figure S7. Annotation of γδ and CD8 αβ T cells from porcine-ileum scRNA-seq data.
(A) Two-dimensional t-SNE visualization of 8,905 cells recovered from the porcine ileum via scRNA-seq and classified as γδ T cells, CD8 αβ T cells, or mixed γδ/CD8 αβ T cells in Fig S5C–F. Each point represents a single cell; color of a point corresponds to one of 15 cell clusters a cell belongs to, with more transcriptionally similar cells belonging to the same cell cluster. The number of cells belonging to each cell cluster is listed in the cluster key. (B) Heatmap of top differentially expressed genes within each cell cluster shown in (A). Up to five differentially expressed genes with the highest positive logFC values were selected for each cell cluster. Genes were differentially expressed in a specified cell cluster relative to the average of all other cells in the dataset shown in (A). Gene expression profiles from up to 100 cells of each cluster are shown in the heatmap, with each column representing a single cell. Selected gene names are shown on the y-axis, and cell cluster IDs are shown on the x-axis. Hierarchical relationships of cell clusters are shown using a phylogenetic tree at the top of the heatmap. At the bottom of the heatmap, cells in cluster 31 were annotated as CD2− γδ T cells, and cells in cluster 51 were annotated as SELLhi γδ T cells. (C) Topic weights from topic modeling of cells shown in (A) overlaid onto two-dimensional t-SNE visualization coordinates. Color of a point corresponds to proportional weighting of a topic within a cell, where total weighting across all topics in each cell is equal to one. (D) Gene module detection scores from multidimensional differential gene expression analysis of cells shown in (A) overlaid onto two-dimensional t-SNE visualization coordinates. Color of a point corresponds to detection score for a gene module within a cell. (E) Scatter plot of gene module 3 detection scores (y-axis) versus topic 3 weights (x-axis) for all cells shown in (A), excluding CD2− γδ T cells (cell cluster 31) and SELLhi γδ T cells (cell cluster 51). Each point represents a single cell. Cells with a gene module 3 detection score >0.11 and/or topic 3 weight >0.41 are shown in red and annotated as cycling cells. Remaining cells are shown in black and classified as non-cycling cells. (F) Scatter plots of gene module 1 detection scores (y-axis) versus topic 1 weights (x-axis) for all non-cycling cells shown in (E). Each point represents a single cell. Upper: point fill corresponds to topic 2 weights. Lower: cells with a gene module 1 detection score >0.25 and/or topic 1 weight at least 4× greater than topic 2 weight are shown in red and classified as activated cells. Remaining cells are shown in black and classified as cytotoxic cells. (G) Relative gene expression levels of CD8B (left), TRDC (right), and merged CD8B and TRDC overlaid onto two-dimensional t-SNE visualization coordinates shown in (A). (H) Scatter plot of ratios of log-normalized CD8B/TRDC (y-axis) and TRDC/CD8B (x-axis) counts for all cells shown in (E). Each point represents a single cell. Cells with a TRDC/CD8B ratio >1 are shown in red and classified as γδ T cells. Remaining cells are shown in black and classified as CD8 αβ T cells. (I) γδ and CD8 αβ T cell annotations established from combined classifications in (B, C, D, E, F, G, H) overlaid onto two-dimensional t-SNE visualization coordinates of cells shown in (A). Color of a point corresponds to cell type annotation. The number of cells belonging to each cell type is listed in the color key on the bottom. (J) Overlay of individual cell types onto two-dimensional t-SNE visualization shown in (I). Cell type is indicated in a respective panel by one of eight colors corresponding to cell types shown in (I), whereas all other cells not corresponding to a specified cell type are shown in light gray. scRNA-seq data shown in (A, B, C, D, E, F, G, H, I, J) were derived from the ileum of two 7-wk-old pigs. *Ensembl identifiers found in gene annotation were converted to gene symbols; refer to methods section “Gene name modifications” for more details Abbreviations: logFC, log fold-change; scRNA-seq, single-cell RNA sequencing; t-SNE, t-distributed stochastic neighbor embedding.
ILC annotations
Cells identified as ILCs (clusters 1, 18, 43, 44, and 51; Fig S5) were extracted to create a subset of data (as described in Generating data subsets methods). From this data subset, previous cell cluster assignments (as described in Cell clustering methods), topic modeling with weighted membership to three topics (as described in Topic modeling methods), and multidimensional DGE analysis with detection of three gene modules (as described in Multidimensional DGE analysis methods) were assessed to identify DEGs and associated enriched biological processes by each method (Fig S8A–D and Table S4). Further identification of ILC populations is shown in Fig S8E–H.
Figure S8. Annotation of ILCs from porcine-ileum scRNA-seq data.
(A) Two-dimensional t-SNE visualization of 2,594 cells recovered from the porcine ileum via scRNA-seq and classified as ILCs in Fig S5C–F. Each point represents a single cell; color of a point corresponds to one of five cell clusters a cell belongs to, with more transcriptionally similar cells belonging to the same cell cluster. The number of cells belonging to each cell cluster is listed in the cluster key. (B) Heatmap of top differentially expressed genes within each cell cluster shown in (A). Up to five differentially expressed genes with the highest positive logFC values were selected for each cell cluster. Genes were differentially expressed in a specified cell cluster relative to the average of all other cells in the dataset shown in (A). Gene expression profiles from up to 100 cells of each cell cluster are shown in the heatmap, with each column representing a single cell. Selected gene names are shown on the y-axis, and cell cluster IDs are shown on the x-axis. Hierarchical relationships of cell clusters are shown using a phylogenetic tree at the top of the heatmap. At the bottom of the heatmap, cluster 53 was annotated as cycling group 1 ILCs. (C) Topic weights from topic modeling of cells shown in (A) overlaid onto two-dimensional t-SNE visualization coordinates. Color of a point corresponds to proportional weighting of a topic within a cell, where total weighting across all topics in each cell is equal to one. (D) Gene module detection scores from multidimensional differential gene expression analysis of cells shown in (A) overlaid onto two-dimensional t-SNE visualization coordinates. Color of a point corresponds to detection score for a gene module within a cell. (E) Scatter plots of gene module 3 detection scores (y-axis) versus topic 1 weights (x-axis) for all cells shown in (A). Each point represents a single cell. Left: point fill corresponds to gene module 1 detection score. Right: cells belonging to cluster 43 are shown in red and were annotated as group 3 ILCs. Remaining cells are shown in black and classified as group 1 ILCs. (F) Scatter plots of gene module 2 detection scores (y-axis) versus topic 3 weights (x-axis) for cells belonging to cell clusters 1, 18, or 44 in (A, B). Each point represents a single cell. Upper: point fill corresponds to topic 2 weights. Lower: cells with a topic 3 weight <0.05 and gene module 2 detection scores >0.4 or topic 2 weights >0.9 are shown in red and annotated as activated cells. Remaining cells are shown in black and annotated as cytotoxic cells. (G) ILC annotations established in (B, C, D, E, F) overlaid onto two-dimensional t-SNE visualization coordinates of cells shown in (A). Color of a point corresponds to cell type annotation. The number of cells belonging to each cell type is listed in the color key on the bottom. (H) Overlay of individual cell types onto two-dimensional t-SNE visualization shown in (G). Cell type is indicated in a respective panel by one of four colors corresponding to cell types shown in (G), whereas all other cells not corresponding to a specified cell type are shown in light gray. scRNA-seq data shown in (A, B, C, D, E, F, G, H) were derived from the ileum of two 7-wk-old pigs. *Ensembl identifiers found in gene annotation were converted to gene symbols; refer to methods section “Gene name modifications” for more details. Abbreviations: ILC, innate lymphoid cell; logFC, log fold-change; scRNA-seq, single-cell RNA sequencing; t-SNE, t-distributed stochastic neighbor embedding.
B lineage lymphocyte annotations
Cells identified as B lineage lymphocytes (Figs 1C and S4B and C) were extracted to create a subset of data (as described in Generating data subsets methods). From this data subset, previous cell cluster assignments (as described in Cell clustering methods), topic modeling with weighted membership to three topics (as described in Topic modeling methods), and multidimensional DGE analysis with detection of four gene modules (as described in Multidimensional DGE analysis methods) were assessed to identify DEGs and associated enriched biological processes by each method (Fig S9A–D and Table S5). Further identification of B lineage lymphocyte populations is shown in Fig S9E–G.
Non-lymphocyte annotations
A data subset was created for myeloid lineage leukocytes (clusters 42, 49, 52; Fig S4) as described in Generating data subsets methods. Because non-lymphocytes were not cells targeted for sequencing, cells identified as myeloid lineage leukocytes were annotated based only on cell cluster assignments (as described in Cell clustering methods) and associated hierarchical clustering, DGE, and biological process enrichment analyses (Fig S10A and B and Table S6). Further identification of myeloid lineage leukocyte cell types is shown in Fig S10C and D. Non-leukocytes were similarly annotated based only on cell cluster assignments (as described in Cell clustering methods) and associated hierarchical clustering, DGE, and biological process enrichment analyses (Fig S10E and F and Table S7). Further identification of non-leukocyte cell types is shown in Fig S10G and H.
Reference-based label transfer and mapping
Mapping and cell label prediction of porcine ileal cells to previously annotated cells from scRNA-seq datasets was performed using Seurat v3.9.9.9026 (Butler et al, 2018; Stuart et al, 2019; Hao et al, 2021). Each porcine ileal scRNA-seq sample (n = 6) was treated as an individual query dataset, and scRNA-seq datasets of porcine PBMCs (Herrera-Uribe et al, 2021), human ileum (Elmentaite et al, 2020), and murine ileum (Xu et al, 2019) were treated as reference datasets. Post-quality control UMI count matrices, cell barcodes, and corresponding cell annotations were obtained for each reference dataset, and only cells from animals/patients under nonpathogenic/noninflammatory conditions were used to create reference datasets. The porcine PBMC dataset included cells derived from blood of steady-state pigs ranging in age between 7 wk and 12 mo (n = 7; Herrera-Uribe et al, 2021). The human ileum dataset included cells derived from ileal biopsies of eight children between 4 and 12 yr of age that did not have inflammatory bowel disease (pediatric, non-Crohn’s; treated as control samples in study). Whether human ileal biopsies contained Peyer’s patches was not specified, though sizeable numbers of B cells were still reported (Elmentaite et al, 2020). The murine ileum dataset included cells from the terminal ileum, enriched for cells in Peyer’s patches or lamina propria (though epithelial cells still present) of specific pathogen-free BALB/cJ mice that were ∼8–10 wk old. Only mice that were not induced to mount allergic responses were used (n = 14, treated as control samples in study; Xu et al, 2019). Cells with unknown identities (“unknown” annotation in porcine PBMCs and “unresolved” annotation in the murine ileum) or low UMIs (“low UMI count” designation in the murine ileum) were further removed, leaving a total of 28,684; 11,302; and 27,159 cells from porcine PBMCs, human ileum, and murine ileum, respectively. For comparison between porcine and human or murine data, genes were filtered to contain only one-to-one human-to-pig or mouse-to-pig gene orthologs, as determined with BioMart (Smedley et al, 2015) and as previously described (Herrera-Uribe et al, 2021), within both reference and query datasets. For comparison between porcine ileal and PBMC datasets, genes were not filtered further.
Each reference dataset was next converted into a Seurat object and processed with SCT normalization, integration, scaling of the integrated assay, and PCA calculations as described in preceding methods. Annotation of cell lineage was also added to cells of each reference dataset, and assignment of each annotated cell type to a cell lineage can be seen in Fig S12.
Each query dataset was processed using porcine genes for comparison to porcine PBMCs, only one-to-one human-to-pig gene orthologs for comparison to the human ileum (termed humanized query data), or only one-to-one mouse-to-pig gene orthologs for comparison to the murine ileum (termed murinized query data), resulting in a porcine, humanized, and murinized query dataset for each of the original six ileal scRNA-seq samples (18 query datasets total). Gene names from humanized and murinized query datasets were converted to human and murine gene names, respectively. Each query dataset was then converted into a Seurat object and processed with log normalization/scaling of the RNA assay and PCA calculations as described in preceding methods. Samples of query data were processed individually and not integrated.
Prediction and mapping scores to reference datasets were next generated for query samples using integrated assay data from reference datasets and RNA assay data from query datasets. Transfer anchors between each query with a respective reference dataset were calculated using the function FindTransferAnchors(), with canonical correlation analysis (CCA) reduction (recommended for cross-species comparisons), log normalization, and 30 dimensions specified. Transfer anchors were used as input for the function TransferData() to calculate predictions probabilities (range 0–1) for each query cell at the level of cell type and cell lineage annotations, again using CCA reduction and 30 dimensions. Mapping scores (range 0–1) were also calculated with the function MappingScore() using the calculated transfer anchors, reference dataset cell embeddings, and query dataset neighbors, weights matrix, and embeddings as inputs.
Query data from all six samples were merged back together, and prediction probabilities and mapping scores were summarized for each respective combination of reference identity assignment and query cluster ID. Mapping scores indicated poor (score = 0) versus good (score = 1) representation of query data in a reference dataset. Prediction probabilities indicated most highly probable similar cells in the reference dataset (0 = not highly probable, 1 = highly probable).
Pseudobulk analysis
Pseudobulk RNA-seq datasets were generated from ileum scRNA-seq samples using the Seurat function AverageExpression() to store mean-normalized counts from the RNA assay of Seurat objects. Pseudobulk samples were generated for each of the six ileal scRNA-seq samples either using reads after ambient RNA removal but before gene/cell filtering or using reads from the final dataset of 31,983 cells after gene/cell filtering. Using the R package, edgeR v3.30.3 (Robinson et al, 2010; McCarthy et al, 2012), pseudobulk counts were incorporated into a DGEList object with function DGEList(), and multidimensional scaling (MDS) plots to visualize sample-specific pseudobulk profiles were created with the function, plotMDS().
Differential abundance (DA) analysis
DA analysis of cells originating from PP versus non-PP samples of the ileum was performed using the R package, miloR v1.0.0 (Dann et al, 2021). All cells derived from PP and non-PP samples were taken as a data subset and reprocessed as described in preceding methods. In addition, SNNs were recalculated for the data subset with the Seurat function FindNeighbors(). The Seurat object was converted into a Milo object with incorporation of the SNN data graph calculated in Seurat with nearest neighbors (k) = 20 specified. Cell neighborhoods were created with the miloR function makeNhoods(), using 20% of cells in the dataset, k = 20, and the significant number of PCs calculated for the data subset. Cell neighborhoods were visualized by overlay onto original t-SNE coordinates calculated for the original total dataset (including whole ileum samples) to promote cohesiveness with previous data visualizations. Within each cell neighborhood, the proportions of cells coming from each PP or non-PP sample were calculated. PP and non-PP samples were specified as treatment variables to compare in an experimental matrix, each with two replicates (pig 1 and 2). Distances between cell neighborhoods were calculated with calcNhoodDistance() and the significant number of PCs for the data subset. DA testing was then performed within each cell neighborhood with the function testNhoods(). Cell neighborhoods were further annotated to correspond to the 26 cell types shown in Fig 1D, where a neighborhood was assigned as a cell type if >70% of cells in the neighborhood belonged to a single respective cell type annotation. If <70% of cells in a neighborhood were annotated as a single cell type, the neighborhood was classified as mixed. A neighborhood was considered differentially abundant at a significance level of 0.1 for corrected P-values.
scRNA-seq analysis of PBMC data
PBMC scRNA-seq data were processed independently but in parallel to ileal samples, with analyses mirroring those described for ileal scRNA-seq data analysis. A clustering resolution of 1.5 was used to cluster PBMCs, and cluster numbers were given a “p” prefix (e.g., cluster p1 instead of cluster 1) to differentiate from ileal cell clusters. From PBMC clusters, gene expression was assessed to identify clusters of ILCs, using the same criteria as described for identification of porcine ileal ILCs and with additional query of NK cell genes described previously (Herrera-Uribe et al, 2021). By these criteria, we identified ILCs in PBMCs as cells belonging to clusters p0, p4, p26, p28, and p30 (Fig S27).
The two PBMC samples derived from the same pigs used for ileal scRNA-seq have been published previously in the reference dataset used for porcine PBMC mapping and prediction comparisons described in above methods; however, reads were not corrected and trimmed further as was done in the previously published work (Herrera-Uribe et al, 2021).
scRNA-seq analysis of merged ileum/PBMC ILC data
Total datasets of the porcine ileum and PBMCs were reduced using DietSeurat() to retain only the RNA assay and to remove dimensionality reductions, graphs, and scaled data. The two datasets were then combined with the Seurat function merge(), and clusters identified as ILCs from each dataset (clusters 1, 18, 43, 44, and 53 in the ileum and clusters p0, p4, p26, p28, and p30 in PBMCs) were specified within the function subset() to generate a data subset of only ILCs as described in Generating data subsets for ileal scRNA-seq analysis methods. In addition, SNNs were recalculated for the data subset with the Seurat function FindNeighbors().
DA, multidimensional DGE, and biological process enrichment analyses were performed as described for ileum scRNA-seq data. DA analysis was performed between ILCs originating from ileum versus PBMC samples. Further annotation of cell neighborhoods into specific cell types (e.g., ileal cytotoxic group 1 ILCs, peripheral ILCs) was not performed.
Gene signatures for peripheral ILCs, ileal ILCs, ileal group 1 ILCs, and group 3 ILCs were created by further filtering of genes in modules created through multidimensional DGE analysis (Table S10) that had high detection scores in ILC subsets specified in Fig 6D and E. To be included in a final gene signature, a gene from a specified module had to be expressed by >10% of cells in all target ILC subsets, and for each target ILC subset compared pairwise to each nontarget ILC subset have either (1) at least 2× the percentage of cells in the target ILC subset expressing the gene compared with the nontarget ILC subset or (2) have a larger percentage of cells (but less than 2× as many) expressing the gene and have at least 2× the average log-normalized gene expression count for the target ILC subset compared with the nontarget ILC subset. Summary statistics used for this filtering process are found in Table S10.
From this rationale, genes in gene module 4 were further filtered to identify a peripheral ILC gene signature, where peripheral ILCs were the target subset and ileal cytotoxic group 1 ILCs, ileal cycling group 1 ILCs, ileal non-naive group 1 ILCs, and ileal group 3 ILCs were nontarget subsets. Gene module 5 genes were filtered to identify an ileal ILC gene signature, where ileal cytotoxic group 1 ILCs, ileal cycling group 1 ILCs, ileal non-naive group 1 ILCs, and ileal group 3 ILCs were the target ILC subsets, and peripheral ILCs were the nontarget ILC subset. Genes in gene module 7 were filtered to identify an ileal group 1ILC gene signature, where ileal cytotoxic group 1 ILCs, ileal cycling group 1 ILCs, and ileal non-naive group 1 ILCs were target ILC subsets, and peripheral ILCs and group 3 ILCs were nontarget subsets. The ileal group 3 ILC gene signature was created by filtering genes in gene modules 3 and 8, using ileal group 3 ILCs as the target subset and ileal cytotoxic group 1 ILCs, ileal cycling group 1 ILCs, ileal non-naive group 1 ILCs, and peripheral ILCs as nontarget ILC subsets.
Gene name modifications
Several porcine genes did not have a gene symbol assigned by Ensembl in the genome annotation file used but did have a gene symbol in the NCBI database corresponding to the Ensembl gene identifier. In these cases, we substituted the Ensembl gene identifier with the NCBI gene symbol in our figures and text and have indicated this change with an asterix (*) at the end of the gene symbol used. Genes with duplicated gene symbols were also converted to Ensembl IDs when creating our gene annotation file, and these were converted back to gene symbols in our figures and text and denoted by an asterix (*) as well. The gene annotated as HLA-DRA was also converted to the updated pig-specific gene symbol SLA-DRA. A comprehensive list of all such substitutions used in figures and text are in Table S11.
Table S11 Ensembl identifiers replaced with gene symbols in text and figures. (9.3KB, xlsx)
Flow cytometry
Flow cytometry staining with cell viability dye and antibodies reactive to extracellular epitopes was performed as previously described (Wiarda et al, 2020). For antibody panels with intracellular CD79α detection, intracellular staining was completed after staining for extracellular markers, using the True-Nuclear Transcription Factor Buffer Set (424401; BioLegend) according to manufacturer’s instructions and as previously described (Boettcher et al, 2020a, 2020b). Cell events were acquired on a FACSymphony A5 flow cytometer (BD Biosciences), and data were analyzed with FlowJo v10.6.1 software (FlowJo, LLC) as previously described (Wiarda et al, 2020). Fluorescence-minus-one (FMO) stains were used as controls for applying gating strategies (Fig S29).
Figure S29. Flow cytometry controls used for gating.
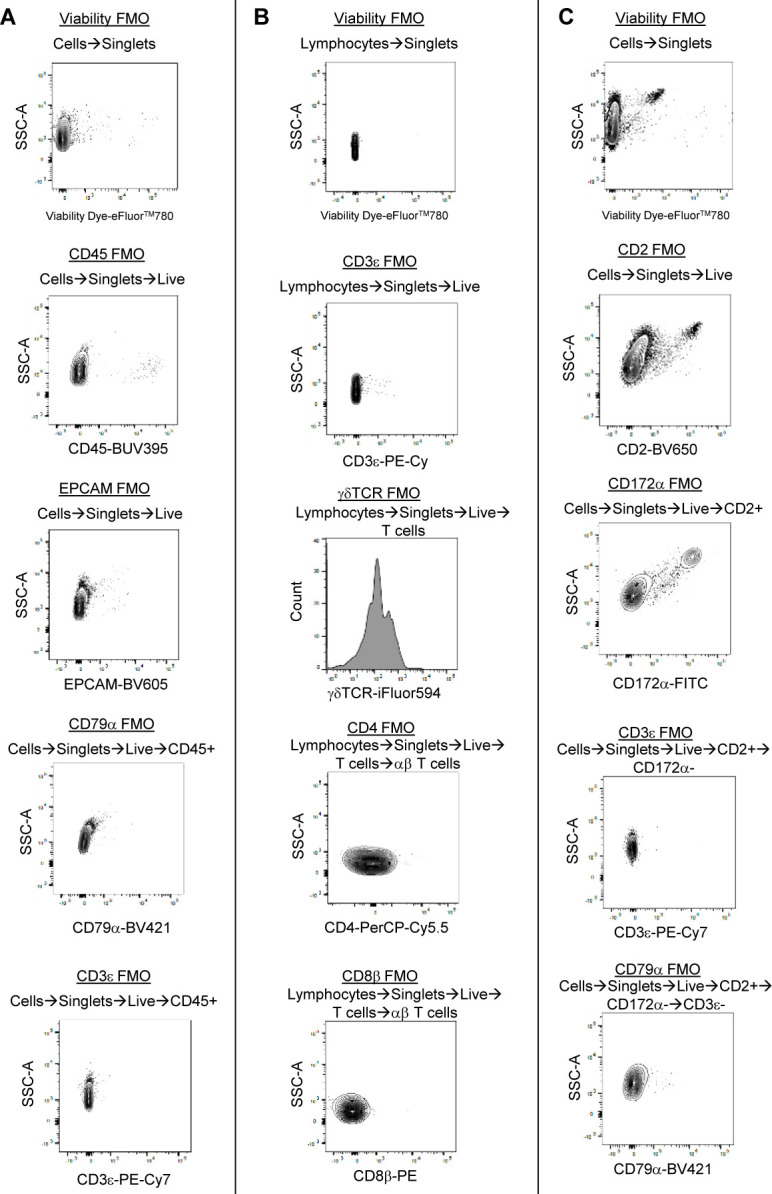
FMO controls for all flow cytometry panels and gating strategies used throughout all main text and supplementary figures. (A) FMO controls corresponding to the flow cytometry panel and gating shown in Figs S2B and S23A. (B) FMO controls corresponding to the flow cytometry panel and gating shown in Fig S23B. (C) FMO controls corresponding to the flow cytometry panel and gating shown in Fig 5A. For each FMO plot, the gating strategy of the shown parent population is listed above each plot and corresponds to the gating strategy used in respective flow cytometry figures. Slight autofluorescence in FITC and BV650 channels of (C) were verified to be absent from final gated populations of CD2+Lin− cells identified in corresponding gating of Fig 5A. Abbreviations: FMO, fluorescence-minus-one; SSC-A, side scatter area.
A panel to identify general populations of leukocytes, epithelial cells, T cells, and B cells (gating strategy shown in Figs S2B and S23A) included Fixable Viability Dye-eFluor780 (65-0865-14; Thermo Fisher Scientific); mouse α-pig CD45 (MCA1222GA; Bio-Rad) detected with rat α-mouse IgG1-BUV395 (740234; BD); rat α-mouse CD326-BV605 (CD326 also known as EPCAM; 118227; BioLegend); mouse α-pig CD3ε-PE-Cy7 (561477; BD); and mouse α-human CD79α-BV421 (566225; BD). A panel to identify γδ, CD4 αβ, and CD8 αβ T cells (gating strategy shown in Fig S23B) included Fixable Viability Dye-eFluor780 (65-0865-14; Thermo Fisher Scientific); mouse α-pig CD3ε-PE-Cy7 (561477; BD); mouse α-pig γδTCR-iFluor594 (primary antibody Washington State University PG2032; custom conjugation to iFluor594 performed by Caprico Biotechnologies); mouse α-pig CD4-PerCP-Cy5.5 (561474; BD); and mouse α-pig CD8β-PE (MCA5954PE; Bio-Rad). A panel to identify ileal ILCs (gating strategy shown in Fig 5A) included Fixable Viability Dye-eFluor780 (65-0865-14; Thermo Fisher Scientific); mouse α-pig CD45 (MCA1222GA; Bio-Rad) detected with rat α-mouse IgG1-BUV395 (740234; BD); mouse α-pig CD2 (PG2009; WSU) detected with rat α-mouse IgG3-BV650 (744136; BD); mouse α-pig CD3ε-PE-Cy7 (561477; BD); mouse α-human CD79α-BV421 (566225; BD); and mouse α-pig CD172α-FITC (MCA2312F; Bio-Rad).
Chromogenic immunohistochemistry
IHC staining for the CD3ε protein was completed on formalin-fixed, paraffin-embedded (FFPE) tissues fixed for 24–30 h in 10% neutral-buffered formalin (NBF; 3.7% formaldehyde) as previously described (Wiarda et al, 2020) with polyclonal rabbit α-human CD3ε antibody (A0452; Dako) and polyclonal goat α-rabbit HRP-conjugated antibody (K4003; Dako). The same protocol was used for CD79α protein staining but replacing the primary antibody incubation with diluted monoclonal mouse α-human CD79α antibody (LS-B4504; LifeSpan BioSciences) for 1 h RT and replacing the secondary antibody incubation with the HRP-labeled polyclonal goat α-mouse antibody (K4000; Dako) for 30 min RT. IHC staining for the pan-cytokeratin protein was carried out similar to CD3ε and CD79α IHC, but antigen retrieval was performed by incubation in Tris–EDTA buffer (10 mM Tris, 1 mM EDTA, pH 9.0; made in-house) for 20 min at 95°C. Primary antibody incubation was performed by incubating slides with diluted monoclonal mouse α-human pan-cytokeratin antibody (MCA1907T; Bio-Rad) overnight at 4°C followed by secondary antibody incubation with HRP-labeled α-mouse antibody for 30 min RT.
Chromogenic RNA in situ hybridization
FFPE tissues were obtained as described in IHC methods. Single-color chromogenic RNA ISH with Sus scrofa-specific channel 1 TRDC probe (553141; Advanced Cell Diagnostics [ACD]) was completed with the RNAscope 2.5 HD Reagent Kit-RED (322350; ACD) as previously described (Palmer et al, 2019) with the following modifications: (1) protease was applied for only 15 min at 40°C, and (2) slides were mounted and coverslipped as described elsewhere (Boettcher et al, 2020a). Dual chromogenic RNA ISH labeling with Sus scrofa-specific channel 1 (CD8B; ACD 815781) and channel 2 (CD4; 491891-C2; ACD) probes was completed with the RNAscope 2.5 HD Duplex Reagent Kit (322430; ACD) as previously described (Boettcher et al, 2020a).
Dual protein immunofluorescence/fluorescent RNA in situ hybridization
FFPE tissues were obtained as described in IHC methods. Dual immunofluorescence (IF) labeling of CD3ε protein and fluorescent RNA ISH of ITGAE or IL22 transcripts was completed according to the RNAscope Multiplex Fluorescent v2 Assay combined with Immunofluorescence-Integrated Co-Detection Workflow (ACD technical note MK 51-150), with some modifications. The RNAscope Multiplex Fluorescent Reagent Kit v2 (323100; ACD) was primarily used for RNA detection, with additional reagents outside of this kit pointed out in subsequent methods descriptions. Slides were baked, deparaffinized, and rehydrated, and hydrogen peroxide incubations were completed as described in RNA ISH methods. Target retrieval was completed by submerging slides in 1× Co-Detection Target Retrieval solution (323165; ACD) for 15 min 95°C, followed by rinsing with distilled water, 0.1% Tween in PBS (PBST), pH 7.2, and drawing of a hydrophobic barrier. CD3ε antibody (as used in IHC) was diluted in Co-detection Antibody Diluent (323160; ACD) and applied to slides for 1 h at RT, followed by washing 2 × 2 min in PBST. Tissues were fixed in 10% NBF (3.7% formaldehyde) for 30 min at RT, then washed 3 × 2 min in PBST. Protease Plus was applied to tissue sections for 15 min at 40°C, followed by rinsing with distilled water. The following steps were next completed sequentially at 40°C, with 2 × 2 min washes in 1× wash buffer between incubations: RNAscope custom channel 1 probe (ITGAE [590581; ACD] or IL22 [590611; ACD]) 2 h, AMP1 30 min, AMP2 30 min, AMP3 15 min, HRP-C1 15 min, Opal 570 (FP1488001KT; Akoya Biosciences) diluted in RNAscope Multiplex TSA Buffer (ACD 322809) 30 min, HRP blocker 15 min, goat α-rabbit IgG (H+L) F(ab′)-Alexa Fluor 488 (A11070; Invitrogen) diluted in Co-detection Antibody Diluent 1 h, DAPI 30 s. After DAPI incubation, DAPI was removed, and slides were mounted with ProLong Gold antifade reagent (P36930; Invitrogen) and #1.5 thickness cover glass (152450; Thermo Fisher Scientific). Slides were dried in the dark at RT, placed in the dark at 4°C overnight, and imaged within 1 wk.
Fluorescent images were acquired with a Nikon A1R Confocal Microscope using a 60× Plan Apo oil objective with numerical aperture 1.40 via hybrid galvano/resonant scanner. Single solid lasers 405, 488, and 561 were used with standard and highly sensitive GaAsP detectors with center wavelength/bandwidth 450/50 (DAPI), 525/50 (Alexa Fluor 488), and 595/50 (Opal 570). 60× large-image acquisition was used to generate a single high magnification, wide field-of-view image by automatically stitching multiple adjacent frames from a multipoint acquisition using the motorized stage on a fully automated inverted Ti2 Nikon microscope. Images were acquired and processed using NIS-Elements software (Nikon).
Data Availability
Final data are available for download and direct query at https://singlecell.broadinstitute.org/single_cell/study/SCP1921/intestinal-single-cell-atlas-reveals-novel-lymphocytes-in-pigs-with-similarities-to-human-cells. Sequencing data are available under GEO accession GSE196388. Scripts for data analyses are available at https://github.com/USDA-FSEPRU/scRNAseq_Porcine_Ileum_PBMC. Seurat objects of processed data used for analyses are available for download and further query/analysis as .h5seurat files at https://data.nal.usda.gov/dataset/data-porcine-intestinal-innate-lymphoid-cells-and-lymphocyte-spatial-context-revealed-through-single-cell-rna-sequencing.
Supplementary Material
Acknowledgements
We thank the following for their excellent contributions to this work: Dr. Kristen Byrne, Zahra Bond, and Sage Becker for technical and laboratory assistance; Dr. David Alt, Dr. Mike Baker, and the Iowa State University DNA Facility for library preparation and sequencing; Drs. Daniel Nielsen and Darrell Bayles for technologic assistance; Samuel Humphrey for flow cytometry expertise and services; Judith Stasko for histology expertise and services; Adrienne Shircliff for confocal microscopy expertise and services; Drs. Nicholas Gabler and Amy Vincent for collection of postmortem samples. Research was supported by appropriated funds from USDA-ARS CRIS 5030-31320-004-00D and an appointment to the Agricultural Research Service (ARS) Research Participation Program administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the United States Department of Energy (DOE) and the United States Department of Agriculture (USDA). ORISE is managed by Oak Ridge Associated Universities (ORAU) under DOE contract number DE-SC0014664. All opinions expressed in this paper are the authors’ and do not necessarily reflect the policies and views of USDA, ARS, DOE, or ORAU/ORISE. This research used resources provided by the SCINet project of the USDA ARS project number 0500-00093-001-00-D.
Author Contributions
JE Wiarda: conceptualization, data curation, software, formal analysis, validation, investigation, visualization, methodology, and writing—original draft.
JM Trachsel: data curation, software, and writing—review and editing.
SK Sivasankaran: data curation, software, and writing—review and editing.
CK Tuggle: conceptualization and writing—review and editing.
CL Loving: conceptualization, resources, supervision, funding acquisition, investigation, methodology, project administration, and writing—review and editing.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
References
- Agarwal S, Cunningham-Rundles C (2019) Gastrointestinal manifestations and complications of primary immunodeficiency disorders. Immunol Allergy Clin North Am 39: 81–94. 10.1016/j.iac.2018.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexa A, Rahnenführer J, Lengauer T (2006) Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics 22: 1600–1607. 10.1093/bioinformatics/btl140 [DOI] [PubMed] [Google Scholar]
- Allman D, Jain A, Dent A, Maile RR, Selvaggi T, Kehry MR, Staudt LM (1996) BCL-6 Expression during B-cell activation. Blood 87: 5257–5268. 10.1182/blood.v87.12.5257.bloodjournal87125257 [DOI] [PubMed] [Google Scholar]
- Annamalai T, Lu Z, Jung K, Langel SN, Tuggle CK, Dekkers JCM, Waide EH, Kandasamy S, Saif LJ (2019) Infectivity of GII.4 human norovirus does not differ between T-B-NK+ severe combined immunodeficiency (SCID) and non-SCID gnotobiotic pigs, implicating the role of NK cells in mediation of human norovirus infection. Virus Res 267: 21–25. 10.1016/j.virusres.2019.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annamalai T, Saif LJ, Lu Z, Jung K (2015) Age-dependent variation in innate immune responses to porcine epidemic diarrhea virus infection in suckling versus weaned pigs. Vet Immunol Immunopathol 168: 193–202. 10.1016/j.vetimm.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio-Domingo P, Romera-Hernandez M, Karrich JJ, Cornelissen F, Papazian N, Lindenbergh-Kortleve DJ, Butler JA, Boon L, Coles MC, Samsom JN, et al. (2015) Type 3 innate lymphoid cells maintain intestinal epithelial stem cells after tissue damage. J Exp Med 212: 1783–1791. 10.1084/jem.20150318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashouri JF, Weiss A (2017) Endogenous Nur77 is a specific indicator of antigen receptor signaling in human T and B cells. J Immunol 198: 657–668. 10.4049/jimmunol.1601301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auray G, Keller I, Python S, Gerber M, Bruggmann R, Ruggli N, Summerfield A (2016) Characterization and transcriptomic analysis of porcine blood conventional and plasmacytoid dendritic cells reveals striking species-specific differences. J Immunol 197: 4791–4806. 10.4049/jimmunol.1600672 [DOI] [PubMed] [Google Scholar]
- Auray G, Talker SC, Keller I, Python S, Gerber M, Liniger M, Ganges L, Bruggmann R, Ruggli N, Summerfield A (2020) High-resolution profiling of innate immune responses by porcine dendritic cell subsets in vitro and in vivo. Front Immunol 11: 1429. 10.3389/fimmu.2020.01429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman NN, Bianchi ATJ, Zwart RJ, Pabst R, Rothkötter HJ (1996) Jejunal and ileal Peyer[R8S2Q1M7]s patches in pigs differ in their postnatal development. Anat Embryol 195: 41–50. 10.1007/s004290050023 [DOI] [PubMed] [Google Scholar]
- Bedford JG, O’Keeffe M, Reading PC, Wakim LM (2019) Rapid interferon independent expression of IFITM3 following T cell activation protects cells from influenza virus infection. PLoS One 14: e0210132. 10.1371/journal.pone.0210132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiki H, Liu H, Huang J, Manchanda N, Nonneman D, Smith TPL, Reecy JM, Tuggle CK (2019) Improved annotation of the domestic pig genome through integration of Iso-Seq and RNA-seq data. BMC Genomics 20: 344. 10.1186/s12864-019-5709-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya D, Cheah MT, Franco CB, Hosen N, Pin CL, Sha WC, Weissman IL (2007) Transcriptional profiling of antigen-dependent murine B cell differentiation and memory formation. J Immunol 179: 6808–6819. 10.4049/jimmunol.179.10.6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binns RM, Licence ST (1985) Patterns of migration of labelled blood lymphocyte subpopulations: Evidence for two types of Peyer[R8S2Q1M7]s patch in the young pig. Adv Exp Med Bio 186: 661–668. 10.1007/978-1-4613-2463-8_81 [DOI] [PubMed] [Google Scholar]
- Boettcher AN, Cino-Ozuna AG, Solanki Y, Wiarda JE, Putz E, Owens JL, Crane SA, Ahrens AP, Loving CL, Cunnick JE, et al. (2020. a) CD3ε(+) cells in pigs with severe combined immunodeficiency due to defects in ARTEMIS. Front Immunol 11: 510. 10.3389/fimmu.2020.00510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher AN, Li Y, Ahrens AP, Kiupel M, Byrne KA, Loving CL, Cino-Ozuna AG, Wiarda JE, Adur M, Schultz B, et al. (2020. b) Novel engraftment and T cell differentiation of human hematopoietic cells in ART-/-IL2RG-/Y SCID pigs. Front Immunol 11: 100. 10.3389/fimmu.2020.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boismenu R, Feng L, Xia YY, Chang JC, Havran WL (1996) Chemokine expression by intraepithelial gamma delta T cells. Implications for the recruitment of inflammatory cells to damaged epithelia. J Immunol 157: 985–992. [PubMed] [Google Scholar]
- Bonnardel J, Da Silva C, Henri S, Tamoutounour S, Chasson L, Montañana-Sanchis F, Gorvel JP, Lelouard H (2015) Innate and adaptive immune functions of Peyer[R8S2Q1M7]s patch monocyte-derived cells. Cell Rep 11: 770–784. 10.1016/j.celrep.2015.03.067 [DOI] [PubMed] [Google Scholar]
- Boos MD, Yokota Y, Eberl G, Kee BL (2007) Mature natural killer cell and lymphoid tissue–inducing cell development requires Id2-mediated suppression of E protein activity. J Exp Med 204: 1119–1130. 10.1084/jem.20061959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breloer M, Kretschmer B, Lüthje K, Ehrlich S, Ritter U, Bickert T, Steeg C, Fillatreau S, Hoehlig K, Lampropoulou V, et al. (2007) CD83 is a regulator of murine B cell function in vivo. Eur J Immunol 37: 634–648. 10.1002/eji.200636852 [DOI] [PubMed] [Google Scholar]
- Butler A, Hoffman P, Smibert P, Papalexi E, Satija R (2018) Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol 36: 411–420. 10.1038/nbt.4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, Lager KM, Splichal I, Francis D, Kacskovics I, Sinkora M, Wertz N, Sun J, Zhao Y, Brown WR, et al. (2009) The piglet as a model for B cell and immune system development. Vet Immunol Immunopathol 128: 147–170. 10.1016/j.vetimm.2008.10.321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caio G, Volta U, Sapone A, Leffler DA, De Giorgio R, Catassi C, Fasano A (2019) Celiac disease: A comprehensive current review. BMC Med 17: 142. 10.1186/s12916-019-1380-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminero A, Pinto-Sanchez MI (2020) Host immune interactions in chronic inflammatory gastrointestinal conditions. Curr Opin Gastroenterol 36: 479–484. 10.1097/mog.0000000000000673 [DOI] [PubMed] [Google Scholar]
- Cano-Gamez E, Soskic B, Roumeliotis TI, So E, Smyth DJ, Baldrighi M, Willé D, Nakic N, Esparza-Gordillo J, Larminie CGC, et al. (2020) Single-cell transcriptomics identifies an effectorness gradient shaping the response of CD4+ T cells to cytokines. Nat Commun 11: 1801. 10.1038/s41467-020-15543-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso R, Lo BC, Núñez G (2020) Host–microbiota interactions in inflammatory bowel disease. Nat Rev Immunol 20: 411–426. 10.1038/s41577-019-0268-7 [DOI] [PubMed] [Google Scholar]
- Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB (1994) Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the αEβ7 integrin. Nature 372: 190–193. 10.1038/372190a0 [DOI] [PubMed] [Google Scholar]
- Clottu AS, Mathias A, Sailer AW, Schluep M, Seebach JD, Du Pasquier R, Pot C (2017) EBI2 expression and function: Robust in memory lymphocytes and increased by natalizumab in multiple sclerosis. Cell Rep 18: 213–224. 10.1016/j.celrep.2016.12.006 [DOI] [PubMed] [Google Scholar]
- Crinier A, Milpied P, Escalière B, Piperoglou C, Galluso J, Balsamo A, Spinelli L, Cervera-Marzal I, Ebbo M, Girard-Madoux M, et al. (2018) High-dimensional single-cell analysis identifies organ-specific signatures and conserved NK cell subsets in humans and mice. Immunity 49: 971–986.e5. 10.1016/j.immuni.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham F, Achuthan P, Akanni W, Allen J, Amode MR, Armean IM, Bennett R, Bhai J, Billis K, Boddu S, et al. (2019) Ensembl 2019. Nucleic Acids Res 47: D745–D751. 10.1093/nar/gky1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabydeen SA, Desai A, Sahoo D (2019) Unbiased Boolean analysis of public gene expression data for cell cycle gene identification. Mol Bio Cell 30: 1770–1779. 10.1091/mbc.e19-01-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann E, Henderson NC, Teichmann SA, Morgan MD, Marioni JC (2021) Differential abundance testing on single-cell data using k-nearest neighbor graphs. Nat Biotechnol 40: 245–253. 10.1038/s41587-021-01033-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denyer MS, Wileman TE, Stirling CMA, Zuber B, Takamatsu HH (2006) Perforin expression can define CD8 positive lymphocyte subsets in pigs allowing phenotypic and functional analysis of Natural Killer, Cytotoxic T, Natural Killer T and MHC un-restricted cytotoxic T-cells. Vet Immunol Immunopathol 110: 279–292. 10.1016/j.vetimm.2005.10.005 [DOI] [PubMed] [Google Scholar]
- Dey KK, Hsiao CJ, Stephens M (2017) Visualizing the structure of RNA-seq expression data using grade of membership models. PLoS Genet 13: e1006759. 10.1371/journal.pgen.1006759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durinck S, Spellman PT, Birney E, Huber W (2009) Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat Protoc 4: 1184–1191. 10.1038/nprot.2009.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton EE, Camelo A, Sleeman M, Herbst R, Carlesso G, Belz GT, Withers DR (2017) Characterisation of innate lymphoid cell populations at different sites in mice with defective T cell immunity. Wellcome Open Res 2: 117. 10.12688/wellcomeopenres.13199.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmentaite R, Kumasaka N, Roberts K, Fleming A, Dann E, King HW, Kleshchevnikov V, Dabrowska M, Pritchard S, Bolt L, et al. (2021) Cells of the human intestinal tract mapped across space and time. Nature 597: 250–255. 10.1038/s41586-021-03852-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmentaite R, Ross ADB, Roberts K, James KR, Ortmann D, Gomes T, Nayak K, Tuck L, Pritchard S, Bayraktar OA, et al. (2020) Single-cell sequencing of developing human gut reveals transcriptional links to childhood Crohn[R8S2Q1M7]s disease. Dev Cell 55: 771–783.e5. 10.1016/j.devcel.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel P, Gribben JG, Freeman GJ, Zhou LJ, Nozawa Y, Abe M, Nadler LM, Wakasa H, Tedder TF (1994) The B7-2 (B70) costimulatory molecule expressed by monocytes and activated B lymphocytes is the CD86 differentiation antigen. Blood 84: 1402–1407. 10.1182/blood.v84.5.1402.1402 [DOI] [PubMed] [Google Scholar]
- Entrican G, Lunney JK, Wattegedera SR, Mwangi W, Hope JC, Hammond JA (2020) The veterinary immunological toolbox: Past, present, and future. Front Immunol 11: 1651. 10.3389/fimmu.2020.01651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farache J, Koren I, Milo I, Gurevich I, Kim KW, Zigmond E, Furtado GC, Lira SA, Shakhar G (2013) Luminal bacteria recruit CD103+ dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity 38: 581–595. 10.1016/j.immuni.2013.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foissac S, Djebali S, Munyard K, Vialaneix N, Rau A, Muret K, Esquerré D, Zytnicki M, Derrien T, Bardou P, et al. (2019) Multi-species annotation of transcriptome and chromatin structure in domesticated animals. BMC Biol 17: 108. 10.1186/s12915-019-0726-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster R, Schubel A, Breitfeld D, Kremmer E, Renner-Müller I, Wolf E, Lipp M (1999) CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99: 23–33. 10.1016/s0092-8674(00)80059-8 [DOI] [PubMed] [Google Scholar]
- Freeman TC, Ivens A, Baillie JK, Beraldi D, Barnett MW, Dorward D, Downing A, Fairbairn L, Kapetanovic R, Raza S, et al. (2012) A gene expression atlas of the domestic pig. BMC Biol 10: 90. 10.1186/1741-7007-10-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD, Cella M, Colonna M (2013) Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-γ-producing cells. Immunity 38: 769–781. 10.1016/j.immuni.2013.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihashi K, Boyaka PN, McGhee JR (2008) Host defenses at mucosal surfaces. In Clin Immunology, 3rd edn, Chapter 19, pp 287–303. 10.1016/B978-0-323-04404-2.10019-3 [DOI] [Google Scholar]
- Fujihashi K, Dohi T, Rennert PD, Yamamoto M, Koga T, Kiyono H, McGhee JR (2001) Peyer[R8S2Q1M7]s patches are required for oral tolerance to proteins. Proc Natl Acad Sci U S A 98: 3310–3315. 10.1073/pnas.061412598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gärdby E, Lycke NY (2000) CD19-deficient mice exhibit poor responsiveness to oral immunization despite evidence of unaltered total IgA levels, germinal centers and IgA-isotype switching in Peyer[R8S2Q1M7]s patches. Euro J Immunol 30: 1861–1871. [DOI] [PubMed] [Google Scholar]
- Gerner W, Käser T, Saalmüller A (2009) Porcine T lymphocytes and NK cells—An update. Dev Comp Immunol 33: 310–320. 10.1016/j.dci.2008.06.003 [DOI] [PubMed] [Google Scholar]
- Giotti B, Chen SH, Barnett MW, Regan T, Ly T, Wiemann S, Hume DA, Freeman TC (2019) Assembly of a parts list of the human mitotic cell cycle machinery. J Mol Cell Biol 11: 703–718. 10.1093/jmcb/mjy063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass DR, Tsai AG, Oliveria JP, Hartmann FJ, Kimmey SC, Calderon AA, Borges L, Glass MC, Wagar LE, Davis MM, et al. (2020) An integrated multi-omic single-cell atlas of human B cell identity. Immunity 53: 217–232.e5. 10.1016/j.immuni.2020.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez LM, Moeser AJ, Blikslager AT (2015) Porcine models of digestive disease: The future of large animal translational research. Transl Res 166: 12–27. 10.1016/j.trsl.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths JA, Richard AC, Bach K, Lun ATL, Marioni JC (2018) Detection and removal of barcode swapping in single-cell RNA-seq data. Nat Commun 9: 2667. 10.1038/s41467-018-05083-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gün G, Kues WA (2014) Current progress of genetically engineered pig models for biomedical research. BioResearch Open Access 3: 255–264. 10.1089/biores.2014.0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R, Lü M, Cao F, Wu G, Gao F, Pang H, Li Y, Zhang Y, Xing H, Liang C, et al. (2021) Single-cell map of diverse immune phenotypes in the acute myeloid leukemia microenvironment. Biomark Res 9: 15. 10.1186/s40364-021-00265-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Qiu J, Tu T, Yang X, Deng L, Anders RA, Zhou L, Fu YX (2014) Induction of innate lymphoid cell-derived interleukin-22 by the transcription factor STAT3 mediates protection against intestinal infection. Immunity 40: 25–39. 10.1016/j.immuni.2013.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gury-BenAri M, Thaiss CA, Serafini N, Winter DR, Giladi A, Lara-Astiaso D, Levy M, Salame TM, Weiner A, David E, et al. (2016) The spectrum and regulatory landscape of intestinal innate lymphoid cells are shaped by the microbiome. Cell 166: 1231–1246.e13. 10.1016/j.cell.2016.07.043 [DOI] [PubMed] [Google Scholar]
- Guy-Grand D, Cerf-Bensussan N, Malissen B, Malassis-Seris M, Briottet C, Vassalli P (1991) Two gut intraepithelial CD8+ lymphocyte populations with different T cell receptors: A role for the gut epithelium in T cell differentiation. J Exp Med 173: 471–481. 10.1084/jem.173.2.471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer SE, Leopold M, Prawits LM, Mair KH, Schwartz JC, Hammond JA, Ravens S, Gerner W, Saalmüller A (2020) Development of a RACE-based RNA-Seq approach to characterize the T-cell receptor repertoire of porcine γδ T cells. Dev Comp Immunol 105: 103575. 10.1016/j.dci.2019.103575 [DOI] [PubMed] [Google Scholar]
- Han L, Jara CP, Wang O, Shi Y, Wu X, Thibivilliers S, Wóycicki RK, Carlson MA, Velander WH, Araújo EP, et al. (2022) Isolating and cryo-preserving pig skin cells for single cell RNA sequencing study. PLoS One 17: e0263869. 10.1371/journal.pone.0263869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Hao S, Andersen-Nissen E, Mauck WM, Zheng S, Butler A, Lee MJ, Wilk AJ, Darby C, Zager M, et al. (2021) Integrated analysis of multimodal single-cell data. Cell 184: 3573–3587.e29. 10.1016/j.cell.2021.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverson K, Rehakova Z, Sinkora J, Sver L, Bailey M (2007) Immune development in jejunal mucosa after colonization with selected commensal gut bacteria: A study in germ-free pigs. Vet Immunol Immunopathol 119: 243–253. 10.1016/j.vetimm.2007.05.022 [DOI] [PubMed] [Google Scholar]
- Haynes NM, Allen CDC, Lesley R, Ansel KM, Killeen N, Cyster JG (2007) Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol 179: 5099–5108. 10.4049/jimmunol.179.8.5099 [DOI] [PubMed] [Google Scholar]
- Hepworth MR, Fung TC, Masur SH, Kelsen JR, McConnell FM, Dubrot J, Withers DR, Hugues S, Farrar MA, Reith W, et al. (2015) Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria–specific CD4+ T cells. Science 348: 1031–1035. 10.1126/science.aaa4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Uribe J, Wiarda JE, Sivasankaran SK, Daharsh L, Liu H, Byrne KA, Smith TPL, Lunney JK, Loving CL, Tuggle CK (2021) Reference transcriptomes of porcine peripheral immune cells created through bulk and single-cell RNA sequencing. Front Genet 12: 689406. 10.3389/fgene.2021.689406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo LG, Einecke G, Allanach K, Halloran PF (2008) The transcriptome of human cytotoxic T cells: Similarities and disparities among allostimulated CD4+ CTL, CD8+ CTL and NK cells. Ame J Transpl 8: 627–636. 10.1111/j.1600-6143.2007.02128.x [DOI] [PubMed] [Google Scholar]
- Hoytema van Konijnenburg DP, Reis BS, Pedicord VA, Farache J, Victora GD, Mucida D (2017) Intestinal epithelial and intraepithelial T cell crosstalk mediates a dynamic response to infection. Cell 171: 783–794.e13. 10.1016/j.cell.2017.08.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryhorowicz M, Lipiński D, Hryhorowicz S, Nowak-Terpiłowska A, Ryczek N, Zeyland J (2020) Application of genetically engineered pigs in biomedical research. Genes 11: 670. 10.3390/genes11060670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington ND, Tabarias H, Fairfax K, Brady J, Hayakawa Y, Degli-Esposti MA, Smyth MJ, Tarlinton DM, Nutt SL (2007) NK cell maturation and peripheral homeostasis is associated with KLRG1 up-regulation. J Immunol 178: 4764–4770. 10.4049/jimmunol.178.8.4764 [DOI] [PubMed] [Google Scholar]
- Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA (1999) ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 397: 263–266. 10.1038/16717 [DOI] [PubMed] [Google Scholar]
- Inoue R, Tsukahara T, Nakatani M, Okutani M, Nishibayashi R, Ogawa S, Harayama T, Nagino T, Hatanaka H, Fukuta K, et al. (2015) Weaning markedly affects transcriptome profiles and Peyer[R8S2Q1M7]s patch development in piglet ileum. Front Immunol 6: 630. 10.3389/fimmu.2015.00630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY (2004) Retinoic acid imprints gut-homing specificity on T cells. Immunity 21: 527–538. 10.1016/j.immuni.2004.08.011 [DOI] [PubMed] [Google Scholar]
- Jaeger N, Gamini R, Cella M, Schettini JL, Bugatti M, Zhao S, Rosadini CV, Esaulova E, Di Luccia B, Kinnett B, et al. (2021) Single-cell analyses of Crohn[R8S2Q1M7]s disease tissues reveal intestinal intraepithelial T cells heterogeneity and altered subset distributions. Nat Commun 12: 1921. 10.1038/s41467-021-22164-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal AI, Dubey C, Swain SL, Croft M (1996) Regulation of CD40 ligand expression on naive CD4 T cells: A role for TCR but not co-stimulatory signals. Int Immunol 8: 275–285. 10.1093/intimm/8.2.275 [DOI] [PubMed] [Google Scholar]
- Jarry A, Cerf-Bensussan N, Brousse N, Selz F, Guy-Grand D (1990) Subsets of CD3+ (T cell receptor α/β or γ/δ) and CD3− lymphocytes isolated from normal human gut epithelium display phenotypical features different from their counterparts in peripheral blood. Euro J Immunol 20: 1097–1103. 10.1002/eji.1830200523 [DOI] [PubMed] [Google Scholar]
- Jin L, Tang Q, Hu S, Chen Z, Zhou X, Zeng B, Wang Y, He M, Li Y, Gui L, et al. (2021) A pig BodyMap transcriptome reveals diverse tissue physiologies and evolutionary dynamics of transcription. Nat Commun 12: 3715. 10.1038/s41467-021-23560-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker Y, Bode H, Wahnschaffe U, Kroesen A, Loddenkemper C, Duchmann R, Zeitz M, Ullrich R (2009) Comparative analysis of mononuclear cells isolated from mucosal lymphoid follicles of the human ileum and colon. Clin Exp Immunol 156: 232–237. 10.1111/j.1365-2249.2009.03883.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käser T (2021) Swine as biomedical animal model for T-cell research—success and potential for transmittable and non-transmittable human diseases. Mol Immunol 135: 95–115. 10.1016/j.molimm.2021.04.004 [DOI] [PubMed] [Google Scholar]
- Kelner GS, Kennedy J, Bacon KB, Kleyensteuber S, Largaespada DA, Jenkins NA, Copeland NG, Bazan JF, Moore KW, Schall TJ, et al. (1994) Lymphotactin: A cytokine that represents a new class of chemokine. Science 266: 1395–1399. 10.1126/science.7973732 [DOI] [PubMed] [Google Scholar]
- Keren DF, Holt PS, Collins HH, Gemski P, Formal SB (1978) The role of Peyer[R8S2Q1M7]s patches in the local immune response of rabbit ileum to live bacteria. J Immunol 120: 1892–1896. [PubMed] [Google Scholar]
- Kilshaw PJ, Murant SJ (1990) A new surface antigen on intraepithelial lymphocytes in the intestine. Euro J Immunol 20: 2201–2207. 10.1002/eji.1830201008 [DOI] [PubMed] [Google Scholar]
- Kim S, Lim B, Mattoo SUS, Oh EY, Jeong CG, Kim WI, Lee KT, Lee SM, Kim JM (2021) Comprehensive transcriptomic comparison between porcine CD8− and CD8+ gamma delta T cells revealed distinct immune phenotype. Animals 11: 2165. 10.3390/ani11082165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HW, Wells KL, Shipony Z, Kathiria AS, Wagar LE, Lareau C, Orban N, Capasso R, Davis MM, Steinmetz LM, et al. (2021) Integrated single-cell transcriptomics and epigenomics reveals strong germinal center-associated etiology of autoimmune risk loci. Sci Immunol 6: eabh3768. 10.1126/sciimmunol.abh3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiriya K, Watanabe N, Nishio A, Okazaki K, Kido M, Saga K, Tanaka J, Akamatsu T, Ohashi S, Asada M, et al. (2007) Essential role of Peyer[R8S2Q1M7]s patches in the development of Helicobacter-induced gastritis. Int Immunol 19: 435–446. 10.1093/intimm/dxm008 [DOI] [PubMed] [Google Scholar]
- Knox JJ, Cosma GL, Betts MR, McLane LM (2014) Characterization of T-bet and Eomes in peripheral human immune cells. Front Immunol 5: 217. 10.3389/fimmu.2014.00217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi E, Hanazono Y, Kunita S (2018) Swine used in the medical university: Overview of 20 years of experience. Exp Anim 67: 7–13. 10.1538/expanim.17-0086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q, Yang X, Zhang H, Liu S, Zhao J, Zhang J, Weng X, Jin J, Liu Z (2020) Lineage specification and pluripotency revealed by transcriptome analysis from oocyte to blastocyst in pig. FASEB J 34: 691–705. 10.1096/fj.201901818rr [DOI] [PubMed] [Google Scholar]
- Kruglov AA, Grivennikov SI, Kuprash DV, Winsauer C, Prepens S, Seleznik GM, Eberl G, Littman DR, Heikenwalder M, Tumanov AV, et al. (2013) Nonredundant function of soluble LTα3 produced by innate lymphoid cells in intestinal homeostasis. Science 342: 1243–1246. 10.1126/science.1243364 [DOI] [PubMed] [Google Scholar]
- Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W (1993) Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75: 263–274. 10.1016/0092-8674(93)80068-p [DOI] [PubMed] [Google Scholar]
- Kumar A, Vlasova AN, Deblais L, Huang HC, Wijeratne A, Kandasamy S, Fischer DD, Langel SN, Paim FC, Alhamo MA, et al. (2018) Impact of nutrition and rotavirus infection on the infant gut microbiota in a humanized pig model. BMC Gastroenterol 18: 93. 10.1186/s12876-018-0810-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwa SF, Beverley P, Smith AL (2006) Peyer[R8S2Q1M7]s patches are required for the induction of rapid Th1 responses in the gut and mesenteric lymph nodes during an enteric infection. J Immunol 176: 7533–7541. 10.4049/jimmunol.176.12.7533 [DOI] [PubMed] [Google Scholar]
- Laroux FS, Norris HH, Houghton J, Pavlick KP, Bharwani S, Merrill DM, Fuseler J, Chervenak R, Grisham MB (2004) Regulation of chronic colitis in athymic nu/nu (nude) mice. Int Immunol 16: 77–89. 10.1093/intimm/dxh006 [DOI] [PubMed] [Google Scholar]
- Lauritsen JPH, Wong GW, Lee SY, Lefebvre JM, Ciofani M, Rhodes M, Kappes DJ, Zúñiga-Pflücker JC, Wiest DL (2009) Marked induction of the helix-loop-helix protein Id3 promotes the γδ T cell fate and renders their functional maturation Notch independent. Immunity 31: 565–575. 10.1016/j.immuni.2009.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavelle EC, Ward RW (2021) Mucosal vaccines—Fortifying the frontiers. Nat Rev Immunol 22: 236–250. 10.1038/s41577-021-00583-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RD, Munro SA, Knutson TP, LaRue RS, Heltemes-Harris LM, Farrar MA (2021) Single-cell analysis identifies dynamic gene expression networks that govern B cell development and transformation. Nat Commun 12: 6843. 10.1038/s41467-021-27232-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Wang Y, Sun Y, Cui H, Zhu SJ, Qiu HJ (2020) Mucosal vaccines: Strategies and challenges. Immunol Lett 217: 116–125. 10.1016/j.imlet.2019.10.013 [DOI] [PubMed] [Google Scholar]
- Ling KL, Dulphy N, Bahl P, Salio M, Maskell K, Piris J, Warren BF, George BD, Mortensen NJ, Cerundolo V (2007) Modulation of CD103 expression on human colon carcinoma-specific CTL. J Immunol 178: 2908–2915. 10.4049/jimmunol.178.5.2908 [DOI] [PubMed] [Google Scholar]
- Linsley PS, Golstein P (1996) Lymphocyte activation: T-cell regulation by CTLA-4. Curr Biol 6: 398–400. 10.1016/s0960-9822(02)00506-7 [DOI] [PubMed] [Google Scholar]
- Liu T, Li J, Yu L, Sun HX, Li J, Dong G, Hu Y, Li Y, Shen Y, Wu J, et al. (2021) Cross-species single-cell transcriptomic analysis reveals pre-gastrulation developmental differences among pigs, monkeys, and humans. Cell Discov 7: 8. 10.1038/s41421-020-00238-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lun ATL, Riesenfeld S, Andrews T, Dao TP, Gomes T, Marioni JC, Marioni JCParticipants in the 1st Human Cell Atlas Jamboree , (2019) EmptyDrops: Distinguishing cells from empty droplets in droplet-based single-cell RNA sequencing data. Genome Biol 20: 63. 10.1186/s13059-019-1662-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist C, Baranov V, Hammarström S, Athlin L, Hammarström ML (1995) Intra-epithelial lymphocytes. Evidence for regional specialization and extrathymic T cell maturation in the human gut epithelium. Int Immunol 7: 1473–1487. 10.1093/intimm/7.9.1473 [DOI] [PubMed] [Google Scholar]
- Lutter L, Hoytema van Konijnenburg DP, Brand EC, Oldenburg B, Van Wijk F (2018) The elusive case of human intraepithelial T cells in gut homeostasis and inflammation. Nat Rev Gastroenterol Hepatol 15: 637–649. 10.1038/s41575-018-0039-0 [DOI] [PubMed] [Google Scholar]
- Mach N, Berri M, Esquerré D, Chevaleyre C, Lemonnier G, Billon Y, Lepage P, Oswald IP, Doré J, Rogel-Gaillard C, et al. (2014) Extensive expression differences along porcine small intestine evidenced by transcriptome sequencing. PLoS One 9: e88515. 10.1371/journal.pone.0088515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair KH, Müllebner A, Essler SE, Duvigneau JC, Storset AK, Saalmüller A, Gerner W (2013) Porcine CD8αdim/-NKp46high NK cells are in a highly activated state. Vet Res 44: 13. 10.1186/1297-9716-44-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroilley T, Berri M, Lemonnier G, Esquerré D, Chevaleyre C, Mélo S, Meurens F, Coville JL, Leplat JJ, Rau A, et al. (2018) Immunome differences between porcine ileal and jejunal Peyer[R8S2Q1M7]s patches revealed by global transcriptome sequencing of gut-associated lymphoid tissues. Sci Rep 8: 9077. 10.1038/s41598-018-27019-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayassi T, Jabri B (2018) Human intraepithelial lymphocytes. Mucosal Immunol 11: 1281–1289. 10.1038/s41385-018-0016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzurana L, Czarnewski P, Jonsson V, Wigge L, Ringnér M, Williams TC, Ravindran A, Björklund ÅK, Säfholm J, Nilsson G, et al. (2021) Tissue-specific transcriptional imprinting and heterogeneity in human innate lymphoid cells revealed by full-length single-cell RNA-sequencing. Cell Res 31: 554–568. 10.1038/s41422-020-00445-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DJ, Chen Y, Smyth GK (2012) Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res 40: 4288–4297. 10.1093/nar/gks042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BD, Bunker JJ, Ishizuka IE, Jabri B, Bendelac A (2014) Elevated T cell receptor signaling identifies a thymic precursor to the TCRαβ+CD4−CD8β− intraepithelial lymphocyte lineage. Immunity 41: 219–229. 10.1016/j.immuni.2014.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meininger I, Carrasco A, Rao A, Soini T, Kokkinou E, Mjösberg J (2020) Tissue-specific features of innate lymphoid cells. Trends Immunol 41: 902–917. 10.1016/j.it.2020.08.009 [DOI] [PubMed] [Google Scholar]
- Meng Q, Chen L, Xiong B, Kang B, Zhang P, Tang S, Han H, Shen W, Feng X, Feng S, et al. (2021) Single-cell transcriptome sequencing and proteomics reveal neonatal ileum dynamic developmental potentials. mSystems 6: e0072521. 10.1128/msystems.00725-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q, Luo Z, Cao C, Sun S, Ma Q, Li Z, Shi B, Shan A (2020) Weaning alters intestinal gene expression involved in nutrient metabolism by shaping gut microbiota in pigs. Front Microbiol 11: 694. 10.3389/fmicb.2020.00694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miragaia RJ, Gomes T, Chomka A, Jardine L, Riedel A, Hegazy AN, Whibley N, Tucci A, Chen X, Lindeman I, et al. (2019) Single-cell transcriptomics of regulatory T cells reveals trajectories of tissue adaptation. Immunity 50: 493–504.e7. 10.1016/j.immuni.2019.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki A, Kandasamy S, Michael H, Langel SN, Paim FC, Chepngeno J, Alhamo MA, Fischer DD, Huang HC, Srivastava V, et al. (2018) Protein deficiency reduces efficacy of oral attenuated human rotavirus vaccine in a human infant fecal microbiota transplanted gnotobiotic pig model. Vaccine 36: 6270–6281. 10.1016/j.vaccine.2018.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeser AJ, Pohl CS, Rajput M (2017) Weaning stress and gastrointestinal barrier development: Implications for lifelong gut health in pigs. Anim Nutr 3: 313–321. 10.1016/j.aninu.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P, Mizoguchi E, Grusby MJ, Glimcher LH, Bhan AK, Tonegawa S (1993) Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell 75: 274–282. 10.1016/0092-8674(93)80069-q [DOI] [PubMed] [Google Scholar]
- Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, von Andrian UH (2003) Selective imprinting of gut-homing T cells by Peyer[R8S2Q1M7]s patch dendritic cells. Nature 424: 88–93. 10.1038/nature01726 [DOI] [PubMed] [Google Scholar]
- Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, Merad M (2014) Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 343: 1249288. 10.1126/science.1249288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat AM, Agace WW (2014) Regional specialization within the intestinal immune system. Nat Rev Immunol 14: 667–685. 10.1038/nri3738 [DOI] [PubMed] [Google Scholar]
- Muramatsu M, Sankaranand VS, Anant S, Sugai M, Kinoshita K, Davidson NO, Honjo T (1999) Specific expression of activation-induced cytidine deaminase (AID), a novel member of the RNA-editing deaminase family in germinal center B cells. Biol Chem 274: 18470–18476. 10.1074/jbc.274.26.18470 [DOI] [PubMed] [Google Scholar]
- Nagai S, Mimuro H, Yamada T, Baba Y, Moro K, Nochi T, Kiyono H, Suzuki T, Sasakawa C, Koyasu S (2007) Role of Peyer[R8S2Q1M7]s patches in the induction of Helicobacter pylori-induced gastritis. Proc Natl Acad Sci U S A 104: 8971–8976. 10.1073/pnas.0609014104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Villagómez D, Van Kaer L (2018) Intestinal intraepithelial lymphocytes: Sentinels of the mucosal barrier. Trends Immunol 39: 264–275. 10.1016/j.it.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondr JK, Pham CTN (2004) Characterization of murine cathepsin W and its role in cell-mediated cytotoxicity. J Biol Chem 279: 27525–27533. 10.1074/jbc.m400304200 [DOI] [PubMed] [Google Scholar]
- Pabst R (2020) The pig as a model for immunology research. Cell Tissue Res 380: 287–304. 10.1007/s00441-020-03206-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer MV, Wiarda J, Kanipe C, Thacker TC (2019) Early pulmonary lesions in cattle infected via aerosolized Mycobacterium bovis. Vet Pathol 56: 544–554. 10.1177/0300985819833454 [DOI] [PubMed] [Google Scholar]
- Pan Z, Yao Y, Yin H, Cai Z, Wang Y, Bai L, Kern C, Halstead M, Chanthavixay G, Trakooljul N, et al. (2021) Pig genome functional annotation enhances the biological interpretation of complex traits and human disease. Nat Commun 12: 5848. 10.1038/s41467-021-26153-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RS, Tomlinson JE, Divers TJ, Van de Walle GR, Rosenberg BR (2021) Single-cell resolution landscape of equine peripheral blood mononuclear cells reveals diverse cell types including T-bet+ B cells. BMC Biol 19: 13. 10.1186/s12915-020-00947-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piriou-Guzylack L, Salmon H (2008) Membrane markers of the immune cells in swine: An update. Vet Res 39: 54. 10.1051/vetres:2008030 [DOI] [PubMed] [Google Scholar]
- Potockova H, Sinkorova J, Karova K, Sinkora M (2015) The distribution of lymphoid cells in the small intestine of germ-free and conventional piglets. Dev Comp Immunol 51: 99–107. 10.1016/j.dci.2015.02.014 [DOI] [PubMed] [Google Scholar]
- Poussier P, Edouard P, Lee C, Binnie M, Julius M (1992) Thymus-independent development and negative selection of T cells expressing T cell receptor alpha/beta in the intestinal epithelium: Evidence for distinct circulation patterns of gut- and thymus-derived T lymphocytes. J Exp Med 176: 187–199. 10.1084/jem.176.1.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poussier P, Julius M (1994) Thymus independent T cell development and selection in the intestinal epithelium. Annu Rev Immunol 12: 521–553. 10.1146/annurev.iy.12.040194.002513 [DOI] [PubMed] [Google Scholar]
- Prazma CM, Yazawa N, Fujimoto Y, Fujimoto M, Tedder TF (2007) CD83 expression is a sensitive marker of activation required for B cell and CD4+ T cell longevity in vivo. J Immunol 179: 4550–4562. 10.4049/jimmunol.179.7.4550 [DOI] [PubMed] [Google Scholar]
- Qi J, Crinier A, Escalière B, Ye Y, Wang Z, Zhang T, Batista L, Liu H, Hong L, Wu N, et al. (2021) Single-cell transcriptomic landscape reveals tumor specific innate lymphoid cells associated with colorectal cancer progression. Cell Rep Med 2: 100353. 10.1016/j.xcrm.2021.100353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahe MC, Murtaugh MP (2017) Interleukin-21 drives proliferation and differentiation of porcine memory B cells into antibody secreting cells. PLoS One 12: e0171171. 10.1371/journal.pone.0171171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Ibeas P, Sang F, Zhu Q, Tang WWC, Withey S, Klisch D, Wood L, Loose M, Surani MA, Alberio R (2019) Pluripotency and X chromosome dynamics revealed in pig pre-gastrulating embryos by single cell analysis. Nat Commun 10: 500. 10.1038/s41467-019-08387-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinette ML, Fuchs A, Cortez VS, Lee JS, Wang Y, Durum SK, Gilfillan S, Colonna M, Shaw L, Yu B, et al. (2015) Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat Immunol 16: 306–317. 10.1038/ni.3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140. 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Gómez IM, Talker SC, Käser T, Stadler M, Reiter L, Ladinig A, Milburn JV, Hammer SE, Mair KH, Saalmüller A, et al. (2019) Expression of T-Bet, Eomesodermin, and GATA-3 correlates with distinct phenotypes and functional properties in porcine γδ T cells. Front Immunol 10: 396. 10.3389/fimmu.2019.00396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothkötter HJ, Pabst R (1989) Lymphocyte subsets in jejunal and ileal Peyer[R8S2Q1M7]s patches of normal and gnotobiotic minipigs. Immunology 67: 103–108. [PMC free article] [PubMed] [Google Scholar]
- Rothkötter HJ (2009) Anatomical particularities of the porcine immune system—A physician[R8S2Q1M7]s view. Dev Comp Immunol 33: 267–272. 10.1016/j.dci.2008.06.016 [DOI] [PubMed] [Google Scholar]
- Rothkötter HJ, Kirchhoff T, Pabst R (1994) Lymphoid and non-lymphoid cells in the epithelium and lamina propria of intestinal mucosa of pigs. Gut 35: 1582–1589. 10.1136/gut.35.11.1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roura E, Koopmans SJ, Lallès JP, Le Huerou-Luron I, De Jager N, Schuurman T, Val-Laillet D (2016) Critical review evaluating the pig as a model for human nutritional physiology. Nutr Res Rev 29: 60–90. 10.1017/s0954422416000020 [DOI] [PubMed] [Google Scholar]
- Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I (1993) Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 75: 253–261. 10.1016/0092-8674(93)80067-o [DOI] [PubMed] [Google Scholar]
- Satoh-Takayama N, Lesjean-Pottier S, Vieira P, Sawa S, Eberl G, Vosshenrich CAJ, Di Santo JP (2010) IL-7 and IL-15 independently program the differentiation of intestinal CD3−NKp46+ cell subsets from Id2-dependent precursors. J Exp Med 207: 273–280. 10.1084/jem.20092029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh-Takayama N, Vosshenrich CAJ, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, et al. (2008) Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 29: 958–970. 10.1016/j.immuni.2008.11.001 [DOI] [PubMed] [Google Scholar]
- Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B (2000) Cxc Chemokine Receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med 192: 1553–1562. 10.1084/jem.192.11.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebzda E, Zou Z, Lee JS, Wang T, Kahn ML (2008) Transcription factor KLF2 regulates the migration of naive T cells by restricting chemokine receptor expression patterns. Nat Immunol 9: 292–300. 10.1038/ni1565 [DOI] [PubMed] [Google Scholar]
- Sedlak C, Patzl M, Saalmüller A, Gerner W (2014) IL-12 and IL-18 induce interferon-γ production and de novo CD2 expression in porcine γδ T cells. Dev Comp Immunol 47: 115–122. 10.1016/j.dci.2014.07.007 [DOI] [PubMed] [Google Scholar]
- Shan Q, Li X, Chen X, Zeng Z, Zhu S, Gai K, Peng W, Xue HH (2021) Tcf1 and Lef1 provide constant supervision to mature CD8+ T cell identity and function by organizing genomic architecture. Nat Commun 12: 5863. 10.1038/s41467-021-26159-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Hou S, Fang Q, Liu X, Liu X, Qi H (2018) PD-1 controls follicular T helper cell positioning and function. Immunity 49: 264–274.e4. 10.1016/j.immuni.2018.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiow LR, Rosen DB, Brdičková N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M (2006) CD69 acts downstream of interferon-α/β to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440: 540–544. 10.1038/nature04606 [DOI] [PubMed] [Google Scholar]
- Simoni Y, Fehlings M, Kløverpris HN, McGovern N, Koo SL, Loh CY, Lim S, Kurioka A, Fergusson JR, Tang CL, et al. (2017) Human innate lymphoid cell subsets possess tissue-type based heterogeneity in phenotype and frequency. Immunity 46: 148–161. 10.1016/j.immuni.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni Y, Newell EW (2017) Toward meaningful definitions of innate-lymphoid-cell subsets. Immunity 46: 760–761. 10.1016/j.immuni.2017.04.026 [DOI] [PubMed] [Google Scholar]
- Sinkora M, Butler JE (2016) Progress in the use of swine in developmental immunology of B and T lymphocytes. Dev Comp Immunol 58: 1–17. 10.1016/j.dci.2015.12.003 [DOI] [PubMed] [Google Scholar]
- Sinkora M, Butler JE (2009) The ontogeny of the porcine immune system. Dev Comp Immunol 33: 273–283. 10.1016/j.dci.2008.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkora M, Sinkorová J, Cimburek Z, Holtmeier W (2007) Two groups of porcine TCRγδ++ thymocytes behave and diverge differently. J Immunol 178: 711–719. 10.4049/jimmunol.178.2.711 [DOI] [PubMed] [Google Scholar]
- Sinkora M, Sinkorová J, Holtmeier W (2005) Development of gammadelta thymocyte subsets during prenatal and postnatal ontogeny. Immunology 115: 544–555. 10.1111/j.1365-2567.2005.02194.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkora M, Stepanova K, Butler JE, Francis D, Santiago-Mateo K, Potockova H, Karova K, Sinkorova J (2011) Ileal Peyer[R8S2Q1M7]s patches are not necessary for systemic B cell development and maintenance and do not contribute significantly to the overall B cell pool in swine. J Immunol 187: 5150–5161. 10.4049/jimmunol.1101879 [DOI] [PubMed] [Google Scholar]
- Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC (2013) Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol 14: 1285–1293. 10.1038/ni.2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedley D, Haider S, Durinck S, Pandini L, Provero P, Allen J, Arnaiz O, Awedh MH, Baldock R, Barbiera G, et al. (2015) The BioMart community portal: An innovative alternative to large, centralized data repositories. Nucleic Acids Res 43: W589–W598. 10.1093/nar/gkv350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, Shibata N, Grunberg S, Sinha R, Zahm AM, et al. (2012) Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science 336: 1321–1325. 10.1126/science.1222551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE, et al. (2013) Innate lymphoid cells—A proposal for uniform nomenclature. Nat Rev Immunol 13: 145–149. 10.1038/nri3365 [DOI] [PubMed] [Google Scholar]
- Stepanova K, Sinkora M (2013) Porcine γδ T lymphocytes can be categorized into two functionally and developmentally distinct subsets according to expression of CD2 and level of TCR. J Immunol 190: 2111–2120. 10.4049/jimmunol.1202890 [DOI] [PubMed] [Google Scholar]
- Stepanova K, Sinkora M (2012) The expression of CD25, CD11b, SWC1, SWC7, MHC-II, and family of CD45 molecules can be used to characterize different stages of γδ T lymphocytes in pigs. Dev Comp Immunol 36: 728–740. 10.1016/j.dci.2011.11.003 [DOI] [PubMed] [Google Scholar]
- Stoeckle C, Gouttefangeas C, Hammer M, Weber E, Melms A, Tolosa E (2009) Cathepsin W expressed exclusively in CD8+ T cells and NK cells, is secreted during target cell killing but is not essential for cytotoxicity in human CTLs. Exp Hematol 37: 266–275. 10.1016/j.exphem.2008.10.011 [DOI] [PubMed] [Google Scholar]
- Strober W, Ehrhardt RO (1993) Chronic intestinal inflammation: An unexpected outcome in cytokine or T cell receptor mutant mice. Cell 75: 203–205. 10.1016/0092-8674(93)80062-j [DOI] [PubMed] [Google Scholar]
- Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM, Hao Y, Stoeckius M, Smibert P, Satija R (2019) Comprehensive integration of single-cell data. Cell 177: 1888–1902.e21. 10.1016/j.cell.2019.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers KM, Bush SJ, Wu C, Su AI, Muriuki C, Clark EL, Finlayson HA, Eory L, Waddell LA, Talbot R (2020) Functional annotation of the transcriptome of the pig, Sus scrofa, based upon network analysis of an RNAseq transcriptional Atlas. Front Genet 10: 1355. 10.3389/fgene.2019.01355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson M, Marsal J, Ericsson A, Carramolino L, Brodén T, Márquez G, Agace WW (2002) CCL25 mediates the localization of recently activated CD8αβ+ lymphocytes to the small-intestinal mucosa. Clin Investig 110: 1113–1121. 10.1172/jci0215988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindle MM, Makin A, Herron AJ, Clubb FJ, Frazier KS (2011) Swine as models in biomedical research and toxicology testing. Vet Pathol 49: 344–356. 10.1177/0300985811402846 [DOI] [PubMed] [Google Scholar]
- Szabo PA, Levitin HM, Miron M, Snyder ME, Senda T, Yuan J, Cheng YL, Bush EC, Dogra P, Thapa P, et al. (2019. a) Single-cell transcriptomics of human T cells reveals tissue and activation signatures in health and disease. Nat Commun 10: 4706. 10.1038/s41467-019-12464-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo PA, Miron M, Farber DL (2019. b) Location, location, location: Tissue resident memory T cells in mice and humans. Sci Immunol 4: eaas9673. 10.1126/sciimmunol.aas9673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talayero P, Mancebo E, Calvo-Pulido J, Rodríguez-Muñoz S, Bernardo I, Laguna-Goya R, Cano-Romero FL, García-Sesma A, Loinaz C, Jiménez C, et al. (2016) Innate lymphoid cells groups 1 and 3 in the epithelial compartment of functional human intestinal allografts. Am J Transpl 16: 72–82. 10.1111/ajt.13435 [DOI] [PubMed] [Google Scholar]
- Talker SC, Käser T, Reutner K, Sedlak C, Mair KH, Koinig H, Graage R, Viehmann M, Klingler E, Ladinig A, et al. (2013) Phenotypic maturation of porcine NK- and T-cell subsets. Dev Comp Immunol 40: 51–68. 10.1016/j.dci.2013.01.003 [DOI] [PubMed] [Google Scholar]
- Tan Z, Wang Y, Yang T, Xing K, Ao H, Chen S, Zhang F, Zhao X, Liu J, Wang C (2017) Differentially expressed genes in the caecal and colonic mucosa of Landrace finishing pigs with high and low food conversion ratios. Sci Rep 7: 14886. 10.1038/s41598-017-14568-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toka FN, Nfon CK, Dawson H, Golde WT (2009) Accessory-cell-mediated activation of porcine NK cells by Toll-like receptor 7 (TLR7) and TLR8 agonists. Clin Vaccin Immunol 16: 866–878. 10.1128/cvi.00035-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA, Gapin L, Glimcher LH (2004) T-bet regulates the terminal maturation and homeostasis of NK and Vα14i NKT cells. Immunity 20: 477–494. 10.1016/s1074-7613(04)00076-7 [DOI] [PubMed] [Google Scholar]
- Tsuji M, Suzuki K, Kitamura H, Maruya M, Kinoshita K, Ivanov II, Itoh K, Littman DR, Fagarasan S (2008) Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity 29: 261–271. 10.1016/j.immuni.2008.05.014 [DOI] [PubMed] [Google Scholar]
- Uehara S, Grinberg A, Farber JM, Love PE (2002) A role for CCR9 in T lymphocyte development and migration. J Immunol 168: 2811–2819. 10.4049/jimmunol.168.6.2811 [DOI] [PubMed] [Google Scholar]
- Van Acker A, Gronke K, Biswas A, Martens L, Saeys Y, Filtjens J, Taveirne S, Van Ammel E, Kerre T, Matthys P, et al. (2017) A murine intestinal intraepithelial NKp46-negative innate lymphoid cell population characterized by group 1 properties. Cell Rep 19: 1431–1443. 10.1016/j.celrep.2017.04.068 [DOI] [PubMed] [Google Scholar]
- Van der Stede Y, Verdonck F, Verfaillie T, Goddeeris BM, Cox E (2005) Porcine-specific CpG-oligodeoxynucleotide activates B-cells and increases the expression of MHC-II molecules on lymphocytes. Vet Immunol Immunopathol 105: 115–124. 10.1016/j.vetimm.2004.12.020 [DOI] [PubMed] [Google Scholar]
- Vandenbon A, Diez D (2020) A clustering-independent method for finding differentially expressed genes in single-cell transcriptome data. Nat Commun 11: 4318. 10.1038/s41467-020-17900-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema WTU, Voskuil MD, Vila AV, van der Vries G, Jansen BH, Jabri B, Faber KN, Dijkstra G, Xavier RJ, Wijmenga C, et al. (2019) Single-cell RNA sequencing of blood and ileal T cells from patients with Crohn[R8S2Q1M7]s disease reveals tissue-specific characteristics and drug targets. Gastroenterology 156: 812–815.e22. 10.1053/j.gastro.2018.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, Nussenzweig MC (2010) Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell 143: 592–605. 10.1016/j.cell.2010.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Burg N, Chappaz S, Baerenwaldt A, Horvath E, Bose Dasgupta S, Ashok D, Pieters J, Tacchini-Cottier F, Rolink A, Acha-Orbea H, et al. (2014) Activated group 3 innate lymphoid cells promote T-cell–mediated immune responses. Proc Natl Acad Sci U S A 111: 12835–12840. 10.1073/pnas.1406908111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldschmidt TJ, Conrad DH, Lynch RG (1988) The expression of B cell surface receptors. I. The ontogeny and distribution of the murine B cell IgE Fc receptor. J Immunol 140: 2148–2154. [PubMed] [Google Scholar]
- Wang J, Chen L, Li P, Li X, Zhou H, Wang F, Li D, Yin Y, Wu G (2008) Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. J Nutr 138: 1025–1032. 10.1093/jn/138.6.1025 [DOI] [PubMed] [Google Scholar]
- Wang S, Xia P, Chen Y, Qu Y, Xiong Z, Ye B, Du Y, Tian Y, Yin Z, Xu Z, et al. (2017) Regulatory innate lymphoid cells control innate intestinal inflammation. Cell 171: 201–216.e18. 10.1016/j.cell.2017.07.027 [DOI] [PubMed] [Google Scholar]
- Wang X, Li S, Wu J, Ding R, Quan J, Zheng E, Yang J, Wu Z (2019. a) A transcriptome analysis identifies biological pathways and candidate genes for feed efficiency in DLY pigs. Genes 10: 725. 10.3390/genes10090725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Peng H, Tian Z (2019. b) Innate lymphoid cell memory. Cell Mol Immunol 16: 423–429. 10.1038/s41423-019-0212-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasowicz K, Winnicka A, Kaleczyc J, Zalecki M, Podlasz P, Pidsudko Z (2018) Neuropeptides and lymphocyte populations in the porcine ileum and ileocecal lymph nodes during postnatal life. PLoS One 13: e0196458. 10.1371/journal.pone.0196458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiarda JE, Trachsel JM, Bond ZF, Byrne KA, Gabler NK, Loving CL (2020) Intraepithelial T cells diverge by intestinal location as pigs age. Front Immunol 11: 1139. 10.3389/fimmu.2020.01139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willinger T, Freeman T, Herbert M, Hasegawa H, McMichael AJ, Callan MFC (2006) Human naive CD8 T cells down-regulate expression of the WNT pathway transcription factors lymphoid enhancer binding factor 1 and transcription factor 7 (T cell factor-1) following antigen encounter in vitro and in vivo. J Immunol 176: 1439–1446. 10.4049/jimmunol.176.3.1439 [DOI] [PubMed] [Google Scholar]
- Winkelmann R, Sandrock L, Porstner M, Roth E, Mathews M, Hobeika E, Reth M, Kahn ML, Schuh W, Jäck HM (2011) B cell homeostasis and plasma cell homing controlled by Krüppel-like factor 2. Proc Natl Acad Sci U S A 108: 710–715. 10.1073/pnas.1012858108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolock SL, Lopez R, Klein AM (2019) Scrublet: Computational identification of cell doublets in single-cell transcriptomic data. Cell Syst 8: 281–291.e9. 10.1016/j.cels.2018.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Ding J, Porter CBM, Wallrapp A, Tabaka M, Ma S, Fu S, Guo X, Riesenfeld SJ, Su C, et al. (2019) Transcriptional atlas of intestinal immune cells reveals that neuropeptide α-CGRP modulates group 2 innate lymphoid cell responses. Immunity 51: 696–708.e9. 10.1016/j.immuni.2019.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye BH, Cattoretti G, Shen Q, Zhang J, Hawe N, Waard RD, Leung C, Nouri-Shirazi M, Orazi A, Chaganti RSK, et al. (1997) The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type inflammation. Nat Genet 16: 161–170. 10.1038/ng0697-161 [DOI] [PubMed] [Google Scholar]
- Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa SI, Gruss P (1999) Development of peripheral lymphoid organs and natural killer cells depends on the helix–loop–helix inhibitor Id2. Nature 397: 702–706. 10.1038/17812 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Honda K, Shinkura R, Adachi S, Nishikawa S, Maki K, Ikuta K, Nishikawa SI (1999) IL-7 receptor α+ CD3– cells in the embryonic intestine induces the organizing center of Peyer[R8S2Q1M7]s patches. Int Immunol 11: 643–655. 10.1093/intimm/11.5.643 [DOI] [PubMed] [Google Scholar]
- Young MD, Behjati S (2020) SoupX removes ambient RNA contamination from droplet-based single-cell RNA sequencing data. GigaScience 9: giaa151. 10.1093/gigascience/giaa151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappia L, Oshlack A (2018) Clustering trees: A visualization for evaluating clusterings at multiple resolutions. GigaScience 7: giy083. 10.1093/gigascience/giy083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Zhang TX, Liu Y, Jia D, Zeng P, Du C, Yuan M, Liu Q, Wang Y, Shi FD (2021. a) B-cell compartmental features and molecular basis for therapy in autoimmune disease. Neurol Neuroimmunol Neuroinflamm 8: e1070. 10.1212/nxi.0000000000001070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhu J, Wang H, Xia J, Liu P, Chen F, Jiang H, Miao Q, Wu W, Zhang L, et al. (2021. b) A high-resolution cell atlas of the domestic pig lung and an online platform for exploring lung single-cell data. J Genet Genomics 48: 411–425. 10.1016/j.jgg.2021.03.012 [DOI] [PubMed] [Google Scholar]
- Zhao J, Zhang S, Liu Y, He X, Qu M, Xu G, Wang H, Huang M, Pan J, Liu Z, et al. (2020) Single-cell RNA sequencing reveals the heterogeneity of liver-resident immune cells in human. Cell Discov 6: 22. 10.1038/s41421-020-0157-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Chen F, Luo L, Wu W, Dai J, Zhong J, Lin X, Chai C, Ding P, Liang L, et al. (2021) Single-cell atlas of domestic pig cerebral cortex and hypothalamus. Sci Bull 66: 1448–1461. 10.1016/j.scib.2021.04.002 [DOI] [PubMed] [Google Scholar]
- Zhu LH, Xu JX, Zhu SW, Cai X, Yang SF, Chen XL, Guo Q (2014) Gene expression profiling analysis reveals weaning-induced cell cycle arrest and apoptosis in the small intestine of pigs. J Anim Sci 92: 996–1006. 10.2527/jas.2013-7551 [DOI] [PubMed] [Google Scholar]
- Ziegler A, Gonzalez L, Blikslager A (2016) Large animal models: The key to translational discovery in digestive disease research. Cell Mol Gastroenterol Hepatol 2: 716–724. 10.1016/j.jcmgh.2016.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Quality control metrics for scRNA-seq samples. (12.7KB, xlsx)
Table S8 Differential gene expression and biological process enrichment results in porcine ileal T/ILCs. (646.1KB, xlsx)
Table S9 Differential gene expression and biological process enrichment results in porcine ileal B/ASCs. (435.6KB, xlsx)
Table S11 Ensembl identifiers replaced with gene symbols in text and figures. (9.3KB, xlsx)
Data Availability Statement
Final data are available for download and direct query at https://singlecell.broadinstitute.org/single_cell/study/SCP1921/intestinal-single-cell-atlas-reveals-novel-lymphocytes-in-pigs-with-similarities-to-human-cells. Sequencing data are available under GEO accession GSE196388. Scripts for data analyses are available at https://github.com/USDA-FSEPRU/scRNAseq_Porcine_Ileum_PBMC. Seurat objects of processed data used for analyses are available for download and further query/analysis as .h5seurat files at https://data.nal.usda.gov/dataset/data-porcine-intestinal-innate-lymphoid-cells-and-lymphocyte-spatial-context-revealed-through-single-cell-rna-sequencing.