Introduction
Primary localized cutaneous nodular amyloidosis (PLCNA) is the rarest form of cutaneous amyloidosis with a predilection for acral sites.1 To our knowledge, PLCNA of the nail bed has not been reported. Here, we present a case of PCLNA arising from the left hallux nail bed and describe the dermoscopic features seen.
Case report
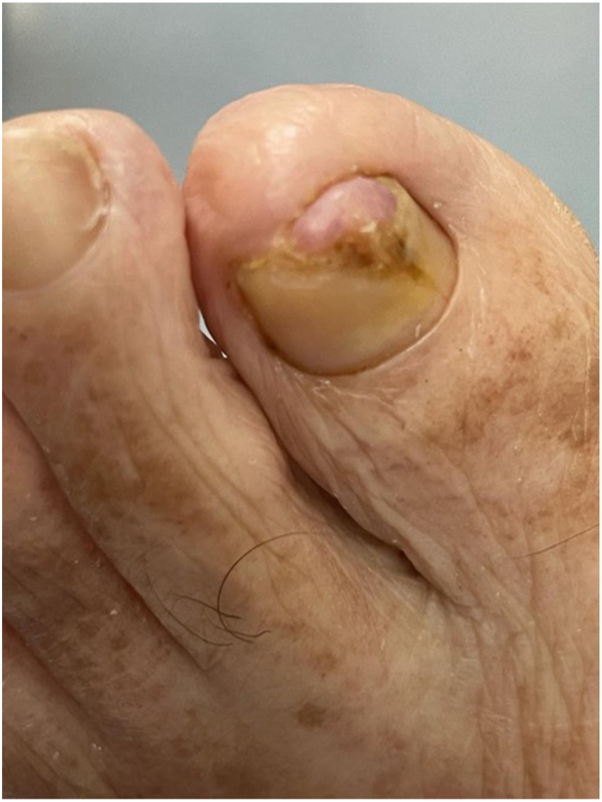
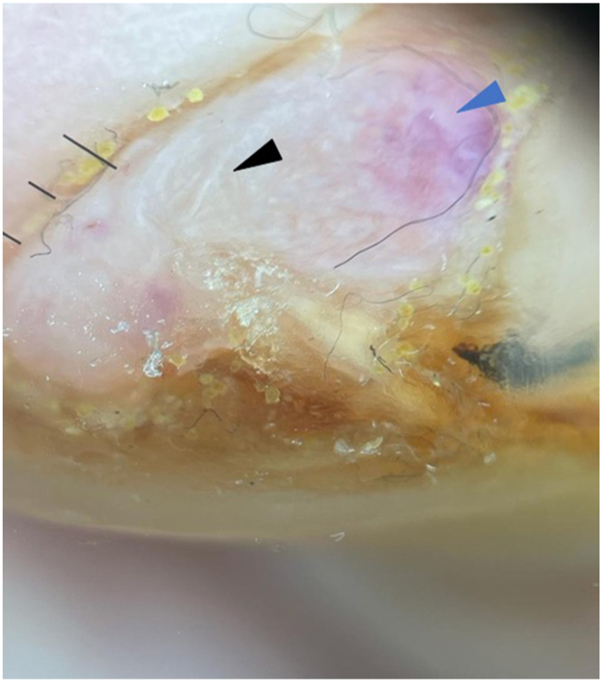
An 89-year-old male with a history of diabetes mellitus presented to us with 1 week of left hallux toenail swelling. He had initially presented to the podiatrist for a right foot injury and was incidentally noted to have a left hallux nail bed nodule. The patient was referred for further assessment of this nodule. Examination showed a 0.7 cm × 0.8 cm pink, irregular tender nodule over the distal left hallux nail bed with onycholysis and destruction of the overlying nail plate (Fig 1). Dermatoscope under polarized light (HEINE DELTAone) showed shiny white lines on a pink-orange background (Fig 2).
Fig 1.
Clinical presentation of a solitary, pink, irregular nodule over the left hallux nail bed with destruction of the overlying nail plate.
Fig 2.
Polarized dermatoscopic image showing shiny white lines (black arrowhead) on an orange-pink background (blue arrowhead) (HEINE DELTAone).
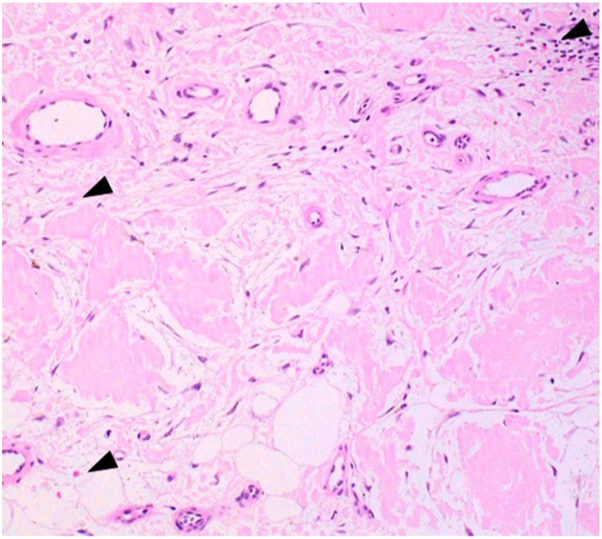
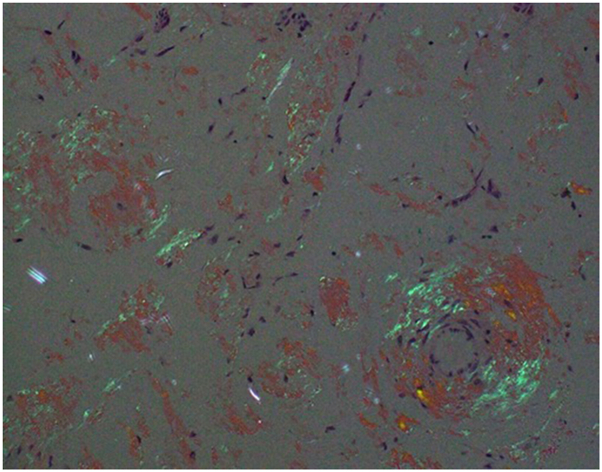
An excision biopsy was performed, and histopathology showed a nodular area of eosinophilic, amorphous extracellular deposits in the dermis, subcutaneous tissue, and blood vessel walls. Some lymphohistiocytic infiltration in the dermis was present (Fig 3). Congo red staining highlighted the extracellular deposits in salmon red color. Under polarization, these deposits demonstrated a bright apple-green birefringence appearance (Fig 4).
Fig 3.
Hematoxylin-eosin–stained skin biopsy specimen showed presence of eosinophilic, amorphous deposition in the stroma and within vessel walls. Congo red stain showed salmon pink deposits in the dermis (black arrowheads) (magnification ×200).
Fig 4.
Under polarization, the Congo red-stained skin biopsy specimen showed a bright apple-green birefringence appearance (magnification ×200).
The patient did not demonstrate any other features of systemic amyloidosis. His serum creatinine, serum and urine protein electrophoresis, and immunofixation results were normal. The diagnosis of PLCNA was established based on clinical and histological features and the absence of systemic manifestations of amyloidosis.
Case discussion
We describe a case of PLCNA arising from the nail bed. This rare condition has a predilection for the face and acral sites. Among the published cases, there have been multiple case reports of lesions on the lower limbs as well as the fourth toe web space,2 but nail bed involvement has not been reported. A list of conditions that can present as a nail bed nodule (and their associated dermoscopic features) is summarized in Table I.
Table I.
Differential diagnosis of nail bed nodules and their dermatoscope findings
| Differential diagnosis of nail bed nodules | Dermatoscope findings |
|---|---|
| Glomus tumor | Discrete linear vascular structures Ramified telangiectasia on nail bed and matrix Pinkish or bluish hue |
| Onychomatrichoma | Longitudinal parallel white lines Splinter hemorrhages Free-edge nail pitting Thickening of the free edge |
| Amelanotic melanoma | Vessels: dotted, hairpin, irregular-linear, polymorphous Pink areas or globules Multiple blue-gray dots Shiny white lines Blue-white veil |
| Squamous cell carcinoma | White, yellow, light brown structureless areas White circles Vessels: glomerular, hairpin, irregular-linear Ulceration |
There is limited literature regarding the dermoscopic findings of PLCNA. Chuang et al3 reported that dermatoscope could be a useful tool to aid in the diagnosis of primary cutaneous amyloidosis and presented the dermoscopic findings of 35 patients with PCA. However, none of them had nodular amyloidosis.
It is interesting to note that our patient had dermoscopic findings of shiny white lines and an orange-pink background, similar to the case presented by Rongioletti et al.4 Sonagara et al5 postulated that the yellowish-orange background may be correlated with deposits of amorphous material within the deep dermis and subcutaneous tissue. Similar conclusions were made by Atzori et al,6 explaining the yellow-orange roundish structures described in their case reports. This is most likely the reason for the orange-pink background in our case (Fig 2), as confirmed by the presence of amorphous eosinophilic deposition in the stroma and within vessel walls (Figs 3 and 4). Skin lesions with an altered composition or orientation of collagen will often reveal shiny white lines under polarized dermatoscope.7 We postulate that this is the same reason we see shiny white lines in our case (Fig 2). Some authors4,6 described hemorrhagic changes and telangiectasias, but these findings were not present in our patient.
Several treatment options have been reported for PLCNA, but there is no consensus on the most effective treatment option.1 Proposed treatment options include surgical excision, electrodesiccation and curettage, carbon dioxide laser, and pulsed dye laser therapy.1,8 Our patient underwent surgical excision of the lesion with good nail bed healing on review 2 months later.
To our knowledge, this is the first reported case of primary cutaneous amyloidosis at the nail bed. This case demonstrates that nodular amyloidosis should be considered as a differential diagnosis for nodules arising from the nail bed and dermatoscope may be a useful aid in the diagnosis. Our case adds to the growing literature on the dermoscopic findings of PLCNA, but further studies will be required to establish a definite correlation between clinical, dermoscopic, and histopathological features of PLCNA.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Woollons A., Black M.M. Nodular localized primary cutaneous amyloidosis: a long-term follow-up study. Br J Dermatol. 2001;145(1):105–109. doi: 10.1046/j.1365-2133.2001.04291.x. [DOI] [PubMed] [Google Scholar]
- 2.Ferreira I.L.O., Fernandes E.L., Lapins J., et al. Primary localised cutaneous nodular amyloidosis on a toe: clinical presentation, histopathology and dermoscopy findings. Dermatol Pract Concept. 2019;9(3):235–236. doi: 10.5826/dpc.0903a18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chuang Y.Y., Lee D.D., Lin C.S., et al. Characteristic dermoscopic features of primary cutaneous amyloidosis: a study of 35 cases. Br J Dermatol. 2012;167(3):548–554. doi: 10.1111/j.1365-2133.2012.11066.x. [DOI] [PubMed] [Google Scholar]
- 4.Rongioletti F., Atzori L., Ferreli C., et al. A unique dermoscopy pattern of primary cutaneous nodular amyloidosis mimicking a granulomatous disease. J Am Acad Dermatol. 2016;74(1):e9–e10. doi: 10.1016/j.jaad.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 5.Sonagara B., Mehta H., Gajjar P. Dermoscopy of localized cutaneous nodular amyloidosis resembling granulomatous disorders. Indian J Dermatopathol Diagn Dermatol. 2019;6:104–106. doi: 10.4103/ijdpdd.ijdpdd_74_18. [DOI] [Google Scholar]
- 6.Atzori L., Ferreli C., Caterina M.C., et al. Primary localised cutaneous nodular amyloidosis and limited cutaneous systemic sclerosis: additional cases with dermatoscopic and histopathological correlation of amyloid deposition. Dermatopathology (Basel) 2021;8(3):229–235. doi: 10.3390/dermatopathology8030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold S.J., Bowling J.C.R. ‘Shiny white streaks’ in lichen amyloidosis: a clue to diagnosis. Australas J Dermatol. 2012;53(4):272–273. doi: 10.1111/j.1440-0960.2011.00871.x. [DOI] [PubMed] [Google Scholar]
- 8.Weidner T., Illing T., Elsner P. Primary localised cutaneous amyloidosis: a systemic treatment review. Am J Clin Dermatol. 2017;18(5):629–642. doi: 10.1007/s40257-017-0278-9. [DOI] [PubMed] [Google Scholar]