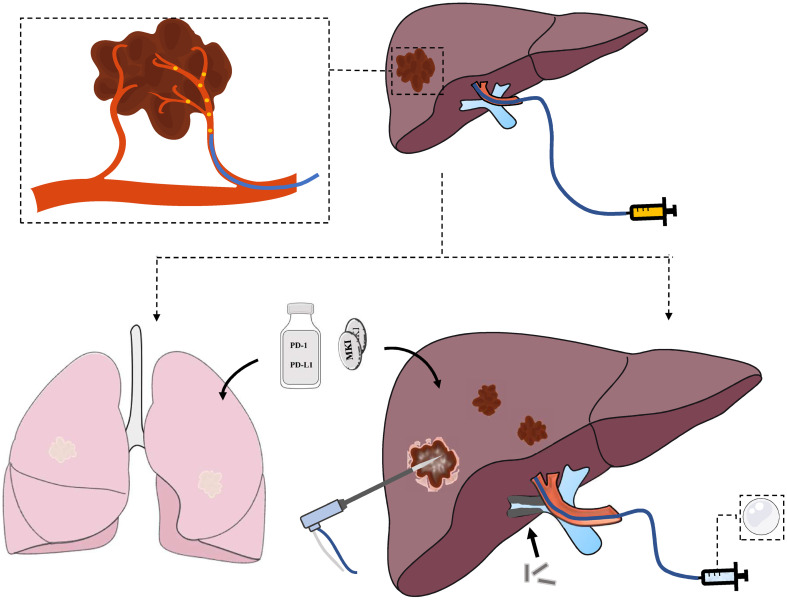
Abstract
Transarterial chemoembolization (TACE) is widely applied for the treatment of hepatocellular carcinoma. Repeat TACE is often required in clinical practice because a satisfactory tumor response may not be achieved with a single session. However, repeated TACE procedures can impair liver function and increase treatment-related adverse events, all of which prompted the introduction of the concept of “TACE failure/refractoriness”. Mainly based on evidence from two retrospective studies conducted in Japan, sorafenib is recommended as the first choice for subsequent treatment after TACE failure/refractoriness. Several studies have investigated the outcomes of other subsequent treatments, including locoregional, other molecular targeted, anti-programmed death-1/anti-programed death ligand-1 therapies, and combination therapies after TACE failure/refractoriness. In this review, we summarize the up-to-date information about the outcomes of several subsequent treatment modalities after TACE failure/refractoriness.
Keywords: Transarterial chemoemboization, Failure, Refractoriness, Subsequent treatment
Graphical abstract
Introduction
Transarterial chemoembolization (TACE) plays a fundamental role in the treatment of unresectable hepatocellular carcinoma (HCC), either as a palliative approach for unresectable condition, as bridging therapy prior to liver transplantation, or for tumor downstaging prior to surgical resection.1–3 According to the global HCC BRIDGE (i.e. Bridge to Better Outcomes in HCC) study involving 18,031 patients in 14 countries, TACE is the most widely applied approach both for intermediate- and advanced-stage HCC.4 Nevertheless, the prognosis of HCC treated with TACE varies because of high patientheterogeneity of patients and biological characteristics of HCC.5–7 Repeat TACE is often recommended because it is sometimes difficult to achieve a satisfactory tumor response with a single session.8 Nonetheless, high frequency and number of repeat TACE procedures can impair liver function and increase treatment-related adverse events, negating the benefits achieved from tumor necrosis. Meticulous assessment of the risks and benefits of repeat TACE is warranted to improve the long-term outcomes of TACE for HCC. The concept of TACE failure/refractoriness was subsequently introduced by several organizations including the Japan Society of Hepatology (JSH), the International Association for the Study of the Liver, and a European expert panel (Table 1).9–11
Table 1. Concepts of TACE failure/refractoriness.
| Organization | Definition |
|---|---|
| JSH criteria, 2010 | (1) Intrahepatic lesion: more than two consecutive incomplete necrosis (depositions ( 50%) of lipiodol) are seen by response evaluation CT within the treated tumors at the 4 weeks after adequately performed TACE; more than two consecutive appearances of a new lesion (recurrence) are seen in the liver by response evaluation CT at the 4 weeks after adequately performed TACE. (2) Appearance of vascular invasion. (3) Appearance of extrahepatic spread continuous elevation of tumor markers even though right after TACE. (4) Tumor marker continuous elevation of tumor markers even though right after TACE |
| JSH–LCSGJ criteria, 2014 | (1) Intrahepatic lesion: two or more consecutive insufficient responses of the treated tumor (viable lesion >50%) even after changing the chemotherapeutic agents and/or reanalysis of the feeding artery seen on response evaluation CT/MRI at 1–3 months after having adequately performed selective TACE; Two or more consecutive progressions in the liver (tumor number increases as compared with tumor number before the previous TACE procedure) even after having changed the chemotherapeutic agents and/or reanalysis of the feeding artery seen on response evaluation CT/MRI at 1–3 months after having adequately performed selective TACE. (2) Continuous elevation of tumor makers immediately after TACE even though slight transient decrease is observed. (3) Appearance of vascular invasion. (4) Appearance of extrahepatic spread |
| International Association for the Study of the Liver | No response after 3 or more TACE procedures within a 6 month period, to the same area |
| Europe | Depending on the purpose of TACE, if TACE is used as palliative therapy, stable lesions can be regarded as effective. Conversely, if TACE is used as a curative therapy, stable lesions are considered as TACE failure |
CT, computed tomography; JSH, Japan Society of Hepatology; LCSGJ, Liver Cancer Study Group of Japan; MRI, magnetic resonance imaging; TACE, transarterial chemoembolization.
Appropriate subsequent treatment after TACE failure/refractoriness is another key point to improve long-term prognosis of patients with HCC. The current TACE failure/refractoriness guidelines recommend sorafenib therapy after occurrence of TACE failure/refractoriness. Notably, this recommendation was mainly based on the results of two retrospective studies with small sample sizes.12,13 Apart from those two studies, several others have investigated the outcomes of other subsequent treatments, including locoregional therapies, other molecular targeted agents (MTAs), anti-programmed death ligand-1/anti-programmed death-1 therapies, and combination therapies after TACE failure/refractoriness. Therefore, we reviewed the available evidence about the efficacy and safety of subsequent therapies after TACE failure/refractoriness in the treatment of HCC.
Search strategy and selection criteria
A comprehensive literature search was performed on the PubMed and Web of Science databases for relevant studies published in English language through April 2021. The search terms were: (“transarterial chemoembolization” OR “transcatheter arterial chemoembolization” OR “TACE”) AND (“failure” OR “refractoriness” OR “refractory”). Only original research articles that focused on the subsequent treatments after TACE failure/refractoriness were included. Duplicate publications, reviews, case reports, conference abstracts, and studies published in languages other than English were excluded.
Subsequent treatment and prognosis after TACE failure/refractoriness
After the introduction of the concept of TACE failure/refractoriness in 2010 by the JSH, several studies have investigated the outcomes of subsequent treatments after TACE failure/refractoriness. A total of 23 studies were finally included in the review (Table 2).12–34 Among them, seven reported outcomes of subsequent treatments without comparison to other treatments. Eight compared the outcomes of sorafenib therapy as subsequent treatment with those of other treatments. Eight studies compared the outcomes of continuation of TACE in combination with systemic therapies with those of other treatments.
Table 2. Studies of treatment and prognosis subsequent to TACE failure/refractoriness.
| StudyRef | Patients, n | BCLC stage | Definition of TACE failure/refractoriness | Subsequent treatment | Median OS (95% CI), months; p-value |
|---|---|---|---|---|---|
| Iwasa et al., 201114 | 84 | C | An increase in size or 25% reduction in size of the hypervascular lesions 1 month after TACE | TAI with cisplatin | – |
| Song et al., 201316 | 10 | A/B | More than two consecutive incomplete necrosis (depositions <50% of lipiodol) | DEB-TACE | 22.2 (N/A) |
| Ikeda et al., 201421 | 114 | N/A | Progression or a tumor shrinkage rate of <25% of the hypervascular lesions 1–3 months after TACE | sorafenib vs. hepatic arterial infusion chemotherapy | 16.4 (N/A) vs. 8.6 (N/A); p<0.01 |
| Ogasawara et al., 201413 | 56 | B | 2010 JSH criteria | sorafenib vs. continued TACE | 25.4 (9.3–41.5) vs. 11.5 (8.3–14.8); p=0.003 |
| Arizumi et al., 201512 | 56 | B | 2014 JSH–LCSGJ criteria | sorafenib vs. continued TACE | 24.7 (17.16–54.7) vs. 13.6 (8.96–17.43); p=0.002 |
| Hatooka et al., 201623 | 96 | B/C | 2010 JSH criteria | HAIC vs. sorafenib | 8 (N/A) vs. 15 (N/A); p=0.021 |
| Huang et al., 201632 | 26 | B | Disease progression or shrinkage of <25% in hypervascular tumor lesions after 1–2 cycles of TACE | S–1 chemotherapy plus TACE vs. TACE monotherapy | 17 (15.6–18.4) vs. 15 (9.2–20.8); p=0.549 |
| Huang et al., 201631 | 26 | B | Disease progression or shrinkage of <25% in hypervascular tumor lesions after 1–2 cycles of TACE | S–1 chemotherapy plus TACE vs. TACE monotherapy | 18 (15.3–24.7) vs. 13 (9.8–16.2); p=0.040 |
| Wu et al., 201728 | 61 | C | No details presented | TACE plus sorafenib vs. TACE | 17.9 (N/A) vs. 7.1 (N/A); p<0.001 |
| Klompenhouwer et al., 201718 | 30 | B/C | Progression or stable disease after one or more sessions of DEB-TACE | TARE | 14.8 (8.33–26.5) |
| Kodama et al., 201822 | 152 | N/A | 2014 JSH–LCSGJ criteria | HAIC vs. sorafenib | 7 vs. 7 for MVI positive; p=0.6710.5 vs. 20 for MVI negative; p=0.001 |
| Qiu et al., 201933 | 58 | B/C | 2014 JSH–LCSGJ criteria | TACE plus apatinib vs. TACE | 17.0 (12.0–22.0) vs. 10.7 (6.4–15.0); p=0.027 |
| Lin et al., 202029 | 66 | A/B/C | Progressive disease after two consecutive of transarterial chemoembolization treatment within 6 months | TACE plus sorafenib vs. TACE | 23.1 (N/A) vs. 11.0 (N/A); p=0.001 |
| Chen et al., 202026 | 44 | B | No less than 2 consecutive ineffective responses of treated tumors (necrotic lesion <50%) or one ineffective response of treated tumors (necrotic lesion <25%) or tumor number increased | MWA vs. sorafenib | Not reached vs. 16.6 (13.4–19.8); p=0.001 |
| Kim et al., 202019 | 60 | A/B/C | No details presented | balloon-occluded TACE | Median TTP: 5.3 (4.0–6.9) |
| Yoo et al., 202024 | 94 | B/C | 2014 JSH–LCSGJ criteria | TACE plus chemotherapy vs. sorafenib | 6.4 (2.9–9.9) vs. 4.1 (2.6–5.6); p=0.355 |
| Shimose et al., 202027 | 171 | B | 2014 JSH–LCSGJ criteria | lenvatinib vs. sorafenib vs. TACE | Median PFS: 5.8 vs. 3.2 vs. 2.8; p=0.01, p<0.001 |
| Villani et al., 202125 | 76 | B | Development of new intrahepatic lesions, the appearance of vascular invasion, the appearance of extrahepatic spread after 3 months from TACE session | continue TACE vs. sorafenib | 10.6 (2.3–14.0) vs. 9.5 (1.7–12.3); p=0.72 |
| Kobayashi et al., 202017 | 27 | B/C | 2014 JSH–LCSGJ criteria | DEB-TACE | 16.3 (8.6–24.0) |
| Zheng et al., 202134 | 51 | B/C | 2014 JSH–LCSGJ criteria | TACE+ICIs+sorafenib vs. TACE+sorafenib | 23.3 (17.56–29.07) vs. 13.8 (9.11–18.50); p=0.012 |
| Kaibori et al., 202130 | 70 | B | 2014 JSH–LCSGJ criteria | TACE+sorafenib vs. TACE | 20.5 vs. 15.4; p=0.009 |
| Hsu et al., 202115 | 87 | C | 2014 JSH–LCSGJ criteria | HAIC | 9.0 (7.6–10.4) |
| Xu et al., 202120 | 19 | A/B | 2014 JSH–LCSGJ criteria | 125I brachytherapy | Median TTP: 8.8 |
BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; DEB-TACE, drug-eluting bead transarterial chemoembolization; HAIC, hepatic arterial infusion chemotherapy; ICIs, immune checkpoint inhibitors; JSH, Japan Society of Hepatology; LCSGJ, Liver Cancer Study Group of Japan; MWA, microwave ablation; OS, overall survival; PFS, progression-free survival; TACE, transarterial chemoembolization; TAI, transcatheter arterial infusion; TARE, transarterial radioembolization; TTP, time to progression.
Subsequent treatments without comparison
Iwasa et al.14 administered transcatheter arterial infusion chemotherapy with a fine-powder formulation of cisplatin for advanced HCC after TACE failure/refractoriness. Notably, the criteria for TACE failure/refractoriness in their study was an increase in size or 25% reduction in the size of hypervascular lesions 1 month after TACE, which was different from the 2010 JSH criteria. The study included 84 patients, and the median overall survival (OS) was 7.1 months. The authors reported only a modest effect of transcatheter arterial infusion chemotherapy using cisplatin, a median progression-free survival (PFS) of only 1.7 months and an objective response rate (ORR) of 3.6%. Besides, adverse events (AEs) occurred frequently during the treatment, and nearly 50% patients experienced grade 3/4 elevations of liver enzymes. Recently, Hus et al.15 reported the outcomes of hepatic arterial infusion chemotherapy (HAIC) with a modified FOLFOX regimen in 87 patients with advanced HCC after TACE failure/refractoriness, based on 2014 JSH–LCSGJ criteria. The majority of patients in the cohort had multinodular HCC and large tumors with a mean diameter of largest tumors of 7.1 cm. The OS, PFS, and time to tumor progression (TTP) were up to 9 months, 3.7 months, and 4.1 months, respectively. However, approximately 29% (25/87) of patients received concurrent treatment with MTAs and the related information was not reported, which may have introduced an element of bias resulting in weaker efficacy of HAIC monotherapy.
In addition, various locoregional therapies including different types of TACE or percutaneous therapies have been investigated. A small pilot study investigated the efficacy of TACE with DC beads (DCB-TACE) in 10 patients with solitary HCC nodules that were insensitive to lipiodol-TACE.16 All tumor nodules responded to DCB-TACE at the 1 month follow-up, and the median TTP was 7.8 months. Unfortunately, the end point of DCB-TACE was not reported and eight of the 10 patients received additional treatments such as HAIC and radiation therapy. Kobayashi et al.17 explored the potential of Hepasphere Microspheres for HCC refractory to conventional TACE following the 2014 JSH–LCSGJ criteria. The targeted tumors had a good response to Hepasphere-TACE, with an ORR of 19.1% and a disease control rate (DCR) of 76.4%. The median PFS and OS in the study were 2.9 and 16.3 months, respectively. Besides, grade 3/4 AEs were not documented, and liver function was preserved during TACE. Transarterial radioembolization (TARE) for HCC after drug-eluting beads TACE (DEB-TACE) failure/refractoriness was reported in 2017.18 The majority of patients (22/30) in the cohort had multinodular HCCs, and the mean diameter of largest tumor was 4.1 cm. The median OS after first TARE was 14.8 months and the safety profile was acceptable. In addition, eleven patients (36.7%) had partial responses and three (10%) were downstaged within the Milan criteria and subsequently received liver transplants. Kim et al.19 reported the outcomes of balloon-occluded TACE for multinodular HCC after conventional TACE. The study included 60 patients with Barcelona Clinic Liver Cancer A/B/C stages and a mean tumor diameter of 3 cm. The primary efficacy outcome of the study was TTP and not OS. The median TTP was 5.3 months and the major complication rate was only 6.7% (4/60). At the first follow-up, tumor response was achieved in all 60 patients, among whom 45 (75%) had complete responses and 15 (25%) had partial responses. Xu et al.20 reported computed tomography-guided Iodine-125 (125I) seed implantation for the treatment of HCC after TACE failure/refractoriness. All 21 patients in their cohort had solitary HCC nodules with a mean diameter of 4.3 cm. The median TTP was 8.8 months and the 6 month ORR of the targeted tumors was 90.5%. The findings suggested that aggressive locoregional therapies, including 125I brachytherapy and thermal ablation can achieve favorable outcomes in residual HCC nodules ≤5 cm, as tumors of that size tend to undergo complete necrosis with minimal postoperative liver damage. However, all were single-arm studies with small sample sizes. Larger studies are required to provide more robust evidence.
Switching to sorafenib and comparison with other treatments
During the past 10 years, sorafenib has been used as the standard treatment for advanced HCC with preserved liver function. Whether it is also the ideal treatment for patients after TACE failure/refractoriness is of considerable interest to the researchers. Ikeda et al.21 compared the efficacy of sorafenib therapy (n=48) with that of HAIC using cisplatin (n=66) in a study of 114 patients with HCC after TACE failure/refractoriness. They defined TACE failure/refractoriness as tumor progression or a tumor shrinkage rate of <25% for hypervascular lesions 1–3 months after TACE. The median OS in the sorafenib group was significantly longer than that in the HAIC group (16.4 vs. 8.6 months; p<0.01). The corresponding DCRs were 60.4% vs. 28.8% (p=0.001) and the median TTPs were 3.9 vs. 2.0 months (p<0.01), which were also significantly better in the sorafenib group. The study results favored sorafenib over HAIC as the subsequent treatment after TACE failure/refractoriness. A large study by Kodama et al.22 compared sorafenib and HAIC as subsequent treatment after TACE failure/refractoriness based on the 2014 JSH–LCSGJ criteria. Patients were divided into two groups by their macroscopic vascular invasion (MVI) status. In the MVI-positive group, the median OS was not significantly different between patients receiving sorafenib and HAIC (7 months in both groups, p=0.67). In the MVI-negative group, the median OS of patients receiving sorafenib was significantly longer than that of patients receiving HAIC (20 months vs. 10.5 months, p=0.001). The authors concluded that sorafenib may be a better choice compared to HAIC for HCC with MVI after TACE failure/refractoriness. Notably, Hatooka et al.23 found that HAIC tended to cause vascular damage and led to drug resistance compared with sorafenib, thereby resulting in a shorter time to treatment failure and shorter median OS for patients with TACE failure/refractoriness. Yoo et al.24 compared the effectiveness of conventional TACE combined with systemic infusion of 5-fluorouracil and sorafenib. Of the 94 patients with unresectable HCC who were refractory to DEB-TACE, 49 received transarterial infusion of epirubicin and cisplatin in a mixture of 5 to 10 mL of iodized oil without gelatin sponge embolization, followed by systemic infusion of 5-FU (200 mg/m2) for 12 h. Although patients treated with TACE combined with systemic chemotherapy had longer median OS, the between-group difference was not statistically significant (6.4 vs. 4.1 months; p=0.355). Notably, grade 3/4 AEs occurred more frequently in the sorafenib group than in the combination group (p=0.024). The authors concluded that TACE combined with HAIC may be a better alternative for patients who experience TACE failure/refractoriness along with sorafenib intolerance. However, more definitive evidence is required to support the efficacy and safety of combination therapy.
Ogasawara et al.13 and Arizumi et al.12 performed similar retrospective studies that compared the treatment efficacy of sorafenib monotherapy and continued TACE for intermediate HCC after TACE failure/refractoriness in 2014 and 2015, respectively. Both studies demonstrated the superiority of sorafenib monotherapy over continued TACE with respect to survival outcomes and preservation of liver function. The study by Ogasawara et al.13 included 56 patients, and approximately 80% patients had ≥3 tumor nodules. Compared with patients who received continued TACE, patients in the sorafenib group (n=36) had significantly longer median OS (25.4 vs. 11.5 months; p=0.003). Simultaneously, time to liver dysfunction, defined as the period from judgment as TACE-refractory until diagnosis of Child-Pugh C disease, was significantly longer in the sorafenib group (29.8 vs. 17 months; p=0.030). A study by Arizumi et al.12 included 32 patients who switched to sorafenib and 24 patients who continued to receive TACE. The median OS in the sorafenib group was significantly longer than that in the TACE group (24.7 vs. 13.6 months; p=0.002). At the 6 month follow-up, the TACE group experienced more serious deterioration of liver function (p=0.005). Nevertheless, as information related to tumor characteristics was not reported, it is debatable whether the difference of OS was related to the between-group differences in the baseline characteristics of patients. Additionally, the median treatment time in the sorafenib group was only 4.13 months, and the survival time after tumor progression was up to 20 months. The long treatment time after tumor progression may have challenged the efficacy of sorafenib. Recently, Villani et al.25 reported results that conflicted with those of the two aforementioned studies. Their study included 76 elderly patients ≥65 years of age with intermediate-stage HCC after TACE failure/refractoriness. The median OS of the two groups were comparable, with an OS of 9.5 months for sorafenib and 10.6 months for continued TACE (p=0.72). The corresponding 1 year survival rates were 43.6% and 32% (p=0.12). Interestingly, microwave ablation (MWA) had better outcomes than sorafenib for viable residual nodules that were insensitive to TACE. Chen et al.26 compared the outcomes of sorafenib and MWA for intermediate HCC with tumor sizes ≤7 cm and tumor numbers ≤5 after TACE failure/refractoriness. The study included 52 patients and after one-to-one propensity score matching (PSM), 22 pairs were enrolled for further analysis. Median OS in the MWA group was significantly longer than that in the sorafenib group both before (48.8 vs. 16.6 months, p=0.001) and after (not reached vs. 16.6 months; p=0.001) PSM. In spite of the favorable OS, the safety of MWA as well as tumor response was not documented in the study.
The REFLECT trial demonstrated the efficacy and safety of lenvatinib for advanced HCC.35 Subsequently, it has been applied as another first-line treatment for advanced HCC. Shimose et al.27 compared sorafenib (n=53), lenvatinib (n=45), and continued TACE (n=73) for intermediate HCC after TACE failure/refractoriness. The definition of TACE failure/refractoriness was based on the 2014 JSH–LCSGJ criteria, and 84% of patients (144/171) were beyond the up-to-seven criteria. The median PFS was significantly longer in the lenvatinib group (5.8 months) than in the sorafenib (3.2 months), and continued TACE (2.8 months) groups (p<0.001). The up-to-seven criteria, albumin-bilirubin (ALBI) grade and treatment modality were prognostic factors for PFS. Building upon the positive outcomes of previous studies, molecular targeted-agent monotherapy may be a good alternative for patients who have TACE failure/refractoriness along with a heavy tumor burden.36
Continued TACE combined with systemic therapies compared with other treatments
Although many randomized controlled trials in addition to the TACTICS trial, failed to demonstrate the efficacy of combination therapy with TACE and sorafenib for intermediate-stage HCC, additional sorafenib administration to patients with TACE refractoriness may potentially improve the prognosis.37–39 In a study by Wu et al.28 TACE-refractory patients treated with TACE plus sorafenib had significantly better median OS than TACE monotherapy, (17.9 vs. 7.1 months, p<0.001). Similarly, the combination treatment group had significantly better median TTP (9.3 vs. 3.4 months, p<0.001). The toxicity of sorafenib was effectively mitigated by lowering the drug dose; only two patients in the combination group experienced severe AEs. A study by Lin et al.29 also supported the efficacy of additional sorafenib administration for patients with TACE refractoriness, with a median OS of 23.1 months with combination therapy and 11.0 months with TACE monotherapy (p=0.001). Furthermore, by analyzing the characteristics of the 202 TACE-refractory patients in the study, a tumor number >3, tumor size ≥5 cm, bilateral tumor extent, or baseline AFP level ≥200 mg/dL were identified as risk factors for TACE refractoriness. Early initiation of combination therapy was recommended for such high-risk patients. In a recent study that supported combination therapy with TACE and sorafenib for intermediate HCC after TACE failure/refractoriness, additional sorafenib administration increased the interval between two TACE sessions, prolonged the maintenance of liver function, prolonged the time to extrahepatic spread, and improved. the transition to post-treatments.30
Huang et al.31,32 performed two similar studies comparing the treatment outcomes of TACE monotherapy and TACE combined with S-1 chemotherapy. Both studies included 26 patients with intermediate HCC, and TACE failure/refractoriness was defined as disease progression or shrinkage of <25% in hypervascular tumor lesions after 1–2 cycles of TACE. Combination treatment had significantly better median OS (18 vs. 13 months; p=0.040) in one of the two studies.31 Interestingly, in the other study, the median OS was not significantly different between the two treatments (17 months for TACE plus S-1 and 15 months for TACE monotherapy, p=0.549).32 The difference in the last day of follow-up may be responsible for the difference clinical outcomes. Qiu et al.33 investigated the treatment outcomes of concurrent apatinib administration for unresectable HCC after TACE failure/refractoriness. A total of 58 patients were included and TACE failure/refractoriness was defined following the 2014 JSH–LCSGJ criteria. Patients treated with TACE combined with apatinib had significantly better median OS compared with those treated with TACE monotherapy (17.0 vs. 10.7 months; p=0.027). Similar results were observed with respect to median PFS (7 vs. 2 months; p<0.001). Three patients in the combination group experienced severe AEs and the remaining 39 experienced a series of apatinib-related AEs that were relieved by symptomatic treatment or dosage reduction. The positive outcomes of combination treatment using apatinib instead of sorafenib were consistent with previous studies that investigated the use of sorafenib as systemic therapy.
Recently, Zheng et al.34 reported the efficacy and safety of TACE combined with immune checkpoint inhibitor (ICI) therapy and sorafenib for patients with TACE failure/refractoriness. A total of 51 patients with unresectable HCC who were refractory to TACE were included and were assigned to treatment with TACE combined with sorafenib (n=29), or TACE combined with sorafenib plus an ICI, either pembroizumab (n=10) or nivolumab (n=12). Patients in the triple combination treatment group had significantly better treatment efficacy compared with those in the TACE combined with sorafenib group, with longer median OS (23.3 vs. 13.8 months; p=0.012), better DCR (81.82% vs. 55.17%; p=0.046), and longer median PFS (16.26 vs. 7.30 months; p<0.001). However, there was no significant difference in median OS or PFS between patients receiving different ICIs. Four patients in the triple combination group and three patients in the TACE combined with sorafenib group required dose reduction or treatment interruption (18.18% vs. 10.34%, p=0.421). The incidence of AEs was comparable in both groups. The study was the first attempt to explore treatment outcomes of TACE combined with recently recommended systemic therapy with ICIs combined with a molecular agent for HCC refractory to TACE. Further studies are required to draw more definitive conclusions.
Discussion
In spite of the multitude of studies of TACE failure/refractoriness, standardized treatment recommendations for patients with TACE failure/refractoriness have not yet been developed. That is largely attributable to two main reasons. Firstly, there is no clear consensus on the definition of TACE failure/refractoriness; a widely-accepted definition is expected to guide study designs and protocols. Notably, seven of the 23 retrieved studies used local tumor response as the sole criterion for judging TACE failure/refractoriness, which may be attributable to the locoregional nature of TACE. It seems to be well-accepted that insufficient radiologic response of treated tumors after repeat TACE sessions is one of the unambiguous definitions of TACE failure/refractoriness (Fig. 1). However, whether portal vein tumor thrombosis, extrahepatic spread, and new lesion(s) are TACE endpoints is still under debate. Secondly, the definition of TACE failure/refractoriness incorporates many situations and considerable patient heterogeneity poses a challenge to the formulation of a unequivocal treatment strategy. In 2020, a nationwide online survey of 257 clinicians treating HCC in 184 hospitals in China was conducted to identify the real-world trends in the clinical application of TACE and recognition of TACE failure/refractoriness.40 Most clinicians (n=229, 89.1%) agreed that TACE was a palliative treatment but can achieve curative effects under certain conditions. Despite the varied treatment outcomes of TACE, nearly all (n=252, 98.1%) still choose TACE as the first-line treatment of patients with intermediate-stage HCC. In addition, 90% (n=226) did not think the current scoring systems, including the ART and ABCR scores or the up-to-seven criteria, were effective in guiding TACE treatment or repeated TACE procedures. Nearly three-quarters (n=199, 74.3%) supported the rationale of the concept of TACE failure/refractoriness, but 91.4% (n=235) did not agree with the current definition of TACE failure/refractoriness. Most participants (n=221, 86%) believed that repeated TACE can be performed for HCC nodules with insufficient necrosis, especially when the tumor-feeding artery was identified by angiography. However, they reported that TACE should be performed no more than three times before assessment of treatment outcome, nearly one-third (n=75, 29.2%) thought that three insufficient TACE sessions was the ideal number to define TACE failure/refractoriness. Notably, only a small proportion (n=42, 16.3%) of participants agreed that appearance of new intrahepatic lesion(s) should be considered as a criterion for TACE failure/refractoriness. Combination therapy, including TACE, was considered as the ideal treatment for new HCC nodule(s). Similarly, as long as patients had well-preserved liver function, over 90% of respondents would choose continuing TACE or TACE-based combination therapy to interfere with intrahepatic tumor progression even in the setting of invasion of portal vein (n=242, 94.2%) or extrahepatic organs (n=253, 98.5%). Based on the evidence from previous studies as well as the survey outcomes, a potentially useful schematic illustration (Fig. 2) is presented to judge TACE failure/refractoriness and to guide subsequent treatment to overcome TACE failure/refractoriness.
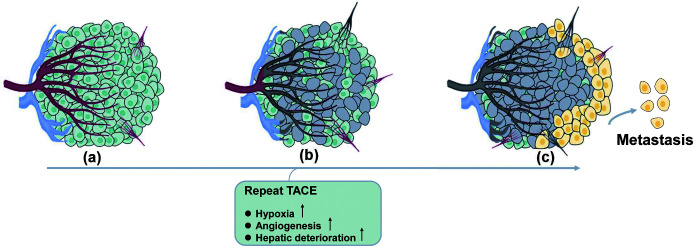
Fig. 1. Pathological and physiological changes in HCCs with consecutive insufficient responses to TACE.
(A) HCCs can be nourished by several potential arteries other than the main feeding artery, and peritumor tissue can be supported by the portal vein as well. (B) After consecutive super selective TACE, HCCs may still be viable or even undergo progression because of a blood supply from delicate collateral arteries or the distal portal vein. Hypovascular HCCs tend to have unsatisfied iodized oil deposition. Under those circumstances, additional TACE is no longer effective, and define TACE failure/refractoriness. (C) Additional TACE sessions after TACE failure/refractoriness not only insignificantly increase tumor response rate but also damage liver function. Frequent interventional insults put pressure on the tumor microenvironment, making the residual HCCs more malignant and aggressive. TACE, transarterial chemoembolization.
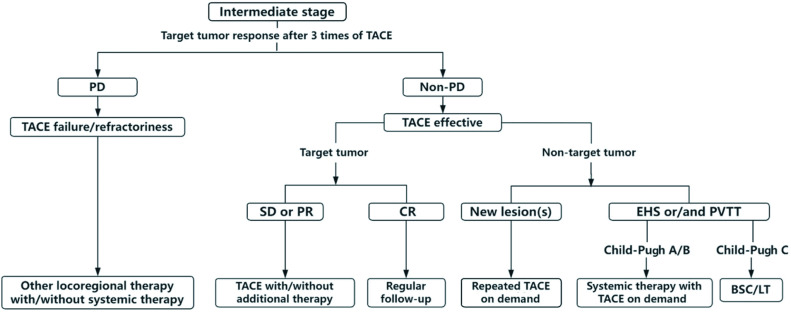
Fig. 2. Algorithm decision tree for judging TACE failure/refractoriness and recommending subsequent treatment.
BSC, best supportive care; CR, complete response; EHS, extrahepatic spread; HCC, hepatocellular carcinoma; LT, liver transplantation; PD, progressing disease; PR, partial response; PVTT, portal vein tumor thrombosis; SD, stable disease; TACE, transarterial chemoembolization.
Conclusion
Overall, not only sorafenib but also other therapies such as DEB-TACE, HAIC, ablation, and TACE combined with systemic therapies are potentially useful as subsequent treatment after TACE failure/refractoriness. However, all the available evidence is based on retrospective studies. Prospective studies are warranted to identify the ideal treatment for HCC after TACE failure/refractoriness.
Abbreviations
- DCB-TACE
DC beads TACE
- DEB-TACE
drug-eluting beads TACE
- HAIC
hepatic arterial infusion chemotherapy
- ICI
immune checkpoint inhibitor
- JSH–LCSGJ
JSH-Liver Cancer Study Group of Japan
- JSH
Japan Society of Hepatology
- MVI
macroscopic vascular invasion
- MWA
microwave ablation
- OS
overall survival
- PFS
progression-free survival
- TACE
transarterial chemoembolization
- TARE
transarterial radioembolization
- TTP
time to progression
References
- 1.Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 2.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 3.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 4.Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35(9):2155–2166. doi: 10.1111/liv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ringelhan M, Pfister D, O’Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19(3):222–232. doi: 10.1038/s41590-018-0044-z. [DOI] [PubMed] [Google Scholar]
- 6.EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 8.Terzi E, Golfieri R, Piscaglia F, Galassi M, Dazzi A, Leoni S, et al. Response rate and clinical outcome of HCC after first and repeated cTACE performed “on demand”. J Hepatol. 2012;57(6):1258–1267. doi: 10.1016/j.jhep.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 9.Kudo M, Matsui O, Izumi N, Kadoya M, Okusaka T, Miyayama S, et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology. 2014;87(Suppl 1):22–31. doi: 10.1159/000368142. [DOI] [PubMed] [Google Scholar]
- 10.Park JW, Amarapurkar D, Chao Y, Chen PJ, Geschwind JF, Goh KL, et al. Consensus recommendations and review by an International Expert Panel on Interventions in Hepatocellular Carcinoma (EPOIHCC) Liver Int. 2013;33(3):327–337. doi: 10.1111/liv.12083. [DOI] [PubMed] [Google Scholar]
- 11.Raoul JL, Gilabert M, Piana G. How to define transarterial chemoembolization failure or refractoriness: a European perspective. Liver Cancer. 2014;3(2):119–124. doi: 10.1159/000343867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arizumi T, Ueshima K, Minami T, Kono M, Chishina H, Takita M, et al. Effectiveness of sorafenib in patients with transcatheter arterial chemoembolization (TACE) refractory and intermediate-stage hepatocellular carcinoma. Liver Cancer. 2015;4(4):253–262. doi: 10.1159/000367743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogasawara S, Chiba T, Ooka Y, Kanogawa N, Motoyama T, Suzuki E, et al. Efficacy of sorafenib in intermediate-stage hepatocellular carcinoma patients refractory to transarterial chemoembolization. Oncology. 2014;87(6):330–341. doi: 10.1159/000365993. [DOI] [PubMed] [Google Scholar]
- 14.Iwasa S, Ikeda M, Okusaka T, Ueno H, Morizane C, Nakachi K, et al. Transcatheter arterial infusion chemotherapy with a fine-powder formulation of cisplatin for advanced hepatocellular carcinoma refractory to transcatheter arterial chemoembolization. Jpn J Clin Oncol. 2011;41(6):770–775. doi: 10.1093/jjco/hyr037. [DOI] [PubMed] [Google Scholar]
- 15.Hsu SJ, Xu X, Chen MP, Zhao ZY, Wang Y, Yin X, et al. Hepatic arterial infusion chemotherapy with modified FOLFOX as an alternative treatment option in advanced hepatocellular carcinoma patients with failed or unsuitability for transarterial chemoembolization. Acad Radiol. 2021;28(Suppl 1):S157–S166. doi: 10.1016/j.acra.2021.01.024. [DOI] [PubMed] [Google Scholar]
- 16.Song DS, Choi JY, Yoo SH, Kim HY, Song MJ, Bae SH, et al. DC bead transarterial chemoembolization is effective in hepatocellular carcinoma refractory to conventional transarteral chemoembolization: A pilot study. Gut Liver. 2013;7(1):89–95. doi: 10.5009/gnl.2013.7.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi S, Tajiri K, Murayama A, Entani T, Futsukaichi Y, Nagata K, et al. Drug-eluting bead-transcatheter arterial chemoembolization for advanced hepatocellular carcinoma refractory to conventional lipiodol-based transcatheter arterial chemoembolization. J Hepatocell Carcinoma. 2020;7:181–189. doi: 10.2147/JHC.S273929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klompenhouwer EG, Dresen RC, Verslype C, Laenen A, De Hertogh G, Deroose CM, et al. Safety and efficacy of transarterial radioembolisation in patients with intermediate or advanced stage hepatocellular carcinoma refractory to chemoembolisation. Cardiovasc Intervent Radiol. 2017;40(12):1882–1890. doi: 10.1007/s00270-017-1739-5. [DOI] [PubMed] [Google Scholar]
- 19.Kim PH, Gwon DI, Kim JW, Chu HH, Kim JH. The safety and efficacy of balloon-occluded transcatheter arterial chemoembolization for hepatocellular carcinoma refractory to conventional transcatheter arterial chemoembolization. Eur Radiol. 2020;30(10):5650–5662. doi: 10.1007/s00330-020-06911-9. [DOI] [PubMed] [Google Scholar]
- 20.Xu X, Ding Y, Pan T, Gao F, Huang X, Sun Q. CT-guided 125I brachytherapy in the treatment of hepatocellular carcinoma refractory to conventional transarterial chemoembolization: A pilot study. Cancer Manag Res. 2021;13:3317–3326. doi: 10.2147/CMAR.S305422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikeda M, Mitsunaga S, Shimizu S, Ohno I, Takahashi H, Okuyama H, et al. Efficacy of sorafenib in patients with hepatocellular carcinoma refractory to transcatheter arterial chemoembolization. J Gastroenterol. 2014;49(5):932–940. doi: 10.1007/s00535-013-0853-7. [DOI] [PubMed] [Google Scholar]
- 22.Kodama K, Kawaoka T, Aikata H, Uchikawa S, Inagaki Y, Hatooka M, et al. Comparison of clinical outcome of hepatic arterial infusion chemotherapy and sorafenib for advanced hepatocellular carcinoma according to macrovascular invasion and transcatheter arterial chemoembolization refractory status. J Gastroenterol Hepatol. 2018;33(10):1780–1786. doi: 10.1111/jgh.14152. [DOI] [PubMed] [Google Scholar]
- 23.Hatooka M, Kawaoka T, Aikata H, Morio K, Kobayashi T, Hiramatsu A, et al. Comparison of outcome of hepatic arterial infusion chemotherapy and sorafenib in patients with hepatocellular carcinoma refractory to transcatheter arterial chemoembolization. Anticancer Res. 2016;36(7):3523–3529. [PubMed] [Google Scholar]
- 24.Yoo SH, Kwon JH, Nam SW, Lee JY, Kim YW, Shim DJ, et al. Transarterial infusion of epirubicin and cisplatin combined with systemic infusion of 5-flurouracil versus sorafenib for hepatocellular carcinoma with refractoriness of transarterial chemoembolization using doxorubicin. Cancer Control. 2020;27(2):1073274820935843. doi: 10.1177/1073274820935843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villani R, Cavallone F, Sangineto M, Fioravanti G, Romano AD, Serviddio G. Management of intermediate-stage hepatocellular carcinoma in the elderly with transcatheter arterial chemoembolization failure: Retreatment or switching to systemic therapy? Int J Clin Pract. 2021;75(4):e13733. doi: 10.1111/ijcp.13733. [DOI] [PubMed] [Google Scholar]
- 26.Chen S, Shi M, Shen L, Qi H, Wan W, Cao F, et al. Microwave ablation versus sorafenib for intermediate-Stage Hepatocellular carcinoma with transcatheter arterial chemoembolization refractoriness: a propensity score matching analysis. Int J Hyperthermia. 2020;37(1):384–391. doi: 10.1080/02656736.2020.1752400. [DOI] [PubMed] [Google Scholar]
- 27.Shimose S, Kawaguchi T, Tanaka M, Iwamoto H, Miyazaki K, Moriyama E, et al. Lenvatinib prolongs the progression-free survival time of patients with intermediate-stage hepatocellular carcinoma refractory to transarterial chemoembolization: A multicenter cohort study using data mining analysis. Oncol Lett. 2020;20(3):2257–2265. doi: 10.3892/ol.2020.11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu J, Li A, Yang J, Lu Y, Li J. Efficacy and safety of TACE in combination with sorafenib for the treatment of TACE-refractory advanced hepatocellular carcinoma in Chinese patients: a retrospective study. Onco Targets Ther. 2017;10:2761–2768. doi: 10.2147/OTT.S131022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin PT, Teng W, Jeng WJ, Hsieh YC, Hung CF, Huang CH, et al. Add-on sorafenib is beneficial for hepatocellular carcinoma patients with transarterial chemoembolization refractoriness: a real-world experience. Eur J Gastroenterol Hepatol. 2020;32(9):1192–1199. doi: 10.1097/MEG.0000000000001637. [DOI] [PubMed] [Google Scholar]
- 30.Kaibori M, Matsushima H, Ishizaki M, Kosaka H, Matsui K, Kariya S, et al. The impact of sorafenib in combination with transarterial chemoembolization on the outcomes of intermediate-stage hepatocellular carcinoma. Asian Pac J Cancer Prev. 2021;22(4):1217–1224. doi: 10.31557/APJCP.2021.22.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang WK, Yang SF, You LN, Liu M, Liu DY, Gu P, et al. Transcatheter arterial chemoembolisation (TACE) plus S-1 for the treatment of BCLC stage B hepatocellular carcinoma refractory to TACE. Contemp Oncol (Pozn) 2016;20(6):468–474. doi: 10.5114/wo.2016.65607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang W, You L, Yang S, Liu D, Liu M, Wang H, et al. Metronomic S-1 chemotherapy plus transcatheter arterial chemoembolization (TACE): a promising treatment of hepatocellular carcinoma refractory to TACE. J BUON. 2016;21(4):909–916. [PubMed] [Google Scholar]
- 33.Qiu Z, Shen L, Chen S, Qi H, Cao F, Xie L, et al. Efficacy of apatinib in transcatheter arterial chemoembolization (TACE) refractory intermediate and advanced-stage hepatocellular carcinoma:A propensity score matching analysis. Cancer Manag Res. 2019;11:9321–9330. doi: 10.2147/CMAR.S223271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng L, Fang S, Wu F, Chen W, Chen M, Weng Q, et al. Efficacy and safety of TACE combined with sorafenib plus immune checkpoint inhibitors for the treatment of intermediate and advanced TACE-refractory hepatocellular carcinoma: A retrospective study. Front Mol Biosci. 2021;7:609322. doi: 10.3389/fmolb.2020.609322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 36.Kudo M, Ueshima K, Chan S, Minami T, Chishina H, Aoki T, et al. Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond up-to-seven criteria and child-pugh A liver function: A proof-of-concept study. Cancers (Basel) 2019;11(8):1084. doi: 10.3390/cancers11081084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69(8):1492–1501. doi: 10.1136/gutjnl-2019-318934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Guglielmi A, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. 2016;64(5):1090–1098. doi: 10.1016/j.jhep.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Meyer T, Fox R, Ma YT, Ross PJ, James MW, Sturgess R, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2(8):565–575. doi: 10.1016/S2468-1253(17)30156-5. [DOI] [PubMed] [Google Scholar]
- 40.Zhong BY, Wang WS, Zhang S, Zhu HD, Zhang L, Shen J, et al. Re-evaluating transarterial chemoembolization failure/refractoriness: A survey by Chinese college of interventionalists. J Clin Transl Hepatol. 2021;9(4):521–527. doi: 10.14218/JCTH.2021.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]