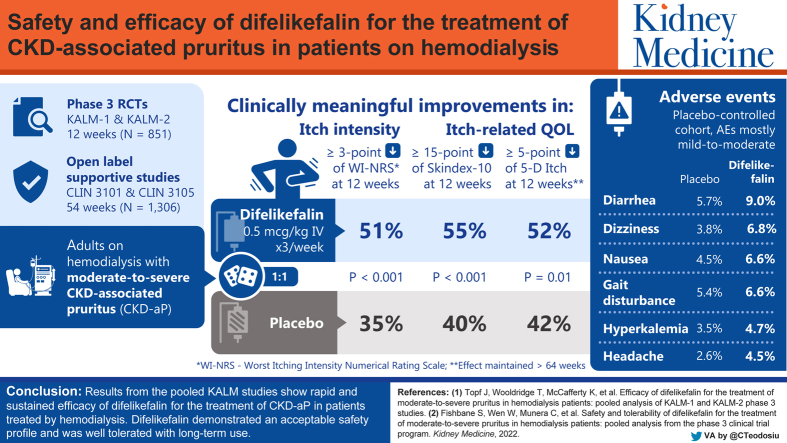
Abstract
Rationale & Objective
Chronic kidney disease–associated pruritus (CKD-aP) in patients treated by hemodialysis (HD) impairs quality of life (QoL). Difelikefalin, a selective κ-opioid receptor agonist, decreased the intensity of CKD-aP in patients undergoing HD. This pooled analysis evaluated difelikefalin’s efficacy and the itch-related QoL overall and in subgroups defined by demographics or disease characteristics.
Study Design
In KALM-1 and KALM-2, participants were randomized (1:1) to receive intravenous difelikefalin or placebo 3 times/wk for 12 weeks, followed by a 52-week open-label extension.
Setting & Participants
Adults with moderate to severe CKD-aP treated by HD in North America, Europe, and the Asia-Pacific region.
Intervention
Intravenous difelikefalin at 0.5 mcg/kg or placebo.
Outcomes
Itch intensity (Worst Itching Intensity Numerical Rating Scale [WI-NRS]) and itch-related QoL (Skindex-10 and 5-D Itch questionnaires).
Results
851 participants were randomized (difelikefalin, n = 426; placebo, n = 425). This pooled analysis demonstrated early (week 1), sustained difelikefalin efficacy, with significantly greater achievement of ≥3-point WI-NRS reduction with difelikefalin (51.1%) versus placebo (35.2%; P < 0.001). Achievement of a ≥4-point WI-NRS reduction was significantly greater with difelikefalin (38.7%) versus placebo (23.4%; P < 0.001). Difelikefalin reduced itch intensity in subgroups based on age, sex, anti-itch medication use, the presence of specific medical conditions, and gabapentin or pregabalin use. More participants receiving difelikefalin versus placebo achieved clinically meaningful decreases of ≥15 points on the Skindex-10 scale (55.5% vs 40.5%, respectively; P < 0.001) and ≥5 points on the 5-D Itch scale (52.1% vs 42.3%, respectively; P = 0.01), with sustained 5-D Itch effects up to 64 weeks.
Limitations
Subgroup samples were small. The WI-NRS, Skindex-10, and 5-D Itch are not used in routine clinical care of dialysis patients; therefore, findings may not reflect the real-world effectiveness of difelikefalin.
Conclusions
Difelikefalin demonstrated rapid, sustained efficacy, with consistent results in diverse populations of patients treated by HD.
Funding
Cara Therapeutics, Inc.
Trial Registration
The KALM-1 trial is registered as NCT03422653 and the KALM-2 trial is registered as NCT03636269.
Index Words: Chronic kidney disease, difelikefalin, efficacy, κ-opioid receptor, pruritus
Visual Abstract
Plain-Language Summary.
Patients with chronic kidney disease treated by hemodialysis (HD) often experience pruritus (itch), a burdensome symptom that negatively impacts quality of life. Difelikefalin is an intravenous drug approved in the United States for the treatment of moderate to severe pruritus in adults treated by HD. In 2 pivotal, phase 3 clinical trials (KALM-1 and KALM-2), difelikefalin significantly decreased pruritus in HD participants with moderate to severe pruritus. Here we report findings of a pooled analysis of efficacy data from KALM-1 and KALM-2. Participants treated with difelikefalin experienced reductions in itch intensity as early as week 1. Clinically meaningful improvements in itch-related quality of life were observed through week 64. These results suggest difelikefalin may bring relief to patients treated by HD who experience pruritus.
Editorial, 100519
Introduction
Pruritus associated with chronic kidney disease (CKD) is a common and often underdiagnosed condition in patients treated by hemodialysis (HD).1, 2, 3 According to the international Dialysis Outcomes and Practice Patterns Study, 26%-48% of patients receiving HD reported they were at least moderately bothered by itchy skin.3 CKD-associated pruritus (CKD-aP) is a highly distressing condition that can substantially impair patients’ quality of life (QoL), causing sleep disturbances, depression, and decreased mental and physical functioning.2,4, 5, 6 CKD-aP has also been associated with an increase in missed dialysis sessions, a higher risk of hospitalization, and an increase in mortality, particularly cardiovascular- and infection-related mortality.4
Despite the need to identify and manage pruritus in patients with CKD, there is a dearth of treatment options.1,7 Off-label treatments may include antihistamines, topical corticosteroids, and gabapentin or pregabalin; although there are reports that treatments like gabapentin and pregabalin are effective at reducing itch, their side effects sometimes prevent their use in this patient population.7,8 No treatments have been approved for CKD-aP in Europe and only 1 treatment, the centrally acting mixed μ-opioid receptor partial agonist and κ-opioid receptor agonist nalfurafine, has been approved in Japan (2009) and South Korea (2013).3,9, 10, 11 A variety of other therapeutics with novel mechanisms of action are being evaluated, but well-controlled clinical studies are still needed to evaluate the efficacy and safety of these therapies for patients with CKD-aP.12
In August 2021, difelikefalin, a novel, selective κ-opioid receptor agonist that works mainly by activating κ-opioid receptors on peripheral sensory neurons and immune cells,13 became the first treatment approved by the US Food and Drug Administration (FDA) for moderate to severe pruritus associated with CKD in adults treated by HD.14,15 With approval by the European Medicines Agency (EMA) in 2022, it is now the first treatment approved for the same patient population in Europe.16 The US approval relied primarily on the evidence from the phase 3 KALM-1 and KALM-2 studies of intravenous (IV) difelikefalin in HD participants with moderate to severe pruritus, in which difelikefalin demonstrated significant reductions in itch intensity versus placebo at week 12.14,17 In both studies, significantly greater proportions of participants in the difelikefalin group achieved ≥3- and ≥4-point reductions in weekly means of daily Worst Itching Intensity Numerical Rating Scale (WI-NRS) scores versus the placebo group.14,18 To obtain a combined estimate of the treatment effects of difelikefalin in HD participants with moderate to severe pruritus, we analyzed pooled data from the KALM-1 and KALM-2 studies, including QoL endpoints. We also analyzed subgroups of the pooled studies based on demographics and participant characteristics at baseline to evaluate the efficacy of difelikefalin in diverse populations.
Methods
Study Design
The study designs and methods for KALM-1 (NCT03422653) and KALM-2 (NCT03636269) have been previously reported.14,17 Additional details of the KALM-1 and KALM-2 studies are presented in Item S1. KALM-1 and KALM-2 were conducted in accordance with ethical principles founded in the Declaration of Helsinki, International Council for Harmonization principles of Good Clinical Practice, and applicable regulations of the countries in which the studies were conducted. Institutional review boards or independent ethics committees reviewed and approved the protocols before the studies commenced (approval numbers available on request). Participants provided written informed consent before participating in the study.
Eligibility Criteria
Eligibility criteria have been previously published for KALM-114 and are presented in Item S1 for KALM-2. Eligible participants were adults (aged 18 years or above in KALM-1 and 18-85 years in KALM-2) treated by HD 3 times per week for ≥3 months before screening. Participants were excluded from the studies if they were scheduled to receive a kidney transplant during the study or if they had a concomitant disease or history of any medical condition that could pose an undue risk to the participant, impede completion of the study procedures, or compromise the validity of the study measurements according to the investigator. Concomitant treatment with stable doses of anti-itch medications (including antihistamines; oral, IV, or topical corticosteroids; gabapentin; and pregabalin) used at the time of the screening visit were permitted; however, the studies did not allow changes to these anti-itch treatment regimens or the use of new anti-itch treatments within 14 days before screening or at any time during double-blind treatment. Also, planned or ongoing ultraviolet B treatment during the study, use of other investigational drugs within 30 days before screening, or planned participation in another clinical study while enrolled in KALM-1 or KALM-2 was not permitted.
Assessments
The primary endpoint in KALM-1 and KALM-2 was the proportion of participants achieving a ≥3-point improvement (reduction) in the weekly mean of daily 24-hour WI-NRS scores at week 12. Each day, participants were asked to indicate, using the WI-NRS, the intensity of the worst itching they had experienced over the past 24 hours, on a scale from 0 (no itching) to 10 (worst itching imaginable; Fig S1). A ≥3-point reduction in the weekly mean WI-NRS score represents a clinically meaningful reduction in itch intensity in patients with moderate to severe CKD-aP,19,20 and proportions of patients achieving a ≥3-point reduction were assessed weekly from week 1-week 12 in the pooled study population. Achievement of a ≥4-point reduction in the weekly mean of daily WI-NRS scores from week 1-week 12 was also assessed in the pooled population.
Additional endpoints evaluated in the pooled population included the proportion of participants achieving a complete WI-NRS response over 12 weeks. For each week, a complete response was defined as reporting 0 or 1 on at least 80% of the daily WI-NRS scores. The cutoff of at least 80% represents 6 of the 7 daily scores collected in 1 week, assuming no data are missing. Achievement of a clinically meaningful improvement in itch-related QoL assessments was evaluated over 12 weeks. The Skindex-10 scale evaluates itch-related QoL across 3 domains (disease, mood or emotional distress, and social functioning), with total scores ranging from 0-60 and higher scores indicating a worse itch-related QoL (Fig S1).21 The 5-D Itch scale evaluates 5 dimensions of itch (duration, degree, direction, disability, and distribution), with total scores ranging from 5-25 and higher scores indicating a worse itch and worse itch-related QoL (Fig S1).22 In patients treated by HD, clinically meaningful thresholds were determined to be a ≥15-point reduction in Skindex-10 and a ≥5-point reduction in 5-D Itch total scores. The long-term impacts of difelikefalin on itch intensity and itch-related QoL were assessed using the 5-D Itch scale during the open-label extension; beyond week 12, participants completed the 5-D Itch scale at weeks 24, 36, and 52 of the open-label phase.
A subgroup analysis of the primary endpoint evaluated efficacy based on the following demographic and baseline characteristics: age, younger than 65 years or 65 years or above; sex, male or female; race, White, Black or African American, or other; and geographic region, United States or non–United States. In addition, efficacy was evaluated in subgroups based on the use of an anti-itch medication at baseline, the presence of specific medical conditions (ie, history of a fall or fracture [related to a fall]; confused state, mental status change, altered mental status, or disorientation; gait disturbance or movement disorder), and prior use of gabapentin or pregabalin.
Statistical Analysis
Efficacy analyses were conducted in the intent-to-treat population from the pooled KALM-1 and KALM-2 studies, which consisted of all randomized participants. Differences between placebo and difelikefalin were analyzed using a logistic regression model containing terms for the treatment group, baseline WI-NRS score, use of an anti-itch medication during the week before randomization, presence of specific medical conditions, and geographic region. For the analysis of the proportions of participants who achieved ≥3-point or ≥4-point reductions in the weekly mean WI-NRS scores, missing weekly WI-NRS scores were imputed by multiple imputation under a missing-at-random assumption. Participants who reported <4 daily WI-NRS scores at week 12 or who discontinued treatment early were considered nonresponders in the analysis of the complete WI-NRS response. Proportions of participants achieving a ≥5-point improvement in the 5-D Itch total score and a ≥15-point improvement in the Skindex-10 total score were analyzed without imputation for missing values. Proportions of participants achieving a ≥5-point improvement in 5-D Itch total score are reported for the pooled population during the placebo-controlled, double-blind period (12 weeks) and the open-label extension period (up to 52 weeks).
Continuous efficacy endpoints were analyzed by a mixed model for repeated measures, with terms for treatment, visit, treatment-by-visit interaction, baseline score, use of an anti-itch medication during the week before randomization, the presence of specific medical conditions, and geographic region. An unstructured covariance structure was applied to model the within-participant errors. Missing values were not imputed. The mean improvements from baseline in 5-D Itch total score are reported for the pooled population during the placebo-controlled, double-blind period (12 weeks) and the open-label extension period (up to 52 weeks).
The subgroup analyses of ≥3-point and ≥4-point reductions from baseline in the weekly mean WI-NRS scores were performed using the same methodology as that employed for the full intent-to-treat population.
Results
Participants
There were 851 randomized participants in the pooled KALM-1 and KALM-2 studies (difelikefalin, n = 426; placebo, n = 425; Fig 1). Of these, 368 (86.4%) participants in the difelikefalin group and 393 (92.5%) participants in the placebo group completed the double-blind treatment period (Fig 1). The most common reason for discontinuation with difelikefalin and placebo was an adverse event (6.3% and 3.8%, respectively; Fig 1).
Figure 1.
Participant dispositions in the pooled KALM-1 and KALM-2 studies. There were 2 participants in the placebo group and 3 participants in the difelikefalin group who discontinued due to a lack of eligibility after randomization.
Demographic and baseline clinical characteristics were comparable in KALM-114 and KALM-217 (Table S1) and between the difelikefalin and placebo groups in the pooled population (Table 1). The proportion of Black or African American participants was slightly greater in the difelikefalin group versus the placebo group in KALM-2 (22.6% [53/235] vs 16.1% [38/236], respectively), similar between the difelikefalin and placebo groups in KALM-1 (43.4% [82/189] vs 39.9% [75/188], respectively), and higher in KALM-1 versus KALM-2.14 Overall, participants had been treated by HD for more than 4 years and experiencing pruritus for more than 3 years (Table 1). At baseline, mean WI-NRS (7.2 and 7.2), 5-D Itch (16.8 and 16.9), and Skindex-10 (35.8 and 36.0) total scores were similar in the difelikefalin and placebo groups, respectively (Table 1). More than one-third of participants reported the use of an anti-itch medication at baseline; the most commonly used (>2%) anti-itch medications were diphenhydramine, hydroxyzine, hydrocortisone, cetirizine, and clemastine (Table 1). Few participants were using gabapentinoids for pruritus (difelikefalin, n = 5 [1.2%]; placebo, n = 5 [1.2%]). Greater proportions of participants in the difelikefalin and placebo groups (n = 87 [20.4%] and n = 74 [17.4%], respectively) were being treated with gabapentin or pregabalin for other conditions.
Table 1.
Baseline Demographics and Disease Characteristics
| Characteristics | Pooled KALM-1 and KALM-2 |
|
|---|---|---|
| Placebo n = 425 | Difelikefalin n = 426 | |
| Age, mean ± SD, years | 58.3 ± 13.5 | 59.1 ± 12.4 |
| Male, n (%) | 258 (60.7) | 249 (58.5) |
| Ethnicity, n (%) | ||
| Not Hispanic or Latino | 287 (67.5) | 287 (67.4) |
| Hispanic or Latino | 136 (32.0) | 133 (31.2) |
| Race, n (%) | ||
| White | 262 (61.6) | 255 (59.9) |
| Black or African American | 114 (26.8) | 135 (31.7) |
| Othera | 49 (11.5) | 36 (8.5) |
| Region, n (%) | ||
| United States | 322 (75.8) | 335 (78.6) |
| Eastern Europe | 60 (14.1) | 54 (12.7) |
| Western Europe | 31 (7.3) | 29 (6.8) |
| Asia | 12 (2.8) | 8 (1.9) |
| Prescription dry body weight, mean ± SD, kg | 82.4 ± 20.6 | 83.4 ± 20.1 |
| Years since diagnosis of ESKD, median (IQR) | 4.1 (5.3) | 3.8 (4.8) |
| Etiology of CKD,b n (%) | ||
| Diabetes | 206 (48.5) | 225 (52.8) |
| Hypertension | 138 (32.5) | 122 (28.6) |
| Glomerulonephritis | 16 (3.8) | 18 (4.2) |
| Cystic kidney | 15 (3.5) | 14 (3.3) |
| Other | 50 (11.8) | 47 (11.0) |
| Years on chronic HD, median (IQR) | 3.9 (5.0) | 3.5 (4.8) |
| Duration of pruritus, median (IQR), years | 2.5 (3.2) | 2.1 (3.2) |
| Blood chemical testingc | ||
| Bilirubin, mean ± SD, mg/dL | 0.5 ± 0.3 | 0.5 ± 0.6 |
| Calcium, mean ± SD, mg/dL | 8.4 ± 0.8 | 8.8 ± 0.8 |
| Phosphate, mean ± SD, mg/dL | 5.6 ± 2.2 | 5.6 ± 1.9 |
| Baseline use of an anti-itch medication, n (%) | 163 (38.4) | 159 (37.3) |
| Most commonly used (>2%) anti-itch medications at baseline, n (%) | ||
| Diphenhydramine | 100 (23.5) | 104 (24.4) |
| Hydroxyzine | 52 (12.2) | 42 (9.9) |
| Hydrocortisone | 16 (3.8) | 11 (2.6) |
| Cetirizine | 10 (2.4) | 7 (1.6) |
| Clemastine | 10 (2.4) | 7 (1.6) |
| Presence of selected medical conditions,d n (%) | 65 (15.3) | 67 (15.7) |
| WI-NRS score, mean ± SD | 7.2 ± 1.5 | 7.2 ± 1.4 |
| Skindex-10 total score, mean ± SD | 36.0 ± 15.1 | 35.8 ± 14.7 |
| 5-D Itch total score, mean ± SD | 16.9 ± 3.5 | 16.8 ± 3.5 |
Note: Percentages are based on the number of participants in each group.
Abbreviations: CKD, chronic kidney disease; ESKD, end-stage kidney disease; HD, hemodialysis; IQR, interquartile range; SD, standard deviation; WI-NRS, Worst Itching Intensity Numerical Rating Scale.
Includes participants who identified as American Indian or Alaska native (placebo, n = 6; difelikefalin, n = 7), Asian (placebo, n = 27; difelikefalin, n = 18), native Hawaiian or other Pacific Islander (placebo, n = 7; difelikefalin, n = 3), unknown (placebo, n = 2; difelikefalin, n = 1), and other (placebo, n = 7; difelikefalin, n = 7).
Diabetes values include patients with diabetes alone; diabetes and hypertension; diabetes, hypertension, and other; or diabetes and other. Hypertension values include patients with hypertension alone or hypertension and other. Glomerulonephritis values include patients with glomerulonephritis alone or glomerulonephritis and other.
Conversion factors for units were as follows: for bilirubin, mg/dL to μmol/L, ×17.1; for calcium, mg/dL to mmol/L, ×0.2495; and for phosphate, mg/dL to mmol/L, ×0.3229.
Specific medical condition values include patients with a history of fall or fracture (related to fall); confused state, mental status change, altered mental status, or disorientation; and gait disturbance or movement disorder.
WI-NRS Outcomes
In the pooled analysis, 51.1% of participants in the difelikefalin group and 35.2% of participants in the placebo group achieved a ≥3-point reduction in the weekly mean of daily WI-NRS scores at week 12 (P < 0.001). The odds of achieving a ≥3-point reduction in the weekly mean WI-NRS score at week 12 were almost twice as great with difelikefalin versus placebo (odds ratio, 1.93; 95% confidence interval [CI], 1.44-2.57]). A significantly greater proportion of participants achieved a ≥3-point reduction in the weekly mean of daily WI-NRS scores with difelikefalin versus placebo, observed as early as week 1 and sustained at all time points up to week 12 (Fig 2A and C). In KALM-1, 50.9% of participants in the difelikefalin group achieved a ≥3-point reduction in the weekly mean WI-NRS score at week 12, versus 28.3% in the placebo group (P < 0.001; Table S2). In KALM-2, 53.4% of participants in the difelikefalin group achieved a ≥3-point reduction in the weekly mean WI-NRS score at week 12, versus 42.6% in the placebo group (P = 0.03; Table S2).
Figure 2.
Proportions of participants with (A) A ≥3-point reduction in the weekly mean of the daily Worst Itching Intensity Numerical Rating Scale (WI-NRS) scores over 12 weeks, (B) A ≥4-point reduction in the weekly mean of the daily WI-NRS scores over 12 weeks, and (C) ≥3-point and ≥4-point reductions in weekly mean WI-NRS scores at week 12. ∗P < 0.05 and ∗∗P < 0.001 difelikefalin versus placebo. Differences between placebo and difelikefalin with respect to proportions were analyzed using a logistic regression model with terms for the treatment group, baseline WI-NRS score, use of an anti-itch medication during the week before randomization, presence of specific medical conditions, and geographic region. Missing weekly WI-NRS scores were imputed by multiple imputation under a missing-at-random assumption. Abbreviations: CI, confidence interval; LS, least squares; WI-NRS, Worst Itching Intensity Numerical Rating Scale.
In the pooled population, achievement of a ≥4-point reduction in the weekly mean of daily WI-NRS scores was significantly greater with difelikefalin versus placebo at all time points from week 3-week 12 (week 12 least-squares mean estimate, 38.7% [95% CI, 32.8%-45.0%] vs 23.4% [95% CI, 18.7%-28.8%], respectively; P < 0.001; Fig 2B and C). Results of this 4-point responder analysis were also statistically significant in the individual studies. In KALM-1, 38.4% of participants in the difelikefalin group achieved a ≥4-point reduction in the weekly mean WI-NRS score at week 12, versus 18.0% in the placebo group (P < 0.001; Table S2). In KALM-2, 37.3% of participants in the difelikefalin group achieved a ≥4-point reduction in the weekly mean WI-NRS score at week 12, versus 26.4% in the placebo group (P = 0.02; Table S2).
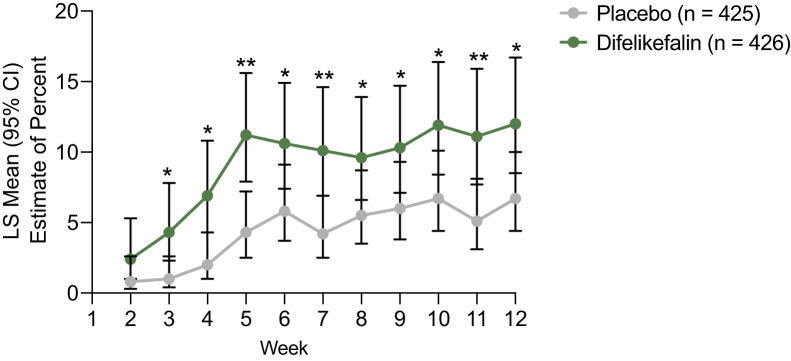
The proportion of participants who achieved a complete response on the WI-NRS was significantly greater with difelikefalin versus placebo at week 12 (12.0% vs 6.7%, respectively; P = 0.006), with significant differences between difelikefalin and placebo starting at week 3 and sustained at all time points up to week 12 (Fig 3). In the individual KALM-1 and KALM-2 studies, achievement of a WI-NRS complete response was numerically higher in the difelikefalin group versus the placebo group (Table S2).
Figure 3.
Achievement of complete response on the Worst Itching Intensity Numerical Rating Scale (WI-NRS) over 12 weeks. ∗P < 0.05 and ∗∗P < 0.001 difelikefalin versus placebo. A complete response was defined as ≥80% of daily WI-NRS scores being equal to 0 or 1 for the preceding week. Differences between placebo and difelikefalin with respect to proportions were analyzed using a logistic regression model containing terms for the treatment group, baseline WI-NRS score, use of an anti-itch medication during the week before randomization, presence of specific medical conditions, and geographic region. Missing weekly WI-NRS scores were imputed by multiple imputation under a missing-at-random assumption. Abbreviations: CI, confidence interval; LS, least squares; WI-NRS, Worst Itching Intensity Numerical Rating Scale.
Itch-Related QoL Outcomes
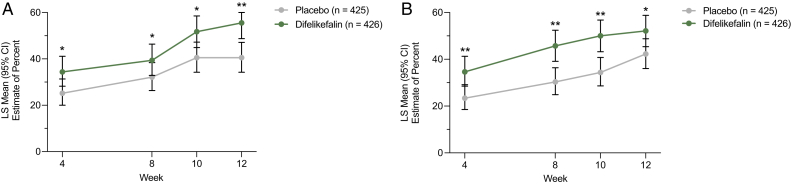
In the pooled analysis, significantly greater proportions of participants in the difelikefalin group achieved clinically meaningful improvements in itch-related QoL versus the placebo group, as measured by ≥15-point improvements in Skindex-10 total scores (55.5% vs 40.5%, respectively, at week 12; P < 0.001) and ≥5-point improvements in 5-D Itch total scores (52.1% vs 42.3%, respectively, at week 12; P = 0.01) over 12 weeks of treatment (Fig 4). Least-squares mean changes from baseline to week 12 in Skindex-10 total scores were −16.9 (95% CI, −18.6 to −15.2) in the difelikefalin group and −13.5 (95% CI, −15.1 to −11.8) in the placebo group (P = 0.001). Least-squares mean changes from baseline to week 12 in 5-D Itch total scores were −4.9 (95% CI, −5.4 to −4.5) in the difelikefalin group and −3.7 (95% CI, −4.1 to −3.3) in the placebo group (P < 0.001). During the open-label extension period (up to an additional 52 weeks), improvements in 5-D Itch total scores emerged in the participants who had switched from placebo to difelikefalin by week 4 and were maintained in the participants who continued difelikefalin treatment without placebo (Fig S2). In addition, the proportion of participants achieving a clinically meaningful 5-D Itch response (≥5-point improvement) was maintained with long-term difelikefalin treatment (Fig 5). In KALM-1 and KALM-2, changes from baseline in Skindex-10 scores for difelikefalin and placebo were similar (Table S2).
Figure 4.
Achievement of clinically meaningful improvements in (A) Skindex-10 and (B) 5-D Itch total scores over 12 weeks. ∗P ≤ 0.05 and ∗∗P ≤ 0.001 difelikefalin versus placebo. Differences between placebo and difelikefalin with respect to proportions were analyzed using a logistic regression model containing terms for the treatment group, baseline score, use of an anti-itch medication during the week before randomization, presence of specific medical conditions, and geographic region. Missing values were not imputed. Clinically meaningful thresholds were determined as ≥15-point reductions in Skindex-10 and ≥5-point reductions in 5-D Itch total scores (unpublished data). Abbreviations: CI, confidence interval; LS, least squares.
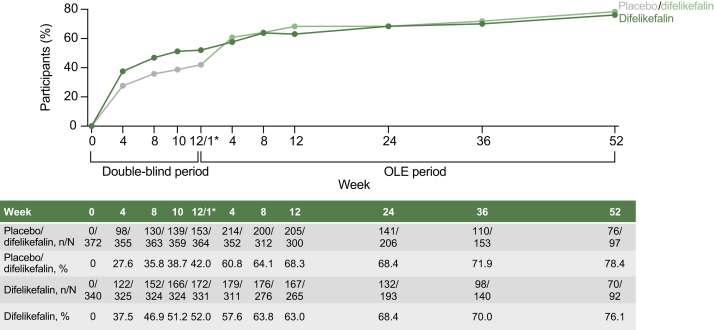
Figure 5.
Achievement of a ≥5-point improvement in 5-D Itch total score in the pooled KALM-1 and KALM-2 studies. Data given as n and N indicate the number of participants who achieved a ≥5-point improvement in the 5-D Itch total score and the total number of participants assessed at each time point, respectively. Data as observed. ∗Week 12 of the double-blind period and week 1 of the open-label extension period, during which participants taking placebo during the double-blind period switched to active treatment with difelikefalin. In KALM-2, in addition to the participants who discontinued from the open-label extension period, 313 of 399 (78.4%) participants could not complete the 52-week open-label extension period because of the sponsor’s decision to stop the study for reasons unrelated to safety or a lack of drug effects. A 2-week discontinuation following the end of the double-blind period of KALM-1 is not pictured in the figure. Abbreviation: OLE, open-label extension.
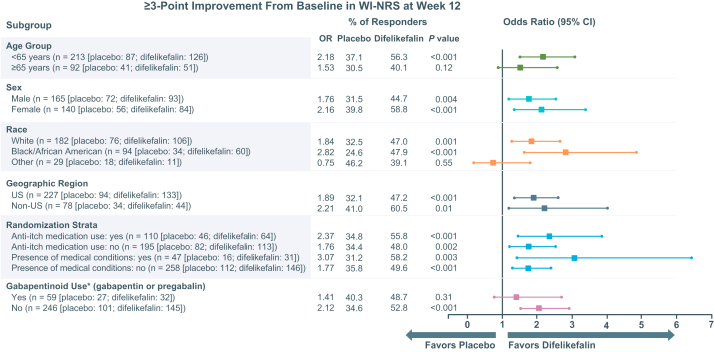
Subgroup Analyses of WI-NRS Improvement
Similar numbers of participants in the difelikefalin and placebo groups were included in each subgroup based on age, race, geographic region, use of an anti-itch medication, the presence of specific medical conditions, and use of gabapentin or pregabalin (Fig 6). When achievement of ≥3-point reductions in the weekly mean WI-NRS scores at week 12 was evaluated in these subgroups, improvements in itch intensity favored difelikefalin versus placebo in all subgroups except for the group of participants who reported their race as “other” (ie, not White or Black or African American; including those who identified as American Indian or Alaska native [n = 13], Asian [n = 45], native Hawaiian or other Pacific Islander [n = 10], unknown [n = 3], and other [n = 14]; Fig 6). Similar findings were observed in subgroup analyses that evaluated achievement of a ≥4-point reduction in the weekly mean WI-NRS score at week 12 (Fig S3).
Figure 6.
Subgroup analyses for ≥3-point Worst Itching Intensity Numerical Rating Scale (WI-NRS) responses at week 12. ∗Prior gabapentinoid use values include participants who used gabapentin or pregabalin for any condition, including itch. Differences between placebo and difelikefalin with respect to proportions were analyzed using a logistic regression model containing terms for the treatment group, baseline WI-NRS score, use of an anti-itch medication during the week before randomization, presence of specific medical conditions, and geographic region. Missing weekly WI-NRS scores were imputed by multiple imputation under a missing-at-random assumption. Abbreviations: CI, confidence interval; OR, odds ratio; WI-NRS, Worst Itching Intensity Numerical Rating Scale.
Discussion
The phase 3 KALM-1 and KALM-2 studies of IV difelikefalin represent the largest worldwide clinical development program to date evaluating a treatment for CKD-aP. This pooled analysis of the phase 3 studies provides compelling evidence of the efficacy of the selective κ-opioid receptor agonist difelikefalin23 in patients undergoing HD with moderate to severe pruritus across multiple regions and in clinically relevant subgroups. In this pooled analysis, difelikefalin at 0.5 mcg/kg IV demonstrated early (week 1) and sustained efficacy, as evidenced by a clinically meaningful reduction in itching intensity (≥3-point WI-NRS score reduction), with the odds of achieving a ≥3-point or ≥4-point score reduction being 2 times greater with difelikefalin versus placebo at week 12. Complete resolution of pruritus was also demonstrated in almost twice the number of participants who received difelikefalin versus placebo. Clinically meaningful improvements in itch-related QoL were also observed and sustained for up to 64 weeks (12-week double-blind and 52-week open-label extension periods).
Although approximately one-third of participants in both treatment groups were receiving an anti-itch medication at baseline, WI-NRS scores at baseline were similar in both treatment groups and indicative of moderate to severe pruritus. These data indicate that many patients with moderate to severe CKD-aP were not receiving sufficient pruritus control from currently available therapies. Mean Skindex-10 and 5-D Itch scores at baseline indicated significant impairments in itch-related QoL, in agreement with findings from the international Dialysis Outcomes and Practice Patterns Study showing that moderate to severe pruritus had a profound impact on the QoL and daily functioning of patients undergoing HD.4
Treatment effects with respect to achievement of ≥3-point and ≥4-point reductions from baseline in the weekly mean of daily WI-NRS scores at week 12 favored difelikefalin across a broad range of patients, independent of age, sex, geographic region, or race. In addition, difelikefalin demonstrated reductions in itch intensity in participants whose itch severity was moderate to severe at baseline despite their use of an anti-itch medication.
In a Cochrane review of interventions for itch in patients with advanced CKD, the authors concluded that gabapentinoids were the most studied therapeutics for the treatment of CKD-aP and show the greatest reductions in itch scores.7,8 It is important to consider that gabapentinoids are not approved anywhere in the world for the treatment of pruritus in patients treated by HD, and the safety and efficacy in this population have not been evaluated in a rigorous clinical development program. In addition, there are varying recommendations for dosing and limitations with respect to the tolerability of gabapentinoids that must be considered in this population.24 In the pooled KALM-1 and KALM-2 studies, few participants were using gabapentin or pregabalin for itch. A subgroup analysis was conducted of participants who were using gabapentinoids for any condition, such as itch and chronic pain, which comprised approximately 20% of participants. Difelikefalin reduced itch intensity regardless of the use of gabapentin or pregabalin, suggesting that use of these agents was not a confounding factor and that difelikefalin may provide additional relief of itch in patients who are treated with gabapentinoids for any condition. Difelikefalin also demonstrated greater achievement of ≥3-point and ≥4-point improvements in the weekly mean WI-NRS scores versus placebo in various subgroups, except for the subgroup of participants who reported their race as “other.” It is difficult to interpret findings for the “other” race subgroup due to heterogeneity, because several subpopulations were represented and could not be assessed separately because of small sample sizes.
Difelikefalin received US FDA approval for the treatment of moderate to severe pruritus associated with CKD in adults treated by HD in August 2021 and received EMA approval in April 2022.15,16 The only other treatment approved for CKD-aP (in Japan) is the centrally acting, mixed μ-opioid receptor partial agonist and κ-opioid receptor agonist nalfurafine. Although both nalfurafine and difelikefalin bind to κ-opioid receptors, the mechanism of action of difelikefalin is a selective κ-opioid receptor agonist located outside of the central nervous system, whereas nalfurafine’s mechanism of action is a centrally acting and potent but nonselective opioid. The present data suggest that activation of κ-opioid receptors in the periphery, located on peripheral nerves and possibly immune cells, is sufficient to reduce itch, bringing new insights to the pathophysiology of CKD-aP.
Difelikefalin was well tolerated in participants undergoing HD.14,17 A thorough report of safety from the phase 3 program of IV difelikefalin in participants with moderate to severe pruritus who are undergoing HD is available in a companion article.25
In addition to the reduction of itch intensity, treatment with IV difelikefalin resulted in clinically meaningful improvements in itch-related QoL measures versus placebo through week 64. Considering that pruritus can be highly burdensome in this patient population,2,4, 5, 6 difelikefalin may help improve itch-related QoL outcomes for patients with CKD-aP in clinical practice.
A notable limitation of this study was the small sample sizes of specific subgroups analyzed based on demographics or disease characteristics. In addition, the assessments (WI-NRS, Skindex-10, and 5-D Itch) used in KALM-1 and KALM-2 are not used in routine clinical care of patients undergoing HD. Therefore, efficacy in these studies may not correspond to or be reflective of the real-world effectiveness of difelikefalin. Finally, patient-reported outcomes such as the WI-NRS are susceptible to placebo effects, because itch is a symptom that is experienced by patients and is assessed in clinical studies using these types of outcomes. Therefore, an appreciable placebo response was expected in these clinical studies and likely impacted the observed treatment effects. However, the proportion of participants who achieved a clinically meaningful reduction in itch intensity with difelikefalin was similar to the response observed with another approved therapeutic for pruritic conditions.26 The placebo response was higher in KALM-2 than in KALM-1 with respect to the proportion of participants who achieved a ≥3-point or ≥4-point reduction from baseline in the weekly mean WI-NRS score at week 12. Although specific reasons for the differences in the placebo effects between the 2 studies are not clear, regional variances and more study sites may have contributed to the higher placebo response observed in KALM-2.
In conclusion, this pooled analysis of the KALM-1 and KALM-2 phase 3 studies demonstrated rapid and sustained reductions in itch intensity that were clinically meaningful and significantly greater with difelikefalin versus placebo in participants with moderate to severe pruritus undergoing HD. These findings were consistent across diverse populations with CKD-aP in multiple participant subgroups. Findings of these pooled analyses suggest that IV difelikefalin has the potential to bring relief to patients treated by HD who experience pruritus and to improve itch-related QoL.
Article Information
Authors’ Full Names and Academic Degrees
Joel Topf, MD, Thomas Wooldridge, MD, Kieran McCafferty, MD, Michael Schömig, MD, Botond Csiky, MD, PhD, Rafal Zwiech, MD, PhD, Warren Wen, PhD, Sarbani Bhaduri, MD, Catherine Munera, PhD, Rong Lin, MD, Alia Jebara, MD, Joshua Cirulli, PharmD, and Frédérique Menzaghi, PhD.
Authors’ Contributions
Research idea and study design: WW, FM; data acquisition: JT, TW, KM, MS, BC, RZ; data analysis: SB, CM, AJ, RL; data interpretation: JT, TW, KM, MS, BC, RZ, WW, SB, CM, AJ, JC, RL, FM. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
The research was sponsored by Cara Therapeutics, Inc. Cara Therapeutics, Inc, employees contributed to the study design, data analysis and interpretation, drafting of the report, and approval to submit the report for publication.
Financial Disclosure
Dr Topf is a KALM-1 principal investigator and on the advisory board for Cara Therapeutics, Inc. Dr McCafferty is a grant holder for AstraZeneca; and has received speaker honoraria and/or travel sponsorship and/or is an advisory board member for AstraZeneca, Napp, Pharmacosmos, and Vifor Fresenius. Dr Zwiech has received grants as an investigator from Cara Therapeutics, Inc. Drs Wen, Munera, Jebara, Cirulli, and Menzaghi are employees of Cara Therapeutics, Inc. Drs Bhaduri and Lin are consultants for Cara Therapeutics, Inc. The remaining authors declare that they have no relevant financial interests.
Acknowledgements
The authors thank the study investigators and patients who participated in these studies. The authors gratefully acknowledge Callie Grimes, PhD, and Illyce Nuñez, PhD (Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ), for medical writing and editorial support, which was funded by Cara Therapeutics, Inc, under the direction of the authors.
Data Sharing
Please contact Cara Therapeutics for data inquiries.
Peer Review
Received December 21, 2021. Evaluated by 2 external peer reviewers, with direct editorial input by the Statistical Editor and an Associate Editor, who served as Acting Editor-in-Chief. Accepted in revised form June 12, 2022. The involvement of an Acting Editor-in-Chief was to comply with Kidney Medicine’s procedures for potential conflicts of interest for editors, as described in the Information for Authors & Journal Policies.
Footnotes
Complete author and article information provided before references.
Figure S1: (A) Worst Itching Intensity Numerical Rating Scale, (B) Skindex-10 scale, and (C) 5-D Itch scale.
Figure S2: Improvement in 5-D Itch total score in the pooled KALM-1 and KALM-2 studies.
Figure S3: Subgroup analyses for ≥4-point WI-NRS response at week 12.
Item S1: Supplementary methods.
Table S1: Demographics and Participant Characteristics at Baseline in KALM-1 and KALM-2 Phase 3 Studies.
Table S2: Summary of Efficacy Outcomes in KALM-1 and KALM-2.
Supplementary Material
Figures S1-S3; Item S1; Tables S1-S2.
References
- 1.Mettang T., Kremer A.E. Uremic pruritus. Kidney Int. 2015;87(4):685–691. doi: 10.1038/ki.2013.454. [DOI] [PubMed] [Google Scholar]
- 2.Pisoni R.L., Wikström B., Elder S.J., et al. Pruritus in haemodialysis patients: international results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2006;21(12):3495–3505. doi: 10.1093/ndt/gfl461. [DOI] [PubMed] [Google Scholar]
- 3.Rayner H.C., Larkina M., Wang M., et al. International comparisons of prevalence, awareness, and treatment of pruritus in people on hemodialysis. Clin J Am Soc Nephrol. 2017;12(12):2000–2007. doi: 10.2215/CJN.03280317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sukul N., Karaboyas A., Csomor P.A., et al. Self-reported pruritus and clinical, dialysis-related, and patient-reported outcomes in hemodialysis patients. Kidney Med. 2021;3(1):42–53.e1. doi: 10.1016/j.xkme.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satti M.Z., Arshad D., Javed H., et al. Uremic pruritus: prevalence and impact on quality of life and depressive symptoms in hemodialysis patients. Cureus. 2019;11(7):e5178. doi: 10.7759/cureus.5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rehman I.U., Lai P.S.M., Lim S.K., Lee L.H., Khan T.M. Sleep disturbance among Malaysian patients with end-stage renal disease with pruritus. BMC Nephrol. 2019;20(1):102. doi: 10.1186/s12882-019-1294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonsen E., Komenda P., Lerner B., et al. Treatment of uremic pruritus: a systematic review. Am J Kidney Dis. 2017;70(5):638–655. doi: 10.1053/j.ajkd.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 8.Hercz D., Jiang S.H., Webster A.C. Interventions for itch in people with advanced chronic kidney disease. Cochrane Database Syst Rev. 2020;12(12):CD011393. doi: 10.1002/14651858.CD011393.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozono H., Yoshitani H., Nakano R. Post-marketing surveillance study of the safety and efficacy of nalfurafine hydrochloride (Remitch® capsules 2.5 μg) in 3,762 hemodialysis patients with intractable pruritus. Int J Nephrol Renovasc Dis. 2018;11:9–24. doi: 10.2147/IJNRD.S145720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phan N.Q., Lotts T., Antal A., Bernhard J.D., Ständer S. Systemic kappa opioid receptor agonists in the treatment of chronic pruritus: a literature review. Acta Derm Venereol. 2012;92(5):555–560. doi: 10.2340/00015555-1353. [DOI] [PubMed] [Google Scholar]
- 11.Seki T., Awamura S., Kimura C., et al. Pharmacological properties of TRK-820 on cloned mu-, delta- and kappa-opioid receptors and nociceptin receptor. Eur J Pharmacol. 1999;376(1-2):159–167. doi: 10.1016/s0014-2999(99)00369-6. [DOI] [PubMed] [Google Scholar]
- 12.Trachtenberg A.J., Collister D., Rigatto C. Recent advances in the treatment of uremic pruritus. Curr Opin Nephrol Hypertens. 2020;29(5):465–470. doi: 10.1097/MNH.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 13.Spencer R.H., Lewis M.E., Stauffer J.W., Mathur V.S., Menzaghi F. Antipruritic effect of the long-acting peripheral kappa opioid receptor agonist CR845: a novel approach for the treatment of uremic pruritus in hemodialysis patients [abstract] J Am Soc Nephrol. 2016;27:338A. [Google Scholar]
- 14.Fishbane S., Jamal A., Munera C., Wen W., Menzaghi F. KALM-1 Trial Investigators. A phase 3 trial of difelikefalin in hemodialysis patients with pruritus. N Engl J Med. 2020;382(3):222–232. doi: 10.1056/NEJMoa1912770. [DOI] [PubMed] [Google Scholar]
- 15.Korsuva (Difelikefalin) [Package Insert]. Cara Therapeutics, Inc.; 2021. https://www.korsuva.com/pi [Google Scholar]
- 16.Kapruvia Summary of Product Characteristics; 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/kapruvia [Google Scholar]
- 17.Wooldridge T.D., Mccafferty K., Schoemig M., et al. Efficacy and safety of difelikefalin for moderate-to-severe CKD–associated pruritus: a global phase 3 study in hemodialysis patients (KALM-2) [abstract FR-OR24] J Am Soc Nephrol. 2020;31(Suppl):22–23. [Google Scholar]
- 18.Wooldridge T.D., Mccafferty K., Schoemig M., et al. Presented at: Annual Meeting of the American Society of Nephrology. October 20-25, 2020. Efficacy and safety of difelikefalin for moderate-to-severe chronic kidney disease–associated pruritus: a global phase 3 study in hemodialysis patients (KALM-2) [Google Scholar]
- 19.Vernon M., Ständer S., Munera C., Spencer R.H., Menzaghi F. Clinically meaningful change in itch intensity scores: an evaluation in patients with chronic kidney disease-associated pruritus. J Am Acad Dermatol. 2021;84(4):1132–1134. doi: 10.1016/j.jaad.2020.06.991. [DOI] [PubMed] [Google Scholar]
- 20.Vernon M.K., Swett L.L., Speck R.M., et al. Psychometric validation and meaningful change thresholds of the Worst Itching Intensity Numerical Rating Scale for assessing itch in patients with chronic kidney disease-associated pruritus. J Patient Rep Outcomes. 2021;5(1):134. doi: 10.1186/s41687-021-00404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathur V.S., Lindberg J., Germain M., et al. A longitudinal study of uremic pruritus in hemodialysis patients. Clin J Am Soc Nephrol. 2010;5(8):1410–1419. doi: 10.2215/CJN.00100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elman S., Hynan L.S., Gabriel V., Mayo M.J. The 5-D itch scale: a new measure of pruritus. Br J Dermatol. 2010;162(3):587–593. doi: 10.1111/j.1365-2133.2009.09586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albert-Vartanian A., Boyd M.R., Hall A.L., et al. Will peripherally restricted kappa-opioid receptor agonists (pKORAs) relieve pain with less opioid adverse effects and abuse potential? J Clin Pharm Ther. 2016;41(4):371–382. doi: 10.1111/jcpt.12404. [DOI] [PubMed] [Google Scholar]
- 24.Ishida J.H., McCulloch C.E., Steinman M.A., Grimes B.A., Johansen K.L. Gabapentin and pregabalin use and association with adverse outcomes among hemodialysis patients. J Am Soc Nephrol. 2018;29(7):1970–1978. doi: 10.1681/ASN.2018010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fishbane S., Wen W., Munera C. Safety and tolerability of difelikefalin for the treatment of moderate-to-severe pruritus in hemodialysis patients: pooled analysis from the phase 3 clinical trial program. Kidney Med. 2022 doi: 10.1016/j.xkme.2022.100513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simpson E.L., Bieber T., Guttman-Yassky E., et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1-S3; Item S1; Tables S1-S2.