Key Points
Question
What are the incidence and clinical features of postacute sequelae of SARS-CoV-2 infection (PASC) in children?
Findings
In this cohort study including 659 286 children tested for SARS-CoV-2 by antigen or polymerase chain reaction, the symptom, condition, and medication with the strongest associations with SARS-CoV-2 infection were loss of taste/smell, myocarditis, and cough and cold preparations, respectively. The incidence proportion of non–multisystem inflammatory syndrome in children–related PASC in the viral test–positive group exceeded the incidence proportion in the viral test–negative group by 3.7%, with increased rates associated with acute illness severity, young age, and medical complexity.
Meaning
PASC in children appears to be uncommon, with features that differ from adults.
This cohort study evaluates diagnosed symptoms, diagnosed health conditions, and medications associated with postacute sequelae of SARS-CoV-2 infection in children.
Abstract
Importance
The postacute sequelae of SARS-CoV-2 infection (PASC) has emerged as a long-term complication in adults, but current understanding of the clinical presentation of PASC in children is limited.
Objective
To identify diagnosed symptoms, diagnosed health conditions, and medications associated with PASC in children.
Design, Setting and Participants
This retrospective cohort study used electronic health records from 9 US children’s hospitals for individuals younger than 21 years who underwent antigen or reverse transcriptase–polymerase chain reaction (RT-PCR) testing for SARS-CoV-2 between March 1, 2020, and October 31, 2021, and had at least 1 encounter in the 3 years before testing.
Exposures
SARS-CoV-2 positivity by viral test (antigen or RT-PCR).
Main Outcomes and Measures
Syndromic (symptoms), systemic (conditions), and medication PASC features were identified in the 28 to 179 days following the initial test date. Adjusted hazard ratios (aHRs) were obtained for 151 clinically predicted PASC features by contrasting viral test–positive groups with viral test–negative groups using proportional hazards models, adjusting for site, age, sex, testing location, race and ethnicity, and time period of cohort entrance. The incidence proportion for any syndromic, systemic, or medication PASC feature was estimated in the 2 groups to obtain a burden of PASC estimate.
Results
Among 659 286 children in the study sample, 348 091 (52.8%) were male, and the mean (SD) age was 8.1 (5.7) years. A total of 59 893 (9.1%) tested positive by viral test for SARS-CoV-2, and 599 393 (90.9%) tested negative. Most were tested in outpatient testing facility settings (322 813 [50.3%]) or office settings (162 138 [24.6%]). The most common syndromic, systemic, and medication features were loss of taste or smell (aHR, 1.96; 95% CI, 1.16-3.32), myocarditis (aHR, 3.10; 95% CI, 1.94-4.96), and cough and cold preparations (aHR, 1.52; 95% CI, 1.18-1.96), respectively. The incidence of at least 1 systemic, syndromic, or medication feature of PASC was 41.9% (95% CI, 41.4-42.4) among viral test–positive children vs 38.2% (95% CI, 38.1-38.4) among viral test–negative children, with an incidence proportion difference of 3.7% (95% CI, 3.2-4.2). A higher strength of association for PASC was identified in those cared for in the intensive care unit during the acute illness phase, children younger than 5 years, and individuals with complex chronic conditions.
Conclusions and Relevance
In this large-scale, exploratory study, the burden of pediatric PASC that presented to health systems was low. Myocarditis was the most commonly diagnosed PASC-associated condition. Acute illness severity, young age, and comorbid complex chronic disease increased the risk of PASC.
Introduction
Scientific and clinical evidence is evolving on the sequelae following COVID-19 caused by SARS-CoV-2. Postacute sequelae of SARS-CoV-2 infection (PASC) has a broad spectrum of clinical manifestations that can affect multiple organ systems. The National Institutes of Health definition of PASC refers to ongoing, relapsing, or new symptoms or other health effects occurring after the acute phase of SARS-CoV-2 infection that is present 4 or more weeks after the acute infection.1 As defined by the US Centers for Disease Control and Prevention, post–COVID-19 conditions include a wide range of new, recurring, or persistent health problems people experience 4 or more weeks after being infected with SARS-CoV-2.2 Alternatively, the World Health Organization defines post–COVID-19 conditions as those occurring 3 or more months from the onset of SARS-CoV-2 infection that cannot be explained by an alternative diagnosis and last for at least 2 months, acknowledging that a separate definition may be applicable for children.3
In adults, PASC is characterized by 2 types of manifestations: (1) persistent, intermittent, or relapsing nonspecific symptoms, such as fatigue, headache, and shortness of breath (ie, a syndromic variant) and (2) conditions that may be a direct result of the acute illness presentation, such as pulmonary fibrosis, or that may arise de novo after infection, such as autoimmune conditions (ie, a systemic variant).4 While more data are accruing in adults,5,6,7,8,9,10,11,12 our understanding of PASC is still limited in children, with the exception of multisystem inflammatory syndrome in children (MIS-C).13 There is an urgent need to elucidate the incidence, clinical features, and duration of PASC in children, with standardized definitions and data collection methods.14
There is wide variability in prevalence estimates of PASC in children ranging from 2% to 66% because of the marked heterogeneity in case ascertainment and methodology of existing studies as well as lack of an adequate comparison group.15,16,17,18,19,20 It remains unclear to what extent children experience SARS-CoV-2 sequelae and how PASC features may differ from those in adults. In this study, our objectives were to (1) identify syndromic (symptoms) and systemic (conditions) features as well as medications used to treat PASC in the 1 to 6 months following SARS-CoV-2 infection in children and adolescents, (2) estimate the incidence of any PASC feature attributable to SARS-CoV-2 infection, and (3) identify risk factors for PASC.
Methods
Data Source
This retrospective cohort study is part of the National Institute of Health Researching COVID to Enhance Recovery (RECOVER) Initiative, which seeks to understand, treat, and prevent PASC.1 We used electronic health record (EHR) data from PEDSnet institutions for the study. PEDSnet is a multi-institutional clinical research network that aggregates EHR data from several of the nation’s largest children’s health care organizations.21,22 Participating institutions included Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania; Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; Children’s Hospital Colorado, Aurora; Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, Illinois; Nationwide Children’s Hospital, Columbus, Ohio; Nemours Children’s Hospital, Wilmington, Delaware; Nemours Children’s Hospital, Orlando, Florida; Seattle Children’s Hospital, Seattle, Washington; and Stanford Children’s Health, Stanford, California. PEDSnet standardizes institutional data to the Observational Medical Outcomes Partnership common data model. PEDSnet has accrued data for more than 8 million children with at least 1 visit from 2009 to 2021 from inpatient and outpatient clinical settings. Reporting of study design and results follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for observational research.23 The Children’s Hospital of Philadelphia’s Institutional Review Board designated this study as not human subjects research and waived informed consent.
We retrieved EHR data from all health care encounters associated with patients who underwent SARS-CoV-2 viral testing (antigen or polymerase chain reaction [PCR]) provided in outpatient, inpatient, and emergency department settings. Data were extracted from the PEDSnet COVID-19 Database version 2022-01-16 on January 25, 2022, and included EHR data with dates of services up to December 31, 2021.
Cohorts
Infected cohorts were individuals younger than 21 years at the time of the health encounter with positive SARS-CoV-2 viral test findings between March 1, 2020, and October 31, 2021. We compared this group with a cohort of children with negative SARS-CoV-2 viral test findings (and no prior or subsequent positive findings) during the same study period. Children with a positive serology test result or with MIS-C/PASC diagnosis codes with negative viral test results were also excluded from the comparison group. Cohort entry was defined by the date of the first positive test result (infected) or first negative test result. We further restricted the sample to those with at least 1 encounter (including telephone or telehealth encounters) within health systems from day 7 to day 1095 before cohort entry to ensure prior care at a PEDSnet institution.
Outcomes
We developed International Statistical Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) syndromic (symptoms), systemic (health conditions), and medication (therapeutic classes) clusters of codes, which served as outcomes. We coded 66 294 ICD-10-CM codes into 439 deduplicated clusters, beginning with the widely used Agency for Healthcare Research and Quality Clinical Classification Software Refined24 and extending the categories to include more refined autoimmune, mental health disorders, and COVID-19–related complication categories. ICD codes were obtained from all service settings, including telephone encounters, inpatient clinics, outpatient clinics, and emergency departments. Syndromic clusters included symptoms such as fever, cough, fatigue, shortness of breath, chest pain, palpitations, chest tightness, headache, and altered smell and taste. Systemic clusters included such conditions as multi-inflammatory syndrome, myocarditis, diabetes, and other autoimmune diseases. We clustered 2172 RxNorm medication ingredient codes into 228 Anatomical Therapeutic Chemical classification level 3 classes for prescribed medications that were listed in the record during the outcome evaluation period.25 Examples include antiarrhythmics, opioids, and systemic corticosteroids. The full list of the code assignments to clusters is publicly available.26
To evaluate their clinical content validity, all clusters were reviewed by experts in pediatric psychiatry, infectious diseases, cardiology, pulmonology, and primary care. We narrowed this list to an a priori set of 121 syndromic and systemic clusters and 30 medication clusters that were clinically predicted to be associated with PASC based on our review of the literature, clinical experience, and potential medications used to treat PASC-associated symptoms and conditions.
The outcome assessment follow-up time period spanned 28 to 179 days after cohort entrance or up to December 31, 2021, whichever event came first. For chronic conditions (eg, asthma, diabetes), we excluded patients from the denominator if they had evidence of that condition in the 18 months before cohort entrance.
Covariates
Age was assessed at cohort entry. Other covariates were sex, race and ethnicity, institution, obesity, testing location (emergency department, inpatient, outpatient clinic, or outpatient testing facility), intensive care unit care 7 days before through 13 days after cohort entrance, and medical complexity. Obesity was defined as the presence of an age- and sex-standardized body mass index z score in the 95th percentile or higher any time before cohort entrance. We used the Pediatric Medical Complexity Algorithm version 2.027 to categorize children as having no chronic condition, noncomplex chronic condition, or complex chronic conditions. We considered diagnoses up to 3 years before cohort entrance. Children were assigned to the complex chronic condition category if they had conditions that affected 2 or more body systems (eg, type 1 diabetes with end-organ complications) or a progressive chronic condition (eg, muscular dystrophy).28
Statistical Analysis
We used descriptive analyses to report characteristics of tested patients, divided into viral test–positive and –negative groups, and contrasted the groups using standardized differences29 and set a cut point of more than 0.20 as a meaningful difference. To explore PASC, we evaluated each clinically predicted symptom, condition, and medication as a separate outcome for a total of 121 syndromic and systemic clusters and 30 classes of medications. This was done using Cox proportional hazards models, adjusting for institution, age group (younger than 1 year, age 1 to 4 years, age 5 to 11 years, age 12 to 15 years, and age 16 to younger than 21 years), sex, race and ethnicity, time period of cohort entrance (March to June 2020, July to October 2020, November 2020 to February 2021, March to June 2021, and July to October 2021), location of testing (emergency department, inpatient, outpatient office, outpatient test only, and other or unknown), and medical complexity. Each regression model produced an adjusted hazard ratio (aHR) and 95% CIs that contrasted the risk (ie, incidence) of the feature in the viral test–positive cohort with the viral test–negative cohort. aHRs for which the 95% CI lower limit was greater than 1.00 were considered to be empirically supported. We displayed these aHRs in forest plots and used this subset in further analyses. This approach permitted evaluation of all encounters in the outcome evaluation period, thus minimizing the risk of bias that may result from children returning from care within the health system vs those who did not. We implemented a false discovery rate adjustment for our P values and 95% CIs in our forest plots. This multiple-testing adjustment procedure controls for the proportion of false coverage statements expressed by the reported CIs to be less than .05.30 All P values were 2-tailed. We additionally produced Kaplan-Meier plots for risk of a syndromic feature (eFigure 2 in the Supplement), systemic feature (eFigure 3 in the Supplement), medication feature (eFigure 4 in the Supplement), or any of the 3 kinds of features (eFigure 5 in the Supplement).
To estimate the incidence of non–MIS-C PASC, we excluded children with MIS-C diagnoses and calculated the difference in the incidence proportions for any clinically predicted and empirically supported syndromic-related, systemic-related, or medication-related PASC feature between children in the SARS-CoV-2 viral test–positive group and those with at least one PASC feature in the SARS-CoV-2 test negative group.
We calculated the standardized morbidity ratio by applying the age, viral testing date, and specific PASC incidence rates (ie, 25 different rates) for the viral test–negative group to the viral test–positive group to obtain expected counts and contrasted those with the number observed. Finally, we evaluated the risk of any syndromic, systemic, or medication feature by age, comorbidity, and illness severity to identify features associated with the risk of non–MIS-C PASC. Analyses were conducted using R version 4.1.2 (The R Foundation).
Results
Cohort Identification
From March 1, 2020, to October 31, 2021, there were 1 782 537 patients younger than 21 years, of which 817 505 patients (45.9%) were tested for SARS-CoV-2 by viral testing (antigen or PCR) across all sites; of these, 659 286 had at least 1 visit in the 7 to 1095 days before the test date. Among 659 286 children in the study sample, 348 091 (52.8%) were male, and the mean (SD) age was 8.1 (5.7) years. A total of 59 893 (9.1%) tested positive by viral testing for SARS-CoV-2, and 599 393 (90.9%) tested negative. The follow-up time during the outcome assessment period for both positive and negative groups were similar (mean [SD] follow-up, 4.6 [0.7] months vs 4.7 [0.7] months). Most patients were tested in outpatient testing facility settings (322 813 [50.3%]) or outpatient clinic settings (162 138 [24.6%]). Viral test–positive patients compared with viral test–negative patients were more likely to be non-Hispanic Black (12 121 of 59 893 [20.2%] vs 92 497 of 599 393 [15.4%]), Hispanic (11 252 [18.8%] vs 91 896 [15.3%]), and older (age 16 to younger than 21 years, 10 416 [17.4%] vs 72 787 [12.1%]; age 12 to 15 years, 11 918 [19.9%] vs 92 552 [15.4%]). The time periods with the highest SARS-CoV-2 percentage of viral test positivity were November 2020 to February 2021 and July to October 2021 (Table 1). From our cohort of 1 782 537 patients, there were 1260 children with a diagnosis of MIS-C (7.1 cases per 10 000 population), of whom 155 tested positive for SARS-CoV-2.
Table 1. Study Sample Characteristics.
| Characteristic | No. (%) | Standardized differencea | ||
|---|---|---|---|---|
| Overall (N = 659 286) | SARS-CoV-2 viral test result | |||
| Negative (n = 599 393) | Positive (n = 59 893) | |||
| Age at cohort entrance, mean (SD), y | 8.1 (5.7) | 7.9 (5.7) | 9.4 (5.9) | .26 |
| Age at cohort entrance, y | ||||
| <1 | 58 660 (8.9) | 54 133 (9.0) | 4527 (7.6) | .25 |
| 1-4 | 195 269 (29.6) | 182 252 (30.4) | 13 017 (21.7) | |
| 5-11 | 217 684 (33.0) | 197 669 (33.0) | 20 015 (33.4) | |
| 12-15 | 104 470 (15.8) | 92 552 (15.4) | 11 918 (19.9) | |
| 16-<21 | 83 203 (12.6) | 72 787 (12.1) | 10 416 (17.4) | |
| Follow-up during outcome assessment period, mean (SD), mo | 4.7 (0.7) | 4.7 (0.7) | 4.6 (0.7) | .13 |
| Sex | ||||
| Female | 311 164 (47.2) | 281 998 (47.0) | 29 166 (48.7) | .03 |
| Male | 348 091 (52.8) | 317369 (53.0) | 30 722 (51.3) | |
| Race and ethnicityb | ||||
| Non-Hispanic Asian or Pacific Islander | 28 832 (4.4) | 27 108 (4.5) | 1724 (2.9) | .19 |
| Non-Hispanic Black | 104 618 (15.9) | 92 497 (15.4) | 12 121 (20.2) | |
| Hispanic | 103 148 (15.6) | 91 896 (15.3) | 11 252 (18.8) | |
| Multiple races | 26 832 (4.1) | 24 485 (4.1) | 2347 (3.9) | |
| Non-Hispanic White | 342 935 (52.0) | 314 782 (52.5) | 28 153 (47.0) | |
| Other race or unknown | 52 921 (8.0) | 48 625 (8.1) | 4296 (7.2) | |
| Institution | ||||
| Site A | 32 922 (5.0) | 31 987 (5.3) | 935 (1.6) | .29 |
| Site B | 83 726 (12.7) | 76 387 (12.7) | 7339 (12.3) | |
| Site C | 41 616 (6.3) | 39 403 (6.6) | 2213 (3.7) | |
| Site D | 125 243 (19.0) | 110 400 (18.4) | 14 843 (24.8) | |
| Site E | 103 830 (15.9) | 94 557 (15.8) | 10 273 (17.2) | |
| Site F | 134 054 (20.3) | 121 725 (20.3) | 12 329 (20.6) | |
| Site G | 97 447 (14.8) | 88 134 (14.7) | 9313 (15.5) | |
| Site H | 39 448 (6.0) | 36 800 (6.1) | 2648 (4.4) | |
| Chronic conditions | ||||
| None | 462 248 (70.1) | 419 152 (69.9) | 43 096 (72.0) | .06 |
| Noncomplex | 104 144 (15.8) | 94 647 (15.8) | 9497 (15.9) | |
| Complex | 92 894 (14.1) | 85 594 (14.3) | 7300 (12.2) | |
| Time period of cohort entrance | ||||
| March 2020-June 2020 | 55 753 (8.5) | 53 590 (8.9) | 2163 (3.6) | .53 |
| July 2020-October 2020 | 146 054 (22.2) | 139 587 (23.3) | 6467 (10.8) | |
| November 2020-February 2021 | 161 986 (24.6) | 138 120 (23.0) | 23 866 (39.8) | |
| March 2021-June 2021 | 129 171 (19.6) | 120 262 (20.1) | 8909 (14.9) | |
| July 2021-October 2021 | 166 322 (25.2) | 147 834 (24.7) | 18 488 (30.9) | |
| Obesity | 164 895 (25.0) | 148 499 (24.8) | 16 396 (27.4) | .06 |
| Admitted to intensive care unit | 14 521 (2.2) | 13 847 (2.3) | 674 (1.1) | .09 |
| Test location | ||||
| Emergency department | 125 797 (19.1) | 114 227 (19.1) | 11 570 (19.3) | .19 |
| Inpatient | 39 513 (6.0) | 37 857 (6.3) | 1656 (2.8) | |
| Outpatient clinic | 162 138 (24.6) | 148 371 (24.8) | 13 767 (23.0) | |
| Outpatient testing facility | 332 813 (50.3) | 298 918 (49.9) | 32 895 (54.9) | |
For continuous variables, the standardized difference was computed as the difference in means divided by the pooled standard deviation. For categorical variables, the standardized difference was calculated from a distance matrix on a vector difference between categories, normalized by a covariate matrix calculated from the rates of categories in each of the 2 groups.
Race and ethnicity data were collected by self-report. The other race category includes patients whose race was categorized as American Indian or Alaska Native (1088 patients) or other (12 004 patients).
Syndromic and Systemic PASC Features
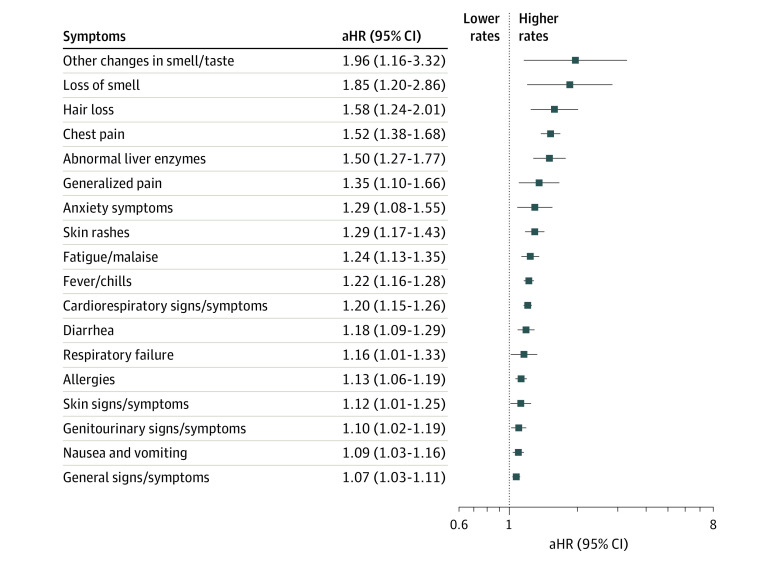
Adjusted analyses of syndromic and systemic features of PASC by cluster category that were clinically predicted and empirically supported are summarized in Figure 1 and Figure 2 (those that were clinically predicted but not empirically supported and vice versa are listed in the eTable in the Supplement). During the outcome assessment period (days 28 to 179), viral test–positive children compared with viral test–negative individuals had higher rates of changes in smell and taste (aHR, 1.96; 95% CI, 1.16-3.32), loss of smell (aHR, 1.85; 95% CI, 1.20-2.86), hair loss (aHR, 1.58; 95% CI, 1.24-2.01), chest pain (aHR, 1.52; 95% CI, 1.38-1.68), abnormal liver enzymes (aHR, 1.50; 95% CI, 1.27-1.77), skin rashes (aHR, 1.26; 95% CI, 1.15-1.38), fatigue and malaise (aHR, 1.24; 95% CI, 1.13-1.35), fever and chills (aHR, 1.22; 95% CI, 1.16-1.28), cardiorespiratory signs and symptoms (aHR, 1.20; 95% CI, 1.15-1.26), and diarrhea (aHR, 1.18; 95% CI, 1.09-1.29) (Figure 1).
Figure 1. Syndromic Features of Postacute Sequelae of SARS-CoV-2 Infection.
Adjusted hazard ratios (aHRs) with associated 95% CIs among patients who tested positive for SARS-CoV-2 infection vs those who tested negative for the risk of each syndromic feature using Cox proportional hazards models. Models were adjusted for age at cohort entrance, sex, race and ethnicity, institution, testing place location, presence of a complex medical condition, and date of cohort entrance.
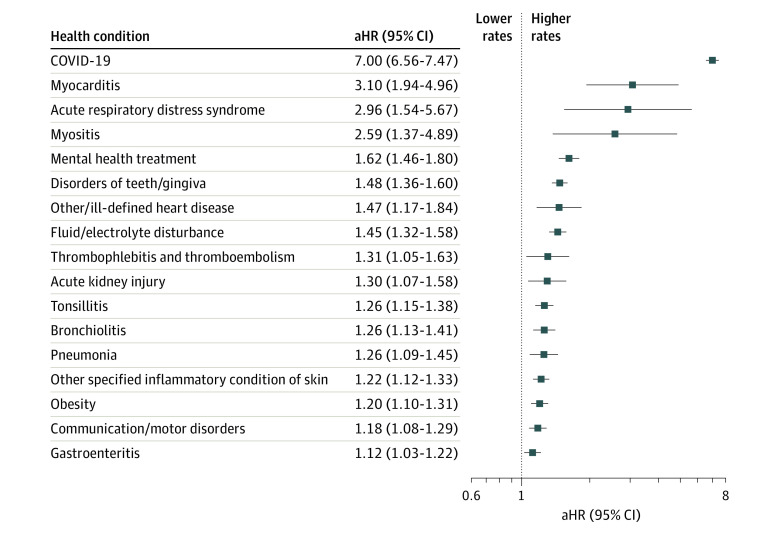
Figure 2. Systemic Features of Postacute Sequelae of SARS-CoV-2 Infection.
Adjusted hazard ratios (aHRs) with associated 95% CIs among patients who tested positive for SARS-CoV-2 infection vs those who tested negative for the risk of each systemic feature using Cox proportional hazards models. Models were adjusted for age at cohort entrance, sex, race and ethnicity, institution, testing place location, and date of cohort entrance. For each health condition evaluated, patients with evidence of that condition 18 months before cohort entrance were excluded from the denominator to identify incident cases. Each ratio compares the risk of the outcome in children who tested positive for SARS-CoV-2 infection vs those who tested negative. The diagnostic cluster for COVID-19 indicates children receiving care for the illness in the postacute period.
Regarding systemic features, infected cohorts had higher rates of myocarditis (aHR, 3.10; 95% CI, 1.94-4.96), acute respiratory distress syndrome (aHR, 2.96; 95% CI, 1.54-5.67), myositis (aHR, 2.59; 95% CI, 1.37-4.89), disorders of teeth or gingiva (aHR, 1.48; 95% CI, 1.36-1.60), other or ill-defined heart disease (aHR, 1.47; 95% CI, 1.17-1.84), and fluid and electrolyte disturbance (aHR, 1.45; 95% CI, 1.32-1.58) (Figure 2). Mental health clusters associated with prior SARS-CoV-2 infection in our analyses were mental health treatment (aHR, 1.62; 95% CI, 1.46-1.80) and anxiety symptoms (aHR, 1.29; 95% CI, 1.08-1.55).
Medications
Medication therapeutic classes clinically predicted and empirically supported for their association with COVID-19 illness are shown in eFigure 1 in the Supplement. The 5 most common were cough and cold preparations, nasal decongestants for systemic use, corticosteroids with antiseptics, opioids, and decongestants.
Incidence of PASC
The incidence proportions for non–MIS-C–related syndromic, systemic, and medication features during the 28 to 179 days after viral testing and their standardized morbidity ratios of testing adjusted for age and date are shown in Table 2. The incidence proportion of at least 1 syndromic, systemic, or medication feature of PASC was 41.9% (95% CI, 41.4-42.4) in the viral test–positive group and 38.2% (95% CI, 38.1-38.4) in the viral test–negative group, for a difference of 3.7% (95% CI, 3.2-4.2). The standardized morbidity ratio, which adjusted for differences in the distributions of age and date of cohort entrance between the 2 groups, was 1.15 (95% CI, 1.14-1.17).
Table 2. Incidence Proportions for Syndromic, Systemic, and Medication Features of Postacute Sequelae of SARS-CoV-2 Infection (PASC) Unrelated to Multisystem Inflammatory Syndrome in Children on Observed Day 28 to 179 After Viral Testing and Standardized Morbidity Ratios Adjusted for Patient Age and Date of Viral Testing.
| PASC feature | Incidences, No. | Persons, No. | Incidence proportion, % (95% CI) | Incidence proportion difference, % (95% CI) | Standardized morbidity ratio | |||
|---|---|---|---|---|---|---|---|---|
| Viral test result positive | Viral test result negative | Viral test result positivea | Viral test result negative | Viral test result positive | Viral test result negative | |||
| Syndromicb | 10 657 | 108 906 | 54 061 | 548 304 | 19.7 (19.4-20.0) | 19.9 (19.8-20.0) | −0.1 (−0.5 to 0.2) | 1.07 (1.05-1.09) |
| Systemicb | 7084 | 50 786 | 52 027 | 529 741 | 13.6 (13.3-13.9) | 9.6 (9.5-9.7) | 4.0 (3.7-4.3) | 1.49 (1.45-1.52) |
| Medication | 7871 | 73 651 | 40 742 | 425 741 | 19.3 (18.9-19.7) | 17.3 (17.2-17.4) | 2.0 (1.6-2.4) | 1.19 (1.16-1.22) |
| Syndromic or systemicc | 14 418 | 133 328 | 50 180 | 510 045 | 28.7 (28.3-29.1) | 26.1 (26.0-26.3) | 2.6 (2.2-3.0) | 1.16 (1.14-1.18) |
| Syndromic, systemic, or medicationd | 17 498 | 162 228 | 41 756 | 424 215 | 41.9 (41.4-42.4) | 38.2 (38.1-38.4) | 3.7 (3.2-4.2) | 1.15 (1.14-1.17) |
Number of viral test–positive and –negative patients differs across PASC features due to washout of chronic conditions.
Any syndromic and any systemic refers to the features in Figures 1 and 2, respectively, that were clinically predicted or plausible and empirically supported in the proportional hazards model.
Either syndromic or systemic refers to the union of the features from any syndromic or systemic.
Syndromic, systemic, or medication refers to the union of the features from any syndromic, systemic, or medication.
Factors Associated With Non–MIS-C–Related PASC
Proportional hazards models for non–MIS-C–related PASC, defined by any clinically predicted and empirically supported syndromic features, systemic conditions, and medication features are shown in Table 3. These analyses demonstrated the highest rate of any PASC feature in children younger than 5 years, requiring intensive care unit–level care during the acute illness episode, or with a complex chronic condition. The risk of non–MIS-C–related PASC was highest during the March to June 2020 time period.
Table 3. Proportional Hazards Models for Postacute Sequelae of SARS-CoV-2 Infection Unrelated to Multisystem Inflammatory Syndrome in Children Defined by Syndromic Features, Systemic Conditions, Medications Features, or All 3a.
| Predictor | Adjusted hazard ratios (95% CI) | |||
|---|---|---|---|---|
| Syndromic | Systemic | Medication | Any syndromic, systemic, or medication | |
| Viral test result and ICU level care | ||||
| Viral test–negative and no ICU care | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Viral test–positive and no ICU care | 1.10 (1.07-1.12) | 1.54 (1.50-1.58) | 1.25 (1.22-1.28) | 1.22 (1.20-1.24) |
| Viral test–negative with ICU care | 1.22 (1.19-1.26) | 1.49 (1.43-1.55) | 2.00 (1.94-2.07) | 1.35 (1.32-1.39) |
| Viral test–positive with ICU care | 1.99 (1.78-2.23) | 3.40 (2.99-3.86) | 2.76 (2.39-3.18) | 2.11 (1.91-2.34) |
| Age, y | ||||
| <1 | 2.35 (2.31-2.39) | 2.08 (2.03-2.14) | 2.15 (2.10-2.20) | 1.86 (1.83-1.89) |
| 1-4 | 1.48 (1.46-1.50) | 1.57 (1.54-1.61) | 1.55 (1.52-1.58) | 1.49 (1.47-1.50) |
| 5-11 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 12-15 | 1.06 (1.04-1.08) | 1.54 (1.50-1.58) | 1.19 (1.16-1.21) | 1.24 (1.22-1.26) |
| 16-20 | 0.99 (0.97-1.01) | 1.42 (1.38-1.46) | 1.21 (1.18-1.24) | 1.16 (1.14-1.18) |
| Sex | ||||
| Female | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Male | 0.93 (0.92-0.94) | 1.05 (1.03-1.07) | 0.98 (0.97-0.99) | 0.97 (0.96-0.98) |
| Race and ethnicityb | ||||
| Asian/Pacific Islander | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Non-Hispanic Black | 1.04 (1.01-1.08) | 1.15 (1.10-1.20) | 1.42 (1.36-1.47) | 1.20 (1.17-1.23) |
| Hispanic | 1.00 (0.97-1.03) | 1.15 (1.10-1.20) | 1.25 (1.20-1.29) | 1.11 (1.08-1.13) |
| Multiple races | 0.96 (0.92-1.00) | 1.03 (0.97-1.09) | 1.03 (0.98-1.08) | 0.98 (0.95-1.02) |
| White | 0.85 (0.83-0.88) | 0.83 (0.80-0.87) | 0.87 (0.84-0.90) | 0.84 (0.83-0.86) |
| Other or unknown | 0.82 (0.79-0.84) | 0.80 (0.76-0.84) | 0.73 (0.70-0.77) | 0.78 (0.76-0.80) |
| Viral testing date | ||||
| March-June 2020 | 1.38 (1.35-1.41) | 1.31 (1.27-1.35) | 1.59 (1.55-1.64) | 1.42 (1.40-1.45) |
| July-October 2020 | 1.13 (1.11-1.15) | 1.06 (1.04-1.09) | 1.12 (1.09-1.14) | 1.12 (1.10-1.13) |
| November 2020-February 2021 | 1.17 (1.15-1.19) | 1.13 (1.10-1.16) | 1.13 (1.10-1.15) | 1.10 (1.09-1.12) |
| March-June 2021 | 1.28 (1.26-1.30) | 1.28 (1.25-1.31) | 1.33 (1.31-1.36) | 1.20 (1.19-1.22) |
| July-November 2021 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Chronic conditions | ||||
| None | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Noncomplex | 1.58 (1.56-1.61) | 1.62 (1.58-1.65) | 1.84 (1.80-1.87) | 1.92 (1.89-1.94) |
| Complex | 3.33 (3.29-3.38) | 3.30 (3.23-3.36) | 3.64 (3.57-3.70) | 3.68 (3.64-3.72) |
Abbreviation: ICU, intensive care unit.
Regression models are adjusted for institution.
Race and ethnicity data were collected by self-report. The other race category includes patients whose race was categorized as American Indian or Alaska Native or as other.
Discussion
In this exploratory study, we identified possible clinical features of PASC, including symptoms, conditions, and medications. Our results showed features that have been well described in adult populations, including changes in taste or smell, chest pain, fatigue or malaise, cardiorespiratory sign or symptoms, and fever or chills. In this pediatric population, however, we also note other features, such as abnormal liver enzymes, hair loss, skin rashes, and diarrhea, which occurred more commonly in children in the 1 to 6 months after SARS-CoV-2 infection compared with viral test–negative patients. Among systemic features, myocarditis was the condition with the strongest association with SARS-CoV-2 infection and is recognized as an important complication in this age group.31,32 Myositis, encounters for mental health treatment, and respiratory infections, including tonsillitis, pneumonia, and bronchiolitis, were also associated with COVID-19.
To estimate the burden of PASC unrelated to MIS-C, we computed the incidence proportion difference of clinically predicted and empirically supported PASC features between viral test–positive and test-negative groups, which was 3.7% (95% CI, 3.2-4.2). This proportion is an estimate of attributable burden of any syndromic-related, systemic-related, or medication-related PASC feature in the sample of children we studied. This estimate should be considered preliminary as it is based entirely on diagnosis and medication codes in EHRs, which depend on clinicians’ coding practices and may exclude children who do not seek health care for PASC symptoms. Nonetheless, the strength of association of PASC increased with acute COVID-19 severity, similar to previous reports,33 and medical complexity.
Early reports of PASC in children reporting higher incidence than in this study were prone to systematic selection biases of cases in the absence of appropriate controls. Patients were recruited from long COVID-19 clinics17,20 or online support groups,34 with data limited to self-reported symptoms, some without a laboratory-confirmed diagnosis of SARS-CoV-2, limiting the ability to differentiate symptoms and conditions attributable to SARS-CoV-2 infection vs illnesses that may have been exacerbated by the situational context of the COVID-19 pandemic (eg, delayed access to health care, prolonged social isolation). Other studies focused on hospitalized patients,35 biasing the sample to patients who were at higher risk of more persistent or severe postacute symptoms. Our study’s strengths lie in capturing patients with viral test–confirmed infection and including a control group of viral test–negative children, thereby minimizing bias from differences in health-seeking behaviors between test-positive and test-negative cohorts. Finally, our population includes children from diverse geographical locations across the US and encompasses ambulatory clinics, outpatient referrals to testing sites, and subspecialty clinics as well as hospitalized patients, which provides a wider spectrum of presentations.
Unlike case series that describe the prevalence of PASC features in a cohort of children previously infected, we compared the incidence rate of these symptoms in test-positive vs test-negative patients to estimate an attributable burden. With this approach, we reported a preliminary estimate of lower incidence of PASC in children compared with adults.5,6,7,8,9 Our findings are similar to recent pediatric studies that use population-based approaches and/or control groups.15,36,37 Notably, the frequency of any PASC-related symptoms is high in both viral test–positive and –negative patients (41.9% [95% CI, 41.4-42.4] vs 38.2% [95% CI, 38.1-38.4]), similar to observations in adult studies.38,39 Nonetheless, the attributable and measurable burden of PASC may still differ in children compared with adults. Potential reasons for these differences may be underrecognition of signs and symptoms associated with PASC because of a dynamic developmental trajectory or age-specific differences in the immune response to infection in PASC related either to ongoing viral replication or an aberrant response.
Limitations
Our findings have limitations that warrant discussion. This EHR-based study identified symptoms, signs, and diagnoses that were significant enough to prompt health service use and be coded by clinicians as a reason for an encounter. Our approach may have missed some findings that may be stored in laboratory, procedural, radiological, and other unstructured text data. The true burden of PASC may be underestimated from EHR data with the potential for infrequent follow-up visits to academic centers of excellence for milder symptoms. For this reason, we limited our cohort to at least 1 visit in the prior 3 years to identify active patients within the health system. Similar strategies have been used in other PEDSnet studies of COVID-1940,41; the benefits of such approaches have been reported previously.42 However, this may underestimate the burden of PASC by excluding previously healthy children who did not have prior health encounters. Next, our test-negative cohort may include individuals with SARS-CoV-2 infection who may have had testing conducted outside our health systems. These limitations may have biased results toward the null. Further, we did not identify specific race or ethnic groups as a risk factor for PASC despite the fact that SARS-CoV-2 disproportionally affects racial and ethnic minority communities, which may reflect differences in care seeking behavior and access to care. Additionally, children who tested positive may be more likely to seek medical care; however, we do not anticipate this to be creating significant bias given our low incident proportion difference, which is similar to findings from other controlled studies.
While there were significant features associated with SARS-CoV-2 infection in our study that overlapped with findings reported in the literature in adults (such as loss of taste and smell43,44), several distinctions are worth noting. First, neurological manifestations, such as headache, vertigo, and paresthesias, which are commonly reported in adults, were not significant findings in our study. In addition, memory loss and brain fog have been documented up to 3 months after SARS-CoV-2 infection in adults6,7,45 but were not identified in our study, possibly due to these symptoms not being adequately captured through diagnosis codes. Next, respiratory symptoms, such as persistent cough and dyspnea, also did not feature as prominently in our study as other observational cohort studies in adults9,46,47,48 and children.17,18,20,37 However, these symptoms were detected through capture of cough and cold preparations in our exploration of medication utilization. Interestingly, during the 1 to 6 months following infection, our study detected increased rates of pneumonia, tonsillitis, and bronchiolitis, which may represent persistent pulmonary symptoms as well as the potential for an increased susceptibility to other infections from lung pathology developing after SARS-CoV-2 infection. While the mechanisms for injury in patients with pulmonary manifestations of PASC are providing some insights in adults,49,50 further research is required to determine how the lung pathophysiology in children differs from adults both in acute COVID-19 infection (for example, increased susceptibility to infection or asthma) and in the mechanisms driving long-term symptoms.
Conclusions
In this large-scale study to understand the features of PASC in children, the burden of PASC appeared to be low and systemic features were predominant and varied. Our findings suggest that the burden and risk windows of PASC may differ between children and adults. Future studies, including long-term prospective studies, such as the National Institutes of Health RECOVER Initiative, are needed to fully elucidate PASC phenotypes.
eTable. Code Sets for Systemic and Syndromic Features of PASC and Medication Therapeutic Classes Associated With PASC
eFigure 1. Medication Therapeutic Classes Associated With PASC
eFigure 2. Kaplan-Meier Plot for Risk of Any Syndromic Feature of PASC
eFigure 3. Kaplan-Meier Plot for Risk of Any Systemic Feature of PASC
eFigure 4. Kaplan-Meier Plot for Risk of Any Medication Feature of PASC
eFigure 5. Kaplan-Meier Plot for Risk of Any Syndromic, Systemic, or Medication Feature of PASC
References
- 1.RECOVER . Homepage. Accessed April 12, 2022, https://recovercovid.org/
- 2.US Centers for Disease Control and Prevention . Interim guidance on evaluating and caring for patients with post-COVID conditions. Accessed December 22, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-index.html
- 3.Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV; WHO Clinical Case Definition Working Group on Post-COVID-19 Condition . A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22(4):e102-e107. doi: 10.1016/S1473-3099(21)00703-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11(1):16144. doi: 10.1038/s41598-021-95565-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626-631. doi: 10.1038/s41591-021-01292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute COVID-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026 [DOI] [PubMed] [Google Scholar]
- 7.Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group . Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. doi: 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C, Huang L, Wang Y, et al. 6-Month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220-232. doi: 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groff D, Sun A, Ssentongo AE, et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open. 2021;4(10):e2128568. doi: 10.1001/jamanetworkopen.2021.28568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lund LC, Hallas J, Nielsen H, et al. Post-acute effects of SARS-CoV-2 infection in individuals not requiring hospital admission: a Danish population-based cohort study. Lancet Infect Dis. 2021;21(10):1373-1382. doi: 10.1016/S1473-3099(21)00211-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radin JM, Quer G, Ramos E, et al. Assessment of prolonged physiological and behavioral changes associated with COVID-19 infection. JAMA Netw Open. 2021;4(7):e2115959. doi: 10.1001/jamanetworkopen.2021.15959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matta J, Wiernik E, Robineau O, et al. ; Santé, Pratiques, Relations et Inégalités Sociales en Population Générale Pendant la Crise COVID-19–Sérologie (SAPRIS-SERO) Study Group . Association of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among French adults during the COVID-19 pandemic. JAMA Intern Med. 2022;182(1):19-25. doi: 10.1001/jamainternmed.2021.6454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos MO, Gonçalves LC, Silva PAN, et al. Multisystem inflammatory syndrome (MIS-C): a systematic review and meta-analysis of clinical characteristics, treatment, and outcomes. J Pediatr (Rio J). 2021;S0021-7557(21)00148-0. doi: 10.1016/j.jped.2021.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munblit D, Sigfrid L, Warner JO. Setting priorities to address research gaps in long-term COVID-19 outcomes in children. JAMA Pediatr. 2021;175(11):1095-1096. doi: 10.1001/jamapediatrics.2021.2281 [DOI] [PubMed] [Google Scholar]
- 15.Radtke T, Ulyte A, Puhan MA, Kriemler S. Long-term symptoms after SARS-CoV-2 infection in children and adolescents. JAMA. 2021;326(9):869-871. doi: 10.1001/jama.2021.11880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buonsenso D, Munblit D, De Rose C, et al. Preliminary evidence on long COVID in children. Acta Paediatr. 2021;110(7):2208-2211. doi: 10.1111/apa.15870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Say D, Crawford N, McNab S, Wurzel D, Steer A, Tosif S. Post-acute COVID-19 outcomes in children with mild and asymptomatic disease. Lancet Child Adolesc Health. 2021;5(6):e22-e23. doi: 10.1016/S2352-4642(21)00124-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brackel CLH, Lap CR, Buddingh EP, et al. Pediatric long-COVID: an overlooked phenomenon? Pediatr Pulmonol. 2021;56(8):2495-2502. doi: 10.1002/ppul.25521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erol N, Alpinar A, Erol C, Sari E, Alkan K. Intriguing new faces of Covid-19: persisting clinical symptoms and cardiac effects in children. Cardiol Young. 2022;32(7):1085-1091. doi: 10.1017/S1047951121003693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashkenazi-Hoffnung L, Shmueli E, Ehrlich S, et al. Long COVID in children: observations from a designated pediatric clinic. Pediatr Infect Dis J. 2021;40(12):e509-e511. doi: 10.1097/INF.0000000000003285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forrest CB, Margolis PA, Bailey LC, et al. PEDSnet: a national pediatric learning health system. J Am Med Inform Assoc. 2014;21(4):602-606. doi: 10.1136/amiajnl-2014-002743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forrest CB, Margolis P, Seid M, Colletti RB. PEDSnet: how a prototype pediatric learning health system is being expanded into a national network. Health Aff (Millwood). 2014;33(7):1171-1177. doi: 10.1377/hlthaff.2014.0127 [DOI] [PubMed] [Google Scholar]
- 23.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. doi: 10.1371/journal.pmed.0040296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grohskopf LA, Alyanak E, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2021-22 influenza season. MMWR Recomm Rep. 2021;70(5):1-28. doi: 10.15585/mmwr.rr7005a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dufort EM, Koumans EH, Chow EJ, et al. ; New York State and Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children Investigation Team . Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383(4):347-358. doi: 10.1056/NEJMoa2021756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.PEDSnet . Updated chronic cluster master. Accessed February 2, 2022. https://github.com/PEDSnet/PASC
- 27.Simon TD, Cawthon ML, Popalisky J, Mangione-Smith R; Center of Excellence on Quality of Care Measures for Children with Complex Needs (COE4CCN) . Development and validation of the Pediatric Medical Complexity Algorithm (PMCA) version 2.0. Hosp Pediatr. 2017;7(7):373-377. doi: 10.1542/hpeds.2016-0173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simon TD, Cawthon ML, Stanford S, et al. ; Center of Excellence on Quality of Care Measures for Children with Complex Needs (COE4CCN) Medical Complexity Working Group . Pediatric medical complexity algorithm: a new method to stratify children by medical complexity. Pediatrics. 2014;133(6):e1647-e1654. doi: 10.1542/peds.2013-3875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flury BK, Riedwyl H. Standard distance in univariate and multivariate analysis. Am Stat. 1986;40(3):249-251. [Google Scholar]
- 30.Benjamini Y, Yekutieli D, Edwards D. False discovery rate: adjusted multiple confidence intervals for selected parameters. J Am Stat Assoc. 2005;100(469):71-93. doi: 10.1198/016214504000001907 [DOI] [Google Scholar]
- 31.Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(14):517-523. doi: 10.15585/mmwr.mm7114e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boehmer TK, Kompaniyets L, Lavery AM, et al. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020-January 2021. MMWR Morb Mortal Wkly Rep. 2021;70(35):1228-1232. doi: 10.15585/mmwr.mm7035e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blomberg B, Mohn KG, Brokstad KA, et al. ; Bergen COVID-19 Research Group . Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021;27(9):1607-1613. doi: 10.1038/s41591-021-01433-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buonsenso D, Pujol FE, Munblit D, Pata D, McFarland S, Simpson FK. Clinical characteristics, activity levels and mental health problems in children with long coronavirus disease: a survey of 510 children. Future Microbiol. 2022;17:577-588. doi: 10.2217/fmb-2021-0285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osmanov IM, Spiridonova E, Bobkova P, et al. ; and the Sechenov StopCOVID Research Team . Risk factors for post-COVID-19 condition in previously hospitalised children using the ISARIC Global follow-up protocol: a prospective cohort study. Eur Respir J. 2022;59(2):2101341. doi: 10.1101/2021.04.26.21256110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borch L, Holm M, Knudsen M, Ellermann-Eriksen S, Hagstroem S. Long COVID symptoms and duration in SARS-CoV-2 positive children—a nationwide cohort study. Eur J Pediatr. 2022;181(4):1597-1607. doi: 10.1007/s00431-021-04345-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molteni E, Sudre CH, Canas LS, et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. Lancet Child Adolesc Health. 2021;5(10):708-718. doi: 10.1016/S2352-4642(21)00198-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18(9):e1003773. doi: 10.1371/journal.pmed.1003773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wanga V, Chevinsky JR, Dimitrov LV, et al. Long-term symptoms among adults tested for SARS-CoV-2—United States, January 2020-April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(36):1235-1241. doi: 10.15585/mmwr.mm7036a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bailey LC, Razzaghi H, Burrows EK, et al. Assessment of 135 794 pediatric patients tested for severe acute respiratory syndrome coronavirus 2 across the United States. JAMA Pediatr. 2021;175(2):176-184. doi: 10.1001/jamapediatrics.2020.5052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forrest CB, Burrows EK, Mejias A, et al. Severity of acute COVID-19 in children <18 years old March 2020 to December 2021. Pediatrics. 2022;149(4):e2021055765. doi: 10.1542/peds.2021-055765 [DOI] [PubMed] [Google Scholar]
- 42.Yu TC, Zhou H. Benefits of applying a proxy eligibility period when using electronic health records for outcomes research: a simulation study. BMC Res Notes. 2015;8:229. doi: 10.1186/s13104-015-1217-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boscolo-Rizzo P, Borsetto D, Fabbris C, et al. Evolution of altered sense of smell or taste in patients with mildly symptomatic COVID-19. JAMA Otolaryngol Head Neck Surg. 2020;146(8):729-732. doi: 10.1001/jamaoto.2020.1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitcroft KL, Hummel T. Olfactory dysfunction in COVID-19: diagnosis and management. JAMA. 2020;323(24):2512-2514. doi: 10.1001/jama.2020.8391 [DOI] [PubMed] [Google Scholar]
- 45.Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77(8):1018-1027. doi: 10.1001/jamaneurol.2020.2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594(7862):259-264. doi: 10.1038/s41586-021-03553-9 [DOI] [PubMed] [Google Scholar]
- 47.Michelen M, Manoharan L, Elkheir N, et al. Characterising long COVID: a living systematic review. BMJ Glob Health. 2021;6(9):e005427. doi: 10.1136/bmjgh-2021-005427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bell ML, Catalfamo CJ, Farland LV, et al. Post-acute sequelae of COVID-19 in a non-hospitalized cohort: results from the Arizona CoVHORT. PLoS One. 2021;16(8):e0254347. doi: 10.1371/journal.pone.0254347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guler SA, Ebner L, Aubry-Beigelman C, et al. Pulmonary function and radiological features 4 months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J. 2021;57(4):2003690. doi: 10.1183/13993003.03690-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Halawa S, Pullamsetti SS, Bangham CRM, et al. Potential long-term effects of SARS-CoV-2 infection on the pulmonary vasculature: a global perspective. Nat Rev Cardiol. 2022;19(5):314-331. doi: 10.1038/s41569-021-00640-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Code Sets for Systemic and Syndromic Features of PASC and Medication Therapeutic Classes Associated With PASC
eFigure 1. Medication Therapeutic Classes Associated With PASC
eFigure 2. Kaplan-Meier Plot for Risk of Any Syndromic Feature of PASC
eFigure 3. Kaplan-Meier Plot for Risk of Any Systemic Feature of PASC
eFigure 4. Kaplan-Meier Plot for Risk of Any Medication Feature of PASC
eFigure 5. Kaplan-Meier Plot for Risk of Any Syndromic, Systemic, or Medication Feature of PASC