Abstract
Central nervous system (CNS) trauma, including traumatic brain injury and spinal cord injury, has a high rate of disability and mortality, and effective treatment is currently lacking. Previous studies have revealed that neural inflammation plays a vital role in CNS trauma. As the initial enzyme in neuroinflammation, cytosolic phospholipase A2 (cPLA2) can hydrolyze membranous phosphatides at the sn-2 position in a preferential way to release lysophospholipids and ω3-polyunsaturated fatty acid dominated by arachidonic acid, thereby inducing secondary injuries. Although there is substantial fresh knowledge pertaining to cPLA2, in-depth comprehension of how cPLA2 participates in CNS trauma and the potential methods to ameliorate the clinical results after CNS trauma are still insufficient. The present review summarizes the latest understanding of how cPLA2 participates in CNS trauma, highlighting novel findings pertaining to how cPLA2 activation initiates the potential mechanisms specifically, neuroinflammation, lysosome membrane functions, and autophagy activity, that damage the CNS after trauma. Moreover, we focused on testing a variety of drugs capable of inhibiting cPLA2 or the upstream pathway, and we explored how those agents might be utilized as treatments to improve the results following CNS trauma. This review aimed to effectively understand the mechanism of cPLA2 activation and its role in the pathophysiological processes of CNS trauma and provide clarification and a new referential framework for future research.
Key Words: autophagy, cytosolic phospholipase A2, drugs, lysosome membrane permeability, mitogen-activated protein kinase, neuroinflammation, spinal cord injury, traumatic brain injury
Introduction
Central nervous system (CNS) trauma, including traumatic brain injury (TBI) and spinal cord injury (SCI), is a leading cause of long-term disability and death worldwide (No authors listed, 2019a). The global burden of disease collaborator group study reported 0.93 million SCI cases and 27.08 million new TBI cases worldwide, while their global prevalence reached 27.04 million and 55.50 million, respectively (No authors listed, 2019b). To date, despite obvious clinical needs, no therapy has significantly improved the long-term rehabilitation outcomes after CNS injury. This may partially reflect an incomplete understanding of the complicated pathobiological mechanisms.
At present, we have knowledge of two events that occur during damage from CNS trauma: the primary mechanical injury involving direct mechanical tissue damage and secondary damage mediated by diverse pathogenic processes such as neuroinflammation, free radicals, calcium overload, and glutamate excitotoxicity (Hall, 1989; Saghazadeh and Rezaei, 2017; Gong et al., 2020). Overlapping boundaries exist between the effects mediated by these factors and other causal links driving the secondary injury (David and López-Vales, 2021). Among these, neural inflammation is an obvious characteristic of the reaction to central nervous system trauma (López-Vales and David, 2019). In addition, it is a vital marker of a variety of neurodegeneration illnesses where inflammatory events facilitate pathological development (Stephenson et al., 2018). CNS trauma-induced neuroinflammatory reactions are intricate and revealing the causal links regulating such inflammatory events is pivotal for the purpose of developing valid therapies (David and López-Vales, 2021).
Cytosolic phospholipase A2 (cPLA2), a key target of inflammatory response, is involved in neuroinflammation in SCI and TBI. Early studies have proven that it exists in both spinal cord and brain neurons (Bonventre, 1996; Ong et al., 1999a). Besides, collective evidence from many recent studies suggests that not only increased cPLA2 activity but also cPLA2-generated mediators play an important role in acute inflammatory responses in the CNS (Farooqui et al., 2006). Biologically active lipids are a large family of multifunctional mediating factors for inflammatory events deserving extreme attention, and they involve prostaglandins and associated eicosanoids known to be predominant inflammatory modulators. Certain enzymes are required to transform aliphatic acids related to the cellular membrane into eicosanoids and the rest of the biologically active lipidic mediating factors (David and López-Vales, 2021). As the first enzyme in such processes, cPLA2 can, in a preferential way, hydrolyze membranous phosphatides at the sn-2 position to produce lysophospholipids and arachidonic acid (AA) that contribute to various aspects of neuroinflammation through cyclooxygenase-2 (COX-2) and lipoxygenase (LOX) pathways after CNS trauma (Li et al., 2019; Sarkar et al., 2020). Under physiological conditions, cPLA2 participates in a variety of significant cellular responses, including phospholipid metabolism, signal transduction, and membrane remodeling (Kita et al., 2019; Wang et al., 2021; Hayashi et al., 2022). Nevertheless, under pathological conditions, elevated cPLA2 activity and excessive free aliphatic acids including AA, proinflammatory mediators, and platelet-activating factor (PAF) might damage lysosome membranes and cause neuroinflammation and oxidative stress (Chuang et al., 2015).
In general, as a rate-limiting step of the inflammatory response, activated cPLA2 will induce the production of more inflammatory factors, creating a cascade effect that promotes the progression of inflammation. Thus, as the critical component of signal transduction in the inflammatory response, cPLA2 vitally influences TBI and SCI pathogenesis (Chao et al., 2018; Stewart et al., 2021). Although an increasing number of studies have confirmed the involvement of cPLA2 in TBI and SCI pathogenesis, few have summarized the association between CNS trauma and cPLA2. In this paper, we have specifically highlighted the effects of cPLA2 and its downstream products on inducing inflammatory events after SCI and TBI. We have also summarized the latest understanding of cPLA2 in CNS trauma, with special focus on the potential effects of cPLA2 in the mediation of secondary damage and the potential therapeutic prospects of cPLA2-related inhibitors.
Retrieval Strategy
Literature review was electronically performed using PubMed database. The following combinations of keywords were used to initially select the articles to be evaluated: cPLA2; domain or structure; traumatic brain injury; spinal cord injury; mechanism or function; MAPK or calcium influx or upstream; brain or spinal. More than half of the selected studies were published from 2017 to 2022. We screened the results of each step by abstract and full title and excluded studies that reported non-cPLA2-related experiments and reviews (Figure 1).
Figure 1.
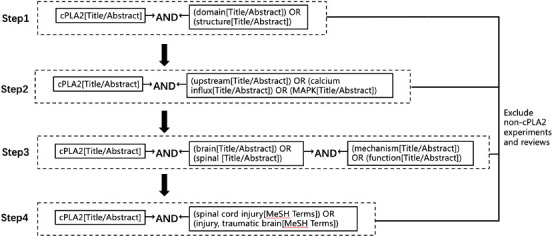
Flowchart for literature retrieval.
cPLA2: Cytosolic phospholipase A2.
Overview of Cytosolic Phospholipase A2
Phospholipase A2 (PLA2) is a family of enzymes with glycerophospholipid decomposition activity. By catalyzing the hydrolysis of phosphatidylcholine, phosphatidylethanolamine, and phosphatidylserine in the sn-2 position of the cell membrane, PLA2 can produce free aliphatic acids and lysophospholipids, which act as lipidic secondary messengers (Peng et al., 2021). The physiological functions of PLA2 include the transformation of the phospholipid structure, promotion of the autologous disappearance of necrotic tissues, and participation in the metabolism of alveolar surfactants (Kita et al., 2019). According to the classification of their biological activity, the PLA2 family is subdivided into approximately four groups (Ong et al., 2010): (i) Secreted phospholipase A2 (sPLA2): sPLA2 is a low molecular mass (14 kDa) enzyme with a rigid tertiary structure configured by disulfide bridges. It needs a millimolar concentration of calcium to exert its enzymatic action and has poor fatty acid selectivity when tested in vitro (Sun et al., 2021). Several studies have suggested that functionally active cPLA2 is a prerequisite for sPLA2-mediated AA release and prostaglandin biosynthesis (Balsinde et al., 1998; Murakami et al., 1998; Shinohara et al., 1999) and that cPLA2 regulates gene expression of sPLA2 (Kuwata et al., 2000). (ii) cPLA2: mainly including cPLA2α, cPLA2β, and cPLA2γ. cPLA2α has a molecular weight of 85 kDa and is widely present in various bodily tissues. It normally requires the participation of a submicromolar Ca2+ concentration and the phosphorylation of upstream kinases during activation to selectively hydrolyze the phospholipid AA sn-2 position. Later, other downstream enzymes, including COX and leukotrienes are responsible for the metabolism of AA to eicosanoids and are located at the sn-2 position too (Farooqui et al., 2006). This gives cPLA2 access to its membrane-associated phospholipid substrate. Since cPLA2α was discovered earlier, the current understanding of cPLA2 mainly comes from cPLA2α. Many studies have found group cPLA2α as the main PLA2 involved in the production of AA and vital for inflammatory events (Sun et al., 2021). Transgenic mice that lack cPLA2α exhibit remarkably harmful phenotypes in inflammatory illnesses such as ischemic cerebral damage, anaphylactic reactions, arthritis, alcohol abuse, and acute lung injury (ALI) (Bonventre et al., 1997; Nagase et al., 2000a, 2002; Kishimoto et al., 2010; Tai et al., 2010; Chuang et al., 2015). (iii) Ca2+-independent phospholipase A2 (iPLA2) exists in various tissues and cells, and its activation does not require the participation of Ca2+ (Sun et al., 2021). It is generally believed that iPLA2 does not directly participate in the release of AA induced by activators (Farooqui et al., 2006). (iv) Plasmalogen-selective PLA2: it has an apparent molecular mass of 39 kDa and mainly exists in the plasma and endarterium (Ong et al., 2010).
A recently published study has shown that iPLA2, cPLA2, and sPLA2 have a unique preference among the specific omega-3 fatty acids—eicosapentaenoic acid and docosahexaenoic acid—or the omega-6 AA, which are the precursors of most pro- and anti-inflammatory factors (Hayashi et al., 2021). Meanwhile, the study discovered that cPLA2 selectively preferred AA, whereas iPLA2 preferred eicosapentaenoic acid, and sPLA2 preferred docosahexaenoic acid as the substrate. Compared to the other three forms, cPLA2 is the best characterized in terms of its enzyme properties and protein structure, while iPLA2, sPLA2, and plasmalogen-selective PLA2 need to be further clarified (Kita et al., 2019). cPLA2 has two independent units that include an N-terminal C2 domain and a catalytic domain, and it is translocated to phosphatide membranes through the C2 domain at the nanomolar level of Ca2+, where it acts on arachidonate-containing phospholipids (Sun et al., 2021). Additionally, increasing evidence has shown that cPLA2 is crucial for the release of AA and lysophospholipids induced by agonists such as cytokines and endotoxins and a series of downstream inflammatory mediators (Sun et al., 2021). Experiments performed using mice with cPLA2 knockout (KO) have shown that cPLA2 is the indispensable PLA2 enzyme required for eicosanoid production in different inflammation illness models (Nagase et al., 2000b). Thus, most researchers now believe that cPLA2 is the “valve” that regulates the inflammatory process (Chatterjee et al., 2021; Duro et al., 2022).
Upstream of Cytosolic Phospholipase A2 Activation
It is generally accepted that the activation of cPLA2 occurs only through the mechanism of postreceptor signal transduction and it depends on the comodulation of many factors (Isenović et al., 2009). The fact that there are many phosphorylation sites on cPLA2 (S431, S454, S505, and S727) showed that cPLA2 was a matrix for the rest of the kinases (de Carvalho et al., 1996). Thus, as a molecule in the complex intracellular regulating network, cPLA2 can give “instructions” by sensing external signals, triggering a series of downstream effects, and playing an important role in connecting these signals with responses (Figure 2).
Figure 2.
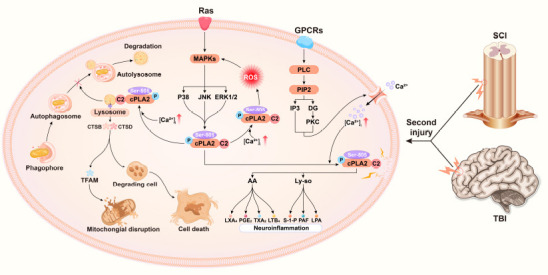
Graphical presentation of the mechanism of cPLA2 in traumatic brain and spinal cord injuries.
The extracellular signal molecule binds to its corresponding G-protein-coupled receptor to activate PLC. PLC cleaves the PIP2 in the cell membrane into DAG and IP3 and then activates PKC to cause a cascade reaction, which opens the calcium channel of the cell membrane and increases the intracellular calcium ion concentration. In addition, a second injury promotes cPLA2 phosphorylation through the ras-MAPK signaling pathway. The increased intracellular calcium promotes the binding of the C2 domain of cPLA2 to membrane phospholipids. Phosphorylated cPLA2 hydrolyses membrane phospholipids, producing downstream products (AA, Ly-so, and their subsequent metabolites) that trigger neuroinflammation. Phosphorylated cPLA2 simultaneously binds to the lysosomal membrane, resulting in increased permeability of the lysosomal membrane. Lysosomal enzymes (CTSB, CTSD) exosmose to degrade TFAM and impair mitochondrial function. Exosmotic lysosomal enzymes break down digestive cells and cause neuronal cell death. Damaged lysosomal enzymes prevent the binding of lysosomal enzymes to autophagosomes and thus hinder the progress of autophagy. [Ca2+]i: Intracellular calcium concentration; AA: arachidonic acid; CTSB: cathepsin B; CTSD: cathepsin D; DAG: diacylglycerol; IP3: inositol triphosphate; LPA: lysophosphatidic acid; LTB4: leukotriene B4; LXA4: lipoxin A4; Ly-so: lysophospholipids; MAPK: mitogen-activated protein kinase; PAF: platelet-activating factor; PGE2: prostaglandin E2; PIP2: phosphati-dylinositol-4,5-bisphosphate; PKC: protein kinase C; PLC: phospholipase C; S-1-P: sphingosine 1 phosphate; TFAM: transcription factor A; TXA2: thromboxane 2.
A previous study showed that there are two classical pathways that can activate cPLA2 (Mouchlis and Dennis, 2019). First, an increase in cytosolic calcium can activate cPLA2 (Sun et al., 2021). In Lin’s research, cPLA2 was stimulated by elevated endocellular Ca2+ levels (Lin et al., 1993). When cells are activated by an acceptor ligand such as platelet-derived growth factor or adenosine triphosphate (ATP), phospholipase C is induced by a G protein-independent/dependent process, resulting in the generation of diacylglycerol and inositol triphosphate. Then, an increase of those intracellular messengers leads to the stimulation of protein kinase C and the mobilization of endocellular Ca2+, or the elevation of endocellular Ca2+ could be a result of Ca2+ influx (Lin et al., 1993). Finally, elevated Ca2+ leads to the movement of cPLA2 from the cytosol to the membranes in which the matrix phosphatide is localized, which is pivotal for cPLA2 stimulation and might explain the partial stimulation of cPLA2 without phosphorylation (Lin et al., 1993).
Extracellular Ca2+ influx has been shown to be dispensable for the activation of cPLA2 (Rzigalinski et al., 1996). The mobilization of low-level AA was realized and followed by the release of Ca2+ stored in endocellular compartments, before extracellular Ca2+ influx. A later AA release was coupled with the extracellular Ca2+ influx and it lasted until maximum [Ca2+]i concentration was reached, suggesting cPLA2-mediated AA release might take part in the modulation of intracellular free Ca2+ content. Therefore, further investigation of the mechanisms of cPLA2 activation by the Ca2+-dependent pathway is necessary.
Another pathway for cPLA2 stimulation is phosphorylation. With the available full sequence of cPLA2, it seems obvious that this enzyme has a consensus phosphorylation motif (involving Ser-505) that is a target of the components of the mitogen-activated protein kinase (MAPK) family (Santerre-Anderson and Werner, 2020). The MAPK family is involved in regulating various neurotransmitters, hormones, growth factors, and cytokines and responding to stress stimulation of the physiological activities of diverse cells (Yuan et al., 2020). It involves three primary components: extracellular signal regulated kinase (ERK), p38, and c-Jun N-terminal kinase (JNK), reflecting three diverse signal transmission cascades (Xue et al., 2020). These three signaling pathways are not completely independent, and they interact with each other to affect the phosphorylation of cPLA2 (Naik et al., 2017). P38 MAPK is considered a stress-triggered kinase and is vital for inflammatory reactions (Kim et al., 2021). It has been established that the phosphorylation of cPLA2 by P38 MAPK on serine-505 increases its catalytic activity (Santerre-Anderson and Werner, 2020). Importantly, ABT-737-induced activation of p38 triggered cPLA2 phosphorylation, and this affected platelet apoptosis (Rukoyatkina et al., 2013). In addition, leptin attenuated lipopolysaccharide-triggered apoptosis damage in murine thymic cells, primarily by decreasing cPLA2 and realizing the cleavage of caspase-3 through the p38-MAPK signaling pathway (Liang et al., 2013).
ERK1/2 is one of the pathways involved in the cellular reaction and sensitivity to stimulus via ultimate control of cellular metabolic and proliferative abilities as well as cellular death (Santerre-Anderson and Werner, 2020). Activation of cPLA2 and generation of the lipidic secondary messenger lysolecithin are considered initiating events (in 2 min) needed for radiation-triggered stimulation of ERK1/2 in vascular endothelial cells (Yazlovitskaya et al., 2008). In a study by Shelat et al. (2008) with primary neurons in cultivation, activation with the ionotropy glutamate receptor agonist caused reactive oxygen species (ROS) generation via the nicotinamide adenine dinucleotide phosphate (NADPH) oxidative enzyme and fast stimulation of ERK1/2 and cPLA2. Further research by Chuang et al. (2015) showed that in both primary and BV-2 microglia stimulated by lipopolysaccharide, the timepoint of increasing p-ERK1/2 was prior to that of p-cPLA2. The associations between p-ERK1/2 and cPLA2 were further verified by U0126, the MEK1/2-ERK1/2 suppressor, which completely blocked cPLA2 phosphorylation. JNK also serves as a component of the MAPK family and mediates various types of cellular signaling processes such as cell proliferation, migration, and apoptosis (Li et al., 2021). Increasing evidence has confirmed that JNK signaling is involved in inflammation and the nervous system (Yang et al., 2009; Nasrazadani and Van Den Berg, 2011). Hernández et al. (1999) found that TNF-α causes early stimulation of p38-MAP kinase and JNK in astrocytic glioma cells, which is before cPLA2 phosphorylation and the release of AA, suggesting that P38 and JNK are both upstream signaling pathways of cPLA2. In addition, an experiment in a liver injury model proved that gigantol ameliorated CCl4 triggered hepatic damage by avoiding stimulation of the JNK/cPLA2/12 LOX inflammation pathway (Xue et al., 2020).
A surprising event that occurred in Kishimoto et al’s research was that the phosphorylation of MAPKs-p38, ERK 1/2, and JNK 1/2 was more remarkable in the cPLA2+/+ ischemia cortex than in the cPLA2–/– ischemia cortex, 6 hours after reinfusion (Kishimoto et al., 2010). The authors believe that the mutual effects may create a positive feedback loop, where cPLA2-reliant ROS elevates kinase stimulation and induces more cPLA2 stimulation. In addition, phosphatidylinositol-4,5-bisphosphate (PIP2), which exists in the plasma membrane, can bind to cPLA2 and activate it. PIP2 promotes the binding of the active parts of the enzyme to the membranous phospholipid matrix by inducing alterations of the cPLA2 conformation (Stahelin et al., 2007). Overall, specific crossover and integration between MAPK pathways and other intracellular pathways constitutes a complex signal network, which indicates that cPLA2 is an active substrate downstream of this vast regulatory network. However, the extent to which they independently affect the phosphorylation of cPLA2 and how they interact with each other still needs further research. Through the coaction of the above pathways, cPLA2 can be activated for a long time and play a “pivotal” role in the various downstream inflammatory factors.
Involvement of Cytosolic Phospholipase A2 in Traumatic Brain Injury and Spinal Cord Injury
Functions of cPLA2 in TBI
More than two decades ago, it was reported that cPLA2 activity was a vital contributing factor to deleterious cellular processes in the CNS (Mori et al., 1996). Later, cPLA2 was found in the gerbil cerebrum and the neuron cultivation of rat cerebrum (Bonventre, 1996). A head trauma model induced in rats by weight drop equipment showed that 15 minutes and 24 hours post trauma, cPLA2 activity increased by 75% and 245% relative to sham, respectively, while there were no significant changes of cPLA2 activity in the uninjured brain parts. It needs to be further investigated whether ischemia gradually develops because of increased production of the AA metabolites produced by cPLA2 (Shohami et al., 1989). [Ca2+]i is reportedly pivotal for the regulation of neuron membrane excitability (Martiszus et al., 2021), and it is vital for maintaining cytoskeleton completeness (Lu et al., 2021). In an in vitro traumatic injury model (stretch-injured neurons), activation of cPLA2 was confirmed to participate in the postponed reinforcement of capacitive Ca2+ influx, which subsequently disrupted the dynamic equilibrium of Ca2+ (Rzigalinski et al., 1996).
Accumulating evidence has proven that ischemia is an important pathophysiological mechanism after TBI, and changes in cerebrovascular physiology further lead to ischemia damage (Veenith et al., 2016; Dobrzeniecki et al., 2021; Weil et al., 2021). A study showed that mice that had encountered TBI in the past had an approximately 4-fold elevation in lesion volume, more edema in the ischemic hemisphere, and functional damage after middle cerebral artery occlusion (MCAO) (Weil et al., 2021). Therefore, improving the symptoms of cerebral ischemia in TBI may also improve the prognosis of TBI.
cPLA2 knockout (KO) mice are protected against postischemic cerebrum damage triggered by MCAO (Bonventre et al., 1997). The sizes of infarctions are decreased in cPLA2 KO mice after reinfusion, which is especially obvious in posterior cerebrum sections. In addition, this work discovered that cPLA2 facilitated early events resulting in damage during the process of cerebral infarction (Kishimoto et al., 2010). These events might magnify the inflammatory cascades and cellular deaths, which define the processes of stroke development. Compared with cPLA2 KO mice, cPLA2 WT mice exhibited more COX-2 expression, prostaglandin E2 (PGE2) generation, and ROS after ischemic events, together with damage to the neuronal morphology, suggesting that cPLA2 is a vital regulator of molecular events that occurred quickly after cerebral ischemia reperfusion.
Zhang et al. (2012) found that cPLA2 inhibitors used in the early stages of stroke can effectively reduce the area and severity of infarcts. AKT (a cPLA2 inhibitor) injections 1 hour prior to and at 1 and 6 hours after the initiation of reinfusion remarkably decreased the infarction sizes in the hemisphere and striatum after 24 hours of reinfusion. However, if ATK was not administered within 6 hours of reperfusion, the injury would not decrease, which means that cPLA2 may take part in the early stage of brain injury.
In another study, Wu et al. (2018) used an effective cPLA2 inhibitor and adenovirus-mediated RNA interference for the treatment of MCAO/R mice. The data indicated that pAd-siRNA-cPLA2 treatment reduced the levels of inflammatory factors, neurological deficits, and tissue damage. Bradykinins (BKs) are reported to be important for the regulation of vasopermeability and inflammation processes after brain damage. In a previous study, controlled cortical impact (CCI) assays were conducted to explore the association between cPLA2 and the BK acceptor pathway in rats (Chao et al., 2018). The data showed cPLA2 upregulation and stimulation after cerebrum damage within the initial 24 hours, particularly for the B2 receptor in the early stage after TBI, which suggested that BK system stimulation was an initial event inducing the stimulation of cPLA2 in the TBI model.
In recent years, additional pathogenetic and molecular mechanisms of cPLA2 in brain injury have been further explored. A recent study demonstrated that lysosome membrane permeability (LMP) increases in neurons following CCI-induced TBI in mice, causing damaged macroautophagy and neuronal deaths (Sarkar et al., 2020). This was the first study to suggest that cPLA2-mediated LMP could facilitate autophagy-lysosome flaws in TBI. However, the specific and systematic mechanisms of cPLA2 in TBI need further research.
Functions of cPLA2 in SCI
In 1999, high levels of cPLA2 activity were noted to exist in the rat spinal cord (Ong et al., 1999b). Later, in an in vitro experiment, cPLA2 immune reactivity was found to be remarkably upregulated in nerve cells undergoing apoptosis (Hornfelt et al., 1999). This finding suggested that cPLA2 was pivotal for certain causal links participating in or tightly associated with neuron death. Later, Liu et al. (2007) found that the usage of annexin A1 (a nonselective PLA2 suppressor) can inhibit SCI-triggered inflammatory events and decrease tissue damage. Another study showed that both cPLA2 and mellitin (an activation agent of endogenetic cPLA2) caused spinal neuron death in vitro, which was remarkably attenuated by mepacrine, a cPLA2 suppressor (Liu et al., 2006). When cPLA2 or mellitin was delivered into the healthy spinal cord via microinjection, the former triggered confined myelinoclasis and the latter caused diffuse tissue necrosis. Both these microinjections caused oxidization, inflammatory events, and tissue damage, leading to relevant electrophysiology and behavioral changes.
It was then revealed that suppressing cPLA2 (AACOCF3) or gene knockout (cPLA2 KO C57Bl/6 mice) can improve motor deficiency and cause less tissue injury after SCI (Liu et al., 2014). In Malada’s study, an in vitro experiment in microglia showed that cPLA2 can upregulate CD40 protein expression by activating the NOX2-NADPH oxidative enzyme and NF-κB. The outcomes indicated that cPLA2 might be an essential magnifier of the inflammatory reaction after CNS trauma (Malada-Edelstein et al., 2017). Recently, a study confirmed that upregulation of cPLA2 can also damage the lysosomal membrane and thus impair autophagic flux (Li et al., 2019), suggesting that activation of cPLA2 by SCI can destabilize the lysosomal membrane. Overall, these data reveal a crucial effect of cPLA2 on regulating oxidation, inflammation, membrane integrity, apoptosis, autophagy, and functional outcomes following SCI.
However, an interesting study discovered there were complicated effects of diverse PLA2 enzymes (cPLA2, sPLA2, and iPLA2) for alleviating or worsening SCI (López-Vales et al., 2011). Although all PLA2s were upregulated after SCI, contray to Liu et al. (2006), cPLA2 was found to benefit SCI, which was identified via special suppressors and cPLA2 KO mice, whereas sPLA2 GIIA and iPLA2 GVIA were deleterious for SCI. López-Vales et al. (2011) speculated that the strain of mice and the genetic background of different strains of mice were the main reasons for the discrepancy. A study focused on lumbar spinal stenosis showed that inhibition of cPLA2 can reduce proinflammatory lipid mediators originating from cauda equina compression-induced injury (Khan et al., 2015). This suggests that cPLA2 is adequately expressed in cells in a wide range of nervous systems, such as the CNS and peripheral nervous system, and it is regulated by cPLA2-related inhibitors.
Specific effects of cPLA2 in cell types after CNS trauma
Once CNS trauma occurs, a variety of numerous cells such as neurons, astrocytes, and microglia will participate in the inflammatory response (Lukacova et al., 2021). The BK receptor is a significant factor within secondary injuries in TBI. A previous study showed that reactive astrocytes (the major component of the glial scar) played a more important role than neurons in BK-cPLA2-AA inflammation (Chao et al., 2018). These authors thought that BK-induced inflammation after TBI primarily occurred with the glia cells, and the glia cells affected the neuron status. In cultured astrocytes and microglia, TNF-α and IL-1β can induce the activation of cPLA2, leading to PGE2 production and AA release (Hernández et al., 1999). AA triggers Ca2+-dependent cell death through mitochondrial permeability transition in both cells (Penzo et al., 2004). PGE2 exerts neurotoxicity via astrocyte glutamate release (Stachowicz, 2021) and microglia cytokine release (Bhatia et al., 2017). Those showed that cPLA2 and its metabolites played different toxicological roles in specific neural cells. In addition, the way to activate cPLA2 may be different in various cells after CNS trauma. In astrocytes, cPLA2 interacts with mitochondrial antiviral-signaling protein to boost nuclear factor kappa B (NF-κB)-driven inflammatory responses (Chao et al., 2019). In microglia, cPLA2 and AA metabolites contribute to ROS and nitric-oxide production during cell activation mainly through the MAPK pathway (Chuang et al., 2015). As mentioned above, although cPLA2 is widely expressed after CNS trauma, studies on the specific role of cPLA2 on different cell types in CNS trauma are limited because of the complex cellular composition of the nervous tissue. However, with recent advances in techniques for separation and isolation of specific cell types in the brain and spinal cord tissue, understanding of the molecular actions of cPLA2 has greatly improved, but the specific underlying mechanism requires further investigations (Sun et al., 2021).
Potential Mechanisms of Cytosolic Phospholipase A2 in Traumatic Brain Injury and Spinal Cord Injury
To date, sufficient evidence has proven that cPLA2 plays a significant role in CNS trauma. However, the mechanism by which activation of cPLA2 affects CNS trauma remains elusive. Some mechanisms have been proposed to explain the cPLA2-mediated damage. These causal links might not exclude each other, and it is possible that every pathway might interact in an independent manner after activation of cPLA2 to cause neuroinflammation and harm the organelles during the inflammatory reactions involved in CNS trauma. Here, we mainly discuss the details of the effect of cPLA2 on the production of metabolites, membrane damage, impaired autophagy flux (Siegrist et al., 2019; Sarkar et al., 2020), and subsequently, impaired SCI and TBI (Figure 2).
Effect of cPLA2 on neuroinflammation in CNS trauma
cPLA2 is vital for the production of diverse inflammatory mediators: a variety of inflammatory mediators are directly or indirectly involved in the pathogenesis of CNS trauma, some of which induce each other and some are antagonistic to each other, thereby jointly building a complex network. A previous study showed that CNS trauma increased cPLA2 metabolites such as free aliphatic acids, eicosanoids, and lipidic peroxides (Abu Hamdeh et al., 2018). First, oxidized free aliphatic acids are a primary product of lipid peroxidation after CNS trauma. In a CCI model, oxidation peaked at 60 minutes after CCI and gradually weakened at the 4- and 24-hour time points (Anthonymuthu et al., 2017). Sixty minutes after TBI, enzyme lipid peroxidation was the primary causal link with 15-LOX, leading to almost complete total oxidation of aliphatic acids. Proinflammatory lipidic mediating factors were elevated at 1 and 4 hours after TBI and were then restored to basic levels after 24 hours, which coincides with the time of early cPLA2-mediated damage (Li et al., 2019; Sarkar et al., 2020). In addition, the metabolism of free aliphatic acids and lysophosphatides leads to the loss of essential phospholipids. Thus, it may also function with a detergent-like effect on the membranes of the neurons and influence nerve conduction (Faure et al., 2014). Moreover, free aliphatic acids could deactivate oxidative phosphorylation (OXPHOS), leading to mitochondrial function disorder (Papa et al., 2012). Arachidonic acid is the predominant product of phospholipids degraded by cPLA2, and subsequently bio-transforms through different pathways into several mediators that are endowed with pivotal roles in the regulation of inflammatory processes (Gorica and Calderone, 2021). For instance, PGE2, as a metabolite of AA, can induce the inflammatory reaction of astrocytes (Song et al., 2021). A closed head injury model showed significant correlation between increased cPLA2 activity and PGE2 at 4 and 24 hours post trauma (Shohami et al., 1989). The elevation of PGE2 production was eliminated when pretreated with 70,000 dextrans, which has been previously shown (Shohami et al., 1989) to inhibit cPLA2 activity. Besides AA can induce mitochondrial swelling in glial cells and damage membrane permeability via channel regulation in brain tissue (Farooqui et al., 1997). Other products induced by AA including TXA2 can facilitate the synthesis of TNF-α and IL-10 (Mitsuhashi et al., 1994), together with LTB4 that promote the ability of mononuclear cells and macrophages to release IL-1, IL-2, and IFN-γ (Filgueiras et al., 2015). The results of a recent study interestingly found that different from other metabolites of AA, lipoxin A4 (LXA4) works as an anti-inflammatory and catabolic lipid mediator that contributes to the resolution of inflammation (Dennis and Norris, 2015). It promotes macrophage recruitment to clear cell debris (Mei et al., 2021) and inhibits macrophage/microglia activation, thus reducing neuropathic pain (Martini et al., 2016). In a TBI model, LXA4 inhibiteds elevation of mRNA and protein levels of TNF-α, IL-1β, and IL-6 and attenuated brain edema and reduced lesion volume (Luo et al., 2013). Besides in a SCI model, Wei et al. (2021) showed that LXA4 exerted a neuroprotective effect through Akt/Nrf2/HO-1 signaling pathway in Erastin-induced ferroptosis of primary spinal cord neurons. According to studies of these researchers, we hypothesized that LXA4, as an anti-inflammatory element in inflammatory response, might be involved in the inhibition of cPLA2 targets based on a negative feedback mechanism.
Apart from AA, another major product mediated by cPLA2 at sn-2 of glycerophospholipids is lysophospholipids, the downstream metabolites of which can also lead to a pro-inflammatory effect. Under physiological conditions, PAF, the product from lysophospholipid acetylation can modulate the function of lipid messenger through the balance between synthesis (via phospholipases) and degradation (via acetylhydrolases) of itself (Piwowarek et al., 2021). However, once this balance is broken under pathological conditions of SCI and TBI (Wang et al., 2016; Yin et al., 2017), PAF will become a proinflammatory mediator and neurotoxic agent. Recently, the downstream product of lysophospholipids, including S-1-P and lysophosphatidic acid (LPA), were found to function as receptor-mediated signaling molecules; this has especially drawn acute scientific interest because of its critical roles in the CNS system (Gaire and Choi, 2021). In cultured microglia, S-1-P can influence ATP release through volume-regulated anionic channel, which is associated with the motion of microglia (Zahiri et al., 2021), suggesting that S-1-P can promote microglial activation and subsequently initiate neuroinflammatory responses. Additionally, López found that LPA was constitutively expressed in the spinal cord parenchyma, and its transcripts were upregulated after contusion injury by microglial cells. LPA-deficient mice showed enhanced motor skills and myelin sparing after SCI, suggesting LPA activation contributes to secondary CNS damage (López-Serrano et al., 2019). Additionally, abundant evidence has shown that LPC can contribute to demyelination (Hamidabadi et al., 2021; Yamazaki et al., 2021), and animal models of LPC-induced focal demyelination have been widely used in experimental research (Kataria et al., 2018).
Regardless of the specific metabolic pathways undertaken by AA and other metabolites, cPLA2 is vital in the first step of the synthesis of these eicosanoids (Wang et al., 2021). cPLA2 can be stimulated by some pivotal damage mediating factors, such as inflammatory cell factors, free radicals, and excitatory amino acids, which are increased after CNS trauma (Vichai et al., 2005; Lin et al., 2015; Wang et al., 2021). Increased cPLA2 activity in turn hydrolyses the neuronal membrane, further increasing the release of inflammatory, oxidative, and excitatory amino acids. This suggests that metabolites of cPLA2 might be pivotal in creating the positive feedback loop induced by trauma injury. The effector system that controls cPLA2 and subsequent mobilization of AA ensures complete regulation of the pathological conditions closely related to arachidonic acid cascade activation.
Effect of cPLA2 on the lysosome membrane function in CNS trauma
Phosphatides serve as the key constituents of neurocyte bilayer membranes (Alashmali et al., 2021). They not only form the backbone of neuronal membranes but also offer membranes the appropriate milieu, fluidity, and ion permeation required for the normal functions of intact membranous proteins, receptors, and ionic channels (Yu et al., 2021). The barrier capability of lysosomal membranes is damaged under multiple pathological conditions including SCI and TBI (Wu and Lipinski, 2019; Ibata and Yuzaki, 2021), resulting in the release of lysosome contents into the cytosol, which, in return, enables the lysosomal lumen to absorb neutral cytosolic solution (Alashmali et al., 2021). The activation of cPLA2 triggers phosphatide decomposition and membranous breakdown via the hydrolytic action of nerve membrane phosphatides, leading to variations in membranous functions such as permeability and fluidity, activities of acceptors and transporters, and ionic homeostasis, and finally contributing to the dysfunction of the membrane (Li et al., 2019; Sun et al., 2021). Once the neural membranes are irreversibly impaired, their function may become completely destroyed. Then, LMP increases, and thus, lysosome proteases such as cathepsin D (CTSD) and cathepsin B (CTSB), which normally exist only in lysosomes, can enter the cytoplasm (Liu et al., 2018).
Mitochondrial transcription factor A (TFAM) was recently reported to be a substrate of CTSB that leaked into the cytosol of microglia (Ni et al., 2019). Under a neutral pH, human recombinant TFAM can be degraded by CTSB, suggesting that CTSB leakage into the cytosol of microglia may result in mitochondrial disruption via a TFAM-dependent degradation pathway (Ni et al., 2019). Previous studies have found that SCI and TBI can alter the subcellular localization of CTSD—diffusive instead of discrete punctate—revealing that LMP enables the leakage of CTSD into the cytosol, inducing reduced lysosome activities after CNS injury (Sarkar et al., 2020). In conclusion, preserving the integrity of the lysosomal membranes is essential not only for maintaining lysosome functions but also for defending cell constituents from exposure to lysosome luminal enzymes (Li et al., 2019). Thus, reducing the activity of cPLA2 after CNS injury by targeting cPLA2 to protect the stability of the lysosome membrane needs further research.
Effect of cPLA2 on autophagy activity in CNS trauma
Autophagy is a lysosome-reliant endocellular process that is involved in the decomposition of cellular proteins and organelles (Klein et al., 2021). Basal levels of autophagy play a significant role in the maintenance of cell homeostasis and seem to be indispensable for normal cell functions and the survival of terminal differentiation cells such as nerve cells. Mice with nerve tissue-specific KO of the vital autophagic genes Atg5 (autophagy-associated 5) or Atg7 (autophagy-associated 7) exhibit serious neural degeneration, with aberrant motor functions and reflexes (Hara et al., 2006; Komatsu et al., 2006). Autophagic damage is associated with neurodegenerative diseases such as Parkinson’s, Alzheimer’s, and Huntington’s and related to lysosomal storage diseases (LSDs) (Shintani and Klionsky, 2004; Klionsky, 2006). Previous studies confirmed that autophagy is inhibited after SCI or TBI, thereby contributing to secondary neuronal cell death (Klionsky and Emr, 2000; Shintani and Klionsky, 2004; Mizushima and Komatsu, 2011; Li et al., 2019).
Autophagy consists of several steps such as the formation of the phagophore, formation of autophagosomes, and fusion of autophagosomes with lysosomes to produce autophagic lysosomes followed by decomposition of the autolysosome (Zhang et al., 2021; Rickman et al., 2022). Autophagy flow is a kinetic process in which these steps occur persistently in cells (Kim et al., 2020). If any obstacle occurs during autophagic flux, the autophagic process cannot be completed. As cPLA2 can exert its lysosomal formation as discussed above, cPLA2-induced lysosomal dysfunction can also impair the autophagy process, thus contributing to cell death. Several recent studies have proven that elevated cPLA2 activities and its transfer to the lysosomal fraction are essential factors during lysosome impairment and the subsequent damage to autophagic flow after CNS trauma (Li et al., 2019; Sarkar et al., 2020). LC3 serves as an autophagic biomarker. In the course of autophagy, cytoplasmic LC3 (LC3-I) enzymatically hydrolyses a small portion of polypeptides and then transforms into the autophagosome membrane type (LC3-II) (Kocak et al., 2021). The LC3-II/I ratio could be utilized to predict autophagy levels. Meanwhile, P62 is a selective autophagy receptor, forming a bridge connecting LC3 with ubiquitinated substrates to be degraded (Liu et al., 2021). P62 binds to ubiquitinated proteins and enters the autophagosome, where it eventually fuses with lysosomes to form autolysosomes and is then cleared. The P62 content increases when autophagic flux is inhibited but it decreases when autophagic flux is activated. After treatment with C-1-p (a cpla2-specific activator), neuroglioma cells exhibited higher LC3II/LC3I and p62 levels. Additionally, upstream autophagy regulators remain unaltered (Sarkar et al., 2020). These results show that cPLA2-induced impairment of autophagy acts mainly by blocking autophagosome lysosome fusion. However, another study showed that cPLA2-activated lipid mediator pathways are involved in autophagy induction (Qi et al., 2011) and confirmed that cPLA2 appears to be able to trigger or amplify autophagy responses. The authors hypothesized that the initiation of cPLA2-induced autophagy may be ATG5-dependent and independent of autophagy flow suppression. It should be noted that this research only detected the autophagy marker LC3 but did not detect p62. LC3II/LC3I levels alone cannot fully reflect changes in autophagic flux. In addition, the differences between these two conclusions may be caused by the different types of cells (one was investigated in neural cells and the other in macrophages). Based on the aforementioned research, the role of cPLA2 in autophagy needs to be explored further. As in the CNS, microglia are the resident immune cells of the brain, which function as macrophages and primarily take part in surveillance and phagocytosis (Stewart et al., 2021). Therefore, more research is required to investigate the effect of cPLA2 on the levels of autophagic flux in microglia after CNS trauma.
Anti-Cytosolic Phospholipase A2 Therapies in Central Nervous System Trauma
Generally, cPLA2 has a great impact on the meditation of inflammation after CNS secondary injury, as discussed above. Therefore, researchers have developed many inhibitors targeting cPLA2. Meanwhile, suppression of the upstream activators and downstream target of cPLA2 has also been explored as a therapeutic possibility. Although there are currently no clinically approved cPLA2 and related inhibitors for the treatment of CNS trauma, many therapies are under development and have shown great promise (Table 1).
Table 1.
Potential drugs target to cPLA2 in central nervous system trauma
| Drug | Target | Disease | Therapeutic effects | References |
|---|---|---|---|---|
| AACOCF3 | cPLA2 | Spinal cord injury | Reducing membrane injury and inflammation, decreasing tissue damage, and improving behavioral recovery | Liu et al., 2014 |
| cPLA2 | CCI induced TBI | Restoring autophagic flow, weakening cortical cellular deaths, and ameliorating movement and cognition functions | Chinmoy et al., 2019 | |
| PACOCF3 | cPLA2 and Ca2+ | Spinal cord injury | Blocking myelin’s detrimental effects of increasing proinflammatory cell factors, reactive oxygen species, and nitric oxide generation in M1 macrophages | Kopper et al., 2021 |
| ANXA1 | Membrane phospholipids | Spinal cord injury | Improving tissue repair, reconstruction, regeneration, increasing white matter sparing, and protecting axons of long descending pathways | Liu et al., 2007 |
| U0126 | ERK1/2 | DCS spinal injury | Attenuating oxidative stress and inflammatory response, and improving motor function by upregulating heat shock protein 32 | Quan et al., 2021 |
| SB203580 | MAPK AP kinase-2&3 | Spinal cord injury | Preventing the delayed progressive degeneration of oligodendrocytes and promoting recovery of motor function | Hideki et al., 2003 |
| Celecoxib | LOX/COX-2 | Spinal cord injury | Attenuating oxidative stress, apoptosis, and inflammation | An et al., 2020 |
| Salsalate | LOX/COX-2 | CCI induced TBI | Blocking pro-inflammatory gene expression and nitrite secretion by microglia | Lagraoui et al., 2017 |
CCI: Controlled cortical impact; COX-2: cyclooxygenase-2; cPLA2: cytosolic phospholipase A2; DCS: decompression sickness; ERK: extracellular signal regulated kinase; LOX: lipoxygenase; TBI: traumatic brain injury.
cPLA2-targeted inhibitors
Arachidonyl trifluoromethyl ketone
The carbochain of arachidonyl trifluoromethyl ketone (AACOCF3) binds in a hydrophobic pocket, and the carbonylic group of AACOCF3 creates a covalent linkage with Ser in the active parts of cPLA2 (Street et al., 1993). AACOCF3 is a 500-fold stronger suppressor of cPLA2 than sPLA2; it inhibits cPLA2 and iPLA2 with IC50 values of 1.5 and 6.0 μM, respectively (Farooqui et al., 2006). Accumulating evidence has shown that after the administration of AACOCF3, lysosomal injury, autophagic suppression, and neuronal death can be alleviated after SCI and TBI (Li et al., 2019; Sarkar et al., 2020). In addition, a study revealed a long-term favorable role of targeting cPLA2 in the anatomical and functional recovery in an SCI model, suggesting that long-term inhibition of cPLA2 sites contributes to recovery from neurological injury (Liu et al., 2014). However, some studies have shown that the concentrations of AACOCF3 used in these experiments may also have a membranotropic effect and suppress the activity of cPLA2 (Dubinin et al., 2016). This should be taken into account when selecting AACOCF3 dosages for research purposes.
Palmityl trifluoromethyl ketone
Palmityl trifluoromethyl ketone (PACOCF3) is synthesized in the same manner as the arachidonoyl analogue (Ackermann et al., 1995), but the COOH group is substituted by trifluoromethyl ketone. A study showed that PACOCF3 blocked myelin’s proinflammatory enhancement of M1 macrophages, suggesting that it may improve inflammation after CNS trauma (Kopper et al., 2021). Although PACOCF3 is a high-selective cPLA2 inhibitor, it has off-target effects, as it can bond with iPLA2 and sPLA2 (Kopper et al., 2021). Besides, Jan et al. (2000)raised an important issue: PACOCF3 may alter cellular functions by affecting Ca2+ signaling in a manner independent of cPLA2 inhibition. Thus, more potential mechanisms of PACOCF3 therapy need to be studied.
Pyrrophenone
Pyrrophenone is an inhibitor of cPLA2 derived from pyrrolidine. A previous study showed that pyrrophenone suppressed sera-activated cPLA2 C2 domain-to-Golgi transfer by blocking calcium mobilization (Yun et al., 2016). It binds to the protein via a variety of hydrophobic pyrrophenone residues located distally from the active parts (Burke et al., 2009). Treatment with pyrrophenone can reduce cPLA2 expression levels and thus suppress lipopolysaccharide- and IFNγ-triggered NO generation within BV-2 cells (Chuang et al., 2015). However, due to its chemical properties (McKew et al., 2008) and the lack of validation in animal models, pyrrophenone needs extensive additional research before its clinical application.
ANXA1
ANXA1 (annexin-A1), a component of the annexin family of Ca2+ and phospholipid-binding proteins, is found in the CNS (Bolton et al., 1990). Recently, in Zachary’s study, the Anxa1 gene was found to participate in tissue repair, re-establishment, and regenerative processes and was strongly associated with repair and regenerative processes after SCI (Fang et al., 2021). The application of ANXA1 remarkably reversed cPLA2-triggered spinal cord neuron death in vitro, decreased tissue injury, and increased white matter sparing in vivo (Liu et al., 2007). Fluorogold retrograde tracing revealed that the application of ANXA1 safeguarded axons of long descending pathways at 42 days after SCI and increased the number of animals responding to tcMMEP. Nevertheless, no detectable behavioral changes were identified in response to ANXA1 treatment.
AX059
AX059 is a highly selective inhibitor of cPLA2, which exhibits > 95% inhibition of cPLA2 at 0.091 mole fraction, while showing 0% inhibition of iPLA2 and sPLA2 (Kalyvas et al., 2009). In Yang’s study, AX059 reduced the onset and progression of experimental autoimmune encephalomyelitis (EAE) in Lewis rats (Yang et al., 2014). In addition, this effect was accompanied by the activation of regulatory T-cell and alterations in the expression of their various cytokines. Based on its strong specificity of inhibiting cPLA2 target and its successful validation in EAE models, we can suppose it is a promising drug that can be administrated in CNS trauma.
AVX001
AVX001, a derivative of PUFA can downregulate cytokine-stimulated PGE2 formation through a mechanism that involves blocking cPLA2-dependent NF-kB activation. Recent studies showed that AVX001 can ameliorate collagen-induced arthritis (Feuerherm et al., 2019) and attenuate inflammation in keratinocytes (Ashcroft et al., 2020) and renal mesangial cells (Huwiler et al., 2012). These data suggest that AVX001 may serve as a novel anti-inflammatory drug in CNS trauma.
cPLA2 upstreaming signal pathway inhibitors
ERK1/2 signal pathway inhibitors
A previous study showed that the ERK1/2 suppressor U0126 decreased the phosphorylation of ERK1/2 and cPLA2 at S505 in rabbit vascular smooth muscle cells, inhibiting AA release (Pavicevic et al., 2008). Later, Xu et al. (2016) found that the use of U0126 can restore spinal cord-damaged neural migratory and adhesive abilities as well as the development of dendritic spines, suggesting that upregulation of the ERK1/2 pathway aggravated SCI. Recently, a study on decompression sickness spinal injury in rats proved that pretreatment with normobaric oxygen and the U0126 combination could effectively attenuate oxidative stress and the inflammatory response and improve motor function (Zhou et al., 2021). All of these data showed effective therapy with U0126. However, the ERK1/2 signaling pathway is responsible for the mediation of a diversity of cellular reactions, such as cellular proliferative, migratory, and differentiation events. Given that it mediates physiological functions in normal cells, additional research is necessary.
P38 signaling pathway inhibitors
SB203580, a suppressor of p38 MAPK, has no reported selectivity for other MAPK signaling pathways and it only blocks MAPK AP kinase-2 and kinase-3 (Braem et al., 2012). In both in vivo and in vitro studies, accumulating evidence has confirmed that the SB203580 inhibitor prevented increased expression of p-cPLA2 and p-p38 (Shibata et al., 2011; Liang et al., 2013). In addition, the p38 MAPK suppressor SB203580 has been applied to CNS trauma models. Intrathecal SB203580 administration into the cerebral ventricle had a neuroprotective effect after tFCI and was correlated with a decrease in iNOS, TNF-α, IL-1β, and COX-2 expression (Piao et al., 2003). In addition, treatment with SB203580 ameliorated hindlimb functions in a slight compression SCI model (Horiuchi et al., 2003) and attenuated BBB extravasation and subsequent edema in a tFCI model in rats (Nito et al., 2008). In contrast to the above consequences, a different study showed that intrathecal SB203580 administration did not ameliorate functional results after a medium contusive SCI (Stirling et al., 2008). The discrepancy between the outcomes may be related to the different degrees of damage used in the different studies.
cPLA2 downstreaming signal pathway inhibitors
Lipoxygenase/COX-2 pathways inhibitors
Experimental evidence has shown that the level of COX-2 was found to be overexpressed primarily in brain and activated macrophages after CNS trauma (Resnick et al., 1998). Celecoxib, a well-known pyrazole-based NSAID and selective COX-2 inhibitor exhibited protective action against SCI via attenuation of COX-2, oxidative stress, apoptosis, and inflammation in male Sprague-Dawley rats (An et al., 2020). The third generation COX2 enzyme inhibitor 5,5-dimethyl-3(3-fluorophenyl)-4(4-methylsulfonyl)phenyl2(5H)-furanone (DFU) altered eicosanoid profiles in the traumatic brain and improved neurological reflexes and memory (Gopez et al., 2005). Furthermore, another study using a CCI mouse model showed that salsalate has a broad anti-inflammatory effect mainly through blocking pro-inflammatory gene expression and nitrite secretion by microglia (Lagraoui et al., 2017). In addition, NSAIDs have been shown to increase myelination of axons and promote axonal elongation and sprouting in SCI (Lambrechts and Cook, 2021). However, owing to the potential complications including gastric ulceration (Kareva, 2020), poor penetrance across the blood-brain barrier into the cerebrospinal fluid (Stasiłowicz et al., 2021), and increased bleeding risk (Murphy et al., 2021), further research is needed to evaluate whether the benefits outweigh the risks of administration of NSAIDs after CNS trauma.
Limitations
This review has several limitations. First, the current review mainly focused on cPLA2α in CNS trauma, and further understanding of other cPLA2 subtypes such as cPLA2β and cPLA2γ are also essential. Second, we discussed the effect of cPLA2 on lysosome membrane in nerve cells following TBI and SCI, but its potential roles on other membranous organelles such as the mitochondria and endoplasmic reticulum, should also be elaborated in further studies. Third, based on the limited literature, the mechanism of cPLA2 on autophagy in CNS trauma was discussed in this review. Many other types of programmed cell death such as ferroptosis, necroptosis, or pyroptosis may be also related to cPLA2, which need extensive investigation. Finally, to determine the effect of cPLA2 in CNS trauma, pharmacological inhibitors of cPLA2 were commonly used in previous studies. However, these inhibitors are not specific. Therefore, using cPLA2-KO mice is a better choice in future research studies.
Conclusion and Perspectives
Accumulating evidence has proven that acute SCI and TBI can cause a secondary injury by several bioprocesses. cPLA2, the heavyweight component of cellular signal transduction in inflammatory responses, acts as a “trigger” in regulating the production of several major inflammatory mediators. In this review, we provide an overview of the cPLA2 family, together with its upstream signaling pathways. We discussed a series of current studies on cPLA2 in SCI and TBI and analyzed the potential pathogenesis of cPLA2. Among them, we focused on exploring the mechanism of cPLA2 and its metabolites that participate in neuroinflammation, causing an increase in lysosomal membrane permeability and inducing damage to neuronal autophagic flux. Next, we listed cPLA2-related inhibitors and their therapeutic effects, but most of these drugs are still in the experimental stage, and very few are currently being used in the clinic.
In addition, several unanswered questions and key questions remain that are likely to guide subsequent studies. Although previous studies have explored the role of cPLA2 and its metabolic products in neuroinflammation in CNS trauma, the specific mechanism of its positive feedback cascade amplification and whether it also depends on upstream MAPK pathways remains unclear. cPLA2 was proven to damage the lysosomal membrane and cause lysosomal enzyme (CTSB and CTSD) extravasation, but whether other contents of the lysosome are released and how they aggravate the damage of neuroinflammation are unknown. Moreover, it is worth noting that the leakage of CTSB caused by lysosomal membrane rupture is one of the mechanisms inducing pyroptosis, and pyroptosis is involved in cell death after CNS trauma (Hu et al., 2020). Moreover, given the heterogeneity of the cerebrum and spinal cord and their different cell populations, the roles of cPLA2 in the brain and spinal cord should be investigated in more detail in a cell type-specific manner. As discussed above, many cPLA2-related drugs are emerging and produce different therapeutic effects through the different targets. Because cPLA2 exerts its effects through phosphorylation and binding with intracellular calcium ion, we hypothesize that the combination of using MAPK inhibitors and calcium ion antagonist (such as pyrrophenone) may be an effective method to thoroughly block cPLA2 activation and produce good curative effect. Early cPLA2-target inhibitors such as AACOCF3 and PACOCF3 for the treatment of SCI and TBI have shown a few adverse reactions due to their off-target effect. Thus, we expect more novel, potent, and highly specific inhibitors of cPLA2 (including AX059 and AVX001) to emerge and be tested in models of CNS trauma. Therefore, more research is required to shed light on these processes. We believe that research regarding the targets of cPLA2 will contribute to the treatment of TBI and SCI, which continue to be serious clinical challenges.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China, No. 82072192 (to KLZ); Public Welfare Technology Research Project of Zhejiang Province, No. LGF20H150003 (to KLZ); the Natural Science Foundation of Zhejiang Province, Nos. LY17H060009 and Y21H060050 (both to WFN); and Wenzhou Science and Technology Bureau Foundation, No. Y20210438 (to KLZ).
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
C-Editor: Zhao M; S-Editor: Li CH; L-Editors: Li CH, Song LP; T-Editor: Jia Y
References
- 1.Abu Hamdeh S, Shevchenko G, Mi J, Musunuri S, Bergquist J, Marklund N. Proteomic differences between focal and diffuse traumatic brain injury in human brain tissue. Sci Rep. 2018;8:6807. doi: 10.1038/s41598-018-25060-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackermann EJ, Conde-Frieboes K, Dennis EA. Inhibition of macrophage Ca(2+)-independent phospholipase A2 by bromoenol lactone and trifluoromethyl ketones. J Biol Chem. 1995;270:445–450. doi: 10.1074/jbc.270.1.445. [DOI] [PubMed] [Google Scholar]
- 3.Alashmali S, Walchuk C, Cadonic C, Albensi B, Aliani M, Suh M. The effect of choline availability from gestation to early development on brain and retina functions and phospholipid composition in a male mouse model. Nutr Neurosci. 2021:1–15. doi: 10.1080/1028415X.2021.1885229. [DOI] [PubMed] [Google Scholar]
- 4.An Y, Li J, Liu Y, Fan M. Neuroprotective effect of novel celecoxib derivatives against spinal cord injury via attenuation of COX-2, oxidative stress, apoptosisand inflammation. Bioorg Chem. 2020;101:104044. doi: 10.1016/j.bioorg.2020.104044. [DOI] [PubMed] [Google Scholar]
- 5.Anthonymuthu T, Kenny E, Amoscato A, Lewis J, Kochanek P, Kagan V, Bayır H. Global assessment of oxidized free fatty acids in brain reveals an enzymatic predominance to oxidative signaling after trauma. Biochim Biophys Acta Mol Basis Dis. 2017;1863(10 Pt B):2601–2613. doi: 10.1016/j.bbadis.2017.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashcroft F, Mahammad N, Midtun Flatekvål H, JullumstrøFeuerherm A, Johansen B. cPLAαenzyme inhibition attenuates inflammation and keratinocyte proliferation. Biomolecules. 2020;10:1402. doi: 10.3390/biom10101402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balsinde J, Balboa M, Dennis E. Functional coupling between secretory phospholipase A2 and cyclooxygenase-2 and its regulation by cytosolic group IV phospholipase A2. Proc Natl Acad Sci U S A. 1998;95:7951–7956. doi: 10.1073/pnas.95.14.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatia H, Roelofs N, Muñoz E, Fiebich B. Alleviation of microglial activation induced by p38 MAPK/MK2/PGE axis by capsaicin:potential involvement of other than TRPV1 mechanism/s. Sci Rep. 2017;7:116. doi: 10.1038/s41598-017-00225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolton C, Elderfield A, Flower R. The detection of lipocortins 1, 2 and 5 in central nervous system tissues from Lewis rats with acute experimental allergic encephalomyelitis. J Neuroimmunol. 1990;29(1-3):173–81. doi: 10.1016/0165-5728(90)90160-o. [DOI] [PubMed] [Google Scholar]
- 10.Bonventre JV. Roles of phospholipases A2 in brain cell and tissue injury associated with ischemia and excitotoxicity. J Lipid Mediat Cell Signal. 1996;14:15–23. doi: 10.1016/0929-7855(96)00503-2. [DOI] [PubMed] [Google Scholar]
- 11.Bonventre JV, Huang Z, Taheri MR, O'Leary E, Li E, Moskowitz MA, Sapirstein A. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390:622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- 12.Braem K, Luyten F, Lories R. Blocking p38 signalling inhibits chondrogenesis in vitro but not ankylosis in a model of ankylosing spondylitis in vivo. Ann Rheum Dis. 2012;71:722–728. doi: 10.1136/annrheumdis-2011-200377. [DOI] [PubMed] [Google Scholar]
- 13.Burke J, Babakhani A, Gorfe A, Kokotos G, Li S, Woods V, McCammon J, Dennis E. Location of inhibitors bound to group IVA phospholipase A2 determined by molecular dynamics and deuterium exchange mass spectrometry. J Am Chem Soc. 2009;131:8083–8091. doi: 10.1021/ja900098y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chao CC, Gutiérrez-Vázquez C, Rothhammer V, Mayo L, Wheeler MA, Tjon EC, Zandee SEJ, Blain M, de Lima KA, Takenaka MC, Avila-Pacheco J, Hewson P, Liu L, Sanmarco LM, Borucki DM, Lipof GZ, Trauger SA, Clish CB, Antel JP, Prat A, et al. Metabolic control of astrocyte pathogenic activity via cPLA2-MAVS. Cell. 2019;179:1483–1498.e22. doi: 10.1016/j.cell.2019.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chao H, Liu Y, Lin C, Xu X, Li Z, Bao Z, Fan L, Tao C, Zhao L, Liu Y, Wang X, You Y, Liu N, Ji J. Activation of bradykinin B2 receptor induced the inflammatory responses of cytosolic phospholipase A after the early traumatic brain injury. Biochim Biophys Acta Mol Basis Dis. 2018;1864(9 Pt B):2957–2971. doi: 10.1016/j.bbadis.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Chatterjee S, Balram A, Li W. Convergence:lactosylceramide-centric signaling pathways induce inflammation, oxidative stress, and other phenotypic outcomes. Int J Mol Sci. 2021;22:1816. doi: 10.3390/ijms22041816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chuang DY, Simonyi A, Kotzbauer PT, Gu Z, Sun GY. Cytosolic phospholipase A2 plays a crucial role in ROS/NO signaling during microglial activation through the lipoxygenase pathway. J Neuroinflammation. 2015;12:199. doi: 10.1186/s12974-015-0419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.David S, López-Vales R. Bioactive lipid mediators in the initiation and resolution of inflammation after spinal cord injury. Neuroscience. 2021;466:273–297. doi: 10.1016/j.neuroscience.2021.04.026. [DOI] [PubMed] [Google Scholar]
- 19.de Carvalho M, McCormack A, Olson E, Ghomashchi F, Gelb M, Yates J, Leslie C. Identification of phosphorylation sites of human 85-kDa cytosolic phospholipase A2 expressed in insect cells and present in human monocytes. J Biol Chem. 1996;271:6987–6997. doi: 10.1074/jbc.271.12.6987. [DOI] [PubMed] [Google Scholar]
- 20.Dennis E, Norris P. Eicosanoid storm in infection and inflammation. Nat Rev Immunol. 2015;15:511–523. doi: 10.1038/nri3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobrzeniecki M, Trofimov A, Martynov D, Agarkova D, Trofimova K, Semenova Z, Bragin D. Secondary cerebral ischemia at traumatic brain injury is more closely related to cerebrovascular reactivity impairment than to intracranial hypertension. Acta Neurochir Suppl. 2021;131:159–162. doi: 10.1007/978-3-030-59436-7_32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubinin M, Astashev M, Penkov N, Gudkov S, Dyachenko I, Samartsev V, Belosludtsev K. Effects of phospholipase A2 inhibitors on bilayer lipid membranes. J Membr Biol. 2016;249:339–347. doi: 10.1007/s00232-016-9872-7. [DOI] [PubMed] [Google Scholar]
- 23.Duro MV, Ebright B, Yassine HN. Lipids and brain inflammation in APOE4-associated dementia. Curr Opin Lipidol. 2022;33:16–24. doi: 10.1097/MOL.0000000000000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang S, Zhong L, Wang AQ, Zhang H, Yin ZS. Identification of regeneration and Hub genes and pathways at different time points after spinal cord injury. Mol Neurobiol. 2021;58:2643–2662. doi: 10.1007/s12035-021-02289-x. [DOI] [PubMed] [Google Scholar]
- 25.Farooqui AA, Ong WY, Horrocks LA. Inhibitors of brain phospholipase A2 activity:their neuropharmacological effects and therapeutic importance for the treatment of neurologic disorders. Pharmacol Rev. 2006;58:591–620. doi: 10.1124/pr.58.3.7. [DOI] [PubMed] [Google Scholar]
- 26.Farooqui AA, Yang HC, Rosenberger TA, Horrocks LA. Phospholipase A2 and its role in brain tissue. J Neurochem. 1997;69:889–901. doi: 10.1046/j.1471-4159.1997.69030889.x. [DOI] [PubMed] [Google Scholar]
- 27.Faure L, Nagarajan S, Hwang H, Montgomery CL, Khan BR, John G, Koulen P, Blancaflor EB, Chapman KD. Synthesis of phenoxyacyl-ethanolamides and their effects on fatty acid amide hydrolase activity. J Biol Chem. 2014;289:9340–9351. doi: 10.1074/jbc.M113.533315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feuerherm AJ, Dennis EA, Johansen B. Cytosolic group IVA phospholipase A2 inhibitors, AVX001 and AVX002, ameliorate collagen-induced arthritis. Arthritis Res Ther. 2019;21:29. doi: 10.1186/s13075-018-1794-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filgueiras LR, Brandt SL, Wang S, Wang Z, Morris DL, Evans-Molina C, Mirmira RG, Jancar S, Serezani CH. Leukotriene B4-mediated sterile inflammation promotes susceptibility to sepsis in a mouse model of type 1 diabetes. Sci Signal. 2015;8:ra10. doi: 10.1126/scisignal.2005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaire B, Choi J. Critical roles of lysophospholipid receptors in activation of neuroglia and their neuroinflammatory responses. Int J Mol Sci. 2021;22:7864. doi: 10.3390/ijms22157864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong L, Lv Y, Li S, Feng T, Zhou Y, Sun Y, Mi D. Changes in transcriptome profiling during the acute/subacute phases of contusional spinal cord injury in rats. Ann Transl Med. 2020;8:1682. doi: 10.21037/atm-20-6519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gopez JJ, Yue H, Vasudevan R, Malik AS, Fogelsanger LN, Lewis S, Panikashvili D, Shohami E, Jansen SA, Narayan RK, Strauss KI. Cyclooxygenase-2-specific inhibitor improves functional outcomes, provides neuroprotection, and reduces inflammation in a rat model of traumatic brain injury. Neurosurgery. 2005;56:590–604. doi: 10.1227/01.NEU.0000154060.14900.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gorica E, Calderone V. Arachidonic acid derivatives and neuroinflammation. CNS Neurol Disord Drug Targets. 2021;21:118–129. doi: 10.2174/1871527320666210208130412. [DOI] [PubMed] [Google Scholar]
- 34.Hall ED. Free radicals and CNS injury. Crit Care Clin. 1989;5:793–805. [PubMed] [Google Scholar]
- 35.Hamidabadi HG, Bojnordi MN, Ehsani S. Recovery potential of transplanted oligoprogenitor cells derived from human dental pulp stem cells in Lysophosphatidyl choline demyelination mode. Bratisl Lek Listy. 2021;122:621–625. doi: 10.4149/BLL_2021_099. [DOI] [PubMed] [Google Scholar]
- 36.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 37.Hayashi D, Mouchlis VD, Dennis EA. Omega-3 versus Omega-6 fatty acid availability is controlled by hydrophobic site geometries of phospholipase A2s. J Lipid Res. 2021;62:100113. doi: 10.1016/j.jlr.2021.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayashi D, Mouchlis VD, Dennis EA. Each phospholipase A(2) type exhibits distinct selectivity toward sn-1 ester, alkyl ether, and vinyl ether phospholipids. Biochim Biophys Acta Mol Cell Biol Lipids. 2022;1867:159067. doi: 10.1016/j.bbalip.2021.159067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hernández M, Bayón Y, Sánchez Crespo M, Nieto ML. Signaling mechanisms involved in the activation of arachidonic acid metabolism in human astrocytoma cells by tumor necrosis factor-alpha:phosphorylation of cytosolic phospholipase A2 and transactivation of cyclooxygenase-2. J Neurochem. 1999;73:1641–1649. doi: 10.1046/j.1471-4159.1999.0731641.x. [DOI] [PubMed] [Google Scholar]
- 40.Horiuchi H, Ogata T, Morino T, Chuai M, Yamamoto H. Continuous intrathecal infusion of SB203580, a selective inhibitor of p38 mitogen-activated protein kinase, reduces the damage of hind-limb function after thoracic spinal cord injury in rat. Neurosci Res. 2003;47:209–217. doi: 10.1016/s0168-0102(03)00216-5. [DOI] [PubMed] [Google Scholar]
- 41.Hornfelt M, Edström A, Ekström PA. Upregulation of cytosolic phospholipase A2 correlates with apoptosis in mouse superior cervical and dorsal root ganglia neurons. Neurosci Lett. 1999;265:87–90. doi: 10.1016/s0304-3940(99)00046-4. [DOI] [PubMed] [Google Scholar]
- 42.Hu X, Chen H, Xu H, Wu Y, Wu C, Jia C, Li Y, Sheng S, Xu C, Xu H, Ni W, Zhou K. Role of pyroptosis in traumatic brain and spinal cord injuries. Int J Biol Sci. 2020;16:2042–2050. doi: 10.7150/ijbs.45467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huwiler A, Feuerherm AJ, Sakem B, Pastukhov O, Filipenko I, Nguyen T, Johansen B. The ω3-polyunsaturated fatty acid derivatives AVX001 and AVX002 directly inhibit cytosolic phospholipase A(2) and suppress PGE(2) formation in mesangial cells. Br J Pharmacol. 2012;167:1691–1701. doi: 10.1111/j.1476-5381.2012.02114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ibata K, Yuzaki M. Destroy the old to build the new:Activity-dependent lysosomal exocytosis in neurons. Neurosci Res. 2021;167:38–46. doi: 10.1016/j.neures.2021.03.011. [DOI] [PubMed] [Google Scholar]
- 45.Isenović ER, Kedees MH, Tepavcević S, Milosavljević T, Korićanac G, Trpković A, Marche P. Role of PI3K/AKT, cPLA2 and ERK1/2 signaling pathways in insulin regulation of vascular smooth muscle cells proliferation. Cardiovasc Hematol Disord Drug Targets. 2009;9:172–180. doi: 10.2174/187152909789007034. [DOI] [PubMed] [Google Scholar]
- 46.Jan CR, Chou KJ, Lee KC, Wang JL, Tseng LL, Cheng JS, Chen WC. Dual action of palmitoyl trifluoromethyl ketone (PACOCF3) on Ca2+signaling:activation of extracellular Ca2+influx and alteration of ATP- and bradykinin-induced Ca2+responses in Madin Darby canine kidney cells. Arch Toxicol. 2000;74:447–451. doi: 10.1007/s002040000130. [DOI] [PubMed] [Google Scholar]
- 47.Kalyvas A, Baskakis C, Magrioti V, Constantinou-Kokotou V, Stephens D, López-Vales R, Lu JQ, Yong VW, Dennis EA, Kokotos G, David S. Differing roles for members of the phospholipase A2 superfamily in experimental autoimmune encephalomyelitis. Brain. 2009;132(Pt 5):1221–1235. doi: 10.1093/brain/awp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kareva EN. NSAID enteropathy. Ter Arkh. 2020;92:85–92. doi: 10.26442/00403660.2020.02.000453. [DOI] [PubMed] [Google Scholar]
- 49.Kataria H, Alizadeh A, Shahriary GM, Saboktakin Rizi S, Henrie R, Santhosh KT, Thliveris JA, Karimi-Abdolrezaee S. Neuregulin-1 promotes remyelination and fosters a pro-regenerative inflammatory response in focal demyelinating lesions of the spinal cord. Glia. 2018;66:538–561. doi: 10.1002/glia.23264. [DOI] [PubMed] [Google Scholar]
- 50.Khan M, Shunmugavel A, Dhammu TS, Matsuda F, Singh AK, Singh I. Oral administration of cytosolic PLA2 inhibitor arachidonyl trifluoromethyl ketone ameliorates cauda equina compression injury in rats. J Neuroinflammation. 2015;12:94. doi: 10.1186/s12974-015-0311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim JM, Im YN, Chung YJ, Youm JH, Im SY, Han MK, Lee HK. Glutamine deficiency shifts the asthmatic state toward neutrophilic airway inflammation. Allergy. 2021 doi: 10.1111/all.15121. doi:10.1111/all.15121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim S, Choi S, Kang D. Quantitative and qualitative analysis of autophagy flux using imaging. BMB Rep. 2020;53:241–247. doi: 10.5483/BMBRep.2020.53.5.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kishimoto K, Li RC, Zhang J, Klaus JA, Kibler KK, Doré S, Koehler RC, Sapirstein A. Cytosolic phospholipase A2 alpha amplifies early cyclooxygenase-2 expression, oxidative stress and MAP kinase phosphorylation after cerebral ischemia in mice. J Neuroinflammation. 2010;7:42. doi: 10.1186/1742-2094-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kita Y, Shindou H, Shimizu T. Cytosolic phospholipase A(2) and lysophospholipid acyltransferases. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:838–845. doi: 10.1016/j.bbalip.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 55.Klein M, Kaleem A, Oetjen S, Wünkhaus D, Binkle L, Schilling S, Gjorgjieva M, Scholz R, Gruber-Schoffnegger D, Storch S, Kins S, Drewes G, Hoffmeister-Ullerich S, Kuhl D, Hermey G. Converging roles of PSENEN/PEN2 and CLN3 in the autophagy-lysosome system. Autophagy. 2021:1–18. doi: 10.1080/15548627.2021.2016232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klionsky DJ. Neurodegeneration:good riddance to bad rubbish. Nature. 2006;441:819–820. doi: 10.1038/441819a. [DOI] [PubMed] [Google Scholar]
- 58.Kocak M, Ezazi Erdi S, Jorba G, Maestro I, Farrés J, Kirkin V, Martinez A, Pless O. Targeting autophagy in disease:established and new strategies. Autophagy. 2021:1–23. doi: 10.1080/15548627.2021.1936359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 60.Kopper TJ, Zhang B, Bailey WM, Bethel KE, Gensel JC. The effects of myelin on macrophage activation are phenotypic specific via cPLA2 in the context of spinal cord injury inflammation. Sci Rep. 2021;11:6341. doi: 10.1038/s41598-021-85863-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuwata H, Yamamoto S, Miyazaki Y, Shimbara S, Nakatani Y, Suzuki H, Ueda N, Yamamoto S, Murakami M, Kudo I. Studies on a mechanism by which cytosolic phospholipase A2 regulates the expression and function of type IIA secretory phospholipase A2. J Immunol. 2000;165:4024–4031. doi: 10.4049/jimmunol.165.7.4024. [DOI] [PubMed] [Google Scholar]
- 62.Lagraoui M, Sukumar G, Latoche JR, Maynard SK, Dalgard CL, Schaefer BC. Salsalate treatment following traumatic brain injury reduces inflammation and promotes a neuroprotective and neurogenic transcriptional response with concomitant functional recovery. Brain Behav Immun. 2017;61:96–109. doi: 10.1016/j.bbi.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lambrechts MJ, Cook JL. Nonsteroidal anti-inflammatory drugs and their neuroprotective role after an acute spinal cord injury:a systematic review of animal models. Global Spine J. 2021;11:365–377. doi: 10.1177/2192568220901689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li X, Cao X, Zhao H, Guo M, Fang X, Li K, Qin L, He Y, Liu X. Hypoxia activates Notch4 via ERK/JNK/P38 MAPK signaling pathways to promote lung adenocarcinoma progression and metastasis. Front Cell Dev Biol. 2021;9:780121. doi: 10.3389/fcell.2021.780121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y, Jones JW, M C, Choi H, Sarkar C, Kane MA, Koh EY, Lipinski MM, Wu J. cPLA2 activation contributes to lysosomal defects leading to impairment of autophagy after spinal cord injury. Cell Death Dis. 2019;10:531. doi: 10.1038/s41419-019-1764-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liang C, Liao J, Deng Z, Song C, Zhang J, Zabeau L, Tavernier J, Zhang K, Xue H, Yan G. Leptin attenuates lipopolysaccharide-induced apoptosis of thymocytes partially via down-regulation of cPLA2 and p38 MAPK activation. Int Immunopharmacol. 2013;15:620–627. doi: 10.1016/j.intimp.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 67.Lin CC, Hsieh HL, Liu SW, Tseng HC, Hsiao LD, Yang CM. BK induces cPLA2 expression via an autocrine loop involving COX-2-derived PGE2 in rat brain astrocytes. Mol Neurobiol. 2015;51:1103–1115. doi: 10.1007/s12035-014-8777-7. [DOI] [PubMed] [Google Scholar]
- 68.Lin LL, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 69.Liu H, Wang C, Yi F, Yeo S, Haas M, Tang X, Guan JL. Non-canonical function of FIP200 is required for neural stem cell maintenance and differentiation by limiting TBK1 activation and p62 aggregate formation. Sci Rep. 2021;11:23907. doi: 10.1038/s41598-021-03404-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu NK, Zhang YP, Titsworth WL, Jiang X, Han S, Lu PH, Shields CB, Xu XM. A novel role of phospholipase A2 in mediating spinal cord secondary injury. Ann Neurol. 2006;59:606–619. doi: 10.1002/ana.20798. [DOI] [PubMed] [Google Scholar]
- 71.Liu NK, Zhang YP, Han S, Pei J, Xu LY, Lu PH, Shields CB, Xu XM. Annexin A1 reduces inflammatory reaction and tissue damage through inhibition of phospholipase A2 activation in adult rats following spinal cord injury. J Neuropathol Exp Neurol. 2007;66:932–943. doi: 10.1097/nen.0b013e3181567d59. [DOI] [PubMed] [Google Scholar]
- 72.Liu NK, Deng LX, Zhang YP, Lu QB, Wang XF, Hu JG, Oakes E, Bonventre JV, Shields CB, Xu XM. Cytosolic phospholipase A2 protein as a novel therapeutic target for spinal cord injury. Ann Neurol. 2014;75:644–658. doi: 10.1002/ana.24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu S, Li Y, Choi HMC, Sarkar C, Koh EY, Wu J, Lipinski MM. Lysosomal damage after spinal cord injury causes accumulation of RIPK1 and RIPK3 proteins and potentiation of necroptosis. Cell Death Dis. 2018;9:476. doi: 10.1038/s41419-018-0469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.López-Serrano C, Santos-Nogueira E, Francos-Quijorna I, Coll-Miró M, Chun J, López-Vales R. Lysophosphatidic acid receptor type 2 activation contributes to secondary damage after spinal cord injury in mice. Brain Behav Immun. 2019;76:258–267. doi: 10.1016/j.bbi.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.López-Vales R, Ghasemlou N, Redensek A, Kerr BJ, Barbayianni E, Antonopoulou G, Baskakis C, Rathore KI, Constantinou-Kokotou V, Stephens D, Shimizu T, Dennis EA, Kokotos G, David S. Phospholipase A2 superfamily members play divergent roles after spinal cord injury. FASEB J. 2011;25:4240–4252. doi: 10.1096/fj.11-183186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.López-Vales R, David S. Bioactive lipids in inflammation after central nervous system injury. Adv Exp Med Biol. 2019;1127:181–194. doi: 10.1007/978-3-030-11488-6_12. [DOI] [PubMed] [Google Scholar]
- 77.Lu F, Li Y, Lin S, Cheng H, Yang S. Spatiotemporal regulation of store-operated calcium entry in cancer metastasis. Biochem Soc Trans. 2021;49:2581–2589. doi: 10.1042/BST20210307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lukacova N, Kisucka A, Kiss Bimbova K, Bacova M, Ileninova M, Kuruc T, Galik J. Glial-neuronal interactions in pathogenesis and treatment of spinal cord injury. Int J Mol Sci. 2021;22:13577. doi: 10.3390/ijms222413577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luo CL, Li QQ, Chen XP, Zhang XM, Li LL, Li BX, Zhao ZQ, Tao LY. Lipoxin A4 attenuates brain damage and downregulates the production of pro-inflammatory cytokines and phosphorylated mitogen-activated protein kinases in a mouse model of traumatic brain injury. Brain Res. 2013;1502:1–10. doi: 10.1016/j.brainres.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 80.Malada-Edelstein YF, Hadad N, Levy R. Regulatory role of cytosolic phospholipase A2 alpha in the induction of CD40 in microglia. J Neuroinflammation. 2017;14:33. doi: 10.1186/s12974-017-0811-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martini AC, Berta T, Forner S, Chen G, Bento AF, Ji RR, Rae GA. Lipoxin A4 inhibits microglial activation and reduces neuroinflammation and neuropathic pain after spinal cord hemisection. J Neuroinflammation. 2016;13:75. doi: 10.1186/s12974-016-0540-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martiszus BJ, Tsintsadze T, Chang W, Smith SM. Enhanced excitability of cortical neurons in low-divalent solutions is primarily mediated by altered voltage-dependence of voltage-gated sodium channels. Elife. 2021;10:e67914. doi: 10.7554/eLife.67914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McKew JC, Lee KL, Shen MW, Thakker P, Foley MA, Behnke ML, Hu B, Sum FW, Tam S, Hu Y, Chen L, Kirincich SJ, Michalak R, Thomason J, Ipek M, Wu K, Wooder L, Ramarao MK, Murphy EA, Goodwin DG, et al. Indole cytosolic phospholipase A2 alpha inhibitors:discovery and in vitro and in vivo characterization of 4-{3-[5-chloro-2-(2-{[(3,4-dichlorobenzyl)sulfonyl]amino}ethyl)-1-(diphenylmethyl)-1H-indol-3-yl]propyl}benzoic acid, efipladib. J Med Chem. 2008;51:3388–3413. doi: 10.1021/jm701467e. [DOI] [PubMed] [Google Scholar]
- 84.Mei HX, Ye Y, Xu HR, Xiang SY, Yang Q, Ma HY, Jin SW, Wang Q. LXA4 inhibits lipopolysaccharide-induced inflammatory cell accumulation by resident macrophages in mice. J Inflamm Res. 2021;14:1375–1385. doi: 10.2147/JIR.S301292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mitsuhashi T, Ikata T, Morimoto K, Tonai T, Katoh S. Increased production of eicosanoids, TXA2, PGI2 and LTC4 in experimental spinal cord injuries. Paraplegia. 1994;32:524–530. doi: 10.1038/sc.1994.84. [DOI] [PubMed] [Google Scholar]
- 86.Mizushima N, Komatsu M. Autophagy:renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 87.Mori M, Aihara M, Kume K, Hamanoue M, Kohsaka S, Shimizu T. Predominant expression of platelet-activating factor receptor in the rat brain microglia. J Neurosci. 1996;16:3590–3600. doi: 10.1523/JNEUROSCI.16-11-03590.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mouchlis VD, Dennis EA. Phospholipase A2 catalysis and lipid mediator lipidomics. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:766–771. doi: 10.1016/j.bbalip.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murakami M, Shimbara S, Kambe T, Kuwata H, Winstead MV, Tischfield JA, Kudo I. The functions of five distinct mammalian phospholipase A2S in regulating arachidonic acid release. Type IIa and type V secretory phospholipase A2S are functionally redundant and act in concert with cytosolic phospholipase A2. J Biol Chem. 1998;273:14411–14423. doi: 10.1074/jbc.273.23.14411. [DOI] [PubMed] [Google Scholar]
- 90.Murphy E, Curneen JMG, McEvoy JW. Aspirin in the modern era of cardiovascular disease prevention. Methodist Debakey Cardiovasc J. 2021;17:36–47. doi: 10.14797/mdcvj.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nagase T, Uozumi N, Ishii S, Kume K, Izumi T, Ouchi Y, Shimizu T. Acute lung injury by sepsis and acid aspiration:a key role for cytosolic phospholipase A2. Nat Immunol. 2000;1:42–46. doi: 10.1038/76897. [DOI] [PubMed] [Google Scholar]
- 92.Nagase T, Uozumi N, Ishii S, Kita Y, Yamamoto H, Ohga E, Ouchi Y, Shimizu T. A pivotal role of cytosolic phospholipase A(2) in bleomycin-induced pulmonary fibrosis. Nat Med. 2002;8:480–484. doi: 10.1038/nm0502-480. [DOI] [PubMed] [Google Scholar]
- 93.Naik MU, Patel P, Derstine R, Turaga R, Chen X, Golla K, Neeves KB, Ichijo H, Naik UP. Ask1 regulates murine platelet granule secretion, thromboxane A(2) generation, and thrombus formation. Blood. 2017;129:1197–1209. doi: 10.1182/blood-2016-07-729780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nasrazadani A, Van Den Berg CL. c-Jun N-terminal kinase 2 regulates multiple receptor tyrosine kinase pathways in mouse mammary tumor growth and metastasis. Genes Cancer. 2011;2:31–45. doi: 10.1177/1947601911400901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ni J, Wu Z, Stoka V, Meng J, Hayashi Y, Peters C, Qing H, Turk V, Nakanishi H. Increased expression and altered subcellular distribution of cathepsin B in microglia induce cognitive impairment through oxidative stress and inflammatory response in mice. Aging Cell. 2019;18:e12856. doi: 10.1111/acel.12856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nito C, Kamada H, Endo H, Niizuma K, Myer DJ, Chan PH. Role of the p38 mitogen-activated protein kinase/cytosolic phospholipase A2 signaling pathway in blood-brain barrier disruption after focal cerebral ischemia and reperfusion. J Cereb Blood Flow Metab. 2008;28:1686–1696. doi: 10.1038/jcbfm.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.No authors listed. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016:a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019a;18:56–87. doi: 10.1016/S1474-4422(18)30415-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.No authors listed. Global, regional, and national burden of neurological disorders, 1990-2016:a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019b;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ong WY, Farooqui T, Farooqui AA. Involvement of cytosolic phospholipase A(2), calcium independent phospholipase A(2) and plasmalogen selective phospholipase A(2) in neurodegenerative and neuropsychiatric conditions. Curr Med Chem. 2010;17:2746–2763. doi: 10.2174/092986710791859289. [DOI] [PubMed] [Google Scholar]
- 100.Ong WY, Sandhya TL, Horrocks LA, Farooqui AA. Distribution of cytoplasmic phospholipase A2 in the normal rat brain. J Hirnforsch. 1999a;39:391–400. [PubMed] [Google Scholar]
- 101.Ong WY, Horrocks LA, Farooqui AA. Immunocytochemical localization of cPLA2 in rat and monkey spinal cord. J Mol Neurosci. 1999b;12:123–130. doi: 10.1007/BF02736926. [DOI] [PubMed] [Google Scholar]
- 102.Papa S, Martino PL, Capitanio G, Gaballo A, De Rasmo D, Signorile A, Petruzzella V. The oxidative phosphorylation system in mammalian mitochondria. Adv Exp Med Biol. 2012;942:3–37. doi: 10.1007/978-94-007-2869-1_1. [DOI] [PubMed] [Google Scholar]
- 103.Pavicevic Z, Leslie CC, Malik KU. cPLA2 phosphorylation at serine-515 and serine-505 is required for arachidonic acid release in vascular smooth muscle cells. J Lipid Res. 2008;49:724–737. doi: 10.1194/jlr.M700419-JLR200. [DOI] [PubMed] [Google Scholar]
- 104.Peng Z, Chang Y, Fan J, Ji W, Su C. Phospholipase A2 superfamily in cancer. Cancer Lett. 2021;497:165–177. doi: 10.1016/j.canlet.2020.10.021. [DOI] [PubMed] [Google Scholar]
- 105.Penzo D, Petronilli V, Angelin A, Cusan C, Colonna R, Scorrano L, Pagano F, Prato M, Di Lisa F, Bernardi P. Arachidonic acid released by phospholipase A(2) activation triggers Ca(2+)-dependent apoptosis through the mitochondrial pathway. J Biol Chem. 2004;279:25219–25225. doi: 10.1074/jbc.M310381200. [DOI] [PubMed] [Google Scholar]
- 106.Piao CS, Kim JB, Han PL, Lee JK. Administration of the p38 MAPK inhibitor SB203580 affords brain protection with a wide therapeutic window against focal ischemic insult. J Neurosci Res. 2003;73:537–544. doi: 10.1002/jnr.10671. [DOI] [PubMed] [Google Scholar]
- 107.Piwowarek K, Rzeszotarska A, Korsak J, Juszkiewicz A, Chciałowski A, Kruszewski J. Clinical significance of plasma PAF acetylhydrolase activity measurements as a biomarker of anaphylaxis:Cross-sectional study. PLoS One. 2021;16:e0256168. doi: 10.1371/journal.pone.0256168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Qi HY, Daniels MP, Liu Y, Chen LY, Alsaaty S, Levine SJ, Shelhamer JH. A cytosolic phospholipase A2-initiated lipid mediator pathway induces autophagy in macrophages. J Immunol. 2011;187:5286–5292. doi: 10.4049/jimmunol.1004004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Resnick DK, Graham SH, Dixon CE, Marion DW. Role of cyclooxygenase 2 in acute spinal cord injury. J Neurotrauma. 1998;15:1005–1013. doi: 10.1089/neu.1998.15.1005. [DOI] [PubMed] [Google Scholar]
- 110.Rickman AD, Hilyard A, Heckmann BL. Dying by fire:noncanonical functions of autophagy proteins in neuroinflammation and neurodegeneration. Neural Regen Res. 2022;17:246–250. doi: 10.4103/1673-5374.317958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rukoyatkina N, Mindukshev I, Walter U, Gambaryan S. Dual role of the p38 MAPK/cPLA2 pathway in the regulation of platelet apoptosis induced by ABT-737 and strong platelet agonists. Cell Death Dis. 2013;4:e931. doi: 10.1038/cddis.2013.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rzigalinski BA, Blackmore PF, Rosenthal MD. Arachidonate mobilization is coupled to depletion of intracellular calcium stores and influx of extracellular calcium in differentiated U937 cells. Biochim Biophys Acta. 1996;1299:342–352. doi: 10.1016/0005-2760(95)00224-3. [DOI] [PubMed] [Google Scholar]
- 113.Saghazadeh A, Rezaei N. The role of timing in the treatment of spinal cord injury. Biomed Pharmacother. 2017;92:128–139. doi: 10.1016/j.biopha.2017.05.048. [DOI] [PubMed] [Google Scholar]
- 114.Santerre-Anderson JL, Werner DF. Ethanol stimulation of microglia release increases ERK1/2-dependent neuronal cPLA2 activity in immature cultured cortical preparations. Neurochem Res. 2020;45:1592–1601. doi: 10.1007/s11064-020-03024-z. [DOI] [PubMed] [Google Scholar]
- 115.Sarkar C, Jones JW, Hegdekar N, Thayer JA, Kumar A, Faden AI, Kane MA, Lipinski MM. PLA2G4A/cPLA2-mediated lysosomal membrane damage leads to inhibition of autophagy and neurodegeneration after brain trauma. Autophagy. 2020;16:466–485. doi: 10.1080/15548627.2019.1628538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shelat PB, Chalimoniuk M, Wang JH, Strosznajder JB, Lee JC, Sun AY, Simonyi A, Sun GY. Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. J Neurochem. 2008;106:45–55. doi: 10.1111/j.1471-4159.2008.05347.x. [DOI] [PubMed] [Google Scholar]
- 117.Shibata N, Kato Y, Inose Y, Hiroi A, Yamamoto T, Morikawa S, Sawada M, Kobayashi M. 4-Hydroxy-2-nonenal upregulates and phosphorylates cytosolic phospholipase A(2) in cultured Ra2 microglial cells via MAPK pathways. Neuropathology. 2011;31:122–128. doi: 10.1111/j.1440-1789.2010.01139.x. [DOI] [PubMed] [Google Scholar]
- 118.Shinohara H, Balboa MA, Johnson CA, Balsinde J, Dennis EA. Regulation of delayed prostaglandin production in activated P388D1 macrophages by group IV cytosolic and group V secretory phospholipase A2s. J Biol Chem. 1999;274:12263–12268. doi: 10.1074/jbc.274.18.12263. [DOI] [PubMed] [Google Scholar]
- 119.Shintani T, Klionsky DJ. Autophagy in health and disease:a double-edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shohami E, Shapira Y, Yadid G, Reisfeld N, Yedgar S. Brain phospholipase A2 is activated after experimental closed head injury in the rat. J Neurochem. 1989;53:1541–1546. doi: 10.1111/j.1471-4159.1989.tb08550.x. [DOI] [PubMed] [Google Scholar]
- 121.Siegrist KJ, Romo D, Upham BL, Armstrong M, Quinn K, Vanderlinden L, Osgood RS, Velmurugan K, Elie M, Manke J, Reinhold D, Reisdorph N, Saba L, Bauer AK. Early mechanistic events induced by low molecular weight polycyclic aromatic hydrocarbons in mouse lung epithelial cells:a role for Eicosanoid signaling. Toxicol Sci. 2019;169:180–193. doi: 10.1093/toxsci/kfz030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Song HH, Song TC, Yang T, Sun CS, He BQ, Li H, Wang YJ, Li Y, Wu H, Hu YM, Wang YJ. High mobility group box 1 mediates inflammatory response of astrocytes via cyclooxygenase 2/prostaglandin E2 signaling following spinal cord injury. Neural Regen Res. 2021;16:1848–1855. doi: 10.4103/1673-5374.303039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stachowicz K. Deciphering the mechanisms of regulation of an excitatory synapse via cyclooxygenase-2. A review. Biochem Pharmacol. 2021;192:114729. doi: 10.1016/j.bcp.2021.114729. [DOI] [PubMed] [Google Scholar]
- 124.Stahelin RV, Subramanian P, Vora M, Cho W, Chalfant CE. Ceramide-1-phosphate binds group IVA cytosolic phospholipase a2 via a novel site in the C2 domain. J Biol Chem. 2007;282:20467–20474. doi: 10.1074/jbc.M701396200. [DOI] [PubMed] [Google Scholar]
- 125.Stasiłowicz A, Tykarska E, Rosiak N, Sałat K, Furgała-Wojas A, Plech T, Lewandowska K, Pikosz K, Pawłowicz K, Cielecka-Piontek J. The inclusion of tolfenamic acid into cyclodextrins stimulated by microenvironmental pH modification as a way to increase the anti-migraine effect. J Pain Res. 2021;14:981–992. doi: 10.2147/JPR.S295795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stephenson J, Nutma E, van der Valk P, Amor S. Inflammation in CNS neurodegenerative diseases. Immunology. 2018;154:204–219. doi: 10.1111/imm.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stewart AN, Lowe JL, Glaser EP, Mott CA, Shahidehpour RK, McFarlane KE, Bailey WM, Zhang B, Gensel JC. Acute inflammatory profiles differ with sex and age after spinal cord injury. J Neuroinflammation. 2021;18:113. doi: 10.1186/s12974-021-02161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stirling D, Liu J, Plunet W, Steeves J, Tetzlaff W. SB203580, a p38 mitogen-activated protein kinase inhibitor, fails to improve functional outcome following a moderate spinal cord injury in rat. Neuroscience. 2008;155:128–137. doi: 10.1016/j.neuroscience.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 129.Street IP, Lin HK, Laliberté F, Ghomashchi F, Wang Z, Perrier H, Tremblay NM, Huang Z, Weech PK, Gelb MH. Slow- and tight-binding inhibitors of the 85-kDa human phospholipase A2. Biochemistry. 1993;32:5935–5940. doi: 10.1021/bi00074a003. [DOI] [PubMed] [Google Scholar]
- 130.Sun GY, Geng X, Teng T, Yang B, Appenteng MK, Greenlief CM, Lee JC. Dynamic role of phospholipases A2 in health and diseases in the central nervous system. Cells. 2021;10:2963. doi: 10.3390/cells10112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tai N, Kuwabara K, Kobayashi M, Yamada K, Ono T, Seno K, Gahara Y, Ishizaki J, Hori Y. Cytosolic phospholipase A2 alpha inhibitor, pyrroxyphene, displays anti-arthritic and anti-bone destructive action in a murine arthritis model. Inflamm Res. 2010;59:53–62. doi: 10.1007/s00011-009-0069-8. [DOI] [PubMed] [Google Scholar]
- 132.Veenith TV, Carter EL, Geeraerts T, Grossac J, Newcombe VF, Outtrim J, Gee GS, Lupson V, Smith R, Aigbirhio FI, Fryer TD, Hong YT, Menon DK, Coles JP. Pathophysiologic mechanisms of cerebral ischemia and diffusion hypoxia in traumatic brain injury. JAMA Neurol. 2016;73:542–550. doi: 10.1001/jamaneurol.2016.0091. [DOI] [PubMed] [Google Scholar]
- 133.Vichai V, Suyarnsesthakorn C, Pittayakhajonwut D, Sriklung K, Kirtikara K. Positive feedback regulation of COX-2 expression by prostaglandin metabolites. Inflamm Res. 2005;54:163–172. doi: 10.1007/s00011-004-1338-1. [DOI] [PubMed] [Google Scholar]
- 134.Wang S, Li B, Solomon V, Fonteh A, Rapoport SI, Bennett DA, Arvanitakis Z, Chui HC, Miller C, Sullivan PM, Wang HY, Yassine HN. Calcium-dependent cytosolic phospholipase A2 activation is implicated in neuroinflammation and oxidative stress associated with ApoE4. Mol Neurodegener. 2021;16:26. doi: 10.1186/s13024-021-00438-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 135.Wang Y, Gao Z, Zhang Y, Feng SQ, Liu Y, Shields LBE, Zhao YZ, Zhu Q, Gozal D, Shields CB, Cai J. Attenuated reactive gliosis and enhanced functional recovery following spinal cord injury in null mutant mice of platelet-activating factor receptor. Mol Neurobiol. 2016;53:3448–3461. doi: 10.1007/s12035-015-9263-6. [DOI] [PubMed] [Google Scholar]
- 136.Wei N, Lu T, Yang L, Dong Y, Liu X. Lipoxin A4 protects primary spinal cord neurons from Erastin-induced ferroptosis by activating the Akt/Nrf2/HO-1 signaling pathway. FEBS Open Bio. 2021;11:2118–2126. doi: 10.1002/2211-5463.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Weil ZM, Karelina K, Whitehead B, Velazquez-Cruz R, Oliverio R, Pinti M, Nwafor DC, Nicholson S, Fitzgerald JA, Hollander J, Brown CM, Zhang N, DeVries AC. Mild traumatic brain injury increases vulnerability to cerebral ischemia in mice. Exp Neurol. 2021;342:113765. doi: 10.1016/j.expneurol.2021.113765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wu H, Liu H, Zuo F, Zhang L. Adenoviruses-mediated RNA interference targeting cytosolic phospholipase A2αattenuates focal ischemic brain damage in mice. Mol Med Rep. 2018;17:5601–5610. doi: 10.3892/mmr.2018.8610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu J, Lipinski MM. Autophagy in neurotrauma:good, bad, or dysregulated. Cells. 2019;8:693. doi: 10.3390/cells8070693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xu D, Cao F, Sun S, Liu T, Feng S. Inhibition of the Ras/Raf/ERK1/2 signaling pathway restores cultured spinal cord-injured neuronal migration, adhesion, and dendritic spine development. Neurochem Res. 2016;41:2086–2096. doi: 10.1007/s11064-016-1921-1. [DOI] [PubMed] [Google Scholar]
- 141.Xue Y, Deng Q, Zhang Q, Ma Z, Chen B, Yu X, Peng H, Yao S, Liu J, Ye Y, Pan G. Gigantol ameliorates CCl(4)-induced liver injury via preventing activation of JNK/cPLA2/12-LOX inflammatory pathway. Sci Rep. 2020;10:22265. doi: 10.1038/s41598-020-79400-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yamazaki R, Osanai Y, Kouki T, Shinohara Y, Huang JK, Ohno N. Macroscopic detection of demyelinated lesions in mouse PNS with neutral red dye. Sci Rep. 2021;11:16906. doi: 10.1038/s41598-021-96395-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yang CS, Yuk JM, Shin DM, Kang J, Lee SJ, Jo EK. Secretory phospholipase A2 plays an essential role in microglial inflammatory responses to Mycobacterium tuberculosis. Glia. 2009;57:1091–1103. doi: 10.1002/glia.20832. [DOI] [PubMed] [Google Scholar]
- 144.Yang D, Ji HF, Zhang XM, Yue H, Lin L, Ma YY, Huang XN, Fu J, Wang WZ. Protective effect of cytosolic phospholipase A2 inhibition against inflammation and degeneration by promoting regulatory T cells in rats with experimental autoimmune encephalomyelitis. Mediators Inflamm 2014. 2014 doi: 10.1155/2014/890139. 890139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yazlovitskaya EM, Linkous AG, Thotala DK, Cuneo KC, Hallahan DE. Cytosolic phospholipase A2 regulates viability of irradiated vascular endothelium. Cell Death Differ. 2008;15:1641–1653. doi: 10.1038/cdd.2008.93. [DOI] [PubMed] [Google Scholar]
- 146.Yin XJ, Chen ZY, Zhu XN, Hu JJ. Loss of PAFR prevents neuroinflammation and brain dysfunction after traumatic brain injury. Sci Rep. 2017;7:40614. doi: 10.1038/srep40614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yu X, Sha L, Liu Q, Zhao Y, Fang H, Cao Y, Zhao J. Recent advances in cell membrane camouflage-based biosensing application. Biosens Bioelectron. 2021;194:113623. doi: 10.1016/j.bios.2021.113623. [DOI] [PubMed] [Google Scholar]
- 148.Yuan J, Dong X, Yap J, Hu J. The MAPK and AMPK signalings:interplay and implication in targeted cancer therapy. J Hematol Oncol. 2020;13:113. doi: 10.1186/s13045-020-00949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yun B, Lee H, Ewing H, Gelb MH, Leslie CC. Off-target effect of the cPLA2αinhibitor pyrrophenone:Inhibition of calcium release from the endoplasmic reticulum. Biochem Biophys Res Commun. 2016;479:61–66. doi: 10.1016/j.bbrc.2016.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zahiri D, Burow P, Großmann C, Müller CE, Klapperstück M, Markwardt F. Sphingosine-1-phosphate induces migration of microglial cells via activation of volume-sensitive anion channels, ATP secretion and activation of purinergic receptors. Biochim Biophys Acta Mol Cell Res. 2021;1868:118915. doi: 10.1016/j.bbamcr.2020.118915. [DOI] [PubMed] [Google Scholar]
- 151.Zhang J, Barasch N, Li RC, Sapirstein A. Inhibition of cytosolic phospholipase A(2) alpha protects against focal ischemic brain damage in mice. Brain Res. 2012;1471:129–137. doi: 10.1016/j.brainres.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 152.Zhang XW, Lv XX, Zhou JC, Jin CC, Qiao LY, Hu ZW. Autophagic Flux detection:significance and methods involved. Adv Exp Med Biol. 2021;1208:131–173. doi: 10.1007/978-981-16-2830-6_9. [DOI] [PubMed] [Google Scholar]
- 153.Zhou Q, Meng X, Huang G, Yi H, Zheng J, Zhang K, Xu W. MEK1/2 inhibition synergistically enhances the preventive effects of normobaric oxygen on spinal cord injury in decompression sickness rats. Front Physiol. 2021;12:674430. doi: 10.3389/fphys.2021.674430. [DOI] [PMC free article] [PubMed] [Google Scholar]


