Abstract
In this work, we have purified the His-tagged oxygenase (ht-oxygenase) component of Rhodococcus globerulus P6 biphenyl dioxygenase. The α or β subunit of P6 oxygenase was exchanged with the corresponding subunit of Pseudomonas sp. strain LB400 or of Comamonas testosteroni B-356 to create new chimeras that were purified ht-proteins and designated ht-αP6βP6, ht-αP6βLB400, ht-αP6βB-356, ht-αLB400βP6, and ht-αB-356βP6. ht-αP6βP6, ht-αP6βLB400, ht-αP6βB-356 were not expressed active in recombinant Escherichia coli cells carrying P6 bphA1 and bphA2, P6 bphA1 and LB400 bphE, or P6 bphA1 and B-356 bphE because the [2Fe-2S] Rieske cluster of P6 oxygenase α subunit was not assembled correctly in these clones. On the other hand ht-αLB400βP6 and ht-αB-356βP6 were produced active in E. coli. Furthermore, active purified ht-αP6βP6, ht-αP6βLB400, ht-αP6βB-356, showing typical spectra for Rieske-type proteins, were obtained from Pseudomonas putida KT2440 carrying constructions derived from the new shuttle E. coli-Pseudomonas vector pEP31, designed to produce ht-proteins in Pseudomonas. Analysis of the substrate selectivity pattern of these purified chimeras toward selected chlorobiphenyls indicate that the catalytic capacity of hybrid enzymes comprised of an α and a β subunit recruited from distinct biphenyl dioxygenases is not determined specifically by either one of the two subunits.
Aryl hydroxylating dioxygenases catalyze the first enzymatic step for most bacterial catabolic pathways involved in the degradation of aromatic compounds (28). They catalyze a dihydroxylation reaction onto vicinal carbons of the aromatic ring. These enzymes can catalyze the hydroxylation of several substrate analogs, which makes them potentially useful for the development of biocatalytic processes to destroy persistent pollutants such as polychlorinated biphenyls (PCBs). Biphenyl dioxygenase (BPH dox) can catalyze the hydroxylation of several PCB congeners, but to extend its capacity to hydroxylate more persistent congeners, new engineered enzymes will need to be developed.
BPH dox has been purified from Comamonas testosteroni B-356 (18, 19) and from Pseudomonas sp. strain LB400 (8, 13, 14). It comprises three components (8, 13, 14, 18, 19): the terminal oxygenase, an iron-sulfur protein (ISPBPH) made up of an α (Mr = 51,000) and a β (Mr = 22,000) subunit; a ferredoxin (FERBPH; Mr = 12,000); and a ferredoxin reductase (REDBPH; Mr = 43,000). The encoding genes for both strain B-356 and strain LB400 are bphA (ISPBPH α subunit), bphE (ISPBPH β subunit), bphF (FERBPH), and bphG (REDBPH) (11, 35). BPH dox hydroxylates vicinal ortho-meta carbons of one of the BPH rings to generate 2,3-dihydro-2,3-dihydroxybiphenyl. FERBPH and REDBPH are involved in electron transfer from NADH to ISPBPH, which is directly involved in the catalytic oxygenation of the molecule (19). The enzyme is also found in the genus Rhodococcus. The four genes that code for Rhodococcus globerulus P6, Rhodococcus strain RHA1, and Rhodococcus sp. strain M5 BPH dox have been sequenced (2, 27, 37). They are designated bphA1 (α subunit), bphA2 (β subunit), bphA3 (FERBPH), and bphA4 (REDBPH) in strain P6 (2).
In previous work, rhodococcal BPH dox was poorly expressed in Escherichia coli (27, 29). However, the genes encoding rhodococcal BPH dox components were expressed in recombinant Pseudomonas (29) and Rhodococcus (27). The substrate selectivity patterns of Pseudomonas putida KT2442 carrying the genes coding for strain P6 BPH dox were analyzed by testing the catalytic capacity of resting cell suspension on 3,4′-dichlorobiphenyl and 2,2′-dichlorobiphenyl. Data suggested that the enzyme metabolizes preferentially the meta-substituted ring over the para-substituted and poorly transformed the double-ortho-substituted congener 2,2′-dichlorobiphenyl (29). Furthermore, P6 BPH dox was unable to catalyze the hydroxylation of 2,2′,5,5′-tetrachlorobiphenyl used to determine the capacity of the enzyme to catalyze the meta-para hydroxylation of the biphenyl molecule (29). Coincidentally, these features are similar to those reported for strain B-356 BPH dox (17). Unlike these two strains, strain LB400 BPH dox shows a preference for 2,2′-dichlorobiphenyl, poorly transforms 3,3′-dichlorobiphenyl, and is able to catalyze the meta-para hydroxylation of 2,2′,5,5′-tetrachlorobiphenyl (15, 17, 22, 30).
Although Pseudomonas pseudoalcaligenes KF707 and LB400 BPH doxes components show a high level of identity, their substrate selectivity patterns toward chlorobiphenyls are quite distinct. Only few amino acid residues located at the N-terminal portion of the terminal oxygenase α subunit were found to determine the substrate specificity of these enzymes (22, 30). Based on sequence comparison between enzymes of several strains, Mondello et al. (30) have identified four regions of the terminal oxygenase α subunit in which specific sequences were consistently associated with either broad (LB400-type) or narrow (KF707-type) PCB substrate specificity. Based on published data of DNA sequence and substrate specificity (2, 29), like B-356 ISPBPH, P6 ISPBPH correlates with the KF707-type strain.
Recently, His-tagged purified chimeras (ht-chimeras) were obtained by exchanging the α and β subunits of LB400 and B-356 BPH dox terminal oxygenases (17). The amino acid residues of the α subunit which were found to determine the substrate selectivity pattern of LB400 and KF707 terminal oxygenase (22, 30) were not found to influence the substrate selectivity pattern of the engineered chimeras (17). Furthermore, the substrate selectivity pattern of ISPBPH chimeras comprised of B-356 α subunit with LB400 β subunit (αB-356βLB400) was very similar to that of LB400 BPH dox, which suggests an involvement of the β subunit on the reactivity pattern of the terminal oxygenase toward PCBs. To extend this study, in this work we purified recombinant P6 ht-ISPBPH expressed from E. coli and Pseudomonas and compared its substrate selectivity pattern toward chlorobiphenyls with that of engineered purified ht-ISPBPH chimeras obtained by exchanging P6 α or β subunit with corresponding peptide of LB400 or B-356 ISPBPH.
MATERIALS AND METHODS
Bacterial strains, culture media, and general protocols.
The bacterial strains used in this study were E. coli M15[pREP4] and SG13009[pREP4] (both from Qiagen Inc., Chatsworth, Calif.), E. coli DH11S (25), P. putida KT2440 (4), C. testosteroni B-356 (1), Pseudomonas sp. strain LB400 (6) (also referred as Burkholderia sp. strain LB400 or Pseudomonas cepacia LB400 [22]), and R. globerulus P6 (2, 3, 12). The media used were Luria-Bertani (LB) broth or solidified with agar (33). Plasmid DNA from E. coli, restriction endonuclease reactions, ligations, agarose gel electrophoresis, and transformation of E. coli cells were done according to protocols described by Sambrook et al. (33). The transformation of P. putida KT2440 was done according to the protocol described by Sambrook et al. (33) for the transformation of E. coli except that Ca2+ was replaced by Rb2+ and the cells were incubated for 2 h at 28°C (instead of 45 min at 37°C) before plating. The transformation rates were approximately 102 transformants per μg of DNA added to the ligation reaction medium. PCR was performed with Pwo DNA polymerase according to the method recommended by Boehringer Mannheim.
Plasmids.
Several vector and plasmids were used in this study. Plasmid pQE31, designed to create His-tagged fused proteins, and pQE51, which is identical to pQE31 except for the lack of the His6-tagged fused gene, were both from Qiagen. Plasmid pYH31 (17, 20) is also designed to create His-tagged fused proteins but is compatible with ColE1-based plasmids. Plasmid pEP31, obtained during this work (Fig. 1A), is a shuttle vector designed to produce fusion ht-protein in Pseudomonas and in E. coli. It confers resistance to both tetracycline and ampicillin. For construction of pEP31, pUCP26 (10, 34), which was graciously provided by H. P. Schweizer (Department of Microbiology, Colorado State University, Fort Collins), was treated with PvuI. The 3.2-kb fragment carrying the rep region (ori [origin of replication] of Pseudomonas aeruginosa PAO, which allows the plasmid to replicate in Pseudomonas) and tetracycline resistance (pALTER-1) was made blunt ended. Similarly, the 2.5-kb PvuII/NdeI fragment of pQE31 carrying the ori for replication, amplicillin resistance, and the promoter-operator region plus the His-tagged fusion gene was made blunt ended and then ligated with the 3.2-kb DNA fragment from pUCP26. Likewise, for construction of pEP51, the 2.5-kb PvuII/NdeI fragment of pQE51 was ligated to the 3.2-kb PvuI fragment of pUCP26. For construction of the 7.8-kb plasmid pQE51[LB400-bphFGBC], a 3.5-kb SmaI/NdeI fragment from pQE51 was ligated to a 4.3-kb BbrPI/NdeI fragment which carries LB400 bphFGBC from pAH17 (graciously provided by V. De Lorenzo, Centro Nacional de Biotecnología, Consejo Superior de Investigaciones Crentíficas Madrid, Spain). Similarly, for construction of the 8.3-kb plasmid pYH31[LB400-bphFGBC], the 4.3-kb BbrPI/NdeI fragment from pAH17 was ligated blunt end to the 4-kb plasmid pYH31 digested with KpnI at its unique KpnI site.
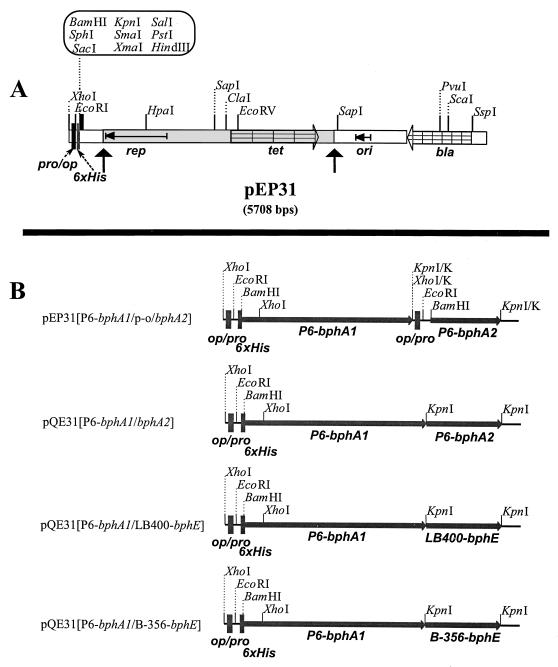
FIG. 1.
(A) Linear map of the E. coli-Pseudomonas shuttle vector pEP31 designed to produce ht-proteins. Only selected restriction sites are shown. The grey and white areas derive from pUCP26 and pQE31, respectively. rep, origin of replication in Pseudomonas; tet, tetracycline resistance; bla, ampicillin resistance; ori, ColE1 origin of replication; pro/op, pQE31 promoter-operator; 6xHis, the six-histidine-tagged fusion gene. The map of pEP51 is identical to that of pEP31 except for the absence of the six-histidine-tagged fusion gene. (B) Constructs used to produce P6 ht-ISPBPH and chimeras derived from it. All constructs except pEP31[P6-bphA1/p-o/bphA2] were made in pQE31, and the cloned DNA fragment was transferred to pEP31 when needed. Details of the strategies used to construct these plasmids are given in Materials and Methods. Only the restriction sites important for the cloning strategies are shown. KpnI/K and XhoI/K indicate sites that were made blunt ended with the Klenow fragment of DNA polymerase I, resulting in their loss in the final construct.
Plasmid constructions used to express P6 ht-ISPBPH and ht-ISPBPH chimeras.
In this work we have produced purified preparations of P6 ht-ISPBPH (ht-αP6βP6) and of all four hybrid combinations between the P6 terminal oxygenase α and β subunits and the corresponding subunits of LB400 and B-356 terminal oxygenases, thus producing ht-αP6βLB400, ht-αLB400βP6, ht-αP6βB-356, and ht-αB-356βP6.
The constructs used to express these enzymes are listed in Table 1. The plasmids and strategies used to obtain these constructs are explained below.
TABLE 1.
Plasmid constructions used to express and produce purified P6 ht-ISPBPH and its chimeras
| ISPBPH | Plasmid construction(s)a | Reference(s) |
|---|---|---|
| ht-αP6βP6 | pQE31[P6-bphA1/bphA2] | This work |
| pYH31[P6-bphA1] and pQE51[P6-phA2] | This work | |
| pEP31[P6-bphA1/p-o/bphA2] | This work | |
| pEP51[P6-bphA1/p-o/bphA2] | This work | |
| ht-αP6βLB400 | pQE31[P6-bphA1/LB400-bphE] | This work |
| pEP31[P6-bphA1/LB400-bphE] | This work | |
| ht-αP6βB-356 | pQE31[P6-bphA1/B-356-bphE] | This work |
| pEP31[P6-bphA1/B-356-bphE] | This work | |
| ht-αLB-400βP6 | pYH31[LB400-bphA] and pQE51[P6-bphA2] | 17, this work |
| ht-αB-356βP6 | pYH31[B-356-bphA] and pQE51[P6-bphA2] | This work |
See text and Fig. 1 for details.
Plasmids pYH31[B-356-bphA] and pYH31[LB400-bphA] were obtained by subcloning the corresponding BamHI/KpnI fragment of pQE31[B-356-bphA] and pQE31[LB400-bphA] described previously (17). The oligonucleotides used to amplify bphA1 from strain P6 were based on known nucleotide sequences (2). They were oligonucleotides I ([BamHI] 5′-CGGGATCCGATGACCAATCAATTGG-3′) and II ([KpnI] 5′-GGGGTACCCCTCAGGCCGAGACATC-3′). P6 bphA1 was cloned as a BamHI/KpnI fragment into corresponding sites of pQE31, pYH31, pEP31, and pEP51 to create pQE31[P6-bphA1], pYH31[P6-bphA1], pEP31[P6-bphA1], and pEP51[P6-bphA1].
pQE51[LB400-bphE] carrying LB400 bphE as a KpnI/KpnI fragment has been described previously (17). B-356 bphE was amplified with oligonucleotides III ([KpnI] 5′CCGGGTACCCATGATATCCACTCCCT3′) and IV ([KpnI] 5′GGGGTACCCCTCAAAAGAACACGCT3′), and the 0.6-kb fragment was cloned into the KpnI site of pQE51 to construct pQE51[B-356-bphE]. Oligonucleotides V ([KpnI] 5′CGAGGTACCTATGACGGACACCGTCG 3′) and VI ([KpnI] 5′CGACGGTACCCTAGAAGAAGAAGCT 3′) were used to amplify strain P6 bphA2. P6 bphA2 was cloned into KpnI-digested pQE51 to construct pQE51[P6-bphA2].
P6 bphA2 was subcloned from pQE51[P6-bphA2] into the unique KpnI site of pQE31[P6-bphA1] to create pQE31[P6-bphA1/bphA2] (Fig. 1B). pQE31[P6-bphA1/B-356-bphE] was obtained by cloning the KpnI/KpnI B-356 bphE fragment into KpnI-treated pQE31[P6-bphA1]. pQE31[P6-bphA1/LB400-bphE] was obtained by cloning the 0.6-kb KpnI/KpnI fragment of pQE51[LB400-bphE] (17) into KpnI-digested pQE31[P6-bphA1].
For the construction of pEP31[P6-bphA1/bphA2], P6 bphA2 was cloned from pQE51[P6-bphA2] into the KpnI site of pEP31[P6-bphA1]. Another construct was made such that both P6 bphA1 and P6 bphA2 were expressed from pEP31 but each was placed immediately downstream of a pQE promoter-operator region (indicated as “p-o” in plasmid designations). pEP31[P6-bphA1/p-o/bphA2] (Fig. 1B) was constructed in the following way. P6 bphA2 was amplified by using oligonucleotides VII ([BamHI] 5′CGGGATCCCATGACGGACACCGTCG 3′) with oligonucleotide VI. The BamHI/KpnI fragment was cloned into pQE51. The resulting plasmid was digested with XhoI/KpnI to release a 0.6-kb fragment carrying the pQE51 promoter-operator region plus P6 bphA2, which was made blunt ended and ligated to the blunt-ended KpnI-digested pEP31[P6-bphA1]. Similar strategies were used to construct pEP51[P6-bphA1/bphA2] and pEP51[P6-bphA1/p-o/bphA2].
For the construction of pEP31[P6-bphA1/B-356-bphE] and pEP31[P6-bphA1/LB400-bphE], the 1.9-kb BamHI/HindIII fragment of pQE31[P6-bphA1/B-356-bphE] or pQE31[P6-bphA1/LB400-bphE] was cloned into appropriately treated pEP31. pEP51[P6-bphA1/B-356-bphE] and pEP51[P6-bphA1/LB400-bphE] were constructed in a similar manner.
The various constructions used in this work were designed to produce ht-purified ISPBPH component carrying the His tag on the α subunit. All constructions were such that the His tag added the same 13 amino acids (MRGSHHHHHHTDP) to the protein at the N-terminal portion.
Expression and purification of P6 ISPBPH and its chimeras in E. coli and P. putida KT2440.
The ht-enzymes were expressed in E. coli cells according to protocols described previously (18, 19). When the proteins were expressed from pEP31 in E. coli cells, the antibiotic concentrations used were 200 μg of ampicillin per ml and 10 μg of tetracycline per ml (tet 10).
Several parameters, such as culture medium, antibiotic concentration, incubation time, temperature, size of the inoculum, concentration of isopropyl-β-d-thiogalactopyranoside (IPTG) as inducer, and cell density at the time of induction, were varied to optimize the expression of ISPBPH from pEP31[bphA1/p-o/bphA2], pEP31[P6-bphA1/LB400-bphE], and pEP31[P6-bphA1/B-356-bphE] in P. putida KT2440. The following optimized protocol was retained for this work. Cells from a frozen culture were grown overnight with shaking at 29°C in LB broth containing 20 μg of tetracycline per ml (tet 20). This culture was used to inoculate two to four 1-liter Erlenmeyer flasks each containing 600 ml of LB broth plus tet 20. The cultures were grown at 250 rpm at 29°C until the optical density reached 0.6 at 600 nm. Then 0.5 mM IPTG was added, and the cultures were incubated in the same conditions for 6 h. Cells were then harvested, washed with piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES; 50 mM, pH 7.4) buffer containing 5% (wt/vol) ethanol, 10% (wt/vol) glycerol, and 300 mM NaCl, and suspended in 5 volumes of the same buffer. This suspension was sonicated on ice until maximum cell breakage. Further purification steps were identical to those described previously for the purification of ht-protein from E. coli cells (19). Under these conditions, approximately 2 mg of purified enzyme was obtained per liter of IPTG-induced culture.
Previously described procedures (19) were used to obtain purified preparations of B-356 ht-FERBPH and B-356 ht-REDBPH from recombinant E. coli cells.
Protein characterization.
Sodium dodecyl sulfate (SDS)-polyacrylamide gels were developed according to method of Laemmli (24). Proteins were stained with Coomassie brillant blue (33). Protein concentrations were estimated by the method of Lowry (26), using bovine serum albumin as a standard. The concentrations of all ht-ISPBPH preparations were also determined spectrophotometrically, using the ɛ455 value of 8,300 M−1 cm−1 established for B-356 ht-ISPBPH (18). The preparations of B-356 ht-FERBPH and B-356 ht-REDRED used in this work were also quantified spectrophotometrically as previously described (19). The Mr of the native proteins was determined by high-pressure liquid chromatography (HPLC) as described previously (18).
Monitoring of enzyme activities and identification of metabolites.
Enzyme assays for BPH dox were performed as described previously (17). The reaction was initiated by adding 50 nmol of biphenyl or 25 nmol of one of the following chlorobiphenyls: 2,2′-, 3,3′-, or 2,5-dichlorobiphenyl or 2,2′,5,5′-tetrachlorobiphenyl (all from ULTRAScientific, Kingstown, R.I.) (added in 2 μl of acetone). Based on previous data showing that the origin of FERBPH did not influence the BPH dox substrate reactivity pattern (17), the reconstituted BPH doxes comprised either P6 ht-ISPBPH or one of the ht-ISPBPH chimeras described above (αP6βLB400, αLB400βP6, αP6βB-356, or αB-356βP6) plus B-356 ht-FERBPH and B-356 ht-REDBPH. The catalytic oxygenation was evaluated by monitoring substrate depletion by HPLC analysis 5 or 10 min after initiation of the reaction, as described previously (17). When 2,2′,5,5′-tetrachlorobiphenyl was the substrate, the catalytic oxygenation was evaluated by monitoring the metabolite production by HPLC using the conditions described previously (5).
A trans-complementation assay was also used to verify whether the P6 ISPBPH component was produced active in E. coli. E. coli DH11S cells carrying pYH31[LB400-bphFGBC] were transformed with pEP31[P6-bphA1/p-o/bphA2] or pQE31[P6-bphA1/bphA2]. The recombinant cells were inoculated on a nitrocellulose membrane placed onto the surface of an LB agar plate. The culture was incubated overnight at 37°C, and then the nitrocellulose membrane was transferred onto fresh LB agar plates containing 0.5 mM IPTG. The culture was incubated for 1 to 2 h and then sprayed with a solution of biphenyl in ether (5%, wt/vol). Positive colonies were recognized by the production of the yellow meta-cleavage metabolite produced from biphenyl. A positive control of E. coli DH11S cells carrying pYH31[LB400 bphFGBC] along with pQE31[B-356-bphAE] was included in the test.
RESULTS
Stability of pEP31 and pEP51 in E. coli and Pseudomonas.
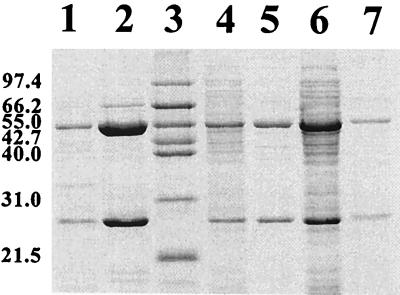
Plasmids pEP31 and pEP51 were maintained for over 50 generations in E. coli DH11S grown on tet 10 as well as in P. putida KT2440 grown on tet 20. As shown by data in Fig. 2, the cloned DNA fragment carrying P6 bphA1/p-o/bphA2 was stably maintained for over 50 generations both in E. coli and in Pseudomonas since both subunits of P6-ht-ISPBPH were produced in those recombinants. Same results were obtained with P6-bphA1/LB400-bphE or P6-bphA1/B-356-bphE.
FIG. 2.
SDS-PAGE of P6 ht-ISPBPH or P6 ISPBPH produced in E. coli or P. putida KT2440 carrying pEP31[P6-bphA1/p-o/bphA2] or pEP51[P6-bphA1/p-o/bphA2]. Lane 1, crude extract (1.9 μg of protein) of E. coli DH11S carrying pYH31[LB400-bphFGBC] plus pEP31[P6-bphA1/p-o/bphA2], where protein induction was achieved as described in Materials and Methods except that the inoculum was a culture grown for 50 generations in LB broth (tet 10); lane 2, purified preparation (1.1 μg of protein) of ht-αP6βP6 obtained from the extract in lane 1; lane 3, Mr marker; lane 4, crude extract (2.4 μg of protein) of P. putida KT2440 carrying pEP31[P6-bphA1/p-o/bphA2] where the inoculum was grown for 50 generations in LB broth (tet 20); lane 5, purified preparation (0.1 μg of protein) of ht-αP6βP6 obtained from the extract shown in lane 4; lane 6, crude extract (8 μg of protein) of P. putida KT2440 carrying pEP51[P6-bphA1/p-o/bphA2] grown for 50 generations as in lane 4; lane 7, crude extract (1 μg of protein) of E. coli DH11S carrying pYH31[LB400-bphFGBC] plus pEP51[P6-bphA1/p-o/bphA2] grown for 50 generations as in lane 1. Sizes are indicated on the left in kilodaltons.
Expression in E. coli and in P. putida KT2440 and purification of P6 ht-ISPBPH.
MacKay et al. (29) observed that P6 biphenyl dioxygenase’s ISPBPH component was not detected in induced E. coli DH5α carrying recombinant P6 bphA1 and bphA2 cloned downstream of the lac or T7 promoter. They suggested that the lack of P6 ISPBPH component in these cells was due to either inefficient translation of bphA1A2 or rapid degradation of the gene product (29).
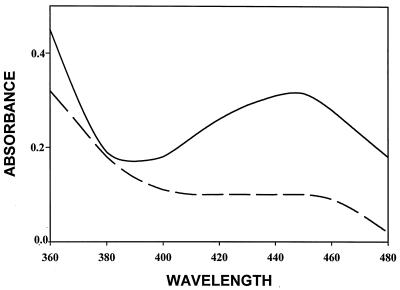
As shown in Fig. 2 (lanes 1 and 2), under our experimental conditions, the P6 ISPBPH α and β subunits were produced in equivalent amounts in E. coli. However, the purified protein was inactive. HPLC analysis showed that like other ISPBPHs the native conformation of P6 ISPBPH was predominantly α3β3 (not shown). However, the UV-visible spectrum was not typical of [2Fe-2S] Rieske-type proteins, showing a broad peak at about 455 nm and lacking the peak around 320 nm (Fig. 3). All attempts to produce active recombinant enzyme either by changing the purification conditions or by changing the culture conditions failed. These attempts included variation of the temperature and of the inoculum size at the time of induction, variation of the concentration of IPTG and the addition of ions such as Fe2+, variation of the type of buffer (phosphate, PIPES, or morpholineethanesulfonic acid) used to break the cells or to elute the enzyme from the Ni+-nitrilotriacetic acid column, and purification under anaerobic conditions.
FIG. 3.
Absorption spectra of ht-P6 ISPBPH (40 μM) obtained from recombinant P. putida KT2440 cells carrying pEP31[P6-bphA1/p-o/bphA2] (solid line) and obtained from recombinant E. coli DH11S cells carrying pEP31[P6-bphA1/p-o/bphA2] (dashed line).
The trans-complementation assay described in Materials and Methods was used to verify whether the enzyme was synthesized inactive in E. coli cells or if it was inactivated after cell breakage. IPTG-induced E. coli DH11S colonies carrying pYH31[LB400-bphFGBC] plus pQE31[B-356-bphAE] immediately turned yellow after spraying with biphenyl, showing the presence of active BPH dox, whereas cells of E. coli DH11S carrying pYH31[LB400-bphFGBC] plus pEP31[P6-bphA1/p-o/bphA2] did not react with biphenyl, showing the lack of active BPH dox (not shown). The fact that E. coli DH11S carrying pYH31[LB400-bphFGBC] plus pEP51[P6-bphA1/p-o/bphA2] did not produce any yellow color from biphenyl in this assay (not shown) but expressed proteins corresponding to bphA1 and bphA2 gene products (Fig. 2, lane 7) shows that the His tag is not the main reason for the inactivity of P6 ISPBPH in E. coli.
Equivalent amounts of α and β subunits were produced in E. coli carrying pEP31[P6-bphA1/bphA2] or pQE31[P6-bphA1/bphA2] (not shown). However, P6 bphA2 was poorly expressed from pEP31[P6-bphA1/bphA2] in P. putida KT2440. Nevertheless, Fig. 2 (lane 5) shows that purified P6 ht-ISPBPH was obtained and both genes were expressed in equivalent ratios (Fig. 2, lane 4) in P. putida KT2440 carrying pEP31[P6-bphA1p-o/bphA2], where each gene was controlled by a promoter-operator.
All purified P6 ht-ISPBPH preparations obtained from P. putida KT2440 carrying pEP31[P6-bphA1/p-o/bphA2] were active. These preparations showed typical spectra for [2Fe-2S] Rieske-type proteins with maximal absorption peaks at 320 and 455 nm and a shoulder at around 575 nm (Fig. 3). Therefore, data confirm that recombinant R. globerulus P6 ISPBPH is not active in E. coli cells (29) but is active when expressed in Pseudomonas. Data also show clearly that both subunits of P6 ISPBPH are expressed in E. coli but the reconstituted enzyme is inactive. Spectral data show that the Rieske cluster on the α subunit is incorrectly assembled (Fig. 3).
Expression in E. coli and purification of ht-αB-356βP6 and ht-αLB400βP6.
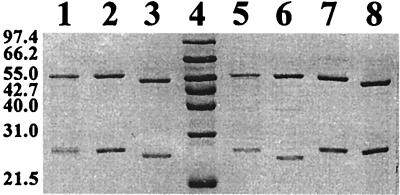
SDS-polyacrylamide gel electrophoresis (PAGE) of purified preparations of ht-αLB-400βP6 and ht-αB-356βP6 showed two single peptide bands of Mr corresponding to that of ht-αB-356 or ht-αLB400 with βP6 (Fig. 4, lanes 7 and 8; compared to lane 1 to 3). Both enzymes showed spectral features typical of Rieske-type proteins. When biphenyl was the substrate, ht-αLB-400βP6 was very poorly active (only traces of the dihydrodiol metabolites were produced from biphenyl), but as shown below, the enzyme was able to transform various chlorobiphenyls efficiently. In this case, however, two of the four purified preparations that we obtained were very poorly active toward all congeners tested, suggesting that the enzyme becomes easily inactivated during purification. On the other hand, ht-αB-356βP6 was stable and catalyzed the hydroxylation of biphenyl at a rate similar to that of ht-αP6βP6.
FIG. 4.
SDS-PAGE of purified active preparations of ISPBPH chimeras. Lane 1, P6 ht-ISPBPH, 50 pmol; lane 2, LB400 ISPBPH, 80 pmol; lane 3, B-356 ISPBPH, 50 pmol; lane 4, Mr marker; lane 5, ht-αP6βLB400, 70 pmol; lane 6, ht-αP6βB-356, 170 pmol; lane 7, ht-αLB400βP6, 60 pmol; lane 8, ht-αB-356βP6, 50 pmol. P6 ht-ISPBPH, ht-αP6βLB400, and ht-αP6βB-356 were produced from P. putida KT2440; the other chimeras were produced from E. coli M15. Sizes are indicated on the left in kilodaltons.
Expression in E. coli and in P. putida and purification of ht-αP6βLB400 and ht-αP6βB-356.
Purified preparations of ht-αP6βB-356 and ht-αP6βLB400 chimeras containing equivalent amounts of each subunit were obtained from E. coli carrying pQE31[P6-bphA1/B-356-bphE] and pQE31[P6-bphA1/LB400-bphE], respectively. However, as for ht-αP6βP6 (see above), the purified enzymes were not active and the spectral features of the purified enzyme preparations were not typical of Rieske-type proteins (not shown).
On the other hand, purified active ht-αP6βB-356 and ht-αP6βLB400 chimeras were obtained when the enzymes were expressed in P. putida KT2440 from pEP31[P6-bphA1/B-356-bphE] and pEP31[P6-bphA1/LB400-bphE], respectively. Both ISPBPH chimeras were active when biphenyl was used as the substrate (Table 2). Spectral features were typical of [2Fe-2S] Rieske-type proteins. SDS-PAGE of purified preparations showed two peptide bands of Mr corresponding to that of ht-αP6 with either βLB400 or βB-356 (Fig. 4, lanes 5 and 6). It is noteworthy that LB400 and B-356 α and β subunits migrate differently although their theoretical Mrs are identical (lanes 2 and 3).
TABLE 2.
Amount of substrate depleted 5 min after initiation of reaction with various enzymes
| Substrate | nmol of substrate depleted or metabolite produced/1.2 nmol of enzymea
|
||||
|---|---|---|---|---|---|
| ht-αP6βP6 ISPBPH | ht-αP6βLB400 ISPBPH | ht-αP6βB-356 ISPBPH | ht-αLB-400βP6 ISPBPH | ht-αB-356βP6 ISPBPH | |
| 2,2′-Dichlorobiphenyl | 8.3 | 0 | 9 | 22.5 | 10.8 |
| 3,3′-Dichlorobiphenyl | 14.5 | 0 | 2.5 | 0 | 6.3 |
| 2,5-Dichlorobiphenyl | 23.3 | 6.8 | 21 | 15.3 | 21.8 |
| 2,2′,5,5′-Tetrachlorobiphenyl | 0 | 1 | 1 | 9 | 0 |
| Biphenyl | 34 | 17 | 5 | Traceb | 43 |
BPH dox reactions were carried out as described in Materials and Methods. The reaction was initiated by adding 25 nmol of substrate except for biphenyl (50 nmol). Values refer to amounts of substrate consumed 5 min after initiation of the reaction when 1.2 nmol of enzyme was present except in the case of 2,2′,5,5′-tetrachlorobiphenyl, values for which refer to nanomoles of 3,4-dihydro-3,4-dihydroxy-2,2′,5,5′-tetrachlorobiphenyl produced per 1.2 nmol of enzyme 10 min after initiation of the reaction.
Trace of 2,3-dihydro-2,3-dihydroxybiphenyl detected by HPLC analysis in the reaction medium.
Activities of purified ht-P6 ISPBPH and chimeras derived from it toward selected chlorobiphenyls.
In a previous investigation (17), purified ht-LB400 BPH dox metabolized 2,2′-dichlorobiphenyl efficiently but 3,3′-dichlorobiphenyl poorly, whereas ht-B-356 BPH dox metabolized 3,3′-dichlorobiphenyl but not 2,2′-dichlorobiphenyl efficiently. Furthermore, unlike ht-LB400 BPH dox, ht-B-356 BPH dox was unable to catalyze the meta-para hydroxylation of 2,2′,5,5′-tetrachlorobiphenyl. In another investigation (29), resting cell suspensions of recombinant P. putida KT2442 carrying P6 bphA1A2A3A4 did not metabolize 2,2′-dichlorobiphenyl or 2,2′,5,5′-tetrachlorobiphenyl.
In this work the catalytic activity of purified ht-P6 ISPBPH and of hybrids obtained by exchanging the α and the β subunits of strain P6, LB400, or B-356 ISPBPH toward chlorobiphenyls was determined (Table 2). Data confirm that P6 ISPBPH is unable to hydroxylate 2,2′,5,5′-tetrachlorobiphenyl. However, unlike the resting cell suspension of P. putida KT2442 carrying P6 bphA1A2A3A4 (29), purified P6 ht-ISPBPH metabolized 2,2′-dichlorobiphenyl. 3,3′-Dichlorobiphenyl was metabolized about twice as fast as 2,2′-dichlorobiphenyl by this enzyme (Table 2). This is a feature that distinguishes P6 BPH dox from both LB400 and B-356 BPH doxes, where the rates of metabolism for these two congeners differed markedly (17).
All the preparations of ht-αLB400βP6 were either inactive or very poorly active when biphenyl was the substrate. However, these preparations could transform 2,2′-dichlorobiphenyl and 2,2′,5,5′-tetrachlorobiphenyl. Table 2 reports the value obtained with the most active preparation. Data show that like LB400 BPH dox, ht-αLB400βP6 shows a marked preference for 2,2′-dichlorobiphenyl and catalyzes the hydroxylation of 2,2′,5,5′-tetrachlorobiphenyl. However, unlike LB400 BPH dox (13), ht-αLB400βP6 was unable to oxygenate naphthalene, as demonstrated by HPLC analysis of the reaction product (data not shown).
On the other hand, unlike both parent enzymes, ht-αP6βLB400 is unable to catalyze the hydroxylation of 2,2′- or 3,3′-dichlorobiphenyl. However, this chimera showed a weak activity on 2,2′,5,5′-tetrachlorobiphenyl. The metabolic capacity of this chimera contrasts with that of ht-αB-356βLB400 (17), which shows features very similar to those of LB400 ISPBPH.
Unlike B-356 BPH dox (17), αB-3566βP6 metabolizes 2,2′-dichlorobiphenyl rapidly. It is also noteworthy that although the rate of transformation is very low, unlike both parents, αP6βB-356 produces small amounts of 3,4-dihydro-3,4-dihydroxy-2,2′,5,5′-tetrachlorobiphenyl from 2,2′,5,5′-tetrachlorobiphenyl. All four chimeras analyzed in this investigation were able to metabolize 2,5-dichlorobiphenyl, in contrast to the data obtained with αLB400βB-356 (17). Altogether, these data indicate that the catalytic capacity of hybrid enzymes comprised of an α and a β subunit recruited from distinct BPH dox is not determined by either one of the two subunits. The catalytic capacity is rather unpredictable and depends on the association between the two subunits.
DISCUSSION
In a previous work (18), we had reported that purified monomeric B-356 ht-ISPBPH α subunit produced alone in recombinant E. coli cells was able to assemble an intact [2Fe-2S] Rieske cluster, showing that the β subunit is not involved in folding the Rieske center. However, the purified B-356 ht-ISPBPH α subunit could not associate in vitro with the purified β subunit to generate an active α3β3 protein. Conversely, the α subunit combined in cell extract with purified exogenous β subunit to generate an active complex, suggesting the presence in E. coli of a cell constituent that interacts with the α subunit to maintain its correct folding in the absence of the β subunit (18). In the present work, we show that rhodococcal ISPBPH subunits produced in E. coli cells are assembled into the correct α3β3 configuration (this was also true for the αP6βLB400 and αP6βB-356 chimeras). However, the [2Fe-2S] Rieske cluster of the αP6 subunit is incorrectly assembled in E. coli but correctly assembled in Pseudomonas. Altogether these observations show that the subunit association is independent of the Rieske center assembly and suggest that the maturation of the ISPBPH [2Fe-2S] cluster, like subunit association, may require the involvement of cell constituents such as chaperones.
ht-P6 ISPBPH is produced active in Pseudomonas cells, showing the importance of choosing the proper organism to express heterologous proteins. Since aryl hydroxylating dioxygenases are found mostly in gram-negative aerobes such as Pseudomonas, Alcaligenes, and Comamonas and in the gram-positive rhodococci, pEP31 and pEP51 constructed during this work provide potentially useful tools to further investigate the biochemical features of these enzymes.
In a recent investigation with B-356 and LB400 ISPBPH, the catalytic features of αB-356βLB400 toward chlorobiphenyls were found to be very similar to those of LB400 BPH dox. On the other hand, with one exception (16), all recent reports (7, 31, 32, 36) indicated that the substrate specificity of the aryl hydroxylating dioxygenase is determined by the terminal oxygenase’s α subunit; the β subunit was not found to contribute to this function. The fact that these investigations were done with whole-cell suspensions of recombinant E. coli clones carrying the four genes required for dioxygenase activity on plasmids preclude direct comparison with data obtained with in vitro-reconstituted purified enzyme. Nevertheless, it was imperative to verify if the behavior of the chimera obtained by exchanging the α and β subunits of LB400 and B-356 ISPBPH was an exception.
Unlike the data obtained with αB-356βLB400 (17), none of the four hybrids described in this work showed features identical to those that characterize the parent which provided the β subunit. Interestingly, the catalytic activity toward chlorobiphenyls of αLB400βP6 was similar to that of LB400 BPH dox. Based on this result, it would be tempting to conclude that the substrate specificity pattern is determined by the α subunit alone and that αB-356βLB400 is an exception. However, it is noteworthy that αLB400βP6 was practically inactive on biphenyl. Furthermore, the catalytic features of the three other chimeras studied in this investigation differed significantly from those of the parent which provided the α subunit. Thus, although 2,2′-dichlorobiphenyl is a very poor substrate for B-356 ISPBPH, αB-356βP6 oxygenate this congener faster than 3,3′-dichlorobiphenyl. Furthermore, unlike both parents, αP6βLB400 was unable to oxygenate 2,2′-dichlorobiphenyl but showed a slight activity toward 2,2′,5,5′-tetrachlorobiphenyl, which was not attacked by P6 ISPBPH. Together these data support the previous conclusion that each new chimera obtained by exchanging the α or β subunit of parent dioxygenases acquires its own new catalytic features that are not determined exclusively by one or the other subunit (16, 17).
Two structural features are essential to obtain an active enzyme. First, the Rieske cluster must be correctly assembled. Second, we have previously reported that ISPBPH must associate into α3β3 heterodimer to be active (18). Based on the present data, it is likely that the three-dimensional structure of the enzyme’s catalytic region, as determined by the association between the α and β subunits, will determine the range of substrates that the enzyme can oxygenate. Selected amino acid residues of the α or β subunits are likely, because of their position or charge, to interfere with the enzyme-substrate interaction. However, depending on the final three-dimensional structure of the catalytic region, the amino acid residues that affect the substrate selectivity in one type of α-β arrangement will not inevitably affect the substrate selectivity in other types of α-β arrangements. This conceptual model explains why the amino acid residues of the oxygenase α subunit that were found to affect the substrate specificity of strain LB400 and KF707 dioxygenases (22, 30) had no effect on the enzyme reactivity pattern of αB-356βB-356 or in αB-356βLB400 (17). However, structure analysis of the biphenyl dioxygenase oxygenase component will be required to assess this hypothesis. Recently, Kauppi et al. (21) reported the three-dimensional structure of the terminal oxygenase component of the homologous naphthalene dioxygenase. Structure analysis shows a major involvement of the α subunit in enzyme catalytic activity. However, so far, structure analysis has not helped to identify the role of the β subunit in enzyme catalytic activity or specificity.
Nevertheless, if structural features of the β subunit influence the enzyme’s specificity, this fact will be of consequence for enzyme-engineering programs designed to create new enhanced enzymes for the degradation of more persistent chlorobiphenyls. Crameri et al. (9) have recently shown that the use of homologous genes to provide functional diversity accelerates the in vitro-directed evolution process based on DNA shuffling. Kumamaru et al. (23) have shuffled strain LB-400 bphA with P. pseudoalcaligenes KF707 bphA1 and successfully obtained mutants expressing phenotypes of both parents. However, our data suggest that the development by molecular evolution of mutants able to catalyze the oxygenation of congeners that both parents are unable to oxygenate may also have to take into consideration other portions of the ISPBPH molecule, including perhaps the β subunit.
ACKNOWLEDGMENTS
This work was supported by grant STP0193182 from the Natural Sciences and Engineering Research Council of Canada. H.C. was a recipient of Bourse d’Excellence for postdoctoral fellows provided by AUPELF-UREF.
REFERENCES
- 1.Ahmad D, Massé R, Sylvestre M. Cloning and expression of genes involved in 4-chlorobiphenyl transformation by Pseudomonas testosteroni strain B-356: homology to polychlorobiphenyl-degrading genes in other bacteria. Gene. 1990;86:53–61. doi: 10.1016/0378-1119(90)90113-6. [DOI] [PubMed] [Google Scholar]
- 2.Asturias J A, Diaz E, Timmis K N. The evolutionary relationship of biphenyl dioxygenase from gram-positive Rhodococcus globerulus P6 to multicomponent dioxygenases from gram-negative bacteria. Gene. 1995;156:11–18. doi: 10.1016/0378-1119(94)00530-6. [DOI] [PubMed] [Google Scholar]
- 3.Asturias J A, Moore E, Yakimov M M, Klatte S, Timmis K N. Reclassification of the polychlorinated biphenyl-degraders Acinetobacter sp. strain P6 and Corynebacterium sp. strain MB1 as Rhodococcus globerulus. Syst Appl Microbiol. 1994;17:226–231. [Google Scholar]
- 4.Bagdasarian M, Lurz R, Ruckert B, Franklin F C, Bagdasarian M M, Frey J, Timmis K N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981;16:237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- 5.Barriault D, Durand J, Maaroufi H, Eltis L D, Sylvestre M. Degradation of polychlorinated biphenyl metabolites by naphthalene-catabolizing enzymes. Appl Environ Microbiol. 1998;64:4637–4642. doi: 10.1128/aem.64.12.4637-4642.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bedard D L, Unterman R, Bopp L H, Brennan M J, Haberl M L, Johnson C. Rapid assay for screening and characterizing microorganisms for the ability to degrade polychlorinated biphenyls. Appl Environ Microbiol. 1986;51:761–768. doi: 10.1128/aem.51.4.761-768.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beil S, Mason J R, Timmis K N, Pieper D H. Identification of chlorobenzene dioxygenase sequence elements involved in dechlorination of 1,2,4,5-tetrachlorobenzene. J Bacteriol. 1998;180:5520–5528. doi: 10.1128/jb.180.21.5520-5528.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broadus R M, Haddock J D. Purification and characterization of the NADH:ferredoxinBPH oxidoreductase component of biphenyl 2,3-dioxygenase from Pseudomonas sp. strain LB400. Arch Microbiol. 1998;170:106–112. doi: 10.1007/s002030050621. [DOI] [PubMed] [Google Scholar]
- 9.Crameri A, Bermudez E, Raillard S, Stemmer W P C. DNA shuffling of a family of genes from diverse species accelerates directed evolution. Nature. 1998;391:288–291. doi: 10.1038/34663. [DOI] [PubMed] [Google Scholar]
- 10.de Kievit T R, Dasgupta T, Schweizer H, Lam J S. Molecular cloning and characterization of the rfc gene of Pseudomonas aeruginosa (serotype O5) Mol Microbiol. 1995;16:565–574. doi: 10.1111/j.1365-2958.1995.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 11.Erickson B D, Mondello F J. Nucleotide sequencing and transcriptional mapping of the genes encoding biphenyl dioxygenase, a multicomponent polychlorinated-biphenyl-degrading enzyme in Pseudomonas strain LB400. J Bacteriol. 1992;174:2903–2912. doi: 10.1128/jb.174.9.2903-2912.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furukawa K, Tonomura K, Kamibayashi A. Effect of chlorine substitution on the biodegradability of polychlorinated biphenyls. Appl Environ Microbiol. 1978;35:223–227. doi: 10.1128/aem.35.2.223-227.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haddock J D, Gibson D T. Purification and characterization of the oxygenase component of biphenyl 2,3-dioxygenase from Pseudomonas sp. strain LB400. J Bacteriol. 1995;177:5834–5839. doi: 10.1128/jb.177.20.5834-5839.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haddock J D, Pelletier D A, Gibson D T. Purification and properties of ferredoxin(BPH), a component of biphenyl 2,3-dioxygenase of Pseudomonas sp. strain LB400. J Ind Microbiol Biotechnol. 1997;19:355–359. doi: 10.1038/sj.jim.2900429. [DOI] [PubMed] [Google Scholar]
- 15.Haddock J D, Horton J R, Gibson D T. Dihydroxylation and dechlorination of chlorinated biphenyls by purified biphenyl 2,3-dioxygenase from Pseudomonas sp. strain LB400. J Bacteriol. 1995;177:20–26. doi: 10.1128/jb.177.1.20-26.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirose J, Suyama A, Hayashida S, Furukawa K. Construction of hybrid biphenyl (bph) and toluene (tod) genes for functional analysis of aromatic ring dioxygenases. Gene. 1994;138:27–33. doi: 10.1016/0378-1119(94)90779-x. [DOI] [PubMed] [Google Scholar]
- 17.Hurtubise Y, Barriault D, Sylvestre M. Involvement of the terminal oxygenase β subunit in the biphenyl dioxygenase reactivity pattern toward chlorobiphenyls. J Bacteriol. 1998;180:5828–5835. doi: 10.1128/jb.180.22.5828-5835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurtubise Y, Barriault D, Sylvestre M. Characterization of active recombinant His-tagged oxygenase component of Comamonas testosteroni B-356 biphenyl dioxygenase. J Biol Chem. 1996;271:8152–8156. doi: 10.1074/jbc.271.14.8152. [DOI] [PubMed] [Google Scholar]
- 19.Hurtubise Y, Barriault D, Powlowski J, Sylvestre M. Purification and characterization of the Comamonas testosteroni B-356 biphenyl dioxygenase components. J Bacteriol. 1995;177:6610–6618. doi: 10.1128/jb.177.22.6610-6618.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurtubise Y, Sylvestre M. pYH31, a ColE1 compatible His-tagged fusion expression vector. Biotechnol Tech. 1999;13:303–307. [Google Scholar]
- 21.Kauppi B, Lee K, Carredano E, Parales R E, Gibson D T, Eklund H, Ramaswamy S. Structure of an aromatic-ring-hydroxylating dioxygenase-naphthalene 1,2-dioxygenase. Structure. 1998;6:571–586. doi: 10.1016/s0969-2126(98)00059-8. [DOI] [PubMed] [Google Scholar]
- 22.Kimura N, Nishi A, Goto M, Furukawa K. Functional analysis of a variety of chimeric dioxygenases constructed from two biphenyl dioxygenases that are similar structurally but different functionally. J Bacteriol. 1997;179:3936–3943. doi: 10.1128/jb.179.12.3936-3943.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumamaru T, Suenaga H, Mitsuoka M, Watanabe T, Furukawa K. Enhanced degradation of polychlorinated biphenyls by directed evolution of biphenyl dioxygenase. Nat Biotechnol. 1998;16:663–666. doi: 10.1038/nbt0798-663. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli U K. Cleavage of structural proteins during the assembly of head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Lin J J, Smith M, Jessee J, Bloom F. DH11S: an Escherichia coli strain for reparation of single-stranded DNA from phagemid vectors. BioTechniques. 1992;12:718–721. [PubMed] [Google Scholar]
- 26.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 27.Masai E, Yamada A, Healy J M, Hatta T, Kimbara K, Fukuda M, Yano K. Characterization of biphenyl catabolic genes of gram-positive polychlorinated biphenyl degrader Rhodococcus sp. strain RHA1. Appl Environ Microbiol. 1995;61:2079–2085. doi: 10.1128/aem.61.6.2079-2085.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mason J R, Cammack R. The electron-transport proteins of hydroxylating bacterial dioxygenases. Annu Rev Microbiol. 1992;46:277–305. doi: 10.1146/annurev.mi.46.100192.001425. [DOI] [PubMed] [Google Scholar]
- 29.McKay D B, Seeger M, Zielinski M, Hofer B, Timmis K N. Heterologous expression of biphenyl dioxygenase-encoding genes from a gram-positive broad-spectrum polychlorinated biphenyl degrader and characterization of chlorobiphenyl oxidation by the gene products. J Bacteriol. 1997;179:1924–1930. doi: 10.1128/jb.179.6.1924-1930.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mondello F J, Turcich M P, Lobos J H, Erickson B D. Identification and modification of biphenyl dioxygenases sequences that determine the specificity of polychorinated biphenyl degradation. J Bacteriol. 1997;163:3096–3103. doi: 10.1128/aem.63.8.3096-3103.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parales J V, Parales R E, Resnick S M, Gibson D T. Enzyme specificity of 2-nitrotoluene 2,3-dioxygenase from Pseudomonas sp. strain JS42 is determined by the C-terminal region of the alpha subunit of the oxygenase component. J Bacteriol. 1998;180:1194–1199. doi: 10.1128/jb.180.5.1194-1199.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parales R E, Emig M D, Lynch N A, Gibson D T. Substrate specificities of hybrid naphthalene and 2,4-dinitrotoluene dioxygenase enzyme systems. J Bacteriol. 1998;180:2337–2344. doi: 10.1128/jb.180.9.2337-2344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 34.Schweizer H P. Escherichia-Pseudomonas shuttle vectors derived from pUC18/19. Gene. 1991;97:109–112. doi: 10.1016/0378-1119(91)90016-5. [DOI] [PubMed] [Google Scholar]
- 35.Sylvestre M, Sirois M, Hurtubise Y, Bergeron J, Ahmad D, Shareck F, Larose A, Barriault D, Guillemette I, Juteau J M. Sequencing of Comamonas testosteroni strain B-356-biphenyl/chlorobiphenyl dioxygenase genes: evolutionary relationships among Gram-negative biphenyl dioxygenases. Gene. 1996;174:195–202. doi: 10.1016/0378-1119(96)00039-x. [DOI] [PubMed] [Google Scholar]
- 36.Tan H M, Cheong C M. Substitution of the ISP alpha subunit of biphenyl dioxygenase from Pseudomonas results in a modification of the enzyme activity. Biochem Biophys Res Commun. 1994;204:912–917. doi: 10.1006/bbrc.1994.2546. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Garnon J, Labbé D, Bergeron H, Lau C K. Sequence and expression of the bpdC1C2BADE genes involved in the initial steps of biphenyl/chlorobiphenyl degradation by Rhodococcus sp. M5. Gene. 1995;164:117–122. doi: 10.1016/0378-1119(95)00448-f. [DOI] [PubMed] [Google Scholar]