Summary
Microglia play a key role in shaping the formation and refinement of the excitatory network of the brain. However, less is known about whether and how they organize the development of distinct inhibitory networks. We find that microglia are essential for the proper development of somatostatin-positive (SST+) cell synapses during the second postnatal week. We further identify a pair of molecules that act antagonistically to one another in the organization of SST+ cell axonal elaboration. Whereas CX3CL1 acts to suppress axonal growth and complexity, CXCL12 promotes it. Assessing the functional importance of microglia in the development of cortical activity, we find that a whisker stimulation paradigm that drives SST+ cell activation leads to reduced cortical spiking in brains depleted of microglia. Collectively, our data demonstrate an important role of microglia in regulating the development of SST+ cell output early in life.
Keywords: microglia, interneurons, inhibitory synapses, cerebral cortex, development, somatostatin
Graphical abstract
Highlights
-
•
Microglia depletion leads to an increase of SST+ cell synapses during development
-
•
Microglia control SST+ cell axonal development through CX3CL1 and CXCL12
-
•
Microglia depletion reduces sensory-driven cortical activation early postnatally
Gesuita et al. demonstrate that microglia contribute to cortical somatostatin-positive (SST+) inhibitory cell development. Early postnatal microglia depletion increases SST+ synapse density and reduces sensory-driven activation of the barrel cortex. Moreover, the chemokines CX3CL1 and CXCL12 control SST+ cell axonal development through microglia-dependent mechanisms.
Introduction
GABAergic inhibitory interneurons (INs) represent 20% of all cortical neurons (Sahara et al., 2012) and regulate excitation within and across cortical areas (Tremblay et al., 2016). During the first postnatal weeks of development, INs experience critical refinement steps, including the remodeling of their axo-dendritic neurites and synaptic connections (Kastli et al., 2020; Marques-Smith et al., 2016; Miyamae et al., 2017; Tuncdemir et al., 2016). Although some factors that regulate IN postnatal development have been revealed, key mechanisms that contribute to their terminal specification are yet to be discovered (Lim et al., 2018a). In the past few years, a growing number of studies have identified microglia as important players in postnatal synaptic remodeling of excitatory connections (Andoh and Koyama, 2021; Miyamoto et al., 2013; Purves and Lichtman, 1980; Schafer and Stevens, 2015). Whether a similar mechanism is in place for inhibitory cells is still largely unknown.
Microglia cells are the resident immune cells of the central nervous system (Ginhoux et al., 2013; Napoli and Neumann, 2009) and participate in both synapse formation (Lim et al., 2013; Miyamoto et al., 2016) and elimination (Hoshiko et al., 2012; Paolicelli et al., 2011; Schafer et al., 2012). Recent work has identified some of the molecular pathways involved in the interaction between microglia and excitatory neurons (Cong et al., 2020; Filipello et al., 2018; Gunner et al., 2019; Lehrman et al., 2018; Li et al., 2020; Paolicelli et al., 2011; Schafer et al., 2012; Stevens et al., 2007). Conversely, only a handful of studies have proposed a role for microglia in shaping inhibitory cell synapses, focusing mainly on parvalbumin-positive (PV+) INs (Favuzzi et al., 2021; Gallo et al., 2022; Squarzoni et al., 2014; Thion et al., 2019). Somatostatin-positive (SST+) INs are the second most numerous class of inhibitory cells; they are the first ones to integrate into cortical circuits (Miyoshi et al., 2007; Oh et al., 2016; Tuncdemir et al., 2016) and are important for feedback inhibition and plasticity (Adler et al., 2019; Kawaguchi and Kubota, 1997; Silberberg and Markram, 2007). Nevertheless, it is currently unknown when and how SST+ axonal projections and synapses are set up during cortical development.
In this study, we focus on the role of microglia in the development of cortical SST+ cells, extending a previous report showing that SST+ synapse number is increased in microglia-depleted animals (Favuzzi et al., 2021). We find that microglia play a central role in SST+ cell development during the second postnatal week by regulating their output at the axonal and synaptic levels, and hence their functional output in the circuit. We further identify a pair of chemokines expressed by SST+ cells, CX3CL1 and CXCL12, whose opposing actions control the development of SST+ cell axons, rather than synapses, in a microglia-dependent manner. Finally, we find that microglia are essential to regulate early postnatal sensory-driven activation of the barrel cortex in vivo.
Results
Microglia regulate the density of SST+ synapses during the second postnatal week
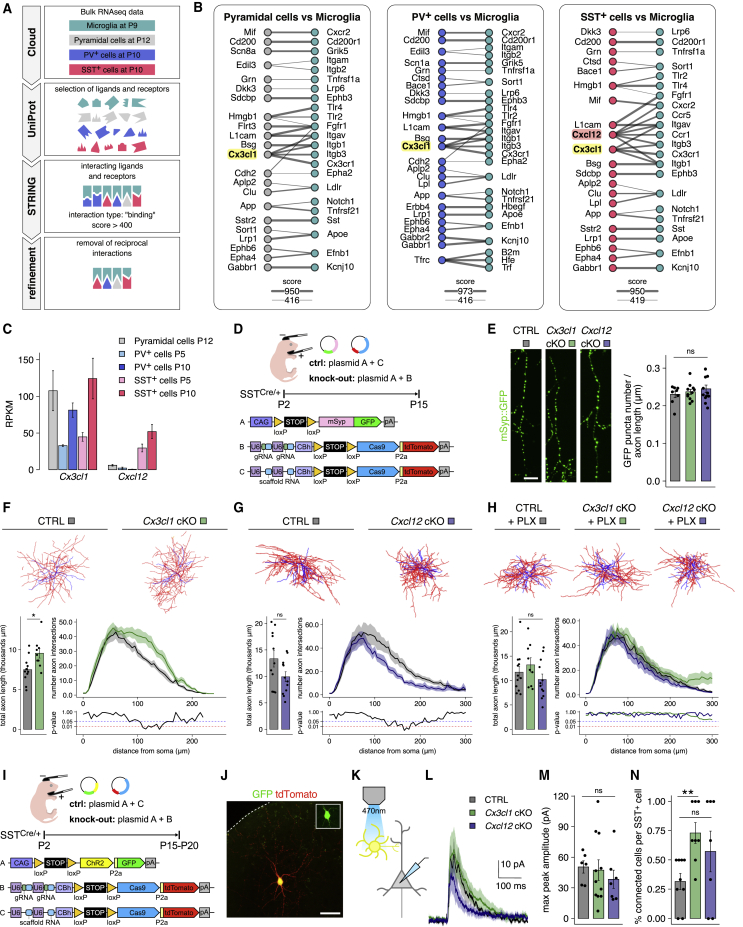
To determine whether microglia interact with cortical SST+ INs, we assessed the proximity of the processes of the two cell types during the first two postnatal weeks of development in the somatosensory cortex (S1) of mice. We focused our analysis on the superficial layers (1–3), where the axons from the majority of the diverse population of cortical SST+ cells ramify (Gesuita and Karayannis, 2021). Our analysis showed that microglia SST+ process appositions peak by postnatal day 8 (P8) (Figure 1A). We then tested if these contact points also involve SST+ synapses and found that microglia contact almost 10% of layer (L) 1 SST+ presynaptic boutons (labeled with GAD65) at P12 and P15 (Figure 1B).
Figure 1.
Microglia regulate the density of SST+ synapses during the second postnatal week
(A) Microglia (Iba1+) staining in S1 L2/3 of a P11 brain section from an SSTCre/+;Ai14Tg/+ mouse (scale bar: 100 μm). In the enlargements of the dashed boxes on the right, an example of microglia process-SST+ process apposition (full arrowhead) and of microglia process-SST+ soma apposition (empty arrowhead) are shown. Putative process-process appositions are quantified as total volume of colocalized signal divided by total SST+ process volume per field of view (each dot is a brain; P4 n = 3, P8 n = 6, P11 n = 3; t test: P4 versus P8 p = 0.0143, P8 versus P11 p = 0.8447, P4 versus P11 p = 0.0044; one-way ANOVA test p = 0.049).
(B) Images and 3D reconstruction of microglia processes contacting SST+ presynaptic boutons (GAD65+ puncta encased in GFP+ axons labeled using a SSTCre/+;RCE mouse) in S1 L1 (scale bar: 1 μm). Percentage of SST+ boutons contacted by microglia (each dot is a brain; P10 n = 6, P12 n = 3, P15 n = 6; t test: P10 versus P12 p = 0.071, P12 versus P15 p = 0.701, P10 versus P15 p = 0.011; one-way ANOVA test p = 0.015).
(C) Microglia staining in S1 of a control and a microglia-depleted brain section at P10 (scale bar: 100 μm; pia surface is indicated by a dotted line).
(D) Images and quantitation of SST+ synapses (GAD65+ and Gephyrin+ puncta encased in GFP+ axons labeled using a SSTCre/+;RCE mouse) in L1 of S1 at P15 (scale bar: 1 μm); arrowheads indicate colocalization (each dot is a brain; P10 control [CTRL] n = 4, PLX n = 7, t test p = 0.1; P12 CTRL n = 3, PLX n = 5, t test p = 0.63; P15 CTRL n = 6, PLX n = 4, t test p = 0.038).
(E) A schematic of subpial electroporation and microglia depletion protocol. The vertical black lines denote the manipulation intitiation (P2) and analysis time points (P10 and P15). Below is the map of the plasmid.
(F) Example of parts of traced axons (scale bar: 12.5 μm). Puncta density is calculated as total number of counted puncta divided by total length of traced axons per cell (each dot is a cell; P10 CTRL n = 9, PLX n = 9, t test p = 0.9; P15 CTRL n = 17, PLX n = 14, t test p = 0.0015; P10 CTRL versus P15 CTRL t test p = 0.00061; P10 PLX versus P15 PLX t test p = 0.64).
(G) Examples of traced cells (axons in red, dendrites in blue).
(H) Total length of traced axons (each dot is a cell; CTRL n = 11, PLX n = 12 cells, t test p = 0.42) and Sholl analysis (t test p values are plotted below; two-way ANOVA repeated measures (RM) test from 8 to 300 μm p = 0.96; CTRL group from Figure 2G, PLX group from Figure 2H).
(I) A schematic representation of the optogenetics experiment. IPSC traces and peak amplitude quantification (each dot is a cell; CTRL n = 15, PLX n = 15, t test p = 0.025).
All data are represented as mean ± SEM, and the exact values are reported in Table S1.
See also Figure S1.
To study whether microglia contribute to SST+ cell synaptic or axonal development, we depleted microglia at early postnatal stages. We used the compound PLX5622, a strong inhibitor of CSF1-receptor tyrosine kinase activity, which efficiently eradicates myeloid cells, including microglia (Dagher et al., 2015). Similar depletion protocols have been efficiently used to assess the critical role of microglia in synaptic maturation and circuit formation (Favuzzi et al., 2021; Paolicelli et al., 2011). Daily administration of PLX5622 between P2 and P14 was efficient in depleting microglia from early postnatal ages (from P4–P5) (Favuzzi et al., 2021) (Figure 1C). Analysis of SST+ cell output synapses in microglia-depleted cortices showed an increased number at P15—but not at P10—compared with control mice (Figure 1D). To assess if microglia also contribute to the maintenance of SST+ output synapses, we shifted their depletion window between P15 and P30. We found that late depletion of microglia does not have a strong effect on SST+ synapse number at P30, suggesting the presence of a critical developmental window between P10 and P15 (Figure S1A).
The effect on SST+ cell synapses we observed during the critical window could not be explained by an alteration in the total number of SST+ cells (Figure S1B) nor by any change in their developmental apoptosis (Southwell et al., 2012) (Figure S1C). Moreover, although a role for microglia in brain vasculature formation has been described (Zhao et al., 2018), we found that blood vessels were largely unaffected, in accordance with data by Paolicelli et al. when studying Cx3cr1 knockout animals (Paolicelli et al., 2011) (Figure S1D).
To assess whether microglia depletion only affects SST+ cell synapses or also impacts axonal development, we sparsely labeled SST+ cells and their output synapses through postnatal sub-pial superficial electroporation (de La Rossa and Jabaudon, 2015; Lim et al., 2018b). This strategy allows for mosaic labeling of a handful of superficial cortical post-mitotic SST+ cells. Specifically, we electroporated SSTCre/+ P1–P2 pups with a Cre-dependent plasmid carrying a tdTomato and the presynaptic protein synaptophysin fused with an enhanced green fluorescent protein (GFP) (Figure 1E). This approach labeled individual SST+ cells in red and their presynaptic boutons in green (Figure S1E). In order to accurately measure the density of the green synaptic puncta, we developed an analysis pipeline that traces the axons and counts the number of presynaptic boutons (Figures S1F–S1H) (further details are reported in the STAR Methods). In line with our previous results, we found that postnatal microglia depletion causes a significant increase in the density of SST+ cell boutons at P15 but not at P10 (Figure 1F). Moreover, our sparse labeling approach allowed for the reconstruction of cell morphology and Sholl analysis of the neurites (Figures 1G and 1H). No change in the expansion or complexity of the axons (Figure 1H) or dendrites (Figure S1I) was detected.
Finally, to functionally assess the impact of the increased synapse density, we used optogenetics coupled with in vitro whole-cell electrophysiology. To activate SST+ cells, we crossed the SST-Cre to the Ai32 mouse line, which expresses Channelrhodopsin (ChR2)-EYFP fusion protein in a Cre-dependent manner. L5 SST+ INs have been shown to exert strong inhibition onto nearby pyramidal cells via their elaborate synaptic contacts in the superficial layers (Silberberg and Markram, 2007). In accordance with these previous findings, we observed light-evoked ChR2-dependent inhibitory postsynaptic currents (IPSCs) onto L5 pyramidal cells of control animals, which were significantly higher in amplitude in microglia-depleted mice (Figure 1I). These findings show that the extra SST+ cell synapses are functional and result in enhanced inhibitory output onto pyramidal cells.
CX3CL1 and CXCL12 are key molecular players for microglia-dependent SST+ cell output development
Previous studies have demonstrated that microglia prune excitatory and inhibitory synapses through neuron-derived signaling molecules (Cong et al., 2020; Favuzzi et al., 2021; Filipello et al., 2018; Gunner et al., 2019; Lehrman et al., 2018; Li et al., 2020; Paolicelli et al., 2011; Schafer et al., 2012; Stevens et al., 2007). To investigate the potential interactome between microglia and SST+ cells, we developed a bioinformatics pipeline to identify all putative ligand-receptor pairs. To this end, we took advantage of recently published RNA sequencing data collected from developing microglia (Matcovitch-Natan et al., 2016) and the two most abundant populations of cortical INs, SST+ and PV+ cells, as well as from pyramidal neurons during the second postnatal week (Favuzzi et al., 2019). We first used the online database Uniprot (The UniProt Consortium, 2017) to identify all the membrane and secreted factors expressed by each population of cells. Subsequently, we used the protein-protein interaction database STRING (Szklarczyk et al., 2019) and selected possible ligand-receptor pairs. Finally, we excluded those interactions where the ligand and the receptor were expressed by both cell types; this last step is based on the hypothesis that the communication between microglia and neurons would be unidirectional (Figure 2A).
Figure 2.
CX3CL1 and CXCL12 are key molecular players of microglia-dependent development of SST+ cells
(A) Schematics of the in silico screening.
(B) Lists of putative interactors. Line thickness is proportional to STRING confidence score (a score of 1,000 indicates the highest confidence of interaction).
(C) Cx3cl1 and Cxcl12 expression levels (RNA sequencing data from Favuzzi et al., 2019).
(D) Schematics of the subpial electroporation protocol and timeline. Below are the maps of the plasmids.
(E) Examples of parts of traced axons (scale bar: 12.5 μm). Puncta density is calculated as total number of counted puncta divided by total length of traced axons per cell (each dot is a cell; CTRL n = 8, Cx3cl1 cKO n = 11, Cxcl12 cKO n = 12; t test: CTRL versus Cx3cl1 cKO p = 0.55, CTRL versus Cxcl12 cKO p = 0.29; one-way ANOVA test p = 0.53).
(F) Examples of traced cells (axons in red, dendrites in blue). Below is the total length of traced axons (each dot is a cell; CTRL n = 11, Cx3cl1 cKO n = 9, t test p = 0.019) and Sholl analysis (t test p values are plotted below; two-way ANOVA RM test from 8 to 200 μm p = 0.018).
(G) Examples of traced cells. Below is the total length of traced axons (CTRL n = 11, Cxcl12 cKO n = 12, t test p = 0.08) and Sholl analysis (t test p-values are plotted below; two-way ANOVA RM test from 90 to 250 μm p = 0.05).
(H) Examples of traced cells. Below is the total length of traced axons (CTRL + PLX n = 12, Cx3cl1 cKO + PLX n = 9, Cxcl12 cKO + PLX n = 12; t test: CTRL + PLX versus Cx3cl1 cKO + PLX p = 0.43, CTRL + PLX versus Cxcl12 cKO + PLX p = 0.37; one-way ANOVA test p = 0.27) and Sholl analysis (t test p values are plotted below; two-way ANOVA RM test from 8 to 300 μm p = 0.3).
(I) Schematics of the subpial electroporation protocol. Below are the maps of the plasmids.
(J) An example of an electroporated cell at P15 (scale bar: 50 μm; pia surface is indicated by the dotted line).
(K) Schematics of the optogenetics experiment.
(L) Average traces of maximum amplitude of evoked IPSCs.
(M) Peak amplitude quantification (each dot is a cell; CTRL n = 6 cells from 4 mice, Cx3cl1 cKO n = 11 cells from 6 mice, Cxcl12 cKO n = 7 cells from 4 mice; t test: CTRL versus Cx3cl1 cKO p = 0.79, CTRL versus Cxcl12 cKO p = 0.29; one-way ANOVA test p = 0.7).
(N) Quantification of the number of pyramidal neurons responding to a single activated SST+ cell (each dot is the percentage of connected pyramidal neurons per single stimulated SST+ cell; CTRL n = 9, Cx3cl1 cKO n = 8, Cxcl12 cKO n = 7; Wilcoxon test: CTRL versus Cx3cl1 cKO p = 0.004, CTRL versus Cxcl12 cKO p = 0.26; Kruskal-Wallis test p = 0.032).
All data are represented as mean ± SEM, and the exact values are reported in Table S1.
See also Figure S2.
Our analysis revealed that, during the second postnatal week, excitatory and inhibitory cells potentially use a very similar set of molecules to communicate with microglia (Figure 2B). As expected, cytokines—a family of molecules used by the immune system for cell-to-cell communication—were the most enriched class of interactors (Figure S2A) (Reimand et al., 2016). Among these, the CX3CL1-CX3CR1 pair stood out as a potential general signal for all neurons. The ligand CX3CL1 is expressed by both excitatory and inhibitory cells (Figures 2C and S2B), while the receptor CX3CR1 is highly enriched in microglia (Kim et al., 2011). This molecular pair has been previously shown to be involved in excitatory synapse elimination in the hippocampus (Paolicelli et al., 2011) and barrel cortex (Gunner et al., 2019) but not in the visual system (Schecter et al., 2017).
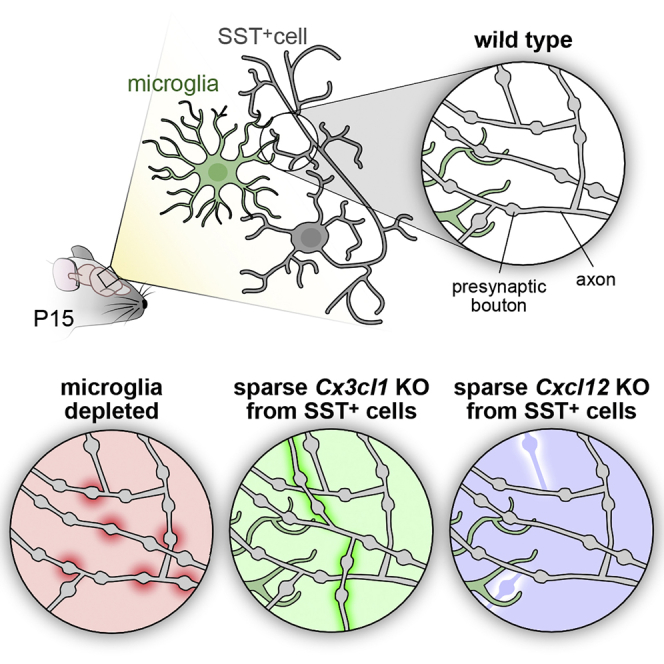
We therefore tested whether CX3CL1 plays a role in SST+ cell synapse number, axonal elaboration, or both. To achieve this, we used the same postnatal electroporation strategy described above to remove CX3CL1 from SST+ cells with CRISPR-Cas9. Specifically, we electroporated P1-2 SSTCre/+ pups with two Cre-dependent plasmids: one coding for mSynaptophysin::GFP fusion protein, and one coding for two distinct guide RNAs complementary to the Cx3cl1 gene, the nuclease Cas9 and a tdTomato protein (Figure 2D). To test the efficiency of the gRNAs in removing the target part of the gene, we proceeded as described in the STAR Methods (see also Figure S2D). Cell-autonomous and sparse removal of Cx3cl1 from SST+ cells led to an increase in total axonal length (Figure 2F), but no observable difference in the density of presynaptic boutons at P15 (Figures 2E and S2F). In addition, Sholl analysis revealed a significant increase in the number of axonal intersections between 100 and 200 μm from the cell body (Figure 2F). In contrast, the dendrites of the cells were unaffected (Figure S2H). This result suggests that CX3CL1, the canonical ligand of microglia CX3CR1 receptor, is a chemokine expressed by developing SST+ cells acting as a negative regulator of their axonal development but not their synapses per se.
Our in silico analysis also identified only one microglia-interacting molecule expressed specifically by SST+ INs but not by PV+ INs or pyramidal cells (Figures 2C and S2C): the chemokine CXCL12. To investigate the role of CXCL12 in the development of SST+ cell axons and synapses, we performed cell-autonomous knockout of Cxcl12 from SST+ cells utilizing the same strategy as for Cx3cl1 (Figures 2D and S2E). Surprisingly, we found that disrupting this gene had an opposite phenotype to the Cx3cl1 conditional knockout (cKO). Morphological reconstruction and Sholl analysis of Cxcl12 cKO SST+ cells revealed a significant decrease in the number of axonal intersections between 100 and 200 μm from the cell body and a trend toward a reduction (non-statistically significant) in total axonal length (Figure 2G). Again, we detected no difference in the density of presynaptic boutons (Figures 2E and S2G), and the dendrites were unaffected by the removal of Cxcl12 (Figure S2I).
To assess whether the two chemokines act in a microglia-dependent manner, we combined the Cx3cl1 and Cxcl12 cKOs with microglia depletion. In PLX-treated animals, neither of the genetic manipulations showed any change in the axonal elaboration of SST+ cells, indicating that both CX3CL1 and CXCL12 signaling act in a microglia-dependent manner (Figures 2H and S2J).
Finally, we evaluated the effect of Cx3cl1 and Cxcl12 cKOs on the functional synaptic output of SST+ cells onto pyramidal neurons. More specifically, we activated superficial SST+ cells through optogenetic stimulation and simultaneously performed electrophysiological recordings on surrounding pyramidal neurons in acute ex vivo brain slices. To achieve this, we electroporated P1–P2 SSTCre/+ pups with a Cre-dependent plasmid coding for ChR2 together with the plasmid used for either Cx3cl1 or Cxcl12 cKOs (Figures 2I and 2J). By utilizing whole-cell voltage-clamp recordings, we measured IPSCs evoked on multiple surrounding pyramidal neurons at P15–P20 by light activation of a single electroporated SST+ cell (Figures 2K and 2L). We found no difference in the amplitude of the evoked IPSCs between the three experimental conditions (Figure 2M). However, the probability of finding connected cells in the surrounding of Cx3cl1 cKO SST+ cells was significantly higher compared with controls; instead, no difference was detected with the connectivity of Cxcl12 cKO SST+ cells (Figure 2N).
Collectively, these results revealed that SST+ cells of superficial cortical layers recruit microglia through a pair of chemokines, CX3CL1 and CXCL12: the first one inhibits axonal growth and complexity, while the second one promotes axonal complexity but does not affect total axonal length. In accordance with these anatomical results, we found that the axonal length increase observed in Cx3cl1 cKO SST+ cells leads to an increase in the number of connected pyramidal cells.
Depletion of microglia in the second postnatal week leads to reduced sensory-driven activation of the barrel cortex
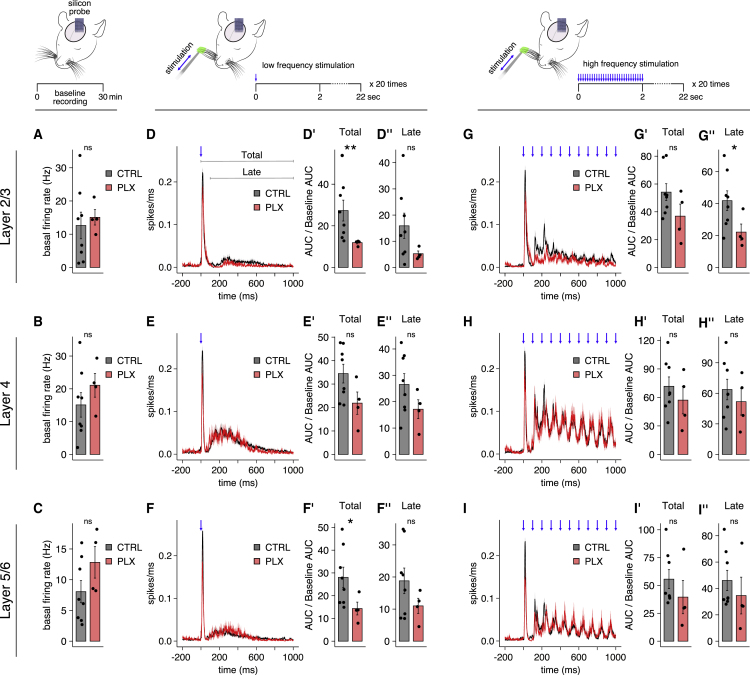
In order to assess the effect of our microglia-depletion protocol on cortical activity in vivo, we performed silicon probe recordings of multi-unit activity (MUA) before and after whisker stimulation in anesthetized P14–P17 mice. Analysis of the MUA at baseline showed no change in microglia-depleted versus control mice for L2/3, L4, and L5/6 (Figures 3A–3C). In contrast, multi-whisker stimulation at low frequency triggered significantly reduced total MUA in both L2/3 (Figure 3D′) and L5/6 (Figure 3F′) but not in L4 (Figure 3E′). In order to gauge the potential contribution of SST+ cells to this reduction, we performed an additional whisker stimulation paradigm at high frequency (10 Hz). This protocol was chosen since SST+ cells, in contrast to PV+ cells, display facilitating recruitment (Natan et al., 2017; Pouille and Scanziani, 2004; Reyes et al., 1998; Seay et al., 2020). Accordingly, after a 10 Hz multi-whisker stimulation, we analyzed the MUA during the late phase (110–1,000 ms) of cortical activation, when SST+ cell contribution should be enhanced and that of PV+ cells reduced. The results showed a significantly stronger inhibition only in the late phase of L2/3 MUA in microglia-depleted animals, which is in line with a stronger contribution of SST+ cells (Figure 3G′ and 3G″). In accordance with this result, no differences were found in late MUA upon low frequency whisker stimulation (Figure 3D″, 3E″, and 3F″).
Figure 3.
Microglia depletion in the second postnatal week leads to reduced sensory-driven activation of the cortical network
(A–C) Average firing rate (Hz) of L2/3, L4, and L5/6 at baseline, without any whisker stimulation.
(D–I) Average traces of MUA evoked by multi-whisker stimulation at low and high frequencies; the blue arrows indicate whisker deflection. “Total” and “late” area under the curves (AUCs) are measured as indicated in the STAR Methods.
Each dot is a mouse. CTRL n = 8, PLX n = 4; (A) t test p = 0.61; (B) t test p = 0.28; (C) t test p = 0.18; (D′) Wilcoxon test p = 0.004; (D′′) t test p = 0.064; (E′) t test p = 0.081; (E′′) t test p = 0.12; (F′) t test p = 0.027; (F′′) t test p = 0.12; (G′) t test p = 0.15; (G′′) t test p = 0.034; (H′) t test p = 0.44; (H′′) t test p = 0.5; (I′) Wilcoxon test p = 0.15; (I′′) Wilcoxon test p = 0.15. Representative examples of silicon probe recordings are reported in Figure S3.
All data are represented as mean ± SEM, and the exact values are reported in Table S1.
Taken together, these data suggest that microglia have a key role in regulating the proper development of cortical inhibition provided by SST+ cells during the second postnatal week, which in turn determines the strength of sensory-evoked activity in barrel cortex.
Discussion
In this study, we focus on SST+ cells whose axons and output synapses reside in the superficial layers of the cortex. These include different SST+ cell types found in L2/3 and L5 (Martinotti and non-Martinotti cells). We reveal that microglia control superficial SST+ cell axonal expansion through two molecules, CX3CL1 and CXCL12, that act in an opposing manner to set up the proper axonal development, but not the synaptic density of SST+ cells (Figure 2). Both molecules necessitate the presence of microglia as the actuator to exert their functions (Figure 2H): this explains why microglia depletion alone does not lead to a net effect on the axonal length or gross ramification (Figures 1G and 1H). We nevertheless also report an increase in the density of SST+ inhibitory synapses in the absence of microglia, suggesting that there is a separate repertoire of molecules responsible for the trimming of individual output synapses (Figures 1D and 1F).
Several studies have explored the role of the ligand CX3CL1 and its receptor CX3CR1 in excitatory synapse refinement (Gunner et al., 2019; Paolicelli et al., 2011; Rogers et al., 2011; Zhan et al., 2014), but not in inhibitory synapses. The published work suggests that microglia prune thalamo-cortical synapses, as labeled by VGLUT2 in the barrel cortex, through CX3CR1-CX3CL1 signaling (Gunner et al., 2019). Nevertheless, whether the reduction in thalamo-cortical VGLUT2 puncta is the result of synaptic versus axonal pruning was not examined. Our sparse labeling and postnatal removal of CX3CL1 from SST+ cells reveal that the gene has no effect on the density of SST+ inhibitory synapses (Figure 2E) but rather on the expansion of their axons (Figure 2F). Intriguingly, our study also identifies CXCL12 as a complementary, but opposite-acting, chemokine, as the cell-autonomous removal of the gene leads to a decrease in axonal complexity of SST+ INs (Figure 2G). Previous work described the importance of CXCL12 for marginal zone migration of Cajal-Retzius cells (Borrell and Marín, 2006; Trousse et al., 2015) and INs prenatally (Li et al., 2008; Lopez-Bendito et al., 2008; Lysko et al., 2014), as well the targeting of PV+ basket IN axons to L5 pyramidal cells of the prefrontal cortex (Wu et al., 2017). Importantly, it has also been reported that microglia are responsive to CXCL12 released by dorsal ventricular zone progenitor cells (Arnò et al., 2014; Lipfert et al., 2013). Our in silico analysis suggests that CXCL12 is recognized by microglia cells through the interaction with either integrins in a CXCR4-independent manner (Fujita et al., 2018) or through the predicted interaction with the chemokine receptors CXCR2, CCR5, and CCR1 (Szklarczyk et al., 2019).
When assessing the ability of Cx3cl1 cKO SST+ cells to inhibit surrounding pyramidal neurons we observe an increase in the number of pyramidal neurons receiving SST+-dependent synaptic inputs compared with control cells (Figure 2N), but find no difference in the amplitude of evoked IPSCs (Figure 2M). Considering that Cx3cl1 cKO does not change the density of SST+ synapses (Figure 2E), we can assume that Cx3cl1 cKO SST+ cells contact more cells through the extension of the axonal arborization (Figure 2F), but do not create more inhibitory synapses on each pyramidal neuron. In contrast, Cxcl12 cKO SST+ cells display decreased axonal complexity, but no significant reduction of axonal length (Figure 2G). In line with the Cx3cl1 cKO results, this manipulation does not lead to any difference in the number of pyramidal neurons inhibited by SST+ cells or in the amplitude of the evoked IPSC (Figures 2N and 2M). These findings support the idea that it is the change in axonal length, rather than a change in axonal complexity, that is involved in the higher number of pyramidal neurons being contacted by Cx3cl1 cKO SST+ cells. On the other hand, in microglia-depleted brains, where there is an increase of synaptic density, but no difference in axonal length, the amplitude of evoked IPSCs onto connected pyramidal cells is increased (Figure 1I).
Our work shows that microglia-dependent events control SST+ cell output during the second postnatal week, when active whisking, exploratory behavior, and, perhaps, higher-order computations begin (van der Bourg et al., 2017; Golshani et al., 2009). We, therefore, assess barrel activity at P14–P17 in control and microglia-depleted animals using in vivo silicon probe recordings. In some accordance with previous studies that have shown increased excitability of the cortical circuit at rest (Merlini et al., 2021), we find a non-statistically significant increase of the baseline spiking activity upon removal of microglia (Figures 3A–3C). It is possible that both excitation and inhibition are increased in the absence of microglia, but the former takes over the latter. In contrast, we report that low-frequency multi-whisker stimulation leads to reduced total MUA in microglia-depleted mice specifically in L2/3 and L5 of barrel cortex (Figure 3D′ and 3F′). This intriguing contradiction could be explained by an increased inhibitory output of any inhibitory cell, especially PV+ and SST+ cells, the two most abundant cortical INs. In contrast, vasoactive intestinal peptide-positive (VIP+) IN activation probably does not participate in our observed results, since multi-whisker activation of VIP+ cells decreases after P12 (Kastli et al., 2020). In an effort to discriminate between PV+ and SST+ contribution, we take into account that SST+ cells increase their spiking upon repetitive stimulation, and hence their effect should be most evident in the late phase of MUA (Pouille and Scanziani, 2004). Indeed, the late cortical MUA shows stronger inhibition in L2/3 of microglia-depleted animals, which could be explained by an effect on SST+ cell output development (Figure 3G’’).
In toto, our data reveal the importance of microglia-dependent mechanisms in the regulation of SST+ cell output and hence proper cortical sensory processing.
Limitations of the study
The use of PLX5622 offers a simple strategy to deplete microglia; nevertheless, the bulk removal of microglia could affect other aspects of brain development beyond the ones we have studied herein. Therefore, part of the observed phenotype could be directly or indirectly caused by microglia depletion.
Our in vivo recordings presented in Figure 3 provide a general investigation about the role of microglia depletion on baseline and evoked cortical activity and also aim to test for the contribution of SST+ cells. Nevertheless, ascertaining the specific role of SST+ cells would require more elaborate experiments.
Finally, in our study, we do not explore the potential receptors of CXCL12 on the microglia side. We merely present a list of potential candidates based on an in silico bioinformatics analysis of available data (Figure 2B).
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-Digoxigenin-POD, Fab fragments | Roche | Cat#11207733910; RRID:AB_514500 |
| chicken anti-GFP | Aves Lab | Cat#1020; RRID:AB_10000240 |
| goat anti-tdTomato | Sicgen | Cat#AB8181, RRID:AB_2722750 |
| mouse anti-GAD65 | Millipore | Cat#MAB351R; RRID:AB_94905 |
| mouse anti-Gephyrin | Synaptic Systems | Cat#147 011; RRID:AB_887716 |
| rabbit anti-Cleaved Caspase-3 (Asp175) | Cell Signaling | Cat#9661; RRID:AB_2341188 |
| rabbit anti-Iba1 | Wako Chemicals | Cat#019–19741; RRID:AB_839504 |
| rabbit anti-mouse Collagen IV | Bio-Rad | Cat#2150–1470; RRID:AB_2082660 |
| Chemicals, peptides, and recombinant proteins | ||
| 4-aminopyradine | Tocris | Cat#0940 |
| Acetic anhydride 98% | Sigma-Merck | Cat#242845 |
| Acrylic dental cement | Kulzer | Cat#K010103 |
| Blocking reagent (Roche) | Roche | Cat#11096176001 |
| CaCl2 | Sigma-Merck | Cat#C8106 |
| Cs-methanosulfonate | Sigma-Merck | Cat#C1426 |
| CsCl | Sigma-Merck | Cat#289329 |
| CsMeSO3 | Sigma-Merck | Cat#C1426 |
| CsOH | Sigma-Merck | Cat#232041 |
| Cyanoacrylate glue | Permabond | Permabond 102 |
| D-(+)-Glucose | Sigma-Merck | Cat#G5767 |
| Denhardt’s solution | Invitrogen | Cat#750018 |
| DiI | Invitrogen | Cat#D282 |
| Dimethyl sulfoxide (DMSO) | Sigma-Merck | Cat#276855 |
| EGTA | Sigma-Merck | Cat#324626 |
| Fast green | Sigma-Merck | Cat#F7252 |
| Fluoromount-G™ Mounting Medium, with DAPI | Invitrogen | Cat#00-4959-52 |
| Formaldehyde solution 36.5–38% | Sigma-Merck | Cat#F8775 |
| Formamide | Acros Organics | Cat#AC181090010 |
| HCl 37% | Sigma-Merck | Cat#339253 |
| HEPES | Sigma-Merck | Cat#H3375 |
| Hydrogen peroxide solution | Sigma-Merck | Cat#107209 |
| KCl | Sigma-Merck | Cat#P9541 |
| Maleic acid | Sigma-Merck | Cat#M0375 |
| Methanol | Sigma-Merck | Cat#34885 |
| MgATP | Sigma-Merck | Cat#A9187 |
| MgCl2 | Sigma-Merck | Cat#M2393 |
| Na2-ATP | Sigma-Merck | Cat#10127531001 |
| Na3-GTP | Sigma-Merck | Cat#G8877 |
| NaCl | Sigma-Merck | Cat#S3014 |
| NaH2PO4 | Sigma-Merck | Cat#S0751 |
| NaHCO3 | Sigma-Merck | Cat#S8875 |
| Normal Donkey Serum | Abcam | Cat#ab7475 |
| OCT | Sakura | Cat#4583 |
| PBS | Sigma-Merck | Cat#P4417 |
| PFA | Sigma-Merck | Cat#441244 |
| Phosphocreatine-Tris | Sigma-Merck | Cat#P1937 |
| PLX5622 powder | Chemgood | Cat#C-1521 |
| Proteinase K | Invitrogen | Cat#AM2548 |
| QX-314-Cl | Tocris | Cat#2313 |
| Ringer Solution | BBraun | Cat#3570010 |
| Salmon sperm | Invitrogen | Cat#15632011 |
| SSC | Invitrogen | Cat#15557–036 |
| Sucrose | Sigma-Merck | Cat#S0389 |
| trans-1,2-Cyclohexanediol (TCHD) | Sigma-Merck | Cat#141712 |
| Triethanolamine >99.5% | Sigma-Merck | Cat#90278 |
| Tris | Biosolve | Cat#0020092391BS |
| Triton™ X-100 | Sigma-Merck | Cat#T8787 |
| TSA Plus Cyanine 5 system | Akoya biosciences | Cat#NEL745001KT |
| TTX | Tocris | Cat#1078 |
| Tween® 20 | Sigma-Merck | Cat#P9416 |
| Yeast tRNA | Invitrogen | Cat#AM7118 |
| Critical commercial assays | ||
| QIAGEN DNeasy Blood & Tissue Kit | QIAGEN | Cat#69504 |
| Deposited data | ||
| Transcriptomic postnatal data of microglia cells | GEO DataSets | GSE79812 |
| Transcriptomic postnatal data of neuronal cell types | GEO DataSets | GSE120161 |
| STRING protein-protein interaction database v11.0 | (Szklarczyk et al., 2019) | https://version-11-0.string-db.org/cgi/download.pl?sessionId=99uSZMsNLaTe |
| Experimental models: Organisms/strains | ||
| Ai14 (B6;129S6-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J) | Jackson Laboratories | Cat#007908; RRID:IMSR_JAX:007908 |
| Ai32 (B6.Cg-Gt(ROSA)26Sortm32(CAG−COP4∗H134R/EYFP)Hze/J) | Jackson Laboratories | Cat#024109; RRID:IMSR_JAX:024109 |
| C57BL/6JRj | Janvier | N/A |
| SST-IRES-Cre (Ssttm2.1(cre)Zjh/J) | Jackson Laboratories | Cat#013044; RRID:IMSR_JAX:013044 |
| Oligonucleotides | ||
|
Cx3cl1 probe FW: 5′-CACAAGGAGGCAGGCAGT-3′ |
Microsynth AG | N/A |
|
Cx3cl1 probe RV (including T7 promoter): 5′-TAATACGACTCACTATAGGGGCACATCCA AGTCTGGGG-3′ |
Microsynth AG | N/A |
|
Cxcl12 probe FW: 5′-AAAGGACTTTCCAGTAGACCCC-3′ |
Microsynth AG | N/A |
|
Cxcl12 probe RV (including T7 promoter): 5′-TAATACGACTCACTATAGGGTCTCAGAC TTGTGTTTTGTGGG-3′ |
Microsynth AG | N/A |
|
Cx3cl1 gRNA a: 5′-GATGACCTCACGAATCCCAG-3′ |
Microsynth AG | N/A |
|
Cx3cl1 gRNA b: 5′-CAGCTAAACCAGGAGTCCTG-3′ |
Microsynth AG | N/A |
|
Cxcl12 gRNA a: 5′-TGAGCTACCGATGCCCCTGC-3′ |
Microsynth AG | N/A |
|
Cxcl12 gRNA b: 5′-TGACGTTGGCTCTGGCGATG-3′ |
Microsynth AG | N/A |
|
Cx3cl1 target DNA amplification FW: 5′-AGGCTGAGACTCTTGCTGTC-3′ |
Microsynth AG | N/A |
|
Cx3cl1 target DNA amplification RV: 5′-GACAGGAAACGCTGGACTTC-3′ |
Microsynth AG | N/A |
|
Cx3cl1 target DNA sequencing FW: 5′-AGGCTGAGACTCTTGCTGTC-3′ |
Microsynth AG | N/A |
|
Cx3cl1 target DNA sequencing RV: 5′-CCATGGGATCTAGTGGGCTG-3′ |
Microsynth AG | N/A |
|
Cxcl12 target DNA amplification FW: 5′-GTTCCTGAGTCTTCCCCACA-3′ |
Microsynth AG | N/A |
|
Cxcl12 target DNA amplification RV: 5′-CAGGACACATCTCTGCCAAG-3′ |
Microsynth AG | N/A |
|
Cxcl12 target DNA sequencing FW: 5′-GTTCCTGAGTCTTCCCCACA-3′ |
Microsynth AG | N/A |
|
Cxcl12 target DNA sequencing RV: 5′-GAAACACTGAGGACATCAGG-3′ |
Microsynth AG | N/A |
| Recombinant DNA | ||
| AAV phSyn1(S)-FLEX-tdTomato-T2A-SypEGFP-WPRE | (Oh et al., 2014) | Addgene #51509; RRID:Addgene_51509 |
| p3E-2a-tdTomato | (Shin et al., 2016) | Addgene #67707; RRID:Addgene_67707 |
| pAAV-EF1a-double floxed-hChR2(H134R)-EYFP-WPRE-HGHpA | a gift from Karl Deisseroth | Addgene #20298; RRID:Addgene_20298 |
| pCAG_loxP-STOP-loxP-ChR2(H134R)-T2A-GFP | this manuscript | N/A |
| pCAG_loxP-STOP-loxP-GFP | this manuscript | N/A |
| pCAG_loxP-STOP-loxP-mSynaptophysin::GFP | this manuscript | N/A |
| pCAG_loxP-STOP-loxP-tdTomato-T2A-mSynaptophysin::GFP | this manuscript | N/A |
| pCAG-GFP | (Matsuda and Cepko, 2004) | Addgene #11150; RRID:Addgene_11150 |
| pCALNL-DsRed | (Matsuda and Cepko, 2007) | Addgene #13769; RRID:Addgene_13769 |
| pX333 | (Maddalo et al., 2014) | Addgene #64073; RRID:Addgene_64073 |
| pX333_U6-gRNA-U6-gRNA-CBh-loxP-STOP-loxP-Cas9-2A-tdTomato | this study | N/A |
| Software and algorithms | ||
| pCLAMP v9.0–10.7.0.3 | Molecular Devices | https://mdc.custhelp.com/app/answers/detail/a_id/18779/∼/axon%E2%84%A2pclamp%E2%84%A2-10-electrophysiology-data-acquisition-%26-analysis-software-download |
| ImageJ – Fiji 2.1.0/1.53c | (Schneider et al., 2012) | https://imagej.nih.gov/ij/ |
| Imaris 9.3.1/9.5.0 | Bitplane | https://imaris.oxinst.com |
| MATLAB R2019a - 2021b | MathWorks | https://www.mathworks.com/ |
| MC_RACK software | Multi Channel Systems | https://www.multichannelsystems.com/software/mc-rack |
| RStudio 1.2.1335 | RStudio Team (2018) | http://www.rstudio.com/ |
| Putative interaction counting code | this manuscript | https://doi.org/10.5281/zenodo.6862093 |
| Synaptic bouton counting code | this manuscript | https://doi.org/10.5281/zenodo.6861757 |
| Computational pipeline for protein-protein interactions | this manuscript | https://doi.org/10.5281/zenodo.6863632 |
| Other | ||
| 64-channel silicon probe | Neuronexus | Cat#A8x8-Edge-5mm-100-200-177-A64 |
| SuperFrost® Plus | Thermo Scientific | Cat#J1800AMNZ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Theofanis Karayannis (karayannis@hifo.uzh.ch).
Materials availability
The plasmids generated in this study are available from the corresponding author on request.
Experimental model and subject details
In this study we used the following mouse lines: SST-IRES-Cre (Jackson Laboratories Cat#013044; RRID:IMSR_JAX:013044), Ai14 (Jackson Laboratories Cat#007908; RRID:IMSR_JAX:007908), Ai32 (Jackson Laboratories Cat#024109; RRID:IMSR_JAX:024109) and wild type C57BL/6JRj animals (Janvier).
All used animals were between P1 and P30. The specific age of the animals at time of each experimental use is reported in the result section and in the corresponding figures.
Animal experiments were approved by the Cantonal Veterinary Office of Zurich, the University of Zurich and Harvard University. Animals were housed in a 12 h reverse dark-light cycle at 24°C and variable humidity and received water and food ad libitum. Both male and female mice were used for all experiments.
Method details
Microglia depletion in postnatal pups
PLX5622 stock was dissolved in DMSO at a final concentration of 100mg/mL. Pups were injected intraperitoneally every day, from P2 until the day before brain collection, with 0.5μL/g of PLX5622 stock solution diluted in at least an equal volume of DMSO (PLX5622 final concentration is 50μg/g of mouse). Control group was injected with an equal volume of DMSO.
Antibodies
In this study, the following antibodies were used: rabbit anti-Iba1 (1:500, Wako Chemicals Cat#019–19741), mouse anti-GAD65 (1:500, Millipore Cat#MAB351R), chicken anti-GFP (1:1000, Aves Lab Cat#1020), mouse anti-Gephyrin (1:500, Synaptic Systems Cat#147 011), rabbit anti-mouse Collagen IV (1:500, Bio-Rad Cat#2150–1470), rabbit anti-Cleaved Caspase-3 (Asp175) (Cell Signaling, Cat#9661), goat anti-tdTomato (1:500, Sicgen Cat#AB8181), anti-Digoxigenin-POD, Fab fragments (1:500, Roche Cat #11207733910).
Immunohistochemistry
For immunohistochemistry in Figures 1A and 1C, S1C, S1D, postnatal mice were perfused with 5mL of phosphate saline buffer (PBS) 1× and 10mL of paraformaldehyde 4% in phosphate buffer 0.1M (PFA 4%); brains were dissected and then post-fixed in PFA 4% for 2 h. Fixed tissue was cryoprotected with sucrose 30% in PBS 1× over two nights and then embedded in OCT and stored at −80°C. Brains were sectioned (20–25 μm) with a cryostat (Leica) on slides (Superfrost Plus, Thermo Scientific) and stored at −80°C. Slides were defrosted, air dried for 30 min and washed 3 times in PBS 1×. Tissue was incubated with blocking solution (donkey serum 10% and Triton™ X-100 0.2% in PBS 1×) for 1 h at room temperature. Sections were incubated overnight at 4°C with primary antibody diluted in blocking solution. Sections were washed 3 times in PBS 1× and then incubated with secondary antibody diluted in blocking solution. Sections were washed 3 times in PBS 1× and mounted with Fluoromount-G™ mounting medium with DAPI.
For immunohistochemistry in Figures 1B and 1D and S1A, brains were processed as described before (Favuzzi et al., 2021).
Imaging and analysis of stained sections
For counting putative interactions (Figure 1A), sections were imaged with an Olympus FV1000 confocal microscope, using a UPlanFL N 40×/1.30 oil immersion objective, a z-stack thickness of 0.5 μm and a resolution of 1600 × 1600 pixel. To automatically count microglia putative interactions with SST+ processes, we first segmented SST+ cell bodies and SST+ processes separately. While cell bodies are blob-like, processes are tubular-like. We enhanced blob-like and tubular-like signals using LoG (Kong et al., 2013) and Jerman vesselness (Jerman et al., 2015) methods respectively. Then, we segmented processes with Otsu thresholding (Otsu, 1979). To improve cell body detection, we then performed connected component analysis of all segmented pieces and extracted structural features (such as roundness, area, mean intensity, etc.) and clustered into two classes using k-means method (Hartigan and Wong, 1979). We manually determined the class boundary to separate cell bodies from other false-positives and trained a support vector machine (Scholkopf and Smola, 2018) classifier. To segment microglia processes, we used adaptive k-means clustering (k-means and Otsu gives similar results under Euclidean distance metric) (Bishop, 2006). After the segmentation of all structures separately from maximum intensity projected images, we turned these 2-dimensional segmentations into 3-dimensions by thresholding each z-profile that falls under a positively segmented pixel. The final colocalization was performed by simple logical operators in 3-dimensions afterwards. Analysis was performed in MATLAB (MathWorks) and the code is publicly available at https://doi.org/10.5281/zenodo.6862093.
For synaptic markers (Figures 1D and S1A) sections were imaged on an upright ZEISS LSM 800 confocal microscope using a 40× oil immersion objective, 1.4 NA, 2.5 digital zoom, 1024 × 1024 pixels (∼0.22 μm resolution using 510 nm emission). Images were analyzed using a custom script in Fiji (ImageJ) software as described before (Favuzzi et al., 2021). Briefly, processing of all channels included noise reduction and smoothing. All single channel images were converted to RGB. Next, a color threshold was automatically set to identify the axon, its area was automatically measured and a masked binary image with axon only was created. For bouton segmentation, a watershed-based method was used such that boutons were separated based on the local minima of the pixel gray values. For the presynaptic boutons (GAD65) or postsynaptic clusters (Gephyrin), a color threshold was selected to segment boutons as isolated puncta. The area of each presynaptic bouton was 0.2–0.8 μm2 whereas postsynaptic clusters measured 0.1–0.3 μm2. The “Analyze Particles” (where the minimum size for presynaptic and postsynaptic structures was 0.20 μm and 0.10μm, respectively) and “Watershed” tools were applied and a mask was generated. A merged image from all masks was created, converted to an 8-bit image and, using an automatic threshold, the overlap between presynaptic boutons, postsynaptic clusters and axon was automatically detected as particles with a size greater than 0.05 μm2 in the “analyze particles” tool.
For analysis of microglia-synapse contacts (Figure 1B), confocal stacks of ∼10–15 μm were acquired with a 0.2 μm step size. These were analyzed with IMARIS 9.3.1 or 9.5.0 software using a MATLAB (MathWorks) script to automatize the analysis as described before (Favuzzi et al., 2021).
For SST+ and Cas3+ cells counting (Figures S1B and S1C), pictures from motor to visual cortex, from L1 to L6, were acquired with a Zeiss Axio Scan.Z1 slidescanner and counted in Fiji (ImageJ) both manually (“cell counter” plug-in) and by using the “analyze particle” and “watershed” tools after selecting the proper color threshold.
Subpial electroporation
Electroporations were done as previously published (de La Rossa and Jabaudon, 2015; Lim et al., 2018b). In brief, plasmids were diluted to 3500ng/ul in PBS 1×, together with Fast green 0.05% and trans-cyclohexane-1,2-diol (TCHD) 1mg/mL. P1-2 pups were anesthetized with isoflurane. Between 0.5 and 1 μL of plasmid solution was injected using sharp pulled PCR glass capillaries below pial surface. Plasmids were electroporated placing the negative paddle of the tweezertrodes on the injection site. Two trains of 10 pulses at 99V, 50ms long and 950ms between each pulse, were applied with 3 s between each train (ECM399, Harvard apparatus).
Synaptic boutons imaging and counting
For synaptic boutons imaging (Figures 1F and 2E and S1E), postnatal mice were perfused with 5mL of PBS 1× and 10mL of PFA 4%; brains were dissected and then post-fixed in PFA 4% overnight. Brains were stored in PBS 1× at 4°C until vibratome sectioning. Brains were sectioned (80 μm thick) with vibratome (Leica). Fluorescent cells were imaged with an Olympus FV1000 confocal microscope, using a UPlanFL N 40×/1.30 oil immersion objective, a z-stack thickness of 0.5 μm and a resolution of 1600 × 1600 pixel. For synaptic boutons counting, the mSyp::GFP channel was filtered using the Wiener filtering followed by bilateral filtering for denoising (the Wiener filter performs adaptive noise removal through low-pass filtering of the image that has been corrupted by a stationary noise (Lim, 1990); the bilateral filter smoothens the signal while preserving the edges (Sokullu et al., 2020; Tomasi and Manduchi, 1998)). Filtered images were segmented using Otsu thresholding (Otsu, 1979). Connected puncta were separated using distance transformation based watershed algorithm (Cuisenaire, 1999). The tdTomato channel was used for tracing processes using accurate fast marching (Hassouna and Farag, 2007; Kroon Dirk-Jan, 2021). Tracing was performed both from source to sink and from sink to source to improve the centerline tracing. We calculated the puncta density by finding puncta profiles falling onto 3D centerline profiles (Figure S1G). The distribution of puncta sizes is plotted in Figure S1H. Puncta smaller than 0.5μm (yellow column) were removed from the total count as they appear as background signal. Any observation classified as a suspected outlier according to the interquartile range criterion (red columns) corresponds to a cluster of puncta too close to be distinguishable by the program. In order to be able to use these data points and estimate the number of puncta composing the cluster, we divided each outlier by the mean size of true detected puncta (white columns). Puncta analysis was performed in MATLAB (MathWorks); puncta sorting was performed in RStudio. The code is publicly available at https://doi.org/10.5281/zenodo.6861757.
Optogenetics and electrophysiology in SSTCre;Ai32 mice
P15 mice were deeply anesthetized with isoflurane, brains were removed and 300 μm coronal slices were cut using a vibratome (Leica) in ice-cold artificial cerebrospinal fluid (ACSF) + Sucrose of the following composition: NaCl 87 mM, NaHCO3 26 mM, KCl 2.5 mM, NaH2PO4 1.25 mM, CaCl2 0.5 mM, MgCl2 4 mM, Glucose 10 mM, Sucrose 75 mM, saturated with 95% O2, 5% CO2 at pH 7.3–7.4. Slices were then transferred to a heated chamber at 34°C with oxygenated ACSF recording solution, where they underwent recovery for 30 min. Slices were then moved to ACSF recording solution at room temperature, where they remained for at least an hour before recording. For recordings, slices were transferred to the recording chamber of an up-right microscope (Zeiss Axioskop 2). All recordings were carried out at a constant temperature (30°C). Slices were perfused with ACSF of the following composition: NaCl 125 mM, KCl 2.5 mM, MgCl2 1 mM, NaH2PO4 1.25 mM, NaHCO3 25 mM, Glucose 20 mM, CaCl2 2 mM, saturated with 95% O2, 5% CO2 at pH 7.3–7.4. The recording pipettes were pulled from borosilicate glass (Harvard Apparatus) to obtain a tip resistance of 3–5 MOhm. Only cells with access resistance <30MOhm were accepted. The access resistance was monitored throughout the recording and any cells where access resistance deteriorated and changed more than 20% were discarded. Evoked IPSCs were recorded by clamping the cells at +0 mV. The following internal solution was used: Cs-methanosulfonate 130 mM, CsCl 5 mM, HEPES 10 mM, EGTA 0.2 mM, MgATP 4 mM, Na-GTP 0.3 mM, Phosphocreatine-Tris 8 mM, QX-314-Cl 5 mM, equilibrated with CsOH at pH 7.3. Data were acquired at a 20 kHz sampling rate using a MultiClamp 700B amplifier (Molecular Devices) and were filtered at 10 kHz. Optogenetic stimulation was conducted under wide-field photostimulation through a 40× water immersion objective. The recorded neuron was centered in the field of view and a 470 nm LED light was triggered to deliver a square-shaped pulse of 1 ms-long illumination at 50% of the maximum stimulation intensity (∼1 mW/mm2). Light pulses eliciting IPSCs were delivered every 15 s. The LED output was driven using a digital output from the Clampex software of the pCLAMP 9.0 program suite (Molecular Devices) controlling a BioLED controller (Mightex). To verify monosynaptic connections, TTX (1 μM) and 4-aminopyradine (1 mM) were applied in some of the optogenetics recordings.
In situ hybridization + immunohistochemistry
For in situ hybridization + immunohistochemistry (Figures S2B and S2C), postnatal mice were perfused with 5mL of PBS 1× and 10mL of PFA 4%; brains were dissected and then post-fixed in PFA 4% for 2 h. Fixed tissue was cryoprotected with sucrose 30% in PBS 1× over two nights and then embedded in OCT and stored at −80°C. Brains were sectioned (25 μm) with a cryostat (Leica) on slides (Superfrost Plus, Thermo Scientific) and stored at −80°C. Slides were defrosted, air dried for 1 h, fixed in formaldehyde 4% in PBS 1× for 10 min, washed twice in PBS 1× for 5 min, fixed in 1.5% H2O2 in methanol for 15 min, washed twice in PBS 1× for 5 min, treated with 0.2M HCl for 8 min, washed twice in PBS 1× for 5 min, digested with Proteinase K (10μg/mL in PBS 1×) for 5 min, washed once in PBS 1× for 5 min, incubated in acetylation solution (for 200 mL of solution: 2.66 mL triethanolamine >99.5%, 0.32 mL HCl 37%, 0.5 mL acetic anhydride 98%) for 10 min, washed three times in PBS 1× for 5 min. Slides were then placed in a humid chamber and covered with hybridization solution (formamide 50%, SSC 5×, yeast tRNA 0.1 mg/mL, Denhardt’s solution 1×, salmon sperm 0.1 mg/mL) for at least 2 h and then hybridized with RNA probes overnight at 65°C. Prior incubation, RNA probes were diluted 1ng/1μL in hybridization solution, heated at 80°C for 5 min and then immediately transferred on wet ice for 3 min. Slides were washed once in SSC 5× for 5 min at 65°C, twice in formamide 50%, SSC 2× for 30 min at 65°C, once in SSC 2× for 15 min at 37°C, once in SSC 0.1× for 15 min at 37°C and once in TN buffer (Tris-HCl pH 7.5 0.1M and NaCl 0.15M). Slides were moved to a humid chamber and blocked with blocking solution (Maleic Acid Buffer 1×, blocking reagent (Roche) 2%, Tween®20 0.3%) for 1 h and finally incubated overnight at 4°C with anti-Dig-POD and goat anti-tdTomato diluted 1:500 in blocking solution. Slides were washed in TNT buffer (TN buffer, Tween®20 0.05%) three times for 5 min and once for 1.5 h. Dig-RNA probes were developed with Cy5-tyramide (or FITC-tyramide) 1:100 in amplification reagent for 20 min. Slides were washed in TNT buffer three times for 5 min, incubated for 1.5 h with a secondary antibody diluted in blocking solution, washed in TNT buffer three times for 5 min, washed once in TN buffer for 5 min and mounted with Fluoromount-G™ mounting medium with DAPI.
Cx3cl1 and Cxcl12 DIG-labelled probes were transcribed from mouse cDNA using the primers provided from http://portal.brain-map.org/.
Plasmids and cloning
To obtain the pCAG_loxP-STOP-loxP-tdTomato-T2A-mSynaptophysin::GFP plasmid, the tdTomato-T2A-mSynaptophysin::GFP ORF was cloned by PCR from Addgene #51509 (Oh et al., 2014) and inserted into a pCALNL vector from Addgene #13769 (Matsuda and Cepko, 2007) (between XmaI and NotI restriction sites). This plasmid was used to label and count presynaptic boutons in CTRL versus microglia-depleted animals.
To obtain the pCAG_loxP-STOP-loxP-mSynaptophysin::GFP plasmid, the mSynaptophysin::GFP ORF was cloned from Addgene #51509 and inserted into a pCALNL vector from Addgene #13769 (between XmaI and NotI restriction sites). This plasmid was used to label and count presynaptic boutons in CTRL versus Cx3cl1 cKO and Cxcl12 cKO cells. Moreover, it was also used to reconstruct cell morphology in CTRL versus Cx3cl1 cKO cells.
To obtain the pCAG_loxP-STOP-loxP-GFP plasmid, the GFP ORF was cut from Addgene #11150 (Matsuda and Cepko, 2004) with XmaI and NotI and inserted into a pCALNL vector from Addgene #13769 (between XmaI and NotI restriction sites). This plasmid was used to reconstruct cell morphology in CTRL versus Cxcl12 cKO cells and in CTRL + PLX versus Cx3cl1 cKO + PLX and Cxcl12 cKO + PLX.
To obtain the pX333_U6-gRNA-U6-gRNA-CBh-loxP-STOP-loxP-Cas9-2A-tdTomato plasmid, the 2A-tdTomato ORF was cloned from Addgene #67707 (Shin et al., 2016) and inserted in frame with Cas9-NLS (without stop codon) of the pX333 vector (Addgene #64073) (Maddalo et al., 2014). Cx3cl1 and Cxcl12 gRNAs were designed with Synthego CRISPR Design Tool (www.synthego.com) and cloned between BbsI and BsaI restriction sites as previously described (Ran et al., 2013).
Cx3cl1 gRNA a: 5′-GATGACCTCACGAATCCCAG-3′
Cx3cl1 gRNA b: 5′-CAGCTAAACCAGGAGTCCTG-3′
Cxcl12 gRNA a: 5′-TGAGCTACCGATGCCCCTGC-3′
Cxcl12 gRNA b: 5′-TGACGTTGGCTCTGGCGATG-3′
To obtain the pCAG_loxP-STOP-loxP-ChR2(H134R)-T2A-GFP plasmid, the ChR2(H134R) ORF was cloned from Addgene #20298 (pAAV-EF1a-double floxed-hChR2(H134R)-EYFP-WPRE-HGHpA was a gift from Karl Deisseroth), while the GFP ORF was cloned from Addgene #11150 (the T2A sequence was added directly through PCR amplification): both fragments were inserted into a pCALNL vector from Addgene #13769 (between XmaI and NotI restriction sites).
Cx3cl1 and Cxcl12 knock-out validation
C57BL/6JRj wild type pregnant females (15.5 days of embryonic development) have been in utero electroporated with pX333_U6-gRNA-U6-gRNA-CBh-loxP-STOP-loxP-Cas9-2A-tdTomato with either Cx3cl1 or Cxcl12 gRNAs as previously described (Meyer-Dilhet and Courchet, 2020). Cortical electroporated region was rapidly dissected, single cell suspension was prepared and fluorescent tdTomato+ cells were FACS sorted as previously described (Jaeger et al., 2020). Genomic DNA was extracted using QIAGEN DNeasy Blood & Tissue Kit (Cat#69504).
A 887 bp genomic sequence containing the Cx3cl1 target DNA was PCR-amplified using the following set of primers:
forward: 5′-AGGCTGAGACTCTTGCTGTC-3′
reverse: 5′-GACAGGAAACGCTGGACTTC-3′
A portion of the amplified fragment was sequenced by Sanger sequencing using the following set of primers:
forward: 5′-AGGCTGAGACTCTTGCTGTC-3′
reverse: 5′-CCATGGGATCTAGTGGGCTG-3′
A 768 bp genomic sequence containing the Cxcl12 target DNA was PCR-amplified using the following set of primers:
forward: 5′-GTTCCTGAGTCTTCCCCACA-3′
reverse: 5′-CAGGACACATCTCTGCCAAG-3′
A portion of the amplified fragment was sequenced by Sanger sequencing using the following set of primers:
forward: 5′-GTTCCTGAGTCTTCCCCACA-3′
reverse: 5′-GAAACACTGAGGACATCAGG-3′
DNA sequences were aligned to the reference genomic sequence using Benchling (https://www.benchling.com). The results are reported in Figures S2D and S2E.
Computational pipeline to identify protein-protein interactions
Transcriptomic postnatal data of neuronal cell types were downloaded from GEO DataSets, accession number GSE120161. Transcriptomic postnatal data (P9) of microglia cells were downloaded from GEO DataSets, accession number GSE79812. Only genes expressed over 30 normalized counts have been considered.
A list of mouse ligand proteins was downloaded from Uniprot website (www.uniprot.org) by inserting the two following commands into the search bar:
-
•
(cc_scl_term:SL-0039) AND (ft_topo_dom:extracellular) AND (reviewed:true) AND (organism_id:10090) NOT (keyword:KW-0675)
-
•
(cc_scl_term:SL-0243) AND (reviewed:true) AND (organism_id:10090)
A list of mouse receptors was downloaded from Uniprot website by inserting the following command into the search bar:
-
•
(cc_scl_term:SL-0039) AND (ft_topo_dom:extracellular) AND (reviewed:true) AND (organism_id:10090) AND (keyword:KW-0675)
These lines filter for specific annotated structural properties and subcellular locations.
Mouse STRING interactions list (version 11.0) was downloaded from STRING website (https://version-11-0.string-db.org/cgi/download.pl?sessionId=99uSZMsNLaTe). Only interactions with an experimental score different from zero and a general score bigger than 400 (medium confidence) were considered. Moreover, only interactions labelled as “binding” type were considered. In addition, for those chemokines expressed by either SST+, PV+ or Pyramidal cells, the interactions annotated in Uniprot, but not in STRING, were added with an arbitrary score of 701.
STRING interactions list was subset first for complementary neuronal receptors and microglia ligands and then for complementary neuronal ligands and microglia receptors.
The code is publicly available at https://doi.org/10.5281/zenodo.6863632.
Morphological reconstruction and sholl analysis
Brains were sectioned with vibratome (Leica) (80 μm thick in Cx3cl1 cKO and its control group, 130 μm thick in Cxcl12 cKO and its control group, 130 μm thick in Cx3cl1 cKO + PLX, Cxcl12 cKO + PLX and their control group). Cells were imaged with a Leica SP8 confocal microscope, using a HC PL APO CS2 40× water immersion objective, a z-stack thickness of 0.5 μm and a resolution of 1600 × 1600 pixel. Morphological reconstruction and Sholl analysis were performed with SNT plugin in Fiji (ImageJ) (Ferreira et al., 2014; Schneider et al., 2012).
Single cell optogenetics and electrophysiology on electroporated cells
P15-20 mice were deeply anesthetized with isoflurane, brains were removed and 270 μm thick coronal slices were cut using a vibratome (Leica) in ice-cold artificial cerebrospinal fluid (ACSF) of the following composition: NaCl 115 mM, KCl 3.5 mM, NaH2PO4 1.2 mM, MgCl2 1.3 mM, CaCl2 2 mM, NaHCO3 25 mM and Glucose 25 mM, saturated with 95% O2, 5% CO2. Slices were then transferred to a chamber with oxygenated ACSF at room temperature, where they underwent recovery for at least 30 min. Slices were then moved to the recording chamber of an up-right microscope and perfused with ACSF. All recordings were carried out at a constant temperature (29.8 C°).
The recording pipettes were pulled from borosilicate glass (Harvard Apparatus) to obtain a tip resistance of 3–4 MOhm. Οnly cells with access resistance <30 MOhm were accepted. Access resistance was monitored throughout the recording; any cell where the access resistance changed more than 20% were discarded. Evoked IPSCs were recorded by clamping the cells at +0 mV. The following internal solution was used: CsMeSO3 130 mM, CsCl 5 mM, NaCl 5 mM, MgCl2 2 mM, EGTA 0.1 mM, HEPES 10 mM, CaCl2 0.05 mM, Na2-ATP 2 mM, Na3-GTP 0.4 mM (pH 7.3, 280–290 mOsm/kg). Data were acquired at 10 kHz sampling rate using an Axopatch 200B amplifier controlled by pCLAMP v10.7.0.3 (Molecular Devices) and were filtered at 2 kHz (Digidata 1440A, Molecular Device). Data are reported without corrections for liquid junction potentials.
Optogenetic stimulation was conducted under wide-field photostimulation through a 40× water immersion objective (40×/0.8, Zeiss). Isolated fluorescent L2/3 SST+ cells were checked for tdTomato and GFP expression, placed at the center of the field of view and stimulated using light pulses (from 20 to 250 ms at 470 nm) of LED light delivered through the microscope objective (Polygon400, Mightex Systems). The stimulation protocol consisted of 10 sequential acquisitions of 5 s each. A single light pulse of a specific duration was triggered 2 s after the beginning of each acquisition. Light pulse duration was increased from 20 to 250 ms (specifically, we used the following steps: 20, 30, 40, 50, 60, 80, 100, 150, 200, 250 ms). This sequence of 10 acquisitions was repeated 9 times. This incremental protocol was chosen to ensure the adequate light-induced activation of cells with variable expression of Channelrhodopsin-2. We did not observe any consistent run down of the synaptic responses with increasing the pulse duration.
Between 2 and 3 pyramidal neurons surrounding the single SST+ cell (around 2 cell bodies of distance) were patched one after the other. We calculated the average of the 9 sweeps for each light pulse duration. Then, we selected the average showing the maximum amplitude of evoked IPSC and used it for statistical comparison. The analysis of the connectivity was expressed as the ratio of the number of pyramidal neurons showing evoked IPSCs over the total number of pyramidal neurons recorded around one single SST+ cell. Outliers were checked and removed according to the interquartile range criterion.
In vivo silicon probe experiments: animal surgery
P14-P17 mice were anesthetized with a first intraperitoneal injection of urethane (1.2g/kg), followed by a second injection 40 min later (10% of the first dose). The head was shaved and sterilized with Betadine®. EMLA cream 5% (lidocaine/prilocaine) was applied on the skin before surgical incision. Vitamin A ointment was applied to protect the eyes. Body temperature was kept at 37°C with a heating pad. Hydration level was checked regularly and maintained by subcutaneous injections of Ringer Solution (1.5g/kg).
After cutting the skin and cleaning the skull, a custom-built head plate was glued to the skull over the right hemisphere with glue and acrylic dental cement. A cranial window (2-5mm wide) was opened above the center of the barrel columns (from Bregma, AP: 1.7 ML: 3.3) with a sharp razor blade; a second single burr hole (needed to insert a silver wire as reference electrode) was drilled at the level of the cerebellum.
For multi-electrode recordings, the dura was penetrated. Neural activity was recorded with a 64-channel silicon probe (Neuronexus) inserted into barrel cortex. Each of the 8 shanks (5 mm long and 200 μm distant from each other) contained 8 recording sites (177 μm2 surface area per recording site) spaced 100 μm apart. Before insertion, the probe was impregnated with DiI (1,1′-dioctadecyl-3,3,3′3′-tetramethylindocarbocyanine) in order to mark the insertion site. The recording of multi-unit activity (MUA) started 1 h after the insertion. All data were continuously digitized at 20 kHz and stored for offline analysis using MC_RACK software (Multi Channel Systems).
After each experiment, the animal was deeply anesthetized with isoflurane and perfused with PBS 1×. The brain was carefully removed from the skull and kept in PFA 4% for 24 h at 4°C. The brain was rinsed once and stored overnight in PBS 1×. Brains were sectioned in 200 μm thick tangential sections and checked for the insertion site marked with DiI.
In vivo silicon probe experiments: whisker stimulation protocols
A stainless-steel rod (1 mm diameter), connected to a miniature solenoid actuator (about 1 mm displacement), was placed 1 mm from the snout. Stimulator movement was calibrated as previously described (Yang et al., 2017). Whiskers were glued all together and stuck to the end of the rod. Two different stimulation paradigms were applied: low frequency, where whiskers were deflected once every 22 s (repeated for 20 trials) and high frequency, where whiskers were deflected repeatedly at 10 Hz (20 deflections in 2 s) every 20 s (repeated for 20 trials); see schematics in Figure 3.
In vivo silicon probe experiments: data analysis
The different cortical layers were estimated based on the Current Source Density (CSD) map computed from local field potentials (LFPs) (van der Bourg et al., 2017). For each mouse, all the recordings (baseline, low and high frequency stimulation protocols) were merged and evaluated together for spike detection. Each merged group includes 64 recording sites. The recording site showing the lower activity in basal condition (without whisker stimulation protocol) was chosen as reference and subtracted from each channel. Spike detection was performed using amplitude-thresholding in the negative range (−6 times the standard deviation of the signal) (van der Bourg et al., 2017). All sampled amplitude values in a time range from −0.5 to +0.5 milliseconds relative to the waveform negative peak were extracted. After the spike selection, the merged group data were split back into the corresponding recordings. For baseline recordings, we calculated the average firing rate (Hz) over 30 min. For recordings upon whisker stimulation protocols, spikes were represented as smoothed averaged peri-stimulus time histograms using 1 ms time bins (Figures 3D–3I). AUC before stimulation was calculated from −199 to −20 ms (stimulus comes at 0 ms). “Total” MUA was calculated as AUC from 5 to 1000 ms divided by AUC before stimulation. “Late” MUA was calculated as AUC from 110 to 1000 ms divided by AUC before stimulation. Data analysis of spikes was performed in MATLAB (MathWorks).
Quantification and statistical analysis
A detailed report of sample sizes, statistical tests used and p-values is reported at the end of each figure legend and in Table S1. We used R Studio to generate plots and perform statistical tests. We checked the normal distribution of data points using the Shapiro-Wilk Normality Test. For Sholl analysis, we used two-sided T-test in 5 μm bins. In addition, we used MATLAB (MathWorks) to perform two-way repeated measures ANOVA (see fitrm and ranova) and post-hoc tests using Bonferroni correction for multiple comparisons (see multcompare). In figures, ns: p-value > 0.05; ∗: p-value < 0.05; ∗∗: p-value < 0.01; ∗∗∗: p-value < 0.001.
Acknowledgments
We are grateful to Gord Fishell for his precious feedback and for his valuable comments on previous versions of this manuscript. We would also like to thank all members of the Karayannis laboratory for stimulating discussions. Imaging was performed with equipment maintained by the Center for Microscopy and Image Analysis, University of Zurich. L.G. was supported by the Forschungskredit of the University of Zurich, grant no. FK-20-029. E.F. was supported by the Charles A. King Trust Postdoctoral Research Fellowship Program, Bank of America, N.A., Co-Trustees. L.A.I. was supported by FY19 Hearst Fellowship. Research in the M.G. lab was supported by the European Research Council under the European Union’s Horizon 2020 Research and Innovation Program (ERC, 819229) and the Swiss National Science Foundation (SNSF, BSSGI0_155832). Research in the T.K. lab was supported by grants from the European Research Council (ERC, 679175) and the Swiss National Science Foundation (SNSF, 31003A_170037).
Author contributions
Conceptualization, L.G. and T.K.; methodology: L.G., A.Ö.A., A.C., E.F., S.U., and M.G.; software, A.Ö.A. and L.G.; formal analysis, L.G., A.Ö.A., A.C., E.F., and L.A.I.; investigation, L.G., A.C., E.F., L.A.I., T.J.S., M.D.G., and S.U.; resources, M.G. and T.K.; data curation, L.G., A.C., and A.Ö.A.; writing – original draft, L.G. and T.K.; writing – review & editing, L.G., A.C., A.Ö.A., E.F., L.A.I., T.J.S., M.G., and T.K.; visualization, L.G., A.C., and E.F.; supervision, T.K.; project administration, L.G.; funding acquisition, M.G. and T.K.
Declaration of interests
The authors declare no competing interests.
Published: August 16, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2022.111209.
Supplemental information
Data and code availability
-
•
This paper analyzes existing, publicly available RNA sequencing (GEO DataSets accession number GSE79812 and GSE120161) and protein-protein interaction data from the STRING database https://version-11-0.string-db.org/cgi/download.pl?sessionId=99uSZMsNLaTe. These accession numbers are also listed in the key resources table. All other data reported in this paper will be shared by the lead contact upon request.
-
•
The original code for putative interaction counting between microglia and SST+ cell processes (Figure 1A) has been deposited at https://doi.org/10.5281/zenodo.6862093. The original code for synaptic bouton counting (Figures 1F and 2E and S1E) has been deposited at https://doi.org/10.5281/zenodo.6861757. The computational pipeline to identify protein-protein interactions between microglia and other neuronal cell types (Figures 2A and 2B) has been deposited at https://doi.org/10.5281/zenodo.6863632. All codes are publicly available. DOIs are also listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Adler A., Zhao R., Shin M.E., Yasuda R., Gan W.B. Somatostatin-Expressing interneurons enable and maintain learning-dependent sequential activation of pyramidal neurons. Neuron. 2019;102:202–216.e7. doi: 10.1016/j.neuron.2019.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoh M., Koyama R. Microglia regulate synaptic development and plasticity. Dev. Neurobiol. 2021;81:568–590. doi: 10.1002/dneu.22814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnò B., Grassivaro F., Rossi C., Bergamaschi A., Castiglioni V., Furlan R., Greter M., Favaro R., Comi G., Becher B., et al. Neural progenitor cells orchestrate microglia migration and positioning into the developing cortex. Nat. Commun. 2014;5:5611. doi: 10.1038/ncomms6611. [DOI] [PubMed] [Google Scholar]
- Bishop C.M. 1st edn. 2006. Pattern Recognition and Machine Learning (Information Science and Statistics) corr. 2nd printing edn. Machine Learning 128. [Google Scholar]
- Borrell V., Marín O. Meninges control tangential migration of hem-derived Cajal-Retzius cells via CXCL12/CXCR4 signaling. Nat. Neurosci. 2006;9:1284–1293. doi: 10.1038/nn1764. [DOI] [PubMed] [Google Scholar]
- van der Bourg A., Yang J.W., Reyes-Puerta V., Laurenczy B., Wieckhorst M., Stüttgen M.C., Luhmann H.J., Helmchen F. Layer-specific refinement of sensory coding in developing mouse barrel cortex. Cereb. Cortex. 2017;27:4835–4850. doi: 10.1093/cercor/bhw280. [DOI] [PubMed] [Google Scholar]
- Cong Q., Soteros B.M., Wollet M., Kim J.H., Sia G.M. The endogenous neuronal complement inhibitor SRPX2 protects against complement-mediated synapse elimination during development. Nat. Neurosci. 2020;23:1067–1078. doi: 10.1038/s41593-020-0672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuisenaire O. Université catholique de Louvain; 1999. Distance Transformations: Fast Algorithms and Applications to Medical Image Processing. [Google Scholar]
- Dagher N.N., Najafi A.R., Kayala K.M.N., Elmore M.R.P., White T.E., Medeiros R., West B.L., Green K.N. Colony-stimulating factor 1 receptor inhibition prevents microglial plaque association and improves cognition in 3xTg-AD mice. J. Neuroinflammation. 2015;12:139. doi: 10.1186/s12974-015-0366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirk-Jan Kroon. 2021. Accurate Fast Marching. MATLAB Central File Exchange. [Google Scholar]
- Favuzzi E., Deogracias R., Marques-Smith A., Maeso P., Jezequel J., Exposito-Alonso D., Balia M., Kroon T., Hinojosa A.J., Maraver E.F., et al. Neurodevelopment: distinct molecular programs regulate synapse specificity in cortical inhibitory circuits. Science. 2019:363. doi: 10.1126/science.aau8977. [DOI] [PubMed] [Google Scholar]
- Favuzzi E., Huang S., Saldi G.A., Binan L., Ibrahim L.A., Fernández-Otero M., Cao Y., Zeine A., Sefah A., Zheng K., et al. GABA-receptive microglia selectively sculpt developing inhibitory circuits. Cell. 2021;184:4048–4063.e32. doi: 10.1016/j.cell.2021.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira T.A., Blackman A.v., Oyrer J., Jayabal S., Chung A.J., Watt A.J., Sjöström P.J., van Meyel D.J. Neuronal morphometry directly from bitmap images. Nat. Methods. 2014;11:982–984. doi: 10.1038/nmeth.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipello F., Morini R., Corradini I., Zerbi V., Canzi A., Michalski B., Erreni M., Markicevic M., Starvaggi-Cucuzza C., Otero K., et al. The microglial innate immune receptor TREM2 is required for synapse elimination and normal brain connectivity. Immunity. 2018;48:979–991.e8. doi: 10.1016/j.immuni.2018.04.016. [DOI] [PubMed] [Google Scholar]
- Fujita M., Davari P., Takada Y.K., Takada Y. Stromal cell-derived factor-1 (CXCL12) activates integrins by direct binding to an allosteric ligand-binding site (site 2) of integrins without CXCR4. Biochem. J. 2018;475:723–732. doi: 10.1042/BCJ20170867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo N.B., Berisha A., van Aelst L. Microglia regulate chandelier cell axo-axonic synaptogenesis. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2114476119. e2114476119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesuita L., Karayannis T. A ‘Marginal’ tale: the development of the neocortical layer 1. Curr. Opin. Neurobiol. 2021;66:37–47. doi: 10.1016/j.conb.2020.09.002. [DOI] [PubMed] [Google Scholar]
- Ginhoux F., Lim S., Hoeffel G., Low D., Huber T. Origin and differentiation of microglia. Front. Cell Neurosci. 2013 doi: 10.3389/fncel.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golshani P., Gonçalves J.T., Khoshkhoo S., Mostany R., Smirnakis S., Portera-Cailliau C. Internally mediated developmental desynchronization of neocortical network activity. J. Neurosci. 2009;29:10890–10899. doi: 10.1523/JNEUROSCI.2012-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunner G., Cheadle L., Johnson K.M., Ayata P., Badimon A., Mondo E., Nagy M.A., Liu L., Bemiller S.M., Kim K.W., et al. Sensory lesioning induces microglial synapse elimination via ADAM10 and fractalkine signaling. Nat. Neurosci. 2019;22:1075–1088. doi: 10.1038/s41593-019-0419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartigan J.A., Wong M.A. Algorithm as 136: a K-means clustering algorithm. Appl. Stat. 1979;28:100. doi: 10.2307/2346830. [DOI] [Google Scholar]
- Hassouna M.S., Farag A.A. Multistencils fast marching methods: a highly accurate solution to the Eikonal equation on Cartesian domains. IEEE Trans. Pattern Anal. Mach. Intell. 2007;29:1563–1574. doi: 10.1109/TPAMI.2007.1154. [DOI] [PubMed] [Google Scholar]
- Hoshiko M., Arnoux I., Avignone E., Yamamoto N., Audinat E. Deficiency of the microglial receptor CX3CR1 impairs postnatal functional development of thalamocortical synapses in the barrel cortex. J. Neurosci. 2012;32:15106–15111. doi: 10.1523/JNEUROSCI.1167-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger B.N., Yángüez E., Gesuita L., Denoth-Lippuner A., Kruse M., Karayannis T., Jessberger S. Miniaturization of smart-seq2 for single-cell and single-nucleus RNA sequencing. STAR Protoc. 2020;1:100081. doi: 10.1016/j.xpro.2020.100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerman T., Pernuš F., Likar B., Špiclin Ž. Beyond Frangi: an improved multiscale vesselness filter. Proc. SPIE. 2015;9413:94132A. doi: 10.1117/12.2081147. [DOI] [Google Scholar]
- Kastli R., Vighagen R., van der Bourg A., Argunsah A.Ö., Iqbal A., Voigt F.F., Kirschenbaum D., Aguzzi A., Helmchen F., Karayannis T. Developmental divergence of sensory stimulus representation in cortical interneurons. Nat. Commun. 2020;11:5729. doi: 10.1038/s41467-020-19427-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y., Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb. Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kim K.W., Vallon-Eberhard A., Zigmond E., Farache J., Shezen E., Shakhar G., Ludwig A., Lira S.A., Jung S. In vivo structure/function and expression analysis of the CX3C chemokine fractalkine. Blood. 2011;118:e156–e167. doi: 10.1182/blood-2011-04-348946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong H., Akakin H.C., Sarma S.E. A generalized laplacian of Gaussian filter for blob detection and its applications. IEEE Trans. Cybern. 2013;43:1719–1733. doi: 10.1109/TSMCB.2012.2228639. [DOI] [PubMed] [Google Scholar]
- de La Rossa A., Jabaudon D. In vivo rapid gene delivery into postmitotic neocortical neurons using iontoporation. Nat. Protoc. 2015;10:25–32. doi: 10.1038/nprot.2015.001. [DOI] [PubMed] [Google Scholar]
- Lehrman E.K., Wilton D.K., Litvina E.Y., Welsh C.A., Chang S.T., Frouin A., Walker A.J., Heller M.D., Umemori H., Chen C., Stevens B. CD47 protects synapses from excess microglia-mediated pruning during development. Neuron. 2018;100:120–134.e6. doi: 10.1016/j.neuron.2018.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Adesnik H., Li J., Long J., Nicoll R.A., Rubenstein J.L.R., Pleasure S.J. Regional distribution of cortical interneurons and development of inhibitory tone are regulated by cxcl12/cxcr4 signaling. J. Neurosci. 2008;28:1085–1098. doi: 10.1523/JNEUROSCI.4602-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Chiou B., Gilman C.K., Luo R., Koshi T., Yu D., Oak H.C., Giera S., Johnson-Venkatesh E., Muthukumar A.K., et al. A splicing isoform of GPR56 mediates microglial synaptic refinement via phosphatidylserine binding. EMBO J. 2020;39:e104136. doi: 10.15252/embj.2019104136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J.S. Prentice Hall; 1990. Two-dimensional Signal and Image Processing. [Google Scholar]
- Lim S.H., Park E., You B., Jung Y., Park A.R., Park S.G., Lee J.R. Neuronal synapse formation induced by microglia and interleukin 10. PLoS ONE. 2013;8:e81218. doi: 10.1371/journal.pone.0081218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L., Mi D., Llorca A., Marín O. Development and functional diversification of cortical interneurons. Neuron. 2018;100:294–313. doi: 10.1016/j.neuron.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L., Pakan J.M.P., Selten M.M., Marques-Smith A., Llorca A., Bae S.E., Rochefort N.L., Marín O. Optimization of interneuron function by direct coupling of cell migration and axonal targeting. Nat. Neurosci. 2018;21:920–931. doi: 10.1038/s41593-018-0162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipfert J., Ödemis V., Wagner D.C., Boltze J., Engele J. CXCR4 and CXCR7 form a functional receptor unit for SDF-1/CXCL12 in primary rodent microglia. Neuropathol. Appl. Neurobiol. 2013;39:667–680. doi: 10.1111/nan.12015. [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito G., Sánchez-Alcañiz J.A., Pla R., Borrell V., Picó E., Valdeolmillos M., Marín O. Chemokine signaling controls intracortical migration and final distribution of GABAergic interneurons. J. Neurosci. 2008;28:1613–1624. doi: 10.1523/JNEUROSCI.4651-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysko D.E., Putt M., Golden J.A. SDF1 reduces interneuron leading process branching through dual regulation of actin and microtubules. J. Neurosci. 2014;34:4941–4962. doi: 10.1523/JNEUROSCI.4351-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddalo D., Manchado E., Concepcion C.P., Bonetti C., Vidigal J.A., Han Y.C., Ogrodowski P., Crippa A., Rekhtman N., de Stanchina E., et al. In vivo engineering of oncogenic chromosomal rearrangements with the CRISPR/Cas9 system. Nature. 2014;516:423–427. doi: 10.1038/nature13902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Smith A., Lyngholm D., Kaufmann A.K., Stacey J.A., Hoerder-Suabedissen A., Becker E.B.E., Wilson M.C., Molnár Z., Butt S.J.B. A transient translaminar GABAergic interneuron circuit connects thalamocortical recipient layers in neonatal somatosensory cortex. Neuron. 2016;89:536–549. doi: 10.1016/j.neuron.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matcovitch-Natan O., Winter D.R., Giladi A., Vargas Aguilar S., Spinrad A., Sarrazin S., Ben-Yehuda H., David E., Zelada González F., Perrin P., et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science. 2016:aad8670. doi: 10.1126/science.aad8670. [DOI] [PubMed] [Google Scholar]
- Matsuda T., Cepko C.L. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc. Natl. Acad. Sci. USA. 2004;101:16–22. doi: 10.1073/pnas.2235688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T., Cepko C.L. Controlled expression of transgenes introduced by in vivo electroporation. Proc. Natl. Acad. Sci. USA. 2007;104:1027–1032. doi: 10.1073/pnas.0610155104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlini M., Rafalski V.A., Ma K., Kim K.Y., Bushong E.A., Rios Coronado P.E., Yan Z., Mendiola A.S., Sozmen E.G., Ryu J.K., et al. Microglial Gi-dependent dynamics regulate brain network hyperexcitability. Nat. Neurosci. 2021;24:19–23. doi: 10.1038/s41593-020-00756-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Dilhet G., Courchet J. Utero cortical electroporation of plasmids in the mouse embryo. STAR Protoc. 2020;1:100027. doi: 10.1016/j.xpro.2020.100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamae T., Chen K., Lewis D.A., Gonzalez-Burgos G. Distinct physiological maturation of parvalbumin-positive neuron subtypes in mouse prefrontal cortex. J. Neurosci. 2017;37:4883–4902. doi: 10.1523/JNEUROSCI.3325-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto A., Wake H., Moorhouse A.J., Nabekura J. Microglia and synapse interactions: fine tuning neural circuits and candidate molecules. Front. Cell. Neurosci. 2013;7:70. doi: 10.3389/fncel.2013.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto A., Wake H., Ishikawa A.W., Eto K., Shibata K., Murakoshi H., Koizumi S., Moorhouse A.J., Yoshimura Y., Nabekura J. Microglia contact induces synapse formation in developing somatosensory cortex. Nat. Commun. 2016;7:12540. doi: 10.1038/ncomms12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G., Butt S.J.B., Takebayashi H., Fishell G. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J. Neurosci. 2007;27:7786–7798. doi: 10.1523/JNEUROSCI.1807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli I., Neumann H. Microglial clearance function in health and disease. Neuroscience. 2009;158:1030–1038. doi: 10.1016/j.neuroscience.2008.06.046. [DOI] [PubMed] [Google Scholar]
- Natan R.G., Rao W., Geffen M.N. Cortical interneurons differentially shape frequency tuning following adaptation. Cell Rep. 2017;21:878–890. doi: 10.1016/j.celrep.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S.W., Harris J.A., Ng L., Winslow B., Cain N., Mihalas S., Wang Q., Lau C., Kuan L., Henry A.M., et al. A mesoscale connectome of the mouse brain. Nature. 2014;508:207–214. doi: 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh W.C., Lutzu S., Castillo P.E., Kwon H.B. De novo synaptogenesis induced by GABA in the developing mouse cortex. Science. 2016:1037–1040. doi: 10.1126/science.aaf5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 1979;9:62–66. doi: 10.1109/TSMC.1979.4310076. [DOI] [Google Scholar]
- Paolicelli R.C., Bolasco G., Pagani F., Maggi L., Scianni M., Panzanelli P., Giustetto M., Ferreira T.A., Guiducci E., Dumas L., et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Pouille F., Scanziani M. Routing of spike series by dynamic circuits in the hippocampus. Nature. 2004;429:717–723. doi: 10.1038/nature02615. [DOI] [PubMed] [Google Scholar]
- Purves D., Lichtman J.W. Elimination of synapses in the developing nervous system. Science. 1980:153–157. doi: 10.1126/science.7414326. [DOI] [PubMed] [Google Scholar]
- Ran F.A., Hsu P.D., Wright J., Agarwala V., Scott D.A., Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimand J., Arak T., Adler P., Kolberg L., Reisberg S., Peterson H., Vilo J. g:Profiler-a web server for functional interpretation of gene lists (2016 update) Nucleic Acids Res. 2016;44:W83–W89. doi: 10.1093/NAR/GKW199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A., Lujan R., Rozov A., Burnashev N., Somogyi P., Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nat. Neurosci. 1998;1:279–285. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- Rogers J.T., Morganti J.M., Bachstetter A.D., Hudson C.E., Peters M.M., Grimmig B.A., Weeber E.J., Bickford P.C., Gemma C. CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J. Neurosci. 2011;31:16241–16250. doi: 10.1523/JNEUROSCI.3667-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahara S., Yanagawa Y., O'Leary D.D.M., Stevens C.F. The fraction of cortical GABAergic neurons is constant from near the start of cortical neurogenesis to adulthood. J. Neurosci. 2012;32:4755–4761. doi: 10.1523/JNEUROSCI.6412-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D.P., Stevens B. Microglia function in central nervous system development and plasticity. Cold Spring Harb. Perspect. Biol. 2015;7:a020545. doi: 10.1101/cshperspect.a020545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D.P., Lehrman E.K., Kautzman A.G., Koyama R., Mardinly A.R., Yamasaki R., Ransohoff R.M., Greenberg M.E., Barres B.A., Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter R.W., Maher E.E., Welsh C.A., Stevens B., Erisir A., Bear M.F. Experience-dependent synaptic plasticity in V1 occurs without microglial CX3CR1. J. Neurosci. 2017;37:10541–10553. doi: 10.1523/JNEUROSCI.2679-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholkopf B., Smola A.J. MIT Press; 2018. Learning with Kernels: Support Vector Machines, Regularization, Optimization, and beyond. [Google Scholar]
- Seay M.J., Natan R.G., Geffen M.N., Buonomano D.v. Differential short-term plasticity of PV and SST neurons accounts for adaptation and facilitation of cortical neurons to auditory tones. J. Neurosci. 2020;40:9224–9235. doi: 10.1523/JNEUROSCI.0686-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin M., Male I., Beane T.J., Villefranc J.A., Kok F.O., Zhu L.J., Lawson N.D. Vol. 143. Development; 2016. Vegfc Acts through ERK to Induce Sprouting and Differentiation of Trunk Lymphatic Progenitors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg G., Markram H. Disynaptic inhibition between neocortical pyramidal cells mediated by martinotti cells. Neuron. 2007;53:735–746. doi: 10.1016/j.neuron.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Sokullu E., Pinsard M., Zhang J., Plathier J., Kolhatkar G., Blum A.S., Légaré F., Ruediger A., Ozaki T., Gauthier M.A. Plasmonic enhancement of two-photon excitation fluorescence by colloidal assemblies of very small AuNPs templated on M13 phage. Biomacromolecules. 2020;21:2705–2713. doi: 10.1021/acs.biomac.0c00401. [DOI] [PubMed] [Google Scholar]
- Southwell D.G., Paredes M.F., Galvao R.P., Jones D.L., Froemke R.C., Sebe J.Y., Alfaro-Cervello C., Tang Y., Garcia-Verdugo J.M., Rubenstein J.L., et al. Intrinsically determined cell death of developing cortical interneurons. Nature. 2012;491:109–113. doi: 10.1038/nature11523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squarzoni P., Oller G., Hoeffel G., Pont-Lezica L., Rostaing P., Low D., Bessis A., Ginhoux F., Garel S. Microglia modulate wiring of the embryonic forebrain. Cell Rep. 2014;8:1271–1279. doi: 10.1016/j.celrep.2014.07.042. [DOI] [PubMed] [Google Scholar]
- Stevens B., Allen N.J., Vazquez L.E., Howell G.R., Christopherson K.S., Nouri N., Micheva K.D., Mehalow A.K., Huberman A.D., Stafford B., et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Szklarczyk D., Gable A.L., Lyon D., Junge A., Wyder S., Huerta-Cepas J., Simonovic M., Doncheva N.T., Morris J.H., Bork P., et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The UniProt Consortium UniProt: the universal protein knowledgebase. Nucleic Acids Res. 2017;45:D158–D169. doi: 10.1093/nar/gkw1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thion M.S., Mosser C.A., Férézou I., Grisel P., Baptista S., Low D., Ginhoux F., Garel S., Audinat E. Biphasic impact of prenatal inflammation and macrophage depletion on the wiring of neocortical inhibitory circuits. Cell Rep. 2019;28:1119–1126.e4. doi: 10.1016/j.celrep.2019.06.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi C., Manduchi R. Proceedings of the IEEE International Conference on Computer Vision. 1998. Bilateral filtering for gray and color images; pp. 839–846. [Google Scholar]
- Tremblay R., Lee S., Rudy B. GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron. 2016;91:260–292. doi: 10.1016/j.neuron.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trousse F., Poluch S., Pierani A., Dutriaux A., Bock H.H., Nagasawa T., Verdier J.-M., Rossel M. CXCR7 receptor controls the maintenance of subpial positioning of cajal–retzius cells. Cereb. Cortex. 2015;25:3446–3457. doi: 10.1093/cercor/bhu164. [DOI] [PubMed] [Google Scholar]
- Tuncdemir S.N., Wamsley B., Stam F.J., Osakada F., Goulding M., Callaway E.M., Rudy B., Fishell G. Early Somatostatin interneuron connectivity mediates the maturation of deep layer cortical circuits. Neuron. 2016;89:521–535. doi: 10.1016/j.neuron.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P.R., Cho K.K.A., Vogt D., Sohal V.S., Rubenstein J.L.R. The cytokine CXCL12 promotes basket interneuron inhibitory synapses in the medial prefrontal cortex. Cereb. Cortex. 2017;27:4303–4313. doi: 10.1093/cercor/bhw230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.W., Prouvot P.H., Reyes-Puerta V., Stüttgen M.C., Stroh A., Luhmann H.J. Optogenetic modulation of a minor fraction of parvalbumin-positive interneurons specifically affects spatiotemporal dynamics of spontaneous and sensory-evoked activity in mouse somatosensory cortex in vivo. Cereb. Cortex. 2017;27:5784–5803. doi: 10.1093/cercor/bhx261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y., Paolicelli R.C., Sforazzini F., Weinhard L., Bolasco G., Pagani F., Vyssotski A.L., Bifone A., Gozzi A., Ragozzino D., Gross C.T. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 2014;17:400–406. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- Zhao X., Eyo U.B., Murugan M., Wu L.J. Microglial interactions with the neurovascular system in physiology and pathology. Dev. Neurobiol. 2018;78:604–617. doi: 10.1002/dneu.22576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
This paper analyzes existing, publicly available RNA sequencing (GEO DataSets accession number GSE79812 and GSE120161) and protein-protein interaction data from the STRING database https://version-11-0.string-db.org/cgi/download.pl?sessionId=99uSZMsNLaTe. These accession numbers are also listed in the key resources table. All other data reported in this paper will be shared by the lead contact upon request.
-
•
The original code for putative interaction counting between microglia and SST+ cell processes (Figure 1A) has been deposited at https://doi.org/10.5281/zenodo.6862093. The original code for synaptic bouton counting (Figures 1F and 2E and S1E) has been deposited at https://doi.org/10.5281/zenodo.6861757. The computational pipeline to identify protein-protein interactions between microglia and other neuronal cell types (Figures 2A and 2B) has been deposited at https://doi.org/10.5281/zenodo.6863632. All codes are publicly available. DOIs are also listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.